Supplemental Digital Content is available in the text.
Keywords: coronavirus, inpatient, ischemic stroke, pandemics, tomography
Background and Purpose:
The coronavirus disease 2019 (COVID-19) pandemic has created challenges in the delivery of acute stroke care. In this study, we analyze the characteristics, evaluation, treatment, and in-hospital outcomes of patients presenting with acute ischemic stroke (AIS) pre-COVID-19 and during COVID-19.
Methods:
Get With The Guidelines-Stroke is a national registry of adults with stroke in the United States. Using this registry, we identified patients with a diagnosis of AIS before (n=39 113; November 1, 2019–February 3, 2020) and after (n=41 971; February 4, 2020–June 29, 2020) the first reported case of COVID-19 in the registry. Characteristics, treatment patterns, quality metrics, and in-hospital outcomes were compared between the 2 groups.
Results:
Stroke presentations decreased by an average of 15.3% per week in the during COVID-19 time period when compared with similar months in 2019. Compared with patients with AIS in the pre-COVID-19 era, patients in the COVID-19 time period had similar rates of intravenous alteplase and endovascular therapy, and similar door to computed tomography, door to needle, and door to endovascular therapy times. In adjusted models, inpatient mortality was similar between those presenting with AIS pre-COVID-19 and during COVID-19 (4.8% versus 5.2%; odds ratio, 1.05 [95% CI, 0.97–1.13]).
Conclusions:
Among hospitals participating in Get With The Guidelines-Stroke, patients presenting with AIS during COVID-19 received, with few exceptions, similar quality care and experienced similar risk-adjusted outcomes when compared with patients with AIS presenting pre-COVID-19. These findings demonstrate that stroke care in the United States remains robust during the COVID-19 pandemic.
Stroke serves as a leading cause of disability and the second leading cause of death worldwide.1 Advances in care, including intravenous thrombolysis and mechanical thrombectomy, have dramatically improved outcomes of patients with stroke, though they require timely presentation and action to be effective.2–4 Given the time sensitive nature of stroke management, there has been growing concern regarding its diagnosis and treatment in the coronavirus disease 2019 (COVID-19) era.5 While some studies demonstrate no difference in diagnostic and treatment metrics, others show significant delays and even increases in mortality among those presenting with stroke during the pandemic.6,7 In addition, global presentations for acute stroke have declined, with some cohorts showing drops in stroke volume on the order of 30% to 40%.8,9
Given the significant morbidity and mortality associated with stroke, and the major disruptions to stroke care during the pandemic, it is essential to understand stroke diagnostic and outcome patterns during COVID-19. Here, using the national Get With The Guidelines-Stroke (GWTG-Stroke) registry, we analyze the characteristics, treatment patterns, quality metrics, and in-hospital outcomes of patients presenting with acute ischemic stroke (AIS) during COVID-19 and compare them to those of patients presenting before the pandemic.
Methods
Study Population
GWTG-Stroke is a voluntary, national inpatient stroke registry currently in use at over 2000 hospitals in the United States.10 Data were abstracted by trained hospital personnel. The validity and reliability of data collection have been previously reported.11 COVID-19 specific elements were added to the registry after the start of the pandemic.12 The final study cohort consisted of 81 084 patients with AIS enrolled between November 1, 2019, and June 29, 2020, from 458 participating hospitals with at least one patient with positive COVID-19. This final cohort was divided into pre-COVID-19 and during COVID-19 time periods. Characteristics, treatment patterns, and outcomes of the cohort were analyzed by comparing those with a diagnosis of AIS between November 1, 2019 and February 3, 2020 (before first diagnosis of COVID-19 in the registry; n=39 113) and those with a diagnosis of AIS between February 4, 2020 and June 29, 2020 (after first diagnosis of COVID-19 in the registry; n=41 971; Figure I in the Data Supplement). The time period immediately before the first COVID-19 registry case was chosen as the comparison group to optimize chances of detecting changes in stroke care directly related to the pandemic. Prior work has shown minimal effect of season on stroke outcomes in GWTG-Stroke.13 In sensitivity analysis, these analyses were repeated comparing a later during COVID-19 time period (April 1, 2020–June 29, 2020) to the pre-COVID-19 time period (November 1, 2019–February 3, 2020). Weekly decrease in stroke presentations were calculated by comparing the number of acute stroke presentations from February 3, 2020 to May 24, 2020, to February 4, 2019, to May 26, 2019. Data from late May and June 2020 were not included in this comparison due to potential lags in data entry, which would cause a false impression of decreased stroke presentations. Symptom onset was defined as last known well time. Each participating hospital received either human research approval to enroll patients without individual consent under the Common Rule or a waiver of authorization and exemption from subsequent review by their institutional review board. IQVIA, Inc (Parsippany, NJ) serves as the data collection/coordination center. Duke Clinical Research Institute (Durham, North Carolina) serves as the data analysis center. This study was approved by the institutional review board of Duke University.
