Abstract
Background
Implantable vagus nerve stimulation (VNS) devices can be used to treat epilepsy in dogs. Adverse effects and short‐term complications associated with delivering suggested therapeutic electrical stimulation (>1.5 mA) are not well‐described.
Objectives
To compare complications and adverse effects observed with standard and rapid protocols of current increase.
Animals
Sixteen client‐owned dogs with idiopathic epilepsy.
Methods
Nonrandomized, nonblinded prospective cohort study. Surgical complications, stimulation‐related adverse effects, modifications to stimulator settings, number of hospital visits, and time to reach 1.5 mA stimulation current without intolerable adverse effects were described in dogs receiving current increases every 1 to 3 weeks (slow ramping) and dogs receiving current increases every 8 to 12 hours (fast ramping).
Results
Self‐resolving surgery site seromas formed in 6 dogs. No other surgical complications were observed. Fourteen dogs reached 1.5 mA. Coughing (11/14 dogs; 5 slow, 6 fast ramping) was the most common adverse effect. Intolerable coughing that limited current increases despite changing other stimulus parameters occurred in 6/7 of the fast‐ramping group and in none of the slow‐ramping group. Median time to 1.5 mA was 72 days (range, 28‐98) in the slow‐ramping group and 77 days (range, 3‐152) in the fast‐ramping group. Median number of clinic visits was 6 for the slow‐ramping group (range, 5‐6) and 3 for the fast‐ramping group (range, 1‐7).
Conclusions and Clinical Importance
Coughing is a common adverse effect of VNS in dogs and generally is well tolerated, particularly if current is increased slowly and other stimulation parameters are adapted for effect.
Keywords: dog, epilepsy, vagus nerve
ABBREVIATION
- VNS
vagus nerve stimulation/stimulator
1. INTRODUCTION
Electrical stimulation of the vagus nerve has been shown to decrease seizure frequency and severity in experimental animals and human patients.1, 2, 3 The postulated therapeutic mechanism is that repeated depolarization of the myelinated afferent A or B fibers in the nerve causes an increase in monoamine activity within the brain, particularly norepinephrine and serotonin.4, 5, 6 The vagus nerve also contains large efferent myelinated A fibers and small, unmyelinated C fibers.7 Depolarization of these fibers is thought to cause the common adverse effects of dysphonia, dysphagia, cough, dyspnea, paresthesia, headache, and pain seen in humans.8, 9
The stimulation settings available for manipulation to increase effectiveness and decrease adverse events are the current delivered (mA) in rectangular pulses, the duration of these pulses (pulse width, μs), the frequency of these pulses (stimulation frequency, Hz), the duration or bursts of these pulses delivered (on time, seconds), and the pause between delivering bursts (off time, minutes). Tolerance to adverse effects also can increase over time without changing these parameters, and it is recommended to increase the current delivered slowly to a recommended therapeutic level.10 The minimum current required to depolarize all of the myelinated fibers (ie, a therapeutic current) is estimated to be 1.50 mA10 and the manufacturer's recommendation is to increase up to this current by 0.25 mA steps slowly every 1 to 3 weeks after implantation (ramping), although early trials of vagus nerve stimulation (VNS) in humans increased the current to the maximum tolerable level much more rapidly (over the first 24 hours after implantation).11
Vagus nerve stimulation using implantable devices has been reported as a treatment for epilepsy in pet dogs.12 In this population, coughing was described as a common adverse effect, and dogs only were stimulated to a level at which coughing was not observed. No data regarding the optimal stimulation settings to avoid these adverse events are available. For example, it is not well‐established if such a slow‐ramping regimen decreases adverse effects in dogs when compared to a more rapid increase in current. Additionally, non‐stimulation‐related complications (eg, implant damage) have been described in experimental Beagles.13
Our aims were to report the stimulation and non‐stimulation‐related adverse effects of VNS implantation in client‐owned epileptic dogs during the initial phase of current increase to 1.5 mA, and to describe adverse effect frequency in dogs in which stimulation current was increased quickly or slowly after implantation.
2. MATERIALS AND METHODS
Dogs were prospectively recruited for implantation of vagus nerve stimulators if they satisfied the criteria for a tier 2 diagnosis of epilepsy, as defined by the International Veterinary Epilepsy Task Force.14 These were: ≥2 unprovoked seizures >24 hours apart, age at onset of >6 months to <6 years, normal interictal neurological and physical examination findings, no clinically relevant abnormalities on CBC or serum biochemistry profile (including preprandial and postprandial serum bile acid concentrations), normal magnetic resonance imaging of the brain, and normal cisternal cerebrospinal fluid examination findings.
