Abstract
Human infection with Cryptosporidium parvum usually elicits characteristic immunoglobulin G (IgG), IgA, and IgM antibody responses against two sporozoite surface antigens with apparent molecular masses of approximately 27 and 17 kDa. We have determined that these two antigens are actually complex families of related antigens. We have developed two new enzyme-linked immunosorbent assays (ELISAs) for the detection and quantitation of serum IgG antibodies against both antigens. The assays utilize a recombinant form of the 27-kDa antigen and a partially purified native fraction isolated from sonicated whole oocysts that contains 17-kDa antigen. An immunoblot assay previously developed in our laboratory served as the reference, or “gold standard,” seroassay for the assessment of the new ELISAs. Positive responses with the recombinant-27-kDa-antigen ELISA were correlated with the immunoblot results for the 27-kDa antigen, with a sensitivity and specificity of 90 and 92%, respectively. Similarly, positive responses with the partially purified native-17-kDa-antigen ELISA correlated with the immunoblot results for the 17-kDa antigen, with a sensitivity and specificity of 90 and 94%, respectively. For both ELISAs the median IgG antibody levels for serum sets collected during outbreaks of waterborne C. parvum infection were at least 2.5-fold higher than the levels determined for a nonoutbreak set. Using the immunoblot as the “gold standard,” the new ELISAs were more specific and, in the case of the 27-kDa-antigen ELISA, more sensitive than the crude oocyst antigen ELISA currently in use. These assays will be useful in future epidemiologic studies.
Cryptosporidium parvum, a coccidian parasite of the mammalian intestinal epithelium, has been identified as the agent responsible for numerous outbreaks of diarrheal disease (5, 7, 8, 11, 13). In immunocompetent hosts, the disease is self-limiting, but in immunocompromised individuals, the disease can become chronic and debilitating (4, 6, 23). Infection of both humans and animals with C. parvum elicits the development of characteristic immunoglobulin G (IgG), IgA, and IgM antibody responses against low-molecular-mass parasite antigens in the 27- and 17-kDa ranges (12, 15–17, 20, 21, 25, 26). Preliminary work has suggested that the presence of antibody against these two immunodominant antigens is associated with protection from symptoms during infection (17). These antigens may be important for invasion of the host cell, since oral administration of monoclonal antibodies against the 27-kDa antigen is capable of reducing the infection load in neonatal mice (2).
In the past, serum antibody responses to C. parvum infection have been tracked by using a crude extract of disrupted oocysts as antigen with either an enzyme-linked immunosorbent assay (ELISA) (28) or a Western blot assay (15, 16, 27). Recent work has shown that the crude oocyst antigen ELISA is not a sensitive means of detecting antibodies against the immunodominant low-molecular-mass C. parvum antigens (16, 17). Although the Western blot assay is quite sensitive (it serves as the reference, or “gold standard,” in our work), it suffers from a limited linear range and the antibody levels are difficult to quantitate by densitometry. The assay is also technically challenging and labor intensive, in that a gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel is required for optimal antigen separation. A new assay capable of high sample throughput and easy quantitation is required for planned population-based studies of the risk factors for C. parvum infection in both immunocompetent and immunocompromised persons. We report here the development of ELISA-based techniques for the detection and quantitation of serum IgG antibodies against the 27- and 17-kDa C. parvum antigens.
MATERIALS AND METHODS
Preparation and purification of native antigen.
C. parvum isolates from Maine and Iowa were maintained by serial passage in Holstein calves (12, 13). Oocysts were isolated from collected feces by discontinuous sucrose gradient centrifugation as described by Arrowood and Sterling (3). A crude antigen supernatant fraction was prepared by sonication and freezing-thawing of purified oocysts followed by centrifugation for 30 min at 24,000 × g, as described elsewhere (14).
A partially purified fraction of the 17- and 27-kDa antigens was prepared from crude antigen by a modification of the Triton X-114 phase partition extraction protocol described by Ko and Thompson (9). Briefly, crude antigen at 1 to 3 mg/ml was mixed with an equal volume of extraction buffer to achieve final concentrations of 20 mM HEPES at pH 7.4, 150 mM NaCl, 2% Triton X-114, 1 mM phenylmethylsulfonyl fluoride, 1 mM p-chloromercuribenzenesulfonic acid, and 5 mM EDTA. After a 30-min incubation at 4°C, insoluble material was removed by centrifugation at 12,000 × g for 15 min at 4°C. The supernatant was frozen for 24 h at −20°C, thawed at 4°C, mixed well, and subjected to two rounds of phase partitioning at 37°C for 10 min. The detergent-rich phase from the final partition was dissolved in 20 mM HEPES and 150 mM NaCl and centrifuged at 12,000 × g for 15 min at 4°C. Partially purified antigens in the collected supernatant were precipitated with 4 volumes of acetone at −20°C overnight. The precipitated proteins were collected by centrifugation at 12,000 × g for 15 min at 4°C and dried at room temperature. The pellet was dissolved either in a nonreducing buffer, for SDS-polyacrylamide gel electrophoresis (10), or in a minimum volume of buffer containing 0.5% SDS and 20 mM HEPES at pH 7.4, for use in ELISA. Both solutions were heated at 95°C for 5 min to insure solubilization.
Crude antigen from the Iowa isolate was used for all of the Western blot analyses of serum samples. Crude antigen from both the Iowa and Maine isolates was used for the development of the Triton X-114 extraction procedures. Triton X-114-purified antigen from the Maine isolate was used for all of the ELISAs.
Recombinant protein expression.
