PURPOSE
We investigated the impact of the CD33-targeted agent gemtuzumab ozogamicin (GO) on survival in pediatric patients with KMT2A-rearranged (KMT2A-r) acute myeloid leukemia (AML) enrolled in the Children's Oncology Group trial AAML0531 (NCT01407757).
METHODS
Patients with KMT2A-r AML were identified and clinical characteristics described. Five-year overall survival (OS), event-free survival (EFS), disease-free survival (DFS), and relapse risk (RR) were determined overall and for higher-risk versus not high-risk translocation partners. GO's impact on response was determined and outcomes based on consolidation approach (hematopoietic stem cell transplant [HSCT] v chemotherapy) described.
RESULTS
Two hundred fifteen (21%) of 1,022 patients enrolled had KMT2A-r AML. Five-year EFS and OS from study entry were 38% and 58%, respectively. EFS was superior with GO treatment (EFS 48% with GO v 29% without, P = .003), although OS was comparable (63% v 53%, P = .054). For patients with KMT2A-r AML who achieved complete remission, GO was associated with lower RR (40% GO v 66% patients who did not receive GO [No-GO], P = .001) and improved 5-year DFS (GO 57% v No-GO 33%, P = .002). GO benefit was observed in both higher-risk and not high-risk KMT2A-r subsets. For patients who underwent HSCT, prior GO exposure was associated with decreased relapse (5-year RR: 28% GO and HSCT v 73% No-GO and HSCT, P = .006). In multivariable analysis, GO was independently associated with improved EFS, improved DFS, and reduced RR.
CONCLUSION
GO added to conventional chemotherapy improved outcomes for KMT2A-r AML; consolidation with HSCT may further enhance outcomes. Future clinical trials should study CD33-targeted agents in combination with HSCT for pediatric KMT2A-r AML.
INTRODUCTION
Chromosomal rearrangements involving KMT2A on chromosome band 11q23 (hereafter KMT2A-rearranged [KMT2A-r]) occur in approximately 20% of pediatric acute myeloid leukemia (AML) cases and represent the most common recurrent cytogenetic abnormality.1–3 More than 80 fusion partners of KMT2A have been characterized,4 and clinical outcome varies depending upon the translocation partner. Specifically, event-free survival (EFS) rates of 34%-61% and overall survival (OS) of 44%-64% have been reported, although outcomes are markedly inferior for higher-risk (HR) translocations.1,2,4–7 A recent analysis of 1,257 heterogeneously treated children with KMT2A-r AML demonstrate 5-year EFS of 46% and OS of 62%.8 Given these suboptimal outcomes, novel treatment approaches are needed.
CONTEXT
Key Objective
Pediatric KMT2A-rearranged (KMT2A-r) acute myeloid leukemia (AML) is a heterogenous disease with suboptimal outcome and thus, novel therapeutic approaches. Within the context of Children's Oncology Group protocol AAML0531, a phase III randomized trial of the CD33-targeted agent gemtuzumab ozogamicin (GO) in combination with conventional chemotherapy, we studied whether GO provided therapeutic benefit in KMT2A-r AML, both overall and within higher- and lower-risk translocation partners.
Knowledge Generated
GO significantly improved event-free survival and reduced relapse risk in KMT2A-r AML, both overall and in higher- and lower-risk KMT2A-subsets. Although intensity of CD33 expression affected GO response, even patients with lower CD33 expression benefited from GO. GO in combination with hematopoietic stem cell transplant may provide additive clinical benefit; however, this needs to be studied further prospectively.
Relevance
Treatment of KMT2A-r AML should include the CD33-targeting agent GO; future trials should study second-generation CD33-targeting agents and further define the role of hematopoietic stem cell transplant in this disease subset.
CD33 is 67-kDA transmembrane glycoprotein present on the majority of AML blasts. Higher CD33 expression correlates with negative prognostic features and significantly lower OS and disease-free survival (DFS) from complete remission (CR).9,10 CD33 is the target of gemtuzumab ozogamicin (GO; Mylotarg, Pfizer, New York, NY), a toxin-conjugated humanized IgG4 anti-CD33 monoclonal antibody. GO is US Food and Drug Administration–approved for treatment of adult and pediatric de novo AML based on previous studies demonstrating safety and efficacy when used as monotherapy or in combination with conventional chemotherapy.11–29
The Children's Oncology Group (COG) Trial AAML0531 (NCT01407757) was a phase III study in which 1,070 de novo pediatric AML patients received a conventional chemotherapy backbone and were randomly assigned to GO. Patients with high-risk disease underwent hematopoietic stem cell transplant (HSCT) with an optimal donor source; intermediate-risk (IR) patients went to HSCT if a matched family donor (MFD) was available. For the 1,022 evaluable patients, GO significantly improved 3-year EFS (GO 53% v 47%, P = .04) but not OS (69% v 65%, P = .39). The lack of OS benefit may have reflected the increased toxic mortality observed in patients who received post-remission GO (7% v 4%, P = .09). Notably, relapse risk (RR) was significantly reduced among GO recipients (33% v 41%, P = .006), which translated into improved DFS (61% v 55%, P = .07).27 In a multivariable model, high CD33 expression was a negative predictor of outcome9 but imparted a more favorable response to GO.10 Specifically, patients with higher CD33 expression who received GO had significantly reduced RR (GO: 32% v patients who did not receive GO [No-GO]: 49%, P < .001) and improved EFS (GO: 53% v No-GO 41%, P = .005). This differential effect was observed in all cytogenetic or molecular risk groups.10
As pediatric KMT2A-r AML is characterized by higher CD33 expression compared with KMT2A wild-type (WT) AML,9,10 we wanted to determine if the addition of GO conferred survival benefit for patients with KMT2A-r AML enrolled on AAML0531 and, if so, whether GO benefit was seen in both HR and lower risk KMT2A-r subsets and/or was influenced by the degree of CD33 expression present. Moreover, as AAML0531 prospectively prescribed use of HSCT for patients with KMT2A-r AML with an MFD or co-occurring HR features, we explored whether GO followed by HSCT had additive clinical impact.
METHODS
Patients and Treatment
Pediatric patients with de novo AML enrolled in the COG trial AAML0531 (August 2006-June 2010) were eligible for this analysis. Details of the treatment regimen used in AAML0531 have been described previously.27 In brief, patients were treated with five cycles of anthracycline and cytarabine–based chemotherapy, with the randomized addition of GO in the experimental arm. GO 3 mg/m2 (0.1 mg/kg if body surface area < 0.6 m2) was given by intravenous injection on day 6 of induction 1 and day 7 of intensification 2. Patients with high-risk features, defined by presence of monosomy 7, monosomy 5/5q deletion, or persistent morphologic disease at end of induction 1 (EOI1), received allogeneic HSCT following the third course of chemotherapy and thus did not receive a second GO dose. Patients with KMT2A-r AML without other high-risk features were allocated to the IR group and received HSCT if an MFD was available. All KMT2A-r samples from patients enrolled in AAML0531 were eligible for correlative study (eg, CD33 expression determination) if consent was obtained. The institutional review boards of all participating institutions approved the clinical protocol and the COG Myeloid Disease Biology Committee approved this research.
Cytogenetic Classification
Local laboratories performed conventional (G-banded) analyses of bone marrow or peripheral blood as well as fluorescence in situ hybridization (FISH) using a series of probes that included KMT2A. For normal conventional karyotype but abnormal interphase FISH showing a KMT2A-r, metaphase FISH was performed to characterize the fusion pattern and enable detection of cryptic signal deletion. All reports were reviewed centrally by COG cytogeneticists (University of Minnesota and St Jude Children's Research Hospital). The International System for Human Cytogenetic Nomenclature-2013 was used to interpret and report results.
