Abstract
The clinical relevance of alexithymia, a condition associated with difficulties identifying and describing one’s own emotion, is becoming ever more apparent. Increased rates of alexithymia are observed in multiple psychiatric conditions, and also in neurological conditions resulting from both organic and traumatic brain injury. The presence of alexithymia in these conditions predicts poorer regulation of one’s emotions, decreased treatment response, and increased burden on carers. While clinically important, the aetiology of alexithymia is still a matter of debate, with several authors arguing for multiple ‘routes’ to impaired understanding of one’s own emotions, which may or may not result in distinct subtypes of alexithymia. While previous studies support the role of impaired interoception (perceiving bodily states) in the development of alexithymia, the current study assessed whether acquired language impairment following traumatic brain injury, and damage to language regions, may also be associated with an increased risk of alexithymia.
Within a sample of 129 participants with penetrating brain injury and 33 healthy controls, neuropsychological testing revealed that deficits in a non-emotional language task, object naming, were associated with alexithymia, specifically with difficulty identifying one’s own emotions. Both region-of-interest and whole-brain lesion analyses revealed that damage to language regions in the inferior frontal gyrus was associated with the presence of both this language impairment and alexithymia. These results are consistent with a framework for acquired alexithymia that incorporates both interoceptive and language processes, and support the idea that brain injury may result in alexithymia via impairment in any one of a number of more basic processes.
Keywords: Alexithymia, Language, Traumatic brain injury, Anterior insula, Inferior frontal gyrus
1. Introduction
Alexithymia is a sub-clinical condition characterised by a difficulty identifying and expressing one’s emotions, accompanied by a pattern of externally oriented thinking (Taylor et al., 1991). The clinical significance of alexithymia is increasingly being appreciated, in part due to its high rates of co-occurrence with a wide range of psychiatric conditions, including autism, eating disorders, schizophrenia, alcohol abuse and substance abuse (Bird and Cook, 2013; Eizaguirre et al., 2004; Pinard et al., 1996; Thorberg et al., 2009; van’t Wout et al., 2007). An increased prevalence of alexithymia, relative to rates observed in the general population, is also seen in neurological conditions including Multiple Sclerosis (Chahraoui et al., 2008), Parkinson’s Disease (Costa et al., 2010), and following traumatic brain injury (TBI) (Henry et al., 2006; Wood and Williams, 2007), providing evidence of “acquired alexithymia” following presumed typical development. Alexithymia has been found to increase the likelihood of a number of other socio-emotional deficits, including difficulties recognising emotions from both faces (Cook et al., 2013; Grynberg et al., 2012; although see McDonald et al., 2011) and voices (Heaton et al., 2012), reduced levels of empathy (Bird et al., 2010), and difficulties regulating one’s own emotion (Pandey et al., 2011). Alexithymia is also associated with impaired learning and decision-making (Bibby and Ferguson, 2011; Ferguson et al., 2009; Kano et al., 2011), increased self-harm (Norman and Borrill, 2015), and negatively impacts the effectiveness of most psychotherapy (Lumley et al., 2007; Mccallum et al., 2003).
While the impact of alexithymia on functioning and treatment efficacy is becoming better understood, the nature and aetiology of alexithymia in both clinical and non-clinical populations is still unclear. In particular, there has been a great deal of debate over whether alexithymia is a unitary construct, or whether subtypes of alexithymia exist. Several subtypes have been proposed –some defined by the form of alexithymic deficit, and others by aetiology. With regard to subtypes of form, much debate has centred around the question of whether some individuals with alexithymia are impaired in the affective and cognitive domain, while others are impaired in the cognitive domain only (Bermond, 1997; Parker et al., 1993), with current data suggesting alexithymia may be a unitary condition, at least in terms of its reportable behavioural characteristics (Bagby et al., 2009).
With respect to aetiological subtypes, while it has been argued that an interoceptive deficit may give rise to alexithymia over development (Brewer et al., 2015; Murphy et al., 2017a), and evidence of impaired interoception in alexithymic individuals supports this conjecture (Brewer et al., 2016; Gaigg et al., 2016; Herbert et al., 2011; Murphy et al., 2017a; Shah et al., 2016), interoceptive deficit may not be the only route by which one may develop alexithymia. Although data addressing the question of multiple aetiological routes to alexithymia is not plentiful, several authors have suggested such a possibility. For instance, Messina and colleagues argue that there may be “primary” and “secondary” forms of alexithymia, where primary alexithymia is a developmental condition and secondary alexithymia a reaction to trauma occurring later in life (Messina et al., 2014).
One strategy to address this possibility is to examine co-occurring deficits in alexithymic individuals; different patterns of co-occurring deficits may suggest different aetiologies. For example, work suggests that alexithymia following HIV infection may be distinct from that found in healthy individuals. Specifically, in patients infected with HIV (which is associated with widespread neurological deterioration and disruption to brain functioning even in the early stages of disease progression; Ernst et al., 2002; Jernigan et al., 1993; Thompson et al., 2005), levels of alexithymia were related to performance on tests of attention, executive function and visuospatial ability, whereas alexithymia severity was unrelated to these factors in healthy control participants (Bogdanova et al., 2010).
1.1. The role of language in alexithymia
While it is somewhat intuitive that general cognitive factors such as attention and executive function may impact upon alexithymia, and previous study of alexithymia has highlighted the importance of interoception, it is even more intuitive that language impairment is likely to lead to alexithymia, particularly following organic or traumatic brain injury. Given that “alexithymia” is literally translated as “no words for feelings”, and core components are a difficulty identifying (i.e. labelling) and expressing these feelings to others, it is logical that impairment of language function following brain injury would lead to alexithymia. Such a hypothesis was discounted early in alexithymia research however, based on the finding that alexithymic individuals show emotion processing deficits on nonverbal as well as verbal tasks (Lane et al., 1996; Wagner and Lee, 2008). This interpretation ignores, however, the pervasive effects of language on seemingly “nonverbal” tasks, and the fact that tasks that do not use linguistic stimuli can still be affected by language processes. While theorists have argued that language processes have an influential role on many perceptual and cognitive tasks (Lupyan, 2012), with regard to emotion, it is thought that verbal labels are likely to contribute to the development of clearly defined emotional categories (see Barrett et al., 2007), and may be used even in non-verbal tasks, such as when one is required to match visual emotional stimuli. Indeed, such an effect was demonstrated by Lindquist et al. (2006), who showed that when access to emotional verbal labels was disrupted using a technique known as semantic satiation, participants were slower and less accurate when required to judge whether two faces depicted the same emotion (a task where verbal labelling of emotion was not explicitly required). Other studies have also shown effects of verbal processing on emotion categorization tasks that do not explicitly require verbal labelling of emotions (Roberson and Davidoff, 2000; Roberson et al., 2010). Further support for the role of language in the alexithymia deficit seen on ‘non-verbal’ emotional tasks is provided by the finding that, in some samples but not all, the facial emotion recognition deficit observed in alexithymic individuals can be completely explained by differences in verbal IQ. For example, in one such study, the effect of alexithymia on the recognition of facial expressions of emotion was found to be no longer significant after verbal IQ was statistically controlled for (Montebarocci et al., 2011; see also Hsing et al., 2013).
While the relationship between language and alexithymia has received limited direct study, available evidence supports the notion that language processes contribute to alexithymia. In a study of 59 post-war veterans, alexithymia was found to be associated with measures of verbal performance (Lamberty and Holt, 1995). Furthermore, Henry et al. (2006) assessed verbal fluency and alexithymia in patients with TBI and found that difficulty identifying feelings, one of the three core features of alexithymia, was correlated with performance on fluency tasks, such that greater difficulty with identifying feelings was related to poorer fluency. While suggestive, fluency measures tap a range of executive processes and therefore the relationship between difficulty identifying feelings and verbal fluency provides only limited evidence for the role of language functioning in alexithymia. Nonetheless, in a subsequent investigation by Wood and Williams (2007), verbal abilities (measured using vocabulary, verbal similarities, and comprehension tasks) were significantly poorer in patients who were alexithymic than in those who were not.
Developmental investigations have also suggested links between language and alexithymia, as early delays in speech development are associated with alexithymia later in life (Karukivi et al., 2012; Kokkonen et al., 2003). Importantly, these relationships are observed with general language skills; suggesting that links between alexithymia and language are not limited to language for emotions or internal states. Alexithymia itself has not been studied in children with language impairment. Nonetheless, these children do show worse emotion regulation abilities, reduced emotional well-being, and impairments on emotion processing tasks such as those requiring the recognition of emotional facial expressions,or inference of the emotional states of others from non-facial cues (Botting and Conti-Ramsden, 2008; Ford and Milosky, 2003; Fujiki et al., 2002; Merkenschlager et al., 2012; Nelson et al., 2011). These difficulties are consistent with elevated rates of alexithymia in this population. Relatedly, children with language impairment have shown deficits on a task comparable to the ‘nonverbal’ emotion task employed by Lane et al. (1996) on which alexithymic adults are impaired. Ford and Milosky (2003) presented children with scenarios (in verbal only format, visual only, and in combined verbal and visual format) and asked what the character in the scenario would feel. Children with language impairment made more errors on the task than their age-matched peers, and were more likely to report that the character would feel an emotion with a valance opposite to the correct emotion (e.g. responding that a character would feel happy instead of angry), regardless of the mode of presentation.
Such evidence supports constructionist theories that argue that language has a central role in emotion. For example, the Conceptual Act Theory (CAT) of emotion (Barrett, 2006; Lindquist et al., 2015) argues that emotions are perceived via an automatic process of categorization. “Core affect”, the constant stream of transient alterations in an organism’s neurophysiological state, is categorized, leading to the feeling of distinct emotion categories, such as “fear”, “envy”, “anger”, and so on. CAT posits that a complete failure of categorization would result in alexithymia (Barrett, 2006). Language is seen to play a key role in both the development and execution of the categorization process, as language supports the acquisition and use of conceptual knowledge about emotion. Indeed, developmental evidence suggests that caregivers provide verbal labels for infants’ and young children’s emotions, based on the facial expressions produced by the child, making it possible for the child to associate verbal labels with particular emotional states (Malatesta and Wilson, 1988). CAT is a developmental model, and its proponents draw largely on developmental evidence (Lindquist et al., 2015). Nonetheless, the basic assumptions of CAT would still predict that an impairment in the categorization process, even if sustained in adulthood, would result in similar impairment. Thus, certain acquired language impairments might be expected to result in acquired alexithymia.
