Abstract
Background
Approaches to the evaluation of pulmonary arterial hypertension (PAH) in premature calves by using lung‐specific epithelial and endothelial biomarkers are needed.
Objective
To investigate the evaluation of PAH in premature calves with and without respiratory distress syndrome (RDS) by using lung‐specific epithelial and endothelial biomarkers and determine the prognostic value of these markers in premature calves.
Animals
Fifty premature calves with RDS, 20 non‐RDS premature calves, and 10 healthy term calves.
Methods
Hypoxia, hypercapnia, and tachypnea were considered criteria for RDS. Arterial blood gases (PaO2, PaCO2, oxygen saturation [SO2], base excess [BE], and serum lactate concentration) were measured to assess hypoxia. Serum concentrations of lung‐specific growth differentiation factor‐15 (GDF‐15), asymmetric dimethylarginine (ADMA), endothelin‐1 (ET‐1), vascular endothelial growth factor (VEGF), and surfactant protein D (SP‐D) were measured to assess PAH.
Results
Arterial blood pH, PaO2, SO2, and BE of premature calves with RDS were significantly lower and PaCO2 and lactate concentrations higher compared to non‐RDS premature and healthy calves. The ADMA and SP‐D concentrations of premature calves with RDS were lower and serum ET‐1 concentrations higher than those of non‐RDS premature and healthy calves. No statistical differences for GDF‐15 and VEGF were found among groups.
Conclusions and Clinical Importance
Significant increases in serum ET‐1 concentrations and decreases in ADMA and SP‐D concentrations highlight the utility of these markers in the diagnosis of PAH in premature calves with RDS. Also, we found that ET‐1 was a reliable diagnostic and prognostic biomarker for PAH and predicting mortality in premature calves.
Keywords: endothelial biomarkers, premature calf, pulmonary epithelium, pulmonary hypertension, RDS
Abbreviations
- ADMA
asymmetric dimethylarginine
- ARDS
acute respiratory distress syndrome
- BE
base excess
- ET‐1
endothelin‐1
- FiO2
fraction of inspired oxygen
- GDF‐15
lung‐specific growth differentiation factor‐15
- iNOS
nitric oxide synthase
- NO
nitric oxide
- non‐RDS
without respiratory distress syndrome
- PaCO2
partial pressure of carbon dioxide
- PAH
pulmonary arterial hypertension
- PaO2, PHT
pulmonary hypertension; partial pressure of oxygen
- RDS
respiratory distress syndrome
- ROC
receiver operating characteristic curve
- SO2
oxygen saturation
- SP‐D
surfactant protein‐D
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
In cows and heifers, average perinatal mortality on dairy farms ranges from 2% to 20%, and in developed countries between 5% and 8%. Premature birth remains an important and common cause of calf mortality in the world.1, 2, 3, 4 The most important problems in premature infants is respiratory distress syndrome (RDS) that leads to increased respiratory effort and inadequate oxygen exchange.5, 6, 7, 8 The RDS caused by surfactant insufficiency is associated with pulmonary hypertension (PHT) in lambs.9 In premature newborns, the lung lobes cannot inflate effectively because of surfactant deficiency, and hypoxia develops because gas exchange does not occur adequately. As a result, alveolar and interstitial edema develop related to interstitial inflammation and endothelial and epithelial damage. Developing hypoxia and alveolar and interstitial edema cause narrowing of the pulmonary arteries and therefore development of pulmonary arterial hypertension (PAH).10, 11, 12 Studies in premature infants have shown that the development of PAH substantially increases mortality.13, 14 Recently, in human medicine, a noninvasive method for determining PAH employs biomarkers specific to pulmonary epithelial and endothelial damage.13, 15, 16, 17, 18, 19
Growth differential factor‐15 (GDF‐15), asymmetric dimethylarginine (ADMA), endothelial‐1 (ET‐1), vascular endothelial growth factor (VEGF), and surfactant protein‐D (SP‐D) concentrations were found to change significantly in infants with PHT.15, 18, 20, 21, 22, 23 The concentration of GDF‐15 is used as a biomarker to evaluate the prognosis of PHT.21, 23 These biomarkers are used to determine the proliferation and apoptosis of endothelial cells in PHT cases.23 Asymmetric dimethylarginine is a naturally occurring amino acid that prevents production of nitric oxide (NO), an end product of oxidative stress. Increased concentrations of ADMA attenuate NO concentrations leading to an increase in vascular tone. Therefore, it has been suggested that ADMA can be considered a useful biomarker in PHT cases.18
Endothelial‐1 is a peptide abundantly present in the human lung and plays an important role in the development of PHT because of the presence of endothelin receptors on vascular smooth muscle cells.13 Earlier studies indicated that the concentration of ET‐1 in plasma and lung tissue is significantly increased in patients with PHT.15, 22 Vascular endothelial growth factor and its receptor concentrations were found to be increased with damage to pulmonary vessels in patients with PHT.15 Furthermore, VEGF concentrations also increased significantly during hypoxic conditions.24 Surfactant protein‐D is secreted by type II pneumocytes and plays an important role in ensuring and maintaining the surface integrity of alveoli. In patients with acute RDS (ARDS), SP‐D concentrations decreased with destruction of type II pneumocytes in the lungs depending on the severity of damage to the lung.25 The changes in the SP‐D concentration in ARDS patients could provide valuable information about the prognosis of the disease.16, 26
Therefore, we aimed to determine: (a) whether PAH had developed in premature calves with and without RDS by evaluating lung epithelial and endothelial damage biomarkers and (b) the importance of these biomarkers in predicting mortality in affected calves.
2. MATERIALS AND METHODS
2.1. Animals
The experimental groups consisted of 50 premature calves with RDS (28 Holstein, 19 Simmental, 2 Brown‐Swiss) and 20 premature calves without RDS (12 Holstein, 7 Simmental, 1 Brown‐Swiss) admitted to the Selcuk University Large Animal Hospital of the Faculty of Veterinary Medicine were enrolled in the study.
Ten healthy normal‐term calves (6 Holstein, 2 Simmental, 2 Brown‐Swiss) from the Faculty Farm also were examined. The gestational age range for premature calves was 230 to 255 days and >280 days for healthy calves.
