Abstract
Background
Angiotensin‐converting enzyme inhibitors (ACEIs) are commonly prescribed in dogs, but the ideal dosage is unknown.
Hypothesis/Objectives
In dogs with cardiac disease, a dose‐response relationship exists for ACEIs with respect to long‐term outcome.
Animals
One hundred forty‐four dogs with cardiac disease, 63 with current or prior congestive heart failure.
Methods
Retrospective medical record review. Cox proportional hazards models were used to determine variables associated with 2‐year survival or survival from first‐onset congestive heart failure (CHF).
Results
Median initial ACEI dosage was 0.84 (interquartile range [IQR], 0.56‐0.98) mg/kg/day, and 108/144 (75%) of dogs received q12h dosing. No clinically relevant changes in renal function test results, serum electrolyte concentrations, or blood pressure occurred between initial prescription of ACEI and first reevaluation (median, 14 days later). In univariable analysis, higher ACEI dose was associated with increased survival from first‐onset CHF (P = .005), and within the subgroup of dogs in CHF at the time of ACEI prescription, higher ACEI dose was associated with improved survival at 2 years (P = .04). In multivariable analysis, q12h dose frequency of ACEI (hazard ratio [HR], 0.30; 95% CI, 0.10‐0.88; P = .03) and higher serum potassium concentration at visit 1 (HR, 0.39; 95% CI, 0.16‐0.97; P = .04) were predictive of 2‐year survival. The ACEIs were well‐tolerated, with only 8/144 (5.6%) dogs having ACEI dose decreased or discontinued because of adverse effects.
Conclusions and Clinical Importance
Twice daily dose frequency might optimize the cardioprotective benefit of ACEIs.
Keywords: benazepril, congestive heart failure, dilated cardiomyopathy, enalapril, myxomatous mitral valve disease
Abbreviations
- ACEI
angiotensin‐converting enzyme inhibitor
- ACVIM
American College of Veterinary Internal Medicine
- BP
blood pressure
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- HR
hazard ratio
- IQR
interquartile range
- MMVD
myxomatous mitral valve disease
- MRSI
mitral regurgitation severity index
- PD
pharmacodynamic
- PK
pharmacokinetic
- RAAS
renin‐angiotensin‐aldosterone system
1. INTRODUCTION
Angiotensin‐converting enzyme inhibitors (ACEIs), such as enalapril and benazepril, are commonly used in both human and veterinary medicine for treatment of cardiac disease,1, 2, 3, 4 systemic hypertension,5, 6 and proteinuria.5, 7, 8, 9 They mitigate effects of the renin‐angiotensin‐aldosterone system (RAAS) by inhibiting the action of angiotensin converting enzyme (ACE), which catalyzes the conversion of angiotensin I into angiotensin II. Decreased production of angiotensin II (and consequently decreased release of aldosterone) results in balanced vasodilatation, decreased sodium and water retention, dilatation of renal efferent arterioles, and decreased cardiac fibrosis and remodeling.10, 11
Although ACEIs frequently are prescribed in dogs, no consensus exists regarding the ideal dosage of ACEI in this species. Pharmacokinetic (PK) and pharmacodynamic (PD) studies comparing various doses of ACEIs in healthy dogs have not provided consistent recommendations. One study in dogs showed that a single PO dose of benazepril effectively suppressed ACE activity for up to 24 hours, and that ACE inhibition in plasma was independent of dosage ≥0.25 mg/kg.12 However, subsequent reanalysis of these data using PK modeling suggested that twice daily (q12h) dosing (as opposed to once daily [q24h] dosing) would achieve greater inhibition of ACE with the same total daily dose.13 Furthermore, a different study of single‐dose enalapril and benazepril at a dosage of 0.5 mg/kg indicated a much shorter duration of effect, with ACE suppression lasting <12 hours.14 These PK/PD studies of ACEIs are limited by the use of plasma ACE activity as a suboptimal surrogate marker for RAAS suppression, because numerous studies in humans and dogs have shown a lack of correlation between circulating ACE concentration and angiotensin II activity.15, 16, 17 These experimental models also fail to take into account the chronobiology of the RAAS, and previous research has shown that biomarkers of the renin pathway are subject to circadian variation in dogs.18, 19, 20
Regardless of PK/PD, the extent and duration of RAAS suppression required to achieve a cardioprotective clinical benefit also is unknown. Different dosages of ACEIs have been used in clinical trials of dogs with naturally‐occurring cardiovascular disease. In early studies demonstrating benefits of ACEIs in dogs with CHF secondary to myxomatous mitral valve disease (MMVD) or dilated cardiomyopathy (DCM), enalapril initially was prescribed at a dosage of 0.5 mg/kg q24h, with clinicians having the option to increase dose frequency to q12h at their discretion.1, 21, 22 Three large‐scale clinical trials have investigated the use of ACEIs in dogs with preclinical MMVD, prescribing enalapril at mean dosages of 0.37 mg/kg23 or 0.46 mg/kg24 q24h, or benazepril at 0.3 mg/kg q24h in combination with spironolactone.25 However, none of these clinical trials compared different ACEI dosages in a single investigation.
The primary objective of our retrospective study was to determine whether a dose‐response relationship exists for ACEIs in dogs with cardiac disease with respect to long‐term outcome. We hypothesized that higher total daily dose of ACEI or q12h dosing would be associated with longer time to all‐cause mortality compared to lower total daily dose or q24h dosing.
2. MATERIALS AND METHODS
A retrospective medical record search was performed to identify dogs prescribed enalapril or benazepril at Iowa State University Lloyd Veterinary Medical Center between October 19, 2005 and October 3, 2018. Inclusion criteria were: (a) dogs were prescribed ACEIs for the treatment of cardiac disease (echocardiogram was required to confirm and characterize underlying structural heart disease); (b) dose and frequency of ACEI were recorded at the time of initial prescription and again during at least 1 subsequent reevaluation; and (c) dogs had not received an ACEI before prescription at Iowa State University Lloyd Veterinary Medical Center. Dogs that were prescribed ACEI solely for the treatment of systemic hypertension or proteinuria were excluded. Approval from an institutional animal care and use committee or other ethical oversight body was not required for this retrospective study.
