PURPOSE
Endocrine therapy resistance in advanced breast cancer remains a significant clinical problem that may be overcome with the use of histone deacetylase inhibitors such as entinostat. The ENCORE301 phase II study reported improvement in progression-free survival (PFS) and overall survival (OS) with the addition of entinostat to the steroidal aromatase inhibitor (AI) exemestane in advanced hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer.
PATIENTS AND METHODS
E2112 is a multicenter, randomized, double-blind, placebo-controlled phase III study that enrolled men or women with advanced HR-positive, HER2-negative breast cancer whose disease progressed after nonsteroidal AI. Participants were randomly assigned to exemestane 25 mg by mouth once daily and entinostat (EE) or placebo (EP) 5 mg by mouth once weekly. Primary end points were PFS by central review and OS. Secondary end points included safety, objective response rate, and lysine acetylation change in peripheral blood mononuclear cells between baseline and cycle 1 day 15.
RESULTS
Six hundred eight patients were randomly assigned during March 2014-October 2018. Median age was 63 years (range 29-91), 60% had visceral disease, and 84% had progressed after nonsteroidal AI in metastatic setting. Previous treatments included chemotherapy (60%), fulvestrant (30%), and cyclin-dependent kinase inhibitor (35%). Most common grade 3 and 4 adverse events in the EE arm included neutropenia (20%), hypophosphatemia (14%), anemia (8%), leukopenia (6%), fatigue (4%), diarrhea (4%), and thrombocytopenia (3%). Median PFS was 3.3 months (EE) versus 3.1 months (EP; hazard ratio = 0.87; 95% CI, 0.67 to 1.13; P = .30). Median OS was 23.4 months (EE) versus 21.7 months (EP; hazard ratio = 0.99; 95% CI, 0.82 to 1.21; P = .94). Objective response rate was 5.8% (EE) and 5.6% (EP). Pharmacodynamic analysis confirmed target inhibition in entinostat-treated patients.
CONCLUSION
The combination of exemestane and entinostat did not improve survival in AI-resistant advanced HR-positive, HER2-negative breast cancer.
INTRODUCTION
Endocrine therapy is the backbone of systemic treatment for advanced hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer.1 Treatment resistance, however, is a significant clinical problem that may be overcome by combining endocrine therapies with agents targeting resistance mechanisms.2 In recent years, a number of agents that improve patient outcomes have been approved by regulatory agencies in this setting, including those targeting the mammalian target of rapamycin, phosphatidylinositide 3-kinase, and cyclin-dependent kinase (CDK) pathways.3-5 However, ongoing development of novel strategies is essential as drug resistance ultimately develops despite this broadening treatment portfolio.
CONTEXT
Key Objective
E2112 was a double-blind placebo-controlled phase III trial that investigated whether the addition of the histone deacetylase inhibitor entinostat to exemestane would improve progression-free and/or overall survival in patients with advanced hormone receptor–positive, human epidermal growth factor receptor 2–negative breast cancer resistant to a nonsteroidal aromatase inhibitor.
Knowledge Generated
Despite supporting preclinical and phase II clinical data, the trial did not meet either coprimary end point and thus does not support a role for entinostat in this setting. Pharmacodynamic analysis confirmed target inhibition in entinostat-treated patients.
Relevance
Results from ongoing correlative analysis and other clinical trials may clarify a role for histone deacetylase inhibitors in a biomarker-selected population or in other breast cancer settings.
Epigenetic modification alters gene expression leading to endocrine therapy resistance and may be reversed by epigenetic modifiers such as histone deacetylase (HDAC) inhibitors.6-8 Entinostat, an oral HDAC inhibitor, induces protein lysine acetylation in preclinical models, which leads to downregulation of estrogen-independent growth factor signaling pathways and normalization of estrogen receptor levels.9,10 Entinostat has also overcome endocrine therapy resistance in letrozole-resistant mouse models.7 On the basis of these promising data, the ENCORE301 randomized phase II study was conducted in patients with advanced HR-positive, HER2-negative breast cancer.11 An improvement in progression-free survival (PFS) and overall survival (OS) was observed with the addition of entinostat to the steroidal aromatase inhibitor (AI) exemestane. Interestingly, protein lysine acetylation in peripheral blood mononuclear cells (PBMCs) was associated with prolonged PFS in the entinostat arm.
In the phase III confirmatory E2112 trial, we hypothesized that the addition of entinostat to exemestane would improve PFS and/or OS in patients with advanced breast cancer resistant to a nonsteroidal AI (NCT02115282).
PATIENTS AND METHODS
Eligibility
Eligible patients were adult women and men who had histologically confirmed invasive adenocarcinoma of the breast, metastatic or locally advanced and not amenable to local therapy with curative intent. Tumors (from primary or metastatic sites) must have expressed estrogen receptor and/or progesterone receptor, with ≥ 1% staining of cells being considered positive, and be HER2-negative defined by international guidelines.12,13 Study participants must have experienced disease progression after nonsteroidal AI use in the adjuvant (progression on or within ≤ 12 months of completion) or metastatic setting.
One prior chemotherapy for metastatic disease, prior treatment with fulvestrant, and prior CDK inhibitor were permitted but not required and must have been completed two weeks before random assignment. Eastern Cooperative Oncology Group performance status 0-1 with measurable or nonmeasurable (limited to 20% of the study population) disease was required. Exclusion criteria included a history of prior exemestane use (other than within 4 weeks of study entry), HDAC inhibitor use (eg, valproic acid), and central nervous system metastases. Participants signed a written informed consent approved by the Central Institutional Review Board of the National Cancer Institute or by the participating institution's local Institutional Review Board.
Study Design
In this international, double-blind, placebo-controlled phase III trial, participants were equally randomly assigned to treatment with exemestane and entinostat (EE) or placebo (EP). The treatments were assigned using permuted blocks within strata, with dynamic balancing within main institutions and their affiliate networks. The coprimary end points of the trial were PFS based on central review, and OS. Secondary end points included toxicity, time to treatment deterioration (TTD), objective response rate (ORR), patient-reported outcomes (PROs), adherence to protocol therapy, and association between lysine acetylation change and PFS or OS. Stratification factors included the setting in which resistance to prior nonsteroidal AI developed (adjuvant or metastatic), geographic region, presence of visceral disease, and prior fulvestrant use; the latter added as a stratification factor after a Protocol amendment permitted prior fulvestrant.
