Abstract
Chemical reactions of N-(4-acetylphenyl)-N-(diphenylphosphino)amine ligand (1) with three group 6B metal hexacarbonyls produced cis-M(CO)4[1-k2P,P][ M = Cr(2), Mo(3), W(4)], respectively. The novel complexes 2–4 were isolated and their structures were elucidated by multinuclear NMR spectroscopy (1H, 13C, 31P NMR) and elemental analysis. Crystal-structure determination using single-crystal X-ray diffraction was carried out on complex 2.
Keywords: Tetracarbonyl; Hexacarbonyl; Group 6B; Chlorodiphenylphosphine; P,P-bidentate
Tetracarbonyl; Hexacarbonyl; Group 6B; Chlorodiphenylphosphine; P,P-bidentate.
1. Introduction
The ongoing significance and increasing relevance of aminophosphine RNH(PR2) and bis(phosphino)amine RN(PR2)2 ligands incorporating direct P–N bonds and their analogues in catalysis and coordination chemistry has been the subject of several review articles [1, 2, 3, 4, 5, 6, 7, 8]. The synthesis of polydentate aminophosphine ligands bearing more than one N(PR2)2 unit and their applications have also been reported [9]. Considering bis(phosphino)amines with P–N–P skeletons, coordination occurs mainly through the two phosphorus atoms in k2P,P-bidentate mode due to the low basicity of the amine nitrogen caused by the π interaction in the P–N unit [10]. Several bis(phosphino)amine ligands as well as their complexes have been studied in a wide range of catalytic processes [1], particularly in ethylene oligo/polymerization [11] and C–C cross coupling reactions [12]. These ligands are also used as assembling ligands for the formation of multinuclear complexes, due to the multiple donor atoms [13], and some of them and their derivatives have also found applications in surface and materials sciences [14, 15]. Moreover, the chalcogenide forms of this type of ligands are also becoming increasingly important in view of their potential applications in catalysis [16].
In continuation of our work in this research area [17] and to contribute to the interest and need of others [18] in designing aminophosphines and phosphorus based ligands for transition metal chemistry and catalytic application, herein we report the chemical synthesis and spectroscopic properties of the cis-chelate complexes M(CO)4[1-k2P,P][ M = Cr(2), Mo(3), W(4)] as well as the crystal structure of 2.
2. Results and discussion
2.1. Chemical synthesis of 2-4
Reaction of equimolar amounts of N-(4-acetylphenyl)-N-(diphenylphosphino)amine ligand [(p-CH3CO)C6H4N(PPh2)2] (1) (see 31P NMR spectrum of 1-(4-(bis(diphenylphosphaneyl)amino)phenyl)ethan-1-one (1) (Fig. S3)) [19], prepared previously from 4-acetylaniline and chlorodiphenylphosphine, with group-6B metal hexacarbonyls in refluxing toluene gave the cis-chelate complexes M(CO)4[1-k2P,P][ M = Cr(2), Mo(3), W(4)], respectively (Figure 1). The new compounds 2–4 were isolated and structurally elucidated by standard spectroscopic (IR and multinuclear NMR spectroscopy: 1H, 13C, and 31P NMR) and analytical tools (elemental analysis). The molecular structure of complex 2 has been established crystallographically using single-crystal X-ray diffraction.
Figure 1.
Preparation of the cis-chelate complexes M(CO)4[1-k2P,P] [M = Cr(2), Mo(3), W(4)].
2.2. Spectroscopic properties
2.2.1. 1H, 13C, 31P NMR, and IR spectra
In the 1H NMR spectrum, the acyl group methyl protons comprise the most distinctive signal, appearing as a singlet resonating at δ 2.41 ppm (2) (see 1H NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]chromium(0) (2) (Fig. S4)), 2.40 ppm (3) (see 1H NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] molybdenum (0) (3) (Fig. S5)), and 2.50 ppm (4) (see 1H NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] tungsten (0) (4) (Fig. S6)). All these signals fall at higher field than those of precursor 1 (2.38 ppm). The 1H NMR signals of the aromatic rings of 2-4 fall in the expected chemical shift range (δ 6.5–7.8 ppm).
The most diagnostic signals in the 13C NMR spectrum are those related to the acyl group (CH3 and C=O carbon atoms). These appear as singlets at δ 26.3/196.6 (2) (see 13C NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]chromium(0) (2) (Fig. S7)), 25.8/195.0 (3) (see 13C NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] molybdenum (0) (3) (Fig. S8)), and 26.0/195.2 (4) ppm (see 13C NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] tungsten (0) (4) (Fig. S9)). The 13C NMR spectra of complexes 2–4 showed two non-equivalent signals for the C≡O ligands in agreement with trans- and cis-C≡O groups oriented to the P-atoms (see Expanded 13C NMR spectrum (CO region) of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]chromium(0) (2) (Fig. S10) and Expanded 13C NMR spectrum (CO region) NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] molybdenum (0) (3) (Fig. S11)). Also, the 13C chemical shifts decreased in the order Cr > Mo > W, in parallel with the increasing number of electrons around the metal [17d, 17f].
