Abstract
Background
While it is now apparent clinical sequelae (long COVID) may persist after acute COVID-19, their nature, frequency and aetiology are poorly characterised. This study aims to regularly synthesise evidence on long COVID characteristics, to help inform clinical management, rehabilitation strategies and interventional studies to improve long-term outcomes.
Methods
A living systematic review. Medline, CINAHL (EBSCO), Global Health (Ovid), WHO Global Research on COVID-19 database, LitCovid and Google Scholar were searched till 17 March 2021. Studies including at least 100 people with confirmed or clinically suspected COVID-19 at 12 weeks or more post onset were included. Risk of bias was assessed using the tool produced by Hoy et al. Results were analysed using descriptive statistics and meta-analyses to estimate prevalence.
Results
A total of 39 studies were included: 32 cohort, 6 cross-sectional and 1 case–control. Most showed high or moderate risk of bias. None were set in low-income countries and few included children. Studies reported on 10 951 people (48% female) in 12 countries. Most included previously hospitalised people (78%, 8520/10 951). The longest mean follow-up time was 221.7 (SD: 10.9) days post COVID-19 onset. Over 60 physical and psychological signs and symptoms with wide prevalence were reported, most commonly weakness (41%; 95% CI 25% to 59%), general malaise (33%; 95% CI 15% to 57%), fatigue (31%; 95% CI 24% to 39%), concentration impairment (26%; 95% CI 21% to 32%) and breathlessness (25%; 95% CI 18% to 34%). 37% (95% CI 18% to 60%) of patients reported reduced quality of life; 26% (10/39) of studies presented evidence of reduced pulmonary function.
Conclusion
Long COVID is a complex condition with prolonged heterogeneous symptoms. The nature of studies precludes a precise case definition or risk evaluation. There is an urgent need for prospective, robust, standardised, controlled studies into aetiology, risk factors and biomarkers to characterise long COVID in different at-risk populations and settings.
PROSPERO registration number
CRD42020211131.
Keywords: COVID-19, public health, systematic review
Key questions.
What is already known?
A significant number of people continue to describe ongoing symptoms long after the acute phase of COVID-19, often referred to as long COVID.
Long COVID is a heterogeneous condition with an uncertain prevalence, for which there is currently no precise case definition.
What are the new findings?
The breadth of reported symptoms suggests a complex, heterogeneous condition affecting both those who were hospitalised and those managed in the community.
Our review identifies weakness (41%; 95% CI 25% to 59%), general malaise (33%; 95% CI 15% to 57%), fatigue (31%; 95% CI 24% to 39%), concentration impairment (26%; 95% CI 21% to 32%) and breathlessness (25%; 95% CI 18% to 34%) as the most common symptoms reported.
What do the new findings imply?
The current evidence base of the clinical spectrum of long COVID is limited, based on heterogenous data, and vulnerable to biases, hence caution should be used when interpreting or generalising the results.
Our review identifies areas where further long COVID research is critically needed to help characterise long COVID in different populations and define its aetiology, risk factors and biomarkers, as well as the impact on variants of concern and vaccination on long-term outcomes.
Introduction
SARS-CoV-2 first emerged in December 2019 causing a widespread pandemic. Most people experience asymptomatic or mild-to-moderate acute COVID-19 symptoms, while around 15% of people are estimated to progress to more severe disease requiring hospitalisation and approximately 5% become critically ill.1
While the acute phase of the disease was characterised early, there are still limited data on long-term outcomes.2 Symptoms of long-lasting COVID-19 sequelae and complications, termed long COVID by people living with long COVID,3 have been reported worldwide. Yet the underlying aetiology behind prolonged or fluctuating symptomatology is limited and there is no widely accepted uniformed case definition.4 Instead, long COVID has been defined pragmatically as ‘not recovering for several weeks or months following the start of symptoms’.4 Others have distinguished between postacute COVID-19, referring to symptoms beyond 3 weeks, and chronic COVID-19, referring to symptoms beyond 12 weeks,5 while the National Institute for Health and Care Excellence distinguishes between ongoing symptomatic COVID-19 lasting from 4 to 12 weeks and post COVID-19 syndrome continuing for over 12 weeks.6
The number of people living with long COVID is unknown. Attempts to quantify the prevalence of long COVID use different methods, including national surveys and patient-led studies, making it difficult to compare across studies. The UK’s Office for National Statistics has estimated that on average 1 in 5 people have symptoms beyond 5 weeks, while 1 in 10 have symptoms persisting over 12 weeks.7 A patient-led survey found that in survival analysis, the chance of full recovery by day 50 was smaller than 20%8 and a COVID-19 symptom app study found that 13.3% (558/4182) patients had symptoms lasting 28 days or more, 4.5% (189/4182) patients had symptoms for 8 or more weeks and 2.3% (95/4182) patients had symptoms lasting over 12 weeks.9
The symptoms of long COVID are equally ill-defined, with patients describing it as a fluctuating illness of disparate symptoms.8 10 Indeed, the National Institute for Health Research has suggested that postacute COVID-19 may consist of several distinct clinical syndromes including: a postintensive care syndrome, chronic fatigue syndrome, long-term COVID-19 syndrome and disease from SARS-CoV-2 inflicted organ damage.11 Additionally, even with an expanding knowledge of risk factors in the acute phase, little is currently known on predictive factors for developing long COVID.9 Despite suggested classifications, there is yet no clear consensus.
Our early understanding of long COVID has been accumulated from case reports and cross-sectional online survey studies as the pandemic global research focus has largely been on studies of hospitalised patients during the acute phase. As the pandemic progresses, emerging studies have followed up patients to present the fluctuating multiorgan sequelae of acute COVID-19, yet evidence is still scarce. There continues to be a call to further understand and acknowledge this condition by incorporating patient knowledge and experiences, together with standardised studies, exploring underlying aetiologies behind different syndromes.12 13
Given the enormous number of people worldwide who have suffered from COVID-19, it is essential to establish a precise categorisation of long COVID. Such categorisation will not only help people better understand their symptoms but also direct research into prevention, treatment and support, ultimately allowing us to understand and prepare to respond to the long-term consequences inflicted by the COVID-19 pandemic. Our review seeks to synthesise and continually update the evidence on the character and prevalence of long COVID.
