PURPOSE
Arsenic combined with all-trans retinoic acid (ATRA) is the standard of care for adult acute promyelocytic leukemia (APL). However, the safety and effectiveness of this treatment in pediatric patients with APL have not been reported on the basis of larger sample sizes.
METHODS
We conducted a multicenter trial at 38 hospitals in China. Patients with newly diagnosed APL were stratified into two risk groups according to baseline WBC count and FLT3-ITD mutation. ATRA plus arsenic trioxide or oral arsenic without chemotherapy were administered to the standard-risk group, whereas ATRA, arsenic trioxide, or oral arsenic plus reduced-dose anthracycline were administered to the high-risk group. Primary end points were event-free survival and overall survival at 2 years.
RESULTS
We enrolled 193 patients with APL. After a median follow-up of 28.9 months, the 2-year overall survival rate was 99% (95% CI, 97 to 100) in the standard-risk group and 95% (95% CI, 90 to 100) in the high-risk group (P = .088). The 2-year event-free survival was 97% (95% CI, 93 to 100) in the standard-risk group and 90% (95% CI, 83 to 96) in the high-risk group (P = .252). The plasma levels of arsenic were significantly elevated after treatment, with a stable effective level ranging from 42.9 to 63.2 ng/mL during treatment. In addition, plasma, urine, hair, and nail arsenic levels rapidly decreased to normal 6 months after the end of treatment.
CONCLUSION
Arsenic combined with ATRA is effective and safe in pediatric patients with APL, although long-term follow-up is still needed.
INTRODUCTION
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia that is relatively less common in the pediatric population.1 APL has become a curable disease with the application of all-trans retinoic acid (ATRA) combination chemotherapy or arsenic trioxide (ATO),2-5 with complete remission (CR) of 90% to 100% of patients in clinical trials and an overall survival (OS) rate between 86% and 97% reported in several large multicenter trials.3,6-12 However, ATO must be infused in the hospital, whereas oral arsenic can be administered in an outpatient context, which is more cost-effective and convenient. The only commercially available oral agent, named the Realgar-Indigo naturalis formula (RIF), has been verified to achieve a similar outcome to that of treatment with intravenous (IV) ATO, with the advantage of increased cost-effectiveness because of reduced medical costs and shorter hospitalizations.13-16
CONTEXT
Key Objective
Since acute promyelocytic leukemia (APL) has become a curable disease, clinicians have focused on simplifying the treatment of APL. Previous studies have revealed the safety and effectiveness of arsenic trioxide and oral arsenic in childhood APL on the basis of the addition of arsenic to all-trans retinoic acid plus anthracycline in a small sample size. To the best of our knowledge, our study is the first to report the efficacy and safety of a chemotherapy-free and chemotherapy-reduced Protocol in pediatric patients with APL on the basis of a large sample size.
Knowledge Generated
Arsenic combined with all-trans retinoic acid in chemotherapy-free or chemotherapy-reduced treatment are effective and safe in pediatric patients with APL in standard-risk or high-risk groups.
Relevance
The treatment of pediatric APL could be further simplified, and oral arsenic, which is more convenient and economical, could be an alternative to the intravenous arsenic as the prognosis is similar in pediatric patients with APL.
Since randomized controlled trials13,16 have shown that the oral RIF plus ATRA was not inferior to IV ATO plus ATRA for the treatment of adult patients with non–high-risk APL in China, with excellent outcomes, and APL in children is relatively less common,17 we designed a single-arm trial in children with APL as recommended by experts in our clinical epidemiology and evidence-based medicine center. Here, we report the 2-year outcomes in a large cohort of 193 children with APL treated with the mainstay therapy of arsenic plus ATRA. This study was registered at the Chinese Clinical Trial Registry (ChiCTR-OIN-17011227).
METHODS
Study Design and Participants
The study was a multicenter, single-arm, clinical trial performed at 38 hospitals in China. Patients with newly diagnosed APL, the t(15;17) and/or PML-RARA fusion gene, age younger than 18 years, and normal cardiac function were enrolled and treated by the Chinese Children's Leukemia Group (CCLG)-APL2016 Protocol (online only). Patients who were allergic to arsenic, were unable to follow the trial Protocol, had contraindications to anthracycline-based chemotherapy, or were participating in other trials at the same time were excluded. Informed consent was obtained from all patients or legal guardians in accordance with the principles of the Declaration of Helsinki.