Statistical Analyses
Patients were divided into groups based on time of presentation (pre-COVID-19 versus during COVID-19). Clinical characteristics were compared between the 2 groups using absolute standardized differences, with an absolute standardized difference ≥10 used as the threshold for statistical significance. Treatment patterns and process measures were compared between the 2 groups using χ2 and Kruskal Wallis tests, respectively. To further evaluate the impact of time period on AIS outcomes, logistic regression models using generalized estimating equations to account for hospital clustering of patients were used. Models were adjusted for demographics (age, sex, race/ethnicity, insurance status), medical history (atrial fibrillation, diabetes, heart failure, prior stroke, prior transient ischemic attack, chronic renal insufficiency, hypertension, coronary artery disease, peripheral vascular disease), clinical characteristics (National Institutes of Health Stroke Scale, medications before admission), and hospital characteristics (rural setting, number of beds, teaching hospital, hospital region, primary stroke center, comprehensive stroke center). Rural setting was defined by the United States Office of Management and Budget as areas other than metropolitan statistical areas. Only insurance status, past medical history, and home medications were imputed using simple imputation before inclusion into univariate and multivariate models. Missing insurance was imputed to not documented. Missing patient medical history and home medications were imputed as not present and not taking, respectively. Hospital variables and outcomes were not imputed. Rates of missingness are described in Table I in the Data Supplement. To account for multiple comparisons, the level of significance was set to P<0.01. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC). A Reporting of Studies Conducted Using Observational Routinely Collected Data checklist can be found in the supplement. Data, analysis plans, and statistical code used for this study may be requested through an application process at www.heart.org/qualityreasearch.
Results
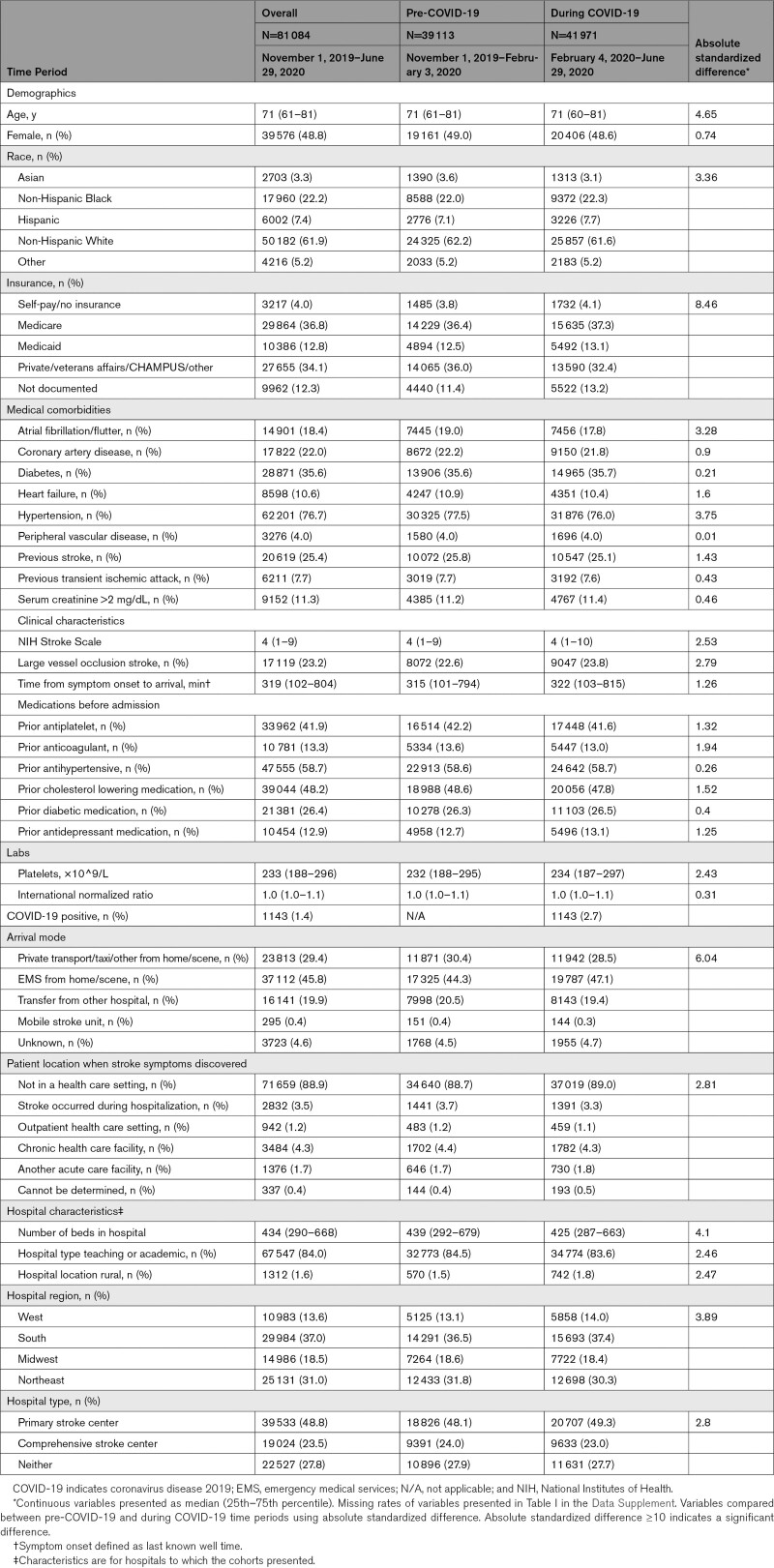
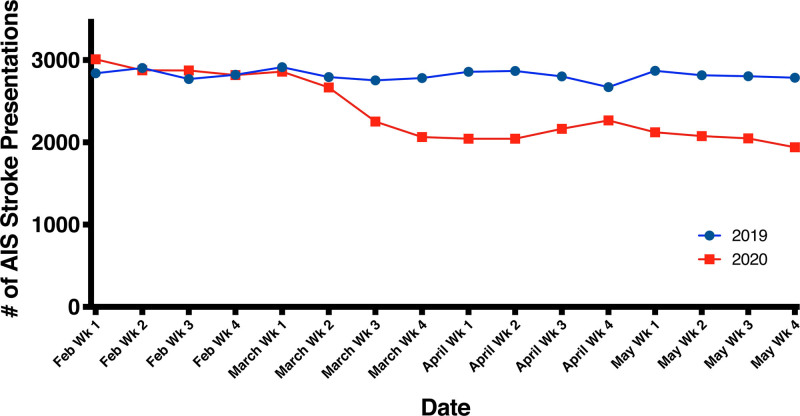
