Supplemental Digital Content is available in the text.
Keywords: acute myocardial infarction, cardiogenic shock, Horowitz index, Pao2/Fio2 ratio, venoarterial extracorporeal membrane oxygenation
OBJECTIVES:
Venoarterial extracorporeal membrane oxygenation treatment in patients with severe cardiogenic shock can cause or aggravate acute lung injury. In our retrospective analysis, we aimed at identifying markers for acute lung injury after arrival on ICU, which predict mortality of those patients.
DESIGN:
Observational, monocentric, retrospective analysis.
SETTING:
Cardiac ICU of Ludwig Maximilian University Hospital, Munich, Germany.
PATIENTS:
Two-hundred eleven patients undergoing venoarterial extracorporeal membrane oxygenation treatment for severe cardiogenic shock were included into this analysis. Patients who died within 24 hours after venoarterial extracorporeal membrane oxygenation implantation were excluded.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
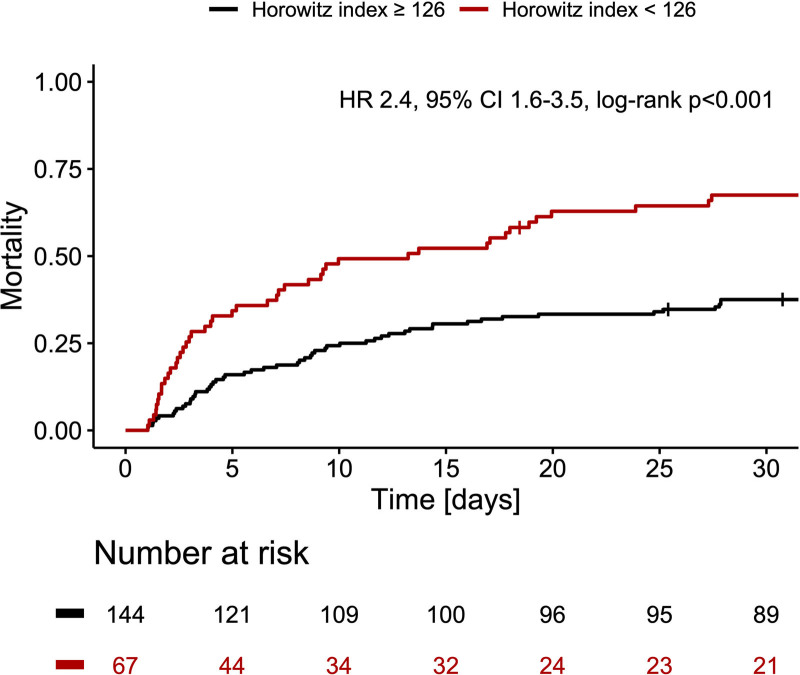
To determine lung injury, we investigated the influence of Pao2/Fio2 ratio (Horowitz index). The lowest Horowitz index was measured on ICU 6–12 hours after venoarterial extracorporeal membrane oxygenation implantation. An optimal cutoff value of less than 126 for Horowitz index to predict mortality was identified via receiver operating characteristic analysis (area under the curve, 0.62). Patients with Horowitz index less than 126 had a 30-day mortality rate of 67.5% compared to 37.5% with a Horowitz index greater than or equal to 126 (p < 0.001). Multivariate analysis identified Horowitz index less than 126 as an independent risk factor of ICU mortality (odds ratio, 3.6; 95% CI, 1.8–7.3; p < 0.001).
CONCLUSIONS:
In this hypothesis-generating analysis, a Horowitz index less than 126 is associated with increased mortality in patients with cardiogenic shock and venoarterial extracorporeal membrane oxygenation, which may serve as a threshold for further therapeutic interventions.
Despite many therapeutic and technical advances, cardiogenic shock remains an important cause of mortality in intensive care medicine. Large-scale randomized trials (1) and contemporary registries (2) report a 30-day mortality rate around 50%. In advanced cardiogenic shock stages, like stage E (extremis) according to Society for Cardiovascular Angiography and Interventions, guideline-directed medical and volume therapy often fails to stabilize this critical condition (3). These patients require mechanical circulatory support such as venoarterial extracorporeal membrane oxygenation (VA-ECMO) (3), which is currently investigated in the large-scale randomized trials Testing the Value of Novel Strategy and Its Cost Efficacy in Order to Improve the Poor Outcomes in Cardiogenic Shock and Extracorporeal Life Support in Cardiogenic Shock.
