Abstract
Background:
Macrophages are involved in the pathogenesis of idiopathic pulmonary fibrosis, partially by activating lung fibroblasts. However, how macrophages communicate with lung fibroblasts is largely unexplored. Exosomes can mediate intercellular communication, whereas its role in lung fibrogenesis is unclear. Here we aim to investigate whether exosomes can mediate the crosstalk between macrophages and lung fibroblasts and subsequently induce fibrosis.
Methods:
In vivo, bleomycin (BLM)-induced lung fibrosis model was established and macrophages infiltration was examined. The effects of GW4869, an exosomes inhibitor, on lung fibrosis were assessed. Moreover, macrophage exosomes were injected into mice to observe its pro-fibrotic effects. In vitro, exosomes derived from angiotensin II (Ang II)-stimulated macrophages were collected. Then, lung fibroblasts were treated with the exosomes. Twenty-four hours later, protein levels of α-collagen I, angiotensin II type 1 receptor (AT1R), transforming growth factor-β (TGF-β), and phospho-Smad2/3 (p-Smad2/3) in lung fibroblasts were examined. The Student's t test or analysis of variance were used for statistical analysis.
Results:
In vivo, BLM-treated mice showed enhanced infiltration of macrophages, increased fibrotic alterations, and higher levels of Ang II and AT1R. GW4869 attenuated BLM-induced pulmonary fibrosis. Mice with exosomes injection showed fibrotic features with higher levels of Ang II and AT1R, which was reversed by irbesartan. In vitro, we found that macrophages secreted a great number of exosomes. The exosomes were taken by fibroblasts and resulted in higher levels of AT1R (0.22 ± 0.02 vs. 0.07 ± 0.02, t = 8.66, P = 0.001), TGF-β (0.54 ± 0.05 vs. 0.09 ± 0.06, t = 10.00, P < 0.001), p-Smad2/3 (0.58 ± 0.06 vs. 0.07 ± 0.03, t = 12.86, P < 0.001) and α-collagen I (0.27 ± 0.02 vs. 0.16 ± 0.01, t = 7.01, P = 0.002), and increased Ang II secretion (62.27 ± 7.32 vs. 9.56 ± 1.68, t = 12.16, P < 0.001). Interestingly, Ang II increased the number of macrophage exosomes, and the protein levels of Alix (1.45 ± 0.15 vs. 1.00 ± 0.10, t = 4.32, P = 0.012), AT1R (4.05 ± 0.64 vs. 1.00 ± 0.09, t = 8.17, P = 0.001), and glyceraldehyde-3-phosphate dehydrogenase (2.13 ± 0.36 vs. 1.00 ± 0.10, t = 5.28, P = 0.006) were increased in exosomes secreted by the same number of macrophages, indicating a positive loop between Ang II and exosomes production.
Conclusions:
Exosomes mediate intercellular communication between macrophages and fibroblasts plays an important role in BLM-induced pulmonary fibrosis.
Keywords: Angiotensin-converting enzyme (ACE)/angiotensin II (Ang II)/angiotensin II type 1 receptor (AT1R) axis, Exosomes, Idiopathic pulmonary fibrosis, Lung fibroblasts, Macrophages
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and lethal lung disease characterized by inflammation and excessive extracellular matrix (ECM) deposition.[1] Lung fibroblasts play a key role in the initiation and progression of pulmonary fibrosis via its proliferation and synthesis of ECM components.[2] Inhibiting activation of fibroblasts may be a promising treatment for pulmonary fibrosis.
Exosomes are nanometer-sized vesicles that originate from the inward budding of endosomal membranes. They are constitutively secreted by almost all cell lineages, and carry bioactive proteins, lipids, RNA, as well as DNA molecules.[3,4] Exosomes mediated material transfer has been recognized as an important route for intercellular communication.[5,6] Increasing evidence demonstrates exosomes are involved in the progression of chronic inflammatory lung diseases including asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis.[7] For example, exosomes in IPF patients contain decreased levels of anti-fibrotic microRNAs (miRNAs) and increased levels of fibrogenic miRNAs compared with control.[8] However, the detailed mechanisms of its role in lung fibrosis are unknown.
Chronic inflammatory infiltration is universally present in fibrotic lesions.[9] As an essential inflammatory regulator, macrophages have been evidenced to promote fibrogenesis in cardiac muscle,[10] kidneys,[11] liver,[12] and lungs.[13] In fibrotic lung diseases, previous studies have proved that macrophages can exert their pro-fibrotic effects partially by activating lung fibroblasts.[13–15] However, whether exosomes mediate the interaction of macrophages and fibroblasts has not been reported.
Accumulating evidence shows angiotensin II (Ang II)/angiotensin II type 1 receptor (AT1R) axis is upregulated and involved in lung fibrosis[16,17] and increased Ang II/AT1R levels can exacerbate pulmonary fibrosis.[18] A recent discovery demonstrates that cardiac fibroblast-derived exosomes increase Ang II production and its receptor expression in cardiomyocytes, resulting in pathological cardiac hypertrophy.[19] Therefore, we hypothesized that macrophage-derived exosomes may promote collagen synthesis in lung fibroblasts via upregulating Ang II/AT1R axis.
In the present study, we explored a novel paracrine mechanism between macrophages and lung fibroblasts in Ang II/AT1R axis-mediated pathophysiological activation process leading to pulmonary fibrosis in vivo and in vitro. We demonstrated that Ang II stimulated macrophages to release AT1R-enriched exosomes, which promoted fibroblasts activation and lung fibrosis via transforming growth factor-β (TGF-β)/smad2/3 pathway by directly transferring AT1R to lung fibroblasts.
Methods
Ethical approval
All experimental procedures on mice were approved by the Committee on the Ethics of Animal Experiments of Southern Medical University (No. NFYY-2016-04) and were performed in accordance with the international ethics for animal use.
Materials
Ang II, irbesartan (IR), and GW4869, an exosome inhibitor, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bleomycin (BLM) was purchased from Nippon Kayaku (Tokyo, Japan). The other reagents are described below.
Animals
Male C57 mice (6–8 weeks old) were purchased from the Central Animal Care Facility of Southern Medical University (Permission No. SCXK 2009-015). Mice were housed in a specific pathogen-free room with 12-h light/dark cycle and controlled temperature and humidity, with free access to water and food.
Experimental design
We used male mice to establish three animal models. In the first model, 30 male mice were randomly divided into three groups (ten mice per group): the control group, the BLM group, and the BLM + GW4869 group. The two BLM groups received 100 μL sterile saline that contained 3.5 mg/kg BLM and the control group received equal volume of sterile saline. The BLM + GW4869 group was injected with GW4869 (2.5 mg/kg) twice a week and the other two groups received equal volume of sterile saline. The mice were sacrificed 4 weeks after BLM or BLM+GW4869 treatment, and lung tissues, bronchoalveolar lavage fluid (BALF), and serum were collected. Hematoxylin and eosin staining, Masson trichrome collagen staining, Ashcroft score, hydroxyproline contents and α-collagen I protein level determined by immunohistochemistry were used to confirm the successful establishment of BLM-induced pulmonary fibrosis model and verify if GW4869 could reduce the BLM-induced pulmonary fibrosis. AT1R protein and Ang II level (in serum and BALF) were detected by immunohistochemistry and ELISA respectively to assess the Ang II/AT1R axis.