KMT2A-r AML: Risk Classification of Recurrent Translocation Partners
HR KMT2A translocation partners were defined as 6q27, 10p11.2, 10p12, 4q21.3, and 19p13.3 based on previously published data.1,5,7,8 The non-HR (NHR) cohort included the remaining KMT2A-r cases but excluded other partners (defined as a NHR translocation with fewer than five cases) as their rarity precluded analysis of the impact of the fusion partner on prognosis, and the unknown partners, given the unclear origin of the fusion partner.
Assessment of CD33 Expression
Using difference from normal flow cytometry, CD33 expression was defined by mean fluorescence intensity (MFI) of leukemic blasts, as described previously.9,10,30–32 CD33 expression data were then compared both overall and by KMT2A-r risk group. For univariable and multivariable analyses, the quartile of CD33 expression assigned for a given patient in the overall AAML0531 CD33 analysis10 was used to determine whether GO response was affected by CD33 expression.
Statistical Analyses
Data on clinical outcomes for patients in AAML0531 were analyzed as of March 31, 2020. The median (range) follow-up time for patients alive at last contact was 9.3 (0.02-13.3) years. Significance of the observed difference in proportions was tested by the Pearson's χ2 test or Fisher's exact test when data were sparse. The Kruskal-Wallis test was used to test differences in medians across multiple groups; the Mann-Whitney test was used when comparing two groups. CR was defined as < 5% blasts by morphology and absence of extramedullary disease. Minimal measurable residual disease (MRD) was determined by detecting flow cytometry-based disease and was typically defined as > 0.02% disease detected in the bone marrow by central difference from normal (ΔN) flow cytometry analysis.33,34 The Kaplan-Meier method was used to estimate 5-year EFS, OS, and DFS.35 Estimates are reported with corresponding log-log 95% CIs. EFS was defined as the time from study entry until death, induction failure, or relapse of any type; OS was defined as the time from study entry to death; and DFS as time from EOI1 for patients in CR until death or relapse. RR was defined as the time from EOI1 for patients in CR to relapse, where deaths without a relapse were considered competing events.36 Treatment-related mortality (TRM) was defined as the time from EOI1 for those who continued therapy until death, where relapses were considered competing events.36 To compare the consolidation approach (HSCT v chemotherapy), 5-year DFS and RR were also compared from end of intensification 1 in subset analyses. Differences between groups of patients were tested by the logrank test for OS, EFS, and DFS. Gray's test was used to test the significance of RR and TRM. Cox proportional hazard models were used for OS, EFS, and DFS, whereas competing risk regression models were used for RR to estimate hazard ratios with 95% CIs for univariate and multivariable analyses.37 Patients lost to follow-up were censored at the date of last known contact. An alpha level of 0.05 was used for P value significance.
RESULTS
Clinical Characteristics and Responses by KMT2A Cytogenetic Classification
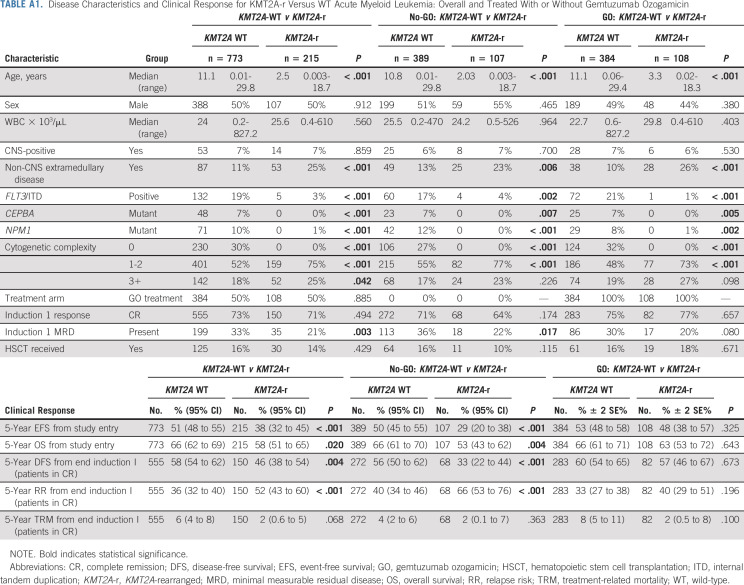
Of 1,022 evaluable patients enrolled in AAML0531, 988 had evaluable cytogenetic data for central review and 215 (21%) had KMT2A-r AML (Appendix Fig A1, online only). Appendix Table A1 (online only) describes the differences in clinical characteristics and outcome for KMT2A-r versus KMT2A WT disease. Patients with KMT2A-r disease were younger and less likely to have clinically relevant co-occurring mutations than KMT2A WT patients and more likely to have cytogenetic complexity and non–central nervous system extramedullary disease, such as soft tissue chloromas or skin involvement (Appendix Table A1). Multivariable Cox regression models containing KMT2A WT versus KMT2A-r, treatment arm (GO v No-GO), and the corresponding interaction term yielded a significant interaction term for EFS (P = .022) and DFS (P = .020), suggesting a different GO treatment effect for KMT2A-WT and KMT2A-r AML for EFS and DFS but not for OS (P = .119) and RR (P = .066).
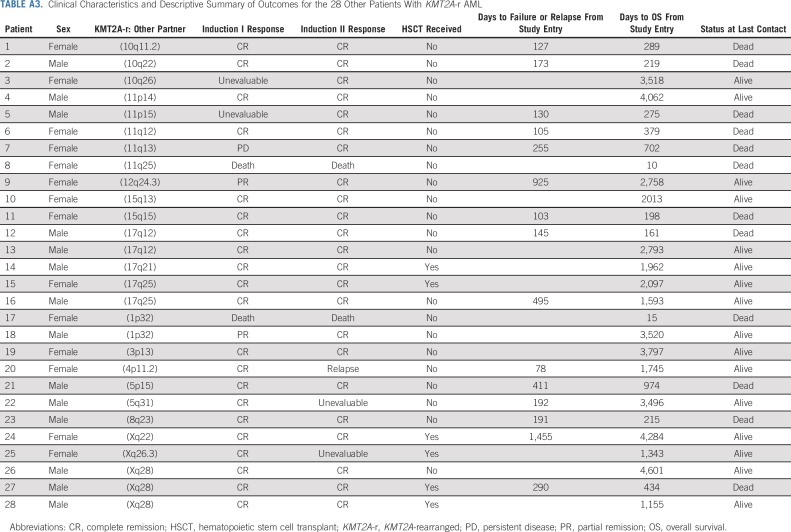
Comparison of disease characteristics across 11 KMT2A-r subgroups, including nine specific partner groups, other, and unknown KMT2A-r partners, revealed significant differences by age at presentation, non-CNS extramedullary AML, and presence of the FLT3/ITD mutation. GO exposure was equally distributed across the KMT2A-r subsets (Appendix Table A2, online only). Given the rarity of published data regarding the 28 patients in the other KMT2A-r subset, their clinical characteristics are further described in Appendix Table A3 (online only).
