Abstract
Depression is a common, often recurrent disorder that causes substantial disease burden worldwide, and this is especially true for women following the pubertal transition. According to the Social Signal Transduction Theory of Depression, stressors involving social stress and rejection, which frequently precipitate major depressive episodes, induce depressive symptoms in vulnerable individuals in part by altering the activity and connectivity of stress-related neural pathways, and by upregulating components of the immune system involved in inflammation. To test this theory, we recruited adolescent females at high and low risk for depression and assessed their psychological, neural, inflammatory, and genomic responses to a brief (10 minute) social stress task, in addition to trait psychological and microbial factors affecting these responses. We then followed these adolescents longitudinally to investigate how their multi-level stress responses at baseline were related to their biological aging at baseline, and psychosocial and clinical functioning over one year. In this protocol paper, we describe the theoretical motivations for conducting this study as well as the sample, study design, procedures, and measures. Ultimately, our aim is to elucidate how social adversity influences the brain and immune system to cause depression, one of the most common and costly of all disorders.
Keywords: Adolescent, Depression, Social rejection, Neural, fMRI, Neuroimaging, Immune, Cytokine, Inflammation, Microbiome, Telomere, Biological aging, Risk, Disease, Health
Highlights
-
•
Depression often emerges in adolescence and causes substantial disease burden worldwide.
-
•
Here, we describe a study examining preclinical disease processes that may be involved.
-
•
Participants were never-depressed adolescent females at high and low risk for depression.
-
•
Their psychological, neural, inflammatory, and genomic responses to social stress were assessed.
-
•
The results will help elucidate how social stress affects the brain and immune system to increase risk for depression.
Experiences of social stress can have a profound impact on mental health (Meyer, 2003). Within this broad category of life stress, stressors involving social rejection have been found to be especially impactful and to represent one of the strongest proximal precipitants of major depressive disorder (MDD; Monroe et al., 1999; Slavich, 2016a; Slavich et al., 2010). This is especially true in early adolescence, a developmental period during which time peer relationships and relative social standing in peer groups become increasingly important (Nolan et al., 2003; Somerville, 2013; Wang et al., 2009). MDD, in turn, is associated with increased risk for self-harm and suicide (Nock et al., 2013), and several chronic diseases that presage early mortality, including obesity, diabetes, heart disease, and neurodegenerative disorders (Patten et al., 2008; Whooley, 2006).
We have hypothesized that experiences of social stress may exert long-term effects on mental and physical health in part by heightening neural responses to social threat and activating innate immune system processes, especially inflammation, which in turn evoke symptoms of depression in vulnerable individuals and contribute to the development of somatic disease conditions that frequently co-occur with MDD (Slavich and Cole, 2013; Slavich and Irwin, 2014; Slavich and Sacher, 2019; Slavich, 2020a, 2020b). To date, however, only a few studies have assessed both neural and immunologic responses to social stress (e.g., Muscatell et al., 2015, 2016), and we are not aware of any studies that have linked interactions between these two systems to psychosocial or clinical features of depression. Moreover, there is a distinct absence of studies that have examined neural-inflammatory dynamics in adolescents at varying risk for depression even though investigating such processes in early life could help reveal pre-clinical disease processes that could be potentially targeted to prevent MDD, one of the most common and costly of all psychiatric disorders (Ferrari et al., 2013).
In this protocol paper, we first summarize research on three key biological processes that are responsive to social stress and increase risk for depression—namely, neural activity and connectivity, inflammatory activity, and the gut microbiome. Second, we discuss the importance of studying how these processes interact and increase risk for MDD in youth who have not yet had their first major depressive episode (MDE). Third, we describe our integrative, multi-level study on this topic, aimed at elucidating stress-related processes that affect risk for depression. By providing these details, our aim is to describe our methodology and demonstrate the utility of studying depression and related disorders using an integrative, multi-level approach.
Neural risk processes in depression
With respect to the neurobiology of depression, experiences of social stress during adolescence may influence risk for depression and depression-related disorders by altering the activity and/or connectivity of neural systems that respond to stress and regulate inflammatory activity (Boyce et al., 2012; McEwen and Gianaros, 2010; Nelson et al., 2005). Research on this topic has revealed a network of brain regions, sometimes referred to as the amygdala network or more broadly as the threat network (Kennedy and Adolphs 2012), which are engaged by physical threats (e.g., snakes, spiders; Mobbs et al., 2010) as well as social threats (e.g., social evaluation, rejection; Muscatell and Eisenberger, 2012). Although the primary site of neural activation differs depending on the type of threat, the regions constituting this network broadly include the amygdala, subgenual region of the anterior cingulate cortex (sgACC), dorsal anterior cingulate cortex (dACC), and anterior insula (Bishop, 2008; Eisenberger, 2012; O'Donovan et al., 2013; Woody and Szechtman, 2011). For example, exposure to a brief episode of social rejection appears to consistently engage the dACC and anterior insula in adults (e.g., Eisenberger et al., 2003; Kross et al., 2011) and the sgACC, anterior insula, and amygdala in adolescents (e.g., Lau et al., 2012; Masten et al., 2009). Moreover, activity in these regions has been related to feelings of distress during laboratory-based social rejection stressors in both adolescents and adults (Eisenberger et al., 2003, Eisenberger et al., 2007), and to feelings of social rejection in daily life (Way et al., 2009). Finally, demonstrating the relevance of this neural network for health, activity in these brain regions is heightened in anxiety disorders (Etkin, 2010) and depression (Hamilton et al., 2012; Dedovic et al., 2016), and is related to several health-related outcomes including depression severity (Gong et al., 2017), physiological stress responding (Eisenberger et al., 2007), psychopharmacological treatment response (Korb et al., 2011), and heart disease risk (Gianaros and Sheu, 2009). Together, these findings suggest that the threat network may be one neural system linking experiences of social stress with depression and depression-related health problems.
Inflammation and depression
In terms of immunological processes involved in depression, recent research has suggested that components of the immune system that mediate inflammation may be a common mechanism underlying risk for both depression and other health problems that frequently co-occur with depression (Miller et al., 2009a; O'Donovan et al., 2013; Slavich and Irwin, 2014). Mediators of the systemic inflammatory response, namely pro-inflammatory cytokines, are frequently elevated in persons with depression (Dowlati et al., 2010; Howren et al., 2009; Yirmiya et al., 2000) and in several disease conditions that co-occur with MDD, including asthma, heart disease, chronic pain, and autoimmune and neurodegenerative disorders (Furman et al., 2019; Miller et al., 2011). Moreover, animal model and human studies have shown that pro-inflammatory cytokines are involved in the pathophysiology of each of the above-mentioned conditions (Allan and Rothwell, 2001; Antoni et al., 2006; Hansson, 2005). Furthermore, research has demonstrated that pro-inflammatory cytokines can communicate with the central nervous system to induce “sickness behaviors” such as social withdrawal, reduced appetite, dysregulated sleep, psychomotor slowing, and fatigue, which are similar to several depressive symptoms and behaviors (Dantzer et al., 2008; Miller et al., 2009a; Raison et al., 2006).
With respect to stress-inflammation links, naturalistic and experimental studies have shown that psychological stressors are a potent activator of the innate immune and inflammatory response (Glaser and Kiecolt-Glaser, 2005; Segerstrom and Miller, 2004). Moreover, this activating effect appears to be especially strong for stressors that involve interpersonal adversity or social rejection (Dickerson et al., 2009; Kiecolt-Glaser et al., 2005; Miller et al., 2009b; Murphy et al., 2013, Murphy et al., 2015). These immunological effects are evident at the protein level as indexed, for example, by the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), and also at the genomic level, as indexed by the expression of the immune response genes TNF, IL1B, IL6, and IL8 (for reviews, see Dieckmann et al., 2020; Slavich and Cole, 2013; Slavich and Irwin, 2014). Moreover, these immunological processes appear to mediate social stress-related increases in disease risk (Cole et al., 2010).
Given the independent body of research showing that stressors involving social rejection are the strongest proximal risk factor for MDD in both adolescence (Monroe et al., 1999) and adulthood (Farmer and McGuffin, 2003; Kendler et al., 2003), one possibility is that social rejection-related increases in inflammation play a pathophysiological role in MDD, especially for vulnerable individuals. Indeed, this is a key hypothesis of the Social Signal Transduction Theory of Depression (Slavich and Irwin, 2014; Slavich and Sacher, 2019), which describes how social adversity may be represented by the brain and upregulate inflammatory processes that promote depressive symptoms among individuals at risk for MDD. As reviewed by Slavich and Irwin (2014), there is substantial evidence in support of the general tenets of this theory. Because very few studies have assessed social, neural, and inflammatory processes in the same individuals, however, much of this evidence is derived from separate lines of research. This is unfortunate given that studying neural-immune responses to social stress, and how these responses in turn relate to depression, likely represents a fruitful approach for elucidating multi-level mechanisms underlying risk for depression as well as other psychiatric and somatic disease conditions that have an inflammatory component.
