Abstract
The metabolic charts memorized in early biochemistry courses, and then later forgotten, have come back to haunt many immunologists with new recognition of the importance of these pathways. Metabolites and the activity of metabolic pathways drive energy production, macromolecule synthesis, intracellular signalling, post-translational modifications and cell survival. Immunologists who identify a metabolic phenotype in their system are often left wondering where to begin and what does it mean? Here, we provide a framework for navigating and selecting the appropriate biochemical techniques to explore immunometabolism. We offer recommendations for initial approaches to develop and test metabolic hypotheses and how to avoid common mistakes. We then discuss how to take things to the next level with metabolomic approaches, such as isotope tracing and genetic approaches. By proposing strategies and evaluating the strengths and weaknesses of different methodologies, we aim to provide insight, note important considerations and discuss ways to avoid common misconceptions. Furthermore, we highlight recent studies demonstrating the power of these metabolic approaches to uncover the role of metabolism in immunology. By following the framework in this Review, neophytes and seasoned investigators alike can venture into the emerging realm of cellular metabolism and immunity with confidence and rigour.
Although the study of cell metabolism is not a new pursuit, metabolism-focused research has boomed within the past 25 years. Foundational work by biochemists over the past century established a framework for interdisciplinary research, transformed and driven forwards by the creativity and collaborative efforts of immunologists, cancer biologists, geneticists, systems biologists, structural biologists and microbiologists. Recent appreciation for metabolism stems from a multitude of realizations, with the common theme being that cellular metabolism does much more than simply provide cells with energy in the form of ATP and/or biomass (BOX 1). Indeed, metabolites and metabolic fluxes can modulate cell signalling pathways and post-translational modifications (PTMs)1. For example, metabolic regulation of PTMs on DNA and histones impact subsequent gene expression2,3, and metabolic activities regulate apoptosis sensitivity4–6. Metabolic enzymes can also serve as cellular or pathogen-derived RNA-binding proteins7 and have been reported to exhibit ‘moonlighting’ functions8,9. Finally, metabolites can act directly as signalling molecules to influence both pro-inflammatory and anti-inflammatory outcomes1,10–12. For example, glutamate can have systemic effects on distant receptors such as those connected to the microbiota-gut-brain axis13 or be used in cell-to-cell nutrient warfare or competition for immunosuppression14. Immunologists have been particularly interested in the finding that metabolic reprogramming is required for immune cell differentiation, function and fate15,16, which has unleashed an array of important findings that have deepened our understanding of the basic biology of different immune cell subsets and their unique metabolic profiles17–19.
Box 1 |. Cellular metabolism: the basics.
Cellular metabolism is a coordinated process that produces ATP for energy, biosynthetic intermediates for macromolecule synthesis (nucleotides, amino acids and lipids) and reducing equivalents (NADPH and FADH2) for redox balance. Glycolysis and mitochondrial oxidative phosphorylation (OXPHOS) are the major bioenergetic pathways that produce ATP. During aerobic glycolysis, glucose is first converted into pyruvate and then secreted as lactate, creating a favourable redox environment that supports subsequent rounds of glycolysis. In doing so, glycolytic intermediates can fuel subsidiary pathways such as the pentose phosphate pathway and one-carbon metabolism for nucleotide synthesis and antioxidant activity. Alternatively, through OXPHOS, pyruvate can be oxidized to acetyl-CoA and enter the mitochondrial tricarboxylic acid (TCA) cycle, which generates reducing equivalents necessary for the electron transport chain to produce mitochondrial ATP. In addition to pyruvate, the end products of amino acid metabolism and fatty acid oxidation (the breakdown of long-chain fatty acids to acetyl-CoA) can replenish the TCA cycle and support OXPHOS activity. This type of TCA cycling is cataplerotic and inputs carbon in equal proportions to the CO2 released. Cataplerotic metabolism is contrasted by anaplerotic functioning of the TCA cycle, in which carbon in excess of that burned by cycling is used to produce biosynthetic intermediates to support cell growth and proliferation.
Technological advances in measuring metabolites and metabolic activities, as well as new single-cell and genetic techniques such as CRISPR–Cas9 editing, have given researchers more tools than ever before to understand and test the complexities of immunometabolism. Advances in single-cell technologies in particular are pushing the boundaries of what is possible for analysing metabolism within heterogeneous cell populations. In this Review, we make an effort to demystify and simplify the navigation of the numerous technologies and strategies available for metabolism research. Although we focus primarily on the field of immunology and the metabolism of immune cells, the concepts and recommendations discussed here can also be applied to other biological fields and cell types.
Immunometabolism: where to start?
Metabolic phenotypes are ubiquitous in biomedical research. This is not surprising given the interplay of cellular metabolism with signalling pathways, gene regulation, effector function and cell fate. As such, there are numerous situations in which researchers unknowingly identify a phenotype anchored in metabolism. Perhaps the analysis of an RNA-sequencing data set uncovers a striking gene-set enrichment in a metabolic programme. Or the targeting of a seemingly non-metabolic gene of interest results in different proliferation rates, morphology or a medium colour change suggesting acidification due to lactate secretion. Should this or a similar situation occur, the researcher may be left scratching their head as to how to probe metabolic changes in their system.
Extracellular flux analysis
If preliminary evidence suggests altered metabolic programmes, extracellular flux analysis (EFA) — for example, using the Agilent Seahorse XF analyser — can characterize and validate broad differences in bioenergetic activity (FIG. 1). To do this, EFA simultaneously measures the activity of the primary bioenergetic pathways, namely mitochondrial oxidative phosphorylation (OXPHOS) and glycolysis. This enables the characterization of the metabolic phenotype and can focus efforts on the relevant bioenergetic and biosynthetic metabolic pathways. Thus, EFA can offer a great starting place for investigating the baseline metabolic wiring of your cells of interest (FIGS 2,3).
Fig. 1 |. A general immunometabolism workflow.

The discovery phase of an immunometabolism project can originate from a multitude of techniques. Extracellular flux analysis (EFA) assays are useful to build a foundation to determine whether metabolic differences are involved in your phenotype of interest. These are especially effective when paired with normalization imaging technology. Next, metabolic phenotypes can be pursued with additional methods. Flow cytometric assays with metabolic dyes and lactate assays are user friendly and provide easily accessible options for most labs. In some instances, these assays also provide single-cell information. Some users may find flow cytometry-based methods more accessible for preliminary validations of a metabolic phenotype, followed by phenotype assessment with EFA. Next, metabolites may be assayed directly using targeted metabolomics. Flux measurements paired with metabolomics provide a detailed overview for metabolomics research and offer a wealth of mechanistic details underlying a metabolic phenotype. Finally, analysis of the phenotype in vivo using a genetic approach provides further support for the phenotype. CyTOF, cytometry by time of flight; log2FC, log2 fold change; RNA-seq, RNA sequencing.
Fig. 2 |. A guide to using extracellular flux assays for glycolysis.
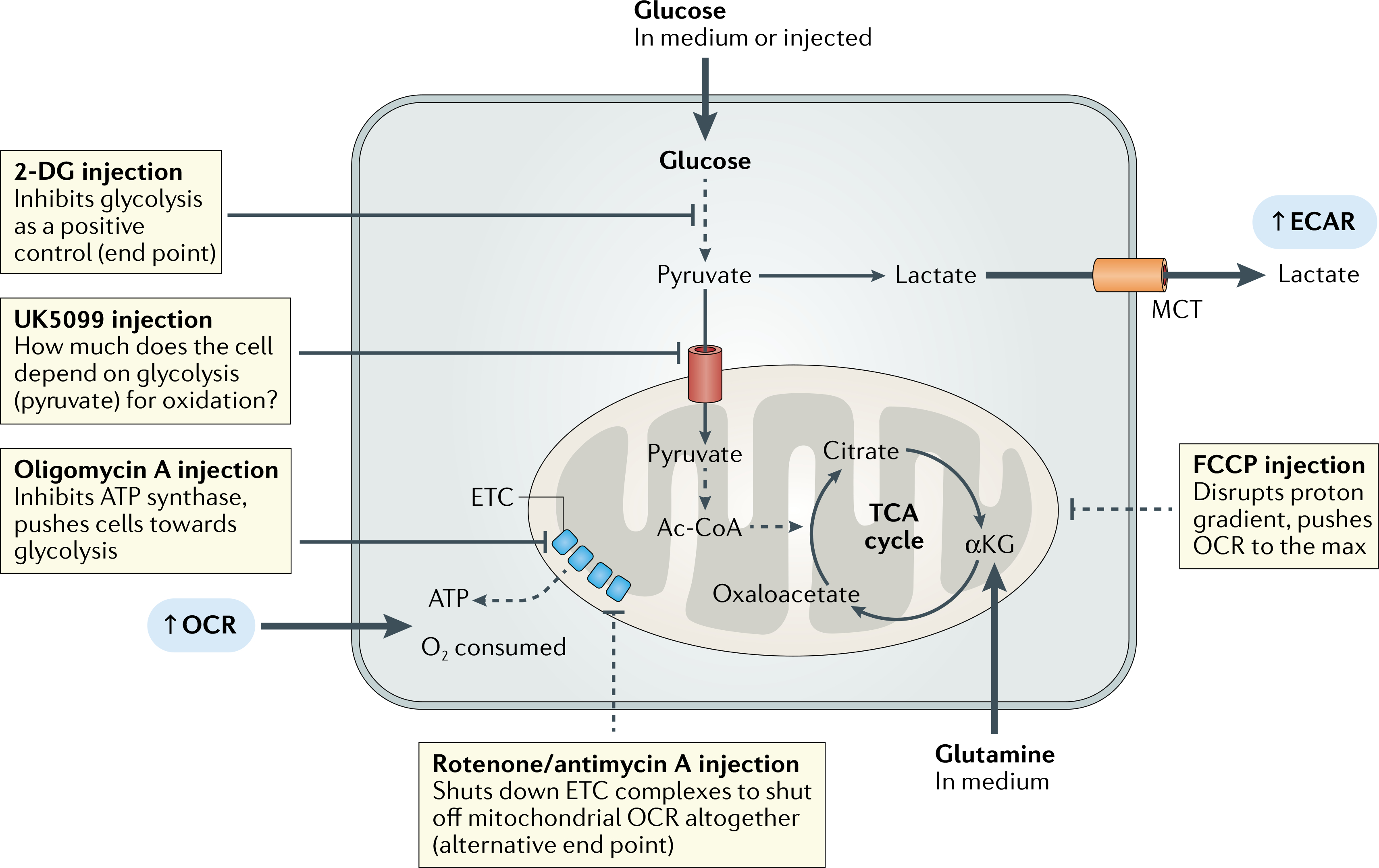
Glycolysis can be probed qualitatively or quantitatively and with respect to oxidative metabolism when paired with a mitochondrial stress test. If the cellular response to acute glucose availability is unknown, the extracellular acidification rate (ECAR) can be monitored after glucose is injected. Ultimately, a rise in ECAR due to lactate accumulation is dependent on lactate excretion via monocarboxylate transporters (MCTs). Glucose may be injected as a first step to monitor glucose consumption in real time, followed by an oligomycin A injection to force glycolytic function (glycolysis stress test). Next, 2-deoxy-d-giucose (2-DG) can be used as an assay end point to get a full picture of the glycolytic capacity of the cells. If the user is interested in the importance of glycolysis with respect to oxidative metabolism, UK5099 can be used to block pyruvate import through the mitochondrial pyruvate carrier (MPC), which prevents pyruvate from being converted into acetyl-CoA (Ac-CoA) and fuelling the tricarboxylic acid (TCA) cycle. Mitochondrial stress test injections in MPC-inhibited cells are used to compare rates of maximal mitochondrial respiration between UK5099-treated samples and untreated controls. αKG, α-ketoglutarate; ETC, electron transport chain; FCCP, carbonyl cyanide-4-trifluoromethoxyphenylhydrazone; OCR, oxygen consumption rate.