Impact of GO on CR and Outcome in KMT2A-r AML
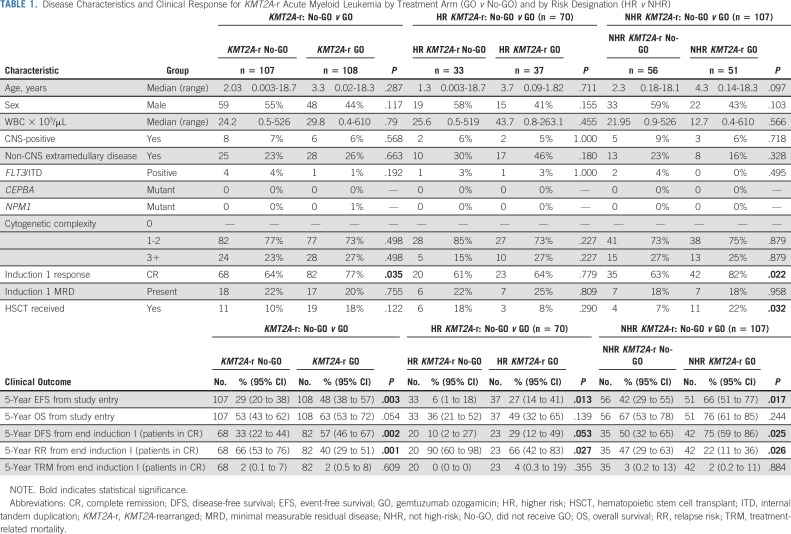
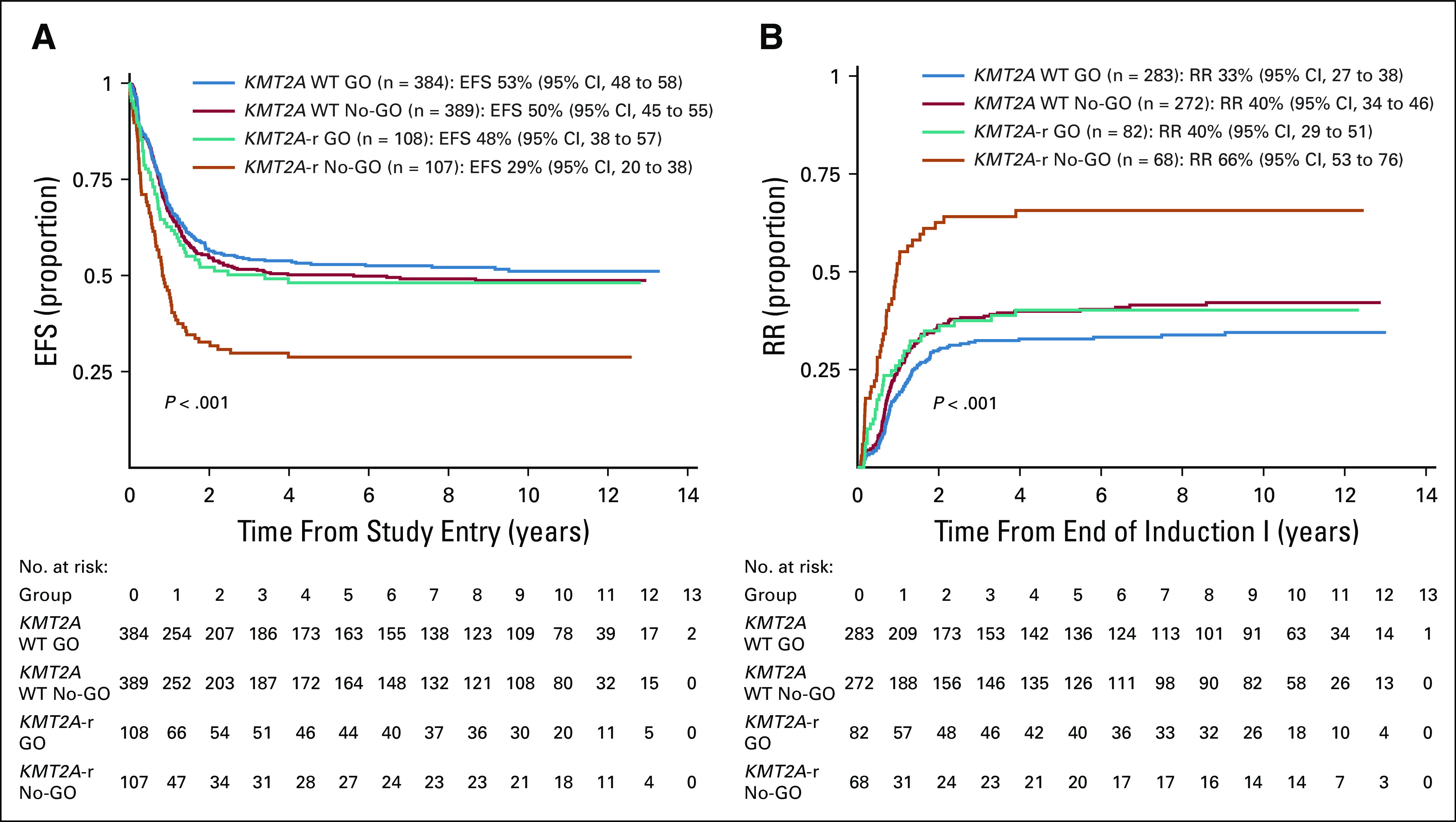
Table 1 compares disease characteristics and induction response of patients with KMT2A-r AML treated with and without GO. Clinical characteristics were similar for the two treatment arms and for HR versus NHR KMT2A-r AML treated with and without GO (Table 1). Patients with KMT2A-r AML treated with GO had higher rates of EOI1 morphologic CR (77%) versus those treated without GO (64%, P = .035, Table 1) but comparable rates of EOI1 MRD (Table 1). GO use was associated with significant improvements in long-term clinical outcomes for patients with KMT2A-r AML. Specifically, patients with KMT2A-r AML who received GO had 5-year EFS of 48% (95% CI, 38 to 57) versus 29% (95% CI, 20 to 38) for the No-GO cohort (P = .003, Table 1, Fig 1A) and RR of 40% (95% CI, 29 to 51) versus 66% (95% CI, 53 to 76, P = .001, Table 1 and Fig 1B). Although OS was not statistically different between the two arms, DFS was superior for patients treated with GO and rates of TRM were comparable (Table 1). Notably, patients with KMT2A-r AML treated with GO had, in general, comparable outcomes to KMT2A WT patients regardless of GO exposure (Appendix Table A1, Figs 1A and 1B).
TABLE 1.
Disease Characteristics and Clinical Response for KMT2A-r Acute Myeloid Leukemia by Treatment Arm (GO v No-GO) and by Risk Designation (HR v NHR)
FIG 1.
Outcomes for patients with KMT2A-r versus KMT2A WT outcome by GO exposure. (A) Five-year EFS from study entry and (B) 5-year RR from CR. CR, complete remission; EFS, event-free survival; GO, gemtuzumab ozogamicin; KMT2A-r, KMT2A-rearranged; No-GO, not receiving GO; RR, relapse risk; WT, wild-type.
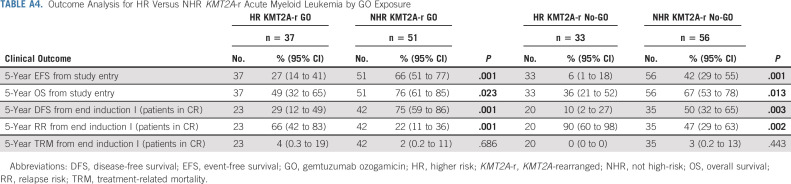
Comparison of outcomes for historically defined HR versus NHR KMT2A-r AML revealed inferior EFS, OS, DFS, and RR for HR subsets (Appendix Table A4, online only, Fig 2). Specifically, EFS for patients with HR translocations was significantly better for those treated with GO (27%; 95% CI, 14 to 41) versus No-GO (6%; 95% CI, 1 to 18, P = .013, Table 1, Fig 3A). In addition, DFS trended toward superiority with GO (Table 1) and RR was significantly reduced (GO: 66%; 95% CI, 42 to 83% v no-GO: 90%; 95% CI, 60 to 98; P = .027; Table 1, Fig 3B). For the NHR subset (n = 107), GO improved EFS (GO: 66%; 95% CI, 51 to 77 v no-GO: 42%; 95% CI, 29 to 55; P = .017; Fig 3A), DFS (GO: 75%; 95% CI, 59 to 86 v no-GO: 50%; 95% CI, 32 to 65%; P = .025), and RR (GO: 22%; 95% CI, 11 to 36 v no-GO: 47%; 95% CI, 29 to 63; P = .026; Table 1, Fig 3B). Although GO improved outcomes for patients within both HR and NHR subsets (Table 1), outcomes remained significantly worse for GO-exposed HR versus NHR patients (Appendix Table A4).
