PURPOSE
Primary or secondary mutations in KIT or platelet-derived growth factor receptor alpha (PDGFRA) underlie tyrosine kinase inhibitor resistance in most GI stromal tumors (GISTs). Avapritinib selectively and potently inhibits KIT- and PDGFRA-mutant kinases. In the phase I NAVIGATOR study (NCT02508532), avapritinib showed clinical activity against PDGFRA D842V–mutant and later-line KIT-mutant GIST. VOYAGER (NCT03465722), a phase III study, evaluated efficacy and safety of avapritinib versus regorafenib as third-line or later treatment in patients with unresectable or metastatic GIST.
PATIENTS AND METHODS
VOYAGER randomly assigned patients 1:1 to avapritinib 300 mg once daily (4 weeks continuously) or regorafenib 160 mg once daily (3 weeks on and 1 week off). Primary end point was progression-free survival (PFS) by central radiology per RECIST version 1.1 modified for GIST. Secondary end points included objective response rate, overall survival, safety, disease control rate, and duration of response. Regorafenib to avapritinib crossover was permitted upon centrally confirmed disease progression.
RESULTS
Four hundred seventy-six patients were randomly assigned (avapritinib, n = 240; regorafenib, n = 236). Median PFS was not statistically different between avapritinib and regorafenib (hazard ratio, 1.25; 95% CI, 0.99 to 1.57; 4.2 v 5.6 months; P = .055). Overall survival data were immature at cutoff. Objective response rates were 17.1% and 7.2%, with durations of responses of 7.6 and 9.4 months for avapritinib and regorafenib; disease control rates were 41.7% (95% CI, 35.4 to 48.2) and 46.2% (95% CI, 39.7 to 52.8). Treatment-related adverse events (any grade, grade ≥ 3) were similar for avapritinib (92.5% and 55.2%) and regorafenib (96.2% and 57.7%).
CONCLUSION
Primary end point was not met. There was no significant difference in median PFS between avapritinib and regorafenib in patients with molecularly unselected, late-line GIST.
INTRODUCTION
GI stromal tumors (GISTs) are the most common sarcoma of the GI tract,1,2 with estimated incidence between 1.0 and 1.5/100,000 per year; current estimates suggest prevalence increasing to almost 10-fold greater than incidence.2,3 Most GISTs are driven by activating oncogenic mutations in receptor kinase KIT (approximately 80%) or platelet-derived growth factor receptor alpha (PDGFRA; approximately 5%-10%).1,2,4
CONTEXT
Key Objective
The phase III VOYAGER study evaluated efficacy and safety of avapritinib versus regorafenib as third-line or later treatment in patients with molecularly unselected unresectable or metastatic GI stromal tumor (GIST). To our knowledge, VOYAGER is the first randomized study in late-line GIST to use an active comparator as a control arm.
Knowledge Generated
Avapritinib was not superior to regorafenib in terms of median progression-free survival (PFS; primary end point) in third-line or later treatment of patients with unresectable or metastatic GIST. Most patients enrolled had KIT-mutant tumors. Consistent with previous experience with avapritinib, response rates and PFS remained high and durable in 3% of patients with platelet-derived growth factor receptor alpha D842V–mutant tumors.
Relevance
As no PFS benefit was observed with avapritinib over regorafenib (intention-to-treat population), our findings do not suggest any change in later-line treatment paradigms for KIT-mutant GIST. However, avapritinib remains the most active available agent for patients with platelet-derived growth factor receptor alpha D842V–mutant GIST.
Treatment guidelines recommend tyrosine kinase inhibitor (TKI) imatinib as first-line standard of care for patients with unresectable or metastatic (U/M) GIST, followed by TKIs sunitinib, regorafenib, and ripretinib as second-line, third-line, and fourth-line therapies.5,6 Despite available treatment, U/M GIST remains an incurable disease and new therapies are needed.7-10 The emergence of heterogeneous tumor subclones harboring secondary KIT mutations in the ATP-binding pocket (exons 13 and 14) or the activation loop (exons 17 and 18) of the KIT kinase domain confers resistance to imatinib.11-14 Activation loop mutations are detectable in approximately 44%-67% of KIT-mutant GIST after treatment with imatinib and are increased to approximately 82% after treatment with imatinib and sunitinib.11,13,15,16 In approximately 5%-6% of patients with U/M GIST, primary activation loop mutations in PDGFRA amino acid 842, particularly D842V, confer primary resistance to imatinib and other TKIs.17,18
Avapritinib (formerly BLU-285; Blueprint Medicines Corporation, Cambridge, MA) is a potent, selective inhibitor of KIT and PDGFRA tyrosine kinases, with high potency for KIT D816V–mutant and PDGFRA D842V–mutant kinases.18 In a phase I study (NAVIGATOR; NCT02508532), avapritinib demonstrated objective response rates (ORRs) of 21% as fourth-line or later therapy in patients with advanced molecularly unselected GIST and 88% in patients with advanced PDGFRA D842V–mutant GIST, regardless of previous therapy.19,20 Findings from NAVIGATOR formed the basis for US Food and Drug Administration approval of avapritinib in the United States and European Medicines Agency approval in Europe for treatment of adults with U/M GIST harboring a PDGFRA exon 18 mutation, including D842V mutations.21,22
On the basis of data from NAVIGATOR, a phase III clinical study to assess the efficacy and safety of avapritinib versus regorafenib (VOYAGER; NCT03465722) was initiated in patients with molecularly unselected U/M GIST previously treated with imatinib and one or two other TKIs. Here we present outcomes of VOYAGER, which to our knowledge is the first randomized study in late-line U/M GIST that uses an active comparator (regorafenib) as a control arm.
PATIENTS AND METHODS
Study Design
VOYAGER was an open-label, randomized, multicenter phase III study (NCT03465722) comparing avapritinib with regorafenib in patients with U/M GIST previously treated with imatinib and one or two additional TKIs. Eligible patients were randomly assigned 1:1 to receive either oral avapritinib 300 mg once daily in continuous 28-day cycles or oral regorafenib 160 mg once daily in 28-day, 3 weeks on and 1 week off cycles (Fig 1). Random assignment was stratified by TKI treatment (third-line v fourth-line), geographic region (Asia v other countries), and PDGFRA D842V status (mutation present v absent) measured by circulating tumor DNA (ctDNA). Patients who received avapritinib had the option to escalate to 400 mg once daily at the investigator's discretion after specific criteria were met (per the Protocol, online only). Crossover from regorafenib to avapritinib was allowed for patients with centrally confirmed radiologic disease progression.