2.2. Clinical evaluation
Upon admission of premature calves to the clinic, history was taken, and live weight, age, and breed were recorded. Routine clinical examinations of all calves were performed. According to clinical observations and blood gas analysis, premature calves that met the criteria for RDS were enrolled in the trial group, premature calves without RDS were enrolled in the positive control group, and healthy normal‐term calves were enrolled in the negative control group. The criteria for prematurity were decreased gestation period (<255 days), low body weight (BW), a short silky hair coat, incomplete eruption of incisors, soft hooves, and weak or no suckling reflex.7, 8, 27
2.3. Criteria for definition of RDS
Hypoxia (PaO2 < 60 mm Hg), hypercapnia (PaCO2 > 45 mm Hg), tachypnea (breaths per minute >45/min), and abdominal respiration with wheezing were the criteria for distinguishing between calves with or without RDS.7, 8, 27, 28 A PaO2 <60 mm Hg and at least 2 other criteria described above were required for a case to be diagnosed as RDS.
2.4. Collection of blood samples
Blood samples were collected from the calves for arterial blood gas analysis and lung‐specific biomarker measurements at the time of admission. For uniformity among the groups, all blood samples were taken within the first 12 hours after birth. Blood samples for serum were taken from jugular vein and for blood gas measurement from auricular arteries. Nonanticoagulant tubes were used for serum and sodium heparin‐containing plastic syringes were used for blood gas measurement. Blood samples taken for biochemical analyses were kept at room temperature for 15 minutes, then centrifuged at 2000g for 10 minutes. Sera were removed and stored at −80°C. Blood gas measurements were performed within 5 to 10 minutes of collection.
2.5. Blood gas analysis
Heparinized arterial blood pH, PaCO2, PaO2, oxygen saturation (SO2), base excess (BE), and lactate concentration were measured using a GEM Premier Plus 3000 analyzer (74351, Blood Gas/ Electrolyte Analyzer, Model 5700; Instrumentation Laboratories, Massachusetts).
2.6. Lung‐specific endothelial and epithelial biomarker analyses
Serum GDF‐15, ADMA, ET‐1, VEGF, and SP‐D concentration of all calves were measured using commercial bovine‐specific ELISA test kits according to the manufacturer's instructions (Bioassay Technology Laboratory, Shanghai, China): bovine growth differentiation factor ELISA kit (Bioassay Technology Laboratory, LOT:202006012), bovine asymmetrical dimethylarginine ELISA kit (Bioassay Technology Laboratory, LOT:202006012), bovine endothelin‐1 ELISA kit (Bioassay Technology Laboratory, LOT:202006012), bovine vascular endothelial cell growth factor ELISA kit (Bioassay Technology Laboratory, LOT:202006012), and bovine SP‐D ELISA kit (Bioassay Technology Laboratory, LOT:202006012). Intra‐assay coefficients of variation, inter‐assay coefficients of variation, and detectable ranges were ≤8%, ≤10%, and 7‐1500 ng/L for GDF‐15; ≤8%, ≤10%, and 0.05‐10 nmol/mL for ADMA; ≤8%, ≤10%, and 2‐600 ng/L for ET‐1; ≤8%, ≤10%, and 15‐3000 ng/L for VEGF; and ≤8%, ≤10%, and 1‐400 ng/L for SP‐D, respectively.
2.7. Treatment protocol
In premature calves without RDS, only the standard treatment protocol was applied whereas premature calves with RDS were treated using a standard treatment protocol along with oxygen application and nebulizer treatment.
2.7.1. Standard treatment
Standard treatment consisted of 5 mL calcium (Calcio PH, Fatro, Istanbul, Turkey), 3 mL phosphorus (Metafos, Teknovet, Istanbul, Turkey) and 3 mL vitamin C (Vita‐C Vetoquinol, Novakim, Kocaeli, Turkey) q12h for 3 days, 10 mg/kg BW erythromycin (Erivet, Biomed, Istanbul, Turkey) q24h for 3 days, vitamins A, D, and E (Adesol AD3E, Topkim, Istanbul, Turkey), and selenium and vitamin E (Selephos, Topkim, Istanbul, Turkey) 1 mL once IM. A 1.3% solution of NaHCO3 (Carbotek, Teknovet, Istanbul, Turkey; 250‐500 mL) was administered to calves with base deficits, and 5% dextrose (5% Dextrose Mediflex, Istanbul, Turkey) solution (100‐350 mL) was administered to calves with hypoglycemia IV. In addition, calves received septiserum once SC (Septicol, Adıyaman, Turkey).
2.7.2. Oxygen application
Oxygen was administered to premature calves with RDS. Oxygen was passed through water to humidify it and given to the calves using a suitable oxygen mask. Oxygen initially was administered intranasally 15 minutes at a flow rate of 5 to 6 L/min for 3 hours with a 10‐minute break after each 15 minutes. After 3 hours, the oxygen flow rate was decreased to 3 to 4 L/min. Oxygen treatment was continued until SO2 reached 80%.7, 8
2.7.3. Nebulizer application
An ultrasonic nebulization device (NebuTech Brand SoHuMa II model, Elsenfeld, Germany) and combinations of formoterol, furosemide, and fluticasone were administered to calves with RDS at doses mentioned below. After each use and before use in a new patient, the device was cleaned, and sterilization procedures were performed in accordance with the procedure.
2.7.4. Doses of inhalation drugs
The nebulization form of fluticasone (fluticasone propionate, Flixotide, GlaxoSmithKline, Istanbul, Turkey) was diluted with 2.5 mL saline solution and administered at a dosage of 15 μg/kg BW over 5 minutes by nebulization for 3 days with 12 hours between treatments.7, 8 The nebulization form of formoterol (Foradil, Novartis, Ankara, Turkey) was diluted with 2.5 mL saline solution and administered at a dosage of 12 μg/kg BW over 5 minutes by nebulization for 3 days with 12 hours between treatments.8 The parenteral form of furosemide (Desal, Munir Sahin İlac, Istanbul, Turkey) was diluted with 2.5 mL saline solution and administered at a dosage of 1 mg/kg BW over 5 minutes by nebulization for 3 days with 12 hours between treatments.7, 8
2.8. Statistical analysis
The statistics program SPSS 25 (IBM Corp. 2017) was used to evaluate the data. The Kolmogorov‐Smirnov test was used to determine the normality of variables and the homogeneity of variances. Parametric data were evaluated using one‐way analysis of variance (ANOVA) and the post hoc Tukey test using mean ± SD and nonparametric data were evaluated using the Mann‐Whitney U test as median (minimum/maximum). For detection of correlation between variables, Spearman correlation test and linear regression analysis were used. The prognostic value of ET‐1 was evaluated using receiver operating characteristic (ROC) curve analysis to determine the prognostic cutoffs for the best differentiation between survivors and nonsurvivors. P < .05 and P < .01 were considered significant.