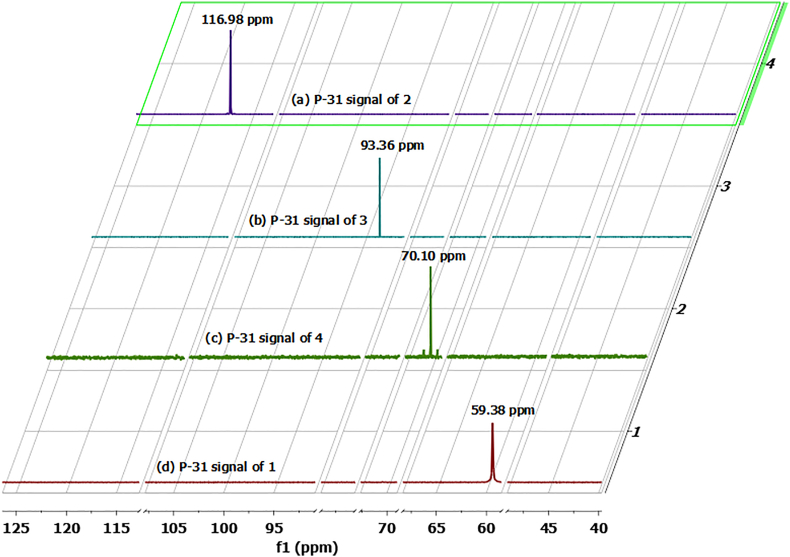
The 31P NMR spectra (Figure 2) show one signal at 116.98 ppm (2) (Figure 2a) (also see 31P NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]chromium(0) (2) (Fig. S12)), 93.36 ppm (3) (Figure 2b)) (also see 31P NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] molybdenum (0) (3) (Fig. S13)), and 70.10 ppm (4) (Figure 2c) (also see 31P NMR spectrum of cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP] tungsten (0) (4) (Fig. S14)), i.e. shifted downfield relative to the parent organic ligand 1(59.38 ppm) (Figure 2d) and fall within the same chemical shift range reported for related species described in the literature [1, 17d, 17e, 17f]. The 31P NMR signal of 4 (Figure 2c) is flanked by two 185W-satellites with 1JW–P coupling constant of 207 Hz, which is in good agreement with structurally-related tungsten complexes [17f].
Figure 2.
Expanded and truncated 31P NMR stack spectra (161.98 MHz) for the phosphorus atom resonances for ligand 1 and the corresponding complexes 2–4. (a) 31P NMR spectrum for complex 2 (116.98 ppm); (b) 31P NMR spectrum for complex 3 (93.36 ppm); (c) 31P NMR spectrum for complex 4 {(70.10 ppm (1JW–P = 207.0 Hz)}; (d) 31P NMR spectrum for parent ligand 1 (59.38 ppm).
The IR in the carbonyl region of complexes 2–4 exhibit υ(C≡O) bands in the range of 1845–2018 cm−1 typical for cis-[M(CO)4L2] [17d, 20].