1.2. Aims of the current study
This study analysed data from a large population of individuals with penetrating traumatic brain injury to consider the potential role of language in alexithymia. Data from patients enrolled in the Vietnam Head Injury Study (VHIS) has previously been used to support the role of the insula in alexithymia (Hogeveen et al., 2016). In the current study, we assessed the association between two widely used behavioural measures of language, the Boston Naming Task and the Token Test, and alexithymia, as well as the relationship between lesion characteristics, language ability, and alexithymia using both region of interest and whole-brain approaches. Two key language regions of interest were selected to be analysed for their association with alexithymia: the superior temporal gyrus, and inferior frontal gyrus. In the classic Geschwind-Wernicke model of language processing, these two areas were considered the dominant areas for language processing (Geschwind, 1970). It is now appreciated that language involves distributed processing across a network of areas, and these two regions are best understood as parts of a complex system (e.g. Friederici, 2012; for a review see Price, 2012). Nonetheless, these regions continue to be considered key language areas of the brain.
Wernicke’s area is situated in the superior temporal gyrus (STG). Historically, it has been considered to play a key role in language comprehension, although the STG is considered to underlie aspects of both speech perception and production, particularly phonological processing (Buchsbaum et al., 2001). Wernicke’s aphasia, caused by lesions to this region, is characterised by deficits in comprehension, and fluent but disordered speech production.
Broca’s area is situated in the inferior frontal gyrus (IFG), and includes two distinct sub-regions; the pars opercularis and the pars triangularis (BA44 and BA45, respectively) (Amunts and Zilles, 2012). Patients suffering from Broca’s aphasia typically have impaired speech production with comparably preserved comprehension. While Broca’s area is often considered to comprise the two regions BA44 and BA45, evidence from both lesions and neuroimaging studies suggests that these areas perform distinct roles, with BA45 being more involved in semantic aspects of language processing, while BA44 is more involved in syntactic or articulatory processes (Amunts et al., 2004; Friederici, 2012; Paulesu et al., 1997). While historically Broca’s area was thought crucial for production, this region has also been shown to have important roles in language comprehension (e.g. Bedny, Hulbert, & Thompson-Schill, 2007; Rogalsky & Hickok, 2011), and to play a general executive function role, specifically in tasks that require selecting between competing representations (see Novick, Trueswell, & Thompson-Schill, 2010 for a review). This executive function role may contribute to both language production (Schnur et al., 2009) and language comprehension (Bedny et al., 2007; Novick, Trueswell, & Thompson-Schill, 2005), but also be recruited during tasks that do not use linguistic stimuli, or where verbal responses are not required (Kemmotsu, Villalobos, Gaffrey, Courchesne, & Müller, 2005; see also Jonides & Nee, 2006, in which it is discussed to what extent stimuli must be verbalizable to trigger the involvement of the IFG).
Since language and emotional processing both rely on a complex network of brain regions (Price, 2012; Lindquist et al., 2012), in addition to our hypothesis-driven ROI approach, we also performed exploratory whole brain voxel-based lesion-symptom mapping (VLSM) analyses. The aim of the VLSMs was to outline the network of brain regions that, when damaged, are associated with both behavioural language deficits and alexithymia.
2. Method
2.1. Participants
The VHIS population has been written about extensively. Following return from combat after serving in the Vietnam war, these veterans were enrolled in a longitudinal study, and have been followed up through four phases. The data collection during Phase 4 (2008–2012) occurred approximately 40 years post-injury. Raymont et al. (2011) provide a good overview of the tasks this population has completed over the four phases of study. This study used data from the same set of patients described in Hogeveen et al. (2016), which included 129 patients with focal TBI and 33 control participants who were also veterans but who had not sustained TBI during combat. Data from the control group feature in results reported in Sections 3.1 and 3.2, while Sections 3.3 and 3.4 include the TBI sample only. The TBI and control groups were closely matched with respect to age (TBI: M = 63.29 years, SD = 2.89 versus Control: M = 63.33 years, SD = 3.80; t(160) = −0.06, p = .948), handedness (TBI: 82% right-handed, 17% left-handed, 1% ambidextrous versus Control: 79% right-handed, 15% left-handed, 6% ambidextrous; X2 = 2.24, p = .33), years of education (TBI: M = 14.55, SD = 2.27 versus Control: M = 15.06, SD = 2.12; t(160) = −1.17, p = .244), and pre-injury intelligence (Armed-Forces Qualification Test (AFQT-7A) percentile scores, TBI: M = 64.37, SD = 17.06 versus Control: M = 72.91, SD = 23.29; t(136) = −1.64, p = .104). Participants provided their written informed consent to take part in the VHIS, and all study protocols were approved by an Institutional Review Board at the National Institute of Neurological Disorders and Stroke.
2.2. Structural imaging of lesions
Patient lesions were mapped using Computerized Tomography (CT) scans. Patients did not undergo MRI scanning due to a high risk of retained metal fragments as a result of their injury and subsequent surgical procedures. The majority of TBI patients were scanned during Phase 3 of the VHIS (2003–2006), but six participants in the present sample did not take part in Phase 3 and were scanned during Phase 4. Scanning took place an average of 38.18 years (SD 7.96 years) after the brain injury was incurred. Non-contrast axial CT scans in helical mode were acquired using a GE Medical Systems Light Speed Plus CT scanner, with a voxel size of 0.4 × 0.4 mm and a 1 mm slice interval. Brain extraction was performed using a Tcl/Tk script in MEDx 3.44 (Medical Numerics Inc., Seterling, VA. USA), and lesion location and volume were identified in the Analysis of Brain Lesion (ABLe) software module implemented in MEDx (Solomon et al., 2007). Lesions were mapped by a trained neuropsychiatrist who manually traced the lesion locations in native space, with these tracings being confirmed by a researcher (author JG) who was blind to the results of neuropsychological testing. CT images were then spatially normalized to MNI space, and the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was used to define the extent of damage to each of the regions-of-interest. Damage to pars operculars, pars triangularis (collectively, Broca’s Area), and superior temporal gyrus (Wernicke’s Area) were calculated by summing the number of lesioned voxels in the AAL regions “Frontal_Inf_Oper,” “Frontal_Inf_Tri,” and “Temporal_Sup,” respectively, multiplying by slice thickness, and then dividing by the number of voxels contained in each AAL region. Total percent volume loss was calculated by summing the number of voxels in the traced lesion area and dividing by total brain volume.
In order to consider the effect of damage to a particular region on alexithymia scores, patients were sorted into three groups; those with no damage to a particular region, those with less than 15% volume loss to a region, and those with 15% or more volume loss to a region (Hogeveen et al., 2016; Koenigs et al., 2007; Tranel et al., 2005). Following significant findings for Broca’s area damage, subsequent analyses were also performed examining specific damage to the two subregions that comprise Broca’s area – the pars triangularis (BA45) and the pars opercularis (BA44). Traditionally, language has been considered the remit of the left hemisphere, however there is now mounting evidence that the right hemisphere also carries out important language functions (Lindell, 2006). Therefore, in all analyses considering volume loss to brain regions, for each patient an average volume loss value across the two hemispheres was calculated.
2.3. Language and behavioural measures
At Phases 2, 3 and 4, versions of the Token Test and Boston Naming task were administered, offering longitudinal data on the patients’ comprehension and lexical access abilities. In the Token Test, patients hear and perform a set of instructions (“Put the blue circle on top of the white square”). In the Boston Naming task, patients see degraded line drawings and must name the item in the picture. Examples of test items include “tree”, “igloo”, or “sphinx”. All are concrete nouns, and not emotion related (i.e. there are no emotion words to name). The naming task at Phase 2 was based on the earlier experimental version of the Boston Naming task, using half of the original 85 line drawings, while the more recent 60-item version of the Boston Naming task was administered at Phases 3 and 4; while there are some differences in the stimuli used, the tests have been found to be very similar (Thompson and Heaton, 1989). All analyses concerning the behavioural language tasks were conducted on raw scores.
A large speech and language battery was administered at Phase 2 with the aim of investigating recovery from chronic aphasia (the findings of this study and descriptions of the tasks used can be found in Ludlow et al. (1986)). However, in the interests of not over-analysing the data by considering too many variables at once (increasing the risk of Type 1 error), we opted to consider data from the Boston Naming and Token Tests, only. These were also the only tests for which data were available for all three Phases.
Alexithymia was assessed at Phase 4, using the Toronto Alexithymia Scale (TAS-20), the standard self-report questionnaire used to measure alexithymia. As well as the summary score, the TAS-20 also contains three subscales which can be considered individually; difficulty identifying feelings (DIF); difficulty expressing feelings (DEF); and externally oriented thinking (EXO). Considering the hypothesis that damage to language regions is associated with alexithymia, it would seem probable that such damage may be related to high scores on the first two subscales in particular. Therefore, as well as considering total TAS-20 scores, we also considered the patients’ scores on each subscale.
Alexithymia has been shown to be related to a number of mental health difficulties, so it important to control for the potential effects of psychiatric conditions such as depression, anxiety and PTSD, all of which can be expected to be especially prevalent in this sample of war veterans. The Beck Depression Inventory (Beck, 1996), State-Trait Anxiety Inventory (Spielberger et al., 1983), and Mississippi Scale for Combat-Related PTSD (Keane et al., 1988) were all administered at Phase 4 when the TAS-20 was given, and these questionnaires are used in our analyses to control for the possible effects of these mental health conditions. This was done by regressing the TAS-20 scores with depression, anxiety and PTSD measures as predictors, and saving the TAS-20 scores as standardised variables. All analyses were conducted upon these standardised TAS-20 scores, rather than the raw scores.
