Abstract
Objective:
To examine the association between ambient temperature and antral follicle count (AFC), a standard measure of ovarian reserve.
Design:
Prospective cohort study
Setting:
Northeastern United States
Patients:
631 women attending the Massachusetts General Hospital Fertility Center (2005–2015) who participated in the Environment and Reproductive Health (EARTH) Study.
Intervention(s):
Daily temperature at the women’s residential address was estimated for the 90 days prior to their antral follicle scan using a spatially refined gridded climate data set. We evaluated the associations between temperature and AFC using Poisson regression with robust standard errors, adjusting for relative humidity, fine particulate matter exposure, age, education, smoking status, year and month of AFC, and diagnosis of diminished ovarian reserve and ovulation disorders.
Main Outcome Measures:
AFC as measured with transvaginal ultrasonography.
Results:
A 1°C increase in average maximum temperature during the 90 days prior to ovarian reserve testing was associated with a −1.6% (95% CI −2.8, −0.4) lower AFC. Associations remained negative, but were attenuated, for average maximum temperature exposure in the 30 days (−0.9% 95% CI −1.8, 0.1) and 14 days (−0.8 95% CI −1.6, 0.0) prior to AFC. The negative association between average maximum temperature and AFC was stronger November through June than during the summer months, suggesting that timing of heat exposure and acclimatization to heat may be important factors to consider in future research.
Conclusions:
Exposure to higher temperatures was associated with lower ovarian reserve. These results raise concern that rising ambient temperatures worldwide may result in accelerated reproductive aging among women.
Keywords: temperature, fertility, climate change, environment, fecundity, ovarian reserve
Capsule:
Exposure to higher ambient temperatures in the 90 days prior to ovarian reserve testing was associated with lower antral follicle counts.
Introduction.
Climate change is now widely recognized as the greatest global threat of the 21st century (1). The most immediate and direct impact of a changing global climate on human health is seen in the steady increase in global average temperature, and the increased frequency, intensity, and duration of extremes of heat (2). By 2070, the risk of exposure to extreme heat for the average U.S. citizen is expected to increase four to six-fold relative to the late twentieth century (3). While the hazards of increasing ambient temperatures on human health are widely recognized (4, 5), there is also mounting evidence linking maternal heat exposure to increased risk of stillbirth, preterm birth, low birth weight, and birth defects (6, 7). Natural gestational changes in thermoregulation make pregnant women vulnerable to heat exposure (8); however the exact biological mechanisms underlying the adverse effects of heat on pregnancy outcomes are not yet clear and are likely differ for different outcomes. For example, heat exposure is associated with higher circulating markers of inflammation and oxidative stress (9), which may impair placental vascular development and decrease uterine and placental–fetal blood flow. Maternal heat exposure may also lead to the production of heat-shock proteins (10), which could disrupt protein homeostasis and potentially alter fetal development.
Little is known regarding the relationship between ambient temperature and fertility in humans. Demographic studies suggest that hot weather causes a significant decline in birth rates 8 to 10 months later (11), yet the drivers of this association are unclear. The animal literature has long documented a link between maternal hyperthermia induced by high ambient temperatures and reduced fertility, largely mediated through effects on oocyte developmental capacity (12, 13). In dairy cows, the proportion of oocytes that develop into competent embryos is lower when exposed to high temperatures in vivo or in vitro (14, 15). Moreover, this deleterious impact of heat stress on follicular growth and oocyte competence carries over into the subsequent cooler months, indicating a long-lasting effect of heat stress on the ovarian pool of oocytes (16). On a molecular level, heat stress can impair oocyte growth and competence through disrupting steroid hormones biosynthesis (17, 18), impairing maternal transcripts involved in oocyte maturation (15, 19), and enhancing the production of reactive oxygen species (20, 21). While the animal literature is compelling, it is unclear whether heat stress has a similar impact on the follicular development of humans.
Given the parallel trends of rising temperatures induced by climate change and the increasing number of women delaying motherhood to 35 years and older (22), when a sharp decline in ovarian function occurs, understanding the environmental drivers of ovarian aging, such as ambient temperature, is becoming increasingly important. Any associations between ambient temperature and female fertility would also have important implications for future population size and structure (23), an essential input for models estimating the health burdens associated with climate change (24). Therefore, our objective was to examine the association between ambient temperature and antral follicle counts (AFC), a standard measure of ovarian reserve (25), among women residing in New England, presenting to an academic fertility center in Boston, MA. We additionally evaluated whether the association between temperature and AFC varied by month of exposure as previous research has shown that the effects of temperature are highly time dependent and that temporal acclimation over the course of a season may greatly influence a human’s response to a given temperature (26).
Materials and Methods.
Study Population.
Women included in this study were participants in the Environment and Reproductive Health (EARTH) Study, a prospective cohort designed to evaluate environmental and dietary determinants of fertility (27). In brief, women aged 18 to 45 years presenting for infertility evaluation and treatment at the Massachusetts General Hospital (MGH) Fertility Center were eligible for the study and approximately 60% of women contacted by the research nurses enrolled. The EARTH Study was approved by the Human Studies Institutional Review Boards of MGH and the Harvard T.H. Chan School of Public Health. Of the 954 antral follicle scans from 806 women initially available for analysis, we excluded scans that were performed while the woman was on leuprolide acetate (n=42), that were missing data on one or more ovaries (n=18), that had one or more polycystic ovaries (n=58), whose ovaries were difficult to visualize (n=12), and that were repeat scans from the same woman (n=102). From this pool of 722 eligible women, we further restricted our analysis to women with complete information on fine particulate matter (PM2.5) exposure, a key covariate, which reduced the sample size to 632 women. Women missing data on PM2.5 exposure had antral follicle scans that were performed after December 31st, 2015 or resided outside the US. From there, we additionally excluded one woman whose primary residence was outside of New England, which brought the final total to 631 women.