The following data were obtained for each dog on the date that enalapril or benazepril was prescribed (Visit 1): signalment, body weight, cardiovascular physical examination findings, noninvasively determined systolic blood pressure (BP), serum biochemistry panel results, classification of underlying structural cardiac disease (MMVD, DCM, or other), American College of Veterinary Internal Medicine (ACVIM) cardiac disease stage4 with DCM staged similarly to MMVD, presence or absence of concurrent or previous CHF, type of ACEI prescribed (enalapril or benazepril), dose and frequency of ACEI, and other cardiovascular medications (furosemide, pimobendan, spironolactone, amlodipine) already being administered at the time of ACEI prescription or prescribed concurrently with the ACEI. For MMVD dogs, mitral regurgitation severity index (MRSI; Clarke Atkins, personal communication) was calculated as (100 × [heart rate/120] × echocardiographic left atrium‐to‐aorta ratio [LA : Ao] × [dog age/10]). The following information was obtained at the first reevaluation after prescription of the ACEI (Visit 2): whether the owner was administering the ACEI as prescribed, BP, serum biochemistry panel results, any changes to ACEI dose or frequency, and any other medications added or changed.
To assess long‐term outcomes, the following information was obtained from the medical record for all visits occurring after Visit 2: (a) any subsequent changes to ACEI dose or frequency and final dose of ACEI administered after dose adjustments, (b) any other medications added or changed, (c) date of eventual development of CHF (for dogs not in CHF at Visit 1), (d) date and cause of death (for deceased dogs), and (e) date of last follow‐up (for dogs alive or lost to follow‐up). Dogs were considered lost to follow‐up (and were excluded from long‐term survival analyses) if they had no additional visits recorded after Visit 2. Dogs with clinical information available after Visit 2 were included in survival analyses and classified either as dead (if date of death was known) or alive at the end of the study (with date of last follow‐up).
3. STATISTICAL ANALYSIS
Statistical analysis was performed using R software (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria). Summary statistics were calculated for all variables for each timepoint and cardiac disease group, and normality was assessed using the Shapiro‐Wilk test. All data are presented as median (interquartile range [IQR]). T tests (normally distributed continuous data), Wilcoxon rank‐sum tests (nonnormally distributed continuous data), or Chi‐squared or Fisher's exact tests (categorical data) were performed to compare variables between cardiac disease groups. Paired t tests or paired Wilcoxon ranked tests were performed to compare variables between Visit 1 and Visit 2, with P values adjusted using the Benjamini‐Hochberg correction method.
Cox proportional hazards modeling was used to determine variables associated with long‐term outcomes, including 2‐year all‐cause survival from Visit 1 and time from first‐onset CHF to all‐cause death. These outcomes were chosen to represent clinically relevant timeframes within a population of dogs with variable heart disease severity and thus variable likelihood of experiencing cardiac complications during a typical lifespan. The following variables were chosen to investigate for potential association with outcome based on clinical relevance: type of structural cardiac disease (DCM vs MMVD), ACVIM cardiac disease stage (B2 vs C), heart rate, blood pressure, MRSI (for MMVD dogs only), serum sodium and potassium concentrations, total daily ACEI dose, ACEI dose frequency, and concurrent prescription of pimobendan. For clinical variables, results from Visit 1 were utilized; for treatment variables, final medications and doses (as recorded in the last follow‐up visit) were used. A binary classification of total daily ACEI dose was achieved by defining “high‐dose” as ≥0.5 mg/kg/day (corresponding to ACEI doses higher than those used in published clinical trials in dogs) and “low‐dose” as <0.5 mg/kg/day (corresponding to the dose range utilized in previous clinical trials).
Univariable analysis initially was performed to obtain P values for each covariate, and P values were adjusted using a Benjamini‐Hochberg correction. Kaplan‐Meier curves (R package survival [3.2‐7] and survminer [0.4.8]) were produced for variables with significant P values in univariable analysis, and log‐rank testing was used to analyze differences in survival based on these variables, with continuous variables dichotomized at the sample mean. Variables with adjusted P values <.1 in univariable analysis were included for multivariable model selection along with total daily ACEI dose and ACEI dose frequency. Multivariable modeling was performed using backward selection utilizing the Akaike information criterion, and hazard ratios (HR) were reported for all variables included in the final model. Normality of continuous variables offered to the multivariable model were confirmed using visual inspection of Q‐Q plots. Assumptions of proportionality and model fit were confirmed using Schoenfeld residuals. Multivariable models also were repeated using time (date of Visit 1) as a frailty term.
4. RESULTS
4.1. Study sample and clinical data at Visit 1 and Visit 2
One‐hundred forty‐four dogs met the inclusion criteria, including 70 castrated males, 7 intact males, 64 spayed females, and 3 intact females. The most commonly represented breeds were mixed breed (n = 25), Cavalier King Charles spaniel (13), Schnauzer (9 miniature, 2 standard), Labrador retriever (9), Chihuahua (5), Yorkshire terrier (5), Maltese (5), Boxer (5), golden retriever (4), Shih tzu (4), Great Dane (3), Cocker spaniel (3), Boston terrier (3), rat terrier (3), and Jack Russell terrier (3). An additional 37 breeds were represented by ≤2 dogs each.
The study sample consisted of 101 dogs diagnosed with MMVD, 28 dogs with DCM, and 15 dogs with other cardiac diseases. Four of the dogs diagnosed with DCM were Boxers with arrhythmogenic right ventricular cardiomyopathy and DCM phenotype. Other cardiac diagnoses included congenital heart disease (n = 10; 4 patent ductus arteriosus, 3 atrioventricular valve dysplasia, 2 pulmonic stenosis, 1 subaortic stenosis); pulmonary hypertension with associated right heart remodeling (n = 3), and endo/myo‐carditis (n = 2). Demographic data and cardiovascular physical examination findings at Visit 1 for dogs with different cardiac disease categories are presented in Table 1. Arrhythmias recorded at Visit 1 included atrial fibrillation (n = 14), ventricular ectopy (6), supraventricular ectopy (3), combination of ventricular and supraventricular ectopy (2), and combination of atrial fibrillation and 3rd degree atrioventricular block (1).
TABLE 1.