2.4. Voxel-Based Lesion-Symptom Mapping (VLSM) analyses
Whole-brain VLSM analyses were conducted to isolate the network of brain regions associated with each measure, and to determine whether damage to a common network of brain regions resulted in impaired performance on multiple measures. The Total (W = 0.99, p = .375), DIF (W = 0.99, p = .22), DEF (W = 0.99, p = .73), and EXO (W = 0.99, p = .35) measures were normally distributed, and therefore the corresponding VLSM analyses employed t-tests to contrast patients with damage to each voxel of the brain to non-brain injured control participants. Inferences in the language task VLSMs were made using Mann-Whitney U-tests since the data were not normally distributed (Token Test: W = 0.30, p < .001; Boston Naming Test: W = 0.73, p < .001). All VLSM analyses were directional in nature (i.e. increased DIF scores were a sign of impaired alexithymia, reduced BN scores were a sign of impaired lexical access, etc.), and a two-tailed 5% false discovery rate (FDR) was employed to correct for multiple comparisons. Each comparison required a minimum cluster size of 10 voxels, and only considered voxels that were damaged in at least four patients (Gläscher et al., 2010). Gray matter volumes were identified using the AAL atlas (Tzourio-Mazoyer et al., 2002), and the ICBM-DTI-81 atlas was used to identify white matter tracts (Mori et al., 2005). Both the DIF and Boston Naming Test VLSMs isolated significant lesion clusters associated with alexithymia and lexical retrieval impairments, respectively, and a conjunction image was created to examine whether a common network of brain regions is associated with both patterns of impairment (Fig. 3).
Fig. 3.
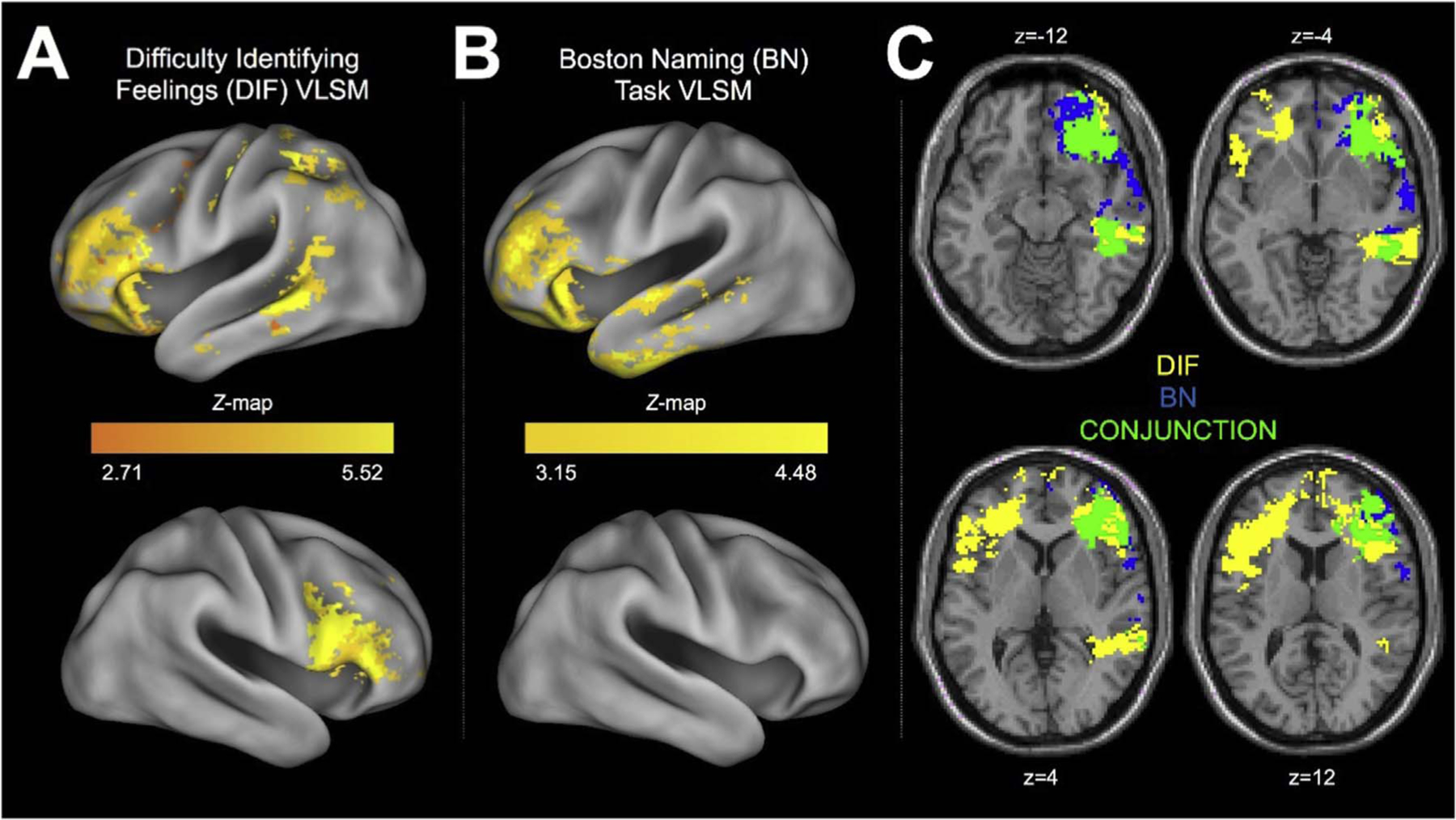
Results from the VSLM and conjunction analyses. Panel A shows the areas implicated in difficulties identifying feelings. Panel B shows the areas implicated in poor performance on the Boston Naming Task. Panel C shows the conjunction analysis, showing the overlapping regions implicated in both performance on the Boston Naming Task and difficulty identifying feelings. The regions in blue were implicated in Boston Naming task performance, the regions in yellow are implicated in difficulty identifying feelings, and the green regions highlight the significant overlap between the two. The conjunction analysis images are shown in radiologic convention (i.e., left is right).
3. Results
3.1. Association between alexithymia and behavioural language assessments
The language tasks exhibited strong ceiling effects, particularly from the later Phases 3 and 4, by which time many participants had received decades of therapy and recovery time. This made conducting ANOVAs inappropriate, due to the limited variance. Thus, in order to examine the relationship between language functioning and alexithymia, participants were grouped as “impaired” or “unimpaired” on a given language task. To be “impaired”, the participant’s score had to be 1.5 SD below the whole-sample mean (this still results in very small “impaired” groups; sample sizes are given below). As three time points were considered for the language measures, a corrected significance threshold would be a = 0.017 (or a = 0.008 for six comparisons, as there are two tests at each of these three time points).
Independent samples t-tests were conducted to compare impaired and unimpaired groups’ alexithymia scores. The results of these tests are summarised in Table 1. For the language tests administered at Phase 4 (the same Phase the alexithymia measures were also taken), the group impaired on Boston Naming (N = 7) scored more highly on the TAS-20 total (p = .018) (though this effect would not survive correction for multiple comparisons) and TAS DIF (p = .001) than the unimpaired group. For the Token Test, there were no differences between the impaired (N = 3) and unimpaired groups. Historic language functioning from previous Phases was also considered. For language performance at Phase 3, for the Boston Naming task (impaired group N = 10), DIF subscale scores were significantly higher in the impaired group than the unimpaired group (p = .007), but not for the other scales, including the total (p = .071). There was no difference in alexithymia scores between groups impaired or not on the Token Test (impaired group N = 3). Finally, for language functioning at Phase 2, considering performance on the Kaplan Naming task (an early version of the Boston Naming task), the impaired group (N = 11) scored more highly on the total TAS-20 score (p = .032) (though this effect would not survive correction for multiple comparisons) and DIF subscale (p = .014). There was again no difference in alexithymia when comparing those impaired (N = 6) and not impaired on the Token Test.
Table 1.
Independent t-tests comparing patients grouped by their performance in the language tests. Bracketed n values represent the number of impaired patients at each time point. TAS DIF, TAS DEF and TAS EXO scores refer to the Difficulty Identifying Feelings, Difficulty Expressing Feelings, and Externally- Oriented Thinking subscales of the Toronto Alexithymia Scale. Note that standardised (z) scores are presented after controlling for depression, anxiety and Post-Traumatic Stress Disorder symptom severity.
| TAS Total | TAS DIF | TAS DEF | TAS EXO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Task | t (df) | p | Mean (SD) Unimpaired/ Impaired |
t (df) | p | Mean (SD) Unimpaired/ Impaired |
t (df) | p | Mean (SD) Unimpaired/ Impaired |
t (df) | p | Mean (SD) Unimpaired/ Impaired |
|
| Phase 2 | BN | −2.16 (133) | .032 | −0.06 (0.96) | −2.49 (133) | .014* | −0.08 (0.93) | −1.20 (133) | .234 | −0.03 (0.96) | −1.19 (133) | .236 | −.007 (1.00) |
| (n = 11) | 0.61 (1.15) | 0.66 (1.13) | 0.33 (1.20) | 0.37 (0.97) | |||||||||
| TT | −0.23 (133) | .822 | −0.03 (0.97) | −1.40 (133) | .163 | −0.06 (0.97) | 1.05 (133) | .296 | −0.01 (0.98) | −0.003 (133) | .998 | 0.008 (0.99) | |
| (n = 6) | 0.07 (1.14) | 0.49 (0.59) | −0.44 (1.30) | 0.009 (1.31) | |||||||||
| Phase 3 | BN | −2.07 (148) | .040 | −0.05 (0.95) | −2.73 (148) | .007** | −0.06 (0.94) | −1.21 (148) | .228 | −0.03 (0.93) | −0.737 (148) | .462 | −0.02 (1.00) |
| (n = 16) | |||||||||||||
| 0.47 (1.07) | 0.62 (1.01) | 0.27 (1.03) | 0.17 (0.93) | ||||||||||
| TT | −1.13 (150) | .260 | −0.01 (0.97) | −1.94 (150) | .054 | −0.01 (0.96) | −0.38 (150) | .703 | −0.004 (0.97) | −0.20 (150) | .841 | −0.004 (0.99) | |
| (n = 3) | 0.63 (1.30) | 1.08 (1.12) | 0.21 (0.70) | 0.11 (1.13) | |||||||||
| Phase 4 | BN | −2.39 (157) | .018 | 0.04 (0.96) | −3.34 (157) | .001** | −0.06 (0.97) | −1.01 (157) | .315 | −0.01 (0.96) | −0.97 (157) | .332 | −.01 (0.98) |
| (n = 7) | |||||||||||||
| 0.86 (0.97) | 1.18 (0.80) | 0.36 (0.84) | 0.36 (1.11) | ||||||||||
| TT (n = 3) | −1.93 (157) | .055 | −0.02 (0.96) | −1.56 (157) | .121 | −0.03 (0.98) | −1.26 (157) | .208 | −0.01 (0.55) | −1.51 (157) | .134 | −0.01 (0.98) | |
| 1.07 (1.44) | −0.01 (0.70) | 0.70 (1.03) | 0.86 (1.46) | ||||||||||
indicates p < .008
indicates p < .017, where these p values correspond to those necessary for significance after correction for multiple comparisons.