FIG 1.
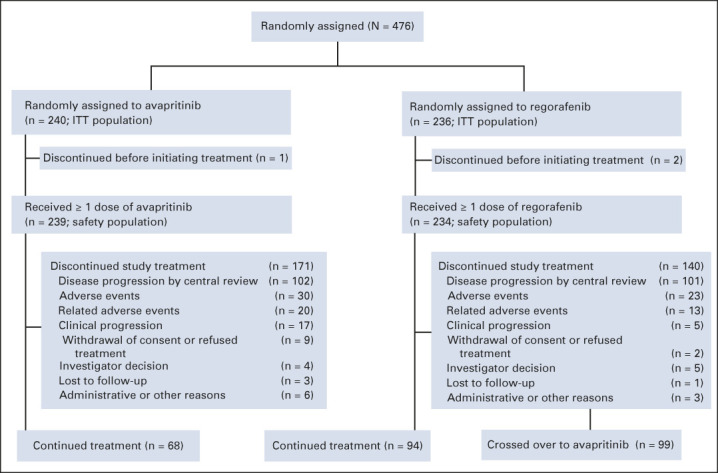
CONSORT diagram for the phase III VOYAGER study. ITT, intention-to-treat.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The Protocol was approved by the institutional review board or independent ethics committee at each study center. All patients provided written informed consent for data collection supporting these analyses.
Eligibility Criteria
Eligible patients (age ≥ 18 years) had histologically confirmed U/M GIST, previously treated with imatinib and one or two additional TKIs, and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were excluded if they previously received avapritinib, regorafenib, or > 3 different TKIs, systemic anticancer therapy within 2 weeks (1 week following Protocol amendment) before random assignment, or radiotherapy within 2 weeks before random assignment or had a known risk for intracranial bleeding (ICB) within 1 year before random assignment.
Outcomes
The primary end point was progression-free survival (PFS) on the basis of central radiologic assessment by RECIST version 1.1 modified for GIST (mRECIST v1.1).7 Key secondary end points were ORR by mRECIST v1.1 and overall survival (OS); other secondary end points included safety, disease control rate (DCR; rate of complete responses and partial responses [PRs] or stable disease [SD] lasting ≥ 16 weeks), and duration of response (DOR) by central radiology per mRECIST v1.1. A post hoc analysis of PFS per investigator assessment in patients who crossed over from regorafenib to avapritinib was conducted.
Assessments
Tumor assessments, by computed tomography with intravenous contrast or magnetic resonance imaging, were performed at baseline and then every 8 weeks (± 1 week) counting from Cycle 1 Day 1 until disease progression was confirmed by central radiology review. Patients who discontinued treatment because of reasons other than disease progression were followed for tumor assessments until disease progression or death (or loss to follow-up). Target and nontarget lesions were identified and assessed according to mRECIST v1.1 by central radiology review.7 Blood samples for characterizing mutation status by ctDNA were collected at screening and at Day 1 of all cycles up to the end of the treatment. Samples for ctDNA analysis were not collected following crossover from regorafenib to avapritinib. Samples were sent to a central laboratory in the United States for analysis.
Adverse events (AEs) were evaluated at each visit from the start of study drug administration up to 30 days after the final dose and were recorded and coded according to the Medical Dictionary for Regulatory Activities v18.1. The severity of AEs was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events. Cognitive effects were defined as cognitive disorder, memory impairment, confusional state, or encephalopathy. ICB was defined as cerebral hemorrhage, intracranial hemorrhage, or subdural hematoma.
Statistical Methods
The sample size was based on the assumption that the median PFS (mPFS) for regorafenib was approximately 5 months.23 A sample size of 460 patients (approximately 230 patients per arm) and a minimum of 264 PFS events were predicted to provide 90% power at a two-sided α value of .05, assuming a PFS hazard ratio (HR; avapritinib v regorafenib) of 0.67.
Efficacy was evaluated in all randomly assigned patients (intention-to-treat [ITT]), and safety was evaluated in all patients who received ≥ 1 dose of avapritinib or regorafenib. Kaplan-Meier (K-M) estimates were used to assess PFS and OS. Median follow-up was calculated using reverse K-M estimates. The Cox regression model was used to assess HR and 95% CI. For ORR, 95% CI was estimated using the Clopper-Pearson method; treatment difference was estimated using a stratified Cochran-Mantel-Haenszel test. K-M estimates were used to descriptively summarize DOR. For DCR, 95% CI was estimated for the ITT population using the Clopper-Pearson method. The cutoff date for these analyses was March 9, 2020.
RESULTS
Patients
Overall, 476 patients were randomly assigned between March 26, 2018, and November 15, 2019, in North America, Europe, Australia, and Asia; 240 patients received avapritinib, and 236 patients received regorafenib. All patients on avapritinib started on 300 mg, with four patients escalated to avapritinib 400 mg; all patients on regorafenib started on 160 mg. Of the patients randomly assigned to regorafenib, 41.9% (99 of 236) of patients crossed over to avapritinib treatment (Fig 1).
