Abstract
Objective:
To examine the association between neonatal cranial ultrasound abnormalities among infants born extremely preterm and neurodevelopmental outcomes at ten years of age.
Study design:
In a multi-center birth cohort of infants born at < 28 weeks’ gestation, 889 of 1198 survivors were evaluated for neurological, cognitive, and behavioral outcomes at 10 years of age. Sonographic markers of white matter damage (WMD) included echolucencies in the brain parenchyma and moderate to severe ventricular enlargement. Neonatal cranial ultrasound findings were classified as: intraventricular hemorrhage (IVH) without WMD, IVH with WMD, WMD without IVH, and neither IVH nor WMD.
Results:
WMD without IVH was associated with an increased risk of cognitive impairment (OR 3.5, 95% CI 1.7, 7.4), cerebral palsy (OR 14.3, 95% CI 6.5, 31.5), and epilepsy (OR 6.9; 95% CI 2.9, 16.8). Similar associations were found for WMD accompanied by IVH. Isolated IVH was not significantly associated these outcomes.
Conclusions:
Among children born extremely preterm, cranial ultrasound abnormalities, particularly those indicative of WMD, are predictive of neurodevelopmental impairments at 10 years of age. The strongest associations were found with cerebral palsy.
Keywords: extreme prematurity, neurodevelopmental outcomes, intraventricular hemorrhage, perinatal brain injury, ultrasound, white matter injury
Compared with children born at term, those born extremely preterm (<28 weeks’ gestation) are at increased risk for long-term neurodevelopmental impairments.(1–3) Among preterm infants, certain prenatal and neonatal factors are predictive of adverse neurodevelopmental outcomes, but early and accurate prediction of such impairments remains an important challenge in neonatology.
Prior studies in preterm born children have indicated that cranial ultrasound (CUS) abnormalities, such hypoechoic (echolucent) areas in the white matter predict cognitive impairment,(4–6) cerebral palsy,(7, 8) and autism spectrum disorder(9). The most prominent limitation of existing studies is that only a few have followed children until middle childhood when many neurodevelopmental outcomes, such as executive function and social responsiveness, are more accurately assessed and more stable than when evaluated in infancy or early childhood.(10–12) Moreover, identifying longer-term functional developmental outcomes of neonatal cranial ultrasound abnormalities may provide pediatricians, parents/caregivers, and other stakeholders a clearer understanding of what remedial therapies and educational supports are likely to be needed for an individual infant born extremely preterm. Here we describe the relationship between CUS abnormalities identified during the initial neonatal intensive care hospitalization and neurodevelopmental outcomes identified at ten years of age, with a focus on ultrasound findings after the initial several postnatal weeks.
Methods
This analysis was based on data collected for the Extremely Low Gestational Age Newborn (ELGAN) Study, a prospective longitudinal study in which 1506 neonates born extremely preterm (23–27 full weeks of gestation) were enrolled at birth from 14 hospitals in five states in the United States between 2002–2004.(13) Of 1198 surviving children, 966 were eligible for follow-up assessment at 10 years of age based on availability of data on levels of protein biomarkers in the first two postnatal weeks. Of 966 eligible children, 889 (92%) participated in comprehensive neurodevelopmental and neurobehavioral assessments at 10 years of age.(14) The institutional review boards of all participating institutions approved the study. At enrollment all mothers provided informed consent for their infant’s participation; at follow-up evaluation at 10 years of age a parent or guardian provided informed consent and children provided assent. A flow chart of participant enrollment is included as Figure 1 (available at www.jpeds.com). Maternal (eg, education and marital status) and neonatal data (eg, bronchopulmonary dysplasia and other morbidities) were obtained by a structured maternal interview during the birth hospitalization and review of maternal and neonatal medical records by a trained research coordinator during the infant’s stay in the NICU.
