SUMMARY
Plant-based dietary patterns are associated with improved cardiometabolic health, but causal dietary components are unclear. Protein has been proposed to play a role, but the importance of protein quantity vs quality remains unknown. We investigated the contributions of total protein amount, amino acid (AA) composition and plant vs animal source. Analysis of total protein and AA composition of food items and dietary patterns revealed differences between individual food items, but few differences between AA profiles of vegan vs omnivorous dietary patterns. Effects of protein quantity, but not quality, on cardiometabolic health markers were observed in mice using semi-purified diets with crystalline AAs in plant vs animal-based ratios and naturally-sourced diets with whole food ingredients. Our data show relatively little difference in protein quality between plant-based and omnivorous dietary patterns, and that reduced total protein intake in plant-based dietary patterns may be a contributor to the benefits of plant-based diets.
Graphical Abstract
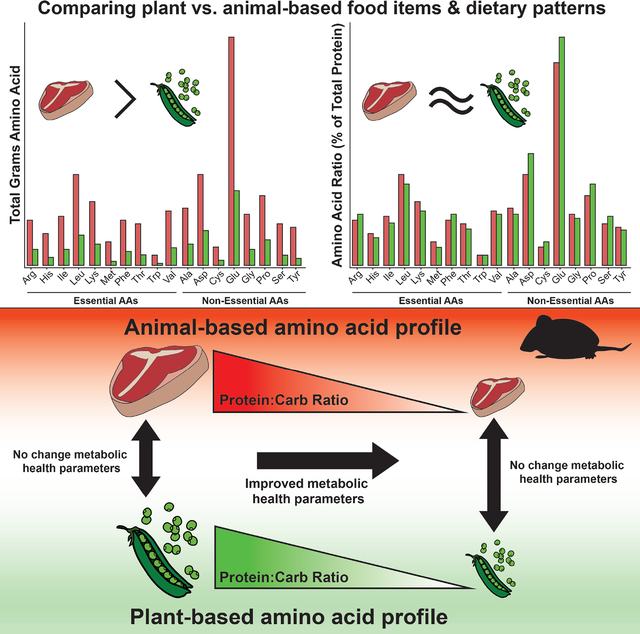
eTOC Blurb
MacArthur et al. show that although vegan diets have lower protein, vegan and omnivorous dietary patterns display similar amino acid intake ratios. They show that total protein, not plant versus animal amino acid composition, drives metabolic health effects in mice, implicating reduced total protein intake in the health benefits of plant-based diets.
INTRODUCTION
Plant-based dietary patterns, including vegetarianism and veganism, are associated with improvements in biomarkers of cardiometabolic health including body mass index(Bujnowski et al., 2011; Rosell et al., 2006) and insulin sensitivity (Kahleova et al., 2018b), and reduced risk of cardiovascular disease (CVD) (Kelemen et al., 2005), type 2 diabetes mellitus (Patel et al., 2017; Sluijs et al., 2010; van Nielen et al., 2014) and all-cause mortality (Budhathoki et al., 2019; Key et al., 2003; Song et al., 2016).
There are a number of significant differences between vegan or vegetarian and omnivorous dietary patterns that could contribute to the observed benefits of plant-based nutrition (Farmer et al., 2011; Kahleova et al., 2018b). These include but are not limited to differences in energy density, macronutrient content and ratios (protein, carbohydrate, fat), vitamins (e.g. A, E, C, folate), micronutrients (e.g. calcium), fiber and polyphenol abundance. Fiber (Lie et al., 2018) and polyphenols (Scalbert et al., 2005), which are both associated with health benefits, are more abundant in plant-based diets and thus may contribute to benefits of plant based diets (Kasahara et al., 2018; Roopchand et al., 2015; Zang et al., 2006). Dietary fats including saturated fats and cholesterol, which are lower in plant-based diets, have been studied extensively in association with the detrimental effects of red meat consumption and potential benefits of plant-based diets. However, the specific contribution of fat to health benefits of plant-based diets is difficult to ascertain due to high variability in total fat intake as well as in the specific type of fats consumed with plant-based diets (Schwab et al., 2014).
More recent data from diverse epidemiological and preclinical studies support a role specifically for protein in benefits of plant-based dietary patterns. In epidemiological studies adjusted for other potential confounding dietary and lifestyle factors, plant protein intake is associated with reduced risk of total and CVD mortality (Budhathoki et al., 2019; Song et al., 2016), while total and/or animal protein intake is associated with increased risk of weight gain (Halkjaer et al., 2011), diabetes (Levine et al., 2014; Sluijs et al., 2010; van Nielen et al., 2014) and CVD mortality (Song et al., 2016). Importantly, isocaloric substitution of plant protein for animal protein, while controlling for other potentially confounding dietary factors, is associated with reduced CVD (Kelemen et al., 2005) and all-cause mortality (Budhathoki et al., 2019; Song et al., 2016), suggesting an intrinsic difference between animal and plant protein that could contribute to health benefits. Experimentally, isolated plant protein in purified, hydrolysate, or constituent amino acid (AA) form reduces cholesterol and is less atherogenic in rabbits than animal proteins (Huff and Carroll, 1980; Huff et al., 1977; Morita et al., 1997).
An obvious potential difference between plant and animal protein is AA composition, or ‘protein quality’. Plant-based protein sources tend to have lower ratios of EAA to NEAA, which have been suggested as mediators of the benefits of plant-based diets. For example, reduced lysine to arginine or methionine to glycine ratios in plant proteins have been associated with reduced glucagon:insulin ratios and cholesterol synthesis (Gorissen et al., 2018; Katan et al., 1982; Sanchez et al., 1988) that could underlie anti-atherogenic effects in preclinical models (Kritchevsky et al., 1982; Morita et al., 1997) although data in humans is equivocal (Vega-Lopez et al., 2010). Dietary restriction of specific AAs that tend to be higher in animal-based food items is also associated with pleiotropic health benefits in rodent models (McCarty, 1999; McCarty et al., 2009). For example, restriction of methionine is associated with health benefits ranging from improved lipid and glucose homeostasis to increased stress resistance and extended longevity in rodent models (Miller et al., 2005; Orentreich et al., 1993). Restriction of BCAAs is also associated with improved glucose homeostasis and metabolic fitness (Fontana et al., 2016). Thus, differences in AA composition can clearly impact numerous health measures, but whether these differences seen with severely restricted semi-purified diet are causative of benefits in whole food plant-based dietary patterns remains unclear.
Other studies have not found differences in health outcomes due specifically to protein quality (Lamming et al., 2017; Levine et al., 2014), instead highlighting the non-mutually exclusive role of reduced protein quantity associated with plant-based diets. In rodents, benefits of reduced protein intake without malnutrition include extended longevity (Solon-Biet et al., 2014), reduced tumor growth (Dunaif and Campbell, 1987; Fontana et al., 2013; McCarty, 1999; McCarty et al., 2009) and improved markers of metabolic fitness (Laeger et al., 2014; Solon-Biet et al., 2015; Treviño-Villarreal et al., 2018).
Epidemiological studies are also consistent with benefits of low protein intake on all-cause mortality (Levine et al., 2014). Individuals consuming plant-based diets consume less protein when adjusted for both calorie intake and body weight; however, the relative contribution of protein quantity vs quality remains unclear, as correction for plant vs animal source attenuates health differences in some studies (Budhathoki et al., 2019; Levine et al., 2014) but not others (Song et al., 2016) and most experimental studies on reduced protein intake use only a single source of protein.
Here we sought to critically evaluate the potential of both protein quantity and quality to contribute to health benefits of plant-based dietary patterns. To this end, we investigated whether individuals adhering to plant-based dietary patterns consume a different AA profile vs those who do not. We also directly assessed effects on health markers in controlled rodent studies using isocaloric diets in which we modulated both total protein and AA composition using either crystalline AA or naturally-sourced protein ingredients. We found surprisingly few differences between AA profiles of vegans and vegetarians vs omnivores, but large effects of total protein amount independent of source in controlled rodent studies, suggesting a potential effect of total protein rather than AA composition in health benefits of plant-based diets.
RESULTS
To quantify differences between plant and animal-based AA profiles on the level of individual food ingredients, we compiled data from the USDA National Nutrient Database (NDB) for Standard Reference (release SR28). Of 4,676 food items with available protein (by weight and % energy) and AA content, 2,328 were animal-based (sub-categories including dairy and egg, poultry, beef, pork, finfish and shellfish, and lamb) and 1,162 were plant-based (sub-categories including fruits, vegetables, nuts and seeds, legumes and grains). In the database there are a high number of redundant food items with nearly identical amino acid profiles (ex. YOGURT, GREEK, NONFAT, VANILLA versus YOGURT, GREEK, NONFAT, STRAWBERRY). To reduce these redundancies, we performed hierarchical clustering on amino acid profiles within food item subcategories. This resulted in 37 animal and 38 plant-based food item clusters which represent the diversity of amino acid profiles across food item subgroups. These clusters were then used as the units of analysis for further comparisons.
Animal-based food items tended to have more total protein by weight (g protein/100g food item, Fig 1A) and as a percent of energy (Fig S1A), and significantly more of all AAs by weight (g AA/100g food item, Fig 1B, S1B). Following normalization of each AA to total protein (ratio of g AA/g total AA) (Fig 1C) seven amino acid had a significantly higher proportional abundance in animal-based food items (Ile, Leu, Lys, Met, Ala, Gly, Tyr) and two in plant-based food items (Asp, Glu) (Fig 1D).