2.3. Molecular structure of 2
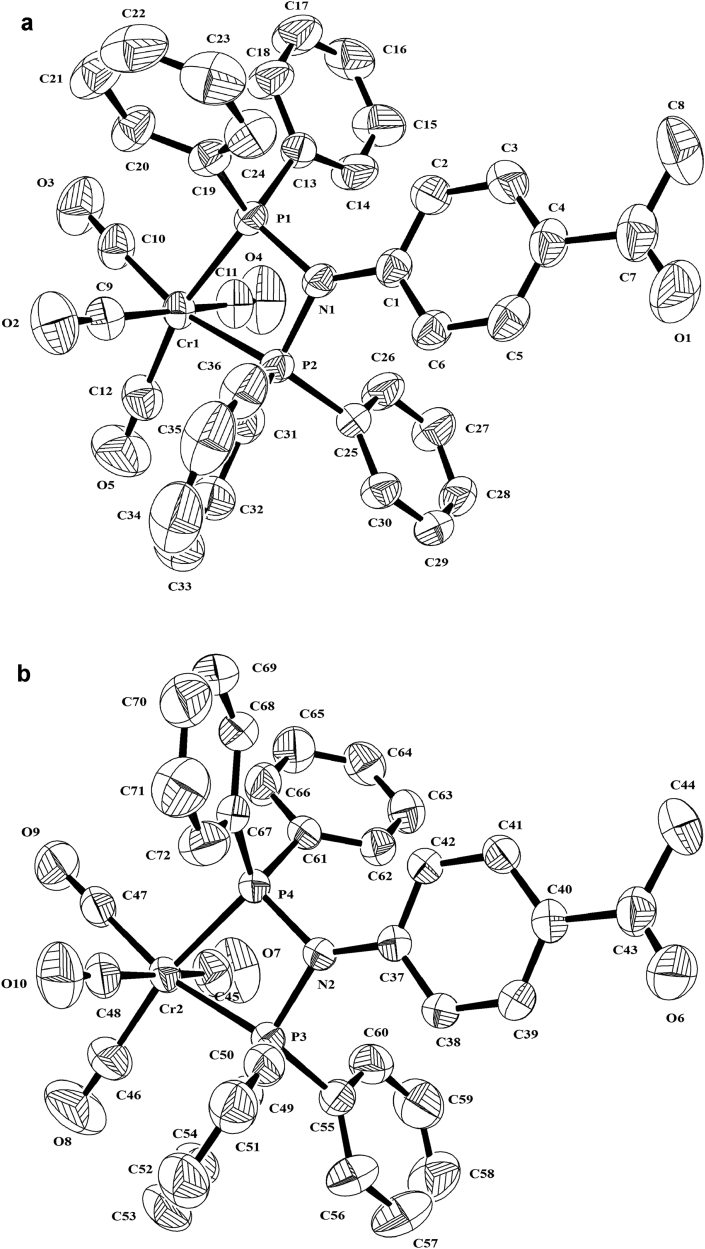
Crystals of 2 were obtained as described in the experimental section. Compound 2 crystallizes in the monoclinic space group P21/n. The molecular structure is depicted in Figure 3.
Figure 3.
Molecular structure of the two independent molecules 2a (above) and 2b (down) (hydrogen atoms are omitted for clarity). Selected bond distances [Å] and angles [°] 2a: Cr(1)–C(10) 1.846(3); Cr(1)–C(12) 1.861(3); Cr(1)–C(11) 1.865(3); Cr(1)–C(9) 1.890(3); Cr(1)–C(9) 1.890(3); Cr(1)–P(1) 2.3220(10); Cr(1)–P(2) 2.3270(8); P(1)–N(1) 1.7306(18); P(1)–C(19) 1.821(3); P(1)–C(13) 1.830(2); P(1)–P(2) 2.6249(9); P(2)–N(1) 1.7245(19); P(2)–C(31) 1.823(2); P(2)–C(25)1.830(2); C(10)-Cr(1)-P(1) 96.50(10); C(12)-Cr(1)-P(1) 164.59(10); C(11)-Cr(1)-P(1) 93.59(10); C(9)-Cr(1)-P(1) 92.93(8); C(10)-Cr(1)-P(2) 165.23(10); C(12)-Cr(1)-P(2) 95.87(10);C(11)-Cr(1)-P(2) 95.07(9); C(9)-Cr(1)-P(2) 92.37(8); P(1)-Cr(1)-P(2) 68.75(3); P(2)-N(1)-P(1) 98.88(9); N(1)-P(1)-C(19) 107.83(11); N(1)-P(1)-C(13) 109.63(10); N(1)-P(1)-Cr(1) 96.01(7); C(19)-P(1)-C(13)103.08(11); N(1)-P(2)-C(31) 108.65(11); N(1)-P(2)-C(25) 105.46(10); C(31)-P(2)-C(25) 105.55(11); N(1)-P(2)-Cr(1) 96.00(7). Selected bond distances [Å] and angles [°] 2b: Cr(2)–C(47) 1.840(3); Cr(2)–C(46) 1.866(3); Cr(2)–C(45) 1.876(3); Cr(2)–C(48) 1.895(3); Cr(2)–P(4) 2.3183(8); Cr(2)–P(3) 2.3376(7); P(3)–N(2) 1.7332(19); P(3)–C(49) 1.824(3); P(3)–C(55) 1.829(3); P(3)–P(4) 2.6364(11); P(4)–N(2) 1.7280(18); P(4)–C(61) 1.822(2); P(4)–C(67) 1.829(3); C(47)-Cr(2)-P(4) 97.92(9); C(46)-Cr(2)-P(4) 168.42(10); C(45)-Cr(2)-P(4) 90.92(9); C(48)-Cr(2)-P(4) 93.39(9); C(47)-Cr(2)-P(3) 166.82(9); C(46)-Cr(2)-P(3) 99.49(10); C(45)-Cr(2)-P(3) 94.57(9); C(48)-Cr(2)-P(3) 91.18(8); P(4)-Cr(2)-P(3) 68.98(3); N(2)-P(3)-C(49) 107.41(11); N(2)-P(3)-C(55) 104.93(10); C(49)-P(3)-C(55) 106.35(12); N(2)-P(3)-Cr(2) 95.48(6); N(2)-P(4)-C(61) 107.76(10); N(2)-P(4)-C(67) 105.59(10); C(61)-P(4)-C(67) 105.59(11); N(2)-P(4)-Cr(2) 96.31(7); P(4)-N(2)-P(3) 99.23(9).