The final cohort consisted of 81 084 patients, with 39 113 patients in the pre-COVID-19 group and 41 971 patients in the during COVID-19 group. In the during COVID-19 group, 1143 (2.7%) patients had a diagnosis of COVID-19. Patient enrollment by week is shown in the Figure and Table II in the Data Supplement. There was a decrease in patients with AIS enrolled following the first COVID-19 registry case, with an average decrease of 15.3% per week during the pandemic when compared with a similar time period the year prior (Figure). Patient characteristics, stratified by pre-COVID-19 or during COVID-19 time period, are displayed in Table 1. The overall median age (25th–75th percentile) was 71 (61–81) years, with 48.8% and 61.9% of the cohort being female, and White, respectively. There were no significant differences in the general characteristics of the cohort (Table 1).
Table 1.
Characteristics of the Cohort Stratified by Pre-COVID-19 and During COVID-19 Time Period
Figure.
Acute ischemic stroke (AIS) presentations in the 458 Get With The Guidelines-Stroke hospitals February to May 2019 vs February to May 2020.
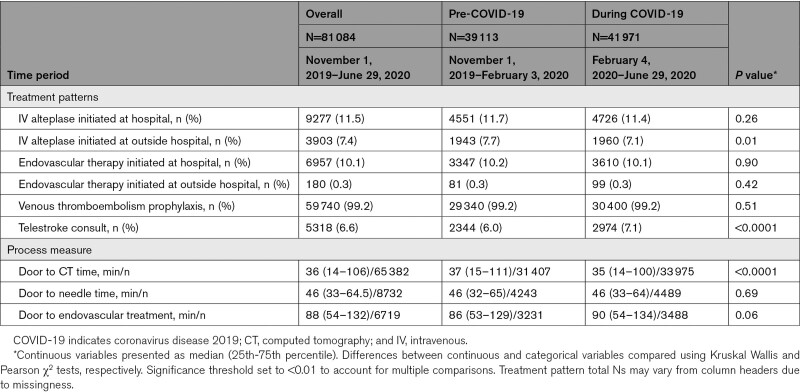
Treatment patterns of the cohort are shown in Table 2. There were no significant differences between the pre-COVID-19 and during COVID-19 time periods in the proportions of patients who received intravenous alteplase (11.7% versus 11.4%, P=0.26) or endovascular therapy (10.2% versus 10.1%, P=0.90). Door to needle and door to endovascular times were not different between the 2 groups. Door to computed tomography (CT) time was slightly shorter during the COVID-19 time period (median, 35 [14–100] versus 37 [15–111] minutes, P<0.001). Need for personal protective equipment for suspected/confirmed disease was listed as a reason for delay in 13.1% and 13.4% of thrombolysis and thrombectomy patients, respectively, in the during COVID-19 time period (Table III in the Data Supplement). With regards to GWTG-Stroke quality measures, there were slight decreases in rates of timely intravenous alteplase administration, prescription of antithrombotics at discharge, dysphagia screen, smoking cessation counseling, stroke education, and rehabilitation consideration in the during COVID-19 group. There was also a slight decrease in the composite defect free quality metric in the during COVID-19 group (Table IV in the Data Supplement).
