ABSTRACT
Streptococcus suis is an emerging zoonotic pathogen that causes severe swine and human infections. Metals are essential nutrients for life; however, excess metals are toxic to bacteria. Therefore, maintenance of intracellular metal homeostasis is important for bacterial survival. Here, we characterize a DtxR family metalloregulator, TroR, in S. suis. TroR is located upstream of the troABCD operon, whose expression was found to be significantly downregulated in response to excess manganese (Mn). Deletion of troR resulted in reduced growth when S. suis was cultured in metal-replete medium supplemented with elevated concentrations of zinc (Zn), copper (Cu), or cobalt (Co). Mn supplementation could alleviate the growth defects of the ΔtroR mutant under Zn and Co excess conditions; however, it impaired the growth of the wild-type (WT) and complemented (CΔtroR) strains under Cu excess conditions. The growth of ΔtroR was also inhibited in metal-depleted medium supplemented with elevated concentrations of Mn. Moreover, the ΔtroR mutant accumulated increased levels of intracellular Mn and Co, rather than Zn and Cu. Deletion of troR in S. suis led to significant upregulation of the troABCD operon. Furthermore, troA expression in the WT strain was induced by ferrous iron [Fe(II)] and Co and repressed by Mn and Cu; the repression of troA was mediated by TroR. Finally, TroR is required for S. suis virulence in an intranasal mouse model. Together, these data suggest that TroR is a negative regulator of the TroABCD system and contributes to resistance to metal toxicity and virulence in S. suis.
IMPORTANCE Metals are essential nutrients for life; however, the accumulation of excess metals in cells can be toxic to bacteria. In the present study, we identified a metalloregulator, TroR, in Streptococcus suis, which is an emerging zoonotic pathogen. In contrast to the observations in other species that TroR homologs usually contribute to the maintenance of homeostasis of one or two metals, we demonstrated that TroR is required for resistance to the toxicity conferred by multiple metals in S. suis. We also found that deletion of troR resulted in significant upregulation of the troABCD operon, which has been demonstrated to be involved in manganese acquisition in S. suis. Moreover, we demonstrated that TroR is required for the virulence of S. suis in an intranasal mouse model. Collectively, these results suggest that TroR is a negative regulator of the TroABCD system and contributes to resistance to metal toxicity and virulence in S. suis.
KEYWORDS: Streptococcus suis, TroR, TroABCD, repression, metal toxicity, virulence
INTRODUCTION
Metals, such as iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu), are essential nutrients for almost all organisms because of their role as enzymatic cofactors or protein structural components (1, 2). Humans and other mammals can sequester and/or mobilize essential metals to make them unavailable to invading pathogens (3). This process, termed nutritional immunity, is an important strategy used by the host to respond to bacterial infection (3, 4). To overcome this and infect the host, bacteria have developed high-affinity metal transporters for acquiring metals from diverse sources (5, 6). Although the effective uptake of metals is critical for bacterial survival within the host, the overaccumulation of metals is detrimental to bacterial cells (6). Emerging evidence indicates that hosts also utilize metal toxicity as an immune defense mechanism against bacterial pathogens (7, 8). As a countermeasure, bacteria have evolved complex mechanisms, such as the efflux of excess metals, to maintain metal homeostasis (8). As such, there is increasing evidence that metal homeostasis is essential for the physiology and/or pathogenesis of bacterial pathogens (9–16).
Streptococcus suis is an emerging zoonotic bacterial pathogen that is associated with severe swine and human diseases, such as meningitis, septicemia, and endocarditis (17, 18). Among the 29 serotypes of S. suis (1 to 19, 21, 23 to 25, 27 to 31, and 1/2) described according to the capsular polysaccharides, S. suis serotype 2 (S. suis 2) is the most prevalent serotype and is the most common cause of swine and human infections worldwide (19). S. suis was the most prevalent bacterial pathogen to be isolated from pig farms in China from 2013 to 2017 (isolation rate, 16.9%) and the third most common bacterial pathogen to be isolated from porcine laboratory submissions in New Zealand from June 2003 to February 2016 (isolation rate, 5.5%) (20, 21). Moreover, in one study, it could be isolated from 95% of the sampled Swedish grower pigs (22). While S. suis is responsible for huge economic losses in the swine industry worldwide, it poses serious threats to public health. Since the report of a human case of S. suis infection in Denmark in 1968, human cases have reached over 1,600 worldwide by the end of 2013; some of these were fatal (23). Sporadic cases of S. suis infections in humans have been frequently reported worldwide in recent years as well (24–29). Thus, a better understanding of the physiology and pathogenesis of S. suis is urgent for the control of the infection caused by this bacterium.
It has been established that metal acquisition and the maintenance of metal homeostasis are important for the physiology and pathogenesis of S. suis. Dpr-mediated removal of intracellular ferrous iron [Fe(II)] protects S. suis against H2O2-induced oxidative stress (30). The Fe transporter FeoB and two cation-uptake regulators (AdcR and Fur) contribute to the virulence of S. suis (31, 32). Both the Mn acquisition system TroA and efflux system MntE are involved in counteracting oxidative stress and virulence in S. suis (33, 34). Recently, we showed that PmtA, an Fe(II) and cobalt (Co) efflux system in S. suis, plays a role in resistance to H2O2-induced oxidative stress (16). We also demonstrated that CopA, a Cu-transporting ATPase, protects S. suis against Cu-induced bactericidal effect (35). In addition, Zur, a Zn uptake regulator, is involved in S. suis response to Zn toxicity (36). Despite some progress, the mechanisms underlying metal acquisition and the maintenance of metal homeostasis in S. suis remain poorly understood. For example, little is known about the regulators by which S. suis senses and responds to excess metals other than Zn.
In attempts to explore the transcriptome change in S. suis in response to excess Mn and to elucidate the mechanisms underlying the maintenance of Mn homeostasis, we identified a DtxR family metalloregulator, TroR, in S. suis. Unlike the homologs in other species that are generally specific to Mn, S. suis TroR contributes to resistance to the toxicity conferred by Mn, Zn, Cu, and Co. We also investigated the regulon of TroR in S. suis and found that TroR negatively regulates the troABCD operon. More importantly, TroR is required for the virulence of S. suis in an intranasal mouse model.
RESULTS
S. suis downregulates the expression of the troABCD operon in response to excess Mn.
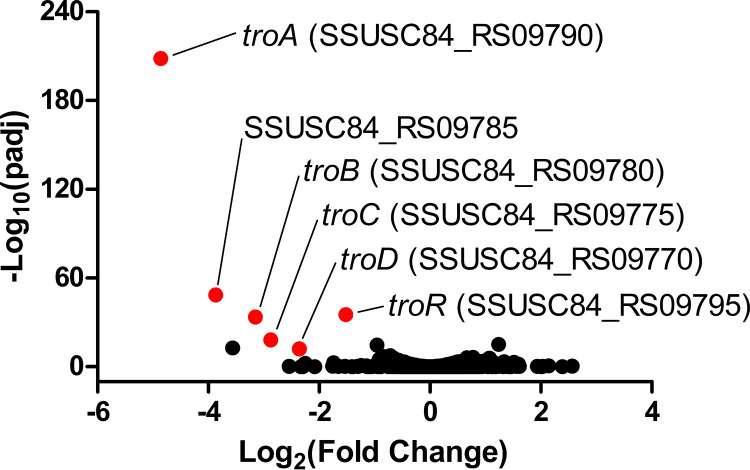
To explore the mechanisms underlying the maintenance of Mn homeostasis, we performed transcriptome analysis of S. suis cultured in the presence of 1 mM MnSO4 in comparison with that cultured in the presence of water by RNA sequencing. Most S. suis genes exhibited no significant difference in expression levels following treatment with Mn. Of the 25 genes that were significantly differentially expressed (fold change of >2, adjusted P value [Padj] of <0.05), 16 genes were upregulated, and the remaining nine genes were downregulated (see Table S1 in the supplemental material). There was a 5.12- to 29.05-fold decrease in the expression levels of genes encoding a well-characterized ABC transporter, TroABCD (Fig. 1 and Table S1). Moreover, there was a 2.87-fold decrease in the expression level of troR, a gene predicted to encode a transcriptional repressor of the troABCD operon, after treatment with Mn (Fig. 1 and Table S1). TroA has the ability to bind Mn and Zn and is required for Mn acquisition in S. suis (33, 37). We hypothesized that the repression of the TroABCD system by TroR is an important mechanism by which S. suis maintains Mn and Zn homeostasis.
FIG 1.
The S. suis transcriptome is changed in response to excess Mn. The S. suis strain SC19 was grown to the mid-exponential phase and exposed to 1 mM MnSO4 or deionized water. After 15 min of treatment, RNA was isolated and subjected to RNA sequencing analysis. The figure shows the transcription profile of S. suis in the presence of Mn in comparison with that in the presence of water. The genes with a fold change of >2 and an adjusted P value (Padj) of <0.05 were defined as differentially expressed genes.
In silico analysis of TroRABCD-like systems in S. suis and other species.
TroR is one of the DtxR family metalloregulators; members of this family have been extensively characterized in streptococci and several other prokaryotes (38). Phylogenetic comparison of S. suis TroR with the previously identified DtxR family metalloregulators revealed that S. suis TroR is very closely related to its homologs in streptococci, including Streptococcus pyogenes MtsR (39–41), Streptococcus pneumoniae PsaR (42–45), Streptococcus gordonii ScaR (46, 47), Streptococcus parasanguinis FimR (48), and Streptococcus mutans SloR (49–51) (see Fig. S1 in the supplemental material). Protein sequence alignment analysis revealed that S. suis TroR shares approximately 54 to 60% amino acid identity to its homologs in these streptococci.