Figure 1; online:
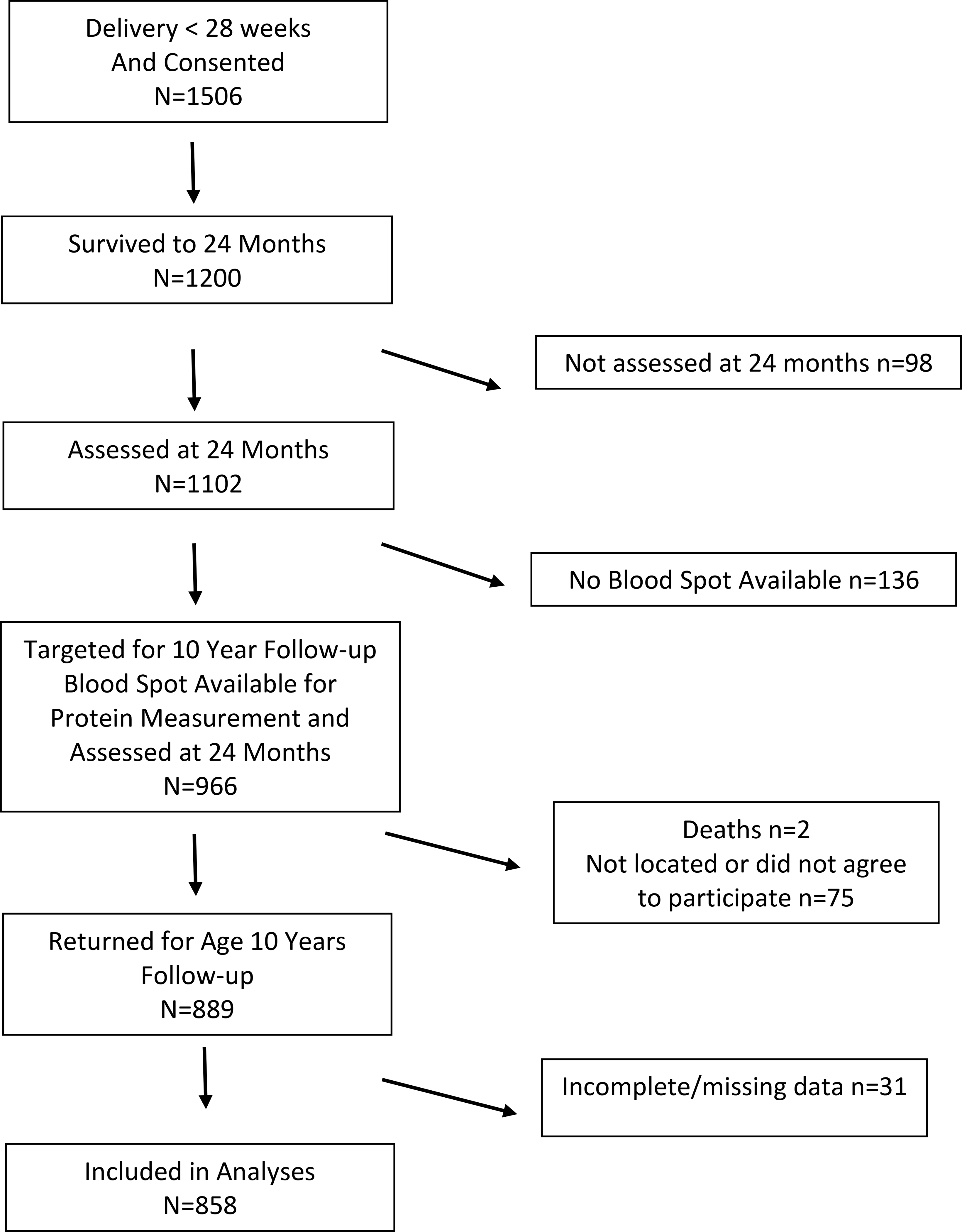
Flowchart of study participant enrollment
Neonatal Cranial Ultrasound Scans
Neonatal CUS scans were obtained as a component of standard care.(15) The anterior fontanelle was used as the sonographic window. Scans were performed with digital high-frequency transducers (7.5 and 10 megahertz). All scans included the 6 standard quasi-coronal views and 5 sagittal views; mastoid window views of the cerebellum were not routinely obtained. This study focused on CUS scans that were obtained between the 15th postnatal day and 40 weeks post-menstrual age.(15)
To minimize observer bias, all scans were read by two independent radiologists unaware of clinical information. A third reader, unaware of the initial two reads, was used as a tie-breaker when the initial two readers differed in their recognition of intraventricular hemorrhage (IVH), echolucent (hypoechoic) or echodense (hyperechoic) parenchymal lesions, or moderate to severe ventriculomegaly. For the current study, we defined white matter damage as the presence of either parenchymal echolucency (hypoechoic zone) and/or moderate to severe ventriculomegaly on a “late” scan (performed after the first two postnatal weeks).