Participants received exemestane 25 mg by mouth once daily and entinostat or placebo 5 mg by mouth once every week (28-day cycle) until disease progression or unacceptable toxicity. Entinostat or placebo was taken on an empty stomach, at least 1 hour before and 2 hours after a meal or snack. A dosage of 3.6 mg of goserelin was administered subcutaneously to pre- and perimenopausal female and male participants on day 1 of each cycle. Dose modifications are detailed in the study Protocol (online only). Dose reduction of entinostat or placebo to 3 mg because of toxicity was permitted; protocol therapy was permanently discontinued if more than two doses of entinostat or placebo were omitted in a single cycle because of entinostat- or placebo-related toxicity. Exemestane dose reductions were not permitted.
Tumor status was assessed at baseline with computed tomography of chest and computed tomography or magnetic resonance imaging of abdomen and pelvis, and bone scan, with reassessment every 12 weeks while on treatment and until first progression of disease. Adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events (version 4.0) during study treatment and at 30 days after last dose of protocol therapy. Peripheral blood samples were obtained at baseline and 15 days after treatment initiation (C1D15) for lysine acetylation analysis by multiparameter flow cytometry of PBMCs (integrated biomarker).11 The PRO assessment schedule is outlined in the study Protocol and will be reported separately (Protocol).
The trial was conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki. The conduct of the trial was monitored by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Data and Safety Monitoring Committee twice annually, including safety, study progress, and interim efficacy results.
Statistical Considerations
A sample size of 600 participants and 410 deaths were required for 80% power to detect a 25% reduction in the OS failure hazard rate (median OS: 22 v 29.3 months), with one-sided type I error of 2.4%. The PFS end point was tested in the first 360 participants and was based on central imaging review by the American College of Radiology Center for Research and Innovation. With 247 PFS events, the study had 88.5% power to detect a 42% reduction in the PFS failure hazard rate (median PFS: 4.1 v 7.1 months), with one-sided type I error of 0.1%. PFS was monitored using a linear 20% inefficacy boundary method, and OS was monitored using group sequential method. An interim futility analysis plan for PFS was incorporated in the study design (linear 20% inefficacy boundary method), as well as an interim efficacy and futility analysis plan for OS (truncated Lan-DeMets spending function corresponding to the O'Brien-Fleming boundary).
The primary analysis of PFS in the intent-to-treat population included follow-up through the 247th PFS event defined by central review within the first 360 patients enrolled. PFS was defined to be time from random assignment to the earliest documented disease progression as defined by RECIST version 1.1 criteria, new primary breast cancer, or death without progression. Cases with incomplete follow-up or without adequate disease evaluations were censored at the date last documented to be free of progression, regardless of whether nonprotocol anticancer therapy was started or not. Patients without any postrandomization imaging were censored at random assignment. OS was defined to be time from random assignment to death from any cause. Cases still alive were censored at the date last known alive. The distributions of PFS and OS were estimated using the Kaplan-Meier method, with 95% CIs calculated using Greenwood's formula. Sensitivity analyses performed for PFS end point included analyses of eligible patients and consideration of those receiving nonprotocol therapy before disease progression as PFS events. Difference in treatment effect was tested using stratified log-rank tests, and treatment effect was estimated via stratified Cox proportional hazard models.
The incidence of treatment-related AEs of any grade and grades 3-5 was summarized and compared between treatment arms using Fisher's exact test. TTD was defined as time from random assignment to disease progression, death, or worsening of symptoms, whichever occurred first. ORR was defined as proportion of patients with best overall response of complete response or partial response among patients with measurable disease. ORR was summarized along with the exact binomial 95% CI and compared between the two arms using Fisher's exact tests. In all analyses, P values were two-sided. A level of .002 was considered statistically significant for stratified log-rank test for the primary PFS analysis. For the OS comparison, the nominal significance level for stratified log-rank test was .037. A level of .05 was used for statistical significance for all other tests. All analyses were conducted using STATA v16.
This report was based on data available as of May 5, 2020, except for the PFS end point. The central review data for the PFS end point was finalized on September 4, 2018, after 249 PFS events in the first 360 patients. The research Protocol and article were written by the authors and reviewed by the pharmaceutical funders, who were not involved in study analysis or interpretation of results.
RESULTS
Patient Characteristics
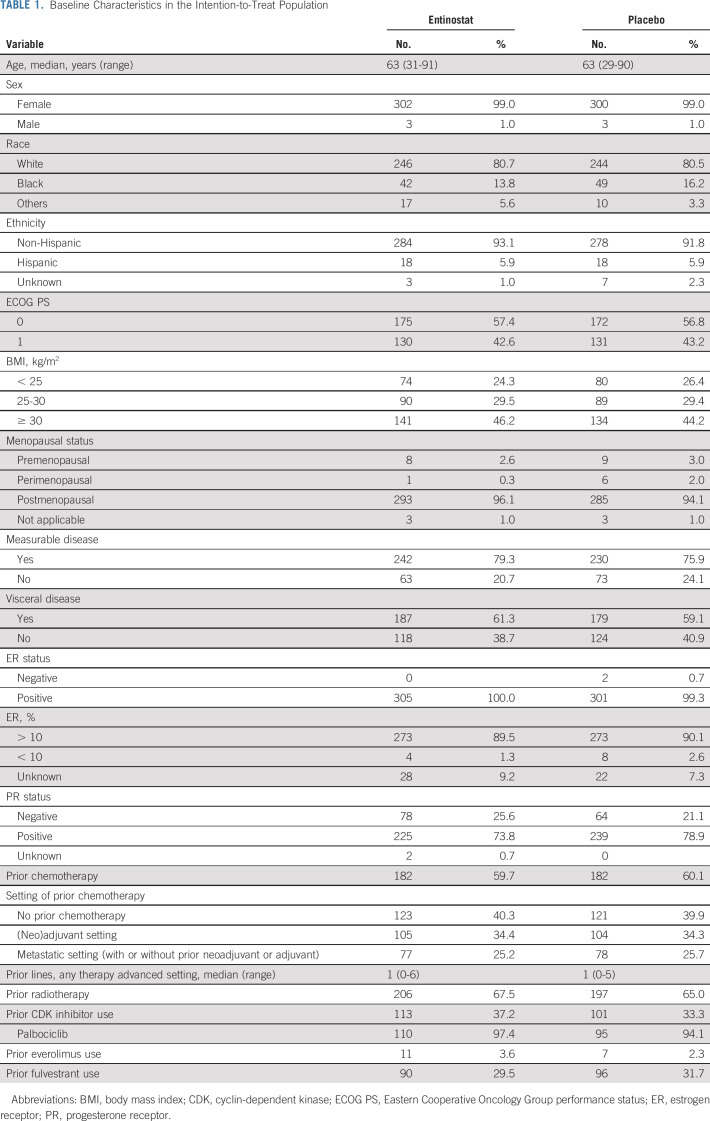
From March 2014 to October 2018, 608 participants (305 EE arm and 303 EP arm) were enrolled from 111 centers in the United States and South Africa (n = 9), including 86 ineligible participants (Fig 1). Baseline characteristics were well-balanced between the arms (Table 1). Median age was 63 years (range: 29-91), 60% of participants had visceral disease, and a majority (84%) had disease resistant to AI in the metastatic setting at study entry. Regarding prior treatment; 60% had received prior chemotherapy (25% had received in the advanced setting), 35% prior CDK inhibitor, and 30% prior fulvestrant.