In the second model (six mice per group at each time point), BLM group received 100 μL sterile saline that contained 3.5 mg/kg BLM and the control group received equal volume of sterile saline. Then mice were sacrificed at day 3, day 7, day 14, and day 28, respectively, and the lung tissues and BALF were obtained. Hematoxylin and eosin staining, Masson trichrome collagen staining, Ashcroft score and hydroxyproline contents were used to confirm the successful establishment of BLM-induced pulmonary fibrosis model at different time points. Expression of F4/80 (a macrophage marker) and the number of macrophages in BALF were used to evaluate macrophages changes in pulmonary fibrosis.
In the third model, 18 male mice were randomly divided into three groups (six mice per group): the control group, the exosomes group, and the exosomes + IR group. The two exosome groups received Ang II-stimulated macrophage exosomes (1 g/kg) through tail vein injection and the control group received equal volume of phosphate buffer saline once a week. Meanwhile, the exosomes + IR group received IR (2 mg/kg) in drinking water. The mice were sacrificed 4 weeks after BLM or exosomes treatment, and lung samples, BALF and serum were collected. Hematoxylin and eosin staining, Masson trichrome collagen staining, hydroxyproline contents and α-collagen I protein level determined by immunohistochemistry were used to assess the role of exosomes in lung fibrosis. The expression of F4/80 was used to evaluate the macrophage infiltration. AT1R protein and Ang II (in serum and BALF) were detected.
In the in vitro experiment, macrophages were stimulated with Ang II and the exosomes were isolated from condition media. Transmission electron microscopy, nanoparticle tracking analysis (NTA) and exosomal markers were used to demonstrate if the isolated vesicles were exosomes. PKH67-labeled macrophages or PKH67-labeled exosomes co-cultured with lung fibroblasts to confirm if the exosomes can be taken up by lung fibroblasts. Then, the lung fibroblasts were stimulated by the exoAng II-Mϕ 10 μg, exoAng II-Mϕ 30 μg or left untreated. The α-collagen I levels were detected to confirm whether macrophage exosomes can promote α-collagen I synthesis.
The lung fibroblasts were stimulated by the exoAng II-MΦ (exosomes derived from Ang II-stimulated macrophages) with untreated lung fibroblast as negative control and Ang II-stimulated lung fibroblasts as positive control. The expression of α-collagen I, AT1R, TGF-β, phospho-Smad2/3 (p-Smad2/3), Smad2/3 was detected by western blot. In addition, the lung fibroblasts were treated with exoAng II-Mϕ, exoAng II-Mϕ + IR or left untreated, and the levels of α-collagen I, AT1R, TGF-β, p-Smad2/3 and Smad2/3 were detected.
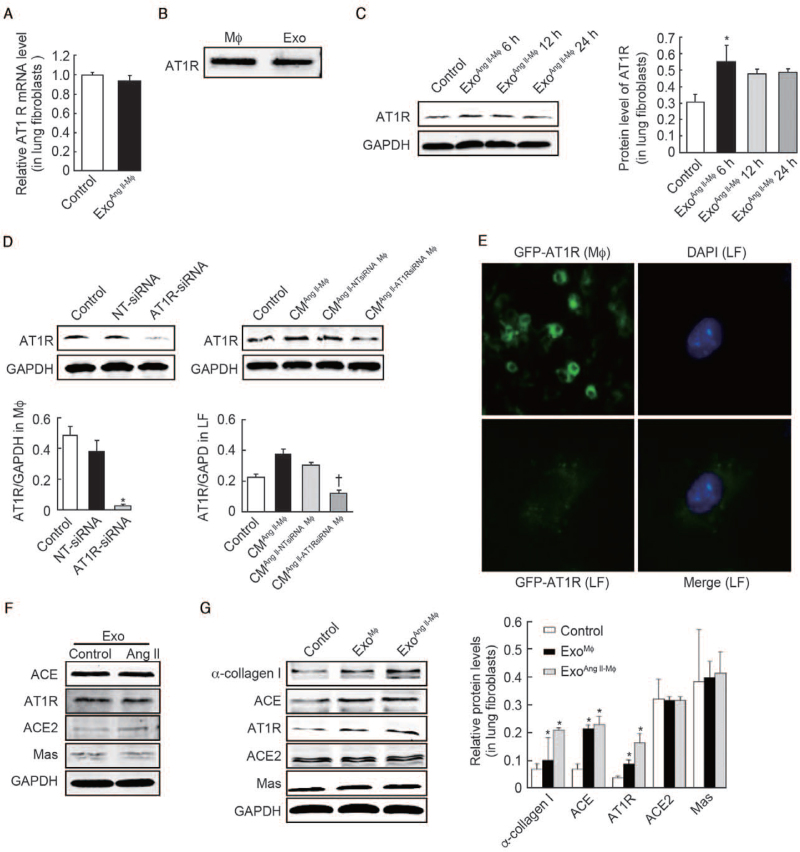
The lung fibroblasts were treated with exoAng II-MΦ or left untreated. The AT1R mRNA in lung fibroblasts and AT1R protein levels in exosomes were detected by PCR and western blot, respectively. Then, lung fibroblasts were treated with exosomes at different time points or left untreated (control, exoAng II-MΦ 6 h, exoAng II-Mϕ 12 h, exoAng II-Mϕ 24 h) and the levels of AT1R proteins were detected to confirm if the AT1R increase is directly derived from exosomes or not.
Macrophages were transfected with AT1R siRNA, NT-siRNA or left untreated and the AT1R protein expression was detected. Then, the macrophages were co-cultured with fibroblasts (control, condition medium [CM]Ang II-Mϕ, CMAng II-NT-siRNA Mϕ, CMAng II-AT1R-siRNA Mϕ) and the AT1R protein was detected to identify if the conditioned medium can induce AT1R expression after silencing AT1R in macrophages. Furthermore, AT1R in macrophages was tagged by GFP, and the exosomes derived from macrophages were co-cultured with fibroblasts. Fluorescence microscope analysis was performed to confirm if the macrophage exosomes directly transported AT1R to the lung fibroblasts.
Exosomes derived from macrophages were treated with Ang II or left untreated. Western blot was performed to examine the levels of ACE, AT1R, ACE2 and Mas protein. Moreover, lung fibroblasts were treated with exoMϕ, exoAng II-Mϕ or left untreated. The levels of α-collagen I, ACE, AT1R, ACE2, and Mas were detected to confirm if exosomes derived from macrophages treated with Ang II or not can promote α-collagen I synthesis.
Macrophages were treated with Ang II or left untreated, and the Alix and AT1R were detected. Moreover, the exosomes were collected from the same amount of macrophages stimulated with and without Ang II. The Alix and AT1R expression in exosomes was detected by western blot.