The genetic structures of TroRABCD-like systems vary across streptococci (Fig. 2). In S. suis and S. pyogenes, the troR homologs and troABCD-like operons are adjacent, but transcribed in opposite directions (Fig. 2). In S. pneumoniae and S. gordonii, the troR homologs are far away from the troABCD-like operons (Fig. 2). Unlike the genetic organization in these species, the troR homologs and troABCD-like operons are adjacent and transcribed in the same direction in S. parasanguinis and S. mutans (Fig. 2). In S. pneumoniae, S. gordonii, and S. parasanguinis, pepO (encoding metalloendopeptidase) and tpx (encoding thiol peroxidase) are located upstream and downstream of the troABCD-like operons, respectively (Fig. 2). While only pepO is located downstream of the troABCD operon in S. suis, neither of the two genes is located upstream or downstream of the troABCD-like operons in S. pyogenes and S. mutans (Fig. 2).
FIG 2.
Genetic structures of TroRABCD-like systems in streptococci. Arrows indicate the direction of transcription, and break lines represent great distance in the genome. Genetic structure analyses were according to the following genomes: S. suis SC84, NC_012924.1; S. pyogenes M1, NC_002737.2; S. pneumoniae D39, NC_008533.2; S. gordonii CH1, NC_009785.1; S. parasanguinis FW213, NC_017905.1; and S. mutans UA159, NC_004350.2.
TroR is required for S. suis resistance to Zn, Co, and Cu toxicity during growth in metal-replete medium.
To investigate the role of TroR, we created a troR deletion mutant (ΔtroR) (see Fig. S2A in the supplemental material) from the S. suis 2 strain SC19 (52). A functional complementation strain of the mutant (CΔtroR), in which troR was placed under its native promoter, was also generated. The two strains were verified by PCR (Fig. S2B), reverse transcription-PCR (Fig. S2C), and DNA sequencing (data not shown).
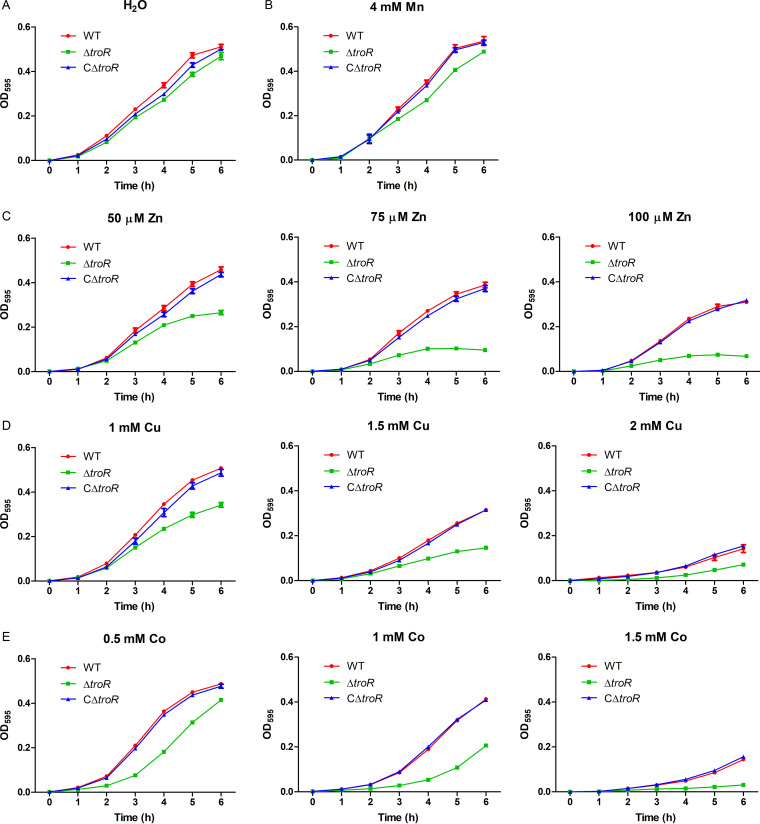
TroA, a lipoprotein potentially repressed by TroR, can interact with Mn and Zn and is involved in Mn acquisition in S. suis (33, 37). The homologs of TroR control Mn homeostasis in some prokaryotes, such as S. pyogenes (39), Staphylococcus aureus (11), Mycobacterium tuberculosis (53), and Treponema denticola (54). Considering these observations, we first assessed the role of TroR in Mn and Zn homeostasis in S. suis. The wild-type (WT), ΔtroR mutant, and CΔtroR complemented strains were grown in TSBS (tryptic soy broth supplemented with newborn bovine serum) medium (metal replete) supplemented with or without elevated concentrations of Mn/Zn, and their growth was evaluated. In the absence of metal supplementation, the three strains exhibited similar growth curves (Fig. 3A). Compared with the WT and CΔtroR strains, the ΔtroR strain exhibited only a moderate growth defect in the presence of a high concentration of Mn (4 mM) (Fig. 3B). However, the ΔtroR strain showed a growth defect in the presence of as little as 50 μM Zn; the level of inhibition was more severe in the presence of higher concentrations of Zn (Fig. 3C).
FIG 3.
TroR is required for S. suis growth in metal-replete medium supplemented with excess Zn, Cu, or Co. The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium supplemented with deionized water (A), 4 mM Mn (B), or various concentrations of Zn (C), Cu (D), or Co (E). The strains were grown in 96-well plates (200 μl per well) at 37°C with linear shaking, and the OD595 values were measured hourly. At least three independent experiments were performed; the data shown are the means ± standard deviations (SDs) from three wells in a representative experiment.
To assess whether TroR is also involved in resistance to the toxicity conferred by other metals, the growth curves of the three strains were also assessed in the presence of elevated concentrations of Fe(II), Cu, Co, nickel (Ni), calcium (Ca), or magnesium (Mg). The ΔtroR strain displayed obvious growth inhibition when supplemented with Cu or Co (Fig. 3D and E). However, it showed only a moderate growth defect in the presence of high concentrations of Fe(II), Ni, Ca, or Mg (4 mM) (see Fig. S3 in the supplemental material).
Taken together, the ΔtroR mutant exhibited obviously impaired growth in TSBS medium supplemented with Zn, Co, and Cu, indicating that TroR protects S. suis against Zn, Co, and Cu toxicity under metal-replete conditions.
Mn supplementation improves the growth of ΔtroR under Zn or Co excess conditions.
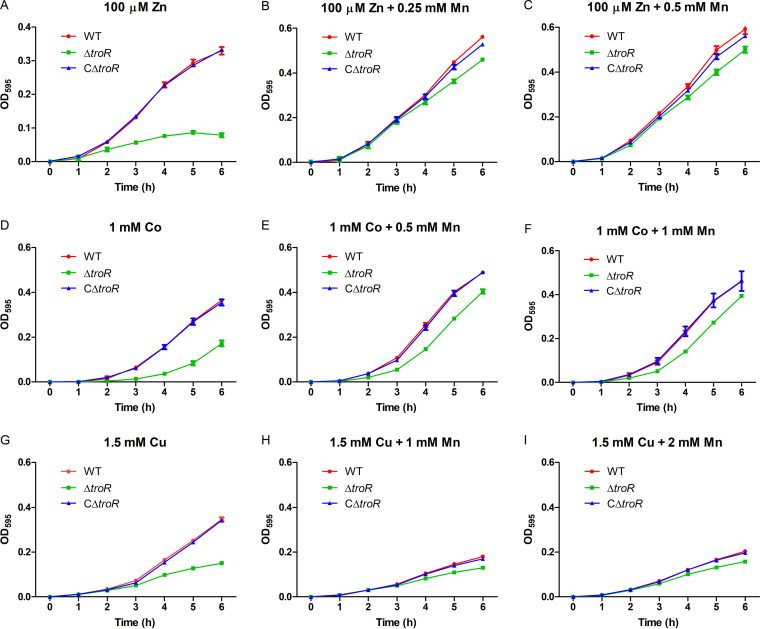
In a previous study, we found that the growth defect of a ΔpmtA mutant under Fe(II) or Co excess conditions could be alleviated by the addition of Mn (16). Accordingly, we sought to determine whether Mn supplementation could alleviate the growth defect of the ΔtroR mutant under Zn, Cu, and Co conditions. While the growth of the ΔtroR mutant was markedly inhibited by 100 μM Zn, Mn supplementation improved the growth of the ΔtroR strain under Zn excess conditions (Fig. 4A to C). Similarly, Mn supplementation alleviated the growth defect of the ΔtroR strain under Co excess conditions; however, the effect was not as significant as that under Zn excess conditions (Fig. 4D to F). Unlike the observations under Zn and Co excess conditions, Mn supplementation had no rescue effect on the growth defect of the ΔtroR strain under Cu excess conditions. Moreover, Mn addition resulted in impaired growth of the WT and CΔtroR complemented strains (Fig. 4G to I).
FIG 4.
Mn supplementation rescues the growth defect of the ΔtroR mutant under Zn or Co excess conditions. (A to C) The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium supplemented with 100 μM Zn alone (A), 100 μM Zn with 0.25 mM Mn (B), or 0.5 mM Mn (C). (D to F) The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium supplemented with 1 mM Co alone (D), 1 mM Co with 0.5 mM Mn (E), or 1 mM Mn (F). (G to I) The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium supplemented with 1.5 mM Cu alone (G), 1.5 mM Cu with 1 mM Mn (H), or 2 mM Mn (I). The strains were grown in 96-well plates (200 μl per well) at 37°C with linear shaking, and the OD595 values were measured hourly. At least three independent experiments were performed; the data shown are the means ± SDs from three wells in a representative experiment.
TroR contributes to S. suis growth under Mn excess conditions when cultured in metal-depleted medium.