Thus, while alexithymia scores did not differ between patients who had impaired and intact comprehension skills, a consistent relationship did emerge between naming skills and alexithymia, in particular difficulty identifying feelings. Differences on this subscale survived conservative significance thresholds at Phases 3 and 4. Differences in alexithymic traits between impaired and unimpaired groups at Phase 2 did not survive the most stringent corrections – although given that the time between the Kaplan Naming test and the TAS-20 was around 20 years, its non-significance is perhaps unsurprising.
3.2. Effect of damage to language regions on levels of alexithymia
Separate one-way ANOVAs for each brain region and alexithymia subscale, with percentage volume loss (no damage, little damage (less than 15% volume reduction), moderate damage (15% volume reduction or more)) as the independent variable, were carried out to examine the effects of damage to language regions on alexithymia scores. Table 2 contains the means and standard deviations of the different damage groups’ alexithymia scores. There was no significant effect of bilateral damage to superior temporal cortex, on total alexithymia scores (F (3,158) = 0.236, p = .871), nor any of the three subscales of the TAS-20 (DIF: F (3,158) = 0.1.08, p = .359; DEF: F (3,158) = 0.383, = 0.766; EXO: F (3,158) = 0.460, p = .711). There was, however, a significant effect of bilateral damage to Broca’s region, for both the total alexithymia score (F (3,158) = 2.851, p = .039, η2 = 0.051), and the DIF subscale (F (3,158) = 4.301, p = .006, η2 = 0.075). Differences on the two other scales were not significant (DEF: F (3,158) = 1.252, p = .293; EXO: F (3,158) = 1.425, p = .237). Post hoc Bonferroni-corrected comparisons showed that for total TAS-20 score, the group with moderate (≥15% damage) to Broca’s areas was significantly higher scoring than TBI patients who had suffered no damage to these regions (p = .024). The differences between the moderate group and the group with little ( < 15% damage) damage, and healthy control participants did not reach significance (p = .071 and p = .078, respectively). Considering the DIF subscale, the moderate damage group were significantly more alexithymic than all other groups (No damage TBI group: p = .022; little damage group: p = .018; healthy controls: p = .003).
Table 2.
Means and standard deviations for alexithymia scales for the different damage groups. TAS DIF, TAS DEF and TAS EXO scores refer to the Difficulty Identifying Feelings, Difficulty Expressing Feelings, and Externally-Oriented Thinking subscales of the Toronto Alexithymia Scale. Note that standardised (z) scores are presented after controlling for depression, anxiety and Post-Traumatic Stress Disorder symptom severity.
| Lesion Volume | N | Total TAS | DIF | DEF | EXO | |
|---|---|---|---|---|---|---|
| Healthy controls | 33 | −0.03 (0.98) | −0.27 (0.81) | 0.09 (0.94) | 0.13 (1.11) | |
| Broca’s area | No damage | 68 | −0.12 (0.98) | 0.01 (1.06) | −0.12 (1.01) | −0.15 (0.90) |
| Little damage ( < 15%) | 51 | −0.003 (0.97) | −0.03 (0.98) | −0.002 (1.04) | 0.03 (1.01) | |
| Moderate damage (> 15%) | 10 | 0.85 (0.91) | 0.97 (0.89) | 0.50 (0.68) | 0.45 (1.05) | |
| BA44 | No damage | 87 | −0.09 (0.96) | 0.05 (0.98) | −0.13 (0.98) | −0.15 (0.90) |
| Little damage ( < 15%) | 35 | 0.05 (1.01) | −0.10 (1.09) | 0.11 (1.07) | 0.11 (1.01) | |
| Moderate damage (> 15%) | 7 | 1.05 (0.90) | 1.09 (0.58) | 0.62 (0.77) | 0.65 (1.21) | |
| BA45 | No damage | 76 | −0.14 (0.97) | −0.07 (1.03) | −0.10 (1.01) | −0.15 (0.94) |
| Little damage ( < 15%) | 37 | −0.09 (0.87) | −0.04 (0.82) | −0.14 (0.97) | −0.03 (0.98) | |
| Moderate damage (> 15%) | 16 | −0.03 (0.98) | 0.99 (0.99) | 0.63 (0.86) | 0.52 (0.88) | |
| Wernicke’s area | No damage | 82 | −0.05 (0.95) | 0.04 (0.97) | −0.08 (1.02) | −0.08 (0.95) |
| Little damage ( < 15%) | 40 | 0.11 (1.06) | 0.12 (1.02) | 0.04 (1.01) | 0.07 (1.00) | |
| Moderate damage (> 15%) | 7 | 0.09 (1.20) | 0.04 (1.69) | 0.23 (0.91) | −0.04 (0.91) |
Two further ANOVAs were conducted to examine the relative contributions of bilateral damage to the two Broca’s sub-regions, BA44 and BA45 (pars opercularis and pars triangularis) to alexithymia. For BA44, there was a significant effect of damage on alexithymia total score (F(3, 158) = 3.003, p = .032, η2 = 0.054) and DIF subscale (F(3, 158) = 4.069, p = .008, η2 = 0.072). In Bonferroni-corrected post hoc comparisons, the moderate damage group had significantly higher scores than the no damage group (p = .020), but differences with the other two groups did not reach significance (little damage group: p = .085; healthy controls p = .052). For the DIF subscale, the moderate damage group had significantly higher scores than all other groups: no damage p = .040, little damage p = .020, healthy controls = 0.005.
For damage to BA45, there was a significant effect of damage on total alexithymia score (F (3,158) = 5.916, p = .001, η2 = 0.10). There were also significant effects on the DIF subscale (F (3,158) = 6.969, p < .001, η2 = 0.12), and on the DEF subscale (F (3,158) = 2.829, p = .040). Effects on the EXO subscale did not reach statistical significance (F (3,158) = 2.312, p = .078). In Bonferroni-corrected post hoc comparisons, the moderate damage group scored significantly more highly than all others on total TAS score: versus the no damage group, p < .001; the little damage group, p = .002; and the healthy control group, p = .006. For the DIF subscale, the moderate damage group scored significantly more highly than all other groups: versus the no damage group, p < .001; the little damage group, p = .002; the healthy control group, p < .001. For DEF subscale, the moderate damage group scored significantly more highly than the no damage TBI group (p = .045). Differences with the little damage group were near significant (p = −.054), but not with the healthy controls (p = .429).
3.3. The independent contributions of anterior insula damage and inferior frontal gyrus damage to alexithymia
These initial analyses suggest that damage to language regions in the IFG is associated with alexithymia. However, it was unclear to what extent these effects were driven by co-occurring damage to the anterior insula (AI), a region that is neuroanatomically very close, and has previously been shown to be associated with acquired alexithymia in this sample (Hogeveen et al., 2016). In order to identify the independent contributions of AI and IFG damage to alexithymia, linear regression analyses were performed. Total volume loss was also included as a regressor in the model to ensure that any observed effects were specific to our a priori regions-of-interest, and not simply associated with increased lesion volumes across the brain.
When only damage to BA44 or BA45 and overall percentage loss to the whole brain were entered as predictors, damage to BA44 was not found to predict total TAS-20 score, or DIF subscale score (see Table 3). Damage to BA45, however, was found to be a significant predictor, of both Total TAS-20 and DIF scores (see Table 4). Damage to BA45 remained a significant predictor of total TAS-20 score when damage to AI was also added to model as a third variable, but not of the DIF subscale.
Table 3.
Results of regression analysis with Total TAS and DIF subscale scores as the dependent variables, with BA44 damage and overall percentage loss as two predictors. VIF = Variance Inflation Factor.
| Regression results for BA44 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA |
BA44 damage |
Overall percentage loss |
||||||||
| F | P | Beta | t | P | VIF | Beta | t | P | VIF | |
| Total TAS | 4.455 | 0.014 | 0.232 | 1.881 | 0.062 | 2.045 | 0.034 | 0.276 | 0.783 | 2.045 |
| DIF subscale | 4.871 | 0.009 | 0.076 | 0.622 | 0.535 | 2.045 | 0.208 | 1.695 | 0.093 | 2.045 |
Table 4.
Results of regression analysis with Total TAS and DIF (difficulty identifying feelings) subscale scores as the dependent variables, with BA45 damage and overall percentage loss as two predictors, followed by regression analyses with AI damage also included as a third predictor. Note that the alexithymia scores used in the regressions are standardised (z) scores, after controlling for depression, anxiety and Post-Traumatic Stress Disorder symptom severity. VIF = Variance Inflation Factor.
| Regression results for BA45 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA |
BA45 damage |
Overall percentage loss |
||||||||
| F | p | Beta | T | p | VIF | Beta | t | p | VIF | |
| Total TAS | 8.344 | < .001 | .354 | 3.318 | .001 | 1.620 | −.019 | −.180 | .857 | 1.620 |
| DIF | 7.079 | .001 | .228 | 2.121 | .036 | 1.620 | .121 | 1.130 | .261 | 1.620 |
| Regression results with AI damage included | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA |
BA45 damage |
Overall percentage loss |
AI damage |
|||||||||||
| F | p | Beta | T | p | VIF | Beta | t | p | VIF | Beta | t | p | VIF | |
| Total TAS | 5.533 | .001 | .376 | 2.406 | .018 | 3.452 | −.009 | −.076 | .939 | 2.003 | −.034 | −.195 | .846 | 4.252 |
| DIF | 4.7 | .004 | .253 | 1.609 | .110 | 3.452 | .133 | 1.109 | .269 | 2.003 | −.039 | −.222 | .825 | 4.252 |
Importantly, damage to the AI was not a significant predictor in either of these models.
The extent of damage to BA45 and AI regions was very highly correlated (see Table 5). The non-significance of AI damage after entering BA45 damage into the model may, therefore, reflect multi-collinearity issues, rather than AI damage not impacting upon alexithymic traits in this sample. Variance inflation factors (VIF) are included in Tables 3 and 4 to quantify the collinearity between the predictors. Additionally, the regression results when only AI and overall loss are included as predictors (without either BA45 or BA44) may be found in Table 6 in the Supplementary material.
