Abstract
Background
Optimal ω-6/ω-3 polyunsaturated fatty acids ratio (PUFAR) is reported to exert protective effects against chronic diseases. However, data on PUFAR and diabetic retinopathy (DR) remains scarce. We aimed to thoroughly quantify whether and how PUFAR was related to DR as well as its role in DR detection.
Methods
This two-centre case-control study was conducted from August 2017 to June 2018 in China, participants were matched using a propensity score matching algorithm. We adopted multivariable logistic regression models and restricted cubic spline analyses to estimate the independent association of PUFAR with DR, adjusting for confounders identified using a directed acyclic graph. The value of PUFAR as a biomarker for DR identification was further evaluated by receiver operating characteristic analyses and Hosmer-Lemeshow tests.
Findings
An apparent negative relationship between PUFAR and DR was observed. Adjusted odds of DR decreased by 79% (OR: 0·21, 95% CI: 0·10–0·40) with an interquartile range increase in PUFAR. Similar results were also obtained in tertile analysis. As compared to those in the 1st tertile of PUFAR, the adjusted odds of DR decreased by 76% (OR: 0·24, 95% CI: 0·08–0·66) and 93% (OR: 0·07, 95% CI: 0·03–0·22) for subjects in the 2nd and 3rd tertiles, respectively. Good calibration and discrimination of the PUFAR associated predictive model were detected and PUFAR = 35 would be an ideal cut-off value for DR identification.
Interpretation
Our results suggest that serum PUAFR is inversely associated with DR. Although PUFAR-alteration is not observed amongst different stages of DR, it can serve as an ideal biomarker in distinguishing patients with DR from those without DR.
Funding
This study was funded by Natural Science Foundation of Zhejiang Province, Zhejiang Basic Public Welfare Research Project, the Major Project of the Eye Hospital of Wenzhou Medical University, and the Academician's Science and Technology Innovation Program in Zhejiang province. Part of this work was also funded by the National Nature Science Foundation of China, and Research Project for College Students in Wenzhou Medical University.
Keywords: ω-6 /ω-3 PUFAs ratio, Diabetic retinopathy, Propensity score matching, Case control study
Research in context.
Evidence before this study
Diabetic retinopathy (DR) has emerged as a leading cause of visual impairment and blindness in the working-age population worldwide. Concerns have been raised about the potential impact of ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) on DR. However, available evidence remains inconclusive, and evaluation of PUFAs mainly relies on self-reported dietary intake, which inevitably leads to unsatisfactory precision of PUFAs measurements and reduces credibility of the conclusions. Besides, ω-3 and ω-6 PUFAs are considered to be competitors combining the same desaturase and elongase in the process of metabolism. Only placing emphasis on a single type of PUFAs is insufficient. Objectively measured ω-6/ω-3 polyunsaturated fatty acids ratio (PUFAR) may help to clarify these associations.
Added value of this study
To the best of our knowledge, this is the first propensity score matching based study to thoroughly examine the relationship between DR and serum PUFAR as well as ω-6 and ω-3 families of PUFAs. The levels of PUFAs are carefully determined by an ultra-performance liquid chromatography-electrospray ionisation-tandem mass spectrometry (UPLC-MS/MS) system which showed great advantages of high sensitivity and selectivity as compared to the self-reported results.
Implication of all the available evidence
The results of this study not only suggest that higher serum PUFAR was associated with lower odds of DR and can serve as a potential biomarker in distinguishing early DR patients from their counterparts, but also promote a further understanding of the possible differential links between serum PUFAR and different disease statuses of diabetes.
Alt-text: Unlabelled box
1. Introduction
Despite recent advances in medicine, there has been a dramatic increase in diabetes mellitus (DM) over the past ten years worldwide, from 285 million in 2009 [1] to 463 million in 2019, and the number is expected to rise to more than 690 million by 2045 [2]. If poorly treated, DM can lead to multiple organ damages, which will inevitably reduce quality of life and increase early mortality. As a frequent complication of DM, diabetic retinopathy (DR) is a chronic DM-induced eye disease and a leading cause of global visual impairment and blindness burdens [3]. Pang et al. [4] have reported that nearly all type 1 and the majority (over 60%) of type 2 diabetic (T2D) patients eventually suffer from some degree of DR after 20 years of diabetes. Besides, due to its silent clinical characteristics, almost all DR patients are diagnosed at a moderate or advanced stage of disease [5]. This not only greatly decreases its clinical efficacy, but also leads to a huge waste of limited healthcare budgets. So, we need a simple and effective approach to distinguish T2D patients with DR from those without DR in time, which will be fruitful for its early diagnosis, timely treatment, and clinical administration.
Previous studies have recognised the critical role of dyslipidemia in neovascular eye diseases, including DR [6]. And available evidence suggests that lipids metabolism may play a central role in the initiation and progression of DR [7]. However, routine lipid profiles such as low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglyceride (TG) are inadequate to serve as sensitive and specific biomarkers for the timely detection of DR because of the complex relationships between lipids and DR [8]. Available lines of evidence suggest that polyunsaturated fatty acids (PUFAs) are closely linked to the risk of DR. Nevertheless, overall results from both intervention and observational studies remains inconclusive. Although it is generally conceptualised that ω-3 PUFAs are beneficial [9], no association between the supplement of ω-3 PUFAs and DR has been detected in several studies [10]. While ω-6 PUFAs are reported to be positively associated with the risk of DR due to their inductions of adhesion molecule expression and leucocyte adhesion in human retinal endothelial cells [11]. However, two clinical studies conducted in Europe reveal that ω-6 PUFAs were inversely associated with DR occurrence [12,13]. In a recently published paper, ω-6 and ω-3 PUFAs are considered to be competitors combining the same desaturase and elongase in the process of metabolism [14], and different ω-6/ω-3 PUFAs ratio (PUFAR) may result in different severity of inflammation and angiogenesis [15]. All these findings strongly indicate that PUFAR may be linked to the pathogenesis of DR. To test this hypothesis and avoid potential underfitting or overfitting as well as collinearity, the association of PUFAR instead of individual PUFAs with DR should be carefully investigated. However, to the best of our knowledge, studies on the relationship between PUFAR and DR are rare. Limited evidence only comes from observational studies and levels of PUFAs are mainly evaluated by self-reported dietary intake, which inevitably leads to unsatisfactory precision of PUFAs measurements and significantly reduces the credibility of relevant conclusions.
