Abstract
Background
Infective endocarditis (IE) in dogs is associated with severe disease and a high case fatality rate but often presents with nonspecific clinical signs.
Hypothesis/Objectives
Serum concentration of cardiac troponin‐I (cTnI) is elevated in dogs with IE and can differentiate dogs with IE from dogs with other diseases with similar clinical features. Concentration of serum cTnI is negatively correlated with survival time in dogs with IE.
Animals
Seventy‐two client‐owned dogs; 29 with IE, 27 with stage‐B myxomatous mitral valve disease (MMVD), and 16 with immune‐mediated disease (IMD).
Methods
Retrospective clinical cohort study. Concentration of serum cTnI was measured in all dogs at time of diagnosis. Clinical findings and echocardiographic interpretation were also recorded. Statistical analyses included Kruskal‐Wallis test, pairwise Mann‐Whitney U tests, receiver operator characteristic, and Cox proportional hazards.
Results
Serum concentration of cTnI was significantly higher in the IE group (0.69 ng/mL [0.03‐80.8]) than in the MMVD (0.05 ng/mL [0.02‐0.11], P < .001) and IMD groups (0.05 ng/mL [0.03‐0.57], P < .001). Increased cTnI was a moderately accurate predictor of IE (area under the curve 0.857 (95% confidence interval [CI] 0.745‐0.968, P < .001). A cTnI cutoff of 0.625 ng/mL had 100% specificity (95% CI 90%‐100%) and 52% sensitivity (95% CI 33%‐70%) in this study sample. There was no association between cTnI concentration and survival time in dogs with IE (hazard ratio 1.013, 95% CI 0.993‐1.034, P = .2).
Conclusions and Clinical Importance
Cardiac troponin‐I concentrations are higher in dogs with IE compared to dogs with preclinical MMVD or IMD. In dogs with a compatible clinical presentation, serum cTnI concentrations >0.625 ng/mL are supportive of IE.
Keywords: cardiac biomarkers, clinical pathology, echocardiography, general practice, inflammation
Abbreviations
- cTnI
cardiac troponin‐I
- IE
infective endocarditis
- IMD
immune‐mediated disease
- IMHA
immune‐mediated hemolytic anemia
- ITP
immune‐mediated thrombocytopenia
- MMVD
myxomatous mitral valve disease
- ROC
receiver operator characteristic
- SRMA
steroid‐responsive meningitis arteritis
1. INTRODUCTION
Infective endocarditis (IE) is a microbial infection causing inflammation of the endothelial surface of at least 1 of the heart valves or the endocardial layer of the heart chambers1, 2 (Figure 1). Despite the severe disease and high case fatality rate associated with IE in dogs, a definitive diagnosis can often be challenging antemortem because of nonspecific clinical signs,1, 2, 3 although gross and histopathologic examination of the affected tissue can prove conclusive. Antemortem diagnosis commonly relies upon satisfaction of the modified Duke criteria,4 a scoring system that has been adapted from human medicine5, 6 and consists of major and minor criteria. Depending on which criteria are met, the diagnosis of IE is classified as definitive, possible, or rejected.
FIGURE 1.
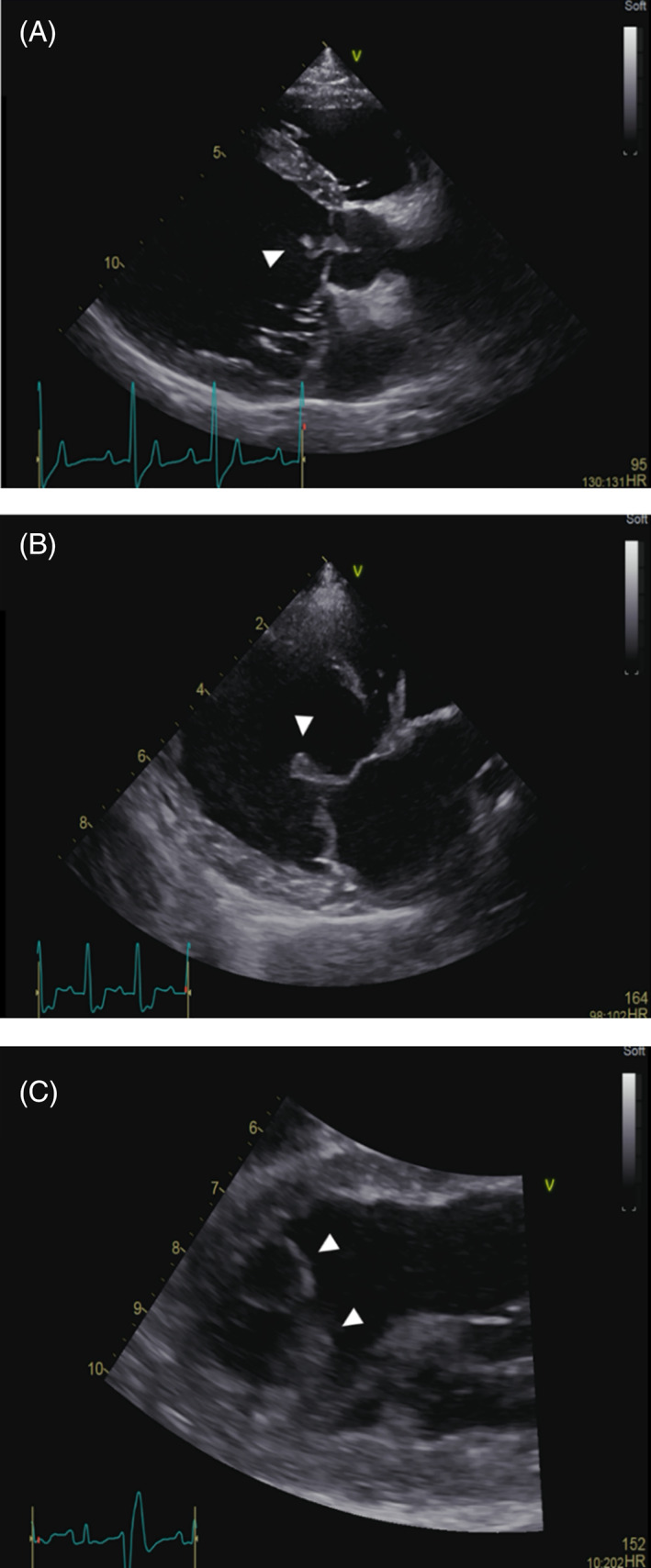
Endocarditis lesions in 3 dogs showing: A, a typical lesion of the aortic valve (arrowhead); B, a lesion of the cranial mitral valve leaflet (arrowhead); and C, two mural lesions of the endocardial surface of the left ventricular apex (arrowheads)
Although the modified Duke criteria represent a practical and reasonable approach to the diagnosis of IE in dogs, there are inevitable limitations. For instance, 1 of the major criteria, novel valve insufficiency, has poor specificity, in part because of the high prevalence of myxomatous mitral valve disease (MMVD) in dogs.7 The presence of MMVD can also make an echocardiographic diagnosis more challenging, as subtle IE lesions can be confused with or obscured by myxomatous changes. Additionally, the minor criteria mostly consist of systemic clinical signs, including pyrexia, and evidence of thromboembolic disease. These signs are nonspecific and can be present in many noncardiac diseases, most notably immune‐mediated disease (IMD), which is itself a minor criterion. Thus, making an antemortem diagnosis of IE is challenging, particularly as the clinical findings overlap considerably with those of MMVD and IMD.
