Abstract
Background:
Smoking prevalence in individuals with opioid use disorder (OUD) is over 80%. Research suggests that opioid use significantly increases smoking, which could account for the strikingly low smoking-cessation rates observed in both methadone- and buprenorphine-maintained patients, even with the use of first-line smoking-cessation interventions. If opioids present a barrier to smoking-cessation, then better smoking outcomes should be observed in OUD patients treated with extended-release naltrexone (XR-NTX, an opioid antagonist) compared to those receiving buprenorphine (BUP-NX, a partial opioid agonist).
Methods:
The current study is a secondary analysis of a 24-week, multi-site, open-label, randomized clinical trial conducted within the National Drug Abuse Treatment Clinical Trials Network comparing the effectiveness of XR-NTX vs. BUP-NX for adults with OUD. Longitudinal mixed effects models were used to determine if there was a significant reduction in cigarette use among daily smokers successfully inducted to treatment (n = 373) and a subset of those who completed treatment (n = 169).
Results:
Among daily smokers inducted onto OUD medication, those in the XR-NTX group smoked fewer cigarettes per day (M = 11.36, SE = 0.62) relative to smokers in the BUP-NX group (M = 13.33, SE = 0.58) across all study visits, (b (SE) = −1.97 (0.55), p < .01). Results were similar for the treatment completers.
Conclusions:
OUD patients treated with XR-NTX reduced cigarette use more than those treated with BUP-NX, suggesting that XR-NTX in combination with other smoking cessation interventions might be a better choice for OUD smokers interested in reducing their tobacco use.
Keywords: Naltrexone, Buprenorphine, Opioid use disorder, Cigarettes, Smoking
1. Introduction
Approximately 83–98% of adults in opioid use disorder (OUD) treatment report current cigarette smoking (Baldassarri et al., 2019; Chisolm et al., 2013; Do et al., 2017; Guydish et al., 2016). Among opioid users, the mortality rate of cigarette smokers is four times greater than that of nonsmokers (Hser, McCarthy, & Anglin, 1994). Hypotheses about the strong association between nicotine and opioids include socioeconomic factors (Do et al., 2017), personality traits (Cooperman, Lu, Richter, Bernstein, & Williams, 2018), and genetic and biological pathways (Reyes-Gibby, Yuan, Wang, Yeung, & Shete, 2015). Given the prevalence and consequences of smoking in individuals in OUD treatment (Rajabi, Dehghani, Shojaei, Farjam, & Motevalian, 2019), it is imperative to understand the link between OUD treatments and tobacco use outcomes.
Cigarette users in OUD treatment report high rates of interest in quitting smoking (Bowman et al., 2012; Clemmey, Brooner, Chutuape, Kidorf, & Stitzer, 1997) yet smoking-cessation interventions yield modest effect sizes and quit rates among smokers in OUD treatment (Navhi, Ning, Segal, Richter, & Arnsten, 2014; Stein et al., 2013; Reid et al., 2008). Poor smoking cessation outcomes may be at least partially attributed to the administration of opioid medications, such as buprenorphine and methadone, to treat OUD, as they have been linked to increases in cigarette smoking (Chait & Griffiths, 1984; Hall et al., 2018; Mello, Lukas, & Mendelson, 1985; Patrick et al., 2014; Winhusen, Theobald, & Lewis, 2016). Conversely, non-opioid medications, such as the opioid antagonist naltrexone, have been shown to decrease smoking frequency and subjective ratings of smoking satisfaction among both opioid users (Wang, Shi, Elman, & Langleben, 2020) and non-opioid users (Epstein & King, 2004; Wewers, Dhatt, & Tejwani, 1998). Additional randomized clinical studies are needed to help determine if continued opioid-exposure is a barrier to smoking cessation among individuals with OUD.
1.1. Current study
The aim of this secondary data analysis of the National Drug Abuse Treatment Clinical Trials Network (CTN) protocol was to evaluate whether treatment (extended-release naltrexone [XR-NTX] versus buprenorphine-naloxone [BUP-NX]) was associated with a reduction in the average number of cigarettes per day for patients who were daily smokers at baseline and successfully inducted onto their study medication. As effective OUD treatments, both XR-NTX and BUP-NX should serve to decrease illicit opioid use. However, opioid-exposure is continued for individuals treated with BUP-NX (a partial agonist opioid medication) but not for those treated with XR-NTX (an opioid antagonist). It was thus hypothesized that participants successfully inducted on to XR-NTX would display a greater decrease in cigarettes per day over time relative to those inducted on to BUP-NX.