3. RESULTS
3.1. Clinical findings
The mean weights of the calves were 22.32 ± 4.90 kg in the RDS group, 23.35 ± 3.70 kg in the non‐RDS calf group, and 44.60 ± 2.83 kg in the control group. Thirty‐five (70%) of the 50 calves in the RDS group, and 19 (95%) of the 20 calves in the non‐RDS group survived, whereas 15 calves in the RDS group and 1 calf in the non‐RDS group died within 48 hours.
Clinical findings such as short gestational period, low BW, teeth not fully separated from the gums, short and soft hair, soft hooves, weakness in muscles and tendons, and weak or no suckling reflex were observed in the premature calves. Additionally, apnea or tachypnea, abdominal or wheezing respirations, cyanotic or pale mucous membranes, prolonged capillary refill time, and hypothermia were present in premature calves with RDS.
Some calves in the RDS group also had abdominal distention after PO feeding and could not eliminate meconium without enema assistance. Treated premature calves had increased interest in the environment, attempted to maintain sternal position with support, and experienced decreased wheezing respirations within 24 hours. After 48 hours of treatment, increased suckling reflexes, efforts to stand up with support, and costo‐abdominal respiration were observed. After 72 hours, premature calves had good suckling reflex and were able to stand up and walk.
3.2. Blood gas analysis
Arterial blood gas parameters of premature and healthy calves are presented in Table 1. In the RDS group, pH, PaO2, SO2, and BE were significantly lower whereas PaCO2 and lactate concentrations were higher than in the non‐RDS and control groups. Compared to the control group, PaO2 was significantly lower (P < .05) in the non‐RDS group (Table 1).
TABLE 1.
Arterial blood gas parameters of premature and healthy calves
| Parameters | RDS group (n:50) | Non‐RDS group (n: 20) | Control group (n: 10) |
|---|---|---|---|
| pH | 7.14 ± 0.16B | 7.40 ± 0.66A | 7.40 ± 0.43A |
| PaCO2 (mm Hg) | 56.76 ± 11.19A | 39.02 ± 4.48B | 40.90 ± 4.33B |
| PaO2 (mm Hg) | 28.76 ± 8.10C | 52.11 ± 11.57B | 63.80 ± 11.07A |
| SO2% | 52.88 ± 23.59B | 86.31 ± 9.48A | 90.90 ± 4.33A |
| Lac (mmol/L) | 7.30A (3‐23) | 2.95BC (0.9‐9.20) | 3.60C (1.90‐6.9 |
| BE (mmol/L) median/(min/max) | −6.50A (−29.00 to 11.20) | −0.82B (−9.50 to 5.70) | 1.15B (−3.70 to 6.30) |
Note: Different letters in the same line are statistically significant (P < .05).
Abbreviations: BE, base deficit; Lac, lactate; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; SO2, oxygen saturation.
3.3. Biomarker analysis
Serum biomarker concentrations in the premature and healthy calves are presented in Table 2. Serum ADMA and SP‐D concentrations of the RDS group were found to be significantly lower than those of the control group (P < .05). Serum ET‐1 concentration of the RDS group were significantly higher than those of the non‐RDS and control groups (P < .05). No statistical differences (P > .05) were found among all groups for GDF‐15 and VEGF concentrations (Table 2). Correlations between arterial blood gas parameters and ADMA, ET‐1, and SP‐D concentrations in the premature and healthy calves are presented in Table 3. Positive correlations between blood pH and PaO2, SO2, and BE were found whereas a negative correlation was found among PaCO2, lactate, and ET‐1 concentrations (P < .01). A positive correlation between blood PaCO2 and lactate concentrations (P < .01) and ET‐1 (P < .05) and negative correlations among PaO2, SO2, and BE (P < .01) were identified. Positive correlations between blood PaO2 and SO2 (P < .01), BE (P < .05), and SP‐D (P < .05), and negative correlation between lactate and ET‐1 concentrations (P < .01) were found. A negative correlation between lactate concentration and BE and a positive correlation with ET‐1 concentration (P < .01) were found. A negative correlation was identified between blood BE and ET‐1 concentrations (P < .01).
TABLE 2.
Biomarker concentrations measured in premature and healthy calves
| Variables | RDS group (n: 50) | Non‐RDS group (n: 20) | Control group (n: 100) |
|---|---|---|---|
| ADMA (nmol/mL) | 0.48 ± 0.09B | 0.52 ± 0.06AB | 0.56 ± 0.09A |
| ET‐1 (ng/L) median/(min/max) | 18.25A (0.08‐49.40) | 7.59BC (0.08‐25.88) | 5.56C (3.71‐12.12) |
| GDF‐15 (ng/L) | 62.50 ± 12.94 | 68.14 ± 11.72 | 64.73 ± 9.36 |
| SP‐D (ng/mL) | 42.52 ± 11.40B | 49.35 ± 6.87AB | 51.28 ± 10.58A |
| VEGF (ng/L) | 109.22 ± 20.02 | 116.83 ± 13.05 | 115.87 ± 19.26 |
Note: Different letters in the same line are statistically significant (P < .05).
Abbreviations: ADMA, asymmetric dimethylarginine; ET‐1, endothelin‐1; GDF‐15, growth differentiation faktor‐15; SP‐D, surfactant protein; VEGF, vascular endothelial growth factor.
TABLE 3.