Lung fibroblasts were treated with exoAng II-Mϕ 10 μg, exoAng II-Mϕ 30 μg or left untreated. The Ang II levels in LF-CM were detected by enzyme-linked immunosorbent assay (ELISA).
Histological and immunochemical assessment
Lung tissues were fixed in 4% paraformaldehyde solution and processed into paraffin sections (5-mm thick). Then the sections were subjected to hematoxylin and eosin, immunohistochemistry and Masson trichrome collagen staining. The histopathological scoring of pulmonary fibrosis was performed according to the method reported by Ashcroft et al (Grade of fibrosis: 0-8; the higher the score, the more severe the fibrosis).[20] The concentration of hydroxyproline was measured according to the manufacturer's instructions (Hydroxyproline Assay Kit, Sigma, Nanjing, China).
For immunohistochemical staining, sections were stained with anti-F4/80, anti-AT1R, anti-TGF-β, and anti-collagen antibodies in a dilution of 1:200. The process of the experiment was performed as previously described.[18]
Cell lines and cell culture
Commercial RAW246.7 cell lines were purchased from American Type Culture Collection (ATCC) (Rockville, MD, USA) and cultured according to the manufacturer's instructions. Pulmonary primary fibroblasts were isolated from mice of 6 to 8 weeks old. The cells were cultured in Dulbecco's modified Eagle medium (GIBCO BRL, Life Technologies, Inc., Rockville, MD, USA) supplemented with 10% fetal bovine serum. In co-culture experiments, macrophages were cultured in 0.4 μm porous Transwell® inserts (Corning, New York, NY, USA) suspended over fibroblasts for 24 h.
Lentivirus transduction and RNA interference (RNAi)
AT1R-green fluorescent protein (GFP) labeled RAW264.7 was created using AT1R-GFP HIV lentivirus vector purchased from Genechem (Shanghai, China). Briefly, 20,000 cells were plated in each well of a 24-well plate and lentivirus was added 24 h later utilizing a multiplicity of infection of 100. Cells were left undisturbed for 96 h, then moved to flask and treated with puromycin (5 μg/mL) until all remaining cells were fluorescent and thereafter cultured normally.
RNAi was performed by the transfection of small interfering RNAs (siRNA) oligos using the Lipofectamine™ RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The sequence of the AT1R siRNA oligos was: CACTCAAGCCTGTCT. Macrophages were transfected with AT1R siRNA and the cultured medium was used to co-culture with fibroblasts, then the AT1R protein expression was detected.
Isolation and characterization of exosomes
For exosome isolation, cells were incubated in serum free medium for 12 h. Extraction and purification were conducted as previously described.[21] Exosome morphology was identified through transmission electron microscopy (JEM-2100F, Tokyo, Japan). The expression of Alix, CD9, and CD63 (surface marker of exosomes), was analyzed through western blot. The nanoparticle tracking analysis (NTA) (NS3000, Worcestershire, UK) was used for exosome tracking, distribution, and particle number counting.
Western blot and enzyme-linked immunosorbent assay (ELISA)
For western blot, proteins from cells or exosomes were loaded and separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies at 4°C overnight, including anti-collagen I, anti-AT1R, anti-Alix, anti-CD9, anti-CD63, anti-calnexin (1:1000, Proteintech, Wuhan, China), anti-TGF-β, anti-p-Smad2/3, anti-Smad2/3 (1:1000, Cell Signaling Technology, Inc., Danvers, MA, USA), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:7500, Ray Antibody Biotech, Beijing, China), and then incubated with anti-rabbit or anti-mouse near-infrared secondary antibodies (1:15,000, LI-COR, Inc., Lincoln, UK) for 1 h. The membrane was exposed to Odyssey® CLx Imager (LI-COR), and Odyssey Software (LI-COR) was used for capturing images and the data analysis.
Cell culture supernatants, BALF, and serum were analyzed for Ang II using Ang II ELISA kits (Suzhou Calvin Biotechnology Co., Ltd, Suzhou, China), according to the manufacturer's instructions.
Reverse transcription-polymerase chain reaction (RT-PCR) and RNA collection
The total RNA of cultured cells was isolated by using Trizol reagent (Invitrogen Life Technologies, Grand Island, NY, USA), according to the manufacturer's instructions. Reverse transcription was done using an RNA Reverse Transcription Kit (TAKARA, Kusatsu, Japan) for real time polymerase chain reaction (PCR). Primers for mouse AT1R were designed and synthesized by TAKARA. Real-time analysis was performed using SYBR Green PCR Master Mix (TAKARA) for real-time PCR. The messenger RNA (mRNA) expression of the target gene was normalized to GAPDH, and the 2-ΔΔCT method was used to calculate relative changes in gene expression.
Statistical analysis
All continuous data with normal distribution were shown as means ± standard deviation from three independent experiments performed in triplicate. The P values were calculated using Student's t test or one-way analysis of variance. A P value of <0.05 was considered to indicate a statistically significant result. Statistical analyses were performed using SPSS® software, version 13.0 (SPSS Inc, Chicago, IL, USA).
Results
Exosomes inhibitor attenuated BLM-induced pulmonary fibrosis in vivo
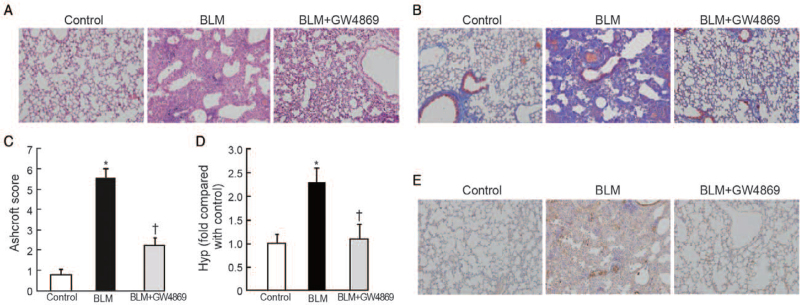
In the first animal model, GW4869, an exosome inhibitor,[22] markedly attenuated BLM-induced pulmonary fibrosis [Figure 1A and 1B] and decreased Ashcroft scores (2.25 ± 0.36 vs. 5.56 ± 0.41, t = 19.29, P < 0.001) [Figure 1C] and level of hydroxyproline (1.12 ± 0.36 vs. 2.26 ± 0.31, t = 7.00, P < 0.001) compared with the BLM group [Figure 1D]. Similarly, the increased protein level of collagen was abrogated by GW4869 infusion [Figure 1E]. Taken together, these results indicated that exosomes play a pro-fibrotic effect in BLM-induced pulmonary fibrosis model.
Figure 1.