Demographic and cardiovascular physical examination findings in 144 dogs receiving angiotensin‐converting enzyme inhibitors (ACEIs) for cardiac disease
| All dogs | MMVD | DCM | Other | P‐values | |
|---|---|---|---|---|---|
| Number | 144 | 101 | 28 | 15 | – |
| Age (years) | 9 (7‐11) | 10 (9‐11)a,b | 8 (4.25‐9.75)a | 7 (1‐7)b |
a <.001 b <.001 |
| Weight (kg) | 11.3 (7‐29) | 9.2 (6.5‐14.2)a | 37.7 (31.1‐52.8) | 12.2 (5.7‐25.5)b |
a <.001 b <.001 |
| Murmur (n, %) | 124/142 (87%) | 97/99 (98%)a,b | 15 (54%)a | 12 (80%)b |
a <.001 b .01 |
| Arrhythmia (n, %) | 26/143 (18%) | 9/100 (9%)a | 14 (50%)a | 3 (20%) | a <.001 |
| CHF (n, %) | 63 (44%) | 42 (42%) | 15 (54%) | 6 (40%) |
Note: Continuous data are presented as median (IQR) and categorical data are presented as number (%). Superscript letters within a row indicate significant differences between cardiac disease groups (adjusted P‐value <.05). For variables with missing data (presence of murmur and arrhythmia), the denominator indicates the number of dogs with data available; if no denominator is listed, no data were missing in that group.
Abbreviations: CHF, congestive heart failure; DCM, dilated cardiomyopathy; MMVD, myxomatous mitral valve disease.
The subsequent follow‐up visit (Visit 2) occurred a median of 14 days (IQR, 9‐71) after Visit 1. Cardiovascular and clinicopathologic results for dogs at Visit 1 and Visit 2, separated into subgroups with vs without CHF at Visit 1, are presented in Table 2.
TABLE 2.
Cardiovascular and clinicopathologic results at Visit 1 (initial prescription) and Visit 2 (subsequent recheck examination, median 14 days later) in 144 dogs receiving angiotensin‐converting enzyme inhibitors (ACEIs) for treatment of cardiac disease, 63 of which had current or prior congestive heart failure (CHF) at the time of Visit 1 and 81 of which did not
| Visit 1 | Visit 2 | P‐value: Visit 1 vs 2 | ||||
|---|---|---|---|---|---|---|
| Variable | No CHF | CHF | No CHF | CHF | No CHF | CHF |
| Heart rate (bpm) (n = 143) | 120 (90‐139) | 151 (129‐170) | 120 (100‐140) | 132 (120‐140) | .88 | .07 |
| Blood pressure (mm Hg) (n = 127) | 151 (130‐170) | 140 (117‐156) | 148 (130‐163) | 130 (114‐160) | .21 | .78 |
| Blood urea nitrogen (mg/dL) (n = 106) | 16 (11‐20) | 18 (14‐22) | 18 (14‐25) | 24 (16‐30) | .09 | .05* |
| Creatinine (mg/dL) (n = 105) | 0.9 (0.7‐1.1) | 0.9 (0.8‐1.3) | 0.9 (0.7‐1.1) | 1.1 (0.8‐1.3) | .96 | .36 |
| Sodium (mEq/L) (n = 104) | 147 (145.5‐149.5) | 145 (144‐148) | 146 (144‐148) | 145 (143‐149) | .07 | .88 |
| Potassium (mEq/L) (n = 104) | 4.8 (4.4‐5.1) | 4.75 (4.1‐4.9) | 4.8 (4.4‐5.2) | 4.6 (4.1‐5.0) | .73 | .96 |
| Chloride (mEq/L) (n = 98) | 114 (112‐117) | 114 (110‐118) | 114 (111‐116) | 111 (108‐114) | .70 | .01* |
| Bicarbonate (mEq/L) (n = 96) | 23 (20.9‐24.1) | 22 (19‐25) | 23 (21‐24) | 24 (22‐27) | .86 | .06 |
| Calcium (mg/dL) (n = 104) | 10.7 (10.1‐11.0) | 10.2 (9.8‐10.6) | 10.7 (10.2‐11.1) | 10.8 (10.2‐11.0) | .73 | .004* |
| Phosphorus (mg/dL) (n = 104) | 4.1 (3.6‐5.0) | 4.5 (4‐5.5) | 4.2 (3.7‐4.9) | 4.5 (4‐5.2) | .78 | .56 |
Note: Data are presented as median (IQR). Number of patients is provided in italics for variables lacking complete data sets at both visits. Significant differences (adjusted P‐value <.05) between Visit 1 and Visit 2 for each subgroup, based on paired t‐tests or paired Wilcoxon ranked tests with Benjamini‐Hochberg correction, are noted with bolding and an asterisk (*).
4.2. ACEI prescription practices
Drug prescription practices at each study timepoint are summarized in Table 3. The relationship between ACEI dose and frequency is depicted in Figure 1. Sixteen dogs had their total daily dose of ACEI increased at Visit 2, either by increasing dose frequency from q24h to q12h (n = 10) or increasing the dose of ACEI at the previous dose frequency (n = 6). Reasons for ACEI dose escalation at Visit 2 included increased or high normal blood pressure (n = 7), clinician preference for q12h dosing (n = 7), or change in ACEI formulation resulting in dose increase (n = 2). Total daily ACEI dose was decreased at Visit 2 in 5 dogs, either by decreasing dose frequency from q12h to q24h (n = 3) or decreasing the dose at the previous dose frequency (n = 2). Reasons for ACEI dose decrease at Visit 2 included borderline hypotension (systolic blood pressure, 90‐100 mm Hg; n = 3), increased renal function test results (n = 1), and owner noncompliance (n = 1). The ACEI were discontinued at Visit 2 in 3 dogs: 1 because of increased renal function test results, 1 because of lethargy and ataxia (presumed hypotension), and 1 because of reversed remodeling of prior cardiac disease. An additional 2 dogs were switched from enalapril to benazepril at Visit 2, both because of increased renal function test results; 1 of these dogs had the same total daily ACEI dose continued, whereas the other dog was changed from q12h to q24h dosing (50% dose decrease). Between Visit 2 and the last follow‐up visit when medications were recorded for each dog, ACEI dose was increased in an additional 11 dogs (6, increased dose; 5, increased frequency), an additional 2 dogs were switched from enalapril to benazepril, and the ACEI was discontinued in 4 dogs (because of reversed remodeling of prior cardiac disease [n = 2], increased renal function test results [n = 1], and owner noncompliance [n = 1]). Overall, 8/144 dogs (5.6%) had their ACEI dose decreased or discontinued because of adverse effects at some point during the study period (4 for increased renal function test results and 4 for hypotension).
