Abstract
Acquired cervical scoliosis previously has been reported in dogs as a clinical sign associated with Chiari‐like malformation and syringomyelia but has not been described with inflammatory central nervous system disease. A 9‐month‐old Flat‐Coated Retriever was presented with an acute onset of cervical scoliosis with no other neurological deficits. Magnetic resonance imaging identified a focal, poorly defined intramedullary lesion within the cranial cervical spinal cord. Cerebrospinal fluid (CSF) analysis indicated mononuclear pleocytosis consistent with a diagnosis of meningomyelitis of unknown etiology. A second dog, a 3‐year‐old female spayed German Shepherd, developed an acute onset of cervical scoliosis with mild generalized proprioceptive ataxia 2 months after commencing immunosuppressive corticosteroid treatment for presumed steroid‐responsive meningitis‐arteritis. Magnetic resonance imaging at the time of diagnosis disclosed a similar intramedullary lesion within the cranial cervical spinal cord, with a neutrophilic pleocytosis on CSF analysis. Both dogs were treated with immunosuppressive dosages of prednisolone, along with cytosine arabinoside in the first dog, with resolution of cervical scoliosis seen in both. To our knowledge, this is the first report of acute onset acquired, reversible cervical scoliosis in dogs with presumed immune‐mediated meningomyelitis.
Keywords: gray matter, meningomyelitis of unknown etiology, spinal cord, steroid‐responsive meningitis‐arteritis, torticollis
Abbreviations
- CSF
cerebrospinal fluid
- IgA
immunoglobulin A
- MMUE
meningomyelitis of unknown etiology
- MRI
magnetic resonance imaging
- RBCs
red blood cells
- RI
reference interval
- SM
syringomyelia
- SRMA
steroid‐responsive meningitis‐arteritis
- TNCC
total nucleated cell count
1. INTRODUCTION
Cervical scoliosis, or torticollis, is an uncommon clinical sign in dogs characterized by lateral curvature of the cervical vertebral column.1 Scoliosis can either be congenital, related to vertebral malformations present at birth, or acquired as a rare consequence of spinal cord disease.1 Acquired cervical scoliosis has been reported as a chronic clinical sign secondary to syringomyelia (SM) in dogs and humans.2, 3, 4 As a consequence of SM, syrinx formation can lead to damage to lower motor neurons in the ventral horn of the spinal cord gray matter. This can lead to paresis and denervation atrophy of the corresponding paraspinal musculature and an imbalance of muscle tone and strength. In rare cases, if asymmetrical and severe enough, this imbalance can result in lateral deviation of the body or neck.5, 6
In alpacas and horses, acute onset acquired cervical scoliosis also has been associated with meningomyelitis secondary to parasitic migration of Parelaphostrongylus tenuis within the cervical spinal cord.7, 8 After ingestion, larvae migrate through the gastrointestinal wall and through the peritoneal cavity to enter the spinal cord via the dorsal roots and develop further in dorsal horn gray matter.9, 10 The larvae then can interfere with bilateral proprioceptive inputs from neuromuscular spindles involved with stretch reflexes necessary to maintain muscle tone.7, 8 The interruption of these afferents on 1 side of the body causes disruption of the normal balance in muscle tone and a deviation toward the opposite side of the lesion.1 To our knowledge, there have been no previous reports of similar acquired cervical scoliosis secondary to myelitis in dogs. We report 2 dogs that were presented with acute onset of acquired, reversible cervical scoliosis presumed to be secondary to immune‐mediated meningomyelitis.
2. CASES
This case report was approved by the institutional ethical review board (URN: SR2021‐0101).