All dogs had coil electrodes (model 302 or 303 lead, LivaNova Inc, Houston, Texas) placed around the cervical portion of the left vagus nerve as described previously.13 Generator (Pulse 102, Demipulse 103, or Aspire HC, LivaNova Inc) placement was SC either over the dorsal cervical region or over the lateral cranial thorax just caudal to the position of the scapula. In cases with thoracic placement, the leads extended caudally across the scapula from the electrode implantation side. All devices were evaluated intraoperatively using a current of 1.0 mA delivered for 30 seconds with a signal frequency of 30 Hz and pulse width of 250 μs. Any cardiac abnormalities noted on continuous ECG were recorded. Postoperatively, incision sites were monitored for seroma formation, signs of infection or dehiscence, and these were recorded if observed.
The severity of coughing and any other current‐related adverse effects (eg, dysphonia, dysphagia, and apparent pain during stimulation) was monitored and recorded postoperatively. Coughing was graded using the following definitions:
Mild: coughs during stimulation a few times per day (rarely). Considered tolerable and no change was made to the stimulator settings.
Moderate: coughs during stimulation several times per day (often). Also considered tolerable.
Severe: coughs harshly or retches on most stimulations. Considered intolerable and settings were changed if encountered.
To increase the output current (ramping) to a suggested therapeutic range of >1.5 mA as quickly as possible, 2 regimens (slow or fast ramping, Figure 1) were offered to the owners and they selected the regimen they preferred. In the slow‐ramping regimen, dogs were to be discharged postoperatively at 0.25 mA current per stimulation and then increased by 0.25 mA every 1 to 3 weeks until either severe coughing occurred during stimulation or 1.5 mA was achieved. Under the fast‐ramping regimen, dogs were to be hospitalized postoperatively and current increased by 0.25 mA every 8 to 12 hours until severe coughing was encountered or 1.5 mA was achieved. Dogs were allocated to the slow or fast regimens in a nonrandomized manner based on owner preference and neither owners nor clinicians were blinded to the choice.
FIGURE 1.
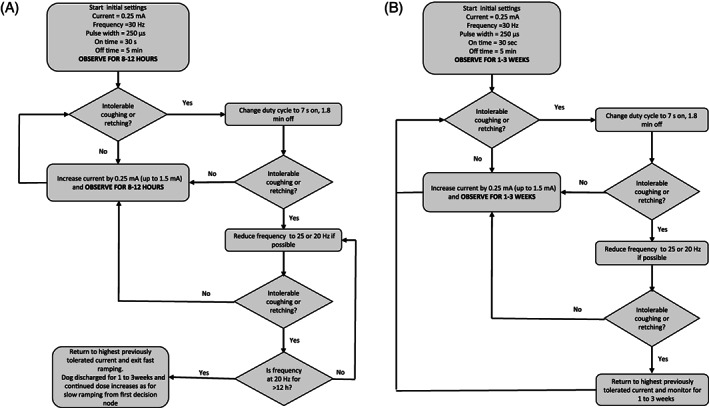
Flow diagrams outlining the (A) fast‐ and (B) slow‐ramping regimes
For both ramping regimens, we planned for identical strategies to be used when severe coughing was encountered, based on published guidelines for humans (Figure 1).15 First, the duration of on and off time (the duty cycle) was to be changed from 30 seconds on and 5 minutes off to 7 seconds on and 1.8 minutes off if the dog was not on this duty cycle already. Second, the stimulation frequency was to be decreased from 30 to 25 Hz or from 25 to 20 Hz. Thirdly, if all of these changes were made, the current was to be decreased to the previous setting at which coughing was mild or moderate and attempts were made to increase the current in 8 to 12 hours (fast ramping during hospitalization) or in 1 to 3 weeks (slow‐ramping or fast‐ramping postdischarge). If the current could not be increased in 8 to 12 hours without severe coughing in the fast‐ramping group during hospitalization, these dogs were to be left at their maximum tolerable current and fast ramping was to be ended and current to be increased as done for the slow‐ramping group. If we could not increase current despite waiting 1 to 3 weeks and all other changes had been made, dogs were to remain at the maximum tolerable current and 1 to 3 weeks allowed to elapse before attempting a subsequent current increase.
3. RESULTS
3.1. Perioperative adverse effects
Sixteen dogs had VNS placed between February 2017 and April 2019. Dogs 1 and 2 had a Pulse 102 generator with a 303 lead. Dog 3 had a Demipulse 103 with a 303 lead. All other dogs had an Aspire HC 105 generator with a 302 lead. The choice of generator or lead was dependent on what unit was available at the time of implantation with the exception of the Demipulse 103 model, which was selected for a small Jack Russell Terrier (dog 3) because it was smaller than the Pulse 102 model.
All dogs except dog 15 initially had thoracic generator placement. Dog 15 had a cervical generator placement, as did dog 11, at reimplantation. In dog 11, the cervical site was chosen because we speculated the original lead may have been damaged by the dog's harness as it passed over the scapula from the thoracic site. In dog 15, the cervical site was chosen because it was implanted immediately after the reimplantation for dog 11 and was the owner's preference after discussion about previous complications we had encountered.
No dog showed evidence of bradycardia during implantation and testing. No abnormalities of the vagus nerve were seen and all lead and generator placements were uneventful except for dog 8, which suffered cardiac arrest at the start of surgery before implantation. This dog was successfully resuscitated and the stimulator placed 6 weeks later with no cardiac abnormalities recorded.