The circulating serum concentration of cardiac troponin‐I (cTnI) often increases with myocardial inflammation and necrosis8, 9 and to a lesser extent with acquired heart diseases such as MMVD10 and dilated cardiomyopathy.11 Other possible causes of abnormally high serum cTnI concentration in dogs include azotemia12 and systemic diseases that might result in secondary myocardial injury, such as gastric dilatation volvulus,13 pyometra,14 and adder envenomation.15
Increases in circulating cTnI concentrations are common in humans with IE, in whom abnormal cTnI concentrations are also associated with poor outcomes.16, 17, 18, 19 However, the potential utility of cTnI as a diagnostic or prognostic marker in dogs with IE has not been reported.
The primary aim of this retrospective study was to determine whether the serum concentration of cTnI could differentiate dogs with IE from 2 control groups, 1 with MMVD and another with IMD. A secondary objective was to determine whether serum concentration of cTnI in dogs with IE was associated with survival time. We hypothesized that serum cTnI concentration would be significantly higher in dogs with IE than those in either control group, and that increased cTnI concentration would be associated with a shorter survival time in dogs with IE.
2. METHODS
Computerized records from 2 veterinary teaching hospitals (Langford Vets, University of Bristol, UK; Department of Small Animal Clinical Science, School of Veterinary Science, University of Liverpool, UK) were retrospectively searched to identify dogs diagnosed with IE, on the basis of either the modified Duke criteria (Table 1) or the opinion and experience of the attending cardiology diplomate. Dogs with MMVD or IMD were all recruited from a single center. Dogs from all groups were eligible for inclusion if they had either serum cTnI measured at the time of diagnosis, or if they had a blood sample taken at diagnosis which was stored and available for subsequent analysis. Dogs were excluded if the blood sample was taken more than 7 days before or after diagnosis. Stored serum samples were kept at −80°C. In the MMVD group, dogs with current or previous signs of congestive heart failure7 were excluded. Other exclusion criteria across all groups were a concurrent diagnosis of neoplasia, azotemia (creatinine >1.40 mg/dL), or any other known disease which could affect cTnI concentration.
TABLE 1.
Suggested modified Duke criteria for diagnosis of infective endocarditis in dogs (adapted from MacDonald et al).1 A definitive diagnosis can be made if 2 major or 1 major and 2 minor criteria are fulfilled. Diagnosis is classified as possible if 1 major and 1 minor criteria are met, or if 3 minor criteria are met, or rejected if a firm alternative explanation of clinical signs are made or clinical signs resolve with fewer than 4 days of treatment
| Criteria | Specific findings |
|---|---|
| Major | 1. Echocardiographic valve lesion with typical characteristics of endocarditis: |
| |
| |
| 2. Positive blood cultures: | |
| |
| |
| 3. Evidence of new valve insufficiency | |
| Minor | Pyrexia of unknown origin |
| Subaortic stenosis | |
| Evidence of embolic signs | |
| Evidence of systemic immune‐mediated disease | |
| Medium to large breed dog (weight >15 kg) | |
| Positive blood cultures not meeting major criteria | |
| Bartonella spp serology ≥1:1024 |
Diagnoses of IE or MMVD were made by a cardiology diplomate, or resident under supervision, and echocardiography was performed in all patients. Dogs in the IMD group were only included if their clinical signs subsequently responded to steroid treatment. They did not need to have undergone an echocardiogram in order to be included. However, IMD patients were excluded if they had clinical signs that were subsequently found to be caused by cardiac disease. Each diagnosis of IMD was made by an internal medicine diplomate or resident under supervision.