Blockage of exosome secretion reversed BLM-induced pulmonary fibrosis. (A) Representative microphotographs of lung sections from controls, BLM, BLM + GW4869 (n = 10 mice per group, sacrificed at day 28) stained with (A) hematoxylin and eosin (H&E) and (B) Masson trichrome. Original magnification, ×200. (C) Morphological changes in fibrotic lungs were assessed using Ashcroft score. n = 10 mice per group, ∗P < 0.001 vs. control; †P < 0.001 vs. BLM. (D) Hydroxyproline contents in different groups. n = 10 mice per group, ∗P < 0.001 vs. control; †P < 0.001 vs. BLM. (E) Expression of α-collagen I proteins was detected using immunohistochemistry. Original magnification, ×200. BLM: Bleomycin; GW4869: An exosome inhibitor; Hyp: Hydroxyproline.
Number of macrophages was increased during lung fibrosis
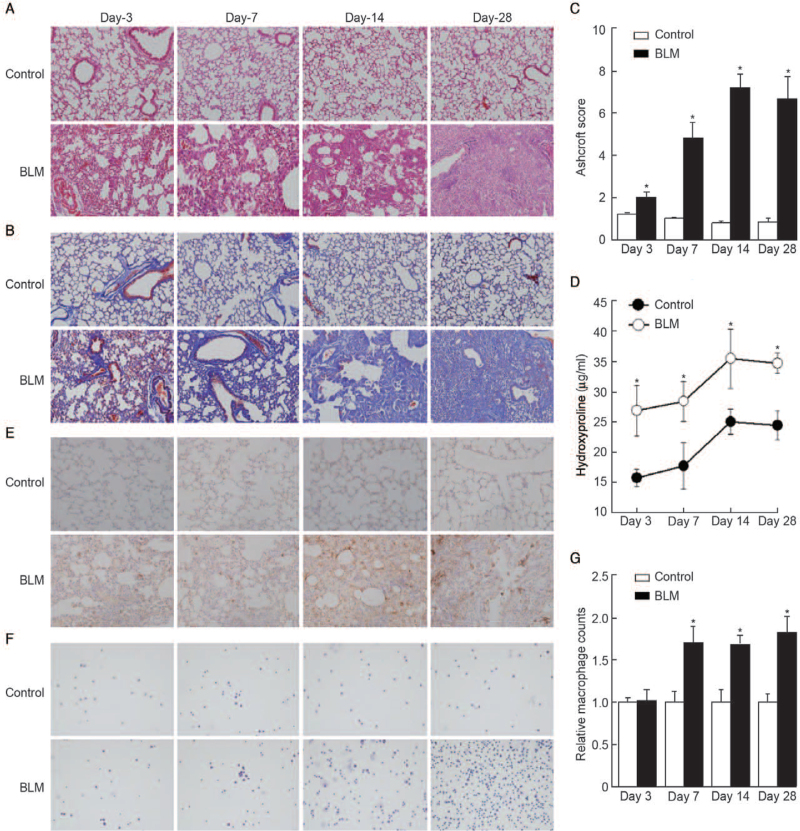
Macrophages play a key role in lung fibrosis. To evaluate the levels of macrophages in lung lesions at various phases in the development of lung fibrosis, BLM-induced fibrotic mice were sacrificed on day 3, day 7, day 14, and day 28 in the second animal model. Compared with the control, BLM-treated mice showed enhanced infiltration of inflammatory cells, the loss of normal alveolar structure, and extensive thickening of alveolar septa in a time-dependent manner [Figure 2A]. The fibrotic alterations became prominent over time, as demonstrated by Masson trichrome staining [Figure 2B], Ashcroft scores, and level of hydroxyproline (P < 0.05 compared with the control on day 3, day 7, day 14, and day 28, respectively) [Figure 2C and 2D]. The infiltrating macrophages were also measured by F4/80 staining, which indicated that BLM administration can cause a significant increase in the infiltration number of macrophages both in lung tissue [Figure 2E] and BALF (P < 0.05 compared with the control on day 7, day 14, and day 28, respectively) [Figure 2F and 2G].
Figure 2.
Macrophage infiltration correlates with lung fibrosis. Representative microphotographs of lung sections from control and BLM (sacrificed at day 3, day 7, day 14, day 28) stained with (A) H&E and (B) Masson trichrome. Original magnification, ×200. (C) Morphological changes in fibrotic lungs were quantified using Ashcroft score. (D) Hydroxyproline contents in different groups. (E) IHC staining was performed to determine the localization and expression of F4/80, a marker of macrophages. Original magnification, ×200. (F and G) The number of macrophages in BALF was measured. Original magnification, ×200. n = 6 mice per group, ∗P < 0.05 vs. control on day 3, day 7, day 14, day 28, respectively. BALF: Bronchoalveolar lavage fluid; BLM: Bleomycin; H&E: Hematoxylin and eosin; IHC: Immunohistochemistry.
Macrophage exosomes trafficked to lung fibroblasts and promoted α-collagen I synthesis
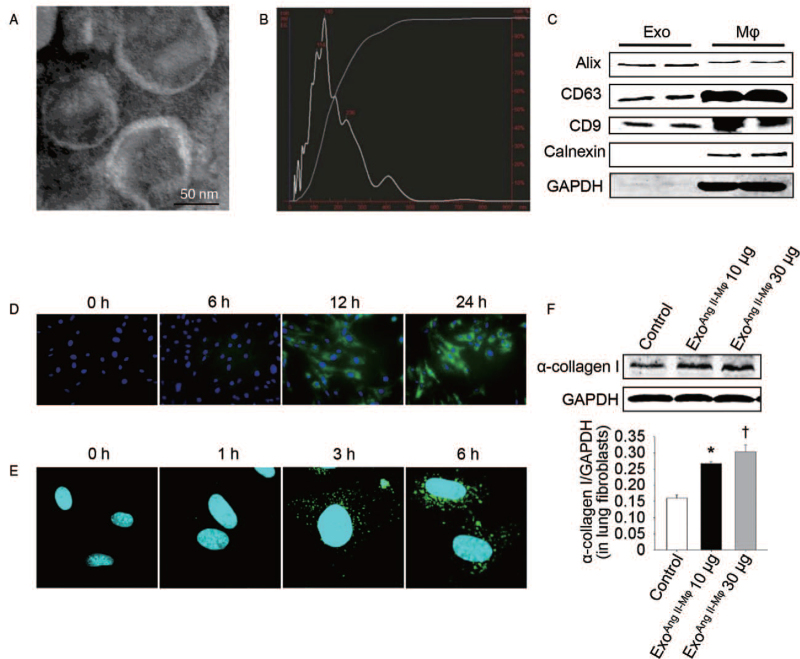
Exosome is an important media of intercellular communication by carrying various contents to recipient cells.[6] To find out whether it can play roles in the pro-fibrotic effects of macrophages by mediating the crosstalk of macrophages and fibroblasts, exosomes secreted from macrophages stimulated with Ang II (exoAng II-Mϕ), a proven pro-fibrotic factor,[17] were isolated from conditioned media using ultracentrifugation and the size and structure were confirmed by transmission electron microscopy [Figure 3A] as well as NTA [Figure 3B]. These isolated particles were round, vesicle-like with a diameter range of 40 to 150 nm, which were characteristic features of most exosomes.[6] Furthermore, they were positive for well-established exosomal makers (eg, Alix, CD9, CD63), but were negative for calnexin, a protein expressed in endoplasmic reticulum but not in exosomes [Figure 3C]. These results demonstrated that the isolated vesicles released from macrophages were exosomes. To figure out the effects of these exosomes on lung fibroblasts, we first tested whether lung fibroblasts could naturally take up macrophage-derived exosomes. PKH67-labeled macrophages and PKH67-labeled exosomes derived from macrophages were incubated with lung fibroblasts. As Figure 3D and 3E shown, macrophage exosomes were taken by lung fibroblasts. Then, lung fibroblasts were stimulated with macrophage exosomes directly and α-collagen I level was assessed. As expected, the expression level of α-collagen I (0.27 ± 0.02 vs. 0.16 ± 0.01, t = 7.01, P = 0.002, compared with the control group; 0.31 ± 0.02 vs. 0.27 ± 0.02, t = 2.94, P = 0.042, compared with the exoAng II-Mϕ10 μg group) was increased significantly in a dose dependent manner [Figure 3F].