Figure 1. Amino acid profiles of plant and animal-based food items.
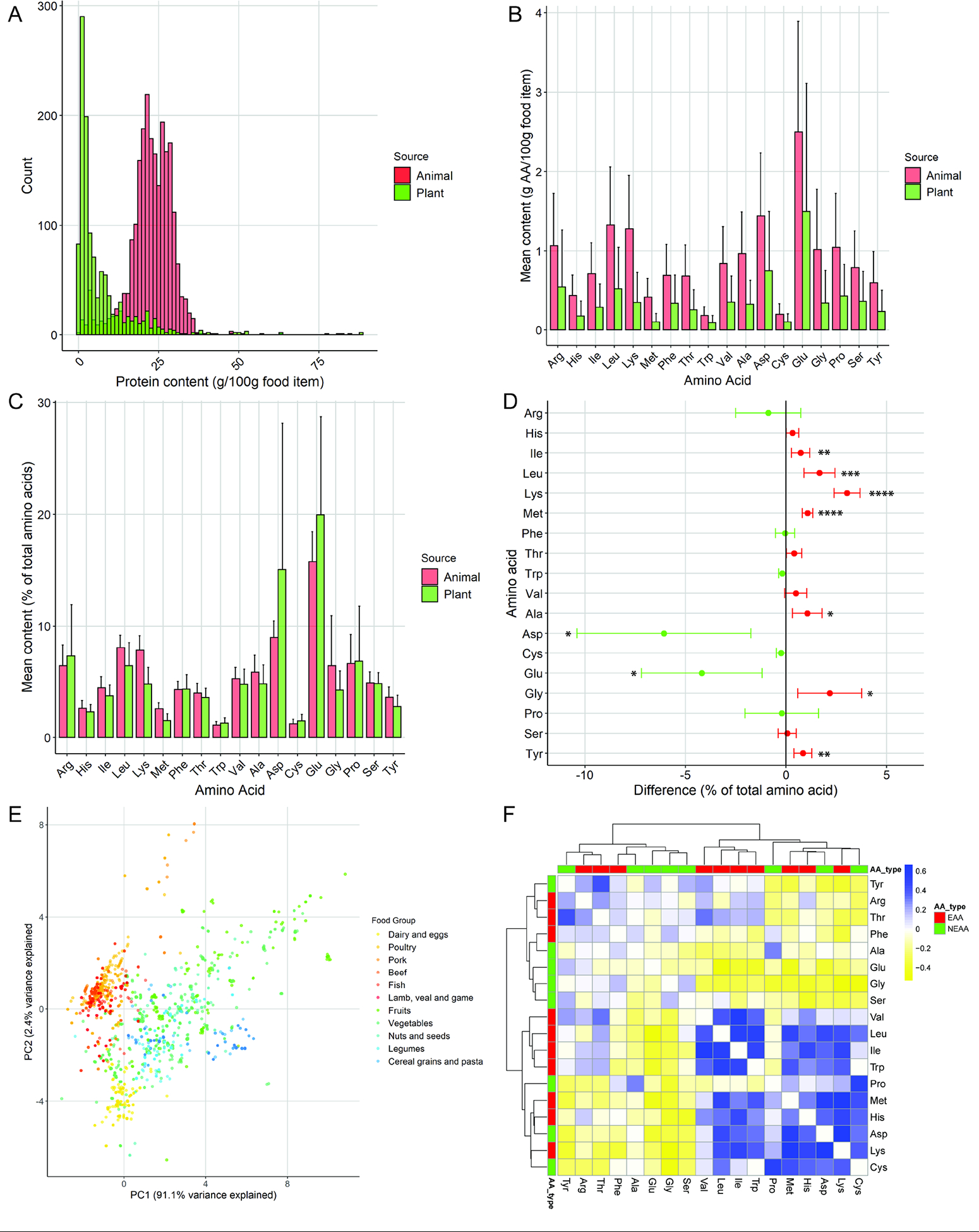
A) Histogram of average protein content (g protein/100g food item) of plant and animal-based food items. B) Amino acid (AA) content of plant vs animal-based food items presented as g AA/100g food item and (C) as percentage of total AAs. D) Difference in percent of total AA (+/− 95% confidence interval) for animal vs plant items. Positive difference (red) indicates higher relative amount in animal food items, negative difference (green) indicates higher in plant food items. E) Principal component plot of AA ratios across multiple plant and animal-based food item subgroups. F) Correlation of individual AA proportions across all food items in the database. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. P values Benjamini-Hochberg adjusted for multiple comparisons. See also Figure S1.
When AAs were grouped as essential vs. non-essential, plant-based food items had significantly less of both groups on a weight basis but on a ratio basis, plant-based food items had proportionally less essential AAs and more non-essential AAs, however neither was significantly different between groups (Fig S1C).
When food items were broken out into their subgroups, greater variability was evident in the AA profiles of plant-based food groups compared to animal-based groups. In principal component plots much greater spread in plant-based food groups/items is evident across the first principal component which captures approximately 91% of the variance in the data (Fig 1E).
Between subgroups, animal-based food groups tended to have higher content of each AA on a weight basis compared to plant groups, with plant-based groups showing greater variability (Fig S1D). When expressed as ratios relative to total protein, the plant-based groups also showed greater variability with all animal-based groups showing similar AA composition except dairy and eggs (Fig S1E). Strong positive and negative correlations between individual AA ratios were evident across all food items (Fig 1F), with the first clustering split discriminating AAs tending to be proportionally higher in animal-based items (Leu, Ile, Met, Lys) versus plant (Asp, Glu).
The in silico analysis above is inherently limited by the potential for bias within the database due to a lack of weighting; all food item clusters receive equal weight even though actual human intakes likely strongly skew towards specific food items/food groups versus others. To account for this potential bias, we next analyzed total protein and AA ratios of typical dietary patterns utilizing data from the National Health and Nutrition Examination Survey (NHANES) from 2005–2012 including two 24-hour dietary recalls per person on a representative sample of the United States population every two years (four distinct cycles of data collection). We first stratified participants (n= 23,245) into three groups based on their dietary intakes over two 24-hour recalls: individuals consuming only plant-based food (vegans; n=67; 0.3%), individuals consuming only dairy or egg animal-based foods (vegetarians; n=1,996; 8.6%) and individuals consuming animal-based foods including meat (omnivores; n=21,182; 91.1%). These proportions of American participants self-reporting a vegan or lacto-ovo vegetarian dietary pattern are similar to those reported independently in a Gallup poll(Gallup, 2018).
Individuals adhering to a vegan dietary pattern consumed less protein both as a percentage of energy and as grams normalized to body weight than either vegetarians or omnivores (% energy 11.4, 16.5 and 16.2% and g/kg 0.64, 0.95 and 1.04 g/kg respectively, Fig 2A–B). Individuals consuming a vegan diet replaced calories from protein entirely with calories from carbohydrate and also tended to consume fewer calories from fat than vegetarians or omnivores (carb 62.3, 49.6 and 49.9% and fat 26.1, 31.9 and 33% respectively, Fig S2A–C). Individuals consuming a vegan diet consumed significantly less of all AAs than omnivores on a weight basis (Fig 2C, S2D).
Figure 2. Amino acid profiles of dietary patterns.