The following two deoxyoligonucleotides were designed for the directional cloning of the C. parvum 27-kDa antigen (22) (GenBank accession no. U34390) into the BamHI and EcoRI restriction enzyme sites of the pGEX 4T-2 expression vector (Pharmacia Biotech, Uppsala, Sweden): Cp23-5′ primer (5′-CGC GGA TCC ATG GGT TGT TCA TCA TCA AAG-3′) and Cp23-3′ primer (5′-GCG GAA TTC ATT AGG CAT CAG CTG GCT TG-3′). The 27-kDa-antigen coding sequence was amplified from 260 ng of genomic DNA by using 100 μM concentrations of Cp23-5′ and Cp23-3′ and AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Norwalk, Conn.) as directed by the manufacturer. The following amplification protocol was used: 30 cycles of 94°C for 1 min, 55°C for 2 min, and 72°C for 3 min, followed by 1 cycle of 72°C for 15 min. Plasmids containing inserts were transformed into Escherichia coli HB101 cells (Life Technologies, Frederick, Md.). The sequence of the resulting clone was confirmed by automated DNA sequencing. A recombinant C. parvum 27-kDa antigen–Schistosoma mansoni glutathione-S-transferase (GST) fusion protein was purified from isopropyl-β-d-thiogalactopyranoside (IPTG)-induced cell cultures by using glutathione Sepharose 4B as directed by the manufacturer (GST bulk purification module; Pharmacia Biotech). The C. parvum protein with an additional GlySer dipeptide at the amino terminus was released by overnight cleavage with thrombin at room temperature and then separated from uncleaved fusion protein and the GST cleavage product by passage over glutathione Sepharose 4B resin. Protein purity was monitored by both SDS-polyacrylamide gel electrophoresis and Western blotting with a monoclonal antibody against the native 27-kDa antigen (C6B6 [12]) and with serum samples from infected humans.
Western blot assay.
Crude oocyst proteins from the Iowa isolate of C. parvum were resolved on gradient SDS-polyacrylamide gels (10 to 22.5% acrylamide) with the buffer system of Laemmli (10). The proteins were electrotransferred onto polyvinylidene difluoride membrane (Immobilon P; Millipore Corp., Bedford, Mass.) and cut into 2-mm-wide strips. Each strip was incubated overnight at 4°C with a 1:100 dilution of serum in phosphate-buffered saline (0.85% NaCl and 10 mM Na2HPO4 at pH 7.2) (PBS) containing 0.3% Tween 20 detergent. Bound antibodies were detected with a biotin-labeled mouse monoclonal antibody against human IgG (clone HP6017; Zymed Laboratories, South San Francisco, Calif.) and alkaline phosphatase-labeled streptavidin (Life Technologies). Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolylphosphate were used to visualize the bound antibodies. The presence or absence of antibodies against the 17- and 27-kDa antigens was determined visually by three independent readers. Eight ambiguous samples (3.3% of the total) were reassayed, and four of these were assayed a third time until a consensus was reached by at least two of the readers.
Western blots that used a mouse monoclonal antibody for visualization of bound antibodies to the 27-kDa antigen (C6B6 [12]) or to the 17-kDa antigen (C6C1 [1]) were developed with a horseradish peroxidase-labeled goat anti-mouse antibody in 0.3% Tween 20-PBS. After incubation for 1 h at room temperature, the antibodies were visualized with diaminobenzidine substrate and H2O2.
ELISA.
Antigens diluted in 0.1 M NaHCO3 buffer (pH 9.6) were used to sensitize 96-well plates (Immulon 2 flat-bottom microtiter immunoassay plates; Dynatech Industries, Inc., McLean, Va.) overnight at 4°C. Each well contained 50 μl of either the recombinant 27-kDa antigen (0.2 μg/ml) or the Triton X-114-extracted antigens (0.14 to 0.28 μg/ml) (BCA protein microassay; Pierce Biotechnology Company, Rockford, Ill.). The plates were blocked with 0.3% Tween 20-PBS for 1 h at 4°C. After a series of three washes (subsequent washes were all with 0.05% Tween 20-PBS), 50-μl aliquots of serum diluted 1:50 with wash buffer were added to all wells. All serum samples were tested in duplicate. A twofold serial dilution (1:50 to 1:6,400) of a strong positive control was used to generate a standard curve on each individual plate. One buffer blank and a battery of seven serum samples known by Western blot assay to be negative for C. parvum antibodies were also included on each plate. The plates were incubated for 2 h at room temperature and then washed four times with wash buffer. A biotinylated mouse monoclonal antibody against human IgG (clone HP6017; Zymed Laboratories) (50 μl of a 1:1,000 dilution in wash buffer) was added to each well and incubated for 1 h at room temperature. Following four washes, the wells were filled with alkaline phosphatase-labeled streptavidin (Life Technologies) (50 μl of a 1:500 dilution in wash buffer) and incubated for an additional hour at room temperature. After four washes (the final wash was for 10 min at room temperature), p-nitrophenylphosphate substrate was added in 3 mM MgCl2 and 10% diethanolamine at pH 10, and the color was allowed to develop until the 1:50 positive control wells had reached an absorbance of about 1.5 at 405 nm. Absorbances were measured with a Molecular Devices UVmax kinetic microplate reader. Antibody levels of the unknown samples were assigned a unit value based on the eight-point positive control standard curve with a four-parameter curve fit. The 1:50 dilution of the positive control was arbitrarily assigned a value of 6,400 U. Unknown samples with absorbance values above the standard curve were diluted further and reassayed. Arbitrary unit values were expressed per microliter of serum.
For the crude oocyst antigen ELISA protocol, a modification of the protocol of Ungar et al. (28) was used. Each well contained 50 μl of crude oocyst antigen in bicarbonate buffer at a protein concentration of 2.0 μg/ml. Test serum samples were diluted 1:50 in 0.05% Tween 20-PBS, and the plates were developed as described above with a biotinylated monoclonal anti-IgG antibody and alkaline phosphatase-labeled streptavidin. Quantitation of the test serum samples was done as described above and was based on the same positive control serum dilution.
Serum samples.
Banked serum specimens were available for analysis, collected in 1988 from 74 employees at the Centers for Disease Control and Prevention who had no history of foreign travel and no documented exposure to C. parvum (referred to as the nonoutbreak serum set). Banked specimens were also available from individuals known to have been exposed to Cryptosporidium during outbreaks of waterborne infection: 129 from the 1987 outbreak in Carrollton, Ga. (7), and 35 from the 1994 outbreak in Walla Walla County, Wash. (5). The 129 serum samples from the Georgia outbreak were divided into two sets. The Georgia “early-outbreak” serum set consisted of 8 samples collected from individuals 2 to 26 days before symptom onset and 25 samples collected from symptomatic individuals at or less than 10 days after the onset of their diarrheal illnesses. Of these 33 samples, 22 were collected from individuals with laboratory-confirmed cases of cryptosporidiosis. The Georgia “late-outbreak” set consisted of 76 samples from patients who met the clinical case definition for cryptosporidiosis (collected between 28 and 66 days after the onset of their diarrheal illness) and 20 samples from asymptomatic individuals who were exposed to the contaminated water supply (collected approximately 4 weeks after the outbreak) (7). Paired samples were available from four symptomatic individuals.