Fig. 3 |. A guide to examination of oxidative phosphorylation and mitochondrial function by extracellular flux assays.
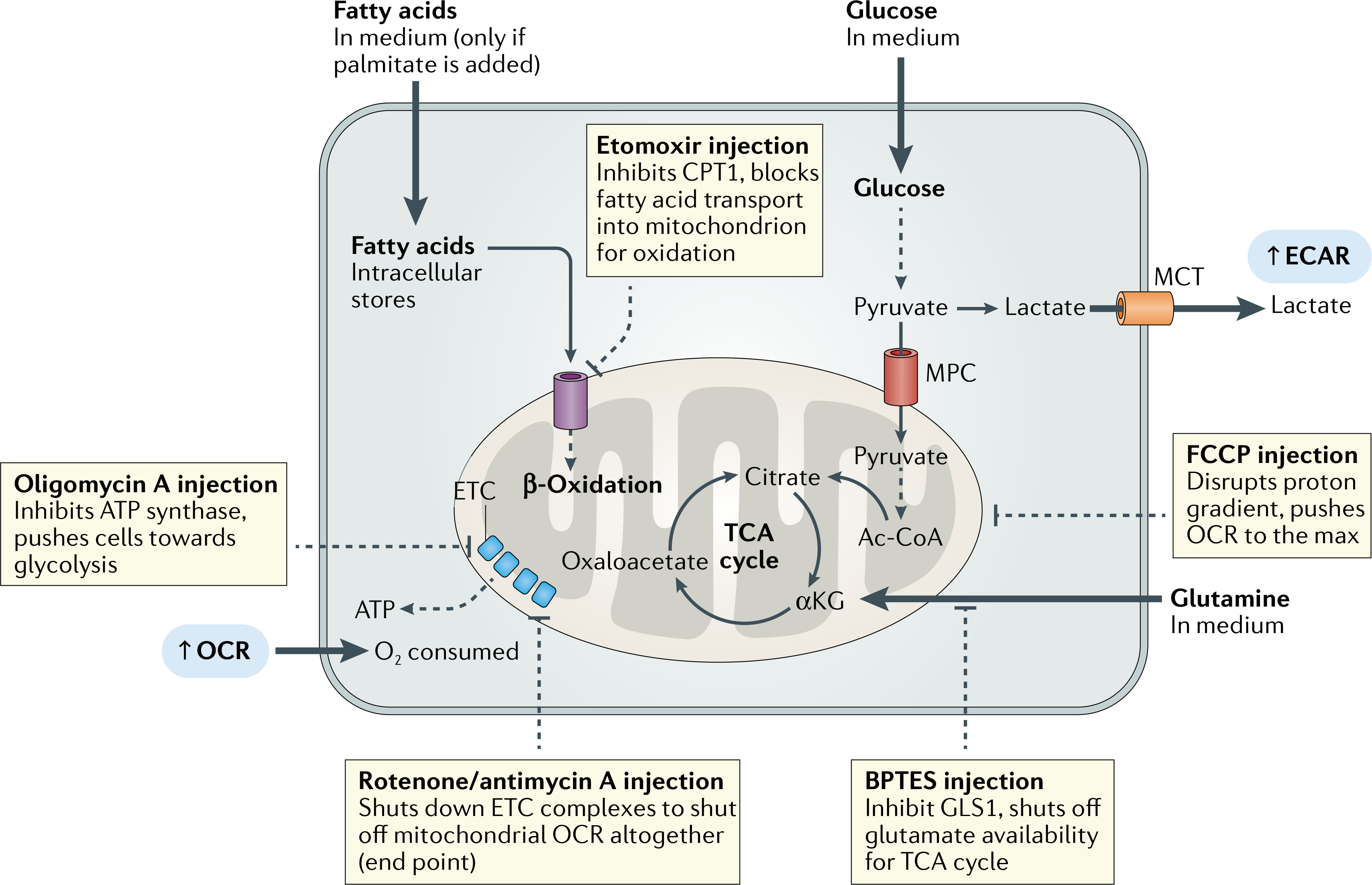
First, the assay conditions such as the formulation of the medium should reflect the question in mind. Fatty acids will be present only in intracellular stores unless assay medium is specifically supplemented with bovine serum albumin-conjugated fatty acids (such as palmitate) or serum. To accurately measure mitochondrial fitness, cell density and inhibitor concentrations should be titrated to ensure quick responses in oxygen consumption rate (OCR) and a well-defined functional maximum if carbonyl cyanide-4-trifiuoromethoxyphenyihydrazone (FCCP) is used. Finally, the normalization strategy should be decided according to the most relevant output parameter (BOX 2). Injection strategies to answer common metabolic questions are described in the flowchart. Mitochondrial stress test injections occur in the following order: oligomycin A, FCCP, rotenone/antimycin A. To investigate the contribution of individual substrates to mitochondrial oxidation, a substrate oxidation stress test can be performed. Etomoxir, glutaminase (GLS1) inhibitor (BPTES) or UK5099 (MPC inhibitor) is injected first followed by Mito Stress Test injections. αKG, α-ketoglutarate; Ac-CoA, acetyl-CoA; CPT1, carnitine palmitoyltransferase 1; ETC, electron transport chain; MPC, mitochondrial pyruvate carrier; MCT, monocarboxylate transporter; TCA cycle, tricarboxylic acid cycle.
To simultaneously measure OXPHOS and glycolysis, EFA uses fibre-optic probes with two sensors that quantify changes in oxygen level and medium pH over time (that is, oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), respectively). Whereas OCR generally reflects mitochondrial OXPHOS, ECAR is predominanty driven by lactic acid release into the medium and serves as a read-out of glycolysis. An added utility of EFA comes with the ability to inject up to four compounds or mixtures into each well during the run and detect the cells’ immediate reactions to these compounds in real time20–22. For example, inhibitors of metabolic pathways of interest can be injected to examine metabolic capacity and adaptation or phorbol 12-myristate 13-acetate (PMA) and ionomycin can be injected to measure metabolic activity upon T cell activation. These experiments have been optimized for both cancer cells and immune cells23,24; however, the list of options available can quickly become daunting to new investigators. Here, we discuss how to choose the most appropriate assay for your experiment with an emphasis on three particularly useful protocols, and how to make this technology work specifically for your interests.
Microbiota–gut–brain axis.
The network that enables bidirectional communication between gut bacteria and the brain.
Choosing and designing the most useful EFA
The glycolytic rate assay and glycolysis stress test.
If prior data implicate altered glucose metabolism, the glycolysis stress test can be used to assess glycolytic activity (FIG. 2). In this assay, samples are first equilibrated under glucose-starvation conditions. Then, glucose is injected to measure basal glycolytic activity in response to glucose availability. Next, to assess the glycolytic capacity (that is, maximal glycolysis), oligomycin is injected to inhibit mitochondrial ATP synthase and enforce the use of glycolysis as the primary source of ATP. The capacity to shift metabolic activity to predominantly rely on glycolysis also informs on the metabolic flexibility of the cell. Last, 2-deoxy-D-glucose (2-DG) is injected to inhibit glycolysis and confirm that ECAR measurements reflect glycolytic activity.
Although ECAR quantifies the degree of media acidification, which is predominantly driven by glycolytic activity (that is, lactate production), the CO2 generated by OXPHOS can readily hydrolyse into bicarbonate and subsequently acidify the medium, thereby confounding ECAR-based analyses. Therefore, the glycolytic rate assay is now a preferred test for glycolytic EFA, as it can differentiate between glycolytic and mitochondrial acidification. This results in more specific and reliable read-outs of glycolysis. In a manner akin to the former assay, the glycolytic rate assay quantifies basal and maximal glycolytic activity, notably under glucose-replete conditions. The linear correlation between O2 consumption and CO2 production by OXPHOS allows for the amount of medium acidification derived from OXPHOS to be subtracted from basal ECAR read-outs, expressed as the glycolytic proton efflux rate (glycoPER). In this way, glycoPER read-outs from the glycolytic rate assay can more precisely quantify glycolytic activity, and ECAR can be used for qualitative assessment of the bioenergetic phenotype.
The mitochondrial stress test.
When OXPHOS is the bioenergetic pathway of interest, the mitochondrial stress test is the appropriate assay to explore mitochondrial metabolism. The mitochondrial stress test can interrogate numerous aspects of mitochondrial biology, through sequential treatment with electron transport chain (ETC) inhibitors (FIG. 3). Following basal OCR measurements, oligomycin is injected to inhibit ATP production, which also serves to back up the ETC and hyperpolarize the proton gradient. Next, an injection of carbonyl cyanide-4-trifluoromethoxyphenylhydrazone (FCCP) depolarizes the mitochondrial membrane to dissipate the proton gradient, forcing maximal ETC activity in an effort to re-establish the electrochemical gradient necessary for mitochondrial function. This OCR measurement quantifies maximal respiration. The difference between maximal and basal OCR represents the spare respiratory capacity, or the amount of energy reserved for response to cellular stress (commonly referred to as metabolic fitness). Finally, the complex I inhibitor rotenone and the complex III inhibitor antimycin A are co-injected to shut down ETC function and as a means to validate the relationship between OCR and ETC activity. Altogether, the mitochondrial stress test characterizes the metabolic phenotype and assesses numerous parameters of mitochondrial function.
Electrochemical gradient.
A gradient of electrochemical potential; in the case of the mitochondrion, to enable protons to move across the inner mitochondrial membrane.
Anaplerotic pathway.
Metabolic pathway, the activity of which replenishes pools of intermediates of the tricarboxylic acid cycle, which can also serve as precursors for other anabolic processes.
The substrate oxidation stress test.
A lead from the OCR assay may prompt a more detailed examination of how mitochondrial metabolism is fuelled. In this case, the substrate oxidation stress test can be used to examine how glucose, glutamine or fatty acids support mitochondrial metabolism. The three primary categories of substrate used for mitochondrial oxidation, particularly in cell culture, are pyruvate (which is imported directly, produced via oxidation of lactate or generated in glycolysis), glutamine-derived α-ketoglutarate and long-chain fatty acids (LCFAs). To determine the utilization of a particular substrate, an inhibitor of the anaplerotic pathway of interest is injected, and then the mitochondrial stress test is applied. Etomoxir, which targets carnitine palmitoyltransferase 1 (CPT1) at low concentrations25, can be used to inhibit LCFA oxidation by blocking the transport of fatty acids into the mitochondrion. UK5099 targets the mitochondrial pyruvate carrier (MPC), which inhibits pyruvate shuttling into the mitochondrion. Finally, BPTES can be used to inhibit glutamine deamidation to glutamate by glutaminase (GLS1). Moreover, following identification of the primary nutrient that drives mitochondrial metabolism, sequential treatment of each pathway inhibitor can assess whether other substrates can compensate to restore mitochondrial respiration.