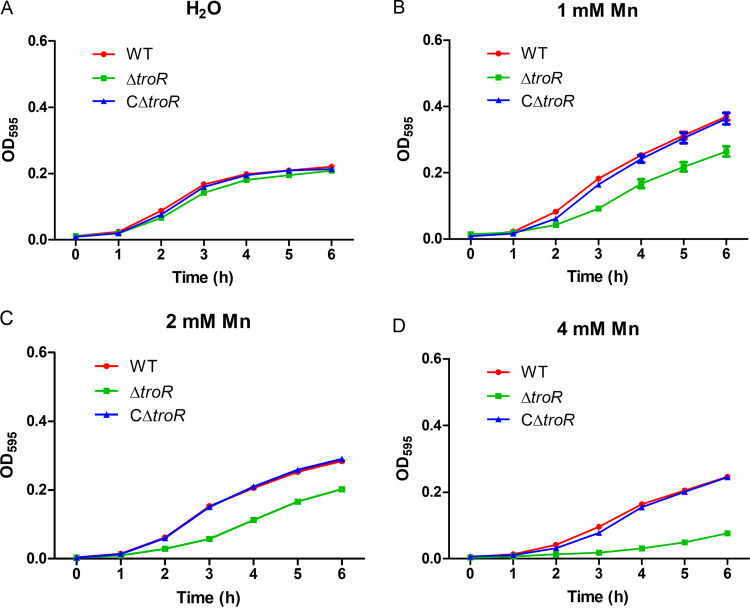
Because TroR homologs contribute to Mn homeostasis in other prokaryotes (11, 39, 53, 54), and the toxicity of a metal might be rescued by another metal, we hypothesized that in metal-replete medium, the effect of Mn supplementation on the growth of the ΔtroR mutant might be masked by other metals existing in this medium. To test this hypothesis, we monitored the growth of the WT, ΔtroR, and CΔtroR complemented strains in Chelex-treated TSBS medium (metal depleted) supplemented with elevated concentrations of Mn. In the absence of Mn, the growth rates of the three strains were almost comparable, with a very slight growth defect being observed in the ΔtroR strain (Fig. 5A). However, when supplemented with various concentrations of Mn, the ΔtroR strain displayed remarkable growth inhibition in comparison with the WT and CΔtroR strains (Fig. 5B to D). These results indicate that TroR is required for S. suis resistance to Mn toxicity during growth under metal-depleted conditions.
FIG 5.
TroR is required for S. suis growth in metal-depleted medium supplemented with excess Mn. The WT, ΔtroR, and CΔtroR strains were grown in Chelex-treated TSBS medium supplemented with deionized water (A), 1 mM Mn (B), 2 mM Mn (C), or 4 mM Mn (D). The strains were grown in 96-well plates (200 μl per well) at 37°C with linear shaking, and the OD595 values were measured hourly. At least three independent experiments were performed; the data shown are the means ± SDs from three wells in a representative experiment.
TroR deletion results in increased levels of intracellular Mn and Co, rather than Zn and Cu.
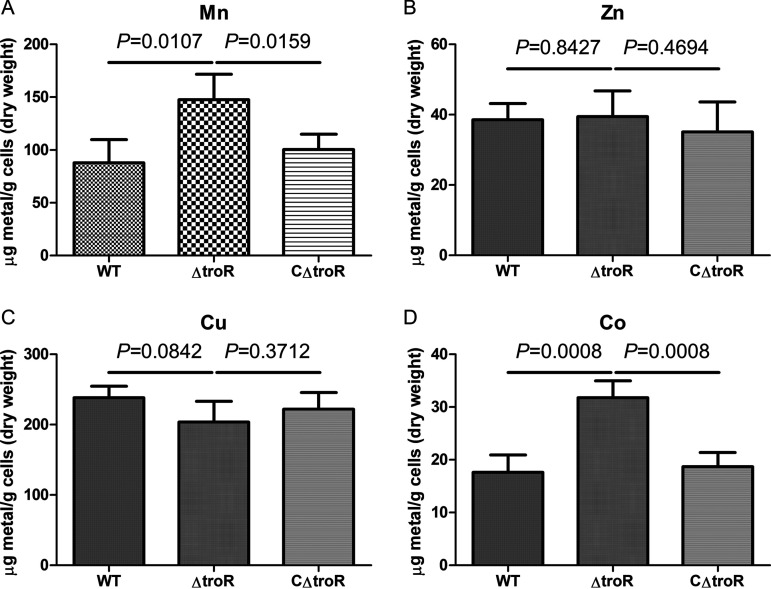
To better understand the mechanism underlying the role of TroR in resistance to metal toxicity, we analyzed the levels of intracellular metals in the WT, ΔtroR mutant, and CΔtroR complemented strains by inductively coupled plasma-optical emission spectroscopy (ICP-OES). We grew the S. suis strains in metal-replete medium to an optical density at 600 nm (OD600) of 0.6, supplemented the medium with Mn, Zn, Cu, or Co, cultured the cells for another 30 min, and collected the cells for ICP-OES analysis. When cultured in the presence of Mn, the ΔtroR mutant accumulated significantly higher levels of intracellular Mn than the WT and CΔtroR complemented strains (Fig. 6A). Similarly, the level of intracellular Co was significantly higher in the ΔtroR strain than in the WT and CΔtroR strains when cultured in the presence of Co (Fig. 6D). Nevertheless, the three strains accumulated comparable levels of intracellular Zn and Cu when cultured in the presence of Zn and Cu, respectively (Fig. 6B and C). Hence, the high susceptibility of the ΔtroR strain to Mn and Co toxicity might be due to the overload of intracellular Mn and Co, respectively.
FIG 6.
The ΔtroR strain accumulates significantly increased levels of intracellular Mn and Co, rather than Zn and Cu. The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium to an OD600 of 0.6 and supplemented with 4 mM Mn (A), 100 μM Zn (B), 1.5 mM Cu (C), or 1 mM Co (D). The cultures were incubated for another 30 min, and bacterial cells were then collected to measure the metal contents. The metal content was expressed as μg of metal per g of cells (dry weight). The data shown are the means ± SDs from four biological samples.
RNA sequencing analysis of the genes regulated by TroR in S. suis.
To identify the regulon of TroR in S. suis and to elucidate the mechanisms underlying TroR-mediated resistance to metal toxicity, we assessed the global gene transcription profiles of the WT and ΔtroR mutant strains grown in TSBS medium by RNA sequencing. In total, 20 genes were significantly differentially expressed in the ΔtroR strain, with 16 genes being upregulated and the remaining four genes being downregulated (Table 1). The gene encoding a serine protease was the most upregulated gene in the ΔtroR strain (183.03-fold) (Table 1). As expected, the genes in the troABCD operon were upregulated by approximately 10.20- to 30.03-fold in the ΔtroR strain (Table 1). The gene encoding CopA, a Cu efflux system (35), was upregulated by approximately 2.29-fold in the mutant (Table 1). Moreover, pmtA, which encodes an Fe(II) and Co efflux pump in S. suis (16), was also upregulated in the ΔtroR strain (3.67-fold) (Table 1). We found that three of the downregulated genes (i.e., GeneID no. SSUSC84_RS08465, SSUSC84_RS08475, and SSUSC84_RS08495) encode enzymes involved in fatty acid biosynthesis (Table 1).
TABLE 1.
Summary of the differentially expressed genes in ΔtroR compared to the WT strain
| GeneID | Read count for: |
Log2 fold change | Padj | Description | |
|---|---|---|---|---|---|
| ΔtroR mutant | WT | ||||
| SSUSC84_RS08475 | 172.496259 | 1,692.640537 | −3.2946 | 4.98E−05 | Enoyl-[acyl-carrier-protein] reductase FabK |
| SSUSC84_RS08465 | 79.42321497 | 776.5504876 | −3.2894 | 0.032699 | 3-Oxoacyl-[acyl-carrier-protein] reductase |
| SSUSC84_RS08495 | 874.0360026 | 2,634.921548 | −1.592 | 1.92E−07 | Enoyl-CoA hydratase |
| SSUSC84_RS07585 | 3,695.542083 | 6,997.785595 | −0.92111 | 0.032699 | Hypothetical protein |
| SSUSC84_RS01215 | 215.337514 | 106.7483999 | 1.0124 | 0.047586 | α,α-Phosphotrehalase |
| SSUSC84_RS05475 | 181.1103214 | 85.95622599 | 1.0752 | 0.038677 | β-Hexosamidase |
| SSUSC84_RS06535 | 1,163.244077 | 508.8348814 | 1.1929 | 0.00072886 | CopA |
| SSUSC84_RS05645 | 319.8083029 | 135.5352753 | 1.2385 | 0.0019577 | Membrane protein |
| SSUSC84_RS05470 | 235.5409301 | 99.05513486 | 1.2497 | 0.0037403 | Glycoside hydrolase family 3 protein |
| SSUSC84_RS07610 | 1,581.638773 | 626.7822994 | 1.3354 | 9.38E−05 | GtrA family protein |
| SSUSC84_RS04025 | 197.6334627 | 72.90937538 | 1.4387 | 0.00033251 | 4-Oxalocrotonate tautomerase |
| SSUSC84_RS01570 | 307.5378467 | 83.89864933 | 1.874 | 0.016174 | PmtA |
| SSUSC84_RS09765 | 88,487.52168 | 8,676.327877 | 3.3503 | 6.53E−32 | PepO |
| SSUSC84_RS09790 | 33,734.07384 | 2,622.592268 | 3.6851 | 4.54E−37 | TroA |
| SSUSC84_RS09785 | 8,337.89467 | 433.2156108 | 4.2665 | 1.34E−46 | Hypothetical protein |
| SSUSC84_RS10665 | 177.4460472 | 7.880123374 | 4.493 | 4.36E−25 | Hypothetical protein |
| SSUSC84_RS09780 | 30,303.83419 | 1,307.754198 | 4.5343 | 3.60E−52 | TroB |
| SSUSC84_RS09775 | 15,491.15677 | 554.916931 | 4.803 | 9.41E−57 | TroC |
| SSUSC84_RS09770 | 16,903.09614 | 562.798094 | 4.9085 | 9.68E−59 | TroD |
| SSUSC84_RS09305 | 46,886.28412 | 256.1670182 | 7.5159 | 9.56E−08 | Serine protease |
Expression of troA in the WT and ΔtroR strains in response to various metals.