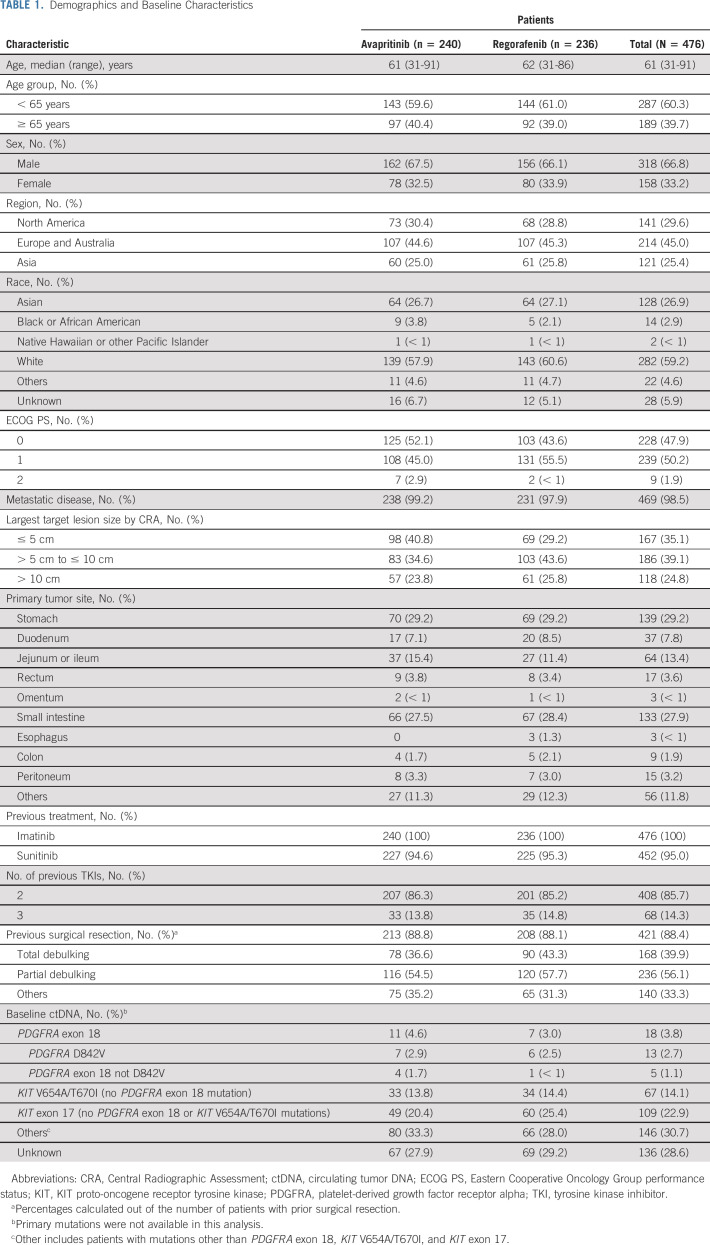
In the ITT population, the median age (range) was 61 (31-91) years, 66.8% (318 of 476) were male, 59.2% (282 of 476) were White, and 25.4% (121 of 476) were recruited from Asia. All patients received previous treatment with imatinib, and 95.0% (452 of 476) of patients received previous treatment with sunitinib. In all, 85.7% (408 of 476) of patients received two distinct previous TKIs and 14.3% (68 of 476) of patients received three distinct previous TKIs. On the basis of baseline ctDNA analysis, a PDGFRA exon 18 mutation was found in 3.8% (18 of 476) of patients, and 30.7% (146 of 476) of patients had mutations other than PDGFRA exon 18, KIT V654A, KIT T670I, or KIT exon 17; 2.7% (13 of 476) of patients had a D842V mutation in the activation loop sequence of PDGFRA exon 18. The mutation status in the ctDNA of 28.6% (136 of 476) of patients was unknown, because of sample unavailability for analysis, primarily since samples could not be shipped outside of China (Table 1).
TABLE 1.
Demographics and Baseline Characteristics
Efficacy
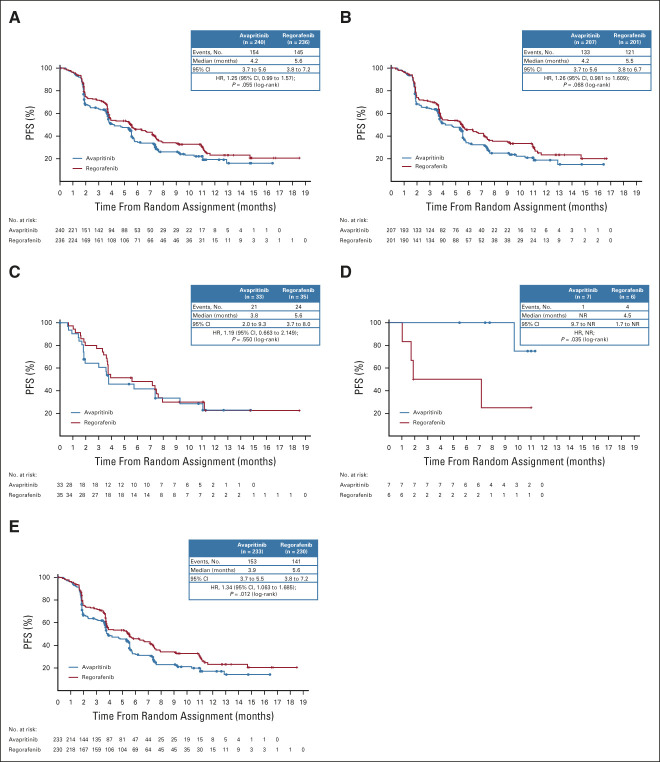
There was no significant difference in mPFS between avapritinib and regorafenib (HR, 1.25; 95% CI, 0.99 to 1.57; mPFS 4.2 v 5.6 months, respectively; P = .055). The shape of the K-M curve was similar in both study arms (Fig 2A). Similarly, there was no significant difference in mPFS between subgroups of patients treated with avapritinib and regorafenib as third-line treatment (HR, 1.26; 95% CI, 0.98 to 1.61; mPFS 4.2 v 5.5 months, respectively; P = .068; Fig 2B) or fourth-line treatment (HR, 1.19; 95% CI, 0.66 to 2.15; mPFS 3.8 v 5.6 months, respectively; P = .550; Fig 2C). mPFS in patients from Asia was similar to that in other countries, with no significant difference in PFS between avapritinib and regorafenib (HR, 1.14; 95% CI, 0.72 to 1.80; mPFS 3.9 v 5.4 months, respectively; P = .583). Among patients with PDGFRA D842V–mutant GIST (n = 13), mPFS was significantly higher for the seven treated with avapritinib (not reached [NR]; 95% CI, 9.7 to NR) compared with the six treated with regorafenib (4.5 months; 95% CI, 1.7 to NR; P = .035; Fig 2D). When excluding these 13 patients from the ITT population, mPFS was statistically higher with regorafenib (HR, 1.34; 95% CI, 1.06 to 1.69; mPFS 3.9 v 5.6 months, respectively; P = .012; Fig 2E). For patients who crossed over from regorafenib to avapritinib (n = 99), mPFS by investigator assessment was 2.6 months (Data Supplement, online only; 95% CI, 1.84 to 3.71) with a 6-month PFS rate of 24.1%.
FIG 2.