TABLE 3.
Prescription practices at various study timepoints in a sample of 144 dogs receiving angiotensin‐converting enzyme inhibitors (ACEIs) for treatment of cardiac disease
| Visit 1 | Visit 2 | Last follow‐up | |
|---|---|---|---|
| Dogs prescribed ACEI (n) | 144 | 141 | 137 |
| ACEI (n, % enalapril) | 132/144 (92%) | 130/141 (92%) | 124/137 (91%) |
| ACEI dose (mg/kg/day) | 0.84 (0.56‐0.98) | 0.86 (0.60‐0.98) | 0.88 (0.62‐1.01) |
| ACEI dose frequency (n, % q12h) | 108/144 (75%) | 114/141 (81%) | 112/137 (82%) |
| ACEI dose increase (n, %) | – | 16/144 (11.1%) | 13/141 (9.2%) |
| ACEI dose decrease (n, %) | – | 5/144 (3.5%) | 0/141 (0%) |
| ACEI discontinued (n, %) | – | 3/144 (2.1%) | 4/141 (2.8%) |
| Furosemide prescribed (n, %) | 63/144 (44%) | 76/144 (53%) | 87/144 (60%) |
| Pimobendan prescribed (n, %) | 96/144 (67%) | 107/144 (74%) | 112/144 (78%) |
| Spironolactone prescribed (n, %) | 4/144 (2.8%) | 22/144 (15%) | 33/144 (23%) |
Note: Continuous data are presented as median (IQR).
Abbreviation: q12h, twice daily dosing.
FIGURE 1.
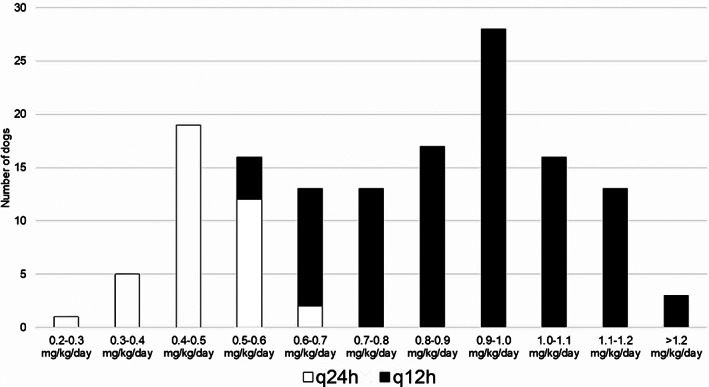
Dose ranges of angiotensin‐converting enzyme inhibitors (ACEIs) prescribed in Visit 1 for 144 dogs receiving an ACEI for treatment of cardiac disease. Dose frequency is indicated by column color. q24h, once daily dosing; q12h, twice daily dosing
4.3. Long‐term outcome
Survival data were available for 108 dogs (49 in CHF at Visit 1, 59 not in CHF at Visit 1), with the remaining 36 dogs lost to follow‐up. Of dogs without CHF at Visit 1, 12/59 (20%) experienced an episode of new CHF during the study period, with onset occurring a median of 246 (IQR, 103‐333) days after Visit 1. Of dogs with follow‐up data that were in CHF at Visit 1, 16/49 (32%) had a relapse of CHF recorded during the study period, occurring a median of 180 (IQR, 61‐383) days after Visit 1.
Of dogs with survival data, 70 still were alive at the end of the study, with median follow‐up time of 221 (IQR, 37.5‐411) days, whereas 38 dogs had died. Of the deceased dogs, 18 experienced confirmed cardiac death (euthanasia for progressive heart disease or sudden cardiac death), whereas 4 had a confirmed noncardiac cause of death; the remaining 16 dogs did not have cause of death recorded.
4.4. Univariable predictor analysis
A subset of clinically relevant variables was explored for association with survival time in univariable analysis (Table 4). When considering 2‐year survival from Visit 1, variables significantly associated with survival included ACVIM stage (P = .03), MRSI (P = .002), and serum potassium concentration (P = .03). Neither total daily ACEI dose nor ACEI dose frequency were predictive of 2‐year survival in the full study sample (P = .1 and .43, respectively; Figure 2). However, ACEI dosage ≥0.5 mg/kg/day was associated with 2‐year survival in the subset of dogs in CHF at Visit 1 (n = 48 dogs; P = .04; Figure 3).
TABLE 4.
Univariable analysis exploring variables associated with outcome in 108 dogs receiving angiotensin‐converting enzyme inhibitors (ACEIs) for treatment of cardiac disease with long‐term follow‐up data available, including 61 dogs who experienced congestive heart failure (CHF) during the study period
| 2‐Year survival from Visit 1 (n = 108) | Survival from first onset of CHF (n = 61) | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P‐value | HR | 95% CI | P‐value |
| Structural heart disease (DCM) | 1.74 | 0.64‐4.70 | .3 | 0.82 | 0.24‐2.81 | .8 |
| ACVIM disease stage (C) | 2.42 | 1.06‐5.51 | .03* | 2.28 | 0.92‐5.69 | .07 |
| Heart rate | 1.006 | 0.998‐1.014 | .16 | 1.0027 | 0.99‐1.01 | .6 |
| Blood pressure | 0.99 | 0.98‐1.002 | .13 | 0.99 | 0.98‐1.01 | .3 |
| MRSI | 1.007 | 1.003‐1.011 | .002* | 1.002 | 0.997‐1.007 | .4 |
| Sodium | 1.02 | 0.91‐1.14 | .8 | 0.98 | 0.87‐1.11 | .8 |
| Potassium | 0.39 | 0.17‐0.90 | .03* | 1.15 | 0.48‐2.77 | .8 |
| Total daily ACEI dose | 0.48 | 0.19‐1.16 | .1 | 0.25 | 0.09‐0.70 | .005* |
| ACEI dose frequency (q12h) | 0.69 | 0.28‐1.71 | .43 | 0.78 | 0.26‐3.03 | .8 |
| Pimobendan (yes) | 0.67 | 0.16‐2.82 | .56 | 0.398 | 0.05‐3.00 | .4 |
Note: Hazard ratios (HR) above 1 indicate that higher values of the indicated variable are associated with an increased risk of death, whereas HR below 1 indicate that higher values of the indicated variable are associated with decreased risk of death. Continuous variables are dichotomized at the sample mean. Variables significant in univariable analysis (P‐value <.05) are noted with bolding and an asterisk (*).