Figure 3.
Exosomes mediate the effect of macrophage-induced α-collagen I synthesis in lung fibroblasts. (A) Morphology of macrophage exosomes determined by transmission electron microscopy (scale bar: 50 nm). (B) Nanoparticle tracking analysis (NTA) for quantitative measurement of isolated exosome particles. (C) Exosomal markers analyzed by western blot. (D) Fibroblasts were co-cultured with PKH67-labeled macrophages or (E) incubated with PKH67-labeled exosomes extracted from macrophages at different time points. Then the fibroblasts were stained with DAPI and analyzed by fluorescent microscopy or confocal microscopy, respectively. (F) Fibroblasts were treated with exosomes from Ang II-stimulated macrophages in doses of 10 μg/mL (exoAng II-Mϕ 10 μg) and 30 μg/mL (exoAng II-Mϕ 30 μg) for 24 h. Western blot was used to detect α-collagen I protein level. n = 3 independent experiments, ∗P < 0.05 vs. control group; †P < 0.05 vs. exoAng II-Mϕ10 μg group. Ang II: Angiotensin II; DAPI: 4-6 Diamidino-2-phenylindole; Exo: Exosomes; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; Mϕ: Macrophages.
Macrophage exosomes promoted α-collagen I synthesis and activated TGF-β/Smad2/3 pathway via upregulating Ang II/AT1R axis
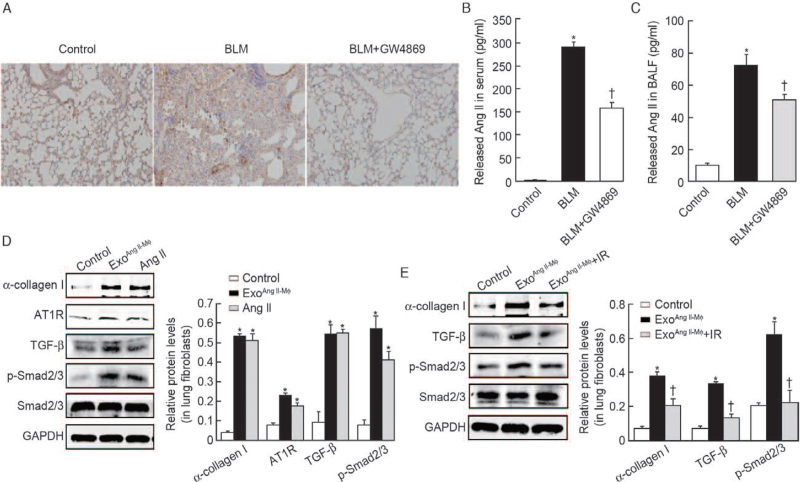
Our previous studies demonstrate that Ang II/AT1R axis plays a key role in the progression of lung fibrosis[2,17] and the TGF-β/Smad2/3 signal pathway is a classical pro-fibrotic pathway.[23] Consistently, we can see AT1R protein in lung tissue [Figure 4A] and the secretion of Ang II in serum (290.54 ± 21.65 vs. 2.98 ± 0.25, t = 41.99, P < 0.001) and BALF (72.93 ± 8.97 vs. 10.06 ± 0.25, t = 22.14, P < 0.001) was markedly enhanced in BLM group, which was markedly reduced by GW4869 treatment (165.84 ± 32.12 vs. 290.54 ± 21.65, t = 10.18, P < 0.001; 55.67 ± 7.43 vs. 72.93 ± 8.97, t = 4.68, P < 0.001) in BLM-induced lung fibrosis model (the first animal model) [Figure 4B and 4C]. As shown in Figure 4D, macrophage exosomes treatment increased the protein level of AT1R, TGF-β, p-Smad2/3 (0.22 ± 0.02 vs. 0.07 ± 0.02, t = 8.66, P = 0.001; 0.54 ± 0.05 vs. 0.09 ± 0.06, t = 10.00, P < 0.001 and 0.58 ± 0.06 vs. 0.07 ± 0.03, t = 12.86, P < 0.001; respectively) of lung fibroblasts in vitro. These results revealed that macrophage exosomes activated Ang II/AT1R axis and TGF-β/Smad2/3 pathway in lung fibroblasts. To investigate the functional link between macrophage exosomes-induced activation of Ang II/AT1R axis and collagen production in lung fibroblasts, we determined the effects of AT1R blocker IR on macrophage exosomes-induced collagen synthesis in lung fibroblasts. As shown in Figure 4E, macrophage exosomes-induced collagen synthesis was reversed by IR in lung fibroblasts, as well as the activation of TGF-β/Smad2/3 pathway (0.19 ± 0.04 vs. 0.38 ± 0.02, t = 6.77, P = 0.003; 0.13 ± 0.02 vs. 0.33 ± 0.01, t = 13.16, P < 0.001; and 0.21 ± 0.09 vs. 0.63 ± 0.08, t = 5.99, P < 0.001; for α-collagen I, TGF-β, p-Smad2/3, respectively). Therefore, macrophage exosomes promoted collagen synthesis and activated TGF-β/Smad2/3 pathway via upregulation of Ang II/AT1R axis in lung fibroblasts.
Figure 4.