Table 2.
Treatment Patterns and Process Measures of the Cohort Stratified by Pre-COVID-19 and During COVID-19 Time Period
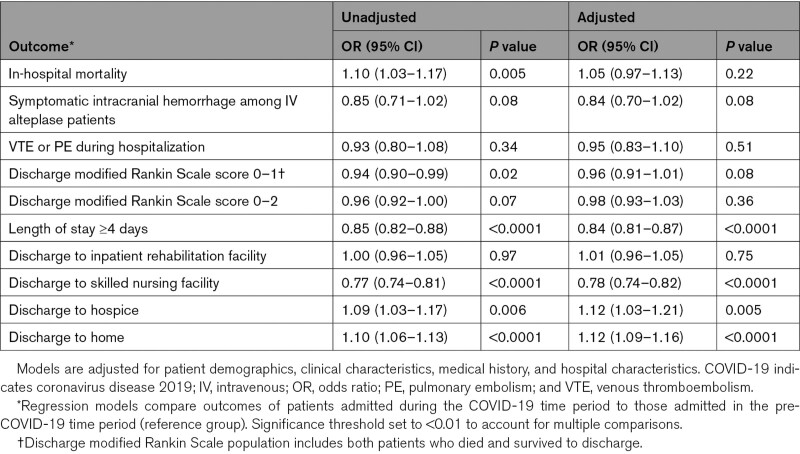
In adjusted models, COVID-19 time period did not significantly associate with odds of inpatient mortality, symptomatic intracranial hemorrhage among intarvenous alteplase patients, venous thromboembolism or pulmonary embolism during hospitalization, low discharge modified Rankin Scale score (0–1 or 0–2), or discharge to inpatient rehabilitation facility (Table 3). Compared with pre-COVID-19, during COVID-19 time period associated with reduced odds of length of stay ≥4 days (odds ratio, 0.84 [95% CI, 0.81–0.87]), reduced odds of discharge to skilled nursing facility (odds ratio, 0.78 [95% CI, 0.74–0.82]), and increased odds of discharge to hospice (odds ratio, 1.12 [95% CI, 1.03–1.21]), and discharge to home (odds ratio, 1.12 [95% CI, 1.09–1.16]).
Table 3.
Association of Time Period (Pre-COVID-19 Versus During COVID-19) With Outcomes Among Patients Presenting With Acute Ischemic Stroke
In sensitivity analysis comparing a later during COVID-19 time period (April 1, 2020–June 29, 2020) to the pre-COVID-19 time period, there were no significant differences in general characteristics of the cohort (Table V in the Data Supplement). Door to CT time was slightly shorter (median, 35 [14–95] versus 37 [15–111] minutes, P<0.001) and door to endovascular treatment time was slightly longer (median, 95 [58–140] versus 86 [53–129] minutes, P=0.001) in the later during COVID-19 time period compared with pre-COVID-19 (Table VI in the Data Supplement). There were no significant differences in in-hospital mortality, symptomatic intracranial hemorrhage among intravenous alteplase patients, or VTE/PE during hospitalization (Table VII in the Data Supplement).
Discussion
In this analysis of 81 084 patients from the GWTG-Stroke registry, we demonstrate an average 15.3% drop in weekly AIS volume in the COVID-19 era when compared with the same time period in 2019. Patients presenting during COVID-19 received high-quality acute evaluation and management, with similar door to CT, door to needle, and door to endovascular times, as well as similar rates of intravenous alteplase therapy and endovascular therapy compared with those presenting pre-COVID-19. Patients had similar adjusted in-hospital mortality in the 2 groups.
