Abstract
Purpose of review
The serotonergic system is implicated in multiple aspects of epilepsy, including seizure susceptibility, sudden unexpected death in epilepsy (SUDEP), and comorbid depression. Despite the complexity of serotonin’s effects on various neuronal networks, ongoing research provides considerable insight into the role of serotonin in human epilepsy. This review explores the potential roles of serotonergic therapies to improve clinical outcomes in epilepsy.
Recent findings
In recent decades research has markedly increased our knowledge of the diverse effects of serotonin on brain function. Animal models of epilepsy have identified the influence of serotonin on seizure threshold in specific brain regions, serotoninergic augmentation’s protective effects on terminal apnea and mortality in SUDEP, and mechanisms underlying behavioral improvement in some models of comorbid depression. Human clinical studies are largely consistent with animal data, but the translation into definitive treatment decisions has moved less rapidly.
Summary
Evidence for serotonergic therapy is promising for improvement in seizure control and prevention of SUDEP. For some epilepsies, such as Dravet syndrome, basic research on serotonin receptor agonists has translated into a positive clinical trial for fenfluramine. The cumulative results of safety and efficacy studies support the routine use of SSRIs for comorbid depression in epilepsy.
Keywords: epilepsy, serotonin, 5-hydroxytrytamine, SUDEP, depression
Introduction
Serotonin, also known at 5-hydroxytrytamine, is a biochemical messenger and regulator synthesized from the essential amino acid L-tryptophan. It was initially described as enteramine in 1937 by Dr. Vittorio Erspamer while studying smooth muscle constrictors of the gut. In 1952 enteramine was demonstrated to be the same substance that Drs. Irvine Page, Maurice Rapport, and Arda Green had called serotonin in their research on vasoconstrictors(1, 2). Serotonin was identified as a neurotransmitter in mammalian brains in 1953 by Dr. Betty Mack Twarog(3). After observing the structural similarity of serotonin and LSD, Dr. D.W. Woolley hypothesized the relationship between mental illness and serotonin in the 1950’s; he further observed that injection of serotonin antagonists into mice “calls forth convulsions much resembling those of human epilepsy (3).” Dr. Page later summarized in his book Serotonin that “the great variety of suggested roles can be said to be a tribute to man’s ingenuity and his unquestionable willingness to write papers…clearly, the field has fallen heir to the current disease of science- too many journals, too many meetings, and too little worth talking about(4).” Despite overestimates of some of the biological roles of serotonin, evidence supporting the association of serotonin and epilepsy has grown rapidly with a greater than five-fold increase in annual publications since 1990 as shown in figure 1. This paper reviews the growing understanding of the modulation of neuronal excitability by serotonin, and highlights recent publications on potential positive effects of serotonergic drugs on seizures, SUDEP, and depression in epilepsy(5).