Macrophage exosomes trigger α-collagen I production and activate TGF-β/Smad2/3 pathway by shifting the RAS toward ACE/Ang II/AT1R axis in lung fibroblasts. (A) IHC to analyze AT1R proteins expression in control, BLM, and BLM + GW4869 groups. Original magnification, ×200. Enzyme linked immunosorbent assay (ELISA) kit was used to determine Ang II level of serum (B) and BALF (C) in control, BLM and BLM + GW4869 groups. n = 10 mice per group; ∗P < 0.001 vs. control, †P < 0.001 vs. BLM, for Ang II in serum; ∗P < 0.001 vs. control, †P < 0.001 vs. BLM, for Ang II in BALF. (D) Primary lung fibroblasts were stimulated with exosomes isolated from Ang II treated-macrophages in a dose of 30 μg/mL. As a positive control, fibroblasts were treated with Ang II. After 24 h, the fibroblasts were subjected to western blot analysis of α-collagen I protein, AT1R, TGF-β, p-Smad2/3, and Smad2/3. n = 3 independent experiments; ∗P < 0.05 vs. control for α-collagen I, AT1R, TGF-β, p-Smad2/3, respectively. (E) Fibroblasts were pretreated with IR for 1 h before stimulation with macrophage exosomes for 24 h. The protein levels of α-collagen I, TGF-β, p-Smad2/3, and Smad2/3 were analyzed by western blot. n = 3 independent experiments; ∗P < 0.05 vs. control; †P < 0.05 vs. exoAng II-Mϕ for collagen I, TGF-β, and p-Smad2/3, respectively. ACE: Angiotensin-converting enzyme; Ang II: Angiotensin II; AT1R: Angiotensin II type 1 receptor; BALF: Bronchoalveolar lavage fluid; BLM: Bleomycin; exo: Exosomes; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GW4869: An exosome inhibitor; IHC: Immunohistochemistry; IR: Irbesartan; p-Smad: Phosphorylated-Smad; RAS: Renin-angiotensin system; TGF-β: Transforming growth factor-β.
Macrophage exosomes directly transported AT1R to the lung fibroblasts
We then wondered how AT1R in lung fibroblasts was upregulated. First, we examined AT1R mRNA level of lung fibroblasts after treated with macrophage exosomes. As Figure 5A shows, AT1R mRNA level remained unchanged compared with control (P > 0.05). Gianluigi et al[24] first demonstrated circulating exosomes induced by cardiac pressure overload contain functional AT1R. Therefore, we hypothesized that macrophage exosomes may contain AT1R and can directly deliver AT1R to lung fibroblasts. Western blot showed that AT1R was abundant in exosomes [Figure 5B]. In addition, the protein level of AT1R in fibroblasts was significantly increased after 6 h of stimulation with macrophage exosomes (0.55 ± 0.11 vs. 0.31 ± 0.05, t = 3.44, P = 0.026) [Figure 5C]. Next, AT1R siRNA was applied to silence AT1R in macrophages. A significant decrease in AT1R expression (0.03 ± 0.01 vs. 0.50 ± 0.05, t = 15.97, P < 0.001) was observed in macrophages with AT1R siRNA [Figure 5D]. After AT1R silence, the conditioned media (CMAng II+AT1RsiRNA-Mϕ) failed to induce AT1R expression in lung fibroblasts (0.12 ± 0.03 vs. 0.23 ± 0.02, t = 5.12, P = 0.007) [Figure 5D]. To further confirm the hypothesis, macrophage AT1R was tagged by GFP and then exosomes were isolated and incubated with lung fibroblasts. As expected, the GFP-tagged AT1R was detected in lung fibroblasts [Figure 5E]. Taken together, these results clearly demonstrated that AT1R protein was delivered into lung fibroblasts by exosomes.
Figure 5.
AT1R-containing exosomes directly deliver AT1R to lung fibroblasts. (A) The AT1R mRNA levels of lung fibroblasts were measured using RT-PCR after treatment with macrophage exosomes. (B) Western blot analysis of AT1R protein level in macrophages and exosomes isolated from activated macrophages. (C) Fibroblasts were treated with exosomes isolated from macrophages. Then the protein level of AT1R was analyzed by western blot at different time points. n = 3 independent experiments, ∗P < 0.05 vs. control. (D) Macrophages were transfected with AT1R siRNA for 48 h, followed by 24 h exposure to 10−7 mol/L Ang II, then the cultured medium was collected for another 24 h and was used to co-culture with fibroblasts. The protein levels of AT1R in macrophages (left) and fibroblasts (right) were measured using western blot. n = 3 independent experiments, ∗P < 0.001, †P < 0.01 vs. control. (E) Fluorescence microscope analysis of macrophages expressing AT1R-green fluorescent protein (GFP) and green fluorescence was observed in lung fibroblasts (LF) when incubated with exosomes collected from overlying media of macrophages expressing AT1R-GFP. (F) Western blot analysis of ACE, AT1R, ACE2, Mas protein levels in exosomes from macrophages treated with or without Ang II. (G) Fibroblasts were treated with exosomes from macrophages treated with Ang II or left untreated. Then the protein levels of α-collagen I, ACE, AT1R, ACE2, Mas were analyzed by western blot. n = 3 independent experiments, ∗P < 0.05 vs. control. ACE: Angiotensin-converting enzyme; ACE2: Angiotensin-converting enzyme 2; Ang II: Angiotensin II; AT1R: Angiotensin II type 1 receptor; CM: Condition media; DAPI: 4-6 diamidino–2–phenylindole; Exo: Exosomes; GAPDH: Glyceraldehyde-3-3phosphate dehydrogenase; GFP: Green fluorescent protein; LF: Lung fibroblasts; Mϕ: Macrophages; mRNA: Messenger RNA; siRNA: Small interfering RNA; RT-PCR: Reverse transcription-polymerase chain reaction.
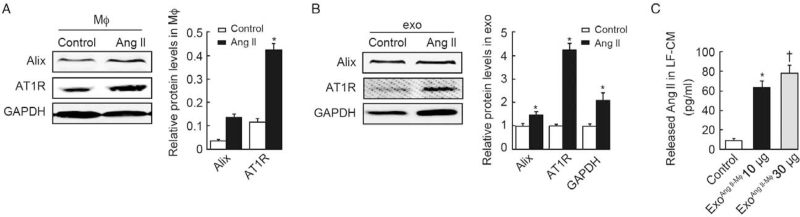
Ang II induced increased production of exosomes by macrophages
Intriguingly, AT1R in the same amount of exosomes from macrophages with or without Ang II stimulation had no significant difference [Figure 5F]. Moreover, the same amount of exosomes from macrophages with or without Ang II was used to treat the lung fibroblasts and exhibited similar impact on collagen synthesis and AT1R expression in fibroblasts [Figure 5G]. As our previous studies showed Ang II infusion can aggravate BLM-induced lung fibrosis[17] and the present results found macrophage exosomes induced collagen synthesis in a dose-dependent manner, we wonder whether Ang II exert its pro-fibrotic effects by increasing the secretion of exosomes. To explore this, we measured the level of Alix and AT1R in Ang II-treated macrophages and found they were increased compared with control (0.14 ± 0.02 vs. 0.04 ± 0.01, t = 7.90, P = 0.001; 0.43 ± 0.04 vs. 0.11 ± 0.02, t = 12.39, P = 0.002, respectively) [Figure 6A]. In addition, after Ang II stimulation, the protein levels of Alix, AT1R, and GAPDH were fold increased in exosomes secreted by the same number of macrophages (1.45 ± 0.15 vs. 1.00 ± 0.10, t = 4.32, P = 0.012; 4.05 ± 0.64 vs. 1.00 ± 0.09, t = 8.17, P = 0.001; 2.13 ± 0.36 vs. 1.00 ± 0.10, t = 5.28, P = 0.006, respectively) [Figure 6B]. It has been reported that cardiac fibroblast-derived exosomes stimulated a dramatic increase in Ang II secretion in cultured neonatal cardiomyocytes.[19] Consistently, we found that the level of Ang II (62.27 ± 7.32 vs. 9.56 ± 1.68, t = 12.16, P < 0.001 compared with the control; 79.79 ± 7.35 vs. 62.27 ± 7.32, t = 2.92, P = 0.043 compared with exoAng II-Mϕ 10 μg; respectively) was markedly increased in the cultural supernatant of lung fibroblasts treated with macrophage exosomes (30 μg/mL) [Figure 6C]. Therefore, there was a positive feedback loop between Ang II generation and exosomes secretion.