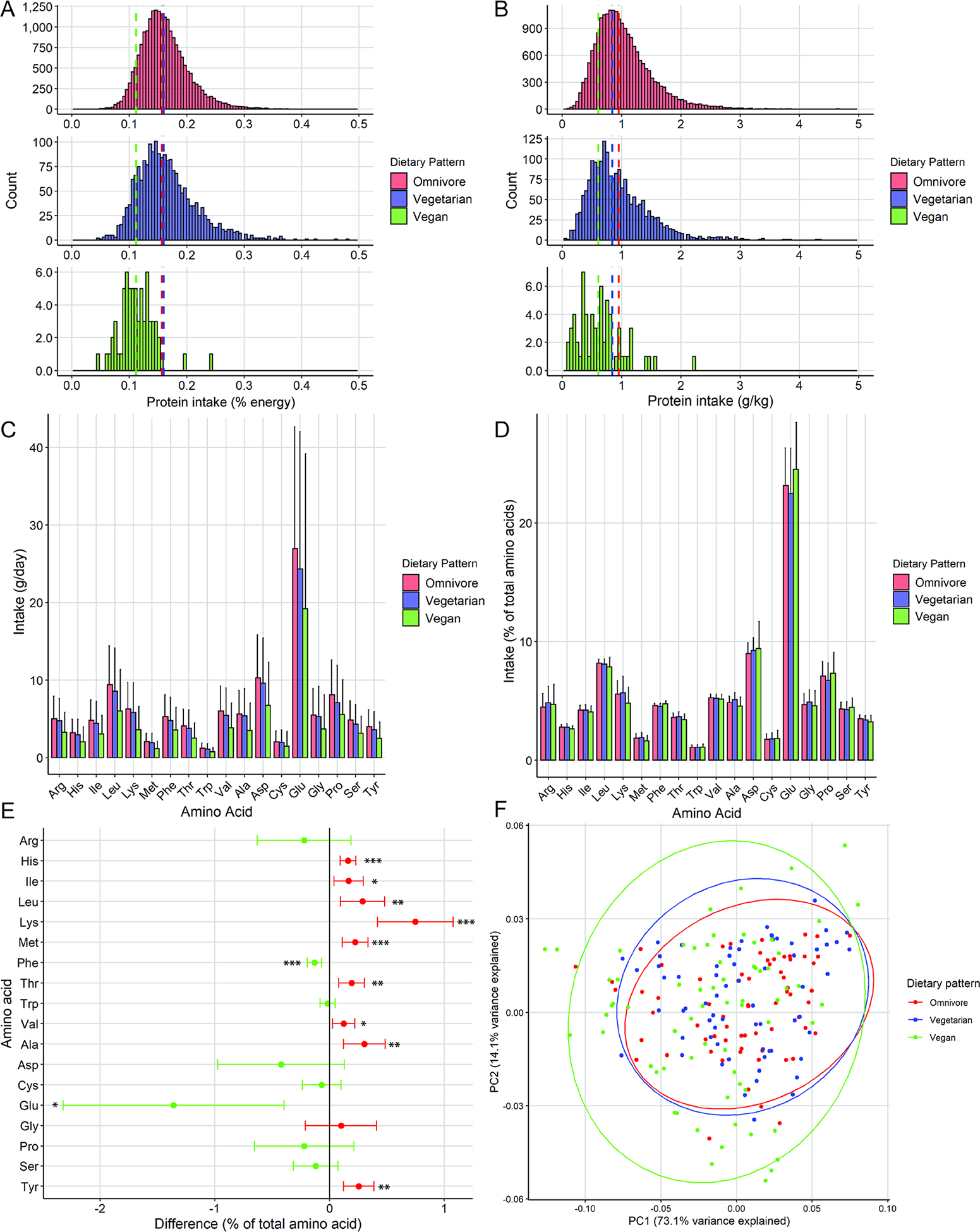
A) Protein intake for vegans (green), lacto-ovo vegetarians (blue) and meat-consuming omnivores (red) presented as g/kg body weight and (B) % of energy intake. C) Amino acid (AA) profiles of the three dietary patterns presented as g/day intake and (D) % of total AA intake. E) Difference in percent of total AA (+/− 95% confidence interval) for omnivore vs vegan dietary pattern. Positive difference (red) indicates higher relative amount in omnivore, negative difference (green) indicates higher vegan. F) Principal component plot of AA ratios with observations colored by dietary pattern. * p<0.05, ** p<0.01, *** p<0.001. P values Benjamini-Hochberg adjusted for multiple comparisons. See also Figure S2.
When AAs were normalized to total AA intake, AA intake ratios between all three diet groups showed a similar general pattern (Fig 2D). Relative intake of nine AAs was significantly higher in omnivores (His, Ile, Leu, Lys, Met, Thr, Val, Ala, Tyr) and two were higher in vegans (Phe, Glu) (Fig 2E). Despite these statistically significant differences, it is notable that the differences in relative intake were less than 0.5% for all but two AAs (Lys and Glu). This suggests that, although the differences are consistent enough to detect statistical significance, the magnitude of the differences are small. This observation was confirmed by a lack of segregation by group in principal component analysis (Fig2F).
Because the magnitude of variation in AA composition between these distinct dietary patterns was so small, we next stratified participants by total protein intake to see if larger differences in AA profiles could be identified in high versus low protein consumers. When participants were stratified by decile of % energy intake from protein, distinct trends emerged but with the same broad patterns intact (Fig 3A). Upon principal component analysis of AA ratios, the first and tenth deciles of percent energy intake from protein showed moderate segregation along PC1 (Fig 3B). Interestingly, individuals consuming low protein tended to replace protein exclusively with carbohydrate, similar to the trend seen with vegans (Fig 3C–D). The trends in differences in proportional AA intake were very consistent and all AAs showed highly statistically significant proportional differences between the first and tenth decile groups with Arg, Hist, Ile, Leu, Lys, Met, Thr, Typ, Val, Ala, Asp, Ser and Tyr higher in high protein consumers and Phe, Cys, Glu, Gly and Pro higher in low protein consumers (Fig 3F). Despite these highly statistically significant differences, there were again only small differences in magnitude of differences, with only three AAs having >1% difference (Lys, Glu, Pro).
Figure 3. Amino acid ratios in high versus low protein consumers.
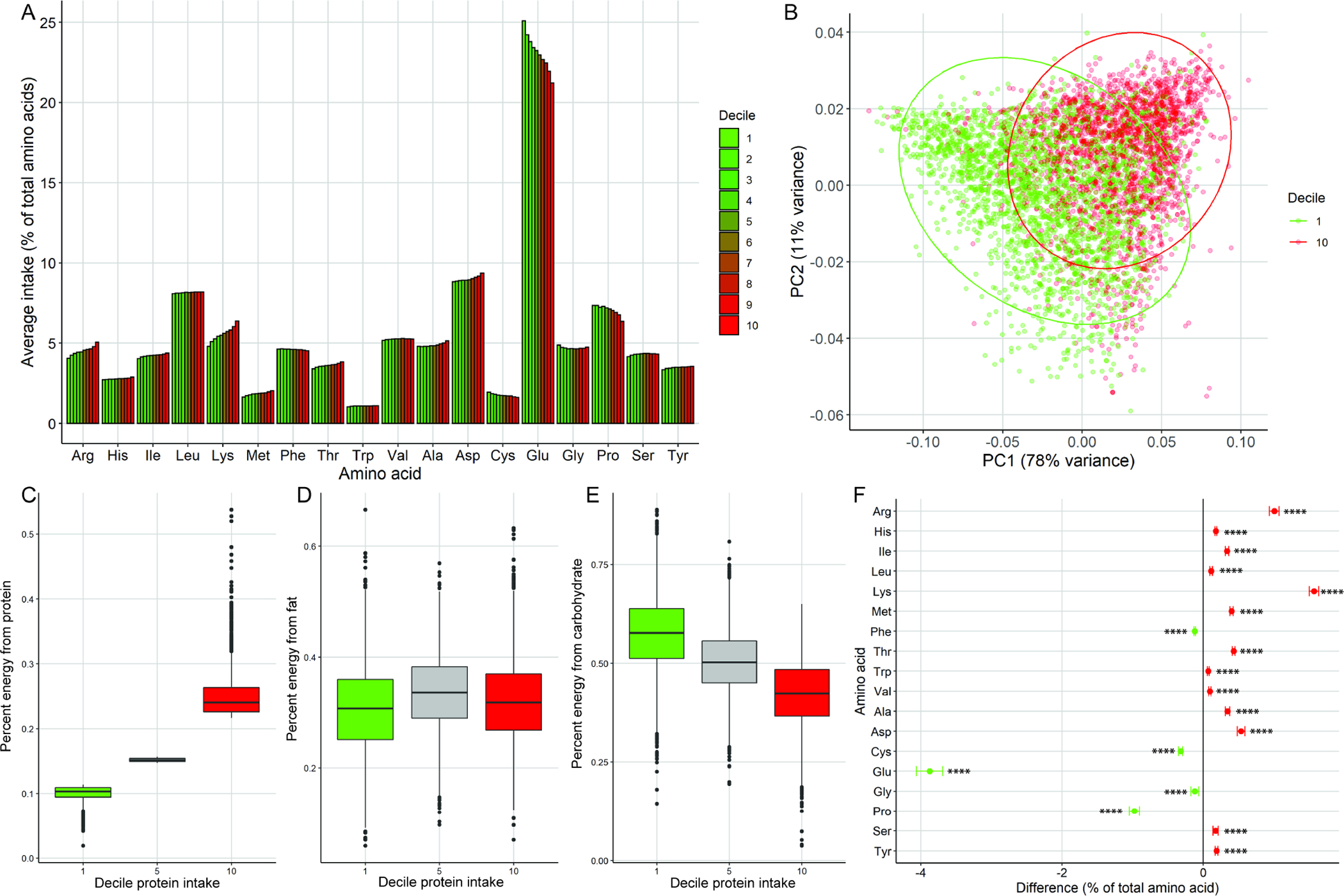
A) Amino acid ratios in individuals across deciles of protein intake as a percent of total energy intake with low-consuming individuals in decile 1 and high consumers in decile 10. B) Principal component plot of amino acid ratios in individuals in the highest (red) and lowest (green) deciles of protein intake. C) Average percent energy intake from protein, fat (D) and carbohydrate (E) for the 1st, 5th and 10th deciles of protein intake. F) Difference in percent of total AA (+/− 95% confidence interval) for 1st vs 10th decile of protein intake. Positive difference (red) indicates higher relative amount in 10th decile, negative difference (green) indicates higher in 1st decile. **** p<0.0001. P values Benjamini-Hochberg adjusted for multiple comparisons.
Our combined in silico analyses of food items and dietary patterns revealed differences in total protein amount in plant vs animal-based food items and dietary patterns. It also revealed differences in the AA profiles of plant versus animal-based food items and dietary patterns and high versus low protein dietary patterns. However, only two AAs had >0.5% difference in relative abundance in vegan versus omnivorous. Thus, we identified AA profiles corresponding to plant versus animal-based dietary patterns, however, we sought to understand whether such small relative differences could explain health differences. To experimentally test the potential contribution of these differences in AA composition relative to differences in total protein intake to the health benefits of plant-based diets, we turned to controlled feeding studies in rodents.