To overcome these limitations, this study aims to comprehensively quantify the association of serum PUFAR with DR, as well as to evaluate its role when using PUFAR as a sensitive and specific biomarker in the detection of DR. An ideal cut-off value of PUFAR is also proposed.
2. Methods
2.1. Study design and participants
This was a two-centre case-control study in China. Detailed information on the study design and participants enrolment could be found in our previous work [16]. In brief, we ascertained 195 type 2 diabetic (T2D) patients (83 with DR and 112 without DR) from two affiliated hospitals of Wenzhou Medical University and Anhui Medical University, respectively, from August 2017 to June 2018. The DR group included 60 patients with non-proliferative diabetic retinopathy (NPDR: 9 mild, 31 moderate, and 20 severe) and 9 patients with proliferative diabetic retinopathy (PDR). DR status was independently assessed by two experienced ophthalmologists strictly following the guidelines for clinical diagnosis and image screening [17]. Details of grading DR were presented in Supplementary Methods. To adjust for the potential impacts due to possible confounders and improve comparability of the results to some extent, 69 pairs of T2D patients with DR and those without DR were matched by age, gender, body mass index (BMI) and glycosylated haemoglobin A1c (HbA1c) at a ratio of 1:1 using propensity score matching (PSM) approach. To fully describe the changes of ω-3 and ω-6 PUFAs in participants with different health states, we additionally matched another 69 healthy volunteers from the routine physical examination cohort from the Second Affiliated Hospital of Wenzhou Medical University with T2D patients by age, gender and BMI via PSM algorithm at a ratio of 1:1 (Supplementary Fig. 1).
2.2. Demographic and clinical covariates
Demographic variables containing age, gender, diabetes duration, smoking, drinking, history of diabetes treatment, hyperlipidaemia and others were obtained via a standardised questionnaire. To improve and guarantee the quality of information acquired, a 10% sub-sample of participants were re-interviewed within 3 weeks of the first interview by a full-time investigator strictly following the protocol of quality control and standardised operation procedure (SOP). Clinical features including fasting plasma glucose (FPG), HbA1c, routine lipid profiles such as HDL-C, LDL-C, TC, TG and others were detected by the automatic biochemical analyser (Roche, Cobas c311).
2.3. Anthropometric measurements
All participants accepted physical examinations by trained investigators according to the SOP. Sitting systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times by standard mercury sphygmomanometers after at least 5 min of rest during the enrolment, and average SBP and DBP values were used for further analyses. In addition, weight and height were also measured to calculate BMI using the following formula: BMI (kg/m2) = weight (kg) / squared height (m2).
2.4. Polyunsaturated fatty acids data
Detailed information on the sample processing and PUFAs determination could be found in our previous work [18]. In brief, serum samples were tested in a random order using an ultra-performance liquid chromatography-electrospray ionisation-tandem mass spectrometry system (UPLC-MS/MS) at the central laboratory of Metware, Inc, a professional and experienced metabolomics institution in China. Technicians were also blind to the characteristics of subjects during the measurement of PUFAs. Individual PUFAs levels were expressed as percentages of the total fatty acids identified (%) and each PUFAs was classified into ω-6 or ω-3 family according to the position of the first double bond from the methyl end of the acyl chain. Total ω-3 and ω-6 PUFAs were determined by the sum of all associated PUFAs in each category, respectively. Finally, the ω-6/ω-3 PUFAs ratio (PUFAR) was calculated using total ω-6 PUFAs divided by total ω-3 PUFAs for clinical interests in balancing the two major PUFAs subclasses.
2.5. Sample size estimation
To ensure the sample size was sufficient to meet statistical requirements and guarantee the reliability of our findings, the sample size and power estimations were carefully estimated using G*Power version 3.1.9.2 (http://stats.idre.ucla.edu/other/gpower/), a statistical power analyses software proposed by scientists from the University of California, Los Angeles. With type I error as 0.05, effect size equals to 0.5, a total sample size of 128 will be needed to achieve a power of 0.8 at the allocation ratio of 1. Please find the details of this part in the supplementary materials.
2.6. Statistical analysis
As missing data would lead to bias and affect the credibility of the conclusion to some extent, data cleaning was carried out to obtain suitable data before final analysis. First, missing values of covariates (the proportion of missing values was less than 20% and considered missing completely at random), were imputed by multiple imputation based on Markov Chain Monte Carlo (MCMC) method with 20 repetitions [19]. Then, population characteristics of participants were compared as follows: (1) Normally distributed variables were described as mean ± standard deviation, and analysis of variance (ANOVA) or student t-test was applied to assess the differences amongst or between groups. While obviously skewed data would be expressed as median (1st quartile, 3rd quartile) and Kruskal-Wallis H test or Mann–Whitney U tests would be performed for the comparisons. (2) All categorical variables were presented as frequency (percentage) and chi-square or Fisher's exact tests were conducted to compare the differences of proportions amongst or between groups.
To investigate the independent association of PUFAR with the odds of DR, multivariable logistic regression models adjusting for confounders screened by a directed acyclic graph (DAG) (Supplementary Fig. 2) were used in the following two ways: with PUFAR as continuous variables [scaled to interquartile range (IQR)] and as categorical variables (tertiles), in which the linear trend tests were also carried out. Furthermore, a potential nonlinear association between PUFAR and the presence of DR was additionally evaluated using the restricted cubic spline (RCS) regression model, with 3 knots at the 5th, 50th, and 95th percentiles. To test the robustness and consistency of the results in different subgroups, we repeated all analyses stratified by age (≤ 55, > 55 years), gender (male, female), BMI (≤ 23.9, > 23.9 kg/m2), FPG (≤ 7, > 7 mmol/L), hypertension (no, yes), and lipid lowering therapy (no, yes), respectively. In addition, possible modifications induced by above stratified factors on the association between PUFAR and DR were also carefully evaluated by including their interaction terms with PUFAR in the logistic regression models. Depending on the potential causal relationships of PUFAR and DR as well as hypertension (Supplementary Fig. 2), we assumed that hypertension might affect the links between PUFAR and DR to some extent. To test our hypothesis, mediation analysis was further performed. Odds ratio (OR) and 95% confidence interval (CI) of the total effect (TE), natural direct effect (NDE), and natural indirect effect (NIE) were estimated by the ‘mediation’ package in R software.