Exposure Assessment.
Upon enrollment into the EARTH Study, all women provided their residential address, which was geocoded using ArcGIS. We estimated daily residential ambient temperatures beginning three months prior to the woman’s AFC date. This time frame corresponds to exposures occurring during the preantral to preovulatory stages of follicular development (approximately 2–4 months) (25). We obtained ambient temperature data from the Parameter-elevation Regressions on Independent Slopes Model (PRISM), which provides daily estimates of minimum (Tmin), maximum (Tmax), and average (Tavg) ambient temperature and mean dew point temperature (Tdew) at a 4 km2 spatial resolution (28–30). The gridded PRISM data set offers more spatially explicit meteorological exposures than observations from individual weather stations (31). Relative humidity (RH) was calculated based on the Magnus approximation: , where b=17.625 and c=243.04.(32) Apparent temperature (Tapp), defined as a person’s perceived air temperature, was calculated using the following formula: (33, 34). Because the Tavg never exceeded 34°C, a wind-speed correction was not required (34).
We averaged the daily ambient temperature values over three time windows that were specified a priori based on the timeframe of follicular development in humans (25) and previous literature from bovines demonstrating that primordial and primary follicles are heat-resistant (12) (Figure 1). These time windows included 1) the three months prior to scan (representing exposure from the preantral to preovulatory stages of development), 2) the month prior to scan (representing exposure from the early antral to preovulatory stages of development), and 3) the two weeks prior to scan (representing exposure during the final stages of antral development). We also calculated the standard deviation of daily ambient temperature values for each of the three time windows as a measure of variability (35).
Figure 1.
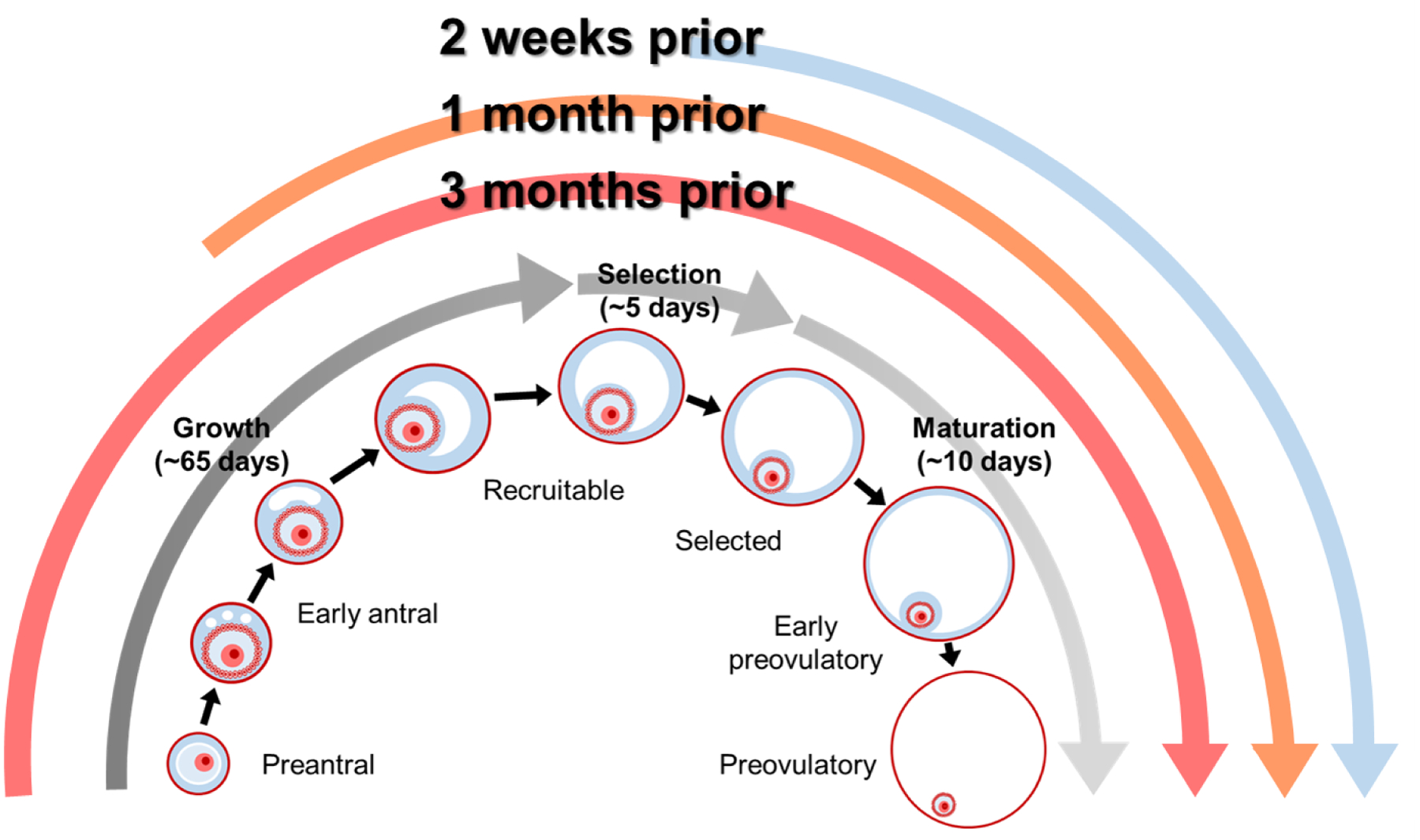
Overview of the timeframe of follicular development in humans and the relevant exposure windows evaluated for the association between ambient temperature and antral follicle count.