Another valuable feature of the EFA is the ability to design your own experimental assay to answer specific questions relevant to each cell and disease model. Modifications can be made to existing protocols, such as rearranging the order of injections26. Alternatively, compounds or ligands of interest can be introduced through the injection ports of the Agilent Seahorse XF analyser to detect changes in real time. Functional antibodies, bead-conjugated antibodies, Toll-like receptor (TLR) ligands, inhibitors, substrates, apoptotic bodies and microorganisms are among some of the possibilities for assay injections, enabling the measurement of various acute metabolic pertubations27–30. These are some of the many possible ways to maximize the capabilities of the EFA and advance your projects by asking more specific questions.
Considerations for effective use of EFA
Interpretation of EFA results can be largely subjective without the adaptation of common statistical and rigour guidelines, and the field has only begun to reach a consensus on statistical methods31. Here, we discuss considerations for obtaining reproducible results, as well as quantification and statistical significance of results in commonly used EFA experimental designs. Before running an experiment, three important experimental variables should be taken into consideration. First, since nutrient availability and concentration directly modulate metabolic activity, the composition of cell culture media should be considered for cell propagation and experimentation (BOX 2). Indeed, cell metabolism is limited by substrate availability and uptake as well as inherent capacity. Second, it is essential to optimize the cell number per well and associated inhibitor concentrations. In general, these need to achieve raw measurements within the detection and sensitivity limits of the analyser. For example, it has been our experience that reproducible results across experiments are achieved when the appropriate cell density results in basal OCR and ECAR measurements of 20–100 (pmol min−1) and 10–90 (mpH min−1), respectively, for the 96-well format. The seeding density to achieve readings in these ranges will be different for each cell type, and depends on factors such as cell size, morphology and differentiated state. Third, the degree to which a metabolic phenotype is able to change within the short incubation period of assay set-up (<2 h) should also be considered. In instances where longer exposure is required to achieve the desired phenotype, pre-treatment before plating can be used. Although this optimization can take time, these initial test runs will help avoid spurious results, large error between technical replicates and poor reproducibility.
Box 2 |. Consider physiologically relevant culture conditions.
Standardized culture media such as RPMI or DMEM artificially supplement cells with the necessary components to robustly facilitate survival, proliferation and differentiation. To this end, each contains an excessive concentration of nutrients that, in many cases, markedly contrasts with nutrient availability in vivo. Cellular metabolism is highly dependent on the availability of these nutrients and can rapidly shift in response to their overabundance or under-abundance. Thus, consideration of culture conditions that are physiologically relevant can result in findings with more meaningful translational potential99,126. As an example, the development of physiological media that recapitulate the nutrient levels found in human plasma is becoming increasingly common in immunometabolic studies127 and cancer128. Using these or similar media in physiological oxygen concentrations can be particularly useful. Detailed below are examples of media formulations that have uncovered striking differences in metabolic phenotypes and drug efficacy when compared with cell culture in classic media.
Physiological assay media
Human plasma-like medium (HPLM) was designed to reflect the polar metabolite composition of human plasma from healthy adults. In vitro culture with HPLM significantly increased de novo pyrimidine synthesis in cancer cells129 and increased activation of human T cells130. Recently, a comparison of HPLM and traditional media also revealed discrepancies in the outcomes of CRISPR-based genetic screens in human cancer cell lines99.
Plasmax, which is similar to HPLM, comprises more than 50 nutrients and metabolites at concentrations normally found in human blood. It was created to better approximate the metabolic profile of breast cancer spheroids compared with in vivo mammary tumours131. Indeed, untargeted metabolomics demonstrated that the culture of breast cancer cells in plasmax instead of DMEM-F12 better recapitulates the metabolic composition of orthotopic xenographs grown in mice128.
AIM-V, X-VIVO and OpTimizer are FDA-approved serum-free media commonly used forthe expansion of humanT cells ex vivo during adoptiveT celltherapies132–134. Although these media demonstrate superior performance in promoting the growth and survival of human tissues and cells, it should be noted that they are not necessarily designed with respect to physiological relevance.
Moving into the actual experiment, variations in cell density will have drastic effects on the resulting OCR and ECAR values and can lead to inappropriate interpretations unless properly normalized. Thus, in addition to accurate cell counting and even cell seeding, a second, post-run normalization method corrects for possible well-to-well variation and provides greater confidence for analysis across independent experiments. This is particularly important for adherent cells (such as macrophages and dendritic cells), where seeding typically occurs the day before the assay, thus allowing a longer time for variations in cell number to occur. Various post-run normalization methods are available (BOX 3), with the most robust and efficient means of normalization involving enumeration through direct imaging of fluorescently stained cells. Although this normalization strategy relies on a dedicated high-throughput imaging instrument, the non-destructive nature makes it compatible with other downstream analyses, such as immunostaining. It is also important to note that this strategy works best when cells are well dispersed and show a clearly defined morphology for the counting to be most accurate.
Box 3 |. Normalization strategies for extracellular flux analysis.
Total cell count
Utilizes bright field imaging. Requires a clear monolayer with non-overlapping cells. May not give accurate cell counts for non-circular, spindle-like morphologies. Greater cell count accuracy of lymphocytes may be possible using high contrast bright field (HCBF) imaging.
Nuclear staining
Requires immunofluorescent imaging. Robust method for counting immune cell types that can irreversibly take up the dye. Not appropriate for multinucleated cells. DAPI will stain nuclei of both live and dead cells, and stains lymphocytes more effectively than Hoescht dye, which can be transported out of some cell types. Can be extended to determine cell cycle distribution32. Alternatively, CyQuant GR dye45 can be used to indirectly quantify cell density if further imaging is not required.
Live cell staining
May be used to distinguish live from dead cells in conjunction with nuclear staining or total cell count. Subtract propidium iodide or 7-AAD+ cells from total cell number (nuclei count) to determine the relative number of live cells. Assumes assay inhibitor treatments do not significantly alter cell viability and may be more applicable to pre-run than post-run quantification.
Total protein content
Accounts for variability in cell size such as resting versus activated cell morphologies. Assumes that assay inhibitor treatments do not significantly alter protein content or mitochondrial biogenesis. May not be appropriate for cell types that differ significantly in extracellular matrix protein content.
NADH or ATP-based fluorescence
Uses a colorimetric or fluorescence-based measurement of NADH or ATP present in supernatant to approximate cell numbers. Assumes that ATP/NADH production and cell number are linearly correlated. Cell Titer Glo or MTT/MTS-based strategies are generally discouraged for normalization of extracellular flux analysis135.
Mitochondrial staining
Aims to characterize number, density, structure and activity of mitochondria. Fixation of cells may be required. MitoTracker Deep Red fluorescence may be used to quantify mitochondrial content32. Granulated and fragmented mitochondria can also be quantified via spot analysis.
Notably, new strategies that complex high-content fluorescent imaging with metabolic flux assays can provide simultaneous analysis of cellular and structural features. By coupling the post-run, cell enumeration method for normalization with additional imaging and fluorescent dyes specific for mitochondrial content, fragmentation state, membrane potential and mitochondrial reactive oxygen species, a wealth of new data can be gained in parallel with EFA32. Importantly, this enables examination of differences in metabolic activity that are due to or normalized by cellular characteristics, such as the health, structure or quantity of mitochondria. This enables users to adapt and modify parameters in ways that are most appropriate and useful for their biological question. Application of such a strategy can provide a wealth of additional data in parallel without the requirement for additional experimentation.
Fragmentation state.
The status of elongated or fused mitochondria versus smaller mitochondria as a result of fission.
EFA interpretation
Following normalization, interpretation of downstream EFA can be misleading or confusing to new users. To confirm whether a metabolic phenotype is significantly altered, most users can define the OCR to ECAR ratio to define cells as mostly ‘glycolytic’ or ‘oxidative’. However, this measurement does not reflect individual pathway activity, and the reality is that cell metabolism is a continuum of these processes rather than black and white states. Having a low OCR to ECAR ratio does not negate oxidative activity. Nor does a low ECAR on its own demonstrate a low glycolytic rate. It may be instead that glucose is converted into pyruvate through glycolysis and subsequently oxidized instead of being converted into lactate33. EFA is best suited to characterize and compare the bioenergetic phenotype across samples, which is defined by the relative ratio of OCR to ECAR. Samples with a higher OCR to ECAR ratio are defined as oxidative, relative to samples with a lower OCR to ECAR ratio, which characterizes a glycolytic phenotype. In other words, the OCR to ECAR ratio identifies the primary bioenergetic pathway used but does not negate the activity and importance of the secondary bioenergetic pathway. This concept is exemplified by newly activated T cells, which exhibit a dramatic rise in both ECAR and OCR upon T cell receptor activation34. In instances when OXPHOS and glycolytic activity both increase or decrease, these are considered to be metabolically active or metabolically quiescent, respectively. This divergence in the degree of metabolic activity may suggest differences in proliferation, and thus the need to engage both pathways to increase energy and biomass. This is exemplified by metabolic and corresponding functional differences between activated T cells and naive T cells.
For those who wish to more specifically analyse the relative contributions of glycolysis and OXPHOS to ATP production, recent improvements to calculations from baseline analyses are available to address these concerns23,24,26,35. The glycolytic index, or the proportion of ATP from glycolysis, can be used as a means of categorizing whether cells are primarily glycolytic or oxidative. And, for direct comparison, the rates of the two pathways should be converted into the same units. Only then can you determine if the majority of ATP is coming from glycolysis rather than oxidative metabolism to make it truly ‘very glycolytic’. For a more thorough review of these considerations, we suggest additional reading26.
Metabolons.
Non-covalent complexes of metabolic enzymes in a metabolic pathway, resulting in increased spatiotemporal efficiency.
Current limitations to EFA technology
Increased OCR is assumed to be due to cellular consumption of oxygen for mitochondrial respiration. Although this reasoning is generally appropriate, oxygen consumption can occur in multiple cellular compartments and is not limited to mitochondria. For example, oxygen is used by peroxisomes to oxidize fatty acids36,37. Oxygen is also used by non-mitochondrial NADPH oxidases, which may be significant for some cell types such as macrophages or neutrophils. This concept is acknowledged and termed non-mitochondrial oxygen consumption during data analysis and may have an impact on specific experimental conditions with sensitive cell types. In addition, EFA characterizes the metabolic phenotype from the point of view of populations. Although flow cytometry-mediated cell sorting can increase the purity of the population of interest, EFA technology is not able to capture single-cell metabolic information. Along these lines, phenotypes can be obscured if samples are heterogeneous either metabolically or in terms of cell composition. Imaging of cells following EFA can help to identify cellular heterogeneity and is an important follow-up. Furthermore, metabolism inside a single cell can differ considerably within different subcellular compartments. In some instances, rapid isolation of organelles such as mitochondria may be possible. However, as appreciation of subcellular compartmentalization of metabolic processes grows, this introduces another layer of complexity for multiple metabolic pathways, especially with regard to their regulation. Although useful as a starting point for investigating basic metabolic phenotypes, more advanced techniques with subcellular resolution will be necessary to understand how metabolons are linked to other cellular functions and immunity38.