To develop a simple and effective predictive model for evaluating the value of PUFAR in DR detection, 69 pairs of cases and controls was randomly split into independent training and testing sets at a ratio of 7:3 by scikit-learn package of Python version 3.8.8 (Copyright © 2001–2021 Python Software Foundation). Goodness-of-fit of models were assessed by calibration curves and Hosmer-Lemeshow tests. While discrimination ability of the predictive model was evaluated by the area under the receiver operating curve (AUC). Then, depending on the cut-off value determined by RCS, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were also calculated in both the training and testing sets.
Furthermore, sensitivity analyses were also conducted to examine the robustness and consistency of our results. First, we repeated the analysis using data without any missing values in covariates to assess the influence of missing data on our findings. Second, single imputation approach, median for continuous variables and mode for categorical ones, was also performed to assess the influence of different imputation methods. Third, covariates associated with metabolic syndrome such as dyslipidemia and hypertension were further controlled to avoid their possible confounding influences on our results. Forth, dietary information such as different types of cooking oils, consumption of oils and salt were also adjusted to evaluate whether the association of PUFAR with DR remained robust when controlling for dietary variations.
Data management and statistical analyses were carried out using Stata/MP 15.1 for windows (© 1985–2017 Stata Corp LLC, College Station, Texas 77,845, USA), Python Version 3.8.8 (© 2001–2021 Python Software Foundation), and R Version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided and the significance level was set as p ≤ 0·05.
2.7. Ethic statement and approval
The protocol of the present study had been approved by the Eye hospital of Wenzhou Medical University ethics committee (Number: KYK [2017] 46). All participants were informed about the study, participation was voluntary, and all signed written informed consent.
2.8. Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all data in the current study and had final responsibility for the decision to submit for publication.
3. Result
3.1. Characteristics of study population
To briefly illustrate the characteristics of study population, the demographic, clinical, and anthropometric features of enroled subjects were summarised in Table 1. When comparing these features in participants with different health statuses, T2D patients with DR were quite comparable to those without DR in almost all features since a PSM approach was conducted during the study design period. However, DR patients were more likely to have higher SBP and longer diabetes duration as compared with other T2D patients.
Table 1.
General characteristics and clinical features of the study population.
| Variables | Control (n = 69) | DM (n = 69) | DR (n = 69) | p value |
| Demographic characteristics | ||||
| Male, n (%) | 53(76·81) | 38(55·07) | 36(52·17) | 0·01 a |
| Age, years | 56·0(54·0,62·0) | 53·0(48·0,61·0) | 56·0(51·0,65·0) | 0·01 b |
| Research centre, n (%) | <0·0001a | |||
| Wenzhou | 100(100·00) | 36(52·17) | 48(69·57) | |
| Hefei | 0(0·00) | 33(47·83) | 21(30·43) | |
| Hypertension history, n (%) | 0·13 a | |||
| No | 50(72·46) | 36(52·17) | 29(42·03) | |
| Yes | 19(27·54) | 33(47·83) | 40(57·97) | |
| Diabetes duration, years | NA | 8·0(4·0,13·0) | 12·0(8·0,17·0) | 0·01 c |
| Lipid-lowering medication, n (%) | 0·56 a | |||
| No | NA | 58(84·06) | 53(76·81) | |
| Yes | NA | 8(11·59) | 12(17·39) | |
| Missing | NA | 3(4·35) | 4(5·80) | |
| Ever insulin therapy, n (%) | 0·17 a | |||
| No | NA | 46(66·67) | 55(79·71) | |
| Yes | NA | 19(27·54) | 10(14·49) | |
| Missing | NA | 4(5·80) | 4(5·80) | |
| Anthropometric Examination | ||||
| Body Mass Index, kg/m2 | 24·47(23·36,26·73) | 23·91(22·22,26·40) | 24·10(22·41,26·64) | 0·52 b |
| Systolic blood pressure, mmHg | NA | 124(118,139) | 135(122,148) | 0·01 c |
| Diastolic blood pressure, mmHg | NA | 79(74,86) | 76(70,85) | 0·20 c |
| Biological measurements | ||||
| Glycated haemoglobin,% | 5·70(5·40,6·05) | 9·90(8·30,11·80) | 9·80(8·90,10·90) | <0·0001 b |
| Fasting plasma glucose, mmol/L | 5·39(5·11,6·06) | 8·35(6·92,12·00) | 8·50(6·31,10·17) | <0·0001 b |
| Triglyceride, mmol/L | 1·95(1·48,2·99) | 1·61(1·04,2·17) | 1·39(1·03,1·86) | <0·0001 b |
| Total cholesterol, mmol/L | 5·00 ± 0·93 | 4·72 ± 1·14 | 4·51 ± 1·40 | 0·05 d |
| Low density lipoprotein, mmol/L | 2·82 ± 0·78 | 2·65 ± 1·00 | 2·55 ± 1·07 | 0·25 d |
| High density lipoprotein, mmol/L | 1·09(0·98,1·26) | 1·02(0·80,1·34) | 1·09(0·86,1·32) | 0·20 b |
Control: healthy control; DM: type 2 diabetic patients without diabetic retinopathy; DR: type 2 diabetic patients with diabetic retinopathy; NA: not applicable.
Data were presented as mean ± standard deviation for normal or similar normal distributed variables, number (percentage) for categorical variables, or median (25th,75th percentiles) for variables having skewed distribution.
p values were derived from Chi-Square tests.
p values for Kruskal-Wallis H tests.
p values for Mann–Whitney U tests.
p values for F tests (ANOVA).
