Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT) has been reported in a patient after receiving two vaccines with a recombinant adenoviral vector encoding the spike glycoprotein of coronavirus disease (COVID-19) in Europe and the United States [1, 2]. Among approximately 12.7 million people who received ChAdOx1 nCov-19 (AstraZeneca) vaccines in Korea [3], we detected two cases of cerebral venous sinus thrombosis together with throm-bocytopenia and positive results for platelet factor 4 (PF4)–heparin antibody using enzyme-linked immunosorbent assay (ELISA). The fatality rate in our study was 50%. This study aimed to analyze a suitable prognostic factor through a central review of domestic cases and a literature review of overseas reports.
Clinical and laboratory characteristics of 44 VITT patients
We reviewed two Korean patients and previously reported 42 patients [1, 4-6] with VITT onset after receiving AstraZeneca vaccines in this study. We excluded one reported case from England because PF4-heparin antibody was not detected using two ELISA methods [5]. In total, 44 patients were selected for inclusion in this analysis (Table 1), composed of 17 and 27 male and female patients, respectively, showing a female predominance, and with a median age of 36 years (range, 21–77 yr). Initial symptoms appeared at a median of 10 days after vaccination (range, 5–24 days). Symptoms were analyzed in only 10 patients; headache was the most common symptom related to cerebral venous thrombosis (CVT). CVT was detected in 29 patients, including the 2 Korean cases, which was the most frequent site in 66% of the total patients. Six patients showed combined cerebral hemorrhage among 29 CVT cases. Pulmonary embolism was diagnosed in 12 (27%) patients, and arterial and splanchnic vein thromboses were detected in 10 (23%) and 8 (18%) patients, respectively.
Table 1.
Clinical and laboratory characteristics of patients with vaccine-induced immune thrombotic thrombocytopenia (VITT).
| Patient No. | Age, years | Sex | Symptom onset (N of days after vaccination) | Symptoms | CVT | Splan-chnic vein throm-bosisa) | Pulm-onary embo-lism | Other throm-bosis or hemorr-hage | Platelet initial (per mL) | Platelet nadir (per mL) | D-dimer, peak (mg/L) | INR peak | PTT peak (sec) | Fibrino-gen nadir (mg/dL) | PF4–heparin ELISA (optical density) | Heparin treatment | Other medical conditions | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Korean 1 | 33 | M | 11 | Headache, seizure | Yes | No | No | Cerebral hemorrhage | 77,000 | 65,000 | >20 | 1.01 | 35.7 | 197.9 | 3.10 | No | ACL-Ab | Full recovery |
| Korean 2 | 33 | M | 8 | Headache, hemiparesis, drowsiness | Yes | No | No | Cerebral hemorrhage | 14,000 | 10,000 | >35.2 | 1.51 | 60.7 | 77 | 0.72 | No | No | Fatal |
| Ref 1-1 | NA | NA | 5 | Chills, fever, nausea, and epigastric discomfort | Yes | Yes | Yes | Aortoiliac artery thrombosis | NA | 13,000 | 142 | 1.4 | 41.6 | 78 | 3.16 | Yes | No | Fatal |
| Ref 1-2 | NA | NA | 6 | NA | No | No | Yes | No | NA | 107,000 | 1.8 | 1.12 | 29 | 568 | 3.08 | LMWHe) | No | Recovering |