Of the 35 samples in the Washington outbreak serum set, 25 were collected from individuals who met the clinical case definition for infection with C. parvum and 10 were collected from exposed individuals who were asymptomatic or who had mild symptoms that did not meet the case definition (5). Four of the serum donors who met the clinical case definition had Cryptosporidium oocysts detected in their stools. All of the samples in the Washington outbreak set were collected approximately 6 weeks after the peak of the epidemic.
Statistical analysis.
ELISA absorbance values for the various sample sets were converted into unit values as described above, and geometric means were calculated. Geometric means were then compared by using a multiple-comparison t test. Blot positives were compared between groups with the Freeman-Tukey multiple-comparison test.
RESULTS
Partial purification of native antigens.
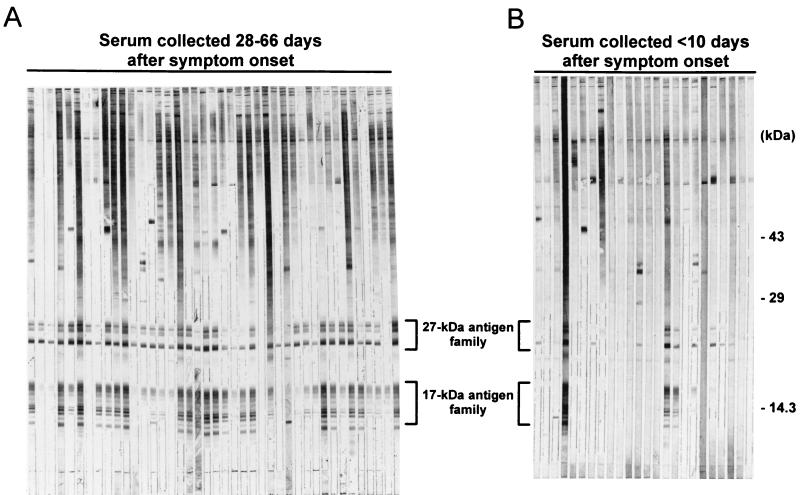
During the course of infection with C. parvum, the host’s IgG antibody response is directed against two low-molecular-mass sporozoite antigens. Using an optimized gradient SDS-polyacrylamide gel system, we have determined that the 27-kDa-antigen family contains approximately 5 proteins in the 23- to 27-kDa range and the 17-kDa-antigen family contains approximately 10 proteins in the 15- to 17-kDa range (Fig. 1) (see below). As shown by the representative Western blots in Fig. 1A, IgG antibodies against the 27- and 17-kDa-antigen families were consistently detected in late-outbreak serum samples collected from the Georgia patients 28 to 66 days after the onset of diarrhea. No other antigens were as consistently recognized by this battery of serum samples. Furthermore, antibodies against these antigens were detected more frequently in the late-outbreak sera than in early-outbreak sera (Fig. 1B) collected from Georgia patients. These observations suggested that antibodies against the two antigens might serve as useful markers for past infection with the parasite.
FIG. 1.
Western blot of serum samples from symptomatic and asymptomatic Carrollton, Ga., residents. A crude antigen supernatant prepared from sonicated oocysts was resolved on an SDS–10 to 22.5% polyacrylamide gel under nonreducing conditions (600 ng/mm of gel width). Following electrotransfer to Immobilon P, 2-mm-wide strips of membrane were incubated overnight at 4°C with serum from individual patients with cryptosporidiosis at a dilution of 1:100 in 0.3% Tween 20-PBS. The blots were developed with a biotinylated anti-human IgG monoclonal antibody and alkaline phosphatase-labeled streptavidin as described in Materials and Methods. (A) Antigens recognized by serum collected from patients with cryptosporidiosis 28 to 66 days after symptom onset (late outbreak). (B) Antigens recognized by sera collected from patients less than 10 days after symptom onset (early outbreak). The locations of the 27- and 17-kDa-antigen families are indicated.
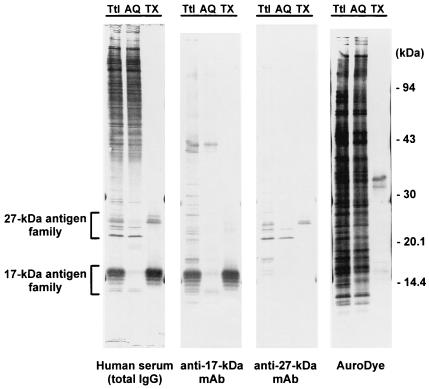
Both the 17- and the 27-kDa antigens have been detected on the sporozoite surface by immunolocalization studies (12). As a first step in the purification of the antigens and to determine whether they are membrane associated, Triton X-114 phase partition extraction was performed on a crude sonicate of oocysts. As shown in Fig. 2, the 17- and 27-kDa antigens recognized by serum samples from an infected patient were partitioned unequally between the two phases following detergent extraction. Of the family of 10 17-kDa antigens recognized by the C6C1 monoclonal antibody, the five largest proteins were completely extracted into the detergent phase, leaving five proteins in the 15-kDa range in the aqueous phase. The proteins were recognized by the monoclonal antibody even after total chemical deglycosylation with anhydrous trifluoromethanesulfonic acid (24). Similarly, of the family of five 27-kDa antigens recognized by the C6B6 monoclonal antibody, the three proteins with the highest apparent molecular masses were extracted into the detergent phase, leaving two lower-molecular-mass proteins in the aqueous phase. These proteins were also recognized by the monoclonal antibody following total chemical deglycosylation (24).
FIG. 2.