We designed AA profiles corresponding to plant vs animal-based dietary patterns using results from the analysis of the USDA food item database. Diets were made with total AA content between 0–18% of energy and AA ratios corresponding to either a plant- or animal-based profile by adding crystalline AAs of the desired content and ratio to a semi-purified, protein-free base mixture (Table S1). As dietary protein content was reduced, sucrose was added to maintain isocaloric diets and to reflect the observed tendency of vegans to replace calories from protein with carbohydrate. All diets were fed ad libitum.
We fed these diets to male B6D2/F1 mice for either one week or six weeks and measured multiple biomarkers of cardiometabolic health. Data were then analyzed for main effects of total AA amount, AA composition (plant vs animal) and AA amount by composition interaction by 2-way ANOVA.
After one week of feeding, body weight, body fat percent, fasting blood glucose, insulin and triglycerides were all significantly decreased as a function of reduced dietary AA intake (Fig 4A–E) with significant main effects of AA amount but not composition (Fig 4I). Interestingly, there was a significant interaction effect (p < 0.05) between AA content and composition for body weight, body fat and triglycerides, although in each case AA amount accounted for the majority of variation (Fig 4I). Serum FGF-21 was significantly increased as a function of reduced AA content (Fig 4F), but without significant effects of AA composition or interaction (Fig 4H).
Figure 4. Titration of protein in plant and animal-based ratios using semi-purified diets for one week.
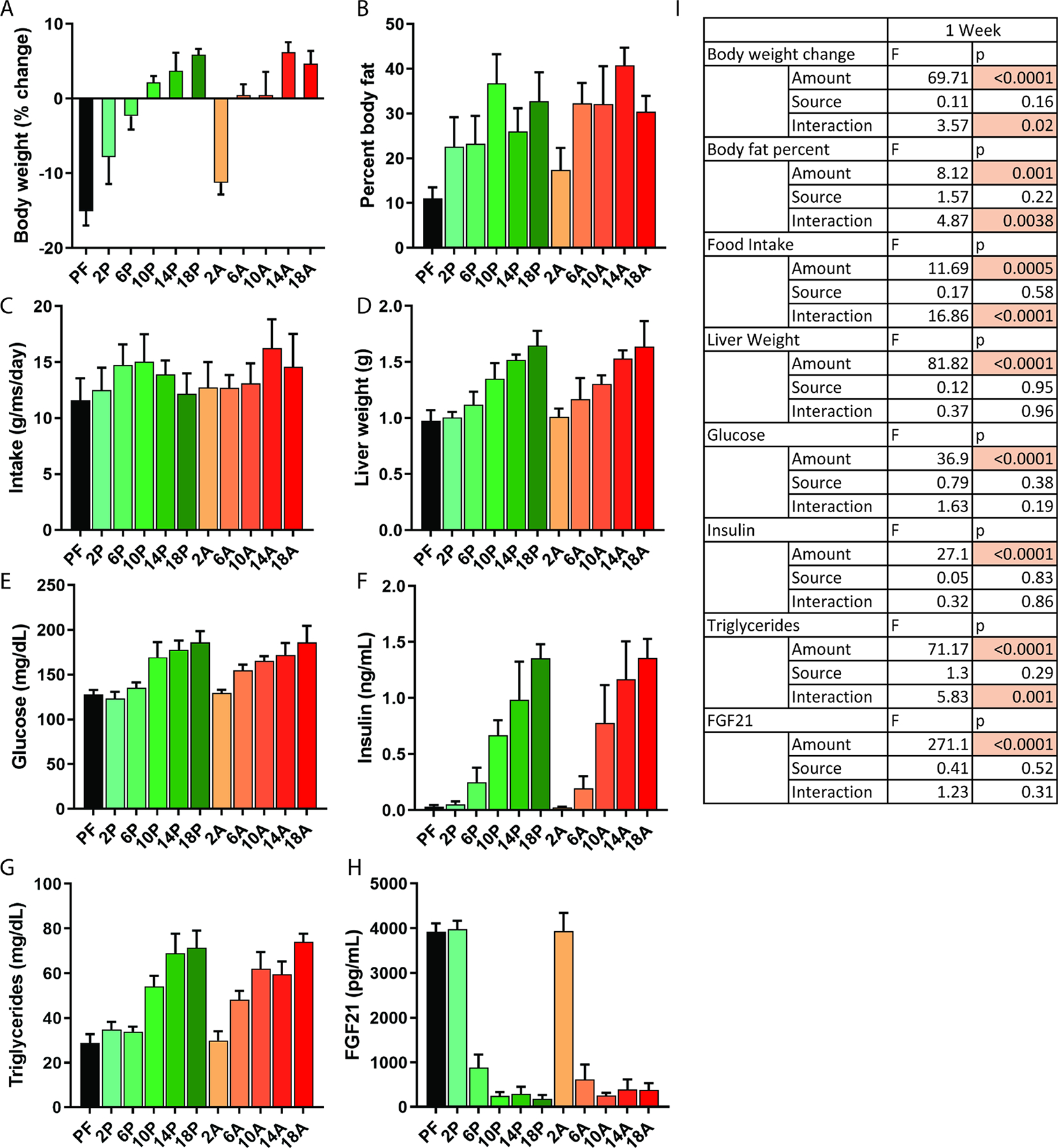
A) Percent change in body weights, B) body fat percentage, C) food intake, D) liver weight, E) blood glucose F) serum insulin, G) serum triglycerides and H) serum FGF21 in male B6D2F1 mice fed for seven days with semi-purified diets containing varying total AA content I) Two-way ANOVA statistics for main effects of protein amount and protein source (plant vs animal composition), and for protein amount by source interaction, p indicates p value and F indicates F statistic. P indicates plant and A indicates animal-based amino acid profiles from crystalline amino acids. PF = 0% protein. 2–18 indicate percent of energy from protein. n=4 mice per group. Error bars indicate standard deviation. See also Table S1.
Over the course of a longer six-week treatment period we only used levels of total AA which were sustainable (6, 10 or 18% of energy), as the very low levels (0 and 2%) result in non-sustainable reductions in lean mass. At these sustainable levels, body weight tended to decrease, while food intake and adiposity tended to increase as a function of decreasing dietary protein content (Fig 5A–C). Liver weight, fasting blood glucose, insulin and triglycerides also all tended to decrease as a function of decreasing protein (Fig 5D–G). FGF21 tended to increase as a function of decreasing protein (Fig 5H). There were no significant main effects of source or interaction effects for any of these variables (Fig 5I).
Figure 5. Titration of protein in plant and animal-based ratios using semi-purified diets for six weeks.
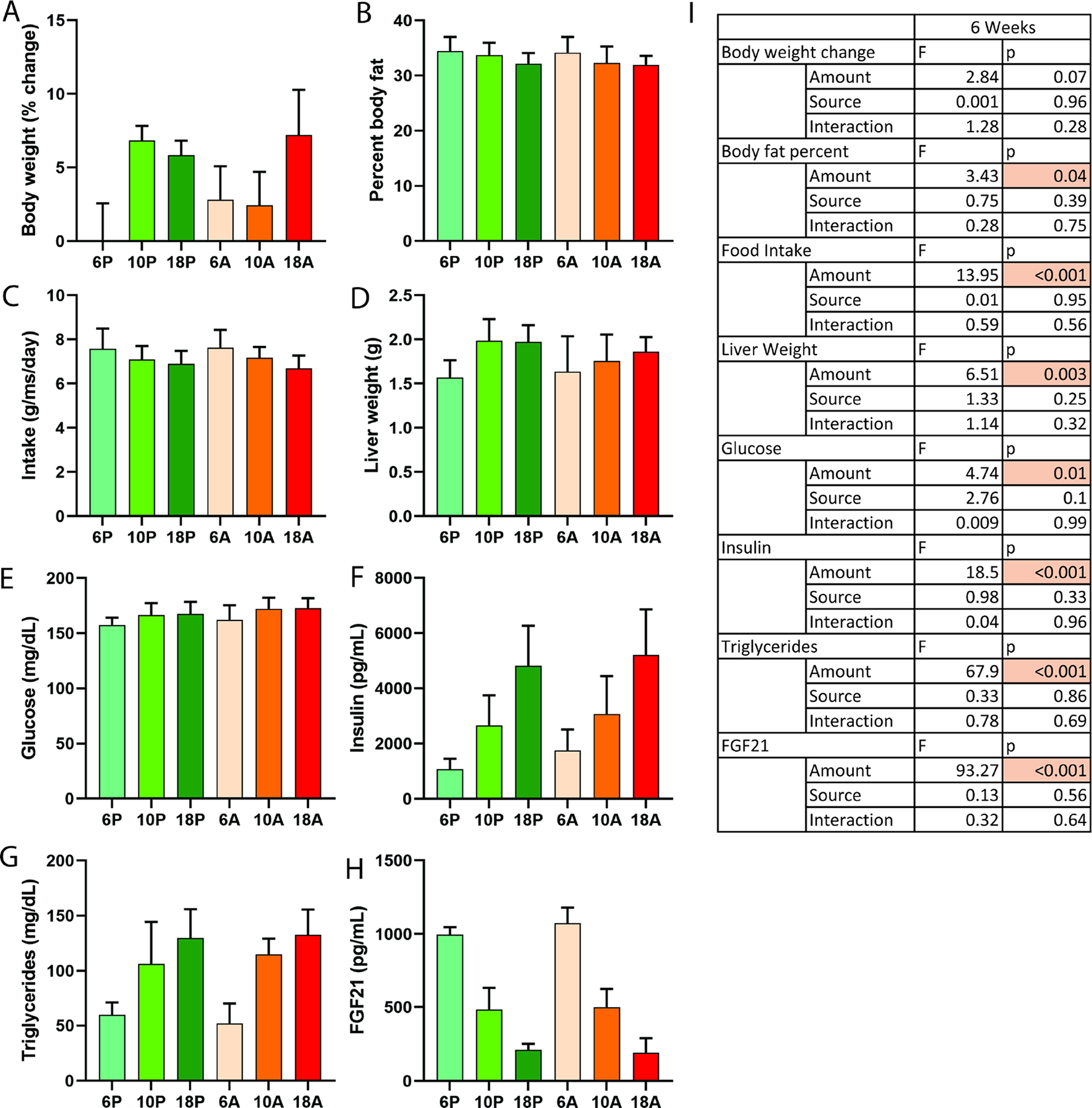
A) Percent change in body weights, B) body fat percentage, C) food intake, D) liver weight, E) blood glucose F) serum insulin, G) serum triglycerides and H) serum FGF21 in male B6D2F1 mice fed for six weeks with semi-purified diets containing varying total AA content. I) Two-way ANOVA statistics for main effects of protein amount and protein source (plant vs animal composition), and for protein amount by source interaction, p indicates p value and F indicates F statistic. P indicates plant and A indicates animal-based amino acid profiles from crystalline amino acids. 6–18 indicate percent of energy from protein. n=8 mice per group. Error bars indicate standard deviation. See also Table S1.