| Ref 1-3 | NA | NA | 9 | NA | Yes | No | No | No | NA | 60,000 | 13 | NA | NA | NA | 3.5 | Unknown | No | Unknown |
| Ref 1-4 | NA | NA | 7 | NA | Yes | No | No | No | NA | 9,000 | NA | 1.66 | 46.6 | NA | 3.4 | Yes | CND | Fatal |
| Ref 1-5 | NA | NA | 13 | NA | Yes | Yes | Yes | Right intra-ventricular, iliofemoral vein, IVC thrombi | NA | 23,000 | NA | 1.25 | 64.8 | 173 | 1.2 | Yes | VWD-I; FVL; ACL-Abs | Recovering |
| Ref 1-6 | NA | NA | 7 | NA | Yes | No | No | No | NA | 75,000 | 2.6 | 1.05 | 23 | NA | NA | Unknown | No | Recovering |
| Ref 1-7 | NA | NA | 8 | NA | Yes | No | No | No | NA | 29,000 | >33.0 | 1.34 | 45 | 210 | NA | Yes | No | Recovering |
| Ref 1-8 | NA | NA | 8 | NA | Yes | No | No | Widespread microvascular thrombi (brain, lungs, and kidneys)d) | NA | 16,000 | NA | NA | NA | NA | 2.02 | No | No | Fatal |
| Ref 1-9 | NA | NA | 16 | NA | Yes | No | No | Multiple organ thrombid) | NA | 13,000 | 21 | 1.7 | 46.1 | 40 | 3.51 | No | No | Fatal |
| Ref 1-10 | NA | NA | 11 | NA | Yes | Yes | No | No | NA | 8,000 | >35.0 | NA | NA | 80 | 2.35 | No | No | Fatal |
| Ref 1-11 | NA | NA | 12b) | NA | Pendingc) | No | No | Cerebral hemorrhagec) | NA | NA | NA | NA | NA | NA | 2.16 | No | Unknown | Fatal |
| Ref 4-1 | 37 | F | 8 | Fever, headache, visual disturbances | Yes | No | No | No | NA | 22,000 | >35 | 1.2 | 25 | 210 | 3.7 | Initial low dose of LMWH | NA | Fatal |
| Ref 4-2 | 42 | F | 10 | Headache, drowsiness | Yes | No | No | No | NA | 14,000 | >35 | 1 | 31 | 80 | 35.93.4 | Reduced dose of LMWH | NA | Fatal |
| Ref 4-3 | 32 | M | 7 | Back pain | No | Yes | No | Azygos vein, hemiazygos vein, and several basivertebral veins’ thrombi | NA | 10,000 | >35 | 1.1 | 25 | 230 | 3.6 | Reduced dose of LMWH | NA | Full recovery |
| Ref 4-4 | 39 | F | 10 | Headache, abdominal pain | Yes | No | No | No | NA | 70,000 | 13 | 1.3 | 25 | 120 | 3.8 | Reduced dose of LMWH | NA | Full recovery |
| Ref 4-5 | 54 | F | 7 | Headache, hemiparesis | Yes | No | No | No | NA | 19,000 | >35 | 1.1 | 29 | 120 | 2.9 | Heparin (5,000 IU) | NA | Fatal |
| Ref 5-1 | 30 | F | 13 | NA | Yes | Yes | Yes | Ischemic bowel with infarction | 27,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-2 | 55 | F | 6 | NA | No | Yes | No | Acute aortic thrombosis and cerebral hemorrhage | 11,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Died |
| Ref 5-3 | 26 | F | 12 | NA | Yes | No | No | No | 64,000 | NA | NA | NA | NA | NA | 2.45 | NA | NA | Survived |
| Ref 5-4 | 52 | F | 10 | NA | Yesd) | No | Yesd) | Cerebral hemorrhaged) | 31,000 | NA | NA | NA | NA | NA | 2.26 | NA | NA | Died |
| Ref 5-5 | 38 | M | 14 | NA | No | No | Yes | No | 16,000 | NA | NA | NA | NA | NA | 2.84 | NA | NA | Died |
| Ref 5-6 | 49 | F | 15 | NA | Yes | No | Yes | Cerebral hemorrhage | 14,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-7 | 25 | M | 9 | NA | Yes | No | No | No | 19,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Died |