The X-ray structure of 2 contains two crystallographically independent molecules, 2a and 2b (Figure 3), in the asymmetric unit. These differ in the orientation of the phenyl groups. The chromium(0) atom is six coordinated via four terminal carbon monoxide ligands and two phosphorus centers, forming an octahedral geometry. The P,P-chelating behavior of ligand 1 to the Cr-metal results in the formation of four-membered ring metallacycle, i.e., P–Mo–P–N (Figure 4), with smaller P–Cr–P [2a: 68.75(3; 2b: 68.98(3)°] bite angle and larger P–N–P [2a: 98.88(9); 2b: 99.23(9)°] bond angle.
Figure 4.
Core center of 2a.
A comparison of the structural data of the P–Cr–P and P–N–P bond angles in 2 with the four available literature structural data on analogous cis-chelated tetracarbonylchromium(0) cis-[Cr(CO)4{C10H7-1-N(PPh2)2}] (5) [21], cis-[Cr(CO)4{((o-MeOC6H4)2P)2NCH3}] (6) [22], cis-[Cr(CO)4{(Ph2P)2NiPr}] (7) [23], and cis-[Cr(CO)4{Ph2P)2NH}] (8) [24a,b], showed that the P–Cr–P bite angle in 2 [2a: 68.75(3); 2b: 68.98(3)°] is slightly larger than those in 5 [67.54(2)°], 6 [67.54(2)°], 7 [67.82(4)°], 8 [68.58(2)°], and significantly lower than the ideal 90° in a regular square-planar geometry. The P–N–P bond angle in 2 [2a: 98.88(9); 2b: 99.23(9)°] is smaller than those in 5 [ 100.76(8)°], 6 [101.24(7)°], 8 [103.24(9)°], and slightly similar to these in 7 [99.86(11)°]. The average P–N [av. 1.7291 Å] bond distance in 2 is slightly larger than those in 5 [av. 1.721 Å], 6 [av. 1.699 Å], 7 [av. 1.713 Å], 8 [av. 1.692 Å], and slightly shorter than those in the free diphosphinoamine ligands [24, 25] which clearly indicate an enhancement of π-character in the P–N unit. The average Cr–P [av. 2.326 Å] bond distance in 2 is smaller than the corresponding bond lengths found in 5 [av 2.347 Å], 6 [av. 2.364 Å], 7 [av. 2.350 Å], and 8 [av. 2.354 Å]. It is worth mentioning that the average Cr–C bond distance for C≡O groups trans to the phosphines is 1.853 Å, whereas those cis to phosphines the distance is 1.882 Å. This bond lengthening results from the differing strength of the metal-to-ligand π bonding [21]. The aromatic rings in 2 as expected have usual bond lengths and angles.
3. Experimental section
3.1. General Remarks
All experimental manipulations were performed under purified dry nitrogen using standard Schlenk and vacuum line techniques. Solvents were dried and freshly distilled under an atmosphere of nitrogen prior to use [26]. The chemicals Mo(CO)6, W(CO)6, Cr(CO)6, chlorodiphenylphosphine, and p-aminoacetophenone were purchased from Aldrich and used as received. N-(4-acetylphenyl)-N-(diphenylphosphino)amine ligand (1) was previously prepared [19]. Infra-red spectra were recorded with a PerkinElmer System 2000 FT-IR spectrometer between 4000 and 400 cm−1 using KBr disks. Microanalyses were performed on a Flash 2000 elemental analyzer. Infra-red spectra were recorded on a Shimadzu FTIR-8400S spectrometer between 4000-400 cm−1 using KBr disks. The NMR spectra were recorded at 25 °C on a Bruker-Avance-DRX-400 MHz NMR spectrometer operating at 400.17 (1H), 100.63 (13C), and 161.98 (31P) using tetramethylsilane for 1H and 85% H3PO4 for 31P NMR as external standards. Melting points were carried out on a Gallenkamp apparatus with open capillaries.