CCLG-APL2016 Protocol
Patients were stratified into two risk groups: those with a WBC count < 10 × 109/L but without FLT3-ITD mutation were classified as being in the standard-risk group (SR), and those with a WBC count ≥ 10 × 109/L or with FLT3-ITD mutation were classified as being in the high-risk group (HR) (Appendix 1, online only).18,19
Chemotherapy-free treatment was administered to the SR group, and treatment with reduced chemotherapy was administered to the HR group (Fig 1). RIF (60 mg/kg daily in two or three divided oral doses) or ATO (one IV dose of 0.15 mg/kg daily) plus ATRA (25 mg/m2 in two or three divided oral doses) were administered during induction in the SR group. Additional anthracycline (idarubicin 10 mg/m2 or daunorubicin 40 mg/m2 per day every other day, 2-3 doses) was injected into HR patients during the induction phase. When the peripheral blood leukocyte count decreases and the absolute number of neutrophils is < 500/μl, anthracyclines should be stopped. Although oral RIF could be prescribed for outpatient administration, all patients were admitted to the hospital during induction therapy until the recovery from coagulopathy and the return of the platelet count to within normal limits.
FIG 1.
CCLG-APL2016 Protocol. If patients fail to get molecular complete remission before maintenance therapy or experienced molecular relapse during maintenance phase or after the completion of therapy, they were removed from the trial. APL, acute promyelocytic leukemia; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; DNR, daunorubicin; IDA, idarubicin; RIF, Realgar-Indigo naturalis formula.
The consolidation therapy included RIF (60 mg/kg daily in three divided oral doses) or ATO (one IV dose of 0.15 mg/kg daily) and ATRA (25 mg/m2 in two or three divided oral doses) for 14 days in the SR group. In the HR group, ATRA (25 mg/m2 in two or three divided oral doses) was administered for 14 days, and anthracycline (idarubicin 10 mg/m2 or daunorubicin 40 mg/m2 daily, every other day) was administered three times. Repeat once if molecular CR is not achieved.
The maintenance therapy included RIF (60 mg/kg daily in two divided oral doses) or ATO (one IV dose of 0.15 mg/kg daily) in a 2 weeks on and 2 weeks off regimen and ATRA (25 mg/m2 in two divided oral doses) in a 1 week on and 1 week off regimen. Eight weeks of treatment constituted one cycle. The patients in the SR group received four cycles (approximately 8 months), and patients in the HR group received five cycles (approximately 10 months).
Response Evaluation During and After Treatment
The primary end points were event-free survival (EFS) and OS at 2 years. Events were defined as follows: the lack of complete molecular remission before maintenance therapy, molecular relapse, hematologic relapse, or death from any cause. OS was calculated from the date of study entry to the date of death from any cause or last follow-up for surviving patients. Secondary end points were the proportion of patients achieving a complete hematologic remission, the proportion of patients achieving a complete molecular response, the incidence of early death, the cumulative incidence of relapse, and safety. Hematologic complete remission was defined as normally regenerating bone marrow with < 5% blasts according to morphology. Molecular complete remission (MCR) was defined as the absence of PML-RARA according to bone marrow reverse transcriptase polymerase chain reaction or quantitative reverse transcriptase polymerase chain reaction, with an assay sensitivity of at least 1 × 10−4. Toxic effects were graded according to the NCI Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
The trial was designed to analyze EFS and OS at 2 years after start of induction therapy. Patients who had major Protocol violations were excluded from the analysis. Descriptive analyses are presented as the means and standard deviations for normally distributed variables and the medians (minimum and maximum) for skewed distribution variables. The survival curves were estimated using the Kaplan-Meier method, and differences in the OS and EFS rates between the SR and HR groups were compared by the log-rank test, and the cumulative incidence of relapse was compared using Fine-Gray test, in which patients with early death was a competing risk event to the relapse. Dichotomous variables were compared with the corrected χ2 test, and continuous variables were compared with the Wilcoxon rank test or pairwise Wilcoxon test. All statistical tests were two-tailed with a significance level of 0.05. The data were analyzed using SPSS Statistics software, version 26 (IBM).