For the primary analysis we categorized all participants into four distinct and mutually exclusive groups based on the available sonographic information: (1) children with neither IVH nor WMD; (2) children with IVH but no WMD (Isolated IVH); (3) children with WMD but no IVH (Isolated WMD); and (4) children with IVH and WMD. When classifying study participants with regard to ultrasound abnormalities we did not consider information about isolated parenchymal echodensity (hyperechoic area) without echolucency or ventriculomegaly, isolated frontal lobe cysts, or isolated germinal matrix hemorrhage. The clinical significance of these abnormalities, compared with IVH and WMD, is less clear (6, 7, 16–18) and the radiographic identification of these abnormalities is less reliable.(15) Furthermore, most cerebral echodensities have either resolved or become echolucent after the first several postnatal weeks. Thirty-one study participants (3.5%) had missing information about “late” ventricular enlargement and therefore could not be categorized in regard to their ultrasound findings and were excluded from the analyses reported here. The specific forms of white matter injury that were noted in the “Isolated WMD” and “WMD + IVH” groups can be found in Table 1 (available at www.jpeds.com). In Table 2 (available at www.jpeds.com) outcomes are presented for the different definitions of white matter injury: the definition on which we focused in this study (cerebral echolucency or ventricular enlargement on an ultrasound performed after the first two postnatal weeks); echolucency or ventricular enlargement, regardless of the postnatal age when noted; and echolucency or echodensity or ventricular enlargement, regardless of the postnatal age when noted.
Table 1; online:
Specific forms of white matter injury noted on cranial ultrasounds classified as WMD
| Form of white matter injury | Isolated WMD % (N) | IVH + WMD % (N) |
|---|---|---|
| Late echolucency only | 7 (2) | 27 (17) |
| Late moderate-to-severe ventriculomegaly only | 70 (21) | 25 (16) |
| Both findings | 23 (7) | 48 (30) |
| TOTAL | 30 | 63 |
Abbreviations: WMD- white matter damage
Table 2; online:
Frequencies of different outcomes for infants with variably-defined WMD findings.
| Definition of WMD | Number with WMD / Total (%) | No neurodevelopme ntal burden %(N) | Normal cognition with CP, ASD, and/or epilepsy % (N) | Cognitive impairment % (N) |
|---|---|---|---|---|
| Echolucency OR late ventriculomegaly* | 93 / 858 (10.5) | 52.7 (49) | 17.2 (16) | 30.1 (28) |
| Echolucency OR ventriculomegaly (includes early and late) | 123 / 889 (13.8) | 44.7 (55) | 17.1 (21) | 38.2 (47) |
| Echolucency OR echodensity OR ventriculomegaly (includes early and late) | 188 / 889 (21.2) | 41.0 (77) | 14.9 (28) | 44.2 (83) |
Abbreviations: ASD- autism spectrum disorder; CP- cerebral palsy; WMD – white matter damage
31 infants did not undergo late ultrasound and could not be classified in regard to presence or absence of late ventriculomegaly
We did not consider information about cerebellar abnormalities. Although existing studies suggest that these abnormalities are clinically significant, without mastoid views we were not confident that our ascertainment of cerebellar abnormalities was accurate.