The gut microbiome, immune system, and depression
One key factor other than life stress that can influence the activity of the brain and immune system, and thus risk for depression, is the gut microbiome. Recent research has suggested that the gut microbiome likely plays a role in both psychological and neurodegenerative disorders for several reasons including the fact that the gut contains the second most neurons in the body after the brain, is the largest immune and endocrine organ in the human body, and has known bidirectional connections with the brain (Martin et al., 2018). Moreover, the gut microbiome can influence the immune system and immune cell trafficking (Schirmer et al., 2016), synthesize neurotransmitters and neuroactive metabolites that cross the blood-brain barrier (Foster and Neufeld, 2013), and activate the vagus nerve (Fülling et al., 2019). In animal models, researchers have shown that changes in the gut microbiome can lead to hyperactivation of the immune system, creating a pro-inflammatory cytokine profile akin to what is seen in depression (Wong et al., 2016). In addition, the gut microbiota has been shown to promote exaggerated hypothalamic-pituitary-adrenal (HPA) axis reactivity to stress in mice (Sudo et al., 2004). As alluded to here, however, the majority of studies on this topic have been conducted in animal models with human studies generally lacking.
Studying high-risk populations
One potentially promising strategy for investigating the role that psychological, neural, inflammatory, and genomic processes play in increasing risk for depression involves studying adolescents at varying risk for MDD as a result of their familial risk status (i.e., high-risk family design). Importantly, by studying adolescents who are at high risk for depression but who have never themselves had the disorder, it is possible to identify pre-clinical disease processes—such as social stress-related changes in psychosocial, neural, inflammatory, or genomic functioning—that precede the onset of depression and are associated with prospective risk for MDD, as opposed to those that are simply concomitant with the disorder.
In this context, it is notable that having a mother with a history of depression is one of the strongest predictors of risk for MDD in youth, and this is especially true for adolescent females who, during this developmental period, become markedly more likely to experience depression as compared to their male counterparts (Hammen et al., 2004; Hankin et al., 1998; Weissman et al., 1987). Furthermore, a history of maternal depression is associated with both a younger age of depression onset and increased severity of depression in daughters of depressed mothers (Lieb et al., 2002). For these reasons, even in a never-depressed adolescent population, having a maternal history of MDD can help distinguish adolescents at high risk for eventually developing depression (i.e., adolescent females at high maternal risk) from those at low risk for developing depression (i.e., adolescent females with no maternal history of depression).
PSY SAD study
The Psychobiology of Stress and Adolescent Depression (PSY SAD) Study aims to integrate the historically disparate lines of research described above to test hypotheses derived from the Social Signal Transduction Theory of Depression. The study does this by characterizing psychological, neural, inflammatory, and genomic responses to acute social stress in adolescent females at low versus high risk for depression. In examining neural processes associated with inflammation and risk for depression, the PSY SAD study goes beyond existing research that has used static or non-personally relevant stimuli such as emotional faces or sad film clips to induce neural responses (Joormann et al., 2012; Mannie et al., 2011; Monk et al., 2008) by exposing adolescent females to a brief, standardized social stressor in the fMRI scanner. In terms of inflammation, many studies have investigated inflammatory responses to laboratory-based stressors (e.g., Aschbacher et al., 2012; Carroll et al., 2011; Dickerson et al., 2009; Quinn et al., 2020). However, much of this research has focused on adult populations or youth who have already developed MDD, which limits the ability to investigate the temporal order of increases in inflammation vis-à-vis the development of depressive symptoms. Further, of the few studies that have examined stress-related differences in inflammatory activity in younger samples (Danese et al., 2011; Slopen et al., 2013), few have used a standardized stress-induction paradigm.
In the PSY SAD study, therefore, we assessed youths' inflammatory responses to an fMRI-based social stressor by measuring both their cytokine and gene expression levels once before and twice following the stressor. In addition, we assessed trait psychological (e.g., emotion regulation, social support) and microbial (i.e., gut microbiota composition & diversity) factors affecting these responses, as well as how these responses related to participants’ biological aging, as indexed by telomere length. As such, this is the first study, to our knowledge, to examine psychological, neural, inflammatory, and genomic responses to an ecologically valid social stressor in adolescent females who were either at high risk for developing MDD (i.e., no personal history of MDD, but a maternal history of the disorder) or low risk for developing MDD (i.e., no personal or maternal history of the disorder).
Method
Participants
Participants were recruited using flyers posted in community locations (e.g., libraries, businesses, schools, churches), online advertisements, social media posts, word of mouth, and announcements made at public and private middle and high schools located throughout the greater Los Angeles area. Because our aim was to study adolescents’ natural responses to social stress, it was important that potential participants not know that the study would involve a laboratory-based social stressor. Consequently, mothers and daughters were told that the study, advertised as the “UCLA Autobiographical Memory Study,” was designed to examine the types of memories that adolescent girls have, how the brain recalls these memories, and how these processes might be influenced by depression.
To be eligible, daughters had to be between 12 and 16 years old, English-speaking, right-handed, not claustrophobic, free of bodily metal (except dental fillings) and other contraindications for MRI, living with their biological mother, and have no current or past history of any Diagnostic and Statistical Manual-IV (DSM-IV) Axis I affective disorder. We focused on young women in this age group because it is a critical period when risk for MDD increases significantly but before most adolescent females experience their first MDE (Angold et al., 1998). In addition, daughters must not have had any recent alcohol or substance use or dependence, not have been pregnant as verified with a pregnancy kit, and not have had any history of head trauma or a learning disability. Finally, daughters had to be free of factors that are known to influence inflammation, including: past or current inflammatory illness, major sleep disturbance, tobacco use, prescription drug use, excessive caffeine use (i.e., >8 beverages per day), or a body mass index of ≥30 (O'Connor et al., 2009).
Procedure
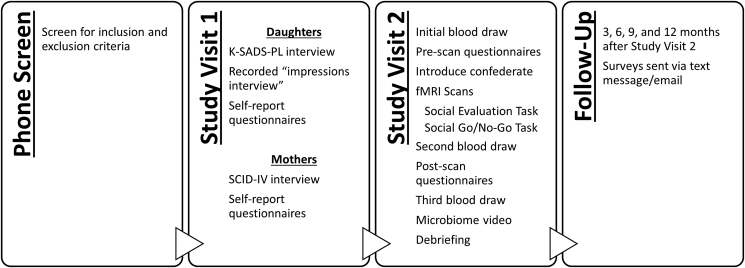
Data collection involved an initial phone screen, two in-person study visits, and four online follow-up assessments (see Fig. 1). All procedures were pre-approved by the Institutional Review Board at UCLA and are described below.
Fig. 1.
The four stages of data collection and the procedures that took place at each time point.
Initial Phone Screen. Mothers and daughters who expressed interest in the study first participated in a phone screen that primarily involved the adolescents’ mothers. The purpose was threefold: (a) describe the study to the mothers and answer any questions about the study procedures, (b) determine whether the mothers and their biological daughters would be likely to meet all of the inclusion and exclusion criteria, and (c) among those believed to be eligible (i.e., those who had not already met any exclusion criteria), schedule a 1.5-hour intake session with both the mothers and their daughters.
Study Visit One (Intake Session). During the intake session, mothers and daughters were given an overview of the study, including a detailed summary of the study procedures and a description of the risks and benefits, followed by an opportunity to ask questions. After consent and assent were obtained, mothers and daughters were separately screened by trained diagnostic interviewers to ensure that they met the inclusion and exclusion criteria, and to determine their MDD risk group.
Each daughter's diagnostic status was evaluated using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL; Kaufman et al., 1997). The K-SADS-PL comprehensively assessed daughters' depressive symptoms and also screened for mania, psychotic disorders, generalized anxiety disorder, panic disorder, and eating disorders. Daughters with a lifetime history of any of these disorders were excluded.
Each mother's diagnostic status was assessed using the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1995) modules for mood episodes, psychotic screening, mood disorders, substance use disorders, anxiety disorders, obsessive-compulsive and related disorders, and trauma and stressor-related disorders. MDD risk status was then determined based on the mother's diagnostic status. Adolescent females with no current or past history of any DSM-IV Axis I affective disorder, but who had a biological mother with a history of MDD, were categorized as high risk, and adolescent females with no personal or maternal history of any Axis I disorder were categorized as low risk. Low-risk mothers could not have a lifetime history of any assessed disorder. High-risk mothers, in turn, must have had a lifetime history of at least one MDE and were allowed to have co-morbid affective diagnoses, given the very high co-morbidity rates between anxiety and depression (Kessler et al., 2005). To maintain diagnostic fidelity, G.M.S. oversaw weekly diagnostic training meetings and independently evaluated a random selection of 25% of cases from the high- and low-risk groups (κ = 1.0).
Once the diagnostic interviews were completed, if the diagnostic inclusion and exclusion criteria were met, mothers and daughters completed self-report questionnaires assessing their demographics, psychiatric symptoms, and trait characteristics (see Table 1 and Table 2).
Table 1.
