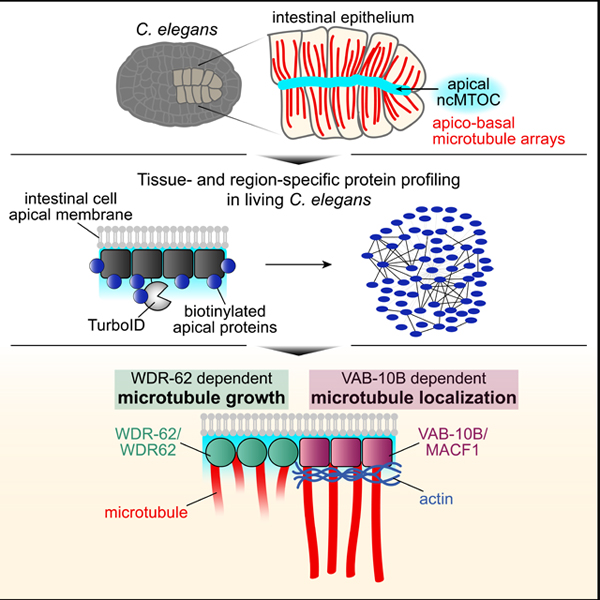
Summary:
Microtubules are polarized intracellular polymers that play key roles in the cell including in transport, polarity, and cell division. Across eukaryotic cell types, microtubules adopt diverse intracellular organization to accommodate these distinct functions coordinated by specific cellular sites called microtubule-organizing centers (MTOCs). Over 50 years of research on MTOC biology has focused mainly on the centrosome, however most differentiated cells employ non-centrosomal MTOCs (ncMTOCs) to organize their microtubules into diverse arrays which are critical to cell function. To identify essential ncMTOC components, we developed the biotin ligase-based proximity labeling approach TurboID for use in C. elegans. We identified proteins proximal to the microtubule minus end protein PTRN-1/Patronin at the apical ncMTOC of intestinal epithelial cells, focusing on two conserved proteins: spectraplakin protein VAB-10B and WDR-62, a protein we identify as homologous to vertebrate primary microcephaly disease protein WDR62. VAB-10B and WDR-62 do not associate with the centrosome and instead specifically regulate non-centrosomal microtubules and the apical targeting of microtubule minus end proteins. Depletion of VAB-10B resulted in microtubule mislocalization and delayed localization of a microtubule nucleation complex γ-TuRC, while loss of WDR-62 decreased the number of dynamic microtubules and abolished γ-TuRC localization. This regulation occurs downstream of cell polarity and in conjunction with actin. As this is the first report for non-centrosomal roles of WDR62 family proteins, we expand the basic cell biological roles of this important disease protein. Our studies identify essential ncMTOC components and suggest a division of labor where microtubule growth and localization are distinctly regulated.
Keywords: MTOC, ncMTOC, TurboID, microtubules, spectraplakin, WD40 repeat protein, C. elegans, differentiation, epithelial cell
eTOC blurb
Differentiated cells use non-centrosomal microtubule organizing centers (ncMTOCs) to build microtubule arrays. Sanchez et al. apply proximity labeling in living C. elegans to generate the first proteomic profile of an ncMTOC. Depletion of ncMTOC components reveals functionally distinct modules controlling microtubule growth and localization.
Graphical Abstract
Introduction:
Microtubules are polarized polymers critical for eukaryotic cell functions including intracellular transport, organelle positioning, cell polarity, and cell shape. Early electron microscopy studies identified subcellular sites to which microtubules localize, termed microtubule-organizing centers (MTOCs).1,2 MTOCs are broadly defined as sites that grow and localize microtubules from their minus ends, greatly contributing to minus end stability and to the ability of plus ends to dynamically probe their environment and build specific spatial patterns. The best-studied MTOC is the centrosome, a non-membrane bound organelle that patterns microtubules into a radial array in animal mitosis and in some specialized cell types. However, in many types of differentiated cells, microtubules no longer associate with the centrosome but rather with a non-centrosomal MTOC (ncMTOC) to achieve cell-type specific organization. For example, intestinal and tracheal epithelial cells organize microtubules at the apical membrane into parallel arrays along the apicobasal axis, neurons organize longitudinal microtubule arrays down axons and dendrites in some cases around endosomes, and muscle cells can organize microtubules from Golgi outposts or the nuclear envelope.3–7 Despite the ubiquity of ncMTOCs across cell types and organisms, their molecular components and mechanisms of formation remain largely unknown.
The ability of MTOCs to grow, stabilize, and anchor microtubules is imparted by molecules acting alone or in functional complexes at microtubule minus ends, only a handful of which has been identified: The ɣ-tubulin ring complex (ɣ-TuRC) functions as a microtubule nucleator and minus end stabilizer;8,9 the CAMSAP/Patronin/Nezha family of proteins functions as a conserved microtubule minus end stabilizer;10–13 and Ninein/NOCA-1 is a proposed microtubule anchoring protein.14,15 The prevalence of these molecules and their contribution to MTOC function vary by cell type and the depletion of one or all of these components frequently does not result in elimination of all microtubules.16–19 For example, depletion of ɣ-TuRC, PTRN-1/Patronin, and NOCA-1 simultaneously in C. elegans intestinal epithelial cells did not affect the gross morphology or density of non-centrosomal microtubules.20 Thus, essential ncMTOC components have yet to be determined.
Essential centrosome components have been defined over the last two decades by various genetic and proteomic approaches,21,22 however such approaches have not been used to systematically identify ncMTOC components. Traditional biochemical techniques require physical disruption of cells leading to a loss of in vivo spatial information and ephemeral interactions between proteins. These issues can be circumvented by biotin ligase-based proximity labeling (PL) techniques, which fuse a protein of interest to a promiscuous labeling enzyme to then mark proximal proteins with a biotin tag. Biotinylated proteins are then isolated and identified by mass spectrometry, providing a “snapshot” of protein interaction networks in vivo. PL techniques historically presented severe limitations in multicellular organisms due to slow labeling times and toxic reagents required to catalyze the necessary reactions.23,24 We previously demonstrated biotin ligase-based PL in C. elegans using the newly-engineered PL enzyme TurboID.25
Here, we use TurboID to identify ncMTOC components in living C. elegans intestinal cells. C. elegans embryonic intestinal cells are well-suited for studying ncMTOCs as MTOC function is reassigned to the apical membrane (apical ncMTOC) following cell division to organize microtubules into parallel apicobasal arrays (Figure S1).7 To probe the composition of the apical ncMTOC, we expressed TurboID fused to PTRN-1 in embryonic intestinal cells. We identified 69 proteins proximal to PTRN-1, validated the localization of five proximal interactors, and focused on identifying functions of two conserved proteins: VAB-10B/MACF1 and WDR-62/H24G06.1, a protein we identify as a homolog of vertebrate WDR62. We report that VAB-10B and WDR-62 specifically localize to the apical ncMTOC in differentiated intestinal cells and in other epithelia but not to the centrosome. Using tissue-specific degradation, we found essential roles for VAB-10B and WDR-62 at the apical ncMTOC: VAB-10B is required for apical microtubule localization in part through a role of actin in microtubule anchoring; WDR-62 is required for apical microtubule growth and for apical ɣ-TuRC localization. As WDR-62 is required for VAB-10B localization, WDR-62 regulates microtubule localization through VAB-10B and microtubule growth through a distinct pathway. The role of VAB-10B and WDR-62 at the apical ncMTOC is downstream of the apical polarity program. Thus, our data support a model where two essential MTOC functions, microtubule growth and localization, are regulated by non-centrosomal factors WDR-62 and VAB-10B. Together, this work identifies essential ncMTOC regulators, highlights that ncMTOCs can be physically and functionally distinct from centrosomes, and demonstrates that ncMTOCs can be composed of different functional modules.
Results:
Tissue-specific expression of TurboID in C. elegans identifies proximity interactors of PTRN-1/Patronin
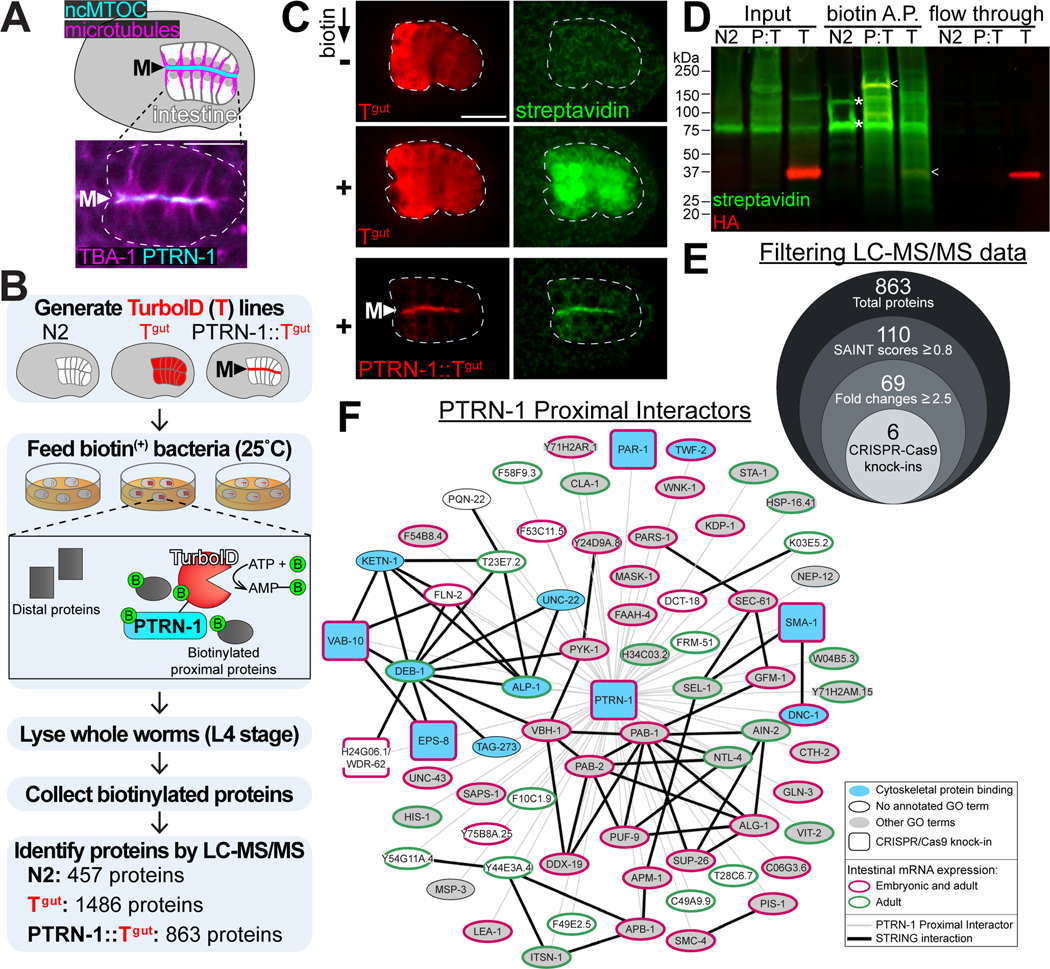
To identify components of the apical ncMTOC, we sought to identify proteins proximal to PTRN-1, a microtubule minus end binding protein that localizes exclusively to the apical ncMTOC in embryonic intestinal cells throughout development (Figure 1A). We ectopically expressed HA-tagged TurboID fused to PTRN-1 in intestinal cells (‘PTRN-1::TurboIDgut’, Figure 1B, 1C). To control for non-specific biotinylation, we ectopically expressed HA-tagged TurboID alone (‘TurboIDgut’). We also analyzed a wild-type (N2) strain not expressing TurboID to control for endogenously biotinylated proteins.
Figure 1. Identification of PTRN-1 proximity interactors.
(A) Dorsal view of C. elegans bean stage embryo indicating the localization of the apical ncMTOC and microtubules at the midline (‘M’) in the polarized intestine. Inset shows live imaging of mCherry::TBA-1/α-tubulin and PTRN-1::GFP in intestine (white dotted line). (B) Experimental workflow for TurboID proximity labeling in the C. elegans intestine for wild-type (N2), cytoplasmic TurboID (‘Tgut’), and TurboID targeted to the ncMTOC (‘PTRN-1::Tgut’). (C) Immunofluorescence of fixed embryos marking TurboID (anti-HA, red) and biotinylated proteins (streptavidin-488, green) for indicated genotypes. Scale bar = 10 μm. (D) Immunoblotting for TurboID (anti-HA, red) and biotinylated proteins (streptavidin-488, green) in whole worm lysates (Input) of wild-type (N2), PTRN-1::TurboIDgut (‘P:T’), or TurboIDgut (‘T’) that were subjected to affinity purification with streptavidin-covered magnetic beads (‘biotin A.P.’). TurboID (arrowheads) and endogenously biotinylated proteins (asterisks) are indicated. Note the absence of proteins in the flow through lane showing proteins not captured by streptavidin beads. (E) Diagram of workflow to filter LC-MS/MS data and corresponding number of proteins for each step (see STAR Methods). (F) Interaction network of 69 PTRN-1 proximal interactors (STRING32, p = 8.06e-10). The GO term “cytoskeletal protein binding” was significantly enriched (11 of the 69 genes, p = 5.3e-10).
Embryos expressing TurboIDgut exhibited strong biotinylation signal in the intestine,25 whereas embryos expressing PTRN-1::TurboIDgut exhibited apically enriched biotinylation activity, indicating that TurboID can mediate regional and spatial control of biotinylation in vivo (Figure 1C). Additionally, streptavidin blots of whole worm lysates showed that a wide range of proteins was biotinylated in worms expressing TurboIDgut or PTRN-1::TurboIDgut compared to the endogenously biotinylated proteins found in wild-type worms (Figure 1D). Importantly, biotinylated proteins could be enriched from whole worm lysates, bypassing the need to perform tissue dissections or fractionation of the ncMTOC structure.
Following identification of biotinylated proteins by mass spectrometry, the 863 total proteins from the PTRN-1::TurboIDgut dataset were filtered using SAINT probabilistic scoring26 (Data S1, Figure 1E). The 69 resulting ‘PTRN-1 proximal interactors’ included VAB-10B (a spectraplakin) and SMA-1 (a β-spectrin), homologs of which have been shown to immunoprecipitate with Patronin/CAMSAP in other systems.27–29 The isolation of homologs of known PTRN-1 interactors highlights the effectiveness of our approach despite our stringent filtering. The absence of some proteins known to localize to the ncMTOC (e.g. ɣ-TuRC and NOCA-1) indicates either the restricted labeling radius of PTRN-1::TurboIDgut, an absence of available lysines to biotinylate, a shortage of biotin, or an inability of TurboID to reach proteins that are sterically inaccessible.
We cross-referenced our list of proximal interactors with other datasets (Figure 1F, see STAR Methods). First, 63 of the 69 PTRN-1 proximity interactors are expressed in the intestine30,31 indicating that our method enriches TurboID labeling activity in a tissue-specific manner, although we cannot pinpoint the developmental stages at which labeling occurred. Second, PTRN-1 proximal interactors were significantly enriched for interactions using STRING32, a database of known and predicted physical and functional protein-protein interactions. Third, our dataset was significantly enriched for the GO term “cytoskeletal protein binding”, consistent with reported functions of PTRN-1. Lastly, 57 of the 69 proteins have predicted human orthologs, suggesting that our knowledge of ncMTOC composition is far from complete across organisms.