FIG 1.
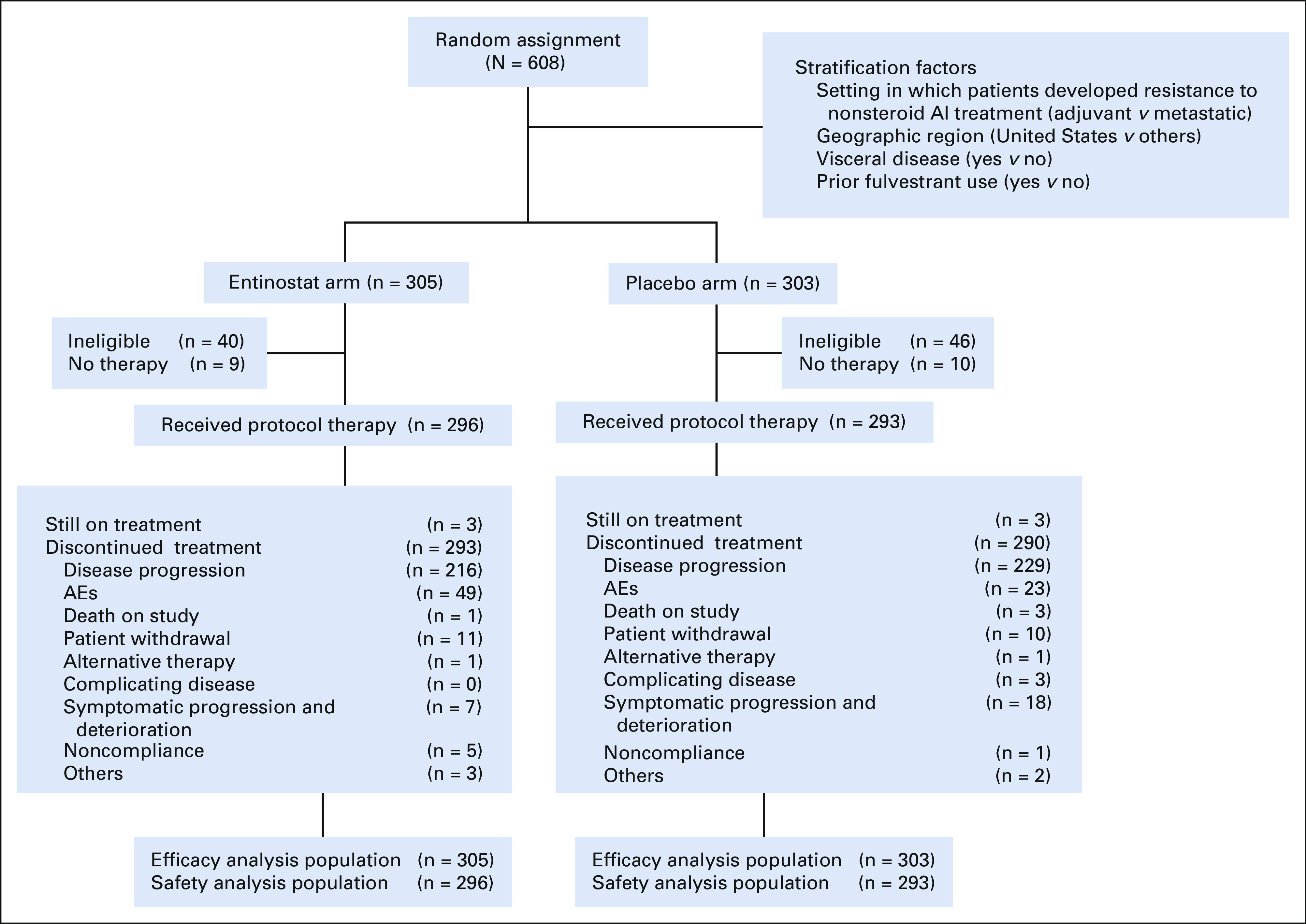
CONSORT diagram. AEs, adverse events; AI, aromatase inhibitor.
TABLE 1.
Baseline Characteristics in the Intention-to-Treat Population
Treatment and Treatment Safety
Of the 608 participants randomly assigned, 589 started protocol therapy (296 EE arm and 293 EP arm; Fig 1). At the data cutoff date (May 2020), six patients were still on treatment (three on each arm). The median number of treatment cycles received was three on both arms (range: 1-43 EP arm and 1-49 EE arm). Dose modification for entinostat or placebo (eg, discontinuation, delay, hold, missed, and reduced) occurred in 70% (206 of 296) on EE arm and 47% (138 of 293) on EP arm. Dose reduction of entinostat or placebo was required in 30% of patients (88 of 296) on EE arm and 3% (8 of 293) on EP arm; discontinuation of therapy because of AEs was more common in patients receiving EE than EP (16% v 8%, P = .002). The main reason for discontinuation of protocol therapy was disease progression for both arms (74% EE arm v 79% EP arm).