Extraction of 27- and 17-kDa antigens by Triton X-114. A crude antigen supernatant prepared from sonicated oocysts was fractionated by Triton X-114 phase partition extraction as described in Materials and Methods. Total unfractionated proteins (Ttl), proteins found in the detergent-depleted aqueous phase (AQ), and proteins extracted into the Triton X-114 detergent-rich phase (TX) were resolved by SDS-polyacrylamide gel electrophoresis as described in the legend to Fig. 1. The proteins were blotted onto Immobilon P and incubated overnight at 4°C with either a 1:100 dilution of human serum from a patient with cryptosporidiosis in 0.3% Tween 20-PBS (Human serum), a 1:1 dilution of tissue culture supernatant in 0.3% Tween 20-PBS containing a monoclonal antibody (C6C1) against the 17-kDa antigen (anti-17-kDa MAb), a 1:1 dilution of tissue culture supernatant in 0.3% Tween 20-PBS containing a monoclonal antibody (C6B6) against the 27-kDa antigen (anti-27-kDa MAb), or AuroDye-forte to stain all proteins (AuroDye). The blots were developed with either a biotinylated anti-human IgG monoclonal antibody and streptavidin-labeled alkaline phosphatase (human serum) or a horseradish peroxidase-labeled goat anti-mouse polyclonal antibody (anti-17-kDa MAb and anti-27-kDa MAb) as described in Materials and Methods. The positions of the molecular mass markers and the 27- and 17-kDa-antigen families are indicated.
Surprisingly, the majority of the oocyst antigens that were recognized by the antibodies in human postinfection serum remained in the aqueous phase following extraction. Many of these antigens are clearly glycosylated, since cleavage of the carbohydrate epitopes with periodate significantly reduced the Western blot reactivity in the high-molecular-mass range without affecting serum antibody binding to the 17- and 27-kDa antigens (24). An AuroDye-stained blot of the Triton X-114 extraction (Fig. 2) suggested that a total of about 10 to 20 proteins were partitioned into the detergent phase. This represents about 3% of the total protein present in the initial oocyst sonicate (Micro BCA protein assay; Pierce).
Expression of recombinant 27-kDa antigen.
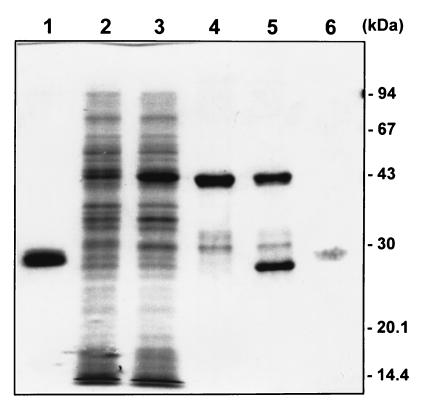
The 11.2-kDa coding sequence identified by Perryman et al. (22) as the 27-kDa antigen was cloned into the pGEX expression vector in frame with GST. Induction with IPTG resulted in the appearance of a fusion protein band at an apparent molecular mass of 43 kDa (Fig. 3, lanes 2 and 3). In contrast to the native antigen, the fusion protein was not membrane associated in the bacterial lysate nor could it be extracted into Triton X-114 (data not shown). The fusion protein bound to the glutathione Sepharose 4B column and could be eluted with only a few minor contaminants in the 30- to 32-kDa range (Fig. 3, lane 4). Cleavage of the glutathione Sepharose 4B-bound fusion protein with thrombin (Fig. 3, lane 5) resulted in a preparation that contained a single, weakly staining protein band at an apparent molecular mass of 27 kDa (Fig. 3, lane 6). The approximate yield of this purified protein was 0.2 mg (Micro BCA assay) per liter of E. coli culture.
FIG. 3.
Purification of recombinant 27-kDa protein. The protein sequence of the 27-kDa antigen was cloned into the pGEX expression system. Proteins from various stages of the expression and purification were resolved on an SDS–12% polyacrylamide gel and stained with Coomassie brilliant blue R-250. Lanes: (1) purified GST; (2) E. coli cells containing the plasmid construct before IPTG induction; (3) the same cells 4 h after IPTG induction; (4) purified fusion protein; (5) purified fusion protein after partial thrombin cleavage; (6) 2 μg of recombinant 27-kDa antigen after removal of uncleaved fusion protein and GST. Lane 6 was digitally enhanced to improve the visibility of the weakly staining recombinant 27-kDa protein band. The positions of the molecular mass markers are indicated.
The 27-kDa apparent molecular mass of the purified, thrombin-cleaved recombinant antigen was unexpected, given the length of the coding sequence that was cloned into the expression vector. A monoclonal antibody (C6B6) raised against the native 27-kDa antigen was used in a Western blot of the proteins resolved as shown in Fig. 3 to confirm that the protein band at 27 kDa was indeed the recombinant 27-kDa antigen (data not shown). The fusion protein, like the purified antigen, reacted with the monoclonal antibody, but no reactivity was noted in the lane loaded with GST alone (data not shown). The weak staining with Coomassie blue and the aberrant migration may reflect the high alanine, proline, and acidic-residue content of the cloned protein sequence (25% Ala, 19% Pro, and 20% Asp plus Glu). As with the fusion protein, the purified recombinant antigen could not be extracted into Triton X-114 detergent (data not shown).
Western blot analysis of serum sets.
Serum sets that were available for use in the ELISA sensitivity and specificity determinations were assayed for the presence of anti-C. parvum antibodies by using a modification of the Western blot assay of Moss et al. (16). The Western blots shown in Fig. 1 are representative of the early- and late-outbreak serum sets available from Carrollton, Ga. Of the early-outbreak serum samples, 33% were positive by immunoblotting for IgG antibodies to the 17-kDa antigen and 52% were positive for antibodies to the 27-kDa antigen (Table 1). In contrast, 95 and 99% of the late-outbreak serum samples were positive for antibodies against the 17- and 27-kDa antigens, respectively (Table 1). No significant differences were observed between the Western blots of the serum samples from symptomatic individuals and those from asymptomatic individuals in the Georgia late-outbreak set. The Washington outbreak serum set had frequencies of positive blots similar to those observed for the Georgia late-outbreak set: 80 and 97% were positive for antibodies against the 17- and 27-kDa antigens, respectively (Table 1). As with the Georgia late-outbreak set, no significant differences were observed between the Western blots of the serum from symptomatic and asymptomatic individuals in the Washington set. The nonoutbreak serum set had an intermediate frequency of positives for antibodies against the 17-kDa antigen (62%) and a high frequency of positives for antibodies against the 27-kDa antigen (92%).