2. Materials and methods
2.1. Participants and study sites
In the parent study (X:BOT; Lee et al., 2018; Nunes et al., 2016), participants (N = 570) were randomized to a 24-week, open label trial comparing the effectiveness of XR-NTX versus BUP-NX for patients with OUD. Eight CTN-affiliated community treatment programs with high volumes of opioid detoxification admissions and outpatient medical management capabilities were selected as study sites. Participants at each site were primarily recruited after detoxification admission and were typically unaware of the study before admission. Community advertising and outreach efforts were also utilized but varied between sites. All sites obtained local Institutional Review Board approval.
Eligible participants were 18 years of age or older, English speaking, met criteria for Diagnostic and Statistical Manual of Mental Disorder-5 OUD and had used non-prescribed opioids in the past 30 days. Further details regarding inclusion/exclusion criteria of the parent study are provided elsewhere (Lee et al., 2018; Nunes et al., 2016). For the purposes of the current analysis, data were constrained to participants who reported smoking cigarettes every day and were successfully inducted to treatment (n = 373; Sample 1) and to the sub-sample completing the study (n = 169; Sample 2).
2.2. Procedures
Participants were randomized in a 1:1 ratio to either XR-NTX or BUP-NX. XR-NTX (4 mL, about 380 mg naltrexone base) was Vivitrol (Alkermes, Dublin, Ireland), a long-acting-injectable antagonist. Before XR-NTX induction, participants had to be detoxified (≥3 days from last opioid use), have opioid-negative urine, and a negative naloxone challenge. Subsequent XR-NTX injections were scheduled for every 28 days.
BUP-NX was Suboxone (Indivior, Slough, UK) sublingual film at 4 mg/1 mg and 8 mg/2 mg strengths. BUP-NX was dispensed to participants by the study team at weeks 0, 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 20 for self-administered daily doses of 8–24 mg (dosage adjusted per clinical status). Typically, induction included observed dosing on the detoxification unit once substantial withdrawal symptoms emerged.
Study medications were discontinued following a relapse event, at the end of 24 weeks, or per safety concerns or participant preference. Medical management was provided by physicians or nurses and voluntary psychosocial counseling was available at all study sites. Research visits occurred at baseline (i.e., prior to medication induction), weekly during treatment, and after treatment at weeks 28 and 36. Additional details regarding the study design are described elsewhere (Lee et al., 2016; Nunes et al., 2016).
2.3. Measures
2.3.1. Demographic information
A demographic information form was administered at baseline and included questions about gender, age, race/ethnicity, education, employment and marital status.
2.3.2. Tobacco use
The Tobacco Use History (TUH) questionnaire was administered at baseline and included questions regarding patterns and characteristics of cigarette use; individuals who endorsed smoking “every day” on the TUH question were categorized as daily smokers.
The Visual Analogue Scale (VAS) was administered at baseline, and every 4 weeks during the treatment phase of the study to assess cravings for alcohol and drugs, including tobacco (Wewers & Lowe, 1990). The open-ended question: “In the past 4 weeks, on average, how many cigarettes did you smoke per day?” was used to measure the primary outcome, average cigarettes per day.
2.4. Statistical analysis
Descriptive statistics were computed for the total analysis sample and then by treatment assignment with differences between groups assessed with chi-square tests for categorical measures and t-test for continuous measures. To assess the effect of treatment assignment on cigarette smoking among baseline daily smokers inducted onto treatment, longitudinal mixed effects models were fit to estimate the average number of cigarettes per day among those randomized to XR-NTX relative to BUP-NX across the 24-week study period. The models utilized an identity link function to match the normal distribution of the outcome (one outlier value of 200 cigarettes per day at week 24 was removed from the models). The longitudinal model was fit using a generalized estimating equation with an autoregressive covariance structure to account for within-subject correlations over time and contained the effects of treatment (XR-NTX versus BUP-NX), study visit (4, 8, 12,16, 20, 24), and their 2-way interaction. If the two-way interaction was not significant, it was omitted from the final model and only the main effects of treatment and study visit were assessed. The model controlled for site as a random effect and for baseline number of cigarettes smoked per day as a fixed effect.