Similar to prior studies, we report a decrease in stroke presentations in the months following the onset of the COVID-19 pandemic.6,8,9,14–18 From a patient standpoint, fear of contracting the virus in the community or hospital setting is likely playing a significant role. In a national poll of 2201 adults conducted by the American College of Emergency Physicians, 70% of adults reported being concerned about contracting COVID-19 if they were to seek care from their doctor for a condition not related to severe acute respiratory syndrome coronavirus 2, 80% reported concern about contracting COVID-19 from another patient or visitor if they had to go to an emergency room, and 29% of adults reported actively delaying or avoiding seeking medical care due to concerns about contracting COVID-19.19 The initial wave of COVID-19 overwhelmed medical systems around the world, raising the possibility that decreased stroke presentations may partially reflect a lack of capacity in overburdened health systems. While some studies have demonstrated steep increases in calls to EMS, most show that stroke cases have also decreased in medical systems that have not been overwhelmed.20–22 In the United States, for example, 911 calls for emergency medical services dropped by almost 26.1% since the start of the pandemic, with many stroke centers reporting adequate resources to effectively manage stroke patients.21–23 Conversely, an overburdened health care system in Lombardy, Italy, actually saw increases in the number of ischemic stroke admissions at the height of the pandemic.24
Shelter in place and social distancing orders, while essential to curb the spread of the disease, may also be contributing to decreases in stroke presentation. In a large Northern California cohort, weekly stroke volume and stroke discharges significantly declined after the announcement of shelter in place orders, with volumes increasing again after the initiation of a gradual reopening phase.18 Social distancing also inherently results in increased social isolation for some, making it more likely for stroke symptoms to be missed or overlooked. Concomitantly, social distancing may be leading to fewer strokes happening to begin with due to factors such as decreases in pollution and work-related stress.25 While decreases in stroke occurrence may be partly contributing, it is unlikely that they alone account for the significant fall observed. In addition, there was significant heterogeneity across the United States as the virus spread over the Spring and Summer months, and some of the variations in case volume may be less prominent when analyzing the country as a whole. Last, our findings could be due to lag in AIS entries into the GWTG-Stroke database due to the administrative burden of COVID-19. While this certainly may be playing a role for data from more recent months (eg, June 2020), data entry from earlier months is likely to be more complete. Further, stroke centers around the world have observed similar findings, suggesting that there is likely a real signal of decreased stroke presentations during the pandemic.
For patients who do present to the hospital, rates of intravenous alteplase therapy, endovascular therapy, and door to CT, needle and endovascular treatment times were similar between the pre-COVID-19 and during COVID-19 groups suggesting that those who do present to the hospital do not experience delays in diagnosis or deficiencies in care. Aside from a slightly longer, likely clinically insignificant, time from door to endovascular treatment in the later during COVID-19 group, these findings remained similar when comparing those presenting in the later COVID-19 time period to those presenting pre-COVID-19. Prior studies demonstrate inconsistent findings regarding delayed presentation times, though for the most part show door to CT, needle, and endovascular times remain similar during the pandemic when compared with the pre-COVID-19 era.7,15,26–30 Although we expected delays for thrombolysis and thrombectomy in our during COVID-19 cohort due to the need for additional personal protective equipment, the relatively preserved door to diagnosis and door to intervention times suggest the donning of personal protective equipment did not lead to delayed patient care.
With regards to GWTG-Stroke achievement and quality measures, there was a 1.8% decrease in intravenous alteplase administered within 4.5 hours in those who arrived to the hospital within 3.5 hours of last known well time and around a 1% decrease in dysphagia screen, smoking cessation counseling, stroke education, and rehabilitation consideration in the during COVID-19 group. Though slightly lower in the during COVID-19 cohort, these quality measures remained above the 85% target, further suggesting maintenance of quality care during the pandemic. These results underscore the importance of the creation and maintenance of robust systems of stroke care such as GWTG-Stroke.
From an outcomes perspective, COVID-19 time period did not associate with in-hospital mortality after risk adjustment. The COVID-19 time period was also not associated with increased symptomatic intracranial hemorrhage among intravenous alteplase patients or venous thromboembolism/pulmonary embolism during hospitalization. These results are consistent with prior published studies that demonstrate no increase in in-hospital mortality among stroke patients during COVID-19, further supporting the notion that stroke care has been relatively well preserved during the pandemic.15,18,27,31
In terms of disposition, we demonstrate that similar numbers of patients were discharged to inpatient rehabilitation, more to home and to hospice, and less to skilled nursing facilities during COVID-19 compared with the pre-COVID-19 time period. COVID-19 time period was also associated with decreased odds of length of stay ≥4 days. These trends likely reflect patient and provider hesitancy toward prolonged hospital stays and desire to triage patients away from high-risk environments. They may also reflect competing pressures on beds in both hospital and skilled nursing facilities during the pandemic. Given the limited follow-up available, we are unable to determine at this time how these disposition changes will ultimately affect stroke long-term outcomes.
