Abstract
Systemic sclerosis (SSc) is a heterogeneous multisystem autoimmune disease whereby its main pathological drivers of disability and damage are vascular injury, inflammatory cell infiltration, and fibrosis. These mechanisms result in diffuse and diverse impairments arising from ischemic circulatory dysfunction leading to painful skin ulceration and calcinosis, neurovascular aberrations hindering gastrointestinal (GI) motility, progressive painful, incapacitating or immobilizing effects of inflammatory and fibrotic effects on the lungs, skin, articular and periarticular structures, and muscle. SSc-related impairments impede routine activities of daily living (ADLs) and disrupt three critical life areas: work, family, social/leisure, and also impact on psychological well-being.
Physical activity and exercise are globally recommended; however, for connective tissue diseases, this guidance carries greater impact on inflammatory disease manifestations, recovery, and cardiovascular health. Exercise, through myogenic and vascular phenomena, naturally targets key pathogenic drivers by downregulating multiple inflammatory and fibrotic pathways in serum and tissue, while increasing circulation and vascular repair.
G-FoRSS, The Global Fellowship on Rehabilitation and Exercise in Systemic Sclerosis recognizes the scientific basis of and advocates for education and research of exercise as a systemic and targeted SSc disease-modifying treatment. An overview of biophysiological mechanisms of physical activity and exercise are herein imparted for patients, clinicians, and researchers, and applied to SSc disease mechanisms, manifestations, and impairment. A preliminary guidance on exercise in SSc, a research agenda, and the current state of research and outcome measures are set forth.
Keywords: Scleroderma, Disability, Exercise, Physical activity, Myokine, Muscle, Pulmonary rehabilitation, Interstitial lung disease, Pulmonary hypertension, Health-related quality of life, Breathlessness, Disease activity, Physical function, Symptom burden, Systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a heterogeneous multisystem autoimmune disease with main pathological drivers of disability/damage being vascular injury, inflammatory cell infiltration, and fibrosis [1]. Skin thickening varies widely and its distribution creates a rudimentary subtyping: sine scleroderma (without skin thickening), limited cutaneous (lcSSc) (skin thickening of face and distal to elbows/knees), and diffuse cutaneous (dcSSc) (includes proximal skin thickening above elbows/knees) [2]. However, systemic manifestations are the true hallmark of SSc. Sine and lcSSc have more vasculopathic tendencies; but any SSc manifestation occurs in all subtypes, and all subtypes being potentially lethal and associated with severe and multiple disabilities [2–5].
In SSc, physical function, a predictor of health-related quality of life (HRQoL) and survival can be severely diminished by diffuse, diverse impairments arising from ischemic circulatory dysfunction (leading to painful skin ulceration and calcinosis, to neurovascular aberrations hindering gastrointestinal (GI) motility), to progressive painful, incapacitating or immobilizing effects of inflammatory and fibrotic effects on the lungs, skin, articular, and periarticular structures and muscle [4,5]. SSc-related impairments impede routine activities of daily living (ADLs), disrupt critical life areas: work, family, social/leisure, and also deteriorate psychological well-being [6,7].
Despite physical activity being vital to general health, muscle function, physical function, and aerobic capacity; attention to these cornerstone therapeutic concepts are often overshadowed by patients/clinicians feeling overwhelmed by other SSc medical management or fearful of overexertion [8,9]. Exercise, through myogenic and vascular phenomena, naturally targets key SSc pathogenic drivers by downregulating multiple inflammatory and fibrotic pathways [10,11] in serum and tissue [12,13] while increasing circulation and vascular repair. Based on evidence in SSc and other connective tissue diseases (CTDs), patients with SSc can benefit from exercise through reduced disease activity, systemic inflammation, pain and fatigue as well as improved muscle function, joint and bone strength, aerobic capacity/cardiopulmonary function, and HRQoL [14]. While SSc notoriously erodes body image, physical activity is elemental to embodiment practices, allays biochemical impact on depression/anxiety, stress, and physical pain burden, and improves self-esteem [15–18]. Myogenic and vascular mechanisms likely contribute to exercise’s beneficial impact on sleep, pain, fatigue, and GI health and gut flora [19,20].
As a global collaborative, relying on scientific evidence and expert experience of developing exercise programs in SSc and other serious health conditions, we describe the current state of exercise as it relates to SSc disability and disease activity. We propose strategies targeting pathological SSc disease mechanisms and manifestations with the goal of reducing disease activity and optimizing physical function that include supervised-, home-, land-, water-based, pulmonary rehabilitative exercise, and newer evidence-based technologies [15–18,21–44]. Future research priorities in SSc rehabilitation medicine are highlighted.
Physical activity and exercise as medicine
Physical activity encompasses any bodily movement by skeletal muscle requiring energy expenditure above resting levels [45,46]. Exercise, a subset of physical activity, is planned, structured, repeated, and targets improvement or maintenance of physical fitness, performance, or health. “Physical activity/exercise,” herein refers to all the following: workouts, training, rehabilitation, physical therapy, including modalities such as martial arts, singing, yoga, and dance for which growing evidence supports improved articular and respiratory health [45].
A physically active lifestyle is essential for health, with higher aerobic fitness being strongly associated with the absence of disease [47]. Recommendations to improve muscle strength/endurance and aerobic capacity define exercise type and dosing (frequency, duration, and intensity) (Fig. 1 and Tables 1–4) [48–51], which correspond to health benefits, e.g., in obesity, type II diabetes, hypertension, cardiovascular disease (CVD), some forms of cancer [46,52], and in CTDs associated with increased risk of CVD, such as SSc. Exercise benefits extend to muscle, bone density, nerve and joint health, HRQoL, sleep quality, and mental health – areas that are more highly impacted in CTDs; while physical inactivity rapidly erodes these benefits.
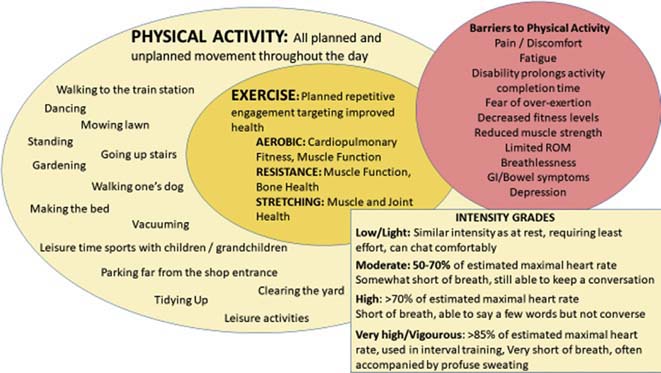
Fig. 1.
All movement is healthful. This diagram perspects the relation of exercise in the larger arena of physical activity with the description of activity intensity by estimated heart rate and lay description (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
Table 1.
Summary of definitions and recommendations for physical activity, related to health benefits and exercise [45,48–50,282] (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
| Physical Activity (PA) | ||||
|---|---|---|---|---|
|
| ||||
| Definition of Physical Activity | Any everyday activity producing increased energy expenditure above resting (sitting) levels. The antithesis of sedentary/nonmotion moments | |||
| An unrestricted spectrum of activity, including exercise, household and employment tasks, mobility, leisure activity such as sports, hobbies, and singing | ||||
| Occurs in varying intensities: light/low, moderate, high, and vigorous | ||||
| Any PA is healthy and contributes to fitness; general health is more favorably impacted with increasing time and intensity of relevant PA | ||||
| Recommended Weekly Dosage of Physical Activity | 150 min of moderate-intensity PA weekly for ≥10 min at a time | |||
| OR | ||||
| 75 min of vigorous-intensity PA weekly for ≥10 min at a time | ||||
| OR | ||||
| an equivalent combination of moderate- and vigorous-intensity PA for ≥10 min at a time | ||||
| PLUS | ||||
| -Strengthening exercise involving major muscle groups (legs, back, chest, abdomen, shoulders, and arms) ≥2 days weekly | ||||
| * Additional health benefits can be attained by doubling the minutes per week above | ||||
|
| ||||
| Physical Activity for Special Populations | For Adults ≥ 65 Years Old | People with Chronic Illness | ||
| Same recommendations as above. | ||||
| OR | ||||
| If limited by health condition, engagement in PA as abilities and conditions allow | ||||
| PLUS | ||||
| Limit amount of sedentary time, replacing it with PA of any intensity (including light intensity) | ||||
| PLUS | ||||
| If mobility is impaired, PA to enhance balance and prevent falls for ≥3 days weekly | ||||
|
| ||||
| Intensity of Physical Activity | Light/Low | Moderate | High | Vigorous |
| Similar intensity as rest Minimal effort Able to chat easily |
50%–70% of max HR 3–6x effort as rest A bit difficult to converse |
>70% of max HR 6–10x effort as rest Difficult to converse |
>85% max HR >10x effort as rest Unable to converse |
|
| Even light intensity PA can support cardiovascular health and can help with weight loss | ||||
| An activity’s intensity is dependent on an individual’s baseline fitness level | ||||
| An activity’s intensity decreases with an individual’s increasing fitness | ||||
| Definition of Exercise (a subset of PA) | A subset of PA that is repeated over time with specified intensity, duration, and frequency | |||
| Directed targets improvement of any aspect of physical fitness: cardiorespiratory, mobility, muscle strength, general health, wound healing, psychological, cognitive, etc. | ||||
| Recommendations for exercise to improve aerobic fitness is 30 min/day for ≥3–5 days weekly at ≥ moderate intensity, at least a bit difficult to converse | ||||
| Recommendations for exercise to improve muscle strength (weights allowing a maximum of 8–12 repetitions) or muscle endurance (weights allowing a maximum of 15–25 repetitions) is 2–3 days weekly with a rest period of 48–72 h | ||||
Table 4.
SSc disease mechanisms and drivers targeted by exercise.
| Circulation |
| Heat generation |
| Nutrient delivery |
| Aerobic exchange |
| Toxin clearance |
| Vascular repair |
| Vascular responsiveness |
| Systemic Inflammation |
| Downregulation of inflammatory cytokines |
| Downregulation of inflammatory cell recruitment |
| Resultant decreased triggering of fibrotic pathways |
| Fibrosis |
| Degradation of fibrosis |
| Possible halting or reversing of fibrosis in evolution |
A phenomenal rate of emerging evidence points toward muscle and muscle contraction being pivotal and pervasive on vital pan-systemic function (Fig. 2). The muscle’s paracrine, endocrine, and cytokine regulation across multiple systems (brain, skin, cardiovascular, vascular, gastrointestinal, osseous, immunological, endocrine, and pulmonary), is increasingly prompting the consideration of muscle as an endocrine organ beyond and its roles of motility/mobility [12–14,19,33,45–47,50,53–60].
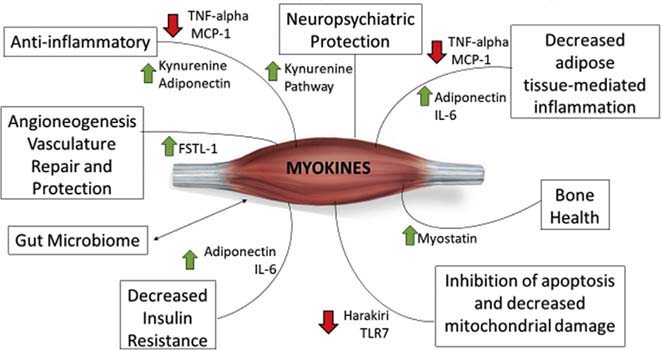
Fig. 2.
Muscle Contraction-Induced Myogenic Mechanisms Facilitating Health. FSTL-1: Follistatin-like 1 IL: interleukin, MCP: monocyte chemoattractant protein-1, TLR-7: Toll-Like Receptor-7, and TNF: Tumour Necrosis Factor (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
Longstanding inflammation in CTDs heightens CVD event risk, bone and muscle loss, pain, stiffness, poor sleep, and fatigue predisposing to further inactivity and muscle disuse. Aerobic and resistance training reduce ESR and CRP in several CTDs [61,62] with improvement in fatigue, pain, and stiffness. Muscle contraction activates multimodal cascades through mechanical and chemical mechanisms that systemically downregulates circulating and tissue inflammation while upregulating vascular support and repair that activate an anti-inflammatory cascade - thus interrupting this vicious inflammatory cycle [54]. Exercise mechanisms are numerous with instantaneous and lasting inflammatory transformations e.g., myriad myokine circuits through e.g., IL-6, IL-1, IL-10, IL-15, FSTL-1, etc., muscle-fat mass distribution impacting adipokine-inflammasome dynamics, mediate long-term reduced risk of cardiovascular disease [12,13,57], and endothelial function and repair [55–57] – a crucial dysfunctional pathway of SSc pathology.
A growing body of evidence supports the safety and efficacy of exercise in systemic inflammatory conditions such as myositis, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) with fatigue and depression reduction [61,62]. In myositis, exercise effects include: a) upregulation of anti-inflammatory genes and genes related to muscle growth and vascularization, with downregulation of proinflammatory genes and fibrosis; b) increased capillary density in the muscle tissue along with improved mitochondrial enzyme function [58,63–65]; and c) the prevention of mitochondrial damage and myofiber apoptosis through exercise-induced reduction of myositis-related Harakiri and TLR7 expression [58,66]. We submit that these clinical and preclinical exercise effects on inflammation and vascular repair and function also occur in SSc and could favorably impact disease manifestations related to these drivers.
Direct and indirect costs related to SSc and other CTDs are significant with reduced labor productivity that results in striking losses [67]. Physical inactivity contributes significantly to overall SSc disease burden [67–71] with exercise being a potent antidote improving function and potentially diminishing disease-related economic burden. Exercise intervention has demonstrated a cost benefit in other conditions such as pulmonary hypertension (PH) and RA, when compared with medical treatment alone [72–74]. Evidence suggests analyses of exercise-related economic benefit in SSc and other CTDs are warranted [72–74].
Disease mechanisms in SSc treatment paradigms
Autoimmunity, inflammation, vasculopathy, and fibrosis in SSc
Inflammation is the hallmark of many autoimmune diseases which includes SSc. Unless halted, ongoing inflammation spurs further proinflammatory pathways and recruitment of immune cells that assault host tissue with histotoxic infiltrates, which result in tissue damage. Inflammation can be rapidly or slowly progressive or even punctuated by periods of inactivity. Quieting inflammation may decelerate disease progression and reduce symptoms. Ongoing inflammatory mechanisms trigger downstream pathways that lead to fibrosis, the final transition from reversible inflammation to permanent damage.
Diffuse vascular/endothelial injury, dysfunction, and disrepair occur in SSc. However, it is yet unclear if interruption of the inflammatory/autoimmune – fibrosis trajectory with pharmaceuticals also deters progressive vascular disease. If so, exercise hypothetically targets vascular SSc manifestations through both anti-inflammatory/anti-fibrosis and circulation mechanisms, including mitigating the endothelial shear stress of SSc vasculopathy [75,76] (Table 5).
Table 5.
Potential impact of exercise on SSc disease manifestations (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
| SSc Manifestation | Potential Impact of Exercise |
|---|---|
| Inflammation | Downregulated with exercise |
| Immunosuppression/Immune dysfunction | Probable improved immune function |
| Fatigue | Reduction of multidimensional fatigue |
| Sleep | Improved sleep after exercise Sleep quality correlates to inflammation fatigue and HRQoL |
| Psychological Impact | Improved confidence |
| Increased self-esteem Improved mood | |
| Self-perceptions of healthy/“not sick” | |
| Reduced stress, anxiety, and worries | |
| Reduced depression | |
| Improved HRQoL | |
| Cold sensation/cold injury | Increased heat to core body |
| Increased distal extremities | |
| Ischemia/RP | Increased oxygenation/gas exchange to extremities |
| Improved wound healing | |
| Possibly reduced DUs, calcinosis, and osteolysis | |
| Possibly improved sexual function | |
| Gastrointestinal Dysmotility | Reduced nausea |
| Reduced bloating | |
| Enhanced peristalsis/digestion and reduced constipation | |
| Diarrhea | Improved microbiome profile |
| Arthropathy | Joint lubrication |
| Decreased stiffness | |
| Decreased inflammation | |
| Increased mobility, flexibility, and dexterity | |
| Strengthening of periarticular muscle support | |
| Skin tightness | Possible reduced skin tightening |
| Possible degree of preservation of skin function e.g., sweating | |
| Possible reduction of subcutaneous edema | |
| Myopathy | Improved muscle strength |
| Myositis | Improved muscle endurance |
| Atrophic myopathy | Improved aerobic capacity and vascularization of muscle |
| Reduced muscle inflammation | |
| Enhanced muscle recovery | |
| Increased muscle mass, with correlates to decreased inflammation | |
| Oral aperture | Improved mouth opening |
| Oral health improvement | |
| Facial changes | Improve mouth function |
| Possibly improved facial expression and verbal communication | |
| Pain | Pain reduction |
| Decreased stiffness | |
| Respiratory capacity and breath phrasing | Improved tolerance to dyspnea |
| Improved exercise tolerance | |
| Need for pharmaceutical treatment | Less need of pharmaceuticals to improve blood circulation |
Treatment in the context of the inflammation-fibrosis trajectory and circulation
SSc symptoms and impairments can result from currently active drivers of disease, which may recede with systemic treatment or from tissue damage that remains after the resolution of active disease. Ideally, SSc is pharmacologically treated in the inflammatory predominant, more reversible early stages to prevent permanent disability. Less ideal, but still important, is salvaging functional tissue by reversing resident inflammation in areas of coexistent fibrotic damage to prevent further fibrotic damage-related disability. SSc progression can span widely between rapidly progressive (robust inflammatory driver) or indolently progressive (subtle inflammatory driver) transformation to fibrosis. Newer antifibrotic medications decelerate fibrosis-transforming pathways, but are unable to reverse end fibrosis or significantly reduce inflammation.
Patient-reported experience and perceptions of exercise
Successful patient engagement requires respectful understanding of hopes, fears, needs, experiences, perceptions, priorities, and barriers of an intervention from the perspective of the targeted population. Limited yet substantive work investigate patients’ perspective of exercise on SSc-related manifestations [77–80] with diverse impairments reported in physical capacity, which includes reduced muscle strength, impaired mobility, cardiopulmonary problems [78], substantial diseaserelated economic burden, and poor HRQoL [81,82].
Participants consistently expressed hopeful perceptions, “The more I exercise, the more improved my health and the chance to survive longer …. ” Inactivity was consistently connected with further decline in health status: “… because of my lung disease … I’ve been close to death a couple of times, so I notice a big difference between exercising and not exercising. It’s as different as night and day.” [77] However, despite perceiving exercise as essential for life and health [77], patients also report not engaging in exercise [77,78]. Multifactorial demotivators include: a) manifestation-related e.g., digital ulcers (DUs), joint pain, and restricted motion; b) constitutional effects of disease e.g., pain and fatigue; c) psychosocial struggles of living with SSc d) fear and lack of exercise safety knowledge, and e) logistical burden of preparation and participation [77].
Patient self-knowledge and experience provide a roadmap for researchers to understand both cohort and individually targeted interventions. In separate studies, patient report corroborated by physiotherapist assessment with markedly reduced muscle endurance in shoulder and hip flexion as well as reduced lower extremity strength [83]. Fatigue, Raynaud’s phenomenon (RP), physical limitations, joint problems, and DUs persist as unmet needs in SSc care [77,80,84,85], though each is potentially modifiable by exercise [77]. SSc symptoms, such as pain, fatigue, breathlessness, and impaired hand function, influence self-rated work ability and employment status [86–89]. Raising awareness of SSc-related work impediments, such as these, may identify interventions for physical function that provide a path to diminish economic burden [77] and improve perceived well-being [89].
Patients report major exercise benefits being improved blood circulation particularly in hands, feet, and prolonged core-warming, breathing, fatigue, pain, sleep, vitality and musculoskeletal function [77]. Whether exercise exerts an effect on socially stigmatizing disease-related barriers, such as DUs [77,90] or body dissatisfaction, deserves investigative attention.
Patients conveyed that planning, adaptation, and post-exercise recovery time were important considerations particularly with severe lung disease or after vigorous exercise [77]. Patients report adapting intensity and activity type to facilitate exercise during inclement weather or increased symptoms. Furthermore, patients conveyed that healthcare professional counselling on exercise instruction, benefits, and cautions might be helpful, particularly early in the disease [77].