Methods
Systematic reviews conducted early during the COVID-19 pandemic soon became redundant due to the rapidity with which new research was released. In recognition of this, many reviewers have moved towards the concept of a ‘living systematic review’ (LSR), which compared with traditional systematic reviews has in-built mechanisms for regular update and renewal.14 15 We conducted a ‘living’ systematic review to provide frequently updated evidence on the symptoms and complications of long COVID. This review was developed in collaboration with infectious disease clinicians, public health professionals, information specialists, review methodologists with experience in clinical epidemic research and members of the global Long COVID Support Group, which includes people living with long COVID. This is the first version of this LSR, which will be updated approximately every 6 months as new evidence emerges, using the established protocol and review platform. The updates will be led by the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) systematic review team in collaboration with members of Long COVID Support. Previous versions will be archived in online supplemental materials. The findings will be disseminated via BMJ Global Health and on a dedicated webpage with infographics and a brief summary for lay people and professionals.
bmjgh-2021-005427supp001.pdf (9.7MB, pdf)
Protocol registration
This report was structured according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines.16 The protocol was registered with PROSPERO and published in a peer-reviewed journal.17
Search strategy
The following databases were searched: Medline and CINAHL (EBSCO), Global Health (Ovid), WHO Global Research Database on COVID-19 and LitCovid from 1 January 2020 to 17 March 2021. Additionally, we searched Google Scholar on 17 March 2021, screening the first 500 titles. A ‘backwards’ snowball search was conducted of the references of systematic reviews. Full search terms are included in online supplemental file 1. The search terms and inclusion criteria have, for this first version, been designed to cast a wide net and will be modified in line with new evidence, research priorities and clinical and policy needs.
Eligibility criteria
Peer-reviewed studies were considered eligible if they included at least 100 people with laboratory confirmed and/or clinically diagnosed COVID-19. Without a clear, internationally agreed case definition, we included studies that reported symptoms or outcomes assessed at 12 or more weeks post COVID-19 onset.6 There were no language restrictions. Reviews and opinion pieces were excluded. Studies were excluded if they included fewer than 100 participants, to avoid small study effects,18 or the follow-up was unclear or less than 12 weeks post onset.
Screening
Screening was performed independently by two systematic reviewers. Any disagreements were resolved via consensus or a third reviewer. Non-English articles were translated using Google Translate and assessed by a systematic reviewer with good knowledge of the language. The data were managed using the review software Rayyan.19
Data extraction
Data extraction was performed using Microsoft Excel. A data extraction template informed by a previous review20 was reviewed, updated and piloted before being finalised. Data extracted included study design, population characteristics, outcomes, prevalence, duration of symptoms and risk factors. Data extraction was performed by one systematic reviewer and checked by a second reviewer. Disagreements were resolved through consensus. To avoid duplication of data in future updates and ensure robustness, data extraction was not performed for non-peer-reviewed preprints.
Risk of bias assessment
The included studies were assessed for risk of bias using the tool produced by Hoy et al21 (online supplemental file 2). This assessment checklist is a validated tool for assessing risk of bias in prevalence studies. The checklist has 10 domains for assessing risk of bias, used to calculate a cumulative overall risk of bias for the whole study.
Data analysis
We undertook individual descriptive analysis for each study. We presented symptom proportions by different settings, as presented in the individual studies: hospitalised, non-hospitalised or a mix of both populations if no subset data were available. Symptoms were broadly grouped into physiological clusters through discussion with clinicians. Proportion of symptoms and its 95% CIs were estimated using the exact method.22 If there were two or more studies for each symptom, a meta-analysis was performed using a random intercept logistic regression model with Hartung-Knapp modification due to the heterogeneity and skewed sample sizes.23 24 Heterogeneity between estimates was assessed using the I2 statistic.25 Additional subgroup analysis was conducted to explore the modification of the following factors on proportion of symptoms: hospitalisation, settings, continents and follow-up timing. We also conducted meta-regression analysis on the percentage of females and intensive care unit (ICU) patients where there were more than 10 studies for the symptom. Sensitivity analyses were conducted to examine the impact of high risk of bias studies and statistical methods, Freeman-Tukey double arcsine transformation using inverse variance meta-analysis, on the estimates. Funnel plots were plotted using proportion of the symptom against the precision and sample sizes22 where there were more than 10 studies for the symptom to explore risk of publication bias. All analysis and data presentation were performed using metaprop26 and ggplot227 in R (V.4.0.5) via RStudio (V.1.3.1093).28 The data are presented using a combination of infographics, prepared by a design company (Design Science29) and scientific tables to facilitate interpretation by different stakeholders, including non-specialists.
Patient and public involvement
The study team includes members who have been affected by long-term COVID-19 sequalae, including members of Long COVID Support,10 a patient support group with global reach, with approximately 40 000 members.
They actively contributed to the development of the study protocol, to inform the research questions and interpretation and presentation of the findings and to communicate the results to different audiences. The results of this LSR will be disseminated to long COVID patient forums for discussion and feedback to inform research priorities and updates.
Results
We identified 6459 studies, of which 39 met the inclusion criteria (online supplemental file 3), all of which were published in English. Of these, 32 were included in the meta-analysis. The remaining studies include single symptoms or imaging and diagnostics and are presented narratively.
Characteristics of included studies
Most studies were set in Europe (62%, 24/39), followed by Asia (23%, 9/39), North America (8%, 3/39) and the Middle East 8% (3/39) (figure 1). There was no study set in a low-middle income country.30 Most were cohort studies (82%, 32/39), followed by cross sectional studies (15%, 6/39) and a case–control study (3%, 1/39). These studies present data on 10 951 (range: 100–1733) people in 12 countries, aged from 9 months to 93 years old and 48% (5206/10 951) were females.
Figure 1.
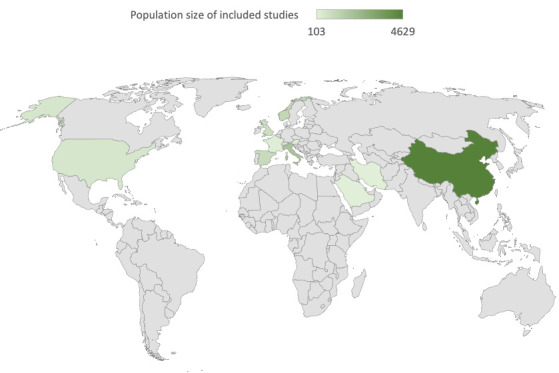
Map of study distribution.