The serum concentration of cTnI was measured using 1 of 2 assays (IMMULITE 2000 Troponin I, a two‐site chemiluminescent immunoassay developed for cTnI measurement from Siemens Healthcare Diagnostics Inc, Tarrytown, NY, USA; AIA‐900 troponin‐I, a second‐generation immunoassay from Tosoh Bioscience, Tessenderlo, Belgium), depending on which center the patient was recruited from. Both assays used chemiluminescent immune‐assay technology, and the reference interval for each center was considered <0.1 ng/mL.20, 21
Data recorded included patient body weight (classified as >15 kg yes/no), age, breed, sex, concurrent disease, the presence of pyrexia (rectal temperature >39.4°C) and echocardiographic interpretation (MMVD, IE, or normal). For the IE group, endocarditis lesion location, predisposing factors identified (immunosuppressive treatment, congenital disease of the aortic valve, or left ventricular outflow tract), the presence of a novel heart murmur, results of blood culture and Bartonella henselae antibody titers (normal <1:120), and the presence of immunologic phenomena or thromboembolic disease were also recorded where available, as were date of last contact and status (alive or dead) in order to calculate survival time after diagnosis.
2.1. Statistical analysis
For continuous data, normality was assessed graphically and using a Shapiro‐Wilk test. Categorical data were compared using a Chi‐square or Fisher's exact test. Serum concentration of cTnI, body weight, sex, age, and rectal temperature were compared across the 3 groups using Kruskal‐Wallis tests, with post hoc pairwise Mann‐Whitney U tests for between‐group comparisons with Bonferroni corrections. Receiver operator characteristic (ROC) curve analysis was performed to produce a range of cutoff values for serum cTnI concentration to discriminate dogs with IE from those with MMVD and IMD. Finally, Cox proportional hazards analysis was performed to explore associations between serum cTnI concentration and survival time in patients with IE. All statistical analyses were performed using commercially available software (IBM SPSS 26 for Mac 10, IBM (UK) Ltd, Portsmouth; GraphPad Prism 8 for Mac, GraphPad Software Inc., San Diego, CA); all tests were 2‐tailed and significance was set at P < .05.
3. RESULTS
The study included 29 dogs with endocarditis, 27 dogs with MMVD, and 16 dogs with IMD: a total of 72 dogs comprised of 17 different breeds (Table 2). The IMD group consisted of patients with immune‐mediated hemolytic anemia (IMHA; n = 5), steroid‐responsive meningitis arteritis (SRMA; n = 5), immune‐mediated thrombocytopenia (ITP; n = 3), or a combination of IMHA and ITP (n = 3). Within the MMVD group, 16 patients were stage B1 and 11 were stage B2.7 Fifteen MMVD patients had no concurrent disease and the remainder included those with neurological (n = 4), endocrine (n = 3) orthopedic (n = 3), dermatological (n = 1), and gastrointestinal (n = 1) disease.
TABLE 2.
Sample characteristics and basic clinical variables for the study sample consisting of dogs with IE (n = 29), MMVD (n = 27), and IMD (n = 16)
| IE | MMVD | IMD | P value | |
|---|---|---|---|---|
| Number of dogs | 29 | 27 | 16 | |
| Male (%) | 15 (52%) | 21 (78%) | 8 (50%) | .08 |
| Age (years) | 7.05 (1.86‐12.01) | 7.84 (3.07‐13.59) | 4.47 (1.01‐12.24) | .007 |
| BW (>15 kg) | 21 (72.4%) | 3 (11/.1%) | 9 (56.3%) | <.001 |
| Temperature >39.4°C (%) | 18 (62%) | 0 (0%) | 8 (50%) | <.001 |
| cTnI (ng/mL) | 0.69 (0.03‐80.8) | 0.05 (0.02‐0.11) | 0.05 (0.03‐0.57) | <.001 |
Abbreviations: BW, body weight; cTnI, serum cardiac troponin‐I; IE, infective endocarditis; IMD, immune mediated disease; MMVD, myxomatous mitral valve disease; RT, rectal temperature.
In the IE group, 14/29 dogs (48%) had isolated mitral valve lesions, 7/29 dogs (24%) had isolated aortic valve lesions, 5/29 dogs (17%) had both aortic and mitral valve lesions, and 3/29 dogs (10%) had a mural lesion. Twenty‐six of the 29 dogs met the modified Duke criteria, for a definitive diagnosis of IE. The remaining 3 dogs were diagnosed with IE by the attending cardiology diplomate. Two of these dogs had mural lesions which were considered unlikely to be a thrombus based on changes in appearance over time and evidence of erosion. The third dog had a mitral lesion in the absence of mitral regurgitation. There was no significant difference in serum cTnI concentrations based on IE lesion location (P = .47). None of the dogs in the IE group had experienced congestive heart failure.