For most experimental designs, increased ECAR, as a result of decreased pH, is assumed to result from lactate secretion generated from pyruvate produced at the end of the glycolysis pathway, but the ECAR may not necessarily reflect glycolysis directly33,39. In fact, because the glycolytic pathway ends with pyruvate, glycolytic cells do not always excrete lactate into the extracellular space, and instead might shuttle pyruvate into different pathways40,41. Therefore, it is possible that cells in one experimental condition do have more or less glucose uptake and glycolysis, but ECAR levels appear identical. To complicate matters, some immune cell subsets, such as regulatory T (Treg) cells, exhibit differential abilities to consume extracellular lactate42. To address this concern, L-lactate can be measured directly from supernatants with commercially available fluorescence-based assays or ELISAs. Alternatively, a glycolytic rate assay should be performed to quantify acidification due specifically to glycolysis.
Single-cell approaches
Assessing heterogeneous cell populations
One of the biggest technological advances of the past several years has been the rapid adoption of high-dimensional single-cell analyses that overcome limitations inherent in bulk cell assays that obscure cell heterogeneity. Although efforts can be made through fluorescence-activated cell sorting (FACS) to isolate specific cell types of interest for downstream assays, the handling of the cells during these enrichment processes can also perturb their metabolic signalling and metabolite abundance43,44. The presence of such cell types can also be inherently low in physiology or pathophysiology, limiting applicability in metabolic assays, and/or suitable isolation markers may not exist. Therefore, methodologies that can capture single-cell pictures within cell populations have been the focus of many groups in the field45,46.
Advances in technology for single-cell resolution of mechanistic target of rapamycin (mTOR) signalling and other metabolic markers have taken a large step forwards in the past couple of years47,48. First, high-dimensional cytometry by fluorescence or mass cytometry (cytometry by time of flight (CyTOF)) can simultaneously capture multiple markers of immune cell function and metabolic markers48–50. Second, single-cell RNA sequencing now enables unbiased gene expression to be determined in single cells to identify metabolic programmes without prior knowledge of cell types and populations51. Here, we introduce some of these advancements as well as simpler options for more efficient, although less-dimensional answers using flow cytometry.
Flow cytometry and CyTOF
One of the simplest and most informative single-cell-based methodologies for analysis of metabolic features is flow cytometry. Commercially available fluorescent dyes and/or analogues for metabolites are abundant and relatively easy to use. Immune cells incubated with a fluorescently labelled substrate will take up the substrate with endogenous transporters. After washing any exogenous substrate in the medium, flow cytometry of the cells enables the quantification of metabolite uptake of that substrate. These assays have the added advantage of requiring small cell numbers, making flow cytometry a popular go-to for quick validation of differences in metabolic activity. Among some examples are 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)-2-deoxyglucose (2-NBDG) for glucose uptake, BODIPY dyes for fatty acids and glutamine tracing52. Additionally, a novel cysteine-fluorescein isothiocyanate (FITC) probe demonstrated activation-induced cysteine uptake in human T cells and B cells, which was specific to the cysteine transporter xCT and could be quantified by both traditional flow cytometry and imaging flow cytometry51. Signalling through the mTOR and AMPK pathways has crucial roles in regulating immunometabolism and can be monitored by phospho-flow for active kinases and phosphorylated substrates53. Although fluorescent metabolite analogues have been widely used in metabolism research, we urge an extensive use of positive and negative controls wherever possible. A notable example considers the reliability of the widely used glucose analogue 2-NBDG, where comparison with multiple orthogonal glucose uptake assays revealed considerable discrepancy in mouse T cells54.
CyTOF enables the multiplexing of flow cytometry, enhancing the dimensionality permitted by analysis with antibody-based metabolic markers, indicators of cell types and activation of signalling states. With up to approximately 40 available measurements, surface markers can be used to identify cell subsets alongside intracellular staining for metabolic markers, cytokines and activation markers55–57. This approach has recently allowed single-cell metabolic profiling of human CD8+ cytotoxic T cells in colorectal carcinoma48 and improved metabolic profiling of immune cells within the tumour microenvironment (TME). The TME can be complex and can contain heterogeneous cell populations including cancer cells, lymphocytes, myeloid cells and other supporting cells. CyTOF allows for simultaneous identification of these cell types and characterization of the expression of key metabolic regulatory enzymes, transporters and transcription factors in mice and in samples from human patients. For example, CD8+ T cells infiltrating clear cell renal cell carcinoma were found to be phenotypically distinct from peripheral CD8+ T cells, which was further correlated to functional and metabolic impairment58,59. As immunotherapy research for solid tumours and other tissues accelerates, this is a powerful tool for assessing the efficacy of potential anti-cancer therapeutics for their capacity to induce metabolic reprogramming in cell populations of interest, such as reactivated immune cells. CyTOF has also been used for simultaneous analysis of histone acetylation marks and cell lineage markers, enabling detection of histone acetylation changes in heterogeneous samples60. Therefore, CyTOF panels that combine acetylation marks, metabolic signalling and lineage markers can ultimately uncover a wealth of novel connections between metabolism and epigenetic regulation in complex immune cell populations.
Single-cell RNA sequencing
Single-cell RNA sequencing (scRNA-seq) has recently become readily available to many researchers. Its application in immunology has uncovered novel immune cell populations and lineages. For example, scRNA-seq of T helper 17 (TH17) cells present in the central nervous system or draining lymph nodes at the peak of experimental autoimmune encephalomyelitis revealed both known and novel regulators of pathogenicity61,62. Among novel regulators of TH17 cell pathogenicity was the glycosphingolipid receptor Gpr65 and a regulator of lipid metabolism, CD5-like (CD5L)63. Interestingly, these regulators identified by scRNA-seq were found to alter the pathogenicity of TH17 cells by modifying intracellular lipid composition. Indeed, metabolic enzymes that are highly expressed in active immune cells can be well quantified by scRNA-seq technology and can uncover novel regulators of immune cell function and/or fate. As exemplified by this study, scRNA-seq will continue to better characterize not only immune cell subsets and their metabolic gene signatures but also the key regulators that are involved in their stability and function.
Variations on scRNA-seq can further enrich this approach. Another strategy known as simultaneous overview of tri-molecule biosynthesis (SOM3B) now offers single-cell resolution of de novo DNA, RNA and protein synthesis in heterogeneous samples64. With these new technologies, it is highly likely that investigators will uncover previously missed metabolic qualities of immune cell subsets in different disease models and in patient samples. Additionally, flow cytometry-based methods of measuring metabolic markers such as CyTOF and Met-flow65, a new high-parameter method focused on measuring rate-limiting metabolic proteins, have a large advantage over scRNA-seq technology. Although scRNA-seq technology is powerful, some metabolic genes and pathways may not be adequately captured within a single cell; therefore, enrichment of specific metabolic pathways is not always readily distinguishable between cell clusters. To this end, recent pipelines have been developed to improve analysis of metabolic gene expression at single-cell level and may aid identification of metabolic programmes in heterogeneous data sets66. However, by using targeted single-cell flow cytometric approaches, the focus can be placed on metabolic genes of interest, offering more depth and a clear view of metabolic perturbations.
Metabolomics
Assessing the functional output of metabolism
The techniques described above address metabolic pathway activity or gene expression; however, they are unable to directly analyse the output of metabolism — namely, the metabolites themselves. Metabolite levels and metabolic fluxes are what ultimately control cellular functions imposed by gene and protein expression. As pathway activity can be dynamically controlled by enzyme-mediated PTMs, subcellular location or allosteric regulation, metabolic flux analyses can be used to identify a particular metabolic intersection responsible for cellular function to provide information that cannot be readily inferred from gene or protein expression alone.
Mass spectrometry-based metabolomics — hereinafter referred to as metabolomics — has the capacity to profile and quantify metabolites (FIG. 4). Owing to its sensitivity in detection, the advantage of metabolomics is the ability to measure hundreds or thousands of metabolites within a sample. Against this backdrop, the identification of altered metabolite levels can inform studies about the role of metabolites in regulating cell function. Additionally, when the fates of nutrients are traced using stable isotope-labelled (that is, non-radioactive) metabolites to assess nutrient fuelling of pathways, metabolomics can map metabolic networks and quantify pathway activities. Here, we describe common metabolomic platforms used to explore the role of metabolites as immunoregulators.
Fig. 4 |. Metabolomics workflow.
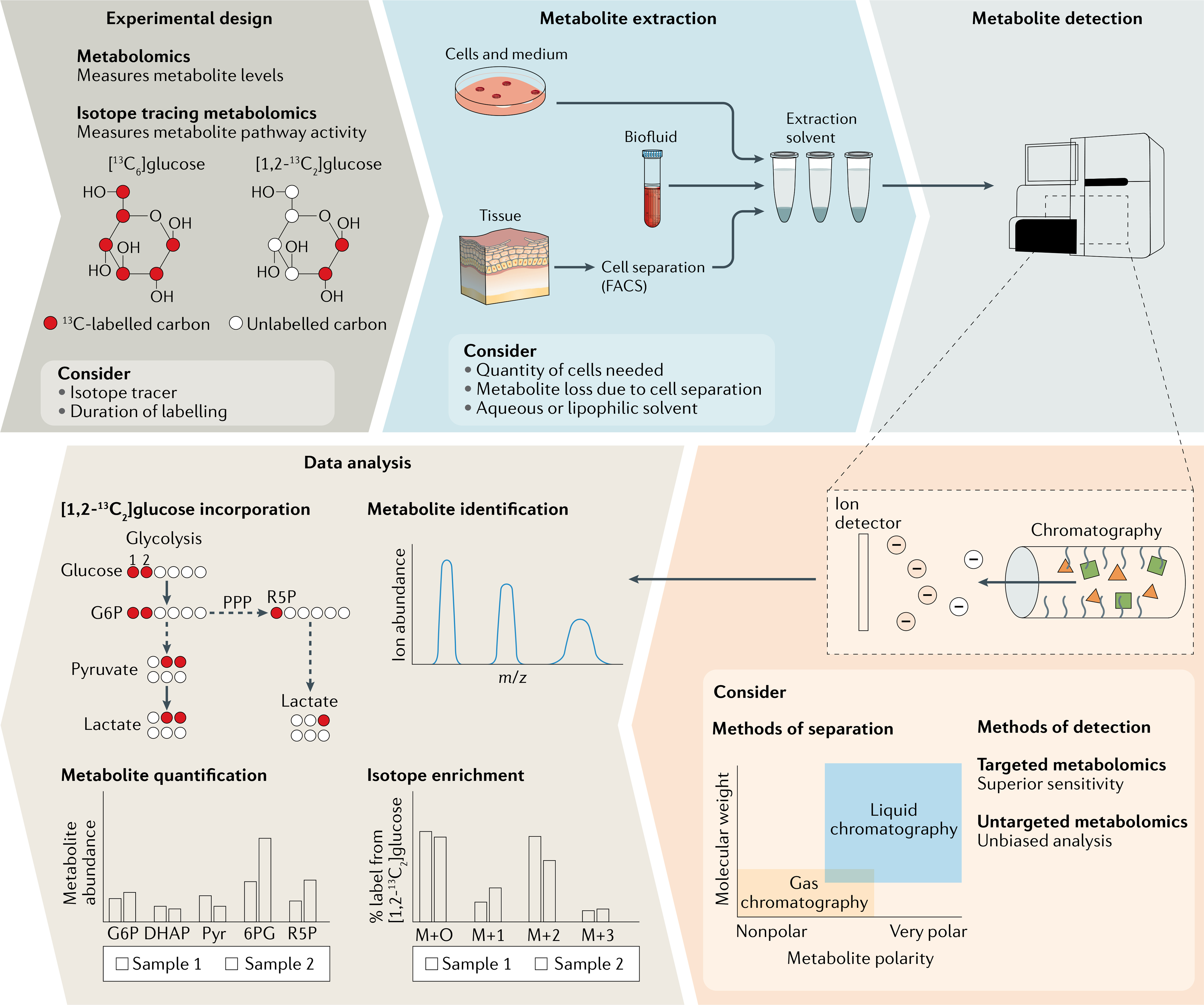
Experimental design: serial sampling or isotope tracing techniques measure pathway activities. Sample preparation and duration of isotope labelling depend on the metabolites and metabolic pathways of interest. Metabolomics at a single time point provides static metabolite levels. Flux measurements require collection of samples as a function of time. Metabolite extraction: after sample harvest, near instantaneous enzyme inactivation is crucial to achieve a representative metabolite profile. Metabolite detection: metabolite extracts are subjected to chromatography-coupled mass spectrometry to separate metabolites based on chemical properties and m/z, followed by detection and quantification. Data analysis: schematic example of [1,2-13C2]glucose incorporation into glycolysis and the pentose phosphate pathway (PPP). 13C-[abeWed carbons are depicted in red; unlabelled carbons in white. Metabolites are identified based on expected m/z. Absolute or relative quantification shows changes in metabolite levels and can reveal pathways of interest. For tracing experiments, tracer incorporation and pathway activity are assessed by the isotope labelling pattern. Glucose incorporation into glycolysis results in partially labelled lactate (M+2) whereas glucose-derived lactate from the PPP results in M+1 lactate. DHAP, dihydroxyacetone phosphate; FACS, fluorescence-activated cell sorting; G6P, glucose 6-phosphate; Pyr, pyruvate; R5P, ribose 5-phosphate; 6PG, 6-phosphogluconate.
