Abstract
Background
Verbal and visual memory deficits are prominent trait markers for schizophrenia, with impairments also observed in first-degree relatives [Snitz, B.E., Macdonald, A.W., 3rd, & Carter, C.S. (2006). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull, 32(1), 179–194]. It remains unclear whether deficits lie in encoding or savings, and whether the deficit is heritable.
Objective
To determine which features of memory performance are impaired in both patients and their healthy siblings, possibly reflecting shared genetic effects.
Method
We tested episodic memory using Logical Memory (LM) and Visual Reproduction (VR) tasks of the Wechsler Memory Scale (Revised). Participants included patients with schizophrenia (n=162), their nonpsychotic siblings (n=146), and controls (n=205), recruited for the “CBDB/NIMH Sibling Study”. We assessed immediate encoding and 30 minute and 24 hour delayed recall as well as savings scores for the “short delay” (immediate to 30 min) and “long delay” (30 min to 24 h) intervals.
Results
We observed marked verbal recall deficits in both patients and siblings compared to controls for all stages (p<.0001). Only patients experienced significant verbal and visual savings deficits over short delays (p<.0001) as well as verbal deficits over long delays (p<.005). In siblings, no saving score difficulty was apparent for either measure.
Conclusions
Our results confirm shared impairment in verbal learning, but not memory, for both patients and siblings, therefore marking it as a potential schizophrenia-associated intermediate phenotype. The results implicate neural systems involved in immediate encoding and stabilization of memory representations in genetic risk for schizophrenia. In contrast, visual recall and savings impairments appear to be illness, i.e. state, deficits.
Keywords: Schizophrenia, Memory, Learning, Cognition, Genetics, Verbal, Visual, Episodic, Family, Phenotype, Endophenotype, Wechsler Memory Scale, Recall, Savings, Retention
1. Introduction
Multiple meta-analytic studies have demonstrated that impairments of episodic memory are among the most profound cognitive deficits documented in schizophrenia research literature (Heinrichs and Zakzanis, 1998). Substantial impairments have been described for both immediate and 30 minute recall of verbal and visual materials, with effect sizes of d=1.27 and 1.00 for immediate encoding and d = 1.2 and 1.09 for 30 minute recall in verbal and visual tasks, respectively (Aleman et al., 1999). Given robust impairment in patients, measures of episodic memory are attractive as potential intermediate phenotypes, i.e. nondiagnostic indicators of genetic liability for the illness. Indeed, meta-analytic reviews of the cognitive performance of family members of patients have shown that verbal memory impairments are among the most reliable deficits (Snitz et al., 2006). Visual memory has been studied less frequently than verbal memory in patients (Snitz et al., 2006), and impairments in the visual domain among family members appear to be somewhat less severe than in the verbal domain (Delawalla et al., 2006; Heinrichs and Zakzanis, 1998; Whyte et al., 2005).
On the Wechsler Memory Scale (Revised), subjects are presented with stories or visual figures and are asked for immediate recall of encoded information. This “immediate encoding” performance is likely to involve a mix of material from short- and long-term memory. Thus, in order to assess long-term memory per se, it is necessary to examine delayed recall and savings over time.
The literature examining delayed recall in schizophrenia is far less extensive and consistent than the immediate encoding literature. Most studies have examined verbal memory following 30 minute delays and calculated savings scores (delayed recall/immediate encoding) and found that patients indeed retained less than controls (Calev et al., 1991; Cirillo and Seidman, 2003; Heinrichs and Zakzanis, 1998; Toulopoulou et al., 2003b). However, longer delay intervals produce less evidence of impairment (Braff et al., 1991). Thus, the evidence for impaired savings in schizophrenia, and whether impairment spans both verbal and visual materials, is surprisingly sparse. The savings issue has rarely been studied in relatives. Two studies (Laurent et al., 1999; Cirillo and Seidman, 2003) found immediate encoding, but not savings score deficits, in relatives. Thus, available evidence suggests that the deficit in relatives may be confined to immediate encoding and spare actual savings/memory.
Our analyses were designed to address the limitations of the available patient and family member literatures by examining immediate encoding as well as 30 minute and 24 hour recall and savings for both verbal and visual materials. The analysis of patient performance for long delays was intended to further define the clinical memory phenotype. The analysis of sibling performance was intended to explore which aspects of the impairments observed in patients also occurred in siblings and might therefore be considered as marking an intermediate phenotype. Based on the literature, we predicted that logical memory measures would likely be intermediate phenotype markers. Our approach to visual reproduction performance was exploratory as the literature does not support a clear prediction. We expected the strongest shared deficits to occur in the immediate encoding and short delay savings, with the expectation that long delay savings might be intact in relatives, and possibly patients.