The map shows the global distribution of the studies identified and the shading shows the combined studies population size by country.
Most studies included adults, while 10% (4/39) also included children.31–34 Only 15% (6/39) of studies reported ethnicity of the participants,35–40 but without stratification. Table 1 presents the included study characteristics.
Table 1.
Study characteristics
| Study | Design | Country | Population size | Age (years) |
Sex (% female) |
COVID-19 confirmation method | Follow-up time (days) | Follow-up timepoint | Follow-up mode |
| Non-hospitalised | |||||||||
| Hopkins et al58 | Cross sectional | UK | 434 | Median (range): 40 (19–77) | 75 | PCR or serological assays (26.3%) | 6 months | First survey | Electronic survey |
| Klein et al47 | Cohort (P) | Israel | 103 | Mean (SD): 35 (12) | 38 | PCR (RT-PCR) | 6 months | Onset | Phone interview |
| Petersen et al32 | Cohort (P) | Faroe Islands | 180 | Mean (SD; range): 39.9 (19.4; 0–93) | 54 | PCR (RT-PCR) | Mean (SD) 125 (17) | Onset | Phone interview |
| Stavem et al68 | Crosssectional | Norway | 451 | Mean (SD): 49.8 (15.2) | 56 | PCR (RT-PCR) | Median (range): 117 (41–193) | Onset | Outpatient visit and survey |
| Non-hospitalised and hospitalised | |||||||||
| Parente-Arias et al55 | Cohort (P) | Spain | 151 | Mean (range): 55.2 (18–88) | 65 | PCR (RT-PCR) | Mean (SD): 100.5 (3.3) | Admission | Phone interview |
| Venturelli et al60 | Cohort (P) | Italy | 767 | Mean (SD): 63 (13.6) | 33 | PCR (RT-PCR) (94%); serology (5%) Clinician diagnosis (1.2%) |
Median (IQR): 105 (84–127) | Onset | Outpatient visit |
| Anastasio et al41 | Cohort (P) | Italy | 379 | Median (IQR; range): 56 (49–63; 20–80) | 54 | PCR (RT-PCR) | Median (IQR): 135 (102–175) | Onset | Outpatient visit |
| Einvik et al67 | Crosssectional | Norway | 538 | Mean (SD) 57.7 (14.2) (hospital) 49.6 (15.3) |
42 (hospital) 56 |
PCR (RT-PCR) | Mean (SD): 112 (30) (hospital) 118 (27) |
Onset | Outpatient visit and survey |
| Jacobson et al40 | Cohort (P) | USA | 118 | Mean (SD): 43.3 (14.4) | 47 | PCR (RT-PCR) | Mean (SD): 119.3 (33) | Diagnosis | Outpatient visit |
| Logue et al35 | Cohort (P) | USA | 177 21 (C) |
Mean (SD): 48 (15.2) | 57 | Lab confirmed | Median (range): 169 (31–300) | Onset | Electronic survey |
| Mazza et al70 | Cohort (P) | Italy | 226 | Mean (SD; range): 58 (12.8; 26–87) | 34 | PCR (RT-PCR) | Mean (SD): 90 (13.4) | Discharge | Phone interview |
| Rass et al50 | Cohort (P) | Austria | 135 | Median (IQR; range) 56 (48–68; 19–87) | 39 | PCR (RT-PCR) | Median (IQR): 102 (91–110) | Onset | Outpatient visit |
| Sonnweber et al48 | Cohort (P) | Austria | 145 | Mean (SD): 57 (14) | 43 | PCR (RT-PCR) | Mean (SD): 103 (21) | Diagnosis | Outpatient visit |
| Hospitalised | |||||||||
| Alharthy et al54 | Cohort (P) | Saudi Arabia | 127 | Mean (SD): 47 (11.38) | 21 | PCR (RT-PCR) | 4 months | Discharge | Outpatient visit |
| Arnold et al37 | Cohort (P) | UK | 110 | Median (IQR): 60 (46–73) | 38 | PCR or radiological diagnosis | Median (IQR): 90 (80–97) | Onset | Outpatient visit |
| Baricich et al63 | Crosssectional | Italy | 204 | Mean (SD): 57.9 (12.8) | 40 | NR | Mean (SD): 124.7 (17.5) | Discharge | Outpatient visit |
| Bellan et al42 | Cohort (P) | Italy | 238 | Median (IQR): 61 (50–71) | 40 | PCR (RT-PCR) (97.5%); bronchoalveolar lavage (0.4%); serology/radiological (2.1%) | 3–4 months | Discharge | Outpatient visit |
| Blanco et al38 | Cohort (P) | Spain | 100 | Mean (SD) TLco<80: 54.98 (10.72) TLco>80: 54.75 (9.83) |
36 | PCR (RT-PCR) | Median (IQR): 104 (89.25–126.75) | Onset | Outpatient visit |
| Doyle et al66 | Cohort (P) | UK | 129 | Mean: 62 (Cambridge) 56 (London) |
31 (Cambridge) 27 (London) |
PCR (RT-PCR) | Median (range): 113 (96–138) | Discharge | NR |
| Garrigues et al65 | Cohort (P) | France | 120 | Mean (SD): 63.2 (15.7) | 38 | PCR (RT-PCR) | Mean (SD): 110.9 (11.1) | Admission | Phone interview |
| Gherlone et al57 | Cohort (P and R) | Italy | 122 | Median (IQR): 62.5 (53.9–74.1) | 25 | PCR (RT-PCR) | Median (IQR): 104 (95–132) | Discharge | Outpatient visit |
| Han et al46 | Cohort (P) | China | 114 | Mean (SD; range): 54 (12; 24–82) | 30 | PCR (RT-PCR) | Mean (SD): 175 (20) | Onset | Outpatient visit |
| Huang et al56 | Cohort (P and R) | China | 1733 | Median (IQR): 57 (47–65) | 48 | Lab confirmed | Median (IQR): 186 (175–199) | Onset | Outpatient visit |
| Zhang et al31 | Cohort (R/S) | China | 527 | Median (IQR; range): 42.5 (32–54; 0–91) | 44 | NR | 6 months | Discharge | Outpatient visit |