Figure 1.
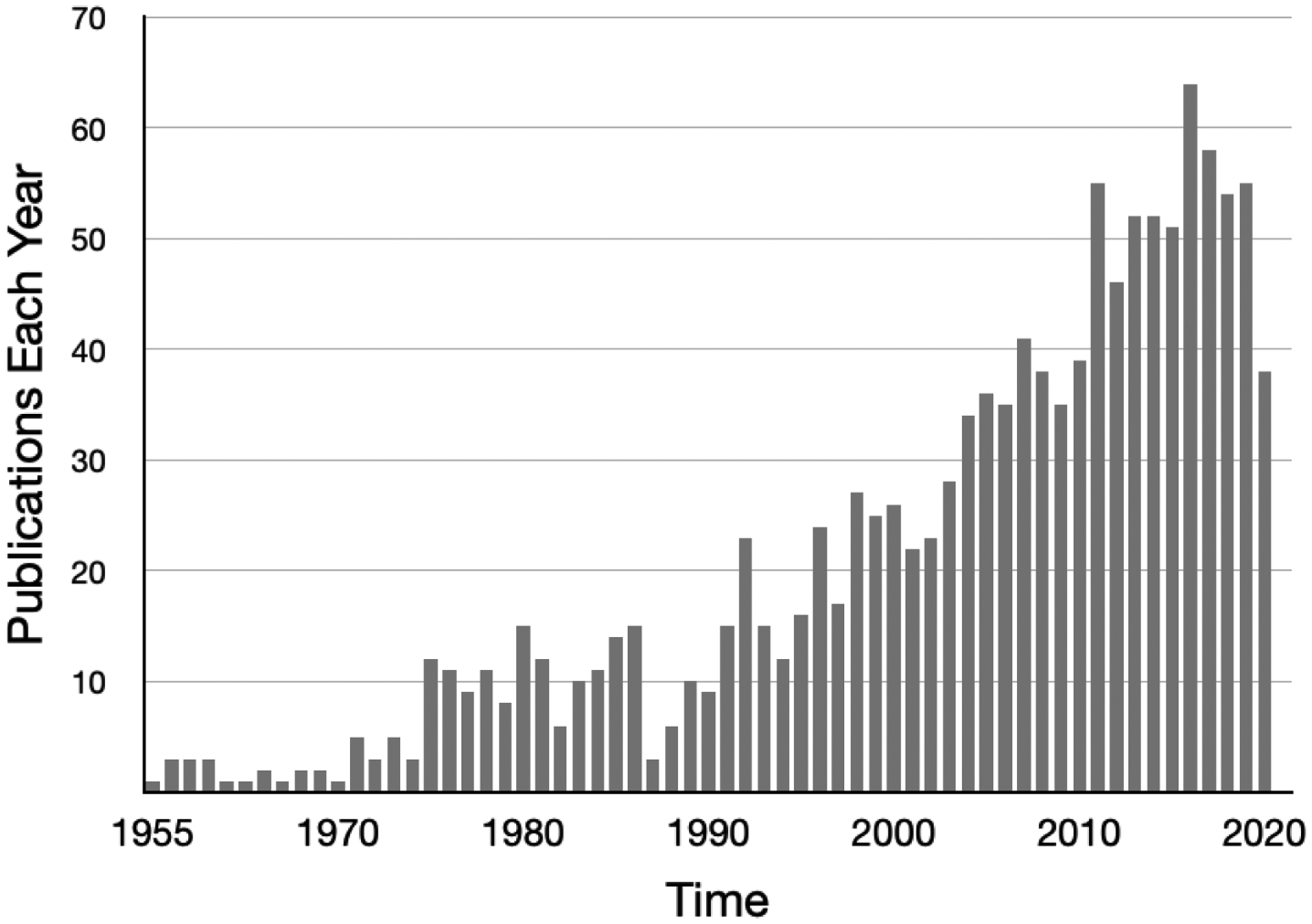
Results of PumMed.gov Search for Serotonin and Epilepsy.
Serotonin modulates neuronal excitability
Serotonergic cell bodies of the nervous system are predominantly located in the raphe nucleus, and project to the medulla and spinal cord, cerebellum, limbic system and striatum, and cerebral cortex. Although serotonergic neurons represent less than 1% of all neurons in the brain, they exhibit diverse effects through complex mechanisms involving seven identified subtypes of G-protein-coupled receptors, 5HT1A–1F, 5HT2A–2C, 5HT3, 5HT4, 5HT5A–5C, 5HT6 and 5HT7(6, 7). Clinically these effects have been found to regulate at varying degrees sleep, memory, reward, pain, appetite, sexuality, movement, mood, and seizure susceptibility(6). The mechanisms of serotonin’s effects are multifaceted, with some receptor subtypes hyperpolarizing and others depolarizing neuron membranes involving glutamatergic or GABAergic neurons(8). For example, activation of 5HT1A receptors causes hyperpolarization of neurons in the hippocampus, whereas 5HT7 may augment depolarization in certain neurons(9). The specific effect of serotonin on excitability and subsequent seizure susceptibility, mood, or respiration therefore depends on the subtype of 5HT receptors and types of neurons (e.g., pyramidal versus interneuron) that are involved, and these effects can vary by brain region.
Serotonergic drugs inhibit seizures
In 1957 Bonnycastle et al proposed a relationship between serotonin and epilepsy inhibition based on the observation that many antiseizure medications increase the concentration of serotonin in rodent brain(10). Cerebrospinal fluid studies found elevated markers of serotonin metabolism in patients with epilepsy that were being treated with epilepsy medications compared to untreated patients and non-epilepsy controls; serotonin levels were highest in patients on toxic medication doses(11). Early mouse experiments demonstrated that intracranial injection of serotonin protected against pentylenetetrazol and audiogenic seizures, similar to the protective effect of GABA(12). However, initial investigations of electroshock seizures did not find that serotonin modulated seizure thresholds(13). Subsequent studies with other models such as pilocarpine-induced status epilepticus and kainic-acid have demonstrated that serotonergic drugs reduce seizure frequency in chronic epilepsy(14, 15). Confusion has occurred due to reports that some tricyclic antidepressants with serotonergic mechanisms lowered the seizure threshold, but investigations of specific tricyclic drugs in primates such as photosensitive baboons concluded that the seizure effects were not due to serotonin system augmentation(16). More recent studies of serotonergic effects in rodent epilepsy models have demonstrated significant reduction in seizures(17, 18), including optogenetic activation of serotonin neurons in the dorsal raphe nucleus(19).