Though an SSc-specific patient-reported experience measure remains to be developed [91,92], a patient activation measure [93] assessing levels of disease knowledge, motivation, and support can be used to facilitate patient engagement in exercise protocols.
SSc manifestations and physical activity/exercise
The following section outlines the current state of exercise safety, efficacy, and assessment crossed with SSc symptoms and manifestations (Tables 6–10 and Fig. 3). It is not exhaustive nor comprehensive in potential benefits nor in the science that predicts the ability of exercise to prevent disease progression and disability in SSc.
Table 6.
Eligibility and safety of exercise in SSc (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
| Who can Exercise: | |
|---|---|
| SSc patients without pulmonary involvement | Concluded to partake without restriction [120] |
| SSc patient with cardiopulmonary involvement | Considered feasible, safe, and effective [118,283]regardless of underlying diagnosis (e.g., ILD and PH) [120] |
| SSc patients with mild pulmonary involvement | Safely able to engage in moderate aerobic intensity with moderate-load resistance exercises [120] |
| SSc patients with myopathy and cardiopulmonary involvement | May warrant special attention focusing on strengthening |
| Pre-Exercise Screening for: | |
| ILD | - Symptomatology |
| - Serial FVC | |
| - Serial DLCO | |
| - 6MWT for desaturation | |
| +/− HRCT of Chest | |
| PH | - Symptomatology |
| - Annual echocardiogram at rest and with exercise | |
| - Serial DLCO | |
| - 6MWT for desaturation | |
| - Right heart catheterization (gold standard) if symptomatology or testing is concerning | |
| Further screening | Cardiac magnetic resonance imaging (CMR) is the gold standard to assess: |
| - right and left ventricular systolic function (LVSF) | |
| - myocardial fibrosis | |
| - pericardial disease [284] | |
| Safety Parameters | - Regular monitoring of blood pressure, heart rate and preferably, forehead oximetry [123] with formal exercise programs. |
| - Severe symptoms and exercise-induced desaturation require individualized modification of intensity and duration [285] | |
| - Supplemental oxygen [118,285] is required for abnormal desaturation Forehead oximetry [123] over digital oximetry SpO2 may reduce Raynaud-related falsely low readings | |
| - Borg CR-10-scale to assess dyspnea and leg tiredness [286] as well as the Borg RPE-scale, Rating of Perceived Exertion can inform baseline and follow-up assessments [287] | |
| - The treating physician should always be notified of any unexpected abnormal assessments, e.g., heart rate, oxygen saturation, etc. or large drops in saturation | |
| Programmatic Considerations | Regardless of SSc manifestations, load intensity, and repetitions are adjusted according to symptoms and tolerance (ref 7). |
| Diverse effective modalities such as continuous versus interval aerobic training can be intensified up to 75%–80% of the patienťs projected maximal load based on their physical condition and comorbidities while monitoring intensity parameters e.g., breathlessness severity, leg fatigue, and heart rate. | |
| Combined aerobic, resistance, and respiratory muscle training induces the strongest improvement in functional capacity reflected by 6MWD and VO2 peak [104] | |
| In severe cardiopulmonary disease, unilateral resistance training may be more accessible over dynamic resistance training. | |
| In exercise-related induced sPAP elevation, interval training is the preferred safer approach due to load reduction on the vessels of the systemic and pulmonary circulation. | |
| In ILD, initiating endurance training between 70% and 80% of max exercise capacity. | |
| Interval training may be an alternative in ILD with periods of relative high-intensity training interspersed with periods of rest/low-intensity training allowing for time to recuperate and lessen breathlessness and fatigue [285] | |
| In PH, it is advisable to avoid interval training due to the associated risk ofrapid changes in pulmonary hemodynamics and risk of syncope [288–290] However, new research and guidelines may bring clarity to optimal exercise strategies in both ILD, PH and the combination of ILD and PH. | |
Table 10.
Potential exercise enhancing qualities of water-based exercise and therapies.
| Possible effects of aquatic exercise in general populations [271–273] | |
|---|---|
| Improved domains | HRQoL |
| Pain | |
| Fatigue | |
| Muscle function, strength, and endurance | |
| Aerobic capacity | |
| Physical function | |
| Range of motion | |
| Stiffness | |
| Muscle spasm | |
| Circulation | |
| Reduced disease activity in some Inflammatory diseases | |
| Safety | Water properties minimize the risk of injury/re-injury |
| Buoyancy and immersion anti-gravity offloading effects provide protective measure | |
| Advisement on pacing to protect against unintended overexertion | |
| Tolerability | Increase ability to focus body movement |
| Stretching more tolerable | |
| Increased exercise duration | |
| Increased exercise intensity | |
| Patient-centered considerations | |
| Skin | Rinsing after chlorinated and salted water |
| Moisturizing skin post-exercise to minimize skin dryness/irritation | |
| Temperature | Aqueous and ambient temperatures facility and changing room require assurance of warmth |
| Logistical feasibility and patient effort | Planning time, energy, and assistance for pre/post preparations. Patients report feeling more exhausted by logistics of clothes change than the exercise, and state exercise benefit is worth it but needs to be addressed. |
| Consider attendants to assist | |
| Mobility | Support for descending/ascending into pool |
Abbreviations: AROM, active range of motion and HRQoL, health-related quality of life.
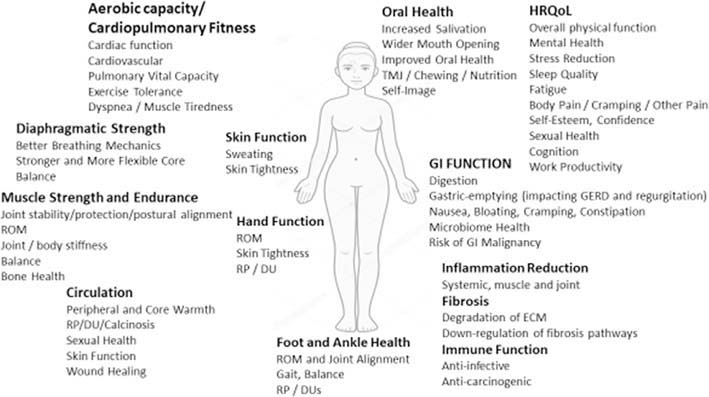
Fig. 3.
Overview of Potential Exercise Benefit relational to anatomical manifestations of SSc in Male and Female subjects. Abbreviations: DU: Digital Ulcer, ECM: Extracellular Matrix, GERD: gastroesophageal reflux; GI: Gastrointestinal, HRQoL: Health-Related Quality of Life, ROM: Range of Motion, RP: Raynaud Phenomenon, and TMJ: Temporal-Mandibular Joint (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
Cardiopulmonary Involvement in SSc: is common with pulmonary parenchymal involvement/interstitial lung disease (ILD) being the leading cause of SSc death, followed by pulmonary vascular involvement/PH. Cylindrical bronchiectasis and severe esophageal reflux may also affect airways in SSc [94]. Cardiac decompensation may occur secondary to SSc pulmonary manifestations, pericardial disease, or malignant hypertension (renal crisis); however, primary SSc-cardiac manifestations can arise from muscle, microvascular, and neuronal dysfunction [95]. Resultant clinical correlates of cardiac involvement in SSc include diastolic dysfunction from either myofibrosis or microcirculatory insufficiency, dilated cardiomyopathy, or arrhythmias. Breathlessness is a common feature of cardiopulmonary manifestations of SSc, along with exercise intolerance, diminished HRQoL, and occasionally pleural or pericardial pain [96–98]. In ILD, a disabling inspiratory cough can exacerbate breathlessness [99]. In early stages, symptoms may be mild, and by unconsciously restricting or slowing activity levels many patients do not recognize early symptoms. Musculoskeletal impairments restricting activity also make the recognition of cardiopulmonary limitations less apparent.
Aerobic and muscle-strengthening exercises significantly improve [100] HRQoL as well as cardiovascular, endothelial, metabolic/glandular, muscle structure and function, lung mechanics, mobility, and systemic inflammation [95,100–103] with overall positive effect on the physiological and psychological components [101,104–106]. Exercise cultivates a fitness that can offset cardiopulmonary deficiency by facilitating greater ease, capacity, and reserve as patients interface with life activities [6]. For example, exercise promotes stronger, more supple, and neurologically responsive feet, important for efficient rising, balancing, propelling, and mobilizing the body. Diaphragmatic strengthening fortifies respiratory dynamics and improves attributes that support respiratory capacity, e.g., balance, core strength, and lower back health [107–109]. As intercostal and accessory muscles achieve improved postural strength and flexibility, increasingly easier bending, reaching, and twisting during activities is possible with less breathlessness. Furthermore, exercise practices movement coordinated with breath, thus increasing skilled capacity for complex, weighty, or increasingly intense activities. Strategies such as Singing for Lung Health [30,35,110–114], yogic breathing, Tai Ch’i [115,116] as well as some yoga and dance techniques, support healthier breath patterns and efficiency, e.g., abdominal relaxation increasing inspiratory capacity, stronger and efficient oral musculature, etc.
Pulmonary rehabilitation is supported as feasible, safe, and effective [117,118] regardless of underlying diagnosis (e.g., ILD and PH) and can improve breathing, exercise tolerance, fatigue, and cough [119]. Exercise safety in SSc-cardiopulmonary involvement [79,120–123] are outlined in Tables 7a and 7b, Tables 8–10 as are programmatic considerations and enhancements [105,106,121]. Cardiopulmonary-related breathlessness carries neurophysiological, cognitive, and emotional distress different from other exertional breathlessness [16,29,124]. Worries over breathlessness and what breathlessness might signify hinders exercise. Patient-clinician discussions are key to successful, confident engagement in exercise [16,99,124,125]. Patients require reassurance that breathlessness and desaturation are distinct attributes often independent of each other. Desaturation is a chemical phenomenon, while breathlessness is a complex multifactorial, multidimensional experience, and of itself is not physiologically harmful [124]. We explain the following to patients who feel frightened or frustrated: “Being physically unfit causes breathlessness and fatigue,” “Exercise treats unfitness, exercise also causes breathlessness, but can be done in non-distressing manners that will diminish breathlessness over time.”
Table 7a.
Stratifies patients with SSc for engagement in exercise (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
| Strata of Engagement | Advisement | Comments |
|---|---|---|
| No pulmonary involvement | Unrestricted but targeted to patient needs and tolerance | Gradual increment of intensity, repetitions, and duration |
| Mild cardiopulmonary symptoms | Moderate aerobic intensity with moderate-load resistance exercises | Gradual increment of intensity, repetitions, and duration |
| Severe cardiopulmonary symptoms | Individualized modification of intensity and duration, with supplemental oxygen as needed [118,290] | Can be intensified up to 75%–80% of patient’s projected maximal load |
| Desaturation with exercise | As above for severe cardiopulmonary symptoms | |
| Increase of systolic pulmonary artery pressure with exercise | Load reduction on systemic and pulmonary circulation is an important consideration | Rapid changes in pulmonary hemodynamics Interval training may increase the risk of syncope [290] |
Table 7b.
General considerations for exercise in SSc* (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
| Concept | Advisement | Comments |
|---|---|---|
| Exercise Initiation | All patients screened for clinically significant ILD and PH Assess current activity levels with FITT Consider assessing patient goals with PSFS |
|
| Aerobic and muscle testing prior to start of exercise | Submaximal ergometer cycle test or treadmill test and muscle tests like TST, 30-sec CST, and FI-2 | |
| Sustaining exercise | Anticipatory guidance of fluctuating fatigue/pain challenging exercise. | Encourage mindfulness practice and pleasure principals during exercise to redirect frustration and disappointments. |
| Education on stretching safety | Emphasis on consistency of practice and expectations of incremental improvement. | |
| Developing alternate options for inclement weather or GI exacerbations | Adaptations Indoor options Online class options |
|
| Consider monitoring achievement with PSFS | ||
| Start gently and escalate with improvement | ||
| Recommendation for home general physical activity (e.g., walks) 30 min/5 days weekly | ||
| Aerobic and muscle testing after exercise period to evaluate intervention | Submaximal ergometer cycle test or treadmill test and muscle tests like TST, 30-sec CST, and FI-2 | |
| Myopathy | Screen for myopathy | In tandem with rheumatologistyet, determine if targeted strengthening required |
| Assess degree of myopathy preferably using FI-2 or FI-3 | ||
| Nutritional status | Assess for low nutritional status | Consider adjustment of calorie intake to exercise-related consumption |
| Face, Mouth, Hand, Wrist, and Shoulder ROM exercises | Ideally encouraged to be daily or twice daily “ritual” | Frequency may be increased at induction and decreased for maintenance. |
| Patients can also implement throughout the day as needed for relief. | ||
| Raynaud | Indoor temperature Outdoor temperature Gripping equipment Erroneous oximetry results |
Alternate plans for inclement weather Use of exercise gloves Forehead oximetry to avoid falsely low readings |
| Digital ulcers | Require bandaging and protective gloves prior to handling equipment Require bandaging and gloving with paraffin wax emersion |
|
| Warming | Hands for improved exercise performance | Consider paraffin bath immersion |
| Sauna | To improve core warmth prior exercise, both in water and on land | |
| Feet | Screen for foot pain | |
| Assess degree and cause of foot pain | Advise on proper footwear with insoles or referral to podiatry where necessary | |
| Cardiopulmonary | Screen for extent and severity Interval monitoring pulse, blood pressure and forehead oximetry Adjust exercise for symptom severity Use supplemental oxygen to keep levels appropriate for exercise [285,291] in ILD [290] and PH [285,290] Combined aerobic, resistance, and respiratory muscle training induces the strongest improvement in functional capacity |
|
| Dynamic resistance training in severe cardiopulmonary disease may require a switch to isolated, unilateral resistance training | ||
| Stretching | Stretching ideally is done regularly to condition anatomic proprioception, balance, and muscular responsiveness. Stretching is essential to maintain ROM and protect the muscle from excessive force | Stretching is also performed before and after other forms of exercise to increase circulation into the muscle, warm muscle, and prepare the muscle to be responsive enough to protect itself and articular structures by the regulation of force and stretch |
| Each flexibility exercise is held for a total of 30–60 s (e.g., 45 s once or 15 s thrice), preferably synchronized with breath cycles, to allow for muscle fibers to relax into its optimal length | Bouncing or pulsing a stretch is considered unsafe. Stretching the point of a “resistance sensation” or “pleasurable pain” but not a “bad pain” to avoid damage ≥30 s may be more beneficial | |
| Warming the area of the body prior to stretching with loose fluid movements, sauna, or warm wax | Moving the general body area for about a minute with arm circles shaking, marching, etc., warms and relaxes muscle before stretching. | |
| Aquatic exercise | Rinse off chlorinated or salted water Moisturize skin post-exercise | |
| Exhaustion may occur with changing of clothes and being wet at room air for extended length of time | Provide attendant support | |
| Water temperature between 30 °C −34 °C and 86 °F −93 °F | Water is a rapid conductor of heat. Lower temperature water draws heat from the body | |
| Advisement on pacing with gradual increase of duration and intensity | To protect against unintended overexertion |
Abbreviations: FITT: frequency, intensity, time duration and type; FI-2: functional index 2; FI-3: functional index 3; ILD: interstitial lung disease; PH: pulmonary hypertension; PSFS: Patient-Specific Function Scale; TST: Timed-stands test; and 30-s CST: 30-s chair stands test.
Treating physician must be apprised of any new oxygen requirement or new cardiopulmonary symptomatology e.g., arrhythmia, syncope/pre-syncope, etc.
Table 8.
Comprehensive profile of published exercise studies in SSc to date.
| Publication | Population/Dropouts | Interventions and Exercise Type | Primary outcomes | Secondary outcomes | Adverse events |
|---|---|---|---|---|---|
| Aerobic and Muscle Performance | |||||
| Oliveira et al. (2009) [292] | 7 SSc(2 dcSSc and 5 lcSS) and 7 healthy controls. No pulmonary involvement on CT or PASP ≤40 mmHG or FVC and DLCO <75% of predicted. 2 dropouts in SSc. |
Aerobic exercise 2x/wk for 8 wks of 40 min on treadmill. |
VO2peak improved significantly in both groups. |
Improved peak exercise oxygen saturation in SSc after it was compared to baseline. | No adverse effects reported. |
| Pinto et al. (2011) [121] | 11 SSc (8 dcSSc and 3 lcSSc). No evidence of moderate or severe pulmonary involvement or PASP ≤40 mmHG or FVC and DLCO <75% of predicted. |
Aerobic exercise 2x/wk for 12 wks of 20 min on treadmill. Resistance training for 30 min for 5 main muscle groups in 8–12 reps x 4 sets. |
VO2peak Improved. | Significant improvements in muscle strength. | No adverse effects reported. |
| Alexanderson et al. (2014) [293] | SSc, n = 4. 2 with lung fibrosis with FVC 50% resp 80% while the other 2 had FVC 100%. |
Aerobic exercise on ergometer cycling of 30 min. Muscular endurance exercise of shoulder and hip flexors for 30–50 min 3x/wk for 8 wks. |
6MWT, no significant change. | Muscle function: (Functional Index 2, shoulder flexion and hip flexion) improved significantly in 3 patients. No significant changes, but trend of reduced fatigue in 3 patients. |
No exercise-related adverse events. |
| Mitropoulos et al. (2018) [100] | LcSSc, n=34. No lung involvement nor myositis or NYHA class 3–4. Dropouts: 1 patient in each group. |
Aerobic exercise with ACE, n = 10 or CE n = 10. ACE = 2 days/wk for 12 wks of 30 min sessions of30 s HIIT followed with 30 s passive recovery. CE = 2 days/wk for 12 w of 30 min with bouts of 30 s HIIT followed with 30 s passive recovery. CG, n = 11, and no exercise. |
VO2peak improved significantly in ACE and CE after intervention. | Life-satisfaction improved in both ACE and CE. Discomfort and Raynaud’s pain decreased in both ACE and CE as well as improved life satisfaction. |
No exercise-related adverse events. |
| Mitropoulos et al. (2019) [294] | LcSSc, n = 32. No PAH or ILD or myositis or NYHA class 3 or 4. |
Aerobic Exercise (n = 16), ACE = 2 days/w for 12 wks of 30 min sessions of 30 sec HIIT followed with 30 sec passive recovery. Resistance training, 5 upper body exercises in 10 reps x 3 sets. CG, n = 16, and no exercise. |
VO2peak improved statistically in ACE compared with CG. | Improved endothelia-dependent reactivity in ACE as well as improved transcutaneous oxygen pressure in finger. | No adverse events reported. |
| Filippetti et al. (2019) [103] | SSc, n = 44, and 22 each in both IG and CG No PH, VC ≤50%, and DLCO ≤30% or NYHA class 3 or 4. Dropouts in IG n = 6 and CG n = 5. |
A 6 months, 3 days/wk, minimally supervised home rehabilitation program in IG, no exercise in CG. Aerobic Exercise, CG, stationary bike at 60% intensity for 15 min, 3 min rest, and 15 min bike. Muscular endurance exercise for upper limbs with load of 60% of 1RM. Stretching for hands. |
6 MWD improved statistically in IG compared with CG. | Improved q-ceps, biceps, and grip strength as well as improved physical score in SF-36 in IG when compared with CG. | 3 patients in IG dropped-out due to pain in joints and other parts. |
| Oro-Facial Exercises | |||||
| Pizzo et al. (2003) [295] | 10 SSc with oral aperture <30 mm. | Mouth stretching and oral augmentation exercises for 15 min twice a day for 18 wks. | Mouth opening significantly increased; subjective improvements in eating, speaking, and ability to perform oral hygiene. | N/A. | Mild muscle fatigue in cheeks. |
| Poole et al. (2010) [296] | 17/21 SSc (9 dcSSc and 8 lcSSc) completed all visits. | Mouth exercises and oral augmentation exercises in combination with education on brushing and flossing teeth and adapted dental appliances. | Dental hygiene improved significantly for decreased bleeding, supragingival calculus, and increases in caries. No significant improvement in mouth opening. | N/A. | None reported. |
| Yuen et al. (2011, 2012) [297,298] | IG: 26(13 dcSSc and 13 lcSSc). CG: 22 (8 dcSSc and 14 lcSSc). |
IG: powered toothbrush and flosser plus mouth stretching and oral augmentation exercises held for 15–20 s, 3 times each, 2 times/day for 6 months if oral aperture <40 mm CG: manual toothbrush and dental floss 2 times/ day for 6 months. | Oral aperture significantly increased as compared to controls at 3 mo but not at 6 mo. Gingival inflammation was significantly reduced in both groups. IG showed a significantly larger reduction in inflammation than CG. |
N/A. | None reported. |
| Hand Exercises | |||||
| Mugii et al. (2006; 2019) [299,300] | 45 SSc (32 dcSSc and 13 lcSSc). 2 dropouts from yr 1 to yr 9. |
Stretching exercises for joints of the hand. Position held 10 s with 3–10 repetitions. | TPM improved at 1 month postintervention, which improved or was maintained at 1 yr. No change in HAQ but individual item score improved. |
N/A. | None reported. |
| At 3-yr follow-up, TPM improved and was maintained or improved at 9 yrs after the first visit. No change in HAQ at 9 yrs except in patients who also had decreased TPM related to worse skin scores. | |||||
| Piga et al. (2014) [301] | IG: 10 SSc (2 dcSSc and 8 lcSSc). CG: 10 SSc (2 dcSSc and 8 lcSSc). No irreversible anatomical damage, active arthritis, or digital ulcers. 2 dropouts in CG. |
IG: 5 mobility and 3 strengthening exercises for the hand to be done at home 5 d/wk for 12 wks monitored remotely for number of repetitions, force, speed, and correctness. | No group differences. Both groups showed improvements in hand function; HAQ and HAMIS for the R hand improved significantly only in IG. | No group differences. Pinch strength and MCP ROM for R hand increased significantly in both groups; no increases in pain, global health, or SF-36. |
None reported. |
| CG: Home program of 3 mobility and 3 strengthening hand exercises to be done 5 d/wk for 12 wks. | |||||
| Stefanantoni et al. (2016) [158] | 15 IG (7 dcSSc and 8 lcSSc). 16 CG (5 dcSSc and 10 lcSSc). No active synovitis, DU. |
Hand exercises tailored to participants’ goals; met at 1 mo and 3 mo with weekly phone calls between sessions. CG: general instruction to do exercises 1x/day. | IG had significant improvements with perceived satisfaction and performance of daily tasks (COPM), HAQ and SF-36 mental health at 3 months. Significant differences between IG and CG at 3 months was perceived performance on daily tasks. | N/A. | None reported. |
| Landim et al. (2017; 2020) [184,186] | IG: 40 (22 lcSSc and 18 dcSSc). CG: 17 (11 lcSSc and 6 dcSSc). No previous hand rehab, hand disease not due to SSc, or unable to perform exercises. |
IG: Home hand exercise program consisting of booklet and video disc; revaluated at 4,8, and 24 wks. CG: usual care; revaluated at 24 wks. | VAS-pain, CHFS, ΔFTP, grip, and tip and pinch strength increased significantly in IG but not in the CG. | SHAQ and SF-36 improved in IG but not in CG. | None. |
ACE, arm crank ergometer; CE, cycle ergometer; CG, control group; CHFT, Cochin Hand Function Test; COPM, Canadian Occupational Performance Measure; CT, computerized tomography; dcSSc, diffuse cutaneous SSc; DLCO, diffusing capacity for carbon monoxide; DU, digital ulcers; ΔFTP change finger to palm; FVC: forced vital capacity; HAMIS, hand mobility in scleroderma; HAQ, health assessment questionnaire; HIIT; high-intensity interval training; IG, intervention group; ILD: interstitial lung disease; lcSSc, limited cutaneous SSc; MCP ROM: metacarpophalangeal range of motion; N/A, not applicable; NYHA: New York heart association (class 1e4); 1RM, one repetition maximum; PAH: pulmonary arterial hypertension; PASP: pulmonary artery systolic pressure; PH: pulmonary hypertension; R hand, right hand; SF-36: Medical Outcomes Survey Short Form; SHAQ, Scleroderma HAQ; 6MWT: Six-minute walk test; TPM, total passive range of motion; VAS: visual analogue scale; VC: vital capacity; q-ceps: quadriceps muscle; and VO2peak: peak oxygen uptake.