Metabolomics concepts and approaches
Advancements in metabolomics have allowed for the simultaneous measurement of tens to thousands of metabolites and the discovery of metabolites with new roles in biology67–70. To start, metabolomics can be performed using targeted or untargeted approaches, and workflows can be adapted for the detection of certain sets or classes of metabolite, depending on the biological question (BOX 4). The goal of targeted metabolomics is to quantify the levels of known metabolites in an experimental setting71. Targeted platforms are optimized for the detection of a set of predetermined metabolites, in which absolute abundances can be measured with increased sensitivity and dynamic range. As such, this approach is hypothesis driven and best suited when pathways of interest are known. Targeted approaches are limited to analysis of well-characterized metabolites and the availability of internal standards necessary for accurate absolute quantification72. In contrast, untargeted metabolomics is discovery driven and examines all detectable metabolite species in a sample, providing a profile of metabolites, known and unknown. Although untargeted analysis can be hypothesis generating and harnessed for discovery purposes, it requires expertise in metabolite identification and can be limited in sensitivity owing to the general goal of detecting as many metabolic features as possible73. In other words, although thousands of species may be detected, only a minority may be identified74. This can, however, lead to new discoveries and is well suited for biomarker development.
Box 4 |. Metabolomics nomenclature Targeted metabolomics.
Assessment of known metabolites based on parameters established with standards. Absolute quantification is achieved by interpolating the amount of a metabolite in an experimental sample from parallel data with metabolite standards or by spiking internal standards into the experimental sample at the time of metabolite harvest. Relative quantification is achieved by comparing ion intensities across samples within a batch.
Untargeted metabolomics
Profiling of known and unknown metabolites in a given sample, the identity of which are established with standards. Untargeted approaches are often used for metabolite discovery (for example, identifying biomarkers). To bridge the gap between targeted and untargeted analysis, instrumentation that employs targeted methods can be modified to broaden metabolite coverage72,76.
Stable isotope labelling metabolomics
Assessment of nutrient utilization in a pathway with stable, non-radioactive isotopically labelled substrates. Stable isotope (for example, 2D, 13C, 15N, 18O) labelling of nutrients (for example, carbohydrates, amino acids and lipids) can be used to assess whether and how a nutrient is used in metabolic pathways. Additionally, positionally labelled tracers can be used to identify which pathways are active and provide relative quantification of pathway activities through expected differences in labelling patterns associated with active pathways.
Flux measurements with metabolomics
Quantification of pathway activity by measuring isotope incorporation as a function of time. Through dynamic labelling techniques, the enrichment of labelled metabolites in a pathway can be quantified as a function of time to measure pathway activity, that is, flux.
Fluxomics
Systems biology approach to map the global, steady-state incorporation of a labelled nutrient input into the metabolic networks within a cell. This technique, not to be confused with a flux measurement, is the untargeted metabolomic profiling of an isotopically labelled nutrient substrate69.
Quenching, extraction and separation.
Metabolite turnover can occur on a timescale of seconds to minutes. Thus, rapid sample collection and metabolism quenching are crucial for accurate metabolite measurements75. This entails minimal perturbations to samples before collection and immediate enzymatic inactivation during harvest to capture the metabolome reflective of the biological system. This is a notable challenge for immunology studies, as it is routinely necessary to purify immune populations of interest using flow cytometry-based sorting or magnetic beads, processes that can take a considerable amount of time and are performed in low-nutrient buffers, allowing metabolite exchange and diffusion. The incubation of isolated cells for short periods under standard culture conditions may provide the nutrients needed to reinstate metabolic homeostasis following sorting. Alternatively, magnetic bead-mediated enrichment may allow for quicker purification methods with minimal metabolite loss. This challenge is an area of active development as it limits the scope of in vivo immunometabolism.
Consideration of the methods involved in metabolite extraction and processing can ensure the detection of metabolites of interest76. For instance, aqueous metabolites are readily extracted using an aqueous/ polar solvent, such as methanol and water; nonpolar metabolites in butanol and methanol; whereas a mixture of methanol and chloroform results in extraction of both polar and nonpolar metabolites with lipophilic properties. Metabolite extracts can be extremely complex. Chromatography deconvolutes metabolite mixtures by separating analytes based on physicochemical properties. For example, common methods of liquid chromatography entail reconstitution of metabolite extracts, followed by separation based on polarity or hydrophobicity. For gas chromatography, metabolites are vapourized and separated based on volatility.
Detection.
Mass spectrometry detects charged molecules. Thus, analytes are ionized to enable detection by mass spectrometry based on their relative mass and charge, followed by quantification of analyte abundances. The ability to separate different analytes with similar mass-to-charge — m/z — ratios is key for increased resolution and detection sensitivity. Routine mass spectrometry instruments are classified as TOF, ion trap (IT) and triple quadrupole (QqQ). Owing to the diversity and complexity of the metabolome, instrumentation and associated techniques are commonly optimized to detect metabolites of interest. For instance, QqQ instruments are highly selective for the detection of predefined analytes, and offer the best sensitivity for quantification of known metabolites. By contrast, TOF and IT instruments can be used for untargeted analysis to profile a wider range of metabolites in a given sample, in a non-predetermined fashion. Of note, no singular technique, or even combination of techniques, is able to assess all of the metabolites in a given sample. As there are additional types of mass spectrometry each with selective advantages and resolving power, consideration of the instrumentation available and metabolites of interest can provide opportunities to adapt workflows most suitable for one’s biological question.
Metabolome.
The complete set of metabolites present in a cell, biological fluid or tissue sample.
Data analysis and interpretation.
Metabolomics performed at a single time point measure static metabolite levels, and the associated analyses need to be considered accordingly (BOX 4). For example, increase in a metabolite pool size may reflect either increased production or decreased consumption. Three techniques can be employed to examine pathway activities69. First, metabolite sampling at multiple time points (flux analysis) can assess changes in metabolite levels over time. Second, isotope tracing metabolomics can quantify pathway activities that contribute to the level of a particular metabolite. In this technique, nutrient inputs are composed of non-radioactive isotope tracers, such as 13C, 15N or 2H; each of these isotopes is one Dalton heavier than its naturally occurring counterpart (that is, 12C, 14N or 1H). Isotope tracers routinely used in metabolomics studies include uniformly labelled glucose (13C6-U-glucose) and glutamine (13C5-U-glutamine)70. Enrichment into metabolism is assessed by their isotope labelling patterns, which can identify pathways that are active and contribute to the accumulation of a particular metabolite. To compare differential pathway activities, positionally labelled tracers can reveal the partitioning of a labelled substrate, as seen by the enrichment of [1,2-13C]glucose into ribose as a read-out of pentose phosphate pathway activity. When paired with immunophenotyping, isotope tracing metabolomics has been successful in determining the functional importance of glycolytic intermediates77,78, glutamine18, arginine79, acetate80,81, methionine82,83 and one-carbon metabolism84,85 as modulators of immune cell activity.
Lastly, fluxomics quantifies metabolic pathway activity by integrating isotope tracing and flux analysis with computational modelling of biochemical reactions86,87. In short, intracellular and extracellular metabolites are quantified at time points that capture tracer incorporation and turnover by a particular pathway, known as dynamic labelling. By integrating data from transcriptomics and proteomics with metabolomics, a systems biology approach is used to infer the route and rate of conversion from the tracer to a particular metabolite88–90. Although fluxomics is best-suited for relating metabolite concentrations to metabolic activity, it can be technically challenging, as dynamic labelling depends on the tracer employed and pathway probed. For example, in cultured cancer and immune cells, it has been our experience that [13C]glucose is rapidly incorporated into glycolysis69. As such, samples need to be collected at a 10 s timescale to capture glycolytic flux. Incorporation into the tricarboxylic acid (TCA) cycle is somewhat slower, and samples are recommended to be interrogated on the order of tens of minutes. In vivo flux analysis is feasible, but more reliable when measuring metabolites and pathways with long half-lives41. Thus, through careful experimental planning, isotope tracing metabolomics can provide relative assessments of pathway activities, which may provide sufficient information to address one’s scientific question.
Genetic approaches in metabolic studies
The crosstalk and flexibility of cellular metabolism can challenge mechanistic studies and efforts to determine causation. Many of the measurements described above may yield fundamentally descriptive results. To define an association or causation of a specific metabolic programme with a specific cell state, it is important to disrupt or alter that pathway or metabolite to test how the perturbation affects the specific cell phenotype. On this note, metabolic redundancy and plasticity may complicate efforts to block a particular metabolite transporter or metabolic enzyme. It is also important to consider in vivo and microenvironmental conditions that can influence cell metabolism and fate.
A direct means to test the role of an enzyme and metabolic pathway in vivo is to directly disrupt that pathway genetically. Reverse genetic approaches, in which individual genes selected from given metabolic pathways are targeted or altered to determine the phenotype and role of that pathway, have revealed key in vivo functions of specific metabolic programmes in distinct cell populations. These strategies continue to be mainstays in understanding metabolic signalling and causation in immunometabolism, including the demonstration that while effector T cells require the glucose transporter GLUT1, Treg cells can function independently of GLUT1 in vivo91. This and related approaches have also corrected some previous assumptions made from small-molecule inhibitor-based studies. A recent example is the inhibitor of CPT1, etomoxir. It was previously understood that LCFA oxidation supported the survival of memory T cells and Treg cells, because etomoxir treatment limited their differentiation and function. However, a T cell-specific genetic model showed that CPT1A is dispensable for the formation of Treg cells and memory T cells, and that the effects previously noted with etomoxir were due to alternative and CPT1A-independent effects25. In a study of restimulation-induced cell death of human T cells, inhibition of fatty acid synthase (FASN) with C75 significantly protected T cells from apoptosis, but inhibition of acetyl-CoA carboxylase (ACC) did not, despite it being a rate-limiting enzyme along the same metabolic pathway92. These examples, among others, underscore the importance of genetic approaches to validate phenotypes whenever possible.