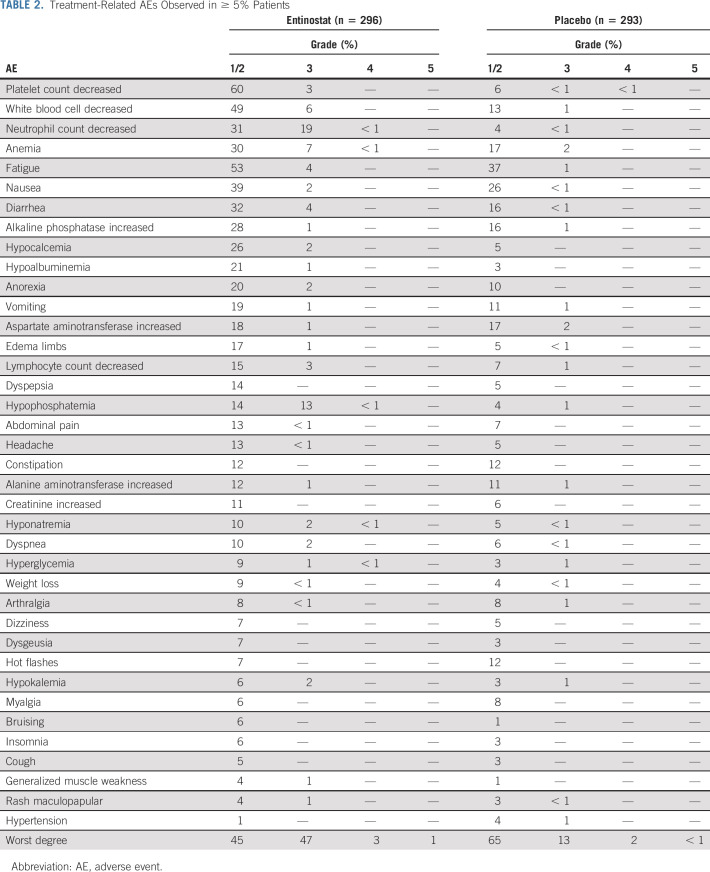
The most common grade 3 and 4 AEs in the EE arm were neutropenia (20%), hypophosphatemia (14%), anemia (8%), leukopenia (6%), fatigue (4%), and diarrhea (4%; Table 2). The incidence of grade 3 or higher toxicity was 33% (95% CI, 30 to 37) in the overall population: 51% on EE arm (95% CI, 45 to 57) and 16% on EP arm (95% CI, 12 to 20; P < .001). A total of 28 (13 EP arm and 15 EE arm) grade 5 or fatal AEs occurred on the trial, with four of these deemed possibly related to the protocol treatment, including heart failure, pneumonitis, and hepatic failure (EE arm) and myocardial infarction (EP arm).
TABLE 2.
Treatment-Related AEs Observed in ≥ 5% Patients
Treatment Efficacy
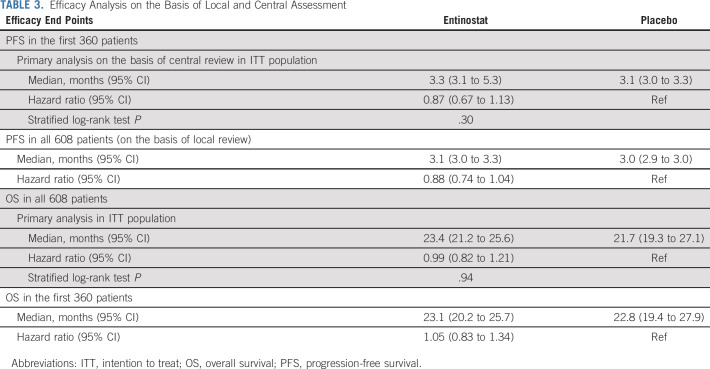
Of the first 360 patients for the PFS end point (the 360th patient was randomly assigned on October 28, 2016), 16 patients did not have any postrandomization scans and could not be centrally reviewed (seven died, eight withdrew consent, and one patient was alive as of September 4, 2018, the data cutoff date for PFS). Of the 344 patients with central review data on their disease status, 249 patients had experienced disease progression or died. The 247th PFS event occurred on November 22, 2017, and all follow-up through that date was included in the final analysis for PFS end point. The median PFS was 3.3 months (95% CI, 3.1 to 5.3) in the EE arm and 3.1 months (95% CI, 3.0 to 3.3) in the EP arm. There was no significant difference in PFS between the arms (two-sided stratified log-rank P = .30; hazard ratio = 0.87 for EE and EP; 95% CI, 0.67 to 1.13; Table 3 and Fig 2). Sensitivity analyses found similar results (data not shown).
TABLE 3.
Efficacy Analysis on the Basis of Local and Central Assessment
FIG 2.