Despite the antiseizure effects of serotonin and related agonists, no antiseizure medications approved by the FDA have had serotoninergic effects as their primary mechanism of action. However, several early clinical observations suggested that serotonergic drugs may reduce seizures(20–22). The development of fenfluramine, which increases serotonin release and inhibits transporter re-uptake, has provided strong pharmacological evidence for serotonergic therapy efficacy for epilepsy(23). Aicardi and Gastaut initially used fenfluramine for self-induced absence seizures based on “some success from a variety of psychiatric disorders,” reporting the ablation of a photoconvulsive response in a small series(24). Several subsequent observational studies supported efficacy of fenfluramine for children with encephalopathy and severe epilepsy, including repeated challenges due to drug shortages(25–28). Several children participating in these studies were confirmed to have SCNIA mutations, which initiated interest in serotonergic therapies for seizures in Dravet Syndrome(29).
The observational and experimental studies of seizure inhibition by fenfluramine culminated in a randomized, blinded, placebo-controlled trial for seizures in Dravet Syndrome(30). The 14-week trial demonstrated a median reduction of motor seizures of 74.9% in the 0.7 mg/kg fenfluramine group, 42.3% in the 0.2mg/kg fenfluramine group, and 19.2% in the placebo group. Both fenfluramine groups had significant seizure reductions compared to placebo (p<0.0001 and p<0.02). As anticipated by the serotonergic effects in the brain and gut, the most frequent adverse effects were decreased appetite, diarrhea, fatigue, and somnolence. Echocardiography did not find evidence for valvular dysfunction or pulmonary hypertension. Similar efficacy and safety results were obtained in a small prospective, open-label pilot study of Lennox-Gastaut syndrome(31).
Multiple additional drugs modulating serotonin signaling, including clemizole, locaserin, and trazodone, have emerged as antiseizure treatments for Dravet syndrome and possibly other epilepsies. Zebrafish with SCN1A homologue mutations develop motor seizures that have been used for phenotypic screening of drug libraries(32). Baraban and colleagues published a series of studies demonstrating that serotonin receptor agonists inhibit seizures in a zebrafish model of Dravet syndrome, which they have translated into small clinical studies that provide preliminary support for the antiseizure efficacy of lorcaserin(33). Their zebrafish Dravet model also allowed development of receptor binding affinity models of 28 analogues of clemizole; three of these analogues had serotonin receptor binding that exerted strong antiepileptic activity(34). These studies provide powerful support that 5HT2B agonists inhibit neuronal hyperexcitability and seizures in a disease caused by a specific sodium channelopathy. Additional research is needed to determine the potential efficacy of serotonergic therapy for more common epilepsies with diverse etiologies.
Serotonin dysfunction may contribute to SUDEP
Premature mortality is significantly greater in epilepsy than the age-matched general population, with standard mortality rates for young adult epilepsy patients estimated to be 6.4 to 8.5 based on a recent systematic review sponsored by the International League Against Epilepsy(35). Annual mortality for pharmacoresistant epilepsy may be as high as 1–1.5%(36, 37). Studies have consistently found SUDEP to be the most common cause of death in epilepsy, and SUDEP is approximately 25-fold higher in people with epilepsy compared to the general population(38, 39). Some risk factors for SUDEP have emerged through epidemiological and observational studies, such as ≥3 generalized tonic-clonic seizures (GTCS) per year and lack of caregiver assistance during a seizure, but the specific mechanisms of SUDEP in humans are not known(40). A retrospective analysis of SUDEP events in epilepsy monitoring units found that all cases followed a secondarily GTCS leading to apnea within three minutes postictally and preceding asystole(41). Although available data are not sufficient to determine whether postictal respiratory dysfunction is central or obstructive, respiration has been found to be altered in clinical and animal models of epilepsy. Furthermore, respiratory dysfunction during seizures has been associated with serotonin abnormalities and may be prevented by serotonergic medications.
Although the definitive causes of SUDEP are not yet known, a recent extensive review highlights the potential roles of serotonin dysregulation(42). In addition to the evidence for serotonin depletion or antagonism prolonging or worsening seizures in animal models of epilepsy, serotonin also regulates arousal and respiratory drive. Maximal Electroshock (MES)-induced seizures in mice often result in terminal apnea that precedes asystole for which death can prevented by mechanical ventilation(43). DBA/1 and DBA/2 mice display respiratory arrest and death following audiogenic seizures; respiratory arrest in this epilepsy model can be prevented by mechanical respiration and by serotonergic augmentation with fenfluramine or optogenetic activation of serotonin neurons in the dorsal raphe nucleus(19, 44).
Multiple studies in humans have informed our understanding of seizure effects of breathing. Oxygen saturation below 90% occurs in one third of focal and generalized seizures(45). Postconvulsive central apnea is observed in 22% of patients with focal and generalized epilepsies, and may be a biomarker for near-SUDEP and SUDEP(46). Higher interictal serum serotonin levels are associated with shorter duration of postictal EEG suppression, and a greater increase in interictal to ictal serotonin level is associated with a shorter tonic phase of tonic-clonic seizures(47). In a subsequent study a greater change in interictal to postictal serotonin was associated with less apneas, further supporting that serotonin may have a protective effect on seizure-related respiratory events associated with SUDEP(48). Lack of permeability of the blood brain barrier (BB) to serotonin may challenge these observations, but epilepsy and seizures have been associated with BBB dysfunction.