3.2. Comparison of PUFAs composition amongst groups
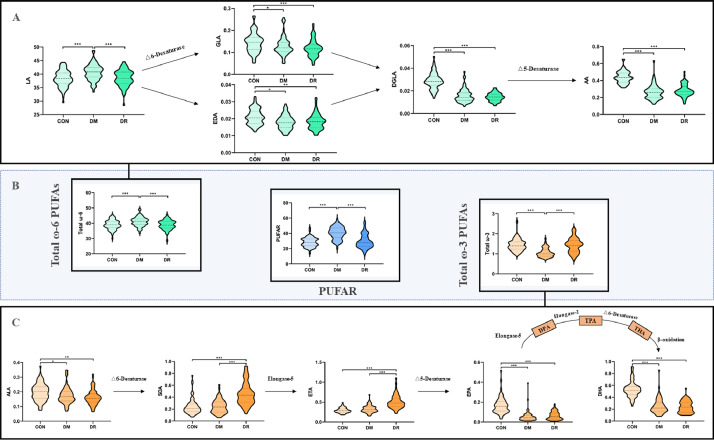
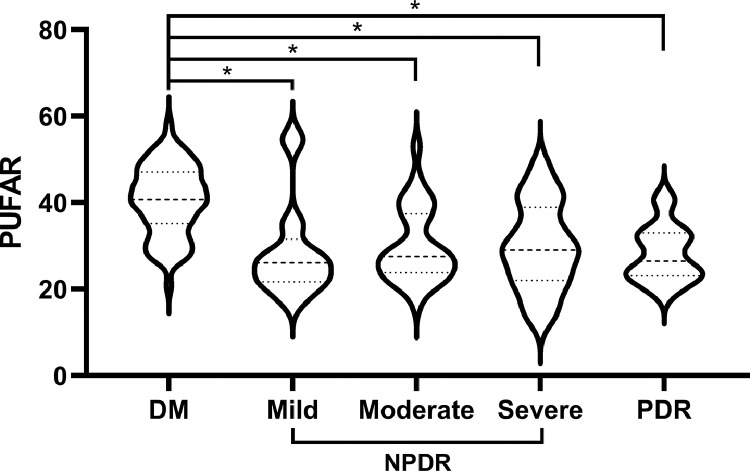
In this study, ten PUFAs including five ω-6 PUFAs (linoleic acid (LA), gamma-linolenic acid (GLA), eicosadienoic acid (EDA), dihomo-gamma-linolenic acid (DGLA), and arachidonic acid (AA)) and five ω-3 PUFAs (alphalinolenic acid (ALA), stearidonic acid (SDA), eicosatetraenoic acid (ETA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA)) were identified by UPLC-MS/MS system. Comparisons of individual serum PUFAs and PUFAR amongst the three groups were shown in Fig. 1 and Supplementary Table 2. Amongst all detected PUFAs, LA was the chief component and counted for about 40% of all fatty acids. For other ω-6 PUFAs, T2D patients (both DM and DR) tended to have lower levels of EDA, GLA, DGLA, and AA when comparing with healthy controls. The levels of ω-3 PUFAs were much lower than those in ω-6 family and only counted for about 1%. However, differences in each ω-3 PUFAs amongst the three groups were statistically significant. From healthy controls to DM to DR, apparent monotonous upward trends in SDA and ETA as well as monotonous downward trends in ALA, EPA, and DHA could be observed respectively. In addition, T2D patients without DR were more likely to have higher PUFAR than their counterparts and patients with increasing levels of DR did not have decreasingly lower PUFAR-levels (Fig. 2).
Fig. 1.
Biosynthesis pathways and relative concentration of PUFAs as well as PUFAR amongst the three groups.
CON: healthy control; DM: type 2 diabetic patients without diabetic retinopathy; DR: type 2 diabetic patients with diabetic retinopathy; PUFAs: serum polyunsaturated fatty acids; LA: linoleic acid; GLA: γ-Linolenic acid; EDA: eicosadienoic acid; DGLA: dihomo-γ-linolenic acid; AA: arachidonic acid; ALA: α-Linolenic acid; SDA: stearidonic acid; ETA: eicosatetraenoic acid; EPA: eicosapentaenoic acid; DPA: docosapentaenoic acid; TPA: 12–0-tetradecanoylphorbol-13-acetate; THA: tetracosahexaenoic acid; DHA: docosahexaenoic acid; Total ω-6 PUFAs: the sum of LA, GLA, EDA, DGLA, and AA; Total ω-3 PUFAs: the sum of ALA, SDA, ETA, EPA, and DHA; PUFAR: serum ω-6/ω-3 polyunsaturated fatty acids ratio. The colour in green, orange, and blue refer to the family of ω-6, ω-3 PUFAs, and PUFAR, respectively. The shade of colour represents the disease status. * p < 0·05; ** p < 0·01; *** p < 0·001 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Fig 2.
Comparison of PUFAR amongst DM and different DR statuses.
PUFAR: serum ω-6/ω-3 polyunsaturated fatty acids ratio; DM: type 2 diabetic retinopathy patients without diabetic retinopathy; DR: type 2 diabetic patients with diabetic retinopathy; NPDR: nonproliferative DR; PDR: proliferative DR. * p < 0·001.
3.3. Association of ω-6, ω-3 PUFAs and PUFAR with DR
Table 2 revealed that both total ω-6 PUFAs and total ω-3 PUFAs were significantly associated with the presence of DR after adjusting for some confounders. With per standard deviation (SD) elevation of total ω-6 PUFAs, adjusted odds ratio (OR) of DR averagely decreased by 50% (OR: 0.50, 95% CI: 0.33–0.75), which strongly indicated that ω-6 PUFAs, especially LA, would be independent protective factors for DR since higher ω-6 PUFAs were significantly associated with decreased likelihood of DR. Meanwhile, a statistically positive association between total ω-3 PUFAs and the presence of DR was also detected. With per interquartile range (IQR) increase of total ω-3 PUFAs, the risk of developing DR averagely increased by 434% (OR: 5.34, 95% CI: 2.48–11.52), which clearly suggested that ω-3 PUFAs, especially SDA and ETA, would be independent risk factors for DR.
Table 2.
ORs (95%CI) for the presence of DR with individual PUFAs.
| PUFAs | Crude a | Adjusted b | Adjusted c |
| ω-6 family | |||
| Linoleic acid, LA§ | 0·43(0·29,0·64) | 0·42(0·27,0·64) | 0·45(0·30,0·70) |
| γ-Linolenic acid, GLA¶ | 0·78(0·56,1·10) | 0·80(0·56,1·15) | 0·84(0·58,1·22) |
| Eicosadienoic acid, EDA¶ | 1·08(0·79,1·50) | 1·06(0·76,1·49) | 1·03(0·72,1·47) |
| Dihomo-γ-linolenic acid, DGLA¶ | 0·76(0·51,1·13) | 0·68(0·44,1·05) | 0·68(0·43,1·05) |
| Arachidonic acid, AA¶ | 1·26(0·90,1·78) | 1·20(0·84,1·71) | 1·15(0·80,1·65) |
| Total ω-6 PUFAs§ | 0·47(0·32,0·70) | 0·46(0·31,0·69) | 0·50(0·33,0·75) |
| ω-3 family | |||
| α-Linolenic acid, ALA¶ | 0·78(0·52,1·17) | 0·81(0·53,1·25) | 0·87(0·56,1·35) |
| Stearidonic acid, SDA¶ | 6·26(3·19,12·26) | 6·41(3·14,13·06) | 5·97(2·91,12·25) |
| Eicosatetraenoic acid, ETA¶ | 7·48(3·58,15·65) | 7·34(3·41,15·84) | 6·82(3·16,14·70) |
| Eicosapentaenoic acid, EPA¶ | 1·29(0·88,1·89) | 1·16(0·80,1·69) | 1·12(0·76,1·64) |
| Docosahexaenoic acid, DHA¶ | 0·89(0·59,1·36) | 0·73(0·45,1·18) | 0·69(0·43,1·12) |
| Total ω-3 PUFAs¶ | 6·57(3·19,13·56) | 5·80(2·73,12·32) | 5·34(2·48,11·52) |
OR: odds ratio; CI: confidence interval; DR: type 2 diabetic patients with diabetic retinopathy; PUFAs: serum polyunsaturated fatty acids.