Proximity interactors exhibit localization patterns similar to PTRN-1
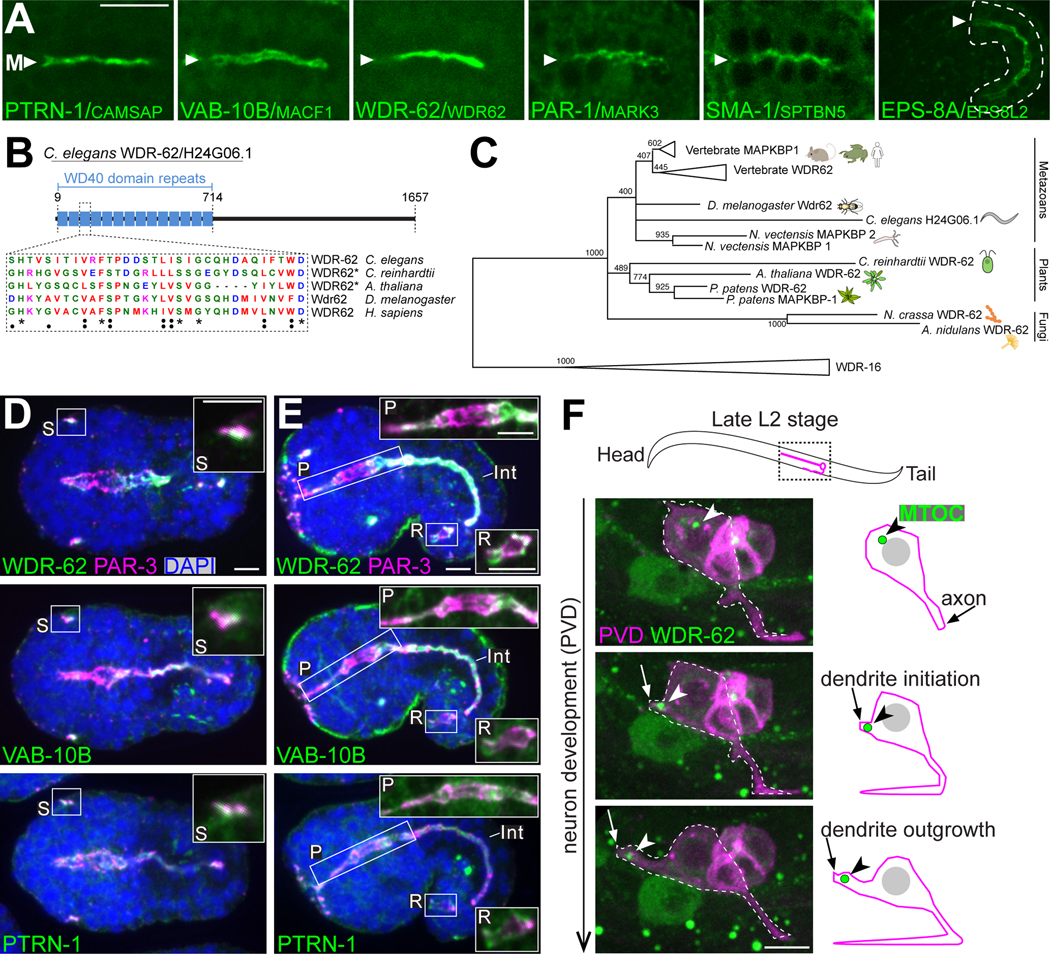
To further validate that biotinylation activity was occurring at the apical ncMTOC, we probed the localization of five PTRN-1 proximal interactors that begin mRNA expression in the embryonic intestine31 and whose respective homologs have cytoskeleton-related functions. Using CRISPR/Cas9, we inserted a fluorescent tag at the endogenous locus encoding VAB-10B, PAR-1, SMA-1, EPS-8A, and H24G06.1, an uncharacterized protein (Figure 2A, S2, S3). We found that like PTRN-1, all five proteins localized to the apical membranes of the embryonic intestine (Figure 2A, Figure S2, S3), further underscoring the effectiveness of our proximity labeling approach. Due to their specific timing of expression and localization (Figure 2, S2, S3) and cytoskeletal related functions in other organisms, we focused our attention on two proteins: VAB-10B, a spectraplakin33, and H24G06.1, which we identified as a homologue of WDR62 and its paralog MAPKBP1 (Figure 2B, 2C, S4, Table S1).34 Mutations in WDR62 are the second most common cause of autosomal recessive primary microcephaly in human patients,35 but the cellular roles of WDR62 remain incompletely understood with the vast majority of studies focusing on a role for WDR62 at centrosomes.36–41
Figure 2. Localization patterns of PTRN-1 proximity interactors.
(A) Dorsal view live imaging of endogenously ZF::GFP tagged PTRN-1 proximity interactors localizing to the intestine midline (‘M’). All intestines are from embryonic bean stage with the exception of EPS-8A, which begins expression at comma stage (see Figure S1A, S3D). Scale bar = 10 μm. (B) Diagram of the domain structure of WDR-62/H24G06.166 (see Table S1). Amino acid conservation shown for one WD40 repeat. (C) Collapsed phylogenetic tree constructed from the WD40 repeat region of eukaryotic WDR62 and WDR16 homologs (see Figure S4). Maximum likelihood support values are indicated for conserved regions within WD40 repeats. Scale bar represents 0.4 substitutions per site. (D-E) Immunofluorescence imaging of fixed late bean stage (D) or comma stage embryos (E) marking PAR-3 (anti-PAR-3, magenta), WDR-62::ZF::GFP, VAB-10B::ZF::GFP or PTRN-1::GFP (anti-GFP, green), and DAPI (blue) with intestine indicated (‘Int’). Insets are higher-magnification views of sensilla (‘S’, ventral embryo view), pharynx (‘P’), and rectum (‘R’). Scale bars = 5 μm. (F) Live imaging of WDR-62::ZF::GFP (green, arrowhead) in the PVD neuron (magenta), as represented in adjacent cartoon.
See also Table S1, S2 and Figures S1-S5.
We found that neither VAB-10B nor WDR-62 localized to centrosomes of the early embryo or in dividing intestinal precursor cells, but rather localized during intestinal cell polarization to the apical ncMTOC (Figure S1, S5). Moreover, VAB-10B and WDR-62 localized to the apical surfaces of other epithelial cell types as indicated by colocalization with the conserved apical polarity protein PAR-3/PAR3 (Figure 2D, 2E). Epithelial expression and apical localization were similar for WDR-62, VAB-10B, and PTRN-1, for example at the apical dendrite tip of developing amphid sensilla neurons (Figure 2D, ‘S’) and in pharyngeal and rectal cells (Figure 2E, ‘P’, ‘R’). As human and mouse WDR62 are known to play prominent roles in neuronal development42, we investigated the localization of WDR-62 in the developing PVD neuron, a highly branched nocioceptor. Intriguingly, we found that WDR-62 localizes to the tip of the outgrowing PVD dendrite (arrowhead, Figure 2F), a site that was recently shown to contain a migrating ncMTOC enriched for microtubules and ɣ-TuRC (Figure 2F).6 Our discovery of WDR-62 in various cell types provided a unique opportunity to study its role beyond the centrosome.
VAB-10B and WDR-62 are required to form non-centrosomal microtubule arrays
The localization patterns of endogenous VAB-10B and WDR-62 and their proximity to PTRN-1 prompted us to investigate their microtubule functions. To do this, we employed the ZF/ZIF-1 tissue-specific protein degradation system whereby proteins containing a ZF degron tag are targeted for degradation by the E3 ligase adapter ZIF-1 (Figure S2C).20,43 We inserted sequences encoding the ZF degron and GFP into the endogenous locus of vab-10b or wdr-62 using CRISPR/Cas9 (Figure S2). The resulting “ZF-tagged” proteins were effectively degraded by ectopically expressing ZIF-1 under the control of the elt-2 promoter, which drives ZIF-1 expression in the intestine starting at the E4 stage, well before apical ncMTOC formation (“gut(−)”, Figure S2C-S2E).20 Intestine-specific loss of VAB-10B (VAB-10Bgut(−)) or WDR-62 (WDR-62gut(−)) did not affect worm viability, accumulation of ɣ-TuRC or mCherry::TBA-1/α-tubulin at centrosomes in dividing intestinal precursors, nor intestinal cell divisions (Figure S6A-S6C). Loss of VAB-10B and WDR-62 did perturb apical nuclear positioning (Figure S6D), a process that is known to require microtubules.7 Despite the apparent loss of GFP following degradation of VAB-10B and WDR-62 (Figure S2D, S2E), a caveat of this approach is that it relies on our ability to tag all isoforms of the resulting proteins and on the kinetics of degradation. Therefore, degradation may not produce null phenotypes. Indeed, we were unable to edit the wdr-62 locus in a location that tagged all isoforms; thus, this depletion case is likely hypomorphic (Figure S2A).
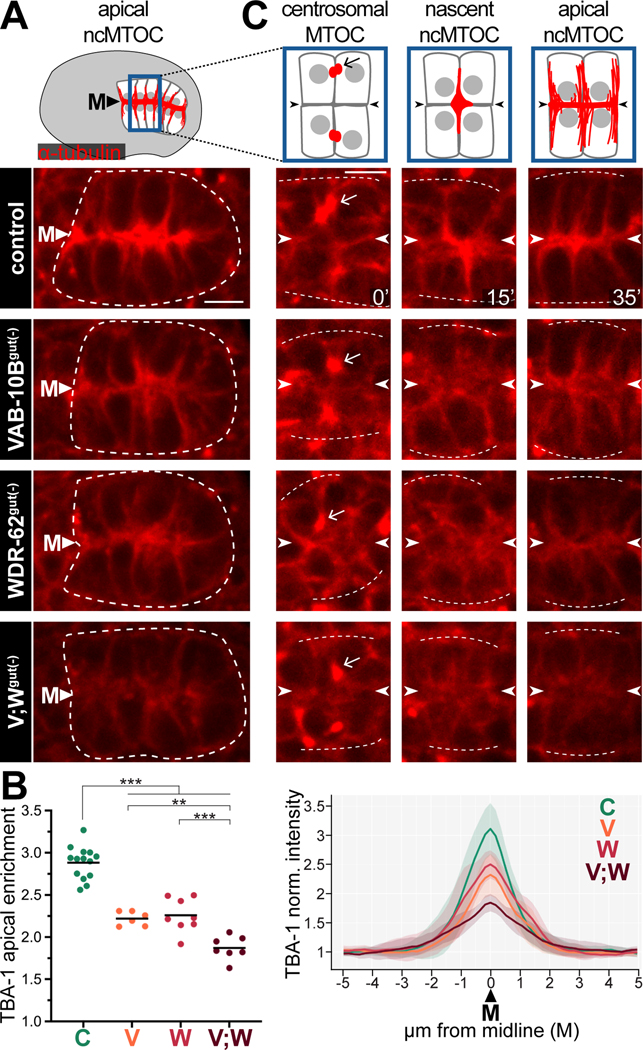
Using our ZF-tagged alleles, we probed apical microtubule organization following the removal of WDR-62 and/or VAB-10B. In control embryos, mCherry::TBA-1 was strongly enriched at apical membranes (‘M’), signifying apical microtubule organization (Figure 3A, 3B).7 By contrast, mCherry::TBA-1 was significantly reduced at apical membranes in VAB-10Bgut(−) and WDR-62gut(−) embryos (Figure 3A, 3B). Co-depleting VAB-10B and WDR-62 ([VAB-10B;WDR-62]gut(−)) resulted in an even more severe depletion of mCherry::TBA-1 at the apical membranes (Figure 3A, 3B), potentially highlighting the incomplete depletion of WDR-62 due to isoforms that are untagged by our degron (Figure S2A).
Figure 3. VAB-10B and WDR-62 are required for non-centrosomal microtubule organization.
(A) Dorsal view of polarized intestine (white dotted line) from live imaging of mCherry::TBA-1/α-tubulin in control, VAB-10Bgut(−), WDR-62gut(−), or [VAB-10B; WDR-62]gut(−) (V;Wgut(−)) embryo. Cartoon of embryonic intestine (top) represents microtubules (red), nuclei (gray), midline (‘M’) in control embryos. (B) Quantifications of mCherry::TBA-1 in polarized intestines as in (A). Left: Apical enrichment of mCherry::TBA-1. Each dot represents a single embryonic intestine and horizontal black lines indicate mean value; **p < 0.01, ***p < 0.001. Right: Normalized mCherry::TBA-1 fluorescence signal along a line perpendicular to the midline of anterior intestine; lighter shading indicates standard deviation. For both graphs: control (‘C’) n = 15; VAB-10Bgut(−) (‘V’) n = 8; WDR-62gut(−) (‘W’) n = 9; V;Wgut(−) (‘V;W’) n = 8. (C) Live imaging of mCherry:TBA-1 in cells exiting E8-E16 division (t = 0’), polarizing E16 cells (t = 15’), and newly polarized E16 cells (t = 35’). Arrow points to presumptive centrosomes and arrowheads indicate future apical surface. White dotted lines mark approximation of intestinal edge. Scale bars = 5 μm.
See also Figures S1-S3, S5, and S6.
In order to determine the time at which microtubule phenotypes arise, we tracked microtubule localization during ncMTOC formation. We have previously found that microtubules follow a stereotyped pattern of localization in polarizing intestinal cells (Figure 3C, S1B).7 First, E16 intestinal cells exit mitosis and centrosomes and associated microtubules (arrow) localize to lateral membranes (Figure 3C, t = 0’). Next, non-centrosomal microtubules emerge at a nascent ncMTOC which moves toward the presumptive apical surfaces, first localizing at cell vertices (t = 15’) before finally spreading along the midline (‘M’, arrowheads) and orienting microtubules along the apicobasal axis (t = 35’). In VAB-10Bgut(−), WDR-62gut(−), and [VAB-10B;WDR-62]gut(−) embryos, centrosomal microtubules (Figure 3C, arrow, t = 0’) appeared unaffected, but non-centrosomal microtubules were initially diffuse (t = 15’) and reduced at the apical membrane, with the greatest reduction seen in [VAB-10B;WDR-62]gut(−) embryos (t = 35’). These data indicate that VAB-10B and WDR-62 regulate non-centrosomal microtubules at the start of ncMTOC formation.
WDR-62 and VAB-10B control dynamic microtubule growth and localization
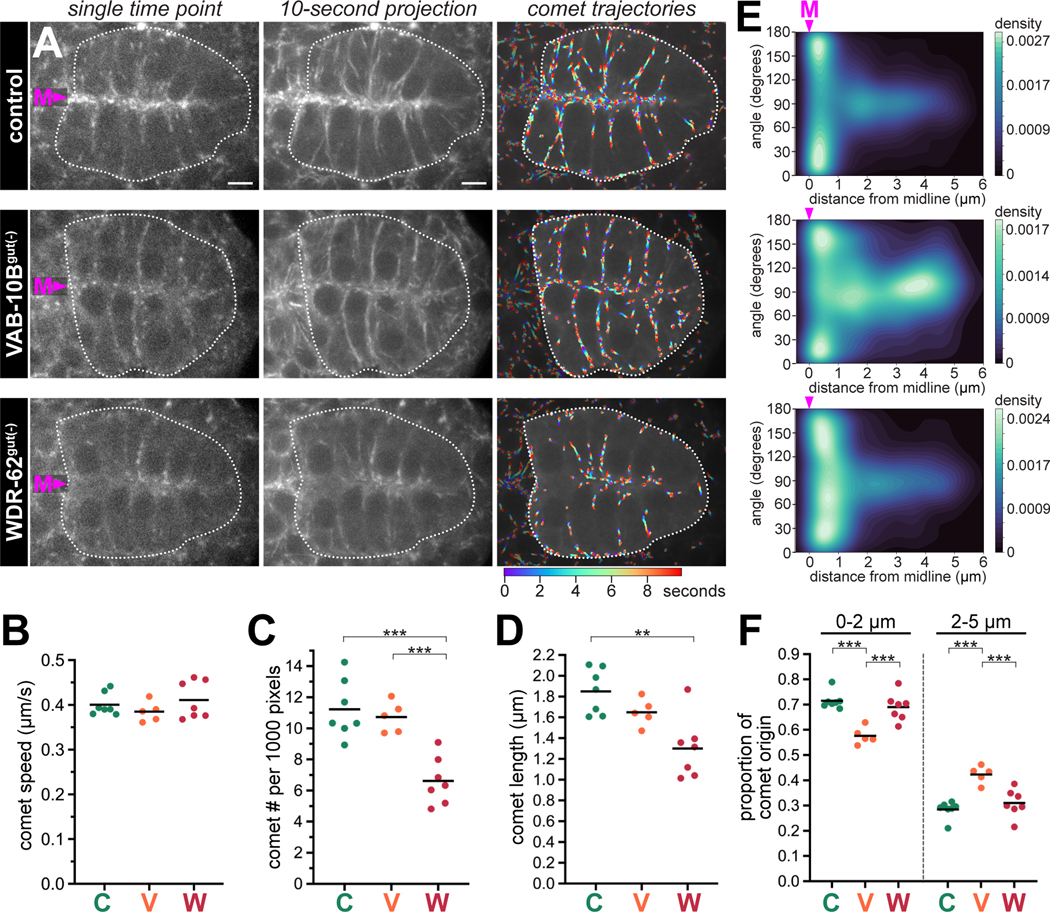
To provide deeper insight into the roles of VAB-10B and WDR-62, we investigated microtubule dynamics in VAB-10Bgut(−) and WDR-62gut(−) embryos by analyzing the endogenous localization of the microtubule plus end binding protein EBP-2/EB1. EBP-2::GFP originates at the apical membranes showing a high enrichment of comets moving toward the basal surface, as has been previously shown (Video S1, Figure 4A).20 Removal of VAB-10B or WDR-62 in intestinal cells resulted in decreased enrichment of EBP-2 at the midline (Figure 4A, Video S1). We next quantified EBP-2 comet speed and direction using custom-built segmentation and tracking algorithms (‘comet trajectories’, Figure 4A, see STAR Methods). Overall, the average microtubule comet speed did not significantly differ between control, VAB-10Bgut(−), and WDR-62gut(−) (Figure 4B). However, EBP-2 comet number, length, and trajectory lifetime were significantly reduced in WDR-62gut(−) embryos, but not in VAB-10Bgut(−) embryos compared to control (Figure 4C, 4D, S6E-S6G). Although VAB-10B depletion did not change the overall number of dynamic microtubules, a higher proportion of comets initiated away from the midline in VAB-10B(gut-) embryos (Figure 4E, 4F, S6H). These data suggest that VAB-10B does not function in microtubule growth, but rather to localize microtubules to the apical membrane, whereas WDR-62 plays a major role in the production and/or stability of microtubules. 245
Figure 4. Depletion of VAB-10B or WDR-62 differentially affect dynamic microtubules.