Oxygen desaturation of <85% was found to be significantly less in focal seizures in patients taking SSRIs during video/EEG monitoring, but not in secondarily GTCS(49). Analysis of 476 seizures in 204 patients from nine epilepsy monitoring units found that patients with chronic use of SSRIs had half the risk of ictal central apnea and less oxygen desaturation compared to controls not using SSRIs or benzodiazepines(50).
Altered arousal regulated by serotonin has also been implicated as a possible mechanism for SUDEP. Hypercapnia-induced arousal is an important response for normal sleep homeostatis(51, 52). Mice with marked reduction in serotonin neurons do not arouse with hypercapnic challenges, but do with hypoxic, auditory, and tactile stimuli(53, 54). Furthermore, some data indicate that the “vigilance state” may be protective against SUDEP. For example, MES seizures in mice during REM sleep are universally fatal(55). The additional observation that serotonin neuronal activity is maximal during wakefulness, reduced during NREM sleep, and nearly absent during REM sleep is consistent with nocturnal seizures being a risk factor for SUDEP(56). Some authors have suggested that serotonin system dysfunction with altered arousal, sleep, and postictal obstructive apnea in the prone position may be a particularly lethal combination that explains many cases of SUDEP(42).
Serotonin may modulate depression in epilepsy
Depression and epilepsy are considered to have a bidirectional relationship based on increased risk of occurrence and severity of one in the presence of the other(57–59). The prevalence of depression is higher in epilepsy than other chronic disabling illnesses, suggesting a neurobiological influence(60). Depression precedes the first seizure at higher rates than controls, possibly indicating a common pathogenic mechanism(61, 62). These clinical observations are supported by a rat model bred for depressive behaviors that also develop increased seizure susceptibility(63).
Animal models of the comorbidity of epilepsy and depression have found evidence for multiple potential mechanisms that include most neurotransmitter systems(64). Although multiple etiologies probably contribute to the comorbidity, clinical and animal research support that serotonergic therapies can benefit comorbid depression and epilepsy. The pilocarpine-induced status epilepticus model of spontaneous seizures results in depressive behaviors including increased immobility on the forced swim test (FST) and decreased saccharin ingestion(65). These behaviors are associated with decreased hippocampal serotonin concentrations, turnover, and release. Compounds that produced antidepressant and anticonvulsant effects in this pilocarpine model, including citalopram, imipramine, and fluoxetine, also increased hippocampal serotonin release(66). It is noteworthy that SSRIs such as fluoxetine may not consistently improve depressive behaviors in the pilocarpine seizure model, and may be more effective in combination with a norepinephrine reuptake inhibitor(65, 67). Despite the complexity of the potential mechanisms underlying depression and epilepsy, a recent authoritative review concluded that compromised serotonin transmission was the probable common final pathway and is “the most immediately relevant neurotransmitter system” to understand their comorbidity(68). Human imaging studies of depression in epilepsy using serotonin ligands have identified abnormalities in limbic regions such as the hippocampus, insula, and cingulate gyrus, providing further support for involvement of serotonergic system dysfunction(69–72).
An early trial of depression treatment in epilepsy found no difference between antidepressants and placebo, concluding that “in patients with depression and epilepsy, immediate prescription with antidepressants may not be indicated(73).” Based largely on single case reports, concern emerged about the induction of seizures by SSRIs(74). Subsequent studies supported the safety of SSRIs related to seizure occurrence, including in epilepsy patients(75, 76). Several small studies of depressed epilepsy patients further supported safety and efficacy for SSRIs(77, 78). A recent larger randomized comparative effectiveness trial found that over one half of depressed patients with active epilepsy achieved remission after treatment with either sertraline or cognitive behavior therapy (CBT)(79). The trial was powered to detect a clinically relevant increase in GTCS, and found that sertraline was not associated with increased seizures or suicidality compared to CBT. Consistent with the bidirectional relationship, patients that achieved remission from depression had a significant reduction in GTCS compared to continued depression.
Conclusion
Serotonin regulates complex neuronal networks involved in seizure susceptibility, breathing and arousal mechanisms possibly contributing to SUDEP, and comorbid depression, as summarized in the schematic in figure 2. Animal models of epilepsy consistently indicate that serotonergic drugs inhibit seizures, but the clinical efficacy in humans is less robust except for some specific epilepsies such as Dravet syndrome. Animal models of SUDEP demonstrate less respiratory arrest and mortality with serotonergic interventions, and human studies indicate that higher serum serotonin concentration and SSRIs are associated with less central apnea and oxygen desaturation. Animal and human studies of SSRIs for the comorbidity of depression and epilepsy indicate efficacy for mood and behavior improvement, safety for seizures and suicidality, and possibly reduction of GTC seizures during remission from depression.