In some cases, metabolic phenotypes may be informed from naturally occurring genetic polymorphisms or disruptions. Recent characterizations of such phenotypes have revealed underlying and/or accommodating genetic immunodeficiencies, and these may offer novel targets for therapy. For example, naive T cells from patients with an autosomal dominant combined immunodeficiency, known as activated phosphoinositide 3-kinase-δ syndrome (APDS), were recently shown to exhibit enhanced glycolytic capacity and reduced mitochondrial respiration, leading to disrupted T cell homeostasis93. In another example, IL-10-producing regulatory B cells depended on cholesterol metabolism to induce IL-10 expression94. Interestingly, patients with the severe autoinflammatory disease mevalonate kinase deficiency who carry partial loss-of-function mutations in a gene involved in the metabolic pathway also exhibited a defect in regulatory B cell IL-10 production. These findings suggest that dysregulated cholesterol metabolism results in imbalanced B cell responses in patients with MKD, and could be attenuated by supplementation with the metabolic intermediate geranylgeranyl pyrophosphate (GGPP). Therefore, metabolic targeting has the potential to reduce disease severity in various immunodeficiencies. Complementary to this, investigation of genetic disruptions in patients with immunodeficiency has the potential to shed light on underappreciated metabolic contributions to immune function.
In addition, forward genetic strategies can screen sets of genes, or even the entire genome, for effects on specific phenotypes95,96. Advances in CRISPR guide RNA (gRNA) sequence algorithms, have greatly improved on-target efficiencies and decreased off-target effects. By delivering Cas9 and pooled panels of gRNAs to haematopoietic stem cells (HSCs) or immune cells, metabolic pathways of interest can be disrupted and rapidly analysed by high-throughput screening to establish enrichment or depletion of guides that target genes that direct specific phenotypes97,98. Notably, different target genes may result from the same gene library screen if selected in different media formulations99. Otherwise, these approaches are limited only by the array of target genes included in the screen. While these technologies continue to emerge and improve, including the use of protein barcodes (Pro-Codes)100 for single-cell CRISPR screening and cell phenotypic analysis, early findings suggest that these approaches will provide a powerful means to associate specific metabolic genes and events with immune cell states and functions. Ultimately, most is gained when a cycle of metabolic measurements and genetic modulations is employed to directly establish how each step in a pathway affects cell metabolism and fate in vivo.
Key lessons from immunometabolism studies
An immune response is dynamic and consists of phases of cellular activation, inflammation and resolution. It is now clear that immune cell activity can be regulated, in part, by metabolic rewiring driven by cell-intrinsic factors and extracellular nutrient availability. Indeed, advancements in metabolic platforms, amenable for real-time and dynamic metabolic sampling of immune cells, have greatly enhanced our understanding of the complex metabolic regulation underlying immunity. Here, we highlight emerging concepts in the immunometabolism field, uncovered through the use of experimental strategies like those in the roadmap detailed above. Below, we describe additional techniques and workflow adaptations, fine-tuned for the experimental question in mind, that aided in the elucidation of novel metabolic mechanisms. Moreover, we discuss active developments and needed advancements in metabolic techniques that will deepen our understanding of metabolism within rare immune cell populations and in complex cellular networks.
Metabolic pathway activity regulates immune cell function.
Major headway has been made in understanding immune metabolism, perhaps most notably in T cells. Early reports using EFA revealed that naive, effector and memory T cells adapt distinct bioenergetic phenotypes that correlate with their differentiated states. Metabolic comparison across CD8+ and CD4+ T cell subsets ignited two conceptual frameworks. First, that activation and cytokine conditions directly control T cell metabolism. Second, that metabolic activity can control T cell differentiation and function. From this, an early view of metabolic programming was that effector T cells induce and rely on glycolysis, then switch to fatty acid oxidation (FAO) and OXPHOS to support memory T cell formation and function. We now understand that conclusions such as this (that some immune cells rely only on glycolysis or OXPHOS) were oversimplified. The reality is that each metabolic pathway provides unique inputs into the overall function of the cell, despite changes in the degree to which glycolysis and OXPHOS are used. Owing to the application of genetic methods that manipulate specific pathway activities and metabolomics analyses, there is also a greater appreciation of the off-target effects of common metabolic inhibitors, especially in memory CD8+ T cells25,101,102.
Although memory CD8+ T cells have been shown in multiple settings to utilize lipid-based oxidative metabolism, these cells remain metabolically flexible. Indeed, many of the metabolic changes in CD8+ T cells can be viewed as transitions from catabolic states as resting and memory cells to anabolic states to support proliferation and effector functions. EFA of CD8+ T cells exhibiting constitutive glycolytic activity, driven by conditional deletion of the hypoxia-inducible factor (HIF) regulator VHL, demonstrated that glycolysis can support memory formation and recall responses in vivo101. In support of this, isotope tracing metabolomics of [13C]palmitate in memory CD8+ T cells with deletion of Cpt1a (encodes the rate-limiting enzyme of FAO) revealed comparable TCA fuelling to that of control wild-type memory T cells, in line with the similar memory responses shown by Cpt1a−/− and wild-type T cells in vivo25. Together, these studies demonstrate that FAO of LCFAs is not required in memory CD8+ T cells, but instead other nutrient sources, such as short-chain fatty acids, can fuel their metabolic activity and cellular function. In addition to casting new light on the metabolic flexibility of memory CD8+ T cells, these studies exemplify how EFA can be used to identify the differences in metabolic phenotypes, followed by metabolomics for analysis of pathway activities and metabolic validation.
Indeed, metabolomics approaches have uncovered how seemingly nuanced differences in metabolic activity can drastically alter CD4+ T cell lineage commitment and the development of associated inflammatory diseases. For instance, although TH1 and TH17 cells exhibit similar bioenergetic phenotypes, each subset produces distinct effector cytokines, which suggests different metabolic demands necessary for the execution of lineage-specific function. Accordingly, isotope tracing metabolomics of [13C]glutamine revealed that TH1 cells predominantly use glutamine to fuel the TCA cycle, whereas TH17 cells require glutamine for the generation of glutathione to limit oxidative stress during differentiation18. By tracking the fate of heavy labelled glucose, it was shown that TH1 cells and Treg cells, but not TH17 cells, preferentially used glucose-derived pyruvate to fuel the TCA cycle77. This finding was crucial for implicating glucose metabolism in Treg cell identity and function, despite their oxidative phenotype demonstrated by EFA103.
In another example, isotopomer glucose tracing in B cells revealed unexpected fates of glucose after naive B cell activation104. Although activated B cells did increase glucose uptake, glucose-derived carbons (and subsequently, labelled pyruvate) were not fully incorporated into lactate. These findings also shed light on opposing conclusions in the B cell literature, in which B cells demonstrate increased ECAR after activation, but direct measurement of lactate in the medium does not show increases and pyruvate may instead fuel increased mitochondrial oxygen consumption and increased OCR. Therefore, combination of both EFA and glucose tracing was necessary for an accurate picture of glycolysis in activated B cells. Together, these findings highlight how isotope tracing techniques can uncover the divergent use of nutrient sources that enable subset-specific immune cell processes.
Finally, given the dynamic and diverse nature of T cell responses in vivo, accumulating evidence suggests that the in vitro metabolic profile of T cells may not reflect their metabolic activity in vivo41,105. Whereas ex vivo analysis of metabolic activity is a facile approach for immune studies, in vivo metabolomic profiling remains an area of development41,68,106. One approach that has established use in clinical practice is 18F-deoxyglucose (FDG) positron emission tomography (PET) imaging, which utilizes the increased rate of glucose uptake of tumours for cancer detection and monitoring. Recently, measurement of in vivo FDG uptake in mouse tumours combined with ex vivo cell sorting showed that myeloid cells in tumours consumed more glucose than cancer cells on a per cell basis107. This challenges the assumption that nutrient scarcity in the TME drives immune cell dysfunction and suggests that cellular metabolic programmes may have a larger role in partitioning of select nutrients. Thus, in vivo radioisotope tracing may be one method to circumvent the limitations of in vitro metabolic studies and offer new insights.
Metabolites that function as enzymatic substrates influence gene expression.
During immune cell activation, metabolic reprogramming coincides with epigenome remodelling, wherein the addition or removal of epigenetic marks alters chromatin accessibility, thereby influencing gene transcription2,3,108. Importantly, epigenetic modifiers are catalysed by the availability of substrates derived from metabolic pathways and regulated by metabolites that competitively inhibit substrate utilization. For example, α-ketoglutarate (αKG) derived from the TCA cycle is required for histone demethylase activity, which can be inhibited by high levels of succinate, fumarate or 2-hydroxyglutarate (2HG). Thus, the dependence on distinct metabolic pathways can regulate the abundance of metabolites required to maintain or reprogramme the epigenome, resulting in modulation of immune cell identity and function.
To assess the functional importance of metabolism on epigenome remodelling, methods to manipulate and measure metabolite levels are crucial for the identification of metabolites that control the activity of epigenetic modifiers. For example, metabolomic profiling of CD8+ T cells uncovered altered 2HG production induced by HIF activity109. Absolute quantification revealed that 2HG was produced at millimolar (physiological) concentrations, suggesting that 2HG may modulate the activity of demethylases. Indeed, 2HG accumulation was found to alter the levels of DNA and histone methylation and influence terminal differentiation in CD8+ T cells. Additionally, adaption of this similar experimental framework to CD4+ T cells uncovered the role of 2HG-mediated epigenetic reprogramming in supporting TH17 cell lineage commitment110 and Treg cell function111. These studies exemplify the power of metabolomic workflows in relating altered metabolite levels to epigenetic remodelling, resulting in regulation of T cell fate.
The ability of certain metabolites to regulate the epigenome has focused efforts on understanding how metabolic decisions prime immune cells for periods of differentiation, cytokine production and survival. In a manner akin to cytokine signalling, immune cell-generated metabolites — those that display functions outside of ATP generation — may serve as an axis to fine tune immune responses12. For example, through isotope tracing techniques, the availability of extracellular acetate80,81 and methionine82,83,112 was found to regulate the extent of histone acetylation and methylation, respectively. By modulating the intracellular pools of metabolites required for epigenetic activity, the availability of these nutrients affected the capacity to carry out epigenetic modifications, resulting in altered effector cytokine expression. Along these lines, recent work on T cell exhaustion implicated epigenetic modifications in regulating the expression of exhaustion markers113,114. Considering that isolation of exhausted T cells requires extensive processing with limited yield, advancements in cell isolation will be critical for examining the role of metabolic dysfunction in regulating the epigenome of exhausted T cells, an area that remains largely under-studied but of great interest.
Metabolic pathways control antitumour immune responses.