RESULTS
Patient Characteristics
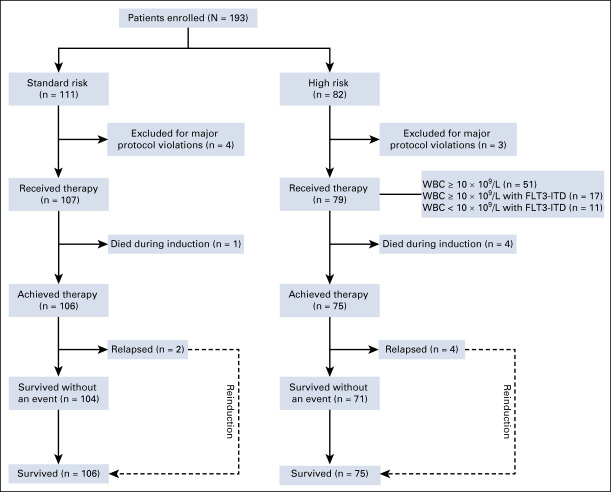
From November 23, 2016 to November 25, 2018, a total of 193 patients were enrolled in this clinical trial. The median follow-up time was 28.9 months (0.2-42.9 months), and 76.3% of patients have been followed for 24 months. Seven patients who had poor compliance and failed to adhere to the Protocol were excluded (Fig 2). The baseline characteristics of the remaining 186 patients are shown in Table 1. The median WBC and platelet counts were 3.6 (1.0-321.0) ×109/L and 28 (2-211) ×109/L, respectively. Twenty-eight patients (15%) had FLT3-ITD gene mutations, of which 11 patients had a lower WBC count (< 10 × 109/L). All 186 eligible patients were evaluated for their response to induction therapy (Table 2). The overall incidence of early death was 3% (5/186). Of the five patients who had early death, three patients died of severe disseminated intravascular coagulation (DIC) with intracranial hemorrhage (one in the SR group and two in the HR group) and two died of severe infections with coagulopathy and pulmonary hemorrhage.
FIG 2.
CONSORT diagram.
TABLE 1.
Baseline Characteristics of the Study Population

TABLE 2.
Clinical Outcomes of 186 Patients With Pediatric APL
Two-Year Outcomes
With a median follow-up time of 28.9 months, the 2-year OS rates were 99% (95% CI, 97 to 100) in the SR group and 95% (95% CI, 90 to 100) in the HR group (P = .088; Fig 3B). The 2-year EFS rates were 97% (95% CI, 93 to 100) in the SR group and 90% (95% CI, 83 to 96) in the HR group (P = .252; Fig 3D). There was no significant difference in 2-year OS and EFS between the SR group and HR group. The 2-year cumulative incidence of relapse was 2% (95% CI, 0 to 6) in the SR group and 6% (95% CI, 2 to 11) in the HR group (P = .921; Fig 3F). Of these six relapse patients, two patients in the SR group relapsed during follow-up (one experienced molecular relapse at 19.5 months, and the other experienced hematologic and molecular relapse at 23.5 months after MCR). Four patients in the HR group relapsed during follow-up (two experienced molecular relapse at 10.6 months and 15.5 months after MCR and two experienced hematologic and molecular relapse at 12.2 months and 19.0 months after MCR). ATRA and arsenic agents plus anthracycline were used as salvage therapies, and all of them achieved a second CR. Only one patient in the HR group had CNS leukemia during hematologic and molecular relapse at 12.2 months after MCR.
FIG 3.

Kaplan-Meier plots of (A and B) OS, (C and D) EFS, and (E and F) the cumulative incidence of relapse. EFS, event-free survival; OS, overall survival.
Treatment Toxicities
During the induction phase, the incidence of DIC was 52% (97 out of 186), and grade 2, grade 3, grade 4, and grade 5 DIC accounted for 16% (29 out of 186), 29% (54 out of 186), 6% (11 out of 186), and 2% (3 out of 186), respectively. Three patients died of severe DIC with intracranial hemorrhage. Thus, severe DIC was still the leading cause of early death in patients with APL. Differentiation syndrome, including its moderate and severe forms,20 developed in 41% (76 out of 186) of the patients. No differentiation syndrome–related deaths occurred.