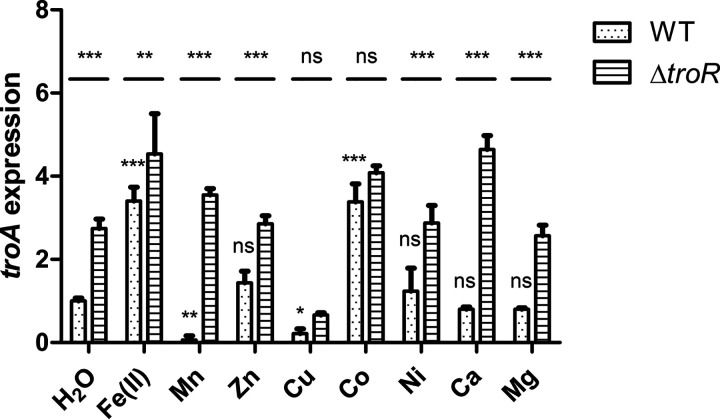
To determine which metals are correlated with troA expression and to further assess the role of TroR in the repression of the troABCD operon, the expression levels of troA in the WT and ΔtroR strains treated with various metals were determined by reverse transcription-quantitative PCR analysis. As reported previously (33, 55), troA expression in the WT strain was significantly induced by Fe(II) and repressed by Mn (Fig. 7). Although TroA has the ability to bind Zn (37), no significant difference in levels of troA expression was detected when the WT strain was treated with Zn (Fig. 7). Similar to the case of Fe(II), Co treatment resulted in approximately 3-fold upregulation of troA in the WT strain (Fig. 7). In contrast, the expression level of troA in the WT strain was significantly downregulated by Cu treatment (Fig. 7). Nevertheless, Ni, Ca, and Mg treatments had no significant effect on the expression level of troA in the WT strain (Fig. 7). TroR functions as a repressor of the troABCD operon; thus, the deletion of troR resulted in the derepression of the troABCD operon. Correspondingly, the expression level of troA in the ΔtroR mutant was commonly higher than that in the WT strain following treatment with or without the metals, with the exception of Cu and Co treatments (Fig. 7). These results indicate that troA expression is induced by Fe(II) and Co and repressed by Mn and Cu and that the repression is mediated by TroR.
FIG 7.
troA expression in the WT and ΔtroR strains in response to various metals. The WT and ΔtroR strains were grown in TSBS to an OD600 of 0.6 and treated for 15 min with 2 mM Fe(II), 2 mM Mn, 50 μM Zn, 1 mM Cu, 1 mM Co, 1 mM Ni, 2 mM Ca, 2 mM Mg, or deionized water. RNA was isolated to perform reverse transcription-quantitative PCR analysis. The data shown are the means ± SDs from three biological triplicates. troA expression in the WT strain in response to the metals was analyzed by one-way analysis of variance with Bonferroni’s posttest. Two-way analysis of variance with Bonferroni’s posttest was used for comparison of troA expression in the ΔtroR mutant with that in the WT strain. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significant difference.
troR deletion attenuates S. suis virulence in an intranasal mouse model.
TroR homologs have been shown to be critical for the virulence of certain bacterial species (39, 44, 56–58). Therefore, we assessed the role of TroR in the virulence of S. suis using two murine infection models. In a mouse model of intraperitoneal infection, four groups of BALB/c mice (10 mice per group) were infected by intraperitoneal injection of 3 × 108 CFU of the WT, ΔtroR mutant, or CΔtroR complemented strain or with phosphate-buffered saline (PBS). After the injections, no clinical symptom was observed in the mice in the PBS-treated group. In contrast, the mice infected with S. suis in the remaining three groups exhibited typical clinical symptoms within 12 h after infection. The levels of the signs of infection were almost comparable among the three groups. Finally, 50% of the mice in the WT group and 40% in the ΔtroR and CΔtroR groups survived over the course of the experiment (see Fig. S4 in the supplemental material). There were no significant differences in the survival rates between the ΔtroR and WT groups (P = 0.7984) and between the ΔtroR and CΔtroR groups (P = 0.7246). Thus, TroR plays no significant role in S. suis virulence in the mouse intraperitoneal infection model.
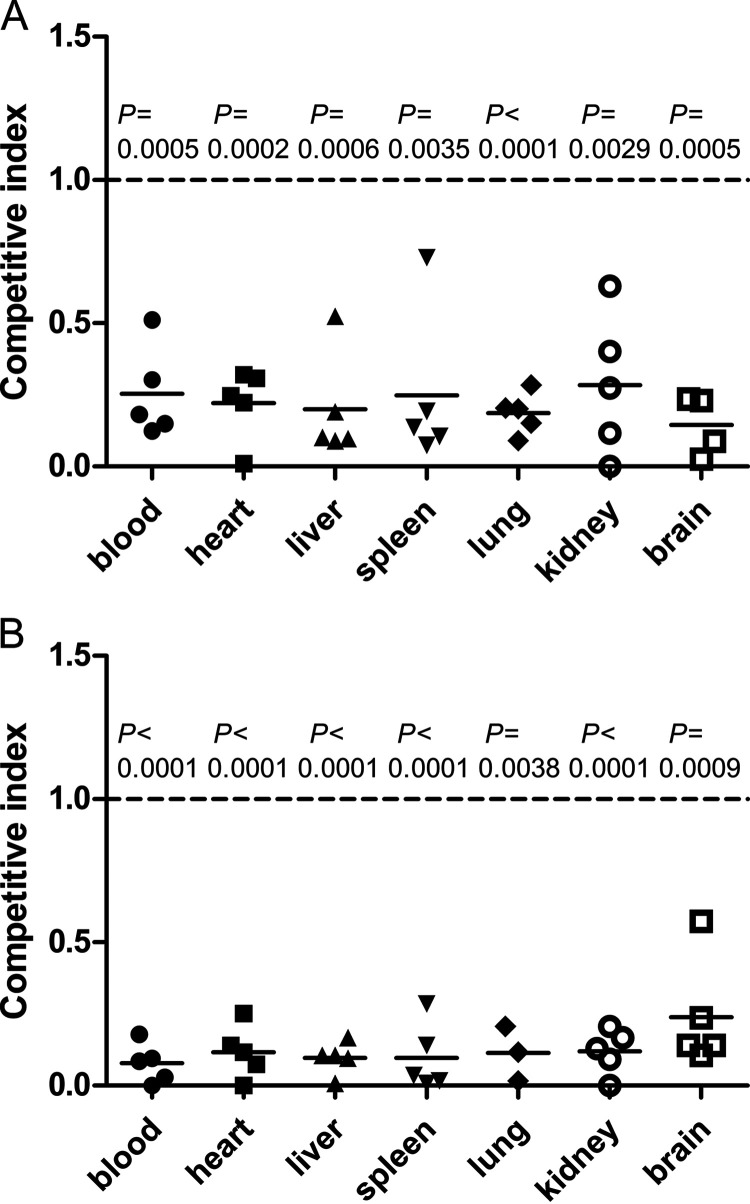
In the competitive-infection assay using a mouse intranasal infection model, groups of five BALB/c mice were intranasally infected with a 1:1 mixture of either the ΔtroR and WT strains or the ΔtroR and CΔtroR strains. The blood, heart, liver, spleen, lung, kidney, and brain samples from the infected mice were collected at 12 h after infection. For each sample collected from mice infected with the mixture of the ΔtroR and WT strains, a total of 70 to 80 colonies were analyzed by colony PCR to determine the competitive index (CI: the ratio of ΔtroR mutant to WT in each sample divided by that in the inoculum). For each sample collected from mice infected with the mixture of the ΔtroR and CΔtroR strains, a total of 70 to 80 colonies were analyzed for spectinomycin susceptibility to determine the CI (the ratio of the ΔtroR mutant to CΔtroR complemented strain in each sample divided by that in the inoculum). The results showed that the CI values were significantly less than 1 for all the samples examined (Fig. 8), suggesting that the ΔtroR mutant had reduced ability to colonize various tissues of mice in this model compared with the WT and CΔtroR strains. Thus, the virulence of the ΔtroR mutant was significantly attenuated in the mouse intranasal infection model.
FIG 8.
Competitive index of the ΔtroR mutant against the WT strain (A) and CΔtroR complemented strain (B) in an intranasal mouse model. Groups of five BALB/c mice were intranasally infected with a 1:1 mixture of either the ΔtroR mutant and the WT strain (group 1) or the ΔtroR and CΔtroR strains (group 2). The blood, heart, liver, spleen, lung, kidney, and brain samples from the infected mice were collected at 12 h after infection. Bacteria recovered from the samples of mice in group 1 were analyzed by colony PCR to determine the competitive index (CI: the ratio of ΔtroR mutant to WT in each sample divided by that in the inoculum). Bacteria recovered from the samples of mice in group 2 were analyzed for spectinomycin susceptibility to determine the CI (the ratio of ΔtroR mutant to CΔtroR complemented strain in each sample divided by that in the inoculum). Mean CI values were compared to 1 (equal competitiveness) using the two-tailed paired t test to determine whether the difference in competitiveness was significant. As there were not enough bacterial cells recovered from the brain of one mouse in group 1, the data shown for this tissue are from four mice. As bacteria recovered from the lungs of two mice in group 2 were contaminated with other microbes, the data shown for this tissue are from three mice.
Taken together, TroR is required for S. suis virulence in an intranasal mouse model, despite the fact that it plays no apparent role in the virulence in the mouse intraperitoneal infection model.
DISCUSSION
As essential nutrients for life, metals play a pivotal role in the survival of bacteria within the host. Nevertheless, excess accumulation of intracellular metals is toxic to bacteria. Hence, it is not surprising that the vertebrate host develops strategies involving both metal limitation and metal excess to control bacterial infections (59). As a countermeasure, bacteria employ high-affinity metal transporters to compete with the host for metals and metal efflux pumps to export redundant metals (8). Typically, the genes encoding metal transporters and metal efflux pumps are controlled by metalloregulators, which can sense and bind metals (59). To date, the biological roles of metalloregulators in S. suis have received little attention. Only three metalloregulators (Zur, AdcR, and Fur) have been described in S. suis so far, yet their roles are not entirely elucidated (32, 36).