Having observed that reduced AA content was the significant driver of cardiometabolic benefits, with only very minor effects of plant vs animal AA composition in the context of highly controlled semi-purified diet experiments, we next tested whether these two major findings apply to protein derived from naturally-sourced ingredients with their numerous potential confounding factors (e.g. different lipid species, differing polyphenol content, etc).
To this end, we made diets using up to 16 whole food items varying in total protein amount and source (FigS3A–C). This number of food items was chosen as it approximately represents the number of unique food items consumed by an individual per day in the NHANES database. Animal or plant-based protein mixtures were added to a low protein base consisting of ~2.5% of energy from plant-based protein sources to achieve diets with 6, 10 or 18% of energy from protein primarily from plant or animal sources (Fig S3D). Food items were chosen which could be found in normal human intake, but also had to be selected to control for extreme differences in energy density which are often present between plant and animal-based food items. Calculated diet compositions were relatively well-matched for macronutrient composition, energy density and fiber content (Fig S3A) and with AA ratios emulating previously observed differences in plant vs. animal-based dietary patterns particularly with regard to the observed Glu vs Lys tradeoff (Fig S3C). Distinct visual appearances were apparent that could not be controlled for (Fig S3B). Chemical analysis of the highest protein plant and animal diets confirmed the accuracy of our calculations in terms of macronutrient breakdown, energy density (Fig S3E) and AA ratios (Fig S3F). All diets were fed ad libitum.
Male B6D2/F1 mice were fed one of the six naturally sourced diets varying in total protein content and source for six weeks and cardiometabolic markers were analyzed as before. Mice on lower protein diets tended to have similar body weights but higher adiposity (Fig 6A–B), with a significant main effect of protein amount on adiposity (Table S1). There was not an observable significant effect of protein amount on food intake, but there was a significant effect of source and a borderline significant (p=0.05) source by amount interaction effect, with the high protein animal group tending to eat more (Fig 6C). There were significant main effects of protein amount and source on liver weight with higher protein increasing liver weight and plant source tending to be higher than animal (Fig 6D). There were significant main effects of protein amount for glucose, insulin and triglycerides with higher protein tending to increase all three (Fig 6E–G). There was also a significant interaction effect for insulin and trend to significant interaction effect for glucose and triglycerides (p<0.1), with higher animal protein tending to increase values more than higher plant protein (Fig 6E–G). FGF21 showed a significant effect of protein amount with no effects of source or interaction (Fig 6H). Glucose tolerance measured by oral glucose tolerance test showed a significant effect of protein amount and trend to significant effect of source (p=0.06) and amount by source interaction (p=0.1) with higher animal protein groups having lower glucose tolerance (Fig 6I)
Figure 6. Titration of protein in plant and animal-based ratios using naturally-sourced diets for six weeks.
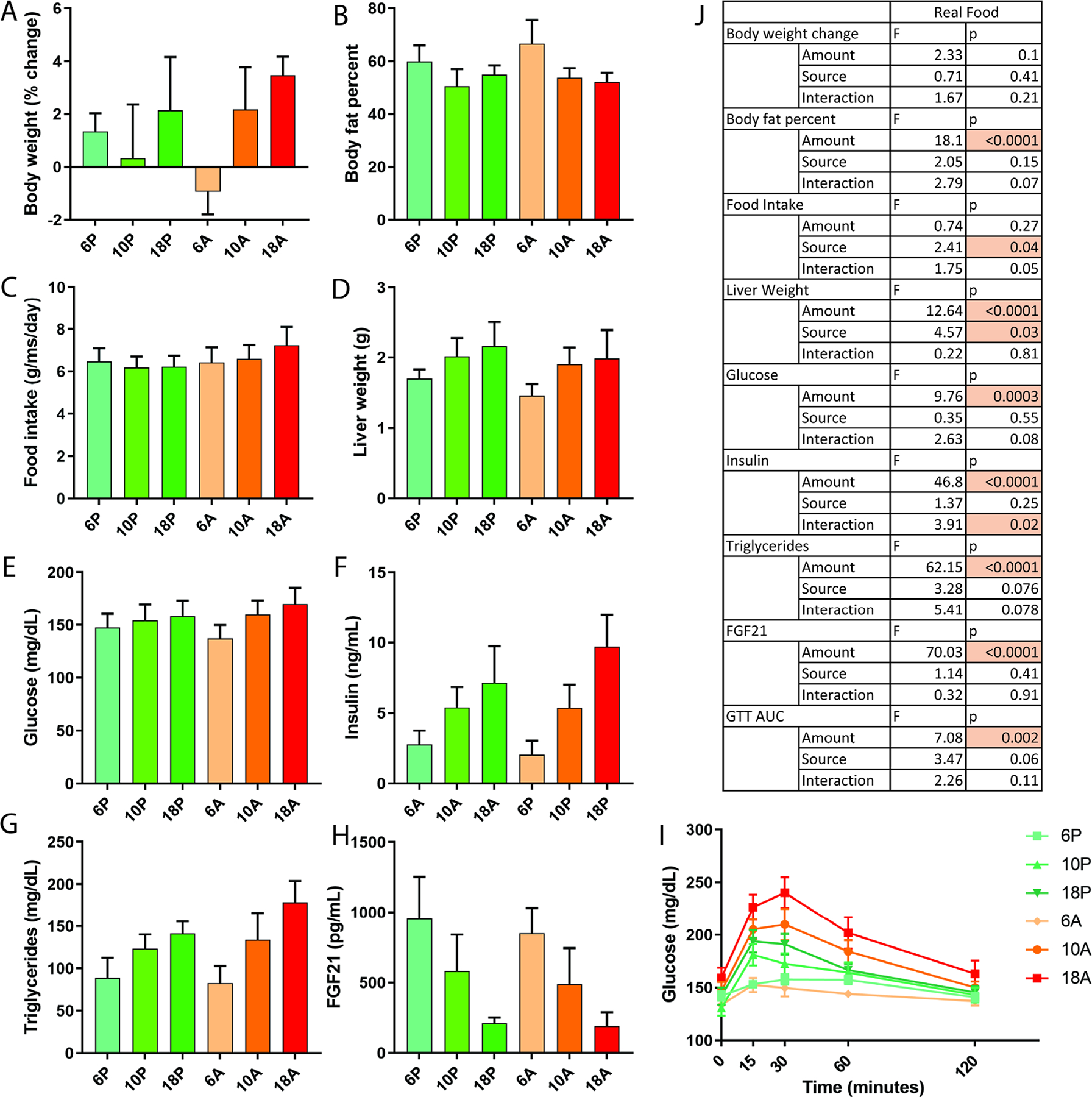
A) Percent change in body weights, B) body fat percentage, C) food intake, D) liver weight, E) blood glucose F) serum insulin, G) serum triglycerides, H) serum FGF21 and I) oral glucose tolerance test curves in male B6D2F1 mice fed for six weeks with naturally-sourced diets containing varying total protein content from plant or animal natural sources. J) Two-way ANOVA statistics for main effects of protein amount and protein source (plant vs animal products), and for protein amount by source interaction, p indicates p value and F indicates F statistic. P indicates plant and A indicates animal food sources. 6–18 indicate percent of energy from protein. n=6–8 mice per group. Error bars indicate standard deviation. See also Figure S3, Table S2.