Grade 3-4 neutropenia occurred in 80% (149 out of 186) of patients, and in 29% (43 out of 149) of them, it lasted for more than 14 days during induction treatment (Appendix Table A1, online only). Grade 3-4 thrombocytopenia occurred in 89% (166 out of 186) of patients, and in 52% (87 out of 186), it lasted for more than 14 days (Appendix Table A1).
For the toxicity in liver and heart, 3% (5 out of 186) of patients had grade 3 elevated serum ALT or AST levels and 8% (15 out of 186) of patients had grade 1-2 hyperbilirubinemia. Only 1% (1/-186) of patients had decreased left ventricular ejection fraction, and no patients had a prolonged corrected QT interval (Appendix Table A1). These adverse events could be recovered after receiving supportive treatment.
In addition, 73% (135 out of 186) of patients developed infections, of which 35% (65 out of 186) were pneumonia, 19% (35 out of 186) upper respiratory tract infections, and 18% (34 out of 186) septicemia.
Arsenic Retention on Follow-Up
The plasma, urine, hair, and nail arsenic concentrations were measured in 34 patients at different time points (Appendix 2, online only). The plasma arsenic level was significantly elevated after 7 days of arsenic administration compared with the level before the administration of arsenic (median, 42.9 ng/ml [range, 27.3-82.8 ng/mL] v 0.7 ng/mL [range, 0.2-8.4 ng/mL]; P < .0001). The median plasma arsenic level was maintained at a stable effective range of levels (10-100 ng/mL),13 ranging from 42.9 to 63.2 ng/mL on the seventh, 14th, and 28th day of arsenic administration and the 10 weeks of maintenance therapy. Furthermore, the plasma arsenic levels rapidly decreased to within normal limits after 6 months without arsenic administration compared with the level before the administration of arsenic (median, 0.9 ng/mL [range, 0.4-2.5 ng/mL] v 0.7 ng/mL [range, 0.2-8.4 ng/mL]; P = .333) (Fig 4A).The urine arsenic level was significantly elevated after arsenic administration, ranging from 1506.1 to 2,166.9 ng/mL on the seventh, 14th, and 28th day of arsenic administration and the 10 weeks of maintenance therapy (Fig 4B).
FIG 4.

Arsenic retention in 34 patients during follow-up. The kinetics of arsenic concentrations in the (A) plasma, (B) urine, (C) hair, and (D) nails of 34 patients. D0, before the administration of arsenic; D7, D14, and D28, on the seventh, 14th, and 28th day of arsenic administration, respectively; off arsenic, immediately after the cessation of arsenic therapy; off arsenic 6 months, 6 months after the cessation of therapy; off arsenic 12 months, 1 year after the cessation of therapy; W10, during the 10 weeks of maintenance therapy.
Hair arsenic (median, 7,004.0 ng/g [range, 650.8-14,683.7 ng/g]) and nail arsenic levels (median, 18,556.1 ng/g [range, 400.0-30,334.0 ng/g]) peaked at the time of the cessation of therapy and rapidly decreased to within normal limits after 6 months without arsenic administration compared with the levels before the administration of arsenic (hair: 268.4 ng/g v 115.6 ng/g, P = .214; nail: 487.6 ng/g v 165.7 ng/g, P = .110) (Figs 4C and 4D, respectively). The common signs of chronic arseniasis, such as cardiovascular events, chronic renal insufficiency, diabetes, or neurologic dysfunction, were not observed so far.
DISCUSSION
Previous studies have shown the safety and effectiveness of ATO21,22 and oral RIF in childhood APL,23 which were based on the addition of arsenic to ATRA combined with anthracycline in a small sample size. Here, our study reports the safety and effectiveness of a chemotherapy-free and chemotherapy-reduced Protocol in pediatric patients with APL on the basis of a larger sample size, which has been identified in adult APL.13,16 In addition, since the literature has reported that the oral arsenic agent RIF showed the same potency but is more convenient and economical than IV infusion with ATO,13,15,16 our study did not design a comparison of the effectiveness of oral and IV arsenic agent. The doctors prescribed the oral or IV arsenic agent depending on the agents' availability in their local hospitals.