Abbreviations: ACVIM, American College of Veterinary Internal Medicine; CI, confidence interval; q12h, twice daily dosing.
FIGURE 2.
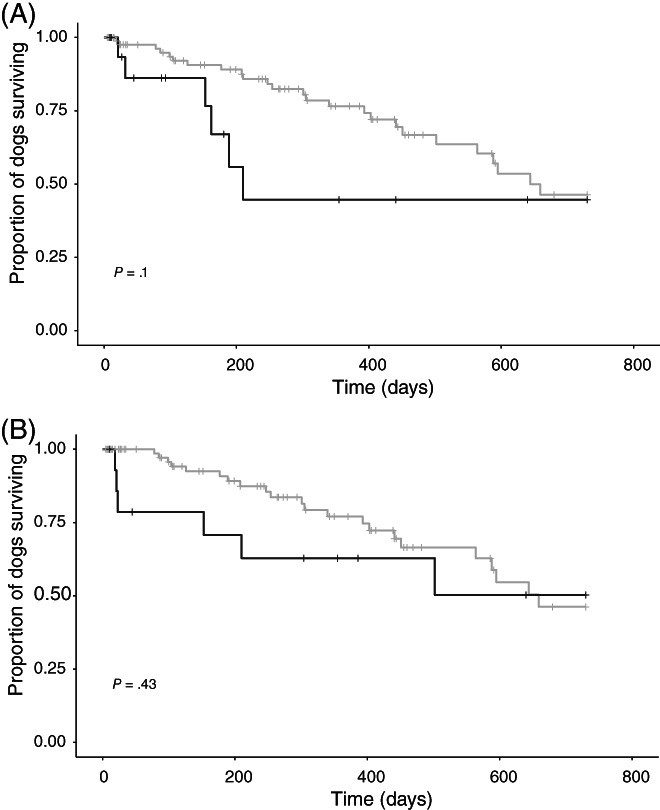
Kaplan‐Meier curves depicting 2‐year survival from Visit 1 in a study sample of 108 dogs receiving angiotensin‐converting enzyme inhibitors (ACEIs) for treatment of cardiac disease with long‐term follow‐up data available, comparing dogs receiving high‐dose (≥0.5 mg/kg/day, gray line) vs low‐dose (<0.5 mg/kg/day, black line) ACEI (A) and dogs receiving q12h (gray line) vs q24h (black line) ACEI dosing (B). Neither ACEI dose (P = .1, A) nor dose frequency (P = .43, B) were significant predictors of 2‐year survival in the full study sample
FIGURE 3.
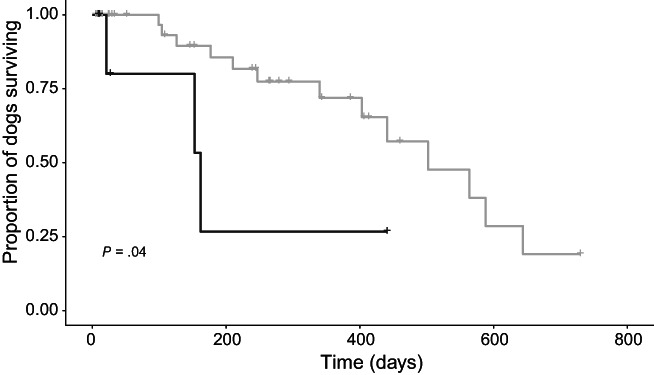
Kaplan‐Meier curve depicting 2‐year survival from Visit 1 for the subset of 48 dogs who were in congestive heart failure at Visit 1 and had long‐term outcome data available, comparing dogs receiving high‐dose angiotensin‐converting enzyme inhibitors (ACEIs; ≥0.5 mg/kg/day, gray line) vs low‐dose (<0.5 mg/kg/day, black line). Higher ACEI dose was associated with higher probability of survival 2 years (P = .04)
Survival analyses also were performed considering time from first onset of CHF to all‐cause death, considering dogs that were in CHF at Visit 1 as well as those that experienced CHF later in the study period (n = 61 dogs, 23 of which died). An ACEI dosage ≥0.5 mg/kg/day was associated with increased survival compared to doses <0.5 mg/kg/day (P = .005; Table 4 and Figure 4).
FIGURE 4.
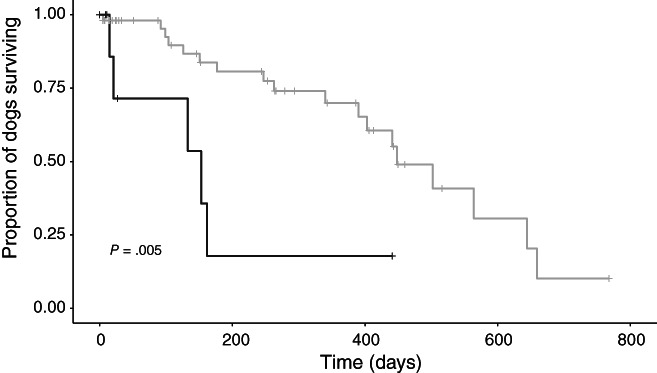
Kaplan‐Meier curve depicting time from first‐onset congestive heart failure (CHF) to all‐cause mortality for the subset of 61 study dogs with survival data that experienced CHF at some point during the study period (at or after Visit 1). Dogs receiving high‐dose angiotensin‐converting enzyme inhibitors (ACEIs; ≥0.5 mg/kg/day, gray) had longer survival from first‐onset CHF compared to dogs receiving lower ACEI doses (black; P = .005)
4.5. Multivariable predictor analysis
All variables with adjusted P values <.1 in the univariable analysis, along with ACEI total daily dose (mg/kg/day) and dose frequency (q24h vs q12h), were considered as candidate predictors in building multivariable models. Variables entered into multivariable modeling for 2‐year survival from Visit 1 were ACVIM stage, MRSI, serum potassium concentration at Visit 1, ACEI total daily dose, and ACEI dose frequency. In the final multivariable model, 2 variables were predictive of improved 2‐year outcome: higher serum potassium concentration at Visit 1 (concentration above vs below sample mean; HR, 0.39; 95% CI, 0.16‐0.97; P = .04) and q12h dosing of ACEI (compared to q24h dosing; HR, 0.30; 95% CI, 0.10‐0.88, P = .03). Inclusion of time as a frailty term did not affect the significance of variables in the final model, nor did it change the log likelihood of the model (−60.3 both with and without the frailty term). Variables entered into multivariate modeling for all‐cause survival from first‐onset CHF were ACVIM Stage, ACEI dose and frequency, and none were found to be significant in the final multivariable analysis utilizing the Akaike information criterion.