Outcome Assessment.
Ovarian AFC, defined as the sum of antral follicles in both ovaries, was measured by a reproductive endocrinologist using transvaginal ultrasonography on the 3rd day of an unstimulated menstrual cycle or on the 3rd day of a progesterone withdrawal bleed. No fertility medications were used in the cycle prior to the antral follicle scan. Even though we excluded women with a diagnosis of polycystic ovary syndrome (PCOS) in their medical records, there were still 15 women (2% of population) with an AFC >30. In order to reduce the influence of these very high AFCs, we truncated AFC at 30.
Covariate Assessment.
Date of birth was collected at entry and weight and height were measured by trained study staff to calculate body mass index (BMI) (kg/m2). A detailed take-home questionnaire contained questions on lifestyle factors, occupation, reproductive health, and medical history. On this questionnaire, women were asked, “What is the typical temperature in your workplace?” with response options of “Room temperature (68°–73°F)”, “Above room temperature”, “Below room temperature”, and “Combination of temperatures”. Women reporting an above room temperature work environment were defined as having a workplace heat exposure. Infertility diagnosis was abstracted from electronic medical records. Daily PM2.5 exposure was estimated during the same time periods as temperature using a validated hybrid model of satellite–derived aerosol optical depth measurements and land-use terms with 1 km2 spatial resolution (36).
Statistical Analysis.
Spearman correlation coefficients were used to measure the strength of association between the different temperature exposures as well as within the temperature exposures over time. Poisson regression models were used to estimate the association of temperature exposures with AFC. Non-linearity was assessed with restricted cubic splines, which used the likelihood ratio test comparing the model with the linear term to the model with the linear and the cubic spline terms (37). Results are presented as either adjusted % change in AFC per unit increase in temperature exposure or population marginal means at the mean level of continuous covariates and most common level of categorical covariates.
Confounding was assessed based on biological relevance and descriptive statistics from our study population. Final models were adjusted for average relative humidity and PM2.5 exposure during the same time period, age, highest education level, smoking status, year and month of AFC, and diagnosis of diminished ovarian reserve (DOR) and ovulation disorders. We adjusted for month of AFC to account for seasonal changes in temperature since our goal was to assess the effect of deviations in temperature from the monthly average rather than seasonal differences.
Since there is evidence that exposure to higher temperatures may be more detrimental to health outcomes outside of the summer season due to acclimatization (26), we evaluated effect modification according to the month and season of antral follicle scan by adding cross product terms to the final multivariate model. We calculated a P for interaction by comparing the nested models using a likelihood ratio test. We also evaluated effect modification by age (<35 years vs. ≥35 years), BMI (<25 kg/m2 vs. ≥25 kg/m2), smoking status (never vs. ever smoker), and infertility diagnosis (female vs. male/unexplained), since all of these parameters are all well-documented predictors of ovarian reserve. Finally, we evaluated effect modification by workplace heat exposure as we hypothesized that the effect of ambient temperature on AFC could be amplified among women with warmer microenvironments.
Results.
The 631 women in our analysis had a median AFC of 12 with a range from 1 to 30. Mean AFC varied by age, education level, infertility diagnosis, and year and month of AFC assessment (Table 1). On average, AFCs were higher among women who were younger, had a lower education, were diagnosed with ovulatory infertility, and had their antral follicle scan performed in July and more recent years. Women resided in Massachusetts (96%), New Hampshire (2%), Rhode Island (1%), and Maine (<1%). Variation in temperature exposure was largely driven by time of year with peak maximum temperatures occurring in July (median: 28.2°C) and lowest temperatures occurring in January (median: 3.5°C).
Table 1.
Baseline demographic and reproductive characteristics among 631 women in the EARTH Study.