Total ω-6 PUFAs: the sum of LA, GLA, EDA, DGLA, and AA; Total ω-3 PUFAs: the sum of ALA, SDA, ETA, EPA, and DHA.
per SD increase of PUFAs.
per IQR increase of PUFAs.
Unadjusted for potential confounders.
Adjusted for diabetes duration, ever insulin therapy.
Adjusted for diabetes duration, ever insulin therapy, and systolic blood pressure.
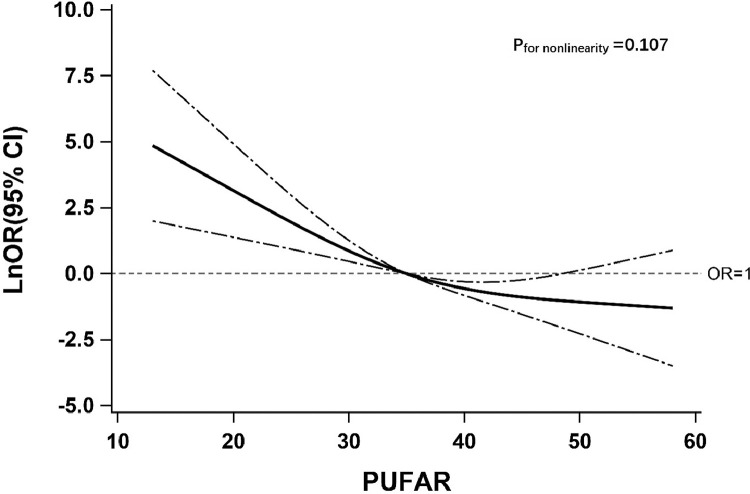
In addition, the association of DR with PUFAR was also carefully investigated (Table 3, Fig. 3). With per IQR increase of PUFAR, adjusted likelihood of DR decreased by 79% (OR: 0.21, 95% CI: 0.10–0.40). Similar results were also found when modelling the PUFAR as tertiles. Comparing with subjects in the lowest tertile, the adjusted ORs of DR decreased by 76% (OR: 0.24, 95% CI: 0.08–0.66) and 93% (OR: 0.07, 95% CI: 0.03–0.22) for those in the 2nd and 3rd tertile of PUFAR, respectively. Meanwhile, an apparent linear trend of DR with PUFAR was additionally detected (ptrend < 0.0001) and results of all sensitivity analyses (Supplementary Table 4) as well as findings from the RCS model (Fig. 3) were highly consistent. To further examine the association of PUFAR with DR, we split 69 pairs of participants into two groups in accordance with the reference point (PUFAR = 35) selected by the RCS model and repeated the association assessment. Compared with participants whose PUFAR was ≤ 35, the likelihood of DR tended to decrease by 87% (OR: 0.13, 95% CI: 0.06–0.31) in those whose PUFAR was > 35. Similar results were also observed in a series of subgroup analyses (Supplementary Fig. 3). Although no significant modifications on the above association were induced by all stratified factors (pinteraction > 0.05), adjusted odds of DR were consistently associated with PUFAR.
Table 3.
Association between PUFAR and the presence of DR.
| PUFAR | n | Cases (%) | Model 1 a |
Model 2 b |
Model 3 c |
|||
| OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | |||
| Per IQR=15·89 | 138 | 69(50·00) | 0·18(0·09,0·34) | <0·0001 | 0·19(0·10,0·38) | <0·0001 | 0·21(0·10,0·40) | <0·0001 |
| Tertiles | ||||||||
| T1(13·02ཞ) | 46 | 38(82·60) | 1·00(1·00,1·00) | Ref. | 1·00(1·00,1·00) | Ref. | 1·00(1·00,1·00) | Ref. |
| T2(28·79ཞ) | 46 | 21(45·70) | 0·18(0·07,0·46) | <0·0001 | 0·20(0·07,0·54) | 0·01 | 0·24(0·08,0·66) | 0·01 |
| T3(40·39, 58·04) | 46 | 10(21·70) | 0·06(0·02,0·17) | <0·0001 | 0·07(0·02,0·19) | <0·0001 | 0·07(0·03,0·22) | <0·0001 |
| Ptrendd | <0·0001 | <0·0001 | <0·0001 | |||||
PUFAR: serum ω-6/ω-3 polyunsaturated fatty acids ratio; DR: type 2 diabetic patients with diabetic retinopathy; n: numbers of subjects in each stratum; Case (%): numbers with DR and percentage; OR: odds ratio; CI: confidence interval; T1, T2, and T3: the 1st, 2nd, and 3rd tertile of PUFAR, respectively.
Unadjusted for potential confounders.
Adjusted for diabetes duration, ever insulin therapy.
Adjusted for diabetes duration, ever insulin therapy, and systolic blood pressure.
p values for testing the linear trend between DR and PUFAR.
Fig. 3.
The restricted cubic spline for the association between PUFAR and odds ratio (natural log-transformed) of DR.
PUFAR: serum ω-6/ω-3 polyunsaturated fatty acids ratio; DR: type 2 diabetic patients with diabetic retinopathy; LnOR: natural log-transformed odds ratios; CI: confidence interval. Knots were located at the 5th, 50th, and 95th percentiles of PUFAR. The solid line indicates LnORs and dashed lines indicate 95%CI. Reference point at PUFAR is 35. Adjusted confounders were diabetes duration, ever insulin therapy, and systolic blood pressure.
3.4. Mediation analysis of hypertension on the association of PUFAR with DR
Based on the criteria of mediation analyses [20], only systolic blood pressure (SBP) was considered as a mediator of the association between serum PUFAR and DR in this study. However, we observed that only a small proportion of the negative association of PUFAR with DR was mediated by SBP after adjusting for diabetes duration and ever insulin therapy (Supplementary Table 3).