Figure 2.
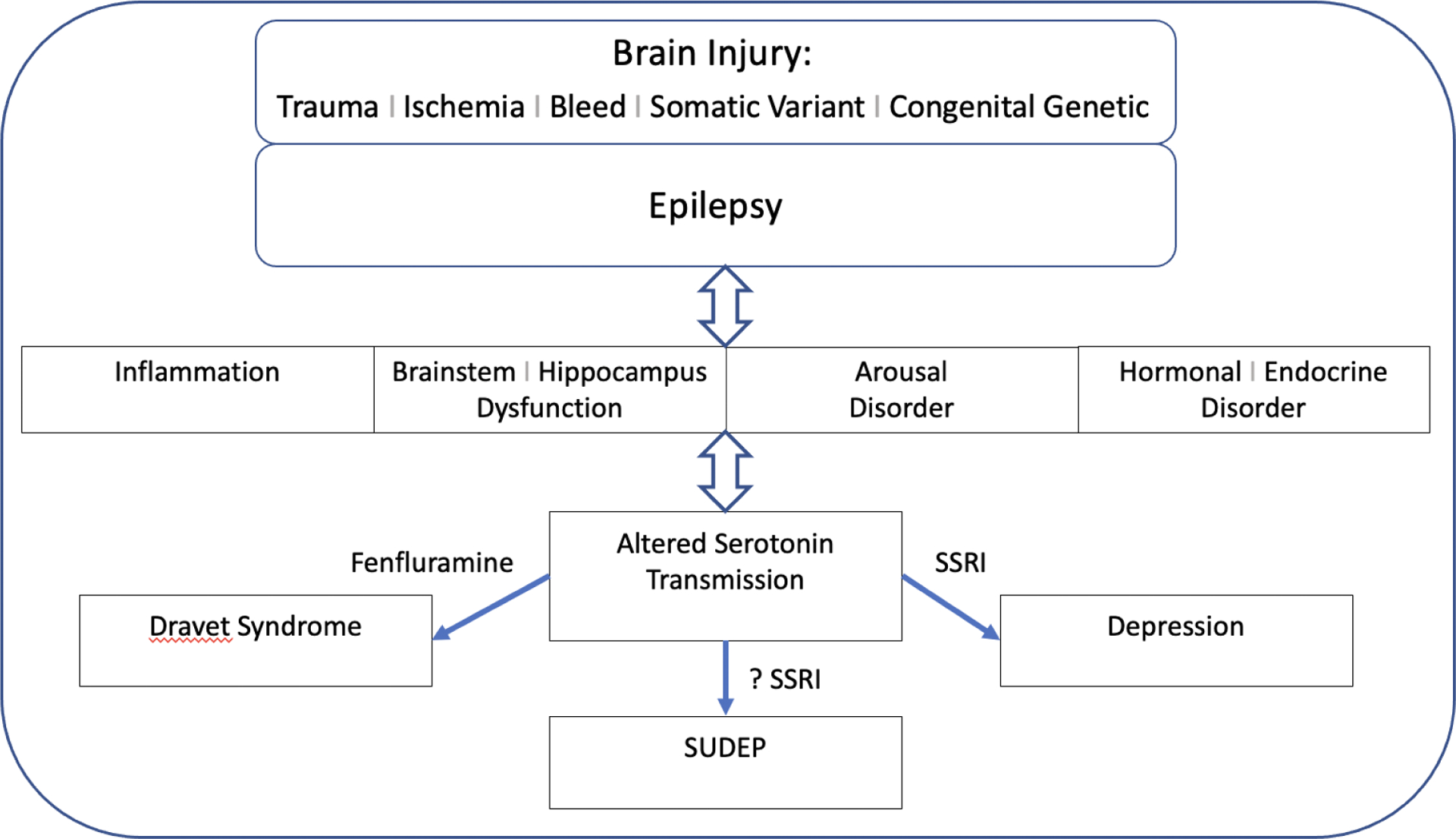
Schematic of brain injury, epilepsy, associated disorders and subsequent altered serotonin transmission resulting in depression, worsened seizures, and possibly SUDEP.
Key points.
Although the effects of serotonin on neurons are complex, the net influence appears to be inhibitory for most brain networks.
Animal models have consistently shown that augmentation of brain serotonin protects against seizures.
Serotonin agonists and reuptake transporter inhibitors prevent seizures in animal models of the sodium channel defect disease Dravet syndrome, and a randomized, blinded trial of children with Dravet syndrome demonstrated a marked reduction in motor seizures with fenfluramine compared to placebo.
Ictal central respiratory suppression is a possible cause of SUDEP and may be regulated by brainstem serotonin; selective serotonin reuptake inhibiters (SSRIs) appear to protect against periictal hypoxia.
Depression is common in epilepsy and serotonin may mediate their bidirectional relationship; despite frequent dysfunction of serotonin networks in epilepsy, SSRIs are effective and safe for depression, and seizures may improve after depression remission.