| Lerum et al61 | Cohort (P) | Norway | 103 | Median (25th–75th percentile): 59 (49–72) | 48 | Nasopharyngeal swab | 3 months | Discharge | Outpatient visit |
| Méndez et al49 | Cohort (R/S) | Spain | 215 | Median (IQR): 55 (47–66) | 40 | Lab confirmed | Median (IQR): 87 (62–109) | Discharge | Outpatient visit |
| Nguyen et al33 | Cohort (P) | France | 125 | Median (IQR; range): 36 (27–48; 16–85) | 55 | PCR (RT-PCR) | Mean (SD): 221.7 (10.9) | Onset | Phone interview |
| Nugent et al36 | Cohort (R/S) | USA | 182 1430 (C) |
Median (IQR): 67.4 (58.3–80.1) | 47 | PCR (RT-PCR) | Median (IQR): 92.9 (52.5–127.7) | Discharge | Outpatient visit |
| Qin et al53 | Cohort (P) | China | 647 | Mean (SD): 58 (15) | 56 | PCR (RT-PCR) | 90 | Discharge | Outpatient visit |
| Qu et al34 | Cohort (P) | China | 540 | Median (IQR): 47.50 (37–57) | 50 | PCR (RT-PCR) | 3 months | Discharge | Electronic survey |
| Sibila et al51 | Cohort (P) | Spain | 172 | Mean (SD): 56.1 (19.8) | 43 | NR | Mean (SD): 101.5 (19.9) | Discharge | Outpatient visit |
| Simani et al59 | Cohort (P) | Iran | 120 | Mean (SD): 54.62 (16.94) | 33 | PCR or radiological diagnosis | 6 months | Discharge | Outpatient visit |
| Suárez-Robles et al64 | Crosssectional | Spain | 134 | Mean (SD): 58.53 (18.53) | 54 | PCR (RT-PCR) | 90 | Discharge | Phone survey |
| Sykes et al39 | Cohort (P) | UK | 134 | Median (range): 58 (25–89) | 34 | PCR (RT-PCR) | Median (range): 113 (46–167) | Discharge | Outpatient visit |
| Taboada et al62 | Cross sectional | Spain | 183 | Mean (SD): 65.9 (14.1) | 40 | PCR (RT-PCR) | 6 months | Discharge | Unstructured interview |
| Weng et al45 | Cohort (P) | China | 117 | 45.3%≥60 years | 44 | Viral nucleic acid test | 90 | Discharge | Phone interview |
| Xiong et al44 | Cohort (P) | China | 538 184 (C) |
Median (IQR; range): 52 (41–62; 22–79) | 55 | PCR (RT-PCR) | Median (IQR; range): 97.0 (95.0–102.0; 91–116) |
Discharge | Phone interview |
| Xu et al43 | Case–control | China | 103 27 (C) |
Median (IQR) M/M: 56 (45–63) S/C: 61 (55–68) |
M/M: 58.8 S/C: 53.6 |
NR | 3 months | Discharge | Outpatient visit |
| Zhang et al52 | Cohort (P) | China | 310 | Median (IQR): 51 (31.8–61) | 50 | PCR (RT-PCR) | Median (IQR): 92.0 (90–100) | Discharge | Outpatient visit |
C, control group; M/M, mild/moderate; NR, not reported; P, prospective; PCR, polymerase chain reaction; R, retrospective; RT, Reverse transcription; S/C, severe/critical; TLco, carbon monoxide transfer factor.
Most studies (67%, 26/39) were cohorts of hospitalised patients post discharge, 10% (4/39) followed up people who were not hospitalised, while 23% (9/39) included both (hospitalised and non-hospitalised populations). Of the inclusions in this review, 78% (8520/10 951) were previously hospitalised during the acute COVID-19 phase. Twenty-two studies included people requiring ICU admission during the acute phase.31 33–35 37 38 40–55
The longest follow-up period in any study was a mean of 221.7 (SD: 10.9) days post onset. Only 56% (22/39) of studies specified COVID-19 severity,31 33–35 37 38 40–55 31% (12/39) treatment received during the acute phase36 40 41 45 46 50 53 56–60 and 62% (24/39) described ventilation support requirements.36–42 45 46 48–51 53 54 56 57 60–66 Pre-existing comorbidities were reported in the majority of studies (85%, 33/39), with hypertension and diabetes most commonly documented.33 35–57 59–63 65 67–69
Risk of bias
Overall, 12 studies were assessed as high risk of bias, 22 as moderate risk of bias and 5 as low risk of bias. Most studies had a high risk of bias with regard to the generalisability of their results to the wider population with COVID-19. High risk of bias ratings were most common for external validity, with item 1 (representation of target population) and item 3 (random selection) having the most high risk of bias ratings (online supplemental file 2). Further, the recruitment process and response rates were often not well described and several studies applied different data collection methods. Although many studies applied validated measurement methods to assess participants, most were not designed to detect symptoms arising from COVID-19. Only four studies included a comparative control group.35 36 43 44
Symptoms and signs
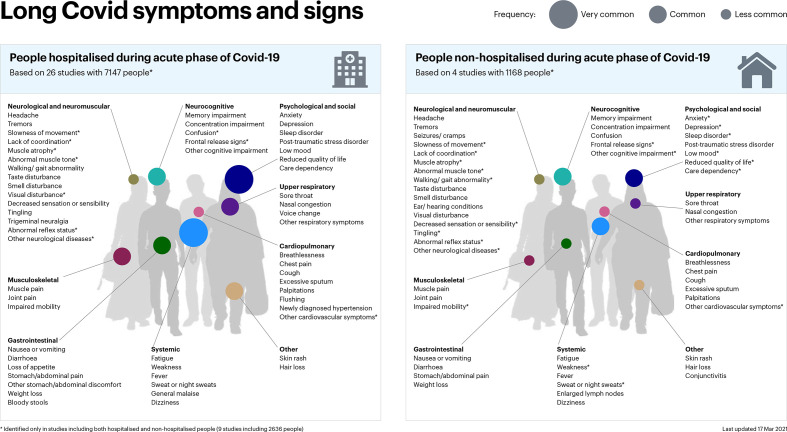
Patients suffering from long COVID report a wide range of new or persistent symptoms, in both the hospitalised and non-hospitalised populations. Symptoms were broadly organised into physiological ‘clusters’ for the purpose of presentation and interpretation of this review (figure 2).