Along these lines, patients with underlying cardiopulmonary conditions, may be at a higher risk of dysfunctional breathing patterns such as hyperventilating or breath-holding, which contribute toward additional neurophysiological mechanisms related to breathlessness sensations [126]. Breathing pattern disorders can be rehabituated to healthier breathing patterns with practiced breath regulation that is strengthened by exercise.
Gastrointestinal Tract in SSc: is the most common internal organ involved in SSc, which affects >90% of patients with SSc. SSc can impair function from mouth to anus, hypothetically by the same vicious cycle of inflammation, vascular insufficiency and leak, immune dysfunction, and disrepair [127] as other organ systems. Myomucosal fibrotic infiltration and neuronal rarefaction results in damage, which include salivary glandular dysfunction, pan- or partial-GI dysmotility, and loss of GI sphincter muscle tone and dysmotility. Symptoms from these pathological processes include difficulty with ingesting food and mastication, acid reflux predisposing to esophagitis, dysphagia, esophageal stricture and malignancy, bloating, cramping, early satiety, nausea, emesis, regurgitation, constipation, bacterial overgrowth, diarrhea, fecal leakage or frank incontinence, and malnutrition. Symptoms should be managed early and aggressively [4,128,129]. GI symptoms severely diminish HRQoL greatly interfering with life participation [130] and potentially resulting in depression [131,132] and self-imposed isolation.
Though not expressly intuitive, exercise impacts GI function and symptoms via multimodal mechanisms [133–137]. Mouth exercise and physical activity correlate with improved salivation, oral health, and function [133,137]. Even minor physical activity such as walking stimulates digestion, reduces nausea, and promotes motility. Physical activity is linked to decreasing digestive system cancers, decreasing proinflammatory gut microbiota, and gut restoration of health-promoting microbiota resulting also in favorable effects on cognition and mental health [19,138–141]. Microbiome restoration could be a key influencer of lower GI health [20] as dysbiosis appears to be a feature of the SSc disease state [142–144].
In exercise, the large diaphragmatic muscle draws downward for chest expansion and lung aeration, exerting rhythmic mechanical massaging forces on the abdominal cavity contents; also creating pronounced intra-abdominal pressure differentials stimulating neuronal networks and parasympathetic action. These actions assist in GI motility and function and in reducing symptoms e.g., nausea, bloating, and constipation. Singing, chanting, martial arts, and yogic breathwork emphasize these actions; but all exercise potentially encourages synchronized breathwork that benefit GI health.
Online home-based exercise platforms are increasingly available, particularly for patients uncomfortable travelling due to diarrhea or fecal soilage.
Face Involvement in SSc: can change in appearance from the tightening and fibrous transformation of facial skin, mask-like (mauskopf) appearance, and perioral wrinkling with retracting lip-thinning and hollowed appearance of the cheeks. The changes can have devastating effects on body image, self-esteem, and well-being [145,146]. A mix of manual techniques and home exercise had a positive effect on mouth opening as compared to only home exercise [147]. Though the impact of facial exercises on self-esteem has not been directly examined, exercise leads to increased self-esteem and feelings of well-being [77,100,148]. Application of sauna techniques to optimize exercise is yet unstudied. In addition to optimizing breath, diaphragmatic strength, and GI dynamics, singing may have similar impact on oral/facial musculature, salivation, and circulation [133,137].
Mouth Involvement in SSc: can impair mouth opening, often noticed during dental examinations, oral care, eating and chewing [149]. The fibrous transformation of the facial skin, muscles, lips, and palatal structures with teeth shifting can lead to multiple oral problems such as reduced oral aperture, decreased salivary production, and increase in dental caries, pain with chewing, and tooth loss. Oral care requires heightened attention from diagnosis throughout the disease course. Oral stretching along with facial exercises [147] and massage have been shown to increase mouth opening with possible other structural, vascular, and glandular benefits and sometimes lead to better oral health [150,151].
Skin in SSc: portends diffuse functional impairment to the body beyond anatomical restriction. SSc disrupts the skin’s superficial and deep architecture of sweat and sebaceous glands, nerves, and blood vessels with biochemical, hormonal, glandular, neurological, immune, circulatory and thermoregulatory, and wound-healing dysfunction [152].
Exercise and manual manipulation increases blood flow to the skin, which provides nourishment, oxygenation, toxin removal, and warmth; muscle activity stimulates mitochondrial function in the skin essential for wound healing [8,153,154]. Exercise hastens lymphatic drainage of edema [147], important in early diffuse disease. Exercise increases circulation and sweating in those able to still sweat. Sweat facilitates toxin and inflammatory cytokine release through the skin.
Hands in SSc: are particularly subject to diffuse morphological changes, impairment, and pain due to inflammatory assault, vascular insufficiency and injury, and fibrous infiltration and damage. These pathological processes may result in infection, ulceration, calcinosis, acro-osteolysis, flexion contractures, carpal tunnel syndrome, cold sensitivity and RP, synovitis, tendinopathy, and amputation [155].
Hand and wrist impairment impacts remunerative and household work, self-care, nutrition, and the handling of exercise equipment. DUs and calcinosis are described as not only painful but also socially stigmatizing during exercise due to visible lesions or the need for bandages and/or gloves for hand protection [77]. Certified hand therapists/OTs can address hand strength, mobility, and contractures; and provide tools for improvement in self-care and work capacity.
The role of hand exercises in SSc cannot be overstated. The hands are often involved early and rapidly lose range of motion (ROM) and strength. Encouraging home-based “ritualised” practice has multiple benefits [156–158]. Exercise supports circulation [100], healthy vasculature, skin repair, and warmth - important factors in RP, DUs, and calcinosis. Exercise increases hand/wrist muscle strength and efficiency, adding to the already intrinsic benefits on local inflammation, stiffness, and joint lubrication [150,151]. Preventive strategies to maintain hand warmth and adjuvant, preparatory strategies described below, such as paraffin, sauna, and water-based exercise, optimize tolerability and outcomes [156,159]. Exercise gloves may improve handgrip when performing muscle strengthening exercises, particularly when handling/gripping cold metal. Grip and hand placement techniques can also be modified to enable the performance of particular exercises [77]. Assessment of hand/arm function at baseline and intervals (Table 9) can provide direction and encouragement [160–175].
Table 9.
Clinically feasible assessments in patients with SSc.
| Quality to Assess | Test | Description | Comments |
|---|---|---|---|
| Range of motion | Functional Shoulder Assessment (FSA) [302] | Assessment of 6 functional movements of the upper extremity. | |
| Muscle endurance | Functional Index 2 (FI-2) [213] | Dynamic assessment of quantity of repetitions before exertion in 7 muscle groups. | 2FI-2 is feasible, good to excellent inter- and intrarater reliability in SSc (unpublished data, Pettersson). |
| FI-3 [303] revised shorter version of FI-2, feasible for clinic visit physical exam | As above, examining 1 to 3 muscle groups. | Especially hip, shoulder, or neck flexion. | |
| Muscle strength | Timed-Stands Test TST [78,215], | Time required to rise from chair 10 times. | Only valid if patient can do all 10 reps. |
| 30-s Chair-Stands Test CST [304], | Number of full rises from chair during 30 s. | Particularly for patients unable to complete TST. | |
| Aerobic capacity | Treadmill, Ebbelings test [51] | Submaximal aerobic test, walks for 4 min at a selfselected pace followed by 4 min, at the same pace, but with a 5% elevation. | More suitable for older/ more lung-impaired patients. |
| Cycle ergometer test, Astrand test [305] | Submaximal aerobic test, 6 min ergometer biking. | ||
| Functional capacity | 6MWT, 6-min walk test [306] | Walking distance on flat ground for 6 min. | Functional capacity test for patients with cardiopulmonary problems. |
| Hand grip force | Grippit [173] | An electronic handheld dynamometer to measure grip strength (finger flexion force). | |
| Jamar Dynamometer [168] | An hydraulic handheld dynamometer to measure grip strength (finger flexion force). | ||
| Upper Extremity Activity limitation | Disabilities of the Arm, Shoulder, and Hand (DASH) [169] | Assesses the disability of the upper extremity and can monitor change in symptoms and upper limb function over time. | |
| Patient Specific Functional Scale (PSFS) [166] | Patients identify up to five important activities they are having difficulty with as a result of their disease. | ||
| Scleroderma Health Assessment Questionnaire SHAQ [160,164,171] | Self-reported questionnaire assessing disability and function, adopted for SSc with VASs for pain, DU, RP, breathing, and GI problems | ||
| Cochin Hand Function Test (CHFT) [161,171,172] (aka Duruoz Hand Index) Abilhand [171,172,174] | 18-item questionnaire measuring manual ability of daily activities. 26-item questionnaire assessing level of difficulty of upper extremity tasks. |
||
| Hand function and motion | Hand Mobility in Scleroderma (HAMIS) [170] | 9 items designed to measure all movements assessed in an ordinary ROM-measured hand test. | |
| Hand Anatomic Index (HAI) [162,163] | Measurement of open hand span minus closed hand span/lateral height of hand. | ||
| Hand Function Index (HFI) [175] | 9 items of the Keital Functional Test that assess global hand and wrist mobility. | Requires less than 1 min. | |
| Mouth Function | Mouth Handicap in Systemic Sclerosis Scale (MHISS) [149] | 12-item scale representing impairment related to mouth opening, sicca, and esthetic concerns. |
Abbreviations: DUs, digital ulcers; GI, gastrointestinal; ROM, range of motion; RP, Raynaud's phenomenon; Sicca, oral dryness; and VAS: visual analog scale.
Feet in SSc: often receive little attention although patients experience significant SSc-related challenges, including RP, ischemic injury, contractures, plantar rigidity, fat pad atrophy, and pain with impact on gait ability and pattern [77], comfort, balance, and other domains of mobility [176,177]. Furthermore, compromised strength of the lower extremity musculature and decreased ankle motion may create higher risk for falls [178]. Pedal soft tissue damage/loss renders standing and exercising painful for some patients, making good footwear or orthotic insoles an important consideration.
Developing strong supple, responsive feet and ankles through exercise and physical activity increases pedal circulation and improves performance efficiency of body mobility (rising, stairs, and walking) and balance. These are all important factors for people already limited by cardiopulmonary impairment, but also for anyone who negotiates rough or unexpected terrain [77].
Joint Involvement in SSc: is diverse both in distribution that involves large or small joints and in mechanism with intrinsic musculoskeletal pathology (inflammatory arthritis, tendonitis, etc) and overt changes (ulceration, calcinosis, inflammation, and fibrosis in overlying skin and fascial layers). Difficulty managing ADLs makes hand, finger, wrists, and elbow joints impairment more readily apparent, but the less overt lower extremity joint involvement of feet, hips, knees, and ankles interfere with mobility and balance [178–180]. Furthermore, downward cycle of inactivity gradually compounds intra- and peri-articular adhesion of fibrous tissue that exacerbates joint stiffness and impairment.
Exercise can impede the impairment trajectory [181]. Joint activity instigates blood circulation that supports tissue health and repair. Myogenic activity decreases local and systemic inflammation. Motion mitigates tissue adhesion, relieves stiffness, and malalignment through lubricating and strengthening of periarticular, articular, and bony structures while promoting repair and regeneration of cartilage [181]. Repetitive exercise strengthens muscle, ligaments, tendons, and their insertion into the bone [182].
Counselling patients on ROM early in the disease course may preserve function, particularly in dcSSc [183–186]. Warm paraffin hand immersion prior to hand exercise sessions may significantly improve active ROM, reduce stiffness and hand dryness [187] as well as increase activity performance and participation [188–190] but these results are not reproduced consistently [189,190]. Sauna, often available at pool facilities and gyms, is reported to improve inner core temperature, decrease RP, and facilitate stretching before/during/after exercise, including aquatic exercise.
Bone Involvement in SSc: results from inflammation, vascular complications e.g., calcinosis/osteolysis as well as decreased physical activity, circulation, muscle mass, and nutrition leading to low bone density, fracture, and avascular necrosis [191–197]. Physical activity invokes muscle contraction and circulatory mechanisms setting off multiple pathways of benefits for strength and vascularization of the underlying bone as well as systemic skeletal structure [198–202].
Muscle Involvement in SSc: is under-recognized, but commonly found on physical exam and multifactorial with histological examination ranging from microangiopathy, inflammation, fibrosis, necrosis, or atrophy [203–205]. Muscle weakness and reduced muscle endurance are hallmark features of most myositis subsets [206] and reported in all serological subsets of SSc [207–210].
Proximal muscle weakness limits physical function in up to 20% of patients with SSc [78,205,211]. Muscle endurance is markedly reduced in both shoulder (53% expected) and hip flexion (40% expected) as measured by Functional Index-2 (FI-2) [212,213], proximating polymyositis and dermatomyositis impairment [214]. Regardless of subtype or degree of lung involvement, lower extremity functional muscle strength in SSc is significantly worse than standard values [212,215], while muscle endurance is lower in moderate to severe as compared to no or mild lung involvement [83,215]. Muscle involvement is associated with and possibly predicts cardiac abnormalities [216–224]. Upon exclusion of medication culprits, (e.g., statins, steroids, and hydroxychloroquine) appropriate, timely management of myopathy includes exercise and rehabilitation to improve long-term disability and function outcomes [225,226].
Health-related quality of life in SSc and the role of exercise
HRQoL is the interface of combined symptom distress and impairment with real-life. Numerous studies demonstrate, independent of traditional disease severity markers, the inverse correlation of HRQoL and survival and survival improvement with HRQoL-targeted interventions [4,52,128,227–235]. Whether SSc-specific manifestations or less well-defined symptoms such as fatigue, pain, psychic/cognitive discomfort, or sexual dysfunction [236], are the prominent aspects of symptom distress, targeted intervention may markedly improve HRQoL [103]. The information given below address less SSc-specific, but no less important, areas of symptom distress.
Pain in SSc: limits physical capacity in 39% of patients [78]. It is often multifactorial, diffuse, and unfortunately defaults to an inaccurate diagnosis of “fibromyalgia” [4,237]. RP, joint, and/or muscle pain, pruritus, skin tightening and subcutaneous pressure, calcinosis, and ulceration are common causes of SSc pain [4,238]. Though, empiric exercise impedes mechanisms driving these discomforting manifestations through multiple pathways [4,181], distinguishing causes of pain and tailoring interventions accordingly may hasten function [4,181]. Analgesia can improve engagement in those with disabling pre-/post-exercise pain [181].
Fatigue in SSc: may be multidimensional with mental/cognitive, motivational, physical, and muscular domains [239,240]. Although a nonspecific symptom often related to hypothalamic effects of systemic inflammation, fatigue in SSc often has other factors that include deconditioning, pain, and inflammation. Serious SSc complications associated with fatigue such as GI bleeding or undiagnosed PH or ILD or non-SSc comorbidities, such as clinically significant CVD, must be addressed medically prior to beginning an exercise program. Malnutrition and poor sleep quality cause fatigue warranting corrective intervention to optimize health and benefits of exercise [241].
Exercise can increase endurance improving fatigue associated with dyspnea and cough and ameliorate their psychological burdens of depression and anxiety [63]. Recalcitrant persistent fatigue, as well as sleep, physical function, and self-perceived general health, improves with exercise in other conditions [242].
Anxiety and Depression in SSc: have psychological and emotional consequences for individuals and their families. SSc requires continued adaptation to changing physiological burden and symptom severity, e.g., fatigue, pain, respiratory, as well as socioeconomic, self-image, and survival uncertainties resulting in elements of psychological distress. Adaptive psychological distress can give way to clinically significant anxiety and depression alongside impaired cognition and motivation.
“Watchful waiting” may be an acceptable strategy for mild depression [243] whereby symptoms are anticipated as transient. However, psychological symptoms can interfere with treatment adherence and key self-management strategies that optimize health and prevent complications. Non-pharmacological approaches, such as exercise, may require adjuvant anti-depressant medications even temporarily while patients adjust.