| Ref 5-8 | 32 | M | 19 | NA | Yes | No | No | No | 87,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-9 | 35 | F | 9 | NA | Yes | No | No | No | 65,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-10 | 77 | M | 8 | NA | No | No | Yes | No | NA | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-11 | 66 | M | 12 | NA | No | No | No | Deep vein thrombosis, adrenal hemorrhage | 34,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-12 | 34 | M | 14 | NA | Yes | No | No | No | 23,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-13 | 54 | M | 10 | NA | No | Yes | No | Myocardial infarction | 71,000 | NA | NA | NA | NA | NA | 0.76 | NA | NA | Died |
| Ref 5-14 | 71 | F | 14 | NA | No | No | No | Hemorrhagic symptoms only | 17,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-15 | 22 | F | 10 | NA | Yes | No | No | Cerebral hemorrhage | 100,000 | NA | NA | NA | NA | NA | 1.4 | NA | NA | Died |
| Ref 5-16 | 39 | F | 10 | NA | No | No | No | Cerebral infarction | 57,000 | NA | NA | NA | NA | NA | 1.4 | NA | NA | Survived |
| Ref 5-17 | 70 | F | 17 | NA | No | No | Yes | Deep vein thrombosis | 28,000 | NA | NA | NA | NA | NA | Pos | NA | NA | Survived |
| Ref 5-18 | 21 | M | 10 | NA | No | No | No | Cerebral infarction | 113,000 | NA | NA | NA | NA | NA | 2.8 | NA | NA | Survived |
| Ref 5-19 | 46 | F | 14 | NA | Yes | No | No | No | 7,000 | NA | NA | NA | NA | NA | >3.00 | NA | NA | Survived |
| Ref 5-20 | 32 | F | 12 | NA | Yes | No | No | No | 98,000 | NA | NA | NA | NA | NA | 2.17 | NA | NA | Died |
| Ref 5-21 | 48 | M | 14 | NA | Yes | No | No | No | 16,000 | NA | NA | NA | NA | NA | 2.45 | NA | NA | Survived |
| Ref 5-22 | 49 | F | 24 | NA | No | No | Yes | No | 61,000 | NA | NA | NA | NA | NA | >3.00 | NA | NA | Survived |
| Ref 6-1 | 72 | F | 7 | Leg pain, claudication | No | No | No | Peripheral artery thromboses | 36,000 | 39,000 | >20 | NA | NA | 237 | 2.70 | Yes | NA | Full recovery |
| Ref 6-2 | 63 | M | 18 | Leg clamping | No | No | Yes | Peripheral artery thromboses, deep vein thrombosis | 36,000 | 26,000 | >10 | 1.3 | NA | 140 | 1.78 | Yes | NA | Full recovery |
| Ref 6-3 | 69 | M | 12 | Headache, confusion | Yes | Yes | Yes | Cerebral infarction, internal jugular vein thrombosis | 35,000 | 29,000 | 3.35 | 1.4 | NA | 210 | 2.69 | No | NA | Recovering |
a)Splanchnic-vein thrombosis indicates thrombosis of the portal, mesenteric, splenic, or hepatic veins.
b)The day when the body of the deceased was found.
c)Brain neuropathological results were pending at time of this report; CVT had not been ruled out.
d)These were the postmortem findings.
e)Treatment with low-molecular-weight heparin was associated with clinical improvement and increasing platelet counts (107,000 to 132,000 over 3 days). The patient’s drug was then switched to a direct oral anticoagulant when the ELISA showed positive results for antibodies against PF4–heparin, with further clinical and platelet-count recovery.
Abbreviations: ACL-Abs, anticardiolipinantibodies; CVT, cerebral venous (sinus) thrombosis of the cortical vein; ELISA, enzyme-linked immunosorbent assay; F, female; FVL, factor V Leiden; INR,
international normalized ratio; IVC, inferior vena cava; LMWH, low-molecular-weight heparin; M, male; NA, not available; PF4, platelet factor 4; Pos, positive; PTT, partial thromboplastin time; VWD-I,
type 1 von Willebrand disease.