Neurodevelopmental Outcomes
Cognitive function:
To determine cognitive functioning at 10 years of age, we evaluated study participants with the School-Age Differential Ability Scales-II (DAS-II) and the “Developmental NEuroPSYchological Assessment” tool (NEPSY-II). We employed latent profile analysis to identify subgroups of extremely preterm (EP) children with similar profiles on 9 domains measuring verbal and nonverbal IQ (DAS-II Verbal and Nonverbal Reasoning scales), working memory (DAS-II Recall of Digits Backwards, Recall Sequential Order), concept generation and mental flexibility (NEPSY-II Animal Sorting), auditory attention and set switching (NEPSY-II Auditory Attention, Response Set), and simple inhibition and inhibition shifting (NEPSY-II Inhibition Inhibition and Inhibition Switching). Using this approach, four neurocognitive profiles were identified : 1) “normal” - mean IQ and executive function scores within the normal range on all measures; 2) “low-normal”- mean IQ and executive function scores ranged from 0.5–1 standard deviations below the mean in the normative sample; 3) “moderately impaired” - mean IQ and executive function scores between 1.5 and 2.5 standard deviations below the mean in the normative sample; and 4) “severely impaired” - mean IQ and executive function scores between 3 to 4 standard deviations below the mean in the normative sample.(19)
Cerebral palsy:
Cerebral palsy was diagnosed using a standardized neurological examination and an algorithm applied to the examination findings.(20, 21) In this analysis we classified children as having moderate or severe cerebral palsy if they had a Gross Motor Function Classification System level of 2 or greater.(22)
Epilepsy:
Identification of children with seizures or epilepsy involved a two-stage process using a validated seizure screen, completed by the parent or guardian, followed by a clinical interview with a pediatric epileptologist.(23) At age 10 years, a research assistant surveyed parents using part one of the screen, which asks 11 broad questions about seizure symptoms. A yes response to any of these questions prompted a pediatric epileptologist to schedule a structured telephone interview to determine whether a reported event was indeed a seizure. A second pediatric epileptologist independently reviewed interview responses and similarly rated the event type. When the two epileptologists disagreed on the presence of seizures, a third pediatric epileptologist reviewed the interview responses and made the final seizure determination. Epilepsy was defined as having two or more unprovoked seizures at any time prior to the study participant’s evaluation at ten years of age. Electroencephalography data were not obtained. (24)
Autism spectrum disorder (ASD) and social impairment:
Children were screened for ASD with the Social Communication Questionnaire (SCQ).(25) Those who met a lenient SCQ cut-off score (>11) were evaluated with the Autism Diagnostic Interview-Revised (ADI-R).(26) The respondent for these measures was the parent or guardian who accompanied the child to the study visit. Children who met ADI-R criteria for ASD (27) were then administered the Autism Diagnostic Observation Schedule-2 (ADOS-2).(28) Children were classified as having ASD if they met standardized research criteria for ASD on the ADOS-2. We also evaluated sub-clinical social impairment using the Social Responsiveness Scale (SRS), completed by the parent or guardian.(29, 30)
Anxiety and Depression:
To identify children with anxiety and/or depression/dysthymia, we used the Child Symptom Inventory – 4 (CSI-4)(31, 32), which was completed by both the parent or guardian as well as the child’s school teacher. Anxiety disorders included generalized anxiety disorder, separation anxiety disorder, and social phobia. Children were classified as having anxiety or depression/dysthymia if they screened positive for these disorders by report of either the parent or the teacher.
Attention deficit hyperactivity disorder (ADHD):
To identify children with ADHD, we used the CSI-4, completed by parents and the child’s teacher, as well as a parental report of their child as having been diagnosed with ADHD. As previously described, children were classified as having ADHD if this diagnosis was supported by at least two of the three sources of information (parent and teacher CSI-4 and physician diagnosis).(33, 34)
Global outcome measures:
In addition to individual impairments, we classified children at age 10 according to a composite outcome of neurodevelopmental burden as described previously(35): (1) Children without neurodevelopmental burden who were free from cognitive impairment, cerebral palsy, autism spectrum disorder, and epilepsy; (2) children without cognitive impairment but with one or more of the following neurologic morbidities: cerebral palsy, autism spectrum disorder, or epilepsy; and (3) children with cognitive impairment, with or without other neurologic impairments. To evaluate quality of life outcomes we used the Pediatric Quality of Life Inventory-Version 4, which was completed by the parent or guardian who accompanied the child to the study visit.(36)
Statistical Analyses
We examined descriptive statistics for the total sample and separately for each type of CUS abnormality. Chi square tests (for categorical variables) were used to examine univariate associations and logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI). When estimating multivariable OR for associations between ultrasound findings and outcomes, we adjusted for gestational age, birthweight z score, sex, maternal education, bronchopulmonary dysplasia, sepsis, necrotizing enterocolitis (Bell Stage 2 or 3), and severe retinopathy of prematurity. Generalized estimating equations (GEE) were conducted to estimate ORs that accounted for clustering from participants who were non-singleton births. GEE analyses were conducted in R using the geepack package.(37–39) For comparison of IQ scores as a continuous measure we used the Wilcoxon rank sum test. Sensitivity, specificity, positive predictive values, negative predictive values, likelihood ratios, and confidence intervals for these test characteristics, were obtained using the online application https://www.medcalc.org/calc/diagnostic_test.php.