DISCUSSION
Benefits of plant-based diets on cardiometabolic health are widely acknowledged, but the underlying mechanisms, including the potential role of protein quantity and/or quality, remain unclear. Here we used in silico and experimental approaches to interrogate the specific contributions and potential interactions between total protein content and AA composition of plant vs. animal-based dietary patterns. In silico analyses demonstrated that while consumers of plant-based dietary pattern consume less protein and more carbohydrate, there are only small differences in AA composition between plant and animal based dietary patterns. Animal studies demonstrated the outsized role of protein:carbohydrate ratio (protein amount) versus protein source or AA composition on cardiometabolic markers. These main effects of protein amount were confirmed under highly controlled experimental conditions in mice using semi-purified diets with crystalline AAs in plant vs. animal-based ratios as the sole protein source. For some parameters, a significant interaction effect between AA profile and AA amount was observed, but the variance explained by these interactions was consistently much lower than that explained by total AA amount, and these significant interactions were only apparent at one week and tended go away after six weeks on diet.
The main effects of protein quantity were also robustly observed using naturally sourced diets composed of at least 15 different whole food ingredients. This result was surprising given how many uncontrolled variables were introduced in this approach, including in non-caloric components such as phytonutrients as well as different lipid profiles. While these results are not mutually exclusive with roles of nonproteinaceous components in benefits of plant-based diets, they do support the notion of protein intake as a major factor in cardiometabolic benefits especially at low levels of total protein intake.
Our data are consistent with and expand upon findings regarding the larger effect sizes of protein quantity over quality specifically at low levels of total protein intake (Brandhorst and Longo, 2019). Levine et al. found that protein amount is more important than single plant (soy) vs. single animal (casein) source in promoting tumor growth in mice (Levine et al., 2014), while Fontana et al. found evidence of a threshold between 10% and 20% protein below which single plant vs. single animal source is less important than the protein content in a mouse model of human xenograft prostate tumor growth (Fontana et al., 2013). One of the few prospective clinical trials comparing vegan to control diets with a specific focus on the protein component found benefits on body composition and insulin sensitivity in a 16 week intervention (Kahleova et al., 2018a; Kahleova et al., 2018b). The authors attributed these benefits to reduced animal protein intake and increased plant protein intake, and in particular a proportional reduction in leucine (8.0% vs 7.7% protein energy in control vs. vegan diets, respectively) and histidine (2.7% vs. 2.6% protein energy in control vs. vegan diets, respectively). However, while these associations were corrected for BMI and energy intake, they were not corrected for total protein intake, which was also significantly reduced (17% vs. 12% total daily energy), and thus more likely to explain changes in fat mass and insulin resistance than the small changes in AA composition (Kahleova et al., 2018a).
It is important to note that while our model places reduced protein quantity over altered quality (plant vs. animal protein source or AA composition) in relative importance when comparing health effects of plant-based vs. omnivorous dietary patterns, the presumed mechanism is still one of restriction of one or more EAA below some threshold, rather than total AA or nitrogen restriction (Yap et al., 2020). Clearly changes in AA composition, which invariably result in alteration of AA ratios, can affect health outcomes even at relatively high total protein intake. For example, restriction of sulfur AA at 14% total protein, or restriction of BCAA at 21% total protein (Fontana et al., 2016), both improve cardiometabolic endpoints in mice, similar to total protein restriction. Although future work is required to identify which specific AAs are implicated in the case of low protein diets, the observation here suggests that AA composition does not vary widely enough between dietary patterns enriched in plant vs animal protein sources to cause such a restriction threshold to be met.
An unexpected finding from our in silico analyses was the lower than expected variation in aggregate AA profiles of different food items and intake patterns. Despite observing the expected increase in the NEAA:EAA ratio in plant-based protein (Fig S1C), and some variability between AA ratios in plant-based food groups (Fig1E, S1E), the overall pattern of AA ratios between plant and animal-based food items on the whole was striking. More importantly, a similar phenomenon was observed in the context of dietary patterns, where we found strikingly little variation in the AA profiles of different dietary patterns from participants across multiple cycles of NHANES. Whether by comparing vegans and omnivores who had substantial differences in the macronutrient profile of their diets, or by stratifying individuals by their percent energy intake from protein, we observed that most AAs only varied by less than 0.5% in terms of their relative abundance despite the considerable variation in total protein intake. We also note that comparing the extremes of dietary protein intake (top and bottom 10% of protein energy intake), neither of which is typical for the general US population, probably serves to overestimate the true differences in AA profiles between dietary patterns but still only showed differences of less than 1% in terms of relative abundance of most AAs.
Limitations of the study
We note the following limitations of our study that impact our interpretation that the low protein content of plant-based diets is a major driver of health benefits. First, our experimental data in mouse models show that the most drastic changes in biomarkers of cardiometabolic health occur at protein levels below 10% of energy. In human cohorts, protein intake is largely fixed between 12–18% with little deviation outside this range. While there are some exceptions, including Okinawan populations that eat less than 10% protein calories in a largely plant based diet (Le Couteur et al., 2016), limited sample sizes available in population studies make it difficult to assess if humans react similarly to mice when exposed to low protein intake. Interestingly, some short-term prospective clinical interventions with protein content around 10% are consistent with improved metabolic markers (Fontana et al., 2016). Although it has been hypothesized that increased calorie consumption with low-protein diets due to protein leverage contributes to obesity and metabolic disease, it is not clear whether this model holds true in humans consuming meals with less than 10% of energy from protein (Gosby et al., 2011; Gosby et al., 2016).
Second, as with any epidemiologic study, the association of a single macronutrient with health parameters is tenuous due to extreme confounding by variable lifestyle and other dietary factors including which macronutrient is substituted when protein is reduced. In the NHANES data vegans tended to substitute protein for carbohydrate, which informed our mouse models. However, the effect of protein for fat substitution is less well studied. Furthermore, although protein energy intake can be treated as a single variable, it is in reality a combination of AA ratios that cannot be fixed, even experimentally, and thus represent a complex multidimensional landscape which complicates drawing conclusions about the effects of dietary protein in free-living humans.
Third, because the maximum amount of dietary protein intake studied here in mice was 18%, which is in the standard range for commercial, naturally sourced chow for rodents and close the median intake for humans according to the NHANES database, our data say nothing about the potential of plant vs. animal protein to affect health outcomes at higher levels of protein intake. Very high levels of protein intake in plant-based dietary patterns has previously been unusual. However, the recent rise in popularity of high protein plant-based meat alternative makes this a newly relevant question.
Our mouse data using naturally sourced diets do suggest that some interactions between protein source and amount may exist at the higher end of the protein:carb ratio. A number of studies support the notion of health benefits at high levels of protein intake, in part due to suppression of overall energy intake (Berryman et al., 2016; Eisenstein et al., 2002), although other studies point out that benefits on cardiometabolic markers such as insulin sensitivity don’t match those expected from the resultant weight loss (Berryman et al., 2016; Smith et al., 2016) and that high protein diets may not have robust health effects in individuals with metabolic disease (Raben et al., 2020). Furthermore, experimental studies using high protein diets (25% energy) in rabbits provide clear evidence of differential atherogenic and cholesterolemic effects of single protein sources depending on plant vs. animal origin (Huff and Carroll, 1980; Huff et al., 1977), which could be in part attributed to differences NEAA/EAA ratios or more specifically to differences in arginine and lysine levels in casein vs soy protein, respectively (Vahouny et al., 1985). What molecular mechanisms underlie these anti-atherogenic effects and whether they require high plant protein intake both remain unclear, but with important implications given the recent increase in popularity of plant-based meat substitutes engineered to resemble the taste, texture and macronutrient profile of meat. Whether these high energy density, high protein options will increase total protein intake, and whether this will be associated with the benefits of plant-based dietary patterns, or loss of benefits due to increased protein intake as predicted by our data here, remains to be seen.
STAR METHODS
Resource Availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Michael R. MacArthur (mmacarthur@ethz.ch)
Materials availability
This study did not generate new unique reagents.
Data and code availability
Code relating to database analysis will be made available upon request.
Experimental Model and Subject Details
Mice.
For all experiments 16-week-old male B6D2/F1 hybrids were purchased from Jackson Labs (strain no. 100006). Mice were acclimated in the facility for at least one week prior to experiments and allowed ad libitum access to food (Purina 5053 chow) and water during acclimation. Mice were maintained at 22°C with 12 hr light-dark cycles and 30–50% relative humidity. All experiments were approved by the respective IACUC. Mouse numbers were n=4 per group for the 1 week semi-purified titration experiment and n=6–8 per group for the 6 week semi-purified titration and naturally sourced titration experiments. Mice were observed daily to ensure good health status. Specific pathogen checks were performed quarterly in the facility.
Diets.
For semi-purified titration experiments, isocaloric diets were made with crystalline AA added to generate either a ‘plant’ or ‘animal’ based profile which was determined by taking the average AA ratios of plant vs animal food items from the USDA NDB. For titration studies, AAs were isocalorically replaced with sucrose. The AAs/sucrose were added to Research Diets D12450BSpx base in final 1% agar to form a homogenous solid diet. The exact compositions of semi-purified diets are described in supplemental table 1.
For naturally sourced titration experiments, whole-food items listed in Fig S3D were mixed to achieve the macronutrient profiles listed in Fig S3A. The items were blended in water/agar to achieve a final 0.25% agar concentration to form a homogenous solid diet. The exact compositions of naturally sourced diets are described in supplemental table 2. Calculated dietary composition including AA profiles, macronutrient composition and energy density were confirmed by HPLC and bomb calorimetry measurements at N.P. Analytical Laboratories.