Limitations
Our study is limited by its retrospective, observational nature, and therefore, can evaluate associations but not causality. Though the validity and reliability of data collection in GWTG-Stroke have been previously reported, and though data were abstracted by trained hospital personnel, we are unable to validate the accuracy of data collection for the data specifically used in this study.11 Descriptive statistics performed are hypothesis generating. Not all data were complete, and imputation was used for select variables (described in methods). Though logistic regression models were adjusted for patient and hospital characteristics, the chance for residual unmeasured confounding remains. Postdischarge data from the during COVID-19 cohort are not yet available and so we are unable to report on long-term outcomes of this population. Our findings may not be generalizable to hospitals that differ from GWTG-Stroke and international cohorts given data were derived from 458 hospitals in the United States participating in GWTG-Stroke. Reported COVID-19 prevalence may be underestimated given the availability and extent of COVID-19 testing at the 458 hospitals is not known. As mentioned above, the decline in observed AIS patients during the pandemic may in part be due to lags in data entry during COVID-19.
Conclusions
This analysis of a cohort of 81 084 patients with AIS from 458 GWTG-Stroke hospitals demonstrates preserved AIS care quality in the pre-COVID-19 and during COVID-19 time periods with similar door to CT, door to needle, and door to endovascular times as well as similar rates of intravenous alteplase therapy, endovascular therapy, and adjusted in-hospital mortality. These findings suggest that stroke management has not deteriorated in the United States during COVID-19, and further validate longstanding private and governmental efforts to establish robust systems of stroke care.
Sources of Funding
This work was sponsored by a research contract from Genentech, Inc—A Member of the Roche Group. The coauthor employed by Genentech (MDP) contributed to the study design, interpretation of data, and writing the report. The decision to submit the study for publication was mutually agreed upon by the authors and the sponsor. The Get With The Guidelines–Stroke (GWTG-Stroke) program is provided by the American Heart Association/American Stroke Association. GWTG-Stroke is sponsored, in part, by Novartis, Boehringer Ingelheim and Eli Lilly Diabetes Alliance, Novo Nordisk, Sanofi, AstraZeneca, Bayer, and Portola Pharmaceuticals.
Disclosures
C. Rutan, J.G. Walchok, J.H. Williams, and Dr Alger employed by the American Heart Association. Dr Smith reports consulting Bayer, Biogen, Javelin; Associate Editor for Stroke; royalties UpToDate. Dr Fonarow reports Consulting Abbott, Amgen, CHF Solutions, Janssen, Medtronic, Merck, Novartis. Dr de Lemos reports Income DSMB or Steering Committees for Amgen, Regeneron, Eli Lilly, Consulting Jannsen. Dr Schwamm reports consulting Medtronic, LifeImage, Genentech; DSMB for Genentech, Penumbra, Diffusion Pharma; Grant funding from Medtronic, PCORI, NINDS; study drug donation from Genentech. Dr Elkind reports study drug in kind from BMS-Pfizer Alliance for Eliquis, ancillary research funding from Roche for National Institutes of Health-funded trial of stroke prevention; royalties UpToDate for stroke and COVID-19 chapters. American Heart Association officer. Dr Decker-Palmer employed by Genentech, Inc. Dr Messé reports Research funding WL Gore, Novartis, Biogen, Mallinkrodt; Personal compensation for participating in clinical event committees for Yale Cardiovascular Research Group; Co-founder, Neuralert Technologies; royalties UpToDate. S. Zhang and Dr Alhanti employed Duke Clinical Research Institute. Dr Xian reports Research funding to Duke Clinical Research Institute from the American Heart Association and Genentech. The other authors report no conflicts.
Supplemental Materials
Online Tables I–VII
Online Figure I
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- COVID-19
- coronavirus disease 2019
- CT
- computed tomography
- GWTG-Stroke
- Get With The Guidelines-Stroke
This article was sent to Jaroslaw Aronowski, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.034414.
For Sources of Funding and Disclosures, see page 3231–3232.
Contributor Information
Pratyaksh K. Srivastava, Email: psrivastava@mednet.ucla.edu.
Shuaiqi Zhang, Email: shuaiqi.zhang@duke.edu.
Ying Xian, Email: ying.xian@duke.edu.
Hanzhang Xu, Email: hanzhang.xu@duke.edu.
Christine Rutan, Email: Christine.rutan@heart.org.
Heather M. Alger, Email: heather.alger@heart.org.