2.1. Case 1
A 9‐month‐old female intact Flat‐Coated Retriever was presented to the Royal Veterinary College Small Animal Referrals Hospital for investigation of a 2‐day history of mild right thoracic and pelvic limb lameness and 1‐day history of nonpainful, abnormal fixed deviation of the head and neck laterally toward the left. Single doses of buprenorphine (0.02 mg/kg), meloxicam (0.1 mg/kg), and amoxicillin/clavulanic acid (12.5 mg/kg) had been administered SC at the primary care practice before referral. Neurological examination showed marked cervical scoliosis with the neck deviated to the left (Figure 1). The dog was otherwise ambulatory with no circling, paresis or ataxia, and no other neurological deficits. Complete blood count and serum biochemical profile were within normal limits apart from mildly increased creatine kinase activity (1765 U/L, reference interval [RI] = 67‐446 U/L).
FIGURE 1.

Photographs of dog 1 at the onset of cervical scoliosis dorsal view (A) and front view (B). Note the marked curvature of the neck toward the left
Under general anesthesia for imaging, the neck could be straightened without resistance. Magnetic resonance imaging (MRI) of the neck identified multiple, intramedullary, asymmetrical lesions within the cervical spinal cord, with the largest lesion extending between the C1 and the middle of the C2 vertebral bodies. Compared to normal spinal cord, the lesions were T2‐weighted hyperintense and T1‐weighted isointense with no associated contrast enhancement and predominantly affected the dorsal gray matter (Figure 2). The surrounding paravertebral musculature showed T2‐weighted and short tau inversion recovery (STIR) hyperintense, T1‐weighted hypointense lesions with irregular contrast enhancement. Analysis of cerebrospinal fluid (CSF) collected from cisternal and lumbar punctures disclosed mild mononuclear pleocytosis from the cisternal sample (total nucleated cell count [TNCC]: 16/mm3, RI < 5; red blood cells [RBCs]: 0/mm3, RI < 5; protein concentration: 0.47 g/L,RI < 0.25 g/L) and marked mononuclear pleocytosis with lymphocyte predominance from lumbar sample (TNCC: 3465/mm3, RI < 5; RBCs: 23/mm3, RI < 5]; protein concentration: 1.44 g/L, RI < 0.4). Serology for infectious diseases (toxoplasmosis, neosporosis, distemper) was negative. Serum immunoglobulin A (IgA) concentrations were within normal limits (34 μg/mL; RI < 110 μg/mL) and CSF IgA concentration was increased (0.36 μg/mL; RI < 0.2 μg/mL).
FIGURE 2.
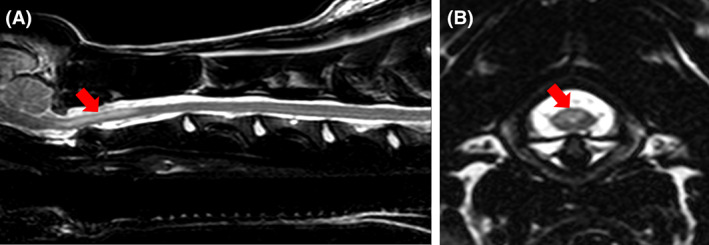
T2‐weighted sagittal (A) and transverse magnetic resonance images of the cervical spine of dog 1 showing an intramedullary hyperintensity (red arrows) at the level of cranial C2 vertebral body (A). The lesion is asymmetrical, affecting predominantly dorsal column gray matter (B)
Based on the presence of multifocal intramedullary spinal cord lesions with marked mononuclear pleocytosis on CSF analysis and negative infectious disease titers, the dog was given a presumptive diagnosis of meningomyelitis of unknown etiology (MMUE). After general anesthesia, the dog deteriorated and became obtunded with nonambulatory tetraparesis. Dexamethasone was administered IV (0.3 mg/kg) along with cytosine arabinoside administered as a single IV constant rate infusion (200 mg/m2), and daily physiotherapy and hydrotherapy sessions were instituted. The dog's mentation, cervical scoliosis, and ambulation improved markedly over the following days and the dog was discharged 2 weeks after initial presentation ambulatory and with mild tetraparesis on prednisolone 1.75 mg/kg PO q24h. Reexamination 4 weeks after discharge indicated marked improvement with only mild left‐sided cervical scoliosis and no paresis or ataxia. The prednisolone was gradually decreased over the following 6 months by approximately 20% each month until a dosage of 0.5 mg/kg PO q48h was reached, and then this medication was discontinued. On follow‐up telephone consultation 24 months after discharge, the dog was reportedly normal with no neurological deficits appreciable and no ongoing treatment.