Figure 2.
Long COVID signs and symptoms.
The focus of each study included in our analysis varied. Some authors focused solely on a specialty, such as dentistry, or a specific symptom, such as cognition, making comparative analysis difficult. Even among those studies which took a broad approach, the prevalence of symptoms was diverse. Similarly, the prevalence of the more commonly reported symptoms varied markedly.
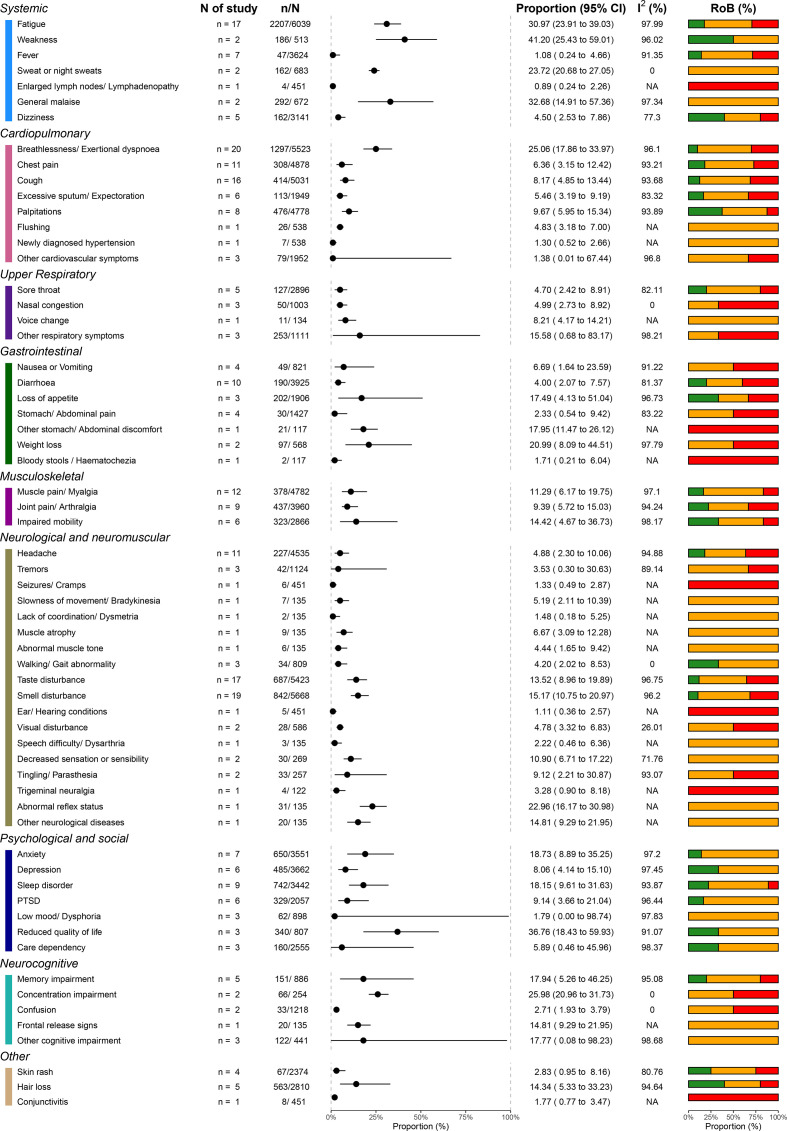
Within these limitations, we performed a meta-analysis of the most commonly reported symptoms and signs of long COVID. The most commonly described symptoms (with prevalence of 25% or greater) were weakness (41%, 95% CI 25.43 to 59.01), general malaise (33%, 95% CI 14.91 to 57.36), fatigue (31%, 95% CI 23.91 to 39.03), concentration impairment (26%, 95% CI 20.96 to 31.73) and breathlessness (25%, 95% CI 17.86 to 33.97). Across studies, 37% (95% CI 18.43 to 59.93) of patients reported reduced quality of life. Although high I2 values (>80%) were observed, they resulted from narrow dispersions in the estimates and well-separated estimates and CIs between studies (online supplemental file 4). The differences between these symptoms and the heterogeneity within them are likely to be, to some extent, due to other factors (eg, study settings, populations and different measurement tools used).
Patients also reported a diverse array of less prevalent symptoms and signs, including sweating, chest pain, sore throat, anxiety and headaches, among others. The prevalence of these symptoms was lower, usually less than 20%. Figure 3 presents the range of documented patient symptoms and signs, including all the studies.
Figure 3.
Signs and symptoms in all studies. RoB, risk of bias.
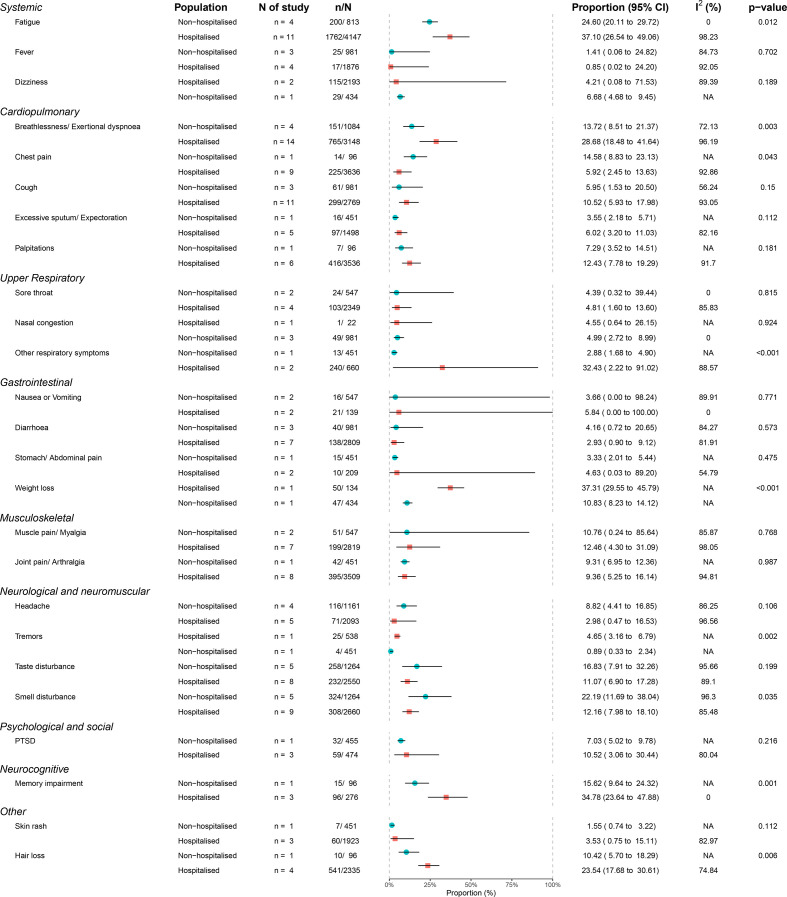
Figure 4 displays these data by population, including the studies that specified hospitalised and non-hospitalised cohorts. We also performed subgroup analysis based on setting (hospitalised vs non-hospitalised) and follow-up time. In several symptoms and signs, the heterogeneity of the results was found to be associated with level of hospitalisation, hospital settings, location of the studies and follow-up timing using subgroup analysis (online supplemental files 5-8). Using meta-regression, the proportion of female patients in the studies was positively associated with headache and smell and taste disturbance (online supplemental file 9), while the proportion of ICU patients in the studies was positively associated with muscle pain (online supplemental file 10). No major difference was found in the sensitivity analyses (online supplemental files 11 and 12). Asymmetries found in the funnel plots suggest reporting biases and poor methodological quality in the included studies (online supplemental file 13).