Jason G. Walchok, Email: jason.walchok@heart.org.
Joseph H. Williams, Email: Joseph.williams@heart.org.
James A. de Lemos, Email: james.delemos@utsouthwestern.edu.
Marquita R. Decker-Palmer, Email: deckerpm@gene.com.
Brooke Alhanti, Email: brooke.alhanti@duke.edu.
Mitchell S.V. Elkind, Email: mse13@columbia.edu.
Steve R. Messé, Email: steven.messe@pennmedicine.upenn.edu.
Eric E. Smith, Email: eesmith@ucalgary.ca.
Lee H. Schwamm, Email: LSCHWAMM@mgh.harvard.edu.
References
- 1.Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018; 38:208–211. doi: 10.1055/s-0038-1649503 [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. ; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. ; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Neurological Disorders Stroke rt PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 5.AHA/ASA Stroke Council Leadership. Temporary emergency guidance to us stroke centers during the coronavirus disease 2019 (covid-19) pandemic: on behalf of the american heart association/american stroke association stroke council leadership. Stroke. 2020; 51:1910–1912. doi: 10.1161/STROKEAHA.120.030023 [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Scher E, Rossan-Raghunath N, Marolia D, Butnar M, Torres J, Zhang C, Kim S, Sanger M, Humbert K, et al. Acute stroke care in a New York City comprehensive stroke center during the COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2020; 29:105068. doi: 10.1016/j.jstrokecerebrovasdis.2020.105068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jasne AS, Chojecka P, Maran I, Mageid R, Eldokmak M, Zhang Q, Nystrom K, Vlieks K, Askenase M, Petersen N, et al. Stroke code presentations, interventions, and outcomes before and during the COVID-19 pandemic. Stroke. 2020; 51:2664–2673. doi: 10.1161/STR.0000000000000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diegoli H, Magalhães PSC, Martins SCO, Moro CHC, França PHC, Safanelli J, Nagel V, Venancio VG, Liberato RB, Longo AL. Decrease in Hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke. 2020; 51:2315–2321. doi: 10.1161/STROKEAHA.120.030481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esenwa C, Parides MK, Labovitz DL. The effect of COVID-19 on stroke hospitalizations in New York City. J Stroke Cerebrovasc Dis. 2020; 29:105114. doi: 10.1016/j.jstrokecerebrovasdis.2020.105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The American Heart Association’s Get With the Guidelines (GWTG)-Stroke development and impact on stroke care. Stroke Vasc Neurol. 2017; 2:94–105. doi: 10.1136/svn-2017-000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012; 163:392–8, 398.e1. doi: 10.1016/j.ahj.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Alger HM, Williams JH, IV, Walchok JG, Bolles M, Fonarow GC, Rutan C. Role of data registries in the time of COVID-19. Circ Cardiovasc Qual Outcomes. 2020; 13:e006766. doi: 10.1161/CIRCOUTCOMES.120.006766 [DOI] [PubMed] [Google Scholar]
- 13.Chu SY, Cox M, Fonarow GC, Smith EE, Schwamm L, Bhatt DL, Matsouaka RA, Xian Y, Sheth KN. Temperature and precipitation associate with ischemic stroke outcomes in the United States. J Am Heart Assoc. 2018; 7:e010020. doi: 10.1161/JAHA.118.010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajdu SD, Pittet V, Puccinelli F, Ben Hassen W, Ben Maacha M, Blanc R, Bracco S, Broocks G, Bartolini B, Casseri T, et al. Acute stroke management during the COVID-19 pandemic: does confinement impact eligibility for endovascular therapy? Stroke. 2020; 51:2593–2596. doi: 10.1161/STROKEAHA.120.030794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altschul DJ, Haranhalli N, Esenwa C, Unda SR, Garza Ramos R, Dardick J, Fernandez-Torres J, Toma A, Labovitz D, Cheng N, et al. The impact of COVID-19 on emergent large-vessel occlusion: delayed presentation confirmed by ASPECTS. AJNR Am J Neuroradiol. 2020; 41:2271–2273. doi: 10.3174/ajnr.A6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyer C, Ebert A, Huttner HB, Puetz V, Kallmünzer B, Barlinn K, Haverkamp C, Harloff A, Brich J, Platten M, et al. Acute stroke in times of the COVID-19 pandemic: a multicenter study. Stroke. 2020; 51:2224–2227. doi: 10.1161/STROKEAHA.120.030395 [DOI] [PubMed] [Google Scholar]