2.2. Case 2
A 3 year 8‐month‐old female neutered German Shepherd dog was presented to the Royal Veterinary College Small Animal Referrals Hospital with a 5‐day history of progressive neck pain and pyrexia. The dog previously had been treated with a 2‐week course of antibiotics for an anal sac abscess 3 weeks before referral. Neurological examination identified marked neck and head pain with lowered head carriage but otherwise normal gait and no other neurological deficits. General physical examination identified a small perianal fistula consistent with the previously identified anal sac abscess and was otherwise unremarkable. Complete blood count, serum biochemical profile, and urinalysis were within normal limits.
Magnetic resonance imaging of the brain and neck identified a focal intramedullary lesion within the cranial cervical spinal cord, extending from the cranial aspect of C1 to the middle of the C2 vertebral body. The lesion was T2‐weighted hyperintense and T1‐weighted isointense compared to normal spinal cord, affecting predominantly the dorsal gray matter, with a mild degree of lateralization toward to the right side (Figure 3). Although the intramedullary lesion did not enhance with contrast, there was diffuse meningeal contrast enhancement of the surrounding meninges as well as the leptomeninges surrounding the brain stem (Figure 3). Computed tomography imaging of the cervical vertebral column, thorax and abdomen did not identify any abnormalities other than changes consistent with the previously noted left anal sac abscess. Cisternal CSF analysis identified a marked neutrophilic pleocytosis, with a TNCC of 7500/mm3 (RI < 5) and total protein concentration of 8.62 g/L (RI < 0.25 g/L). No microorganisms were seen on cytology and urine, blood, and CSF cultures were negative for bacterial growth.
FIGURE 3.
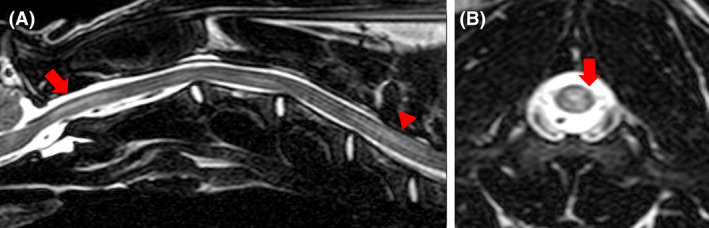
T2‐weighted sagittal (A) and transverse magnetic resonance images of the cervical spine of dog 2 showing an intramedullary hyperintensity (red arrows) extending from the level of the foramen magnum to mid‐C2 vertebral body (A). The lesion is asymmetrical, affecting predominantly dorsal column gray matter (B)
Given the history of recent pyrexia and cervical spinal hyperesthesia in combination with neutrophilic pleocytosis on CSF analysis, differential diagnoses included bacterial meningomyelitis, an atypical form of steroid‐responsive meningitis‐arteritis (SRMA; given the age of the dog), or an unusual neutrophilic form of MMUE. Given concern for a possible bacterial cause, initial treatment consisted of gabapentin (20 mg/kg PO q24h) and broad spectrum antibiotics (amoxicillin/clavulanic acid, 20 mg/kg PO q12h; enrofloxacin, 5 mg/kg PO q24h; and metronidazole, 7 mg/kg PO q12h for 14 days, with the first 5 days IV). After lack of improvement over the next 7 days and given the negative bacterial culture results, an immune‐mediated cause such as SRMA or MMUE was considered more likely and prednisolone (1 mg/kg PO q12h) treatment was commenced. The dog's condition improved markedly and at reexamination 5 weeks after initial presentation it was neurologically normal with no head or neck pain, at which point the prednisolone dosage was decreased to 1 mg/kg PO q24h. Eight days later, however, the owner reported development of a large lump on the side of the dog's neck. Reexamination identified marked cervical scoliosis with the neck deviated laterally to the left (Figure 4). No pain response was elicited on examination, and the neck could not be straightened manually with the dog awake. Examination also disclosed mild generalized proprioceptive ataxia, but was otherwise unremarkable. Additional investigation, including repeated advanced imaging, at this stage was declined by the owner. Corticosteroid treatment was continued and gradually tapered. Reexamination 11 weeks later disclosed mild generalized proprioceptive ataxia and mild, markedly improved cervical scoliosis to the left. Continuation of corticosteroid tapering and restricted exercise with hydrotherapy were recommended and telephone update from the owner 3 months after discharge indicated no concerns, and corticosteroid treatment therefore was discontinued. The dog again was presented to the referring veterinarian 6 years after initial presentation with hemoabdomen, at which point the dog was euthanized on request of the owner. Normal neck posture was reported at the time of death.