5. DISCUSSION
Our retrospective study indicated an association between ACEI dose frequency and long‐term outcome in a sample of dogs with cardiac disease. In multivariable analysis, q12h dosing was associated with higher probability of 2‐year survival in the full study cohort. These results suggest that the clinical benefit of ACEI might be dose‐dependent, with q12h dosing conferring superior cardioprotection. Additional PK/PD studies and prospective clinical trials are needed to confirm this association and determine the ideal dosage of ACEI for dogs with cardiac disease.
Our findings could provide a potential explanation for the failure of previous clinical trials to demonstrate long‐term benefit in dogs with cardiac disease when utilizing ACEI dosages of 0.25‐0.5 mg/kg q24h. Our study sample reflects a limited repertoire of ACEI prescribing practices that is consistent with current consensus guidelines4 but differs from previous clinical trials of ACEIs in veterinary cardiology. The median initial total daily dose of ACEI in our study was 0.84 mg/kg/day, nearly twice the dose utilized in prospective trials of ACEIs in dogs. In addition, >75% of dogs received q12h dosing throughout the study, whereas a minority of dogs received q12h dosing in previous trials.1, 2, 22, 23, 24, 25, 26, 27 Our results indicated a dose‐dependent effect on survival even within this narrow dosing range, even though limited prescribing practices could have increased the risk of type II error. Over 90% of dogs in our study received enalapril (as opposed to benazepril), making it impossible to draw any conclusions about 1 ACEI vs the other.
Importantly, our study could not differentiate the effects of ACEI dose vs frequency on long‐term outcomes. Substantial overlap existed between total daily dose and dose frequency in our study such that all dogs receiving low‐dose ACEIs (<0.5 mg/kg/day) were receiving q24h dosing. It is therefore impossible to determine whether the survival benefit found in our study was associated with higher ACEI dose, q12h dose frequency, or both. Indeed, total daily dose was a more consistent predictor of survival than was dose frequency in univariable analysis, whereas dose frequency was significant in multivariable analysis. Given the high correlation between these predictors, it is not surprising that the multivariable analysis prioritized them differently. Also, univariable statistical methods assess significance based on P values only, whereas the multivariable analysis method selects the best model based on a combination of likelihood and number of variables in the model. Although our study cannot separate the effects of total daily dose from dose frequency, 2 lines of evidence suggest that q12h dosing might be the more clinically relevant factor. First, PK/PD studies in dogs show that duration of RAAS inhibition after ACE administration might be <12 hours14, 17 and that splitting a given ACEI dose into q12h administration could achieve better RAAS inhibition than the full dose given q24h.13 Second, studies in healthy dogs indicate that the circadian rhythm of the RAAS is influenced by time of feeding (ie, sodium intake) such that for dogs fed in the morning, peak RAAS activation occurs approximately 12 hours later during the evening and overnight.18, 19, 20 These results indicate that with an q24h dosing schedule, evening dosing could be more effective than morning dosing, a phenomenon that has been identified in humans receiving ACEIs for systemic hypertension.28, 29, 30 Because the recommended timing of q24h dosing is rarely standardized in veterinary prescriptions, q12h dosing may be superior by ensuring consistent RAAS suppression regardless of chronobiology or individual feeding schedule. Regardless of the relative importance of dose vs frequency, our results suggest that dosages utilized in previous clinical trials in dogs with cardiac disease (<0.5 mg/kg q24h) might not optimize the potential cardioprotective effect of ACEIs, and provide rationale to support the higher ACEI dosage recommended in current consensus guidelines (0.5 mg/kg q12h).4
In addition to the total daily dose of ACEI, our study also explored other clinical variables associated with long‐term outcome in dogs with cardiac disease. Not surprisingly, ACVIM disease stage was predictive of survival, with stage C dogs (current or prior CHF) having worse outcome than stage B2 (advanced preclinical) dogs. Previous studies have identified advanced age, higher heart rate, and murmur intensity as negative prognostic indicators in MMVD,23, 24, 31, 32 whereas presence of tachyarrhythmias is associated with poorer outcome in dogs with DCM.33, 34 These variables were not predictive of all‐cause survival in our study, perhaps because the study sample included dogs both with MMVD and DCM. Lower serum potassium concentration at Visit 1 was predictive of nonsurvival in our study, consistent with findings in humans with heart failure.35, 36 Proposed mechanisms for this association include hypokalemia‐induced abnormalities in myocardial metabolism or increased risk of ventricular arrhythmias. The MRSI is a novel clinical index of MMVD severity created by the primary author of the VETPROOF study24 with the goal of stratifying cardiovascular risk in dogs with MMVD. The MRSI incorporates 3 variables previously shown to be predictive of outcome in MMVD (age, heart rate, and left atrial dimension indexed to aortic dimension), with age and heart rate normalized based on average values from the VETPROOF dataset. The MRSI performed well as a significant predictor of all‐cause mortality within the subset of MMVD dogs in our study, suggesting that this index might have prognostic utility in dogs with MMVD. Definitive conclusions cannot be drawn however because assessment of this index was not a primary aim of our study.