RESULTS
Study participants
Compared with the 309 study participants who were not evaluated at ten years but were presumed to be alive, the 889 study participants who were evaluated at ten years of age were more likely to be white race and more likely to have mothers who had attended college but were less likely to have received public health insurance during the pregnancy that resulted in the ELGAN Study participant’s birth (Table 3; available at www.jpeds.com). Neonatal characteristics, such as gestational age, sepsis, and bronchopulmonary dysplasia, were similar for participants who were evaluated at ten years and those who were not evaluated. Of the 889 study participants who were evaluated at ten years of age, 858 (96.5%) underwent ultrasound scanning at least once after the second postnatal week and were included in the analysis reported here. 641 infants (75%) had neither IVH nor WMD, 124 (14%) had IVH without WMD, 30 (3.5%) had WMD without IVH, and 63 (7.3%) had both IVH and WMD. Males and those born 23–24 weeks gestation were overrepresented among children who had both IVH and WMD (Table 4).
Table 3;online:
Prenatal and neonatal attributes of study participants who were evaluated at ten years and study participants who were not evaluated at ten years. Data are column percentages.
| Not evaluated at 10 years | Evaluated at 10 years | |
|---|---|---|
| Female | 46 | 49 |
| Non-white race | 50 | 37 |
| Hispanic ethnicity | 20 | 10 |
| Medicaid eligible | 53 | 35 |
| Maternal education (years) | ||
| ≤ 12 | 52 | 41 |
| >12, < 16 | 24 | 23 |
| ≥ 16 | 24 | 35 |
| Gestational age (weeks) | ||
| 23–24 | 19 | 21 |
| 25–26 | 50 | 45 |
| 27 | 32 | 34 |
| Sepsis | 28 | 29 |
| Chronic lung disease | 45 | 52 |
| TOTAL | 309 | 889 |
Table 4:
Prenatal and neonatal attributes of study participants and their mothers. Data are column percentages with number of participants in parenthesis.
| Ultrasound findings % (Ns) | ||||
|---|---|---|---|---|
| Neither IVH nor WMD | Isolated IVH | Isolated WMD | IVH and WMD | |
| Female | 51 (328) | 44 (54) | 57 (17) | 33 (21) |
| Non-white race | 36 (231) | 29 (36) | 40 (12) | 44 (28) |
| Hispanic ethnicity | 10 (65) | 6 (7) | 17 (5) | 11 (7) |
| Gestational age (weeks) | ||||
| 23–24 | 18 (113) | 27 (34) | 17 (5) | 46 (29) |
| 25–26 | 45 (290) | 51 (63) | 47 (14) | 37 (23) |
| 27 | 37 (238) | 22 (27) | 37 (11) | 17 (11) |
| Birthweight z-score | ||||
| ≤ −2 | 7 (46) | 2 (3) | 3 (2) | |
| < −1 | 15 (93) | 15 (18) | 13 (4) | 6 (4) |
| ≥ −1 | 78 (502) | 83 (103) | 83 (25) | 90 (57) |
| Multiple gestation | 33 (213) | 35 (43) | 27 (8) | 33 (21) |
| Medicaid eligible | 34 (216) | 31 (39) | 46 (13) | 35 (22) |
| Maternal education (years) | ||||
| ≤ 12 | 41 (256) | 38 (47) | 42 (11) | 39 (24) |
| >12, < 16 | 23 (140) | 22 (27) | 38 (10) | 31 (19) |
| ≥ 16 | 36 (226) | 40 (50) | 19 (5) | 30 (18) |
| Receipt of antenatal steroids | 89 (566) | 93 (112) | 87 (26) | 89 (55) |
| Cesarean delivery | 58 (72) | 50 (15) | 59 (37) | |
| Indication for preterm delivery | ||||
| 44 (280) | 48 (59) | 60 (18) | 60 (38) | |
| pPROM | 22 (141) | 22 (27) | 17 (5) | 21 (13) |
| Preeclampsia | 15 (97) | 8 (10) | 3 (1) | 5 (3) |
| Abruption | 11 (68) | 15 (18) | 3 (1) | 2 (1) |
| Cervical insufficiency | 4 (28) | 6 (8) | 7 (2) | 10 (6) |