In SLE, inactivity is associated with tripled incidence of depression [70]. Exercise has been repeatedly demonstrated to improve cognition, depression, anxiety, self-image, work performance, and coping. Even a simple change in body positions and postures profoundly and swiftly shifts mood/affect [102,244–248]. Exercise significantly improves sleep quality that is foundational to cognitive and psychological health. Evidenced mechanisms through which exercise affects mental conditioning are numerous and continually growing including microbiome-gut-brain axis [19,139,249,250] enrichment, facilitating brain’s discarding of depressive chemicals [251,252] and upregulating antidepressant chemicals [181]. The impact of yoga, singing [253], and gentle exercise positively impacts both physical and psychological outcomes [30,35,111–116,254].
Initiation, engagement, and exercise types
Documenting exercise/physical activity routinely along with medication history, during clinic visits provides opportunities for continued encouragement, education, and review of patient goals and priorities in relation to SSc care (Fig. 4).
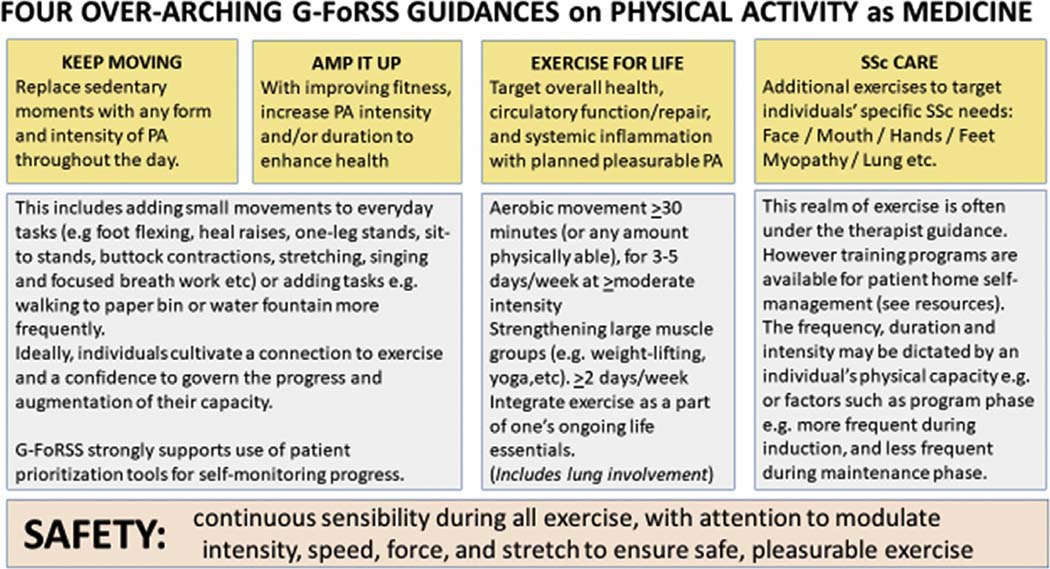
Fig. 4.
G-FoRSS preliminary recommendations for physical activity and exercise in SSc. Based on evidence collated from the WHO, SSc disease mechanisms and health promoting mechanisms of exercise (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
Exercise interventions are most effective when guided by patient goals and their life priorities [90]. Tools such as the Patient-Specific Function Scale PSFS [165,166] support patients to monitor their own progress. Preventive exercise strategies initiated early in the disease course, ideally supported by Physical, Occupational, and Respiratory therapist education on the consistency of practice, stretching safety, and incremental progress, may preserve physical function and HRQoL [77] by offsetting progressive loss of flexibility and mobility. Screening for SSc-cardiopulmonary involvement is essential.
SSc presents additional physical and psychological taxes on motivation [16,255,256] for exercise. Early on this may include diagnosis-related trauma [15–18], which exercise may relieve. Both supervised and self-managed exercise initiation strategies require individualization and flexibility according to levels of fatigue, pain, muscle, skin, joint, and vital organ involvement. Anticipatory guidance helps patients manage fears, frustrations, and disappointments, particularly regarding fluctuating fatigue and pain, while sustaining commitment to exercise. Possible supports include apps/videos or exercise buddies [77]. RP may deter outdoor exercise in cold temperatures [77] and even indoor exercise that involves gripping equipment; but is potentially mitigated with exercise glove use.
Integrating mindfulness techniques and nurturing perceptions of exercise as a friendly, pleasurable experience, may lessen immobilizing tendencies such as perceived lack of control, self-consciousness, and defeatism [15–18]. Noticing pleasurable exercise sensations (e.g., air on skin, massage sensation, etc) may help cultivate parasympathetic (polyvagal) self-regulation, holistic “core” muscle recruitment, and integration resulting in improved musculoskeletal performance, balance, joint and postural alignment, and more rapid escalation of exercise dose (frequency, intensity, time duration, and type (FITT)) [30,257].
Exercise dose begins and escalates gently with a goal of 30 min [49,258] 3–5 days a week. Fluctuations in disease behavior may require dose adjustment under therapist supervision. Tables 6, 7a and 7b define exercise approaches, including ROM, muscle strength, muscle endurance, aerobic capacity, and functional capacity. Tables 8, 9 summarize, respectively, cumulative SSc exercise studies and preferential baseline and follow-up assessments [259].
Incremental increases in nonexercise physical activity, e.g., standing, walking, and as much body movement throughout the day enhances health benefits, physical function, and HRQoL [48,49]. But while patients with SSc may dedicate time to exercise for health improvement; nonexercise physical activity throughout the day may be impeded by fatigue, pain, GI symptoms, or disability prolonging time to complete a routine activity.
Stretching: is a naturally occurring phenomenon that regulates muscle fiber length and excess force for optimal muscle tone and protection of periarticular structures (e.g., tendon, muscle, and ligaments). Stretching is a pivotal conditioning mechanism for ROM, balance, global and limb proprioception, and movement efficiency [260,261]. Stretching elicits vasodilatation intensifying blood flow to muscle, increasing oxygenation, warmth and tissue waste removal, and relieving stiffness. Habitual stretching induces proangiogenic factors increasing neo-angiogenesis and capillary density [262], potentially impacting multiple SSc therapeutic avenues. Effective stretching occurs when muscle fiber exceeds its optimal length and/or the magnitude of stretch is guided by ROM and pain limitations Implementation is gentle, and held for 30–60 seconds synchronized with breath cycles for sufficient relaxation [50]. Long-term benefits of therapeutic hand stretching with marked improvement in hand, arm, and overall function in SSc [156] warrant further investigation in diverse SSc manifestations.
Water-based: Water’s properties of buoyancy, depth-graded hydrostatic pressure, viscosity, surface tension, hydromechanics, thermodynamics, and density [263–270] allow for uniquely efficient rehabilitation strategies (Table 11). Growing high-level evidence of aquatic exercise in autoimmune and other conditions demonstrates safety without neuromusculoskeletal exacerbations or other adverse effects. Additionally, demonstrated are efficacy in diminishing stiffness, pain, muscle spasm, fatigue, and improving cardiovascular endurance as well as physical function, including ROM, balance, and walking [183,263,264,266–269,271–278]. Despite strong rationale only one study [183] as yetinvestigated aquatic exercise in SSc, which demonstrated significantly improved HRQoL, physical function, and activity [183].
Table 11.
Preliminary research agenda for the investigation of exercise inSSc as advocated by The Global Fellowship on Rehabilitation and Exercise in Systemic Sclerosis (G-FoRSS)
| QUALITATIVE INVESTIGATIONS |
Patient experiences of exercises prior to and concurrent with SSc diagnosis: Personal feelings before SSc diagnosis |
| Activity profile | |
| Observation of sweating in SSc | |
| Type of exercises | |
| Pleasure | |
| Preferential time of day and frequency | |
| Exercise adherence to sustain benefit over time | |
| Home-based versus hospital-based settings for exercise | |
| Patient perceived impact of exercise on: | |
| SSc Manifestation Domains: | |
| Raynaud, circulation, and sustaining core warmth | |
| Wound healing | |
| Calcinosis | |
| Lung symptoms | |
| Gastrointestinal: SICCA, bloating, and constipation | |
| Muscle function | |
| Articular function | |
| Sexual function, e.g., erectile dysfunction improvement after aerobic exercise | |
| Symptom Domains: | |
| Dyspnea | |
| Cough | |
| Pain | |
| Fatigue | |
| Sleep | |
| HRQoL Domains: | |
| Body Image and Self-esteem | |
| Well-being and vitality | |
| Depression/Anxiety | |
| Meaningful activities | |
| Worker productivity/performance | |
| Body perception | |
| Patient perceptions of engaging in exercise: | |
| Fears and worries | |
| Hopes, goals, and benefits | |
| Benefits of individual or group PT led “SSc School on Exercise" | |
| Home-based versus hospital-based settings for exercise | |
| Goals are to fulfil anticipated benefits and provide knowledge to address concerns | |
| Patient-perceived barriers/hindrances to initiating and to sustaining exercise practice: | |
| Logistical management (oxygen, changing clothes/shoes, physical, and work) | |
| Time restraints (work and family) | |
| Time management/prioritizations | |
| Climate | |
| Access to pleasurable self-directed exercise | |
| Access to home or hospital-based exercise | |
| Modality of delivery: | |
| Digital/Print | |
| Audio/Visual Recording | |
| Community/collective learning | |
| Community/collective exercise | |
| Combined approaches | |
| Medication side effects | |
| Presence of pain | |
| QUANTITATIVE INVESTIGATIONS | Characterizing FITT in both aerobic and resistance exercise to target circulatory, antiinflammatory, and respiratory effects. Best submaximal exercise testing method in SSc – with special focus on different degrees of lung involvement |
| Priority systemic exercise types (e.g., resistance, aerobic, etc.) in SSc | |
| As a general approach to SSc | |
| With and without lung/heart involvement: | |
| - In severe pulmonary disease | |
| - Across varying degrees of lung involvement? | |
| - In combined PH and ILD | |
| - Respiratory training – inspiratory muscle training and/or positive expiratory pressure | |
| With further definition in land- and water-based applications | |
| The use of minimally invasive muscle biopsies to evaluate response to exercise | |
| Home-based versus hospital-based settings for exercise | |
| Effects of exercise on: | |
| Raynaud phenomenon | |
| Sexual function | |
| Circulatory and hemodynamic effects | |
| Worker productivity/performance | |
| Serum and histological biomarkers: | |
| Muscle tissue mRNA expression of inflammatory and fibrotic pathways | |
| Inflammatory serum biomarkers | |
| Angiogenesis | |
| NT-Pro-BNP and Uric acid | |
| SSc Manifestation Domains: | |
| Raynaud, circulation, and sustaining core warmth | |
| Wound-healing | |
| Calcinosis | |
| Skin tightening | |
| Gastrointestinal: sicca (oral dryness), bloating, and constipation | |
| Muscle function | |
| Articular function: strength and AROM/PROM | |
| Symptom Domains: | |
| Dyspnea | |
| Cough | |
| Pain | |
| Fatigue | |
| Sleep | |
| HRQoL Domains: | |
| Body Image and Self-esteem | |
| Well-being and vitality | |
| Depression/Anxiety | |
| Meaningful activities and participation | |
| Self-perception of general health | |
| Effects of stretching/AROM/PROM (as above) | |
| HANDS: | Optimal treatment protocols |
| Comparative efficacy of different delivery methods | |
| Hand function and joint motion | |
| Optimal time in disease progression to emphasize hand exercises | |
| Impact of hand exercise on: | |
| Raynaud | |
| Digital ulcers/Wound healing/Calcinosis/Infection rate | |
| Contracture development/improvement | |
| Skin tightening | |
| Manual dexterity | |
| Worker productivity/performance | |
| Self-esteem | |
| OROFACIAL: | Impact of orofacial exercises on: |
| Prevention or delay of facial changes in people with SSc | |
| Dental/oral hygiene | |
| Dental/palatal structure changes | |
| Oral aperture: diameter and mobility | |
| Changes in lip thickness | |
| Nutritional intake/status | |
| Salivary production | |
| Well-being/self-esteem | |
| Progression of telangiectases | |
| Sauna as adjuvant to exercise | |
| HEALTH ECONOMIC PERSPECTIVE | Cost-efficiency of supervised or educational interventions for patients Cost return on PT/OT/RT supervised structured exercise retreats in warmer climates to develop exercise safety and efficacy knowledge, intensify patient interest, and empowerment, and explore variance of self-directed exercise Patient costs related to supervised or patient-directed exercise interventions Hospital visits and need of medical treatment |
| SUSTAINABILITY | Patient general knowledge regarding exercise programs and continuance |
| Patient-reported experiences of successful maintenance prior to and concurrent with SSc diagnosis | |
| Patient perceptions of potential external strategies to sustain exercise | |
| Patient perceptions of self-regulating strategies to sustain exercise | |
| SAFETY | Screening for safety |
| Screening for individually tailored safety modifications – baseline assessment – to tailor exercise and safety measures to the individual | |
| Range of safe parameters in: | |
| - non-cardiopulmonary involvement | |
| - ILD, PH or combined ILD/PH or heart failure | |
| - patients with other vital organ involvement | |
| Self-monitoring devices e.g., oximetry | |
| - Guidance on efficient use | |
| - Distress related to use | |
| PROGRAMMATIC EXERCISE DESIGN | Intensity |
| Session duration | |
| Frequency | |
| Escalation protocols | |
| Safety assessments | |
| Equipment | |
| General vs. SSc Manifestation Targeted | |
| Transition to Patient-managed continuance | |
| INVESTIGATION OF EXERCISE TYPES and QUALITIES | Patient Preference/Aspirations |
| Patient Experience | |
| Types Deconstructed by Programmatic Components | |
| Resistance | |
| Aerobic | |
| Singing | |
| Water-based | |
| Yoga | |
| Balance | |
| Combined approaches with borrowed elements to maximize benefit | |
| PATIENT SELF-ASSESSMENT | Patient perceptions and patient experience of utility |
| Patient experience of self-governance | |
| Track own metrics – authentic supported self-management | |
| Patient as their own control | |
| Metrics that are personalized to patient context | |
| - Patient-specific activity profile | |
| - Mouth diameter card | |
| - Hand tracings for extension | |
| - Duration, Intensity, and Load of selected exercise | |
| - VAS for fatigue, pain, RP, and muscle tiredness and dyspnea | |
| - Patient-Specific Functional Scale (PSFS) | |
| - VAS for patient-selected goal activities | |
| TRIAL DESIGN | Relative importance of cohort assembly |
| SSc criteria confirmation | |
| Targeted manifestation (ILD, PH, DU, etc) | |
| Phenotype (e.g., sine, dcSSc, and lcSSc) | |
| Degree of lung involvement (e.g., No – Mild vs Moderate – End-stage) | |
| Acceptable blinding and randomization procedures Optimal study duration for efficacy | |
| Harmonized patient support/check-ins across trials | |
| Standardized adherence monitoring | |
| Safety assessment | |
| Procedures for selecting primary, co-primary, secondary, and exploratory endpoints | |
| Standardized components of warm-up, cool-down, and stretching of muscles/skin | |
| Qualitative collection of patient experience data related to intervention and trial | |
| Global consensus on harmonization of outcome measures | |
| Promote interstudy comparative potential | |
| Ensure COSMIN thresholds of reliable performance and patient-reported outcome measures | |
| - Identification of optimal existing outcome measures | |
| - New instrument development or modification where needed e.g., Activity Profile | |
| - Acceptability to patients | |
| - Defining interval outcome measure assessment | |
| - Define best practice of pulse oximetry during exercise | |
| MODALITY | How people learn |
| Hard copy of learning resources versus web-based resources | |
| What impacts preferred modalities of learning and participation – age, gender, etc. | |
| Inherent challenges in home-based / telehealth /virtual sessions: | |
| - measuring range of motion e.g. joints of hands | |
| - predicting in home safety e.g. tripping hazards | |
| Use of apps, pictures, and videos to collect outcome data. |
Abbreviations: AROM, active range of motion; dcSSc, diffuse cutaneous SSc; COSMIN, Consensus-based Standards for the selection of health Measurement Instruments; DU, digital ulcers; HRQoL, health-related quality of life; ILD, interstitial lung disease; lcSSc, limited cutaneous SSc; mRNA, messenger ribonucleic acid; NT-pro-BNP, N-terminal prohormone ofbrain natriuretic peptide; OT, occupational therapist; PH, pulmonary hypertension; PROM, passive range of motion; PT, physiotherapist; RT, respiratory therapist; and VAS, visual analog scale.
Though a more easy exercise medium [266], water deceptively requires greater muscle activity and provides an optimal environment for wide-ranging therapeutic interventions, including strength-training, cardiopulmonary rehabilitation, and for circulatory disorders [272,279]. Joint mobilization and stretching, balance, gait, resistance, strength [269] and endurance training, and weight-bearing activities are more easily modified in water to accommodate a spectrum of abilities. Patients explore movement strategies and patterns against gravity without anxiety or fear of falling.
Water temperatures between 30 °C and 34 °C/86 °F –93 °F preserves optimal body heat, exercise tolerability, and HRQoL effects [273–277]; cooler/cold water can induce RP and increase joint and muscle stiffness. Water bidirectionally conducts heat 25 times faster than air facilitating deeper, more rapid tissue penetration [263,264], but also more rapid body heat dissipation, thus enabling increased exercise duration and intensity with less fatigue [267,270]. These sublime factors can unwittingly lead to overexertion. Patient logistical concerns regarding oxygenation, fatigue, and mobility challenges with dressing, drying, and skincare are important considerations [280].
G-FoRSS preliminary research agenda
Despite significant evidence of exercise effects on inflammation and circulatory flow and repair, further work is needed to define exercise as a medicinal disease-modifying intervention with patient-centered strategies that support sustained patient engagement. We provide a roadmap to examining exercise in SSc (Table 11) with the hopes of building collaboration and to stimulate interest in the wider research community in the disease-modifying and HRQoL-preserving effects of exercise in SSc. These include examining, and ultimately, prescribing FITT in terms of quantifying exercise intensity, duration, and type to target circulatory, anti-inflammatory, and respiratory effects; along with how these effects translate to meaningful changes for patients in terms of physical and psychological function and HRQoL. The potential impact of both general and targeted exercise on skin tightening, RP, wound healing, and mobility is compelling for both the prevention and treatment of SSc complications.
With few therapeutics demonstrating improvement of SSc manifestations, exercise and physical activity demonstrate convincing mechanisms of action, with virtually no downside. Instead exercise consistently promises major and diverse benefits, and deserves robust research attention. Furthermore, engaging in exercise allows patients to foster some control and self-efficacy in managing a frightening disease.
G-FoRSS is committed to supporting research efforts in addition to the following:
Patient experiences of exercises prior to and concurrent with SSc diagnosis:
Patient perceived and quantitative impact of exercise on SSc manifestation, symptom, and HRQoL domains
Patient perceptions, fears, hopes, and worries related to engaging in exercise
Patient perceived barriers/hindrances to initiating and to sustaining exercise practice
Patient perception and quantified impact of early ROM intervention on face, mouth, hands, shoulders, and feet
Characterizing optimal FITT in both aerobic and resistance exercise on land and in water, in patients without/with cardiopulmonary involvement
The use of minimally invasive / sutureless muscle biopsies to evaluate response to exercise
A focus on optimal hand exercise regimens and implementations
A focus on the impact of facial exercises on oral health, salivation, and nutrition
A focus on the impact of exercise, including singing and diaphragmatic strength on GI symptoms and GI-related quality of life
Examining the impact of exercise interventions from a health economics perspective
Establishing clear safety parameters for exercise in relation to the presence and extent of cardiopulmonary involvement
Examining treatment effects of exercise on SSc-myopathy
Assessing patient-preferred education and engagement modes for initiation and sustained exercise
Summary
Exercise possesses essential health benefits for everyone and perfectly addresses the World Health Organization’s preamble to their constitution to ‘address health as a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’ [281]. The therapeutic underpinnings of exercise target the specific mechanisms behind the pervasive SSc-disease biophysical and psychosocial manifestations. Exercise engagement should be a routine treatment-based discussion with respect to patients’ goals and clinical assessment.