Blood cultures were obtained in 17/29 dogs diagnosed with IE, of which 5 dogs (29%) had a positive blood culture with 4 meeting the major modified Duke criterion and 1 meeting the minor criterion. The organisms cultured were Actinomyces odontolyticus, Gram‐negative bacilli, Corynebacterium spp, Pasteurella multocida, and Streptococcus spp.
Dogs in the MMVD group were older than those with IMD (7.84 [3.07‐13.59] years vs 4.47 [1.01‐12.24] years, respectively; P = .01). Fewer dogs weighing >15 kg were present in the MMVD group (11%) compared to the IE group (72%, P < .001) and IMD group (56%, P = .002). No difference in proportion of dogs presenting with pyrexia was detected between the dogs in IE and IMD groups (62% IE vs 50% IMD, P = .27).
Significant differences in serum cTnI concentrations were detected between the 3 groups (P ≤ .001) with cTnI concentrations being higher in dogs with IE than in those with MMVD (0.69 ng/mL [0.03‐80.8] vs 0.05 ng/mL [0.02‐0.11], P < .001) or those with IMD (0.69 ng/mL [0.03‐80.8] vs 0.05 ng/mL [0.03‐0.57], P < .001). Additionally, serum cTnI concentration was also significantly higher in dogs with IE when compared to dogs with stage B2 MMVD (n = 11) only (0.69 ng/mL [0.03‐80.8] vs 0.05 ng/mL [0.04‐0.11], P < .05). Cardiac troponin‐I concentrations were not significantly different between the 2 control groups (P = .28; Figure 2).
FIGURE 2.
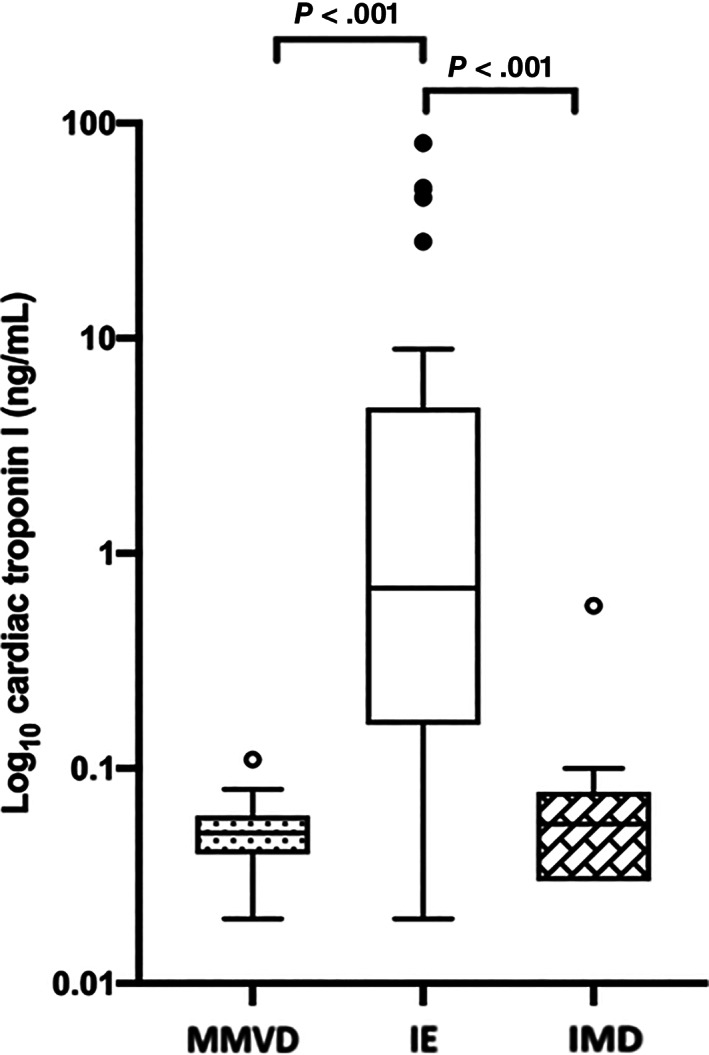
Box and Whisker plot showing the serum concentration of cardiac troponin‐I (cTnI; log transformed for illustration) in dogs with infective endocarditis (IE, n = 29), myxomatous mitral valve disease (MMVD, n = 27), and immune‐mediate diseases (IMD, n = 16). The concentration of cTnI was not significantly different between the 2 non‐IE groups (P = .28)
Serum concentration of cTnI exceeded the reference interval (<0.1 ng/mL) in 23/29 (79%) dogs with IE, a proportion that was higher than that observed for any of the individual major or minor modified Duke criteria other than “typical echocardiographic lesion” (Table 3). Of the combined control group dogs (n = 43), serum cTnI concentrations were >0.1 ng/mL in 4 animals: 1 from the MMVD group and 3 from the IMD group.
TABLE 3.