Dog 1 developed pyrexia and neutrophilia the day after surgery associated with an infected IV catheter site. No implant infection was noted after a 2‐week course of cephalexin at 20 mg/kg PO q12h. A seroma developed at the generator site in 6 dogs, a soft swelling in 2 dogs, and no reaction in 8 dogs.
3.2. Early Implant failures
One of 16 dogs had implant failure within 3 months. Dog 11 had a seroma at initial implantation and then was noted to have a bunched, abnormal lead at examination 21 days later. The lead impedance decreased from 1917 to <600 Ω when tested on day 36. Surgical exploration 40 days postimplantation indicated that the lead was twisted and broken halfway along its length. The lead was replaced and both lead and generator appeared functional despite this dog also suffering wound dehiscence over the lead reimplantation site 5 days postoperatively. The 302 lead initially placed in this dog was replaced with a 303 lead.
3.3. Outcome of fast ramping
The owners of 7 dogs chose fast ramping. All 7 eventually tolerated a current of 1.5 mA (Figure 2A). Only 2 dogs (12 and 7) achieved 1.5 mA during initial hospitalization. Dog 12 was the only dog in this group that never coughed and current increase was uneventful, and dog 7 was discharged with moderate coughing at 1.5 mA but subsequently had current decreased to 1.0 mA because of severe stimulation‐related adverse effects (reported below). The remaining 6 dogs all developed severe cough that limited current increases in the hospital before reaching 1.5 mA despite modifications of other parameters.
FIGURE 2.
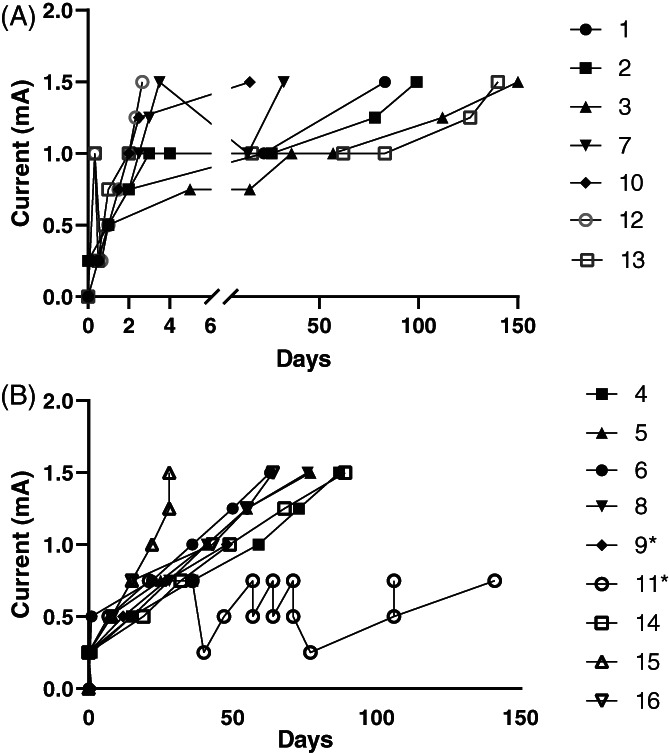
Current modifications over time in all dogs in the (A) fast‐ and (B) slow‐ramping groups. Note the broken time axis in the fast‐ramping group. * indicates the dogs where 1.5 mA was not achieved. Dog 11 was consistent with the rest of the slow‐ramping group until lead reimplantation at day 40
Two dogs that developed severe coughing were managed by changes in the duty cycle. The first 3 dogs implanted (1–3) had an initial duty cycle of 30 seconds on and 5 minutes off. It was apparent in dogs 1 and 2 at 1.0 mA that severe coughing started after approximately 20 seconds of stimulation. A modified duty cycle of 7 seconds on and 1.8 minutes off was instituted because it delivers charge to the brain for 10% of the time and has been suggested to increase tolerability without decreasing effectiveness in human patients.15 This change markedly improved coughing in both dogs but did not allow an immediate increase in current and thus both were discharged and started on the slow‐ramping protocol in which current was changed every 1 to 3 weeks to allow an increase to 1.5 mA without severe coughing. The owner of dog 3 requested a change to the modified duty cycle based on our experience with dogs 1 and 2 before developing severe coughing. All dogs after dog 3 were started on the modified duty cycle.
The remaining 4 dogs with severe coughing in the fast‐ramping group were managed by changes to the stimulus frequency. Severe coughing was encountered at currents >0.75, 1.25, 1.25, and 1 mA for dogs 3, 7, 10, and 13, respectively. All had stimulus frequency decreased to 20 Hz, which improved the coughing encountered at the highest tolerable current but did not allow further current increase at that time and all were discharged and changed to the slow‐ramping protocol, which allowed current increases every 1 to 3 weeks in 2 dogs (dogs 7 and 10), maintained on 25 and 30 Hz, respectively, between attempts.