Figure 6.
Ang II induces increased production of exosomes by macrophages. Western blot analyzed the protein levels of Alix and AT1R in (A) macrophages or (B) exosomes derived from the same amount of macrophages treated with Ang II or left untreated. n = 3 independent experiments, ∗P < 0.05 vs. control. (C) Assessment of Ang II levels in LF-CM after exosomes exposure. n = 3 independent experiments; ∗P < 0.001 vs. control; †P < 0.05 vs. exoAng II-Mϕ 10 μg. LF-CM: Condition media of lung fibroblasts; exo: Exosomes; Ang II; Angiotensin II; AT1R: Angiotensin II type 1 receptor; Mϕ: Macrophages; GAPDH: Glyceraldehyde-3-3phosphate dehydrogenase.
Exosomes-derived from macrophages promoted inflammation of lung via upregulating Ang II/AT1R axis
To further demonstrate the effects of exosomes derived from activated macrophages and the detailed molecular mechanism, we injected macrophage exosomes into the tail vein of mice once a week. Four times later, we observed changes in the margin area [Supplementary Figure 1A and 1B] with elevated hydroxyproline level (5.14 ± 0.95 vs. 2.87 ± 0.42, t = 5.34, P < 0.001 compared with control) [Supplementary Figure 1C] and more macrophages infiltration [Supplementary Figure 1D], which can be prevented by IR, an AT1R inhibitor (3.62 ± 0.89 vs. 5.14 ± 0.95, t = 2.86, P = 0.017 compared with exoAngII-Mϕ) [Supplementary Figure 1C]. We also found the Ang II (66.52 ± 6.87 vs. 51.94 ± 1.98, t = 59.38, P < 0.001 in serum; 42.80 ± 3.51 vs. 7.34 ± 2.05, t = 5.22, P < 0.001 in BALF; Supplementary Figure 1E and 1F)/AT1R axis and TGF-β pathway [Supplementary Figure 1G] were activated in exosomes treated mice as that in BLM-induced pulmonary fibrosis model. In this section, these results confirmed that macrophage exosomes promote lung inflammatory response and up-regulate Ang II/AT1R axis.
Discussion
In this study, for the first time, we demonstrated that AT1R-containing exosomes derived from macrophages exhibited pro-fibrotic effects via upregulating the Ang II/AT1R axis in lung fibroblasts or fibrotic lung tissue. In turn, increased Ang II caused macrophages to increase the production of exosomes. The principal findings obtained included the following aspects: (1) GW4869 exhibited excellent anti-fibrotic effect. (2) Macrophage exosomes contained AT1R and directly delivered AT1R to lung fibroblasts, resulting in collagen synthesis via upregulating Ang II/AT1R/TGF-β axis.
Exosomes, one form of extracellular vesicles, are increasingly regarded as playing crucial roles in cell-cell communications.[25] Resent research shows exosomes are involved in the pathological and physiological process in the fibrotic diseases of multiple organs, such as heart, liver, and kidney.[4,25,26] In recent years, researchers found that exosomes play a variety of roles in lung fibrosis.[8,27,28] However, little is known regarding the effects of exosomes in animal models of pulmonary fibrosis. In our observations, we found the changes associated with fibrosis can be reversed by exosome inhibitor GW4869 in BLM-induced pulmonary fibrosis models. The anti-fibrotic effect of GW4869 was amazing and caught our attention. Previous studies showed that GW4869, a neutral sphingolipase inhibitor, plays pivotal roles in various cellular processes, such as inflammation and apoptosis.[29,30] Thus, the precise molecular mechanism by which it exerted this effect is complicated and needs to be elucidated. In this paper, we focus on the role of exosomes.
Increasing evidence confirms macrophages play a pivotal role in the pathogenesis of lung fibrosis.[14,31]In vivo, we found that macrophages infiltration significantly increased during the whole process of BLM-induced lung fibrosis. It has been reported that macrophages affected the function of fibroblasts through the paracrine activity of various cytokines. However, it remained unknown whether exosomes can mediate the crosstalk between macrophages and fibroblasts. In our observations, we found macrophage-secreted exosomes can be taken up by lung fibroblasts and promote collagen expression. In vivo, macrophage exosomes injection promoted inflammation in mouse lung. Thus, it was clear that exosomes derived from macrophages acted as a pro-fibrotic mediator in lung tissue. Although the pro-fibrotic effect of macrophage exosomes was clear, the precise molecular mechanism by which it exerted this effect needs to be elucidated.
The angiotensin-converting enzyme2 (ACE2)/angiotensin (1-7)/Mas axis and the ACE/Ang II/AT1R axis are main components of renin-angiotensin system (RAS) and the two axes have opposite effects. Imbalance of the two axes, especially overactivation of the ACE/Ang II/AT1R axis, plays a vital role in the fibrosis of different organs including the lung.[2,32] In support of these findings, we found that AT1R and Ang II levels were significantly increased in BLM-induced pulmonary fibrosis model, as well as the macrophage exosomes treated mice. Moreover, we found that the cargos of macrophage exosomes contained the components of RAS, such as ACE, AT1R, ACE2, and Mas. Especially, the Ang II/AT1R axis was highly activated in lung fibroblasts when stimulated with exosomes derived from macrophages. The pro-fibrotic effects of macrophage exosomes and the hyperactive Ang II/AT1R axis were neutralized by IR. In brief, macrophage exosomes promoted collagen synthesis and exacerbated pulmonary fibrosis via activating Ang II/AT1R axis.
TGF-β/Smad2/3 signal pathway has been heavily implicated in the development of fibrosis in various organs, including heart, kidneys, liver, and lungs.[33] In this study, macrophage exosomes activated lung fibroblasts, up-regulated TGF-β/Smad2/3 pathway, and promoted collagen synthesis. By inhibiting AT1R, IR reversed macrophage exosomes-induced upregulation of TGF-β/Smad2/3 pathway, as well as the production of collagen. Thus, macrophage exosomes promoted collagen synthesis via the TGF-β/Smad2/3 pathway by up-regulating Ang II/AT1R axis.