In the present study, we identified a metalloregulator, TroR, in S. suis. Both troR and the troABCD operon were significantly downregulated when S. suis was treated with Mn. A similar observation has been made for the homologous system (SloABCR) in S. mutans (60). In S. mutans, the binding of SloR to Mn promotes SloR dimerization and facilitates SloR binding to the promoter of the sloABC locus, thus blocking sloABC transcription, presumably via exclusion of RNA polymerase (61, 62). We speculated that in S. suis, Mn represses transcription of the troABCD operon via a similar mechanism. In addition, the promoter of troR is adjacent to the promoter of the troABCD operon in the genome of S. suis. We speculated that following Mn treatment, the binding of TroR to the promoter of troABCD affected RNA polymerase binding to the promoter of troR; thus, troR was also downregulated in the presence of excess Mn.
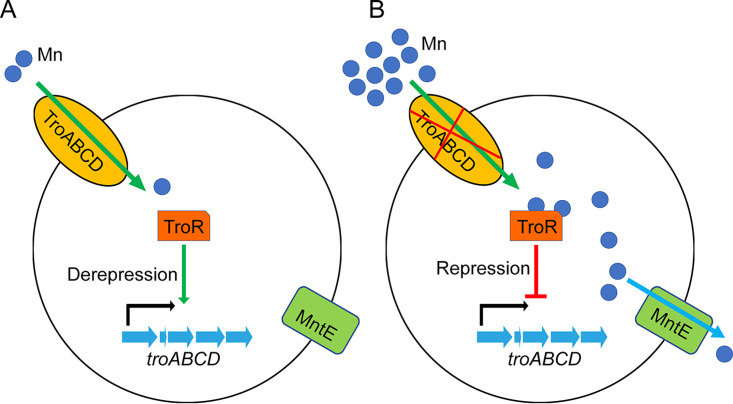
TroR is required for S. suis resistance to the toxicity conferred by Mn, Zn, Cu, and Co. The contribution of TroR homologs to Mn homeostasis has been well established in other species (11, 39, 53, 54). Similarly, we found that the ΔtroR mutant exhibited decreased resistance to Mn toxicity when cultured under metal-depleted conditions. Although a higher level of intracellular Mn was accumulated in the ΔtroR strain, it exhibited only a moderate growth defect when cultured in metal-replete medium supplemented with Mn. As observed in the present study and previous studies (10, 16), the toxicity of a metal may be rescued by another metal. The effect of Mn against the ΔtroR strain in metal-replete medium may be rescued by trace amounts of other metals. RNA sequencing analysis revealed that the troABCD operon is repressed by TroR. Moreover, TroA has been shown to contribute to Mn acquisition in S. suis (33). Thus, it was not surprising that the ΔtroR mutant, in which troA was significantly upregulated, accumulated a higher level of intracellular Mn than the WT and CΔtroR complemented strains. In a previous study, we demonstrated that the Mn efflux system MntE is involved in S. suis resistance to Mn toxicity (34). Taken together, S. suis could coordinate Mn import and export by the repression of the troABCD operon and activation of mntE in response to Mn toxicity (Fig. 9).
FIG 9.
Proposed model for S. suis resistance to Mn toxicity. Under Mn limitation conditions (A), TroR derepresses the transcription of the troABCD operon, and the TroABCD system is expressed. Mn enters S. suis cells via the TroABCD system. Under Mn excess conditions (B), the expression of the TroABCD system is repressed by TroR, and Mn import is prevented. Moreover, the Mn efflux system MntE is activated; intracellular Mn can be exported outside the cells by MntE.
It has been well established that TroR homologs contribute to Mn homeostasis (11, 39, 53, 54). However, the involvement of TroR homologs in bacterial resistance to Zn, Cu, and Co toxicity has rarely been reported. It is unexpected that S. suis TroR contributes to Zn toxicity, as the levels of intracellular Zn in the WT, ΔtroR, and CΔtroR strains were comparable following Zn treatment. Considering that troA is significantly upregulated in the ΔtroR mutant and that TroA has the ability to bind Mn and Zn with nanomolar affinity (37), it is also confusing that the three strains could accumulate equal concentrations of Zn. In S. pneumoniae, Zn is not transported via the PsaABC system, despite the fact that PsaA could bind Zn (63). Accordingly, we speculated that the TroABCD system of S. suis could not transport Zn; thus, the WT, ΔtroR, and CΔtroR strains had similar accumulations of intracellular Zn. Consistent with this inference, troA expression in the WT strain was not significantly downregulated in response to Zn. Moreover, TroA exhibited no apparent role in Zn acquisition in a previous study (33). In S. pneumoniae, extracellular Zn can inhibit intracellular Mn accumulation with little effect on intracellular Zn (63). In Escherichia coli, Zn excess perturbs Fe and Cu homeostasis (64). It is reasonable to speculate that excess Zn results in disorder of metal homeostasis in S. suis. A possible explanation for the higher susceptibility of the ΔtroR mutant to Zn is that deletion of troR reduces the ability of S. suis to respond to the disorder of metal homeostasis.
Similar to the case of Zn, Cu treatment resulted in a growth defect in the ΔtroR strain, despite comparable Cu accumulation in the mutant. In S. aureus, a ΔmntR mutant accumulated increased levels of intracellular Cu and showed decreased growth in the presence of Cu (65). The observed growth defect of the mutant under Cu excess conditions is consistent with our result; however, we did not observe higher Cu accumulation in the ΔtroR mutant. As troA expression was significantly downregulated when the WT strain was treated with Cu, it is reasonable to speculate that TroA can import Cu. The expression of copA (encoding a Cu efflux system) (35) was significantly upregulated in the ΔtroR strain, revealing that the mutant suffered Cu stress. Taken together, we speculated that the ΔtroR mutant indeed imports a higher level of Cu; however, the effect could be masked by the overexpression of the Cu efflux system. The ΔtroR mutant also exhibited decreased resistance to Co toxicity. In line with this result, a higher level of intracellular Co was accumulated in the mutant. Moreover, the expression of the gene encoding PmtA, an Fe(II) and Co efflux pump (16), was upregulated in the ΔtroR mutant.
Growth defects resulting from metal toxicity can often be attributed to mismetallation of metalloproteins with nonactivating metals (59, 66). In certain bacteria, the deletion of the efflux pump for a metal has been found to result in a growth defect in the presence of that metal, which can usually be alleviated by the addition of another metal (10, 16, 67, 68). In line with these findings, we showed that the growth defect of the ΔtroR mutant under Zn and Co excess conditions could be partly rescued by Mn supplementation. However, the growth inhibition of the ΔtroR strain resulting from excess Cu was not alleviated by Mn supplementation. Moreover, the growth of the WT and CΔtroR complemented strains was severely inhibited following Mn supplementation. According to the Irving-Williams stability series on protein-metal affinity [Mg/Ca < Mn < Fe(II) < Co < Ni < Cu > Zn] (69), Cu has the highest affinity to proteins. Hence, we speculated that if metalloproteins are mismetallated by Cu, Mn would not have the advantage of competing with Cu; instead, Mn itself would exert a toxic effect. The growth of the ΔtroR mutant was severely inhibited by Cu. Hence, Mn supplementation did not enhance the growth defect of the ΔtroR strain. Nevertheless, the WT and CΔtroR strains grew much better than the ΔtroR mutant in the presence of Cu; thus, the toxic effect should be more prominent.
The regulon of TroR homologs appears to be species and strain specific. In S. suis, TroR repressed the expression of the troABCD operon. This result is consistent with previous observations in several species, such as S. pneumoniae and S. pyogenes serotypes M1 and M3 (39, 44, 70). Nevertheless, mtsA transcripts were not changed in the mtsR mutant of S. pyogenes serotype M49 (40). In S. pyogenes serotype M49, an increase in the transcription of genes encoding the metal exporters PmtA and CopA was observed in the mtsR mutant (40). In line with this result, the expression of pmtA and copA was significantly upregulated in the ΔtroR mutant of S. suis. The gene encoding serine protease was the most upregulated gene in the ΔtroR mutant. Similarly, the homologous gene in S. pneumoniae was significantly upregulated in a ΔpsaR mutant (56). In addition, we found that in S. suis, troA expression was upregulated in response to Fe(II) and Co and downregulated in response to Mn and Cu. The effect of metal treatment on the expression of troA homologs has been well characterized in S. pneumoniae (42–45). While Mn negatively affects the expression of psaBCA, Zn, Co, and Ni have the opposite effect on the expression of this operon (42–45). The impact of Mn and Co on troA expression is consistent with the findings in S. pneumoniae (44, 45); however, we did not observe any role of Zn and Ni in troA expression. According to the mechanisms of metal sensing and regulation by TroR homologs in S. pyogenes and S. mutans (39, 61, 62), we speculated that Fe(II) and Co could compete with Mn for binding to TroR, affecting TroR binding to the promoter of troABCD; thus, troA was upregulated in the presence of excess Fe(II) and Co. Interestingly, troA expression was significantly repressed by Cu, indicating that TroA plays a potential role in Cu import, which has also been suggested by another study (65). Since Cu represses troA expression even in the ΔtroR mutant, it is likely that other regulators, such as CopY, which is involved in Cu homeostasis (36), might also participate in regulating the troABCD operon.