Frequency with which clinical findings were met for each of the modified Duke criteria in the group of 29 dogs with endocarditis, in addition to the 2 proposed cutoffs for cardiac troponin‐I concentrations (the upper reference limit of the assays at >0.1 ng/mL, and the suggested cut‐off of >0.625 ng/mL)
| Modified Duke criteria | Number of dogs with IE |
|---|---|
| Echocardiographic evidence | 29 (100%) |
| Novel valve insufficiency | 22 (76%) |
| Major blood culture | 6 of 17 (35%) |
| Temperature >39.4°C | 18 (62%) |
| Weight >15 kg | 21 (72%) |
| Predisposing factor | 7 (24%) |
| Thromboembolic disease | 10 (36%) |
| Immune‐mediated disease | 7 (24%) |
| Minor blood culture | 3 of 17 (18%) |
| Bartonella serology | 1 of 5 (20%) |
| cTnI (>0.1 ng/mL) | 23 (79%) |
| cTnI (>0.625 ng/mL) | 15 (52%) |
Abbreviations: cTnI, serum cardiac troponin‐I; IE, infective endocarditis.
Receiver operator characteristic curve analysis demonstrated that elevated serum cTnI was a moderately accurate predictor of endocarditis in this study sample (area under the curve 0.857 [95% confidence interval [CI] 0.745‐0.968], P < .001; Figure 3). A serum cTnI concentration of ≥0.125 ng/mL yielded the highest combined sensitivity (79%; 95% CI 60%‐91%) and specificity (98%; 95% CI 86%‐100%). A serum cTnI concentration cutoff of 0.625 ng/mL yielded a sensitivity of 52% (95% CI 33%‐70%) and a specificity of 100% (95% CI 90%‐100%).
FIGURE 3.
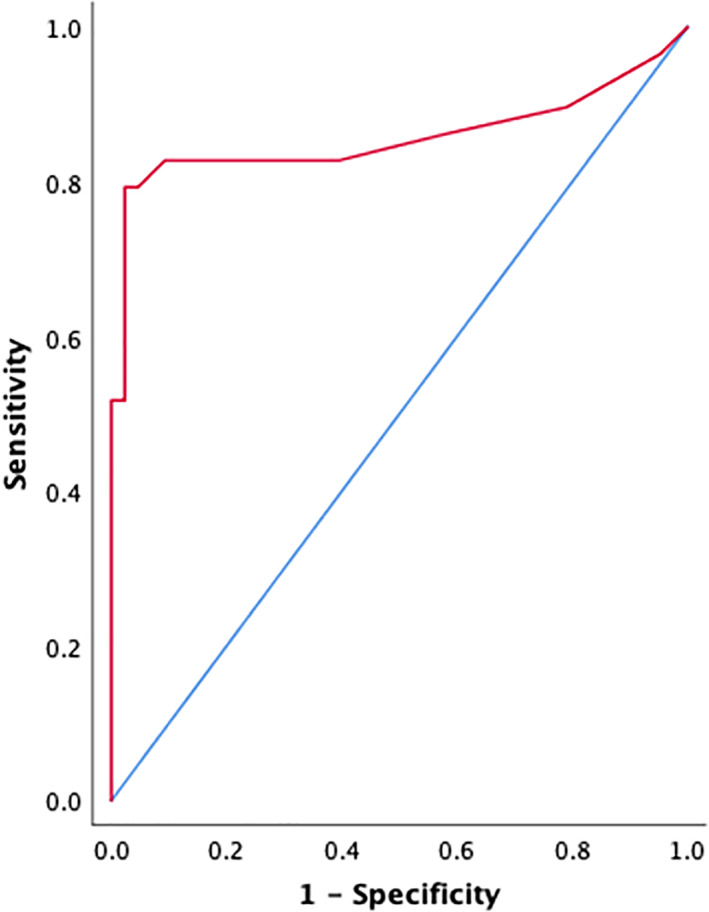
Receiver operator characteristic curve, demonstrating diagnostic performance of serum cardiac troponin‐I in dogs with endocarditis. This was a moderately accurate predictor of endocarditis in this sample (area under the curve 0.857 [95% confidence interval (CI) 0.745‐0.968], P < .001)
Median follow‐up time for dogs in the present study was 47 days (0‐1370 days). At the time of follow‐up, 20/29 (69%) of the patients with endocarditis had died, with euthanasia prompted by clinical signs the cause of death in all cases. All dogs with MMVD and IMD were alive at time of follow‐up. Median follow‐up times for MMVD and IMD patients were 182 days (0‐1143 days) and 47 days (3‐139 days), respectively. Median survival time for dogs in the IE group was 63 days (0‐1370 days). Cox proportional hazards analysis revealed no significant association between survival time and serum cTnI concentration in patients with endocarditis (hazard ratio 1.013, 95% CI 0.993‐1.034, P = .2).
4. DISCUSSION
In this sample of dogs, cTnI proved to be a useful tool to help distinguish dogs with IE when compared to control groups of dogs with other echocardiographic valve lesions (MMVD) and dogs with systemic inflammatory disease (IMD). Specifically, a cutoff value of 0.625 ng/mL had 100% specificity in this study sample. There was no association between serum cTnI concentrations and survival time in dogs with IE.