Our study also provides an opportunity to assess the safety and tolerability of ACEIs in dogs with cardiac disease. Enalapril and benazepril were well‐tolerated in our study sample, with only 8/144 (5.6%) dogs experiencing adverse effects that prompted dose decrease or discontinuation. Indeed, ACEI dose escalation was over 3 times more common (19% of dogs) than dose decrease or discontinuation in our study. This high tolerability is consistent with prospective clinical trials of ACEIs in dogs with cardiac disease, which consistently report no difference in adverse effects between ACEIs and either placebo1, 2, 22, 23, 24, 27 or positive control.26, 37 The most common adverse effects prompting ACEI dose adjustment in our study were increased renal function test results and hypotension, although it is impossible to determine if these adverse effects were truly drug‐related without a comparator control group. No statistically significant changes were found in BP, renal function test results, or serum electrolyte concentrations between Visits 1 and 2 in our study, except in the subgroup of dogs with CHF (that were concurrently receiving furosemide, and experienced an increase in BUN and decrease in serum chloride concentration). Lack of significant change in BP after ACEI administration is not surprising, because ACEIs are known to have only weak antihypertensive effect in healthy dogs (systolic blood pressure decrease of approximately 10 mm Hg).6, 38 Furthermore, some dogs in our study were in active CHF at Visit 1, and thus might have had decreased cardiac output that improved with CHF treatment by Visit 2. Consistency in renal function test results during ACEI administration also has been reported previously. Although azotemia is a recognized potential adverse effect of ACEIs in dogs,1, 22 studies have found no difference in renal function test results between dogs treated long‐term with ACEIs compared to placebo39 or pimobendan.37 Overall, our findings support the assertion that ACEIs are well‐tolerated with low risk of worsening renal function in dogs with cardiovascular disease.
In addition to the previously mentioned confounding of ACEI dose and frequency, several other limitations must be considered when interpreting the results of our study, particularly those inherent to any retrospective design. All dogs in the study received an ACEI; there was no control group receiving placebo or no treatment. Treatment protocols were not standardized, and ACEI dose and frequency were determined by individual clinician preferences. Cases were collected over a wide date range involving multiple clinicians and clinical services at an academic referral hospital. Data relevant to RAAS chronobiology, including time of ACEI dosing, time of feeding, and time of diagnostic testing, were not recorded. Cause of death only was recorded in a small number of dogs. As a result, these analyses only assessed all‐cause death, and we cannot comment on the effect of ACEI or other variables on cardiac death specifically.
Our results suggest that q12h ACEI dosing might be associated with improved long‐term survival compared to q24h dose frequency. Administration of ACEI at a median dose of 0.84 mg/kg/day was well‐tolerated in this large group of dogs with cardiac disease. These findings provide a potential explanation for the failure of clinical trials to identify a clinical benefit of ACEI in dogs with cardiovascular disease, because all such previous trials have utilized ACEI dosages <0.5 mg/kg q24h. Additional PK/PD and prospective clinical studies will be needed to determine the ideal dosage of ACEIs to optimize RAAS suppression and cardioprotective benefit in dogs.
CONFLICT OF INTEREST DECLARATION
Drs. Ward and Mochel have received consulting fees and honoraria from Ceva Sante Animale. Dr. Dorman's work is supported in part by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) Hatch project IOW03617. The content of this paper is however solely the responsibility of the authors and does not represent the official views of Ceva Sante Animale or the NIFA/USDA.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Ward JL, Chou Y‐Y, Yuan L, Dorman KS, Mochel JP. Retrospective evaluation of a dose‐dependent effect of angiotensin‐converting enzyme inhibitors on long‐term outcome in dogs with cardiac disease. J Vet Intern Med. 2021;35(5):2102‐2111. 10.1111/jvim.16236
REFERENCES
- 1.COVE Study Group . Controlled clinical evaluation of enalapril in dogs with heart failure: results of the Cooperative Veterinary Enalapril Study Group. J Vet Intern Med. 1995;9:243‐252. [DOI] [PubMed] [Google Scholar]
- 2.BENCH (BENazepril in Canine Heart disease) Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multicenter, prospective, randomized, double‐blinded, placebo‐controlled, long‐term clinical trial. J Vet Cardiol. 1999;1:7‐18. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Kjekshus J, Snapinn S. Long‐term survival in severe heart failure in patients treated with enalapril: ten year follow‐up of CONSENSUS I. Eur Heart J. 1999;20:136‐139. [DOI] [PubMed] [Google Scholar]
- 4.Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33:1127‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefebvre HP, Toutain PL. Angiotensin‐converting enzyme inhibitors in the therapy of renal diseases. J Vet Pharmacol Ther. 2004;27:265‐281. [DOI] [PubMed] [Google Scholar]
- 6.Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2018;32:1803‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaden SL, Elliott J. Management of proteinuria in dogs and cats with chronic kidney disease. Vet Clin North Am Small Anim Pract. 2016;46:1115‐1130. [DOI] [PubMed] [Google Scholar]
- 8.King JN, Font A, Rousselot JF, et al. Effects of benazepril on survival of dogs with chronic kidney disease: a multicenter, randomized, blinded, placebo‐controlled clinical trial. J Vet Intern Med. 2017;31:1113‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IRIS Canine GN Study Group Standard Therapy Subgroup . Consensus recommendations for standard therapy of glomerular disease in dogs. J Vet Intern Med. 2013;27:S27‐S43. [DOI] [PubMed] [Google Scholar]
- 10.Regulski M, Regulska K, Stanisz BJ, et al. Chemistry and pharmacology of angiotensin‐converting enzyme inhibitors. Curr Pharm Des. 2014;21:1764‐1775. [DOI] [PubMed] [Google Scholar]