Kaplan-Meier analysis of PFS for patients with U/M GIST treated with avapritinib or regorafenib in (A) the ITT population, (B) patients who received the study drug as third line treatment, (C) patients who received study drug as fourth line treatment, (D) patients with PDGFRA D842V–mutant GIST, and (E) patients in the ITT population who did not have PDGFRA D842V–mutant GIST. GIST, GI stromal tumor; HR, hazard ratio; ITT, intention-to-treat; NR, not reached; PDGFRA, platelet-derived growth factor receptor alpha; PFS, progression-free survival; U/M, unresectable or metastatic.
At the cutoff date, OS data were immature with the median follow-up of 8.5 months for avapritinib and 9.6 months for regorafenib. OS estimates at 12 months were similar for avapritinib and regorafenib in the ITT population (68.2% v 67.4%, respectively), among patients who were treated as third-line (67.9% v 68.8%, respectively) or as fourth-line (67.4% v 60.4%, respectively).
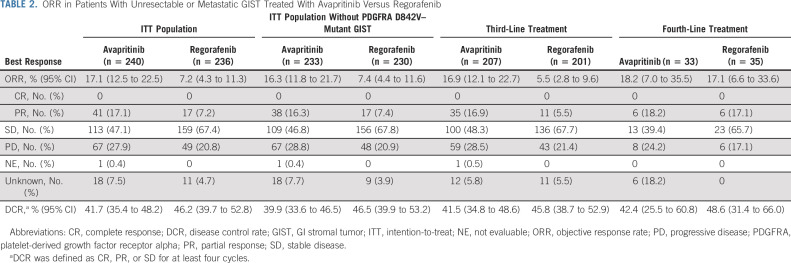
In the ITT population, ORR was significantly higher for avapritinib (17.1%; 95% CI, 12.5 to 22.5; all PR) compared with regorafenib (7.2%; 95% CI, 4.3 to 11.3; all PR; P < .001; Table 2). The median DOR was 7.6 months (95% CI, 5.6 to NR) for avapritinib and 9.4 months (95% CI, 7.4 to NR) for regorafenib. ORR was significantly higher for avapritinib compared with regorafenib even when patients with PDGFRA D842V–mutant GIST were excluded from analysis (P < .003; Table 2) and in patients who received avapritinib or regorafenib as third-line treatment (P < .001; Table 2). There was no difference in ORR among patients who received avapritinib or regorafenib as fourth-line treatment. In patients who received ≥ 1 dose of avapritinib after crossing over from regorafenib (n = 96), the ORR on avapritinib by investigator assessment was 10.4% (95% CI, 5.1 to 18.3) (Data Supplement). Two patients with PDGFRA D842V–mutant GIST crossed over from regorafenib to avapritinib, and both remained on avapritinib treatment at the time of the data cutoff (one was in PR, and the other was yet to have a postavapritinib scan).
TABLE 2.
ORR in Patients With Unresectable or Metastatic GIST Treated With Avapritinib Versus Regorafenib
In the ITT population, 47.1% of patients had SD and 27.9% had progressive disease (PD) as best response with avapritinib, compared with regorafenib with which 67.4% of patients had SD and 20.8% had PD as best response. The DCR was 41.7% (95% CI, 35.4 to 48.2) for avapritinib and 46.2% (95% CI, 39.7 to 52.8) for regorafenib (Table 2). Similarly, the DCR was 39.9% (95% CI, 33.6 to 46.5) for avapritinib and 46.5% (95% CI, 39.9 to 53.2) with regorafenib when patients with PDGFRA D842V–mutant GIST were excluded (Table 2). Among seven patients with PDGFRA D842V–mutant GIST treated with avapritinib, the ORR was 42.9% (95% CI, 9.9 to 81.6; all PR), 57.1% had SD, no patient had PD, and the DCR was 100.0% (95% CI, 59.0 to 100.0). By contrast, none of the six patients with PDGFRA D842V–mutant GIST treated with regorafenib had a radiologic response, 50.0% had SD, 16.7% had PD, and the DCR was 33.3% (95% CI, 4.3 to 77.7; Data Supplement).
Safety
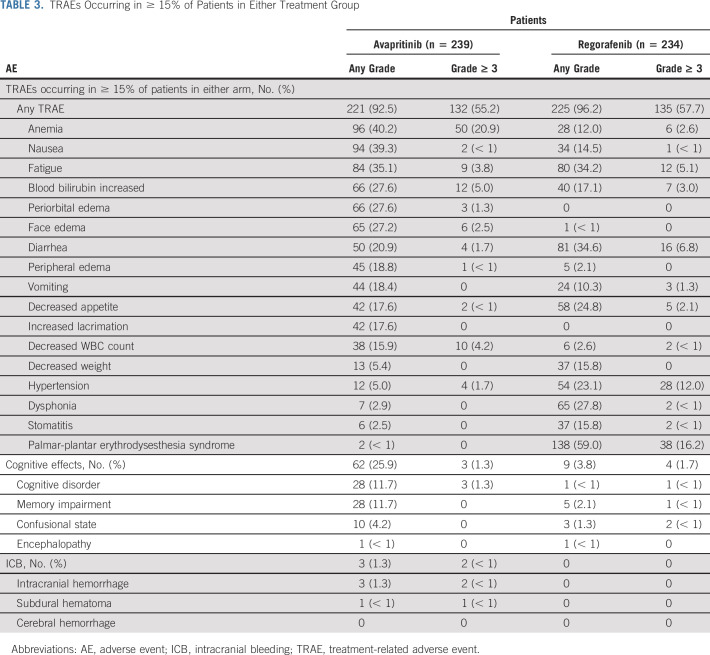
In the safety population, incidences of any-grade treatment-related adverse events (TRAEs) were similar between patients receiving avapritinib (92.5%) and regorafenib (96.2%), with 55.2% and 57.7% reporting grade ≥ 3 TRAEs, respectively (Table 3). The most common any-grade TRAEs occurring in ≥ 30% of patients were anemia (40.2%), nausea (39.3%), and fatigue (35.1%) with avapritinib and fatigue (34.2%), diarrhea (34.6%), and palmar-plantar erythrodysesthesia syndrome (59.0%) with regorafenib. The rate of discontinuation because of TRAEs was 8.3% for avapritinib and 5.6% for regorafenib. Overall, 66% (65 of 99) of patients who crossed over from regorafenib to avapritinib discontinued treatment; reasons for discontinuation included disease progression (36 of 99 [36.4%]), AEs (10 [10.1%] including 3 [3.0%] because of TRAEs), clinical progression (7 [7.1%]), administrative or others (6 [6.1%]), withdrawal of consent (3 [3.0%]), investigator decision (2 [2.0%]), and patient refused treatment (1 [1.0%]).