These analyses were performed on the sample of daily baseline smokers that successfully inducted onto their treatment drug (n = 373; Sample 1), and on the sub-sample completing treatment (n = 169; Sample 2). A11 analyses were run using SAS® version 9.4. All hypothesis tests were performed two-sided with a 5% significance level.
3. Results
3.1. Participants
Table 1 presents descriptive summaries of demographic and clinical characteristics of the daily baseline smokers who successfully inducted onto assigned treatment (n = 373) in total and by treatment arm. At baseline, the average number of cigarettes per day reported was 14.24 (SD = 6.51) in the XR-NTX group and 16.09 (SD = 7.83) in the BUP-NX group. Among the entire sample of everyday smokers and non-smokers (N = 522), preliminary analyses revealed that the interaction between baseline smoking and treatment on induction status was not significant, nor was smoking on induction status controlling for treatment.
Table 1.
Baseline demographic and clinical characteristics of the total analysis sample and by treatment assignment (n = 373).
| By treatment assignment | |||||||
|---|---|---|---|---|---|---|---|
|
|
Total analysis sample (n = 373) |
BUP-NX (n = 208) |
XR-NTX (n = 165) |
Difference between groups |
|||
| Measurea | N | % or M (SD) | N | % or M (SD) | N | % or M (SD) | p-Value |
| Gender (% male) | 259 | 69.4% | 148 | 71.2% | 111 | 67.3% | 0.419 |
| Age (years) | 373 | 32.9 (9.2) | 208 | 32.9 (9.3) | 165 | 32.9 (9.1) | 0.998 |
| Race | 0.652 | ||||||
| White only | 292 | 78.3% | 164 | 78.8% | 128 | 77.6% | |
| Black only | 33 | 8.8% | 16 | 7.7% | 17 | 10.3% | |
| Other | 48 | 12.9% | 28 | 13.5% | 20 | 12.1% | |
| Hispanic ethnicity (% Hispanic) | 58 | 15.5% | 38 | 18.3% | 20 | 12.1% | 0.104 |
| Marital status (% never married) | 250 | 67.0% | 142 | 68.3% | 108 | 65.5% | 0.843 |
| Unemployment (% not employed) | 239 | 64.1% | 139 | 66.8% | 100 | 60.6% | 0.214 |
| IV use (% yes) | 253 | 67.8% | 144 | 69.2% | 109 | 66.1% | 0.515 |
| Primary opioid | 0.867 | ||||||
| Buprenorphine | 3 | 0.8% | 1 | 0.5% | 2 | 1.2% | |
| Opioid analgesics | 61 | 16.4% | 33 | 15.9% | 28 | 17.1% | |
| Methadone | 7 | 1.9% | 4 | 1.9% | 3 | 1.8% | |
| Heroin | 300 | 80.9% | 169 | 81.6% | 131 | 79.9% | |
| Primary opioid cost (dollars/day) | 371 | 92.8 (73.2) | 207 | 96.4 (78.1) | 164 | 88.3 (66.4) | 0.289 |
| Age at onset (years) | 373 | 20.4 (6.6) | 208 | 20.6 (7.1) | 165 | 20.2 (5.9) | 0.553 |
| Duration of opioid use (years) | 373 | 12.5 (8.8) | 208 | 12.3 (8.8) | 165 | 12.7 (8.8) | 0.658 |
| First treatment episode (% yes) | 147 | 39.4% | 80 | 38.5% | 67 | 40.6% | 0.674 |
| Stimulant use (30d prior to adm) (% yes) | 208 | 55.8% | 123 | 59.1% | 85 | 51.5% | 0.141 |
| Sedative use (30d prior to adm) (% yes) | 109 | 29.2% | 68 | 32.7% | 41 | 24.8% | 0.098 |
| Heavy alcohol use (30d prior to adm) (% yes) | 101 | 27.1% | 53 | 25.5% | 48 | 29.1% | 0.436 |