3.5. Identification of DR using PUFAR
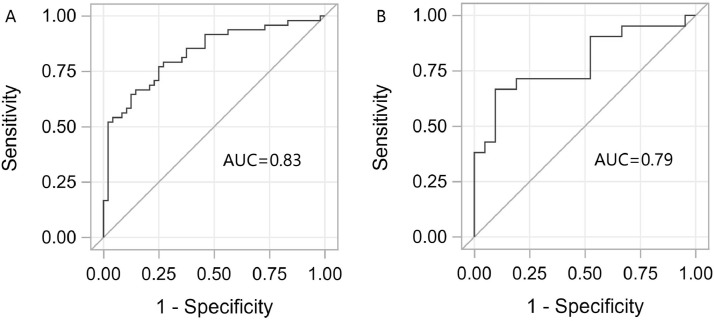
Our overall results suggested that the relationship between PUFAR and DR was strong enough and whether PUFAR could be used as an effective biomarker for DR identification should be further investigated. As was shown in Supplementary Fig. 4, good calibration could be achieved even though only PUFAR was included in the model, and it was consistent in both the training (χ2=8.03, P = 0.43) and testing sets (χ2=7.90, P = 0.44) for H-L tests. According to the discrimination tests in Fig. 4, the area under the curve (AUC) of ROC analysis was 0.83 (95% CI: 0.75, 0.91) in the training set (Fig. 4(A)) and 0.79 (95% CI: 0.65–0.93) in the independent testing set (Fig. 4(B)), respectively. In addition, depending on the cut-off value (PUFAR = 35) determined by RCS, the associated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 75.00%, 77.08%, 76.60%, 75.51% in the training set and 71.43%, 80.95%, 78.95%, 73.91% in the testing set, respectively. These results demonstrated again that PUFAR could serve as a simple and effective biomarker and would play an important role in the identification of DR.
Fig. 4.
ROC curves and AUC showing the discrimination ability of PUFAR to detect DR in both training set (A) and testing set (B).
ROC: receiver operating characteristic; AUC: areas under the ROC curves; PUFAR: serum ω-6/ω-3 polyunsaturated fatty acids ratio; DR: type 2 diabetic patients with diabetic retinopathy.
4. Discussion
To the best of our knowledge, this was the first study to comprehensively quantify the association of PUFAs and PUFAR with DR depending on a multi-centre, PSM designed study, and the levels of PUFAs were determined by UPLC-MS/MS system instead of participants’ self-report. Based on the principle of data mining approach, the potential role of PUFAR in DR detection was also carefully examined. Our findings strongly suggest that exposure to higher PUFAR is an important protective factor of DR, and PUFAR with an appropriate cut-off value (PUFAR ≤ 35) can serve as an ideal biomarker for DR identification though its alteration was not observed amongst different stages of DR. In addition, our findings advance an understanding of the possible differential links between serum PUFAR and different disease statuses of diabetes.
Previous studies have noted the possible relationships between PUFAs and DM [21,22]. However, no relevant study has further explored the possible changes of PUFAs in patients from DM to DR. This study found that the levels of four ω-6 PUFAs (EDA, GLA, DGLA, and AA) and three ω-3 PUFAs (ALA, EPA, and DHA) would be apparently down-regulated from healthy controls to DM and DR while two plant-derived ω-3 PUFAs (SDA and ETA) showed contrary trends. And the relationships of PUFAs with DR was complex, simply linear trends could not account for the variations differ from healthy controls to DM to DR. Although comparison across studies of PUFAs and disease is complicated by differences in study design and exact exposure and outcomes examined, consistent with our findings, results based on previous epidemiological studies have suggested that ALA, an essential precursor of long chain ω-3 PUFAs, has protective effects on improving glucose homoeostasis [23]. EPA and DHA, known as metabolites of ALA, are potent biological regulators with therapeutic and preventive effects on human health. And SDA, an intermediate metabolite of ALA, helps to enhance the tissue levels of EPA and is a potential sustainable source to generate EPA and DHA. However, it is evident that EPA and DHA are more potent than SDA in reducing the risk of chronic diseases [24]. So, negative effects of SDA on DR detected in our study should be interpreted with caution, as it might be due to the inhibition of conversion from SDA to EPA and DHA in DR patients, rather than SDA itself being deleterious. As the potential effect of ETA on DR was rarely explored before, insufficient evidence could be obtained to compare with our findings. Depending on the comparable changes with SDA amongst the three groups in this study, it is possible to hypothesize that ETA has a similar biological effect as SDA on the initiation and development of DR.
Although associations of DR with ω-6 PUFAs are still inconclusive, the relationships observed in this study are in line with pieces of published literature. DGLA and GLA have been reported to possess certain anti-proliferative and anti-inflammatory activities [25,26]. Available evidence from a large European cohort demonstrated the inverse association between EDA and diabetes [27]. Besides, Shen et al. [28] reported that a decrease in AA was closely correlated with a decrease in anti-oxidants and an increase in pro-inflammatory molecules in diabetic patients. Additionally, a parabola trend had also been observed between LA and the progression of the disease. Two clinical trials confirmed that LA-rich diet could inhibit the development of microangiopathy or the deterioration of DR [12,13]. While a cross-sectional study observed that higher LA was positively associated with the risk of DM [29]. This variability between findings can variously be attributed to the complex interplay of metabolic factors and their different disease statuses. It is believed that PUFAs of ω-6 and ω-3 families compete for the same set of enzymes (Δ6 and Δ5 desaturases and elongases) and metabolic pathway [28]. Besides, the conversion of LA (a chief member of ω-6 PUFAs), and ALA (an essential member of ω-3 PUFAs) into their long-chain PUFAs depend on the ratio of ingested ω-6/ω-3 PUFAs [30]. Therefore, it is not strange that PUFAR will be more important in comparison of single PUFAs.