Table 5.
Correlations between percentage of volume lost at different regions. It can be seen that damage to Broca’s area (BA 44 and BA 45) is highly predictive of anterior insula damage.
| BA45 | BA44 | Anterior Insula | Percentage loss to whole brain | |
|---|---|---|---|---|
| BA45 | / | 0.766** | 0.842** | 0.619** |
| BA44 | 0.766** | / | 0.833** | 0.715** |
| Anterior Insula | 0.842** | 0.833** | / | 0.706** |
| Percentage loss to whole brain | 0.619** | 0.715** | 0.706** | / |
Indicates significant at p < .001.
The large change in the beta for AI loss between these regressions is symptomatic of multicollinearity.
While disentangling the relative independent contributions of AI and BA45 damage is extremely difficult in our current dataset, it is interesting to consider the alexithymia scores of groups split by AI and/ or BA45 damage. Figs. 1 and 2 show the mean TAS total or DIF scores for the different groups. As would be expected given the previous regression analyses, groups with moderate damage to BA45 have higher alexithymic traits than other groups with less extensive or no damage to BA45.
Fig. 1.
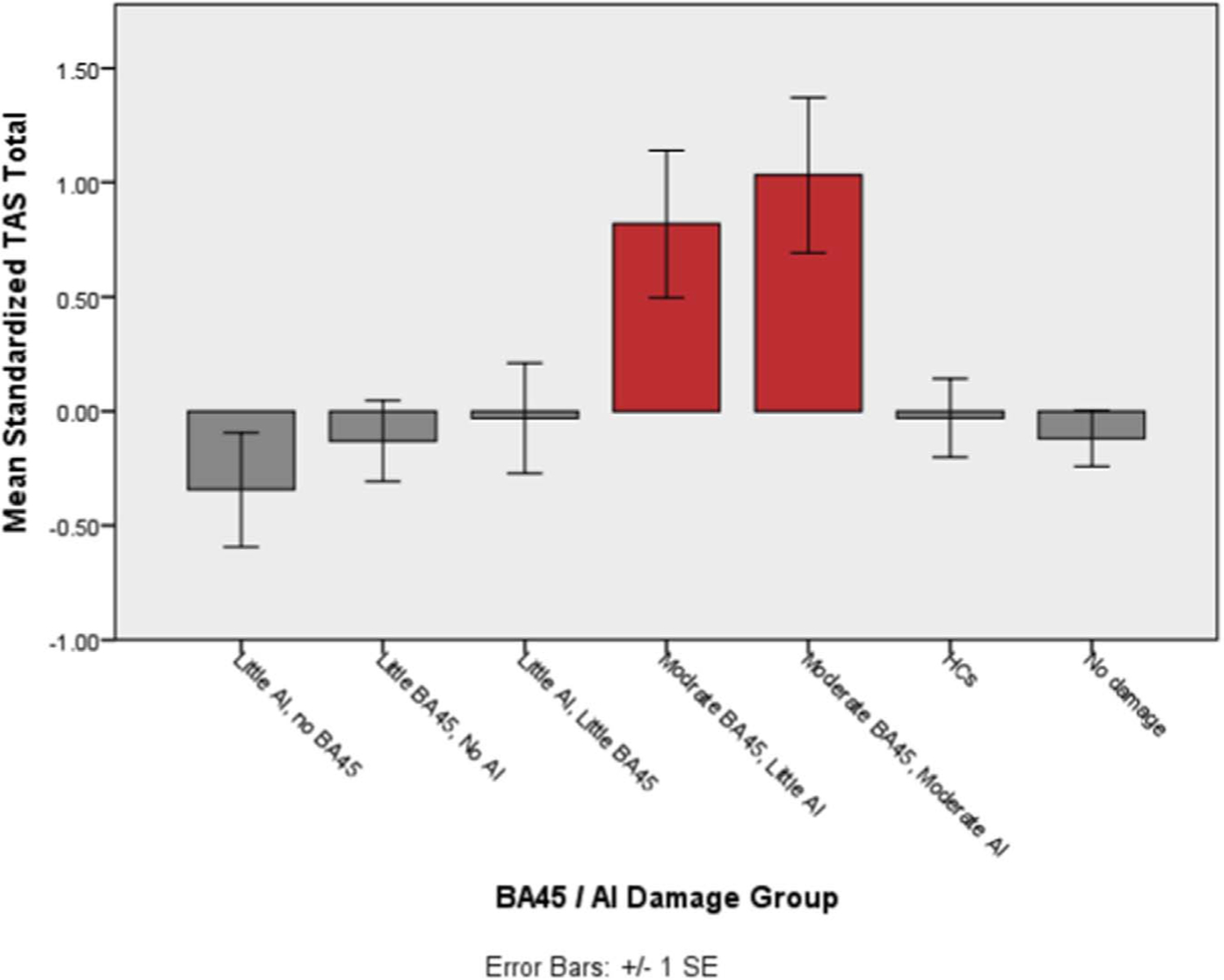
Average TAS total score for groups categorized by AI and BA45 damage. TAS score is the standardized variable, after controlling for depression, anxiety and PTSD symptoms. Red bars highlight groups with > 15% BA45 volume loss.
Fig. 2.
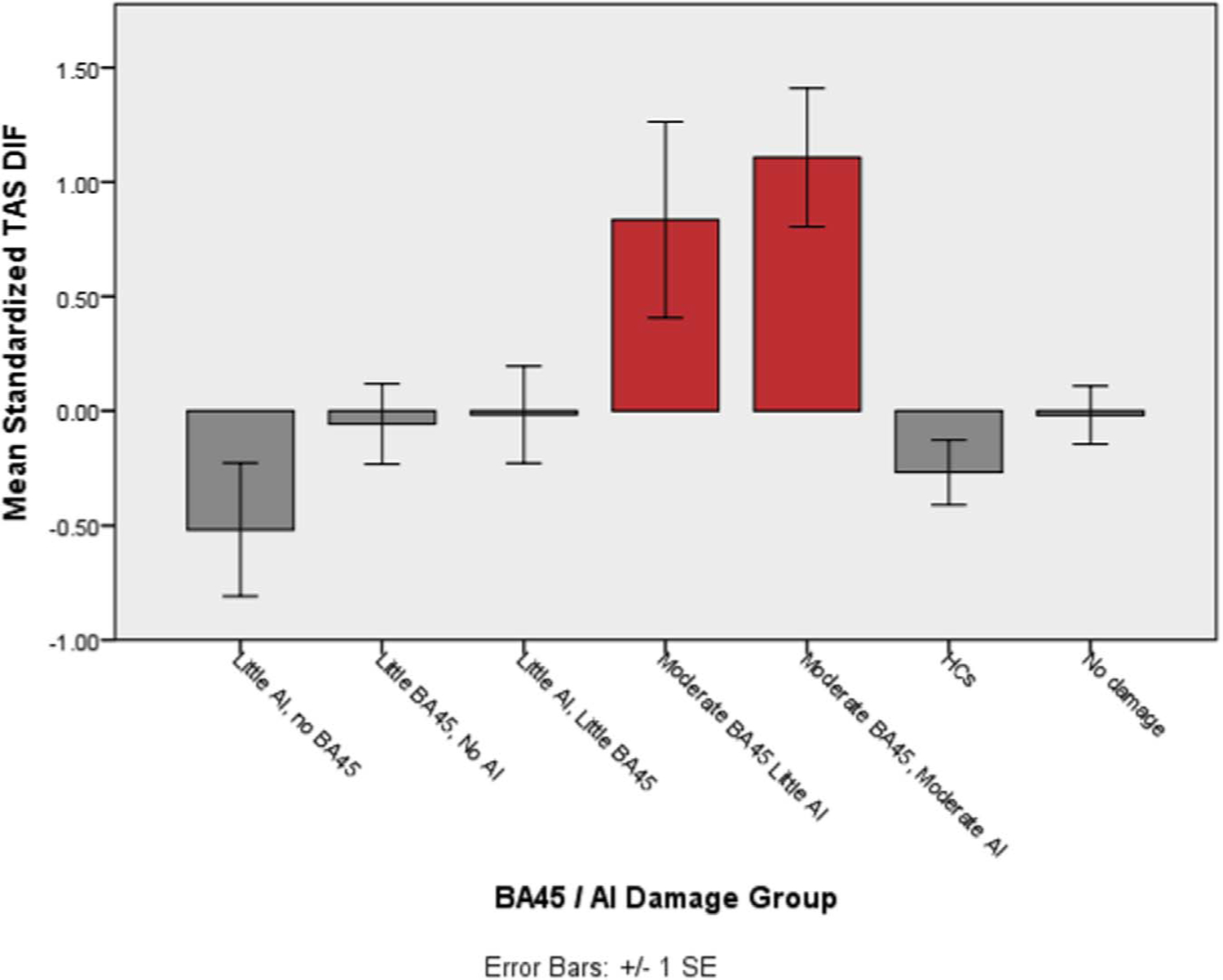
Average DIF score for groups categorized by AI and BA45 damage. DIF score is the standardized variable, after controlling for depression, anxiety and PTSD symptoms. Red bars highlight groups with > 15% BA45 volume loss.
3.4. VSLM and conjunction analyses
In addition to the regression analyses outline above, we examined which brain regions contributed to naming difficulties and DIF scores using a series of voxel-based lesion-symptom mapping (VLSM) analyses. We considered the damage sites that were associated with poor naming and high DIF scores, initially considering these independently. For a voxel to be tested for its association with behavioural performance, at least 4 patients needed to have a lesion at this voxel. Fig. 3A and B show the regions where damage was associated with poorer naming and higher DIF scores, respectively. For the Boston Naming task, the lesion sites implicated were frontal and temporal regions in the left hemisphere, including the orbitofrontal cortex, inferior frontal cortex, insula, and superior and inferior temporal regions. For DIF, a wider network of regions was implicated, including regions in the frontal cortices (including the pars opercularis, orbitalis, and triangularis), and bilateral ACC and bilateral insula. DIF performance was also related to damage in middle inferior and superior temporal regions, extending into parietal regions. Further details of the VLSM results can be found in Tables 7 and 8, in the Supplementary materials.
A conjunction analysis was also run to examine the overlap in lesion sites implicated in both high DIF and poor naming. As can be seen in Fig. 3C, clusters within the left IFC (including the pars opercularis, orbitalis and triangularis) and AI emerged as significant, as well as damage to regions in the inferior and middle temporal cortex.