Since the diagnosis of IE is predominantly based on a scoring system—the modified Duke criteria4—any additional tests which may assist in adding weight to a diagnosis of IE are valuable, especially for vets with limited experience of this disease. In this study, we selected 2 control groups in an attempt to mimic clinical scenarios in which IE might be considered a differential diagnosis. For example, heart murmurs are commonly reported in dogs with IE,1, 22, 23 IMHA,24 and ITP25 and are an essential component of stage‐B MMVD.7 Other commonly shared clinical features of IE and IMD include pyrexia2, 24, 26 and embolic disease,2, 22, 27, 28 which were both observed in IE patients in this study (Table 3).
In a clinical scenario, echocardiography is indicated in a dog presenting with pyrexia of unknown origin, thromboembolic signs, and evidence of IMD to further investigate the possibility of IE; in this case, all 3 signs are minor components of the modified Duke criteria, but are also common in dogs with noncardiac disease. Since echocardiographic differentiation of vegetative and myxomatous lesions is dependent on operator experience and the quality of the ultrasound image obtained (training and machine factors), we propose that the addition of serum cTnI measurement—a widely available and objective assay—could provide valuable further support for a diagnosis of IE, especially if >0.625 ng/mL.
The results of the ROC curve analysis demonstrated that serum cTnI concentrations differentiated dogs with IE from the control groups with moderate accuracy. Furthermore, a high proportion of dogs with IE exhibited increased serum cTnI concentration (23/29, 79%) and 100% specificity was observed when using a cutoff of >0.625 ng/mL. Therefore, we propose that serum cTnI concentration >0.625 ng/mL could be considered as a minor Duke criterion in dogs. However, the low sensitivity (52%) of this cutoff indicates that a serum cTnI concentration below 0.625 ng/mL does not exclude IE from the list of differentials.
A number of disorders can cause increased serum concentrations of cTnI, including primary cardiac disease,8, 10, 11, 29 azotemia and systemic disease,12 pyometra,14 and gastric dilatation and volvulus.13 In this study, no dog with IE had to be excluded because of azotemia (defined as creatinine >1.40 mg/dL) and none had evidence of systemic disease that was not secondary to IE. Indeed, no alternative cause for increases in cTnI could be identified in any dog with IE. The pathophysiologic basis of increases in cTnI in dogs with IE remains unclear. As cTnI is an intracellular myocardial protein,9 its increases might be because of a localized myocarditis caused by inflammatory mediators, septic or thrombotic coronary emboli, or myocardial involvement of the infection itself; all of which have been proposed as possible mechanisms in humans.18, 19
Dogs with MMVD can have increased cTnI concentrations,10, 30 depending on disease stage and severity. Immune‐mediated diseases such as SRMA31 and IMHA32 can also cause an increase in cTnI concentrations which often normalize after the treatment of the primary condition. In our study, although 1/27 dogs with MMVD and 3/16 dogs with IMD had elevated serum cTnI concentrations, significant differences remained detectable between these groups and dogs with IE. Furthermore, the difference in serum cTnI concentration between IE and MMVD groups remained significant when only stage B2 dogs were included, indicating that cTnI is useful in differentiating dogs with IE from more advanced preclinical valvular disease. Concentrations overlapped between all groups and to maximize the predictive value of serum cTnI measurement in the diagnosis of IE, we recommend a cutoff value >0.625 ng/mL as a practically useful tool, as this did not yield any false‐positive results in this study sample.
The diagnostic utility of serum cTnI measurement in our study reflects the results of studies on IE in humans. However, unlike these studies,16, 17, 19 we did not find any association between serum cTnI concentration and outcome. This difference might be because of species differences in host immune response, differences in available treatments, different causative infectious agents associated with IE, or different disease‐lifestyle associations; for example, IE in humans is commonly associated with IV use of recreational drugs.33, 34 However, as the cause of death in all our IE dogs was euthanasia, we must also consider the influence of financial and emotional factors in determining outcome.
Limitations of this study were typical for its retrospective design. Results were obtained from dogs diagnosed in referral centers in 1 country, and therefore sample demographics and the results of survival analysis may not be generalizable to general practice or different geographic regions. However, our sample of dogs represented an authentic selection of naturally occurring cases of IE with no apparent selection bias other than the final diagnosis being made in the referral setting. The IMD group was small and heterogeneous, with only 16 dogs and 3 different diagnoses. Group sizes are relatively small, but the number of dogs with IE is reasonable in the context of the published literature.
To maximize the number of cases within the IE group, dogs were enrolled from 2 centers. Because these centers were located within 200 miles of one another, the differences in the background population were likely to be minimal and therefore comparison with a control group from only 1 center should not affect our analysis. Recruiting IE cases from 2 institutions also meant that 2 different assays for cTnI were used for measurements in the IE group. Although the sample handling and analysis technique were similar, the numbers might not be directly comparable and so our cutoff values established should be considered as estimates rather than a definitive number. As with any test result, veterinarians must contextualize the measurement within the overall clinical picture of the animal under their care. In addition, increased bilirubin concentration can interfere with the measurement of cTnI when using 1 of these 2 assays. In this study, none of the IE dogs were hyperbilirubinemic and, although dogs with IMHA often have an increased concentration of bilirubin, cTnI from all dogs with IMD was measured using the other assay, which does not suffer from the same interference.