Daughter self-report measures and time points for data collection.
| Measures | T1 | T2 | T3 | T4 | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | ✓ | |||||
| Racial/Ethnic Background | ✓ | |||||
| Subjective SES (SSS; Goodman et al., 2007) | ✓ | |||||
| Pubertal Status (Tanner and Davies, 1985) | ✓ | |||||
| Recent Health Experiences Questionnaire | ✓ | |||||
| Health Information | ✓ | |||||
| Perinatal Health – Maternal Report | ||||||
| Mental Health Status | ||||||
| Depression (MFQ; Wood et al., 1995) | ✓ | ✓ | ||||
| Depression – Maternal Report (MFQ; Wood et al., 1995) | ✓ | |||||
| Anxiety (SCARED; Birmaher et al., 1997) | ✓ | ✓ | ||||
| Social Phobia (SPIN; Connor et al., 2000) | ✓ | ✓ | ||||
| Automatic Thoughts (Hollon and Kendall, 1980) | ✓ | |||||
| Trait Characteristics | ||||||
| Social Support (Cutrona and Russell, 1987) | ✓ | |||||
| Loneliness (UCLA-LS; Russell et al., 1980) | ✓ | |||||
| Parental Bonding (Parker et al., 1979) | ✓ | |||||
| Dysfunctional Attitudes (Weissman, 1979) | ✓ | ✓ | ✓ | ✓ | ||
| Hopelessness (Spirito et al., 1988) | ✓ | |||||
| Rumination (Treynor et al., 2003) | ✓ | |||||
| Self-Esteem (RSE; Rosenberg, 1965) | ✓ | |||||
| Impulsivity (UPPS; Whiteside et al., 2005) | ✓ | |||||
| Emotion Regulation (ERQ-CA; Gullone and Taffe, 2012) | ✓ | |||||
| Implicit Theory (Levy et al., 1998) | ✓ | |||||
| Health Risk Behaviors (e.g., Substance Use, Sexual History) | ✓ | |||||
| State Measurements | ||||||
| Positive and Negative Affect Schedule (PANAS; Thompson, 2007) | ✓ | ✓ | ||||
| Shame & Guilt (SSGS; Marschall et al., 1994) | ✓ | ✓ | ||||
| Self-Esteem (CTS; Heatherton and Polivy, 1991) | ✓ | ✓ | ||||
| Social Disconnection (Eisenberger et al., 2010) | ✓ | ✓ | ||||
| Profile of Mood States (POMS-SF; Curran et al., 1995) | ✓ | ✓ | ||||
| Social Evaluation and Rejection (Muscatell et al., 2015) | ✓ | ✓ | ||||
| Stress | ||||||
| Stress and Adversity Inventory for Adolescents (Adolescent STRAIN; Slavich et al., 2019) | ✓ | |||||
| Stress Mindset (Crum et al., 2013) | ✓ | ✓ | ||||
| Perceived Stress (Cohen et al., 1983) | ✓ | |||||
Note. T1 = Intake Session; T2 = Experimental (fMRI) Session, pre-Social Evaluation Task; T3 = Experimental (fMRI) Session, post-Social Evaluation Task; T4 = Follow-up surveys administered at 3, 6, 9, and 12 months following T2 (i.e., the Experimental fMRI Session).
Table 2.
Mother self-report measures and time points for data collection.
| Measures | T1 | FU |
|---|---|---|
| Demographics | ||
| Age | ✓ | |
| Racial/Ethnic Background | ✓ | |
| Subjective SES (SSS; Goodman et al., 2007) | ✓ | |
| Family Income | ✓ | |
| Maternal Education | ✓ | |
| Mental Health Status | ||
| Depression (BDI; (Beck et al., 1996) | ✓ | |
| Anxiety (STAI; (Spielberger et al., 1983) | ✓ | |
| Trait Characteristics | ||
| Social Support (Cutrona and Russell, 1987) | ✓ | |
| Loneliness (UCLA-LS; Russell et al., 1980) | ✓ | |
| Dysfunctional Attitudes (Weissman, 1979) | ✓ | |
| Rumination (Treynor et al., 2003) | ✓ | |
| Self-Esteem (RSE; Rosenberg, 1965) | ✓ | |
| Parental Affection in Childhood (Rossi, 2001) | ✓ | |
| Stress | ||
| Stress and Adversity Inventory for Adults (Adult STRAIN; Slavich and Shields, 2018) | ✓ | |
Note. T1 = Intake Session; FU = Online post-intake follow-up. Maternal questionnaires other than the STRAIN were completed during the intake session. The Adult STRAIN assessing mothers' lifetime stressor exposure was administered online following the intake session, and family income and maternal education status were assessed by mothers' online self-report following study completion.
Next, daughters completed a 10-minute video-recorded “impressions interview” (modified from Muscatell et al., 2015), in which an interviewer asked the daughter 33 questions about themselves in the absence of their mother. As shown in Table 3, the interview focused on the daughter's opinions, feelings, and memories from childhood, and was later used in the Social Evaluation Task. The interview was designed to have a conversational feel and included personally relevant questions such as: “What is your favorite hobby?“, “What are you most afraid of?“, and “What qualities do you value most in a friendship?“.
Table 3.
Interview questions assessing daughters’ interests, opinions, values, & childhood memories.
| 1. What is your favorite hobby? |
| 2. What city in the world would you most want to live in? |
| 3. What do you like to do to relax? |
| 4. How much money do you want to earn in your life? |
| 5. What are your favorite television shows? |
| 6. Do you like your smile? |
| 7. Do you dream frequently? |
| 8. What are you most proud of? |
| 9. Who do you most admire? |
| 10. What is your greatest shortcoming? |
| 11. What do you think people like about you? |
| 12. Do you like being in charge? |
| 13. What is the most inspiring movie you have seen? |
| 14. What do you like to eat? |
| 15. What qualities do you look for in a boyfriend or girlfriend? |
| 16. If you didn't have to have a job in life, what would you do? |
| 17. When are you most likely to procrastinate? |
| 18. What place in the world would you most like to travel to? |
| 19. What are you most afraid of? |
| 20. How do you define success? |
| 21. What is your best quality? |
| 22. How do you feel about cheating? |
| 23. What do you do for fun? |
| 24. How competitive are you? |
| 25. When or if you are in a relationship, are you a good relationship partner? |
| 26. How important is education to you? |
| 27. How important is money to you? |
| 28. Who are your heroes? |
| 29. What makes you happy? |
| 30. What qualities do you value most in a friendship? |
| 31. Now, I would like you to think about your past. When you think about your past, what is the first memory that comes to mind that is not positive or negative, but just neutral. This could be a memory of something you did … or a place you visited … |
| 32. Now I would like you to think about a time in your life when you felt really bad or sad because of something that happened. Tell me about that memory. |
| 33. Now I would like you to think about a time in your life when you felt really good or happy because of something that happened. Tell me about that memory. |
Finally, daughters were asked to provide three photos of female peers in their social network whom they like and three photos of female peers whom they dislike, which were in turn used in a personally relevant Social Go/No-Go Task (see below).
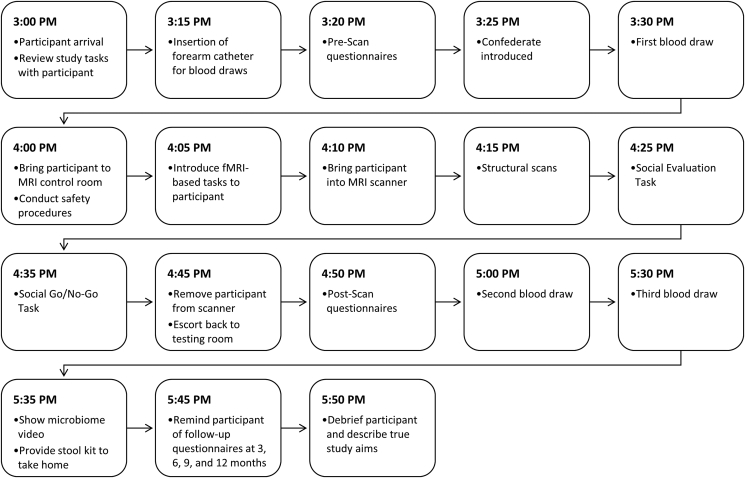
Study Visit Two (Experimental Session). After completing all of the study procedures for the intake session (above), daughters and their mothers were scheduled for a second three-hour experimental session, which generally took place within one month of the intake session (median = 26.5 days). The experimental session involved fMRI-based tasks and blood draws, which occurred once before and twice after the MRI scan. This second session also included an assessment of mothers' and daughters' lifetime stressor exposure, as well as questionnaires assessing daughters’ responses to the fMRI tasks (see Table 1 and Fig. 2).
Fig. 2.
A sample timeline of the events for Study Visit Two (i.e., the experimental session).
Upon arriving for the experimental session, daughters were taken to a private testing room that was adjacent to the MRI scanner to be prepped for their blood draw. For the blood draws, a nurse from the UCLA Clinical and Translational Research Center (CTRC) inserted an MRI-safe indwelling catheter into the participant's non-dominant forearm. Participants were given 15 minutes to acclimate to the catheter, after which their baseline blood sample was drawn.
In the time between needle insertion and the start of the MRI scan, daughters completed questionnaires assessing their current mood states and perceptions (see Table 1). The entire set of surveys took approximately 10–15 minutes to complete. While completing their questionnaires, daughters were introduced to “another participant” (who was, in reality, a confederate) whom they were told was participating in a related study at the same time. The confederate was always a female, college-aged research assistant who dressed and acted like an older adolescent to enhance the believability that she was a participant and to create an experience of social-evaluative threat for the participant.