3.2. Preparation of 2-4
3.2.1. cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]chromium(0) (2)
A solution of N-(4-acetylphenyl)-N-(diphenylphosphino)amine ligand (1) (0.70 g, 1.39 mmol) and Cr(CO)6 (0.31 g, 1.39 mmol) was refluxed in 50 mL toluene for 30 h. The solution was reduced under vacuum to 10 mL and the yellow product precipitated by adding 20 mL n-hexane. Crystals were obtained from a mixture of CH2Cl2 ∖ n-hexane at – 4 °C in 75 % yield. Mp 217–220 °C. 1H NMR (400.17 MHz, CDCl3, δ/ppm): 2.41 (s, 3H, CH3), 6.66 (d, J = 8.8 Hz, 2H), 6.65–7.63 (m, 22H, C6H4 and 4C6H5); 13C NMR (100.62 MHz, CDCl3, δ/ppm): 26.3 (CH3), 121.5 (t, JP–C = 3.5 Hz), 128.9 (t, JP–C = 5.0 Hz), 129.1 (s), 130.9 (t, JP–C = 6.5 Hz), 132.1 (s), 135.3 (s) 135.5 (d, JP–C = 18.1 Hz), 145.7 (t, JP–C = 7.0 Hz) (C6H4 and 4C6H5), 196.6 (C=O), 220.9 (t, cis-C≡O, 2JP–C = 12.1 Hz) and 227.8 (t, trans-C≡O, 2JP–C = 9.1 Hz). 31P NMR (161.97 MHz, CDCl3, δ/ppm): 116.98 (s, 2P). Selected IR (KBr, cm−1): υ (C≡O) = 1845, υ (C≡O) = 1903, υ (C≡O) = 2013, υ (C=O) = 1693. Anal. Calcd. For C36H27NO5P2Cr: C 64.77; H 4.08; N 2.10%. Found: C 64.78; H 4.10; N 2.12 %.
3.2.2. cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]molybdenum(0) (3)
A similar procedure to that described for 2 was used here, except Mo(CO)6 (0.34 g, 1.39 mmol) was employed instead of Cr(CO)6 and the yellow product are obtained in 70% yield. Mp 132−134 °C. 1H NMR (400.13 MHz, CDCl3, δ/ppm): 2.40 (s, 3H, CH3), 6.68 (d, J = 8.8 Hz, 2H), 6.68–7.80 (m, 22H, C6H4 and 4C6H5). 13C NMR (100.62 MHz, CDCl3, δ/ppm): 25.8 (CH3), 112.6 (s), 125.0 (s), 127.3 (t, JP–C = 5.5Hz), 127.6(d, JP–C = 11.1Hz), 129.8 (s), 130.5 (s), 132.1 (t, JP–C = 3.0Hz), 153.2 (s) (C6H4 and 4C6H5), 195.0 (C=O), 207.3 (d, cis-C≡O, 2JP–C = 7.1 Hz) and 210.0 (d, trans-C≡O, 2JP–C = 10.1 Hz). 31P NMR (161.97 MHz, CDCl3, δ/ppm): 93.36 (s, 2P). Selected IR (KBr, cm−1): υ (C≡O) = 1850, υ (C≡O) = 1909, υ (C≡O) = 2018, υ (C=O) = 1692. Anal. calcd. for C36H27NO5P2Mo: C 60.77; H 3.82; N 1.97%. Found: C 60.78; H 3.80; N 1.99 %.
3.2.3. cis-Tetracarbonyl[N-(4-acetylphenyl)-N-(diphenylphosphino-kP)-P,P-diphenylphosphinous amide-kP]tungsten(0) (4)
A similar procedure to that described for 2 was used here, except W(CO)6 (0.49 g, 1.39 mmol) was employed instead of Cr(CO)6 and the yellow product are obtained in 65% yield. Mp 162–163 °C. 1H NMR (400.17 MHz, CDCl3, δ/ppm): 2.50 (s, 3H, CH3) and 6.56–7.76 (m, 24H, C6H4 and 4C6H5).13CNMR (100.62 MHz, CDCl3, δ/ppm): 26.0 (CH3), 114.2 (s), 127.4 (s), 130.0 (s), 130.4 (s), 132.2 (s), 138.5 (s), 139.2 (s), 150.9 (s) (C6H4 and 4C6H5), 195.2 (C=O), 204.0 (cis-C≡O) and 206.1 (trans-C≡O). 31P NMR (161.97 MHz, CDCl3, δ/ppm): 70.10 (s, 2P, 1JW–P = 207.1 Hz). Selected IR (KBr, cm−1): υ (C≡O) = 1860, υ (C≡O) = 1903, υ (C≡O) = 2015, υ (C=O) = 1695. Anal. calcd. for C36H27NO5P2W: C 54.09; H 3.40; N 1.75%. Found: C 54.10; H 3.42; N 1.74 %.