Cardiac troponin‐I has a half‐life of just under 2 hours in the dog35 In the current study, we chose a time period of 7‐days before or after diagnosis in which a sample could be obtained for inclusion in the study. We chose this relatively wide window because IE is not an acute insult, but likely to be associated with more chronic inflammation, over several days or weeks, rather than an acute event, unlike a myocardial infarction in humans, where cTnI would wane within hours of a single insult. Additionally, the clinical signs of IE can be nonspecific,1 and it is common for dogs to remain undiagnosed for days or weeks before investigation by an experienced clinician. Therefore, we propose that our 2‐week window was reasonable and reflective of using the test in general practice.
We chose not to include dogs with stage‐C MMVD in our study. This was because we wanted to investigate whether the measurement of serum cTnI concentration would prove useful in differentiating dogs with IE and dogs with earlier MMVD that had more subtle valve lesions than tend to be present in stage C. Also, overt signs of heart failure might allow more straightforward localization of disease to the heart. This means that we have not accounted for how congestive heart failure might increase serum cTnI concentration in dogs with MMVD and those with IE causing severe enough valve dysfunction to cause congestive signs. However, since none of the dogs within the IE group had presented with signs of congestive heart failure, stage‐B MMVD served as an appropriate comparison.
Despite these limitations, these data suggest that measurement of serum cTnI concentration might be helpful in the diagnosis of IE. Further studies in a wider sample of dogs would be helpful to validate our findings, especially if carried out prospectively using a single assay and standardized sample‐handling technique. It would also be worth investigating if serum cTnI concentration improved diagnostic confidence for IE. This could be performed using a case‐scenario questionnaire to evaluate the decision‐making of veterinarians.
In conclusion, the results of this study demonstrate that serum cTnI concentrations are significantly higher in dogs with IE compared to control samples of dogs with preclinical MMVD or IMD. Care should be taken not to infer a diagnosis of IE based solely on cTnI concentration. However, based on these results, we propose that in dogs with a compatible clinical presentation, serum cTnI concentrations >0.625 ng/mL are supportive of a diagnosis of IE.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by the Langford Trust.
Kilkenny E, Watson C, Dukes‐McEwan J, et al. Evaluation of serum cardiac troponin‐I concentrations for diagnosis of infective endocarditis in dogs. J Vet Intern Med. 2021;35(5):2094‐2101. 10.1111/jvim.16234
REFERENCES
- 1.Macdonald K. Infective endocarditis in dogs: diagnosis and therapy. Vet Clin North Am Small Anim Pract. 2010;40(4):665‐684. [DOI] [PubMed] [Google Scholar]
- 2.Sykes JE, Kittleson MD, Chomel BB, MacDonald KA, Pesavento PA. Clinicopathologic findings and outcome in dogs with infective endocarditis: 71 cases (1992‐2005). J Am Vet Med Assoc. 2006;228(11):1735‐1747. [DOI] [PubMed] [Google Scholar]
- 3.Miller MW, Fox PR, Saunders AB. Pathologic and clinical features of infectious endocarditis. J Vet Cardiol. 2004;6(2):35‐43. [DOI] [PubMed] [Google Scholar]
- 4.Bonagura JD, Twedt DC, Kirk RW. Kirk's Current Veterinary Therapy XV. 15th ed.St. Louis, MO: Elsevier Saunders; 2014:786‐791. [Google Scholar]
- 5.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633‐638. [DOI] [PubMed] [Google Scholar]
- 6.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200‐209. [DOI] [PubMed] [Google Scholar]
- 7.Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33(3):1127‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oyama MA, Sisson DD. Cardiac troponin‐I concentration in dogs with cardiac disease. J Vet Intern Med. 2004;18(6):831‐839. [DOI] [PubMed] [Google Scholar]
- 9.Langhorn R, Willesen JL. Cardiac troponins in dogs and cats. J Vet Intern Med. 2016;30(1):36‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljungvall I, Höglund K, Tidholm A, et al. Cardiac troponin I is associated with severity of myxomatous mitral valve disease, age, and C‐reactive protein in dogs. J Vet Intern Med. 2010;24(1):153‐159. [DOI] [PubMed] [Google Scholar]
- 11.Wess G, Simak J, Mahling M, Hartmann K. Cardiac troponin I in Doberman Pinschers with cardiomyopathy. J Vet Intern Med. 2010;24(4):843‐849. [DOI] [PubMed] [Google Scholar]
- 12.Porciello F, Rishniw M, Herndon WE, Birettoni F, Antognoni MT, Simpson KW. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J. 2008;86(10):390‐394. [DOI] [PubMed] [Google Scholar]