The expanse of exercise as a treatment intervention in SSc warrants dedicated investigation with standardized approaches. This group has convened to prioritize a research agenda for exercise in SSc and accelerate selected research through the development of a global network of interdisciplinary SSc specialists and patient research partners.
Table 2.
Summary of the American College of Sports Medicine exercise recommendations for general population and in rheumatic and musculoskeletal diseases [50,282] (Courtesy of LA Saketkoo on behalf of G-FoRSS, rights reserved).
| Exercise type | Exercise Goal | Intensity | Duration | Frequency |
|---|---|---|---|---|
| Aerobic exercises | Improve aerobic capacity, i.e., cardiopulmonary fitness | 55%–90% of HRmax | 20–90 min | 3–5 days/week |
| Resistance exercises | Improve muscle strength | 60%–85% of 1 RM | 8–12 repetitions, 2–4 sets. Exercises should induce fatigue but not exhaustion | 2–3 days/week |
| Improve muscle endurance | 30%–50% of 1RM | 15–25 repetitions with variable number of sets | 2–3 days/week | |
| Stretching | Improve range of motion and flexibility | Most effective when muscles/tissue is warm, e.g., after exercise and/or external heating (sauna, heat packs, or paraffin bath) | Approximately 10 min | ≥2–3 days/week |
Abbreviations: HRmax: maximum heart rate and RM: repetition maximum.
Table 3.
Overall benefits of exercise in general populations (Courtesy ofLA Saketkoo on behalfofG-FoRSS, rights reserved).
| SYSTEMIC | Brain Health |
| Cardiovascular health | |
| Cardiopulmonary health | |
| Circulatory function and repair | |
| Immune Function | |
| Decreased inflammation | |
| Malignancy prevention | |
| Bone density | |
| Bone circulation | |
| Increased muscle mass | |
| Skin health | |
| Gastrointestinal health | |
| Endocrine health | |
| Exocrine health including sweat and salivation | |
| Malignancy prevention | |
| GLOBAL FUNCTION | Activity capacity |
| Physical conditioning and strength | |
| Health-related Quality of Life | |
| Alertness | |
| Cognition/Reduced Risk of Cognitive Decline | |
| Mobility (walking, climbing, running, and cycling) | |
| Balance | |
| Improved self-care | |
| Workability | |
| Social Function | |
| Sexual health | |
| CONSTITUTIONAL | Decreased anxiety |
| Decreased depression | |
| Decreased pain | |
| Decreased fatigue | |
| Improved sleep | |
| LOCAL | Lung mechanics |
| Chest kinematics | |
| Muscle pathology | |
| Range of motion | |
| Articular strength | |
| Articular and Periarticular Lubrication | |
| Muscle strength | |
| Muscle mass | |
| Decreased inflammation | |
| Decreased stiffness |
Practice points.
Progress in exercise and physical activity should be documented on the history of present illness, with physical activity goals clearly stated
Discussions and counselling on physical activity and exercise are an essential component of SSc care and overall health
Highlighting the impact of exercise on improved circulation and decreased inflammation may support patient engagement in exercise
Explaining the benefits of exercise according to patients’ individual SSc symptoms may support patient engagement
Initiating face, mouth, hand, and possibly shoulder ROM exercises early may prevent disability and impact skin tightness as well as local circulation and inflammation
The need for referral to OT/PT/RT should be considered at each patient visit
Reminding patients at each visit of available online Scleroderma instructional resources e.g., The Scleroderma Foundation
Acknowledgments
G-FoRSS is an open interdisciplinary network of SSc experts including rehabilitation, physical, occupational, speech and respiratory therapists, basic scientists, patients, physicians and SSc organizations convened to accelerate the research and practice of exercise as a disease-modifying treatment in SSc. For more information please contact: lsaketk@tulane.edu.
Funding
AMR is a National Institute of Health Research, Senior Nurse Research Leader. The views expressed in this article are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Swedish Research Council (HA, HP), Swedish Rheumatism Association (HA, HP), Promobilia Foundation (HA) National Institute of Health Research UK (AMR), Pulmonary Fibrosis Trust UK (AMR), Irish Lung Fibrosis Association (ILFA) (AMR), National Institutes of Health: K24 AR060297 (CFB) and L30 HL129466 (MRL), National Heart, Lung, Blood Institute K23HL150237–02 (ERV); Charles and Elizabeth Wetmore Foundation of Greater New Orleans (LAS), and Sarcoidosis Awareness Foundation of Louisiana (SAFOL) (LAS).
In kind support of work related to this report: Scleroderma Foundation, Federation of European Scleroderma Associations (FESCA), Sarcoidosis UK, Scleroderma and Raynauds Society UK (SRUK), and Pulmonary Fibrosis Trust UK; British Lung Foundation Singing for Lung Health Programs, Accessible Yoga International (AY), Alvin Ailey American Dance Theatre, Anna Halprin Dance, Jose Limon Dance Foundation, Martha Graham Dance Company, Iyengar Yoga Center NYC, The Musical Breath UK, and Soundcastle UK.
APPENDIX
SUPPLEMENTAL FILES and RESOURCES:
Patient and Physician Education and Advocacy Organizations:
Scleroderma Foundation: www.scleroderma.org.
Federation of European Scleroderma Associations (FESCA): www.fesca-scleroderma.eu/wordpress/
Scleroderma Australia: https://www.sclerodermaaustralia.com.au/
Scleroderma & Raynaud’s UK (SRUK): https://www.sruk.co.uk/scleroderma/
Swedish National Association of Systemic sclerosis: https://rss.reumatiker.se/
Instructional Resources:
Janet Poole Hands/Face Instructional Links: https://www.youtube.com/watch?v=1F02FxdOgwI
https://www.youtube.com/watch?v=8MztM3zItik
https://www.youtube.com/watch?v=YwWP7mgcYhU.
Stretching exercises for the hand and face. The Scleroderma Foundation, http://www.scleroderma.org/site/DocServer/Form_16c_low_res.pdf?docID=19809&AddInterest=1281.
Taking Charge of Systemic Sclerosis (TOSS): an internet program for systemic sclerosis. https://www.selfmanagescleroderma.com/
Living Well: Heart, Lung, Muscle & Mind: A collection of videos dedicated to yoga rehab and dance rehab for heart, lung, muscle, and autoimmune conditions
https://www.youtube.com/channel/UCRgvkbyzep-Q3LGBiAksQZw/videos.
3–3-1 Exercise Tutorial https://www.youtube.com/watch?v=zsBRxmkzAnM&t=2s.
Move Towards Health: UMC CPHC Instructional Booklet on Safe Home-based Dance Practice https://doi.org/10.13140/RG.2.2.25576.49927.
Sleep Booklet: https://www.dropbox.com/s/0axd782mi818smc/SF%20Arizona%20Conference%20-%20SLEEP%20-%20DOUBLE%20Booklet.docx?dl=0.
Mindfulness Booklet: https://www.dropbox.com/s/mrpl33zxjsk20br/SF%20Arizona%20Conference%20-%20RESTORE%20YOURSELF-%20DOUBLE%20Booklet.docx?dl=0.
Mindfulness in Scleroderma Videos: https://www.youtube.com/watch?v=pNK9RP4Abyw.
Clinical Resources:
Patient Specific Functional Scale (PSFS) User Manual: https://www.physio-pedia.com/Patient_Specific_Functional_Scale.
Functional Index-2: https://www.youtube.com/watch?v=qw4XvWKQErU.
Manual Muscle Test 8 (MMT8): https://www.niehs.nih.gov/research/resources/assets/docs/mmt8_grading_and_testing_procedures_for_the_abbreviated_8_muscle_groups_508.pdf.
Timed Up and Go Test: https://youtu.be/auqAb_AWM1U.
TImed sit to stand test: https://www.youtube.com/watch?v=puJhQXUlbdA.
30-s Sit to Stand Test: https://www.youtube.com/watch?v=PzCTwkJVhWg.
Footnotes
Declaration of competing interest
None of the authors have conflicts to disclose in relation to this publication.
Audience: Rheumatologists and pulmonologists to sensitize to these concepts; therapists to use as broad guidelines/roadmap; patients for inspiration, guidance, and self-management; researchers to sensitize to questions that need investigation; and global collaborators interested in joining forces.
References
- [1].Hochberg MC, Silman AJ, Smolen JS, et al. Rheumatology. Mosby/Elsevier; 2011. [Google Scholar]
- [2].Barnett AJ, Miller MH, Littlejohn GO. A survival study of patients with scleroderma diagnosed over 30 years (1953–1983): the value of a simple cutaneous classification in the early stages of the disease. J Rheumatol 1988;15(2):276–83. [PubMed] [Google Scholar]
- [3].Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007;66(7):940–4. 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saketkoo LA. Wildflowers abundant in the garden of systemic sclerosis research, while hopeful exotics will one day bloom. Rheumatology 2018;57(3):410–3. 10.1093/rheumatology/kex420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sobanski V, Giovannelli J, Allanore Y, et al. Phenotypes determined by cluster Analysis and their survival in the prospective European scleroderma trials and research cohort of patients with systemic sclerosis. Arthritis Rheum 2019; 71(9):1553–70. 10.1002/art.40906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saketkoo LA, Escorpizo R, Keen KJ, et al. On behalf of EUSTAR. International Classification of Functioning, Disability and Health Core Set construction in systemic sclerosis and other rheumatic diseases: a EUSTAR initiative. Rheumatology 2012;51(12):2170–6. 10.1093/rheumatology/kes185. [DOI] [PubMed] [Google Scholar]
- [7].Nakayama A, Tunnicliffe DJ, Thakkar V, et al. Patients’ perspectives and experiences living with systemic sclerosis: a systematic review and thematic synthesis of qualitative studies. J Rheumatol 2016;43(7):1363–75. 10.3899/jrheum.151309. [DOI] [PubMed] [Google Scholar]
- [8].Belz D, Moinzadeh P, Riemekasten G, et al. Large variability of frequency and type of physical therapy in patients in the German network for systemic sclerosis. Arthritis Care Res 2020;72(8):1041–8. 10.1002/acr.23998. [DOI] [PubMed] [Google Scholar]
- [9].Pauling JD, Skeoch S, Paik JJ. The clinicoserological spectrum of inflammatory myopathy in the context of systemic sclerosis and systemic lupus erythematosus. Indian J Rheumatol 2020;15(6):S81–90. 10.4103/injr.injr_136_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mehdipoor M, Damirchi A, Razavi Tousi SMT, et al. Concurrent vitamin D supplementation and exercise training improve cardiac fibrosis via TGF-β/Smad signaling in myocardial infarction model of rats. J Physiol Biochem 2021. 10.1007/s13105-020-00778-6. Published online January 11. [DOI] [PubMed] [Google Scholar]
- [11].Lin Y-Y, Hong Y, Zhou M-C, et al. Exercise training attenuates cardiac inflammation and fibrosis in hypertensive ovariectomized rats. J Appl Physiol 2020;128(4):1033–43. 10.1152/japplphysiol.00844.2019(1985). [DOI] [PubMed] [Google Scholar]
- [12].Bay ML, Pedersen BK. Muscle-organ crosstalk: focus on immunometabolism. Front Physiol 2020;11. 10.3389/fphys.2020.567881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat Rev Rheumatol 2015;11(2):86–97. 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- [14].Sveaas SH, Smedslund G, Hagen KB, et al. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: a systematic review and meta-analysis. Br J Sports Med 2017;51(14):1065–72. 10.1136/bjsports-2016-097149. [DOI] [PubMed] [Google Scholar]
- [15].Koch SC, Riege RFF, Tisborn K, et al. Effects of dance movement therapy and dance on health-related psychological outcomes. A meta-analysis update. Front Psychol 2019;10. 10.3389/fpsyg.2019.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carel H Phenomenology of illness. Oxford University Press. https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780199669653.001.0001/acprof-9780199669653. [Accessed 19 February 2021]. [Google Scholar]
- [17].Gendron LM, Nyberg A, Saey D, et al. Active mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018; 2018(10). 10.1002/14651858.CD012290.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Halprin A. Returning to health: with dance, movement and imagery. LifeRhythm Books; 2002. [Google Scholar]
- [19].Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017;2017. 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Natalello G, Bosello SL, Paroni Sterbini F, et al. Gut microbiota analysis in systemic sclerosis according to disease characteristics and nutritional status. Clin Exp Rheumatol 2020;38(3):73–84. [PubMed] [Google Scholar]
- [21].Hackney ME, Earhart GM. Effects of dance on balance and gait in severe Parkinson disease: a case study. Disabil Rehabil 2010;32(8):679–84. 10.3109/09638280903247905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lapum JL, Bar RJ. Dance for individuals with dementia. J Psychosoc Nurs Ment Health Serv 2016;54(3):31–4. 10.3928/02793695-20160219-05. [DOI] [PubMed] [Google Scholar]
- [23].Salimpoor VN, Benovoy M, Larcher K, et al. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci 2011;14(2):257–62. 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- [24].Witek MAG, Clarke EF, Wallentin M, et al. Syncopation, body-movement and pleasure in groove music. PloS One 2014; 9(4). 10.1371/journal.pone.0094446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tarr B, Launay J, Dunbar RIM. Silent disco: dancing in synchrony leads to elevated pain thresholds and social closeness. Evol Hum Behav 2016;37(5):343–9. 10.1016/j.evolhumbehav.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Douka S, Zilidou VI, Lilou O, et al. Traditional dance improves the physical fitness and well-being of the elderly. Front Aging Neurosci 2019;11. 10.3389/fnagi.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li G, Zhu A, Huang Y, et al. The effect of traditional Tibetan guozhuang dance on vascular health in elderly individuals living at high altitudes. Am J Transl Res 2020;12(8):4550–60. [PMC free article] [PubMed] [Google Scholar]
- [28].Williams S Dance group enriches lives of people living with breathlessness. Lancet Res Med 2020;8(8):763–4. 10.1016/S2213-2600(20)30309-X. [DOI] [PubMed] [Google Scholar]
- [29].Malpass A, Dodd J, Feder G, et al. Disrupted breath, songlines of breathlessness: an interdisciplinary response. Med Humanit 2019;45(3):294–303. 10.1136/medhum-2018-011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saketkoo LA, Alexanderson H, Lammi MR, et al. An ode to the primal tonic of dance—congratulating the Life of Breath project. Lancet Res Med 2020;8(12):e90–1. 10.1016/S2213-2600(20)30466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yoga. What you need to know. NCCIH. https://www.nccih.nih.gov/health/yoga-what-you-need-to-know. [Accessed 14 March 2021]. [Google Scholar]
- [32].Armstrong M, Vogiatzis I. Personalized exercise training in chronic lung diseases. Respirology 2019;24(9):854–62. 10.1111/resp.13639. [DOI] [PubMed] [Google Scholar]
- [33].Gronek P, Wielinski D, Cyganski P, et al. A review of exercise as medicine in cardiovascular disease: pathology and mechanism. Aging Dis 2020;11(2):327–40. 10.14336/AD.2019.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liederbach M, Kremenic IJ, Orishimo KF, et al. Comparison of landing biomechanics between Male and female dancers and athletes, Part 2: influence of fatigue and implications for anterior cruciate ligament injury. Am J Sports Med 2014; 42(5):1089–95. 10.1177/0363546514524525. [DOI] [PubMed] [Google Scholar]
- [35].Oxley R, Harrison SL, Rose A, et al. The meaning of the name of ‘pulmonary rehabilitation’ and its influence on engagement with individuals with chronic lung disease. Chron Respir Dis 2019;16. 10.1177/1479973119847659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Troosters T, Blondeel A, Janssens W, et al. The past, present and future of pulmonary rehabilitation. Respirology 2019; 24(9):830–7. 10.1111/resp.13517. [DOI] [PubMed] [Google Scholar]
- [37].Breath L of. Dance easy videos. Life of breath. Published March 13, 2020, https://lifeofbreath.org/2020/03/dance-easy-breathe-better-and-feel-good/. [Accessed 19 February 2021].
- [38].ChallengeMeThis. Dance your way to health” A short documentary. https://www.youtube.com/watch?v=4bFIuoXKMrA. [Accessed 19 February 2021].
- [39].Move Towards Health Instructional Booklet. https://www.dropbox.com/s/tgdv8dgbg9f4dla/CPHC%20-%20MOVE%20Towards%20Health%20-%20DOUBLE%20Booklet.docx?dl=0. [Accessed 14 March 2021].
- [40].Horosko M Martha Graham: the evolution of her dance theory and training. University Press of Florida; 2002. [Google Scholar]
- [41].Lewi D. The illustrated dance technique of José Limón. Princeton Book Company; 1999. [Google Scholar]
- [42].Perces MB. The dance technique of Lester Horton. Princeton, NJ: Princeton Book Co.; 1992. [Google Scholar]
- [43].Iyengar BKS, Menuhin Y. Light on yoga: the bible of modern yoga. Schocken; 1979. https://www.amazon.com/Light-Yoga-Bible-Modern/dp/0805210318. [Accessed 14 March 2021]. [Google Scholar]
- [44].Living well: heart, lung, muscle & mind. 3–3-1 tutorial = 3 minutes arms/3 minutes legs/1 Minute of Rising from chair - with saketkoo MD. 2020. https://www.youtube.com/watch?v=zsBRxmkzAnM&t=2s. [Accessed 19 February 2021].
- [45].Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Publ Health Rep 1985;100(2):126–31. [PMC free article] [PubMed] [Google Scholar]
- [46].EUPAP – a European model for physical activity on prescription - the public health agency of Sweden. http://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/living-conditions-and-lifestyle/physical-activity/eupap-a-european-model-for-physical-activity-on-prescription/. [Accessed 15 March 2021].