TABLE 3.
TRAEs Occurring in ≥ 15% of Patients in Either Treatment Group
AEs that were considered serious (SAEs) occurred in 41.4% of patients treated with avapritinib and 35.9% of patients treated with regorafenib. Treatment-related SAEs occurred in 19.7% of patients treated with avapritinib and in 14.5% of patients treated with regorafenib. Anemia was the most common treatment-related SAE in patients treated with avapritinib (any grade, 6.7%; grade ≥ 3, 5.9%). In patients treated with regorafenib, the most common any-grade treatment-related SAEs were diarrhea and pyrexia (both 1.7%) and the most common grade ≥ 3 treatment-related SAEs were diarrhea and GI hemorrhage (both 1.3%).
Cognitive effects of any grade occurred in 25.9% of patients treated with avapritinib and in 3.8% of patients treated with regorafenib. Memory impairment and cognitive disorder were the most common any-grade cognitive effects, with higher prevalence in patients treated with avapritinib (both 11.7%) compared with regorafenib (2.1% and < 1%, respectively). Grade ≥ 3 cognitive effects occurred in three (1.3%) patients treated with avapritinib, all of whom had cognitive disorder, and four (1.7%) patients treated with regorafenib (Table 3), of whom two had confusional state, one had cognitive disorder, and one had memory impairment. ICB events of any grade occurred in 3 (1.3%) patients receiving avapritinib. Of the two patients who had a grade ≥ 3 ICB event in the avapritinib arm, one had intracranial hemorrhage and the other had a subdural hematoma, both of which resulted in treatment discontinuation. No patients in the regorafenib arm experienced ICB events (Table 3).
DISCUSSION
To our knowledge, VOYAGER was the first randomized study in patients with molecularly unselected U/M GIST postimatinib comparing a novel compound (avapritinib) with an active comparator (regorafenib). Avapritinib did not meet the primary end point of superior PFS compared with regorafenib (HR, 1.25; 95% CI, 0.99 to 1.57) despite having a significantly higher response rate than regorafenib.19 Similar to observations in the NAVIGATOR study, avapritinib demonstrated antitumor activity in all patients with PDGFRA D842V–mutant GIST.19 Although the response rate among patients with PDGFRA D842V–mutant GIST treated with avapritinib was lower in VOYAGER compared with NAVIGATOR, the median follow-up for OS in VOYAGER was 11.5 months (95% CI, 8.2 to 12.4), shorter than that reported in NAVIGATOR (27.5 months for PFS).24 Avapritinib did not show superiority compared with regorafenib for mPFS in the ITT population, which might be attributed to the different inhibitory spectrum with avapritinib compared with regorafenib. Avapritinib is a potent inhibitor of PDGFRA with the activation mutation D842V (on exon 18) and other primary PDGFRA or KIT mutations (on exon 11 and exon 11/17),18 and of KIT with secondary activation loop mutations. Avapritinib demonstrates less potency against KIT mutations on exons 13 and 14 (ATP binding pocket),18 whereas regorafenib is an inhibitor of KIT with primary (exons 9 and 11) and secondary (exons 14 and 17) mutations.25 The VOYAGER patient population included those with various KIT mutations and patients with PDGFRA exon 18–mutant GIST. As such, the relative efficacy of avapritinib and regorafenib might be influenced by the underlying mutational landscape on an individual basis. Unfortunately, baseline tumor mutation status was not always known and ctDNA data were not available for all patients, limiting the feasibility of evaluating the predictive value of imatinib resistance mutations as detected in plasma. In addition, primary KIT or PDGFRA mutations in tumor samples might not have been detectable by ctDNA. Further analysis of available ctDNA data with respect to efficacy is warranted.
Another consideration is the statistical assumptions regarding PFS made when designing this study. Preliminary data from the NAVIGATOR study in the regorafenib-naïve population showed an mPFS of 8.6 months.26 On the basis of these data, the VOYAGER study design targeted an HR of 0.67 for PFS, which corresponded to an expected improvement in mPFS of 2.5 months, a 50% increase over the 5 months expected with regorafenib.7 We predicted that these statistical assumptions on the basis of early NAVIGATOR data would be replicated in VOYAGER, providing a robust, clinically significant improvement in PFS. This highlights the challenge of designing a phase III study on the basis of early phase I data in GIST, which may be prone to a selection bias if numbers are too low. Notably, mPFS of regorafenib was very similar (3-week difference) to the phase III GRID study.7
Ripretinib was recently approved as fourth-line treatment for GIST, on the basis of the phase III INVICTUS study, in which ripretinib showed an ORR of 9% and a PFS of 6.3 months.27 Although crosstrial comparisons should be made with caution because of differences in study populations and conduct, the PFS for avapritinib and regorafenib as fourth-line treatments in VOYAGER was 3.8 months and 4.5 months, respectively, whereas response rates as fourth-line treatments in VOYAGER were numerically higher at 18.2% and 17.1%, respectively.
In a post hoc analysis of patients who crossed over from regorafenib, the mPFS by investigator assessment was 2.6 months, with a 6-month PFS rate of 24.1%. Although this was not a prespecified analysis, it may still suggest a clinical benefit in a subset of patients who progressed on regorafenib. Although similar efficacy was observed between avapritinib and regorafenib, the AE profile was distinct for each drug. The rate of some AEs (such as anemia, nausea, neurocognitive effects, and edema) was higher with avapritinib, however, the rate of those known to be particularly challenging with regorafenib (such as hypertension, dysphonia, stomatitis, and palmar-plantar erythrodysesthesia syndrome) was higher with regorafenib. The AE profile of avapritinib was consistent with that reported in NAVIGATOR,19 and the AE profile with regorafenib was consistent with the safety profile of oral multikinase inhibitors in patients with GIST.7,28 The rates of grade ≥ 3 cognitive effects were similar between avapritinib (1.3%) and regorafenib (1.7%). The rate of grade 1-2 cognitive effects was higher with avapritinib, consistent with a relationship to study drug. Notably, the rate of cognitive effects with avapritinib in this study (25.1%) was lower than that reported in NAVIGATOR (40.2%).19 In a post hoc analysis of NAVIGATOR, the rate of cognitive effects was found to be associated with cumulative exposure to avapritinib and was higher in patients who started on avapritinib 400 mg versus avapritinib 300 mg once daily.29 Of note, even low-grade cognitive effects may be impactful for patients. Thus, dose interruption until resolution is key to managing these side effects.29 In VOYAGER, the lower rate of cognitive effects could be attributed to a uniform starting dose of avapritinib 300 mg, early recognition of cognitive effects, and rapid intervention, including dose interruption and reduction. Data that inform on possible mechanisms of action for cognitive effects observed with avapritinib are limited. Platelet-derived growth factors and their receptors (PDGFRs) are expressed in several cell types of the nervous system30 and play a role in CNS development, but there are no molecular data providing evidence of PDGFR inhibition as a mechanism of avapritinib-related cognitive effects.