While our focus is on behavior, the distinction between immediate encoding and long delay savings may have important implications for understanding neurobiological and genetic mechanisms. Specifically, there is a great deal of evidence from studies of long-term potentiation – a cellular model of memory – that the mechanisms implicated in the induction of LTP differ from those implicated in the long delay maintenance of LTP (Pastalkova et al., 2006). Glutamatergic transmission and stimulation of NMDA and AMPA receptors are thought to play a critical role in the initial induction of LTP, whereas long-term maintenance involves protein synthesis and structural modification of synapses (Bekinschtein et al., 2007; Raymond, 2007). Behavioral evidence of impairment limited to either short or long delay memory may have important implications for understanding the genetic architecture implicated in schizophrenia.
2. Experimental methods
2.1. Participant inclusion
Participants were recruited to be a part of the “CBDB/NIMH Sibling Study” (D. Weinberger, PI). After complete description of the study to the subjects, written informed consent was obtained. Egan et al. (2001) and Goldberg et al. (2003) provide more detail of methods and possible ascertainment biases. Briefly, we tested schizophrenic patients, their siblings, and healthy controls between 18 and 60 years of age who had a premorbid IQ greater than 70. Participants in all groups were included in the analysis reported below if they passed a rigorous set of medical, psychiatric and neurological inclusion criteria as well as structural brain imaging evaluations. Exclusion criteria included history of closed head injury with a loss of consciousness for longer than 5 min, current medical illness that might impact cognitive function, and alcohol or other drug abuse within the past 6 months.
Families recruited were required to have one schizophrenic patient and at least one sibling who was available for testing (no twins were included). Diagnosis was determined through a Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996) as well as a medical record review for each participant conducted by a psychiatrist or clinical psychologist. Patients were included if they met DSM-IV criteria for schizophrenia or schizoaffective disorder, depressed type. Siblings were included only if they did not receive a diagnosis of any current Axis I disorder, lifetime evidence of any type of psychosis, or a schizophrenia spectrum Axis II disorder. Siblings were included who had a history of a nonpsychotic Axis I disorder (such as mood disorder) if it was diagnosed as being in full remission using SCID criteria. Healthy controls were recruited via radio and print advertisements within Bethesda and surrounding areas. Exclusion criteria consisted of existing medical rule-outs, any active Axis I disorders, or Axis II schizophrenia spectrum disorders.
2.2. Demographics
The demographic features of the groups are shown in Table 1. A total of 162 patients and 146 siblings met inclusion criteria and had completed all three recall stages for the WMS-R Logical Memory subtest. From an original pool of 337 controls, we deleted all of the 132 subjects in the age range of 18 and 24 in order to achieve greater age equivalence across the 3 groups. t-tests for independent groups revealed that there were no age differences, but the patient group had a higher proportion of males than the healthy control group (χ2 = 30.14, p <.0001). The sibling and control groups did not differ in gender composition. In addition, the differences in education completed for both patients and siblings compared to healthy control subjects were significant as assessed by t-test (patients: t =−12.66, p <.0001; siblings: t = −6.49, p <.0001).
Table 1.
Demographics by group
| Patients
|
Siblings
|
Controls
|
||||
|---|---|---|---|---|---|---|
| N=162 (LM); 159 (VR) | N=146 (LM); 143 (VR) | N=205 (LM); 143 (VR) | ||||
| M | SD | M | SD | M | SD | |
| Education (years) | 14.3 * | 2.28 | 15.83 * | 2.35 | 17.6 | 2.6 |
| Fam SES a | 51.45 | 13.7 | 52.68 | 12.12 | 52.02 | 12.79 |
| Age | 34.53 | 9.94 | 36.39 | 10.27 | 35.7 | 8.56 |
| Gender (male/female) | 113/49 | 59/87 | 84/121 | |||
Years of education completed differed significantly between patients vs. controls as well as siblings vs. controls (p<.0001).
Family SES was calculated as a composite score derived from the Four Factor Index of Social Status (Hollingshead, 1975).