FIG 2.
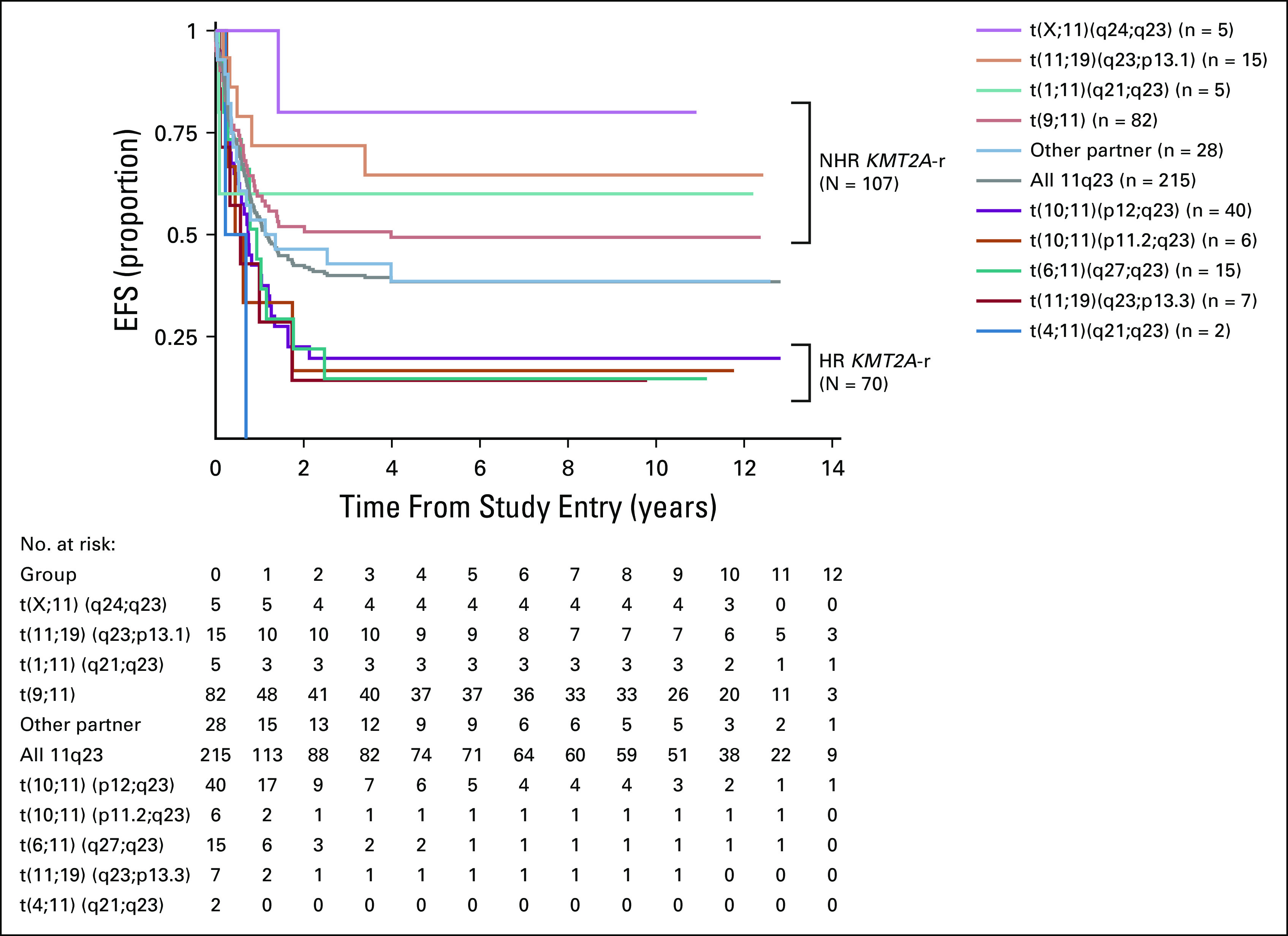
EFS for patients with KMT2A-r acute myeloid leukemia. EFS from study entry for the entire study cohort (n = 215) and by specific translocation partners associated with higher-risk KMT2A-r AML (n = 70), non–high-risk KMT2A-r AML (n = 107), and other KMT2A-r subsets (n = 28). AML, acute myeloid leukemia; EFS, event-free survival; KMT2A-r, KMT2A-rearranged.
FIG 3.
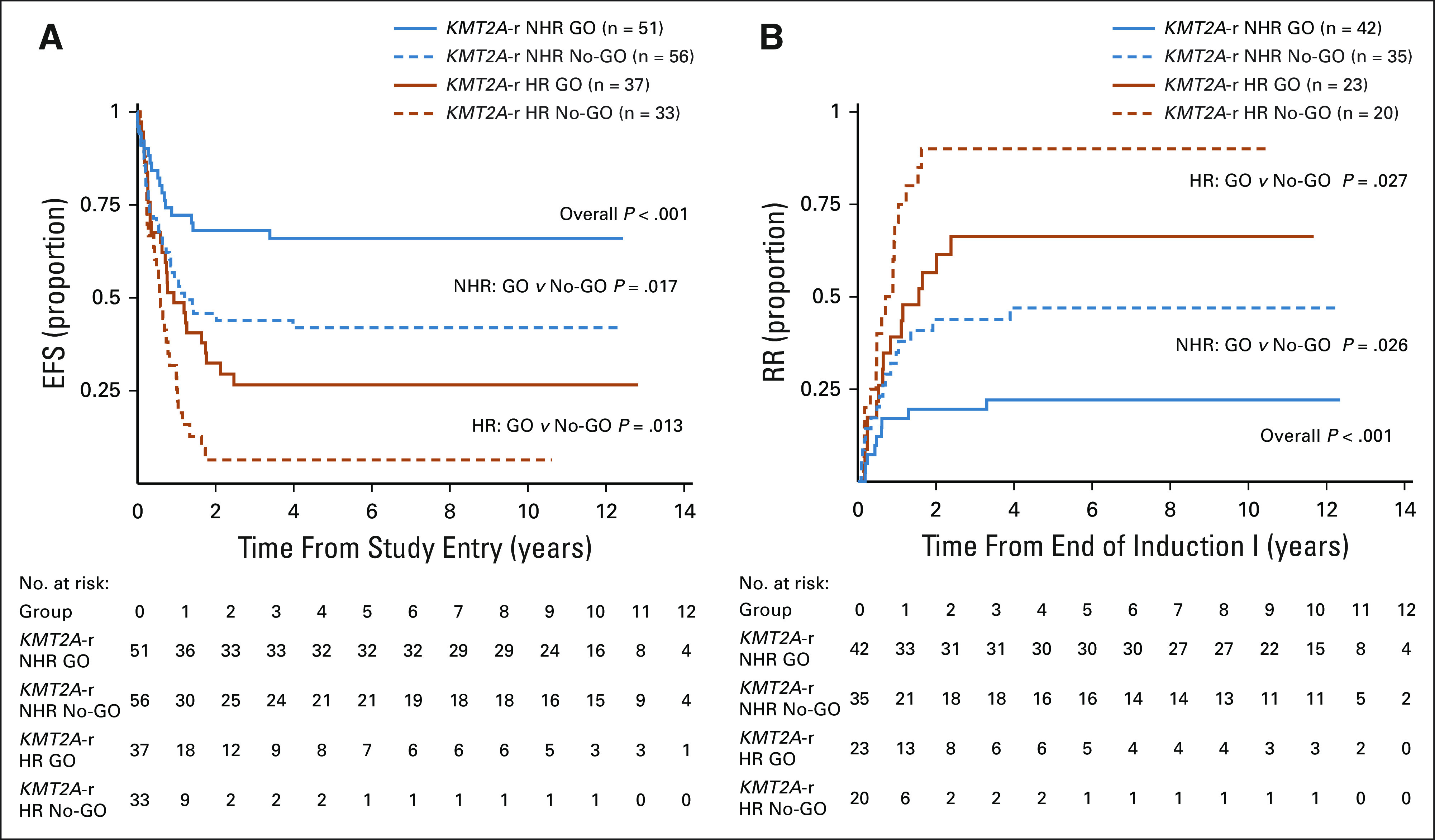
Outcomes for patients with HR versus NHR patients with KMT2A-r AML by GO exposure. (A) EFS from study entry and (B) RR from CR. CR, complete remission; EFS, event-free survival; GO, gemtuzumab ozogamicin; HR, higher risk; KMT2A-r, KMT2A-rearranged; NHR, not high-risk; No-GO, did not receive GO; RR, relapse risk.