Approximately 1 hour after the start of the experimental session, both the participant and the confederate were taken to the MRI scanner control room. Here, the participant and confederate were introduced to the two main tasks of the study: a social “impressions task” (Eisenberger et al., 2011)—herein referred to as the Social Evaluation Task—and a Social Go/No-Go Task (see below). At the conclusion of the MRI scan, participants were escorted back to the testing room, where they completed a second questionnaire packet (see Table 1) designed to assess changes in their current mood state and perceptions.
Participants also provided post-scan blood samples at 35 and 65 minutes after start of the Social Evaluation Task (see Table 4 for details on the timing of the three blood draws). After the final blood sample was obtained, participants were shown a brief informational video on the microbiome (National Public Radio, “The Invisible Universe of the Human Microbiome”) and were given a stool collection kit with instructions on how to use the kit at home.
Table 4.
Timing of neural and biological assessments during the experimental session.
| Assessments | Time Point | ||
|---|---|---|---|
| NeuralStructure and Function | 45 min Scan Time | ||
| Strctural MRI Scans | ✓ | ||
| 10-min Social Evaluation Task | ✓ | ||
| 9-min Social Go/No-Go Task | ✓ | ||
| Blood Drawsfor Immunologic and Genomic Analysis | −55 min | +35 min | +65 min |
| 3 mL, EDTA Vacutainer Tube (e.g., TNF-α, IL-1β, IL-6) | ✓ | ✓ | ✓ |
| 2.5 mL, PAXgene Blood RNA Tube (e.g., gene expression) | ✓ | ✓ | ✓ |
| 8.5 mL, PAXgene Blood DNA Tube (e.g., telomere length) | ✓ | ||
| 8 mL, BD Vacutainer CPT Tube (e.g., cellular analysis) | ✓ | ||
| Gut Microbiome | Post-Visit | ||
| Stool Sample | ✓ | ||
Note. Blood draws took place approximately 55 minutes before the start of the Social Evaluation Task (i.e., 30 minutes prior to the 45-minute scan time), and 35 and 65 minutes after the start of the task (i.e., 15 and 45 minutes, respectively, after the end of scanning). The Social Evaluation Task began approximately 25 minutes into the 45-minute scan time, which consisted of safety procedures (e.g., metal detection, measurement of weight), an explanation of the two fMRI tasks, setting-up the participant in the scanner, structural scans, the 10-minute Social Evaluation Task, the 9-minute Social Go/No-Go Task, and the removal of the participant from the scanner. The Social Evaluation task was introduced at approximately minute 5 of the scan session and was begun at around minute 25.
After all study procedures were completed, participants were debriefed using a script that drew from the work of Ross et al. (1975) on proper debriefing procedures for social psychological experiments involving deception. The primary goals of the debriefing session were to educate participants about the research process, inform participants of the true aims of the experiment, and describe why deception is sometimes necessary in psychology research. Throughout this 15-minute debriefing session, the overarching goal was to make participants feel fully informed and an integral part of the research process. Therefore, daughters were encouraged to ask any questions they had about the study before being asked not to discuss the study with friends or peers.
Follow-up Surveys. Participants were contacted by text message or email at 3 months, 6 months, 9 months, and 12 months following the experimental session and asked to complete a brief online survey assessing their current socioemotional and mental health status (see Table 1).
Social Evaluation Task
As described above, we experimentally induced feelings of social evaluation using a previously validated social impressions task (i.e., “Social Evaluation Task”; Eisenberger et al., 2011; Muscatell et al., 2015). Prior research with young-adult females has shown that this task engages the amygdala and leads to significant increases in IL-6 (Muscatell et al., 2015), which is a key inflammatory cytokine involved in the acute phase response (Irwin and Slavich, 2017; Slavich, 2020a). This task has also been shown to lead to significant increases in self-reported feelings of social evaluation and rejection (Dedovic et al., 2016), which are common features of depression in youth (Platt et al., 2013).
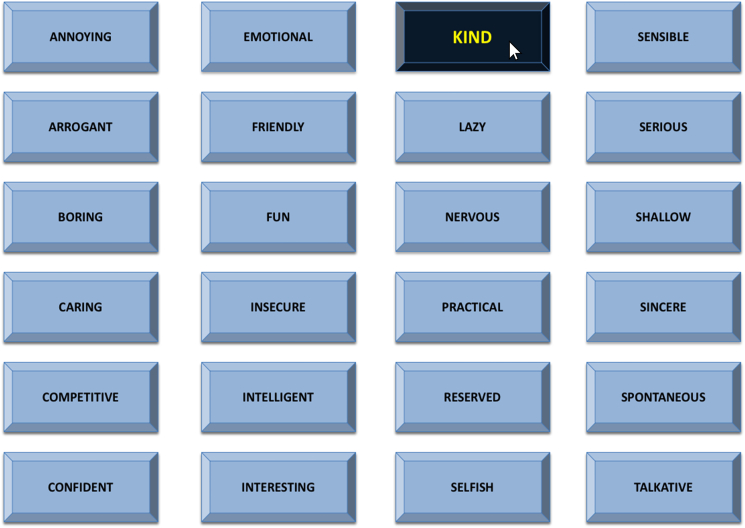
While in the MRI scanner control room, participants were given instructions for the Social Evaluation Task in the presence of the confederate. First, participants were reminded of their 10-minute recorded interview from the intake session and shown a five-second video clip of themselves participating in the interview. Participants were then told that the “other participant” would be watching and judging their video by clicking on 1 of 24 potential adjectives (one-third positive: e.g., “interesting”; one-third neutral: “practical”; one-third negative: “annoying”) displayed on a grid every 10 seconds (see Fig. 3), which the participant would be able to see in real time in the MRI scanner. After being given instructions, both the participant and confederate were asked if they had any questions about the task, and the confederate always asked the clarifying question “how often am I supposed to give a rating?” to enhance the believability of the situation. In reality, all participants watched the same pre-recorded video in which the socially evaluative adjectives were “selected” in pseudorandom order, with no more than two similarly valenced words clicked consecutively. During the task, participants indicated how they felt every time an adjective was clicked using a response box, with possible responses ranging from 1 (very bad) to 4 (very good). The Social Evaluation Task lasted for 10 minutes.
Fig. 3.
A screenshot of the Social Evaluation Task that participants completed in the fMRI scanner. Participants viewed this grid of 24 adjectives. Approximately every 10 seconds, an adjective was “pressed” by a mouse cursor that was supposedly controlled by the “other participant.” In reality, this was a pre-recorded video, and all participants viewed the same video and therefore received the exact same social feedback. Depicted is an example of the positive word “kind” being pressed.
Social Go/No-Go Task
In addition to the Social Evaluation Task, participants completed an adapted Social Go/No-Go Task. Affective go/no-go tasks have been shown to reliably engage limbic regions and are used to study stress-related functional connectivity in adolescents (Hare et al., 2008, Tottenham et al., 2011). We sought to expand this paradigm from emotional expressions of strangers (i.e., the traditional affective go/no-go task) to the peer group context to examine the influence of social information in biasing performance during a go/no-go task. Therefore, prior to their arrival at the scan session, daughters provided three photos of female peers they liked (i.e., “friendly faces”) and three photos of female peers they disliked (i.e., “unfriendly faces”), drawn from their social network. An additional three age- and race-matched faces were selected by the research team for each participant (i.e., “unknown faces”). All photos were gray-scaled and cropped to only include the face. The task thus consisted of three condition blocks: friendly, unfriendly, and unknown. The order of the conditions was randomized across participants. Prior to each block, participants were directed to press a button on the response box as fast as they could when they saw a face that matched that round's condition (“Go” trials) and to not press for the other types of faces (“No-Go” trials). Faces were presented on screen for 500 ms regardless of whether a response was made; between each trial, a fixation cross was displayed for 2000 to 4500 ms. Each block included 27 Go trials and 10 No-Go trials; photos were presented in a pseudorandom order.
fMRI image acquisition & processing
Imaging data were acquired using a Prisma 3.0 T whole-body scanner (Siemens Medical Systems, Iselin, New Jersey) at the Staglin One Mind Center for Cognitive Neuroscience at UCLA. High resolution T1-weighted structural images were acquired using a magnetized prepared rapid acquisition gradient echo (MPRAGE) sequence containing 1.1 mm isotropic voxels, TR/TE/flip angle = 2300 ms/2.95 ms/9°, FOV = 270 mm2, 176 slices. Blood oxygenation level-dependent (BOLD) functional images were acquired containing 3 mm isotropic voxels, TR/TE/flip angle = 2000 ms/34 ms/76°, FOV = 208 mm2, 48 slices. The number of volumes collected for the Social Evaluation Task varied per participant because the end of the task did not coincide exactly with the end of the scanner sequence; the Social Go/No-Go Task was 87 volumes per condition.