2.3. Design
We administered the Wechsler Memory Scale —Revised (WMS-R) Logical Memory (LM) and Visual Reproduction (VR) subtests, with three recall stages: Immediate encoding (LM1), 30 minute recall (LM2), and 24 hour recall (LM3). During the 30 minute period between recall trials, participants completed performance and verbal subtests from the WAIS-R. We calculated savings scores for “short delay” (LM1 to LM2; approximately 30 min) and “long delay” (LM2 to LM3; approximately 24 h) intervals as: (LM2/LM1)×100 and (LM3/LM2)×100, respectively. After immediate encoding, each participant was told that they would be asked about the task a second time; in contrast, no one was given warning that there would also be a 24 hour recall task.
As seen in Table 1, data were available from fewer subjects on VR subtests than for Logical Memory. This is mainly a result of the Sibling Study protocol design in which all subjects were administered Logical Memory, while some controls (76) received an abbreviated one-day battery in which they were contacted the following day by phone and asked to recall the Logical Memory stories only. All patients and siblings, as well as 129 controls, were administered the 24 hour recall testing in person on the second day of testing.
3. Statistical analyses
3.1. Main analysis
All analyses were conducted using Statistica software, version 7.0 (Statsoft Corp, Tulsa, Okla). Independent group t-tests were used to examine group differences in recall and savings. We used analysis of covariance to control for education, gender and age effects, when significant.
3.2. Relative risk
We conducted a relative risk (RR) analysis for the “affected” participants. Relative risk is used to estimate upper limits of heritability (James, 1971) and to determine power in genetic studies (Risch and Merikangas, 1996). Relative risk (for siblings) is the ratio of percent of “affected” siblings/percent of “affected” control subjects (Egan et al., 2001). This approach focused on whether there were an excess number of siblings that demonstrated impairment using different cut-off scores to define impairment (Risch, 2001).
Unlike the description of recruitment into the parent study, not all schizophrenic patients had siblings and vice versa in the present study. Therefore, we examined two cohorts from the study populations. First, we included all patients and siblings (“Cohort A”). Next, we examined intact families only (“Cohort B”); that is, each family consisted of one patient and one unaffected sibling (67 families). Results for both cohort analyses yielded the same pattern; therefore we report on the largest, most representative sample.
We conducted two RR analyses: The first was more inclusive and defined an individual as “affected” by scoring 1 or more Standard Deviations below the control mean. The second analysis captured only individuals who scored 2 or more Standard Deviations (SD) below the control mean. In both analyses, we compared three groups to healthy controls: patients, siblings (λ), and a more familial group of “concordant” siblings (λ′), related to the “affected” patients who met either the 1 or 2 SD impairment criteria (thus, the Ns varied by the number of affected patients for each measure).
4. Results
4.1. Immediate and delayed recall performance in patients and their siblings
See Fig. 1 for t-test results on the recall scores and Table 2 for the effect sizes. Patient performance was significantly lower on raw scores than healthy controls and the sibling group on all three LM (patients vs. controls: t(365)=− 16.0, −16.4, −16.1 for LM1, 2 and 3 respectively; patients vs. siblings: t(306)=−11.3, −11.2, −10.8 for LM1, 2 and 3 respectively; all p<.0001) and VR (patients vs. controls: t(286)=−8.1, t(286)=−10.3, t(285)=−10.1 for VR 1, 2 and 3 respectively; patients vs. siblings: t(300)=−7.3, t(300)=−10.0, t(299)=−8.0 for VR1, 2 and 3 respectively; all p<.0001) recall assessments. In contrast to patients, the sibling group scored significantly lower than controls on all three LM recalls (t(349) = −4.0, −4.0, −4.0 for LM1, 2 and 3; all ps <.0001), but did not differ on VR (t(270)= −1.4, −.9, −.7 for VR1, 2 and 3 respectively). Sibling VR performance virtually matched that of controls, differing by approximately 1/2 point at each test occasion.
Fig. 1.