Significance of GO and HSCT in KMT2A-r AML
Given the observed therapeutic benefit of GO and known benefit of HSCT in some AML subsets, we explored whether pre-HSCT GO affected post-HSCT outcomes. Of 215 patients with KMT2A-r AML, 30 (14%) received HSCT in first CR; 19/30 (63%) of these patients also received GO during induction 1. For HSCT recipients with prior GO exposure, DFS from end of intensification 1 was 72% (95% CI, 45 to 87) versus 27% (95% CI, 7 to 54) for patients in the no-GO cohort (P = .004, Fig 4A). RR was also reduced with GO/HSCT (28% CI, 10 to 50 v 73% CI, 32 to 91 for no-GO and HSCT, P = .006). For patients with KMT2A-r AML receiving chemotherapy without HSCT, there remained a trend toward improved outcome with GO (Fig 4B). The lowest rates of relapse were ultimately seen in patients with KMT2A-r AML who received GO and HSCT (Fig 4C).
FIG 4.
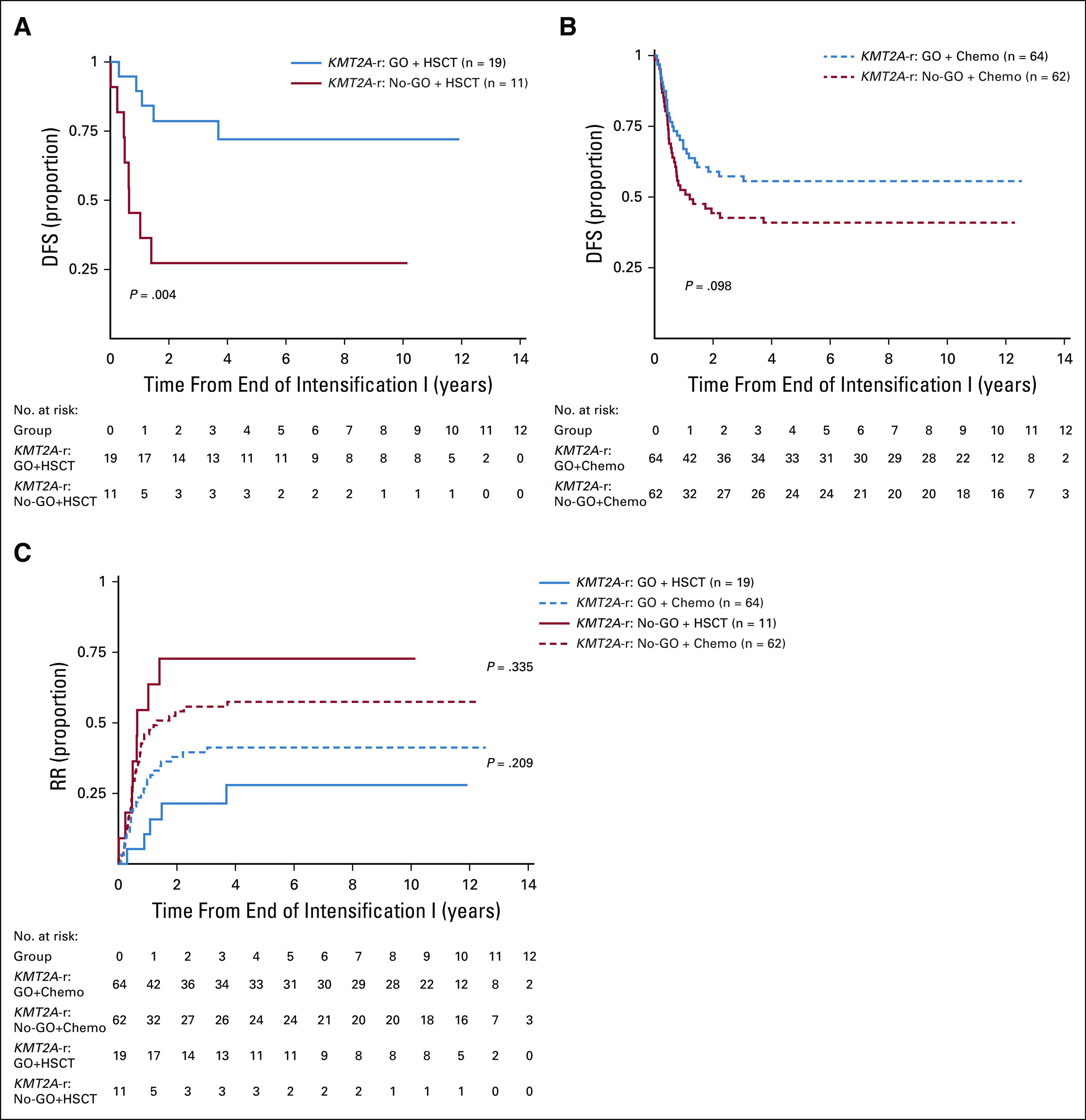
Outcome for KMT2A-r acute myeloid leukemia by GO exposure and consolidation approach. (A) DFS by GO exposure for patients treated with GO and HSCT, (B) DFS by GO exposure for patients treated with chemotherapy only, and (C) RR by GO exposure and consolidation approach. DFS, disease-free survival; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem cell transplant; KMT2A-r, KMT2A-rearranged; No-GO, did not receive GO; RR, relapse risk.