Financial Support and Sponsorship:
This work was supported by the National Institute for Neurological Disorders and Stroke, Bethesda, MD, USA.
Footnotes
Conflicts of Interest: None
References
- 1.Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169(4306):800–1. [DOI] [PubMed] [Google Scholar]
- 2.Whitaker-Azmitia PM. The discovery of serotonin and its role in neuroscience. Neuropsychopharmacology. 1999;21(2 Suppl):2S–8S. [DOI] [PubMed] [Google Scholar]
- 3.Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;175(1):157–61. [DOI] [PubMed] [Google Scholar]
- 4.Page IH. Serotonin. Chicago, IL: Yearbook Medical Publishers, Inc; 1968. [Google Scholar]
- 5.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52Suppl 1:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.De Deurwaerdere P, Di Giovanni G. Serotonin in Health and Disease. Int J Mol Sci. 2020;21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review of serotonin’s role in health and diverse diseases involving the central nervous system.
- 8.Ciranna L Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4(2):101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gharedaghi MH, Seyedabadi M, Ghia JE, et al. The role of different serotonin receptor subtypes in seizure susceptibility. Exp Brain Res. 2014;232(2):347–67. [DOI] [PubMed] [Google Scholar]
- 10.Bonnycastle DD, Giarman NJ, Paasonen MK. Anticonvulsant compounds and 5-hydroxytryptamine in rat brain. Br J Pharmacol Chemother. 1957;12(2):228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chadwick D, Jenner P, Reynolds EH. Serotonin metabolism in human epilepsy: the influence of anticonvulsant drugs. Ann Neurol. 1977;1(3):218–24. [DOI] [PubMed] [Google Scholar]
- 12.Schlesinger K, Stavnes KL, Boggan WO. Modification of audiogenic and pentylenetetrazol seizures with gamma-aminobutyric acid, norepinephrine and serotonin. Psychopharmacologia. 1969;15(3):226–31. [DOI] [PubMed] [Google Scholar]
- 13.Jobe PC, Stull RE, Geiger PF. The relative significance of norepinephrine, dopamine and 5-hydroxytryptamine in electroshock seizure in the rat. Neuropharmacology. 1974;13(10–11):961–8. [DOI] [PubMed] [Google Scholar]
- 14.Vermoesen K, Massie A, Smolders I, et al. The antidepressants citalopram and reboxetine reduce seizure frequency in rats with chronic epilepsy. Epilepsia. 2012;53(5):870–8. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez EJ, Williams PA, Dudek FE. Effects of fluoxetine and TFMPP on spontaneous seizures in rats with pilocarpine-induced epilepsy. Epilepsia. 2002;43(11):1337–45. [DOI] [PubMed] [Google Scholar]
- 16.Trimble M, Anlezark G, Meldrum B. Seizure activity in photosensitive baboons following antidepressant drugs and the role of serotoninergic mechanisms. Psychopharmacology (Berl). 1977;51(2):159–64. [DOI] [PubMed] [Google Scholar]
- 17.Faingold CL, Randall M, Zeng C, et al. Serotonergic agents act on 5-HT3 receptors in the brain to block seizure-induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy Behav. 2016;64(Pt A):166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagdy G, Kecskemeti V, Riba P, et al. Serotonin and epilepsy. J Neurochem. 2007;100(4):857–73. [DOI] [PubMed] [Google Scholar]
- **19.Zhang H, Zhao H, Zeng C, et al. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol Dis. 2018;110:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that controlled activation of brain stem serotonin cells protects against seizures and SUDEP in a model of audiogenic epilepsy.
- 20.Favale E, Audenino D, Cocito L, et al. The anticonvulsant effect of citalopram as an indirect evidence of serotonergic impairment in human epileptogenesis. Seizure. 2003;12(5):316–8. [DOI] [PubMed] [Google Scholar]
- 21.Specchio LM, Iudice A, Specchio N, et al. Citalopram as treatment of depression in patients with epilepsy. Clin Neuropharmacol. 2004;27(3):133–6. [DOI] [PubMed] [Google Scholar]
- 22.Albano C, Cupello A, Mainardi P, et al. Successful treatment of epilepsy with serotonin reuptake inhibitors: proposed mechanism. Neurochem Res. 2006;31(4):509–14. [DOI] [PubMed] [Google Scholar]
- **23.Polster T Individualized treatment approaches: Fenfluramine, a novel antiepileptic medication for the treatment of seizures in Dravet syndrome. Epilepsy Behav. 2019;91:99–102. [DOI] [PubMed] [Google Scholar]; Extensive review of the development of fenfluramine for the treatment of seizures in Dravet syndrome.