In the current study, we observed that PUFAR was positively related to the presence of DM (Supplementary Table 5). This finding was consistent with previous reports [29] while quite contrary to the negative relationship between PUFAR and DR illustrated in our study. It has been generally accepted that high PUFAR promotes the pathogenesis of many chronic diseases [31]. However, type 2 diabetes mellitus is a disturbance of metabolic homoeostasis with highly variable aetiology and progression [32]. The changes in PUFAR caused by metabolic disorders may not be a simple process that continues to increase with disease progression. In addition, the levels of individual PUFAs and PUFAR are maintained in a dynamic equilibrium. The ratio is influenced by individual PUFAs, and the conversion of essential PUFAs, in turn, depends on the ratio [33]. These may account for the results that a parabolic trend of PUFAR was observed with the progression of diabetes, and although the level of PUFAR between the healthy controls and the DR was similar, the composition of individual PUFAs was completely different. On the other hand, the level of PUFAR showed in our study was relatively higher than the others. The main reason was that LA (ω-6 PUFAs) was found to be significantly higher while DHA (ω-3 PUFAs) was significantly lower in comparison of the results showed in other studies. As ω-6 and ω-3 PUFAs are essential fatty acids that must be consumed in the diet and the conversion of LA and ALA to their higher metabolites is limited by the rate-limiting enzyme (△6-desaturase), we speculate that the dietary intake of ω-3 PUFAs, especially DHA, is relatively low in the Chinese population, whereas the dietary intake of ω-6 PUFAs, especially LA, is generally high. Therefore, an optimal threshold of 35 for PUFAR may be more applicable to populations with similar dietary habits as Chinese.
Furthermore, though hypertension has been naturally considered as one of the major risk factors for DR [34], SBP was consistently shown to be associated in the majority of population-based studies, while the association of diastolic blood pressure (DBP) was less consistent [35]. PUFAs were previously found to be significantly associated with SBP [36]. But the possible intermediate effects for SBP between PUFAs and DR have not been recognised yet. Therefore, we conducted mediation analyses of SBP in the relationship between PUFAs and DR in this study and found that LA alone, total ω-6 PUFAs, and PUFAR met the criteria for further mediation analyses. The weak mediation effects observed might be attributed to the actual situation that hypertensive patients included in our study had only a slight increase in blood pressure, or there may be other significant and larger effects to mediate the association between PUFAR and DR. Therefore, further studies are expected to verify the results.
The main strengths of this study may be summarised as follows. Firstly, the patients diagnosed with DR in our study were from endocrinology department and had no evident ocular symptoms, which guaranteed the target of early DR identification. Secondly, the cases and controls were matched by a PSM approach, which might largely increase the comparability of participants in many potential confounding factors. Thirdly, the level of serum PUFAs were determined by UPLC-MS/MS system instead of participants’ self-reports, which greatly facilitated accurate examination of serum PUFAs exposure. Fourthly, study population was enroled from two study centres covered over 150 million people in Zhejiang and Anhui provinces, China, which might decrease potential selection bias to some extent. Fifthly, adjusted confounding variables in the study were screened via a directed acyclic graph (DAG), which strongly indicated that the management for confounders was rigorous and considerate because of the combination of DAG and PSM. Finally, potential mediating effects of hypertension on the association between DR and PUFAR are also firstly investigated. All measures mentioned above will obviously improve the robustness and credibility of our findings.
Our study also inevitably has several limitations. The case-control design prevents us from clarifying the causal relationship and mechanisms between DR occurrence and PUFAR. Although our findings remain to be confirmed by large scale longitudinal studies, the results clearly revealed that serum PUFAs might be linked to the risk of DR since different PUFAs levels were observed in participants from healthy controls to DM and DR. Besides, our sample size was relatively small, which may affect the results to some extent. However, a small sample size does not necessarily mean that the sample size is insufficient. Our results clearly revealed that the current sample size was sufficient enough to meet the requirements of statistics (power >0.8) and to guarantee the reliability of our findings. In addition, the cut-off value of PUFAR at 35 to identify patients at high risk of DR was determined based on a case-control study and needs to be confirmed in additional prospective cohort studies. Moreover, confounding variables attained from the self-reported questionnaire may be at risk of some misclassification which may not be balanced across the groups. And no specific nutrition information was obtained via questionnaire in this study. This may partly prevent us from completely removing the potential influences due to dietary intake on the final conclusion.
In conclusion, this was the first propensity score matching based study to thoroughly qualify the relationship between DR and serum PUFAR as well as ω-6 and ω-3 families of PUFAs. We detected a negative monotonic relationship between PUFAR exposure and the presence of DR. Furthermore, PUFAR could be used as a specific and sensitive biomarker to distinguish type 2 diabetic patients with early DR from those without DR though its alteration was not observed amongst different stages of DR, and PUFAR = 35 may be an optimal cut-off value. These findings may provide new insights into the effective administration of DR prevention and control.
Funding
This study was funded by Natural Science Foundation of Zhejiang Province (LZ19H020001), Zhejiang Basic Public Welfare Research Project (LGF19H260011), the Major Project of the Eye Hospital of Wenzhou Medical University (YNZD201602), and the Academician's Science and Technology Innovation Program in Zhejiang Province (2018R413182). Part of this work was also funded by the National Nature Science Foundation of China (81670777), and Research Project for College Students in Wenzhou Medical University (wyx2020101048).
Contributors
Shuzhen Zhao and Dongzhen Jin conducted the research, analysed the data, and wrote the manuscript. Shengyao Wang, Huihui Li, Yujie Chang, Yange Ma and Yixi Xu analysed the data. Ruogu Huang, Mengyuan Lai, Zhezheng Xia, Mingzhu Che and Jingjing Zuo contributed to the epidemiological investigation, sample handling, data management, and analysis. Chengnan Guo, and Fang Peng repeated the data analysis independently. Guangyun Mao, Chao Zheng, and Depeng Jiang designed the study, thoroughly reviewed and edited the manuscript. All authors contributed to critical revision of the manuscript, and approved the final version. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
We declare no conflicts of interests.
Acknowledgments
The authors would like to thank the team members from Wenzhou Medical University, Anhui Medical University and Zhejiang University School of Medicine for their hard works and collaborations. The authors would like to thank Metware, Inc., for their careful assay of all the serum samples. The authors would like to thank all study participants for donating their contribution and time.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101089.
Contributor Information
Chao Zheng, Email: chao_zheng@zju.edu.cn.
Guangyun Mao, Email: mgy@wmu.edu.cn.