CD33 Expression in KMT2A-r AML
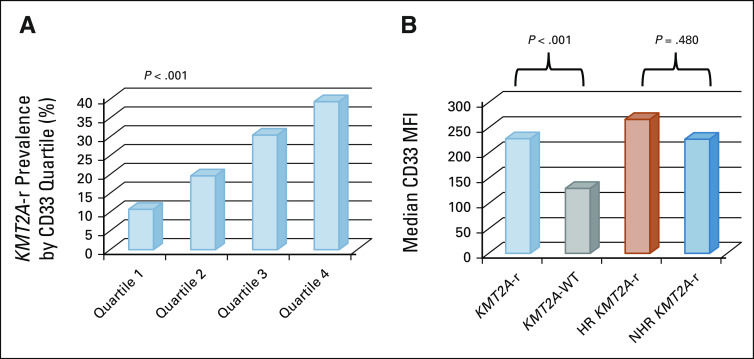
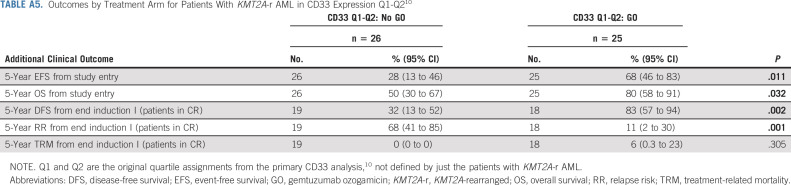
Given the known association between CD33 expression and GO response and previous evidence that patients with KMT2A-r AML have a characteristic phenotype with higher CD33 expression in prospective analysis,9,10,30 we analyzed CD33 expression data for 168 of 215 (78%) patients with KMT2A-r AML with evaluable CD33 data. CD33 MFI was heterogenous in the patients with KMT2A-r AML but tended to cluster in higher AAML0531 CD33 expression quartiles 10 (Appendix Fig A2A, online only). Specifically, median CD33 MFI of leukemic cells isolated from KMT2A-r AML was 229.13 (range 6-1,351) versus 129 (range 2.68-1,225.87) for KMT2A-WT disease (P ≤ .001, Appendix Fig A2B). Interestingly, HR KMT2A-r translocations had a comparable median CD33 MFI (median 267.32; range 22-1,119.5) to that of NHR translocations (median 226.5; range 7.6-1,351; P = .480, Appendix Fig A2B). Importantly, patients with KMT2A-r AML who were in quartile (Q)1 or Q2 (Q1-Q2 median CD33: 84; range 6-146.94) in the composite AAML0531 CD33 analysis10 retained clinical benefit from GO (Appendix Table A5, online only), demonstrating superior EFS and OS from study entry and DFS from CR. RR was also reduced in Q1-Q2 patients who received GO therapy (Appendix Table A5).
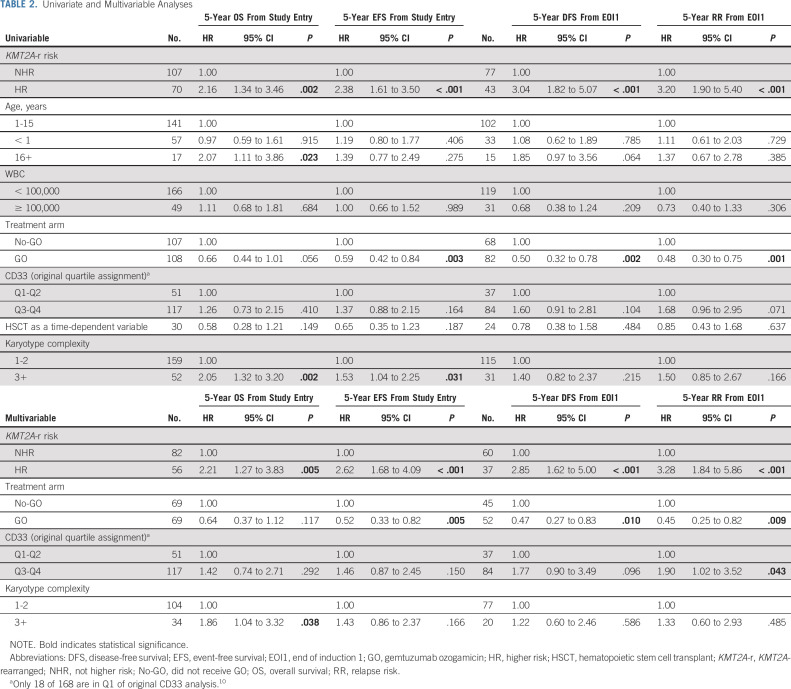
Univariate and Multivariable Analyses
Given the significant association between higher CD33 expression and KMT2A-r AML as well as impact of GO exposure on the KMT2A-r AML response, we performed Cox regression analyses to evaluate whether GO or CD33 expression had an independent impact on clinical outcomes in the context of established prognostic features. Age, presenting WBC count, risk designation of the KMT2A partner (HR v NHR), complex karyotype (≥ 3 cytogenetic abnormalities), GO exposure, HSCT exposure as a time-dependent variable, and CD33 expression, as defined by CD33 quartile classification from the original AAML0531 analysis10 were assessed in a univariate analysis. HR KMT2A-r fusions were associated with inferior EFS and OS as well as DFS and RR. GO treatment was associated with superior EFS, DFS, and lower RR. CD33 expression, as defined by CD33 quartile designation (Q1-2 v Q3-4) was not independently associated with outcome. Older age was associated with inferior OS, and presence of complex karyotype affected OS and EFS (Table 2). In a multivariable model that included KMT2A-r risk group (HR v NHR), treatment arm (GO v no-GO), CD33 quartile assignment, and complex karyotype, GO exposure was independently associated with improved EFS and DFS and reduced RR. Higher CD33 expression (Q3-Q4) retained prognostic significance for RR. In addition, HR KMT2A-r disease was independently associated with reduced EFS, OS, and DFS, as well as higher RR. Complex karyotype was also an independent predictor of inferior OS (Table 2).
TABLE 2.
Univariate and Multivariable Analyses
DISCUSSION
GO significantly improved EFS and DFS in children with KMT2A-r AML enrolled on AAML0531 by reducing rates of relapse without increasing TRM. This effect was observed in both HR and NHR KMT2A-r translocation cohorts. Importantly, the addition of GO abrogated the negative prognostic impact of a KMT2A-r, independent of CD33 expression, and resulted in comparable outcomes to that of KMT2A WT patients treated with or without GO. These findings support use of GO in all patients with KMT2A-r AML treated with a COG backbone of therapy. Moreover, the observation that treatment of KMT2A-r AML with GO followed by HSCT further improved outcomes suggests that GO exposure pre-HSCT may affect post-HSCT prognosis.
Children and adolescents with KMT2A-r AML have generally been treated as IR patients in cooperative group trials,23,27,38–41 although it is clear that outcomes vary for specific translocation partners. The large retrospective analyses by Balgobind et al1 of KMT2A-r pediatric AML demonstrated that patients with translocation partner 1q21 had favorable outcomes, whereas those with partners 10p11.2, 10p12, or 6q27 had markedly poor survival. Subsequent analyses, including our present study, confirmed the unfavorable effect of partners 10p11.2, 10p12, and 6q27, and added 19p13.3 and 4q21.3 as two additional unfavorable partner genes.7,8
Previous studies have also demonstrated that certain AML subsets like FLT3/ITD+ AML have high CD33 expression and that this confers poor outcome.9 Importantly, however, higher CD33 expression is also associated with improved GO response, both overall and in the high-risk FLT3/ITD+ disease subset.9,10,42 Both HR and NHR KMT2A-r subsets had high CD33 expression levels, although notably, our analysis demonstrates that even patients with lower CD33 expression appeared to have clinical benefit from GO. Together, this suggests that additional biologic factors in KMT2A-r AML might contribute to the favorable GO response seen. Surprisingly, despite the poor EFS and high RR in patients with KMT2A-r AML, EOI1 MRD was reported in < 20% of patients at EOI1. Although GO did not appear to decrease rates of MRD detection in our series, its use during induction ultimately affected DFS and RR particularly in patients receiving HSCT, suggesting GO may affect leukemic stem cells of more mature CD33+ origin resulting in additive benefit when used in combination with HSCT.