| Count (%) | Antral Follicle Count (Mean ± SD) | |
|---|---|---|
| Demographic characteristics | ||
| Age, years | ||
| <32 | 127 (20.1) | 17.2 ± 7.0 |
| 32–35 | 202 (32.0) | 14.0 ± 6.5 |
| 36–39 | 197 (31.2) | 12.1 ± 6.0 |
| ≥40 | 105 (16.6) | 9.5 ± 5.5 |
| Race, n (%) | ||
| White | 527 (83.5) | 13.3 ± 6.7 |
| Other | 104 (16.5) | 13.4 ± 6.9 |
| Body Mass Index, kg/m2 | ||
| <18.5 | 12 (1.9) | 16.8 ± 7.1 |
| 18.5–24.9 | 400 (63.4) | 13.3 ± 6.7 |
| 25–29.9 | 146 (23.1) | 13.2 ± 6.7 |
| ≥30 | 73 (11.6) | 13.2 ± 7.1 |
| Smoking status, n (%) | ||
| Never smoked | 461 (73.1) | 13.5 ± 6.8 |
| Ever smoked | 170 (26.9) | 13.0 ± 6.7 |
| Education, n (%) | ||
| < College | 46 (7.3) | 14.5 ± 7.4 |
| College graduate | 268 (42.5) | 13.2 ± 6.7 |
| Graduate degree | 317 (50.2) | 13.2 ± 6.7 |
| State of residence, n (%) | ||
| Massachusetts | 607 (96.2) | 13.3 ± 6.7 |
| New Hampshire | 15 (2.4) | 13.1 ± 7.8 |
| Rhode Island | 8 (1.3) | 12.6 ± 6.8 |
| Maine | 1 (0.2) | - |
| Workplace heat exposure, n (%) | ||
| No | 614 (97.3) | 13.3 ± 6.8 |
| Yes | 17 (2.7) | 12.8 ± 6.0 |
| Reproductive characteristics | ||
| History of being pregnant, n (%) | ||
| No | 346 (54.8) | 13.8 ± 6.8 |
| Yes | 285 (45.2) | 12.7 ± 6.6 |
| Initial infertility diagnosisa, n (%) | ||
| Male factor | 163 (25.9) | 14.4 ± 6.5 |
| Female factor | 209 (33.2) | 11.8 ± 7.5 |
| DOR | 74 (11.8) | 6.9 ± 4.0 |
| Endometriosis | 35 (5.6) | 11.6 ± 6.4 |
| Ovulation Disorders | 57 (9.1) | 17.9 ± 8.2 |
| Tubal | 34 (5.4) | 12.5 ± 5.8 |
| Uterine | 9 (1.4) | 11.0 ± 4.6 |
| Unexplained | 256 (40.7) | 13.9 ± 6.1 |
| Antral follicle scan characteristics | ||
| Year of AFC | ||
| 2005–2007 | 104 (16.5) | 11.3 ± 5.6 |
| 2008–2010 | 234 (37.1) | 13.3 ± 6.8 |
| 2011–2013 | 175 (27.7) | 13.6 ± 6.6 |
| 2014–2015 | 118 (18.7) | 14.7 ± 7.5 |
| Month of AFC, n (%) | ||
| January | 64 (10.1) | 11.8 ± 6.9 |
| February | 63 (10.0) | 13.3 ± 7.1 |
| March | 46 (7.3) | 13.3 ± 5.8 |
| April | 53 (8.4) | 13.6 ± 6.5 |
| May | 52 (8.2) | 13.6 ± 7.5 |
| June | 45 (7.1) | 12.0 ± 5.6 |
| July | 34 (5.4) | 15.9 ± 7.7 |
| August | 43 (6.8) | 13.6 ± 7.4 |
| September | 48 (7.6) | 13.0 ± 6.2 |
| October | 59 (9.4) | 13.9 ± 6.9 |
| November | 75 (11.9) | 13.5 ± 6.7 |
| December | 49 (7.8) | 13.2 ± 6.5 |
Abbreviations: AFC, antral follicle count; DOR, diminished ovarian reserve.
3 women were missing infertility diagnoses
The spearman correlation between average Tmin, Tmax, Tavg, and Tapp exposures within a given time period were all ≥0.98 (Supplemental Table 1). Between time periods, the spearman correlations for temperature exposures were 0.86 for 3 month- vs.1 month-average, 0.77 for 3 month- vs. 2 week-average, and 0.98 for 1 month- vs. 2 week-average. Temperature had weak, positive correlations with relative humidity (ρ=0.24 to 0.32 for 2 weeks to 3 months) and PM2.5 exposure (ρ=0.04 to 0.12 for 2 weeks to 3 months).
Warmer ambient temperatures were associated with lower ovarian reserve (Table 2). There were no consistent associations between relative humidity and AFC or measures of temperature variability and AFC (Supplemental Table 2). Associations between average ambient temperatures and AFC were strongest for Tmax followed by Tavg, Tapp, and Tmin. For example, a 1°C increase in average Tmax over the 3 months prior to antral follicle scan was associated with a 1.6% lower (95% CI −2.8, −0.4%) AFC. The negative associations were similar, although attenuated, for average Tmax exposure in the 1 month (% change= −0.9% 95% CI −1.8, 0.1) and two weeks (% change= −0.8 95% CI −1.6, 0.0) prior to antral follicle scan (Table 2).
Table 2.
Association between average temperature exposure prior to scan and antral follicle counts (AFC) among 631 women in the EARTH Study.
| Adjusted % Change in AFCa | ||
|---|---|---|
| per 1°C increase | Per IQR (15 °C) increase | |
| 3 Months Prior | ||
| Average Temperature, °C | −1.4 (−2.6, −0.2) | −19.1 (−32.4, −3.1) |
| Maximum Temperature, °C | −1.5 (−2.7, −0.3) | −20.5 (−33.8, −4.4) |
| Minimum Temperature, °C | −1.2 (−2.2, −0.2) | −16.4 (−28.5, −2.3) |
| Apparent Temperature, °C | −1.3 (−2.4, −0.1) | −17.7 (−31.0, −1.8) |
| 1 Month Prior | ||
| Average Temperature, °C | −1.0 (−2.0, 0.0) | −14.0 (−25.6, −0.1) |
| Maximum Temperature, °C | −0.9 (−1.9, 0.1) | −12.4 (−24.2, 1.6) |
| Minimum Temperature, °C | −1.0 (−1.9, −0.1) | −14.2 (−25.0, −1.8) |
| Apparent Temperature, °C | −1.3 (−2.3, −0.4) | −18.4 (−29.8, −5.2) |
| 2 Weeks Prior | ||
| Average Temperature, °C | −0.7 (−1.5, 0.1) | −9.9 (−20.7, 2.3) |
| Maximum Temperature, °C | −0.8 (−1.6, 0.0) | −11.8 (−22.0, −0.2) |
| Minimum Temperature, °C | −0.5 (−1.3, 0.3) | −7.5 (−18.0, 4.3) |
| Apparent Temperature, °C | −0.8 (−1.7, 0.0) | −11.8 (−22.3, 0.1) |
Abbreviations: AFC, antral follicle count
Adjusted models account for average relative humidity (continuous) and PM2.5 exposure (continuous) in same time period prior to AFC, age (continuous), education (<college vs. ≥college), smoking status (ever vs. never), year of AFC (continuous), month of AFC (Jan, Feb, Mar, Apr, May, Jun, Jul, Aug, Sep, Oct, Nov, Dec), and diagnosis of diminished ovarian reserve and ovulation disorders.