Two large randomized trials showed that the effect of treatment with the combination of ATO and ATRA without chemotherapy was at least equal to and, with long-term follow-up, even superior to the effects of ATRA-chemotherapy combinations in patients with SR APL.3,9,12 The combination of ATO and ATRA without chemotherapy therefore became the standard therapy for newly diagnosed non–high-risk APL recommended by the NCCN,24 European LeukemiaNet,25 and Chinese Hematological Association.26 ATO and ATRA combinations with reduced chemotherapy also appear very promising in patients with HR APL.9,27 Meanwhile, the therapeutic effects of oral arsenic RIF plus ATRA are not inferior to those of IV ATO plus ATRA, which means that a completely oral, chemotherapy-free model is an alternative to the standard IV treatment of patients with APL.12,15,16,27 However, few studies of the combination of ATO and ATRA have been reported in the treatment of pediatric patients with APL with the continuous measurement of arsenic concentrations. Our single-arm trial showed that arsenic plus ATRA without chemotherapy in the SR group and arsenic plus ATRA with reduced chemotherapy (240 mg/m2) in the HR group achieved similarly excellent outcomes in pediatric patients with APL, with a 98% 2-year EFS rate in the SR group, a 90% 2-year EFS rate in the HR group, a 99% 2-year OS rate in the SR group, and a 95% OS rate in the HR group. An important study18 by Zhu et al showed an estimated 3-year OS rate of 100% in the RIF-ATRA group. The higher OS rate in their study may have been because of the exclusion of patients with HR APL and more active measures taken to reduce the tumor burden in patients with WBC counts < 10 × 109/L there than in our study. Furthermore, 38 hospitals participated in the CCLG-APL2016 Protocol, some of which are in resource-limited provinces with fewer medical resources, which may also have contributed to a slightly worse prognosis. However, APL is a malignancy that can be definitively cured by targeted therapies, namely retinoic acid and/or arsenic regimens, both of which trigger PML-RARA degradation through nonoverlapping pathways,28 explaining the excellent outcome in patients with APL treated with ATRA combined with arsenic (IV ATO or oral RIF). The use of oral RIF in China results in better safety outcomes, better quality of life, and lower medical costs for patients than other treatments.15 The numbers of patients using ATO and RIF during induction and maintenance in our study are shown in Appendix Figure A1 (online only). Approximately 73% (136 out of 186) of patients were administered RIF during maintenance therapy, resulting in improved quality of life and lower medical costs.14 Since our study does not have enough power to compare the outcome of patients with ATO or oral arsenic RIF, the outcomes are simply described in Appendix Figure A2 (online only).
Patients with APL frequently presenting with consumptive coagulopathy can cause a life-threatening hemorrhage.29 Our previous study showed severe DIC is the primary cause of early death.30 In this study, five (3%) patients died early, and three of them had severe coagulopathy, which means early awareness and urgent intervention for life-threatening bleeding are critical factors for reducing early death. In addition to supportive measures, the prompt administration of ATRA can also improve abnormal coagulation. ATRA can downregulate APL cell tissue factor, which can prevent and treat DIC and secondary fibrinolysis.31 Thus, it is necessary to start retinoic acid–induced differentiation as soon as possible in patients with clinically highly suspected APL, even if PML-RARA has not been confirmed.
The toxicity of arsenic is a major concern, especially in growing children. Our study demonstrated that the retention of arsenic did not result in significant arsenic accumulation in the plasma, urine, hair, or nails at 6 months after the cessation of treatment, and the concentrations of arsenic in those tissues declined faster in the children than in adults as reported in previous studies.13,32 The most common adverse event was hepatic damage, and the incidence of hepatic damage was lower than that reported by Zhu et al.16
One limitation of our study is that this is a single-arm trial, and therefore, the grade of evidence is not as high as that obtained from a randomized controlled trial33; however, childhood APL is very rare,34 and the excellent efficacy of arsenic plus ATRA for adult patients with APL has been well-confirmed. This is why our study was designed to be a single-arm trial in children with APL as recommended by experts in our clinical epidemiology and evidence-based medicine center. In addition, because of the impact of the COVID-19 pandemic, specimens at some time points have not yet been tested for arsenic concentrations, resulting in only a few tested samples at some points, such as 12 months after the cessation of arsenic therapy. We will test these samples as soon as possible to ensure that there are sufficient samples at each monitoring time point.