Blood draws & assays
Blood was drawn once prior to the Social Evaluation Task (i.e., a baseline blood draw approximately 55 minutes before the start of the Social Evaluation Task) and twice following the task (i.e., at approximately 35 and 65 minutes after the Social Evaluation Task began). At each time point, 3 mL of blood was drawn into an EDTA Vacutainer Tube for the subsequent quantification of participants’ cytokine and β-endorphin levels. These samples were immediately placed on ice and then transferred by the end of each study session to the UCLA Center for Pathology Research Services, which centrifuged the samples for 15 minutes at 3000 RPMs. Extracted plasma was divided into 1 mL aliquots and frozen at −80 °C until assays were performed by the Olvera Alvarez Lab.
Plasma concentrations of TNF-α, IL-1β, and IL-6 were measured in duplicate using the MILLIPLEX MAP Human High Sensitivity Cytokine panel (catalog # HSCYTMAG-60SK); β-endorphin, in turn, was measured using the Human Neuropeptide Panel (catalog #HNPMAG-35K) from Luminex Corporation (Austin, USA). Samples were thawed for 45 minutes, placed in a vortex for 1 minute, and centrifuged for 10 minutes at 10,000 rpm. For β-endorphin analysis, acetonitrile precipitation was used for sample extraction on 250 μL of plasma. 96-well plates were prepared using an automatic liquid handler (epMotion®5070; Eppendorf, Enfield, CT) programmed to carry out the immunoassay procedure prescribed by the manufacturer. Plates were read on a Luminex 200 analyzer running xPOTENT® Ver 3.1 software (Luminex Corporation, Austin, TX). The Median Fluorescent Intensity data produced from xPONENT® were used to calculate analyte concentrations using a best curve-fitting method in MILLIPLEX Analyst software Ver 5.1 (Vigene Tech, Inc., Carlisle, MA). Analyte concentrations were reported as pg/mL and had the following lower detection limits: TNF-α (0.16 pg/mL), IL-1β (0.14 pg/mL), IL-6 (0.11 pg/mL), β-endorphin (85 pg/mL). All controls were within the expected range. The inter-assay CVs for plasma were 6.74%, 7.65%, 6.17%, and 7.40% for TNF-α, IL-1β, IL-6, and β-endorphin, respectively.
In addition, at each time point, 2.5 mL of blood was drawn into a PAXgene Blood RNA Tube to test for social stress-induced changes in gene expression using genome-wide transcriptional profiling. By the end of each study session, samples from all 3 time points were transferred to the UCLA Center for Pathology Research Services, where blood tubes were frozen at −80 °C. Samples were then transferred to the UCLA Social Genomics Core Laboratory where total RNA was extracted (RNeasy; Qiagen, Valencia, CA), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent Bioanalyzer), converted to barcoded cDNA (Lexogen QuantSeq 3′ FWD), and sequenced on an Illumina HiSeq4000 system (Illumina, San Diego, CA) in the UCLA Neuroscience Genomics Core Laboratory, all following the manufacturer's standard protocols for this workflow. Assays targeted >10 million 65-nt single-stranded sequence reads for each sample, each of which was mapped to the reference human transcriptome using the STAR aligner and quantified as gene transcripts per million mapped reads. Log2-transformed gene expression data were analyzed by standard linear statistical models to identify differentially expressed genes, which can serve as inputs into higher-order bioinformatics analyses to quantify differences in inflammatory activity as indicated by (a) a pre-specified composite score for pro-inflammatory genes, (b) TELiS promoter sequence-based bioinformatics analyses assessing the activity of pro-inflammatory transcription factors (e.g., NF-κB, AP-1), and (c) Transcript Origin Analyses quantifying the relative contribution of classical monocytes, non-classical monocytes, and neutrophil granulocytes to the observed differences in inflammatory gene expression, with all analyses conducted as previously described (see Cole et al., 2020).
Finally, at the baseline only, two additional tubes of blood were collected from participants. For the first blood sample, 8.5 mL of blood was drawn into a PAXgene Blood DNA Tube, which was then transported to the UCLA Cousins Center for Psychoneuroimmunology Inflammatory Biology Core Laboratory following each study visit and immediately stored at −80 °C. DNA were then extracted and used for the subsequent quantification of participants’ leukocyte telomere length as a marker of biological aging following standard real time qPCR methods, as published previously (Carroll et al., 2016, 2020; Robles et al., 2016). Briefly, the telomere assay was performed by the UCLA Aging Biology & Behavior Laboratory using a standard curve method where PCR products are generated for the telomere gene and hemoglobin gene, and cycle threshold values are then plotted on a standard curve of human genomic DNA to estimate ng/microliter concentration values. Telomere length values were expressed as the ratio of the estimated concentration generated for the telomere gene (T) divided by the hemoglobin single (S) copy gene = (T/S). Samples were run in triplicate and assessed for reliability.
For the second blood sample, 8 mL of blood was drawn into a BD Vacutainer CPT Tube (BD Biosciences, San Jose, CA). PBMCs were then isolated by density gradient centrifugation, separated into three aliquots. The first aliquot was processed for telomerase extraction, the second was placed in a RPMI with L-glutamine and 10% FBS solution, frozen first in −20°C to avoid shock, and then transferred to liquid nitrogen for viable cell preservation and long-term storage for use in future studies using these samples. The third aliquot of PBMCs was placed in an RLT buffer plus b-2-mercaptoethanol (2 ME) solution to preserve RNA for potential future use.
Gut microbiome
Stool kits given to each participant at the end of the experimental session included a stool collection vessel for affixing to the toilet, a 101 mm tube containing 5 mL 70% ethanol, a biohazard bag, gloves, a return shipping container, and a pre-paid FedEx label. Participants were instructed to produce a stool sample and aliquot a small portion using the scooper affixed to the lid of the 101 mm tube. The tube was then placed in the biohazard bag and the bag placed into the return shipping box addressed to the Devkota Lab at Cedars-Sinai Medical Center. Participants were instructed to ship their sample within 24 hours and to keep their sample at room temperature until such time. Samples were de-identified and analysis was blinded.
DNA was extracted from 0.5g stool by the Devkota Lab using the Dneasy PowerSoil Kit (QIAGEN, Germantown, MD). Samples were added to lysis tubes with 400 μg proteinase K and homogenized at 5 m/s for 2 min. This was followed by heat treatment at 95 °C for 15 minutes and centrifugation at 16,000×g for 5 minutes at 4 °C. Supernatant was transferred to a new tube and reserved for later use. 300 μL fresh lysis buffer was added back to the lysis tube for a second round of bead beating and heating. Supernatant from both rounds of cell lysis were pooled for DNA isolation as per the manufacturer's protocol. DNA extracts were then submitted to the UCLA Microbiome Center Core for bacterial sequencing of the V4 16S rRNA region on an Illumina HiSeq.
R packages were used to process and analyze 16S rRNA sequencing. Paired-end reads were quality filtered, trimmed, merged, denoised, chimera filtered, and binned into sequence variants using DADA2 v1.5.8 (Callahan et al., 2016). There was an average of 85,473 reads per sample after pre-processing and filtering. Samples with less than 1000 reads were removed from analysis. 16S sequence variants were aligned to the Greengenes reference database v13.8 and taxonomically assigned with a minimum bootstrap confidence level of 80. Sequence variants unresolved for taxonomic classification and singletons were omitted from further analyses. Samples were rarefied to the minimum read count to account for uneven sampling effort. Phyloseq v1.22.3 (McMurdie and Holmes, 2013) was used to assess α and β diversity measures. Bray-Curtis distance between samples were visualized by principal coordinate analysis.
Data analysis
The sample size for the present study was based on prior research using the Social Evaluation Task (Eisenberger et al., 2011) and simulations of fMRI data from standard block designs (Mumford and Nichols, 2008), which suggest that to achieve a one-sided type-I error rate of 0.005 and power of 0.80 for a task with 15 repetitions of each condition, a sample with 18–19 participants per group is required. When factoring in unusable fMRI data for ≤3 participants/group due to head motion, we continued recruiting until we had at least 22 participants per diagnostic group.
Participant characteristics (e.g., trait impulsivity, lifetime stress exposure, demographics) were assessed so these variables could be used as predictors or covariates in relevant analyses. The success of the social rejection induction will be verified by analyzing pre- to post-induction changes in relevant psychological variables, such as self-reported social disconnection and depressed mood. We expect significant increases in these social-emotional variables (e.g., greater social disconnection & depressed mood) for all participants, as well as potential differences between the high- and low-risk girls, with high-risk girls exhibiting worse outcomes. These analyses will be conducted with p < .05 as the criterion for significance.
Neuroimaging data will be processed and analyzed using SPM 12, and functional connectivity with CONN Toolbox v.19c. Pre-processing will include image realignment to correct for head motion, normalization into MNI space (resampled at 3 × 3x3mm), and spatial smoothing using an 8 mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. General linear models will be established for each participant.