There was little evidence of departure from linearity for the association between ambient temperature and ovarian reserve (P for non-linearity=0.17). Rather there was a persistent, negative, linear association between temperature and AFC across the entire range of temperatures observed in our study (Figure 2). There was no evidence of statistically significant effect modification by season (P for interaction=0.65), but there was suggestive effect modification by month (P for interaction=0.07). Associations between maximum temperatures and AFC were strongest for scans conducted from November through June and were weaker during the summer months (July, August, September, and October) (Supplemental Table 3).
Figure 2.
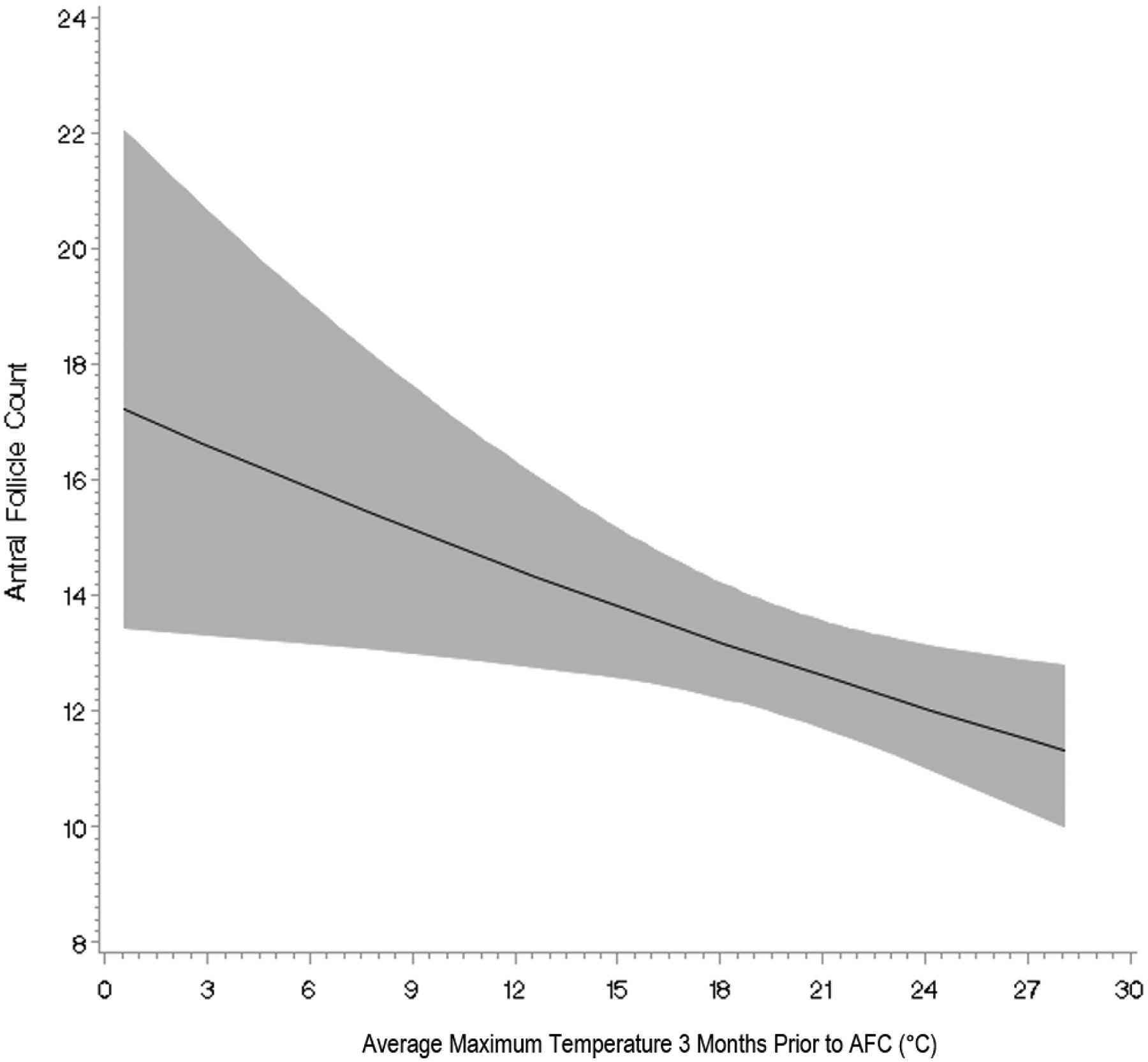
Association between average maximum temperature in the three months prior to scan and antral follicle counts (AFC) among 631 women in the EARTH Study fit with a restricted cubic spline. Models were adjusted for average relative humidity (continuous) and PM2.5 exposure (continuous) in 3 months prior to AFC, age (continuous), education (<college vs. ≥college), smoking status (ever vs. never), year of AFC (continuous), month of AFC (Jan, Feb, Mar, Apr, May, Jun, Jul, Aug, Sep, Oct, Nov, Dec), and diagnosis of diminished ovarian reserve and ovulation disorders.