In summary, our results show that excellent outcomes can be achieved in pediatric SR APL using arsenic plus ATRA in a chemotherapy-free treatment regimen and in patients with HR APL treated with arsenic plus ATRA with reduced chemotherapy. The use of arsenic in pediatric patients with APL is safe. However, long-term follow-up is still needed since the median follow-up time was only 2 years.
ACKNOWLEDGMENT
We thank all patients and parents for their support. We also thank all the staff members of the collaborating institutes. Special thanks are due to Dr Hong-Hu Zhu for the Protocol guidance and treatment consultancies.
APPENDIX 1. Treatment
Standard-Risk Group
Induction: (a) Realgar-Indigo naturalis formula (RIF) 60 mg/kg/d or arsenic trioxide (ATO) 0.15 mg/kg/d, days 1 approximately 28 and (b) all-trans retinoic acid (ATRA) 25 mg/m2/d, days 1 approximately 28.
Consolidation: (a) RIF 60 mg/kg/d or ATO 0.15 mg/kg/d, days 1 approximately 14 and (b) ATRA 25 mg/m2/d, days 1 approximately 14.
Maintenance: (a) RIF 60 mg/kg/d or ATO 0.15 mg/kg/d, 2 weeks on and 2 weeks off, 4 weeks per cycle, for four cycles in total and (b) ATRA 25 mg/m2/d, 1 week on and 1 week off, 4 weeks per cycle, for four cycles in total.
High-Risk Group
Induction: (a) RIF 60 mg/kg/d or ATO 0.15 mg/kg/d, days 1 approximately 28, (b) ATRA 25 mg/m2/d, days 1 approximately 28, and (c) anthracycline: idarubicin 10 mg/m2/d or daunorubicin 40 mg/m2/d, once every other day for 2 approximately 3 days.
Consolidation: (a) anthracycline: idarubicin 10 mg/m2/d or daunorubicin 40 mg/m2/d, once every other day, for 3 days and (b) ATRA 25 mg/m2/d, days 1 approximately 14.
Maintenance: (a) RIF 60 mg/kg/d or ATO 0.15 mg/kg/d, 2 weeks on and 2 weeks off, 4 weeks per cycle, for five cycles and (b) ATRA 25 mg/m2/d, 1 week on and 1 week off, 4 weeks per cycle, for five cycles.
Cytoreductive Therapy During Induction
If the WBC count was > 10 × 109/L before or during induction, one of the following drugs was added:
Hydroxyurea: 10-40 mg/kg/d in two or three divided oral doses for < 2 weeks;
Cytarabine: 40-100 mg/m2, IV, once daily or every 12 hours for less than 1 week;
Homoharringtonine: 1 mg/m2, IV, once daily for fewer than 5 days;
High-risk group: anthracycline added; and
Maintain platelet count > 50 × 109/L and fibrinogen > 1.5 g/L.
PML-RARA Fusion Gene Detection
Induction: day 0, day 28, and day 15 if necessary.
Consolidation: day 28.
Maintenance (two times): week 11 and week 21.
After the cessation of therapy: at the cessation of therapy, then every 6 months for 2 years.
Multicenter Data Quality Control and Management
In this open-label trial, eligible participants were centrally registered and assigned a unique number. Case report forms were used to record the treatment response, and the main outcome indicators were also collected from multiple centers before each annual collaboration working group meeting. An access database was established for the entry of the data from the case report forms. Two persons trained by the project manager were responsible for all data input, and another person corrected any mismatched data and updated the outcomes from multiple centers every 6 months.
APPENDIX 2. Arsenic Retention Evaluation
Arsenic concentration was detected in plasma, urine, hair, and nail samples during and after the cessation of arsenic treatment in 34 patients. The arsenic concentrations in the plasma and urine were measured at nine time points: before the administration of arsenic (D0); on the seventh, 14th, and 28th day of arsenic administration (D7, D14, and D28, respectively); during the 10 weeks of maintenance therapy (W10); at the time of the cessation of therapy; at 6 months after the cessation of therapy; and at 1 year after the cessation of therapy. The arsenic concentrations in the hair and nail samples were measured before the administration of arsenic (D0), on the 28th day of arsenic administration (D28), at the cessation of therapy, at 6 months after the cessation of therapy, and at 1 year after the cessation of therapy.