It is important to stress that these analyses do not pre-select a region of interest, and are based on the whole brain. The significance of regions in the IFC in these analyses thus compliment the findings of the previous regression and ANOVA analyses which found that damage to language regions in the IFG can lead to acquired alexithymia. However, these analyses all implicated both the IFG and the AI, and it is worth noting that these whole-brain analyses may be just as affected by the high comorbidity of AI and IFG damage as the preceding ROI analyses.
4. Discussion
Evidence suggests that the anterior insula, and the interoceptive processes it underpins, play a central role in alexithymia (Brewer et al., 2016; Gu et al., 2013; Herbert et al., 2011; Hogeveen et al., 2016; Murphy et al., 2017b; Shah et al., 2016; Silani et al., 2008), however, language may represent another key skill that affects an individual’s ability to identify and express their own emotions. The current study analysed data from a large sample of TBI patients in order to examine the impact of behavioural language impairments and damage to language regions on alexithymia. The results suggested that impaired object naming ability is associated with alexithymia, specifically with difficulties identifying one’s own emotions. Damage to areas in the inferior frontal gyrus, commonly considered to be an important language region, was also associated with increased alexithymia scores. Furthermore, in addition to these ROI-based analyses, a whole-brain VLSM analysis also identified areas in the inferior frontal cortex associated with difficulty identifying feelings, and conjunction analyses examining lesions sites implicated in both DIF and naming deficits highlighted both the IFC and AI. This study adds to a growing body of evidence for a link between verbal abilities and alexithymia, in both groups with acquired alexithymia and in developmental populations (Henry et al., 2006; Karukivi et al., 2012; Karukivi and Saarijärvi, 2014; Kokkonen et al., 2003; Wood and Williams, 2007). Although it was the case that in some of the analyses the high correlation between IFG and AI damage made identifying their separate effects difficult, these results, in conjunction with previous research, support the notion that alexithymia may arise due to disruption in either interoception or language.
As outlined in the Introduction, whether subtypes of alexithymia exist is currently debated. Although recent factor analytic studies have provided evidence against the existence of the two subtypes suggested by Bermond (i.e. one group of alexithymic individuals characterised by cognitive and affective impairment and another characterised by cognitive impairment only, Bermond, 1997) (Bagby et al., 2009), this is not necessarily at odds with our suggestion that there are multiple routes to alexithymia (aetiological subtypes). First, these factor analyses were conducted on patients without TBI; one possibility is that while alexithymia subtypes do not exist in the general population they may exist in groups that have suffered TBI. Indeed, it has been argued that acquired and developmental alexithymia are different conditions (Becerra et al., 2002). Specifically, Becerra et al. (2002) suggested that acquired alexithymia may result in a ‘more pure’ deficit of emotional awareness, as compared to developmental cases of alexithymia which are usually accompanied by comorbid personality symptoms or other long-standing conditions. The structure of acquired alexithymia following TBI could thus be rather different to developmental alexithymia. More importantly, it is possible that alexithymia (regardless of whether it is developmental or acquired) may manifest similarly at the behavioural level, but have differing cognitive or biological root causes. Individuals could experience difficulties identifying and expressing their own emotion for a number of different underlying reasons, but factor analyses of responses on alexithymia questionnaires will not necessarily reflect these diverse causes.
The evidence for at least partially distinct routes to alexithymia is already supported in other populations, including adult neurodegenerative diseases. For example, in frontotemporal dementia (FTD), both patients suffering from the language-type and behavioural-variant type show deficits in emotion processing (Kumfor and Piguet, 2012; Miller et al., 2012). However, poor performance on emotion tasks can be explained in patients suffering from the semantic dementia subtype by considering these patients’ non-emotional language abilities (e.g. abstract word knowledge, performance on verbal IQ tests) (Hsieh et al., 2012; Miller et al., 2012). In the behavioural-variant of FTD however, social dysfunction can be seen in apparently preserved general cognition, and normal language function (e.g. Lough et al., 2001; Lough and Hodges, 2002), and controlling for language functioning does not remove these patients’ emotion deficits (Miller et al., 2012). Such findings suggest overlapping alexithymic-like emotional problems in semantic dementia and behavioural-variant FTD at the behavioural level, but that the emotional difficulties arise due to different primary deficit. While in semantic dementia the primary cause may be language-related, in behavioural-variant FTD interoception processes may be a more likely candidate, given evidence of reduced responses to pain, eating despite satiety, and increased sugar cravings in this population (Carlino et al., 2010; Miller et al., 1995; Woolley et al., 2007).
It is worth noting that while the current study argues for a role for language impairment in acquired alexithymia, it is impossible to infer from these data whether either language or interoceptive impairment alone is sufficient to cause alexithymia. It is possible that there are some individuals with alexithymia who are impaired in the interoceptive domain only, and others who suffer only from a language impairment, but it is likely that far more individuals experience deficits in both domains. This is because it may be the case that one impairment arises due to the existence of the other. If, for example, one has impaired language, then this is likely to cause difficulties attributing verbal labels to interoceptive (including emotional) states. In turn, this may lead to reduced awareness of, and ability to distinguish between, these states, due to the absence of labels to discriminate between interoceptive categories. On the other hand, if one suffers from impaired interoceptive awareness, then this is likely to make attribution of verbal labels to emotional states difficult (as one’s ability to differentiate these states is poor), leading to deficits of emotional language. The current data showed an association between non-emotional language (in the Boston naming task) and alexithymia. This might suggest that, at least in some individuals with alexithymia, language impairment is not simply a consequence of poor interoception, although it remains possible that this association reflects the high degree of comorbid damage to the AI and language regions in the IFC. The Boston Naming Task draws on a number of cognitive processes including: visual analysis; activating semantic representations (requiring both the selection and retrieval of semantic information); accessing phonological word forms; and motor programming for articulation (Harry and Crowe, 2014). However, only semantic representation and lexical naming are likely to overlap with the difficulty identifying feelings facet of alexithymia; both the identification of one’s own affect and the naming of a visual stimulus require the activation of the semantic representation of the concept, and access to its word form. We therefore propose that it is language-specific difficulties on the naming task that are responsible for its association with difficulties identifying feelings, implicating language impairment in alexithymia.
Drawing our findings and previous evidence together, it appears that a general verbal naming deficit (which could arise due to deficits in a number of different functions which the IFG and its subregions have been proposed to underlie) can result in alexithymic difficulties. Such a hypothesis is in need of direct investigation, but this prediction is in keeping with recent theories about the role of language in emotion processing (Barrett, 2006; Lindquist et al., 2015).
5. Limitations and future directions
Due to the nature of the injuries sustained by the veterans, it was not possible to examine the independent effects of isolated IFG and AI lesions on alexithymic traits. Indeed, there are no patients in the current sample that have moderate damage to BA45 without either small or moderate comorbid damage to the AI, and the regression results indicate some degree of multicollinearity. These individuals’ lesions resulted from penetrating wounds, and for AI damage to occur the path of the projectile is likely to have had to pass through more lateral brain regions such as Broca’s area.
Because damage to the AI and BA45 were so comorbid in the current sample, reliably disentangling the independent contributions of damage to these two areas is problematic. Thus, these results require replication in samples where damage to these two areas can be dissociated, and alternative study populations may present the opportunity to do so. For example, Chen et al. (2016) examined the performance of a group of patients with circumscribed insular glioma, without language impairment, on empathy tasks. This study did not examine alexithymia per se, but did demonstrate that AI damage in the assumed absence of IFG damage is related to deficits known to be associated with alexithymia, including difficulty recognising emotions in others (Cook et al., 2013; Grynberg et al., 2012). Future studies should aim to shed light on the additive and interactive effects of interoception and language difficulties; it is possible that having one ability intact could be sufficient for adequate emotion processing, or allow individuals to develop compensatory strategies that allow them to broadly identify their own emotions. Indeed, the evidence of alexithymic difficulties in FTD, both the behavioural-variant and language variants, and previous research suggesting interoception impairments in behavioural variant-FTD, suggest that this population may be particularly useful in disentangling these effects. Comparing these subtypes to a third group in which interoception and language abilities are spared would allow for the examination of the relative contribution of these processes to alexithymia.
On a related note, this sample was not selected for aphasic symptoms, and future research should examine the prevalence and predictors of alexithymia in those with aphasia following TBI. It would also be useful to test interoception, language, and alexithymia soon after brain injury. A limitation of the current study is that the TBI sample was limited to older veterans, many years after injury. This leads to the possibility that any loss of emotional abilities following brain injury may have been able to be recovered through compensatory strategies. While this possibility cannot be ruled out, other studies have not found a relationship between time since injury and alexithymia (Williams and Wood, 2010).
The relationship between alexithymia and other language skills is also in need of research. The current analyses only examined the relationship between alexithymia and the two language tasks for which data were available from all three Phases, requiring the ability to comprehend instructions and to name objects. However, constructionist models of emotion (Barrett, 2006) would predict that a disruption in emotional categorization could occur for a number of reasons. These would include the poor development or loss of emotion vocabulary. Alternatively, in an acquired disorder group such as this, conceptual knowledge about emotions may remain intact, but a disruption in the semantic retrieval or selection of this knowledge would still lead to alexithymic difficulties. Furthermore, investigating the role of language processes in alexithymia in populations that do not have TBI will be essential in uncovering whether alexithymia can arise due to developmental language difficulties, rather than in acquired language impairments alone. Longitudinal studies in particular are required in these populations, in order to track the development of linguistic impairments from early childhood into adulthood.
While we have interpreted our findings in support of a role for language processes and language-related brain regions in acquired alexithymia, as noted in the Introduction, Broca’s area has also been argued to underpin executive functions in general. In principle then, it is possible that damage to the inferior frontal gyrus may be associated with alexithymia as a consequence of its effect on broader executive function, rather than being due to a specific language impairment. However, no effects of damage to Broca’s area were observed on the Token task which makes at least as much demand on executive functions as the Boston Naming task, making an executive function explanation of these data unlikely. However, it should be acknowledged that the relative sizes of the language-impaired (versus the unimpaired) groups were small, and thus the study may have been under-powered to detect the presence of a (necessarily smaller) effect on the Token task.