FIGURE 4.

Photographs of dog 2 at the onset of cervical scoliosis. As for dog 1, note the marked curvature of the neck toward the left
3. DISCUSSION
The dogs that developed acute cervical scoliosis in our case report had similar intramedullary cranial cervical spinal cord lesions on MRI, as well as markedly inflammatory CSF findings without evidence of clinically relevant infectious organisms on serological testing. Furthermore, both dogs responded positively to immunosuppressive treatment, supporting the diagnosis of immune‐mediated inflammatory central nervous system disease.
Cervical scoliosis can result from damage to either motor or sensory tracts at the level of the gray matter of the cervical spinal cord. As with SM, cervical scoliosis may develop as a result of asymmetrical paraspinal muscle denervation and atrophy secondary to ventral horn damage.11 As SM develops within the cervical spinal cord, the expanding syrinx can extend ventrally causing damage to the lower motor neurons, the cell bodies of which are found in the ventral horn.12 Their axons exit through the white matter into the ventral nerve root to eventually reach the motor end plate of the associated muscle.1 Disruption of the lower motor neurons in the ventral white matter may lead to denervation and ipsilateral atrophy of the paraspinal muscles and an imbalance in muscle tone.7 This imbalance leads to flexion of the neck away from the side of muscle atrophy.5, 6 This pathogenesis can be compared to the lateral deviation of the nose toward the unaffected side seen in horses with facial paralysis.7
For the dogs in our report, MRI identified intramedullary changes affecting predominantly the dorsal gray matter of the cervical spinal cord, suggesting the underlying pathophysiology may be similar to P tenuis myelitis in horses and alpacas. Cervical scoliosis is a clinical sign occasionally seen in horses and alpacas resulting from infection with P tenuis. Development and migration of the larvae within the dorsal gray matter to the subarachnoid space causes necrosis and nonsuppurative inflammation.7 The proposed pathogenesis therefore involves interference with the proprioceptive innervation by interruption of the dorsally located general proprioceptive tracts, the neurons of which originate in sensory receptors in muscles, ligaments, and joints.7 Such input from neuromuscular spindles involved with stretch reflexes is necessary to maintain muscle tone.7, 8 Proprioceptive receptors are abundant in the neck region and have been suggested to play an important role in regulation of posture.13, 14, 15 This unilateral denervation results in unopposed contractions of the contralateral paraspinal muscles and deviation of the cervical vertebrae to the contralateral side of the lesion.1 As seen in our cases, the inflammatory lesions affected the dorsal gray matter within the cervical spinal cord asymmetrically. It is therefore possible that these lesions resulted in an asymmetric interruption to proprioceptive pathways, leading to lack of opposing reflex paraspinal muscle tone and subsequent scoliosis.