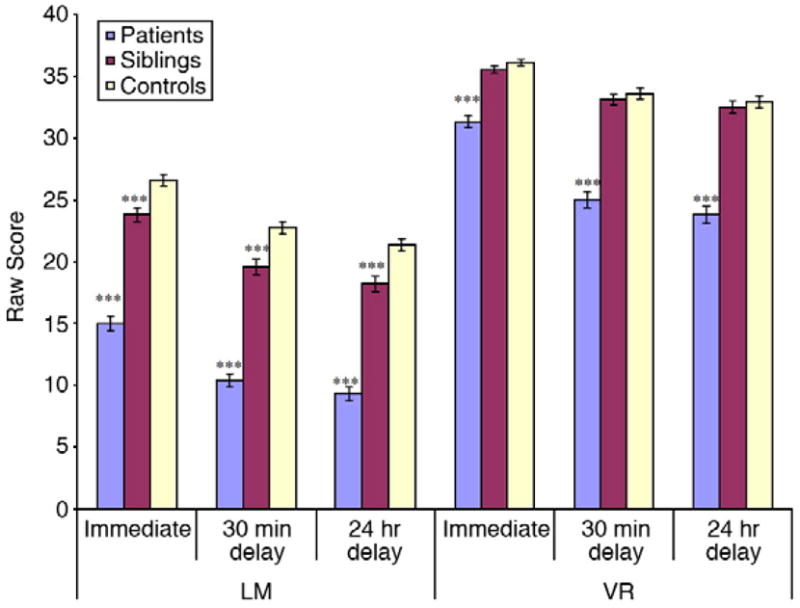
Recall performance by group for Logical Memory and VR subtests. *p<.05; ***p<.0001 (after t-tests and ANCOVA analyses). Siblings differed notably from controls in LM Recall 1–3 (p<.0001); patients were consistently worse than controls for LM and VR recall measures (p<.0001).
Table 2.
Effect sizes for impairment for verbal and visual memory
| Effect size
|
||||
|---|---|---|---|---|
| Patients | Siblings | |||
| d | CI (95%) | d | CI (95%) | |
| LM1 | 1.69 | (1.45–1.92) | .43 | (.21–.64) |
| LM2 | 1.73 | (1.49–1.97) | .43 | (.21–.64) |
| LM3 | 1.69 | (1.45–1.93) | .43 | (.21–.64) |
| %sLM2/1 | .86 | (.64–1.07) | .23 | (.01–.44) |
| %sLM3/2 | .24 | (.03–.45) | −.02 | (−.24–.19) |
| VR1 | .97 | (.21–.64) | .18 | (−.06–.42) |
| VR2 | 1.21 | (.21–.64) | .10 | (−.14–.34) |
| VR3 | 1.19 | (.21–.64) | .07 | (.17–.31) |
| %sVR2/1 | .90 | (.01–.44) | .00 | (−.24–.24) |
| %rsVR3/2 | .16 | (−.24–.19) | .00 | (−.24–.24) |
LM = Logical Memory; VR = Visual Reproduction. 1 = immediate encoding, 2 = 30 minute recall, 3 = 24 hour recall; %s 2/1 = short delay (immediate to 30 min) savings score, %s 3/2 = long delay (30 min to 24 h) savings score.
4.2. Short and long delay savings in patients and their siblings
Fig. 2 contains t-test results for savings scores. In patients, short delay savings scores were significantly lower relative to healthy controls in both LM (t(365)= −8.2, p<.0001) and VR (t(286)=−7.6, p<.0001). At the long delay interval, patients did not differ from controls in their VR savings (t(286)=−1.4, p=.17); a small, but significant difference was observed in 24 hour LM savings (t(365)=−2.3, p<.05). Remarkably, mean patient savings over long delays were 87% for LM and nearly 95% for VR. In contrast, short delay savings scores were 66% for LM and 79% for VR.
Fig. 2.
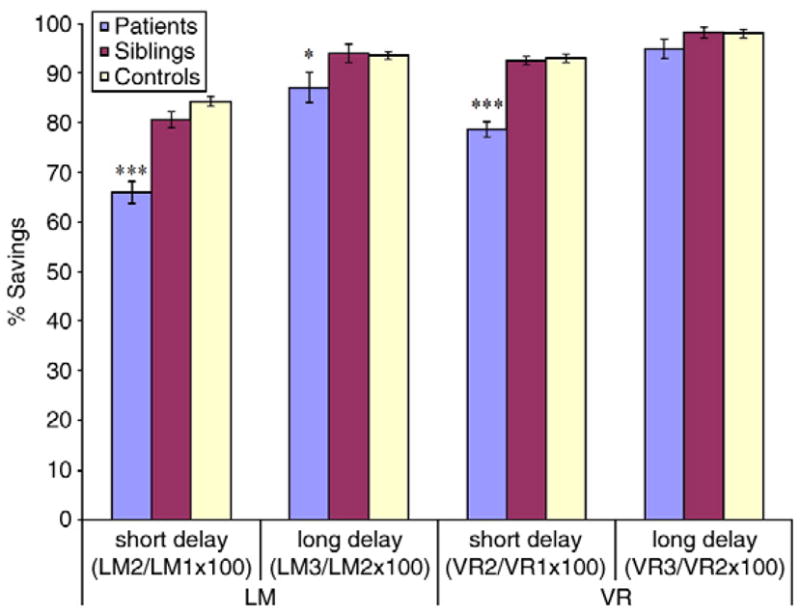
Savings scores by group for LM and VR subtests. *p<.05; ***p<.0001 (after t-tests and ANCOVA analyses). Siblings were not impaired for any savings measure after controlling for covariates. Sibling VR savings were virtually identical to controls. Patients retained significantly less for short delay LM and VR (p<.0001) and long delay LM (p<.05), but did not continue to forget rapidly over long delay VR.