Figure 4.
Sign and symptoms in hospitalised and non-hospitalised cohorts. Note: The data on sign and symptoms from studies with data on hospitalised or non-hospitalised cohorts, it does not include studies that included mixed cohorts without subcategorisation. PTSD, post-traumatic stress disorder.
Imaging and diagnostics
Multiple studies assessed lung sequelae and respiratory performance through outpatient visits follow-up (49%, 19/39).31 37–43 46 48 49 51–54 56 60 61 66 Imaging results were reported in 33% (13/39)31 37–39 43 46 48 52–54 56 61 66 of the cohort studies, with one including controls43 and one with a population including children.31 Authors used heterogenous measurement techniques with an observed tendency towards novel imaging, including artificial intelligence and point‐of‐care ultrasound.43 54 Studies found abnormal CT results, including consolidation, reticulation, residual ground glass opacity, interstitial thickening and fibrotic changes. Some of these studies presented comparisons between initial CT findings and those at follow-up, showing improvements in pulmonary clinical measures and radiologic resolutions at follow-up visits.37 39 46 48 54 One study assessing thrombotic complications in COVID-19 with a minimum of 90-day follow-up from critical care admission found low rates of hospital-associated venous thromboembolism post discharge.66
Pulmonary function tests were reported in 26% (10/39) of studies,37 38 41–43 48 49 51 53 61 including spirometry, diffusion capacity, lung volume and exercise tests. These studies found evidence of altered pulmonary function, most frequently significant reduction of carbon monoxide transfer factor.
One study assessed kidney function in people with COVID-19-associated acute kidney injury (AKI) compared with people with non-COVID-19-associated AKI, found that COVID-19-related AKI was associated with decreased kidney recovery during outpatient follow-up.36
Risk factors
Exploring the literature, we sought to produce a meta-analysis of risk factors for long COVID. We found a considerable diversity of reported risk factors, including age, sex, comorbidities, ethnicity and severity of the acute phase.
Several cohorts (64%, 25/39) assessed whether there was an association between the severity of initial COVID-19, including symptom load, level of hospital care, need for mechanical ventilation and the risk of persisting sequelae. An association between female gender and long COVID risk has also been noted in longitudinal studies (20.5%, 8/39), as has the association between presence of comorbidity,40 55 57 63 68 70 increasing age32 34 50 55 62 63 and minority ethnicity,40 67 with long COVID and long COVID risk.
The limitations of the existing evidence base and inconsistency of reported findings preclude confident conclusions at this time. Instead, we have summarised the reported significant associations to date (online supplemental file 14) and suggest that these associations be explored in prospective controlled trials.
Discussion
Our work represents the most comprehensive review of evidence regarding long COVID yet produced. Accurate to 17 March 2021, this LSR captures the breadth of persistent symptoms reported in 39 studies, including over 10 000 people. These data suggest long COVID is a syndrome affecting both previously hospitalised and non-hospitalised people, characterised by marked fatigue, weakness, general malaise, breathlessness and concentration impairment lasting for a prolonged period of time. Besides these common symptoms, there is a diverse array of secondary symptoms. The findings in this review show symptoms and prevalence aligned to current knowledge on long COVID. The Office for National Statistics (ONS) Cohort Study, including control participants, reports the most common symptoms persisting for 12 or more weeks included fatigue (8.3%), headache (7.2%), cough (7%) and myalgia (5.6%).7
A deeper understanding of long COVID is currently prevented by the limitations of the published literature. The studies included in our review were highly heterogeneous due to differences in their study designs, settings, populations, follow-up time and symptom ascertainment methods. In addition, studies used inconsistent terminology describing symptoms and limited details and stratification on pre-existing comorbidities, the severity of COVID-19 and treatment methods. This inconsistency and limited reporting partly explain the high degree of variability observed. The lack of case–control studies prevent a direct attribution of symptoms solely to COVID-19; larger prospective studies with matched control groups are needed. We note that there are large, robust prospective cohort studies of hospitalised patients71 and non-hospitalised people.72 Simultaneously, qualitative studies are ongoing to better explore the long COVID patient experience.73
The findings have identified several research gaps and priorities. The majority of long COVID cohorts were conducted in Western Europe on patients recently discharged from hospital. There is a paucity of evidence on the long-term effects of COVID-19 in low-to-middle income countries and in people who were not hospitalised. Similarly, there were no studies identified focusing on children, despite evidence showing that children and young people are also affected by long COVID.74 Additionally, no study stratified by ethnicity, an important risk factor for the acute phase.
Our review also highlights a need for standardised and validated COVID-19 research tools to harmonise data collection, improve quality and reduce reporting variability. For instance, fatigue is one of the most commonly reported symptoms of long COVID. However, the symptom alone is not clearly defined and it is open to different interpretations, hence it requires a validated tool such as the Visual Analogue Scale, graded fatigue scale for robust, objective and comparative analysis. ISARIC has developed open access research tools available to sites globally to facilitate standardisation of data collection, analysis and interpretation for adults and children of an age.75 We support the broader use of this tool as well as initiatives to standardise outcome measures for long COVID.