This study is limited as it is a retrospective analysis of a heterogeneous molecular subset within a larger prospective clinical trial that was not specifically designed to address the impact of GO or HSCT in KMT2A-r AML. Moreover, given that the other and unknown variants could not contribute to KMT2A-risk stratification, this missing data further limit the significance of our analyses. Nevertheless, this study includes a relatively large number of pediatric patients with KMT2A-r AML treated on a standard chemotherapy backbone in a randomized controlled trial. Although our analysis suggests that the combination of GO and HSCT may improve outcomes for pediatric KMT2A-r AML further, we concede that the number of patients who received both therapies was small and therefore further prospective studies are needed to explore the additive benefit of GO and HSCT. Importantly, our analysis has influenced KMT2A-r AML risk stratification for the current COG phase III pediatric AML trial, AAML1831, and provides additional rationale for including GO in the backbone of chemotherapy for all patients with KMT2A-r AML enrolled. However, given the higher TRM seen with GO therapy in COG AAML0531 and evidence in the NOPHO AML-2004 study that GO lacked clear benefit when given in consolidation, AAML1831 restricts GO use to the first cycle of treatment.43 Investigation of second-generation CD33-targeting agents is prudent in this disease subset and may further aid identification of KMT2A-r disease features that predict for favorable response in this heterogenous group of patients.
ACKNOWLEDGMENT
The authors thank Vani J. Shanker for scientific editing and the Children's Oncology Group Reference Laboratory, particularly Sommer Castro, for providing diagnostic specimens. They also thank the patients and families who consented to the use of biologic specimens in these trials.
Appendix
FIG A1.
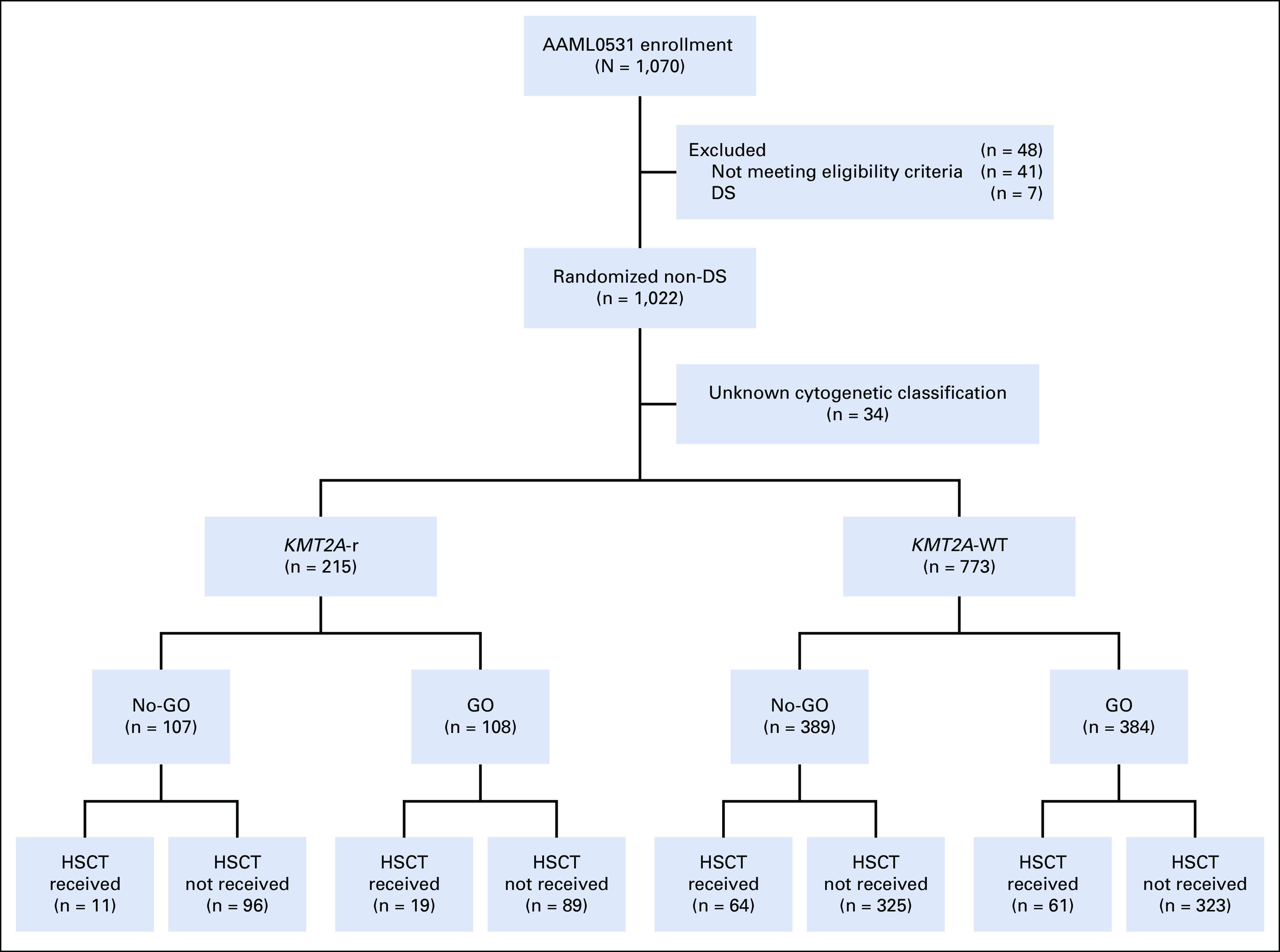
CONSORT diagram of the study population. DS, Down syndrome; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem cell transplant; KMT2A-r, KMT2A-rearranged; No-GO, did not receive GO; WT, wild type.
FIG A2.
CD33 expression in KMT2A-r AML. (A) Distribution of patients with KMT2A-r AML in AAML0531-defined CD33 expression quartiles.10 (B) Median CD33 MFI for KMT2A-r versus KMT2A-WT and for HR versus NHR KMT2A-r AML. AML, acute myeloid leukemia; HR, higher risk; KMT2A-r, KMT2A-rearranged; MFI, mean fluorescence intensity; NHR, non–high-risk; WT, wild-type.
TABLE A1.
Disease Characteristics and Clinical Response for KMT2A-r Versus WT Acute Myeloid Leukemia: Overall and Treated With or Without Gemtuzumab Ozogamicin
TABLE A2.
Disease Characteristics and Induction Response by KMT2A Translocation Partner
TABLE A3.
Clinical Characteristics and Descriptive Summary of Outcomes for the 28 Other Patients With KMT2A-r AML
TABLE A4.
Outcome Analysis for HR Versus NHR KMT2A-r Acute Myeloid Leukemia by GO Exposure
TABLE A5.
Outcomes by Treatment Arm for Patients With KMT2A-r AML in CD33 Expression Q1-Q210
Jessica A. Pollard
Consulting or Advisory Role: Syndax Pharmaceuticals, Kura Oncology
Erin Guest
Consulting or Advisory Role: Syndax Pharmaceuticals
Mike R. Loken
Employment: Hematologics Inc
Leadership: Hematologics Inc
Stock and Other Ownership Interests: Hematologics Inc
Consulting or Advisory Role: Newlink Genetics
Lisa Eidenschink Brodersen
Employment: Hematologics Inc
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
Alan S. Gamis
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
DISCLAIMER
L.E.B. is employed by Hematologics, and M.R.L. is equity owner and employed by Hematologics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part (correlation of 11q23/KMT2A and gemtuzumab ozogamicin response) as an oral abstract at the 57th Annual Meeting of American Society of Hematology, Orlando, FL, December 2015 (Blood 2015 126:23). Additional data on risk stratification of 11q23/KMT2A subsets were presented as an oral abstract at the 58th Annual Meeting of the American Society of Hematology, San Diego, December 2016 (Blood 2016 128:1,211).