(A) Dorsal view of live polarized intestines (white dotted lines, midline (‘M’)) from live imaging of endogenous EBP-2/EB1::GFP in indicated genotypes (see Video S1) of single time points, 10-second time projections, or overlaid comet trajectories. Color map indicates time frame for comet trajectories. Scale bars = 5 μm. (B-F) Quantifications of computationally tracked comet trajectories in the intestine. Control (‘C’) n = 7 embryos, VAB-10Bgut(−) (‘V’) n = 5 embryos, WDR-62gut(−) (‘W’) n = 7 embryos. (B-D, F) Each dot represents a single embryonic intestine and horizontal black lines indicate mean value. Total comets analyzed per intestine: control: 261 – 392; VAB-10Bgut(−): 268 – 320; WDR-62gut(−): 137– 265. **p < 0.01, ***p < 0.001. (B) Average comet speed; (C) average comet number per 1000 pixels; (D) average comet length; (F) average proportion of comets initiating at either 0–2 μm or 2–5 μm from an annotated midline. (E) Density maps of instantaneous comet angles relative to their minimum distance from an annotated midline (0 μm, midline (‘M’)) for each genotype (see STAR Methods). An angle of 90° corresponds to vertical trajectories relative to the annotated midline. All trajectories are 6 frames or longer and originate between 0–5 μm from the annotated midline.
VAB-10B and WDR-62 recruit MTOC proteins to the apical membrane
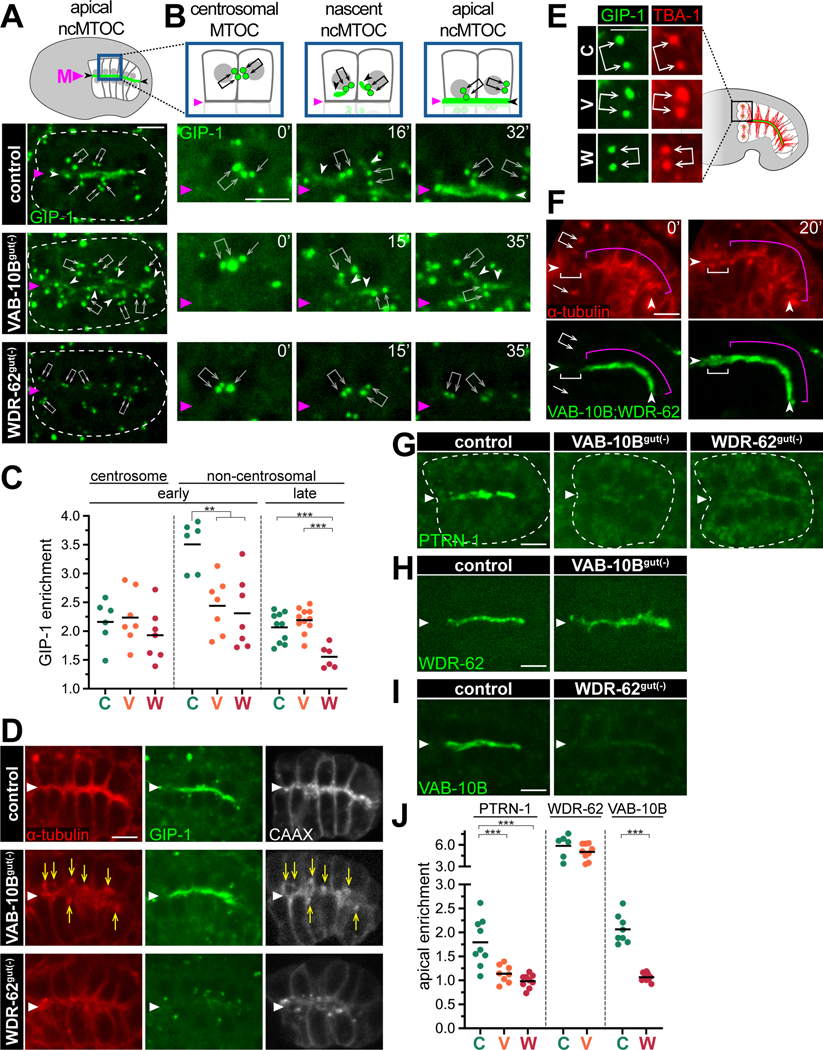
We next sought to understand the roles of VAB-10B and WDR-62 in localizing MTOC components. Intestinal cells polarize after the E8-E16 division and the ɣ-TuRC component GFP::GIP-1/GCP3 localizes to both inactive centrosomes (arrows, Figure 5A) and to the apical ncMTOC (arrowhead).7 The enrichment of GIP-1 to inactive centrosomes was unaffected in VAB-10Bgut(−) and WDR-62gut(−) embryos (arrows, Figure 5A, 5C). In contrast, removal of VAB-10B resulted in mislocalized non-centrosomal GIP-1 (white arrowheads), and more strikingly, removal of WDR-62 exhibited a dramatic decrease of non-centrosomal GIP-1 (magenta arrowhead, Figure 5A, 5C).
Figure 5. VAB-10B and WDR-62 differentially recruit microtubule minus end proteins to the ncMTOC.
(A, B) Dorsal view live imaging of endogenous GFP::GIP-1/GCP3 localized to centrosomes (arrows) or non-centrosomal pools (white arrowheads) in indicated genotypes. Midline indicated with magenta triangle (‘M’). Cartoon represents GFP::GIP-1 (green) and nuclei (gray) in control. (B) Magnified view of cells exiting E8-E16 division (t = 0’), polarizing E16 cells (t = 15’, t = 16’), and newly polarized E16 cells (t = 32’, t = 35’). (C) Quantification of GIP-1 enrichment in polarized intestinal cells at centrosomes (as in A) or at the apical surface at the same ‘early’ stage of polarization (as in A) and ~1 hour later67 (‘late’, as in D) in control (‘C’, n = 6), VAB-10Bgut(−) (‘V’, n = 7), or WDR-62gut(−) (‘W’, n = 7) embryos. (D) Live imaging of mCherry::TBA-1/α-tubulin, GFP::GIP-1, and membrane marker BFP::CAAX in ‘late’ polarized intestinal cells. Yellow arrows indicate examples of mislocalized mCherry::TBA-1. (E) Live imaging of mCherry::TBA-1 and GFP::GIP-1 localization at centrosomes (joined white arrows) in one dividing anterior intestinal cell in indicated genotypes. (F) Live imaging of mCherry::TBA-1 and both endogenous VAB-10B::ZF::GFP and WDR-62::ZF::GFP in comma stage embryonic intestine. Anterior intestinal cells (white bracket) undergo mitosis (t = 0’) and then re-polarize (t = 20’). Presumptive centrosomes (arrows) and apical site (arrowheads) are indicated. Neighboring intestinal cells (magenta bracket) do not divide. (G-I) Live imaging of indicated endogenous protein in polarized intestines: (G) PTRN-1::GFP (control (n = 9), VAB-10Bgut(−) (n = 8), and WDR-62gut(−) (n = 9)); (H) WDR-62::RFP (control (n = 6) and VAB-10Bgut(−) (n = 9)); (I) VAB-10B::GFP (control (n = 8) and WDR-62gut(−) (n = 10)). J) Quantifications of apical enrichment in (G-I) for control (‘C’), VAB-10Bgut(−) (‘V’), and WDR-62gut(−) (‘W’) embryos; For all quantifications, each dot represents a single embryonic intestine and horizontal black lines indicate mean value; **p < 0.01, ***p < 0.001. Scale bars = 5 μm.
To better understand the precise time at which these phenotypes arise, we imaged GIP-1 during ncMTOC formation (Figure S1B). In control embryos, GIP-1 localized to centrosomes at lateral membranes between adjacent non-sister E16 cells as they exited mitosis (arrows, t = 0’, Figure 5B).7 Next, ‘plumes’ of non-centrosomal GIP-1 appeared adjacent to centrosomes (white arrowheads, t = 16’) and then coalesced at the midline (magenta arrowhead, t = 32’).7 We have previously shown that these plumes indicate the nascent ncMTOC.7 In VAB-10Bgut(−) and WDR-62gut(−) embryos, centrosomal GIP-1 appeared normal at mitotic exit (t = 0’, Figure 5B), but soon after their phenotypes diverged; specifically, VAB-10Bgut(−) embryos formed non-centrosomal GIP-1 plumes (t = 15’) that showed a severe delay in their coalescence at the midline, whereas non-centrosomal GIP-1 plumes neither formed (t = 15’) nor appeared at the midline (t = 35’) in WDR-62gut(−) embryos. These data suggest that early in polarization, WDR-62 recruits ɣ-TuRC possibly via nascent non-centrosomal microtubules and VAB-10B links ɣ-TuRC to the apical site.
At a later developmental stage, apically localized GIP-1 and TBA-1 remained reduced in WDR-62gut(−) embryos (Figure 5C ‘late’, 5D). In contrast, VAB-10Bgut(−) embryos showed normal apical GIP-1 localization despite aberrant TBA-1 localization (n = 8/10, yellow arrows), highlighting that ɣ-TuRC is not always a faithful marker of microtubule organization (Figure 5C, 5D). Aberrant TBA-1 foci in VAB-10Bgut(−) embryos were not coincident with centrosomes as indicated by GIP-1 localization, but instead with a CAAX membrane marker (Figure 5D). As the Ras CAAX motif can label vesicles,44 this colocalization with microtubules is consistent with recent reports linking endosomes to MTOC function.6,45,46
We next asked whether WDR-62 and VAB-10B depletions affected ɣ-TuRC localization to mitotic centrosomes. As four polarized intestinal cells re-enter mitosis in late embryogenesis, α-tubulin and ɣ-TuRC are removed from the apical ncMTOC and localize to active centrosomes (Figure 5E).47 Centrosomal accumulation of α-tubulin and ɣ-TuRC were not affected in the dividing cells of VAB-10Bgut(−) or WDR-62gut(−) embryos (Figure 5E), indicating the specificity of microtubule phenotypes to the apical ncMTOC. Consistent with this result, both VAB-10B and WDR-62 left the apical membrane during mitosis (white bracket, Figure 5F), did not accumulate at the centrosomes (arrows, t = 0’), and returned to the apical membrane upon mitotic exit (t = 20’).
In addition to ɣ-TuRC localization, apical PTRN-1 signal was drastically decreased in VAB-10Bgut(−) and WDR-62gut(−) embryos (Figure 5G, 5J). Although PTRN-1 in the embryonic intestine is not required for apical ncMTOC function,20 this result is consistent with reports of spectraplakins targeting Patronin/CAMSAP27,48 and is the first report of a role for WDR62 family proteins in PTRN-1 localization. Finally, we assessed the relationship between VAB-10B and WDR-62. Although WDR-62 showed an apical enrichment in VAB-10Bgut(−) embryos (Figure 5H, 5J), apical VAB-10B localization was severely reduced in WDR-62gut(−) embryos (Figure 5I, 5J) suggesting that WDR-62 functions upstream of VAB-10B.
The conserved PAR polarity complex regulates PTRN-1, WDR-62, and VAB-10B localization
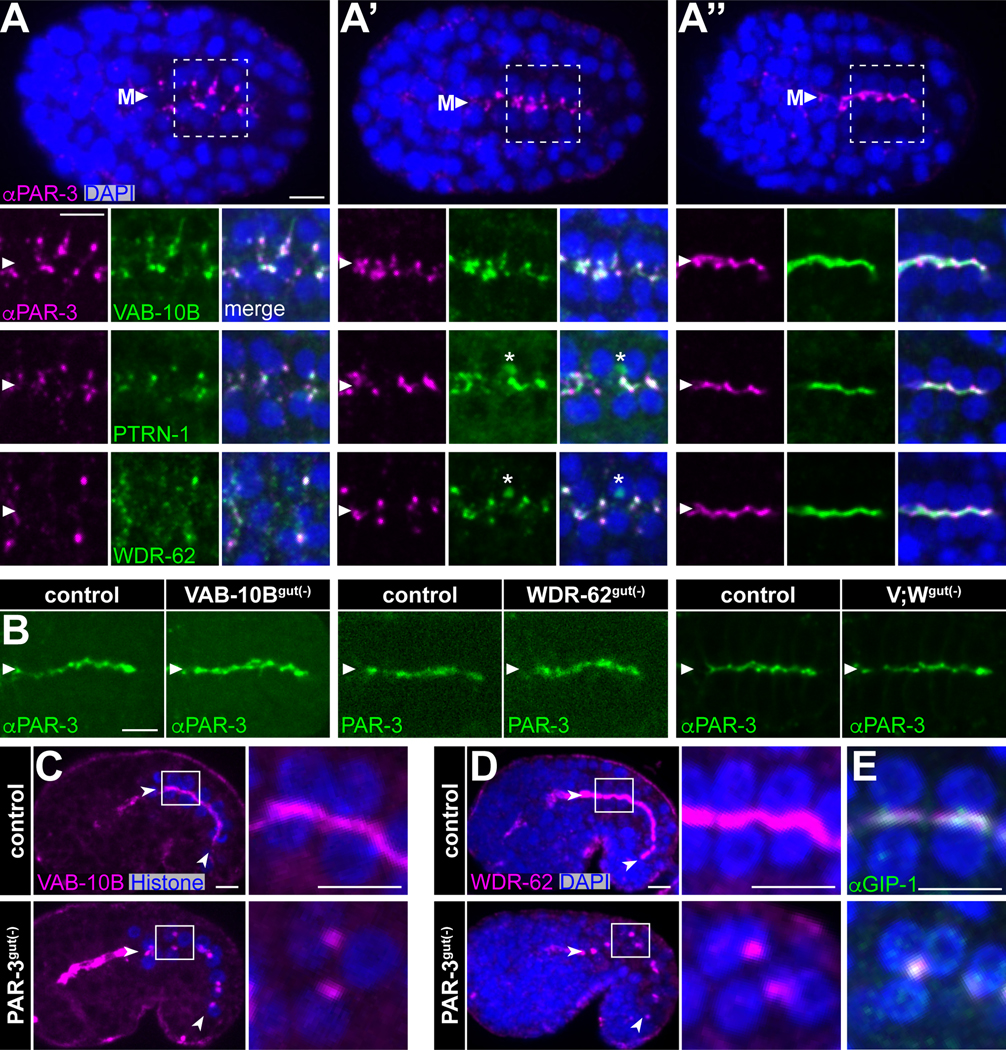
As the movement of the nascent ncMTOC to the midline during E16 intestinal cell polarization is coincident with and controlled by the apical polarity protein PAR-3,7,49 we next investigated the localization of PTRN-1, WDR-62 and VAB-10B relative to PAR-3 (Figure S1B). PTRN-1, WDR-62, and VAB-10B initially appeared as foci on lateral membranes with PAR-3 (Figure 6A), moved together with PAR-3 toward the midline (Figure 6A’), and then spread across the midline to subsequently increase in intensity after cells established an apical surface (Figure 6A”). Importantly, none of these proteins colocalized with centrosomes and instead appeared as part of the nascent ncMTOC (Figure S5). These data led us to ask whether polarity cues control VAB-10B and WDR-62 localization or vice versa. PAR-3 localization was unaffected in VAB-10Bgut(−), WDR-62gut(−), or [VAB-10B;WDR-62]gut(−) embryos (Figure 6B), suggesting that neither VAB-10B nor WDR-62 control the upstream aspects of apical polarization. In contrast, intestine-specific depletion of PAR-3 (PAR-3gut(−)) mislocalized VAB-10B (Figure 6C) and WDR-62 into large patches away from the apical surface (Figure 6D) that co- localized with GIP-1 (Figure 6E),7,49 indicating that VAB-10B and WDR-62 are targeted to the apical surface by PAR-3 and track with the ncMTOC upon its mislocalization.