Acute lung injury and progressive respiratory failure occur in these patients due to backward heart failure with pulmonary congestion, resuscitation, or aspiration. The VA-ECMO ensures the peripheral and end-organ perfusion; however, the routinely implemented retrograde aortic flow per se increases the left ventricular (LV) afterload, which may result in an insufficient LV unloading and thus aggravates pulmonary congestion (4). The Horowitz index (Pao2/Fio2 ratio) is a well-known marker of acute pulmonary injury and predicts mortality in patients with acute respiratory distress syndrome (5). Thus, our hypothesis was that the Horowitz index (Pao2/Fio2 ratio) may also predict mortality in patients with cardiogenic shock and VA-ECMO therapy early after ICU admission.
MATERIALS AND METHODS
Study Population
In compliance with the Declaration of Helsinki and data protection laws, cardiogenic shock patients, who were treated in the cardiac ICU of the Ludwig Maximilian University (LMU) Hospital Munich between January 2010, and March 2021, were included in the LMUshock registry (World Health Organization trial ID DRKS00015860, institutional review board [IRB] board name: “Ethikkommission bei der Medizinischen Fakultät der LMU München”, IRB approval number: 18-001). Cardiogenic shock was defined according to the European Society of Cardiology guidelines (6) and the Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock II trial (1) as follows:
-
Hypotension despite adequate filling status.
Systolic blood pressure less than 90 mm Hg for 30 minutes, or
catecholamine support to maintain systolic blood pressure greater than 90 mm Hg.
Signs of pulmonary congestion.
-
One of the following clinical and laboratory signs of hypoperfusion:
Clinical: altered mental status, dizziness, cold, clammy skin and extremities, oliguria with urine output less than 30 mL/hr, narrow pulse pressure.
Laboratory: metabolic acidosis, elevated serum lactate greater than 2 mmol/L, elevated creatinine, due to primary cardiac dysfunction.
Patients with VA-ECMO (implantation period: up to 1 d before ICU admission until 30 d after ICU admission) and a minimal survival of at least 24 hours after VA-ECMO implantation were included in our study. VA-ECMO implantation before ICU admission was performed in our catheterization laboratory or in an external hospital before transfer to our ICU. VA-ECMO implantation after ICU admission was performed at bedside or in our catheterization laboratory. Patients with incomplete data, venovenous extracorporeal membrane oxygenation or veno-arterial-venous (VAV)-ECMO, were excluded.
VA-ECMO
See Supplemental Material: VA-ECMO (http://links.lww.com/CCX/A796).
Study Endpoints and Variables
The primary endpoint was 30-day mortality rate after ICU admission, and the secondary endpoint mortality per se on ICU. The Horowitz index (Pao2/Fio2 ratio) was defined as the minimum Pao2/Fio2 ratio measured via the right radial artery, if possible, within 6–12 hours after VA-ECMO implantation permitting a potential complex coronary intervention, transfer to the ICU, and initial stabilization of the patient. Bleeding events during the first 30 days were classified according to the bleeding academic research consortium (BARC). Ischemic events (myocardial infarction, stent thrombosis, and stroke) during the first month were obtained and analyzed.
Statistical Analysis
See Supplemental Material: Statistical analysis (http://links.lww.com/CCX/A795).
RESULTS
Study Population and Baseline Characteristics
Two-hundred eleven patients with VA-ECMO therapy, who survived at least 24 hours after VA-ECMO implantation, were included in our analysis (Supplemental Fig. 1, http://links.lww.com/CCX/A797). At ICU admission, the mean age was 57 ± 13 years, and 82% were male. Cardiac arrest occurred in 68% of patients. Predominant cause of cardiogenic shock was ST-elevation myocardial infarction (STEMI) in 44% of patients and non-STEMI in 19%. The baseline characteristics are summarized in Supplemental Table 1 (http://links.lww.com/CCX/A798).
Median duration of ICU stay was 11 days (interquartile range [IQR], 5–17 d), and median Simplified Acute Physiology Score II score 74 (IQR, 67–83). Median first lactate on ICU admission was 8 mmol/L (IQR, 4–10 mmol/L). In 46% of cases, renal replacement therapy was necessary. Concomitant coaxial LV assist device (Impella) treatment was used in 14.7% of patients (combination of a venoarterial extracorporeal life support system and the Impella left ventricular assist device concept) (4). All ICU characteristics are summarized in Supplemental Table 2 (http://links.lww.com/CCX/A799). The vast majority of VA-ECMO implantations was performed at the catheterization laboratory, prior to ICU admission (Supplemental Fig. 2, http://links.lww.com/CCX/A800).