ICB events occurred in three patients (1.3%) treated with avapritinib and were managed by dose interruptions, reductions, and/or discontinuations. There were no ICB events in patients treated with regorafenib. The rate of ICB events in the VOYAGER study was similar to that in the NAVIGATOR study, with ICBs reported in one patient treated with avapritinib 300 mg.19
In conclusion, avapritinib was not superior to regorafenib in terms of mPFS in third-line or later treatment of patients with molecularly unselected U/M GIST in the VOYAGER study. As expected, avapritinib demonstrated a high ORR and prolonged DOR in a subgroup of seven patients with PDGFRA D842V–mutant GIST. The safety profile of avapritinib in VOYAGER was consistent with that reported in NAVIGATOR.19
ACKNOWLEDGMENT
The authors would like to thank the patients, their families, all investigators, clinical research staff, and sites involved in this study. Medical writing support was provided by Miriam Cohen, PhD, and Deborah Cantu, PhD; editorial support was provided by Travis Taylor, BA, all of Paragon, Knutsford, United Kingdom, supported by Blueprint Medicines Corporation. Blueprint Medicines Corporation follows all current policies established by the International Committee of Medical Journal Editors and Good Publication Practice guidelines (http://annals.org/aim/article/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3).
Yoon-Koo Kang
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, Macrogenics, Surface Oncology, Blueprint Medicines
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, Kayothera
Research Funding: Blueprint Medicines, Deciphera, Daiichi Sankyo RD Novare, Merck, Eisai, SpringWorks Therapeutics
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice, WCG
Robin L. Jones
Consulting or Advisory Role: Lilly, Immune Design, Merck Serono, Adaptimmune, Daiichi Sankyo, Eisai, Morphotek, TRACON Pharma, Immodulon Therapeutics, Deciphera, PharmaMar, Blueprint Medicines, Clinigen Group, Epizyme, Boehringer Ingelheim, Bayer, Karma Oncology, UpToDate
Research Funding: GlaxoSmithKline
Travel, Accommodations, Expenses: PharmaMar
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Roche, Lilly, Pfizer, Pierre Fabre, Sanofi, Merck
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen
Speakers' Bureau: Pfizer, Novartis, Lilly
Research Funding: Novartis, Roche, Bristol Myers Squibb
Travel, Accommodations, Expenses: Orphan Europe, Pierre Fabre
Olivier Mir
Stock and Other Ownership Interests: Transgene, Amplitude Surgical, Ipsen
Honoraria: Roche
Consulting or Advisory Role: Lilly, Pfizer, Roche, Lundbeck, Janssen
Speakers' Bureau: Lilly, Roche, Pfizer
Research Funding: Ipsen, AstraZeneca, Blueprint Medicines
Travel, Accommodations, Expenses: Roche, Pfizer
Shreyaskumar Patel
Consulting or Advisory Role: Novartis, Immune Design, MaxiVax, Epizyme, Janssen, Lilly, Daiichi Sankyo, Bayer, Dova Pharmaceuticals, Deciphera
Research Funding: Blueprint Medicines, Hutchinson Med Pharma
Margaret von Mehren
Consulting or Advisory Role: Deciphera, Exelixis
Research Funding: ArQule, Novartis, Blueprint Medicines, Deciphera, Gradalis, Springworks Therapeutics, Lilly, Arog, Genmab, ASCO
Travel, Accommodations, Expenses: Deciphera Pharmaceuticals, NCCN
Other Relationship: NCCN
Peter Hohenberger
Honoraria: Roche, AstraZeneca, GlaxoSmithKline, BLUMedicine, Novartis
Consulting or Advisory Role: Nanobiotix, Pfizer
Research Funding: Novartis, Siemens Healthcare Diagnostics
Travel, Accommodations, Expenses: PharmaMar
Victor Villalobos
Employment: Janssen Oncology
Consulting or Advisory Role: Janssen, Lilly, Novartis, AbbVie, Ignyta, Agios, Epizyme, Blueprint Medicines, Springworks Therapeutics, NanoCarrier, Daiichi Sankyo
Travel, Accommodations, Expenses: Lilly, Janssen, Xencor, GenMab, Epizyme
Mehdi Brahmi
Expert Testimony: Bayer
Travel, Accommodations, Expenses: PharmaMar, Mundipharma
William D. Tap
Leadership: Certis Oncology Solutions, Atropos, Innova Therapeutics
Stock and Other Ownership Interests: Certis Oncology Solutions, Atropos
Consulting or Advisory Role: EMD Serono, Lilly, Daiichi Sankyo, Blueprint Medicines, Agios, NanoCarrier, Deciphera, C4 Therapeutics, Mundipharma, Adcendo, Ayala Pharmaceuticals, Kowa Pharmaceutical, Servier, AbMaxBio
Research Funding: Novartis, Lilly, Plexxikon, Daiichi Sankyo, TRACON Pharma, Blueprint Medicines, Immune Design, BioAtla, Deciphera
Patents, Royalties, Other Intellectual Property: Companion Diagnostic for CDK4 inhibitors—14/854,329, Enigma and CDH18 as companion Diagnostics for CDK4 inhibition—SKI2016-021-03
Jonathan Trent
Consulting or Advisory Role: Novartis, Lilly, Janssen, Blueprint Medicines, Deciphera, Daiichi Sankyo, Epizyme, Agios, C4 Therapeutics, Bayer
Patrick Schöffski
Honoraria: Deciphera, Blueprint Medicines, Boehringer Ingelheim
Consulting or Advisory Role: Blueprint Medicines, Ellipses Pharma, Adaptimmune, Intellisphere, Transgene, Deciphera, Exelixis, Boehringer Ingelheim, Medscape, Guided Clarity, Ysios Capital, Studiecentrum voor Kernenergie
Research Funding: CoBioRes NV, Eisai, G1 Therapeutics, Novartis, PharmaMar
Travel, Accommodations, Expenses: MSD, Ipsen, Boehringer Ingelheim
Kevin He
Employment: Blueprint Medicines, Agios
Stock and Other Ownership Interests: Blueprint Medicines, Agios, Incyte
Paggy Hew
Employment: Blueprint Medicines
Stock and Other Ownership Interests: Blueprint Medicines
Travel, Accommodations, Expenses: Blueprint Medicines
Kate Newberry
Employment: Blueprint Medicines
Stock and Other Ownership Interests: Blueprint Medicines
Maria Roche
Employment: Blueprint Medicines, Epizyme
Stock and Other Ownership Interests: Blueprint Medicines, Epizyme
Michael C. Heinrich
Stock and Other Ownership Interests: MolecularMD
Honoraria: Novartis
Consulting or Advisory Role: MolecularMD, Novartis, Blueprint Medicines, Deciphera, Theseus Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Patent on treatment of GIST-licensed to Novartis
Expert Testimony: Novartis
Sebastian Bauer
Honoraria: Novartis, Pfizer, Bayer, Pharmamar, GlaxoSmithKline
Consulting or Advisory Role: Blueprint Medicines, Bayer, Lilly, Deciphera, Nanobiotix, Daiichi Sankyo, Exelixis, Janssen-Cilag, ADC Therapeutics, Mundipharma, GlaxoSmithKline
Research Funding: Blueprint Medicines, Novartis, Incyte
Travel, Accommodations, Expenses: Pharmamar
No other potential conflicts of interest were reported.