Correlation results between arterial blood gas parameters and ADMA, ET‐1, and SP‐D concentrations in premature and healthy calves (Pearson correlation analysis)
| Parameters | pH | PaCO2 | PaO2 | SO2 | Lac | BE | ADMA | ET‐1 | SP‐D |
|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | −0.76** | 0.51** | 0.63** | −0.80** | 0.85** | 0.09 | −0.38** | 0.16 |
| PaCO2 | 1 | −0.60** | −0.66** | 0.52** | −0.40** | −0.05 | 0.26* | −0.08 | |
| PaO2 | 1 | 0.79** | −0.33** | 0.27* | 0.21 | −0.31** | 0.25* | ||
| SO2 | 1 | −0.46** | 0.39** | 0.09 | −0.44** | 0.18 | |||
| Lac | 1 | −0.82** | −0.09 | 0.38** | −0.17 | ||||
| BE | 1 | 0.07 | −0.37** | 0.14 | |||||
| ADMA | 1 | −0.42** | 0.82** | ||||||
| ET‐1 | 1 | −0.44** | |||||||
| SP‐D | 1 |
Abbreviations: ADMA, asymmetric dimethylarginine; BE, base deficit; ET‐1, endothelin‐1; Lac, lactate; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; SO2, oxygen saturation; SP‐D, surfactant protein.
P < .05.
P < .01.
Correlation results between some arterial blood gas parameters and ET‐1 concentrations in the premature and healthy calves are presented in Table 3 and Figure 1. A negative correlation between ADMA and ET‐1 concentrations (P < .01; Table 3; Figure 1) and a positive correlation with SP‐D concentrations (P < .01) were identified (Table 3; Figure 2). A negative correlation (P < .01) was found between ET‐1 and SP‐D concentrations (Figure 2).
FIGURE 1.
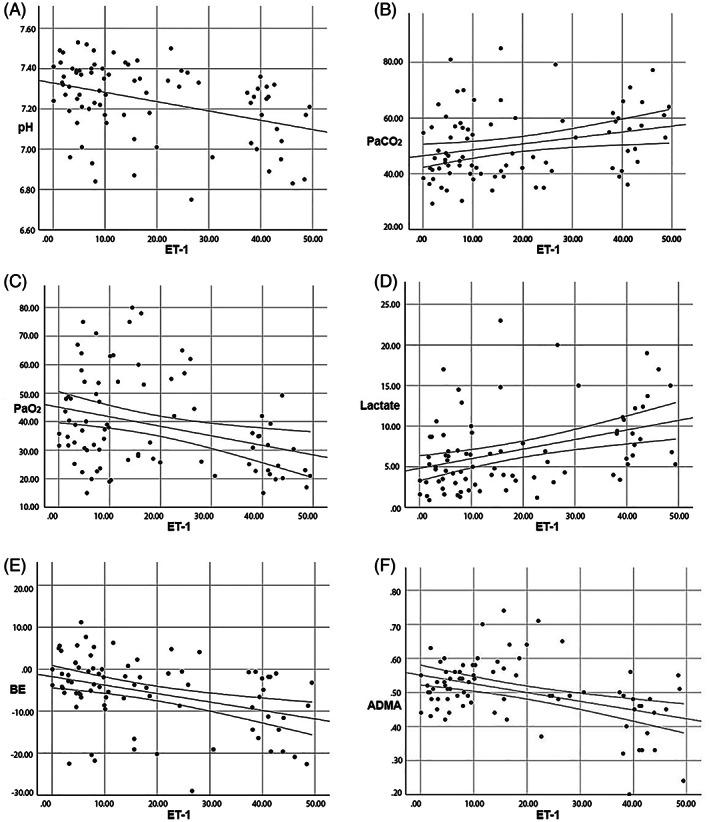
Linear regression analysis graphs between ET‐1 concentrations and pH (A), PaCO2 (B), PaO2 (C), BE (E), and ADMA (F) concentrations. ET‐1 concentrations were determined positive correlation PaCO2 (B) and lactate concentrations (D). ADMA, asymmetric dimethylarginine; BE, base deficit; ET‐1, endothelin‐1; Lac, lactate; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; SO2, oxygen saturation
FIGURE 2.
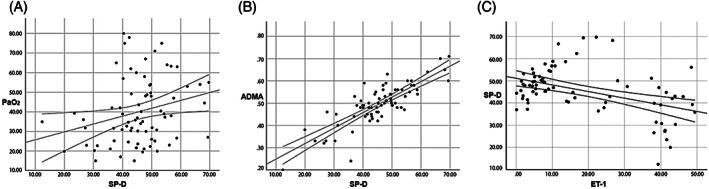
Linear regression analysis graph between SP‐D levels and PaO2, ADMA, and ET‐1 concentrations. A positive correlation between serum SP‐D concentrations and PaO2 (A) and ADMA (B) was determined whereas a negative correlation was found ET‐1 concentrations (C). ADMA, asymmetric dimethylarginine; ET‐1, endothelin‐1; PaO2, arterial partial pressure of oxygen; SP‐D, surfactant protein
The results of the premature calves also showed that the concentrations of ET‐1 from the nonsurvivor calves were significantly higher than those of the survivor calves (P < .001; Table 4). Receiver operating characteristic curve (ROC) analysis for the utility of ET‐1 in differentiating between the survivor and nonsurvivor calves estimated an area under the curve (AUC) of 0.884 (P = .000, 95% confidence interval [CI], 0.806‐0.961; Table 5; Figure 3).
TABLE 4.
Comparison of endothelin‐1 (ET‐1) concentrations in calves that died or survived (Median [min‐max])
| Variable | Survivor (n: 54) | Nonsurvivor (n: 16) | P value |
|---|---|---|---|
| ET‐1(ng/L) | 8.05 (0.08‐48.69) | 39.58 (15.63‐49.40) | .000 |
TABLE 5.
Importance of ET‐1 concentration in mortality prediction as a result of receiver operating characteristic curve (ROC) analysis
| Variable | AUC | SE | P value | 95% Cl | Cutoff value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| ET‐1 (ng/L) | 0.884 | 0.039 | .000 | 0.806 | 0.961 | 34.04 | 87 | 82 |
Abbreviations: AUC, area under the curve, CI, confidence interval; ET‐1; endothelin‐1.
FIGURE 3.