All cases showed decreased platelet counts and elevated D-dimer levels, both at initial presentation [5, 6] and during treatment [1, 4, 6]. The initial and nadir platelet counts were available for 26 and 21 cases, respectively. The median platelet counts (range) initially and at nadir were 35,000 (7,000–113,000) and 23,000 (8,000–107,000) (reference range, 150,000–440,000/mL). All except two cases had positive results for PF4–heparin antibody on ELISA, but the quantitative value did not correlate with the clinical outcome. Data on peak prothrombin
Platelet counts according to clinical outcomes in VITT
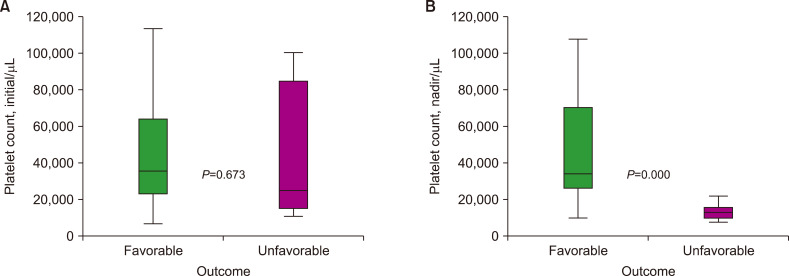
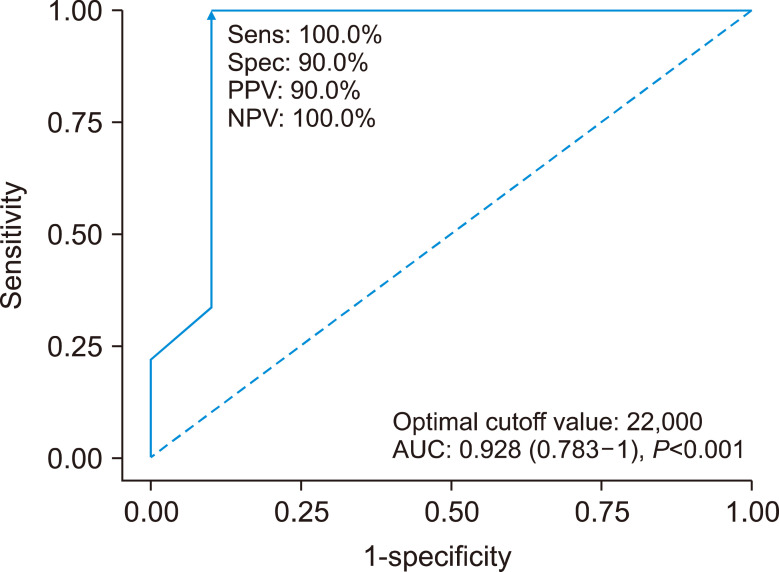
When comparing patients with favorable outcomes (recovery or full recovery) and those with unfavorable outcomes (fatal/died), the initial platelet counts between the groups were not significantly different in 26 patients, as determined using the nonparametric test (median, 35,500 and 23,000/mL). In contrast, the nadir platelet counts during the clinical course in 10 patients with favorable outcomes were significantly higher than those of 9 patients with unfavorable outcomes (median, 34,000 and 13,000/mL) (Fig. 1). The cutoff value for predicting fatality based on the nadir platelet count was 22,000/mL, as determined using a receiver operating characteristic curve, with an area under the curve of 0.928 (Fig. 2).
Fig. 1.
Comparison of the distribution of the initial (A) and nadir (B) platelet counts between patients with favorable outcomes and those with unfavorable outcomes.
Fig. 2.
Receiver operating characteristic curve to predict the prognosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) with the nadir platelet count.
Discussion
In Korea, adenovirus-based COVID-19 vaccines were banned for those in their 20s and permitted only for those ≥30-years-old since April 25, 2021, after performing the risk-benefit assessment when comparing severe hospitalization and mortality rates after COVID-19 with the predicted mortality of VITT [7]. Moreover, an active surveillance system involving a self-monitoring application that enabled early declaration and inspection was maintained for people receiving AstraZeneca and Janssen vaccines. Tests for the PF4-heparin antibody were performed in a central laboratory. Among the 35 suspected thrombocytopenia and/or thrombosis cases, two were confirmed as VITT [8]. The relatively low incidence of two VITT cases among 12.7 million individuals when compared with that in Western countries (348 cases among 14.3 million individuals receiving AstraZeneca vaccination in the UK, 32 cases among 10.2 million individuals receiving Janssen vaccination in the USA) [9, 10] should be considered when preparing the vaccination guidelines for each country.