- 13.Schober KE, Cornand C, Kirbach B, Aupperle H, Oechtering G. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation‐volvulus. J Am Vet Med Assoc. 2002;221(3):381‐388. [DOI] [PubMed] [Google Scholar]
- 14.Pelander L, Hagman R, Häggström J. Concentrations of cardiac Troponin I before and after ovariohysterectomy in 46 female dogs with pyometra. Acta Vet Scand. 2008;50(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segev G, Ohad DG, Shipov A, Kass PH, Aroch I. Cardiac arrhythmias and serum cardiac troponins in Vipera palaestinae envenomation in dogs. J Vet Intern Med. 2008;22(1):106‐113. [DOI] [PubMed] [Google Scholar]
- 16.Thoker ZA, Khan KA, Rashid I, Zafar D. Correlation of cardiac troponin I levels with infective endocarditis & its adverse clinical outcomes. Int J Cardiol. 2016;222:661‐664. [DOI] [PubMed] [Google Scholar]
- 17.Tsenovoy P, Aronow WS, Joseph J, Kopacz MS. Patients with infective endocarditis and increased cardiac troponin I levels have a higher incidence of in‐hospital mortality and valve replacement than those with normal cardiac troponin I levels. Cardiology. 2009;112(3):202‐204. [DOI] [PubMed] [Google Scholar]
- 18.Watkin RW, Lang S, Smith JM, Elliott TSJ, Littler WA. Role of troponin I in active infective endocarditis. Am J Cardiol. 2004;94(9):1198‐1199. [DOI] [PubMed] [Google Scholar]
- 19.Purcell JB, Patel M, Khera A, et al. Relation of troponin elevation to outcome in patients with infective endocarditis. Am J Cardiol. 2008;101(10):1479‐1481. [DOI] [PubMed] [Google Scholar]
- 20.Langhorn R, Yrfelt JD, Stjernegaard CS, Christiansen LB, Olsen LH, Nielsen LN. Analytical validation of a conventional cardiac troponin I assay for dogs and cats. Vet Clin Pathol. 2019;48(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 21.Langhorn R, Willesen JL, Tarnow I, Kjelgaard‐Hansen M. Evaluation of a high‐sensitivity assay for measurement of canine and feline serum cardiac troponin I. Vet Clin Pathol. 2013;42(4):490‐498. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald KA, Chomel BB, Kittleson MD, Kasten RW, Thomas WP, Pesavento P. A prospective study of canine infective endocarditis in northern California (1999‐2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 23.Sisson D, Thomas WP. Endocarditis of the aortic valve in the dog. J Am Vet Med Assoc. 1984;184(5):570‐577. [PubMed] [Google Scholar]
- 24.Orcutt ES, Lee JA, Bianco D. Immune‐mediated hemolytic anemia and severe thrombocytopenia in dogs: 12 cases (2001‐2008). J Vet Emerg Crit Care (San Antonio). 2010;20(3):338‐345. [DOI] [PubMed] [Google Scholar]
- 25.Tipold A, Schatzberg SJ. An update on steroid responsive meningitis‐arteritis. J Small Anim Pract. 2010;51(3):150‐154. [DOI] [PubMed] [Google Scholar]
- 26.Jackson ML, Kruth SA. Immune‐mediated hemolytic anemia and thrombocytopenia in the dog: a retrospective study of 55 cases diagnosed from 1979 through 1983 at the Western College of Veterinary Medicine. Can Vet J. 1985;26(8):245‐250. [PMC free article] [PubMed] [Google Scholar]
- 27.Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune‐mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med. 2002;16(5):504‐509. [DOI] [PubMed] [Google Scholar]
- 28.Klein MK, Dow SW, Rosychuk RA. Pulmonary thromboembolism associated with immune‐mediated hemolytic anemia in dogs: ten cases (1982‐1987). J Am Vet Med Assoc. 1989;195(2):246‐250. [PubMed] [Google Scholar]
- 29.Spratt DP, Mellanby RJ, Drury N, Archer J. Cardiac troponin I: evaluation I of a biomarker for the diagnosis of heart disease in the dog. J Small Anim Pract. 2005;46(3):139‐145. [DOI] [PubMed] [Google Scholar]
- 30.Hezzell MJ, Boswood A, Chang YM, Moonarmart W, Souttar K, Elliott J. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med. 2012;26(2):302‐311. [DOI] [PubMed] [Google Scholar]
- 31.Spence S, French A, Penderis J, et al. The occurrence of cardiac abnormalities in canine steroid‐responsive meningitis arteritis. J Small Anim Pract. 2019;60(4):204‐211. [DOI] [PubMed] [Google Scholar]
- 32.Gow DJ, Gow AG, Bell R, et al. Serum cardiac troponin I in dogs with primary immune‐mediated haemolytic anaemia. J Small Anim Pract. 2011;52(5):259‐264. [DOI] [PubMed] [Google Scholar]
- 33.Crane LR, Levine DP, Zervos MJ, Cummings G. Bacteremia in narcotic addicts at the Detroit Medical Center. I. Microbiology, epidemiology, risk factors, and empiric therapy. Rev Infect Dis. 1986;8(3):364‐373. [DOI] [PubMed] [Google Scholar]
- 34.Weisse AB, Heller DR, Schimenti RJ, Montgomery RL, Kapila R. The febrile parenteral drug user: a prospective study in 121 patients. Am J Med. 1993;94(3):274‐80.35. [DOI] [PubMed] [Google Scholar]
- 35.Dunn ME, Coluccio D, Hirkaler G, et al. The complete pharmacokinetic profile of serum Cardiac Troponin I in the Rat and the Dog. Toxicol Sci. 2011;123 (2):368–373. 10.1093/toxsci/kfr190. [DOI] [PubMed] [Google Scholar]