Appendix. Supplementary materials
References
- 1.International Diabetes Federation . 4th Ed. International Diabetes Federation; Brussels, Belgium: 2009. IDF diabetes atlas. [Google Scholar]
- 2.International Diabetes Federation . 9th Ed. International Diabetes Federation; Brussels, Belgium: 2019. IDF diabetes atlas. [Google Scholar]
- 3.Kozioł M., Nowak M.S., Udziela M. First nation-wide study of diabetic retinopathy in Poland in the years 2013-2017. Acta Diabetol. 2020;57(10):1255–1264. doi: 10.1007/s00592-020-01540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang B., Li M., Song J. Luo Tong formula attenuates retinal inflammation in diabetic rats via inhibition of the p38MAPK/NF-κB pathway. Chin Med. 2020;15:5. doi: 10.1186/s13020-019-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y., Guo C., Ha W., Ding Z. Carnosine improves diabetic retinopathy via the MAPK/ERK pathway. Exp Ther Med. 2019;17(4):2641–2647. doi: 10.3892/etm.2019.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsaidan A.A., Ghoraba M. Awareness of diabetic retinopathy among patients with type 2 diabetes mellitus in primary health care in security forces hospital Riyadh, Saudi Arabia. J Fam Med Prim Care. 2019;8(7):2433–2438. doi: 10.4103/jfmpc.jfmpc_324_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosby-Nwaobi R., Chatziralli I., Sergentanis T. Cross talk between lipid metabolism and inflammatory markers in patients with diabetic retinopathy. J Diabetes Res. 2015;2015 doi: 10.1155/2015/191382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid S., Sas K., Abcouwer S. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539–1549. doi: 10.1007/s00125-019-4959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor K., SanGiovanni J., Lofqvist C. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki M., Kawasaki R., Rogers S. The associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well-controlled diabetes. Investig Ophthalmol Vis Sci. 2015;56(12):7473–7479. doi: 10.1167/iovs.15-17485. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Jump D.B., Grant M.B., Esselman W.J., Busik J.V. Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Investig Ophthalmol Vis Sci. 2003;44(11):5016–5022. doi: 10.1167/iovs.03-0418. [DOI] [PubMed] [Google Scholar]
- 12.Houtsmuller A., Zahn K., Henkes H. Unsaturated fats and progression of diabetic retinopathy. Doc Ophthalmol. 1980;48(2):363–371. doi: 10.1007/BF00141465. [DOI] [PubMed] [Google Scholar]
- 13.Howard-Williams J., Patel P., Jelfs R. Polyunsaturated fatty acids and diabetic retinopathy. Br J Ophthalmol. 1985;69(1):15–18. doi: 10.1136/bjo.69.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behl T., Kotwani A. Omega-3 fatty acids in prevention of diabetic retinopathy. J Pharm Pharmacol. 2017;69(8):946–954. doi: 10.1111/jphp.12744. [DOI] [PubMed] [Google Scholar]
- 15.Gong Y., Fu Z., Liegl R. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am J Clin Nutr. 2017;106(1):16–26. doi: 10.3945/ajcn.117.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Wang T., Zuo J. Association of n-6 PUFAs with the risk of diabetic retinopathy in diabetic patients. Endocr Connect. 2020;9(12):1191–1201. doi: 10.1530/EC-20-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han X., Yang K., Gross R.W. Microfluidics-based electrospray ionization enhances the intrasource separation of lipid classes and extends identification of individual molecular species through multi-dimensional mass spectrometry: development of an automated high-throughput platform for shotgun lipidomics. Rapid Commun Mass Spectrom. 2008;22(13):2115–2124. doi: 10.1002/rcm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo J., Lan Y., Hu H. Metabolomics-based multidimensional network biomarkers for diabetic retinopathy identification in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2021;9(1) doi: 10.1136/bmjdrc-2020-001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenkman H.J.F., Visser E., van Rossum P.S.N. Association between waiting time from diagnosis to treatment and survival in patients with curable gastric cancer: a population-based study in the Netherlands. Ann Surg Oncol. 2017;24(7):1761–1769. doi: 10.1245/s10434-017-5820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valeri L., Vanderweele T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown T.J., Brainard J., Song F. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4697. doi: 10.1136/bmj.l4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian F., Ardisson Korat A.V., Imamura F. n-3 fatty acid biomarkers and incident type 2 diabetes: an individual participant-level pooling project of 20 prospective cohort studies. Diabetes Care. 2021;44(5):1133–1142. doi: 10.2337/dc20-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes P., Hollanda-Miranda W., Beraldo R. Supplementation of α-linolenic acid improves serum adiponectin levels and insulin sensitivity in patients with type 2 diabetes. Nutrition. 2015;31(6):853–857. doi: 10.1016/j.nut.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Prasad P., Anjali P., Sreedhar R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit Rev Food Sci Nutr. 2021;61(10):1725–1737. doi: 10.1080/10408398.2020.1765137. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor R., Huang Y. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7(6):531–534. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
- 26.Okamura T., Nakajima H., Hashimoto Y. Low circulating dihomo-gamma-linolenic acid is associated with diabetic retinopathy: a cross sectional study of KAMOGAWA-DM cohort study. Endocr J. 2021;68(4):421–428. doi: 10.1507/endocrj.EJ20-0564. [DOI] [PubMed] [Google Scholar]
- 27.Forouhi N., Imamura F., Sharp S. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the epic-interact case-cohort study. PLoS Med. 2016;13(7) doi: 10.1371/journal.pmed.1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen J., Bi Y.L., Das U.N. Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch Med Sci. 2014;10(6):1167–1174. doi: 10.5114/aoms.2014.47826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shetty S., Kumari N.S., Shetty P. ω-6/ω-3 fatty acid ratio as an essential predictive biomarker in the management of type 2 diabetes mellitus. Nutrition. 2020:79–80. doi: 10.1016/j.nut.2020.110968. 110968. [DOI] [PubMed] [Google Scholar]
- 30.Harnack K., Andersen G., Somoza V.J.N. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr Metab (Lond) 2009;6:8. doi: 10.1186/1743-7075-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y., Wahlberg K., Love T.M. Associations of blood mercury and fatty acid concentrations with blood mitochondrial DNA copy number in the Seychelles child development nutrition study. Environ Int. 2019;124:278–283. doi: 10.1016/j.envint.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., DuBois D.C., Sukumaran S. Variability in Zucker diabetic fatty rats: differences in disease progression in hyperglycemic and normoglycemic animals. Diabetes Metab Syndr Obes. 2014;7:531–541. doi: 10.2147/DMSO.S69891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strandvik B. The omega-6/omega-3 ratio is of importance! Prostaglandins Leukot Essent Fatty Acids. 2011;85(6):405–406. doi: 10.1016/j.plefa.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Yau J., Rogers S., Kawasaki R. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L., Quang N.D., Banu R. Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS ONE. 2020;15(3) doi: 10.1371/journal.pone.0229665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Sun B., Zhang D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007-2014. Nutrients. 2019;11(6):1312. doi: 10.3390/nu11061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.