Dogs 3 and 13 all still could not tolerate a current increase 1 to 3 weeks later despite the modified duty cycle and reduction to 20 Hz. Dog 3 tolerated increasing above 1 mA 107 days after the end of fast ramping. Dog 13 tolerated increasing above 1 mA 124 days after the end of fast ramping.
Further modifications to the duty cycle were made in 3 dogs in the fast‐ramping group (dogs 1, 2, and 13) after they had been discharged. In all of these dogs an intolerable cough temporarily limited current increase >1.0 mA but they could tolerate a shorter off‐time of 1.1 minutes (dogs 1 and 13) or 0.8 minutes (dog 2). All 3 dogs were perceived to have poor seizure control by their owners at the time of these changes and thus the changes were made as a compromise to increase charge delivered by the stimulator without intolerable adverse effects.
Aside from coughing, the only other adverse event seen in the fast‐ramping group was dog 7, which exhibited rapid movements of the muscles overlying the left shoulder during some stimulations at any level of intensity. This dog reached a current of 1.5 mA 3 days postimplantation and was discharged with moderate coughing. After discharge, this dog was reported to have difficulty swallowing food, was reluctant to eat, and would leave its tongue hanging from its mouth at rest. Its gag reflex was normal on examination. These signs persisted for 14 days until the current was decreased to 1 mA and the stimulus frequency to 25 Hz from 30 Hz. When the current was increased to 1.5 mA 18 days later, moderate coughing occurred, but no abnormalities of eating or tongue movement recurred.
3.4. Outcome of slow ramping
The owners of 9 dogs chose slow ramping. We could not increase the current to 1.5 mA in 2 of these 9 dogs (Figure 2B). One (dog 9) was euthanized 48 days after implantation because of a severe cluster of seizures with moderate coughing at 0.75 mA and no additional attempts to increase current were made. The other (dog 11) initially tolerated the modified duty cycle and no coughing occurred up to 0.75 mA. At this time, the lead became damaged and was replaced. After reimplantation, severe coughing occurred at the previous settings and the dog could not tolerate a current increase above 0.25 mA despite frequency reduction to 20 Hz unless the pulse width also was decreased to 130 μs, at which a current of 0.5 mA could be tolerated with moderate coughing. Frequency was decreased to 10 Hz 30 days later to decrease coughing to a mild level with a current of 0.75 mA.
In the remaining 7 dogs for which 1.5 mA was achieved, 2 never coughed at any level of stimulation (dogs 6 and 15). No dogs developed severe coughing that limited current increases every 1 to 3 weeks that could not be managed by modification of other parameters.
All dogs in the slow‐ramping group were started on the modified duty cycle of 7 seconds on and 1.8 minutes off except for dog 15 that never coughed and was started and maintained with 30 seconds on and 5 minutes off at the owner's request. All dogs were discharged within 24 hours of implantation at an initial current of 0.25 mA with the exception of dog 6, which never coughed and was discharged on 0.5 mA because the owners asked to change from a fast to a slow‐ramping regimen after 2 dose increases 8 hours apart.
Three of the 7 dogs in the slow‐ramping group that reached 1.5 mA did not require any frequency changes to ameliorate coughing. Dogs 6 and 15 never coughed and dog 8 only exhibited mild coughing at 1.0 mA.
Two dogs (dog 4 at 0.5 mA and dog 5 at 0.75 mA) were decreased from 30 to 25 Hz and 2 dogs (dogs 14 and 16 at 1.25 mA) had decreases to 20 Hz during ramping, all of which tolerated a 0.25‐mA increase without severe coughing that was present without the frequency change.
Aside from coughing, the only other adverse event identified in the slow‐ramping group was occasional reluctance to eat that occurred after each dose change but resolved within 24 to 48 hours in dog 14.
3.5. Comparison between slow and fast ramping
For comparison between the groups, we excluded data from the 2 dogs in the slow‐ramping group that did not achieve a current of 1.5 mA because we believed the reasons for this failure were independent of group allocation. Both appeared to be tolerating 1 to 3 weekly increases in current until another factor (euthanasia or device breakage) limited the current increase (Figure 2B).
In the slow group, 2/7 dogs did not cough at any level of stimulation compared to 1/7 in the fast group. The remaining dogs first developed tolerable coughs at a median of 1 mA (range, 0.5‐1.25 mA) in the slow group and 0.75 mA (range, 0.25‐1 mA) in the fast group (Figure 3).
FIGURE 3.