Next, we wonder how macrophage-exosomes increase AT1R expression in lung fibroblasts. In the study of cardiac disease, Lyu et al[19] reported that the mRNA levels of AT1R, angiotensin II type 2 receptor, ACE, ACE2, and angiotensinogen are significantly increased in neonatal rat cardiomyocytes treated with cardiac fibroblasts-derived exosomes. However, in our results, treatment with macrophage exosomes caused little change of AT1R mRNA in lung fibroblasts, while the protein level of AT1R was markedly increased just at 6 h. Thus, we conclude that the increased protein level of AT1R is not due to the increased transcription of AT1R mRNA. Recent review shows protein, RNA, DNA, lipids, and metabolites containing in exosomes can be transferred to recipient cells and exert corresponding functional effects on the pathological and physiological process of multiple diseases.[34,35] What's more, an article published in the Journal of Molecular and Cellular Cardiology[19] shows circulating exosomes induced by cardiac pressure overload contain functional AT1Rs and the AT1R-enriched exosomes mediate the transfer of AT1R to recipient cells. Consistent with these findings, western blot shows AT1R protein was positive and enriched in exosomes derived from macrophages. In addition, GFP-tagged AT1R was detected in lung fibroblasts incubated with exosomes derived from macrophages. Collectively, these results clearly demonstrated that the membrane protein AT1R was delivered into lung fibroblasts by macrophage-exosomes.
Increasing evidence elucidated that Ang II stimulation increased the secretion of exosomes.[19,24,36] Our data showed the relative quantity of exosomes secreted by macrophages was markedly increased after Ang II treatment, and the Ang II level was higher in LF-CM after treatment with exosomes which formed a positive feedback loop with the activation of Ang II/AT1R axis.
Conclusions
In conclusion, our findings demonstrated that AT1R-containing exosomes derived from macrophages can activate TGF-β/Smad2/3 pathway, promote collagen synthesis and mediate BLM-induced pulmonary fibrosis via shifting RAS to Ang II/AT1R axis. Consequently, these results revealed that exosomes can mediate cell to cell communication and participate in the initiation and progression of pulmonary fibrosis, indicating inhibition of Ang II-induced exosomes secretion from macrophages may be a promising approach for prevention and treatment of pulmonary fibrosis.
Funding
This study was supported by the Science and Technology Project in Guangzhou (No. 201904010482) and the National Natural Science Foundation of China (Nos. 81570064, 81870068, and 82070063).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Sun NN, Zhang Y, Huang WH, Zheng BJ, Jin SY, Li X, Meng Y. Macrophage exosomes transfer angiotensin II type 1 receptor to lung fibroblasts mediating bleomycin-induced pulmonary fibrosis. Chin Med J 2021;134:2175–2185. doi: 10.1097/CM9.0000000000001605
Na-Na Sun and Yue Zhang contributed equally to this study.
Supplemental digital content is available for this article.
References
- 1.Ballinger MN, Newstead MW, Zeng X, Bhan U, Mo XM, Kunkel SL, et al. IRAK-M promotes alternative macrophage activation and fibroproliferation in bleomycin-induced lung injury. J Immunol 2015; 194:1894–1904. doi: 10.4049/jimmunol.1402377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang MX, et al. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid Redox Signal 2015; 22:241–258. doi: 10.1089/ars.2013.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobb RJ, Lima LG, Möller A. Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol 2017; 67:3–10. doi: 10.1016/j.semcdb.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 2013; 24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther 2017; 174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 6.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 7.Hough KP, Chanda D, Duncan SR, Thannickal VJ, Deshane JS. Exosomes in immunoregulation of chronic lung diseases. Allergy 2017; 72:534–544. doi: 10.1111/all.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alipoor SD, Mortaz E, Garssen J, Movassaghi M, Mirsaeidi M, Adcock IM. Exosomes and exosomal miRNA in respiratory diseases. Mediators Inflamm 2016; 2016:5628404.doi: 10.1155/2016/5628404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wermuth PJ, Jimenez SA. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med 2015; 4:2.doi: 10.1186/s40169-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang NP, Erskine J, Zhang WW, Zheng RH, Zhang LH, Duron G, et al. Recruitment of macrophages from the spleen contributes to myocardial fibrosis and hypertension induced by angiotensin II. J Renin Angiotensin Aldosterone Syst 2017; 18:1470320317706653.doi: 10.1177/1470320317706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiteras R, Flaquer M, Cruzado JM. Macrophage in chronic kidney disease. Clin Kidney J 2016; 9:765–771. doi: 10.1093/ckj/sfw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bility MT, Nio K, Li F, McGivern DR, Lemon SM, Feeney ER, et al. Chronic hepatitis C infection-induced liver fibrogenesis is associated with M2 macrophage activation. Sci Rep 2016; 6:39520.doi: 10.1038/srep39520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang J, Cheng S, Feng T, Wu Y, Xie W, Zhang M, et al. Neotuberostemonine attenuates bleomycin-induced pulmonary fibrosis by suppressing the recruitment and activation of macrophages. Int Immunopharmacol 2016; 36:158–164. doi: 10.1016/j.intimp.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Boorsma CE, Draijer C, Melgert BN. Macrophage heterogeneity in respiratory diseases. Mediators Inflamm 2013; 2013:769214.doi: 10.1155/2013/769214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol 2019; 20:163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Díez-Freire C, Dooies A, et al. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2010; 182:1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Y, Pan M, Zheng B, Chen Y, Li W, Yang Q, et al. Autophagy attenuates angiotensin ii-induced pulmonary fibrosis by inhibiting redox imbalance-mediated NOD-like receptor family pyrin domain containing 3 inflammasome activation. Antioxid Redox Signal 2019; 30:520–541. doi: 10.1089/ars.2017.7261. [DOI] [PubMed] [Google Scholar]
- 18.Meng Y, Yu CH, Li W, Li T, Luo W, Huang S, et al. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am J Respir Cell Mol Biol 2014; 50:723–736. doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- 19.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, et al. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 2015; 89:268–279. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988; 41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 2017; 8:15287.doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging 2014; 35:1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachapelle P, Li M, Douglass J, Stewart A. Safer approaches to therapeutic modulation of TGF-beta signaling for respiratory disease. Pharmacol Ther 2018; 187:98–113. doi: 10.1016/j.pharmthera.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015; 131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 2014; 124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep 2017; 7:3710.doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Medina A, Lehmann M, Burgy O, Hermann S, Baarsma HA, Wagner DE, et al. Increased extracellular vesicles mediate WNT5A signaling in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2018; 198:1527–1538. doi: 10.1164/rccm.201708-1580OC. [DOI] [PubMed] [Google Scholar]
- 28.Njock MS, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F, et al. Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax 2019; 74:309–312. doi: 10.1136/thoraxjnl-2018-211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, et al. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem 2002; 277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Uhlig S. The role of sphingolipids in respiratory disease. Ther Adv Respir Dis 2011; 5:325–344. doi: 10.1177/1753465811406772. [DOI] [PubMed] [Google Scholar]
- 31.Gwyer Findlay E, Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm 2012; 2012:140937.doi: 10.1155/2012/140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 2013; 169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol 2017; 8:461.doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maas S, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 2017; 27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun 2017; 8:15016.doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Zhang C, Liu L, A X, Chen B, Li Y, et al. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther 2017; 25:192–204. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.