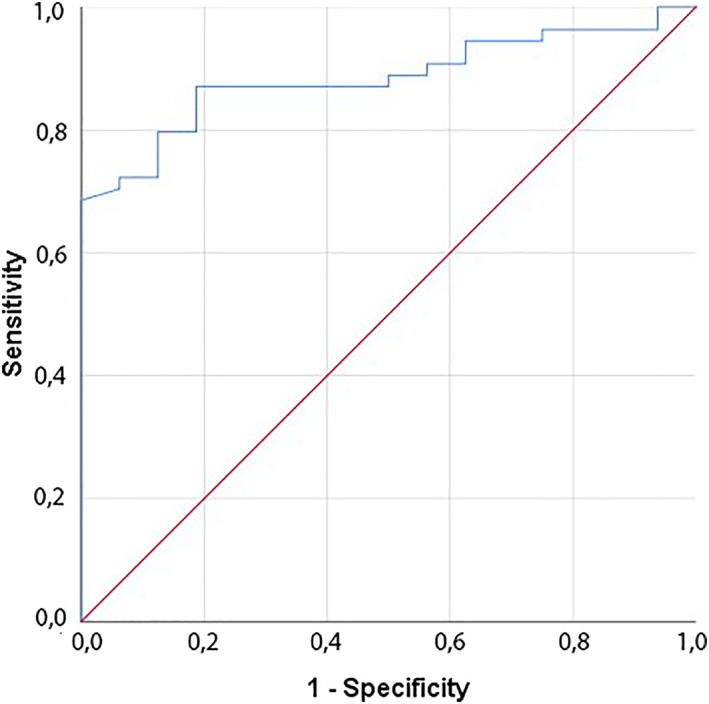
Receiver operator characteristic curve analysis graph based on serum ET‐1 concentration for surviving and dying premature calves. Performed ROC analysis to determine the relationship of ET‐1 concentration with mortality in premature calves determined that the ET‐1 concentration has prognostic importance to determining mortality. ET‐1, endothelin‐1; ROC, receiver operating characteristic curve
4. DISCUSSION
In our study, arterial blood pH, PaCO2, PaO2, SO2, BE, and lactate concentrations of calves were evaluated. The results indicate that pH, PaO2, SO2, and BE concentrations decreased and PaCO2 and lactate concentrations increased in premature calves with RDS compared to the control group whereas no differences were detected in non‐RDS premature calves. According to the results, arterial blood gas measurements were found to be reliable and accurate in determining whether RDS developed in premature calves. As many researchers have reported earlier,7, 8, 27, 29 our study also indicated that pH, PaCO2, PaO2, SO2, and lactate are the best blood gas parameters for the evaluation of lung function in premature calves with RDS.
In RDS caused by interstitial inflammation, excessive strain‐related PHT, interstitial edema, insufficient lung inflation and aeration, and development of hypoxia are the most important causes of mortality. These symptoms develop as a result of disrupted gas exchange with surfactant deficiency associated with pulmonary adaptation disorder caused by lack of lung development in premature calves.7, 8, 27 The causes of high mortality in premature calves with RDS have been reported to be severe hypercapnia and hypoxemia.7, 8, 27, 30, 31, 32 In our study, 15 (30%) of 50 premature calves with RDS died whereas only 1 calf (5%) of the 20 non‐RDS premature calves died, which suggests that RDS in the premature calves was the most important cause of mortality.
Earlier studies on premature calves with RDS have found variable severity of changes in blood gases and acid‐base balance.7, 8, 31 In addition to hypercapnia and hypoxia in premature calves with RDS, mixed acidosis (respiratory‐metabolic acidosis) is common.3, 6, 8 In our study, a positive correlation between pH and PaO2, SO2, and BE and a negative correlation between PaCO2 and lactate concentrations were observed.
Blood pH and BE are commonly accepted as valuable parameters for detecting mixed (respiratory‐metabolic acidosis) acidosis in calves after birth.33, 34 The decrease in blood pH occurs as a result of insufficient removal of CO2 from the lung and accumulation in the blood.35 In addition to PCO2, a high L‐lactate concentration also contributes to the development of acidosis.7, 8, 36 Lactate is produced under hypoxic conditions and poor tissue perfusion, and is used as an indirect marker of tissue hypoxia.5, 7, 8, 37, 38 A study in premature infants reported a strong correlation between lactate concentrations and BE and found that the effect of lactate on blood pH increased mortality rates.39 Base deficit often is used as an indirect indicator of lactic acidosis.40 Lactate plays an important role in the development of acidosis in newborns with asphyxia and is responsible for metabolic acidosis. It remains in the blood at high concentrations much longer than CO2.7, 8, 36 In our study, hypoxia, hypercapnia, a significant increase in lactate concentration, a decrease in BE, and acidosis were identified in premature calves with RDS, whereas the blood gas parameters of non‐RDS premature calves were within normal limits. Our study showed that significant changes occurred in blood gases and acid‐base balance in premature calves with RDS. Changes in blood gases such as an increase in blood PaCO2 and lactate concentrations and decreases in PaO2 and SO2 are the most important indicators of tissue hypoxia during the development of RDS.4, 6, 8, 27, 31, 41 Increased PaCO2 and lactate concentrations and decreased PaO2 and SO2 indicate widespread tissue hypoxia as a result of impaired lung function in premature calves with RDS and indicate severe respiratory acidosis in these cases.
In our study, GDF‐15, ADMA, ET‐1, VEGF, and SP‐D biomarkers were evaluated as indicators of lung endothelial and epithelial damage in the evaluation of PHT in premature calves with and without RDS and in comparison to normal healthy calves.
Endothelin‐1 is a peptide abundantly found in the human lung and has been reported to play an important role in the development of PHT because of the presence of endothelin receptors (ET‐A and ET‐B) on vascular smooth muscle cells.13 Endothelin‐1 concentration was found to increase significantly in cases of pulmonary hypertension.15, 22 A previous study reported higher concentrations of ET‐1 in cases of PHT compared to normal healthy individuals.42 On the other hand, it was determined that the mortality rate was higher in patients with PHT having high concentrations of ET‐1.13 In our study, serum ET‐1 concentrations of premature calves with RDS were significantly increased compared to those of the non‐RDS premature and control group calves. Although ET‐1 can be expressed in numerous tissues and organs, concentrations of ET‐1 mRNA were at least 5‐fold higher in lung than in any other organ.43 These findings suggest the development of PHT in premature calves with RDS as has been confirmed by previous studies in humans.15, 22, 42Also, serum ET‐1 concentrations of nonsurvivor premature calves were found to be higher than those of surviving premature calves (Table 4). Receiver operating characteristic curve analysis to determine the relationship of ET‐1 concentrations with mortality in premature calves determined that the ET‐1 had prognostic importance in determining mortality with a cutoff of 34 ng/L (Table 5). A previous study reported higher mortality rates in people with PHT with high concentrations of ET‐1,12 which is consistent with our hypothesis that RDS‐related PHT could develop in premature calves.