TroR homologs have been shown to be required for the virulence of certain pathogens (39, 44, 56–58). While no apparent role was observed for TroR in S. suis virulence in a mouse intraperitoneal infection model, the ΔtroR mutant had a significantly reduced ability to colonize various tissues of mice in an intranasal mouse model. It is possible that the metal contents are very low in the abdominal cavity of mice; the bacteria might not suffer from metal toxicity when they were inoculated by intraperitoneal injection. The intranasal mouse model is another model widely used for studying the virulence of S. suis (71–73). A well-established competitive-infection assay (74–76) with this model was further performed to compare the virulence of the ΔtroR mutant with that of the WT and CΔtroR complemented strains. The data clearly showed that the ΔtroR strain had reduced ability to colonize various tissues of mice, revealing that the virulence of the ΔtroR strain was attenuated in the intranasal mouse model. It has been demonstrated that mice could increase metal concentrations in various tissues, such as nasopharynx and lung, to respond to intranasal infection with S. pneumoniae (63). Accordingly, we speculated that the levels of metals such as Zn were increased in the nasopharynx of mice in response to S. suis infection. Therefore, the ΔtroR mutant, which was defective in resistance to metal toxicity, exhibited attenuated virulence in the intranasal mouse model.
While some interesting findings have been reported, several questions still need to be answered. Although TroR contributes to S. suis resistance to Zn and Cu toxicity, the real mechanisms remain unknown. The ΔtroR mutant accumulates higher levels of intracellular Co; however, little is known about how Co enters S. suis cells and why the WT and ΔtroR strains uptake different levels of Co. As the gene encoding serine protease was significantly differentially expressed in response to Mn, Fe(II), and Co (55), and it was the most upregulated gene in the ΔtroR strain, evaluation of the roles of serine protease in metal homeostasis would be interesting. In S. pneumoniae, PsaR has a strain-specific impact on global gene expression and bacterial virulence (56). Further studies can be performed to examine the roles of TroR and the TroABCD system in other serotypes of S. suis. Although TroR homologs generally contribute to the homeostasis of one or two metals (38), S. suis TroR plays a role in resistance to the toxicity conferred by multiple metals. A structural basis for metal sensing by TroR is also needed to elucidate the mechanism underlying its function.
In conclusion, the expression of the troABCD operon was significantly downregulated when S. suis was treated with excess Mn. TroR, a metalloregulator that potentially represses the troABCD operon, was hypothesized to play a role in the maintenance of Mn and Zn homeostasis. Functional analysis of TroR revealed that it represses expression of the troABCD operon and contributes to the resistance of S. suis to the toxicity conferred by Mn, Zn, Cu, and Co. TroR is required for S. suis virulence in the mouse intranasal infection model, despite the fact that no apparent role in the virulence was observed in the mouse intraperitoneal infection model.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 2; the primers are listed in Table S2 in the supplemental material. Unless otherwise specified, S. suis strains were cultured at 37°C in tryptic soy broth (Becton, Dickinson and Company) supplemented with 10% (vol/vol) newborn bovine serum (Sijiqing, Hangzhou, China) (TSBS [i.e., metal-replete medium]) or on tryptic soy agar (Becton, Dickinson and Company) supplemented with 10% (vol/vol) newborn bovine serum (TSAS). E. coli strains were cultured in Luria-Bertani (LB) broth or on LB agar. When required, spectinomycin was added to the medium at 100 μg/ml for S. suis or 50 μg/ml for E. coli. Metal-deplete medium was prepared by incubating TSBS with 1% Chelex-100 resin (Sigma-Aldrich) with rotation for 18 h. The metal salts (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were of analytical grade and were dissolved in deionized water to prepare the metal stock, except for Fe(II). As Fe(II) is easily oxidized, Fe(II) solution was prepared before each use.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| SC19 | Virulent S. suis strain isolated from brain of dead pig | Laboratory collection |
| ΔtroR | troR deletion mutant of strain SC19 | This study |
| CΔtroR | Complemented strain of ΔtroR mutant; Spcr | This study |
| DH5α | Cloning host for recombinant vector | Tsingke |
| Plasmids | ||
| pSET4s | Thermosensitive suicide vector; Spcr | 77 |
| pSET4s-ΔtroR | Knockout vector for troR deletion | This study |
| pSET2 | E. coli-S. suis shuttle vector; Spcr | 79 |
| pSET2-troR | pSET2 containing troR and its promoter | This study |
Spcr, spectinomycin resistant.
RNA extraction.
To explore the transcriptomic change in S. suis in response to Mn, the S. suis 2 strain SC19 was grown in TSBS medium to the mid-exponential phase (OD600 of 0.6) and divided into two equal aliquots: one aliquot was supplemented with 1 mM Mn and the other with deionized water. The cultures were incubated for another 15 min and then centrifuged for collecting bacterial cells. The cell pellets were immediately subjected to RNA extraction using the Eastep Super total RNA extraction kit (Promega, Shanghai, China). The RNA concentration and integrity were determined using the Qubit 2.0 fluorometer and the Agilent 2100 Bioanalyzer system, respectively. Three independent experiments were performed to obtain biological triplicate samples.
To compare the differences in the transcriptomes of the WT strain and ΔtroR mutant, the two strains were grown in TSBS medium to an OD600 of 0.6; bacterial cells were collected by centrifugation and then subjected to RNA extraction. RNA extraction and assessment were performed as described above. Three independent experiments were performed to obtain biological triplicate samples.
To evaluate troA expression in the WT strain and ΔtroR mutant in response to various metals, both strains were grown in TSBS medium to an OD600 of 0.6; each culture was then divided into nine equal aliquots, which were supplemented with 2 mM Fe(II), 2 mM Mn, 50 μM Zn, 1 mM Cu, 1 mM Co, 1 mM Ni, 2 mM Ca, 2 mM Mg, or deionized water. After 15 min of incubation, the bacterial cells were collected for RNA extraction. RNA extraction and assessment were performed as described above. Three independent experiments were performed to obtain biological triplicate samples.
RNA sequencing analysis.
RNA sequencing analysis was performed with the help of Novogene Bioinformatics Technology Co., Ltd. cDNA library preparation, sequencing, and data analysis were performed as described previously (55).
Mutant construction and functional complementation.
The ΔtroR mutant was constructed in the S. suis 2 strain SC19 background using the pSET4s suicide plasmid (77), following previously described procedures (78). The CΔtroR complemented strain was generated using the pSET2 plasmid (79), as described previously (80).
Growth curve analyses.
The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium to the early stationary phase (OD600 of 1.2) and then diluted in fresh medium (TSBS or Chelex-treated TSBS) supplemented with various concentrations of the specified metals. In the medium supplemented with Fe(II), 1 g/liter of trisodium citrate dihydrate was added to reduce Fe precipitation (67). The cultures were subpacked in 96-well plates (200 μl/well, three wells per culture). The plates were incubated at 37°C with linear shaking (120 rpm), and the OD595 values were measured hourly using the CMax Plus plate reader (Molecular Devices, Shanghai, China). At least three independent experiments were performed for each condition.
Intracellular metal content measurement.
The WT, ΔtroR, and CΔtroR strains were grown in TSBS medium to an OD600 of 0.6 and then supplemented with metals (4 mM Mn, 100 μM Zn, 1.5 mM Cu, or 1 mM Co). The cultures were incubated for another 30 min and then centrifuged for collection of bacterial cells. The bacterial cells were washed three times with PBS containing 0.25 M EDTA and three times with PBS alone. After drying at 110°C, the cells were weighed, digested in 66% nitric acid at 70°C for 48 h, and diluted to 2% nitric acid with deionized water. Following this, the metal concentration in the cells was analyzed by ICP-OES. The metal content was expressed as μg of metal per g of cells (dry weight). Four independent experiments were performed to obtain four biological samples for each strain and each condition.
Reverse transcription-quantitative PCR analysis.
Approximately 0.2 μg of RNA per sample was converted to cDNA using the NovoScript Plus All-in-One 1st Strand cDNA synthesis SuperMix (gDNA Purge; Novoprotein, Shanghai, China). Quantitative PCR was performed on the StepOnePlus real-time PCR system (Applied Biosystems) using NovoStart SYBR qPCR SuperMix Plus (Novoprotein, Shanghai, China) and the primers specified in Table S2. The relative gene expression level was analyzed using the threshold cycle (2−ΔΔCT) method, with 16S rRNA being used the reference gene (81). The experiments were performed in triplicate using biological triplicate samples.
Virulence study using murine infection models.
The animal studies were approved by the Animal Welfare and Ethics Committees of Yangzhou University. In total, 50 female BALB/c mice (4 to 6 weeks old) were used for the virulence study.
For the mouse intraperitoneal infection model, 40 BALB/c mice were randomly divided into four groups (10 mice per group). The mice in groups 1, 2, and 3 were intraperitoneally infected with 3 × 108 CFU of the WT, ΔtroR, and CΔtroR strains, respectively. The mice in group 4 were intraperitoneally infected with 300 μl PBS, and served as the control. The clinical symptoms and survival rates were recorded twice daily for 7 days.
For the competitive-infection assay with the mouse intranasal infection model, 10 BALB/c mice were randomly divided into two groups (five mice per group). The mice were anesthetized via inhalation of isoflurane and pretreated with 12.5 μl of 1% acetic acid placed in each nostril 1 h prior to intranasal infection. After controlled recovery and additional anesthesia, the mice in group 1 were intranasally infected with a 1:1 mixture of the ΔtroR and WT strains (20 μl in total, 2 × 108 CFU for each strain), and the mice in group 2 were infected with a 1:1 mixture of the ΔtroR and CΔtroR strains (20 μl in total, 2 × 108 CFU for each strain). At 12 h after infection, the blood, heart, liver, spleen, lung, kidney, and brain samples from the infected mice were collected. The blood samples were diluted and plated, while the other samples were homogenized in 1 ml PBS, diluted, and plated. For each sample in group 1, a total of 70 to 80 colonies were analyzed by colony PCR with the primer pair Out1/Out2 to determine the CI. For each sample in group 2, a total of 70 to 80 colonies were analyzed for spectinomycin susceptibility (simultaneously transferred to plates with and without spectinomycin) to determine the CI.
Bioinformatic analysis.
Protein sequences of the DtxR family metalloregulators were obtained by searching the NCBI database using their GenBank accession numbers or locus tags found in the articles cited. The sequences were submitted to Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) to generate the phylogenetic tree, which was further edited using FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). The BPROM program (http://linux1.softberry.com/berry.phtml) was used to predict the promoter of troR.