| Cannabis use (30d prior to adm) (% yes) | 175 | 46.9% | 105 | 50.5% | 70 | 42.4% | 0.122 |
| Cigarettes per day | 373 | 15.3 (7.3) | 208 | 16.1 (7.8) | 165 | 14.2 (6.5) | 0.015 |
| Fagerstrom test for nicotine dependence | 0.532 | ||||||
| Low (0–2) | 63 | 17.1% | 32 | 15.6% | 31 | 18.9% | |
| Moderate (3–5) | 172 | 46.6% | 94 | 45.9% | 78 | 47.6% | |
| High (6+) | 134 | 36.3% | 79 | 38.5% | 55 | 33.5% | |
| HAM-D score (possible range: 0–52) | 372 | 8.8 (6.8) | 207 | 9.2 (6.9) | 165 | 8.3 (6.5) | 0.185 |
| Any psychiatric disorders (% yes) | 252 | 67.6% | 141 | 67.8% | 111 | 67.3% | 0.916 |
| SOWS (possible range: 0–64) | 373 | 15.4 (13.2) | 208 | 15.1 (12.6) | 165 | 15.7 (13.8) | 0.687 |
| Severity | 0.676 | ||||||
| Low | 208 | 55.8% | 114 | 54.8% | 94 | 57.0% | |
| High | 165 | 44.2% | 94 | 45.2% | 71 | 43.0% | |
30d = 30 days.
3.2. Cigarette use among daily smokers successfully inducted on naltrexone and buprenorphine
Among baseline daily smokers who inducted onto their treatment drug (Sample 1), the 2-way interaction between treatment and study visit revealed no significant differences in the treatment effect over time (F(5, 1377) = 0.60, p = .70) and was subsequently removed from the model. In the main effects model, the baseline number of cigarettes smoked per day (b = 0.51, p < .01) and treatment (p < .01) were significantly associated with the number of cigarettes smoked per day during the study. Participants randomized to XR-NTX smoked an average of 11.36 (SE = 0.62) cigarettes per day during the study, while those randomized to BUP-NX smoked an average of 13.33 (SE = 0.58) cigarettes per day during the study (group difference: b (SE) = −1.97 (0.55)). The number of cigarettes smoked did not significantly change across visits (p = .51) during the study. A small number of patients in XR-NTX (11.6%) and BUP-NX (5.5%) achieved sustained abstinence from cigarettes. Fig. 1 illustrates the observed and model estimated means with baseline overall mean adjustment of average number of cigarettes per day across study visits.
Fig. 1.
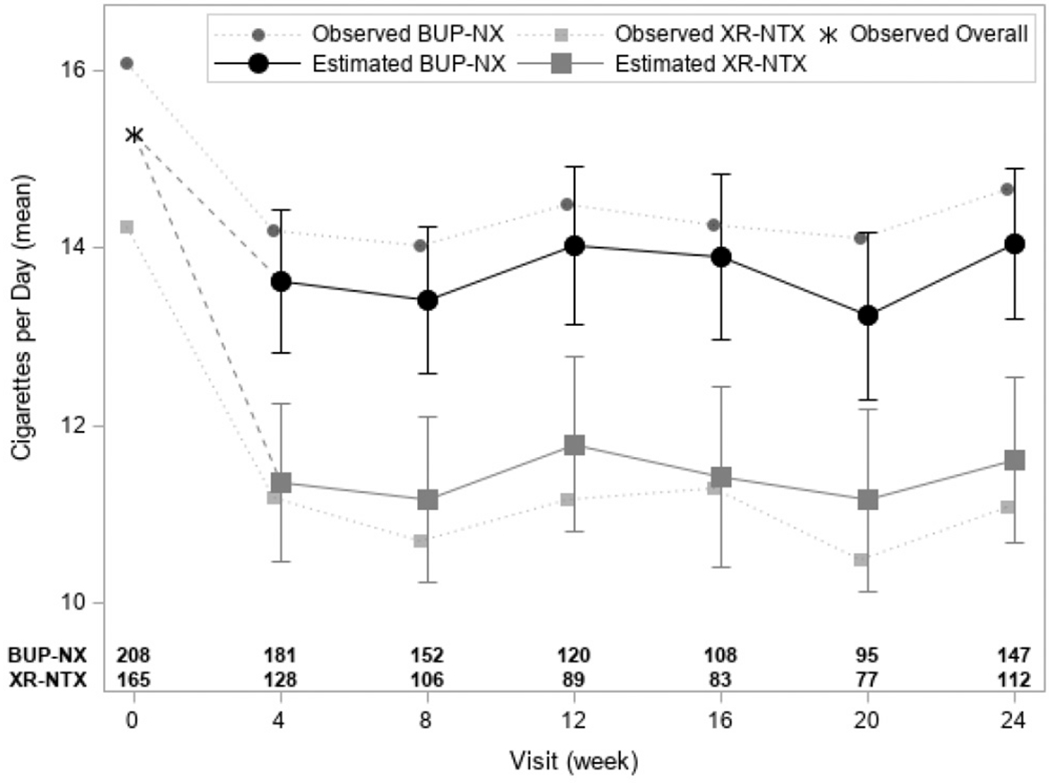
Model estimated means (±1 standard error) with baseline overall mean adjustment of average number of cigarettes per day across study visits among the daily smokers inducted onto study medication (n = 373). Dashed lines show the baseline mean adjustment and solid lines show the model estimated means at study visits. The observed overall baseline mean is provided with an asterisk (*). Dotted lines present observed baseline and study visit treatment group means.
a The 2-way treatment by time interaction was not significant (F(5, 1377) = 0.60, p = .70).
b Sample size of outcome data available by treatment and visit week are provided along the x-axis. Missing data are assumed to be missing at random in analyses. There were no significant differences in missingness by treatment group over time (F(5,1855) = 1.11, p = .35) or by treatment overall (F(1, 371) = 1.74, p = .18).