The correlation between blood gas parameters and ET‐1 also was evaluated. A negative correlation was found between ET‐1 concentrations and pH, PaO2, SO2, and BE, and a positive correlation between PaCO2 and lactate concentrations was observed. In conjunction with the development of PHT in lambs with experimentally induced RDS, plasma ET‐1 concentrations and PaCO2 increased and pH and PaO2 decreased in response to RDS.8 However, in infants with RDS, blood ET‐1 concentrations increased, the mechanism for which has not been fully elucidated. However, it was found in rats that increases in plasma and pulmonary ET‐1 concentrations are positively correlated with the severity of hypoxia and that ET‐1 plays a role in hypoxia‐related pulmonary arterial narrowing or PHT.44 In our study, correlations related to ET‐1 concentrations are consistent with those of earlier studies,9, 44 and we conclude that hypoxia and acidosis during RDS may cause ET‐1 synthesis and release into the bloodstream. The increase in serum ET‐1 concentration observed in premature calves with RDS may be related to the pulmonary vasoconstriction caused by hypoxia.
Nitric oxide (NO) is produced from L‐arginine by NO synthase and plays a central role in maintaining low pulmonary vascular resistance.45, 46, 47 Nitric oxide production is dependent on oxygen, and lack of NO synthesis under hypoxic conditions contributes to chronic hypoxic pulmonary vasoconstriction.48, 49 A significant increase in the concentration of ADMA occurred with endothelial damage.17 Increased ADMA concentrations cause a decrease in NO, which then increases vascular tone. Therefore, ADMA may be a useful biomarker in identifying PHT.18 A previous study determined that the secretion of ADMA decreased the activity of connexin 43, causing pulmonary endothelial dysfunction.50 An increase in the concentration of ADMA is considered to be an indicator of poor prognosis in patients with PHT, congestive heart failure, and portopulmonary hypertension and is negatively correlated with right atrial pressure and positively with venous oxygen saturation.18 Plasma ADMA concentrations were increased in infants with RDS.51 In a study conducted in premature infants with bronchopulmonary dysplasia, plasma ADMA concentrations were found to be higher in preterm infants with PAH than in infants without PAH.14 In PAH, the concentration of ET‐1, as a vasoconstrictor agent, increases and the concentration of NO, as a vasodilator agent, decreases. Endothelin‐1 concentrations in the circulation of PAH patients increase along with pulmonary vascular resistance.43 In our study, serum ADMA concentrations of the calves with RDS were decreased compared to those of the control group. Earlier studies14, 51 found a positive correlation between ADMA and ET‐1 concentrations, whereas we detected a negative correlation. Although our results contradict previous studies, given the central role of NO in maintaining low pulmonary vascular resistance, the low concentration of ADMA in premature calves with RDS was considered to be associated with stimulation of vasodilatation to decrease PAH or hypoxic pulmonary vasoconstriction by increasing NO production.
The main cause of RDS in prematurity is the lack of surfactant because of inadequate development of the lungs. Surfactant protein‐D is secreted by type II pneumocytes and plays an important role in maintaining the surface integrity of alveoli. Studies in infants with RDS determined that, in the first day of life, protein‐A and SP‐D concentrations were significantly lower than those of in healthy infants.52 Research examining genetic predisposition to RDS focused on genes for surfactant protein and found that the lack of SP‐D gene alleles in premature infants was associated with RDS development.53 In rabbits in which surfactant was experimentally removed from the lung, plasma and pulmonary ET‐1 concentrations were increased.54 Mice that lack SP‐D have increased NO production and inducible NO synthase (iNOS) expression in the bronchoalveolar (BAL) fluid.55 In patients with ARDS, BAL fluid SP‐D concentrations of nonsurvivors on day 1 were significantly lower than those of survivors and BAL SP‐D concentrations were associated with arterial‐to‐inspired oxygen (PaO2/FiO2) ratio on days 1 and 3 after the onset of ARDS. In other studies investigating ARDS, it was found that differentiation in SP‐D concentrations may provide important information about the prognosis of the disease.16, 26 It was found that SP‐D concentrations decreased after destruction of type II pneumocytes in the lungs and were closely related to the severity of damage to the lung.25
In our study, serum SP‐D concentrations were significantly lower in premature calves with RDS compared to the control group calves. A positive correlation between SP‐D concentrations and PaO2 and ADMA concentrations was determined whereas a negative correlation between SP‐D and ET‐1 concentrations was detected. The reason for the low concentration of SP‐D in the premature calves with RDS was thought to be related to inadequate development of the lungs and hypoxia‐induced destruction of type II pneumocytes. Previous results25 also support our hypothesis. Identification of a negative correlation between the biomarkers ET‐1 and SP‐D during lung damage confirms this conclusion.
Although our results were similar to those found in human medicine, some limitations still exist. The most important limiting factor in our study is that the presence PAH was not confirmed by invasive or noninvasive methods. Therefore, the biomarker findings of our study should be interpreted carefully until they are evaluated together with direct measurements of PAH.
5. CONCLUSION
Significant changes occurred in blood gases and acid‐base balance in premature calves with RDS. Serum ET‐1 concentrations were found to be high and ADMA and SP‐D concentrations were low in premature calves with RDS. Thus, serum ET‐1 may be useful in the diagnosis of PAH in premature calves with RDS, and a cutoff of 34 ng/mL corresponds to a sensitivity of 87% and a specificity of 82% for prediction of mortality.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Ethics Board of Selcuk University Veterinary Faculty Experimental Animals Production and Research Center (SÜVDAMEK) Ethics Board (approval number: 2019/79).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This research was supported by Selcuk University Scientific Research Project Office with project number 20401007.
Ider M, Naseri A, Ok M, et al. Biomarkers in premature calves with and without respiratory distress syndrome. J Vet Intern Med. 2021;35(5):2524‐2533. 10.1111/jvim.16217
Funding information Selcuk University Scientific Research Project Office, Grant/Award Number: 20401007
Contributor Information
Merve Ider, Email: m.ider@selcuk.edu.tr.