- 11.Toutain PL, Lefèbvre HP. Pharmacokinetics and pharmacokinetic/pharmacodynamic relationships for angiotensin‐converting enzyme inhibitors. J Vet Pharmacol Ther. 2004;27:515‐525. [DOI] [PubMed] [Google Scholar]
- 12.King JN, Mauron C, Kaiser G. Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin‐converting enzyme activity after single and repeated administrations to dogs. Am J Vet Res. 1995;56:1620‐1628. [PubMed] [Google Scholar]
- 13.Toutain PL, Lefebvre HP, King JN. Benazeprilat disposition and effect in dogs revisited with a pharmacokinetic/pharmacodynamic modeling approach. J Pharmacol Exp Ther. 2000;292:1087‐1093. [PubMed] [Google Scholar]
- 14.Hamlin RL, Nakayama T. Comparison of some pharmacokinetic parameters of 5 angiotensin‐converting enzyme inhibitors in normal beagles. J Vet Intern Med. 1998;12:93‐95. [DOI] [PubMed] [Google Scholar]
- 15.van de Wal RMA, Plokker HWM, Lok DJA, et al. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367‐372. [DOI] [PubMed] [Google Scholar]
- 16.Mochel JP, Peyrou M, Fink M, et al. Capturing the dynamics of systemic renin‐angiotensin‐aldosterone system (RAAS) peptides heightens the understanding of the effect of benazepril in dogs. J Vet Pharmacol Ther. 2013;36:174‐180. [DOI] [PubMed] [Google Scholar]
- 17.Mochel JP, Fink M, Peyrou M, Soubret A, Giraudel JM, Danhof M. Pharmacokinetic/pharmacodynamic modeling of renin‐angiotensin aldosterone biomarkers following angiotensin‐converting enzyme (ACE) inhibition therapy with benazepril in dogs. Pharm Res. 2015;32:1931‐1946. [DOI] [PubMed] [Google Scholar]
- 18.Mochel J, Fink M, Bon C, et al. Influence of feeding schedules on the chronobiology of renin activity, urinary electrolytes and blood pressure in dogs. Chronobiol Int. 2014;31:715‐730. [DOI] [PubMed] [Google Scholar]
- 19.Mochel J, Fink M, Peyrou M, et al. Chronobiology of the renin‐angiotensin‐aldosterone system in dogs: relation to blood pressure and renal physiology. Chronobiol Int. 2013;30:1144‐1159. [DOI] [PubMed] [Google Scholar]
- 20.Mochel J, Danhof M. Chronobiology and pharmacologic modulation of the renin‐andiogensin‐aldosterone system in dogs: what have we learned? Rev Physiol Biochem Pharmacol. 2015;169:43‐69. [DOI] [PubMed] [Google Scholar]
- 21.The IMPROVE Study Group . Acute and short‐term hemodynamic, echocardiographic, and clinical effects of enalapril maleate in dogs with naturally acquired heart failure: results of the Invasive Multicenter PROspective Veterinary Evaluation of enalapril study. J Vet Intern Med. 1995;9:234‐242. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger S, Benitz A, Ericsson G, et al. Effects of enalapril maleate on survival of dogs with naturally acquired heart failure. The Long‐Term Investigation of Veterinary Enalapril (LIVE) Study Group. J Am Vet Med Assoc. 1998;213:1573‐1577. [PubMed] [Google Scholar]
- 23.Kvart C, Häggström J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med. 2002;16:80‐88. [PubMed] [Google Scholar]
- 24.Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc. 2007;231:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 25.Borgarelli M, Ferasin L, Lamb K, et al. DELay of Appearance of sYmptoms of canine degenerative mitral valve disease treated with spironolactone and benazepril: the DELAY study. J Vet Cardiol. 2020;27:34‐53. [DOI] [PubMed] [Google Scholar]
- 26.Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22:1124‐1135. [DOI] [PubMed] [Google Scholar]
- 27.O'Grady M, O'Sullivan M, Minors S, Horne R. Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman pinschers. J Vet Intern Med. 2009;23:977‐983. [DOI] [PubMed] [Google Scholar]
- 28.Hermida RC, Ayala DE, Fernández JR, et al. Administration‐time differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int. 2013;30:28‐314. [DOI] [PubMed] [Google Scholar]
- 29.Hermida RC, Ayala DE, Mojón A, Smolensky MH, Portaluppi F, Fernández JR. Sleep‐time ambulatory blood pressure as a novel therapeutic target for cardiovascular risk reduction. J Hum Hypertens. 2014;28:567‐574. [DOI] [PubMed] [Google Scholar]
- 30.Hermida RC, Crespo JJ, Domínguez‐Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia chronotherapy trial. Eur Heart J. 2019;41:4565‐4576. [DOI] [PubMed] [Google Scholar]
- 31.Mattin MJ, Boswood A, Church DB, Brodbelt DC. Prognostic factors in dogs with presumed degenerative mitral valve disease attending primary‐care veterinary practices in the United Kingdom. J Vet Intern Med. 2019;33:432‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattin MJ, Brodbelt DC, Church DB, Boswood A. Factors associated with disease progression in dogs with presumed preclinical degenerative mitral valve disease attending primary care veterinary practices in the United Kingdom. J Vet Intern Med. 2019;33:445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvert CA, Pickus CW, Jacobs GJ, Brown J. Signalment, survival, and prognostic factors in Doberman pinschers with end‐stage cardiomyopathy. J Vet Intern Med. 1997;11:323‐326. [DOI] [PubMed] [Google Scholar]
- 34.Calvert C, Hall G, Jacobs G, Pickus C. Clinical and pathologic findings in Doberman pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure: 54 cases (1984‐1991). J Am Vet Med Assoc. 1997;210:505‐511. [PubMed] [Google Scholar]
- 35.Ahmed A, Zannad F, Love TE, et al. A propensity‐matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishihara T, Tokitsu T, Sueta D, et al. Serum potassium and cardiovascular events in heart failure with preserved left ventricular ejection fraction patients. Am J Hypertens. 2018;31:1098‐1105. [DOI] [PubMed] [Google Scholar]
- 37.Chetboul V, Lefebvre HP, Sampedrano CC, et al. Comparative adverse cardiac effects of pimobendan and benazepril monotherapy in dogs with mild degenerative mitral valve disease: a prospective, controlled, blinded, and randomized study. J Vet Intern Med. 2007;21:742‐753. [DOI] [PubMed] [Google Scholar]
- 38.Sakatani A, Miyagawa Y, Takemura N. Evaluation of the effect of an angiotensin‐converting enzyme inhibitor, alacepril, on drug‐induced renin–angiotensin–aldosterone system activation in normal dogs. J Vet Cardiol. 2016;18:248‐254. [DOI] [PubMed] [Google Scholar]
- 39.Atkins CE, Brown WA, Coats JR, et al. Effects of long‐term administration of enalapril on clinical indicators of renal function in dogs with compensated mitral regurgitation. J Am Vet Med Assoc. 2002;221:654‐658. [DOI] [PubMed] [Google Scholar]