Figure 6. VAB-10B and WDR-62 interface with apical polarity determinants.
(A-A”) Dorsal views from immunofluorescence imaging of fixed embryos marking PAR-3 (anti-PAR-3, magenta), WDR-62::ZF::GFP, VAB-10B::ZF::GFP, or PTRN-1::GFP (anti-GFP, green), or DNA (DAPI, blue) from (A) pre-polarized, (A’) polarizing, and (A”) apically polarized intestines. Higher-magnification views of white boxed region in (A) shown for PAR-3 and VAB-10B and from comparable regions and stages for WDR-62 and PTRN-1. Arrowheads mark the intestinal midline (‘M’). Asterisks mark intruding green signal from ventral germ cells. (B) Immunofluorescence of fixed embryos (anti-PAR-3) or live imaging (PAR-3:mCherry) of endogenous PAR-3 in indicated genotypes. (C-E) Imaging of comma stage embryos. Higher-magnification views of white boxed region in intestinal cells are shown at right. (C) Live imaging of VAB-10B::GFP (magenta) and histone::mCherry (blue). (D, E) Immunofluorescence imaging of fixed embryos marking WDR-62::RFP (magenta) and DAPI (blue). (E) Stage-matched embryos show GIP-1 localization (anti-GIP-1, green). Scale bars = 5 μm.
See also Figures S2 and S5.
Actin filaments regulate non-centrosomal microtubules
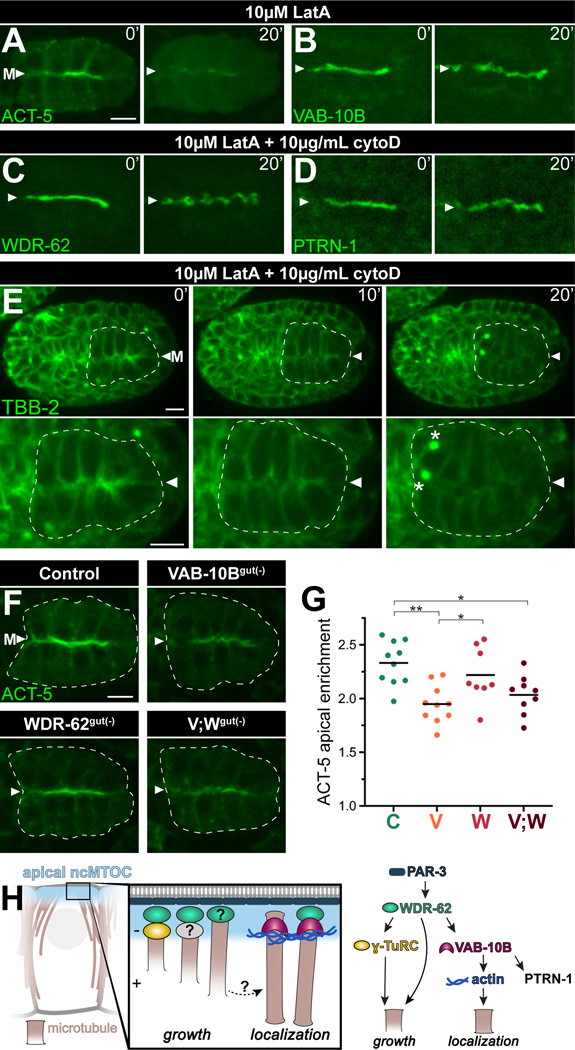
As spectraplakins have been shown to function as physical crosslinkers between actin at the cell cortex and non-centrosomal microtubules minus ends,27,48 we hypothesized that actin could similarly localize VAB-10B and other ncMTOC components to the apical membrane. To test this, we soaked embryos in actin polymerization inhibitors and then removed the impermeable eggshell and vitelline membrane of embryos either mechanically (Figure S7) or by laser permeabilization (Figure 7A-E), allowing all cells to be exposed to the drugs with temporal control. We confirmed the apical enrichment of actin filaments with a transgene encoding YFP::ACT-550 in polarized intestines (Figure 7A, t = 0’) and found a robust depletion after Latrunculin A (LatA) treatment (t = 20’), illustrating the effectiveness of the drug treatment. The apical surfaces of embryos treated with actin inhibitors became gnarled which is consistent with previous actin perturbation observations,51 but neither VAB-10B, WDR-62, nor PTRN-1 were removed from the apical surface (Figure 7B-7D, S7A, S7B). We next treated embryos expressing endogenously tagged GFP::TBB-2/β-tubulin (Figure 7E, S7C, Video S2), endogenously tagged EBP-2::GFP (Figure S7D), or a transgene encoding mCherry::TBA-1/α-tubulin (n = 8, data not shown) with actin inhibitors. Strikingly, in the presence of actin inhibitors, microtubules appeared to be released from apical surfaces in interphase intestinal cells as indicated by the gradual removal of TBB-2 (Figure 7E, t = 10’−20’, Video S2, S7C) and a decrease in EBP-2 apical enrichment (Figure S7D). In stark contrast, the mitotic anterior intestinal cells (Figure 7E, t = 20’, asterisks) normally accumulated microtubules at the centrosome, suggesting a specific non-centrosomal role for actin in microtubule localization.
Figure 7. Actin filaments regulate non-centrosomal microtubules.
(A-D) Time-lapse live imaging of embryos expressing YFP::ACT-5 transgene (n = 3), or endogenously tagged VAB-10B::ZF::GFP (n = 4), WDR-62:ZF::GFP (n = 3) or PTRN-1::GFP (n = 3) beginning several seconds after eggshell permeabilization (t = 0’) in the presence of indicated actin inhibitors Latrunculin A (LatA) and Cytochalasin D (CytoD) for 20 minutes (t = 20’). (E) Time-lapse live imaging of GFP::TBB-2/β-tubulin with intestine (white dotted line), midline (‘M’, white triangle), and active mitotic centrosomes (asterisks) indicated; n = 3; see Video S2. (F) Live imaging of YFP::ACT-5 in control, VAB-10Bgut(−), WDR-62gut(−), or [VAB-10B; WDR-62]gut(−) (V;Wgut(−)) embryo. (G) Quantifications of apical YFP::ACT-5 fluorescent signal enrichment from (F). Each dot represents a single embryonic intestine and horizontal black lines indicate mean value; control (‘C’) n = 10; VAB-10Bgut(−) (‘V’) n = 10; WDR-62gut(−) (‘W’) n = 8; V;Wgut(−) (‘V;W’) n = 9; *p < 0.05; **p < 0.01. (H) Model of apical ncMTOC composition and function. The apical PAR complex lies upstream of divergent microtubule growth and localization modules. Scale bars = 5 μm.
Finally, given the apparent role for actin in apical microtubule localization, we tested the role of VAB-10B or WDR-62 in actin localization. Although WDR-62gut(−) embryos did not show a significant change in accumulation of actin to the apical surface, we saw a striking decrease in apical ACT-5 enrichment in VAB-10Bgut(−) or [VAB-10B;WDR-62]gut(−) embryos (Figure 7F, 7G). These data are consistent with a previous report that RNAi of vab-10B resulted in shorter or absent actin filaments in skin cells52 and additionally suggest that actin functions downstream of VAB-10B to anchor microtubules to the apical surface.
Discussion
Using biotin-based proximity labeling coupled with a tissue-specific degradation system in C. elegans, we addressed fundamental knowledge gaps in ncMTOC biology and present technical and conceptual advances: 1) TurboID is an effective method for identifying proximity interactors in a living multicellular organism, in this case at an ncMTOC; 2) Spectraplakin VAB-10B and WD40 repeat protein WDR-62 are two essential ncMTOC components in vivo; 3) The apical ncMTOC is molecularly distinct from the centrosome; and 4) ncMTOCs can be composed of functionally distinct modules that control microtubule growth and localization (Figure 7H).
We show the first report of biotin-based proximity labeling in C. elegans and the first proteomic profile of an ncMTOC which identified previously uncharacterized H24G06.1/WDR-62. Previous studies of WDR62 have largely focused on centrosomal roles in mitosis as it localizes at spindle poles in dividing cultured cells and is required for the stability of spindle microtubules and for timely cell cycle progression.38 WDR62 also has centrosomal functions in interphase cells as mouse embryonic fibroblasts deficient in Wdr62 show defects in centriole duplication, and Wdr62 maintains interphase centrosomal MTOC activity in Drosophila neuroblasts.39,40 By using C. elegans intestinal cells as a model, we discovered non-mitotic and non-centrosomal roles for WDR-62. These roles are likely not unique to C. elegans as WDR62 is expressed in postmitotic neuronal layers in mouse and human brains36,38,53 and the loss of function of Wdr62 disrupted apical complex proteins in mouse cortical neurons through an unexplored mechanism.40 Furthermore, our phylogenetic analyses suggest that the ancient function of WDR62 proteins may be rooted in more basic microtubule functions as WDR62 is conserved in organisms such as A. thaliana and P. patens that do not have centrosomes but still build patterned microtubule arrays.
Our tissue-specific depletion studies provide evidence for separate functional modules controlling microtubule growth and localization within ncMTOCs (Figure 7H). Conceptually, microtubule growth could be directed by proteins whose function impacts microtubule stabilization and/or nucleation. Wdr62 depletion in Drosophila neuroblasts reduced microtubule localization at interphase centrosomes and microtubules were more sensitive to microtubule depolymerization39, consistent with Wdr62 acting as either a stabilizer or nucleator. We found that WDR-62 depletion reduced the number of dynamic microtubules and abolished localization of the microtubule nucleator ɣ-TuRC, suggesting a role for WDR-62 in nucleation. However, as ɣ-TuRC contributes only a minor amount of microtubules to the apical ncMTOC,20 this role would be to directly or indirectly nucleate ɣ-TuRC-independent microtubules, for example by acting as a scaffold through its WD40 repeat domains which in other systems are required for microtubule association.37,54 WDR-62 depletion also shortened the length and lifetime of EBP-2 comet trajectories, indicating a role in stabilization. As WDR-62 appeared to be restricted to microtubule minus ends, its role in stabilization at the plus ends could be through the loading of specific molecules such as +TIPs or kinesin which could in turn promote growth and stabilization.55,56
The spectraplakin VAB-10B appears to function downstream of WDR-62 in a second functional module that regulates microtubule localization to the apical surface. Although spectraplakins are well-known for binding the microtubule plus-end binding protein EB1 and the microtubule lattice,57–59 the Drosophila spectraplakin Shot and human spectraplakin ACF7 are proposed to interact with and anchor microtubule minus ends.27,48 We found that VAB-10B is proximal to the microtubule minus ends protein PTRN-1, localizes near minus ends, and its depletion affected the localization of microtubules to the apical surface without affecting overall microtubule numbers. These data suggest that VAB-10B localizes microtubules to the apical surface and prevents microtubules from localizing to other cellular sites. We also found that VAB-10B recruits PTRN-1 like in other systems, however the microtubule localizing function of VAB-10B in these cells is not dependent on PTRN-1, as PTRN-1 is not required for microtubule localization in the embryonic intestine.20 Thus, VAB-10B localizes microtubules to the apical surface independently of PTRN-1, potentially through its microtubule binding domain or spectrin repeats acting as a platform to recruit other microtubule regulators.
An understanding of how VAB-10B and WDR-62 becomes apically localized will reveal mechanistic aspects of ncMTOC establishment, as we found that these proteins are essential components of the apical ncMTOC. Given previous reports, we expected actin to localize VAB-10B to the apical membrane.27,48 However, neither VAB-10B nor WDR-62 localization were affected following pharmacological actin inhibition. In contrast and to our surprise, the same pharmacological inhibition of the actin network liberated microtubules from the apical surface. Furthermore, VAB-10B depletion decreased the enrichment of actin at the apical membrane. Together, these data suggest that actin is required downstream of VAB-10B for anchoring microtubules. Actin could mediate the interaction of VAB-10B with microtubules or microtubule binding proteins directly or indirectly by modulating the binding ability of VAB-10B. A similar mechanism was recently proposed for ɣ-TuRC whereby perturbing the interaction between ɣ-TuRC and actin inhibited ɣ-TuRC nucleation activity likely by changing the shape of the complex.60,61
Our data lead to a model where apical microtubules are grown and localized by two centrosome-independent mechanisms, separating these two microtubule functions at ncMTOCs (Figure 7H). This type of division of labor has been suggested for other MTOCs.18,48,62 As our data indicate that WDR-62 acts upstream of VAB-10B, WDR-62 could contribute to both microtubule growth and localization by functioning in distinct complexes. Alternatively, given that depletion of WDR-62 or VAB-10B leads to defect in microtubule growth or localization, respectively, microtubules grown by WDR-62 could be subsequently localized by VAB-10B.
The cellular origin of non-centrosomal microtubules and by extension the function of ncMTOCs is a topic of debate and likely varies by cell type. Different non-mutually exclusive models have been proposed that differ in their involvement of the centrosome.63 Our data strongly indicate that the apical ncMTOC predominantly nucleates and localizes microtubules using molecules that do not have centrosomal origins. First, we showed neither VAB-10B nor WDR-62 localize to centrosomes. Second, depleting VAB-10B and WDR-62 specifically affected non-centrosomal ɣ-TuRC and non-centrosomal microtubules but did not perturb mitosis or the localization of ɣ-TuRC or microtubules to centrosomes. Although we previously found a role for the centrosome in apical ɣ-TuRC localization,7 due to the minor role of ɣ-TuRC at the apical ncMTOC and the observation that ɣ-TuRC can localize to the apical ncMTOC without first localizing to the centrosome,20 we favor a model where the centrosome, perhaps through its astral microtubules, creates an environment that promotes the timely formation of the nascent ncMTOC.
How an ncMTOC is built de novo at the onset of differentiation and how it forms a non-membrane bound functional unit is an open question. The ncMTOC could be a phase-separated structure, as PTRN-1 and several PTRN-1 proximity interactors contain intrinsically disordered regions. Alternatively, and consistent with the colocalization of displaced microtubules with membrane puncta in embryos depleted of VAB-10B, membranous structures could serve as a platform for ncMTOC assembly as Golgi, Golgi outposts, and endosomes have all been shown to associate with non-centrosomal microtubules.5,6,45,64
Diverse non-centrosomal microtubule arrays have long been observed in a wide range of differentiated cell types. Our proximity labeling approach coupled with a tissue specific degradation method has uncovered key ncMTOC components in polarized epithelial cells that will serve as the foundation for our understanding of the fundamental mechanisms cells use to generate specific microtubule patterns.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Jessica Feldman (feldmanj@stanford.edu).