Both dogs described in our report had relaxation of cervical muscle tone under general anesthesia and the neck could be placed in a straight position for advanced imaging. In contrast, a previous case report of severe cervical SM and scoliosis in a dog indicated that the neck could not be straightened under general anesthesia.12 This difference in clinical findings may provide additional information to help differentiate between likely etiologies. Along with the acute onset and resolution of clinical signs with treatment, relaxation of muscle tone under anesthesia in the dogs in our report supports underlying interference with proprioceptive input as the cause of the scoliosis, as opposed to asymmetric denervation atrophy of cervical paraspinal muscles. However, in another case report, easy vertebral column manipulation of an alpaca with acute scoliosis caused by P tenuis infection was possible early in the disease process, whereas after 1.5 months the scoliosis became fixed, and persisted after euthanasia.7 Secondary changes such as fibrosis of joint capsules and gradual remodeling of articular processes with chronic scoliosis therefore could lead to a decrease in cervical spinal range of motion over time, and decreased likelihood of resolution with treatment.7
Dog 1 had T2‐weighted and STIR hyperintense, T1‐weighted hypointense lesions with irregular contrast enhancement in the surrounding paravertebral musculature. This finding previously has been associated with the presence of inflammatory CNS disease, consistent with the findings in our case.16 It is also possible the T2‐weighted and STIR hyperintense lesions with contrast enhancement on T1‐weighted sequences could reflect acutely denervated muscle associated with the adjacent cervical spinal cord gray matter lesions.17 The combination of intramedullary spinal cord lesions on MRI and CSF mononuclear pleocytosis, without evidence of an infectious cause, in this dog were considered most consistent with a diagnosis of MMUE.18 Dog 2 had signs of pyrexia and cervical hyperesthesia, as well as marked neutrophilic pleocytosis on CSF analysis, most consistent with a diagnosis of SRMA.19 However, the age of the dog, normal CBC findings count, and intramedullary lesion noted on MRI were less consistent with SRMA, raising the possibility of an unusual form of MMUE. Although neutrophilic CSF is an unusual finding in MMUE, it has been estimated that <10% of suspected meningoencephalitis of unknown etiology cases have neutrophils as the predominant cell type.20 The diagnostic findings in our cases serve to illustrate the sensitive but nonspecific nature of CSF analysis in spinal cord disease. Although the specific diagnosis cannot be confirmed in the absence histopathological examination, the long‐term positive response of both dogs to immunosuppressive treatment supports a suspicion of immune‐mediated meningomyelitis.
The characteristic clinical and diagnostic imaging findings in our cases suggest that acute onset of cervical scoliosis may be seen in dogs with lesions affecting the cranial cervical spinal cord gray matter, and that inflammatory CNS disease should be considered. Scoliosis in both dogs resolved after treatment of their presumed immune‐mediated meningomyelitis, suggesting that acquired cervical scoliosis may be a reversible clinical sign in such cases.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Royal Veterinary College, University of London, institutional ethical review board (URN: SR2021‐0101).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Poad L, De Decker S, Fenn J. Acquired cervical scoliosis in two dogs with inflammatory central nervous system disease. J Vet Intern Med. 2021;35(5):2421‐2426. doi: 10.1111/jvim.16257
REFERENCES