The association between average Tmax over the 3 months prior to antral follicle scan and AFC was similar across age (<35 vs. ≥35 years), BMI (<25 vs. ≥25 kg/m2), and smoking status (never vs. ever) groups; however, the estimated effect of Tmax exposure on AFC was stronger among women whose primary infertility diagnosis was due to a female cause (% change= −1.8% 95% CI −3.0, −0.6%) compared to women with an unexplained or male factor diagnosis (% change= −1.2% 95% CI −2.4, 0.0%) (P for interaction=0.02) (Supplemental Table 4). Within the female factor infertility diagnoses, the strongest associations were observed for uterine (% change= −2.9), followed by endometriosis (% change= −2.0), tubal (% change= −1.9), ovulatory (% change= −1.8), and DOR (% change= −1.6).
Women reporting hotter work environments had a non-significant 11.4% (95% CI −22.7, 1.5) lower AFC compared to women with normal or cooler work environments. Although limited by small sample sizes, there was a suggestion of a slightly stronger association between ambient temperature and AFC in women with hotter work environments (n=17; % change= −1.9 95% CI −3.3, −0.5%); however, this was not statistically significant (P for interaction= 0.47).
Discussion.
In our prospective study of women seeking infertility treatment, we found that exposure to higher ambient temperatures was negatively associated with AFC, an objective marker of ovarian reserve. Our preliminary results suggest that the steady increase in global temperature due to climate change may result in accelerated reproductive aging in women. Our findings also indicate that there may be temporal changes in a woman’s susceptibility to heat over the course of a year as the negative association between temperature and AFC was weaker during the summer months and may be explained by physiological acclimatization or adaptive changes in response to heat.
Previous studies have documented a negative relationship between higher ambient temperatures and lower semen quality (38, 39), a marker of fertility in men; however, less is known about the relationship between temperature and female gametes. In female cows, even a slight degree of maternal hyperthermia induced by high ambient temperatures can compromise fertility and much of this effect appears to be mediated by damage to the follicle-enclosed oocytes (12). The ovarian pool of oocytes in the bovine is highly sensitive to heat shock beginning at the preantral phase. Moreover, even heat-induced alterations that occur at early stages of follicular development can ultimately compromise oocyte maturation and developmental competence (40). In our study, we found similar results with the strongest associations observed for higher exposure to temperature in the 3 months prior to antral follicle scan and weaker associations for the month and two weeks prior to scan. This suggests that cumulative heat exposures experienced throughout the preantral to preovulatory stages of follicular development may be more detrimental than shorter-term exposures in the final stages of antral development (25).
Several molecular and cellular pathways have been proposed to underlie the adverse effects of heat on folliculogenesis in bovines- many of which could have direct relevance to humans. These include disrupted steroid hormones production (17, 18), altered mitochondrial distribution and function (15, 19), heightened activation of apoptotic cascades (41), and enhanced production of reactive oxygen species (20, 21). However, we must be cautious in extrapolating data from domesticated animals used for food production to humans because selection for growth or milk yield increases metabolic rate and exacerbates the problem of body temperature regulation during heat stress. Thus, future research is warranted to determine if these same biological mechanisms apply in humans.
While our finding of a stronger association between temperature and AFC in the non-summer months may at first be counterintuitive, it is consistent with findings on other health outcomes including lung function, hospitalizations, and mortality (26, 42, 43). The most plausible explanation for this observation is temporal acclimatization due to physiological adaptations (e.g. expansion of sweat glands and increase in cardiac output) and/or behavior change (e.g. use of air conditioners) (44). This is the same reasoning behind why we would not necessarily expect to see decreased fertility in equatorial and tropical countries as compared to Scandinavian countries, because women who live in these areas where high temperatures are common adapt to these conditions, lessening the impact of heat. Women experiencing higher than average temperatures in the winter and early spring may be more likely to suffer from uncompensable heat stress, since the body is unable to maintain a thermal steady state possibly leading to worse health outcomes such as impaired follicular growth. By the summer months, when these high temperatures are more common, their body and activities have likely adapted to these conditions, lessening the impact of heat. This same rationale is also used to explain why studies on heat and health outcomes often tend to find greater vulnerability to the heat among populations in the Northeastern, Midwestern, and Pacific parts of the US, (and decreased vulnerability farther south) because people residing in these regions are less accustomed to experiencing high temperatures. We also observed a stronger association between temperature and AFC in women with a female factor infertility diagnosis, suggesting that this subpopulation could be particularly susceptible to the consequences of heat exposure. In a separate paper from this cohort, we similarly found that the effects of PM2.5 exposure on AFC were more pronounced in women with an existing female-specific cause of infertility (45).