- 17.Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of COVID-19 on stroke evaluation in the United States. N Engl J Med. 2020; 383:400–401. doi: 10.1056/NEJMc2014816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen-Huynh MN, Tang XN, Vinson DR, Flint AC, Alexander JG, Meighan M, Burnett M, Sidney S, Klingman JG. Acute stroke presentation, care, and outcomes in community hospitals in Northern California during the COVID-19 pandemic. Stroke. 2020; 51:2918–2924. doi: 10.1161/STROKEAHA.120.031099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Emergency Physicians. Public poll: Emergency care concerns amidst covid-19. 2020. Accessed December 1, 2020. https://www.emergencyphysicians.org/article/covid19/public-poll-emergency-care-concerns-amidst-covid-19
- 20.Rudilosso S, Laredo C, Vera V, Vargas M, Renú A, Llull L, Obach V, Amaro S, Urra X, Torres F, et al. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke. 2020; 51:1991–1995. doi: 10.1161/STROKEAHA.120.030329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao J, Sayles E, Antzoulatos E, Stanton RJ, Sucharew H, Broderick JP, Demel SL, Flaherty ML, Grossman AW, Kircher C, et al. Effect of COVID-19 on emergent stroke care: a regional experience. Stroke. 2020; 51:e2111–e2114. doi: 10.1161/STROKEAHA.120.030499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khot UN, Reimer AP, Brown A, Hustey FM, Hussain MS, Kapadia SR, Svensson LG. Impact of COVID-19 pandemic on critical care transfers for ST-segment-elevation myocardial infarction, stroke, and aortic emergencies. Circ Cardiovasc Qual Outcomes. 2020; 13:e006938. doi: 10.1161/CIRCOUTCOMES.120.006938 [DOI] [PubMed] [Google Scholar]
- 23.Lerner EB, Newgard CD, Mann NC. Effect of the coronavirus disease 2019 (covid-19) pandemic on the u.S. Emergency medical services system: a preliminary report. Acad Emerg Med. 2020; 27:693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benussi A, Premi E, Pilotto A, Libri I, Pezzini A, Paolillo C, Borroni B, Magoni M, Padovani A. Effects of COVID-19 outbreak on stroke admissions in Brescia, Lombardy, Italy. Eur J Neurol. 2021; 28:e4–e5. doi: 10.1111/ene.14505 [DOI] [PubMed] [Google Scholar]
- 25.Urrutia-Pereira M, Mello-da-Silva CA, Solé D. COVID-19 and air pollution: a dangerous association? Allergol Immunopathol (Madr). 2020; 48:496–499. doi: 10.1016/j.aller.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neves Briard J, Ducroux C, Jacquin G, Alesefir W, Boisseau W, Daneault N, Deschaintre Y, Eneling J, Gioia LC, Iancu D, et al. Early impact of the covid-19 pandemic on acute stroke treatment delays. Can J Neurol Sci. 2020; 48:122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisullo G, Brunetti V, Di Iorio R, Broccolini A, Caliandro P, Monforte M, Morosetti R, Piano C, Pilato F, Calabresi P, et al. ; STROKE TEAM Collaborators. Effect of lockdown on the management of ischemic stroke: an Italian experience from a COVID hospital. Neurol Sci. 2020; 41:2309–2313. doi: 10.1007/s10072-020-04545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plumereau C, Cho TH, Buisson M, Amaz C, Cappucci M, Derex L, Ong E, Fontaine J, Rascle L, Riva R, et al. Effect of the COVID-19 pandemic on acute stroke reperfusion therapy: data from the lyon stroke center network. J Neurol. 2020; 268:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchino K, Kolikonda MK, Brown D, Kovi S, Collins D, Khawaja Z, Buletko AB, Russman AN, Hussain MS. Decline in stroke presentations during COVID-19 surge. Stroke. 2020; 51:2544–2547. doi: 10.1161/STROKEAHA.120.030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegler JE, Zha AM, Czap AL, Ortega-Gutierrez S, Farooqui M, Liebeskind DS, Desai SM, Hassan AE, Starosciak AK, Linfante I, et al. Influence of the covid-19 pandemic on treatment times for acute ischemic stroke: the society of vascular and interventional neurology multicenter collaboration. Stroke. 2020; 52:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tejada Meza H, Lambea Gil Á, Sancho Saldaña A, Martínez-Zabaleta M, Garmendia Lopetegui E, López-Cancio Martínez E, Castañón Apilánez M, Herrera Isasi M, Marta Enguita J, Gómez-Vicente B, et al. ; NORDICTUS Investigators. Impact of COVID-19 outbreak in reperfusion therapies of acute ischaemic stroke in northwest Spain. Eur J Neurol. 2020; 27:2491–2498. doi: 10.1111/ene.14467 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.