DISCLAIMER
The sponsor was involved in the study design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors. M.C.H. received partial salary support from a Merit Review grant from the Department of Veterans Affairs (2I01BX000338-05).
CLINICAL TRIAL INFORMATION
Y.-K.K. and S.G. contributed equally; M.C.H. and S.B. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Yoon-Koo Kang, Suzanne George, Robin L. Jones, Margaret von Mehren, William D. Tap, Jonathan Trent, Patrick Schöffski, Kevin He, Maria Roche, Michael C. Heinrich, Sebastian Bauer
Financial support: Jonathan Trent
Administrative support: Suzanne George, Robin L. Jones, Peter Hohenberger, Jonathan Trent
Provision of study materials or patients: Yoon-Koo Kang, Suzanne George, Robin L. Jones, Piotr Rutkowski, Lin Shen, Olivier Mir, Shreyaskumar Patel, Yongjian Zhou, Margaret von Mehren, Peter Hohenberger, Victor Villalobos, Mehdi Brahmi, William D. Tap, Jonathan Trent, Patrick Schöffski, Michael C. Heinrich
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yoon-Koo Kang
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, Macrogenics, Surface Oncology, Blueprint Medicines
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, Kayothera
Research Funding: Blueprint Medicines, Deciphera, Daiichi Sankyo RD Novare, Merck, Eisai, SpringWorks Therapeutics
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice, WCG
Robin L. Jones
Consulting or Advisory Role: Lilly, Immune Design, Merck Serono, Adaptimmune, Daiichi Sankyo, Eisai, Morphotek, TRACON Pharma, Immodulon Therapeutics, Deciphera, PharmaMar, Blueprint Medicines, Clinigen Group, Epizyme, Boehringer Ingelheim, Bayer, Karma Oncology, UpToDate
Research Funding: GlaxoSmithKline
Travel, Accommodations, Expenses: PharmaMar
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Roche, Lilly, Pfizer, Pierre Fabre, Sanofi, Merck
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen
Speakers' Bureau: Pfizer, Novartis, Lilly
Research Funding: Novartis, Roche, Bristol Myers Squibb
Travel, Accommodations, Expenses: Orphan Europe, Pierre Fabre
Olivier Mir
Stock and Other Ownership Interests: Transgene, Amplitude Surgical, Ipsen
Honoraria: Roche
Consulting or Advisory Role: Lilly, Pfizer, Roche, Lundbeck, Janssen
Speakers' Bureau: Lilly, Roche, Pfizer
Research Funding: Ipsen, AstraZeneca, Blueprint Medicines
Travel, Accommodations, Expenses: Roche, Pfizer
Shreyaskumar Patel
Consulting or Advisory Role: Novartis, Immune Design, MaxiVax, Epizyme, Janssen, Lilly, Daiichi Sankyo, Bayer, Dova Pharmaceuticals, Deciphera
Research Funding: Blueprint Medicines, Hutchinson Med Pharma
Margaret von Mehren
Consulting or Advisory Role: Deciphera, Exelixis
Research Funding: ArQule, Novartis, Blueprint Medicines, Deciphera, Gradalis, Springworks Therapeutics, Lilly, Arog, Genmab, ASCO
Travel, Accommodations, Expenses: Deciphera Pharmaceuticals, NCCN
Other Relationship: NCCN
Peter Hohenberger
Honoraria: Roche, AstraZeneca, GlaxoSmithKline, BLUMedicine, Novartis
Consulting or Advisory Role: Nanobiotix, Pfizer
Research Funding: Novartis, Siemens Healthcare Diagnostics
Travel, Accommodations, Expenses: PharmaMar
Victor Villalobos
Employment: Janssen Oncology
Consulting or Advisory Role: Janssen, Lilly, Novartis, AbbVie, Ignyta, Agios, Epizyme, Blueprint Medicines, Springworks Therapeutics, NanoCarrier, Daiichi Sankyo
Travel, Accommodations, Expenses: Lilly, Janssen, Xencor, GenMab, Epizyme
Mehdi Brahmi
Expert Testimony: Bayer
Travel, Accommodations, Expenses: PharmaMar, Mundipharma
William D. Tap
Leadership: Certis Oncology Solutions, Atropos, Innova Therapeutics
Stock and Other Ownership Interests: Certis Oncology Solutions, Atropos
Consulting or Advisory Role: EMD Serono, Lilly, Daiichi Sankyo, Blueprint Medicines, Agios, NanoCarrier, Deciphera, C4 Therapeutics, Mundipharma, Adcendo, Ayala Pharmaceuticals, Kowa Pharmaceutical, Servier, AbMaxBio
Research Funding: Novartis, Lilly, Plexxikon, Daiichi Sankyo, TRACON Pharma, Blueprint Medicines, Immune Design, BioAtla, Deciphera
Patents, Royalties, Other Intellectual Property: Companion Diagnostic for CDK4 inhibitors—14/854,329, Enigma and CDH18 as companion Diagnostics for CDK4 inhibition—SKI2016-021-03
Jonathan Trent
Consulting or Advisory Role: Novartis, Lilly, Janssen, Blueprint Medicines, Deciphera, Daiichi Sankyo, Epizyme, Agios, C4 Therapeutics, Bayer
Patrick Schöffski
Honoraria: Deciphera, Blueprint Medicines, Boehringer Ingelheim
Consulting or Advisory Role: Blueprint Medicines, Ellipses Pharma, Adaptimmune, Intellisphere, Transgene, Deciphera, Exelixis, Boehringer Ingelheim, Medscape, Guided Clarity, Ysios Capital, Studiecentrum voor Kernenergie
Research Funding: CoBioRes NV, Eisai, G1 Therapeutics, Novartis, PharmaMar
Travel, Accommodations, Expenses: MSD, Ipsen, Boehringer Ingelheim
Kevin He
Employment: Blueprint Medicines, Agios
Stock and Other Ownership Interests: Blueprint Medicines, Agios, Incyte
Paggy Hew
Employment: Blueprint Medicines
Stock and Other Ownership Interests: Blueprint Medicines
Travel, Accommodations, Expenses: Blueprint Medicines
Kate Newberry
Employment: Blueprint Medicines
Stock and Other Ownership Interests: Blueprint Medicines
Maria Roche
Employment: Blueprint Medicines, Epizyme
Stock and Other Ownership Interests: Blueprint Medicines, Epizyme
Michael C. Heinrich
Stock and Other Ownership Interests: MolecularMD
Honoraria: Novartis
Consulting or Advisory Role: MolecularMD, Novartis, Blueprint Medicines, Deciphera, Theseus Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Patent on treatment of GIST-licensed to Novartis
Expert Testimony: Novartis