Similarly, our study highlights the need for further research to refine the many circulating interim case definitions and precisely characterise long COVID, including the potential impacts of variants of concern and vaccination on long COVID.
As this is an LSR, emerging themes from this first version will inform future updates. The LSR will be updated periodically, as new research is published internationally, in order to provide relevant up to date information for clinicians, patients, researchers, policy-makers and health-service commissioners. Version changes will be identified and previous reports will be archived.
Conclusion
This LSR summarises published evidence on the spectrum of long-term COVID-19-associated symptoms and sequelae (as of 17 March 2021). It is clear that long COVID affects different populations, with a wide range of symptomatology. Our findings suggest this multiorgan syndrome is characterised by fatigue, weakness, malaise, breathlessness and concentration impairment, among other less frequent symptoms. Currently, the strength of the available evidence is limited and prone to bias. The long-term effects of COVID-19, in both hospitalised and non-hospitalised individuals, including children and at-risk populations, should be a priority for future research using standardised and controlled study designs. Robust research is needed to characterise and define long COVID and identify risk factors and underlying aetiology, in order to inform prevention, rehabilitation, clinical and public health management to improve recovery and long-term COVID-19 outcomes. This LSR will be updated approximately every 6 months as new evidence emerges for up to 2 years.
Acknowledgments
The authors would like to thank the members of the Long Covid Support Group and the International Severe Acute Respiratory and emerging Infection Consortium Global Support Centre.
Footnotes
Handling editor: Seye Abimbola
LM, NE, VC and AD contributed equally.
Contributors: MM, CS, VC and LS developed the concept of the study and led on the development of the protocol in collaboration with EH and members of the Long Covid Support Group (CH, MO, JSuett). MM, CS and LS led on the drafting of the manuscript with contributions from all coauthors. EH, VC and MM performed the online searches. MM, CS, NE and LM screened the articles for inclusion. MM, CS and PB extracted data from the included articles. DD, IR and CS critically appraised the studies. VC led on the meta-analysis and presentation of the figures, in collaboration with MM, DD, VC, NE, LM, CH, MO, JScott, DD, PB, IR, DM, AJEB, CF, GC, PO, CS and LS, who helped inform the analysis, interpretation of the results and formulation of recommendations. All coauthors reviewed and approved the manuscript.
Funding: This work was supported by the UK Foreign, Commonwealth and Development Office and Wellcome (215091/Z/18/Z), the Bill & Melinda Gates Foundation (OPP1209135) and the EU FP7 project PREPARE (602525).
Competing interests: JSuett declares he is an individual living with long-term symptoms of probably COVID-19. All other authors declare no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 2020. [Google Scholar]
- 2.ONS . The prevalence of long COVID symptoms and COVID-19 complications - Office for National Statistics, 2020. Available: https://www.ons.gov.uk/news/statementsandletters/theprevalenceoflongcovidsymptomsandcovid19complications [Accessed 14 Aug 2021].
- 3.Callard F, Perego E. How and why patients made long Covid. Soc Sci Med 2021;268:113426. 10.1016/j.socscimed.2020.113426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabavi N. Long covid: how to define it and how to manage it. BMJ 2020;370:m3489. 10.1136/bmj.m3489 [DOI] [PubMed] [Google Scholar]
- 5.Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020;370:m3026. 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 6.NICE . Overview COVID-19 rapid guideline: managing the long-term effects of COVID-19 | guidance, 2020. Available: https://www.nice.org.uk/guidance/ng188 [Accessed 05 May 2021]. [PubMed]
- 7.ONS . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK - Office for National Statistics, 2021. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 [Accessed 03 May 2021].
- 8.Patient Led Research . Report: what does COVID-19 recovery actually look like? Available: https://patientresearchcovid19.com/research/report-1/ [Accessed 29 Oct 2020].
- 9.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long Covid Support Group . Facebook groups. Available: https://www.facebook.com/groups/longcovid/ [Accessed 29 Oct 2020].
- 11.NIHR Evidence - Living with Covid19 - Informative and accessible health and care research. Available: https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ [Accessed 29 Oct 2020].
- 12.Callard F, Perego E. How and why patients made long Covid. Soc Sci Med 2021;268:113426. 10.1016/j.socscimed.2020.113426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet 2021;397:455–7. 10.1016/S0140-6736(20)32705-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.REH-cover - rapid living systematic reviews. Available: /covid-19/reh-cover-rapid-living-systematic-reviews [Accessed 09 Nov 2020].
- 15.Elliott JH, Synnot A, Turner T, et al. Living systematic review: 1. introduction-the why, what, when, and how. J Clin Epidemiol 2017;91:23–30. 10.1016/j.jclinepi.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michelen M, Sigfrid L, Manoharan L, et al. What are the long-term symptoms and complications of COVID-19: a protocol for a living systematic review. F1000Res 2020;9:1455. 10.12688/f1000research.27284.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29. 10.1016/s0895-4356(00)00242-0 [DOI] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michelen M, Jones N, Stavropoulou C. In patients of COVID-19, what are the symptoms and clinical features of mild and moderate cases? CEBM. Available: https://www.cebm.net/covid-19/in-patients-of-covid-19-what-are-the-symptoms-and-clinical-features-of-mild-and-moderate-case/ [Accessed 29 Oct 2020].
- 21.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13. 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 23.Jackson D, Law M, Rücker G, et al. The hartung-knapp modification for random-effects meta-analysis: a useful refinement but are there any residual concerns? Stat Med 2017;36:3923–34. 10.1002/sim.7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, et al. Seriously misleading results using inverse of freeman-tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019;10:476–83. 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham H. Ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag, 2009. [Google Scholar]
- 28.RStudio Team . RStudio: integrated development for R, 2020. Available: https://support.rstudio.com/hc/en-us/articles/206212048-Citing-RStudio [Accessed 14 Aug 2021].
- 29.Design science: bring knowledge to life. Available: https://design-science.org.uk/ [Accessed 14 Aug 2021].
- 30.World bank country and lending groups – world bank data help desk. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Accessed 4 Dec 2020].