- [47].Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory fitness and cardiovascular disease prevention: an update. Curr Atherosclerosis Rep 2018;20(1):1. 10.1007/s11883-018-0711-4. [DOI] [PubMed] [Google Scholar]
- [48].Organization WH. WHO guidelines on physical activity and sedentary behaviour: at a glance. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/337001. [Accessed 15 March 2021]. [Google Scholar]
- [49].NCDs Global action plan on physical activity 2018–2030: more active people for a healthier world. WHO. http://www.who.int/ncds/prevention/physical-activity/global-action-plan-2018-2030/en/. [Accessed 15 March 2021]. [Google Scholar]
- [50].Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334–59. 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- [51].Ebbeling C, Ward A, Puleo E, et al. Development of a single-stage submaximal treadmill walking test. Med Sci Sports Exerc 1991;23(8):966–73. [PubMed] [Google Scholar]
- [52].Ninot G, Flori N, Huteau M-E, et al. Activités physiques et cancers : des bénéfices prouvés pendant et aprés les traitements. Bull Canc 2020;107(4):474–89. 10.1016/j.bulcan.2019.11.017. [DOI] [PubMed] [Google Scholar]
- [53].Cronin O, Keohane DM, Molloy MG, et al. The effect of exercise interventions on inflammatory biomarkers in healthy, physically inactive subjects: a systematic review. QJM 2017;110(10):629–37. 10.1093/qjmed/hcx091. [DOI] [PubMed] [Google Scholar]
- [54].Pedersen BK. The diseasome of physical inactivity – and the role of myokines in muscle–fat cross talk. J Physiol 2009; 587(Pt 23):5559–68. 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Oshima Y, Ouchi N, Sato K, et al. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 2008;117(24):3099–108. 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ouchi N, Oshima Y, Ohashi K, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 2008;283(47): 32802–11. 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kollet DP, Marenco AB, Bellé NL, et al. Aerobic exercise, but not isometric handgrip exercise, improves endothelial function and arterial stiffness in patients with myocardial infarction undergoing coronary intervention: a randomized pilot study. BMC Cardiovasc Disord 2021;21. 10.1186/s12872-021-01849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Boehler JF, Horn A, Novak J, et al. Mitochondrial dysfunction and role of harakiri in the pathogenesis of myositis. J Pathol 2019;249(2):215–26. 10.1002/path.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Suriano F, Van Hul M, Cani PD. Gut microbiota and regulation of myokine-adipokine function. Curr Opin Pharmacol 2020; 52:9–17. 10.1016/j.coph.2020.03.006. [DOI] [PubMed] [Google Scholar]
- [60].Sakurai T, Ogasawara J, Kizaki T, et al. The effects of exercise training on obesity-induced dysregulated expression of adipokines in white adipose tissue. Int J Endocrinol 2013;2013. 10.1155/2013/801743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Alexanderson H, Boström C. Exercise therapy in patients with idiopathic inflammatory myopathies and systemic lupus erythematosus – a systematic literature review. Best Pract Res Clin Rheumatol 2020;34(2):101547. 10.1016/j.berh.2020.101547. [DOI] [PubMed] [Google Scholar]
- [62].Swärdh E, Brodin N. Effects of aerobic and muscle strengthening exercise in adults with rheumatoid arthritis: a narrative review summarising a chapter in Physical activity in the prevention and treatment of disease (FYSS 2016). Br J Sports Med 2016;50(6):362–7. 10.1136/bjsports-2015-095793. [DOI] [PubMed] [Google Scholar]
- [63].Munters LA, Dastmalchi M, Andgren V, et al. Improvement in health and possible reduction in disease activity using endurance exercise in patients with established polymyositis and dermatomyositis: a multicenter randomized controlled trial with a 1-year open extension followup. Arthritis Care Res 2013;65(12):1959–68. 10.1002/acr.22068. [DOI] [PubMed] [Google Scholar]
- [64].Alemo Munters L, Dastmalchi M, Katz A, et al. Improved exercise performance and increased aerobic capacity after endurance training of patients with stable polymyositis and dermatomyositis. Arthritis Res Ther 2013;15(4):R83. 10.1186/ar4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Munters LA, Loell I, Ossipova E, et al. Endurance exercise improves molecular pathways of aerobic metabolism in patients with myositis. Arthritis Rheumatol 2016;68(7):1738–50. 10.1002/art.39624. [DOI] [PubMed] [Google Scholar]
- [66].Boehler JF, Hogarth MW, Barberio MD, et al. Effect of endurance exercise on microRNAs in myositis skeletal muscle—a randomized controlled study. PloS One 2017;12(8). 10.1371/journal.pone.0183292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].López-Bastida J, Oliva-Moreno J, Linertová R, et al. Social/economic costs and health-related quality of life in patients with rare diseases in Europe. Eur J Health Econ 2016;17(1):1–5. 10.1007/s10198-016-0780-7. [DOI] [PubMed] [Google Scholar]
- [68].Brown H, Roberts J. Exercising choice: the economic determinants of physical activity behaviour of an employed population. Soc Sci Med 2011;73(3):383–90. 10.1016/j.socscimed.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hammam N, Ezeugwu VE, Rumsey DG, et al. Physical activity, sedentary behavior, and long-term cardiovascular risk in individuals with rheumatoid arthritis. Physician Sportsmed 2019;47(4):463–70. 10.1080/00913847.2019.1623995. [DOI] [PubMed] [Google Scholar]
- [70].Patterson SL, Trupin L, Yazdany J, et al. Physical inactivity independently predicts incident depression in a multi-racial/ ethnic systemic lupus cohort. Arthritis Care Res 2021. 10.1002/acr.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Legge A, Blanchard C, Hanly JG. Physical activity, sedentary behaviour and their associations with cardiovascular risk in systemic lupus erythematosus. Rheumatology 2020;59(5):1128–36. 10.1093/rheumatology/kez429. [DOI] [PubMed] [Google Scholar]
- [72].Ehlken N, Verduyn C, Tiede H, et al. Economic evaluation of exercise training in patients with pulmonary hypertension. Lung 2014;192(3):359–66. 10.1007/s00408-014-9558-9. [DOI] [PubMed] [Google Scholar]
- [73].Brodin N, Lohela-Karlsson M, Swärdh E, et al. Cost-effectiveness of a one-year coaching program for healthy physical activity in early rheumatoid arthritis. Disabil Rehabil 2015;37(9):757–62. 10.3109/09638288.2014.940429. [DOI] [PubMed] [Google Scholar]
- [74].Williams MA, Williamson EM, Heine PJ, et al. Strengthening and stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomised controlled trial and economic evaluation. Health Technol Assess 2015;19(19):1–222. 10.3310/hta19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Frech TM, Machin DR, Murtaugh MA, et al. Implications of endothelial shear stress on systemic sclerosis vasculopathy and treatment. Clin Exp Rheumatol 2018;36(Suppl 113):175–82. [PMC free article] [PubMed] [Google Scholar]
- [76].Maiorana A, O’Driscoll G, Taylor R, et al. Exercise and the nitric oxide vasodilator system. Sports Med 2003;33(14): 1013–35. 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- [77].Pettersson H, Nordin A, Svenungsson E, et al. Experiences of physical activity and exercise in individuals with systemic sclerosis: a qualitative study. Muscoskel Care 2020;18(2):150–60. 10.1002/msc.1447. [DOI] [PubMed] [Google Scholar]
- [78].Pettersson H, Åkerström A, Nordin A, et al. Self-reported physical capacity and activity in patients with systemic sclerosis and matched controls. Scand J Rheumatol 2017;46(6):490–5. 10.1080/03009742.2017.1281436. [DOI] [PubMed] [Google Scholar]
- [79].Liem SIE, Vliet Vlieland TPM, Schoones JW, et al. The effect and safety of exercise therapy in patients with systemic sclerosis: a systematic review. Rheumatol Adv Pract 2019;3(2). 10.1093/rap/rkz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bérezné A, Seror R, Morell-Dubois S, et al. Impact of systemic sclerosis on occupational and professional activity with attention to patients with digital ulcers. Arthritis Care Res 2011;63(2):277–85. 10.1002/acr.20342. [DOI] [PubMed] [Google Scholar]
- [81].Morrisroe K, Huq M, Stevens W, et al. Determinants of unemployment amongst Australian systemic sclerosis patients: results from a multicentre cohort study. Clin Exp Rheumatol 2016;34(5):79–84. [PubMed] [Google Scholar]
- [82].Morrisroe K, Sudararajan V, Stevens W, et al. Work productivity in systemic sclerosis, its economic burden and association with health-related quality of life. Rheumatology 2018;57(1):73–83. 10.1093/rheumatology/kex362. [DOI] [PubMed] [Google Scholar]
- [83].Pettersson H, Boström C, Bringby F, et al. Muscle endurance, strength, and active range of motion in patients with different subphenotypes in systemic sclerosis: a cross-sectional cohort study. Scand J Rheumatol 2019;48(2):141–8. 10.1080/03009742.2018.1477990. [DOI] [PubMed] [Google Scholar]
- [84].Stöcker JK, Vonk MC, van den Hoogen FHJ, et al. Room for improvement in non-pharmacological systemic sclerosis care? — a cross-sectional online survey of 650 patients. BMC Rheumatol 2020;4. 10.1186/s41927-020-00142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Poole JL, Newbill SL, Serrano J, et al. Use of focus groups and patient partners to revise an internet self-management program for people with systemic sclerosis. Patient Exp J 2019;6(2):75–82. [Google Scholar]
- [86].Sandqvist G, Scheja A, Hesselstrand R. Pain, fatigue and hand function closely correlated to work ability and employment status in systemic sclerosis. Rheumatology 2010;49(9):1739–46. 10.1093/rheumatology/keq145. [DOI] [PubMed] [Google Scholar]
- [87].Mendelson C, Poole JL, Allaire S. Experiencing work as a daily challenge: the case of scleroderma. Work 2013;44(4): 405–13. 10.3233/WOR-2012-1420. [DOI] [PubMed] [Google Scholar]
- [88].Poole JL, Anwar S, Mendelson C, Allaire S. Workplace barriers encountered by employed persons with systemic sclerosis. Work 2016;55(4):923–9. 10.3233/WOR-162448. [DOI] [PubMed] [Google Scholar]
- [89].Sandqvist G, Scheja A, Eklund M. Working ability in relation to disease severity, everyday occupations and well-being in women with limited systemic sclerosis. Rheumatology 2008;47(11):1708–11. 10.1093/rheumatology/ken359. [DOI] [PubMed] [Google Scholar]
- [90].Harb S, Cumin J, Rice DB, et al. Identifying barriers and facilitators to physical activity for people with scleroderma: a nominal group technique study. Disabil Rehabil 2020:1–8. 10.1080/09638288.2020.1742391. [DOI] [PubMed] [Google Scholar]
- [91].Beckers E, Webers C, Boonen A, et al. Validation and implementation of a patient-reported experience measure for patients with rheumatoid arthritis and spondyloarthritis in The Netherlands. Clin Rheumatol 2020;39(10):2889–97. 10.1007/s10067-020-05076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Saketkoo LA, Scholand MB, Lammi MR, Russell A-M. Patient-reported outcome measures in systemic sclerosis-related interstitial lung disease for clinical practice and clinical trials. J Scleroderma Relat Disord 2020;5(2 Suppl):48–60. 10.1177/2397198320904178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient Activation measure. Health Serv Res 2005;40(6 Pt 1):1918–30. 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Andonopoulos AP, Yarmenitis S, Georgiou P, et al. Bronchiectasis in systemic sclerosis. A study using high resolution computed tomography. Clin Exp Rheumatol 2001;19(2):187–90. [PubMed] [Google Scholar]
- [95].Janosik DL, Osborn TG, Moore TL, et al. Heart disease in systemic sclerosis. Semin Arthritis Rheum 1989;19(3):191–200. 10.1016/0049-0172(89)90032-2. [DOI] [PubMed] [Google Scholar]
- [96].Battaglia S, Bellia M, Serafino-Agrusa L, et al. Physical capacity in performing daily activities is reduced in scleroderma patients with early lung involvement. Clin Res J 2017;11(1):36–42. 10.1111/crj.12299. [DOI] [PubMed] [Google Scholar]
- [97].Lima TRL, Guimarães FS, Silva LA, et al. Relationship between functional capacity, joint mobility and pulmonary function in patients with systemic sclerosis. J Bodyw Mov Ther 2015;19(1):17–24. 10.1016/j.jbmt.2014.01.002. [DOI] [PubMed] [Google Scholar]
- [98].Lima TRL, Guimarães FS, Neves RS, et al. Scleroderma: assessment of posture, balance and pulmonary function in a cross-sectional controlled study. Clin BioMech 2015;30(5):438–43. 10.1016/j.clinbiomech.2015.03.013. [DOI] [PubMed] [Google Scholar]
- [99].Saketkoo L, Mittoo S, Frankel S, et al. Reconciling healthcare professional and patient perspectives in the development of disease activity and response criteria in connective tissue disease related interstitial lung diseases. J Rheumatol 2014; 41(4):792–8. 10.3899/jrheum.131251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mitropoulos A, Gumber A, Crank H, et al. The effects of upper and lower limb exercise on the microvascular reactivity in limited cutaneous systemic sclerosis patients. Arthritis Res Ther 2018;20(1):112. 10.1186/s13075-018-1605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gilson M, Zerkak D, Wipff J, et al. Prognostic factors for lung function in systemic sclerosis: prospective study of 105 cases. Eur Respir J 2010;35(1):112–7. 10.1183/09031936.00060209. [DOI] [PubMed] [Google Scholar]
- [102].Mura G, Bhat KM, Pisano A, et al. Psychiatric symptoms and quality of life in systemic sclerosis. Clin Pract Epidemiol Ment Health 2012;8:30–5. 10.2174/1745017901208010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Filippetti M, Cazzoletti L, Zamboni F, et al. Effect of a tailored home-based exercise program in patients with systemic sclerosis: a randomized controlled trial. Scand J Med Sci Sports 2020;30(9):1675–84. 10.1111/sms.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Waller L, Krüger K, Conrad K, et al. Effects of different types of exercise training on pulmonary arterial hypertension: a systematic review. J Clin Med 2020;9(6). 10.3390/jcm9061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Varga J, Porszasz J, Boda K, et al. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med 2007;101(11):2297–304. 10.1016/j.rmed.2007.06.017. [DOI] [PubMed] [Google Scholar]
- [106].Kerti M, Balogh Z, Kelemen K, Varga JT. The relationship between exercise capacity and different functional markers in pulmonary rehabilitation for COPD. Int J Chronic Obstr Pulm Dis 2018;13:717–24. 10.2147/COPD.S153525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mohan V, Paungmali A, Sitilerpisan P, et al. Respiratory characteristics of individuals with non-specific low back pain: a cross-sectional study. Nurs Health Sci 2018;20(2):224–30. 10.1111/nhs.12406. [DOI] [PubMed] [Google Scholar]
- [108].Yoon HS, Cha YJ, You J, Sung H. Effects of dynamic core-postural chain stabilization on diaphragm movement, abdominal muscle thickness, and postural control in patients with subacute stroke: a randomized control trial. NeuroRehabilitation 2020;46(3):381–9. 10.3233/NRE-192983. [DOI] [PubMed] [Google Scholar]
- [109].Son MS, Jung DH, You J, et al. Effects of dynamic neuromuscular stabilization on diaphragm movement, postural control, balance and gait performance in cerebral palsy. NeuroRehabilitation 2017;41(4):739–46. 10.3233/NRE-172155. [DOI] [PubMed] [Google Scholar]
- [110].Moss H, Lynch J, O’Donoghue J. Exploring the perceived health benefits of singing in a choir: an international cross-sectional mixed-methods study. Perspect Public Health 2018;138(3):160–8. 10.1177/1757913917739652. [DOI] [PubMed] [Google Scholar]
- [111].Lewis A, Cave P, Hopkinson N. Singing for lung health: service evaluation of the British lung foundation programme. Perspect Public Health 2018;138(4):215–22. 10.1177/1757913918774079. [DOI] [PubMed] [Google Scholar]
- [112].Kang J, Scholp A, Jiang JJ. A review of the physiological effects and mechanisms of singing. J Voice 2018;32(4):390–5. 10.1016/j.jvoice.2017.07.008. [DOI] [PubMed] [Google Scholar]
- [113].Fu MC, Belza B, Nguyen H, et al. Impact of group-singing on older adult health in senior living communities: a pilot study. Arch Gerontol Geriatr 2018;76:138–46. 10.1016/j.archger.2018.02.012. [DOI] [PubMed] [Google Scholar]
- [114].Idrose AM, Juliana N, Azmani S, et al. Singing improves oxygen saturation in simulated high-altitude environment. J Voice 2020. 10.1016/j.jvoice.2020.06.031. Published online July 29. [DOI] [PubMed] [Google Scholar]
- [115].Qiu Z-H, Guo H-X, Lu G, et al. Physiological responses to Tai Chi in stable patients with COPD. Respir Physiol Neurobiol 2016;221:30–4. 10.1016/j.resp.2015.10.019. [DOI] [PubMed] [Google Scholar]
- [116].Zhu S, Shi K, Yan J, et al. A modified 6-form Tai Chi for patients with COPD. Compl Ther Med 2018;39:36–42. 10.1016/j.ctim.2018.05.007. [DOI] [PubMed] [Google Scholar]
- [117].Tonelli R, Cocconcelli E, Lanini B, et al. Effectiveness of pulmonary rehabilitation in patients with interstitial lung disease of different etiology: a multicenter prospective study. BMC Pulm Med 2017;17. 10.1186/s12890-017-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Grünig E, MacKenzie A, Peacock AJ, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J 2020. 10.1093/eurheartj/ehaa696.ehaa696. [DOI] [PubMed] [Google Scholar]
- [119].Holland AE, Watson A, Glaspole I. Comprehensive pulmonary rehabilitation for interstitial lung disease: a consensus approach to identify core education topics. Patient Educ Counsel 2019;102(6):1125–30. 10.1016/j.pec.2019.01.010. [DOI] [PubMed] [Google Scholar]
- [120].de Oliveira NC, Portes LA, Pettersson H, et al. Aerobic and resistance exercise in systemic sclerosis: state of the art. Muscoskel Care 2017;15(4):316–23. [DOI] [PubMed] [Google Scholar]
- [121].Pinto AL, Oliveira NC, Gualano B, et al. Efficacy and safety of concurrent training in systemic sclerosis. J Strength Condit Res 2011;25(5):1423–8. 10.1519/JSC.0b013e3181d6858b. [DOI] [PubMed] [Google Scholar]
- [122].Mahlen DA. The measurement of dyspnea during exercise in patients with lung disease. Chest 1992;101(5):242S–7S. 10.1378/chest.101.5_Supplement.242S. [DOI] [PubMed] [Google Scholar]
- [123].Wilsher M, Good N, Hopkins R, et al. The six-minute walk test using forehead oximetry is reliable in the assessment of scleroderma lung disease. Respirology 2012;17(4):647–52. 10.1111/j.1440-1843.2012.02133.x. [DOI] [PubMed] [Google Scholar]
- [124].Herigstad M, Faull OK, Hayen A, et al. Treating breathlessness via the brain: changes in brain activity over a course of pulmonary rehabilitation. Eur Respir J 2017;50(3). 10.1183/13993003.01029-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Mittoo S, Frankel S, LeSage D, et al. Patient perspectives in OMERACT provide an anchor for future metric development and improved approaches to healthcare delivery in connective tissue disease related interstitial lung disease (CTD-ILD). Curr Respir Med Rev 2015;11(2):175–83. 10.2174/1573398X11666150619182624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bruton A, Lee A, Yardley L, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med 2018;6(1):19–28. 10.1016/S2213-2600(17)30474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kumar S, Singh J, Rattan S, et al. Review article: pathogenesis and clinical manifestations of gastrointestinal involvement in systemic sclerosis. Aliment Pharmacol Ther 2017;45(7):883–98. 10.1111/apt.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jaeger VK, Distler O, Maurer B, et al. Functional disability and its predictors in systemic sclerosis: a study from the DeSScipher project within the EUSTAR group. Rheumatology 2018;57(3):441–50. 10.1093/rheumatology/kex182. [DOI] [PubMed] [Google Scholar]
- [129].Yang H, Xu D, Tao Li M, et al. Gastrointestinal manifestations on impaired quality of life in systemic sclerosis. J Dig Dis 2019;20(5):256–61. [DOI] [PubMed] [Google Scholar]
- [130].Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology 2009;48. 10.1093/rheumatology/ken485(suppl_3):iii36-iii39. [DOI] [PubMed] [Google Scholar]
- [131].Nietert PJ, Mitchell HC, Bolster MB, et al. Correlates of depression, including overall and gastrointestinal functional status, among patients with systemic sclerosis. | the Journal of Rheumatology. J Rheumatol 2005;32(1):51–7. [PubMed] [Google Scholar]