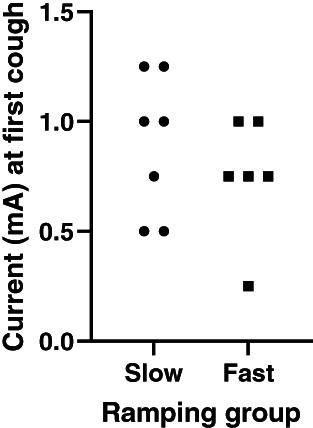
Comparison of current at first recorded cough. The 3 dogs which did not cough at any stimulation (2 slow, 1 fast) are not represented but the 2 dogs from the slow‐ramping group that did not achieve 1.5 mA (because of euthanasia or lead breakage and complications after reimplantation) are included
The time to reach 1.5 mA was similar in both the slow‐ and fast‐ramping groups, but the route to reach that current was quite different. Only 1/7 dogs in the fast‐ramping group could tolerate a current of 1.5 mA during hospitalization; the remaining 6 dogs developed current‐limiting severe coughs and needed to wait several weeks before coughing subsided and they could tolerate a further increase (Figure 2A). In contrast, no dogs in the slow‐ramping group developed coughing that was unmanageable by altering other parameters and their rate of current increase was more predictable (Figure 2B). Mean time to reach 1.5 mA was 72 days in the slow‐ramping group and 77 days in the fast‐ramping group. The median number of clinic visits was 6 for the slow‐ramping group (range, 5‐6) and 3 for the fast‐ramping group (range, 1‐7; Figure 2).
3.6. Settings and adverse effects postramping
In the 14 dogs that tolerated a current of 1.5 mA, the other parameters at the time this current was achieved are summarized in Table 1. The most frequent settings ultimately used to achieve a tolerable current of 1.5 mV (5/14 dogs) were 250 μs pulse width, 20 Hz pulse frequency, 7 seconds on time and 1.8 minutes off. These parameters were within published guidelines for treatment of human patients.15
TABLE 1.
Summary of the parameter settings used in dogs to allow a simulation current of 1.5 mA and a tolerable cough. * indicates dogs that did not cough. Dog 9 was euthanized before achieving 1.5 mA and dog 11 could not tolerate a current >0.5 mA
| Pulse width (μs) | Pulse frequency (Hz) | On time (s) | Off time (min) | Dogs | |
|---|---|---|---|---|---|
| Slow ramping | Fast ramping | ||||
| 250 | 20 | 7 | 1.8 | 4, 5, 14, 16 | 3 |
| 250 | 30 | 7 | 1.8 | 6*, 8 | 10, 12* |
| 250 | 20 | 7 | 1.1 | 1, 13 | |
| 250 | 25 | 7 | 1.8 | 7 | |
| 250 | 20 | 7 | 0.8 | 2 | |
| 250 | 25 | 30 | 5.0 | 15* | |
At the end of ramping, 5 dogs were not coughing (3 slow, 2 fast), 6 experienced moderate coughing (4 fast, 2 slow), and 3 experienced mild coughing (2 slow, 1 fast).
3.7. Lead impedance
In the 9 dogs for which data were available for both time points, mean impedance at implantation was 2184 Ω (SD, 581; range, 1060‐2900) and 2368 Ω 3 to 4 months postimplantation (SD, 716; range, 1506‐3576). Impedance increased between measurements in 5 dogs and decreased in 4 (Figure 4).
FIGURE 4.
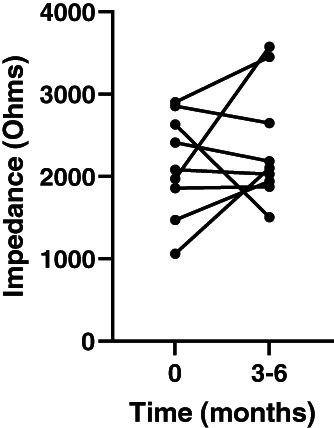
Vagus nerve stimulation (VNS) circuit impedance at implantation and at 1.5 mA (3‐6 months) for the 9 dogs where this was recorded at both time points
Lead impedance for all 4 dogs that never coughed was checked multiple times to ensure circuit failure was not the reason for the lack of coughing. Each measurement was verified by the programming software used (manufacturer reference interval 600‐5300 Ω) and all measurements were between 1600 and 2200 Ω when measured.
4. DISCUSSION
We found that stimulation‐related adverse effects occurred in approximately 80% (13/16) of dogs. The main adverse effect was coughing during stimulation and was present in approximately 64% of dogs at a suggested therapeutic current of 1.5 mA (9/14). Coughing appeared to be dose‐related and occurred at currents >0.75 mA in 9/14 dogs and could be decreased in severity by alterations in other stimulation parameters, particularly duty cycle and pulse frequency. These findings are similar to those in humans with VNS10 and suggest that current recommendations for humans15 can be adapted for dogs. Our findings suggest that tolerability may be improved by using an initial duty cycle of 7 seconds on and 1.8 minutes off. This change has not been shown to impact effectiveness in humans,15 but no information is available on the effect of this alteration on seizure control in dogs.
The slow‐ramping protocol appeared to have some advantages over the fast protocol. Discounting the 2 dogs that could not reach a therapeutic current, the number of dogs not coughing at 3 months was similar between groups (3/7 vs 2/7), but the current at first cough appeared higher for the slow group (Figure 1) and the number of dogs for which intolerable coughing prevented a current increase was lower (0/7 slow vs 6/7 fast). This current limitation meant that the dogs in both groups reached 1.5 mA at a similar time (72 vs 77 days) but in the fast ramping group 2 dogs required longer times (140 and 152 days) because their adverse effects substantially limited the current increases.