For the Social Evaluation Task, the presentation of each feedback word and its on-screen duration will be modeled as an event and convolved with a canonical hemodynamic response function. Our regressor-of-interest will code for the type of feedback presented (i.e., positive, neutral, negative). For each model, the time-series will be high-pass filtered using a 128hz function (or, in functional connectivity analyses, band-pass filtered to remove frequencies below 0.008 Hz or above 0.09 Hz), and serial autocorrelation will be modeled as an AR (1) process. Following estimation, linear contrasts will be computed for each participant to compare BOLD signal during the negative vs. neutral feedback trials. Contrast images for each participant will then be entered into random effect analyses at the group level for statistical inference. Analyses testing for potential group differences will first be evaluated at p < .05 using a priori, anatomically defined ROIs in the threat network (Eisenberger and Cole, 2012; Irwin and Cole, 2011). Next, coupling between these nodes will be examined using functional connectivity analyses (Rissman et al., 2010; Gee et al., 2013; Toga et al., 2006). Finally, multi-voxel pattern analyses (Dosenbach et al., 2010; Turk-Browne, 2013; Shirer et al., 2012) will be used to determine whole-brain functional connectivity patterns that distinguish participants based on MDD risk status (i.e., high vs. low). All analyses will control for age and adjusted for multiple comparisons (FDR correction corresponding to p < .05) when relevant.
To examine impairment of inhibitory control while processing socially unfriendly vs. friendly faces, we will assess errors of commission in trials where unfriendly faces represent no-go stimuli and compare them to errors of commission in trials where unknown faces represent no-go stimuli. Similarly, we will assess errors of commission in trials where friendly faces represent no-go stimuli and compare them to errors of commission in trials where unknown faces represent no-go stimuli. We will calculate mean errors of commission on each trial, and we expect mean errors of commission to be greater in response to negative social stimuli than unknown stimuli. Moreover, we expect this social-related impairment to be greater for high-risk vs. low-risk girls.
To investigate whether high-risk youth exhibit greater inflammatory responses to social stress than their low-risk counterparts, a series of growth-curve models with hierarchical linear modeling will be estimated. In the within-person (Level 1) models, cytokine level trajectories will be estimated as a function of time and a residual term. These models will yield a series of person-specific intercepts reflecting cytokine levels at baseline and person-specific slopes reflecting rates of change in TNF-α, IL-1β, and IL-6 over the study. In the between-person (Level 2) models, intercept and slope values will be estimated for each participant as a function of relevant covariates (i.e., age, ethnicity, BMI, socioeconomic status) and MDD Risk Group status (high risk vs. low risk). For the gene expression data, genes that show a ≥20% increase in expression from pre- to post-stress will be identified, and differential gene expression scores for each individual will be computed.
To test associations between neural and cytokine and genomic responding to stress for high- and low-risk adolescents, additional growth-curve models will be estimated. However, ROI and ROI × Risk Group predictors will be added at Level 2. Next, to examine associations between the functional connectivity and inflammatory responding data, multiple linear regression analyses will be conducted with the connectivity correlations as predictor variables, and the cytokine and gene expression pre- to post-stress difference scores as outcome variables. Connectivity Strength × Risk Group interaction terms will be included in these models to test for functional connectivity-inflammatory responding differences between the high- and low-risk groups.
To test for potential differences in microbial factors (i.e., gut microbiota composition & diversity), DESeq2 v 1.24.0 (Love et al., 2014) with Benjamini-Hochberg correction will be used to identify bacterial groups that are differentially abundant between high- and low-risk daughters. Pearson and Spearman correlations will be used to examine the association between bacterial relative abundances and participants’ psychosocial, emotional, and clinical characteristics.
Finally, exploratory analyses will be conducted to evaluate potential moderating factors that could influence the associations described above, such as lifetime stress exposure (Mayer et al., 2019; Slavich et al., 2019; Stewart et al., 2019) and relevant social-psychological traits (e.g., emotion regulation, social support), as well as how group differences may relate to health-relevant markers such as telomere length.
Discussion
Although an abundance of research has demonstrated that major life stressors, especially those involving social rejection, increase risk for depression and depression-related health problems (Slavich, 2016b), researchers still lack a clear mechanistic understanding of how social adversity induces biological changes that lead to these conditions. The overarching aim of this study is to help advance research on this important topic by leveraging state-of-the-art neuroimaging, immunologic, and genomic methods and data analysis techniques to characterize adolescents’ psychological, neural, inflammatory, and genomic responses to an ecologically valid social stressor and, moreover, to examine how these responses differ for youth at high- vs. low-risk for depression. We also seek to better understand a variety of psychosocial and biological factors that may moderate these social-biological-clinical associations.
Although this work could be carried out using many different populations, we believe that adolescent girls represent a logical and important starting place for at least two major reasons. First, although depression is a highly burdensome disorder in general, females disproportionately suffer given their much greater likelihood of experiencing MDD, and this is especially true of youth who grow up with a depressed mother. Second, studying teenage adolescents who are at risk for—but have not yet developed—MDD means that the resulting discoveries will help elucidate pre-clinical disease processes that could prospectively predict the initial emergence of disease and, in addition, potentially be targeted to reduce risk for depression and other burdensome, immune-related disorders that frequently co-occur with MDD.
The organizing framework for this study comes from the Social Signal Transduction Theory of Depression (Slavich and Irwin, 2014; Slavich and Sacher, 2019; see also Quinn et al., 2020; Seiler et al., 2020; Slavich et al., 2020). Briefly, this formulation describes how experiences of social stress and rejection get represented by the brain, and how the brain in turn governs physiologic, molecular, and genomic changes that can promote depressive symptoms, especially for persons at risk for MDD. By comprehensively assessing the psychological, neural, inflammatory, microbial, and genomic mechanisms that underlie risk for depression in adolescent females, we aim not only to test hypotheses derived from this theory but to better understand neurocognitive and immunologic dynamics that are activated by stress, and how the activity and connectivity of these systems differ as a function of risk for depression and a selection of potential moderating factors, including relevant psychological traits and the gut microbiome. Most importantly, by investigating these links, we strive to inform the development of new strategies that might one day help reduce risk for depression and related disorders using precision medicine or similar approaches (Schüssler-Fiorenza Rose et al., 2019; Williams and Hack, 2020).
Looking forward, our hope is that by pursuing this line of research, we may help develop a more comprehensive understanding of the pathophysiology of depression, one of the most common and costly of all disorders worldwide (Ferrari et al., 2013). More broadly, this work may contribute to the development of new theories that help explain the social and biological bases of human health and behavior (Slavich, 2020b). Presently, we have evidence that psychosocial interventions can reduce inflammatory processes that degrade health (Shields et al., 2020). However, much remains unknown about how experiences of the social world affect the brain and body, and how interventions designed to improve health can be personally tailored to maximally reduce disease risk and improve wellbeing over the life course.
Data availability
Data will be made available and can be requested by contacting the corresponding author.
Funding
This research was supported by a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant 23958 from the Brain & Behavior Research Foundation, and National Institutes of Health (NIH) grant K08 MH103443 to GMS. SS was supported by National Science Foundation GRFP Grant DGE-2034835. The UCLA CTRC is supported by NIH grant UL1 TR001881. These organizations were not involved with the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of this manuscript; or decision to submit this article for publication.
Declaration of competing interest
The authors declare no conflicts of interest with regard to this work.
Acknowledgments
We thank the mothers and daughters who participated in this study. We also thank the many students, trainees, and fellows who helped with various aspects of this study, including: Jacob Allely, Sammy Benavidez, Kaitlyn Breiner, Ashley Chipoletti, Kelly Costa, Desiree Delavary, Micah Dombroe, Kishan Ghadiya, Kirsten Gimse, Connie Ha, Marzia Hazara, Kean Hsu, Ashley Huynh, Mark Libowitz, Roman Liccini, Kristy Lin, Abigail Looi, Oria Mimi Lu, Kaivalya Molugu, Riya Mukhopadhyay, Rachel Ogata, Kelly Sun, Evelyn Valencia, Ruben Valentin, Kevin Walsh, and Hilary Wilson. Finally, we thank Keely Muscatell for providing the Social Evaluation Task, Nim Tottenham for providing the adapted Social Go/No-Go Task, and several centers at UCLA for supporting the study including the Cousins Center for Psychoneuroimmunology, Staglin One Mind Center for Cognitive Neuroscience, UCLA Clinical and Translational Research Center (CTRC), UCLA Neuroscience Genomics Core, and Center for Pathology Research Services.