Statistical analysis.
GraphPad Prism 5 was used for data analysis. The differences in metal content were analyzed with the two-tailed unpaired t test. troA expression in the WT strain in response to various metals was analyzed by one-way analysis of variance with Bonferroni’s posttest. Two-way analysis of variance with Bonferroni’s posttest was used for comparison of troA expression in the ΔtroR mutant with that in the WT strain. The two-tailed paired t test was used to analyze the data in the competitive-infection assay. The log rank test was used to analyze the murine survival rates.
Data availability.
RNA sequencing data have been submitted to the NCBI Gene Expression Omnibus (GEO) under accession no. GSE164206 and GSE164207.
ACKNOWLEDGMENTS
We thank T. Sekizaki (National Institute of Animal Health, Japan) for supplying the pSET4s and pSET2 plasmids.
This research was supported by the National Natural Science Foundation of China (no. 31802210, to C. Zheng), China Postdoctoral Science Foundation (no. 2018M630615, to C. Zheng), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, to C. Zheng), and the Interdisciplinary Project from Veterinary Science of Yangzhou University (yzuxk202002, to C. Zheng).
Footnotes
Supplemental material is available online only.
Contributor Information
Xiaohui Zhou, Email: xiaohui.zhou@uconn.edu.
Xinan Jiao, Email: jiao@yzu.edu.cn.
Charles M. Dozois, INRS—Institut Armand-Frappier
REFERENCES
- 1.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. 2008. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218. 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 2.Barondeau DP, Getzoff ED. 2004. Structural insights into protein-metal ion partnerships. Curr Opin Struct Biol 14:765–774. 10.1016/j.sbi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Skaar EP, Raffatellu M. 2015. Metals in infectious diseases and nutritional immunity. Metallomics 7:926–928. 10.1039/c5mt90021b. [DOI] [PubMed] [Google Scholar]
- 4.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry RD, Bobrov AG, Fetherston JD. 2015. The role of transition metal transporters for iron, zinc, manganese, and copper in the pathogenesis of Yersinia pestis. Metallomics 7:965–978. 10.1039/c4mt00332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honsa ES, Johnson MD, Rosch JW. 2013. The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae. Front Cell Infect Microbiol 3:92. 10.3389/fcimb.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djoko KY, Ong CL, Walker MJ, McEwan AG. 2015. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem 290:18954–18961. 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner AG, Ong CY, Walker MJ, Djoko KY, McEwan AG. 2017. Transition metal homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae. Adv Microb Physiol 70:123–191. 10.1016/bs.ampbs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 9.VanderWal AR, Makthal N, Pinochet-Barros A, Helmann JD, Olsen RJ, Kumaraswami M. 2017. Iron efflux by PmtA is critical for oxidative stress resistance and contributes significantly to group A Streptococcus virulence. Infect Immun 85:e00091-17. 10.1128/IAI.00091-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ. 2015. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio 6:e00278-15. 10.1128/mBio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunenwald CM, Choby JE, Juttukonda LJ, Beavers WN, Weiss A, Torres VJ, Skaar EP. 2019. Manganese detoxification by MntE is critical for resistance to oxidative stress and virulence of Staphylococcus aureus. mBio 10:e02915-18. 10.1128/mBio.02915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. 2014. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J Infect Dis 209:1500–1508. 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 13.Frawley ER, Karlinsey JE, Singhal A, Libby SJ, Doulias PT, Ischiropoulos H, Fang FC. 2018. Nitric oxide disrupts zinc homeostasis in Salmonella enterica serovar Typhimurium. mBio 9:e01040-18. 10.1128/mBio.01040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M. 2011. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci USA 108:1621–1626. 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alquethamy SF, Khorvash M, Pederick VG, Whittall JJ, Paton JC, Paulsen IT, Hassan KA, McDevitt CA, Eijkelkamp BA. 2019. The role of the CopA copper efflux system in Acinetobacter baumannii virulence. Int J Mol Sci 20:575. 10.3390/ijms20030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng C, Jia M, Gao M, Lu T, Li L, Zhou P. 2019. PmtA functions as a ferrous iron and cobalt efflux pump in Streptococcus suis. Emerg Microbes Infect 8:1254–1264. 10.1080/22221751.2019.1660233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. 2007. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis 7:201–209. 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, Wang C. 2014. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 5:477–497. 10.4161/viru.28595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura M, Fittipaldi N, Calzas C, Gottschalk M. 2017. Critical Streptococcus suis virulence factors: are they all really critical? Trends Microbiol 25:585–599. 10.1016/j.tim.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Ku X, Yu X, Sun Q, Wu H, Chen F, Zhang X, Guo L, Tang X, He Q. 2019. Prevalence and antimicrobial susceptibilities of bacterial pathogens in Chinese pig farms from 2013 to 2017. Sci Rep 9:9908. 10.1038/s41598-019-45482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley CB, Chidgey KL, Bridges JP, Gordon E, Lawrence KE. 2020. Isolates, antimicrobial susceptibility profiles and multidrug resistance of bacteria cultured from pig submissions in New Zealand. Animals (Basel) 10:1427. 10.3390/ani10081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werinder A, Aspán A, Backhans A, Sjölund M, Guss B, Jacobson M. 2020. Streptococcus suis in Swedish grower pigs: occurrence, serotypes, and antimicrobial susceptibility. Acta Vet Scand 62:36. 10.1186/s13028-020-00533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M. 2014. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect 3:e45. 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romay-Lema EM, Ventura-Valcárcel P, Iñiguez-Vázquez I, García-Pais MJ, García-Garrote F, Rabuñal-Rey R, Alonso MP, Corredoira-Sánchez J. Streptococcus suis spondylodiscitis: 2 new cases and a literature review. Enferm Infecc Microbiol Clin, in press. 10.1016/j.eimc.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Agoston Z, Terhes G, Hannauer P, Gajdacs M, Urban E. 2020. Fatal case of bacteremia caused by Streptococcus suis in a splenectomized man and a review of the European literature. Acta Microbiol Immunol Hung 67:148–155. 10.1556/030.2020.01123. [DOI] [PubMed] [Google Scholar]
- 26.Jiang F, Guo J, Cheng C, Gu B. 2020. Human infection caused by Streptococcus suis serotype 2 in China: report of two cases and epidemic distribution based on sequence type. BMC Infect Dis 20:223. 10.1186/s12879-020-4943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olearo F, Marinosci A, Stephan R, Cherkaoui A, Renzi G, Gaia N, Leo S, Lazarevic V, Schrenzel J. 2020. First case of Streptococcus suis infection in Switzerland: an emerging public health problem? Travel Med Infect Dis 36:101590. 10.1016/j.tmaid.2020.101590. [DOI] [PubMed] [Google Scholar]
- 28.Liu SS, Wang Y, Xue L, Ma C, Li CH. 2019. Hemophagocytic lymphohistiocytosis due to Streptococcus suis in a 12-year-old girl: a case report. Medicine (Baltimore, MD) 98:e15136. 10.1097/MD.0000000000015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Németh A, Knausz M, Schmidt P. 2019. Special case of purulent meningitis caused by Streptococcus suis. Case report. Orv Hetil 160:30–34. 10.1556/650.2019.31243. [DOI] [PubMed] [Google Scholar]
- 30.Pulliainen AT, Kauko A, Haataja S, Papageorgiou AC, Finne J. 2005. Dps/Dpr ferritin-like protein: insights into the mechanism of iron incorporation and evidence for a central role in cellular iron homeostasis in Streptococcus suis. Mol Microbiol 57:1086–1100. 10.1111/j.1365-2958.2005.04756.x. [DOI] [PubMed] [Google Scholar]
- 31.Aranda J, Cortes P, Garrido ME, Fittipaldi N, Llagostera M, Gottschalk M, Barbe J. 2009. Contribution of the FeoB transporter to Streptococcus suis virulence. Int Microbiol 12:137–143. [PubMed] [Google Scholar]
- 32.Aranda J, Garrido ME, Fittipaldi N, Cortes P, Llagostera M, Gottschalk M, Barbe J. 2010. The cation-uptake regulators AdcR and Fur are necessary for full virulence of Streptococcus suis. Vet Microbiol 144:246–249. 10.1016/j.vetmic.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Schreur PJW, Rebel JMJ, Smits MA, van Putten JPM, Smith HE. 2011. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol 193:5073–5080. 10.1128/JB.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Zheng C, Cao M, Zeng T, Zhao X, Shi G, Chen H, Bei W. 2017. The manganese efflux system MntE contributes to the virulence of Streptococcus suis serotype 2. Microb Pathog 110:23–30. 10.1016/j.micpath.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Li M, Zhang H, Zheng B, Han H, Wang C, Yan J, Tang J, Gao GF. 2008. Functional definition and global regulation of Zur, a zinc uptake regulator in a Streptococcus suis serotype 2 strain causing streptococcal toxic shock syndrome. J Bacteriol 190:7567–7578. 10.1128/JB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng C, Jia M, Lu T, Gao M, Li L. 2019. CopA protects Streptococcus suis against copper toxicity. Int J Mol Sci 20:2969. 10.3390/ijms20122969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng BW, Zhang QM, Gao J, Han HM, Li M, Zhang JR, Qi JX, Yan JH, Gao GF. 2011. Insight into the interaction of metal ions with TroA from Streptococcus suis. PLoS One 6:e19510. 10.1371/journal.pone.0019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merchant AT, Spatafora GA. 2014. A role for the DtxR family of metalloregulators in Gram-positive pathogenesis. Mol Oral Microbiol 29:1–10. 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Do H, Makthal N, Chandrangsu P, Olsen RJ, Helmann JD, Musser JM, Kumaraswami M. 2019. Metal sensing and regulation of adaptive responses to manganese limitation by MtsR is critical for group A Streptococcus virulence. Nucleic Acids Res 47:7476–7493. 