TABLE 1.
Western blot assay characterization of serum samples
| Serum set | No. of samples available | No. blot positive (%)a
|
|
|---|---|---|---|
| 17 kDa | 27 kDa | ||
| Georgia nonoutbreak | 74 | 46 (62)b | 68 (92)e |
| Georgia early outbreak | 33 | 11 (33)cd | 17 (52)efg |
| Georgia late outbreak | |||
| Total | 96 | 91 (95)bc | 95 (99)f |
| Symptomatic | 76 | 73 (96) | 76 (100) |
| Asymptomatic | 20 | 18 (90) | 19 (95) |
| Washington late outbreak | |||
| Total | 35 | 28 (80)d | 34 (97)g |
| Symptomatic | 25 | 22 (88) | 25 (100) |
| Asymptomatic | 10 | 6 (60) | 9 (90) |
Frequencies indicated by the same footnote letters (b to g) were found to have significant differences by the Freeman-Tukey test.
P = 0.0001.
P = 0.0001.
P = 0.0018.
P = 0.0002.
P = 0.0001.
P = 0.0001.
ELISA analysis of serum sets.
The serum sets were assayed by ELISA with both the Triton X-114-extracted antigens and the recombinant 27-kDa antigen. The mean values, medians, and ranges (in arbitrary units) for the two assays are given in Table 2. The Georgia early-outbreak set had the lowest mean reactivity for both the Triton antigen (11.5 U) and the recombinant 27-kDa antigen (102.5 U). The mean ELISA values were significantly lower than those determined for the Georgia nonoutbreak set (P = 0.0001 and 0.0012, respectively) and the Georgia late-outbreak and Washington outbreak sets (P = 0.0001 for all comparisons). The mean Triton antigen and recombinant-27-kDa-antigen ELISA values for the nonoutbreak set were seven- and fourfold higher, respectively, than those for the Georgia early-outbreak set but were still significantly lower than those determined for the Georgia late-outbreak (P = 0.0001 for both assays) and Washington outbreak (P = 0.0104 and 0.0226, respectively) sets.
TABLE 2.
ELISA characterization of serum samples
| Serum set | No. of samples available | Mean ELISA responsea (median/range)
|
|
|---|---|---|---|
| Triton 17 kDa | Recombinant 27 kDa | ||
| Georgia nonoutbreak | 74 | 80.3 (106.5/0–1,293)c | 444.0 (537.5/0–6,430)d |
| Georgia early outbreak | 32b | 11.5 (10/0–3,426)c | 102.5 (274.5/0–3,740)d |
| Georgia late outbreak (total) | 96 | 384 (591/0–7,878) | 1,503 (1,696/0–34,456) |
| Washington late outbreak (total) | 35 | 32.2 (491/0–6,400) | 1,321 (1,338/91–13,528) |
Arbitrary units.
One sample with responses of 43,136 U and 46,336 U to the Triton and recombinant 27-kDa antigens, respectively, was considered an outlier and omitted.
Mean values were significantly different from those of other serum sets and from each other when a multiple-comparison t test was used. The P values for all comparisons were <0.0104.
Mean values were significantly different from those of other serum sets and from each other when a multiple-comparison t test was used. The P values for all comparisons were <0.0226.
In contrast to the qualitative assessment of the 27-kDa group in the Western blot assay, the recombinant-27-kDa-antigen ELISA was able to demonstrate a significant difference (P = 0.0243) between symptomatic and asymptomatic members of the Georgia late-outbreak set. Symptomatic individuals had a mean value of 2,015.7 arbitrary units (median = 2,060; range (R) = 87 to 34,456) compared to 492.6 U for the asymptomatic individuals (median = 940; R = 0 to 4,599). Similar trends were noted for the Triton antigen ELISA (mean values of 454.0 and 203.2 for symptomatic and asymptomatic subsets, respectively) and for the symptomatic and asymptomatic subsets from the Washington outbreak (recombinant-27-kDa-antigen ELISA mean values of 1,854.7 and 565.1; Triton antigen ELISA values of 439.2 and 147.0, respectively), but the differences did not approach statistical significance. Neither ELISA assay detected a significant difference between the Georgia late-outbreak and Washington outbreak sets.
Sensitivity and specificity of ELISAs.
Based on an overall comparison of the ELISA responses and the Western blotting results (which served as the reference, or gold standard), positive threshold unit values were chosen for both ELISAs so as to maximize the sensitivity and specificity of the ELISAs relative to the Western blot. These values were generally greater than the cutoff values that would have been assigned based on a mean plus 3 standard deviations of the ELISA responses of the seven Western blot-negative controls that were included on each ELISA plate. Using a positive cutoff of 206 U, the recombinant-27-kDa-antigen ELISA was able to predict 90% of the samples that were positive by blotting for antibodies against the 27-kDa antigen and 92% of the samples that were negative by blotting. Using a positive cutoff of 76 U for the Triton-purified antigen ELISA, the assay was able to correctly identify 90% of those samples that were positive by blotting for antibodies against the 17-kDa antigen and 94% of the samples that were negative by blotting. We do not yet know if these values will be applicable to other serum sets and to other lots of purified and recombinant proteins.
The Triton antigen ELISA response did not correlate well with the 27-kDa-antigen blot response even though the 27-kDa antigen was present in the partially purified preparation used to sensitize the plates. Of 39 samples that were positive by blotting for antibodies against the 27-kDa antigen but negative by blotting for antibodies against the 17-kDa antigen, only 3 (8%) were defined as positive by the Triton-purified-antigen ELISA. Antibodies against the 27-kDa antigen were certainly present in most of these samples, since 30 (77%) were correctly identified as positive when the recombinant-27-kDa-antigen ELISA was used. Thus, the 17-kDa antigen appears to be responsible for most of the response seen in the ELISA with the partially purified Triton fraction.