The analysis of savings in siblings revealed only one significant contrast with controls, short delay savings for LM (t(349)=− 2.1, p<.05). However, the main effect of group did not remain significant in the ANOVA model that controlled for education and gender: F(1, 348)= 2.1, p=.15. Further, sibling savings scores for VR did not differ from controls for short and long delays.
4.3. Correlations
To explore possible effects of IQ on performance, we examined the relationship between full scale IQ (FSIQ) and WMS performance across groups. We found that FSIQ was significantly correlated with LM1 in all groups (r=.44, .41, and .31 in patients, siblings, and controls respectively). Interestingly, FSIQ had no relationship to short or long delay LM savings in patients (r=.06 and .09 respectively), with significant but very small correlations with short delay savings in siblings and controls (r=.23 and .15 respectively), a relationship that was absent altogether for long delay savings in siblings and controls (r=.08, and .02 respectively). The picture is somewhat different with VR: the VR1 correlations were significant in patients and siblings (r=.43, .40) but not in controls (r=.13). In short delay savings the FSIQ correlations were .30, .16 and .19 in patients, siblings and controls respectively. These decreased in magnitude at the long delay interval, where none of the correlations were significant (r=−.10, .08, and .16 in patients, siblings, and controls respectively).
4.4. Relative risk and chi-square analyses
Table 3 shows the results of relative risk and chi-square analyses for the total patient and sibling groups using cut-off scores of performing 1 and 2 SD below the healthy comparison group mean. As expected, the observed relative risks are higher using the more specific 2 SD cut-off. The chi-square analysis of the patient data is generally consistent with the t-test results: significant chi squares were observed for all recalls of verbal and visual material. However, unlike the t-tests, patients demonstrated a significant deficit in 24 hour VR savings. It is important to note, however, that this reflects severe impairment in a small minority of patients for this measure.
Table 3.
Relative risk estimates for impairment on verbal and visual memory
| Measure | % Affected
|
Patients
|
All siblings (λ)
|
Concordant Siblings (λ')
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Siblings | Controls | RR | p(χ2) | RR | p(χ2) | N | RR | p(χ 2) | ||
| LM1 | 1SD | 78.40% | 27.40% | 16.10% | 4.9 | <.0001 (142.8) | 1.7 | .01 (6.6) | 86 | 1.7 | .02 (5.4) |
| 2SD | 41.40% | 6.20% | 1.50% | 28.3 | <.0001 (93.3) | 4.2 | .01 (5.7) | 45 | 4.6 | .04 (4.3) | |
| LM2 | 1SD | 79.00% | 28.80% | 17.60% | 4.5 | <.0001 (138.3) | 1.6 | .01 (6.2) | 83 | 1.9 | .005 (7.8) |
| 2SD | 43.20% | 8.90% | 2.00% | 22.2 | <.0001 (98.1) | 4.6 | .003 (8.9) | 45 | 5.7 | .003 (8.9) | |
| LM3 | 1SD | 80.90% | 28.80% | 17.60% | 4.6 | <.0001 (146.2) | 1.6 | .01 (6.2) | 85 | 1.7 | .02 (5.1) |
| 2SD | 41.40% | 6.90% | 1.00% | 42.4 | b.0001 (96.7) | 7 | .003 (8.9) | 43 | 7.2 | .01 (6.5) | |
| %R LM2/1 | 1SD | 50.60% | 22.00% | 13.20% | 3.8 | <.0001 (60.8) | 1.7 | .03 (4.67) | 50 | 2 | .03 (5.0) |
| 2SD | 31.50% | 11.60% | 2.00% | 16.1 | <.0001 (61.9) | 6 | .0002 (14.2) | 28 | 7.3 | .0008 (11.3) | |
| %R LM3/2 | 1SD | 34.80% | 21.90% | 15.20% | 2.3 | <.0001 (18.9) | 1.4 | .11 (2.6) | 40 | 1.2 | .71 (.14) |
| 2SD | 24.10% | 6.90% | 4.00% | 6.1 | <.0001 (32.5) | 1.8 | .22 (1.5) | 30 | 1.9 | .49 (.48) | |
| VR1 | 1SD | 51.30% | 18.20% | 10.10% | 5.1 | <.0001 (54.5) | 1.8 | .06 (3.6) | 39 | 2.5 | .01 (6.1) |
| 2SD | 30.10% | 3.50% | 5.40% | 5.6 | <.0001 (28.1) | 1.2 | .76 (.09) | 27 | 2.7 | .08 (3) | |