Materials availability
DNA constructs and transgenic C. elegans strains generated in this study are available from the Lead Contact, Dr. Jessica Feldman, upon request.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
All original code is available at https://github.com/JacobsWagnerLab/published/tree/master/Sanchez_2020 and is publicly available as of the date of publication. All scripts are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rat anti-HA | Roche | Cat#11867423001 |
| goat anti-rat IRDye 680RD | Licor | Cat#925-68076; Lot#C61115-06 |
| streptavidin-IRDye 800CW | Licor | Cat#925-32230; Lot#C60913-04 |
| mouse anti-HA | Abcam | Cat#ab130275 [16B12]; Lot#GR250145-5 |
| mouse anti-Myc | Abcam | Cat#ab32[9E10]; Lot#GR3272830 |
| rabbit anti-GFP | Abcam | Cat#ab6556; Lot#6R3271077-1 |
| mouse anti-PAR-3 | DSHB | Cat#p4a1; RRID: AB528424 |
| rabbit anti-GIP-1 | GenScript7 | N/A |
| CY3-anti-mouse | Jackson Immunoresearch Laboratories | Cat#115-165-166; Lot#117091 |
| streptavidin Alexa Fluor 488 | Invitrogen | Ref#532354; Lot#1719656 |
| DAPI | Sigma | N/A |
| 647-anti-rabbit | Jackson Immunoresearch Laboratories | Cat#711-605-152 |
| 488-anti-mouse | Jackson Immunoresearch Laboratories | Cat#115-545-166 |
| 488 anti-rabbit | Thermofisher scientific | Cat#A11034 |
| Cy5 anti-mouse | Jackson Immunoresearch Laboratories | Cat#715-175-151 |
| Bacterial and virus strains | ||
| E. coli (MG1655 bioB::kan) biotin auxotrophic food | Dr. John E. Cronan, University of Illinois | N/A |
| E. coli OP50 standard food | CGC | N/A |
| E. coli NA22 standard food | CGC | N/A |
| Critical commercial assays | ||
| Pierce 660nm Protein Assay Kit | Thermo Fisher Scientific | Cat#22662 |
| Pierce Streptavidin Magnetic Beads | Thermo Fisher Scientific | Cat#88817 |
| Q5® Site-Directed Mutagenesis kit | New England BioLabs | Cat#E0554S |
| NEBuilder® HiFi DNA Assembly | New England BioLabs | Cat#E5520S |
| Deposited data | ||
| control_df (compressed.csv with microtubule positions and angles in the wild type C. elegans embryos) | This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/control_df |
| VAB_10Bgut_minus_df (compressed.csv with microtubule positions and angles in the VAB-10Bgut(−) C. elegans embryos) | This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/VAB_10Bgut_minus_df |
| WDR_62gut_minus_df (compressed.csv with microtubule positions and angles in the WDR-62gut(−) C. elegans embryos) | This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/WDR_62gut_minus_df |
| Experimental models: Organisms/strains | ||
| C. elegans strain N2 wild-type | CGC | N/A |
| C. elegans strain JLF696: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; ptrn-1(wow4[ptrn-1::gfp]) X; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF291: wowEx68(myo-2p::mCherry::unc-54 3’UTR; ges-1p::3xHA::TurboID::unc-54 3’UTR) | 25 | CGC |
| C. elegans strain JLF337: wowIs24(myo-2p::mCherry::unc-54 3’UTR;ges-1p::ptrn-1a::3xHA::TurboID::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF371: wowIs26(myo-2p::mCherry::unc-54 3’UTR; ges-1p::ptrn-1a::3xHA::TurboID::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF372: wowIs17(myo-2p::mCherry::unc-54 3’UTR; ges-1p::3xHA::TurboID::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF525: vab-10b(wow80[vab-10b::zf::gfp]) I; zif-1(gk117) III | This study | N/A |
| C. elegans strain JLF551: zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF504: zif-1(gk117) III; par-1(wow87[par-1::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF690: zif-1(gk117) III; sma-1(wow102[sma-1::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF621: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; eps-8(wow101[eps-8a::zf::gfp]) IV | This study | N/A |
| C. elegans strain JLF15: ptrn-1(wow4[ptrn-1::gfp]) X | This study | N/A |
| C. elegans strain TV26135: zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wyIs900(unc-122p::rfp; lin-32p::mCherry::PLCdeltaPH) | This study | N/A |
| C. elegans strain JLF644: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF589: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF666: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF685: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF701: ebp-2(wow47[ebp-2::gfp]) II; par-3(it300[par-3::mCherry]) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study/CGC | N/A |
| C. elegans strain JLF599: vab-10b(wow80[vab-10b::zf::gfp]) I; ebp-2(wow47[ebp-2::gfp]) II; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF718: ebp-2(wow47[ebp-2::gfp]) II; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF667: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; gip-1[wow3(gfp::gip-1)] zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF597: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; gip-1[wow3(gfp::gip-1)] zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF658: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; gip-1[wow3(gfp::gip-1)] zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF699: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; ptrn-1(wow4[ptrn-1::gfp]) X; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF700: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; wdr-62(wow94[wdr-62::zf::gfp]) V; ptrn-1(wow4[ptrn-1::gfp]) X; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF714: vab-10b(wow80[vab-10b::zf::gfp]) I; zif-1(gk117) III; wdr-62(wow111[wdr-62::tag-rfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF439: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; zif-1(gk117) III | This study/CGC | N/A |
| C. elegans strain JLF473: vab-10b(wow80[vab-10b::zf::gfp]) I; ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; zif-1(gk117) III | This study | N/A |
| C. elegans strain JLF657: tbb-2(tj26[gfp::tbb-2]) unc-119(ed3) par-3(it300[par-3::mCherry]) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF757: wowIs28(myo-2p::mCherry::unc-54 3’UTR ;elt-2p::zif-1::unc-54 3’UTR;end-1p::histone1::mCherry)II; par-3(wow59[par-3::zf::gfp]) zif-1(gk117) III/qC1; wdr-62(wow111[wdr-62::tag-rfp]) V | This study | N/A |
| C. elegans strain JLF442: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study/CGC | N/A |
| C. elegans strain SA899: tbb-2(tj26[gfp::tbb-2]) unc-119(ed3) III; tjIs72[pie-1p::cherry::h2b, pie-1p::cherry::tbg-1, unc-119(+)] | Asako Sugimoto/CGC | N/A |
| C. elegans strain JLF673: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF681: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; sma-1(wow102[sma-1::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF616: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II ;zif-1(gk117) III; sma-1(wow102[sma-1::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF694: eps-8(wow101[eps-8a::zf::gfp]) IV | This study | N/A |
| C. elegans strain JLF692: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; eps-8(wow101[eps-8a::zf::gfp])/tmC9 IV | This study | N/A |
| C. elegans strain JLF1024: zif-1(gk117) III; wow112(zf::gfp::wdr-62) V | This study | N/A |
| C. elegans strain JLF648: sma-1(wow102[sma-1::zf::gfp]) V; TH110[pie-1p::par-6::mCherry] | This study | N/A |
| C. elegans strain JLF1021: vab-10b(wow157[vab-10b::gfp::sqt-1::hygR]) I; wowIs28(myo-2p::mCherry::unc-54 3’UTR; elt-2p::zif-1::unc-54 3’UTR; end-1p::histone1::mCherry) II; par-3(wow59[par-3::zf::gfp]) zif-1(gk117) III/qC1 | This study | N/A |
| C. elegans strain JLF1026: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1027: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1028: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1029: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1030: vab-10b(wow157[vab-10b::gfp::sqt-1::hygR]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF1031: vab-10b(wow157[vab-10b::gfp::sqt-1::hygR]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| Oligonucleotides | ||
| Sequences for CRISPR/Cas9 edits, see Table S4 | This paper | N/A |
| Recombinant DNA | ||
| Plasmid pAS31 [ges-1p::3xHA::TurboID::unc-54 3’UTR] | Addgene | Addgene plasmid # 118220; http://n2t.net/addgene:118220; RRID: Addgene_118220 |
| Plasmid pAS33 [ges-1p::ptrn-1a::3xHA::TurboID::unc-54 3’UTR] | This study | N/A |
| Plasmid SA109 [elt-2p::zif-1::unc-54 3’UTR] | Addgene | Addgene plasmid # 59783; http://n2t.net/addgene:59783; RRID: Addgene_59783 |
| Plasmid pMP1 [end-1p::mtag::bfp::caax] | This study | N/A |
| Plasmid pCFJ90 [myo-2p::mcherry::unc-54 3’UTR] | Addgene | Addgene plasmid # 19327; http://n2t.net/addgene:19327; RRID:Addgene_19327 |
| Plasmid pJF248 [end-1p::histone1::mcherry] | This study | N/A |
| Plasmid pJL188 [lin-32p::mcherry::PLCΔPH] | This study | N/A |
| Plasmid pDD162 [eft-3p::Cas9 + Empty sgRNA] | Addgene | Addgene plasmid # 47549; http://n2t.net/addgene:47549; RRID: Addgene_47549 |
| Plasmid pJF250 [ZF::GFP::3xFlag Empty repair template] | 20 | N/A |
| Plasmid pDD282 [GFP::3xFlag Empty repair template] | Addgene | Addgene plasmid # 66823; http://n2t.net/addgene:66823; RRID: Addgene_66823 |
| Plasmid pDD286 [TagRFP-T::3xMyc Empty repair template] | Addgene | Addgene plasmid # 70684; http://n2t.net/addgene:70684; RRID: Addgene_70684 |
| Plasmid pAS45 [eft-3p::Cas9 + vab-10b sgRNA] | This paper | N/A |
| Plasmid pAS62 [eft-3p::Cas9 + wdr-62 sgRNA] | This paper | N/A |
| Plasmid pAS78 [eft-3p::Cas9 + wdr-62 sgRNA] | This paper | N/A |
| Plasmid pMP44 [eft-3p::Cas9 + par-1 sgRNA] | This paper | N/A |
| Plasmid pMP15 [eft-3p::Cas9 + par-3 sgRNA] | This paper | N/A |
| Plasmid pLC003 [eft-3p::Cas9 + sma-1 sgRNA] | This paper | N/A |
| Plasmid pLC007 [eft-3p::Cas9 + eps-8 sgRNA] | This paper | N/A |
| Plasmid pAS46 [ZF::GFP::3xFlag with vab-10b repair template] | This paper | N/A |
| Plasmid pAS61 [ZF::GFP::3xFlag with wdr-62 repair template] | This paper | N/A |
| Plasmid pAS82 [ZF::GFP::3xFlag with wdr-62 repair template] | This paper | N/A |
| Plasmid pAS77 [TagRFP-T::3xMyc with wdr-62 repair template] | This paper | N/A |
| Plasmid pMP43 [ZF::GFP::3xFlag with par-1 repair template] | This paper | N/A |
| Plasmid pMP14 [ZF::GFP::3xFlag with par-3 repair template] | This paper | N/A |
| Plasmid pLC002 [ZF::GFP::3xFlag with sma-1 repair template] | This paper | N/A |
| Plasmid pLC006 [ZF::GFP::3xFlag with eps-8 repair template] | This paper | N/A |
| Plasmid pAS57 [GFP::3xFlag with vab-10b repair template] | This paper | N/A |
| Software and algorithms | ||
| Image J (version 2.1.0) | NIH | https://imagej.nih.gov/ij/ |
| Prism 9 for macOS (version 9.1.0) | GraphPad | https://www.graphpad.com |
| NIS elements | Nikon Instruments | https://www.microscope.healthcare.nikon.com/products/software/nis-elements |
| Illustrator | Adobe | https://www.adobe.com |
| InDesign | Adobe | https://www.adobe.com |
| Photoshop | Adobe | https://www.adobe.com |
| WDSPdb 2.0 | 66 | http://www.wdspdb.com/wdsp/ |
| JalView v2.11.1.0 | 83 | https://www.jalview.org |
| Clustel Omega v1.2.2 | 82 | https://www.ebi.ac.uk/Tools/msa/clustalo/ |
| Gblocks v0.91 | 84 | http://molevol.cmima.csic.es/castresana/Gblocks.html |
| PhyML 3.0 | 85 | http://www.atgc-montpellier.fr/phyml/ |
| SMS for PhyML | 86 | http://www.atgc-montpellier.fr/sms/ |
| FigTree v1.4.4 | Andrew Rambaut | http://tree.bio.ed.ac.uk/software/figtree/ |
| Worm tissue expression prediction server | 30 | http://worm.princeton.edu/ |
| GO term finder | 71 | https://go.princeton.edu/cgi-bin/GOTermFinder |
| Biomart | 73 | http://biomart.org |
| STRING | 32 | http://string-db.org |
| Python 3.7, Sanchez_etal_python_environment.yaml The Python 3.7 environment used to run all the analysis. This.yaml file includes all the package versions and dependencies. |
https://www.anaconda.com | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/Sanchez_etal_python_environment.yaml |
| Python 3.7, microtubule_tracking_class.py A class that includes all the microtubule segmentation and tracking functions |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/microtubule_tracking_class.py |
| Python 3.7, run_microtubule_tracking_class_example.py An example on how to use the ‘microtubule_tracking_class’ |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/run_microtubule_tracking_class_example.py |
| Python 3.7, Sanchez_etal_figure_functions.py The functions used to plot Figures 4E, S6G-H |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/Sanchez_etal_figure_functions.py |
| Python 3.7, Sanchez_etal_plot_figures.py The script which runs the ‘Sanchez_etal_figure_functions’ to plot the data |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/Sanchez_etal_plot_figures.py |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
C. elegans strains and maintenance
Nematodes were cultured and manipulated68. Unless otherwise noted, strains were cultured and maintained at 20°C on E. coli OP50 bacteria. To deplete animals of excess biotin, animals were grown on E. coli (MG1655 bioB::kan)69 that was first grown in LB growth medium with kanamycin (100 μg/mL), then washed three times in 1x M9 buffer. The strains used in this study are listed in the key resources table (organisms/strains).
METHOD DETAILS
TurboID Proximity-Dependent Protein Labeling Method
Plasmids and strains
C. elegans codon-optimized ligase gene TurboID (containing 3 worm introns present in GFP) is as previously published25. All expression constructs were cloned into pJF241 to produce plasmids pAS31 (ges-1p::3xha::turboID::unc-54) and pAS33 (ges-1p::ptrn-1a::3xha::turboID::unc-54). Transgenic worms were generated by injecting 50 ng/μL plasmid and 2.5 ng/μL of the co-injection marker myo-2p::mCherry into one day old N2 hermaphrodites. Extrachromosomal arrays were integrated with gamma irradiation (total dose of 3800 radians) using a cesium irradiator. Expression from a high copy array is necessary in this tissue and stage as we did not see appreciable biotinylation from a single copy insertion of the same transgene (data not shown).
Mass spectrometry sample preparation
Embryos of each genotype were plated on XL peptone-rich plates coated in NA22 bacteria and grown until L4 stage at 25°C. Worms were harvested via M9 washes and frozen in 500 μL pellets at −80°C and unless otherwise noted, all following steps were done at 4°C. On the day of sample preparation, frozen worm pellets were thawed to 4°C in 600 μL of high SDS RIPA buffer (50 mM Tris-HCl pH 8.0; 150 mM NaCl; 1% SDS; 0.5% sodium deoxycholate; 1% TritonX-100; 1mM PMSF; 1x HALT; 2.5 mg/mL Leupeptin; 5 mg/mL Pepstatin), transferred to bead beating tubes (lysing matrix C, 1.0 mm silica spheres, MP Biomedicals), and agitated at 6.5 m/s, 20 sec pulse (3x) with 5 minute breaks on 4°C ice (FastPrep-24 Classic). Samples were transferred to sonication tubes and sonicated at 18% at 5 second intervals with 5 second breaks for 50 seconds total in a 4°C bath (Branson Sonifier SFX250). SDS-free RIPA buffer was added to achieve a final SDS concentration of 0.2%. Rotate lysate for 1 hour at 4°C. Samples were transferred to ultracentrifuge tubes (13×51MM), and spun for 30 minutes at 51,000 RPM in a TLA-100.3 ultracentrifuge rotor (Beckman). The liquid layer was collected, avoiding the lipid layer, and 12 mg of total input protein was rotated overnight at 4°C with washed streptavidin beads (Pierce Streptavidin Magnetic Beads, Thermo Fisher Scientific, #88817) at a ratio of 8 μg protein/1 μL beads. After binding, the samples were washed for 2 minutes each with the following solutions and indicated number of repeats: RIPA buffer (2x), 1M KCl (1x), 0.1M Na2CO3 (1x), 2M urea (1x), 4M urea (1x), RIPA (2x), PBS (5x).
Western Blotting
Lysate was loaded onto a 4–20% Mini-PROTEAN TGX PAGE gel (Bio-Rad), transferred to a nitrocellulose membrane (0.4 μm, Bio-Rad). Blots were blocked in 5% milk PBST solution, probed with anti-HA (1:5000, rat monoclonal, Roche), and detected with secondary antibody (1:5000, goat anti-rat IRDye 680RD, Licor) and streptavidin-IRDye (1:5000, 800CW, Licor). Blots were imaged on LI-COR Odyssey CLx. Western blot data presented in Figure 1D is representative of three independent experiments.
Mass Spectrometry
Biotinylated samples were processed for mass spectrometry on streptavidin beads. Beads were re-suspended in 50 mM ammonium bicarbonate and then reduced with 10 mM DTT at 55°C for 5 min., followed by 25 min. at room temperature. Alkylation was performed with 30 mM acrylamide for 30 min. at room temperature. Digestion was performed with Trypsin/LysC (Promega) in the presence of 0.02% protease max (Promega) in a standard overnight digest at room temperature on a head-over-head mixer. The digestion reaction was quenched with 1% formic acid and peptides were de-salted with C18 Monospin reversed phase columns (GL Sciences). De-salted peptides were dried in a speed vac. Samples were reconstituted in 20 μl reconstitution buffer (2% acetonitrile with 0.1% Formic acid), 2 μl of which was injected on the instrument.
Mass spectrometry experiments were performed using an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific, San Jose, CA) with liquid chromatography using a Nanoacquity UPLC (Waters Corporation, Milford, MA). For a typical LCMS experiment, a flow rate of 450 nL/min was used, where mobile phase A was 0.2% formic acid in water and mobile phase B was 0.2% formic acid in acetonitrile. Analytical columns were prepared in-house with an I.D. of 100 microns pulled to a nanospray emitter using a P2000 laser puller (Sutter Instrument, Novato, CA). The column was packed using C18 reprosil Pur 1.8 micron stationary phase (Dr. Maisch) to a length of ~25 cm. Peptides were directly injected onto the analytical column using a gradient (2–45% B, followed by a high-B wash) of 80 min. The mass spectrometer was operated in a data dependent fashion using CID fragmentation for MS/MS spectra generation.