- [132].Bodukam V, Hays RD, Maranian P, et al. Association of gastrointestinal involvement and depressive symptoms in patients with systemic sclerosis. Rheumatology 2011;50(2):330–4. 10.1093/rheumatology/keq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kim H-J, Lee J-Y, Lee E-S, et al. Simple oral exercise with chewing gum for improving oral function in older adults. Aging Clin Exp Res 2020. 10.1007/s40520-020-01606-z. Published online May 31. [DOI] [PubMed] [Google Scholar]
- [134].Huang J, Liao J, Fang Y, et al. Six-Week exercise training with dietary restriction improves central hemodynamics associated with altered gut microbiota in adolescents with obesity. Front Endocrinol 2020;11. 10.3389/fendo.2020.569085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhong F, Wen X, Yang M, et al. Effect of an 8-week exercise training on gut microbiota in physically inactive older women. Int J Sports Med 2020. 10.1055/a-1301-7011. Published online December 15. [DOI] [PubMed] [Google Scholar]
- [136].Barber TM, Kyrou I, Randeva HS, Weickert MO. Mechanisms of insulin resistance at the crossroad of obesity with associated metabolic abnormalities and cognitive dysfunction. Int J Mol Sci 2021;22(2). 10.3390/ijms22020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lee K-H, Jung E-S, Choi Y-Y. Effects of lingual exercises on oral muscle strength and salivary flow rate in elderly adults: a randomized clinical trial. Geriatr Gerontol Int 2020;20(7):697–703. 10.1111/ggi.13944. [DOI] [PubMed] [Google Scholar]
- [138].Singh S, Devanna S, Edakkanambeth Varayil J, et al. Physical activity is associated with reduced risk of esophageal cancer, particularly esophageal adenocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol 2014;14:101. 10.1186/1471-230X-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fretheim H, Chung BK, Didriksen H, et al. Fecal microbiota transplantation in systemic sclerosis: a double-blind, placebo-controlled randomized pilot trial. PloS One 2020;15(5). 10.1371/journal.pone.0232739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Codella R, Luzi L, Terruzzi I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis 2018;50(4):331–41. 10.1016/j.dld.2017.11.016. [DOI] [PubMed] [Google Scholar]
- [141].Papadimitriou N, Dimou N, Tsilidis KK, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun 2020;11. 10.1038/s41467-020-14389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Volkmann ER, Hoffmann-Vold A-M. Gastrointestinal tract microbiota modifications in systemic sclerosis. Eur J Rheumatol 2020;7(Suppl 3):S228–36. 10.5152/eurjrheum.2019.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Volkmann ER, Hoffmann-Vold A-M, Chang Y-L, et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol 2017;4(1). 10.1136/bmjgast-2017-000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Volkmann ER, Chang Y-L, Barroso N, et al. Systemic sclerosis is associated with a unique colonic microbial consortium. Arthritis Rheum 2016;68(6):1483–92. 10.1002/art.39572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Amin K, Clarke A, Sivakumar B, et al. The psychological impact of facial changes in scleroderma. Psychol Health Med 2011;16(3):304–12. 10.1080/13548506.2010.540250. [DOI] [PubMed] [Google Scholar]
- [146].van Lankveld WGJM, Vonk MC, Teunissen H, van den Hoogen FHJ. Appearance self-esteem in systemic sclerosis—subjective experience of skin deformity and its relationship with physician-assessed skin involvement, disease status and psychological variables. Rheumatology 2007;46(5):872–6. 10.1093/rheumatology/kem008. [DOI] [PubMed] [Google Scholar]
- [147].Maddali-Bongi S, Landi G, Galluccio F, et al. The rehabilitation of facial involvement in systemic sclerosis: efficacy of the combination of connective tissue massage, Kabat’s technique and kinesitherapy: a randomized controlled trial. Rheumatol Int 2011;31(7):895–901. 10.1007/s00296-010-1382-9. [DOI] [PubMed] [Google Scholar]
- [148].Azar M, Rice DB, Kwakkenbos L, et al. Exercise habits and factors associated with exercise in systemic sclerosis: a Scleroderma Patient-centered Intervention Network (SPIN) cohort study. Disabil Rehabil 2018;40(17):1997–2003. 10.1080/09638288.2017.1323023. [DOI] [PubMed] [Google Scholar]
- [149].Mouthon L, Rannou F, Bérezné A, et al. Development and validation of a scale for mouth handicap in systemic sclerosis: the Mouth Handicap in Systemic Sclerosis scale. Ann Rheum Dis 2007;66(12):1651–5. 10.1136/ard.2007.070532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Mouthon L, Poole JL. Physical and occupational therapy. In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, editors. Scleroderma: from pathogenesis to comprehensive management. Springer International Publishing; 2017. p. 603–13. 10.1007/978-3-319-31407-5_44. [DOI] [Google Scholar]
- [151].Varga J, Denton C, Wigley FM, Allanore Y, Kuwana M, editors. Scleroderma: from pathogenesis to comprehensive management. second ed. Springer International Publishing; 2017. 10.1007/978-3-319-31407-5. [DOI] [Google Scholar]
- [152].Czirják L, Foeldvari I, Müller-Ladner U. Skin involvement in systemic sclerosis rheumatology oxford academic. Rheumatol Adv Prac 2008;47(5):44–5. [DOI] [PubMed] [Google Scholar]
- [153].Crane JD, MacNeil LG, Lally JS, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015;14(4):625–34. 10.1111/acel.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bugaj O, Zieliński J, Kusy K, et al. The effect of exercise on the skin content of the reduced form of NAD and its response to transient ischemia and reperfusion in highly trained athletes. Front Physiol 2019;10. 10.3389/fphys.2019.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Freire V, Bazeli R, Elhai M, et al. Hand and wrist involvement in systemic sclerosis: US features. Radiology 2013;269(3): 824–30. 10.1148/radiol.13121994. [DOI] [PubMed] [Google Scholar]
- [156].Horváth J, Bálint Z, Deiszinger A, et al. Efficacy of intensive hand physical therapy in patients with systemic sclerosis. Clin Exp Rheumatol 2017;35(4):159–66. [PubMed] [Google Scholar]
- [157].Vannajak K, Boonprakob Y, Eungpinichpong W, et al. The short-term effect of gloving in combination with Traditional Thai Massage, heat, and stretching exercise to improve hand mobility in scleroderma patients. J Ayurveda Integr Med 2014;5(1):50–5. 10.4103/0975-9476.128859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Stefanantoni K, Sciarra I, Iannace N, et al. Occupational therapy integrated with a self-administered stretching program on systemic sclerosis patients with hand involvement. Clin Exp Rheumatol 2016;34(5):157–61. [PubMed] [Google Scholar]
- [159].Lehmann JF, Masock AJ, Warren CG, Koblanski JN. Effect of therapeutic temperatures on tendon extensibility. Arch Phys Med Rehabil 1970;51(8):481–7. [PubMed] [Google Scholar]
- [160].Steen VD, Medsger TA. The value of the health assessment questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997;40(11):1984–91. 10.1002/art.1780401110. [DOI] [PubMed] [Google Scholar]
- [161].Rannou F, Poiraudeau S, Berezné A, et al. Assessing disability and quality of life in systemic sclerosis: construct validities of the Cochin hand function scale, health assessment questionnaire (HAQ), systemic sclerosis HAQ, and medical outcomes study 36-item short form health survey. Arthritis Care Res 2007;57(1):94–102. 10.1002/art.22468. [DOI] [PubMed] [Google Scholar]
- [162].Roberts-Thomson AJ, Massy-Westropp N, Smith MD, et al. The use of the hand anatomic index to assess deformity and impaired function in systemic sclerosis. Rheumatol Int 2006;26(5):439–44. 10.1007/s00296-005-0058-3. [DOI] [PubMed] [Google Scholar]
- [163].Roberts-Thomson AJ, Englert H, Ahern MJ, et al. A modified hand anatomic index to assesss hand deformity in scleroderma. Rheumatol Int 2009;29(7):847–8. 10.1007/s00296-008-0777-3. [DOI] [PubMed] [Google Scholar]
- [164].Smyth AE, MacGregor AJ, Mukerjee D, et al. A cross-sectional comparison of three self-reported functional indices in scleroderma. Rheumatology 2003;42(6):732–8. 10.1093/rheumatology/keg145. [DOI] [PubMed] [Google Scholar]
- [165].Patient specific functional scale. Physiopedia. https://www.physio-pedia.com/Patient_Specific_Functional_Scale. [Accessed 18 March 2021].
- [166].Stratford P, Gill C, Westaway M, Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiother Can 1995;47(4):258–63. 10.3138/ptc.47.4.258. [DOI] [Google Scholar]
- [167].Kennedy CA, Beaton DE, Solway S, et al. Disabilities of the arm, shoulder, and hand (DASH). In: DASH and QuickDASH outcome measure user’s manual. third ed. 2011. https://dash.iwh.on.ca/dash-manual. [Accessed 18 March 2021]. [Google Scholar]
- [168].Bohannon R, Bear-Lehman J, Desrosiers J, et al. Average grip strength: a meta-analysis of data obtained with a jamar dynamometer from individuals 75 Years or more of age. J Geriatr Phys Ther 2007;30(1):28–30. [PubMed] [Google Scholar]
- [169].Varjú C, Bálint Z, Solyom AI, et al. Cross-cultural adaptation of the disabilities of the arm, shoulder, and hand (DASH) questionnaire into Hungarian and investigation of its validity in patients with systemic sclerosis. Clin Exp Rheumatol 2018;26(5):776–83. [PubMed] [Google Scholar]
- [170].Sandqvist G, Eklund M. Hand Mobility in Scleroderma (HAMIS) test: the reliability of a novel hand function test. Arthritis Care Res 2000;13(6):369–74. [PubMed] [Google Scholar]
- [171].Vanthuyne M, Smith V, Arat S, et al. Validation of a manual ability questionnaire in patients with systemic sclerosis. Arthritis Care Res 2009;61(5):695–703. 10.1002/art.24426. [DOI] [PubMed] [Google Scholar]
- [172].Brower LM, Poole JL. Reliability and validity of the Duruöz Hand Index in persons with systemic sclerosis (scleroderma). Arthritis Care Res 2004;51(5):805–9. 10.1002/art.20701. [DOI] [PubMed] [Google Scholar]
- [173].Nordenskiöld UM, Grimby G. Grip force in patients with rheumatoid arthritis and fibromyalgia and in healthy subjects. A study with the grippit instrument. Scand J Rheumatol 1993;22(1):14–9. 10.3109/03009749309095105. [DOI] [PubMed] [Google Scholar]
- [174].Ahrens HC, Siegert E, Tomsitz D, et al. Digital ulcers score: a scoring system to assess digital ulcers in patients suffering from systemic sclerosis. Clin Exp Rheumatol 2016;34(5):142–7. [PubMed] [Google Scholar]
- [175].Eberl DR, Fasching V, Rahlfs V, et al. Repeatability and objectivity of various measurements in rheumatoid arthritis. A comparative study. Arthritis Rheum 1976;19(6):1278–86. 10.1002/art.1780190608. [DOI] [PubMed] [Google Scholar]
- [176].Alcacer-Pitarch B, Buch MH, Gray J, et al. Pressure and pain in Systemic sclerosis/Scleroderma - an evaluation of a simple intervention (PISCES): randomised controlled trial protocol. BMC Muscoskel Disord 2012;13:11. 10.1186/1471-2474-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Maddali Bongi S, Ravenni G, Ciampi B, et al. Biomechanical podiatric evaluation in an Italian cohort of patients with systemic sclerosis: a pilot study. Eur J Rheumatol 2016;3(4):169–74. 10.5152/eurjrheum.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Poole JL, Brandenstein J. Difficulty with daily activities involving the lower extremities in people with systemic sclerosis. Clin Rheumatol 2016;35(2):483–8. 10.1007/s10067-015-3137-1. [DOI] [PubMed] [Google Scholar]
- [179].Bálint Z, Farkas H, Farkas N, et al. A three-year follow-up study of the development of joint contractures in 131 patients with systemic sclerosis. Clin Exp Rheumatol 2014;32(6):68–74. [PubMed] [Google Scholar]
- [180].Avouac J, Walker U, Tyndall A, et al. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: results from the EULAR scleroderma trial and research group (EUSTAR) database. J Rheumatol 2010; 37(7):1488–501. 10.3899/jrheum.091165. [DOI] [PubMed] [Google Scholar]
- [181].Borisovskaya A, Chmelik E, Karnik A. Exercise and chronic pain. In: Xiao J, editor. Physical Exercise for human health. Advances in experimental medicine and biology. Springer; 2020. p. 233–53. 10.1007/978-981-15-1792-1_16. [DOI] [PubMed] [Google Scholar]
- [182].Buckwalter JA. Activity vs. rest in the treatment of bone, soft tissue and joint injuries. Iowa Orthop J 1995;15:29–42. [PMC free article] [PubMed] [Google Scholar]
- [183].Maddali Bongi S, Del Rosso A, Galluccio F, et al. Efficacy of a tailored rehabilitation program for systemic sclerosis. Clin Exp Rheumatol 2009;27(3):44–50. [PubMed] [Google Scholar]
- [184].Landim SF, Bertolo MB, Del Rio AP, et al. Sustained efficacy of a concise self-management programme for hands in systemic sclerosis: a longitudinal case–control observational study. Rheumatology 2020;59(11):3330–9. 10.1093/rheumatology/keaa140. [DOI] [PubMed] [Google Scholar]
- [185].Antonioli CM, Bua G, Frigé A, et al. An individualized rehabilitation program in patients with systemic sclerosis may improve quality of life and hand mobility. Clin Rheumatol 2009;28(2):159–65. 10.1007/s10067-008-1006-x. [DOI] [PubMed] [Google Scholar]
- [186].Landim SF, Bertolo MB, Marcatto de Abreu MF, et al. The evaluation of a home-based program for hands in patients with systemic sclerosis. J Hand Ther 2019;32(3):313–21. 10.1016/j.jht.2017.10.013. [DOI] [PubMed] [Google Scholar]
- [187].Sandqvist G, Åkesson A, Eklund M. Evaluation of paraffin bath treatment in patients with systemic sclerosis. Disabil Rehabil 2004;26(16):981–7. 10.1080/09638280410001702405. [DOI] [PubMed] [Google Scholar]
- [188].Mancuso T, Poole JL. The effect of paraffin and exercise on hand function in persons with scleroderma: a series of single case studies. J Hand Ther 2009;22(1):71–8. 10.1016/j.jht.2008.06.009. [DOI] [PubMed] [Google Scholar]
- [189].Gregory WJ, Wilkinson J, Herrick AL. A randomised controlled trial of wax baths as an additive therapy to hand exercises in patients with systemic sclerosis. Physiotherapy 2019;105(3):370–7. 10.1016/j.physio.2018.08.008. [DOI] [PubMed] [Google Scholar]
- [190].Kristensen LQ, Oestergaard LG, Bovbjerg K, et al. Use of paraffin instead of lukewarm water prior to hand exercises had no additional effect on hand mobility in patients with systemic sclerosis: a randomized clinical trial. Hand Ther 2019;24(1): 13–21. 10.1177/1758998318824346. [DOI] [Google Scholar]
- [191].Chen J, Lei L, Pan J, Zhao C. A meta-analysis of fracture risk and bone mineral density in patients with systemic sclerosis. Clin Rheumatol 2020;39(4):1181–9. 10.1007/s10067-019-04847-0. [DOI] [PubMed] [Google Scholar]
- [192].Sampaio-Barros MM, Castelo Branco LCM, Takayama L, et al. Acroosteolysis and bone metabolism parameters distinguish female patients with limited systemic sclerosis with and without calcinosis: a case control study. Clin Rheumatol 2019; 38(11):3189–93. 10.1007/s10067-019-04637-8. [DOI] [PubMed] [Google Scholar]
- [193].Thietart S, Louati K, Gatfosse M, et al. Overview of osteo-articular involvement in systemic sclerosis: specific risk factors, clinico-sonographic evaluation, and comparison with healthy women from the French OFELY cohort. Best Pract Res Clin Rheumatol 2018;32(4):591–604. 10.1016/j.berh.2019.01.008. [DOI] [PubMed] [Google Scholar]
- [194].Fauny M, Bauer E, Albuisson E, et al. Vertebral fracture prevalence and measurement of the scanographic bone attenuation coefficient on CT-scan in patients with systemic sclerosis. Rheumatol Int 2018;38(10):1901–10. 10.1007/s00296-018-4139-5. [DOI] [PubMed] [Google Scholar]
- [195].Ruaro B, Casabella A, Paolino S, et al. Correlation between bone quality and microvascular damage in systemic sclerosis patients. Rheumatology 2018;57(9):1548–54. 10.1093/rheumatology/key130. [DOI] [PubMed] [Google Scholar]
- [196].Bruni C, Guiducci S, Bellando-Randone S, Matucci-Cerinic M. Avascular bone necrosis: an underestimated complication of systemic sclerosis. Semin Arthritis Rheum 2017;47(1):e3–5. 10.1016/j.semarthrit.2017.03.015. [DOI] [PubMed] [Google Scholar]
- [197].Sampaio-Barros MM, Alvarenga JC, Takayama L, et al. Distal radius and tibia bone microarchitecture impairment in female patients with diffuse systemic sclerosis. Osteoporos Int 2019;30(8):1679–91. 10.1007/s00198-019-04965-0. [DOI] [PubMed] [Google Scholar]
- [198].Gomarasca M, Banfi G, Lombardi G. Chapter Four - myokines: the endocrine coupling of skeletal muscle and bone. In: Makowski GS, editor. Advances in clinical chemistry. vol. 94. Elsevier; 2020. p. 155–218. 10.1016/bs.acc.2019.07.010. [DOI] [PubMed] [Google Scholar]
- [199].Kirk B, Feehan J, Lombardi G, Duque G. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep 2020;18(4):388–400. 10.1007/s11914-020-00599-y. [DOI] [PubMed] [Google Scholar]
- [200].Guo B, Zhang Z-K, Liang C, et al. Molecular communication from skeletal muscle to bone: a review for muscle-derived myokines regulating bone metabolism. Calcif Tissue Int 2017;100(2):184–92. 10.1007/s00223-016-0209-4. [DOI] [PubMed] [Google Scholar]
- [201].Dolan E, Dumas A, Keane KM, et al. The influence of acute exercise on bone biomarkers: protocol for a systematic review with meta-analysis. Syst Rev 2020;9. 10.1186/s13643-020-01551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Buccoliero C, Oranger A, Colaianni G, et al. The effect of Irisin on bone cells in vivo and in vitro. Biochem Soc Trans 2021; 49(1):477–84. 10.1042/BST20200978. [DOI] [PubMed] [Google Scholar]
- [203].Walker UA, Clements PJ, Allanore Y, et al. Muscle involvement in systemic sclerosis: points to consider in clinical trials. Rheumatology 2017;56(Suppl 5):v38–44. 10.1093/rheumatology/kex196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Clements PJ, Furst DE, Campion DS, et al. Muscle disease in progressive systemic sclerosis. diagnostic and therapeutic considerations. Arthritis Rheum 1978;21(1):62–71. 10.1002/art.1780210111. [DOI] [PubMed] [Google Scholar]
- [205].Medsger TA, Rodnan GP, Moossy J, Vester JW. Skeletal muscle involvement in progressive systemic sclerosis (scleroderma). Arthritis Rheum 1968;11(4):554–68. 10.1002/art.1780110405. [DOI] [PubMed] [Google Scholar]
- [206].Vencovský J, Alexanderson H, Lundberg IE. Idiopathic inflammatory myopathies. Rheum Dis Clin N Am 2019;45(4): 569–81. 10.1016/j.rdc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- [207].Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 2005;35(1):35–42. 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [208].Kaji K, Fertig N, Medsger TA, et al. Autoantibodies to RuvBL1 and RuvBL2: a novel systemic sclerosiserelated antibody associated with diffuse cutaneous and skeletal muscle involvement. Arthritis Care Res 2014;66(4):575–84. 10.1002/acr.22163. [DOI] [PubMed] [Google Scholar]
- [209].Pauling JD, Salazar G, Lu H, et al. Presence of anti-eukaryotic initiation factor-2B, anti-RuvBL1/2 and anti-synthetase antibodies in patients with anti-nuclear antibody negative systemic sclerosis. Rheumatology 2018;57(4):712–7. 10.1093/rheumatology/kex458. [DOI] [PubMed] [Google Scholar]
- [210].Fertig N, Domsic RT, Rodriguez-Reyna T, et al. Anti-U11/U12 RNP antibodies in systemic sclerosis (SSc): a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum 2009;61(7):958–65. 10.1002/art.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Justo AC, Guimarães FS, Ferreira AS, et al. Muscle function in women with systemic sclerosis: association with fatigue and general physical function. Clin BioMech 2017;47:33–9. 10.1016/j.clinbiomech.2017.05.011. [DOI] [PubMed] [Google Scholar]
- [212].Pettersson H, Boström C, Bringby F, et al. Muscle endurance, strength, and active range of motion in patients with different subphenotypes in systemic sclerosis: a cross-sectional cohort study. Scand J Rheumatol 2019;48(2):141–8. 10.1080/03009742.2018.1477990. [DOI] [PubMed] [Google Scholar]
- [213].Alexanderson H, Broman L, TollbÄck A, et al. Functional index-2: validity and reliability of a disease-specific measure of impairment in patients with polymyositis and dermatomyositis. Arthritis Care Res 2006;55(1):114–22. 10.1002/art.21715. [DOI] [PubMed] [Google Scholar]