Sebastian Bauer
Honoraria: Novartis, Pfizer, Bayer, Pharmamar, GlaxoSmithKline
Consulting or Advisory Role: Blueprint Medicines, Bayer, Lilly, Deciphera, Nanobiotix, Daiichi Sankyo, Exelixis, Janssen-Cilag, ADC Therapeutics, Mundipharma, GlaxoSmithKline
Research Funding: Blueprint Medicines, Novartis, Incyte
Travel, Accommodations, Expenses: Pharmamar
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. : Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 6:e20294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson B, Bumming P, Meis-Kindblom JM, et al. : Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 103:821-829, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Soreide K, Sandvik OM, Soreide JA, et al. : Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 40:39-46, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, von Mehren M, Antonescu CR, et al. : NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8:S1-S41, 2010. (suppl 2); quiz S42-S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casali PG, Abecassis N, Aro HT, et al. : Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv68-iv78, 2018 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Gastrointestinal Stromal Tumors (GISTs) (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf
- 7.Demetri GD, Reichardt P, Kang Y-K, et al. : Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:295-302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetri GD, van Oosterom AT, Garrett CR, et al. : Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368:1329-1338, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, von Mehren M, Blanke CD, et al. : Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472-480, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Smith BD, Kaufman MD, Lu WP, et al. : Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell 35:738-751.e9, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Liegl B, Kepten I, Le C, et al. : Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 216:64-74, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gramza AW, Corless CL, Heinrich MC: Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res 15:7510, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. : Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res 12:1743-1749, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kee D, Zalcberg JR: Current and emerging strategies for the management of imatinib-refractory advanced gastrointestinal stromal tumors. Ther Adv Med Oncol 4:255-270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonescu CR, Besmer P, Guo T, et al. : Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 11:4182-4190, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Heinrich MC, Corless CL, Demetri GD, et al. : Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342-4349, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Cassier PA, Fumagalli E, Rutkowski P, et al. : Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res 18:4458-4464, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Evans EK, Gardino AK, Kim JL, et al. : A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med 9:eaao1690, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Heinrich MC, Jones RL, von Mehren M, et al. : Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol 21:935-946, 2020 [DOI] [PubMed] [Google Scholar]
- 20.George S, Jones RL, Bauer S, et al. : Avapritinib in patients with advanced gastrointestinal stromal tumors following at least three prior lines of therapy. Oncologist 26:e639-e649, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blueprint Medicines Corporation : AYVAKIT (Avapritinib). Prescribing Information. Cambridge, MA, Blueprint Medicines Corporation, 2020 [Google Scholar]
- 22.Blueprint Medicines (the Netherlands) B.V. : AYVAKYT® (Avapritinib). Summary of Product Characteristics. Amsterdam, the Netherlands, Blueprint Medicines (the Netherlands) B.V., 2020 [Google Scholar]
- 23.Bayer Healthcare Pharmaceuticals Inc : VITRAKVI (Larotrectinib). Prescribing Information. Whippany, NJ, Bayer Healthcare Pharmaceuticals Inc, 2020 [Google Scholar]
- 24.Jones RL, Serrano C, von Mehren M, et al. : Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: Long-term efficacy and safety data from the NAVIGATOR phase 1 trial. Eur J Cancer 145:132-142, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrano C, Mariño-Enríquez A, Tao DL, et al. : Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer 120:612-620, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrich M, von Mehren M, Jones RL: Avapritinib is highly active and well-tolerated in patients (pts) with advanced GIST driven by diverse variety of oncogenic mutations in KIT and PDGFRA. Presented at the CTOS Annual Meeting, Rome, Italy, November 14-17, 2018
- 27.Blay JY, Serrano C, Heinrich MC, et al. : Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21:923-934, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giampieri R, Prete MD, Prochilo T, et al. : Off-target effects and clinical outcome in metastatic colorectal cancer patients receiving regorafenib: The TRIBUTE analysis. Sci Rep 7:45703, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph CP, Abaricia SN, Angelis MA, et al. : Optimal avapritinib treatment strategies for patients with metastatic or unresectable gastrointestinal stromal tumors. Oncologist 26:e622-e631, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sil S, Periyasamy P, Thangaraj A, et al. : PDGF/PDGFR axis in the neural systems. Mol Aspects Med 62:63-74, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]