Others (16, 17) have reported that the blot responses to the 27- and 17-kDa antigens are not well correlated with the ELISA response when a crude oocyst antigen preparation is used to sensitize the plates. In our hands a modified version of the crude antigen ELISA (at a 117-U positive cutoff value chosen to optimize sensitivity and specificity relative to the Western blot) had a 91% sensitivity and a 60% specificity for detection of antibodies against the 17-kDa antigen. At the same unit value cutoff, the crude antigen assay accurately predicted 83% of the samples that were positive by blotting for antibodies against the 27-kDa antigen and 69% of those that were negative. However, of 23 samples that were negative by blotting for antibodies against both the 17- and 27-kDa antigens, 5 (22%) appeared to be positive by the crude antigen ELISA. Only one of these same samples was misclassified by the Triton antigen ELISA, and only two were misclassified by the recombinant-27-kDa-antigen ELISA. Of 42 samples that were positive by blotting for only one antigen, the crude antigen ELISA detected 21 (50%) while the recombinant-27-kDa-antigen and Triton-purified-antigen ELISAs together correctly identified 30 (71%).
ELISAs on paired sera.
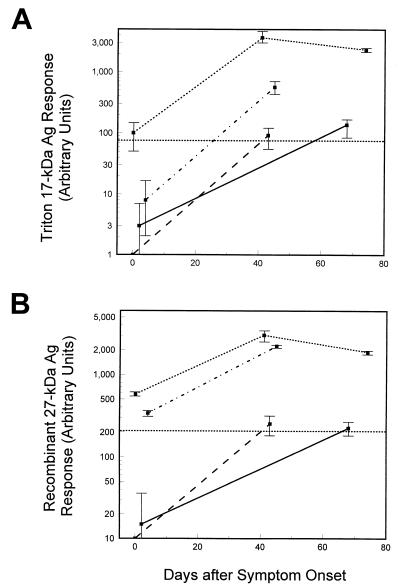
Both ELISAs were used to monitor changes in antibody levels in paired serum samples from four symptomatic individuals (two of whom had laboratory-confirmed cryptosporidiosis) from the Carrollton, Ga., outbreak. The initial serum samples were collected in the early-outbreak period (0 to 4 days after symptom onset), and the follow-up samples were collected in the late-outbreak period (41 to 68 days after symptom onset). A third sample was available from one individual (74 days after onset). As shown in Fig. 4A, two individuals who were initially negative for IgG antibodies by both ELISAs developed positive responses by the second time point. The seroconversion detected by ELISA for these two individuals was also evident by immunoblotting. IgG antibodies against the 27- and 17-kDa antigens as well as IgA antibodies against the 17-kDa antigen and IgM antibodies against the 27-kDa antigen were present in the late-outbreak serum specimens but absent from the early-outbreak samples (data not shown). One individual was ELISA positive by both assays at all time points and had peak levels of antibody approximately 40 days after onset. The remaining individual, who was initially negative for antibody by Triton antigen ELISA but positive by the recombinant-27-kDa-antigen ELISA, was positive by both assays at the later time point. All of the individuals experienced increases in antibody levels by both assays after the initial sample was collected. The Triton antigen ELISA and the recombinant-27-kDa-antigen ELISA were able to track changes in antibody levels in single individuals and to correctly identify those individuals who had anti-17-kDa and anti-27-kDa antibodies by Western blotting in each instance (blots not shown).
FIG. 4.
ELISA responses for paired serum samples. Paired serum samples from patients with cryptosporidiosis were assayed for antigen (Ag)-specific IgG antibodies using the Triton antigen ELISA (A) or the recombinant-27-kDa-antigen ELISA (B) as described in Materials and Methods. The 76-arbitrary unit (A) and 206-unit (B) threshholds for positivity in the two ELISAs are indicated by dotted lines across the graphs. Samples with ELISA responses near or below this cutoff were assayed on three separate occasions, and all others were assayed twice. The mean values and 1 standard deviation are indicated. Each kind of line represents an individual patient.
DISCUSSION
In the original report of the Carrollton, Ga., C. parvum outbreak (7), the IgG antibody seroprevalence was reported to be 76% in symptomatic individuals (n = 68) and 56% in asymptomatic individuals (n = 18) when a crude antigen ELISA was used. Using the Western blot assay, we reexamined this same late-outbreak serum set along with an additional eight samples from symptomatic individuals and two samples from asymptomatic individuals. The seroprevalences for IgG antibodies against the immunodominant 17- and 27-kDa antigens were much higher than anticipated, based upon the earlier ELISA results: 96% for the 17-kDa antigen and 100% for the 27-kDa antigen in the symptomatic individuals and 90 and 95% for these antigens, respectively, in the asymptomatic individuals. Discrepancies between the crude antigen ELISA and the Western blot assay have been described earlier by our laboratory and by others. Moss et al. (16) reported that, of 20 patients with confirmed or probable cryptosporidiosis who were classified as positive for antibodies against the low-molecular-mass antigens by immunoblotting, only 11 showed a significant change in crude antigen ELISA response between initial and follow-up serum samples. Of 14 noninfected individuals who did not meet the case definition for cryptosporidiosis and who were negative by immunoblot, 5 showed a significant change in crude antigen ELISA response between the two time points. Similarly, using a different version of the crude antigen ELISA, Ungar et al. (28) were able to identify only 70% of those individuals who had a 27-kDa-antigen response by Western blotting and 71% of those who were blot negative. Given that the antibody responses to the 27- and 17-kDa C. parvum antigens appear to be characteristic of infection and may be relevant to the development of symptomatic disease (17), a new ELISA with a sensitivity and specificity equivalent to those of Western blotting is required for future work.
A careful analysis of the 17- and 27-kDa antigens demonstrated that they are actually complex families of related proteins, some of which are membrane associated and some of which are soluble. We believe that the 10 antigens in the 17-kDa-antigen family and the 5 in the 27-kDa-antigen family share the same protein backbone but are modified to various extents, perhaps by endopeptidase cleavages and by the addition of carbohydrates and fatty acids. The five soluble antigens in the 15-kDa range and the two soluble antigens in the 23-kDa range probably represent cleaved forms of the 17- and 27-kDa antigens, respectively, that lack carbohydrates and a membrane anchor. The observation that the recombinant form of the 27-kDa antigen runs on SDS-polyacrylamide gels at an apparent molecular mass of 27 kDa but cannot be extracted into Triton X-114 detergent supports this hypothesis. We are currently studying the possibility that both antigens are linked to the membrane via glycosyl phosphatidylinositol anchors.