All diets in all studies were fed ad libitum. Food intake was measured daily at approximately ZT22. Mice were fasted for four hours prior to sacrifice and tissue collection which took place at approximately ZT20.
Method details
Body composition.
Body mass was determined by daily measurement at approximately ZT22. Lean and fat mass were measured in awake mice using an EchoMRI analyzer system.
Metabolic health markers.
Glucose was measured using a Clarity BG1000 handheld glucometer. Insulin was measured using by ELISA following manufacturer protocol (Crystal Chem #90080). FGF21 was measured by ELISA following manufacturer protocol (R&D Systems #MF2100). Serum triglycerides were measured using a colorimetric kit according to manufacturer protocol (MilliporeSigma TRO100–1KT).
Glucose tolerance test (GTT).
Following a 16 hr fast, 1.5 g/kg of D-glucose solution (30%, dissolved in sterile water, Sigma Aldrich, St Louis MO) was administered by oral gavage and blood glucose measured from a small tail nick prior to (time 0) and at 15, 30, 60 and 120 minutes post-injection using a handheld glucometer (Clarity BG1000, VWR, Radnor PA).
Quantification and Statistical Analysis
Food item and database analyses.
AA profiles of individual food items were analyzed using the USDA Food and Nutrient Database SR-28 (NDB). Food items with the food groups codes 100 (dairy and eggs), 500 (poultry), 1000 (pork), 1300 (beef), 1500 (finfish and shellfish) and 1700 (lamb, veal and game) were considered “animal” food items. Food items with the food group codes 900 (fruits), 1100 (vegetables), 1200 (nuts and seeds), 1600 (legumes) and 2000 (cereal grains and pasta) were considered “plant” food items.
AAs in the USDA NDB were extracted in three groups for measurement. The three groups were tryptophan, sulfur-containing AAs and all other AAs. Tryptophan was measured by alkaline hydrolysis followed by HPLC. Sulfur-containing AAs were measured by performic oxidation followed by HPLC. All other AAs were measured by acid hydrolysis followed by HPLC. Values in the database are represented as grams of AA per 100g of food item. Full details on measurement methods and calculation are available in the USDA NDB SR28 documentation.
Food item intake analyses
The National Health and Nutrition Examination Survey is a large observational survey program which releases data from a nationally representative sample of Americans every 2 years. These data include dietary, anthropometric and clinical measures. Dietary data include two 24-hour dietary recalls conducted by trained dietary interviewers. To analyze AA composition of dietary records from the NHANES, AA data from SR28 were linked to NHANES Food and Nutrition Database for Dietary Studies (FNDDS) food items using SR link codes. Individual records were obtained from survey rounds covering from 2005 to 2012, comprising four rounds of data release. Individuals who had two complete 24-hour dietary recalls, were not pregnant and were over the age of 18 were included, leaving at total of 23,245 participants. For all nutrient intake variables including AAs and total protein, the mean value of the two days of dietary records was used. Vegans were categorized as individuals who reported eating no animal products on either of the 24-hour recalls. Vegetarians were categorized as individuals who reported consuming eggs or dairy products, but no meat, fish or poultry on 24-hour recalls. A full description of survey protocols and all data are available at the US Centers for Disease Control (CDC) NHANES website.
Statistical Methods.
Database analyses were performed in R (4.0.3). USDA NDB SR28 ASCII data files were downloaded from the USDA website and imported in R. NHANES XPT data files were downloaded from the CDC NHANES website. Data were imported into R using the sasxport.get function from the Hmisc package. For NHANES AA analysis, nutrient variables from each individual’s two 24 hour recalls were averaged. For food item analysis, food items within each food subgroup (beef, dairy and eggs, finfish and shellfish, lamb, pork, poultry, fruit, grains, legumes, nuts and seeds, vegetables) were clustered based on their relative AA profile in order to account for high levels of redundancy in the database. Optimal cluster numbers were determined using the NbClust package. 37 clusters were identified from animal-based food item groups and 38 were identified from plant-based food item groups. All clustering was performed using a Euclidean distance matrix and unweighted pair group method with arithmetic mean agglomeration method. These clusters were then used as the unit of analysis for further comparisons between plant and animal-based food items. For both USDA NDB and NHANES analyses, significance was assessed by performing Welsh’s t-test between animal/plant food items or omnivore/vegan dietary patterns. Tests were adjusted for multiple comparisons using the Benjamini-Hochberg correction. Principal component analyses were performed using the prcomp function.
For mouse studies, data were analyzed using Graphpad Prism (8.4.3). All experiments were analyzed with two way ANOVA and main effects of total protein amount and protein source/AA profile as well and protein content by protein source/profile interaction were presented. Statistical parameters are listed in the figure legends. Two way ANOVA tests met assumptions for homogeneity of variance and normality using the Levene and Shapiro-Wilk tests respectively.
Supplementary Material
Figure S1 Amino acid profiles of plant and animal-based food items
A) Histogram of percent of energy from protein for plant (green) and animal (red)- based food items. B) Difference in AA content (+/− 95% confidence interval) for animal vs plant items. Positive difference (red) indicates higher amount in animal food items, negative difference (green) indicates higher in plant food items. C) Mean content (+/− SD) of non-essential and essential amino acids in plant versus animal-based food items. D) Mean AA content across various food group categories presented as g/100g food item and (E) % of total AA. ** p<0.01, *** p<0.001, **** p<0.0001. P values Benjamini-Hochberg adjusted for multiple comparisons.
Figure S2 Amino acid profiles of plant and animal-based food items
A) Protein, B) carbohydrate and C) fat intake as a percent of energy in vegan (green), lacto-ovo vegetarians (blue) or omnivores (red). D) Difference in g/day AA intake (+/− 95% confidence interval) for omnivore vs vegan dietary pattern. Positive difference (red) indicates higher intake in omnivore, negative difference (green) indicates higher vegan. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. P values Benjamini-Hochberg adjusted for multiple comparisons.
Figure S3 Composition of naturally source mouse diets
A) Calculated macronutrient composition, fiber content and energy density of naturally-sourced diets. B) Visual appearance of the 18% protein animal (left) and plant (right) diets. C) Amino acid profiles of naturally sourced diets. D) List of ingredients used to generate naturally sourced diets. E) Measured macronutrient composition and energy densities of 18% plant and animal naturally sourced diets. F) Calculated versus measured amino acid profiles of 18% plant and animal naturally sourced diets.
TableS1: Composition of plant and animal-based amino acid mixtures used for semi-purified diet studies in g AA/100g total AA (left) and composition of semi-purified diets (right).
Table S2: Composition of naturally source diet base mixture (left) and overall composition of naturally sourced diets (right)
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| Ultra-sensitive mouse insulin ELISA kit | Crystal Chem | Cat# 90080 |
| Serum triglyceride colorimetric assay kit | Millipore Sigma | Cat# TRO100–1KT |
| Mouse FGF21 ELISA kit | R&D Systems | Cat# MF2100 |
| Experimental models: Organisms/strains | ||
| Mouse strain: B6D2F1/J | The Jackson Laboratory | Cat# JAX:100006 |
| Software and algorithms | ||
| GraphPad Prism v. 9.1 | GraphPad | |
| R v4.0.3 | NA | |
| Other | ||
| Mouse diets (see Tables S1, S2) | This paper | N/A |
HIGHLIGHTS.
Plant vs animal-based food items have different amino acid composition.
Vegans consume less protein but a similar amino acid profile as omnivores.
Total protein, not amino acid composition, drives metabolic health effects in mice.
ACKNOWLEDGEMENTS
This work was supported in part by the NIH/NIDDK/NIA (5R01DK090629-08 to J.R.M. 1F31AG064863-01 to M.R.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors have no competing interests to declare.
REFERENCES
- Berryman CE, Agarwal S, Lieberman HR, Fulgoni VL, and Pasiakos SM (2016). Diets higher in animal and plant protein are associated with lower adiposity and do not impair kidney function in US adults. Am J Clin Nutr 104, 743–749. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, and Longo VD (2019). Protein Quantity and Source, Fasting-Mimicking Diets, and Longevity. Adv Nutr 10, S340–S350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhathoki S, Sawada N, Iwasaki M, Yamaji T, Goto A, Kotemori A, Ishihara J, Takachi R, Charvat H, Mizoue T, et al. (2019). Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality in a Japanese Cohort. JAMA Intern Med 179, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnowski D, Xun P, Daviglus ML, Van Horn L, He K, and Stamler J (2011). Longitudinal association between animal and vegetable protein intake and obesity among men in the United States: the Chicago Western Electric Study. J Am Diet Assoc 111, 1150–1155 e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif GE, and Campbell TC (1987). Dietary protein level and aflatoxin B1-induced preneoplastic hepatic lesions in the rat. J Nutr 117, 1298–1302. [DOI] [PubMed] [Google Scholar]
- Eisenstein J, Roberts SB, Dallal G, and Saltzman E (2002). High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev 60, 189–200. [DOI] [PubMed] [Google Scholar]
- Farmer B, Larson BT, Fulgoni VL 3rd, Rainville AJ, and Liepa GU (2011). A vegetarian dietary pattern as a nutrient-dense approach to weight management: an analysis of the national health and nutrition examination survey 1999–2004. J Am Diet Assoc 111, 819–827. [DOI] [PubMed] [Google Scholar]
- Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, Nguyen H, Vessella R, and Pili R (2013). Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 4, 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, et al. (2016). Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep 16, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup (2018). Snapshot: Few Americans Vegetarian or Vegan.
- Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, and van Loon LJC (2018). Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, Jebb SA, Brand-Miller J, Caterson ID, Raubenheimer D, and Simpson SJ (2011). Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS One 6, e25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Lau NS, Tam CS, Iglesias MA, Morrison CD, Caterson ID, Brand-Miller J, Conigrave AD, Raubenheimer D, and Simpson SJ (2016). Raised FGF-21 and Triglycerides Accompany Increased Energy Intake Driven by Protein Leverage in Lean, Healthy Individuals: A Randomised Trial. PloS one 11, e0161003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkjaer J, Olsen A, Overvad K, Jakobsen MU, Boeing H, Buijsse B, Palli D, Tognon G, Du H, van der AD, et al. (2011). Intake of total, animal and plant protein and subsequent changes in weight or waist circumference in European men and women: the Diogenes project. Int J Obes (Lond) 35, 1104–1113. [DOI] [PubMed] [Google Scholar]
- Huff MW, and Carroll KK (1980). Effects of dietary proteins and amino acid mixtures on plasma cholesterol levels in rabbits. J Nutr 110, 1676–1685. [DOI] [PubMed] [Google Scholar]
- Huff MW, Hamilton RM, and Carroll KK (1977). Plasma cholesterol levels in rabbits fed low fat, cholesterol-free, semipurified diets: effects of dietary proteins, protein hydrolysates and amino acid mixtures. Atherosclerosis 28, 187–195. [DOI] [PubMed] [Google Scholar]
- Kahleova H, Fleeman R, Hlozkova A, Holubkov R, and Barnard ND (2018a). A plant-based diet in overweight individuals in a 16-week randomized clinical trial: metabolic benefits of plant protein. Nutr Diabetes 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahleova H, Tura A, Hill M, Holubkov R, and Barnard ND (2018b). A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, Mehrabian M, Denu JM, Backhed F, Lusis AJ, et al. (2018). Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol 3, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan MB, Vroomen LH, and Hermus RJ (1982). Reduction of casein-induced hypercholesterolaemia and atherosclerosis in rabbits and rats by dietary glycine, arginine and alanine. Atherosclerosis 43, 381–391. [DOI] [PubMed] [Google Scholar]
- Kelemen LE, Kushi LH, Jacobs DR Jr., and Cerhan JR (2005). Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol 161, 239–249. [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Davey GK, Allen NE, Spencer EA, and Travis RC (2003). Mortality in British vegetarians: review and preliminary results from EPIC-Oxford. Am J Clin Nutr 78, 533S–538S. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D, Tepper SA, Czarnecki SK, and Klurfeld DM (1982). Atherogenicity of animal and vegetable protein. Influence of the lysine to arginine ratio. Atherosclerosis 41, 429–431. [DOI] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. (2014). FGF21 is an endocrine signal of protein restriction. J Clin Invest 124, 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Baar EL, Arriola Apelo SI, Tosti V, and Fontana L (2017). Short-term consumption of a plant protein diet does not improve glucose homeostasis of young C57BL/6J mice. Nutr Healthy Aging 4, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Solon-Biet S, Wahl D, Cogger VC, Willcox BJ, Willcox DC, Raubenheimer D, and Simpson SJ (2016). New Horizons: Dietary protein, ageing and the Okinawan ratio. Age Ageing 45, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. (2014). Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie L, Brown L, Forrester TE, Plange-Rhule J, Bovet P, Lambert EV, Layden BT, Luke A, and Dugas LR (2018). The Association of Dietary Fiber Intake with Cardiometabolic Risk in Four Countries across the Epidemiologic Transition. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF (1999). Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity. Med Hypotheses 53, 459–485. [DOI] [PubMed] [Google Scholar]
- McCarty MF, Barroso-Aranda J, and Contreras F (2009). The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med Hypotheses 72, 125–128. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, and Smith-Wheelock M (2005). Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Oh-hashi A, Takei K, Ikai M, Kasaoka S, and Kiriyama S (1997). Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J Nutr 127, 470–477. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, and Zimmerman JA (1993). Low methionine ingestion by rats extends life span. J Nutr 123, 269–274. [DOI] [PubMed] [Google Scholar]
- Patel H, Chandra S, Alexander S, Soble J, and Williams KA Sr. (2017). Plant-Based Nutrition: An Essential Component of Cardiovascular Disease Prevention and Management. Curr Cardiol Rep 19, 104. [DOI] [PubMed] [Google Scholar]
- Raben A, Vestentoft PS, Brand-Miller J, Jalo E, Drummen M, Simpson L, Martinez JA, Handjieva-Darlenska T, Stratton G, Huttunen-Lenz M, et al. (2020). The PREVIEW intervention study: Results from a 3-year randomized 2 × 2 factorial multinational trial investigating the role of protein, glycaemic index and physical activity for prevention of type 2 diabetes. Diabetes Obes Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, and Raskin I (2015). Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 64, 2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell M, Appleby P, Spencer E, and Key T (2006). Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes (Lond) 30, 1389–1396. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Hubbard RW, Smit E, and Hilton GF (1988). Testing a mechanism of control in human cholesterol metabolism: relation of arginine and glycine to insulin and glucagon. Atherosclerosis 71, 87–92. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Johnson IT, and Saltmarsh M (2005). Polyphenols: antioxidants and beyond. Am J Clin Nutr 81, 215S–217S. [DOI] [PubMed] [Google Scholar]
- Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, and Becker W (2014). Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, and van der Schouw YT (2010). Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Gordon I., Yoshino J, Kelly Shannon C., Reeds Dominic N., Okunade A, Patterson Bruce W., Klein S, and Mittendorfer B (2016). High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell Rep 17, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ, and Le Couteur DG (2015). Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell Rep 11, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, and Giovannucci EL (2016). Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern Med 176, 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño-Villarreal JH, Reynolds JS, Bartelt A, Langston PK, MacArthur MR, Arduini A, Tosti V, Veronese N, Bertozzi B, Brace LE, et al. (2018). Dietary protein restriction reduces circulating VLDL triglyceride levels via CREBH-APOA5-dependent and -independent mechanisms. JCI insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahouny GV, Adamson I, Chalcarz W, Satchithanandam S, Muesing R, Klurfeld DM, Tepper SA, Sanghvi A, and Kritchevsky D (1985). Effects of casein and soy protein on hepatic and serum lipids and lipoprotein lipid distributions in the rat. Atherosclerosis 56, 127–137. [DOI] [PubMed] [Google Scholar]
- van Nielen M, Feskens EJ, Mensink M, Sluijs I, Molina E, Amiano P, Ardanaz E, Balkau B, Beulens JW, Boeing H, et al. (2014). Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct Case-Cohort Study. Diabetes Care 37, 1854–1862. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez S, Matthan NR, Ausman LM, Harding SV, Rideout TC, Ai M, Otokozawa S, Freed A, Kuvin JT, Jones PJ, et al. (2010). Altering dietary lysine:arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atherosclerosis 210, 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap YW, Rusu PM, Chan AY, Fam BC, Jungmann A, Solon-Biet SM, Barlow CK, Creek DJ, Huang C, Schittenhelm RB, et al. (2020). Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nature Comm 11, 2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, and Cohen RA (2006). Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55, 2180–2191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Amino acid profiles of plant and animal-based food items
A) Histogram of percent of energy from protein for plant (green) and animal (red)- based food items. B) Difference in AA content (+/− 95% confidence interval) for animal vs plant items. Positive difference (red) indicates higher amount in animal food items, negative difference (green) indicates higher in plant food items. C) Mean content (+/− SD) of non-essential and essential amino acids in plant versus animal-based food items. D) Mean AA content across various food group categories presented as g/100g food item and (E) % of total AA. ** p<0.01, *** p<0.001, **** p<0.0001. P values Benjamini-Hochberg adjusted for multiple comparisons.
Figure S2 Amino acid profiles of plant and animal-based food items
A) Protein, B) carbohydrate and C) fat intake as a percent of energy in vegan (green), lacto-ovo vegetarians (blue) or omnivores (red). D) Difference in g/day AA intake (+/− 95% confidence interval) for omnivore vs vegan dietary pattern. Positive difference (red) indicates higher intake in omnivore, negative difference (green) indicates higher vegan. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. P values Benjamini-Hochberg adjusted for multiple comparisons.
Figure S3 Composition of naturally source mouse diets
A) Calculated macronutrient composition, fiber content and energy density of naturally-sourced diets. B) Visual appearance of the 18% protein animal (left) and plant (right) diets. C) Amino acid profiles of naturally sourced diets. D) List of ingredients used to generate naturally sourced diets. E) Measured macronutrient composition and energy densities of 18% plant and animal naturally sourced diets. F) Calculated versus measured amino acid profiles of 18% plant and animal naturally sourced diets.
TableS1: Composition of plant and animal-based amino acid mixtures used for semi-purified diet studies in g AA/100g total AA (left) and composition of semi-purified diets (right).
Table S2: Composition of naturally source diet base mixture (left) and overall composition of naturally sourced diets (right)
Data Availability Statement
Code relating to database analysis will be made available upon request.