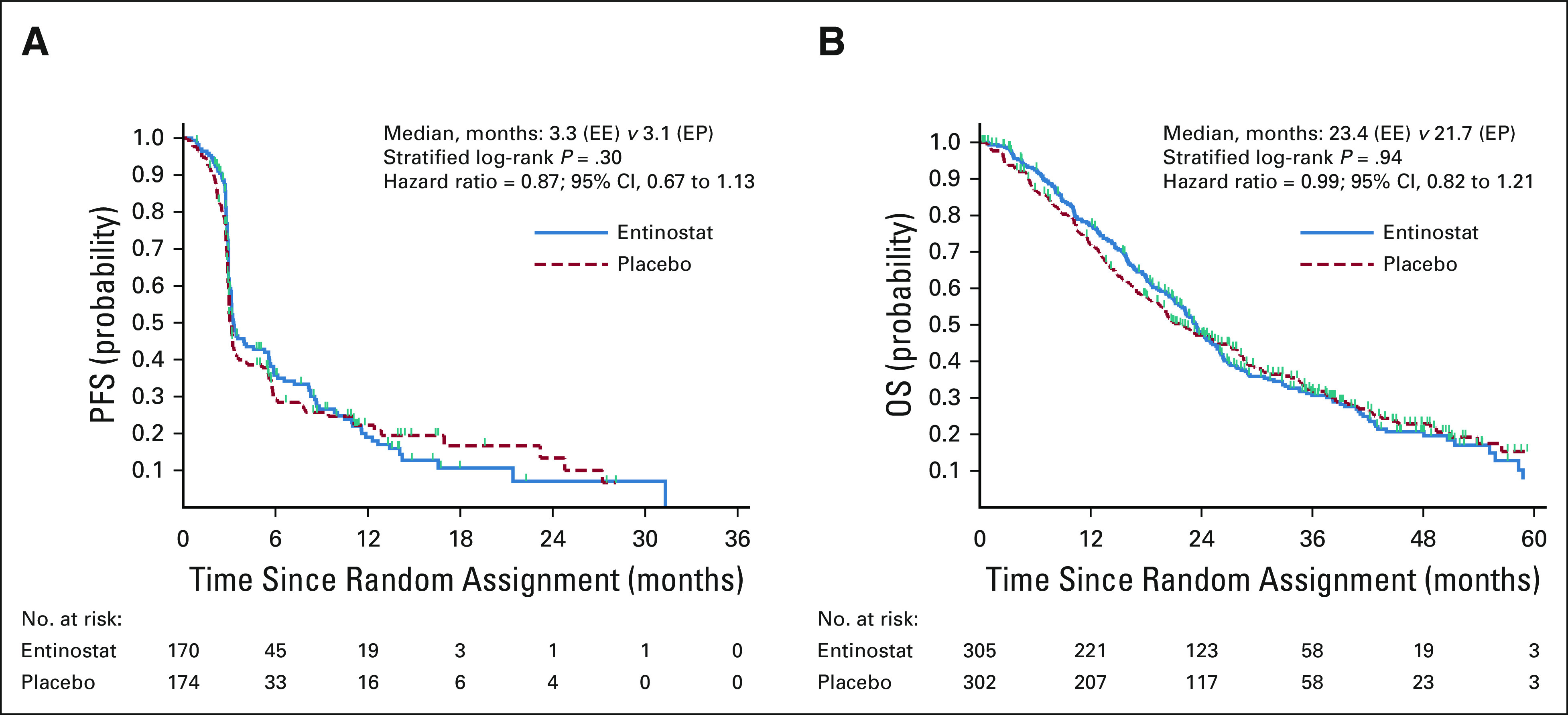
Kaplan-Meier estimates of (A) PFS and (B) OS by treatment arm. EE, exemestane and entinostat; EP, exemestane and placebo; OS, overall survival; PFS, progression-free survival.
As of May 5, 2020, 412 deaths had occurred and the final OS analysis was performed. The median OS was 23.4 months (95% CI, 21.2 to 25.6) on the EE arm and 21.7 months (95% CI, 19.3 to 27.1) on the EP arm (Fig 2). There was no significant difference in OS between the two arms (stratified log-rank P = .94; hazard ratio = 0.99; 95% CI, 0.82 to 1.21). Subgroup analysis showed similar results in all subgroups for PFS and OS (data not shown). The median TTD was 2.9 months (95% CI, 2.8 to 3.1) in the EE arm and 2.9 months (95% CI, 2.8 to 3) in the EP arm, among the 351 participants in the first 360 patients who started protocol therapy. The ORR among 472 of 608 patients with measurable disease was 5.8% (14 of 242; 95% CI, 3.2 to 9.5) in the EE arm and 5.6% (13 of 230; 95% CI, 3.0 to 9.5) in the EP arm.
Pharmacodynamic End Point
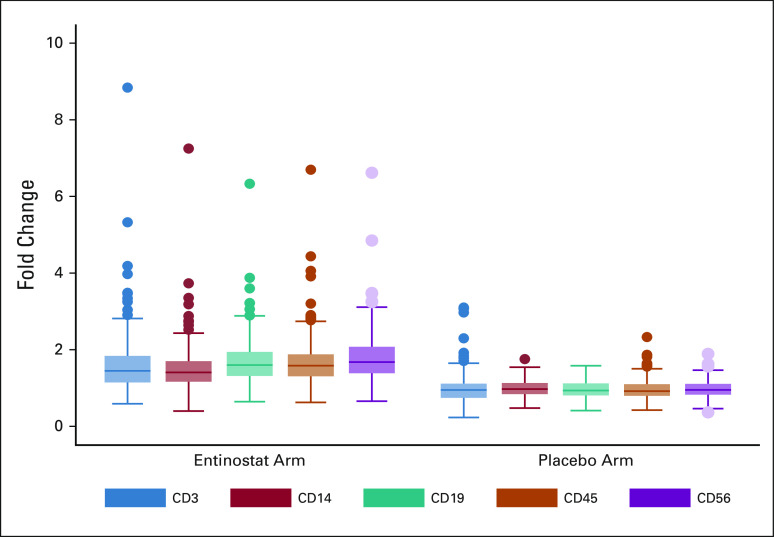
Among the 608 participants enrolled, 505 consented to the integrated biomarker study. Participants on EE had significantly higher increase in lysine acetylation in PBMCs by C1D15 than patients on EP (397 paired samples available for analysis [189 EE and 208 EP], P < .001 for all; Fig 3), indicating HDAC inhibitor target inhibition. The median fold change in lysine acetylation was approximately 1.5 in the EE arm and one in the EP arm. Future analyses will evaluate prognostic information in entinostat-treated patients, alongside pharmacogenomic and pharmacokinetic data.
FIG 3.
Median fold change in lysine acetylation in peripheral blood mononuclear cells by treatment arm.
DISCUSSION
The E2112 trial was designed to test the hypothesis that the addition of entinostat to exemestane would improve PFS and/or OS in patients with AI-resistant HR-positive, HER2-negative advanced breast cancer. This randomized double-blind placebo-controlled phase III trial accrued its target population but did not meet either coprimary end point and thus does not support a role for entinostat in this setting. The most common toxicities observed included fatigue and hematologic, gastrointestinal, and metabolic AEs, similar to previous studies incorporating entinostat.11,14,15 The pharmacodynamic end point showed that participants on EE had significantly higher increase in lysine acetylation in PBMCs by C1D15 than patients on EP, which confirms that the HDAC inhibitor was acting at the intended target.