References
- Allan S.M., Rothwell N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Worthman C.M. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol. Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Antoni M.H., Lutgendorf S.K., Cole S.W., Dhabhar F.S., Sephton S.E., McDonald P.G., Stefanek M., Sood A.K. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Canc. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K., Epel E., Wolkowitz O.M., Prather A.A., Puterman E., Dhabhar F.S. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav. Immun. 2012;26:346–352. doi: 10.1016/j.bbi.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Manual for the Beck Depression Inventory-II. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. Neural mechanisms underlying selective attention to threat. Ann. N. Y. Acad. Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Boyce W.T., Sokolowski M.B., Robinson G.E. Toward a new biology of social adversity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17143–17148. doi: 10.1073/pnas.1121264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.E., Low C.A., Prather A.A., Cohen S., Fury J.M., Ross D.C., Marsland A.L. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav. Immun. 2011;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.E., Esquivel S., Goldberg A., Seeman T.E., Effros R.B., Dock J., Olmstead R., Breen E.C., Irwin M.R. Insomnia and telomere length in older adults. Sleep. 2016;39:559–564. doi: 10.5665/sleep.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.E., Mahrer N.E., Shalowitz M., Ramey S., Dunkel Schetter C. Prenatal maternal stress prospectively relates to shorter child buccal cell telomere length. Psychoneuroendocrinology. 2020;121:104841. doi: 10.1016/j.psyneuen.2020.104841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cole S.W., Arevalo J.M., Takahashi R., Sloan E.K., Lutgendorf S.K., Sood A.K., Sheridan J.F., Seeman T.E. Computational identification of gene-social environment interaction at the human IL6 locus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W., Shanahan M.J., Gaydosh L., Harris K.M. Population-based RNA profiling in Add Health finds social disparities in inflammatory and antiviral gene regulation to emerge by young adulthood. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:4601–4608. doi: 10.1073/pnas.1821367117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K.M., Davidson J.R., Churchill L.E., Sherwood A., Weisler R.H., FOA E. Psychometric properties of the social phobia inventory (SPIN) Br. J. Psychiatr. 2000;176:379–386. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- Crum A.J., Salovey P., Achor S. Rethinking stress: the role of mindsets in determining the stress response. J. Pers. Soc. Psychol. 2013;104:716–733. doi: 10.1037/a0031201. [DOI] [PubMed] [Google Scholar]
- Curran S.L., Andrykowski M.A., Studts J.L. Short form of the profile of mood states (POMS-SF): psychometric information. Psychol. Assess. 1995;7:80–83. [Google Scholar]
- Cutrona C.E., Russell D.W. The provisions of social relationships and adaptation to stress. Advances in Personal Relationships. 1987;1:37–67. [Google Scholar]
- Danese A., Caspi A., Williams B., Ambler A., Sugden K., Mika J., Werts H., Freeman J., Pariante C.M., Moffitt T.E., Arseneault L. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatr. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K., Slavich G.M., Muscatell K.A., Irwin M.R., Eisenberger N.I. Dorsal anterior cingulate cortex responses to repeated social evaluative feedback in young women with and without a history of depression. Front. Behav. Neurosci. 2016;10:64. doi: 10.3389/fnbeh.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Gable S.L., Irwin M.R., Aziz N., Kemeny M.E. Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychol. Sci. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann L., Cole S., Kumsta R. Stress genomics revisited: gene co-expression analysis identifies molecular signatures associated with childhood adversity. Transl. Psychiatry. 2020;10:1–11. doi: 10.1038/s41398-020-0730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Jr., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Cole S.W. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Taylor S.E., Gable S.L., Hilmert C.J., Lieberman M.D. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Mashal N.M., Irwin M.R. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav. Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Muscatell K.A., Byrne Haltom K.E., Leary M.R. The neural sociometer: brain mechanisms underlying state self-esteem. J. Cognit. Neurosci. 2011;23:3448–3455. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr. Top. Behav. Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Farmer A.E., McGuffin P. Humiliation, loss and other types of life events and difficulties: a comparison of depressed subjects, healthy controls and their siblings. Psychol. Med. 2003;33:1169–1175. doi: 10.1017/s0033291703008419. [DOI] [PubMed] [Google Scholar]
- Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J., Vos T., Whiteford H.A. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer M.B., Gibbon M., Williams J.B.W. Biometrics Research Department, New York State Psychiatric Institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Foster J.A., Neufeld K.A.M. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fülling C., Dinan T.G., Cryan J.F.… Gut microbe to brain signaling: What happens in vagus…. Neuron. 2019;101:998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., Miller A.H., Mantovani A., Weyand C.M., Barzilai N., Goronzy J.J., Rando T.A., Effros R.B., Lucia A., Kleinstreuer N., Slavich G.M. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Sheu L.K. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. 2009;47:922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gong L., Yin Y., He C., Ye Q., Bai F., Yuan Y., Zhang H., Lv L., Zhang H., Xie C., Zhang Z. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J. Psychiatr. Res. 2017;84:9–17. doi: 10.1016/j.jpsychires.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Goodman E., Huang B., Schafer-Kalkhoff T., Adler N.E. Perceived socioeconomic status: a new type of identity that influences adolescents' self-rated health. J. Adolesc. Health. 2007;41:479–487. doi: 10.1016/j.jadohealth.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullone E., Taffe J. The emotion regulation questionnaire for children and adolescents (ERQ–CA): a psychometric evaluation. Psychol. Assess. 2012;24:409–417. doi: 10.1037/a0025777. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatr. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hammen C., Shih J.H., Brennan P.A. Intergenerational transmission of depression: test of an interpersonal stress model in a community sample. J. Consult. Clin. Psychol. 2004;72:511–522. doi: 10.1037/0022-006X.72.3.511. [DOI] [PubMed] [Google Scholar]
- Hankin B.L., Abramson L.Y., Moffitt T.E., Silva P.A., McGee R., Angell K.E. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J. Abnorm. Psychol. 1998;107(1):128. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatr. 2008;63:921–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Polivy J. Development and validation of a scale for measuring stat self-esteem. J. Pers. Soc. Psychol. 1991;60:895–910. [Google Scholar]
- Hollon S.D., Kendall P.C. Cognitive self-statements in depression: development of an automatic thoughts questionnaire. Cognit. Ther. Res. 1980;4:383–395. [Google Scholar]
- Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Cole S.W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R., Slavich G.M. In: Handbook of Psychophysiology. fourth ed. Cacioppo J.T., Tassinary L.G., Berntson G.G., editors. Cambridge University Press; New York: 2017. Psychoneuroimmunology; pp. 377–398. [Google Scholar]
- Joormann J., Cooney R.E., Henry M.L., Gotlib I.H. Neural correlates of automatic mood regulation in girls at high risk for depression. J. Abnorm. Psychol. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Hettema J.M., Butera F., Gardner C.O., Prescott C.A. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch. Gen. Psychiatr. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kennedy D.P., Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Loving T.J., Stowell J.R., Malarkey W.B., Lemeshow S., Dickinson S.L., Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatr. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Korb A.S., Hunter A.M., Cook I.A., Leuchter A.F. Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatr. Res. 2011;192:188–194. doi: 10.1016/j.pscychresns.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E., Berman M.G., Mischel W., Smith E.E., Wager T.D. Social rejection shares somatosensory representations with physical pain. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y.F., Guyer A.E., Tone E.B., Jenness J., Parrish J.M., Pine D.S., Nelson E.E. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. Int. J. Behav. Dev. 2012;36:36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S.R., Stroessner S.J., Dweck C.S. Stereotype formation and endorsement: the role of implicit theories. J. Pers. Soc. Psychol. 1998;74:1421–1436. [Google Scholar]
- Lieb R., Isensee B., Höfler M., Pfister H., Wittchen H.U. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch. Gen. Psychiatr. 2002;59:365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie Z.N., Taylor M.J., Harmer C.J., Cowen P.J., Norbury R. Frontolimbic responses to emotional faces in young people at familial risk of depression. J. Affect. Disord. 2011;130:127–132. doi: 10.1016/j.jad.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Marschall D., Sanftner J., Tangney J.P. George Mason University; Fairfax, VA: 1994. The State Shame and Guilt Scale. [Google Scholar]
- Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J.C., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cognit. Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S.E., Prather A.A., Puterman E., Lin J., Arenander J., Coccia M., Shields G.S., Slavich G.M., Epel E.S. Cumulative lifetime stress exposure and leukocyte telomere length attrition: the unique role of stressor duration and exposure timing. Psychoneuroendocrinology. 2019;104:210–218. doi: 10.1016/j.psyneuen.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer I.H. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol. Bull. 2003;129:674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatr. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Rohleder N., Cole S.W. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom. Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Parker K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Rowe J.B., Eich H., FeldmanHall O., Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Klein R.G., Telzer E.H., Schroth E.A., Mannuzza S., Moulton J.L., 3rd, Guardino M., Masten C.L., McClure-Tone E.B., Fromm S., Blair R.J., Pine D.S., Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatr. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monroe S.M., Rohde P., Seeley J.R., Lewinsohn P.M. Life events and depression in adolescence: relationship loss as a prospective risk factor for first onset of major depressive disorder. J. Abnorm. Psychol. 1999;108:606–614. doi: 10.1037//0021-843x.108.4.606. [DOI] [PubMed] [Google Scholar]