Findings were similar among the sub-sample of participants who completed treatment (Sample 2, n = 169).
4. Discussion
Cigarette smoking is prevalent among adults in OUD treatment. The current analysis examined changes in average number of cigarettes per day among daily smokers successfully inducted onto XR-NTX vs. BUP-NX for the treatment of OUD. Using data from a multi-site randomized clinical trial comparing the effectiveness of XR-NTX and BUP-NX for OUD, this study found that ad-libitum daily smokers in XR-NTX smoked fewer cigarettes per day than those in the BUP-NX arm when controlling for baseline average number of cigarettes per day. This finding was consistent among the subset who successfully inducted onto treatment and the subset who successfully completed treatment. Given the greater health issues and mortality among smokers with OUD (Hser et al., 1994; Rajabi et al., 2019), these important data suggest that naltrexone could be helpful to people with OUD who are seeking to reduce their smoking.
These findings replicate prior studies that have also demonstrated reduced cigarette smoking among individuals maintained on either oral or injectable extended release naltrexone (Epstein & King, 2004; Wang et al., 2020; Wewers et al., 1998). Wang et al. (2020) recently found a 29% decline in daily cigarette consumption among smokers with OUD who were receiving XR-NTX. Moreover, the decrease was sustained over time, with approximately 14 (SD = 1.0) cigarettes smoked per day at baseline, 9.8 cigarettes (SD = 1.0) after one month of treatment and 8.6 cigarettes (SD = 1.1) after two months of treatment. Pharmacological studies suggest that opioid antagonists, such as naltrexone, lessen the reinforcing effect of nicotine (Kirshenbaum, Suhaka, Phillips, & de Souza Pinto, 2016; Yoon, Lane, & Weaver, 2015). However, a systematic review of studies evaluating the effectiveness of oral naltrexone in promoting long term smoking cessation found no evidence of an effect of naltrexone on reduced smoking (David, Lancaster, Stead, Evins, & Prochaska, 2013). Treatment compliancy and variability in medication dosage are inherent issues in taking oral naltrexone and should be taken into consideration when interpreting results from studies of oral naltrexone. These findings are consistent with pharmacological studies finding an association between smoking and opioid agonists, such as methadone (Frosch, Shoptaw, Nahom, & Jarvik, 2000; Talka, Tuominen, & Salminen, 2015) and buprenorphine (Gubner, Guydish, Humfleet, Benowitz, & Hall, 2017; Pajusco et al., 2012). It is important to note that, in the current study, buprenorphine did not increase smoking, although the reduction in smoking was not as large as XR-NTX. Additional studies are needed to further explore the link between the type of opioid medication (antagonist versus agonist) and smoking-cessation outcomes.