Strengths of our study include its prospective design, large sample size, robust assessment of ovarian reserve (25), and our comprehensive adjustment for other reproductive and lifestyle factors that enhanced our ability to adjust for confounding. The primary limitation of our study is that we used residence-based ambient temperature for exposure assessment, an approach leading to measurement error in comparison to personal temperature measurements. We tried to minimize this error by using a spatially refined, gridded climate data set rather than data from airport weather stations, which may not fully capture exposures where people tend to live, or individual adaptation strategies. Nevertheless, we expect our results are conservative, given that any potential exposure misclassification would be non-differential and, on average, tend to bias our results towards the null hypothesis of no association. Due to the sole inclusion of women undergoing infertility treatment, it may not be possible to generalize our findings to all reproductive aged women. While previous work has shown that infertile women <40 years have similar AFCs compared with women of the same age with no history of infertility (46), women presenting to fertility clinics tend to have different demographic profiles than the general population (47). For example, due to the homogeneity of our cohort we had limited ability to evaluate how race/ethnicity and markers of socioeconomic status may modify the association between temperature and ovarian reserve as suggested by previous research on preterm birth (48). Future work on this topic in more diverse cohorts is warranted. We also only included data from a single climate region, which may limit generalizability. However, because Northeasterners are less accustomed to heat and have reduced prevalence of adaptations such as air-conditioning, they may be more likely to be adversely affected by extreme heat events (49). For example, a study of over 29 million pregnancies found that ambient temperature was more strongly associated with reduced fetal growth in regions with colder climates such as the Northeastern US (50). Finally, because this is an observational study, residual confounding by other factors, is still possible. For example, we lacked information on viral infections, such as influenza, which are more common during winter months. We purposefully controlled for month in all of our analyses to account for factors that may vary by season and to a finer extent, month. It is also worth noting that we observed, on average, higher AFCs when women were exposed to lower temperatures in the 3 months prior to scan, suggesting that if there was residual confounding resulting from a higher proportion of women experiencing influenza and fevers in the winter months (which may decrease follicular growth) this would be biasing our results downwards, towards the null.
In conclusion, our study provides evidence that exposure to higher temperatures may decrease human fertility by accelerating ovarian aging. In Western countries where the average age at first birth is increasing (51), even a small effect of rising ambient temperatures on female fertility potential could have negative consequences on fertility rates and important implications for future population size and structure. Since direct control over climate is often beyond an individual’s control, advocacy for regulations to curb greenhouse gas emissions and limit the magnitude or rate of global warming is essential.
Supplementary Material
Acknowledgments:
We would like to thank all members of the EARTH study team, specifically principal investigator Russ Hauser, research nurses Myra G. Keller and Jennifer B. Ford, senior research staff Ramace Dadd, the physicians and staff at Massachusetts General Hospital Fertility Center, and all the EARTH study participants.
Study Funding/Competing Interests:
This work was supported by grants R01ES009718, R01ES022955, P30ES000002, and R00ES026648 from the National Institute of Environmental Health Sciences (NIEHS). This publication was also made possible by U.S. Environmental Protection Agency (U.S. EPA): RD-834798 and RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication. All authors report no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W et al. Health and climate change: policy responses to protect public health. Lancet 2015;386:1861–914. [DOI] [PubMed] [Google Scholar]
- 2.Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Belesova K, Boykoff M et al. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet 2019;394:1836–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones B, O’Neill BC, McDaniel L, McGinnis S, Mearns LO, Tebaldi C. Future population exposure to US heat extremes. Nat Clim Change 2015;5:652–5. [Google Scholar]
- 4.Kovats RS, Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health 2008;29:41–55. [DOI] [PubMed] [Google Scholar]
- 5.Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect 2012;120:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw Open 2020;3:e208243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ 2020;371:m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartgill TW, Bergersen TK, Pirhonen J. Core body temperature and the thermoneutral zone: a longitudinal study of normal human pregnancy. Acta Physiol (Oxf) 2011;201:467–74. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Brook RD, Wang T, Song X, Feng B, Yi T et al. Short-term effects of ambient air pollution and outdoor temperature on biomarkers of myocardial damage, inflammation and oxidative stress in healthy adults. Environ Epidemiol 2019;3:e078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett GD. Hyperthermia: malformations to chaperones. Birth Defects Res B Dev Reprod Toxicol 2010;89:279–88. [DOI] [PubMed] [Google Scholar]
- 11.Barreca A, Deschenes O, Guldi M. Maybe Next Month? Temperature Shocks and Dynamic Adjustments in Birth Rates. Demography 2018;55:1269–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth Z Effect of Heat Stress on Reproduction in Dairy Cows: Insights into the Cellular and Molecular Responses of the Oocyte. Annu Rev Anim Biosci 2017;5:151–70. [DOI] [PubMed] [Google Scholar]
- 13.Khan A, Khan MZ, Umer S, Khan IM, Xu H, Zhu H et al. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals (Basel) 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeron Y, Ocheretny A, Kedar O, Borochov A, Sklan D, Arav A. Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 2001;121:447–54. [PubMed] [Google Scholar]
- 15.Gendelman M, Roth Z. In vivo vs. in vitro models for studying the effects of elevated temperature on the GV-stage oocyte, subsequent developmental competence and gene expression. Anim Reprod Sci 2012;134:125–34. [DOI] [PubMed] [Google Scholar]
- 16.Roth Z, Bor A, Braw-Tal R, Wolfenson D. Carry-over effect of summer thermal stress on characteristics of the preovulatory follicle of lactating cows. J Therm Biol 2004;29:681–5. [Google Scholar]
- 17.Bridges PJ, Brusie MA, Fortune JE. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest Anim Endocrinol 2005;29:508–22. [DOI] [PubMed] [Google Scholar]
- 18.Wolfenson D, Lew BJ, Thatcher WW, Graber Y, Meidan R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim Reprod Sci 1997;47:9–19. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira RM, Chiaratti MR, Macabelli CH, Rodrigues CA, Ferraz ML, Watanabe YF et al. The Infertility of Repeat-Breeder Cows During Summer Is Associated with Decreased Mitochondrial DNA and Increased Expression of Mitochondrial and Apoptotic Genes in Oocytes. Biol Reprod 2016;94:66. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa M, Hirabayashi M, Kanai Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction 2002;124:683–9. [DOI] [PubMed] [Google Scholar]
- 21.Nabenishi H, Takagi S, Kamata H, Nishimoto T, Morita T, Ashizawa K et al. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin A. Mol Reprod Dev 2012;79:31–40. [DOI] [PubMed] [Google Scholar]
- 22.Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000–2014. NCHS data brief, no 232. In. Hyattsville, MD: National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 23.Potential impact of later childbearing on future population. Population Division, United Nations Department of Economic and Social Affairs. 2019; No. 2019/5. Date Accessed: November 10th, 2020. Available from: https://www.un.org/en/development/desa/population/publications/pdf/popfacts/PopFacts_2019-5.pdf. [Google Scholar]
- 24.Vicedo-Cabrera AM, Sera F, Gasparrini A. Hands-on Tutorial on a Modeling Framework for Projections of Climate Change Impacts on Health. Epidemiology 2019;30:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril 2010;94:1044–51. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Nordio F, Zanobetti A, Kinney P, Vautard R, Schwartz J. Acclimatization across space and time in the effects of temperature on mortality: a time-series analysis. Environ Health 2014;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messerlian C, Williams PL, Ford JB, Chavarro JE, Minguez-Alarcon L, Dadd R et al. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open 2018;2018(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PRISM Climate Group, Oregon State University. Available: http://prism.oregonstate.edu/recent/ [data accessed March 13th 2019].