| VR2 | 1SD | 61.60% | 15.40% | 13.20% | 4.7 | <.0001 (69.7) | 1.2 | .60 (.27) | 63 | 1.6 | .18 (1.8) |
| 2SD | 37.70% | 3.50% | 5.40% | 7 | <.0001 (41.7) | .6 | .44 (12) | 34 | 1.1 | .92 (.01) | |
| VR3 | 1SD | 62.70% | 11.60% | 11.60% | 5.4 | <.0001 (77.2) | 1.6 | .13 (2.3) | 62 | 1.9 | .05 (3.9) |
| 2SD | 36.10% | 5.60% | 5.40% | 6.7 | <b.0001 (38.5) | 1 | .95 (.00) | 33 | 1.1 | .89 (.02) | |
| %R VR2/1 | 1SD | 53.50% | 14.00% | 14.70% | 3.6 | <.0001 (46.3) | 1 | .87 (.03) | 64 | 1.2 | .66 (.20) |
| 2SD | 35.20% | 3.50% | 4.60% | 7.6 | <.0001 (39.4) | .8 | .63 (.23) | 32 | 1.3 | .71 (.14) | |
| %R VR3/2 | 1SD | 27.20% | 11.20% | 10.10% | 2.7 | .0003 (13.3) | 1.1 | .78 (.09) | 25 | 1.2 | .77 (.08) |
| 2SD | 17.70% | 7.00% | 4.70% | 3.8 | .0007 (11.6) | 1.5 | .41 (.67) | 13 | 3.3 | .11 (2.6) | |
= relative risk for all siblings (N=146 (LM); 143 (VR)); λ′=relative risk for “concordant siblings” subgroup, i.e., siblings of patients with cognitive impairment for test (N indicates total concordant siblings for each measure). All χ2 tests compare distribution in siblings with that in controls (N=205 (LM); 519 (VR)). Criteria used to define “affected” status of subjects are one (1SD) and two (2SD) standard deviations below mean of control group. Bold numbers indicate significance of p≤.05.
Among the siblings, the results of the chi squares were consistent with the pattern of findings documented with t-tests. While there was a nearly significant effect in siblings for the initial recall of visual material using the 1 SD cut-off, it was not apparent using the 2 SD cutoff or t-test analyses, and therefore is not a reliable observation. In the exploratory, within family analysis, the relative risks were almost identical to those observed in the larger sample, suggesting that risk for memory impairment in siblings is not tightly tied to patient performance level.
5. Discussion
5.1. Summary of findings
Our analyses yielded several clear findings. First, patients demonstrated marked recall impairments for both verbal and visual materials at all recall intervals, consistent with the literature (Aleman et al., 1999; Cirillo and Seidman, 2003; Dickinson et al., 2007; Heinrichs and Zakzanis, 1998; Sitskoorn et al., 2004; Snitz et al., 2006; Toulopoulou et al., 2003b). Second, siblings demonstrated recall impairments for verbal materials at all time points, with nearly normal performance levels with visual recall across all trials, unlike those observed in some previous family studies (Sitskoorn et al., 2004; Snitz et al., 2006; Whyte et al., 2005). Third, patients demonstrated impaired short delay savings for both LM and VR, consistent with most other studies (Aleman et al., 1999; Cirillo and Seidman, 2003; Sitskoorn et al., 2004; Snitz et al., 2006; Toulopoulou et al., 2003a,b). Fourth, at 24 hour testing, patient savings scores differed slightly, but significantly from that of healthy controls for LM, but not VR. Finally, siblings displayed normal savings over all intervals for LM and VR, consistent with the few reports in the literature examining short delay savings in relatives (Laurent et al., 2000, 1999; Toulopoulou et al., 2003a,b; Trandafir et al., 2006). While we cannot prove the absence of differences, our study groups are large, providing ample statistical power, and we have provided effect sizes to describe our findings. It is difficult to compare our results for 24 hour LM and VR savings to prior reports in the literature, given methodological differences (Harris et al., 1996). Thus, patients and their siblings shared verbal recall impairment, which appears to mark the intermediate phenotype, whereas visual memory impairment appears to mark the clinical phenotype.