Mass spectrometry analysis
MS/MS data were processed using Byonic (Protein Metrics, San Carlos, CA) to identify peptides and infer proteins using the C. elegans database from Uniprot. Proteolysis with Trypsin/LysC was assumed to be semi-specific allowing for N-ragged cleavage with up to two missed cleavage sites. Precursor mass accuracies were held within 12 ppm, and 0.4 Da for MS/MS fragments. Proteins were held to a false discovery rate of 1%, using standard approaches. Subsequent analyses were done using Uniprot Protein ID and corresponding spectral count after contaminants were removed. Any Uniprot Protein ID not detected for a given sample was assigned a spectral count value of 0 for the purpose of the analysis. For pairwise comparisons, protein length and spectral counts for each sample were uploaded to crapome.org70 (database version v1.1) and the following parameters were used: workflow 3, fold change score options: FC-A(user,default); FC-B(all, stringent), C is control, Saint Expressed SAINT options: Saint Express with user control [(SE)C(user)]; incorporate known data (IKD)=none; number of replicates per bait (NR)=all. SAINT analysis26 score and fold change value for each Uniprot Protein ID were calculated. PTRN-1 proximity interactors were defined by a minimum SAINT score above or equal to 0.8 and fold changes above or equal to 2.5 when the PTRN-1::TurboIDgut dataset was compared to TurboIDgut and wild-type (N2) controls.
Dataset queries
Adult RNAseq30 information was acquired from: http://worm.princeton.edu/ and embryonic RNAseq information was acquired from a published dataset31. GO term analyses were done using GO term finder71 (https://go.princeton.edu/cgi-bin/GOTermFinder) to find shared GO terms with p-values indicated in Data S1. To find predicted human orthologs of C. elegans genes, OrthoList72 and Biomart73 (http://biomart.org) were used. The interaction p-value was calculated via Search Tool for the Retrieval of Interacting Genes/Proteins (STRING32) interactions were assessed from co-expression, experimental evidence, databases, and text-mining.
CRISPR/Cas9
All CRISPR/Cas9-based insertions were achieved using the self-excising cassette (SEC) method74. Plasmid pDD286 was used for insertion of 3xMyc and TagRFP-T or plasmid JF25020 was used for insertion of a ZF domain, 3xFLAG, and GFP. Cas9 and sgRNAs for each edit were expressed from plasmid pDD162, into which the appropriate sgRNA sequence was added with a Q5 Site-Directed Mutagenesis Kit. For each edit, young adult zif-1(gk117) hermaphrodites were injected with the SEC plasmid and Cas9/sgRNA plasmid. Worms were recovered and screened for the expected edit according to a published protocol74. Sequences for sgRNA and homology arms are listed in Table S2.
ZF/ZIF-1 degradation
The ZF/ZIF-1 system20,43 was used as described above (See CRISPR/Cas9): the ZF degron and adjoining GFP were inserted into the endogenous locus of the gene of interest in zif-1(gk117) hermaphrodites. Exogenous ZIF-1 was expressed from either an extrachromosomal array (wowEx111) or by single-copy insertion75 as indicated in the key resources table (organisms/strains). Transgenic worms were generated by injecting 50 ng/μL of the desired plasmids and 2.5 ng/μL of the co-injection marker myo-2p::mCherry into one day old N2 hermaphrodites. To keep degradation methodology consistent, we used the same array across all strains by mating. Degradation of GFP signal is demonstrated in Figure S2. Unless otherwise noted, the degradation experiments were paired with controls expressing the degradation machinery without a ZF tagged allele in zif-1(gk117) or N2 worms (e.g. zif-1(gk117); wowEx111(elt-2p:zif-1)). Two other types of controls were used in the following cases:
ZF-tagged allele without the degradation machinery: Figure 6B (control for V;Wgut(−)), Figures S2D, S2E, S3C, S3E, and S6D.
ZF-tagged allele with non-degron tagged protein provided by a balancer chromosome: Figures 6C–6E and S3E.
Inhibition of actin polymerization
Actin inhibitor experiments were performed in two ways. First (Figure 7), embryos were coated with 0.1% trypan blue and attached to coverslips coated with poly-lysine in SGM buffer7,76 containing either 10μM Latrunculin A or a mixture of 10μM Latruculin A with 10ug/mL Cytochlasin D. The coverslip was inverted over a slide with Teflon spacers and 15 to 20 22.5 μm glass beads (Whitehouse scientific) to prevent the coverslip from bending under the pressure from the objective. The eggshell and vitelline membrane of E16-stage embryos were permeabilized using a Micropoint pulse-nitrogen dye laser (Coumarin dye, Andor). Embryos were imaged before and then immediately after laser permeabilization at 5-minute intervals. Second (Figure S7), embryos were collected in a mouth pipette and then added to an inverted drop of 5–7.5% sodium hypochlorite (Sigma) and 2.5N KOH for 3 minutes to weaken the eggshell. The slide was then inverted while simultaneously adding an equivalent volume of SGM. Embryos were moved through 5 washes of SGM and then into a final drop of SGM+1% DMSO or SGM+10μM Latruculin A. The vitelline membrane was removed mechanically by moving embryos individually through the end of a drawn-out capillary with a mouth pipette. Embryos were then moved to a small drop of equivalent media on a coverslip bottom culture dish for imaging.
Immunofluorescence
One-day-old adults were incubated in 1x M9 solution for 4–5 hours and their embryos were removed, fixed, and stained77. Embryos were attached to microscope slides coated with poly-lysine and containing Teflon spacers. Slides were frozen on dry ice, embryos were permeabilized by freeze-crack method and submerged in 100% MeOH for 5–10 minutes at - 20°C. Embryos were submerged for 5 minutes twice in PBS, then once in PBT (PBS plus 0.1% Tween). Embryos were incubated in primary antibody overnight at 4°C. Embryos were then washed in PBT for 5 minutes three times and then incubated in secondary antibody for 1 hour at 37°C. Embryos were washed once in PBT then twice in PBS, mounted in Vectashield (Vector Laboratories), and stored at 4°C. The following primary antibodies were used: anti-HA primary antibody (Abcam, 1:200), anti-Myc (Abcam, 1:100), anti-GFP (Abcam, 1:200), anti-PAR-3 (DSHB), anti-GIP-1 (GenScript7). The following secondary antibodies were used: CY3-anti-mouse secondary antibody (Jackson Immunoresearch Laboratories, 1:200), Streptavidin Alexa Fluor 488 (Invitrogen, 1:200), DAPI (Sigma, 1:10,000), 647-anti-rabbit (Jackson Immunoresearch Laboratories, 1:50), 488-anti-mouse (Jackson Immunoresearch Laboratories, 1:200), 488 anti-rabbit (Jackson Immunoresearch Laboratories, 1:200), Cy5 anti-mouse (Jackson Immunoresearch Laboratories, 1:50).
Microscopy
For live imaging, embryos were isolated from young hermaphrodites incubated for 4–5 hours in 1x M9 solution at 20°C. Embryos were mounted on a pad made of 3% agarose dissolved in 1x M9 solution and imaged on a Nikon Ti-E inverted microscope (Nikon Instruments, Melville, NY) with a confocal spinning disk head using a 60x or 100x PLAN APO oil objective (NA = 1.4 or NA = 1.45, respectively) controlled using NIS Elements software (Nikon). Images were captured using an Andor Ixon Ultra back thinned EM-CCD camera controlled using NIS Elements software (Nikon) at a z-sampling rate of 0.5 μm and using 488 nm, 561 nm, 405 nm, or 640 nm imaging lasers. Fixed images were acquired using a 60x PLAN APO oil objective (NA = 1.4) on the above Nikon confocal system. All images were processed in NIS Elements software, Fiji/ImageJ, and Adobe InDesign.
Viability assays:
For each indicated strain, one-day old adult worms were singled on separate plates. The adult was removed after laying eggs for 4 hours and the remaining embryos on the plate were counted. Three days later the number of living larvae were counted and viability was calculated by dividing the number of hatched larvae by the number of eggs that were initially laid. Viability for EPS-8Agut(−) and its associated controls was calculated by dividing the number of adult/L4 worms by the number of hatched L1 larvae with the desired genotype.
QUANTIFICATION AND STATISTICAL ANALYSES
Analysis considerations
Embryos were carefully chosen based on the appropriate stage of development and with their dorsal side oriented toward the coverslip. The same microscopy imaging parameters were used across genotypes for a given experiment.
Quantification of apical fluorescence enrichment
Analysis was done as previously described20. Bean-stage embryos were chosen for analysis in Fiji. A membrane marker, nuclear positions, and distance from primordial germ cells were used to select the plane in the Z axis in which to define the intestinal midline. For each embryo, a sum Z-projection of three slices flanking the midline plane was used for the remaining analysis. Boxes 2 μm in width were drawn by hand in intestinal cytoplasmic regions to calculate background fluorescence and one box was drawn at the apical midline to define the region of interest (ROI). For each embryo, apical enrichment was calculated by dividing the mean intensity within the apical ROI by the mean cytoplasmic intensity. To plot line profiles, a 1-μm wide line was hand-drawn perpendicular to the apical midline. Profile plots were generated in R using ggplot2 and all other plots were generated in Prism 9 (version 9.1). Each profile was normalized by dividing each pixel intensity value by the average intensity 3.5 μm to 5.5 μm from the midline. The mean profile was calculated with the intensity value for each distance from the midline.
EBP-2 comet analysis
For the segmentation and tracking of EBP-2 comets, as well as for the estimation of the speed and angle of microtubule polymerization, we used a set of image filters and custom-built functions in Python 3.7.4 (Anaconda Software Distribution, Conda version 4.9.2, https://www.anaconda.com). The SciPy ecosystem78 was used for image processing, the NumPy and Pandas libraries79,80 were used for efficient mathematical operations and data manipulation. The analysis pipeline is organized in the microtub_tracking class, which is available in the GitHub repository https://github.com/JacobsWagnerLab/published/tree/master/Sanchez_2020, together with the Python environment (including the Python version and all related packages and dependencies).
Segmentation of the embryo and the embryonic intestine:
For the segmentation of the embryo and the embryonic intestine, an adaptive filter was used on the maximum intensity projection of the EBP-2::GFP stream acquisition images and the BFP::CAAX intestine specific marker. The image was first smoothed using the gaussian_filter function from the scipy.ndimage submodule. The threshold_local filter from the skimage.filters submodule was then applied on the smoothed image to generate a dynamic threshold. This dynamic threshold, which was estimated on the basis of the local pixel neighborhood, was used to mask the pixels of the entire embryo or of its intestine (pixels higher than the local threshold). The edges of the binary mask were bridged and the holes were filled using the binary_closing and binary_fill_holes functions from the scipy.ndimage submodule. An area threshold in pixels was used to remove small masked regions (noise around the embryo or its intestine). If there was more than one embryo in the field of view, the largest embryo was selected.
Definition of the annotated midline:
To obtain a midline reference point within the intestine, a second-degree univariate spline (scipy.interpolate.UnivariateSpline) was fitted to a set of manually selected mid-gut coordinates. The mid-gut coordinates were selected on the maximum projection of the EBP-2::GFP images for each embryo, using a graphical interface that was constructed as part of our microtub_tracking class in Python. The resulting fitted ‘annotated midline’ had a sub-pixel resolution (0.1 pixels).
Segmentation of EBP-2 comets:
For the detection and segmentation of the EBP-2 comets, a Laplacian of Gaussian (LoG) filter and adaptive thresholding were applied on the EBP-2::GFP images. The LoG filter was constructed by smoothing the image (scipy.ndimage.gaussian_filter) and then applying a Laplace operator (skimage.filters.laplace). A percentile of the brightest pixels was set as a hard threshold on the LoG-transformed image to generate a binary mask. A second binary mask was generated by adaptively thresholding (skimage.filters.threshold_local) the original fluorescence image. The two binary masks were multiplied. As a result, only the pixels that are selected in both masks will correspond to the pixels of the segmented comets. The masked spots in the image were labeled. Labels smaller than 3 pixels, which correspond to noise, were removed from the analysis. To better separate clustered comets (i.e. a mask label containing more than one comet), a new LoG filter with strict thresholding parameters was applied to mask labels larger than 40 pixels. The centroid coordinates of the mask labels, each corresponding to one EBP-2 comet, were used as the location of the comet. The segmentation algorithm was applied to all fluorescence images acquired during the stream image acquisition. The same segmentation parameters were used for all analyzed embryos.
Tracking of the EBP-2 comets:
The segmented microtubule comets were linked and tracked using their distance between subsequent frames. The algorithm linked each EBP-2 comet to another comet in the next time-point, within a specified radius of 4 pixels. If more than one comet was detected, the most proximal one was linked. If no comet was detected within the search radius in the next time-point, the algorithm searched for comets in the subsequent time-point. This procedure resulted in complete comet trajectories even when intermediate comet positions were missed (e.g., due to failed segmentation). The same tracking parameters were used for all analyzed embryos.
Estimation of the comet trajectory angles:
Because the comets are tracked every 100 msec, the displacement between adjacent time-points in the stream image acquisition can be smaller than the comet localization error. In order to improve the comet displacement statistics, we applied a time-lag of four frames in the displacement estimation. As a result, the displacement of the comet position between each frame (n) and 4 frames ahead (n+4) was calculated. All spurious trajectories that included less than four frames were removed from the analysis. The EBP-2 comet extension angle was estimated on the basis of these 4-frame (or 400 msec) intervals.
The angle of each instantaneous comet displacement was calculated as the arc-tangent (inverse tangent) of the slope of the line connecting the comet position at each frame (n) with its position 4 frames ahead (n+4) in the comet trajectory. For each comet displacement, the displacement angle was corrected by subtracting the instantaneous angle of the annotated midline nearest to the comet position. The instantaneous angle of the annotated midline was also calculated as the arc-tangent of its local slope. Specifically, the point on the annotated midline that is closest to the original comet position was selected and the local slope of the annotated midline was calculated by fitting a linear regression to the adjacent line coordinates. Twenty-one points in total were used for the linear fit, which corresponds to approximately 2 pixels given the 0.1 pixels resolution of the annotated midline.
The average angle of each comet trajectory was calculated as the arc-tangent of the slope of the linear regression fitted to all the comet coordinates, from the first to the last frame of the trajectory. Similar to the instantaneous angles, the average angles of the comet trajectories were corrected by the angle of the annotated midline. Since our analysis does not differentiate between the posterior and the anterior end of the gut, all angles were expressed between 0° and 180°. Comet trajectories that move away from the annotated midline were expected to have instantaneous or average angles of approximately 90°. Comet trajectories that extend parallel to the annotated midline are expected to have angles of approximately 0° or 180°.
Visualization of the instantaneous comet angles and positions (see Figure 4E):
The continuous two-dimensional distributions of the instantaneous comet relative angles and positions (relative to the annotated midline) were visualized using a Gaussian kernel density estimation (Seaborn81 data visualization library in Python, kdeplot sub-module). The bandwidth of the kernel density estimator was determined using Scott’s rule and 30 iso-contours were drawn. The density maps were built using 23758 instantaneous displacements from 1365 comet trajectories tracked in 7 wildtype embryos, 11621 instantaneous displacements from 740 comet trajectories tracked in 5 VAB-10Bgut(−) embryos, and 8075 instantaneous displacements from 640 comet trajectories tracked in 7 WDR-62gut(−) embryos.
Statistical analysis
We performed Student’s t-tests for single comparisons using Prism 9 (version 9.1) software. For multiple comparisons, Prism 9 software was used to perform one-way parametric ANOVA followed by the Tukey’s multiple comparisons post-hoc test. Figure legends indicate p values.
Phylogenetic analysis
Representative WDR-16 and WDR-62 homologs from invertebrates (Caenorhabditis elegans H24G06.1a), vertebrates (Homo sapiens WDR16, WDR62, and MAPKBP1), and chytrid fungi (Batrachochytrium dendrobatidis BDEG_26806 and BATDEDRAFT_35991) were used to identify reciprocal best blast hits in species represented in the phylogenetic tree (Table S1). Number of WD40 repeats was determined by WDSP66. Sequences were aligned using Clustal Omega82 in JalView83 before trimming to 230 amino acid positions within the WD40 repeat region using Gblocks v0.9184 with parameters -b3=15 -b4=2 -b5=a. PhyML 3.085 with substitution model (LG+G) selected by Akaike Information Criterion86 was used to generate support values using 1000 bootstraps, and the resulting tree was visualized with FigTree v1.4.4.
ADDITIONAL RESOURCES
This study did not generate any additional resources.