- [214].Alexanderson H, Regardt M, Ottosson C, et al. Muscle strength and muscle endurance during the first year of treatment of polymyositis and dermatomyositis: a prospective study. J Rheumatol 2018;45(4):538–46. 10.3899/jrheum.161183. [DOI] [PubMed] [Google Scholar]
- [215].Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med 1985;78(1):77–81. 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- [216].Allanore Y, Meune C, Vonk MC, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010;69(1): 218–21. 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- [217].Ranque B, Bérezné A, Le-Guern V, et al. Myopathies related to systemic sclerosis: a case–control study of associated clinical and immunological features. Scand J Rheumatol 2010;39(6):498–505. 10.3109/03009741003774626. [DOI] [PubMed] [Google Scholar]
- [218].Ranque B, Authier F-J, Berezne A, et al. Systemic sclerosis-associated myopathy. Ann N Y Acad Sci 2007;1108(1):268–82. 10.1196/annals.1422.029. [DOI] [PubMed] [Google Scholar]
- [219].Ranque B, Authier F-J, Le-Guern V, et al. A descriptive and prognostic study of systemic sclerosis-associated myopathies. Ann Rheum Dis 2009;68(9):1474–7. 10.1136/ard.2008.095919. [DOI] [PubMed] [Google Scholar]
- [220].Follansbee WP, Zerbe TR, Medsger TA Jr. Cardiac and skeletal muscle disease in systemic sclerosis (scleroderma): a high risk association. Am Heart J 1993;125(1):194–203. 10.1016/0002-8703(93)90075-K. [DOI] [PubMed] [Google Scholar]
- [221].West SG, Killian PJ, Lawless OJ. Association of myositis and myocarditis in progressive systemic sclerosis. Arthritis Rheum 1981;24(5):662–7. 10.1002/art.1780240506. [DOI] [PubMed] [Google Scholar]
- [222].Nordin A, Jensen-Urstad K, Björnådal L, et al. Ischemic arterial events and atherosclerosis in patients with systemic sclerosis: a population-based case-control study. Arthritis Res Ther 2013;15(4):R87. 10.1186/ar4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Nordin A, Björnådal L, Larsson A, et al. Electrocardiography in 110 patients with systemic sclerosis: a cross-sectional comparison with population-based controls. Scand J Rheumatol 2014;43(3):221–5. 10.3109/03009742.2013.843720. [DOI] [PubMed] [Google Scholar]
- [224].Yiu KH, Schouffoer AA, Marsan NA, et al. Left ventricular dysfunction assessed by speckle-tracking strain analysis in patients with systemic sclerosis: relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum 2011; 63(12):3969–78. 10.1002/art.30614. [DOI] [PubMed] [Google Scholar]
- [225].Munters LA, Alexanderson H, Crofford LJ, Lundberg IE. New insights into the benefits of exercise for muscle health in patients with idiopathic inflammatory myositis. Curr Rheumatol Rep 2014;16(7):429. 10.1007/s11926-014-0429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lundberg IE, Nader GA. Molecular effects of exercise in patients with inflammatory rheumatic disease. Nat Clin Pract Rheumatol 2008;4(11):597–604. 10.1038/ncprheum0929. [DOI] [PubMed] [Google Scholar]
- [227].Blok IM, van Riel ACMJ, Schuuring MJ, et al. Decrease in quality of life predicts mortality in adult patients with pulmonary arterial hypertension due to congenital heart disease. Neth Heart J 2015;23(5):278–84. 10.1007/s12471-015-0666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Liang JW, Cheung YK, Willey JZ, et al. Quality of life independently predicts long-term mortality but not vascular events: the Northern Manhattan Study. Qual Life Res 2017;26(8):2219–28. 10.1007/s11136-017-1567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Kanwal F, Gralnek IM, Hays RD, et al. Health-related quality of life predicts mortality in patients with advanced chronic liver disease. Clin Gastroenterol Hepatol 2009;7(7):793–9. 10.1016/j.cgh.2009.03.013. [DOI] [PubMed] [Google Scholar]
- [230].Berg SK, Rasmussen TB, Mols RE, et al. Both mental and physical health predicts one year mortality and readmissions in patients with implantable cardioverter defibrillators: findings from the national DenHeart study. Eur J Cardiovasc Nurs 2019;18(2):96–105. 10.1177/1474515118794598. [DOI] [PubMed] [Google Scholar]
- [231].Baekelandt BMG, Hjermstad MJ, Nordby T, et al. Preoperative cognitive function predicts survival in patients with resectable pancreatic ductal adenocarcinoma. HPB 2016;18(3):247–54. 10.1016/j.hpb.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Ratjen I, Schafmayer C, Enderle J, et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: a German cohort study. BMC Canc 2018;18. 10.1186/s12885-018-5075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: potential mechanisms of prolonged survival. Chron Respir Dis 2013;10(1):35–47. 10.1177/1479972312471549. [DOI] [PubMed] [Google Scholar]
- [234].Giese-Davis J, Collie K, Rancourt KMS, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol 2011;29(4):413–20. 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for patients with advanced cancer. J Am Med Assoc 2009;302(7):741–9. 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Gerbild H, Larsen CM, Graugaard C, Areskoug Josefsson K. Physical activity to improve erectile function: a systematic review of intervention studies. Sex Med 2018;6(2):75–89. 10.1016/j.esxm.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Ostuni P, Botsios C, Sfriso P, et al. Prevalence and clinical features of fibromyalgia in systemic lupus erythematosus, systemic sclerosis and Sjögren’s syndrome. Minerva Med 2002;93(3):203–9. [PubMed] [Google Scholar]
- [238].Willems LM, Kwakkenbos L, Leite CC, et al. Frequency and impact of disease symptoms experienced by patients with systemic sclerosis from five European countries. Clin Exp Rheumatol 2014;32(6):88–93. [PubMed] [Google Scholar]
- [239].Knoop V, Cloots B, Costenoble A, et al. Fatigue and the prediction of negative health outcomes: a systematic review with meta-analysis. Ageing Res Rev 2021;67:101261. 10.1016/j.arr.2021.101261. [DOI] [PubMed] [Google Scholar]
- [240].Pattyn N, Van Cutsem J, Dessy E, Mairesse O. Bridging exercise science, cognitive psychology, and medical practice: is “cognitive fatigue” a remake of “the emperor’s new clothes”? Front Psychol 2018;9. 10.3389/fpsyg.2018.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Tan X, van Egmond LT, Cedernaes J, Benedict C. The role of exercise-induced peripheral factors in sleep regulation. Mol Metab 2020;42. 10.1016/j.molmet.2020.101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2017;2017(4). 10.1002/14651858.CD003200.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Thombs BD, Jewett LR, Kwakkenbos L, et al. Major depression diagnoses among patients with systemic sclerosis: baseline and one-month followup. Arthritis Care Res 2015;67(3):411–6. 10.1002/acr.22447. [DOI] [PubMed] [Google Scholar]
- [244].Pasquarelli B, Bull N. Experimental investigation of the body-mind continuum in affective states. J Nerv Ment Dis 1951; 113(6):512–21. [PubMed] [Google Scholar]
- [245].Meyer J, McDowell C, Lansing J, et al. Changes in physical activity and sedentary behaviour due to the COVID-19 outbreak and associations with mental health in 3,052 US adults. 2020. 10.33774/coe-2020-h0b8g. Published online May 12. [DOI] [PMC free article] [PubMed]
- [246].Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatr Res 2017;249:102–8. 10.1016/j.psychres.2016.12.020. [DOI] [PubMed] [Google Scholar]
- [247].Bull N A sequence concept of attitude. J Psychol 1946;22:165–73. 10.1080/00223980.1946.9917303. [DOI] [PubMed] [Google Scholar]
- [248].Awick EA, Ehlers DK, Aguiñaga S, et al. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen Hosp Psychiatr 2017;49:44–50. 10.1016/j.genhosppsych.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Rea K, Dinan TG, Cryan JF. Gut Microbiota: A Perspective for Psychiatrists. NPS 2020;79(1–2):50–62. 10.1159/000504495. [DOI] [PubMed] [Google Scholar]
- [250].Arnoriaga-Rodríguez M, Fernández-Real JM. Microbiota impacts on chronic inflammation and metabolic syndrome - related cognitive dysfunction. Rev Endocr Metab Disord 2019;20(4):473–80. 10.1007/s11154-019-09537-5. [DOI] [PubMed] [Google Scholar]
- [251].Ignácio ZM, da Silva RS, Plissari ME, et al. Physical exercise and neuroinflammation in major depressive disorder. Mol Neurobiol 2019;56(12):8323–35. 10.1007/s12035-019-01670-1. [DOI] [PubMed] [Google Scholar]
- [252].Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017;357(6349). 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- [253].Ronzi S, Orton L, Pope D, et al. What is the impact on health and wellbeing of interventions that foster respect and social inclusion in community-residing older adults? A systematic review of quantitative and qualitative studies. Syst Rev 2018; 7. 10.1186/s13643-018-0680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Lewis A, Cave P, Hopkinson NS. Singing for Lung Health: a qualitative assessment of a British Lung Foundation programme for group leaders. BMJ Open Respir Res 2017;4(1). 10.1136/bmjresp-2017-000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Child Abuse and Neglect Prevention Violence Prevention|Injury Center CDC. Published March 15, 2021, https://www.cdc.gov/violenceprevention/childabuseandneglect/index.html. [Accessed 19 March 2021].
- [256].der Kolk BAV. The body keeps the score: brain, Mind, and body in the healing of trauma. Penguin Books; 2015. [Google Scholar]
- [257].Lesley Ann Saketkoo. Living well: heart, lung, muscle & mind: a collection of videos dedicated to yoga rehab and dance rehab for heart, lung, muscle and autoimmune conditions. 2020. https://www.youtube.com/watch?v=zsBRxmkzAnM&t=2s. [Accessed 19 February 2021].
- [258].Dimitrov S, Hulteng E, Hong S. Inflammation and exercise: inhibition of monocytic TNF production by acute exercise via β2-adrenergic activation. Brain Behav Immun 2017;61:60–8. 10.1016/j.bbi.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Pettersson H, Alexanderson H. Good to excellent inter- and intrarater reliability and proven feasibility in patients with SSc. 2020. [Google Scholar]
- [260].Riley DA, Van Dyke JM. The effects of active and passive stretching on muscle length. Phys Med Rehabil Clin 2012;23(1): 51–7. 10.1016/j.pmr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- [261].Page P Current concepts IN muscle stretching for exercise and rehabilitation. Int J Sports Phys Ther 2012;7(1):109–19. [PMC free article] [PubMed] [Google Scholar]
- [262].Hotta K, Behnke BJ, Arjmandi B, et al. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J Physiol 2018;596(10):1903–17. 10.1113/JP275459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Barczyk K, Zawadzka D, Hawrylak A, et al. The influence of corrective exercises in a water environment on the shape of the antero-posterior curves of the spine and on the functional status of the locomotor system in children with Io scoliosis. Ortop Traumatol Rehabil 2009;11(3):209–21. [PubMed] [Google Scholar]
- [264].Becker BE. Aquatic therapy: scientific foundations and clinical rehabilitation applications. PM&R 2009;1(9):859–72. 10.1016/j.pmrj.2009.05.017. [DOI] [PubMed] [Google Scholar]
- [265].Severin AC, Burkett BJ, McKean MR, et al. Effects of water immersion on squat and split squat kinematics in older adults. J Aging Phys Activ 2019;27(3):398–405. 10.1123/japa.2018-0166. [DOI] [PubMed] [Google Scholar]
- [266].Stuart AR, Doble J, Presson AP, Kubiak EN. Anatomic landmarks facilitate predictable partial lower limb loading during aquatic weight bearing. Curr Orthop Pract 2015;26(4):414–9. 10.1097/BCO.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Salem Y, Scott AH, Karpatkin H, et al. Community-based group aquatic programme for individuals with multiple sclerosis: a pilot study. Disabil Rehabil 2011;33(9):720–8. 10.3109/09638288.2010.507855. [DOI] [PubMed] [Google Scholar]
- [268].Wang T-J, Belza B, Elaine Thompson F, et al. Effects of aquatic exercise on flexibility, strength and aerobic fitness in adults with osteoarthritis of the hip or knee. J Adv Nurs 2007;57(2):141–52. 10.1111/j.1365-2648.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- [269].Lima TB, Dias JM, Mazuquin BF, et al. The effectiveness of aquatic physical therapy in the treatment of fibromyalgia: a systematic review with meta-analysis. Clin Rehabil 2013;27(10):892–908. 10.1177/0269215513484772. [DOI] [PubMed] [Google Scholar]
- [270].Salem Y, Scott A, Belobravka V. Effects of an aquatic exercise program on functional mobility in individuals with multiple sclerosis: a community-based study. J Aquatic Phys Ther 2010;18(1):22–32. [Google Scholar]
- [271].Reichert T, Costa RR, Preissler AAB, et al. Short and long-term effects of water-based aerobic and concurrent training on cardiorespiratory capacity and strength of older women. Exp Gerontol 2020;142:111103. 10.1016/j.exger.2020.111103. [DOI] [PubMed] [Google Scholar]
- [272].Menegatti E, Masiero S, Zamboni P, et al. Randomized controlled trial on Dryland and Thermal Aquatic standardized exercise protocol for chronic venous disease (DATA study). J Vasc Surg Venous Lymphat Disord 2021. 10.1016/j.jvsv.2020.12.078. Published online January 9. [DOI] [PubMed] [Google Scholar]
- [273].Fappiano M, Gangaway JM. Aquatic physical therapy improves joint mobility, strength, and edema in lower extremity orthopedic injuries. J Aquatic Phys Ther 2008;16(1):10–5. [Google Scholar]
- [274].Munguía-Izquierdo D, Legaz-Arrese A. Exercise in warm water decreases pain and improves cognitive function in middleaged women with fibromyalgia. Clin Exp Rheumatol 2007;25(6):823–30. [PubMed] [Google Scholar]
- [275].Watts KE, Gangaway JM. Evidence-based treatment of aquatic physical therapy in the rehabilitation of upper-extremity orthopedic injuries. J Aquatic Phys Ther 2007;15(1):19–26. [Google Scholar]
- [276].Cardoso JR, Atallah AN, Cardoso A. Aquatic therapy exercise for treating rheumatoid arthritis. Cochrane Database Syst Rev 2009. 10.1002/14651858.CD003684. Published online. [DOI] [Google Scholar]
- [277].Broach E, Dattilo J. The effect of aquatic therapy on strength of adults with multiple sclerosis. Ther Recreat J 2003;37(3). https://js.sagamorepub.com/trj/article/view/1015. [Accessed 21 March 2021]. [Google Scholar]
- [278].Severin AC, Burkett BJ, McKean MR, Sayers MGL. Biomechanical aspects of aquatic therapy: A Literature Review on Application and Methodological Challenges 5; 2016. p. 16 (1). [Google Scholar]
- [279].Meffert H, Lemke U, Fehlinger R, et al. Influence of underwater massage on the re-warming, thermal conductivity and blood supply of the skin in progressive scleroderma. Dermatol Monatsschr 1975;161(7):551–5. [PubMed] [Google Scholar]
- [280].Racine M, Hudson M, Baron M, Nielson WR. Canadian scleroderma research group. The impact of pain and itch on functioning and health-related quality of life in systemic sclerosis: an exploratory study. J Pain Symptom Manag 2016; 52(1):43–53. 10.1016/j.jpainsymman.2015.12.314. [DOI] [PubMed] [Google Scholar]
- [281].Constitution. https://www.who.int/about/who-we-are/constitution. [Accessed 15 March 2021].
- [282].Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol 2020;34(2):101504. 10.1016/j.berh.2020.101504. [DOI] [PubMed] [Google Scholar]
- [283].Woolstenhulme JG, Guccione AA, Herrick JE, et al. Left ventricular function before and after aerobic exercise training in women with pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 2019;39(2):118–26. 10.1097/HCR.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Hung G, Mercurio V, Hsu S, et al. Progress in understanding, diagnosing, and managing cardiac complications of systemic sclerosis. Curr Rheumatol Rep 2019;21(12):68. 10.1007/s11926-019-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Nakazawa A, Cox NS, Holland AE. Current best practice in rehabilitation in interstitial lung disease. Ther Adv Respir Dis 2017;11(2):115–28. 10.1177/1753465816676048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Mahler DA. The measurement of dyspnea during exercise in patients with lung disease. Chest 1992;101(5 Suppl): 242S–7S. [PubMed] [Google Scholar]
- [287].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14(5):377–81. [PubMed] [Google Scholar]
- [288].Desai SA, Channick RN. Exercise in patients with pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 2008;28(1): 12–6. 10.1097/01.HCR.0000311502.57022.73. [DOI] [PubMed] [Google Scholar]
- [289].Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114(14):1482–9. 10.1161/CIR-CULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- [290].Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188(8):e13–64. 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- [291].Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med 2020;202(10):e121–41. 10.1164/rccm.202009-3608ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Oliveira NC, dos Santos Sabbag LM, de Sa Pinto AL, et al. Aerobic exercise is safe and effective in systemic sclerosis. Int J Sports Med 2009;30(10):728–32. 10.1055/s-0029-1224180. [DOI] [PubMed] [Google Scholar]
- [293].Alexanderson H, Bergegård J, Björnådal L, Nordin A. Intensive aerobic and muscle endurance exercise in patients with systemic sclerosis: a pilot study. BMC Res Notes 2014;7:86. 10.1186/1756-0500-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Mitropoulos A, Gumber A, Akil M, Klonizakis M. Exploring the microcirculatory effects of an exercise programme including aerobic and resistance training in people with limited cutaneous systemic sclerosis. Microvasc Res 2019;125: 103887. 10.1016/j.mvr.2019.103887. [DOI] [PubMed] [Google Scholar]
- [295].Pizzo G, Scardina GA, Messina P. Effects of a nonsurgical exercise program on the decreased mouth opening in patients with systemic scleroderma. Clin Oral Invest 2003;7(3):175–8. 10.1007/s00784-003-0216-5. [DOI] [PubMed] [Google Scholar]
- [296].Poole J, Conte C, Brewer C, et al. Oral hygiene in scleroderma: the effectiveness of a multi-disciplinary intervention program. Disabil Rehabil 2010;32(5):379–84. 10.3109/09638280903171527. [DOI] [PubMed] [Google Scholar]
- [297].Yuen HK, Marlow NM, Reed SG, et al. Effect of orofacial exercises on oral aperture in adults with systemic sclerosis. Disabil Rehabil 2012;34(1):84–9. 10.3109/09638288.2011.587589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Yuen HK, Weng Y, Bandyopadhyay D, et al. Effect of a multi-faceted intervention on gingival health among adults with systemic sclerosis. Clin Exp Rheumatol 2011;29(2 Suppl 65):S26–32. [PMC free article] [PubMed] [Google Scholar]
- [299].Mugii N, Matsushita T, Oohata S, et al. Long-term follow-up of finger passive range of motion in Japanese systemic sclerosis patients treated with self-administered stretching. Mod Rheumatol 2019;29(3):484–90. 10.1080/14397595.2018.1466635. [DOI] [PubMed] [Google Scholar]
- [300].Mugii N, Hasegawa M, Matsushita T, et al. The efficacy of self-administered stretching for finger joint motion in Japanese patients with systemic sclerosis. J Rheumatol 2006;33(8):1586–92. [PubMed] [Google Scholar]
- [301].Piga M, Tradori I, Pani D, et al. Telemedicine applied to kinesiotherapy for hand dysfunction in patients with systemic sclerosis and rheumatoid arthritis: recovery of movement and telemonitoring technology. J Rheumatol 2014;41(7): 1324–33. 10.3899/jrheum.130912. [DOI] [PubMed] [Google Scholar]
- [302].Boström C, Harms-Ringdahl K, Nordemar R. Relationships between measurements of impairment, disability, pain, and disease activity in rheumatoid arthritis patients with shoulder problems. Scand J Rheumatol 1995;24(6):352–9. 10.3109/03009749509095180. [DOI] [PubMed] [Google Scholar]
- [303].Ernste FC, Chong C, Crowson CS, et al. Functional index-3: a valid and reliable functional outcome assessment measure in patients with dermatomyositis and polymyositis. J Rheumatol 2021;48(1):94–100. 10.3899/jrheum.191374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70(2):113–9. 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- [305].Astrand I Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl 1960; 49(169):1–92. [PubMed] [Google Scholar]
- [306].Enright PL. The six-minute walk test. Respir Care 2003;48(8):783–5. [PubMed] [Google Scholar]