3.3. Data collection and structure determination
Crystallographic data are given in Table 1. Single-crystal X-ray diffraction data were collected using an Oxford Diffraction Supernova dual-source diffractometer equipped with a 135 mm Atlas CCD area detector. Crystals were selected under Paratone-N oil, mounted on micromount loops and quench-cooled using an Oxford Cryosystems open flow N2 cooling device [27]. Data were collected at 150 K using mirror monochromated CuKα radiation (λ = 1.5418 Å) and processed using the CrysAlisPro package, including unit cell parameter refinement and inter-frame scaling (which was carried out using SCALE3 ABSPACK within CrysAlisPro) [28]. Equivalent reflections were merged and diffraction patterns processed with the CrysAlisPro. The structure was subsequently solved using direct methods and refined on F2 using the SHELXL 97-2 package [29]. All non-hydrogen atoms were refined with anisotropic displacement parameters. All H-atoms bonded to carbon atoms were placed in geometrically optimized positions and refined with an isotropic displacement parameter relative to the attached atoms. CCDC- 1979888 contains the supplementary crystallographic data (excluding structure factors) for the structure of 2. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif.
Table 1.
Crystal data and structure refinements of 2.
| 2 | |
|---|---|
| Formula | C72H54Cr2N2O10P4 |
| Mr | 1335.05 |
| Temp [K] | 150(2) |
| Crystal system | monoclinic |
| Space group | P 21/n |
| a [Å] | 13.622(5) |
| b [Å] | 13.060(5) |
| c [Å] | 39.153(5) |
| α [°] | 90 |
| β [°] | 91.511(5) |
| γ [°] | 90 |
| V [Å3] | 6963(4) |
| Z | 4 |
| ρcalcd (Mg m−3) | 1.274 |
| F(000) | 2752 |
| Abs coeff (mm−1) | 3.907 |
| No. of rflns coll. | 49675 |
| No. of indep rflns | 13508 |
| Rint | 0.0318 |
| No. of parameters | 813 |
| R1 (I > 2σ(I)) | 0.0390 |
| wR2 (all data) | 0.1115 |
| (Δ/ρ)max [e.Å−3] | 0.276 |
| (Δ/ρ)min [e.Å−3] | -0.268 |
4. Conclusion
We have shown the successful synthesis of the cis-chelate complexes M(CO)4[1-k2P,P][ M = Cr(2), Mo(3), W(4)] and the crystal structure of 2. The crystallographic study reveals that chromium(0) atom is six coordinated via four terminal carbon monoxide ligands and two phosphorus centers, forming an octahedral geometry. The two Cr–C bonds mutually trans are longer than those trans to Cr–P bonds due to the various strengths of the metal-to-ligand -bonding. The above types of complexes are scant in the literature and will be further investigated for their biological properties and synthetic usefulness and will serve as a steppingstone for further development in the field.
Declarations
Author contribution statement
Harbi T. Al-Masri; Ziad Moussa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by United Arab Emirates University (Grant no. G00003291/Fund no.31S401-12S040/Project 852).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Dr. Sheikh Mobin for collecting the X-ray data. The X-ray of compound 2 is provided by Sophisticated Instrument Center (SIC), India.
Contributor Information
Harbi Tomah Al-Masri, Email: harbialmasri@aabu.edu.jo.
Ziad Moussa, Email: zmoussa@uaeu.ac.ae.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Fliedel C., Ghisolfi A., Braunstein P. Functional short-bite ligands: synthesis, coordination chemistry, and applications of N-functionalized bis(diaryl/dialkylphosphino)amine-type ligands. Chem. Rev. 2016;116:9237–9304. doi: 10.1021/acs.chemrev.6b00153. [DOI] [PubMed] [Google Scholar]
- 2.Meijboom R., Bowen R.J., Berners-Price S.J. Coordination complexes of silver(I) with tertiary phosphine and related ligands. Coord. Chem. Rev. 2009;253:325–342. [Google Scholar]
- 3.Fei Z., Dyson P.J. The chemistry of phosphinoamides and related compounds. Coord. Chem. Rev. 2005;249 2056-2047. [Google Scholar]
- 4.Agbossou F.N., Suisse I. Chiral aminophosphine phosphinite ligands and related auxiliaries: recent advances in their design, coordination chemistry, and use in enantioselective catalysis. Coord. Chem. Rev. 2003;242:145–158. [Google Scholar]