The oncology literature has indicated that positive phase II data are not necessarily replicated in the phase III setting, highlighting the importance of careful phase III study design.16,17 E2112 did not meet its primary objective. This was despite robust supportive preclinical data in AI-resistant breast cancer mouse models,7 and data from the ENCORE301 randomized phase II study, which reported both a PFS and OS improvement in advanced endocrine-resistant breast cancer.11 The E2112 study design mirrored closely that of the ENCORE301 trial in an attempt to replicate the promising results observed. Key eligibility criteria requirements at activation of E2112 included postmenopausal status, prior resistance to AI, allowance of 0-1 prior chemotherapy for metastatic disease, and no prior treatment with fulvestrant. Because of slower than anticipated accrual, a number of eligibility adjustments were made early in the study conduct to permit enrollment of premenopausal patients with concurrent ovarian suppression and prior fulvestrant use. A 20% cap was also placed on enrollment of patients with nonmeasurable disease, in keeping with data from ENCORE301. It is not clear if these changes affected the results, with 92% of E2112 participants being postmenopausal. However, differences in prior treatment may have influenced the final outcome, with approximately 30% having received prior fulvestrant and 35% prior CDK inhibitor.
The E2112 results also contrast with the PFS advantage reported in the phase III randomized placebo-controlled ACE trial, which has led to the regulatory approval of the combination of exemestane and the HDAC inhibitor tucidinostat in China.18 Postmenopausal patients who had experienced disease relapse or progressive disease after at least one endocrine therapy were randomly assigned in a 2:1 ratio to exemestane with tucidinostat or placebo (n = 365). The primary objective of that trial was investigator-assessed PFS, with a 3.6-month difference observed between the arms. The secondary objective of OS has not yet been reported. Important differences in the study population, design, and results between the E2112 and ACE trials can be considered. Genetic and metabolic differences, for example, between North American and Chinese patients may have affected HDAC inhibitor metabolism and individual response to therapy in these trials. The ACE trial patients were less likely to have received prior endocrine therapy in the advanced-disease setting (approximately 50% v 84% in E2112) and were generally younger (median age 55 v 63 years in E2112). The frequency of grade 3 and 4 AEs was also much higher in the investigational arm of the ACE trial (75%) than that observed in E2112 (50%), suggesting a greater degree of HDAC inhibition or differing off-target effects of tucidinostat.19,20 Notably, tucidinostat dosing was twice per week, in contrast to once per week for entinostat. Despite the PFS improvement of 3.6 months in the ACE trial, the lack of improvement with entinostat in E2112 suggests that this point estimate may be higher than the true PFS, calling into question whether true benefit from HDAC inhibition is clinically meaningful in this setting. Finally, as OS was a secondary objective of the ACE trial, longer follow-up is unlikely to yield robust evidence of an OS advantage as the study was not powered to evaluate this.
OS remains the most important end point for the assessment of novel agents in advanced cancers. However, PFS may be viewed as an important intermediate end point that may be meaningful to those living with advanced disease.21 The short median PFS (approximately 3 months) and low ORR (approximately 5%) observed in the overall E2112 study population indicates that better predictors are required to identify those patients who may need more intensive therapies after progression on endocrine-based therapy. Much progress has been made in recent years with the addition of CDK and phosphatidylinositide 3-kinase inhibitors, as examples, to the breast cancer treatment armamentarium.4,22 These agents often delay time to chemotherapy use, although primary or secondary resistance ultimately develops in the majority of patients. Differences in toxicity profiles between such novel agents may also play a role in treatment decision making for an individual patient.1
Strengths of the E2112 study include strong supportive preclinical and clinical rationale, an adequately powered randomized double-blind placebo-controlled design, coprimary objectives of PFS and OS, and accrual of about 600 patients across the National Cancer Institute National Clinical Trials Network. Although the study did not meet its primary objective, the data and biospecimens collected provide a rich resource for further investigation of prognostic and predictive factors in endocrine-resistant advanced breast cancer. The fact that 35% of patients had received prior CDK inhibitor suggests that these results are also relevant to the current standard-of-care approach to managing AI-resistant HR-positive, HER2-negative advanced breast cancer. The incorporation of PRO measures is also a strength of the study, and we anticipate that additional analyses will provide valuable information for future study designs, including those focusing on the area of survivorship in patients with advanced breast cancer.23
In conclusion, the combination of entinostat and exemestane did not improve outcomes in patients with advanced endocrine-resistant breast cancer. It remains to be confirmed if there is a role for HDAC inhibitors in a biomarker-selected population or in other breast cancer settings on the basis of results from ongoing correlative analysis and other clinical trials. Collaborative, multidisciplinary investigations will maximize our ability to provide effective treatment options for patients and ultimately long-term control of advanced breast cancer.
ACKNOWLEDGMENT
We thank Joanne Zujewski, MD, of the National Cancer Institute for encouraging and facilitating the development of the trial; the staff at the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) Coordinating Center and Clinical Trials Support Unit; the members and research teams of the ECOG-ACRIN Cancer Research Group; and Judy Murray, CCRC, for serving as the study liaison for the trial.