Despite trying to use a preplanned protocol for both groups of dogs, we deviated from this protocol in 4 dogs. Dogs 1, 2, and 13 (all fast ramping) had further modifications to duty cycle before a current of 1.5 mA was achieved because they could tolerate these changes but not a current increase and their owners perceived their seizures to be worsening. Dog 11 (slow ramping) had changes to stimulation frequency outside of the protocol after reimplantation to try to achieve a current that was previously well tolerated. We do not believe these deviations altered our conclusions because dogs 1, 2, and 13 were all in the fast‐ramping group, giving further evidence it was less well tolerated, and the difference between dog 11's pre‐ and post‐reimplantation current tolerance was so marked, we felt that these complications were associated with reimplantation rather than initial implantation.
Another important limitation of our study is the lack of statistical analysis of our results. We felt that statistical analysis of our findings in a small sample and without preplanned tests would be inappropriate, and that clear presentation of the study design and results achieved would be preferable.16 Both methods would carry the risk of over‐ or underinterpretation of the reliability of our findings, and although our data might show benefits of slow ramping over fast ramping for tolerability, this observation should be validated in a prospective comparative trial. Using our data as a guide, the minimum sample size to detect a difference similar to that we observed would be 5 dogs in each group (assuming 85% fast‐ramping dogs develop an intolerable cough compared to 15% of slow‐ramping dogs at 80% power and 95% confidence).17
We saw surgery‐site reactions (seroma or soft tissue swelling) at the generator implantation sites in half (8/16) of the dogs at initial implantation. This frequency is similar to previous reports that describe seroma formation in 2/1012 and 7/10 dogs (4 at the site of the generator).13 We chose to place the generator over the lateral thorax rather than the dorsolateral cervical musculature to secure the generator away from the region where a collar would be worn. Comparison of seroma rates between these gropus would be inappropriate because of small sample size, but seroma formation does not appear to be more likely at one particular site. Because these minor complications were self‐limiting, they do not appear to be a reason to choose 1 site over another.
We did not identify any stimulator‐related cardiac adverse effects. The 1 dog in our study that experienced cardiac arrest did so at the time of skin incision and the cardiac arrest could not be related to the device. This rate is the same as encountered in nonepileptic Beagles,13 but lower than in another study, in which 3/10 epileptic dogs experienced bradycardia during intraoperative device testing (1 mA delivered for 30 seconds), including 2 dogs with <30 seconds of self‐limiting asystole.12 The reason for this difference is unclear because dogs in our population had similar impedance testing as the dogs that showed bradycardia, but it does illustrate that those severe cardiac complications are rare in dogs with VNS placement.
A current of 1.5 mA has been recommended as the minimum therapeutic current in humans based on computational modeling.18 This research suggests populations of axons responsible for anticonvulsant effects (myelinated A and B fibers) are likely to be sufficiently depolarized at approximately 0.75 mA but if circuit impedance increases substantially over time after implantation, currents of 1.5 mA may be necessary.
Circuit impedance appeared to increase over the early postimplantation period, but this increase was not uniform and whereas large increases were evident in 4 dogs; the other 5 had small increases or decreases (Figure 4). Impedance is a measure of resistance to current flow through the lead and the vagus nerve between the electrodes. It can increase when the lead is damaged, but the order of magnitude is much more dramatic than we observed (up to >10 000 Ω). Smaller increases (such as we have observed) are thought to be caused by fibrotic cuffs of tissue forming at the electrode‐nerve interface within the first 4 to 8 weeks after implantation.18 Our data suggest that this fibrous tissue formation may not affect the impedance in all dogs and thus a current of 1.5 mA may not be necessary for all dogs.
Circuit impedance also can decrease when the lead or generator is damaged, as was evident in the dog in our study that required reimplantation and had impedance <600 Ω. This situation is similar to previous descriptions of VNS in dogs where impedance <600 Ω was also indicative of lead damage.13
Coughing was the most common adverse effect in our patients (13/16; 81%). The previous report of epileptic dogs with VNS implantation12 did not specify the proportion of dogs that coughed, but did report the maximum current at which no coughing was seen in all 10 dogs, implying that all dogs coughed at currents <1.5 mA. Both reports in the veterinary literature contrast to those in the human medical literature where the most common adverse effect is voice change (62%‐66%) with coughing encountered in approximately 21% to 45% in the first 3 months after implantation.11, 19 The reason for this difference is unclear and it may reflect neuro‐anatomical differences between dogs and humans.
The mechanism of coughing during VNS treatment is not definitively established. We considered the 2 most likely explanations to be direct stimulation of myelinated and unmyelinated afferent fibers in the vagus nerve that contribute to the afferent arm of the brainstem‐mediated cough reflex20 or direct stimulation of the efferent fibers that form the recurrent laryngeal nerve causing rapid movement of the larynx during stimulation.8, 9 This rapid movement also could trigger the cough reflex by stimulation of the cranial laryngeal nerve (which is separate from the vagosympathetic trunk).