Since the 17-kDa antigen has not yet been cloned, we exploited the membrane association of the antigens and their extractability into Triton X-114 detergent in order to partially purify a native protein fraction for use in one of the new ELISAs. The ELISA response with the Triton-extracted antigens correlated well (at least 90% sensitivity and specificity) with the presence or absence of antibodies against the 17-kDa antigen, as determined by Western blotting. The 27-kDa antigen present in the Triton fraction apparently does not contribute significantly to the ELISA response (at least at low antibody titers). This observation may reflect the low concentration of this antigen relative to the 17-kDa antigen. Based on the AuroDye-stained blot of the partially purified proteins, the 27-kDa antigen is probably present at a 10- to 20-fold lower concentration than the 17-kDa antigen. At 14 ng of antigen per well in the Triton ELISA, each well would contain less than 1 ng of the 27-kDa antigen compared with 10 ng per well in the recombinant-27-kDa-antigen ELISA.
Our inability to detect the 27-kDa-antigen response with the Triton-extracted-antigen ELISA led to the development of a second ELISA that uses a recombinant protein based on the 27-kDa-antigen sequence of Perryman et al. (22). Despite the absence of carbohydrates on the recombinant protein, the sensitivity and specificity of the recombinant-27-kDa-antigen ELISA (at least 90% compared to the Western blot assay) were similar to those observed for the native 17-kDa antigen in the Triton-extracted-antigen ELISA. This suggests that a significant proportion of the human antibody response is directed towards the protein component of the 27-kDa antigen. This result is consistent with our observation that antibody recognition of the 27- and 17-kDa antigens by Western blotting is retained following carbohydrate cleavage with periodate or anhydrous trifluoromethanesulfonic acid.
Using the new assays, we were able to demonstrate statistically significant differences in ELISA responses between a nonoutbreak set of serum samples and sets of acute- and convalescent-phase sera from the Carrollton, Ga., outbreak. Late-outbreak serum samples from an outbreak in Washington State had ELISA responses similar to those of the Georgia outbreak. This suggests that there were no gross differences in the antigenic makeup of the isolates that were responsible for the two waterborne infection outbreaks, despite the widely separate geographic locations. We have recently examined serum samples from a food-borne infection outbreak with similar results (24). Surprisingly, we were also able to demonstrate a significant difference between the responses of the nonoutbreak serum set and the early-outbreak set. The early-outbreak set from Carrollton had a mean response by both ELISAs unexpectedly lower than that of the set collected contemporaneously from Centers for Disease Control and Prevention employees in nearby Atlanta. Although these serum sets are not representative of populations, it is interesting to speculate that the residents of Carrollton who became ill may have been at increased risk for symptomatic Cryptosporidium infection because of lower overall antibody titers. Human volunteer studies have suggested that the presence of an IgG antibody response to the 17- and 27-kDa antigens is able to prevent illness but not infection upon oocyst challenge (17). If this hypothesis is correct, the exposed but asymptomatic late-outbreak subsets should contain serum both from individuals who were not infected and from individuals who were infected but who never developed overt symptoms. Indeed, the mean ELISA values for these subsets were between those determined for the nonoutbreak set and those determined for the symptomatic late-outbreak subsets, but the observed differences were only statistically significant for one comparison (symptomatic versus asymptomatic Georgia late-outbreak sera by recombinant-27-kDa-antigen ELISA). Unfortunately, we do not know the levels of anti-17- and anti-27-kDa antibodies in any of the exposed, asymptomatic individuals prior to the two outbreaks, since paired serum samples were not available.
With paired serum specimens we have demonstrated that two symptomatic individuals who lacked an IgG response by both of the new ELISAs and the crude antigen ELISA and lacked IgG, IgA, and IgM responses by Western blotting were able to seroconvert to IgG positive by blotting and by ELISA upon C. parvum infection. IgA and IgM responses were also detected by immunoblot assay. In one of these individuals, infection was confirmed by microscopy of stool after acid-fast staining. These results are consistent with our previous experience with immunoblotting (17, 18). In contrast, Okhuysen et al. (19) were able to detect an IgM and an IgA response but not an IgG response upon primary challenge of volunteers with C. parvum. This apparent discrepancy may reflect the fact that Okhuysen et al. used a crude antigen ELISA for their work and based their assignment of seroconversion on an ELISA change of 0.1 optical density unit between a baseline serum and a postchallenge serum. When the crude antigen ELISA was used on the two individuals who seroconverted in our paired serum study, one demonstrated a 0.15-optical density unit change and the other had only a 0.062-unit change (data not shown). Our results suggest that most infected persons develop IgG antibody responses that can be detected with appropriately sensitive and specific assays.
In future studies, we hope to use these new assays to examine sample sets that are representative of the general population and of outbreak populations in order to determine basal IgG antibody levels and to relate changes in antibody levels to rates of Cryptosporidium exposure and infection.
ACKNOWLEDGMENTS
We are indebted to William MacKenzie for his critical comments and helpful suggestions on the manuscript. We would also like to recognize the assistance provided by Jan Mead and Maria-Teresa Bonafonte during the early stages of this work.