FIG A1.

Number of patients using ATO and RIF during induction and maintenance therapy. ATO, arsenic trioxide; RIF, Realgar-Indigo naturalis formula.
FIG A2.
Kaplan-Meier plots of (A) OS, (B) EFS, and (C) the cumulative incidence of relapse of patients receiving different arsenic dosage forms. Patients were divided into three groups: ATO alone (n = 57), RIF alone (n = 49), and ATO + RIF (n = 80). There was no significant difference in 2-year OS (P = .137), EFS (P = .745), and the cumulative incidence of relapse (P = .085) among these three groups. ATO, arsenic trioxide; EFS, event-free survival; OS, overall survival; RIF, Realgar-Indigo naturalis formula.
TABLE A1.
Incidence of Hematologic and Nonhematologic Toxic Effects During Induction Treatment
SUPPORT
Supported by the National Science and Technology Key Projects (Grant No. 2017ZX09304029004), the Beijing Municipal Administration of Hospitals DengFeng Program (Grant No. DFL20151101), the Capital Health and Development Special Grant (Grant No. 2016-1-2091), and subproject of major Projects of Shanghai Science and Technology Commission (Grant No. 14411950602.2014-2017).
CLINICAL TRIAL INFORMATION
H.Z., H.J., S.H., and N.L. contributed equally to this work.
T.W., of Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, and H.J., of Shanghai Children's Hospital, Shanghai Jiaotong University, contributed equally to this work as co-corresponding authors.
AUTHOR CONTRIBUTIONS
Conception and design: Huyong Zheng, Tianyou Wang, Hui Jiang
Provision of study materials or patients: Jianxin Li, Yunpeng Dai, Xiaoqin Feng, Fu Li, Hui Gao
Collection and assembly of data: Huyong Zheng, Shaoyan Hu, Ning Liao, Diying Shen, Xin Tian, Guoping Hao, Runming Jin, Jianxin Li, Yongjun Fang, Xiuli Ju, Ansheng Liu, Ningling Wang, Xiaowen Zhai, Jiashi Zhu, Qun Hu, Limin Li, Wei Liu, Lirong Sun, Li Wang, Yunpeng Dai, Xiaoqin Feng, Fu Li, Hui Liang, Xinhui Luo, Mei Yan, Qingning Yin, Yan Chen, Yueqin Han, Lijun Qu, Yanling Tao, Hui Gao, Zhixu He, Limin Lin, Jixia Luo, Kaili Pan, Jingrong Zhang, Rong Zhang, Min Zhou, Yuanyuan Zhang, Linya Wang, Ruidong Zhang, Peifang Xiao, Yayun Ling, Xiaoxia Peng, Tianyou Wang, Hui Jiang
Data analysis and interpretation: Huyong Zheng, Linya Wang, Yaguang Peng, Hui Jiang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Arsenic Combined With All-Trans Retinoic Acid for Pediatric Acute Promyelocytic Leukemia: Report From the CCLG-APL2016 Protocol Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Philip AP, David GP: Principles and Practice of Pediatric Oncology (7th ed). Philadelphia, PA, Wolters Kluwer, 2015 [Google Scholar]
- 2.Wang ZY, Chen Z: Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 111:2505-2515, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Lo-Coco F, Avvisati G, Vignetti M, et al. : Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369:111-121, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Chen SJ, Chen Z: Targeting agents alone to cure acute promyelocytic leukemia. N Engl J Med 369:186-187, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Sanz MA, Lo-Coco F: Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol 29:495-503, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Estey E, Garcia-Manero G, Ferrajoli A, et al. : Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107:3469-3473, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, Estey E, Jones D, et al. : Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol 27:504-510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abaza Y, Kantarjian H, Garcia-Manero G, et al. : Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 129:1275-1283, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett AK, Russell NH, Hills RK, et al. : Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet Oncol 16:1295-1305, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Iland HJ, Collins M, Bradstock K, et al. : Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: A non-randomised phase 2 trial. Lancet Haematol 2:e357-366, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Powell BL, Moser B, Stock W, et al. : Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 116:3751-3757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo-Coco F, Di Donato L, Schlenk RF: Targeted therapy alone for acute promyelocytic leukemia. N Engl J Med 374:1197-1198, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Zhu HH, Wu DP, Jin J, et al. : Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: A multicenter randomized controlled trial. J Clin Oncol 