5.2. Effect size differences
Our effect sizes (seen in Table 2) are larger than recent meta-analyses (Aleman et al., 1999); (Dickinson et al., 2007) for LM and somewhat larger for VR tasks. This discrepancy is most likely caused by ascertainment biases. As a tertiary care center, patients admitted to the NIMH could be more severely ill than typical in community settings. Further, our control group was higher functioning and less variable than the general population (WAIS estimated IQ for controls was 107.5, with an SD=10.1). The NIMH Sibling study is demanding, and only highly motivated families are likely to volunteer, possibly resulting in unusually “unaffected” siblings and more highly educated patients. However, the fact that we still detected verbal memory deficits in a less impaired sibling cohort should enhance confidence that verbal recall will prove to be a robust intermediate phenotype in other samples.
How then do we understand the fact that siblings appear to share verbal – but not visual – memory impairments with their ill family member? One possibility is that the two tasks are not matched for discriminating power. That is, between group differences will be larger on the test with better discriminating power even if there is a similar degree of impairment on the abilities required by the two tests. Many more subjects performed at very high accuracy levels (>70% correct) on VR than LM. This was similarly true of siblings and controls: 95% of siblings and 96% of controls scored >70% correct on VR. Nonetheless, no perfect scores were observed in either group. While initial recalls may have approached ceiling, performance at the 30 minute and 24 hour interval moved farther away from ceiling, and should have therefore enhanced sensitivity to group differences. However, we did not see evidence of increasing deficit in the sibling group at later intervals. Siblings performed nearly identically to controls, a result that is hard to explain on the basis of discriminating power, unless one wanted to assert a gross sensitivity discrepancy between LM and VR. While logically possible, we do not think it is consistent with the widespread use and findings involving VR in the clinical and research literatures. Further, the psychometric differences between LM and VR immediate encoding should not undermine the validity of savings analyses: 24 hour savings scores in siblings were consistently high across both tasks. Nonetheless, further research using more challenging visual memory tasks, including measures of recognition performance, is needed to confirm our proposal that siblings perform as strongly as control populations.
Another possible explanation for memory discrepancies between patients and siblings is that the transition to illness involves visual memory mechanisms localized to the right hemisphere, whereas the intermediate phenotype is limited to left hemisphere memory systems. However, the literature on temporal lobe epilepsy and the WMS-R does not provide convincing evidence of differential laterality for the VR subtest, whereas LM may be more reliably compromised in focal left TLE (Barr et al., 1997). Thus, this argument, while appealing, appears to be at odds with the accumulated literature. We are struck by the fact that VR performance is correlated with FSIQ in patients and siblings. Thus, the lower IQ in patients should be accompanied by lower VR, suggesting that the VR deficit in patients (and not observed in well siblings) is part of a more general impairment across multiple cognitive functions. FSIQ had a much stronger relationship with initial encoding than with long delay savings, a pattern consistent with marked impairments in patient initial encoding performance coupled with relatively preserved long delay savings. While the origin of the patient VR deficit (not shared with siblings) remains a matter of speculation and argument, it is apparent that measures of verbal recall are robust indicators of shared impairment as an intermediate phenotype among siblings at risk for schizophrenia.
5.3. Interpretations and implications
There are important biological implications of our finding that both patients and siblings show greatest impairment in initial learning and little impairment in long delay savings. While it is yet to be determined exactly how long term potentiation (LTP) functions in human memory, our observations correspond to findings that the protein synthesis-dependent late phase of LTP (L-LTP) relies heavily on Brain-Derived Neurotrophic Factor (BDNF) (Bekinschtein et al., 2007). The notably small effect sizes in long delay savings in both ill patients and their relatives suggest that the mechanisms required for gene transcription and synaptic remodeling associated with L-LTP are surprisingly intact in schizophrenia. Learning impairments observed here may suggest a common deficit in the induction of LTP, a process where N-methyl-D-aspartic acid (NMDA) receptor activation plays a critical role. Indeed, the fact that NMDA receptor antagonists are capable of impairing memory encoding, but not long delay memory maintenance (Pastalkova et al., 2006) closely models our behavioral data. Further, the fact that many proposed schizophrenia susceptibility genes likely impact glutamatergic and NMDA receptor function (Le-Niculescu et al., 2007; MacDonald and Chafee, 2006), suggests that the learning deficits shared by patients and their siblings may result from shared genes that impact this pathway, and this aspect of behavior. While this formulation is somewhat speculative, we believe that a precise understanding of patient and sibling cognitive performance is needed to constrain consideration of basic biological mechanisms implicated by genetic findings in the illness.