Several limitations are worth noting. First, the parent study was not designed and powered to evaluate the association between OUD medication and tobacco use outcomes. However, these findings provide promising preliminary data for well-powered longitudinal studies designed specifically to capture nuances in the relationship between opioids and cigarettes. Future studies should also include other methods of nicotine delivery, such as e-cigarettes and vaping. Second, the assessment of cigarette smoking was based on self-report, which can be open to response biases. The difference between XR-NTX and BUP-NX in cigarettes per day smoked was small from a clinical perspective, about 2.0 to 2.5 cigarettes per day, and few patients on either medication achieved sustained abstinence from cigarettes. Nonetheless, the findings encourage future studies that combine XR-NTX with behavioral interventions to increase motivation and skills to quit smoking and also with smoking-cessation pharmacotherapies.
This study suggests that in patients with OUD, treatment with an opioid antagonist is associated with larger decreases in smoking than treatment with a partial agonist. Research evaluating the potential synergy of smoking-cessation interventions (e.g., Varenicline or nicotine patches) for cigarette smokers receiving XR-NTX for OUD may be warranted.
Acknowledgments
Funding:
This work was supported by the National Institutes of Health/National Institute on Drug Abuse UG1DA013035 (Pis: Rotrosen, Nunes) and UG1DA013732 (PI: Winhusen). The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinion expressed in this paper is solely those of the authors.
References
- Baldassarri SR, Fiellin DA, Savage ME, Madden LM, Beitel M, Dhingra LK, … Barry DT (2019). Electronic cigarette and tobacco use in individuals entering methadone or buprenorphine treatment. Drug and Alcohol Dependence, 197, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J, Wiggers J, Colyvas K, Wye P, Walsh RA, & Bartlem K (2012). Smoking cessation among Australian methadone clients: Prevalence, characteristics and a need for action. Drug and Alcohol Review, 31, 507–513. [DOI] [PubMed] [Google Scholar]
- Chait LD, & Griffiths RR (1984). Effects of methadone on human cigarette smoking and subjective ratings. The Journal of Pharmacology and Experimental Therapeutics, 229, 636–640. [PubMed] [Google Scholar]
- Chisolm MS, Fitzsimons H, Leoutsakos JM, Acquavita SP, Heil SH, Wilson-Murphy M, … Jones HE (2013). A comparison of cigarette smoking profiles in opioid-dependent pregnant patients receiving methadone or buprenorphine. Nicotine & Tobacco Research, 15, 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape MA, Kidorf M, & Stitzer M (1997). Smoking habits and attitudes in a methadone maintenance treatment population. Drug and Alcohol Dependence, 44, 123–132. [DOI] [PubMed] [Google Scholar]
- Cooperman NA, Lu SE, Richter KP, Bernstein SL, & Williams JM (2018). Pilot study of a tailored smoking cessation intervention for individuals in treatment for opioid dependence. Nicotine & Tobacco Research, 20, 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Lancaster T, Stead LF, Evins AE, & Prochaska JJ (2013). Opioid antagonists for smoking cessation. Cochrane Database of Systematic Reviews, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do HP, Nguyen LH, Thi Nguyen NP, Ngo C, Thi Nguyen HL, Le GT, … Dunne MP (2017). Factors associated with nicotine dependence during methadone maintenance treatment: Findings from a multisite survey in Vietnam. BMJ Open, 7 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, & King AC (2004). Naltrexone attenuates acute cigarette smoking behavior. Pharmacology, Biochemistry, and Behavior, 77, 29–37. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, & Jarvik ME (2000). Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Experimental and Clinical Psychopharmacology, 8, 97–103. [DOI] [PubMed] [Google Scholar]