Amir Naseri, Email: anaseri@selcuk.edu.tr.
Mahmut Ok, Email: mok@selcuk.edu.tr.
Kamil Uney, Email: kuney@selcuk.edu.tr.
Alper Erturk, Email: t.c.alpererturkvet@gmail.com.
Murat K. Durgut, Email: mkaan.durgut@selcuk.edu.tr.
Tugba M. Parlak, Email: tugba.parlak@selcuk.edu.tr.
Nimet Ismailoglu, Email: vthismailoglu@gmail.com.
Muhammed M. Kapar, Email: m.mustafakapar@gmail.com.
REFERENCES
- 1.Mee JF. Why do so many calves die on modern dairy farms and what can we do about calf welfare in the future? Animals. 2013;3(4):1036‐1057. 10.3390/ani3041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornmatitsuk B, Franzén G, Gustafsson H, et al . Endocrine measurements and calving performance of Swedish Red and White and Swedish Holstein dairy cattle with special respect to stillbirth. Acta Vet Scand. 2003;44(1):21‐33. 10.1186/1751-0147-44-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleul U. Respiratory distress syndrome in calves. Vet Clin North Am Food Anim Pract. 2009;25(1):179‐193. 10.1016/j.cvfa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Guzelbektes H, Coskun A, Ok M, et al . Prevalence of gastroesophageal reflux disease in premature calves. J Vet Intern Med. 2012;26(4):1051‐1055. 10.1111/j.1939-1676.2012.00934.x. [DOI] [PubMed] [Google Scholar]
- 5.Karapinar T, Dabak M. Treatment of premature calves with clinically diagnosed respiratory distress syndrome. J Vet Intern Med. 2008;22(2):462‐466. 10.1111/j.1939-1676.2008.0074.x. [DOI] [PubMed] [Google Scholar]
- 6.Yıldız R, Aydogdu U, Guzelbektes H, et al. Venous lactate, pH and partial pressure of carbon dioxide levels as prognostic indicators in 110 premature calves with respiratory distress syndrome. Vet Rec. 2017;180(25):611. 10.1136/vr.103730. [DOI] [PubMed] [Google Scholar]
- 7.Yıldız R, Ok M. Clinical efficacy of combinations of nebulised fluticasone, salbutamol and furosemide on lung function in premature calves with respiratory distress syndrome. Vet Med. 2017;62(10):541‐552. 10.17221/34/2017-Vetmed. [DOI] [Google Scholar]
- 8.Ok M, Yıldız R, Traş B, et al. Effect of nebulized formoterol, ipratropium bromide, and furosemide in combination with fluticasone propionate on arterial blood gases of premature calves with respiratory distress syndrome. J Hell Vet Medical Soc. 2020;71(1):2011‐2018. 10.12681/jhvms.22949. [DOI] [Google Scholar]
- 9.De Vroomen M, Cardozo RHL, Steendijk P, et al. Endothelin‐1 plasma concentration increases in the early phase of pulmonary hypertension development during respiratory distress syndrome: a study in newborn lambs. Early Hum Dev. 2001;63(1):9‐21. 10.1016/S0378-3782(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 10.Gomella TL. Neonatology. 5th ed.New York: Appleton & Lange; 2004. [Google Scholar]
- 11.Fanaroff AA, Martin RJ. Neonatal‐perinatal medicine disease of the fetus and infant. The Respiratory Distress Syndrome and its Management. 6th ed.New York: Mosby; 1998. [Google Scholar]
- 12.Yurdakök M. Respiratuvar distres sendromu ve ventilatör tedavisinin ilkeleri. Katkı Pediatri Dergisi Neonatal Respiratuvar Distres Özel Sayısı. 1991;12(3‐4):299‐370. [Google Scholar]
- 13.Galié N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61(2):227‐237. 10.1016/j.cardiores.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Trittmann JK, Peterson E, Rogers LK, et al. Plasma asymmetric dimethylarginine levels are increased in neonates with bronchopulmonary dysplasia‐associated pulmonary hypertension. J Pediatr. 2015;166(2):230‐233. 10.1016/j.jpeds.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubens C, Ewert R, Halank M, et al. Big endothelin‐1 and endothelin‐1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120(5):1562‐1569. 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 16.Calfee CS, Ware LB, Glidden DV, et al. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med. 2011;39(4):711‐717. 10.1097/CCM.0b013e318207ec3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok FA, Surie S, Kempf T, et al. A simple non‐invasive diagnostic algorithm for ruling out chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Thromb Res. 2011;128(1):21‐26. 10.1016/j.thromres.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Yang T, Xu X, et al. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: a case control study. BMC Pulm Med. 2015;15(1):50. 10.1186/s12890-015-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García‐Laorden MI, Lorente JA, Flores C, et al . Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5(14):283. 10.21037/atm.2017.06.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez JA, Parry AJ, et al . Decreased surfactant proteins in lambs with pulmonary hypertension secondary to increased blood flow. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1264‐L1270. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes CJ, Wharton J, Howard LS, et al . Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart. 2011;97(13):1054‐1060. 10.1136/hrt.2011.224857. [DOI] [PubMed] [Google Scholar]
- 22.Stewart DJ, Levy RD, Cernacek P, et al . Increased plasma endothelin‐1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114(6):464‐469. 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 23.Nickel N, Jonigk D, Kempf T, et al. GDF‐15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res. 2011;12(1):1‐11. 10.1186/1465-9921-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995;95(4):1798‐1807. 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant‐associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160(6):1843‐1850. 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 26.Ware LB, Koyama T, Billheimer DD, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137(2):288‐296. 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yıldız R, Ok M, Ider M, et al. The changes in biomarkers for necrotising enterocolitis in premature calves with respiratory distress syndrome. Vet Med. 2019;64(10):440‐447. 10.17221/37/2019-vetmed. [DOI] [Google Scholar]