- 1.de Lahunta A, Glass E, Kent M. Lower motor neuron: general somatic efferent system. , Veterinary Neuroanatomy and Clinical Neurology. 4th ed.Philadelphia, PA: WB Saunders Co; 2015:103‐105. [Google Scholar]
- 2.Nordwall A, Wikkelsø CA. Late neurologic complication of scoliosis surgery in connection with syringomyelia. Acta Orthop Scand. 1979;50:407‐410. [DOI] [PubMed] [Google Scholar]
- 3.Child G, Higgins RJ, Cuddon P. Acquired scoliosis associated with hydromyelia and syringomyelia in two dogs. J Am Vet Med Assoc. 1986;189:909‐912. [PubMed] [Google Scholar]
- 4.Takagi S, Tsuyoshi K, Ohsaki T, et al. Hindbrain decompression in a dog with scoliosis associated with syringomyelia. J Am Vet Med Assoc. 2005;226:1359‐1363. [DOI] [PubMed] [Google Scholar]
- 5.Isu T, Chono Y, Iwasaki Y, et al. Scoliosis associated with syringomyelia presenting in children. Childs Nerv Syst. 1992;8:97‐100. [DOI] [PubMed] [Google Scholar]
- 6.Rusbridge C, MacSweeny JE, Davies JV, et al. Syringohydromyelia in Cavalier King Charles spaniels. J Am Anim Hosp Assoc. 2000;36(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AL, Lamm CG, Divers TJ. Acquired cervical scoliosis attributed to Parelaphostrongylus tenuis infection in an alpaca. J Am Vet Med Assoc. 2006;229:562‐565. [DOI] [PubMed] [Google Scholar]
- 8.Mittelman NS, Divers TJ, Engiles JB, et al. Parelaphostrongylus tenuis cerebrospinal nematodiasis in a horse with cervical scoliosis and meningomyelitis. J Vet Intern Med. 2017;31:890‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayhew IG, de Lahunta A, Georgi JR. Naturally occurring cerebrospinal parelaphostrongylosis. Cornell Vet. 1976;66:56‐72. [PubMed] [Google Scholar]
- 10.Summers BA, Cummings JF, de Lahunta A. Veterinary Neuropathology. St. Louis, MO: Mosby; 1995:159‐162. [Google Scholar]
- 11.Isu T, Iwasaki Y, Akino M, Abe H. Hydrosyringomyelia associated with a Chiari I malformation in children and adolescents. Neurosurgery. 1990;26:591‐596. [DOI] [PubMed] [Google Scholar]
- 12.Cerda‐Gonzalez S, Dewey CW. Congenital diseases of the craniocervical junction in the dog. Vet Clin North Am Small Anim Pract. 2010;40:121‐141. [DOI] [PubMed] [Google Scholar]
- 13.Richmond FJ, Abrahams VC. Morphology and distribution of muscle spindles in dorsal muscles of the cat neck. J Neurophysiol. 1975;38:1322‐1339. [DOI] [PubMed] [Google Scholar]
- 14.Bakker DA, Richmond FJ. Distribution of receptors around neck vertebrae in the cat [proceedings]. J Physiol. 1980;298:40P‐41P. [PubMed] [Google Scholar]
- 15.Bakker DA, Richmond FJR, Abrahams VC, Courville J. Patterns of primary afferent termination in the external cuneate nucleus from cervical axial muscles in the cat. J Comp Neurol. 1985;241:467‐479. [DOI] [PubMed] [Google Scholar]
- 16.Eminaga S, Cherubini GB, Villiers E, Targett M, Caine A. STIR muscle hyperintensity in the cervical muscles associated with inflammatory spinal cord disease of unknown origin. J Small Anim Pract. 2013;54:137‐142. [DOI] [PubMed] [Google Scholar]
- 17.Russo CP, Smoker WRK, Weissman JL. MR appearance of trigeminal and hypoglossal motor denervation. Am J Neuroradiol. 1997;18:1375‐1383. [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelis I, Volk HA, Van Ham L, De Decker S. Clinical presentation, diagnostic findings and outcome in dogs diagnosed with presumptive spinal‐only meningoencephalomyelitis of unknown origin. J Small Anim Pract. 2017;58:174‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowrie M, Penderis J, McLaughlin M, Eckersall PD, Anderson TJ. Steroid responsive meningitis‐arteritis: a prospective study of potential disease markers, prednisolone treatment, and long‐term outcome in 20 dogs (2006‐2008). J Vet Intern Med. 2009;23:862‐870. [DOI] [PubMed] [Google Scholar]
- 20.Granger N, Smith PM, Jeffery ND. Clinical findings and treatment of non‐infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Vet J. 2010;184:290‐297. [DOI] [PubMed] [Google Scholar]