Supplementary Material
Data S1: PTRN-1 proximal interactors with enriched Gene Ontology Function terms, SAINT and Fold Change scores, related to Figure 1.
Table S1: Phylogenic analysis accession numbers, related to Figure 2.
Video S1. EBP-2::GFP comet dynamics, related toFigure 4. Dorsal view of live, bean stage C. elegans embryos of indicated genotypes with intestine outlined (white dotted line). 10-second stream acquisition of EBP-2::GFP.
Video S2. Actin perturbation liberates apical microtubules, related toFigure 7. Dorsal view of live, bean stage C. elegans embryo expressing GFP::TBB-2/β-tubulin filmed immediately after treatment with 10 Latrunculin A and 10ug/mL Cytochlasin D. Pharynx (blue dotted line) and intestine (white dotted line) are indicated. Note the division of the anterior intestinal cells (asterisk). Scale bar = 10 μm.
Highlights.
Proximity labeling in living C. elegans identifies ncMTOC proteins
WDR-62, VAB-10B, and actin filaments drive ncMTOC function and assembly
Functionally distinct ncMTOC modules control microtubule growth and localization
The apical ncMTOC is physically and functionally distinct from the centrosome
Acknowledgements:
We thank Ashby Morrison, Kristy Red-Horse, Asako Sugimoto, Karen Oegema, Dan Dickinson, and Bob Goldstein for providing reagents, strains, and for CRISPR advice and protocols. We thank Maria Sallee for sharing relevant data65 and members of the Feldman lab for helpful discussions about the manuscript. We thank Andrew McKay for helpful experimental design and data analysis conversations about the manuscript. Some of the nematode strains used in this work were provided by the Caenorhabditis Genetic Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). Mass Spectrometry was performed at Stanford University (Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University Mass Spectrometry). This work was supported by an NIH New Innovator Award DP2GM119136-01 and R01GM133950 awarded to J.L.F. A.D.S. was supported by NIH Training Grant 2T32GM007276. T.C.B. was supported by Dow Graduate Research and Lester Wolfe Fellowships. C.J.W. and K.S. are investigators of the Howard Hughes Medical Institute. A.P. was supported by Rubicon Science 2018-1 research program (019.182EN.018) partly financed by the Dutch Research Council (NWO). K. S. was supported by NIH NS082208. A.Y.T. was supported by NIH R01 DK121409.
Footnotes
Declaration of Interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Porter KR (1966). Cytoplasmic Microtubules and Their Functions.CIBA Foundation Symp. In Principles of Biomolecular Organizations. Edited by Wolstenholme GEW, O’Connor MJ A. London: Churchill Ltd; 308–345. [Google Scholar]
- 2.Pickett-Heaps JD (1969). The evolution of the mitotic apparatus: an attempt at comparative ultrastructural cytology in dividing plant cells. Cytobios., 3: 257–280. [Google Scholar]
- 3.Brodu V, Baffet AD, Le Droguen P-M, Casanova J, and Guichet A. (2010). A developmentally regulated two-step process generates a noncentrosomal microtubule network in Drosophila tracheal cells. Developmental Cell 18, 790–801. [DOI] [PubMed] [Google Scholar]
- 4.Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, and Ralston E. (2013). Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. The Journal of Cell Biology 203, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ori-McKenney KM, Jan LY, and Jan YN (2012). Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang X, Kokes M, Fetter RD, Sallee MD, Moore AW, Feldman JL, and Shen K. (2020). Growth cone-localized microtubule organizing center establishes microtubule orientation in dendrites. eLife Sciences 9, e56547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman JL, and Priess JR (2012). A Role for the Centrosome and PAR-3 in the Hand-Off of MTOC Function during Epithelial Polarization. Current Biology 22, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakley CE, and Oakley BR (1989). Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 338, 662–664. [DOI] [PubMed] [Google Scholar]
- 9.Wiese C, and Zheng Y. (2000). A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nature Cell Biology 2, 358–364. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin SS, and Vale RD (2010). Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng W, Mushika Y, Ichii T, and Takeichi M. (2008). Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 135, 948–959. [DOI] [PubMed] [Google Scholar]
- 12.Baines AJ, Bignone PA, King MDA, Maggs AM, Bennett PM, Pinder JC, and Phillips GW (2009). The CKK domain (DUF1781) binds microtubules and defines the CAMSAP/ssp4 family of animal proteins. Mol. Biol. Evol 26, 2005–2014. [DOI] [PubMed] [Google Scholar]
- 13.Akhmanova A, and Hoogenraad CC (2015). Microtubule minus-end-targeting proteins. Current Biology 25, R162–R171. [DOI] [PubMed] [Google Scholar]
- 14.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, and Bornens M. (2000). Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. 113 ( Pt 17), 3013–3023. [DOI] [PubMed] [Google Scholar]
- 15.Lechler T, and Fuchs E. (2007). Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. The Journal of Cell Biology 176, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannak E, Oegema K, Kirkham M, Gönczy P, Habermann B, and Hyman AA (2002). The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent. The Journal of Cell Biology 157, 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers GC, Rusan NM, Peifer M, and Rogers SL (2008). A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell 19, 3163–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Wu D, Quintin S, Green RA, Cheerambathur DK, Ochoa SD, Desai A, and Oegema K. (2015). NOCA-1 functions with γ-tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in C. elegans. eLife Sciences 4, e08649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Buchwalter RA, Zheng C, Wight EM, Chen JV, and Megraw TL (2020). A perinuclear microtubule-organizing centre controls nuclear positioning and basement membrane secretion. Nature Cell Biology 22, 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallee MD, Zonka JC, Skokan TD, Raftrey BC, and Feldman JL (2018). Tissue-specific degradation of essential centrosome components reveals distinct microtubule populations at microtubule organizing centers. PLOS Biology 16, e2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer M, Cubizolles F, Schmidt A, and Nigg EA (2016). Quantitative analysis of human centrosome architecture by targeted proteomics and fluorescence imaging. The EMBO Journal 35, 2152–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arslanhan MD, Gulensoy D, Firat-Karalar EN (2020). A Proximity Mapping Journey into the Biology of the Mammalian Centrosome/Cilium Complex. Cells 9, 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roux KJ, Kim DI, Raida M, and Burke B. (2012). A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. The Journal of Cell Biology 196, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinke AW, Mak R, Troemel ER, and Bennett EJ (2017). In vivo mapping of tissue- and subcellular-specific proteomes in Caenorhabditis elegans. Sci Adv 3, e1602426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, and Ting AY (2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol 6, e02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi H, Larsen B, Lin Z-Y, Breitkreutz A, Mellacheruvu D, Fermin D, Qin ZS, Tyers M, Gingras A-C, and Nesvizhskii AI (2011). SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nature Methods 8, 70–U100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ning W, Yu Y, Xu H, Liu X, Wang D, Wang J, Wang Y, and Meng W. (2016). The CAMSAP3-ACF7 Complex Couples Noncentrosomal Microtubules with Actin Filaments to Coordinate Their Dynamics. Developmental Cell 39, 61–74. [DOI] [PubMed] [Google Scholar]
- 28.Khanal I, Elbediwy A, Diaz de la Loza MDC, Fletcher GC, and Thompson BJ (2016). Shot and Patronin polarise microtubules to direct membrane traffic and biogenesis of microvilli in epithelia. 129, 2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King MDA, Phillips GW, Bignone PA, Hayes NVL, Pinder JC, and Baines AJ (2014). A conserved sequence in calmodulin regulated spectrin-associated protein 1 links its interaction with spectrin and calmodulin to neurite outgrowth. J. Neurochem 128, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaletsky R, Yao V, Williams A, Runnels AM, Tadych A, Zhou S, Troyanskaya OG, and Murphy CT (2018). Transcriptome analysis of adult Caenorhabditis elegans cells reveals tissue-specific gene and isoform expression. PLoS Genet 14, e1007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimshony T, Feder M, Levin M, Hall BK, and Yanai I. (2015). Spatiotemporal transcriptomics reveals the evolutionary history of the endoderm germ layer. Nature 519, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gally C, Zhang H, and Labouesse M. (2016). Functional and Genetic Analysis of VAB-10 Spectraplakin in Caenorhabditis elegans. Meth. Enzymol 569, 407–430. [DOI] [PubMed] [Google Scholar]
- 34.Pervaiz N, and Abbasi AA (2016). Molecular evolution of WDR62, a gene that regulates neocorticogenesis. Meta Gene 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris-Rosendahl DJ, Kaindl AM (2015). What next-generation sequencing (NGS) technology has enabled us to learn about primary autosomal recessive microcephaly (MCPH). Molecular and Cell Probes, 29: 271–281. [DOI] [PubMed] [Google Scholar]
- 36.Yu TW, Mochida GH, Tischfield DJ, Sgaier SK, Flores-Sarnat L, Sergi CM, Topcu M, McDonald MT, Barry BJ, Felie JM, et al. (2010). Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nature Genetics 42, 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogoyevitch MA, Yeap YYC, Qu Z, Ngoei KR, Yip YY, Zhao TT, Heng JI, and Ng DCH (2012). WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression. J Cell Sci 125, 5096–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J-F, Zhang Y, Wilde J, Hansen KC, Lai F, and Niswander L. (2014). Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nature Communications 5, 3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramdas Nair A, Singh P, Salvador Garcia D, Rodriguez-Crespo D, Egger B, and Cabernard C. (2016). The Microcephaly-Associated Protein Wdr62/CG7337 Is Required to Maintain Centrosome Asymmetry in Drosophila Neuroblasts. CellReports 14, 1100–1113. [DOI] [PubMed] [Google Scholar]
- 40.Jayaraman D, Kodani A, Gonzalez DM, Mancias JD, Mochida GH, Vagnoni C, Johnson J, Krogan N, Harper JW, Reiter JF, et al. (2016). Microcephaly Proteins Wdr62 and Aspm Define a Mother Centriole Complex Regulating Centriole Biogenesis, Apical Complex, and Cell Fate. Neuron 92, 813–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgourdou P, Mishra-Gorur K, Saotome I, Henagariu O, Tuysuz B, Campos C, Ishigame K, Giannikou K, Quon JL, Sestan N, et al. (2017). Disruptions in asymmetric centrosome inheritance and WDR62-Aurora kinase B interactions in primary microcephaly. Sci Rep 7, 43708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shohayeb B, Lim NR, Ho U, Xu Z, Dottori M, Quinn L, and Ng DCH (2018). The Role of WD40-Repeat Protein 62 (MCPH2) in Brain Growth: Diverse Molecular and Cellular Mechanisms Required for Cortical Development. Mol. Neurobiol 55, 5409–5424. [DOI] [PubMed] [Google Scholar]
- 43.Armenti ST, Lohmer LL, Sherwood DR, and Nance J. (2014). Repurposing an endogenous degradation system for rapid and targeted depletion of C. elegans proteins. Development 141, 4640–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, and Philips MR (1999). Endomembrane Trafficking of Ras. Cell 98, 69–80. [DOI] [PubMed] [Google Scholar]
- 45.Hehnly H, and Doxsey S. (2014). Rab11 Endosomes Contribute to Mitotic Spindle Organization and Orientation. Developmental Cell 28, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiner AT, Seebold DY, et al. (2020). Endosomal Wnt signaling proteins control microtubule nucleation in dendrites. PLoS Biol 18, e3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang R, and Feldman JL (2015). SPD-2/CEP192 and CDK Are Limiting for Microtubule-Organizing Center Function at the Centrosome. Curr. Biol 25, 1924–1931. [DOI] [PubMed] [Google Scholar]
- 48.Nashchekin D, Fernandes AR, and St Johnston D. (2016). Patronin/Shot Cortical Foci Assemble the Noncentrosomal Microtubule Array that Specifies the Drosophila Anterior-Posterior Axis. Developmental Cell 38, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Achilleos A, Wehman AM, and Nance J. (2010). PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacQueen AJ, Baggett JJ, Perumov N, Bauer RA, Januszewski T, Schriefer L, and Waddle JA (2005). ACT-5 Is an Essential Caenorhabditis elegans Actin Required for Intestinal Microvilli Formation. Mol. Biol. Cell 16, 3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Furden D, Johnson K, Segbert C, and Bossinger O. (2004). The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Developmental Biology 272, 262–276. [DOI] [PubMed] [Google Scholar]
- 52.Bosher JM, Hahn B-S, Legouis R, Sookhareea S, Weimer RM, Gansmuller A, Chisholm AD, Rose AM, Bessereau J-L, and Labouesse M. (2003). The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J Cell Biol 161, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bilguvar K, Ozturk AK, Louvi A, Kwan KY, Choi M, Tatli B, Yalnizoglu D, Tuysuz B, Caglayan AO, Gokben S, et al. (2010). Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature 467, 207–U293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim NR, Yeap YYC, Zhao TT, Yip YY, Wong SC, Xu D, Ang C-S, Williamson NA, Xu Z, Bogoyevitch MA, et al. (2015). Opposing roles for JNK and Aurora A in regulating the association of WDR62 with spindle microtubules. J Cell Sci 128, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Widmer LA, Stangier MM, Steinmetz MO, Stelling J, and Barral Y. (2019). Remote control of microtubule plus-end dynamics and function from the minus-end. eLife Sciences 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta KK, Alberico EO, Näthke IS, and Goodson HV (2014). Promoting microtubule assembly: A hypothesis for the functional significance of the +TIP network. Bioessays 36, 818–826. [DOI] [PubMed] [Google Scholar]
- 57.Sun D, Leung CL, and Liem RK (2001). Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): identification of a novel group of microtubule associated proteins. J Cell Sci 114, 161–172. [DOI] [PubMed] [Google Scholar]
- 58.Applewhite DA, Grode KD, Keller D, Zadeh AD, Zadeh A, Slep KC, and Rogers SL (2010). The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network. Mol. Biol. Cell 21, 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suozzi KC, Wu X, and Fuchs E. (2012). Spectraplakins: master orchestrators of cytoskeletal dynamics. The Journal of Cell Biology 197, 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu P, Zupa E, Neuner A, Böhler A, Loerke J, Flemming D, Ruppert T, Rudack T, Peter C, Spahn C, et al. (2020). Insights into the assembly and activation of the microtubule nucleator γ-TuRC. Nature 578, 467–471. [DOI] [PubMed] [Google Scholar]
- 61.Consolati T, Locke J, Roostalu J, Chen ZA, Gannon J, Asthana J, Lim WM, Martino F, Cvetkovic MA, Rappsilber J, et al. (2020). Microtubule Nucleation Properties of Single Human gamma TuRCs Explained by Their Cryo-EM Structure. Developmental Cell 53, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muroyama A, Seldin L, and Lechler T. (2016). Divergent regulation of functionally distinct γ-tubulin complexes during differentiation. The Journal of Cell Biology 213, 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez AD, and Feldman JL (2016). Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Current Opinion in Cell Biology 44, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia ARR, McLeod IX, et al. (2007). Asymmetric CLASP-Dependent Nucleation of Noncentrosomal Microtubules at the trans-Golgi Network. Developmental Cell 12, 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sallee MD, Pickett MA, Feldman JF (2020). Apical PAR complex proteins protect against epithelial assaults to create a continuous and functional intestinal lumen. bioRxiv 2020.10.28.359299; doi: 10.1101/2020.10.28.359299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma J, An K, Zhou JB, Wu NS, Wang Y, Ye ZQ (2019). WDSPdb: an updated resource for WD40 proteins. Bioinformatics 35, 4824–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sulston JE, Schierenberg E, White JG, Thomson JN (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100: 64–119. [DOI] [PubMed] [Google Scholar]
- 68.Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortega-Cuellar D, Hernandez-Mendoza A, Moreno-Arriola E, Carvajal-Aguilera K, Perez-Vazquez V, Gonzalez-Alvarez R, and Velazquez-Arellano A. (2010). Biotin starvation with adequate glucose provision causes paradoxical changes in fuel metabolism gene expression similar in rat (Rattus norvegicus), nematode (Caenorhabditis elegans) and yeast (Saccharomyces cerevisiae). J Nutrigenet Nutrigenomics 3, 18–30. [DOI] [PubMed] [Google Scholar]
- 70.Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, Miteva YV, Hauri S, Sardiu ME, Low TY, et al. (2013). The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nature Methods 10, 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, and Sherlock G. (2004). GO::TermFinder--open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 20, 3710–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaye DD, and Greenwald I. (2011). OrthoList: A Compendium of C. elegans Genes with Human Orthologs. PLoS ONE 6, e20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J, Haider S, Baran J, Cros A, Guberman JM, Hsu J, Liang Y, Yao L, and Kasprzyk A. (2011). BioMart: a data federation framework for large collaborative projects. Oxford University Press; 2011, bar038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dickinson DJ, Pani AM, Heppert JK, Higgins CD, and Goldstein B. (2015). Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics 200, 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Tang NH, Lara-Gonzalez P, Zhao Z, Cheerambathur DK, Prevo B, Chisholm AD, Desai A, and Oegema K. (2017). A toolkit for GFP-mediated tissue-specific protein degradation in C. elegans. Development 144, 2694–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shelton CA, and Bowerman B. (1996). Time-dependent responses to glp-1-mediated inductions in early C. elegans embryos. Development 122, 2043–2050. [DOI] [PubMed] [Google Scholar]
- 77.Leung B, Hermann GJ, and Priess JR (1999). Organogenesis of the Caenorhabditis elegans Intestine. Developmental Biology 216, 114–134. [DOI] [PubMed] [Google Scholar]
- 78.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, et al. (2020). SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods 17, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, et al. (2020). Array programming with NumPy. Nature 585, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKinney W. (2010). Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference 445, 51–56. [Google Scholar]
- 81.Waskom ML (2021). seaborn: statistical data visualization. Journal of Open Source Software 6, 3021. [Google Scholar]
- 82.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waterhouse AM, Procter JB, Martin DMA, Clamp M, and Barton GJ (2009). Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Talavera G, and Castresana J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol 56, 564–577. [DOI] [PubMed] [Google Scholar]
- 85.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, and Gascuel O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol 59, 307–321. [DOI] [PubMed] [Google Scholar]
- 86.Lefort V, Longueville J-E, and Gascuel O. (2017). SMS: Smart Model Selection in PhyML. Mol. Biol. Evol 34, 2422–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: PTRN-1 proximal interactors with enriched Gene Ontology Function terms, SAINT and Fold Change scores, related to Figure 1.