- 24.Aicardi J, Gastaut H. Treatment of self-induced photosensitive epilepsy with fenfluramine. N Engl J Med. 1985;313(22):1419. [DOI] [PubMed] [Google Scholar]
- 25.Boel M, Casaer P. Add-on therapy of fenfluramine in intractable self-induced epilepsy. Neuropediatrics. 1996;27(4):171–3. [DOI] [PubMed] [Google Scholar]
- 26.Ceulemans B, Boel M, Leyssens K, et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53(7):1131–9. [DOI] [PubMed] [Google Scholar]
- 27.Ceulemans B, Schoonjans AS, Marchau F, et al. Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia. 2016;57(7):e129–34. [DOI] [PubMed] [Google Scholar]
- 28.Schoonjans A, Paelinck BP, Marchau F, et al. Low-dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol. 2017;24(2):309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Kecskes A, Copmans D, et al. Pharmacological characterization of an antisense knockdown zebrafish model of Dravet syndrome: inhibition of epileptic seizures by the serotonin agonist fenfluramine. PLoS One. 2015;10(5):e0125898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Lagae L, Sullivan J, Knupp K, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10216):2243–54. [DOI] [PubMed] [Google Scholar]; Report of the results of a well designed and executed clinical trial quantifying the efficacy and safety of fenfluramine for seizure control in Dravet Syndrome.
- 31.Lagae L, Schoonjans AS, Gammaitoni AR, et al. A pilot, open-label study of the effectiveness and tolerability of low-dose ZX008 (fenfluramine HCl) in Lennox-Gastaut syndrome. Epilepsia. 2018;59(10):1881–8. [DOI] [PubMed] [Google Scholar]
- 32.Griffin A, Krasniak C, Baraban SC. Advancing epilepsy treatment through personalized genetic zebrafish models. Prog Brain Res. 2016;226:195–207. [DOI] [PubMed] [Google Scholar]
- 33.Griffin A, Hamling KR, Knupp K, et al. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain. 2017;140(3):669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin AL, Jaishankar P, Grandjean JM, et al. Zebrafish studies identify serotonin receptors mediating antiepileptic activity in Dravet syndrome. Brain Commun. 2019;1(1):fcz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurman DJ, Logroscino G, Beghi E, et al. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sperling MR, Feldman H, Kinman J, et al. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46(1):45–50. [DOI] [PubMed] [Google Scholar]
- 37.Sperling MR, Barshow S, Nei M, et al. A reappraisal of mortality after epilepsy surgery. Neurology. 2016;86(21):1938–44. [DOI] [PubMed] [Google Scholar]
- 38.Ryvlin P, Rheims S, Lhatoo SD. Risks and predictive biomarkers of sudden unexpected death in epilepsy patient. Curr Opin Neurol. 2019;32(2):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devinsky O, Ryvlin P, Friedman D. Preventing Sudden Unexpected Death in Epilepsy. JAMA Neurol. 2018;75(5):531–2. [DOI] [PubMed] [Google Scholar]
- 40.Harden C, Tomson T, Gloss D, et al. Practice Guideline Summary: Sudden Unexpected Death in Epilepsy Incidence Rates and Risk Factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr. 2017;17(3):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966–77. [DOI] [PubMed] [Google Scholar]
- **42.Petrucci AN, Joyal KG, Purnell BS, et al. Serotonin and sudden unexpected death in epilepsy. Exp Neurol. 2020;325:113145. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed review of the possible causal associations of serotonin dysfunction and SUDEP, also suggesting potential interventional strategies.
- 43.Buchanan GF, Murray NM, Hajek MA, et al. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592(19):4395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Tupal S, Faingold CL. Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia. 2019;60(3):485–94. [DOI] [PubMed] [Google Scholar]; This study provides further support for the possible efficacy of serotonergic drugs to inhibit SUDEP.
- 45.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131(Pt 12):3239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Vilella L, Lacuey N, Hampson JP, et al. Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology. 2019;92(3):e171–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an important study indicating that postconvulsive central apnea may be a biomarker for SUDEP, and also that laryngospasm was not observed.
- 47.Murugesan A, Rani MRS, Hampson J, et al. Serum serotonin levels in patients with epileptic seizures. Epilepsia. 2018;59(6):e91–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48.Murugesan A, Rani MRS, Vilella L, et al. Postictal serotonin levels are associated with peri-ictal apnea. Neurology. 2019;93(15):e1485–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]; The observations reported in this study indicate that greater increase in serotonin during a seizure may be protective against apnea.
- 49.Bateman LM, Li CS, Lin TC, et al. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51(10):2211–4. [DOI] [PubMed] [Google Scholar]
- **50.Lacuey N, Martins R, Vilella L, et al. The association of serotonin reuptake inhibitors and benzodiazepines with ictal central apnea. Epilepsy Behav. 2019;98(Pt A):73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large sample provinng additional evidence suggesting SSRIs may protect against central apnea and oxygen desaturation.