- 5.Appleby T., Woollins J.D. Inorganic backbone phosphines. Coord. Chem. Rev. 2002;235:121–140. [Google Scholar]
- 6.Ly T.Q., Woollins J.D. Bidentate organophosphorus ligands formed via P–N bond formation: synthesis and coordination chemistry. Coord. Chem. Rev. 1998;176:451–481. [Google Scholar]
- 7.Agbossou F., Carpentier J.F., Hapiot F. The aminophosphine-phosphinites and related ligands: synthesis, coordination chemistry and enantioselective catalysis. Coord. Chem. Rev. 1998;178–180:1615–1645. [Google Scholar]
- 8.Witt M., Roesky H.W. Transition and main group metals in cyclic phosphazanes and phosphazenes. Chem. Rev. 1994;94:1163–1181. [Google Scholar]
- 9.Aydemir M., Baysal A., Gümgüm B. Synthesis and characterization of tris{2-(N,N-bis(diphenylphosphino) aminoethyl} amine derivatives: application of a palladium(II) complex as a pre-catalyst in the Heck and Suzuki cross-coupling reactions. J. Organomet. Chem. 2003;693:3810–3814. [Google Scholar]
- 10.Necas M., Foreman M.R.J., Marek J., Woollins J.D., Novosad J. New mixed-donor unsymmetrical P–N–P ligands and their palladium(II) complexes. New J. Chem. 2001;25:1256–1263. [Google Scholar]
- 11.Britovsek G.J.P., McGuinness D.S., Wierenga T.S., Young C.T. Single- and Double-coordination mechanism in ethylene tri- and tetramerization with Cr/PNP Catalysts. ACS Catal. 2015;5:4152–4166. [Google Scholar]
- 12.Ghisolfi A., Fliedel C., Rosa V., Monakhov K. Yu., Braunstein P. Combined experimental and theoretical study of bis(diphenylphosphino)(N-thioether)amine-type ligands in nickel(II) complexes for catalytic ethylene oligomerization. Organometallics. 2014;33:2523–2534. [Google Scholar]
- 13.Ghisolfi A., Fliedel C., de Frémont P., Braunstein P. Mono- and polynuclear Ag(i) complexes of N-functionalized bis(diphenylphosphino)amine DPPA-type ligands: synthesis, solid-state structures and reactivity. Dalton Trans. 2017;46:5571–5586. doi: 10.1039/c6dt04755f. [DOI] [PubMed] [Google Scholar]
- 14.Fliedel C., Rosa V., Falceto A., Rosa P., Alvarez S., Braunstein P. Unsymmetrical chelation of N-thioether-functionalized bis(diphenylphosphino)amine-type ligands and substituent effects on the nuclearity of iron(II) complexes: structures, magnetism, and bonding. Inorg. Chem. 2016;55:4183–4198. doi: 10.1021/acs.inorgchem.6b02585. [DOI] [PubMed] [Google Scholar]
- 15.Fliedel C., Faramarzi V., Rosa V., Doudin B., Braunstein P. Janus microspheres for visual assessment of molecular interconnects. Chem. Eur. J. 2014;20:1263–1266. doi: 10.1002/chem.201303626. [DOI] [PubMed] [Google Scholar]
- 16.Fliedel C., Poli R. Coordination chemistry of neutral mono-oxide, sulfide and selenide bis(diphenylphosphino)amine (DPPA)-based ligands and their N-substituted/functionalized derivatives. Coord. Chem. Rev. 2018;355:1–26. [Google Scholar]
- 17.a) Al-Masri H.T., Emwas A.H., Al-Talla Z.A., Alkordi M.H. Synthesis and characterization of new N-(diphenylphosphino)naphthylamine chalcogenides: X-ray structures of C10H7-1-HNP(Se)Ph2 and Ph2P(S)OP(S)Ph2phosphorus, Sulfur,Silicon relat. Elements. 2012;187:1082–1090. [Google Scholar]; b) Al-Masri H.T. Synthesis and characterization of new N,N-bis(diphenylphosphino)naphthylamine chalcogenides: X-ray structure of C10H7-1 N{P(S)Ph2}2. Synth. React. Inorg. Met. Org. Chem. 2013;43:102–106. [Google Scholar]; c) Al-Masri H.T. Synthesis, characterization, and structures of palladium(II) and platinum(II) complexes containing N,N-bis (diphenylphosphanyl) naphthylamine. Z. Anorg. Allg. Chem. 2012;638:1012–1017. [Google Scholar]; d) Al-Masri H.T., Mohamed M., Moussa Z., Alkordi M.H. Synthesis and characterization of carbonyl group-6-Metal derivatives with Ligand N,N-bis(diphenylphosphino) naphthalen-1-amine (=N-(diphenylphosphino)-N-naphthalen-1-yl-P,P-diphenylphosphinous amid). Molecular structure of cis-tetracarbonyl[N-(diphenylphosphino-kP)-N-naphthalen-1-yl-P, P-diphenylphosphinous