Unlike lymphoid tissues, which are replete in nutrients and cytokines that can support T cell survival and activation, the TME can be nutrient poor, immunosuppressive and largely restrictive to antitumour T cell responses115,116. The identification of metabolic dependencies required for tumour growth, but not for antitumour immunity, is an emerging therapeutic strategy117–119. Therefore, a major focus of current research is to understand how immune cells are shaped by the TME, in which myriad cell types exist, interact and contribute to the metabolic environment. A straight-forward technique that models a nutrient-poor TME is the use of standardized media depleted of selective nutrients, such as glucose or glutamine. Under nutrient restriction, alternative nutrient sources and pathways that maintain immune activity can be revealed. Recent application of this technique successfully identified the role of lactate42, inosine120, serine121 and methionine82 as essential or alternative nutrient sources for intratumoural T cells. Consequently, a better understanding of how intratumoural cell types contribute and respond to nutrient depletion will form the basis for interventions that aim to modulate the metabolite composition systemically and/or through targeted approaches.
Another technique used to model the interactions between cell types in the TME relies on conditioned medium (supernatant harvested from tumour cell cultures), which reduces the complexity of the model system. For example, the effects of tumour-derived factors on immune cell polarization and function can be examined by culturing immune cells in conditioned medium, which can be subject to heat inactivation or low molecular weight filtration. When a metabolite is the relevant factor, metabolomics can identify and quantify the metabolite of interest. This approach was successful in identifying the role of tumour-associated macrophages (TAMs) in mediating chemoresistance to gemcitabine therapy in pancreatic cancer122. Following polarization of TAMs using pancreatic cancer-conditioned medium, metabolomics revealed that TAMs generate and release numerous pyrimidine nucleosides, and in particular, deoxycytidine. Gemcitabine, an analogue of deoxycytidine, induces cell death by being incorporated into DNA to inhibit synthesis. Consequently, cotreatment of deoxycytidine and isotope-labelled gemcitabine was found to inhibit gemcitabine incorporation into DNA of cancer cells, suggesting that TAM-released deoxycytidine confers chemoresistance by inhibiting gemcitabine uptake and metabolism by pancreatic cancer cells. This model, developed using a facile in vitro co-culture platform, was subsequently validated using genetically engineered mouse models of pancreatic cancer.
Analogous approaches have also been applied to investigate metabolic interactions between immunosuppressive and antitumour cells. For example, conditioned medium generated from ex vivo cultures of apoptotic Treg cells isolated from the TME was found to potently suppress effector T cell proliferation and cytokine production123. Given the variety of suppressive factors that mediate Treg cell suppression, low molecular weight filtration and metabolomic approaches enabled the identification of adenosine as the relevant small molecule that mediates superior immunosuppression by tumour-infiltrating, apoptotic Treg cells.
Similarly, the novel metabolite methylglyoxal was shown to be produced by myeloid-derived suppressor cells (MDSCs) and transferred to T cells, wherein its accumulation inhibited antitumour activity14. Through EFA and metabolic phenotyping by flow cytometry, MDSCs were observed to be less metabolically active than monocyte controls. To understand which metabolite mediates this metabolic phenotype, untargeted metabolomics identified methylglyoxal as a metabolic marker of MDSCs. Notably, a competitive pulse-chase approach using isotope tracing metabolomics demonstrated a branching reaction from glycolysis as the active metabolic pathway responsible for generating methylglyoxal in MDSCs. Last, the use of a fluorescent probe specific for methylglyoxal greatly aided orthogonal approaches that confirmed the immunosuppressive function of methylglyoxal in vitro and ex vivo.
Conclusions
Although it may seem that the rapid expansion in immunometabolism research has hinged on new technologies for advancement, time-tested techniques in biochemistry, pharmacology and immunology continue to play important roles. The increased accessibility, sensitivity and power of metabolic assays have made metabolic testing widely available and comparable across labs. No doubt these approaches will continue to advance the fields of immunology and cell metabolism.
Here, we make efforts to provide a roadmap to integrate established and novel metabolism techniques to investigate a potential metabolic phenotype. Although some of these techniques are not widely available for the average metabolism enthusiast, collaborations may empower advancements in mechanistic studies and increase their quality considerably. Along the way, we highlight potential trip-ups and points of confusion that can arise with the drawbacks of some of these technologies. Importantly, metabolic phenotypes are not ‘black and white’, making interpretation of metabolism read-outs difficult. In fact, most metabolic perturbations are likely to occur as a continuum of activity along a gradient of pathway flux. For this reason, metabolism researchers should be prepared to use multiple strategies to validate and characterize their metabolic findings.
As the size and complexity of multi-omic and other data sets increases, methods to integrate information across platforms are becoming increasingly important. Tools that enable genome-scale models of metabolism and metabolic networks have made numerous advancements in recent years124. Improvements have also been made in constraint-based models for predicting metabolic functions of uncharacterized genes of interest125. Genetic approaches enable mechanistic studies of metabolic genes to assess causation of metabolic phenotypes and cell fates and should take physiological culture conditions into consideration whenever possible. Additionally, technologies with single-cell capability will ultimately be important in advancing metabolic studies of immune cells, given the heterogeneity of immune cell populations and the gradients that exist in their metabolic states.
Finally, this roadmap is only meant to suggest possible routes. There is much uncharted territory and no one ‘right’ way to travel from a metabolic hypothesis to the theoretical end of the road. Therefore, do not be afraid to think outside the box, do what works best for you and enjoy the journey.
Acknowledgements
The authors thank C. Deeter and K. V. Tormos at Agilent for helpful resources and discussion.
Funding
This work was supported by T32 DK101003 (K.V.), T32 DK094775 (H.S.H), K00 CA234920 (J.E.B.), T32 GM007347 (A.S.), 1R37CA237421, R01CA248160, R01CA244931 (C.A.L.) and R01 CA217987 and R01 DK105550 (J.C.R.).
Footnotes
Competing interests
J.C.R. holds stock equity in Sitryx and within the past 2 years has received unrelated research support, travel and honoraria from Incyte, Sitryx, Caribou, Nirogy, Kadmon, Calithera, Tempest, Merck, Mitobridge and Pfizer. The other authors declare no competing interests.
Peer review information
Nature Reviews Immunology thanks N. Chandel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Murphy MP & O’Neill LAJ Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174, 780–784 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Fan J, Krautkramer KA, Feldman JL & Denu JM Metabolic regulation of histone post-translational modifications. ACS Chem. Biol. 10, 95–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron AM, Lawless SJ & Pearce EJ Metabolism and acetylation in innate immune cell function and fate. Semin. Immunol. 28, 408–41 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason EF & Rathmell JC Cell metabolism: an essential link between cell growth and apoptosis. Biochem. Biophys. Acta 1813, 645–654 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voss K, Larsen SE & Snow AL Metabolic reprogramming and apoptosis sensitivity: Defining the contours of a T cell response. Cancer Lett. 408, 190–196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green DR, Galluzzi L & Kroemer G Metabolic control of cell death. Science 345, 1457–1465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim B et al. Discovery of widespread host protein interactions with the pre-replicated genome of CHIKV Using VIR-CLASP. Mol. Cell 78, 624–640 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Y, Tariq M, Guo X, Kanwal S & Esteban MA Intricacies in the cross talk between metabolic enzymes, RNA, and protein translation. J. Mol. Cell Biol. 11,813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tristan C, Shahani N, Sedlak TW & Sawa A The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal. 23, 317–323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollizzi KN & Powell JD Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 14, 435–446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jellusova J Cross-talk between signal transduction and metabolism in B cells. Immunol. Lett. 201, 1–13 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Zasfona Z & O’Neill LAJ Cytokine-like roles for metabolites in immunity. Mol. Cell 78, 814–823 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Baj A et al. Glutamatergic signaling along the microbiota-gut-brain axis. Int. J. Mol. Sci. 20, 1482 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumann T et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat. Immunol. 21,555–566 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Johnson MO, Siska PJ, Contreras DC & Rathmell JC Nutrients and the microenvironment to feed a T cell army. Semin. Immunol. 28, 505–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boothby M & Rickert RC Metabolic regulation of the immune humoral response. Immunity 46, 743–755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerriets VA & Rathmell JC Metabolic pathways in T cell fate and function. Trends Immunol. 33, 168–173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MO et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 175, 1780–1795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung J, Zeng H & Horng T Metabolism as a guiding force for immunity. Nat. Cell Biol. 21,85–93 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Zhang J & Zhang Q Using seahorse machine to measure OCR and ECAR in cancer cells. Methods Mol. Biol. 1928, 353–363 (2019). [DOI] [PubMed] [Google Scholar]
- 21.van der Windt GJW, Chang CH & Pearce EL Measuring bioenergetics in T cells using a seahorse extracellular flux analyzer. Curr. Prot. Immunol. 113, 16B.1–16B.14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelgrom LR, van der Ham AJ & Everts B Analysis of TLR-induced metabolic changes in dendritic cells using the Seahorse XFe96 extracellular flux analyzer. Methods Mol. Biol. 1390, 273–285 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Mookerjee SA, Nicholls DG & Brand MD Determining maximum glycolytic capacity using extracellular flux measurements. PLoS ONE 11, e0152016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mookerjee SA & Brand MD Measurement and analysis of extracellular acid production to determine glycolytic rate. J. Vis. Exp. 106, 53464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raud B et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. CellMetab. 28, 504–515.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mookerjee SA, Gerencser AA, Nicholls DG & Brand MD Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. 292, 7189–7207 (2017). This is a helpful in-depth discussion of using extracellular flux assays and proper interpretation.
- 27.Newling M et al. C-reactive protein promotes inflammation through FcγR-induced glycolytic reprogramming of human macrophages. J. Immunol. 203, 225–235 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Saini V et al. Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis. Nat. Commun. 11,557 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco da Cunha F et al. Extracellular vesicles isolated from mesenchymal stromal cells modulate CD4+ T lymphocytes toward a regulatory profile. Cells 9, 1059 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis KD et al. Glycogen metabolism supports early glycolytic reprogramming and activation in dendritic cells in response to both TLR and Syk-dependent CLR agonists. Cells 9, 715 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG & Brand MD The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 1847, 171–181 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Little AC et al. High-content fluorescence imaging with the metabolic flux assay reveals insights into mitochondrial properties and functions. Commun. Biol. 3, 271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S, Li H, Chen J & Qian Q Lactic acid: no longer an inert and end-product of glycolysis. Physiology 32, 453–463 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Menk AV et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelletier M, Billingham LK, Ramaswamy M & Siegel RM Extracellular flux analysis to monitor glycolytic rates and mitochondrial oxygen consumption. Methods Enzymol. 542, 125–149 (2014). [DOI] [PubMed] [Google Scholar]
- 36.di Cara F et al. Peroxisomes in immune response and inflammation. Int. J. Mol. Sci. 20, 3877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordgren M & Fransen M Peroxisomal metabolism and oxidative stress. Biochimie 98, 56–62 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Shi L & Tu BP Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol. 33, 125–131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks GA Cell-cell and intracellular lactate shuttles. J. Phys. 587, 5591–5600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 21, 298–308 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma EH et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51,856–870.e5 (2019). This paper illustrates the power of 13C tracing to define metabolic pathways in vivo and shows that CD8+ T cells use glucose primarily for biosynthetic pathways rather than conversion into lactate.