- Mumford J.A., Nichols T.E. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage. 2008;39:261–268. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.L., Slavich G.M., Rohleder N., Miller G.E. Targeted rejection triggers differential pro-and anti-inflammatory gene expression in adolescents as a function of social status. Clin. Psychol. Sci. 2013;1:30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.L.M., Slavich G.M., Chen E., Miller G.E. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma. Psychol. Sci. 2015;26:111–121. doi: 10.1177/0956797614556320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I. A social neuroscience perspective on stress and health. Soc. Personal. Psychol. Compass. 2012;6:890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Dedovic K., Slavich G.M., Jarcho M.R., Breen E.C., Bower J.E., Irwin M.R., Eisenberger N.I. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Dedovic K., Slavich G.M., Jarcho M.R., Breen E.C., Bower J.E., Irwin M.R., Eisenberger N.I. Neural mechanisms linking social status and inflammatory responses to social stress. Soc. Cognit. Affect Neurosci. 2016;11:915–922. doi: 10.1093/scan/nsw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nock M.K., Green J.G., Hwang I., McLaughlin K.A., Sampson N.A., Zaslavsky A.M., Kessler R.C. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry. 2013;70:300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan S.A., Flynn C., Garber J. Prospective relations between rejection and depression in young adolescents. J. Pers. Soc. Psychol. 2003;85:745–755. doi: 10.1037/0022-3514.85.4.745. [DOI] [PubMed] [Google Scholar]
- O'Connor M.F., Bower J.E., Cho H.J., Creswell J.D., Dimitrov S., Hamby M.E., Hoyt M.A., Martin J.L., Robles T.F., Sloan E.K., Thomas K.S., Irwin M.R. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A., Slavich G.M., Epel E.S., Neylan T.C. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci. Biobehav. Rev. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G., Tupling H., Brown L.B. A parental bonding instrument. Psychol. Psychother. Theor. Res. Pract. 1979;52:1–10. [Google Scholar]
- Patten S.B., Williams J.V., Lavorato D.H., Modgill G., Jetté N., Eliasziw M. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen. Hosp. Psychiatr. 2008;30:407–413. doi: 10.1016/j.genhosppsych.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Platt B., Kadosh K.C., Lau J.Y. The role of peer rejection in adolescent depression. Depress. Anxiety. 2013;30:809–821. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- Quinn M.E., Stanton C.H., Slavich G.M., Joormann J. Executive control, cytokine reactivity to social stress, and depressive symptoms: testing the social signal transduction theory of depression. Stress. 2020;23:60–68. doi: 10.1080/10253890.2019.1641079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J., Greely H.T., Wagner A.D. Detecting individual memories through the neural decoding of memory states and past experience. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9849–9854. doi: 10.1073/pnas.1001028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles T.F., Carroll J.E., Bai S., Reynolds B.M., Esquivel S., Repetti R.L. Emotions and family interactions in childhood: associations with leukocyte telomere length. Psychoneuroendocrinology. 2016;63:343–350. doi: 10.1016/j.psyneuen.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Princeton University Press; Princeton, NJ: 1965. Society and the Adolescent Self-Image. [Google Scholar]
- Ross L., Lepper M.R., Hubbard M. Perseverance in self-perception and social perception: biased attributional processes in the debriefing paradigm. J. Pers. Soc. Psychol. 1975;32:880–892. doi: 10.1037//0022-3514.32.5.880. [DOI] [PubMed] [Google Scholar]
- Rossi A.S. Caring and doing for others: social responsibility in the domains of family, work, and community. University of Chicago Press; Chicago, IL: 2001. [Google Scholar]
- Russell D., Peplau L.A., Cutrona C.E. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J. Pers. Soc. Psychol. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Schirmer M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., Kurilshikov A. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–1136. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüssler-Fiorenza Rose S.M., Contrepois K., Moneghetti K.J., Zhou W., Mishra T., Mataraso S., Dagan-Rosenfeld O., Ganz A., Dunn J., Hornburg D., Rego S., Perelman D., Ahadi S., Reza Sailani M., Zhou Y., Leopold S., Chen J., Ashland M., Christle J.W., Avina M., Limcaoco P., Ruiz C., Tan M., Butte A.J., Weinstock G.M., Slavich G.M., Sodergren E., McLaughlin T.L., Haddad F., Snyder M.P. A longitudinal big data approach for precision health. Nat. Med. 2019;25:792–804. doi: 10.1038/s41591-019-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom S.C., Miller G.E. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A., von Känel R., Slavich G.M. The psychobiology of bereavement and health: a conceptual review from the perspective of Social Signal Transduction Theory of Depression. Front. Psychiatr. 2020;11:565239. doi: 10.3389/fpsyt.2020.565239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields G.S., Spahr C.M., Slavich G.M. Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2020;77:1031–1043. doi: 10.1001/jamapsychiatry.2020.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebr. Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M. In: The SAGE Encyclopedia of Theory in Psychology. first ed. Miller H.L., editor. SAGE Publications; Thousand Oaks, CA: 2016. Psychopathology and stress; pp. 762–764. [Google Scholar]
- Slavich G.M. Life stress and health: a review of conceptual issues and recent findings. Teach. Psychol. 2016;43:346–355. doi: 10.1177/0098628316662768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M. In: The Oxford Handbook of Stress and Mental Health. Harkness K.L., Hayden E.P., editors. Oxford University Press; New York: 2020. Psychoneuroimmunology of stress and mental health; pp. 519–546. [Google Scholar]
- Slavich G.M. Social safety theory: a biologically based evolutionary perspective on life stress, health, and behavior. Annu. Rev. Clin. Psychol. 2020;16:265–295. doi: 10.1146/annurev-clinpsy-032816-045159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology. 2019;236:3063–3079. doi: 10.1007/s00213-019-05326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Shields G.S. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): an overview and initial validation. Psychosom. Med. 2018;80:17–27. doi: 10.1097/PSY.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., O'Donovan A., Epel E.S., Kemeny M.E. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Stewart J.G., Esposito E.C., Shields G.S., Auerbach R.P. The Stress and Adversity Inventory for Adolescents (Adolescent STRAIN): associations with mental and physical health, risky behaviors, and psychiatric diagnoses in youth seeking treatment. J. Child Psychol. Psychiatry. 2019;60:998–1009. doi: 10.1111/jcpp.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Cole S.W. The emerging field of human social genomics. Clin. Psychol. Sci. 2013;1(3):331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Giletta M., Helms S.W., Hastings P.D., Rudolph K.D., Nock M.K., Prinstein M.J. Interpersonal life stress, inflammation, and depression in adolescence: testing social signal transduction theory of depression. Depress. Anxiety. 2020;37:179–193. doi: 10.1002/da.22987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., Kubzansky L.D., McLaughlin K.A., Koenen K.C. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. The teenage brain: sensitivity to social evaluation. Curr. Dir. Psychol. Sci. 2013;22:121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Spirito A., Williams C.A., Stark L.J., Hart K.J. The Hopelessness Scale for Children: psychometric properties with normal and emotionally disturbed adolescents. J. Abnorm. Child Psychol. 1988;16:445–458. doi: 10.1007/BF00914174. [DOI] [PubMed] [Google Scholar]
- Stewart J.G., Shields G.S., Esposito E.C., Cosby E.A., Allen N.B., Slavich G.M., Auerbach R.P. Life stress and suicide in adolescents. J. Abnorm. Child Psychol. 2019;47:1707–1722. doi: 10.1007/s10802-019-00534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J.M., Davies P.S. Clinical longitudinal standards for height and height velocity for North American children. J. Pediatr. 1985;107:317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- Thompson E.R. Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS) J. Cross Cult. Psychol. 2007;38:227–242. [Google Scholar]
- Toga A.W., Thompson P.M., Mori S., Amunts K., Zilles K. Towards multimodal atlases of the human brain. Nat. Rev. Neurosci. 2006;7:952–966. doi: 10.1038/nrn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognit. Ther. Res. 2003;27:247–259. [Google Scholar]
- Turk-Browne N.B. Functional interactions as big data in the human brain. Science. 2013;342:580–584. doi: 10.1126/science.1238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Iannotti R.J., Nansel T.R. School bullying among US adolescents: physical, verbal, relational, and cyber. J. Adolesc. Health. 2009;45:368–375. doi: 10.1016/j.jadohealth.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way B.M., Taylor S.E., Eisenberger N.I. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. University of Pennsylvania; Philadelphia: 1979. The Dysfunctional Attitude Scale: A Validation Study. (Unpublished Doctoral Dissertation) [Google Scholar]
- Weissman M.M., Gammon G.D., John K., Merikangas K.R., Warner V., Prusoff B.A., Sholomskas D. Children of depressed parents: increased psychopathology and early onset of major depression. Arch. Gen. Psychiatr. 1987;44:847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- Whiteside S.P., Lynam D.R., Miller J.D., Reynolds S.K. Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsivity. Eur. J. Pers. 2005;19:559–574. [Google Scholar]
- Whooley M.A. Depression and cardiovascular disease: healing the broken-hearted. J. Am. Med. Assoc. 2006;295:2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Hack L.M. A precision medicine–based, ‘fast-fail’ approach for psychiatry. Nat. Med. 2020;26:653–654. doi: 10.1038/s41591-020-0854-z. [DOI] [PubMed] [Google Scholar]
- Wong M.L., Inserra A., Lewis M.D., Mastronardi C.A., Leong L., Choo J., Rogers G.B. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A., Kroll L., Moore A., Harrington R. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note. J. Child Psychol. Psychiatry. 1995;36:327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Woody E.Z., Szechtman H. Adaptation to potential threat: the evolution, neurobiology, and psychopathology of the security motivation system. Neurosci. Biobehav. Rev. 2011;35:1019–1033. doi: 10.1016/j.neubiorev.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Yirmiya R., Pollak Y., Morag M., Reichenberg A., Barak O., Avitsur R., Shavit Y., Ovadia H., Weidenfeld J., Morag A., Newman M.E., Pollmächer T. Illness, cytokines, and depression. Ann. N. Y. Acad. Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available and can be requested by contacting the corresponding author.