With both of these mechanisms, it would be expected that stimulation of the vagus nerve would always cause coughing above a certain threshold, but 3/16 dogs did not cough in our study. It may be that dogs and humans that do not cough have a higher threshold for stimulation of their cough reflex or are more likely to consciously inhibit it. This possibility could explain some of the difference in frequency of coughing between dogs and humans if humans are more likely to consciously suppress their reflex than dogs. Irritation of the vagus nerve also potentially could lower the threshold for the cough reflex in humans,21 which may explain why 1 dog in our study was unable to tolerate VNS after re‐implantation if the damaged to the lead also had inflamed the vagus nerve.
Another explanation is that the cough‐stimulating fibers are sometimes not included in the stimulator coil placement. Branching of the left vagus nerve within the carotid sheath has been observed in approximately 12% of humans.22 This anatomic variation conceivably could minimize adverse effects and efficacy if the electrodes encircle a branch that does not contain either the axons responsible for adverse or therapeutic effects. In approximately 0.6% of humans, the nonrecurrent laryngeal nerve23 replaces the recurrent laryngeal nerve but exits the vagus in the superior cervical spine, rather than at the level of the aortic arch. Vagal electrode placement in affected individuals would not stimulate axons innervating the larynx. No branching of the vagus nerve within the carotid sheath was observed in any of the dogs in our study, but further research into the frequency of these variations in dogs and their influence on VNS adverse effects or clinical effectiveness is warranted.
Dog 7 was rapidly increased to 1.5 mA over 3 days and showed some unusual adverse effects of muscle fasciculations over the ipsilateral cervical and shoulder musculature, dysphagia, and abnormal tongue movement. These signs cannot be explained by direct stimulation from the electrodes we placed because vagal innervation of the pharynx by the pharyngeal branch leaves the vagus cranial to the electrodes, motor innervation of the tongue in the dog is exclusively from the hypoglossal nerve, and the vagus does not innervate any muscles of the neck, although another cranial nerve (the accessory nerve) does.
We propose 2 distinct possible mechanisms for these signs. Firstly, the vagus and the accessory nerves run in close proximity as they enter the cranium through the jugular foramen and there is direct communication between the 2 via the internal branch of the accessory nerve. Ephaptic transmission from the vagus to the accessory nerve could cause fasciculation of the trapezius omotransversarius, sternocephalicus, and cleidocervicalis muscles over the shoulder.
Secondly, the dysphagia and abnormal tongue position and movement were persistent between stimulations, suggesting they were caused by prolonged effects on the pharynx and tongue. We could not find an explanation for why the hypoglossal nerve would be affected by stimulation. The hypoglossal nerve provides sensation to the caudal tongue and the vagus and glossopharyngeal nerves provide motor and sensory innervation to the pharynx. We hypothesize that the depolarizations delivered to the brainstem of dog 7 (via the vagus) induced reversible changes to the neurons in the nucleus ambiguus, the nucleus of the solitary tract, or both, altering tongue sensation and pharyngeal sensation or movement to produce the dysphagia and tongue abnormalities seen.
Our data provide information about the outcome of ramping in dogs with VNS. Because our study group was small, we did not perform statistical analysis. We also did not have strict preplanned protocols in place where dogs would be excluded if the protocols were not followed, a control group, or blinded random allocation of treatment types. Because of these design issues, our data cannot be used to determine the optimal parameters to minimize coughing in the ramping phase or provide definitive guidance on adverse effects, and additional work based on our data is needed.
Another limitation to accepting these settings as optimal for use in dogs with VNS is that they were only evaluated by their effect on coughing, not effectiveness at decreasing seizures. Although all final parameters at 1.5 mA were within recommended guidelines for humans, it is not established if changes such as modified duty cycle, decreased current, or pulse frequency will have a clinically relevant impact on the effectiveness of seizure control or battery life. Additional work evaluating the effectiveness of VNS in dogs and the impact of device settings is needed.
5. CONCLUSION
Our study suggests that a stimulation current of 1.5 mA can be tolerated in almost all dogs, but that a modified duty cycle might improve tolerability in dogs that experience coughing when current is increased. Because the time to reach 1.5 mA and the number of clinic visits were similar in both the slow‐ and fast‐ramping groups, but the incidence of intolerable coughing was lower in the slow‐ramping group, we recommend routinely increasing the current at 1 to 3 week intervals, but that a fast‐ramping protocol can be used in some individuals.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Bristol Animal Welfare and Ethics Review Body (AWERB) under the unique veterinary investigation number VIN/20/035.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. The authors acknowledge Mark Lawson (Livanova UK) for technical guidance.