- 29.Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH et al. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. International Journal of Climatology 2008;28:2031–64. [Google Scholar]
- 30.Daly C, Smith JI, Olson KV. Mapping Atmospheric Moisture Climatologies across the Conterminous United States. PLoS One 2015;10:e0141140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spangler KR, Weinberger KR, Wellenius GA. Suitability of gridded climate datasets for use in environmental epidemiology. J Expo Sci Environ Epidemiol 2019;29:777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alduchov OA, Eskridge RE. Improved Magnus’ form approximation of saturation vapor pressure. J Appl Meteorol 1996;35:601–9. [Google Scholar]
- 33.Steadman RG. Part II: effects of wind, extra radiation and barometric pressure on apparent temperature. J Appl Meteorol 1979;18:874–5. [Google Scholar]
- 34.Kalkstein LS, Valimont KM. An evaluation of summer discomfort in the United States using a relative climatological index. Bull Am Meteorol Soc 1986;67:842–8. [Google Scholar]
- 35.Shi L, Kloog I, Zanobetti A, Liu P, Schwartz JD. Impacts of Temperature and its Variability on Mortality in New England. Nat Clim Chang 2015;5:988–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA et al. A New Hybrid Spatio-Temporal Model For Estimating Daily Multi-Year PM2.5 Concentrations Across Northeastern USA Using High Resolution Aerosol Optical Depth Data. Atmos Environ (1994) 2014;95:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Meng T, Wu L, Duan Y, Li G, Shi C et al. Association between ambient temperature and semen quality: A longitudinal study of 10 802 men in China. Environ Int 2020;135:105364. [DOI] [PubMed] [Google Scholar]
- 39.Santi D, Magnani E, Michelangeli M, Grassi R, Vecchi B, Pedroni G et al. Seasonal variation of semen parameters correlates with environmental temperature and air pollution: A big data analysis over 6 years. Environ Pollut 2018;235:806–13. [DOI] [PubMed] [Google Scholar]
- 40.de ST-JJR, de FAPM, de Sa WF, de MFA, Viana JH, Camargo LS et al. Effect of maternal heat-stress on follicular growth and oocyte competence in Bos indicus cattle. Theriogenology 2008;69:155–66. [DOI] [PubMed] [Google Scholar]
- 41.Roth Z, Hansen PJ. Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol Reprod 2004;71:1898–906. [DOI] [PubMed] [Google Scholar]
- 42.Rice MB, Li W, Wilker EH, Gold DR, Schwartz J, Zanobetti A et al. Association of outdoor temperature with lung function in a temperate climate. Eur Respir J 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Q, Li S, Coelho M, Saldiva PHN, Hu K, Abramson MJ et al. Assessment of Intraseasonal Variation in Hospitalization Associated With Heat Exposure in Brazil. JAMA Netw Open 2019;2:e187901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung SS, McLellan TM, Tenaglia S. The thermophysiology of uncompensable heat stress. Physiological manipulations and individual characteristics. Sports Med 2000;29:329–59. [DOI] [PubMed] [Google Scholar]
- 45.Gaskins AJ, Minguez-Alarcon L, Fong KC, Abdelmessih S, Coull BA, Chavarro JE et al. Exposure to Fine Particulate Matter and Ovarian Reserve Among Women from a Fertility Clinic. Epidemiology 2019;30:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A et al. Infertile women below the age of 40 have similar anti-Mullerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod 2016;31:1034–45. [DOI] [PubMed] [Google Scholar]
- 47.Stephen EH, Chandra A. Use of infertility services in the United States: 1995. Fam Plann Perspect 2000;32:132–7. [PubMed] [Google Scholar]
- 48.Basu R, Chen H, Li DK, Avalos LA. The impact of maternal factors on the association between temperature and preterm delivery. Environ Res 2017;154:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGeehin MA, Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect 2001;109Suppl 2:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA. Ambient temperature and preterm birth: A retrospective study of 32 million US singleton births. Environ Int 2019;126:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.SF2.3: Age of mothers at childbirth and age-specific fertility. Organisation for Economic Co-operation and Development (OECD) Family Database. Published: May 29, 2019.Date Accessed: November 11th, 2020. Available from: http://www.oecd.org/els/soc/SF_2_3_Age_mothers_childbirth.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