- 51.Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guyenet PG, Abbott SB. Chemoreception and asphyxia-induced arousal. Respir Physiol Neurobiol. 2013;188(3):333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107(37):16354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao ZQ, Scott M, Chiechio S, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26(49):12781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajek MA, Buchanan GF. Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol. 2016;115(5):2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163(1):135–50. [DOI] [PubMed] [Google Scholar]
- 57.Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52Suppl 1:21–7. [DOI] [PubMed] [Google Scholar]
- 58.Hesdorffer DC, Ishihara L, Mynepalli L, et al. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72(2):184–91. [DOI] [PubMed] [Google Scholar]
- 59.Gilliam FG, Santos J, Vahle V, et al. Depression in epilepsy: ignoring clinical expression of neuronal network dysfunction? Epilepsia. 2004;45Suppl 2:28–33. [DOI] [PubMed] [Google Scholar]
- 60.Ettinger A, Reed M, Cramer J, et al. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63(6):1008–14. [DOI] [PubMed] [Google Scholar]
- 61.Hesdorffer DC, Hauser WA, Olafsson E, et al. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59(1):35–41. [DOI] [PubMed] [Google Scholar]
- 62.Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54(3):388–98. [DOI] [PubMed] [Google Scholar]
- 63.Epps SA, Tabb KD, Lin SJ, et al. Seizure susceptibility and epileptogenesis in a rat model of epilepsy and depression co-morbidity. Neuropsychopharmacology. 2012;37(13):2756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Epps SA, Weinshenker D. Rhythm and blues: animal models of epilepsy and depression comorbidity. Biochem Pharmacol. 2013;85(2):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazarati A, Siddarth P, Baldwin RA, et al. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131(Pt 8):2071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smolders I, Clinckers R, Meurs A, et al. Direct enhancement of hippocampal dopamine or serotonin levels as a pharmacodynamic measure of combined antidepressant-anticonvulsant action. Neuropharmacology. 2008;54(6):1017–28. [DOI] [PubMed] [Google Scholar]
- 67.Kumar U, Medel-Matus JS, Redwine HM, et al. Effects of selective serotonin and norepinephrine reuptake inhibitors on depressive- and impulsive-like behaviors and on monoamine transmission in experimental temporal lobe epilepsy. Epilepsia. 2016;57(3):506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazarati A, Sankar R. Common Mechanisms Underlying Epileptogenesis and the Comorbidities of Epilepsy. Cold Spring Harb Perspect Med. 2016;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lothe A, Didelot A, Hammers A, et al. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PET study. Brain. 2008;131(Pt 10):2765–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giovacchini G, Toczek MT, Bonwetsch R, et al. 5-HT 1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med. 2005;46(7):1128–35. [PMC free article] [PubMed] [Google Scholar]
- 71.Theodore WH, Hasler G, Giovacchini G, et al. Reduced hippocampal 5HT1A PET receptor binding and depression in temporal lobe epilepsy. Epilepsia. 2007;48(8):1526–30. [DOI] [PubMed] [Google Scholar]
- 72.Savic I, Lindstrom P, Gulyas B, et al. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62(8):1343–51. [DOI] [PubMed] [Google Scholar]
- 73.Robertson MM, Trimble MR. The treatment of depression in patients with epilepsy. A double-blind trial. J Affect Disord. 1985;9(2):127–36. [DOI] [PubMed] [Google Scholar]
- 74.Pisani F, Spina E, Oteri G. Antidepressant drugs and seizure susceptibility: from in vitro data to clinical practice. Epilepsia. 1999;40Suppl 10:S48–56. [DOI] [PubMed] [Google Scholar]
- 75.Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345–54. [DOI] [PubMed] [Google Scholar]
- 76.Kanner AM, Kozak AM, Frey M. The Use of Sertraline in Patients with Epilepsy: Is It Safe? Epilepsy Behav. 2000;1(2):100–5. [DOI] [PubMed] [Google Scholar]
- 77.Kuhn KU, Quednow BB, Thiel M, et al. Antidepressive treatment in patients with temporal lobe epilepsy and major depression: a prospective study with three different antidepressants. Epilepsy Behav. 2003;4(6):674–9. [DOI] [PubMed] [Google Scholar]
- 78.Orjuela-Rojas JM, Martinez-Juarez IE, Ruiz-Chow A, et al. Treatment of depression in patients with temporal lobe epilepsy: A pilot study of cognitive behavioral therapy vs. selective serotonin reuptake inhibitors. Epilepsy Behav. 2015;51:176–81. [DOI] [PubMed] [Google Scholar]
- **79.Gilliam FG, Black KJ, Carter J, et al. A Trial of Sertraline or Cognitive Behavior Therapy for Depression in Epilepsy. Ann Neurol. 2019;86(4):552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; A randomized trial reporting that more than half of patients with the comorbidity of major depression and active epilepsy achieve remission from depression with an SSRI or CBT, and that remission is associated with reduction of GTC seizures.