Harcourt‐Brown TR, Carter M. Implantable vagus nerve stimulator settings and short‐term adverse effects in epileptic dogs. J Vet Intern Med. 2021;35(5):2350‐2358. 10.1111/jvim.16226
REFERENCES
- 1.Panebianco M, Rigby A, Weston J, Marson AG. Vagus nerve stimulation for partial seizures. Cochrane Database of Systematic Reviews. 2015;2015(4):CD002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghani S, Vilensky J, Turner B, Tubbs RS, Loukas M. Meta‐analysis of vagus nerve stimulation treatment for epilepsy: correlation between device setting parameters and acute response. Childs Nerv Syst. 2015;31(12):2291‐2304. [DOI] [PubMed] [Google Scholar]
- 3.Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79(3):345‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krahl SE, Clark KB. Vagus nerve stimulation for epilepsy: a review of central mechanisms. Surg Neurol Int. 2012;3(SUPPL4):S255‐S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martlé V, Raedt R, Waelbers T, et al. The effect of vagus nerve stimulation on CSF monoamines and the PTZ seizure threshold in dogs. Brain Stimul. 2015;8(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 6.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part III. Headache. 2016;56(3):479‐490. http://doi.wiley.com/10.1111/head.12649 [DOI] [PubMed] [Google Scholar]
- 7.Yuan H, Silberstein SD. Vagus nerve and Vagus nerve stimulation, a comprehensive review: part I. Headache. 2016;56(1):71‐78. http://doi.wiley.com/10.1111/head.12647 [DOI] [PubMed] [Google Scholar]
- 8.Ardesch JJ, Sikken JR, Veltink PH, van der Aa HE, Hageman G, Buschman HPJ. Vagus nerve stimulation for epilepsy activates the vocal folds maximally at therapeutic levels. Epilepsy Res. 2010;89(2–3):227‐231. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer MJ, Szabo CA, Jackson CE, Simpson CB. Vagal nerve stimulation: clinical and electrophysiological effects on vocal fold function. Ann Otol Rhinol Laryngol. 2005;114(1 I):7‐14. [DOI] [PubMed] [Google Scholar]
- 10.Wheless JW, Gienapp AJ, Ryvlin P. Vagus nerve stimulation (VNS) therapy update. Epilepsy and Behavior. 2018;88S:2‐10. [DOI] [PubMed] [Google Scholar]
- 11.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial‐onset seizures: a randomized active‐control trial. Neurology. 1998;51(1):48‐55. https://n.neurology.org/content/51/1/48 [DOI] [PubMed] [Google Scholar]
- 12.Muñana KR, Vitek SM, Tarver WB, et al. Use of vagal nerve stimulation as a treatment for refractory epilepsy in dogs. J Am Vet Med Assoc. 2002;221(7):977‐983. https://pubmed.ncbi.nlm.nih.gov/12369700/ [DOI] [PubMed] [Google Scholar]
- 13.Martlé V, van Ham LML, Boon P, et al. Vagus nerve stimulator placement in dogs: surgical implantation technique, complications, long‐term follow‐up, and practical considerations. Vet Surg. 2016;45(1):71‐78. [DOI] [PubMed] [Google Scholar]
- 14.de Risio L, Bhatti S, Muñana K, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11(148):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heck C, Helmers SL, DeGiorgio CM. Vagus nerve stimulation therapy, epilepsy, and device parameters: scientific basis and recommendations for use. Neurology. 2002;59(6 SUPPL. 4):S31‐S37. https://n.neurology.org/content/59/6_suppl_4/S31 [DOI] [PubMed] [Google Scholar]
- 16.Jeffery N. Liberating the (data) population from subjugation to the 5% (P‐value). J Small Anim Pract. 2015;56:483‐484. https://www.onlinelibrary.wiley.com/doi/full/10.1111/jsap.12391 [DOI] [PubMed] [Google Scholar]
- 17.Comparing two proportions ‐ sample size ‐ select statistical consultants; 2021. https://select-statistics.co.uk/calculators/sample-size-calculator-two-proportions/
- 18.Helmers SL, Begnaud J, Cowley A, et al. Application of a computational model of vagus nerve stimulation. Acta Neurol Scand. 2012;126(5):336‐343. https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0404.2012.01656.x [DOI] [PubMed] [Google Scholar]
- 19.DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long‐term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41(9):1195‐1200. http://doi.wiley.com/10.1111/j.1528-1157.2000.tb00325.x [DOI] [PubMed] [Google Scholar]
- 20.Haji A, Kimura S, Ohi Y. A model of the central regulatory system for cough reflex. Biol Pharm Bull. 2013;36(4):501‐508. [DOI] [PubMed] [Google Scholar]
- 21.Dandurand C, Champagne PO, Elayoubi K, Weil AG, Lespérence P, Bouthillier A. Vagus nerve stimulator‐related speech/exercise induced cough. J Clin Neurosci. 2017;37:47‐48. [DOI] [PubMed] [Google Scholar]
- 22.Hammer N, Glätzner J, Feja C, et al. Human vagus nerve branching in the cervical region. PLoS One. 2015;10(2):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling XY, Smoll NR. A systematic review of variations of the recurrent laryngeal nerve. Clin Anat. 2016;29(1):104‐110. [DOI] [PubMed] [Google Scholar]