Roisin M. Connolly
Other Relationship: Pfizer
Kathy D. Miller
Consulting or Advisory Role: Merck, Genentech/Roche, Athenex, AstraZeneca, Bristol Myers Squibb/Celgene
Research Funding: Taiho Pharmaceutical, Novartis, Seattle Genetics, Pfizer, Astex Pharmaceuticals, British Biotech, CytomX Therapeutics, Alphamab
Karen L. Smith
Stock and Other Ownership Interests: AbbVie, Abbott Laboratories
Honoraria: ASiM CME
Research Funding: Pfizer
Ursa A. Brown-Glaberman
Consulting or Advisory Role: Novartis, Biotheranostics, Eisai, Seattle Genetics, Taiho Oncology
Research Funding: Seattle Genetics
Bryan A. Faller
Consulting or Advisory Role: L.E.K. Consulting
Travel, Accommodations, Expenses: Genentech, Novartis, EB SQUIBB, Celgene, Boehringer Ingelheim, Eisai, AstraZeneca, Lilly, Amgen, Merck, Takeda
Open Payments Link: https://openpaymentsdata.cms.gov/physician/127090
Adedayo A. Onitilo
Consulting or Advisory Role: Kite, a Gilead Company, Envision Communications
Speakers' Bureau: GlaxoSmithKline, Puma Biotechnology, Kite/Gilead, AbbVie
Mark E. Burkard
Consulting or Advisory Role: Pointcare Genomics, Strata Oncology, Novartis
Research Funding: AbbVie, Strata Oncology, Puma Biotechnology, Loxo, Merck, Arcus, Apollomics, Elevation Oncology, Genentech
Patents, Royalties, Other Intellectual Property: I have a patent for implantable or localized drug delivery device that can sample the tumor microenvironment and deliver drug. I have a patent for a method to detect recombination events with CRISPR-mediated editing. I have a patent for conducting expansion microscopy without specialized equipment
George T. Budd
Honoraria: Deciphera
Consulting or Advisory Role: Deciphera, Epic Sciences
Speakers' Bureau: Deciphera
Research Funding: Genentech/Roche, TRACON Pharma, Daiichi Sankyo/Lilly, Ambrx
Open Payments Link: https://openpaymentsdata.cms.gov/physician/774695/summary
Ellis G. Levine
Research Funding: Oncolytic Biotech
Patents, Royalties, Other Intellectual Property: UpToDate
Peter A. Kaufman
Stock and Other Ownership Interests: Amgen
Honoraria: Lilly
Consulting or Advisory Role: Polyphor, Roche/Genentech, Lilly, Eisai, Macrogenics, Pfizer, Merck, AstraZeneca
Speakers' Bureau: Lilly
Research Funding: Eisai, Polyphor, Roche/Genentech, Lilly, Novartis, Macrogenics, Pfizer, Sanofi
Travel, Accommodations, Expenses: Lilly, Polyphor, Macrogenics
Alexandra Thomas
Stock and Other Ownership Interests: Johnson & Johnson, Gilead Sciences, Bristol Myers Squibb, Pfizer
Consulting or Advisory Role: BeyondSpring Pharmaceuticals, Lilly
Research Funding: Sanofi
Patents, Royalties, Other Intellectual Property: UpToDate Royalties
Travel, Accommodations, Expenses: Genentech
Jane B. Trepel
Research Funding: Syndax, EpicentRX, AstraZeneca
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer and has assigned his rights to JHU and participates in a royalty-sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Joseph A. Sparano
Stock and Other Ownership Interests: Metastat
Consulting or Advisory Role: Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack, Adgero Biopharmaceuticals, Cardinal Health, GlaxoSmithKline, CStone Pharmaceuticals, Epic Sciences, Daiichi Sankyo, BMSi
Speakers' Bureau: Eisai, Certara
Research Funding: Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, Merrimack, Radius Health, Olema Pharmaceuticals
Travel, Accommodations, Expenses: Menarini Silicon Biosystems, Roche/Genentech, Adgero Biopharmaceuticals, Myriad Genetics, Pfizer, AstraZeneca, Rhenium Medical
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
PRIOR PRESENTATION
Presented as an oral presentation at the 2020 San Antonio Breast Cancer Virtual Symposium, December 8-11, 2020 (abstract 86, GS4-02).
SUPPORT
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD, and Mitchell D. Schnall, MD, PhD, Group Cochairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794, CA180802, CA180795, CA180799, CA189956, CA189830, CA189856, CA180888, CA180866, CA180821, CA180868, CA180822, CA189859, CA180853.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.00944.
AUTHOR CONTRIBUTIONS
Conception and design: Roisin M. Connolly, Fengmin Zhao, Kathy D. Miller, Richard L. Piekarz, Jane B. Trepel, Joseph A. Sparano
Administrative support: Kathy D. Miller, Richard L. Piekarz, Joseph A. Sparano
Provision of study materials or patients: Roisin M. Connolly, Karen L. Smith, Ursa A. Brown-Glaberman, Bryan A. Faller, Adedayo A. Onitilo, Mark E. Burkard, George T. Budd, Ellis G. Levine, Peter A. Kaufman, Alexandra Thomas, Jane B. Trepel, Joseph A. Sparano
Collection and assembly of data: Roisin M. Connolly, Fengmin Zhao, Kathy D. Miller, Ursa A. Brown-Glaberman, Jennifer S. Winn, Bryan A. Faller, Adedayo A. Onitilo, Mark E. Burkard, George T. Budd, Melanie E. Royce, Alexandra Thomas, Jane B. Trepel, Antonio C. Wolff, Joseph A. Sparano
Data analysis and interpretation: Roisin M. Connolly, Fengmin Zhao, Kathy D. Miller, Min-Jung Lee, Karen L. Smith, Bryan A. Faller, Adedayo A. Onitilo, George T. Budd, Ellis G. Levine, Peter A. Kaufman, Alexandra Thomas, Jane B. Trepel, Antonio C. Wolff, Joseph A. Sparano
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
E2112: Randomized Phase III Trial of Endocrine Therapy Plus Entinostat or Placebo in Hormone Receptor–Positive Advanced Breast Cancer. A Trial of the ECOG-ACRIN Cancer Research Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Roisin M. Connolly
Other Relationship: Pfizer
Kathy D. Miller
Consulting or Advisory Role: Merck, Genentech/Roche, Athenex, AstraZeneca, Bristol Myers Squibb/Celgene
Research Funding: Taiho Pharmaceutical, Novartis, Seattle Genetics, Pfizer, Astex Pharmaceuticals, British Biotech, CytomX Therapeutics, Alphamab
Karen L. Smith
Stock and Other Ownership Interests: AbbVie, Abbott Laboratories
Honoraria: ASiM CME
Research Funding: Pfizer
Ursa A. Brown-Glaberman
Consulting or Advisory Role: Novartis, Biotheranostics, Eisai, Seattle Genetics, Taiho Oncology
Research Funding: Seattle Genetics
Bryan A. Faller
Consulting or Advisory Role: L.E.K. Consulting
Travel, Accommodations, Expenses: Genentech, Novartis, EB SQUIBB, Celgene, Boehringer Ingelheim, Eisai, AstraZeneca, Lilly, Amgen, Merck, Takeda
Open Payments Link: https://openpaymentsdata.cms.gov/physician/127090
Adedayo A. Onitilo
Consulting or Advisory Role: Kite, a Gilead Company, Envision Communications
Speakers' Bureau: GlaxoSmithKline, Puma Biotechnology, Kite/Gilead, AbbVie
Mark E. Burkard
Consulting or Advisory Role: Pointcare Genomics, Strata Oncology, Novartis
Research Funding: AbbVie, Strata Oncology, Puma Biotechnology, Loxo, Merck, Arcus, Apollomics, Elevation Oncology, Genentech