- 28.Bleul UT, Bircher BM, Kähn WK. Effect of intranasal oxygen administration on blood gas variables and outcome in neonatal calves with respiratory distress syndrome: 20 cases (2004–2006). J Am Vet Med Assoc. 2008;233(2):289‐293. 10.2460/javma.233.2.289. [DOI] [PubMed] [Google Scholar]
- 29.Ok M, Birdane FM, Sen I, et al. Study on some blood biochemical parameters in premature calves. Indian Vet J. 2000;77(10):859‐861. [Google Scholar]
- 30.Sustronck B, Van Loon G, Gasthuys F, et al. Effect of the ınhalation of nitric oxide on 5‐hydroxytryptamine‐ınduced pulmonary hypertension in calves. J Vet Med A. 1996;43(1–10):513‐520. 10.1111/j.1439-0442.1996.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 31.Ok M, Birdane FM. Prematüre buzağılarda kan asit‐baz dengesi, bazı kan gazları ve elektrolit düzeyleri. Vet Bıl Derg. 2000;16(1):147‐150. [Google Scholar]
- 32.Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 2009;56(3):579‐600. 10.1016/j.pcl.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eigenmann UJ, Schoon HA, Jahn D, et al . Neonatal respiratory distress syndrome in the calf. Vet Rec. 1984;114(6):141‐144. 10.1136/vr.114.6.141. [DOI] [PubMed] [Google Scholar]
- 34.Szenci O, Taverne MA. Perinatal blood gas and acid‐base status of caesarean‐derived calves. Zentralbl Veterinarmed A. 1988;A35(8):572. [PubMed] [Google Scholar]
- 35.Bleul U, Lejeune B, Schwantag S, et al . Blood gas and acid‐base analysis of arterial blood in 57 newborn calves. Vet Rec. 2007;161(20):688‐691. 10.1136/vr.161.20.688. [DOI] [PubMed] [Google Scholar]
- 36.Bleul U, Götz E. The effect of lactic acidosis on the generation and compensation of mixed respiratory‐metabolic acidosis in neonatal calves. Vet Rec. 2013;172(20):528. 10.1136/vr.101192. [DOI] [PubMed] [Google Scholar]
- 37.Divers TJ, Peek SF. In Rebhun's Diseases of Dairy Cattle. 2th ed.St Louis, MO; Saunders Elseiver; 2008. [Google Scholar]
- 38.Pirrone A, Mariella J, Gentilini F, et al . Amniotic fluid and blood lactate concentrations in mares and foals in the early postpartum period. Theriogenology. 2012;78(6):1182‐1189. 10.1016/j.theriogenology.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Nadeem M, Clarke A, Dempsey EM. Day 1 serum lactate values in preterm infants less than 32 weeks gestation. Eur J Pediatr. 2010;169(6):667‐670. 10.1007/s00431-009-1085-y. [DOI] [PubMed] [Google Scholar]
- 40.Husain FA, Martin MJ, Mullenix PS, et al . Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185(5):485‐491. 10.1016/S0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt M, Sangild PT, Blum JW, et al . Combined ACTH and glucocorticoid treatment improves survival and organ maturation in premature newborn calves. Theriogenology. 2004;61(9):1729‐1744. 10.1016/j.theriogenology.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Cody RJ, Haas GJ, Binkley PF, et al . Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992;85(2):504‐509. 10.1161/01.cir.85.2.504. [DOI] [PubMed] [Google Scholar]
- 43.Chester AH, Yacoub MH. The role of endothelin‐1 in pulmonary arterial hypertension. Glob Cardiol Sci Pract. 2014;2:29‐78. 10.5339/gcsp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirakami G, Nakao K, Saito Y, et al. Acute pulmonary alveolar hypoxia increases lung and plasma endothelin‐1 levels in conscious rats. Life Sci. 1991;48(10):969‐976. 10.1016/0024-3205(91)90362-f. [DOI] [PubMed] [Google Scholar]
- 45.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32(2):503‐512. 10.1183/09031936.00160307. [DOI] [PubMed] [Google Scholar]
- 46.Cua CL, Rogers LK, Chicoine LG, et al. Down syndrome patients with pulmonary hypertension have elevated plasma levels of asymmetric dimethylarginine. Eur J Pediatr. 2011;170(7):859‐863. 10.1007/s00431-010-1361-x. [DOI] [PubMed] [Google Scholar]
- 47.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188(6):639‐646. 10.1164/rccm.201304-0686pp. [DOI] [PubMed] [Google Scholar]
- 48.Dinh‐Xuan AT. Endothelial modulation of pulmonary vascular tone. Eur Respir J. 1992;5(6):757‐762. [PubMed] [Google Scholar]
- 49.Ozaki M, Kawashima S, Yamashita T, et al. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension. 2001;37(2):322‐327. 10.1161/01.hyp.37.2.322. [DOI] [PubMed] [Google Scholar]
- 50.Giannakoulas G, Mouratoglou SA, Gatzoulis MA, et al . Blood biomarkers and their potential role in pulmonary arterial hypertension associated with congenital heart disease. A systematic review. Int J Cardiol Heart Vasc. 2014;174(3):618‐623. 10.1016/j.ijcard.2014.04.156. [DOI] [PubMed] [Google Scholar]
- 51.Kavurt S, Demirel N, Bas AY, et al . Increased ADMA levels are associated with poor pulmonary outcome in preterm neonates. J Matern Fetal Neonatal Med. 2017;30(7):864‐869. 10.1080/14767058.2016.1190332. [DOI] [PubMed] [Google Scholar]
- 52.Wang JY, Yeh TF, Lin YC, et al . Measurement of pulmonary status and surfactant protein levels during dexamethasone treatment of neonatal respiratory distress syndrome. Thorax. 1996;51(9):907‐913. 10.1136/thx.51.9.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryckman KK, Dagle JM, Kelsey K, et al . Genetic associations of surfactant protein D and angiotensin‐converting enzyme with lung disease in preterm neonates. J Perinatol. 2012;32(5):349‐355. 10.1038/jp.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamiyama J, Jesmin S, Sakuramoto H, et al. Assessment of circulatory and pulmonary endothelin‐1 levels in a lavage‐induced surfactant‐depleted lung injury rabbit model with repeated open endotracheal suctioning and hyperinflation. Life Sci. 2014;118(2):370‐378. 10.1016/j.lfs.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Atochina EN, Beers MF, Hawgood S, et al. Surfactant protein‐D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am J Respir Cell Mol Biol. 2004;30(3):271‐279. 10.1165/rcmb.2003-0091oc. [DOI] [PubMed] [Google Scholar]