- 42.Angelin A et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binek A et al. Flow cytometry has a significant impact on the cellular metabolome. J. Prot. Res. 18, 169–181 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Llufrio EM, Wang L, Naser FJ & Patti GJ Sorting cells alters their redox state and cellular metabolome. Redox Biol. 16, 381–387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu G et al. Dissecting the human immune system with single cell RNA sequencing technology. J. Leukoc. Biol. 107, 613–623 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Ye F & Guo G Revolutionizing immunology with single-cell RNA sequencing. Cell. Mol. Immunol. 16, 242–249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saxton RA & Sabatini DM mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hartmann FJ et al. Single-cell metabolic profiling of human cytotoxic T cells. Nat. Biotechnol. 39, 186–197 (2021). This paper demonstrates the potential of high-dimensional profiling of metabolic proteins in single cells to define metabolic phenotypes.
- 49.Subrahmanyam PB & Maecker HT CyTOF measurement of immunocompetence across major immune cell types. Curr. Protoc. Cytom. 82, 9.54.1–9.54.12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartmann FJ & Bendall SC Immune monitoring using mass cytometry and related high- dimensional imaging approaches. Nat. Rev. Rheum. 16, 87–99 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artyomov MN & van den Bossche J Immunometabolism in the single-cell era. Cell Metab. 32, 710–725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue M, Wei W, Su Y, Johnson D & Heath JR Supramolecular probes for assessing glutamine uptake enable semi- quantitative metabolic models in single cells. J. Am. Chem. Soc. 138, 3085–3093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siska PJ et al. Fluorescence-based measurement of cystine uptake through xCT shows requirement for ROS detoxification in activated lymphocytes. J. Immunol. Methods 438, 51–58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sinclair LV, Barthelemy C & Cantrell DA Single cell glucose uptake assays: a cautionary tale. Immunometabolism 2, e200029 (2020). This paper highlights the contradictory results that can be obtained using fluorescent analogues of glucose to measure glucose uptake.
- 55.Evers TMJ et al. Deciphering metabolic heterogeneity by single-cell analysis. Anal. Chem. 91, 13314–13323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L & Vertes A Single-cell mass spectrometry approaches to explore cellular heterogeneity. Angew Chem. Int. Ed. 57, 4466–4477 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Galler K et al. Making a big thing of a small cell-recent advances in single cell analysis. Analyst 139, 1237–1273 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Siska P J. et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2, e93411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckermann KE et al. CD28 costimulation drives tumor-infiltrating T cell glycolysis to promote inflammation. JCI Insight 5, e138729 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yucel N et al. Glucose metabolism drives histone acetylation landscape transitions that dictate muscle stem cell function. Cell Rep. 27, 3939–3955.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karmaus PWF et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature 565, 101–105 (2019). This study uses scRNA-seq to define in vivo metabolic phenotypes for pathogenic and stem TH17 cells.
- 62.Gaublomme JT et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163, 1400–1412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang C et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimmey SC, Borges L, Baskar R & Bendall SC Parallel analysis of tri-molecular biosynthesis with cell identity and function in single cells. Nat. Commun. 10, 1185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahl P J. et al. Met-Flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun. Biol. 3, 305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao Z, Dai Z & Locasale JW Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 10, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang C, Chen L & Rabinowitz JD Metabolomics and isotope tracing. Cell 173, 822–837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan M et al. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat. Protoc. 14, 313–330 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buescher JM et al. A roadmap for interpreting 13C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 34, 189–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rinschen MM, Ivanisevic J, Giera M & Siuzdak G Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 20, 353–367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patti GJ, Yanes O & Siuzdak G Innovation: metabolomics: the apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 13, 263–269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu H, Zhang P, Zhu J & Raftery D Globally optimized targeted mass spectrometry: reliable metabolomics analysis with broad coverage. Anal. Chem. 87, 12355–12362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sindelar M & Patti GJ Chemical discovery in the era of metabolomics. J. Am. Chem. Soc. 142, 9097–9105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallart-Ayala H, Teav T & Ivanisevic J Metabolomics meets lipidomics: assessing the small molecule component of metabolism. BioEssays 42, 2000052 (2020). [DOI] [PubMed] [Google Scholar]
- 75. Lu W et al. Metabolite measurement: pitfalls to avoid and practices to follow. Annu. Rev. Biochem. 86, 277–304 (2017). A helpful guide for navigating metabolomics of water-soluble metabolites, comparing the strengths and weaknesses of liquid chromatography-tandem mass spectrometry, gas chromatography-mass spectrometry and NMR.
- 76.Lee HJ, Kremer DM, Sajjakulnukit P, Zhang L & Lyssiotis CA A large-scale analysis of targeted metabolomics data from heterogeneous biological samples provides insights into metabolite dynamics. Metabolomics 15, 103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerriets VA et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blagih J et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses invivo. Immunity 42, 41–54 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Geiger R et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balmer ML et al. Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity. 44, 1312–1324 (2016). [DOI] [PubMed] [Google Scholar]
- 81. Balmer ML et al. Memory CD8+ T cells balance pro- and anti-inflammatory activity by reprogramming cellular acetate handling at sites of infection. Cell Metab. 32, 457–467.e5 (2020). This study demonstrates that alternative fuels such as acetate can play key roles in T cell function in infection.
- 82.Bian Y et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 585, 277–282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roy DG et al. Methionine metabolism shapes T helper cell responses through regulation of epigenetic reprogramming. Cell Metab. 31,250–266.e9 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Ron-Harel N et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 24, 104–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma EH et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Niedenfuhr S, Wiechert W & Noh K How to measure metabolic fluxes: a taxonomic guide for 13C fluxomics. Curr. Opin. Biotechnol. 34, 82–90 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Oruganty K, Campit SE, Mamde S, Lyssiotis CA & Chandrasekaran S Common biochemical properties of metabolic genes recurrently dysregulated in tumors. Cancer Metab. 8, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Llufrio EM, Cho K & Patti GJ Systems-level analysis of isotopic labeling in untargeted metabolomic data by X13CMS. Nat. Protoc. 14, 1970–1990 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guijas C, Montenegro-Burke JR, Warth B, Spilker ME & Siuzdak G Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 36, 316–320 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forsberg EM et al. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat. Protoc. 13, 633–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Macintyre AN et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voss K, Luthers CR, Pohida K & Snow AL Fatty acid synthase contributes to restimulation-induced cell death of human CD4 T cells. Front. Mol. Biosci. 6, 106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jia Y et al. Hyperactive PI3Kδ predisposes naïve T cells to activation via aerobic glycolysis programs. Cell. Mol. Immunol. 10.1038/s41423-020-0379-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bibby JA et al. Cholesterol metabolism drives regulatory B cell IL-10 through provision of geranylgeranyl pyrophosphate. Nat. Commun. 11, 3412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webster DE, Roulland S & Phelan JD Protocols for CRISPR-Cas9 screening in lymphoma cell lines. Methods Mol. Biol. 1956, 337–350 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Sanjana NE Genome-scale CRISPR pooled screens. Anal. Biochem. 532, 95–99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LaFleur MW et al. A CRISPR-Cas9 delivery system for in vivo screening of genes in the immune system. Nat. Commun. 10, 1668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bailis W et al. Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature 571,403–407 (2019). These two papers use in vivo pooled CRISPR gene targeting in forward genetic screens to identify key metabolic genes in haematopoietic cells and demonstrate crucial roles for mitochondrial metabolism.
- 99.Rossiter NJ et al. CRISPR screens in physiologic medium reveal conditionally essential genes in human cells. Cell Metab. 10.1016/j.cmet.2021.02.005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wroblewska A et al. Protein barcodes enable high-dimensional single-cell CRISPR screens. Cell 175, 1141–1155.e 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Phan AT et al. Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity 45, 1024–1037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raud B, McGuire PJ, Jones RG, Sparwasser T & Berod L Fatty acid metabolism in CD8+ T cell memory: challenging current concepts. Immunol. Rev. 283, 213–231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerriets VA et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 17, 1459–1466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waters LR, Ahsan FM, Wolf DM, Shirihai O & Teitell MA Initial B cell activation induces metabolic reprogramming and mitochondrial remodeling. iScience 5, 99–109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Franchi L et al. Inhibiting oxidative phosphorylation in vivo restrains Th17 effector responses and ameliorates murine colitis. J. Immunol. 198, 2735–2746 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glick GD et al. Anaplerotic metabolism of alloreactive T cells provides a metabolic approach to treat graft-versus-host disease. J. Pharm. Exp. Ther. 351,298–307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reinfeld BI et al. Cell programmed nutrient partitioning in the tumor microenvironment. Nature 10.1038/s41586-021-03442-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reid MA, Dai Z & Locasale JW The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat. Cell Biol. 19, 1298–1306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tyrakis P A. et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 540, 236–241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu T et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548, 228–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weinberg SE et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature 565, 495–499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng M et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Franco F, Jaccard A, Romero P, Yu YR & Ho PC Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2, 1001–1012 (2020). [DOI] [PubMed] [Google Scholar]
- 114.Blank CU et al. Defining ‘T cell exhaustion’. Nat. Rev Immunol. 19, 665–674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang CH et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lyssiotis CA & Kimmelman AC Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Leone RD et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019). This study demonstrates that glutamine restrains differentiation of TH1 and cytotoxic T cells, and a broad inhibitor of glutamine metabolism can enhance antitumour immunotherapy.
- 118.Bader JE, Voss K & Rathmell JC Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol. Cell 78, 1019–1033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Halbrook CJ & Lyssiotis CA Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 31,5–19 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Wang T et al. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nat. Metab. 2, 635–647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kurniawan H et al. Glutathione restricts serine metabolism to preserve regulatory T cell function. Cell Metab. 5, 920–936.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Halbrook CJ et al. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 29, 1390–1399.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maj T et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 18, 1332–1341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brunk E et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 36, 272–281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bordbar A, Monk JM, King ZA & Palsson BO Constraint-based models predict metabolic and associated cellular functions. Nat. Rev. Genet. 15, 107–120 (2014). [DOI] [PubMed] [Google Scholar]
- 126.Lagziel S, Gottlieb E & Shlomi T Mind your media. Nat. Metab. 2, 1369–1372 (2020). [DOI] [PubMed] [Google Scholar]
- 127.Ackermann T & Tardito S Cell culture medium formulation and its implications in cancer metabolism. Trends Cancer 5, 329–332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Voorde J. vande et al. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 5, eaau7314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. antor JR et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–27 2.e17 (2017). This study illustrates the value of physiological media for in vitro metabolic studies as an approach to more closely model in vivo metabolism.
- 130.Leney-Greene MA, Boddapati AK, Su HC, Cantor JR & Lenardo MJ Human plasma-like medium improves T lymphocyte activation. iScience 23, 100759 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferrick DA, Neilson A & Beeson C Advances in measuring cellular bioenergetics using extracellular flux. Drug Disc. Today 13, 268–274 (2008). [DOI] [PubMed] [Google Scholar]
- 132.Xu H et al. Influence of various medium environment to in vitro human T cell culture. In Vitro Cell. Dev. Biol. Anim. 54, 559–566 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Sato K et al. Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy 11, 936–946 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Medvec AR et al. Improved expansion and in vivo function of patient T cells by a serum-free medium. Mol. Ther. Methods Clin. Dev. 8, 65–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chan G, Kleinheinz T, Peterson D & Moffat J A simple high-content cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assays. PLoS ONE 8, e63583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