Rapid deterioration and fatality due to VITT were the main problems during the early periods, until we elucidated the exact mechanism of the disease. The key patho-physiology causing both thrombosis and bleeding is based on an autoantibody-mediated immune response, similar to heparin-induced thrombocytopenia. Therefore, the induction of immune tolerance through the administration of immunoglobulin and steroids is recommended as an effective treatment. The administration of anticoagulants other than heparin and low-molecular-weight heparin should be considered to improve the clinical course of thrombosis [11]. However, this study revealed that patients with severe thrombocytopenia at presentation or patients who failed to recover their platelet counts during treatment with immunosuppressants and those who bypassed anticoagulants may have a poor prognosis. This is the first important and significant recommendation globally, although only a few VITT cases have been reported, and their numbers are smaller than those reported in each country’s media. Our study findings suggest the following recommendations: 1) Individuals who receive AstraZeneca and Janssen vaccines should be educated about visiting the hospital immediately if they experience symptoms possibly related to thrombosis and thrombocytopenia between 4 days and 4 weeks after vaccination (suspected cases). 2) Medical staff in hospitals, including primary clinics, should immediately request PF4/heparin antibody tests to the central laboratory if they screened cases of thrombocytopenia and thrombosis through blood and imaging tests (presumed cases). 3) Patients with thrombocytopenia and thrombosis with positive results for PF4/heparin antibody should be treated with immuno-globulins, steroids, and substitutive anticoagulants (confirmed cases). In conclusion, we cautiously suggest that early diagnosis of VITT after symptom development is the most important aspect in reducing VITT-related fatality. Furthermore, clinicians should consider more active measures, such as plasma exchange, early when they detect patients whose initial or subsequent platelet counts are less than 22,000/mL.
Acknowledgments
The authors would like to thank the COVID-19 Immunization Safety Management Team of the Korean Disease Control and Prevention Agency and adverse event following immunization response teams of Seoul Metropolitan City and Gyeonggi Province.
Footnotes
Sources of support
This study was supported by a grant from the Seoul National University Bundang Hospital (Grant No. 16-2017- 006).
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–5. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korea Disease Control and Prevention Agency (KDCA), author The current status of Cov-19 vaccination. KDCA; Cheongju, Korea: 2021. [Accessed on June 22, 2021]. at http://kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=713731&cg_code=&act=view&nPage=1 . [Google Scholar]
- 4.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–30. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–11. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct Immune globulin for vaccine-induced thrombotic thrombo-cytopenia. N Engl J Med. 2021 doi: 10.1056/NEJMoa2107051. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korea Disease Control and Prevention Agency (KDCA), author Public Health Weekly Report Vol. 14, No. 17, 2021. KDCA; Cheongju, Korea: 2021. [Accessed on June 9, 2021]. at https://kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=713111&act=view . [Google Scholar]
- 8.Bourguignon A, Arnold DM, Warkentin TE, et al. The diagnosis and treatment guidelines for thrombosis with thrombocytopenia syndrome after Cov-19 vaccination. Korean Disease Control and Prevention Agency; Cheongju, Korea: 2021. [Accessed June 22, 2021]. at https://ncv.kdca.go.kr/board.es?mid=a12101000000&bid=0031#content . [Google Scholar]
- 9.Bourguignon A, Arnold DM, Warkentin TE, et al. Coronavirus vaccine - weekly summary of Yellow Card reporting. Medicines and Healthcare Products Regulatory Agency; London, UK: 2021. [Accessed June 3, 2021]. at https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting . [Google Scholar]
- 10.Bourguignon A, Arnold DM, Warkentin TE, et al. Selected adverse events reported after COVID-19 vaccination. Centers for Disease Control and Prevention; Atlanta, GA: 2021. [Accesed May 27, 2021]. at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html . [Google Scholar]
- 11.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19:1585–8. doi: 10.1111/jth.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]