amid-kP]molybdenum(cis-[Mo(CO)4{C10H7-1-N(PPh2)2}]) Helv. Chim. Acta. 2013;96:738–746. [Google Scholar]; e) Al-Masri H.T., Moussa Z. Synthesis and Spectroscopic Properties of PdII and PtII complexes with monosulfide and monoselenide bis(phosphanyl) amine ligands. Z. Anorg. Allg. Chem. 2016;642:914–920. [Google Scholar]; f) Al-Masri H.T., Mohamed B.M., Moussa Z., Fazal A., Fettouhi M. Molybdenum(0) and tungsten(0) complexes with P,S and P,Se- monooxidized bis(phosphanyl) amine ligands. Z. Anorg. Allg. Chem. 2014;640:469–475. [Google Scholar]; g) Al-Masri H.T., Almejled A.A. Synthesis, X-ray structures, and photoluminescence of the octahedral Cu4I4 Cluster with bulky bidentate bis(phosphanyl) amine ligand. Z. Anorg. Allg. Chem. 2020;646:354–358. [Google Scholar]
- 18.Al-Masri H.T., Sieler J., Lönnecke P., Junk P.C., Hey-Hawkins E. Synthesis, characterization and crystal of novel intramolecularly base-stabilized borane derivatives with six- and seven-membered chelate rings. Inorg. Chem. 2004;43:7162–7169. doi: 10.1021/ic049352n. and references therein. [DOI] [PubMed] [Google Scholar]
- 19.Al-Masri H.T., Moussa Z., Al Masaeid N.M. Synthesis and characterizations of N-(4-acetylphenyl)-N-(diphenylphosphino)-P,P-diphenylphosphinous amide derivatives: application of Pd(ΙΙ) derivative as pre-catalyst in Suzuki cross-coupling reaction. J. Struct. Chem. 2020;61:1837–1846. [Google Scholar]
- 20.Balakrishna M.S., Prakasha T.K., Krishnamurthy S.S., Siriwardane U., Hosmane N.S. Organometallic derivatives of diphosphinoamines, X2PN(R)PX2. Reactions with carbonyl derivatives of group 6 metals and iron pentacarbonyl. The crystal structures of [Mo(CO)4PhN(P(OPh)2)2] and [W(CO)4iPrN(PPh2)2] J. Organomet. Chem. 1990;390:203–2016. [Google Scholar]
- 21.Al-Masri H.T. Synthesis and molecular structure of cis-tetracarbonyl[N-(diphenylphosphino-kP)-naphthalen-1-yl-P,P-diphenylphosphinous amide-kP]chromium(0) J. Crystallog. 2014;2014:1–4. [Google Scholar]
- 22.Knorr M., Strohmann C. Syntheses, structures, and reactivity of dinuclear molybdenum-platinum and tungstenplatinum complexes with bridging carbonyl, sulfur dioxide, isonitrile, and aminocarbyne ligands and a dppa backbone (dppa = Ph2PNHPPh2) Organometallics. 1999;18:248–257. [Google Scholar]
- 23.Agapie T., Day M.W., Henling L.M., Labinger J.A., Bercaw J.E. A chromium-diphosphine system for catalytic ethylene trimerization: synthetic and structural studies of chromium complexes with a nitrogen-bridged diphosphine ligand with ortho-methoxyaryl substituents. Organometallics. 2006;25:2733–2742. [Google Scholar]
- 24.a) Balakrishna M.S., George P.P., Mague J.T. Synthesis and derivatization, structures and transition metal (Mo(0), Fe(II), Pd(II) and Pt(II)) complexes of phenylaminobis-(diphosphonite), PhN{P(OC6H4OMe-o)2}2. J. Organomet. Chem. 2004;689:3388–3394. [Google Scholar]; b) Vladimir K., Peter W.R. Dinuclear potassium-chromium and potassium-tungsten carbonyl complexes. Eur. J. Inorg. Chem. 2004;5:1045–1050. [Google Scholar]
- 25.Biricik N., Kayan C., Gümgüm B., Fei Z., Scopelliti R., Dyson P.J., Gurbuz N., Özdemir I. Synthesis and characterization of ether-derivatized aminophosphines and their application in C-C coupling reactions. Inorg. Chim. Acta. 2010;363:1039–1047. [Google Scholar]
- 26.Perrin D.D., Armarego W.L.F. third ed. Pergamon; New York: 1988. Purification of Laboratory Chemicals. [Google Scholar]
- 27.Cosier J., Glazer A.M. A nitrogen-gas-stream cryostat for general X-ray diffraction studies. J. Appl. Crystallogr. 1986;19:105–107. [Google Scholar]
- 28.CrysAlisPro (Version 1.171.31.7.), (Version 1.171.31.7.) (England: Agilent Technologies).
- 29.a) Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]; b) Sheldrick G.M. Phase annealing in SHELX-90: direct methods for larger structures. Acta Crystallogr. 1990;A46:467–473. [Google Scholar]; c) Sheldrick G.M. University of Göttingen; Germany: 1998. SHELX9: Programs for crystal Structure Analysis (Release 97–2) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.