- Gubner NR, Guydish J, Humfleet GL, Benowitz N, & Hall SM (2017). Nicotine biomarkers and rate of nicotine metabolism among cigarette smokers taking buprenorphine for opioid dependency. Drug and Alcohol Dependence, 178, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Pagano A, Martínez C, Le T, Chun J, Tajima B, Docto L, Garina D, & Delucchi K (2016). An international systematic review of smoking prevalence in addiction treatment. Addiction, 111, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Gasper JJ, Delucchi KL, Hersh DF, & Guydish JR (2018). Cigarette smoking cessation intervention for buprenorphine treatment patients. Nicotine & Tobacco Research, 20, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, & Anglin MD (1994). Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Preventive Medicine, 23, 61–69. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Suhaka JA, Phillips JL, & de Souza Pinto MV (2016). Nicotine enhancement and reinforcer devaluation: Interaction with opioid receptors. Pharmacology, Biochemistry, and Behavior, 150, 17. [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Mpa PN, Bailey GL, Brigham GS, Cohen AJ, … Rotrosen J (2016). NIDA clinical trials network CTN-0051, extended-release naltrexone vs. buprenorphine for opioid treatment (X:BOT): Study design and rationale. Contemporary Clinical Trials, 50, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, … Rotrosen J (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicenter, open-label, randomized controlled trial. Lancet, 391, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, & Mendelson JH (1985). Buprenorphine effects on cigarette smoking. Psychopharm, 86, 417–425. [DOI] [PubMed] [Google Scholar]
- Navhi S, Ning Y, Segal KS, Richter KP, & Arnsten JH (2014). Varenicline efficacy and safety among methadone-maintained smokers: A randomized placebo-controlled trial. Addiction, 109, 1554–1563. 10.1111/add.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EV, Lee JD, Sisti D, Segal A, Caplan A, Fishman M, … Rotrosen J (2016). Ethical and clinical safety considerations in the design of the effectiveness trial: A comparison of buprenorphine versus naltrexone treatment for opioid dependence. Contemporary Clinical Trials, 51, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusco B, Chiamulera C, Quaglio G, Moro L, Casari R, Amen G, … Lugoboni F (2012). Tobacco addiction and smoking status in heroin addicts under methadone vs. buprenorphine therapy. International Journal of Environmental Research and Public Health, 9, 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Dunn KE, Badger GJ, Heil SH, Higgins ST, & Sigmon SC (2014). Spontaneous reductions in smoking during double-blind buprenorphine detoxification. Addictive Behaviors, 39, 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi A, Dehghani M, Shojaei A, Farjam M, & Motevalian SA (2019). Association between tobacco smoking and opioid use: A meta-analysis. Addictive Behaviors, 92, 225–235. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, … Rotrosen J (2008). Smoking cessation treatment in community-based substance abuse rehabilitation programs. Journal of Substance Abuse Treatment, 35, 68–77. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Yuan C, Wang J, Yeung SCJ, & Shete S (2015). Gene network analysis shows immune-signaling and ERK1/2 as novel genetic markers for multiple addiction phenotypes: Alcohol, smoking and opioid addiction. BMC Systems Biology, 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, & Anderson BJ (2013). Varenicline for smoking cessation among methadone-maintained smokers: A randomized clinical trial. Drug and Alcohol Dependence, 133, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talka R, Tuominen RK, & Salminen O (2015). Methadone’s effect on nAChRs—A link between methadone use and smoking? Biochemical Pharmacology, 97, 542–549. [DOI] [PubMed] [Google Scholar]
- Wang AL, Shi Z, Elman I, & Langleben DD (2020). Reduced cigarette smoking during injectable extended-release naltrexone treatment for opioid use disorder. The American Journal of Drug and Alcohol Abuse, 1–6. [DOI] [PubMed] [Google Scholar]
- Wewers ME, & Lowe NK (1990). A critical review of visual analogue scales in the measurement of clinical phenomena. Research in Nursing & Health, 13, 227–236. 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Dhatt R, & Tejwani GA (1998). Naltrexone administration affects ad libitum smoking behavior. Psychopharmacol, 140, 185–190. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Theobald J, & Lewis D (2016). Design considerations for a pilot trial using a novel approach for evaluating smoking-cessation medication in methadone-maintained smokers. Contemporary Clinical Trials, 47, 334–339. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Lane SD, & Weaver MF (2015). Opioid analgesics and nicotine: More than blowing smoke. Journal of Pain & Palliative Care Pharmacotherapy, 29, 281–289. [DOI] [PubMed] [Google Scholar]