31:4215-4221, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Liang GW, Huang XJ, et al. : Reduced medical costs and hospital days when using oral arsenic plus ATRA as the first-line treatment of acute promyelocytic leukemia. Leuk Res 39:1319-1324, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Zhu HH, Hu J, Lo CF, et al. : The simpler the better: Oral arsenic for acute promyelocytic leukemia. Blood 134:597-605, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Wu D, Du X, et al. : Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: A non-inferiority, randomised phase 3 trial. Lancet Oncol 19:871-879, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Gamis AS, Alonzo TA, Perentesis JP, et al. : Children's Oncology Group's 2013 blueprint for research: Acute myeloid leukemia. Pediatr Blood Cancer 60:964-971, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanz MA, Lo CF, Martin G, et al. : Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood 96:1247-1253, 2000 [PubMed] [Google Scholar]
- 19.Testa U, Lo-Coco F: Prognostic factors in acute promyelocytic leukemia: Strategies to define high-risk patients. Ann Hematol 95:673-680, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Montesinos P, Bergua JM, Vellenga E, et al. : Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: Characteristics, outcome, and prognostic factors. Blood 113:775-783, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Zhang L, Wu J, et al. : Long-term prognosis of childhood acute promyelocytic leukaemia with arsenic trioxide administration in induction and consolidation chemotherapy phases: A single-centre experience. Eur J Haematol 91:483-489, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Kutny MA, Alonzo TA, Gerbing RB, et al. : Arsenic trioxide consolidation allows anthracycline dose reduction for pediatric patients with acute promyelocytic leukemia: Report from the Children's Oncology Group phase III historically controlled trial AAML0631. J Clin Oncol 35:3021-3029, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang MH, Wan WQ, Luo JS, et al. : Multicenter randomized trial of arsenic trioxide and Realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: Interim results of the SCCLG-APL clinical study. Am J Hematol 93:1467-1473, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallman MS, Wang ES, Altman JK, et al. : Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17:721-749, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Sanz MA, Fenaux P, Tallman MS, et al. : Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 133:1630-1643, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Society of Hematology, Chinese Medical Association, Chinese Medical Doctor Association : Chinese guidelines for diagnosis and treatment of acute promyelocytic leukemia (2018). Zhonghua Xue Ye Xue Za Zhi 39:179-183, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Liu Y, Jia J, et al. : Oral arsenic and all-trans retinoic acid for high-risk acute promyelocytic leukemia. Blood 131:2987-2989, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Ablain J, Rice K, Soilihi H, et al. : Activation of a promyelocytic leukemia-tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat Med 20:167-174, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Cicconi L, Lo-Coco F: Current management of newly diagnosed acute promyelocytic leukemia. Ann Oncol 27:1474-1481, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang L, Zhang R, et al. : Long-term follow-up of children with acute promyelocytic leukemia treated with Beijing Children’s Hospital APL 2005 protocol (BCH-APL 2005). Pediatr Hemat Oncol 36:399-409, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Lavallée V, Chagraoui J, MacRae T, et al. : Transcriptomic landscape of acute promyelocytic leukemia reveals aberrant surface expression of the platelet aggregation agonist podoplanin. Leukemia 32:1349-1357, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Hu J, Chen L, et al. : The 12-year follow-up of survival, chronic adverse effects, and retention of arsenic in patients with acute promyelocytic leukemia. Blood 128:1525-1528, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld DA, Finkelstein DM, Macklin E, et al. : Design and analysis of a clinical trial using previous trials as historical control. Clin Trials 16:531-538, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Samad A, Pombo-de-Oliveira MS, et al. : Global characteristics of childhood acute promyelocytic leukemia. Blood Rev 29:101-125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]