- 31.Zhang J, Xu J, Zhou S, et al. The characteristics of 527 discharged COVID-19 patients undergoing long-term follow-up in China. Int J Infect Dis 2021;104:685–92. 10.1016/j.ijid.2021.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis 2020;324. 10.1093/cid/ciaa1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen NN, Hoang VT, Lagier J-C, et al. Long-Term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin Microbiol Infect 2021;27:931-932. 10.1016/j.cmi.2020.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu G, Zhen Q, Wang W, et al. Health-related quality of life of COVID-19 patients after discharge: a multicenter follow-up study. J Clin Nurs 2021;30:1742-1750. n/a. 10.1111/jocn.15733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021;4:e210830. 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open 2021;4:e211095. 10.1001/jamanetworkopen.2021.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021;76:399–401. 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco J-R, Cobos-Ceballos M-J, Navarro F, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect 2021;27:892-896. 10.1016/j.cmi.2021.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is Long-COVID and how should we manage it? Lung 2021;199:113–9. 10.1007/s00408-021-00423-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis 2021;73:e826-e829. 10.1093/cid/ciab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-erm impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J 2021. 10.1183/13993003.04015-2020. [Epub ahead of print: 11 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021;4:e2036142. 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Zhou M, Luo P, et al. Plasma metabolomic profiling of patients recovered from COVID-19 with pulmonary sequelae 3 months after discharge. Clin Infect Dis 2021. 10.1093/cid/ciab147. [Epub ahead of print: 17 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021;27:89–95. 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol 2021;6:344–6. 10.1016/S2468-1253(21)00076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han X, Fan Y, Alwalid O, et al. Six-Month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021;299:E177–86. 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect 2021;27:769–74. 10.1016/j.cmi.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J 2021;57. 10.1183/13993003.03481-2020. [Epub ahead of print: 29 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Méndez R, Latorre A, González-Jiménez P, et al. Reduced diffusion capacity in COVID-19 survivors. Ann Am Thorac Soc 2021;18:1253–5. 10.1513/AnnalsATS.202011-1452RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rass V, Beer R, Schiefecker AJ, et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur J Neurol 2021. 10.1111/ene.14803. [Epub ahead of print: 07 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sibila O, Albacar N, Perea L, et al. Lung function sequelae in COVID-19 patients 3 months after hospital discharge. Arch Bronconeumol 2021;57 Suppl 2:59–61. 10.1016/j.arbres.2021.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Zhang C, Li X, et al. Thin-section computed tomography findings and longitudinal variations of the residual pulmonary sequelae after discharge in patients with COVID-19: a short-term follow-up study. Eur Radiol 2021;31:7172–83. 10.1007/s00330-021-07799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin W, Chen S, Zhang Y, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J 2021;58. 10.1183/13993003.03677-2020. [Epub ahead of print: 22 07 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alharthy A, Abuhamdah M, Balhamar A, et al. Residual lung injury in patients recovering from COVID-19 critical illness: a prospective longitudinal point-of-care lung ultrasound study. J Ultrasound Med 2021;40:1823–38. 10.1002/jum.15563 [DOI] [PubMed] [Google Scholar]
- 55.Parente-Arias P, Barreira-Fernandez P, Quintana-Sanjuas A, et al. Recovery rate and factors associated with smell and taste disruption in patients with coronavirus disease 2019. Am J Otolaryngol 2021;42:102648. 10.1016/j.amjoto.2020.102648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 2020;13:2330–9https://imaging.onlinejacc.org/content/early/2020/07/30/j.jcmg.2020.05.004?versioned=true 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gherlone EF, Polizzi E, Tetè G, et al. Frequent and persistent salivary gland ectasia and oral disease after COVID-19. J Dent Res 2021;100:464–71. 10.1177/0022034521997112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hopkins C, Surda P, Vaira LA, et al. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology 2021;59:26–31. 10.4193/Rhin20.544 [DOI] [PubMed] [Google Scholar]
- 59.Simani L, Ramezani M, Darazam IA, et al. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol 2021;27:154–9. 10.1007/s13365-021-00949-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo Province: a post-acute outpatient re-evaluation. Epidemiol Infect 2021;149:e32. 10.1017/S0950268821000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021;57. 10.1183/13993003.03448-2020. [Epub ahead of print: 29 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taboada M, Cariñena A, Moreno E, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect 2021;82:e31–3. 10.1016/j.jinf.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baricich A, Borg MB, Cuneo D, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med 2021;57. 10.23736/S1973-9087.21.06699-5 [DOI] [PubMed] [Google Scholar]
- 64.Suárez-Robles M, Iguaran-Bermúdez MDR, García-Klepizg JL, et al. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J 2020;37:289. 10.11604/pamj.2020.37.289.27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020;81:e4–6. 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doyle AJ, Thomas W, Retter A, et al. Updated hospital associated venous thromboembolism outcomes with 90-days follow-up after hospitalisation for severe COVID-19 in two UK critical care units. Thromb Res 2020;196:454–6. 10.1016/j.thromres.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Einvik G, Dammen T, Ghanima W, et al. Prevalence and risk factors for post-traumatic stress in hospitalized and non-hospitalized COVID-19 patients. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18042079. [Epub ahead of print: 20 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stavem K, Ghanima W, Olsen MK, et al. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021;76:405–7. 10.1136/thoraxjnl-2020-216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 70.Mazza MG, Palladini M, De Lorenzo R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun 2021;94:138–47. 10.1016/j.bbi.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.PHOSP . The Post-hospitalisation COVID-19 study (PHOSP-COVID). Available: http://phosp.org [Accessed 09 Nov 2020].
- 72.de Lusignan S, Lopez Bernal J, Zambon M, et al. Emergence of a novel coronavirus (COVID-19): protocol for extending surveillance used by the royal college of general practitioners research and surveillance centre and public health England. JMIR Public Health Surveill 2020;6:e18606. 10.2196/18606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ladds E, Rushforth A, Wieringa S. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality criteria for services. medRxiv 2020;10:20211854. 10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Counting long covid in children, 2020. Available: https://blogs.bmj.com/bmj/2020/10/16/counting-long-covid-in-children/ [Accessed 29 Oct 2020].
- 75.COVID-19 Long term protocol & survey. ISARIC. Available: https://isaric.org/research/covid-19-clinical-research-resources/covid-19-long-term-follow-up-study/ [Accessed 24 Nov 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-005427supp001.pdf (9.7MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.