Table S1: Phylogenic analysis accession numbers, related to Figure 2.
Video S1. EBP-2::GFP comet dynamics, related toFigure 4. Dorsal view of live, bean stage C. elegans embryos of indicated genotypes with intestine outlined (white dotted line). 10-second stream acquisition of EBP-2::GFP.
Video S2. Actin perturbation liberates apical microtubules, related toFigure 7. Dorsal view of live, bean stage C. elegans embryo expressing GFP::TBB-2/β-tubulin filmed immediately after treatment with 10 Latrunculin A and 10ug/mL Cytochlasin D. Pharynx (blue dotted line) and intestine (white dotted line) are indicated. Note the division of the anterior intestinal cells (asterisk). Scale bar = 10 μm.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
All original code is available at https://github.com/JacobsWagnerLab/published/tree/master/Sanchez_2020 and is publicly available as of the date of publication. All scripts are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rat anti-HA | Roche | Cat#11867423001 |
| goat anti-rat IRDye 680RD | Licor | Cat#925-68076; Lot#C61115-06 |
| streptavidin-IRDye 800CW | Licor | Cat#925-32230; Lot#C60913-04 |
| mouse anti-HA | Abcam | Cat#ab130275 [16B12]; Lot#GR250145-5 |
| mouse anti-Myc | Abcam | Cat#ab32[9E10]; Lot#GR3272830 |
| rabbit anti-GFP | Abcam | Cat#ab6556; Lot#6R3271077-1 |
| mouse anti-PAR-3 | DSHB | Cat#p4a1; RRID: AB528424 |
| rabbit anti-GIP-1 | GenScript7 | N/A |
| CY3-anti-mouse | Jackson Immunoresearch Laboratories | Cat#115-165-166; Lot#117091 |
| streptavidin Alexa Fluor 488 | Invitrogen | Ref#532354; Lot#1719656 |
| DAPI | Sigma | N/A |
| 647-anti-rabbit | Jackson Immunoresearch Laboratories | Cat#711-605-152 |
| 488-anti-mouse | Jackson Immunoresearch Laboratories | Cat#115-545-166 |
| 488 anti-rabbit | Thermofisher scientific | Cat#A11034 |
| Cy5 anti-mouse | Jackson Immunoresearch Laboratories | Cat#715-175-151 |
| Bacterial and virus strains | ||
| E. coli (MG1655 bioB::kan) biotin auxotrophic food | Dr. John E. Cronan, University of Illinois | N/A |
| E. coli OP50 standard food | CGC | N/A |
| E. coli NA22 standard food | CGC | N/A |
| Critical commercial assays | ||
| Pierce 660nm Protein Assay Kit | Thermo Fisher Scientific | Cat#22662 |
| Pierce Streptavidin Magnetic Beads | Thermo Fisher Scientific | Cat#88817 |
| Q5® Site-Directed Mutagenesis kit | New England BioLabs | Cat#E0554S |
| NEBuilder® HiFi DNA Assembly | New England BioLabs | Cat#E5520S |
| Deposited data | ||
| control_df (compressed.csv with microtubule positions and angles in the wild type C. elegans embryos) | This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/control_df |
| VAB_10Bgut_minus_df (compressed.csv with microtubule positions and angles in the VAB-10Bgut(−) C. elegans embryos) | This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/VAB_10Bgut_minus_df |
| WDR_62gut_minus_df (compressed.csv with microtubule positions and angles in the WDR-62gut(−) C. elegans embryos) | This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/WDR_62gut_minus_df |
| Experimental models: Organisms/strains | ||
| C. elegans strain N2 wild-type | CGC | N/A |
| C. elegans strain JLF696: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; ptrn-1(wow4[ptrn-1::gfp]) X; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF291: wowEx68(myo-2p::mCherry::unc-54 3’UTR; ges-1p::3xHA::TurboID::unc-54 3’UTR) | 25 | CGC |
| C. elegans strain JLF337: wowIs24(myo-2p::mCherry::unc-54 3’UTR;ges-1p::ptrn-1a::3xHA::TurboID::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF371: wowIs26(myo-2p::mCherry::unc-54 3’UTR; ges-1p::ptrn-1a::3xHA::TurboID::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF372: wowIs17(myo-2p::mCherry::unc-54 3’UTR; ges-1p::3xHA::TurboID::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF525: vab-10b(wow80[vab-10b::zf::gfp]) I; zif-1(gk117) III | This study | N/A |
| C. elegans strain JLF551: zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF504: zif-1(gk117) III; par-1(wow87[par-1::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF690: zif-1(gk117) III; sma-1(wow102[sma-1::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF621: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; eps-8(wow101[eps-8a::zf::gfp]) IV | This study | N/A |
| C. elegans strain JLF15: ptrn-1(wow4[ptrn-1::gfp]) X | This study | N/A |
| C. elegans strain TV26135: zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wyIs900(unc-122p::rfp; lin-32p::mCherry::PLCdeltaPH) | This study | N/A |
| C. elegans strain JLF644: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF589: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF666: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF685: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF701: ebp-2(wow47[ebp-2::gfp]) II; par-3(it300[par-3::mCherry]) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study/CGC | N/A |
| C. elegans strain JLF599: vab-10b(wow80[vab-10b::zf::gfp]) I; ebp-2(wow47[ebp-2::gfp]) II; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF718: ebp-2(wow47[ebp-2::gfp]) II; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF667: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; gip-1[wow3(gfp::gip-1)] zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF597: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; gip-1[wow3(gfp::gip-1)] zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF658: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; gip-1[wow3(gfp::gip-1)] zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF699: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; ptrn-1(wow4[ptrn-1::gfp]) X; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF700: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; wdr-62(wow94[wdr-62::zf::gfp]) V; ptrn-1(wow4[ptrn-1::gfp]) X; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF714: vab-10b(wow80[vab-10b::zf::gfp]) I; zif-1(gk117) III; wdr-62(wow111[wdr-62::tag-rfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF439: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; zif-1(gk117) III | This study/CGC | N/A |
| C. elegans strain JLF473: vab-10b(wow80[vab-10b::zf::gfp]) I; ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; zif-1(gk117) III | This study | N/A |
| C. elegans strain JLF657: tbb-2(tj26[gfp::tbb-2]) unc-119(ed3) par-3(it300[par-3::mCherry]) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF757: wowIs28(myo-2p::mCherry::unc-54 3’UTR ;elt-2p::zif-1::unc-54 3’UTR;end-1p::histone1::mCherry)II; par-3(wow59[par-3::zf::gfp]) zif-1(gk117) III/qC1; wdr-62(wow111[wdr-62::tag-rfp]) V | This study | N/A |
| C. elegans strain JLF442: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study/CGC | N/A |
| C. elegans strain SA899: tbb-2(tj26[gfp::tbb-2]) unc-119(ed3) III; tjIs72[pie-1p::cherry::h2b, pie-1p::cherry::tbg-1, unc-119(+)] | Asako Sugimoto/CGC | N/A |
| C. elegans strain JLF673: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF681: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; sma-1(wow102[sma-1::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF616: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II ;zif-1(gk117) III; sma-1(wow102[sma-1::zf::gfp]) V | This study | N/A |
| C. elegans strain JLF694: eps-8(wow101[eps-8a::zf::gfp]) IV | This study | N/A |
| C. elegans strain JLF692: ItSi910[elt-2p::vhhgfp4::zif-1::operon-linker::mCherry::histone::tbb-2 3’UTR; cb-unc-119(+)] II; eps-8(wow101[eps-8a::zf::gfp])/tmC9 IV | This study | N/A |
| C. elegans strain JLF1024: zif-1(gk117) III; wow112(zf::gfp::wdr-62) V | This study | N/A |
| C. elegans strain JLF648: sma-1(wow102[sma-1::zf::gfp]) V; TH110[pie-1p::par-6::mCherry] | This study | N/A |
| C. elegans strain JLF1021: vab-10b(wow157[vab-10b::gfp::sqt-1::hygR]) I; wowIs28(myo-2p::mCherry::unc-54 3’UTR; elt-2p::zif-1::unc-54 3’UTR; end-1p::histone1::mCherry) II; par-3(wow59[par-3::zf::gfp]) zif-1(gk117) III/qC1 | This study | N/A |
| C. elegans strain JLF1026: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1027: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1028: zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1029: vab-10b(wow80[vab-10b::zf::gfp]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR); opIs310[ced-1p::yfp::act-5::let-853 3’UTR + unc-119(+)] | This study | N/A |
| C. elegans strain JLF1030: vab-10b(wow157[vab-10b::gfp::sqt-1::hygR]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| C. elegans strain JLF1031: vab-10b(wow157[vab-10b::gfp::sqt-1::hygR]) I; zuIs278(pie-1p::cherry::tba-1::pie-1 3’UTR) II; zif-1(gk117) III; wdr-62(wow94[wdr-62::zf::gfp]) V; wowEx111(myo-2p::mCherry; end-1p::bfp::caax; elt-2p::zif-1::unc-54 3’UTR) | This study | N/A |
| Oligonucleotides | ||
| Sequences for CRISPR/Cas9 edits, see Table S4 | This paper | N/A |
| Recombinant DNA | ||
| Plasmid pAS31 [ges-1p::3xHA::TurboID::unc-54 3’UTR] | Addgene | Addgene plasmid # 118220; http://n2t.net/addgene:118220; RRID: Addgene_118220 |
| Plasmid pAS33 [ges-1p::ptrn-1a::3xHA::TurboID::unc-54 3’UTR] | This study | N/A |
| Plasmid SA109 [elt-2p::zif-1::unc-54 3’UTR] | Addgene | Addgene plasmid # 59783; http://n2t.net/addgene:59783; RRID: Addgene_59783 |
| Plasmid pMP1 [end-1p::mtag::bfp::caax] | This study | N/A |
| Plasmid pCFJ90 [myo-2p::mcherry::unc-54 3’UTR] | Addgene | Addgene plasmid # 19327; http://n2t.net/addgene:19327; RRID:Addgene_19327 |
| Plasmid pJF248 [end-1p::histone1::mcherry] | This study | N/A |
| Plasmid pJL188 [lin-32p::mcherry::PLCΔPH] | This study | N/A |
| Plasmid pDD162 [eft-3p::Cas9 + Empty sgRNA] | Addgene | Addgene plasmid # 47549; http://n2t.net/addgene:47549; RRID: Addgene_47549 |
| Plasmid pJF250 [ZF::GFP::3xFlag Empty repair template] | 20 | N/A |
| Plasmid pDD282 [GFP::3xFlag Empty repair template] | Addgene | Addgene plasmid # 66823; http://n2t.net/addgene:66823; RRID: Addgene_66823 |
| Plasmid pDD286 [TagRFP-T::3xMyc Empty repair template] | Addgene | Addgene plasmid # 70684; http://n2t.net/addgene:70684; RRID: Addgene_70684 |
| Plasmid pAS45 [eft-3p::Cas9 + vab-10b sgRNA] | This paper | N/A |
| Plasmid pAS62 [eft-3p::Cas9 + wdr-62 sgRNA] | This paper | N/A |
| Plasmid pAS78 [eft-3p::Cas9 + wdr-62 sgRNA] | This paper | N/A |
| Plasmid pMP44 [eft-3p::Cas9 + par-1 sgRNA] | This paper | N/A |
| Plasmid pMP15 [eft-3p::Cas9 + par-3 sgRNA] | This paper | N/A |
| Plasmid pLC003 [eft-3p::Cas9 + sma-1 sgRNA] | This paper | N/A |
| Plasmid pLC007 [eft-3p::Cas9 + eps-8 sgRNA] | This paper | N/A |
| Plasmid pAS46 [ZF::GFP::3xFlag with vab-10b repair template] | This paper | N/A |
| Plasmid pAS61 [ZF::GFP::3xFlag with wdr-62 repair template] | This paper | N/A |
| Plasmid pAS82 [ZF::GFP::3xFlag with wdr-62 repair template] | This paper | N/A |
| Plasmid pAS77 [TagRFP-T::3xMyc with wdr-62 repair template] | This paper | N/A |
| Plasmid pMP43 [ZF::GFP::3xFlag with par-1 repair template] | This paper | N/A |
| Plasmid pMP14 [ZF::GFP::3xFlag with par-3 repair template] | This paper | N/A |
| Plasmid pLC002 [ZF::GFP::3xFlag with sma-1 repair template] | This paper | N/A |
| Plasmid pLC006 [ZF::GFP::3xFlag with eps-8 repair template] | This paper | N/A |
| Plasmid pAS57 [GFP::3xFlag with vab-10b repair template] | This paper | N/A |
| Software and algorithms | ||
| Image J (version 2.1.0) | NIH | https://imagej.nih.gov/ij/ |
| Prism 9 for macOS (version 9.1.0) | GraphPad | https://www.graphpad.com |
| NIS elements | Nikon Instruments | https://www.microscope.healthcare.nikon.com/products/software/nis-elements |
| Illustrator | Adobe | https://www.adobe.com |
| InDesign | Adobe | https://www.adobe.com |
| Photoshop | Adobe | https://www.adobe.com |
| WDSPdb 2.0 | 66 | http://www.wdspdb.com/wdsp/ |
| JalView v2.11.1.0 | 83 | https://www.jalview.org |
| Clustel Omega v1.2.2 | 82 | https://www.ebi.ac.uk/Tools/msa/clustalo/ |
| Gblocks v0.91 | 84 | http://molevol.cmima.csic.es/castresana/Gblocks.html |
| PhyML 3.0 | 85 | http://www.atgc-montpellier.fr/phyml/ |
| SMS for PhyML | 86 | http://www.atgc-montpellier.fr/sms/ |
| FigTree v1.4.4 | Andrew Rambaut | http://tree.bio.ed.ac.uk/software/figtree/ |
| Worm tissue expression prediction server | 30 | http://worm.princeton.edu/ |
| GO term finder | 71 | https://go.princeton.edu/cgi-bin/GOTermFinder |
| Biomart | 73 | http://biomart.org |
| STRING | 32 | http://string-db.org |
| Python 3.7, Sanchez_etal_python_environment.yaml The Python 3.7 environment used to run all the analysis. This.yaml file includes all the package versions and dependencies. |
https://www.anaconda.com | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/Sanchez_etal_python_environment.yaml |
| Python 3.7, microtubule_tracking_class.py A class that includes all the microtubule segmentation and tracking functions |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/microtubule_tracking_class.py |
| Python 3.7, run_microtubule_tracking_class_example.py An example on how to use the ‘microtubule_tracking_class’ |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/run_microtubule_tracking_class_example.py |
| Python 3.7, Sanchez_etal_figure_functions.py The functions used to plot Figures 4E, S6G-H |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/Sanchez_etal_figure_functions.py |
| Python 3.7, Sanchez_etal_plot_figures.py The script which runs the ‘Sanchez_etal_figure_functions’ to plot the data |
This paper | https://github.com/JacobsWagnerLab/published/blob/master/Sanchez_2020/Sanchez_etal_plot_figures.py |