SUPPORT
Supported by Chair's Grant of the Children's Oncology Group—U10 CA98543-08 and Statistics and Data Center Grant No CA U10CA180899, NCTN Operations Center Grant U10CA180886, St Baldrick's Foundation, National Cancer Institute Grant No. RO1CA114563 (S.M.), and St Baldrick's Career Development Award (J.P.).
CLINICAL TRIAL INFORMATION
J.P. and E.G. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Jessica A. Pollard, Erin Guest, Todd A. Alonzo, Robert B. Gerbing, Mike R. Loken, E. Anders Kolb, Richard Aplenc, Soheil Meshinchi, Susana C. Raimondi, Betsy Hirsch, Alan S. Gamis
Provision of study materials or patients: Richard Aplenc
Collection and assembly of data: Jessica A. Pollard, Erin Guest, Todd A. Alonzo, Robert B. Gerbing, Mike R. Loken, Lisa Eidenschink Brodersen, E. Anders Kolb, Richard Aplenc, Soheil Meshinchi, Susana C. Raimondi, Betsy Hirsch, Alan S. Gamis
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gemtuzumab Ozogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results From the Phase III Children's Oncology Group Trial AAML0531
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jessica A. Pollard
Consulting or Advisory Role: Syndax Pharmaceuticals, Kura Oncology
Erin Guest
Consulting or Advisory Role: Syndax Pharmaceuticals
Mike R. Loken
Employment: Hematologics Inc
Leadership: Hematologics Inc
Stock and Other Ownership Interests: Hematologics Inc
Consulting or Advisory Role: Newlink Genetics
Lisa Eidenschink Brodersen
Employment: Hematologics Inc
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
Alan S. Gamis
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Balgobind BV, Raimondi SC, Harbott J, et al. : Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: Results of an international retrospective study. Blood 114:2489-2496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison CJ, Hills RK, Moorman AV, et al. : Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol 28:2674-2681, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bolouri H, Farrar JE, Triche T Jr, et al. : The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med 24:103-112, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer C, Burmeister T, Groger D, et al. : The MLL recombinome of acute leukemias in 2017. Leukemia 32:273-284, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coenen EA, Raimondi SC, Harbott J, et al. : Prognostic significance of additional cytogenetic aberrations in 733 de novo pediatric 11q23/MLL-rearranged AML patients: Results of an international study. Blood 117:7102-7111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Raimondi SC, Tong X, et al. : Favorable impact of the t(9;11) in childhood acute myeloid leukemia. J Clin Oncol 20:2302-2309, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Guest EM, Hirsch BA, Kolb EA, et al. : Prognostic significance of 11q23/MLL fusion partners in children with acute myeloid leukemia (AML)—Results from the Children's Oncology Group (COG) trial AAML0531. Blood 128:1211, 2016 [Google Scholar]
- 8.Van Weelderen RE, Klein K, Goemans BF, et al. : Outcome of (novel) subgroups in 1257 pediatric patients with KMT2A-rearranged acute myeloid leukemia (AML) and the significance of minimal residual disease (MRD) status: A retrospective study by the I-BFM-SG. Blood 136:26-27, 2020. (suppl 1) [Google Scholar]
- 9.Pollard JA, Alonzo TA, Loken M, et al. : Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood 119:3705-3711, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollard JA, Loken M, Gerbing RB, et al. : CD33 expression and its association with gemtuzumab ozogamicin response: Results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol 34:747-755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnett AK, Hills RK, Milligan D, et al. : Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J Clin Oncol 29:369-377, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Hills RK, Castaigne S, Appelbaum FR, et al. : Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 15:986-996, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaunay J, Recher C, Pigneux A, et al. : Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study. Blood 118:79, 2011 [Google Scholar]
- 14.Borthakur G, Cortes JE, Garcia-Manero G, et al. : Addition of gemtuzumab ozogamicin (GO) to fludarabine, cytarabine and G-CSF (FLAG) based induction regimen results in better early molecular response and relapse free survival compared to idarubicin (FLAG-Ida) in newly diagnosed core binding factor leukemia. Blood 132, 2018 [Google Scholar]
- 15.Burnett AK, Russell NH, Hills RK, et al. : Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 30:3924-3931, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Walter RB, Appelbaum FR, Estey EH, et al. : Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 119:6198-6208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castaigne S, Pautas C, Terre C, et al. : Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 379:1508-1516, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Burnett AK, Hills RK, Hunter AE, et al. : The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 27:75-81, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Laszlo GS, Estey EH, Walter RB: The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev 28:143-153, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Loke J, Khan JN, Wilson JS, et al. : Mylotarg has potent anti-leukaemic effect: A systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Ann Hematol 94:361-373, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thol F, Schlenk RF: Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin Biol Ther 14:1185-1195, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Aplenc R, Alonzo TA, Gerbing RB, et al. : Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: A report from the Children's Oncology Group. J Clin Oncol 26:2390-3295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubnitz JE, Inaba H, Dahl G, et al. : Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol 11:543-552, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper TM, Franklin J, Gerbing RB, et al. : AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children's Oncology Group. Cancer 118:761-769, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Cowan AJ, Laszlo GS, Estey EH, et al. : Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front Biosci (Landmark Ed) 18:1311-1334, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hear C, Inaba H, Pounds S, et al. : Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer 119:4036-4043, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamis AS, Alonzo TA, Meshinchi S, et al. : Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol 32:3021-3032, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guest EM, Aplenc R, Sung L, et al. : Gemtuzumab ozogamicin in infants with AML: Results from the Children's Oncology Group trials AAML03P1 and AAML0531. Blood 130:943-945, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niktoreh N, Lerius B, Zimmermann M, et al. : Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: A report by Berlin-Frankfurt-Munster study group. Haematologica 104:120-127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voigt AP, Brodersen LE, Alonzo TA, et al. : Phenotype in combination with genotype improves outcome prediction in acute myeloid leukemia: A report from Children's Oncology Group protocol AAML0531. Haematologica 102:2058-2068, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells DA, Ogata K: On flow cytometry in myelodysplastic syndromes, with caveats. Leuk Res 32:209-210, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Walter RB, Gooley TA, van der Velden V, et al. : CD33 expression and P-glycoprotein mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 109:4168-4170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loken MR, Alonzo TA, Pardo L, et al. : Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: A report from Children's Oncology Group. Blood 120:1581-1588, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terstappen L, Loken M: Myeloid cell differentiation in normal bone marrow and acute myeloid leukemia assessed by multi-dimensional flow cytometry. Anal Cell Pathol 2:229-240, 1990 [PubMed] [Google Scholar]
- 35.Kaplan E, Meier P: Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 36.Kalbfleisch J, Prentice RL: The Statistical Analysis of Failure Time Data (ed 2). Hoboken, NJ, John Wiley & Sons, 2002 [Google Scholar]
- 37.Cox D: Regression models and life-tables. J R Stat Soc Series B Stat Methodol 34:187-220, 1972 [Google Scholar]
- 38.Aplenc R, Meshinchi S, Sung L, et al. : Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: A report from the Children's Oncology Group. Haematologica 105:1879-1886, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creutzig U, Zimmermann M, Lehrnbecher T, et al. : Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: Results of AML-BFM 98. J Clin Oncol 24:4499-4506, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Gibson BE, Wheatley K, Hann IM, et al. : Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia 19:2130-2138, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Creutzig U, Zimmermann M, Ritter J, et al. : Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia 19:2030-2042, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Tarlock K, Alonzo TA, Gerbing RB, et al. : Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: A report from the Children's Oncology Group. Clin Cancer Res 22:1951-1957, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasle H, Abrahamsson J, Forestier E, et al. : Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: Results from NOPHO-AML 2004. Blood 120:978-984, 2012 [DOI] [PubMed] [Google Scholar]