Horowitz Index and Mortality Rate
The minimal Horowitz index (Pao2/Fio2 ratio), assessed 6–12 hours after VA-ECMO implantation, was used as key variable of acute lung injury, and a cutoff value predicting mortality was evaluated by receiver operating characteristic analysis, as mentioned above. The estimated optimal cutoff value was found to be at a Pao2/Fio2 ratio of 126 (area under the curve, 0.62). Patients with an index less than 126 experienced a 30-day mortality rate of 67.5% compared with 37.5% at an index greater than or equal to 126 (p<0.001) (Fig. 1). Rate of bleeding events greater than or equal to BARC3 was higher in the group with a Horowitz index less than 126 (Supplemental Fig. 3, http://links.lww.com/CCX/A801). Ischemic events (myocardial infarction, stent thrombosis, and stroke) during ICU stay were similar in both groups (Supplemental Fig. 4, http://links.lww.com/CCX/A802).
Figure 1.
Mortality curves for patients with Horowitz index less than 126 versus Horowitz index greater than or equal to 126 are shown for 30 d after ICU admission. Hazard ratio (HR) and 95% CI are displayed for patients with Horowitz index less than 126.
Horowitz Index as an Independent Risk Factor for ICU Mortality
The Horowitz index (Pao2/Fio2 ratio) as a risk factor of ICU mortality was investigated by using binary logistic regression models. Hence, the index was integrated into previous risk models of patients with cardiogenic shock and VA-ECMO therapy (7, 8). The univariate analysis is shown in Supplemental Table 3 (http://links.lww.com/CCX/A803). Among those, independent predictors of ICU mortality, identified by multivariate analysis, were female gender (odds ratio [OR], 0.428 for male gender; p = 0.035), first lactate measured on ICU (OR, 1.088 per mmol/L; p = 0.007), acute myocardial infarction (OR, 0.407; p = 0.020), and Horowitz index (Pao2/Fio2 ratio) less than 126 (OR, 3.621; p < 0.001) (Supplemental Table 3, http://links.lww.com/CCX/A803).
DISCUSSION AND CONCLUSIONS
This hypothesis-generating study found respiratory failure, characterized by Horowitz index (Pao2/Fio2 ratio) less than 126, as a predictor of mortality in patients suffered from cardiogenic shock and treated by VA-ECMO therapy. The key findings of our study are as follows:
1) Patients with a Horowitz index (Pao2/Fio2 ratio) below 126 experienced a 1.8-fold higher 30-day mortality compared with patients with an index equal or above 126.
2) A Horowitz index (Pao2/Fio2 ratio) less than 126 is an independent predictor of ICU mortality, along with female gender at birth, first lactate measured on ICU, and nonischemic cardiogenic shock.
In this study, we aimed at investigating the Horowitz index (Pao2/Fio2 ratio) as a marker for mortality early after ICU admission in cardiogenic shock patients undergoing VA-ECMO treatment, which may indicate the necessity for further ventilation management optimization, for example, prone position or escalation to VAV-ECMO. This hypothesis should be further tested in dedicated randomized trials.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Scherer and Orban designed the study, interpreted data, and wrote the article. Drs. Lüsebrink, Joskowiak, Feuchtgruber, Petzold, and Peterss collected data and critically revised the article. Drs. Hausleiter, Massberg, and Hagl interpreted data and critically revised the article.
Supported, in part, by Deutsche Forschungsgemeinschaft (413635475), Munich Clinician Scientist Program of Ludwig Maximilian University (LMU) Munich (to Dr. Scherer), and Medical Faculty of LMU Munich (to Drs. Feuchtgruber and Orban).
Drs. Joskowiak and Peterss received speaker honoraria from AstraZeneca. Dr. Hausleiter received speaker honoraria and research support from Abbott Vascular and Edwards Lifesciences, outside the submitted work. Dr. Orban received speaker honoraria from Abbott Medical, AstraZeneca, Abiomed, Bayer vital, Biotronik, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences Services, Sedana Medical, outside the submitted work. The remaining authors have disclosed that they do not have any conflicts of interest.
All ethical standards were met in writing and submitting this correspondence.
Data available on request.
REFERENCES
- 1.Thiele H, Zeymer U, Neumann FJ, et al. ; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367:1287–1296 [DOI] [PubMed] [Google Scholar]
- 2.Rathod KS, Koganti S, Iqbal MB, et al. Contemporary trends in cardiogenic shock: Incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care. 2018; 7:16–27 [DOI] [PubMed] [Google Scholar]
- 3.Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur Heart J. 2019; 40:2671–2683 [DOI] [PubMed] [Google Scholar]
- 4.Lüsebrink E, Orban M, Kupka D, et al. Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur Heart J. 2020; 41:3753–3761 [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012; 122:2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37:2129–2200 [DOI] [PubMed] [Google Scholar]
- 7.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016; 42:370–378 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015; 36:2246–2256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.