A key consideration for future research examining alexithymia in language-impaired populations is that alexithymia questionnaires such as the TAS-20 require some meta-cognitive ability, and some patients may feasibly lack insight into their own emotional difficulties. In the current sample, as also reported in Hogeveen et al. (2016), a sub-sample (N = 135) of the participants also completed the Mayer-Salovey-Caruso Emotional Intelligence Scale (MSCEIT; Mayer et al., 2002), a self-report measure that does not require participants to make judgements about their own emotional abilities. The “perceiving emotions” and “understanding emotions” subscales of the MSCEIT were significantly negatively associated with raw TAS-total scores (perceiving emotions: r = − 0.18, p = .02, 5; understanding emotions: r = −0.24, p = .003), providing construct validity of the TAS-20 in this population.
Finally, our current data and analyses do not allow us to make firm conclusion about the causal direction of the relationship between alexithymia and language. We have assumed language impairment causes alexithymia, and it seems difficult to explain how a specific difficulty with one’s own emotions would lead to an object naming deficit that spans words for non-emotional items, but a relationship in the opposite direction cannot be ruled out.
6. Implications
Together with existing research, the current results suggest that recognition of one’s own emotions relies not on a single mechanism, process or region, but on a set of cognitive abilities and processes, underpinned by a network of brain regions. If this is the case, then we can expect alexithymia to arise in a number of different populations, for a number of different reasons. Those who suffer language impairments may present with similar behavioural difficulties as those who suffer interoceptive impairment, and deficit in one of these domains may eventually lead to deficit in the other. Understanding these distinct aetiologies will be important not only for our theoretical understanding of alexithymia and emotion processes, but also for clinical practice, where a difficulty in identifying and describing one’s feelings has been shown to impact on the success of psychological therapies (Lumley et al., 2007; Mccallum et al., 2003). The potential uses of interoception training continues to be investigated (e.g. Schaefer et al., 2014), and could be effective for conditions in which alexithymia and interoception difficulties are common, such as autism (Berthoz and Hill, 2005; Berthoz et al., 2013; Fiene and Brownlow, 2015; Garfinkel et al., 2016; Hill et al., 2004; Shah et al., 2016). Such research will be crucial in testing the predictions of the alexithymia-interoception model, and applying this model to real world clinical practice. However, a link between language and alexithymia suggests that not all alexithymic difficulties will be due to interoceptive failure, and thus interventions based on improved interoceptive insight may hold limited utility for clinical groups in which language impairments outweigh interoceptive impairments.
7. Summary
This paper sought to explore links between behavioural language impairments, damage to language regions, and alexithymia. Our analyses suggest a possible role for language regions in the IFG, and processes underpinned in part by these areas, including naming. The framework we have described draws together previous theories and experimental evidence. The extent to which AI and IFG damage exhibit independent effects on acquired alexithymia, and the relative importance of damage to these regions, remains unclear. Understanding potential sources of alexithymic difficulties other than interoception deficits, however, will have important implications both theoretically, and for the clinical application of alexithymia research.
Supplementary Material
Acknowledgements
We thank our Vietnam veterans for their participation in the study. We are grateful to V. Raymont, S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for testing and evaluating participants. The National Naval Medical Center and the National Institute of Neurological Disorders and Stroke provided their facilities and supported the research. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government. This research was supported by the Therapeutic Cognitive Neuroscience Fund (BG).
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.neuropsychologia.2017.12.037.
References
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Zilles K, 2004. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space - The roles of Brodmann areas 44 and 45. NeuroImage 22 (1), 42–56. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K, 2012. Architecture and organizational principles of Broca’s region. Trends Cogn. Sci 16 (8), 418–426. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Quilty LC, Taylor GJ, Grabe HJ, Luminet O, Verissimo R, Vanheule S, 2009. Are there subtypes of alexithymia? Personal. Individ. Differ 47 (5), 413–418. [Google Scholar]
- Barrett LF, 2006. Solving the emotion paradox: categorization and the experience of emotion. Personal. Social Psychol. Rev 10 (1), 20–46. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Lindquist KA, Gendron M, 2007. Language as context for the perception of emotion. Trends Cogn. Sci 11 (8), 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra R, Amos A, Jongenelis S, 2002. Organic alexithymia: a study of acquired emotional blindness. Brain Inj 16 (7), 633–645. [DOI] [PubMed] [Google Scholar]
- Beck AT, 1996. Manual for the Beck Depression Inventory-II The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bermond B, 1997. Brain and alexithymia. In: Vingerhoets A, Bussel F, Boelhouwer J (Eds.), The (Non)expression of Emotions in Health and Disease Tilburg University Press, Tilburg, The Netherlands, pp. 115–130. [Google Scholar]
- Berthoz S, Hill EL, 2005. The validity of using self-reports to assess emotion regulation abilities in adults with autism spectrum disorder. Eur. Psychiatry 20 (3), 291–298. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Lalanne C, Crane L, Hill EL, 2013. Investigating emotional impairments in adults with autism spectrum disorders and the broader autism phenotype. Psychiatry Res 208 (3), 257–264. [DOI] [PubMed] [Google Scholar]
- Bibby PA, Ferguson E, 2011. The ability to process emotional information predicts loss aversion. Personal. Individ. Differ 51 (3), 263–266. [Google Scholar]
- Bird G, Cook R, 2013. Mixed emotions: the contribution of alexithymia to the emotional symptoms of autism. Transl. Psychiatry 3 (7), e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T, 2010. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 133 (5), 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova Y, Diaz-Santos M, Cronin-Golomb A, 2010. Neurocognitive correlates of alexithymia in asymptomatic individuals with HIV. Neuropsychologia 48 (5), 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botting N, Conti-Ramsden G, 2008. The role of language, social cognition, and social skill in the functional social outcomes of young adolescents with and without a history of SLI. Br. J. Dev. Psychol 26 (2), 281–300. [Google Scholar]
- Brewer R, Cook R, Bird G, 2016. Alexithymia: a general deficit of interoception. R. Soc. Open Sci 3, 150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R, Happé F, Cook R, Bird G, 2015. Commentary on “Autism, oxytocin and interoception”: alexithymia, not Autism Spectrum Disorders, is the consequence of interoceptive failure. Neurosci. Biobehav. Rev 56, 348–353. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C, 2001. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn. Sci 25 (5), 663–678. [Google Scholar]
- Carlino E, Benedetti F, Rainero I, Asteggiano G, Cappa G, Tarenzi L, Pollo A, 2010. Pain perception and tolerance in patients with frontotemporal dementia. Pain 151 (3), 783–789. [DOI] [PubMed] [Google Scholar]
- Chahraoui K, Pinoit J, Viegas N, Adnet J, Bonin B, Moreau T, 2008. Alexithymie et liens avec la depression et l’anxiété dans la sclérose en plaques. Rev. Neurol 164, 242–245. [DOI] [PubMed] [Google Scholar]
- Chen P, Wang G, Ma R, Jing F, Zhang Y, Wang Y, Zhang X, 2016. Multidimensional assessment of empathic abilities in patients with insular glioma. Cogn., Affect., Behav. Neurosci 1–14. [DOI] [PubMed]
- Cook R, Brewer R, Shah P, Bird G, 2013. Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychol. Sci 24 (5), 723–732. [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Carlesimo GA, Salamone G, Caltagirone C, 2010. Prevalence and characteristics of alexithymia in Parkinson’s disease. Psychosomatics 51 (1), 22–28. [DOI] [PubMed] [Google Scholar]
- Eizaguirre AE, de Cabezon AOS, de Alda IO, Olariaga LJ, Juaniz M, 2004. Alexithymia and its relationship with anxiety and depression in eating disorders. Personal. Individ. Differ 36, 321–331. [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S, 2002. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59 (9), 1343–1349. [DOI] [PubMed] [Google Scholar]
- Ferguson E, Bibby PA, Rosamond S, O’Grady C, Parcell A, Amos C, O’Carroll R, 2009. Alexithymia, cumulative feedback, and differential response patterns on the Iowa gambling task. J. Personal 77 (3), 883–902. [DOI] [PubMed] [Google Scholar]
- Fiene L, Brownlow C, 2015. Investigating interoception and body awareness in adults with and without autism spectrum disorder. Autism Res 8 (6), 709–716. [DOI] [PubMed] [Google Scholar]
- Friederici AD, 2012. The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn. Sci 16 (5), 262–268. [DOI] [PubMed] [Google Scholar]
- Ford JA, Milosky LM, 2003. Inferring emotional reactions in social situations: differences in children with language impairment. J. Speech Lang. Hear. Res 46 (1), 21–30. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Brinton B, Clarke D, 2002. Emotion regulation in children with specific language impairment. Lang. Speech Hear. Serv. Sch 33 (2), 102–111. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Cornell AS, Bird G, 2016. The psychophysiological mechanisms of alexithymia in autism spectrum disorder. Autism [DOI] [PubMed]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, Critchley HD, 2016. Discrepancies between dimensions of interoception in autism: implications for emotion and anxiety. Biol. Psychol 114, 117–126. [DOI] [PubMed] [Google Scholar]
- Geschwind N, 1970. The organization of language and the brain. Science 170 (961), 940–944. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R, 2010. Distributed neural system for general intelligence revealed by lesion mapping. Proc. Natl. Acad. Sci 107 (10), 4705–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg D, Chang B, Corneille O, Maurage P, Vermeulen N, Berthoz S, Luminet O, 2012. Alexithymia and the processing of emotional facial expressions (EFEs): systematic review, unanswered questions and further perspectives. PLoS One 7 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J, 2013. Anterior insular cortex and emotional awareness. J. Comp. Neurol 521 (15), 3371–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry A, Crowe SF, 2014. Is the Boston naming test still fit for purpose? Clin. Neuropsychol 28 (3), 486–504. [DOI] [PubMed] [Google Scholar]