Acknowledgments
We thank our recruiters for their diligence in bringing participants to the clinic year after year. We are also grateful to the families that dedicated their time to participate in our study.
Role of funding source
Funding for this study was provided by NIMH, with IRB protocol number 95-M-0160. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Dr. Daniel Weinberger and Dr. Michael Egan designed the study and wrote the protocol. Dr. Terry Goldberg and Dr. James Gold oversaw testing and data management. Shayna Skelley managed the literature searches, conducted statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Barr WB, Chelune GJ, Hermann BP, Loring DW, Perrine K, Strauss E, et al. The use of figural reproduction tests as measures of nonverbal memory in epilepsy surgery candidates. J Int Neuropsychol Soc. 1997;3(5):435–443. [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53(2):261. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, et al. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry. 1991;48(10):891–898. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- Calev A, Edelist S, Kugelmass S, Lerer B. Performance of long-stay schizophrenics on matched verbal and visuospatial recall tasks. Psychol Med. 1991;21(3):655–660. doi: 10.1017/s0033291700022297. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13(2):43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, et al. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr Bull. 2006;32(3):525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50(2):98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User's Guide for the SCID-I for DSM-IV Axis I Disorders-Research Version 1996 [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Harris JG, Adler LE, Young DA, Cullum CM, Rilling LM, Cicerello A, et al. Neuropsychological dysfunction in parents of schizophrenics. Schizophr Res. 1996;20(3):253–260. doi: 10.1016/0920-9964(96)00009-6. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status [Electronic Version] 1975 [Google Scholar]
- James JW. Frequency in relatives for an all-or-none trait. Ann Hum Genet. 1971;35(1):47–49. doi: 10.1111/j.1469-1809.1956.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Laurent A, Moreaud O, Bosson JL, Naegele B, Boucharlat J, Saoud M, et al. Neuropsychological functioning among non-psychotic siblings and parents of schizophrenic patients. Psychiatry Res. 1999;87(2–3):147–157. doi: 10.1016/s0165-1781(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Laurent A, d'Amato T, Naegele B, Murry P, Baro P, Foussard N, et al. Executive and amnestic functions of a group of first-degree relatives of schizophrenic patients. Encephale. 2000;26(5):67–74. [PubMed] [Google Scholar]
- Le-Niculescu H, Balaraman Y, Patel S, Tan J, Sidhu K, Jerome RE, et al. Towards understanding the schizophrenia code: an expanded convergent functional genomics approach. Am J Med Genet, B Neuropsychiatr Genet. 2007;144(2):129–158. doi: 10.1002/ajmg.b.30481. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Chafee MV. Translational and developmental perspective on N-methyl-D-aspartate synaptic deficits in schizophrenia. Dev Psychopathol. 2006;18(3):853–876. [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30(4):167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Risch N. Implications of multilocus inheritance for gene-disease association studies. Theor Popul Biol. 2001;60(3):215–220. doi: 10.1006/tpbi.2001.1538. [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71(2–3):285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulopoulou T, Morris RG, Rabe-Hesketh S, Murray RM. Selectivity of verbal memory deficit in schizophrenic patients and their relatives. Am J Med Genet, B Neuropsychiatr Genet. 2003a;116(1):1–7. doi: 10.1002/ajmg.b.10027. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Rabe-Hesketh S, King H, Murray RM, Morris RG. Episodic memory in schizophrenic patients and their relatives. Schizophr Res. 2003b;63(3):261–271. doi: 10.1016/s0920-9964(02)00324-9. [DOI] [PubMed] [Google Scholar]
- Trandafir A, Meary A, Schurhoff F, Leboyer M, Szoke A. Memory tests in first-degree adult relatives of schizophrenic patients: a meta-analysis. Schizophr Res. 2006;81(2–3):217–226. doi: 10.1016/j.schres.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM, Toulopoulou T, Rabe-Hesketh S, et al. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;78(1):13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]