10.1093/nar/gkz524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toukoki C, Gold KM, McIver KS, Eichenbaum Z. 2010. MtsR is a dual regulator that controls virulence genes and metabolic functions in addition to metal homeostasis in the group A Streptococcus. Mol Microbiol 76:971–989. 10.1111/j.1365-2958.2010.07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bates CS, Toukoki C, Neely MN, Eichenbaum Z. 2005. Characterization of MtsR, a new metal regulator in group A Streptococcus, involved in iron acquisition and virulence. Infect Immun 73:5743–5753. 10.1128/IAI.73.9.5743-5753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manzoor I, Shafeeq S, Kuipers OP. 2015. Ni2+-dependent and PsaR-mediated regulation of the virulence genes pcpA, psaBCA, and prtA in Streptococcus pneumoniae. PLoS One 10:e0142839. 10.1371/journal.pone.0142839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloosterman TG, Witwicki RM, van der Kooi-Pol MM, Bijlsma JJE, Kuipers OP. 2008. Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae. J Bacteriol 190:5382–5393. 10.1128/JB.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston JW, Briles DE, Myers LE, Hollingshead SK. 2006. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect Immun 74:1171–1180. 10.1128/IAI.74.2.1171-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manzoor I, Shafeeq S, Kloosterman TG, Kuipers OP. 2015. Co2+-dependent gene expression in Streptococcus pneumoniae: opposite effect of Mn2+ and Co2+ on the expression of the virulence genes psaBCA, pcpA, and prtA. Front Microbiol 6:748. 10.3389/fmicb.2015.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoll KE, Draper WE, Kliegman JI, Golynskiy MV, Brew-Appiah RAT, Phillips RK, Brown HK, Breyer WA, Jakubovics NS, Jenkinson HF, Brennan RG, Cohen SM, Glasfeld A. 2009. Characterization and structure of the manganese-responsive transcriptional regulator ScaR. Biochemistry 48:10308–10320. 10.1021/bi900980g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakubovics NS, Smith AW, Jenkinson HF. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol Microbiol 38:140–153. 10.1046/j.1365-2958.2000.02122.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen YYM, Shieh HR, Chang YC. 2013. The expression of the fim operon is crucial for the survival of Streptococcus parasanguinis FW213 within macrophages but not acid tolerance. PLoS One 8:e66163. 10.1371/journal.pone.0066163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crepps SC, Fields EE, Galan D, Corbett JP, Von Hasseln ER, Spatafora GA. 2016. The SloR metalloregulator is involved in the Streptococcus mutans oxidative stress response. Mol Oral Microbiol 31:526–539. 10.1111/omi.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolerson E, Swick A, Newlon L, Palmer C, Pan Y, Keeshan B, Spatafora G. 2006. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. J Bacteriol 188:5033–5044. 10.1128/JB.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol 185:5967–5975. 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teng L, Dong XX, Zhou Y, Li ZW, Deng LM, Chen HC, Wang XH, Li JQ. 2017. Draft genome sequence of hypervirulent and vaccine candidate Streptococcus suis strain SC19. Genome Announc 5:e01484-16. 10.1128/genomeA.01484-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandey R, Russo R, Ghanny S, Huang XJ, Helmann J, Rodriguez GM. 2015. MntR(Rv2788): a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol Microbiol 98:1168–1183. 10.1111/mmi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brett PJ, Burtnick MN, Fenno JC, Gherardini FC. 2008. Treponema denticola TroR is a manganese- and iron-dependent transcriptional repressor. Mol Microbiol 70:396–409. 10.1111/j.1365-2958.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia M, Wei M, Zhang Y, Zheng C. 2020. Transcriptomic analysis of Streptococcus suis in response to ferrous iron and cobalt toxicity. Genes (Basel) 11:1035. 10.3390/genes11091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendriksen WT, Bootsma HJ, van Diepen A, Estevao S, Kuipers OP, de Groot R, Hermans PWM. 2009. Strain-specific impact of PsaR of Streptococcus pneumoniae on global gene expression and virulence. Microbiology (Reading) 155:1569–1579. 10.1099/mic.0.025072-0. [DOI] [PubMed] [Google Scholar]
- 57.Abrantes MC, Kok J, Lopes MD. 2013. EfaR is a major regulator of Enterococcus faecalis manganese transporters and influences processes involved in host colonization and infection. Infect Immun 81:935–944. 10.1128/IAI.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ando M, Manabe YC, Converse PJ, Miyazaki E, Harrison R, Murphy JR, Bishai WR. 2003. Characterization of the role of the divalent metal ion-dependent transcriptional repressor MntR in the virulence of Staphylococcus aureus. Infect Immun 71:2584–2590. 10.1128/IAI.71.5.2584-2590.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandrangsu P, Rensing C, Helmann JD. 2017. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15:338–350. 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kajfasz JK, Katrak C, Ganguly T, Vargas J, Wright L, Peters ZT, Spatafora GA, Abranches J, Lemos JA. 2020. Manganese uptake, mediated by SloABC and MntH, is essential for the fitness of Streptococcus mutans. mSphere 5:e00764-19. 10.1128/mSphere.00764-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spatafora G, Corbett J, Cornacchione L, Daly W, Galan D, Wysota M, Tivnan P, Collins J, Nye D, Levitz T, Breyer WA, Glasfeld A. 2015. Interactions of the metalloregulatory protein SloR from Streptococcus mutans with its metal ion effectors and DNA binding site. J Bacteriol 197:3601–3615. 10.1128/JB.00612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monette P, Brach R, Cowan A, Winters R, Weisman J, Seybert F, Goguen K, Chen J, Glasfeld A, Spatafora G. 2018. Autoregulation of the Streptococcus mutans SloR metalloregulator is constitutive and driven by an independent promoter. J Bacteriol 200:e00214-18. 10.1128/JB.00214-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357. 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Z, Wang P, Wang H, Yu ZH, Au-Yeung HY, Hirayama T, Sun H, Yan A. 2019. Zinc excess increases cellular demand for iron and decreases tolerance to copper in Escherichia coli. J Biol Chem 294:16978–16991. 10.1074/jbc.RA119.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Tameemi H, Beavers WN, Norambuena J, Skaar EP, Boyd JM. 2021. Staphylococcus aureus lacking a functional MntABC manganese import system has increased resistance to copper. Mol Microbiol 115:554–573. 10.1111/mmi.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imlay JA. 2014. The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–28128. 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Arguello JM, Helmann JD. 2015. PfeT, a P1B4-type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol Microbiol 98:787–803. 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner AG, Ong CY, Djoko KY, West NP, Davies MR, McEwan AG, Walker MJ. 2017. The PerR-regulated P1B-4-type ATPase (PmtA) acts as a ferrous iron efflux pump in Streptococcus pyogenes. Infect Immun 85:e00140-17. 10.1128/IAI.00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Counago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O'Mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, McDevitt CA. 2014. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol 10:35–41. 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 70.Hanks TS, Liu M, McClure MJ, Fukumura M, Duffy A, Lei B. 2006. Differential regulation of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters by the metalloregulator MtsR of group A Streptococcus. Infect Immun 74:5132–5139. 10.1128/IAI.00176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seitz M, Beineke A, Seele J, Fulde M, Valentin-Weigand P, Baums CG. 2012. A novel intranasal mouse model for mucosal colonization by Streptococcus suis serotype 2. J Med Microbiol 61:1311–1318. 10.1099/jmm.0.043885-0. [DOI] [PubMed] [Google Scholar]
- 72.Li W, Wan Y, Tao Z, Chen H, Zhou R. 2013. A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet Microbiol 162:186–194. 10.1016/j.vetmic.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Seitz M, Beineke A, Singpiel A, Willenborg J, Dutow P, Goethe R, Valentin-Weigand P, Klos A, Baums CG. 2014. Role of capsule and suilysin in mucosal infection of complement-deficient mice with Streptococcus suis. Infect Immun 82:2460–2471. 10.1128/IAI.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Greeff A, Buys H, van Alphen L, Smith HE. 2002. Response regulator important in pathogenesis of Streptococcus suis serotype 2. Microb Pathog 33:185–192. 10.1016/s0882-4010(02)90526-7. [DOI] [PubMed] [Google Scholar]
- 75.de Greeff A, Buys H, Verhaar R, Dijkstra J, van Alphen L, Smith HE. 2002. Contribution of fibronectin-binding protein to pathogenesis of Streptococcus suis serotype 2. Infect Immun 70:1319–1325. 10.1128/IAI.70.3.1319-1325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng CK, Xu JL, Shi GL, Zhao XG, Ren SJ, Li JQ, Chen HC, Bei WC. 2016. Formate-tetrahydrofolate ligase is involved in the virulence of Streptococcus suis serotype 2. Microb Pathog 98:149–154. 10.1016/j.micpath.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Takamatsu D, Osaki M, Sekizaki T. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46:140–148. 10.1006/plas.2001.1532. [DOI] [PubMed] [Google Scholar]
- 78.Zheng CK, Xu JL, Li JQ, Hu LH, Xia JD, Fan JY, Guo WN, Chen HC, Bei WC. 2014. Two Spx regulators modulate stress tolerance and virulence in Streptococcus suis serotype 2. PLoS One 9:e108197. 10.1371/journal.pone.0108197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takamatsu D, Osaki M, Sekizaki T. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 45:101–113. 10.1006/plas.2000.1510. [DOI] [PubMed] [Google Scholar]
- 80.Zheng CK, Xu JL, Ren SJ, Li JQ, Xia MM, Chen HC, Bei WC. 2015. Identification and characterization of the chromosomal yefM-yoeB toxin-antitoxin system of Streptococcus suis. Sci Rep 5:13125. 10.1038/srep13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S4, Tables S1 and S2. Download AEM.01375-21-s0001.pdf, PDF file, 0.4 MB (425.5KB, pdf)
Data Availability Statement
RNA sequencing data have been submitted to the NCBI Gene Expression Omnibus (GEO) under accession no. GSE164206 and GSE164207.