REFERENCES
- 1.Arrowood M J. Ph.D. thesis. Tucson: University of Arizona; 1988. [Google Scholar]
- 2.Arrowood M J, Mead J R, Mahrt J L, Sterling C R. Effects of immune colostrum and orally administered antisporozoite monoclonal antibodies on the outcome of Cryptosporidium parvum infections in neonatal mice. Infect Immun. 1989;57:2283–2288. doi: 10.1128/iai.57.8.2283-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 4.DuPont H L, Chappel C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin M S, Goldman D P, Wells T G, Kobayashi J M, Herwaldt B L. Cryptosporidiosis in Washington State: an outbreak associated with well water. J Infect Dis. 1996;174:1372–1376. doi: 10.1093/infdis/174.6.1372. [DOI] [PubMed] [Google Scholar]
- 6.Flanigan T, Whalen C, Turneer J, Soave R, Toerner J, Havlir D, Kotler D. Cryptosporidium infection and CD4 counts. Ann Intern Med. 1992;116:840–842. doi: 10.7326/0003-4819-116-10-840. [DOI] [PubMed] [Google Scholar]
- 7.Hayes E B, Matte T D, O’Brien T R, McKinley T W, Logsdon G S, Rose J B, Ungar B L P, Word D W, Pinsky P F, Cummings M L, Wilson M A, Long E G, Hurwitz E S, Juranek D D. Large community outbreak of cryptosporidiosis due to contamination of a filtered public water supply. N Engl J Med. 1989;320:1372–1376. doi: 10.1056/NEJM198905253202103. [DOI] [PubMed] [Google Scholar]
- 8.Juranek D D. Cryptosporidiosis: sources of infection and guidelines for prevention. Clin Infect Dis. 1995;21(Suppl.):S57–S61. doi: 10.1093/clinids/21.supplement_1.s57. [DOI] [PubMed] [Google Scholar]
- 9.Ko Y-G, Thompson G A., Jr Purification of glycosylphosphatidylinositol-anchored proteins by modified Triton X-114 partitioning and preparative gel electrophoresis. Anal Biochem. 1995;224:166–172. doi: 10.1006/abio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.MacKenzie W R, Hoxie N J, Proctor M E, Gradus M S, Blair K A, Peterson D E, Kazmierczak J J, Addiss D A, Fox K R, Rose J B, Davis J P. A massive outbreak of cryptosporidiosis infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 12.Mead J R, Arrowood M J, Sterling C R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988;74:135–143. [PubMed] [Google Scholar]
- 13.Millard P S, Gensheimer K F, Addiss D G, Sosin D M, Beckett G A, Houck-Jankoski A, Hudson A. An outbreak of cryptosporidiosis from fresh-pressed apple cider. JAMA. 1994;272:23–30. [PubMed] [Google Scholar]
- 14.Moss D M, Lammie P J. Proliferative responsiveness of lymphocytes from Cryptosporidium parvum-exposed mice to two separate antigen fractions from oocysts. Am J Trop Med Hyg. 1993;49:393–401. doi: 10.4269/ajtmh.1993.49.393. [DOI] [PubMed] [Google Scholar]
- 15.Moss D M, Bennett S N, Arrowood M J, Hurd M R, Lammie P J, Wahlquist S P, Addiss D G. Kinetic and isotypic analysis of specific immunoglobulins from crew members with cryptosporidiosis on a U.S. Coast Guard cutter. J Eukaryot Microbiol. 1994;41:52S–55S. [PubMed] [Google Scholar]
- 16.Moss D M, Bennett S I, Arrowood M J, Wahlquist S P, Lammie P J. Enzyme-linked immunoelectrotransfer blot analysis of a cryptosporidiosis outbreak on a United States Coast Guard cutter. Am J Trop Med Hyg. 1998;58:110–118. doi: 10.4269/ajtmh.1998.58.110. [DOI] [PubMed] [Google Scholar]
- 17.Moss D M, Chappell C L, Okhuysen P C, DuPont H L, Arrowood M J, Hightower A W, Lammie P J. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J Infect Dis. 1998;178:827–833. doi: 10.1086/515377. [DOI] [PubMed] [Google Scholar]
- 18.Moss, D. M., and P. J. Lammie. Unpublished observations.
- 19.Okhuysen P C, Chappell C L, Sterling C R, Jakubowski W, DuPont H L. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect Immun. 1998;66:441–443. doi: 10.1128/iai.66.2.441-443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega-Mora L M, Troncoso J M, Rojo-Vazquez F A, Gomez-Bautista M. Identification of Cryptosporidium parvum oocyst/sporozoite antigens recognized by infected and hyperimmune lambs. Vet Parasitol. 1994;53:159–166. doi: 10.1016/0304-4017(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 21.Peeters J E, Villacorta I, Vanopdenbosch E, Vandergheynst D, Naciri M, Ares-Mazas E, Yvore P. Cryptosporidium parvum in calves: kinetics and immunoblot analysis of specific serum and local antibody responses (immunoglobulin A [IgA], IgG, and IgM) after natural and experimental infection. Infect Immun. 1992;60:2309–2316. doi: 10.1128/iai.60.6.2309-2316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perryman L E, Jasmer D P, Riggs M W, Bohnet S G, McGuire T C, Arrowood M J. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 23.Pozio E, Rezza G, Boschini A, Pezzotti P, Tamburrini A, Rossi P, Di Fine M, Smacchia C, Schiesari A, Gattei E, Zucconi R, Ballarini P. Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J Infect Dis. 1997;176:969–975. doi: 10.1086/516498. [DOI] [PubMed] [Google Scholar]
- 24.Priest, J. W., and P. J. Lammie. Unpublished data.
- 25.Reperant J-M, Naciri M, Chardes T, Bout D T. Immunological characterization of a 17-kDa antigen from Cryptosporidium parvum recognized early by mucosal IgA antibodies. FEMS Microbiol Lett. 1992;99:7–14. doi: 10.1016/0378-1097(92)90280-2. [DOI] [PubMed] [Google Scholar]
- 26.Reperant J-M, Naciri M, Iochmann S, Tilley M, Bout D T. Major antigens of Cryptosporidium parvum recognised by serum antibodies from different infected animal species and man. Vet Parasitol. 1994;55:1–13. doi: 10.1016/0304-4017(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 27.Ungar B L P, Nash T E. Quantification of specific antibody response to Cryptosporidium antigens by laser densitometry. Infect Immun. 1986;53:124–128. doi: 10.1128/iai.53.1.124-128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ungar B L P, Soave R, Fayer R, Nash T E. Enzyme immunoassay detection of immunoglobulin M and G antibodies to Cryptosporidium in immunocompetent and immunocompromised persons. J Infect Dis. 1986;153:570–578. doi: 10.1093/infdis/153.3.570. [DOI] [PubMed] [Google Scholar]