- Heaton P, Reichenbacher L, Sauter DA, Allen R, Scott S, Hill E, 2012. Measuring the effects of alexithymia on perception of emotional vocalizations in autistic spectrum disorder and typical development. Psychol. Med 42 (11), 2453–2459. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Theodorou G, Summers F, 2006. Cognitive and psychosocial correlates of alexithymia following traumatic brain injury. Neuropsychologia 44 (1), 62–72. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Herbert C, Pollatos O, 2011. On the relationship between interoceptive awareness and alexithymia: is interoceptive awareness related to emotional awareness? J. Personal 79, 1149–1175. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U, 2004. Brief report: cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J. Autism Dev. Disord 34 (2), 229–235. [DOI] [PubMed] [Google Scholar]
- Hogeveen J, Bird G, Chau A, Krueger F, Grafman J, 2016. Acquired alexithymia following damage to the anterior insula. Neuropsychologia 82, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Foxe D, Leslie F, Savage S, Piguet O, Hodges JR, 2012. Grief and joy: emotion word comprehension in the dementias. Neuropsychology 26 (5), 624–630. [DOI] [PubMed] [Google Scholar]
- Hsing CK, Hofelich Mohr A, Brent Stansfield R, Preston SD, 2013. Alexithymia slows performance but preserves spontaneous semantic decoding of negative expressions in the emostroop task. Int. J. Psychol. Res 6 (SPE), 56–67. [Google Scholar]
- Jernigan TL, Archibald S, Hesselink R, Atkinson JH, Velin RA, Mccutchan JA, Group H, 1993. Magnetic Resonance Imaging Morphometric Analysis of Cerebral Volume Loss in Human Immunodeficiency Virus Infection. Arch. Neurol 50 (3), 250–255. [DOI] [PubMed] [Google Scholar]
- Kano M, Ito M, Fukudo S, 2011. Neural substrates of decision making as measured with the Iowa Gambling Task in men with alexithymia. Psychosom. Med 73 (7), 588–597. [DOI] [PubMed] [Google Scholar]
- Karukivi M, Joukamaa M, Hautala L, Kaleva O, Haapasalo-Pesu KM, Liuksila PR, Saarijärvi S, 2012. Deficit in speech development at the age of 5 years predicts alexithymia in late-adolescent males. Compr. Psychiatry 53 (1), 54–62. [DOI] [PubMed] [Google Scholar]
- Karukivi M, Saarijärvi S, 2014. Development of alexithymic personality features. World J. Psychiatry 4 (4), 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Caddell JM, Taylor KL, 1988. Mississippi scale for combat-related posttraumatic stress disorder: three studies in reliability and validity. J. Consult. Clin. Psychol 56, 85–90. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J, 2007. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat. Neurosci 11 (2), 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkonen P, Veijola J, Karvonen JT, Laksy K, Jokelainen J, Jarvelin MR, Joukamaa M, 2003. Ability to speak at the age of 1 year and alexithymia 30 years later. J. Psychosom. Res 54 (5), 491–495. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Piguet O, 2012. Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychol. Rev 22 (3), 280–297. [DOI] [PubMed] [Google Scholar]
- Lamberty GJ, Holt CS, 1995. Evidence for a verbal deficit in alexithymia. J. Neuropsychiatry Clin. Neurosci 7 (3), 320–324. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak a., Schwartz GE, 1996. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosom. Med 58 (3), 203–210. [DOI] [PubMed] [Google Scholar]
- Lindell AK, 2006. In your right mind: right hemisphere contributions to language processing and production. Neuropsychol. Rev 16 (3), 131–148. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Barrett LF, Bliss-Moreau E, Russell JA, 2006. Language and the perception of emotion. Emot 6 (1), 125. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wagner TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci 35, 121–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, MacCormack JK, Shablack H, 2015. The role of language in emotion: predictions from psychological constructionism. Front. Psychol 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough S, Gregory C, Hodges JR, 2001. Dissociation of social cognition and executive function in frontal variant frontotemporal dementia. Neurocase 7 (2), 123–130. [DOI] [PubMed] [Google Scholar]
- Lough S, Hodges JR, 2002. Measuring and modifying abnormal social cognition in frontal variant frontotemporal dementia. J. Psychosom. Res 53 (2), 639–646. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Rosenberg J, Fair C, Buck D, Schesselman S, Salazar A, 1986. Brain lesions associated with nonfluent aphasia 15 years following penetrating head injury. Brain 109, 55–80. [DOI] [PubMed] [Google Scholar]
- Lumley MA, Neely LC, Burger AJ, 2007. The assessment of alexithymia in medical settings: implications for understanding and treating health problems. J. Personal. Assess 89 (3), 230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupyan G, 2012. Linguistically modulated perception and cognition: the label-feedback hypothesis. Front. Psychol 3, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta CZ, Wilson A, 1988. Emotion cognition interaction in personality development: a discrete emotions, functionalist analysis. Br. J. Social Psychol 27 (1), 91–112. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G, 2002. Mayer-Salovey-Caruso Emotional Intelligence Test (Version 2.0) User’s Manual Multi-Health Systems, Toronto, Canada. [Google Scholar]
- Mccallum M, Piper WE, Ogrodniczuk JS, Joyce AS, 2003. Relationships among psychological mindedness, alexithymia and outcome in four forms of short-term psychotherapy. Psychol. Psychother.: Theory, Res. Pract 76, 133–144. [DOI] [PubMed] [Google Scholar]
- McDonald S, Rosenfeld J, Henry JD, Togher L, Tate R, Bornhofen C, 2011. Emotion perception and alexithymia in people with severe traumatic brain injury: One disorder or two? A preliminary investigation. Brain Impair 12 (3), 165–178. [Google Scholar]
- Merkenschlager A, Amorosa H, Kiefl H, Martinius J, 2012. Recognition of face identity and emotion in expressive specific language impairment. Folia Phoniatr. et Logop 64 (2), 73–79. [DOI] [PubMed] [Google Scholar]
- Messina A, Beadle JN, Paradiso S, 2014. Towards a classification of alexithymia: primary, secondary and organic. J. Psychopathol 20 (1), 38–49. [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I, 1995. Dietary changes, compulsion and sexual behaviour in frontotemporal degeneration. Dementia 6, 195–199. [DOI] [PubMed] [Google Scholar]
- Miller LA, Hsieh S, Lah S, Savage S, Hodges JR, Piguet O, 2012. One size does not fit all: face emotion processing impairments in semantic dementia, behavioural-variant frontotemporal dementia and Alzheimer’s disease are mediated by distinct cognitive deficits. Behav. Neurol 25 (1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montebarocci O, Surcinelli P, Rossi N, Baldaro B, 2011. Alexithymia, verbal ability and emotion recognition. Psychiatr. Q 82 (3), 245–252. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher LM, 2005. MRI Atlas of Human White Matter Elsevier. [DOI] [PubMed] [Google Scholar]
- Murphy J, Brewer R, Catmur C, Bird G, 2017. Interoception and psychopathology: a developmental neuroscience perspective. Dev. Cogn. Neurosci 23, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Catmur C, Bird G, 2017b. Alexithymia is associated with a multi-domain, multi-dimensional failure of interoception: evidence from novel tests. J. Exp. Psychol.: Gen in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KE, Welsh JA, Trup EMV, Greenberg MT, 2011. Language delays of impoverished preschool children in relation to early academic and emotion recognition skills. First Lang 31 (2), 164–194. [Google Scholar]
- Norman H, Borrill J, 2015. The relationship between self-harm and alexithymia. Scand. J. Psychol 56 (4), 405–419. [DOI] [PubMed] [Google Scholar]
- Pandey R, Saxena P, Dubey A, 2011. Emotion regulation difficulties in alexithymia and mental health. Eur. J. Psychol 7 (4), 604–623. [Google Scholar]
- Parker JD, Bagby RM, Taylor GJ, Endler NS, Schmitz P, 1993. Factorial validity of the 20-item Toronto Alexithymia Scale. Eur. J. Personal 7 (4), 221–232. [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Fazio F, 1997. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8 (8), 2011–2017. [DOI] [PubMed] [Google Scholar]
- Pinard L, Negrete JC, Annable L, Audet N, 1996. Alexithymia in substance abusers. Am. J. Addict 5, 32–39. [Google Scholar]
- Price CJ, 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62 (2), 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson D, Davidoff J, 2000. The categorical perception of colors and facial expressions: the effect of verbal interference. Mem. Cogn 28 (6), 977–986. [DOI] [PubMed] [Google Scholar]
- Roberson D, Damjanovic L, Kikutani M, 2010. Show and tell: the role of language in categorizing facial expression of emotion. Emotion Rev. 2 (3), 255–260. [Google Scholar]
- Schaefer M, Egloff B, Gerlach AL, Witthöft M, 2014. Improving heartbeat perception in patients with medically unexplained symptoms reduces symptom distress. Biol. Psychol 101 (1), 69–76. [DOI] [PubMed] [Google Scholar]
- Shah P, Hall R, Catmur C, Bird G, 2016. Alexithymia, not autism, is associated with impaired interoception. Cortex 81, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U, 2008. Levels of emotional awareness and autism: an fMRI study. Soc. Neurosci 3 (2), 97–112. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J, 2007. User-friendly software for the analysis of brain lesions (ABLe). Comput. Methods Programs Biomed 86 (3), 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA, 1983. Manual for the State-trait Anxiety Inventory, Second ed. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Taylor GJ, Bagby RM, Parker JD, 1991. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics 32 (2), 153–164. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JS, 2005. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc. Natl. Acad. Sci. USA 102 (43), 15647–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LL, Heaton RK, 1989. Comparison of different versions of the Boston Naming Test. Clin. Neuropsychol 3 (2), 184–192. [Google Scholar]
- Thorberg F, Young R, Sullivan K, Lyvers M, 2009. Alexithymia and alcohol use disorders: a critical review. Addict. Behav 34 (3), 237–245. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A, 2005. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain 128 (12), 2872–2881. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15 (1), 273–289. [DOI] [PubMed] [Google Scholar]
- van’t Wout M, Aleman A, Bermond B, Kahn RS, 2007. No words for feelings: alexithymia in schizophrenia patients and first-degree relatives. Compr. Psychiatry 48 (1), 27–33. [DOI] [PubMed] [Google Scholar]
- Wagner H, Lee V, 2008. Alexithymia and individual differences in emotional expression. J. Res. Personal 42 (1), 83–95. [Google Scholar]
- Williams C, Wood RL, 2010. Alexithymia and emotional empathy following traumatic brain injury. J. Clin. Exp. Neuropsychol 32 (3), 259–267. [DOI] [PubMed] [Google Scholar]
- Wood RL, Williams C, 2007. Neuropsychological correlates of organic alexithymia. J. Int. Neuropsychol. Soc 13 (3), 471–479. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL, 2007. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 69 (14), 1424–1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