Patents, Royalties, Other Intellectual Property: I have a patent for implantable or localized drug delivery device that can sample the tumor microenvironment and deliver drug. I have a patent for a method to detect recombination events with CRISPR-mediated editing. I have a patent for conducting expansion microscopy without specialized equipment
George T. Budd
Honoraria: Deciphera
Consulting or Advisory Role: Deciphera, Epic Sciences
Speakers' Bureau: Deciphera
Research Funding: Genentech/Roche, TRACON Pharma, Daiichi Sankyo/Lilly, Ambrx
Open Payments Link: https://openpaymentsdata.cms.gov/physician/774695/summary
Ellis G. Levine
Research Funding: Oncolytic Biotech
Patents, Royalties, Other Intellectual Property: UpToDate
Peter A. Kaufman
Stock and Other Ownership Interests: Amgen
Honoraria: Lilly
Consulting or Advisory Role: Polyphor, Roche/Genentech, Lilly, Eisai, Macrogenics, Pfizer, Merck, AstraZeneca
Speakers' Bureau: Lilly
Research Funding: Eisai, Polyphor, Roche/Genentech, Lilly, Novartis, Macrogenics, Pfizer, Sanofi
Travel, Accommodations, Expenses: Lilly, Polyphor, Macrogenics
Alexandra Thomas
Stock and Other Ownership Interests: Johnson & Johnson, Gilead Sciences, Bristol Myers Squibb, Pfizer
Consulting or Advisory Role: BeyondSpring Pharmaceuticals, Lilly
Research Funding: Sanofi
Patents, Royalties, Other Intellectual Property: UpToDate Royalties
Travel, Accommodations, Expenses: Genentech
Jane B. Trepel
Research Funding: Syndax, EpicentRX, AstraZeneca
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer and has assigned his rights to JHU and participates in a royalty-sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Joseph A. Sparano
Stock and Other Ownership Interests: Metastat
Consulting or Advisory Role: Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack, Adgero Biopharmaceuticals, Cardinal Health, GlaxoSmithKline, CStone Pharmaceuticals, Epic Sciences, Daiichi Sankyo, BMSi
Speakers' Bureau: Eisai, Certara
Research Funding: Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, Merrimack, Radius Health, Olema Pharmaceuticals
Travel, Accommodations, Expenses: Menarini Silicon Biosystems, Roche/Genentech, Adgero Biopharmaceuticals, Myriad Genetics, Pfizer, AstraZeneca, Rhenium Medical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cardoso F, Paluch-Shimon S, Senkus E, et al. : 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623-1649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner NC, Neven P, Loibl S, et al. : Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 389:2403-2414, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Campone M, Piccart M, et al. : Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520-529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André F, Ciruelos EM, Juric D, et al. : Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann Oncol 32:208-217, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Rossi V, Berchialla P, Giannarelli D, et al. : Should all patients with HR-positive HER2-negative metastatic breast cancer receive CDK 4/6 inhibitor as first-line based therapy? A network meta-analysis of data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT trials. Cancers (Basel) 11:1661, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly R, Stearns V: Epigenetics as a therapeutic target in breast cancer. J Mammary Gland Biol Neoplasia 17:191-204, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabnis GJ, Goloubeva OG, Kazi AA, et al. : HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Mol Cancer Ther 12:2804-2816, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan J, Yin WJ, Lu JS, et al. : ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol 134:883-890, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Connolly RM, Rudek MA, Piekarz R: Entinostat: A promising treatment option for patients with advanced breast cancer. Futur Oncol 13:1137-1148, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah P, Gau Y, Sabnis G: Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 143:99-111, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Yardley DA, Ismail-Khan RR, Melichar B, et al. : Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 31:2128-2135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond MEH, Allison KH, et al. : HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update summary. JCO Oncol Pract 14:437-441, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Allison KH, Hammond MEH, Dowsett M, et al. : Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 38:1346-1366, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Connolly RM, Li H, Jankowitz RC, et al. : Combination epigenetic therapy in advanced breast cancer with 5-azacitidine and entinostat: A phase II National Cancer Institute/Stand Up to Cancer Study. Clin Can Res 23:2691-2701, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore L, Rothenberg ML, O'Bryant CL, et al. : A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin Cancer Res 14:4517-4525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Shaughnessy J, Osborne C, Pippen JE, et al. : Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364:205-214, 2011 [DOI] [PubMed] [Google Scholar]
- 17.O'Shaughnessy J, Hellerstedt B, Schwartzberg L, et al. : Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 32:3840-3847, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Li W, Hu X, et al. : Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:806-815, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Ning ZQ, Li ZB, Newman MJ, et al. : Chidamide (CS055/HBI-8000): A new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol 69:901-909, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Knipstein J, Gore L: Entinostat for treatment of solid tumors and hematologic malignancies. Expert Opin Investig Drugs 20:1455-1467, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Seidman AD, Maues J, Tomlin T, et al. : The evolution of clinical trials in metastatic breast cancer: Design features and endpoints that matter. Am Soc Clin Oncol Ed Book 40:44-54, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Gao JJ, Cheng J, Bloomquist E, et al. : CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol 21:250-260, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar V, Hudgens S, Piault‐Louis E, et al. : Patient‐reported outcomes in oncology clinical trials: Stakeholder perspectives from the accelerating anticancer agent development and validation workshop 2019. Oncologist 25:819-821, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.00944.