| Fetal indication | 4 (27) | 2 (2) | 10 (3) | 3 (2) |
| Late onset sepsis | 24 (152) | 29 (36) | 23 (7) | 38 (24) |
| Necrotizing enterocolitis | 7 (47) | 9 (11) | 17 (5) | 25 (16) |
| Severe retinopathy of prematurity | 12 (75) | 16 (20) | 17 (5) | 25 (16) |
| Bronchopulmonary dysplasia | 50 (327) | 50 (61) | 53 (16) | 73 (46) |
| Maximum Column Total | 641 | 124 | 30 | 63 |
Abbreviations: IVH- intraventricular hemorrhage; pPROM- preterm premature rupture of membranes; WMD- white matter damage defined as cerebral echolucency or late moderate to severe ventriculomegaly
CUS Findings and Neurodevelopmental Outcomes
Cognitive outcomes:
Overall, about three quarters of children were classified as having either normal or low-normal cognitive function at 10 years of age (Table 5). Children with isolated IVH had similar cognitive outcomes as children whose ultrasounds were free from IVH and WMD. The lack of difference was found both when cognitive outcome was modelled as a categorical outcome as well as in analysis where IQ scores were treated as continuous outcomes; specifically children whose ultrasounds had isolated IVH, compared with those whose ultrasounds had neither IVH nor WMD, had similar IQ scores. Unadjusted mean differences between scores for children with IVH versus those without IVH or WMD (95% confidence interval in parentheses) ranged from −3.0 (−6.6, 0.6) for nonverbal IQ to −2.2 (5.7, 1.4) for full scale IQ (data not shown). In contrast, the presence of WMD, whether alone or in combination with IVH was associated with a significant increase in risk of moderate-to-severe cognitive impairment (unadjusted OR 3.5, 95% CI 1.7–7.4 and 5.0, 95% CI 2.9–8.5, respectively). Figure 2 and Table 6 (available at www.jpeds.com) show that these associations persisted after adjusting for other perinatal variables associated with cognitive impairment including gestational age, birthweight z-score, sex, maternal education, bronchopulmonary dysplasia, necrotizing enterocolitis, and severe retinopathy of prematurity. OR estimates similar to those presented in Table 6were obtained in a sensitivity analysis in which we adjusted further for mother’s marital status at birth, mother’s eligibility for Medicaid during her pregnancy, and enrollment site.
Table 5:
Frequencies of various neurodevelopmental outcomes by cranial ultrasound finding
| Outcome | Ultrasound findings % (Ns) | |||
|---|---|---|---|---|
| Neither IVH nor WMD | Isolated IVH | Isolated WMD | IVH and WMD | |
| Neurologic/cognitive outcomes | ||||
| Cognitive function | ||||
| Normal | 37 (235) | 33 (41) | 17 (5) | 13 (8) |
| Low-normal | 43 (269) | 41 (50) | 34 (10) | 30 (18) |
| Moderate impairment | 15 (93) | 20 (24) | 24 (7) | 28 (17) |
| Severe impairment | 6 (35) | 6 (7) | 24 (7) | 30 (18) |
| Cerebral palsy | 6 (37) | 7 (9) | 47 (14) | 51 (32) |
| GMFCS > 0 | 2 (13) | 3 (4) | 33 (10) | 25 (16) |
| Epilepsy | 4 (25) | 9 (11) | 27 (8) | 19 (12) |
| Neuropsychiatric/neurobehavioral outcomes | ||||
| Pediatric quality of life < 70 | 20 (131) | 25 (41) | 40 (12) | 49 (31) |
| Autism spectrum disorder | 7 (42) | 9 (11) | 7 (2) | 6 (4) |
| SRS ≥ 65 among children with IQ > 85, excluding those with ASD | 10 (62) | 11 (14) | 13 (4) | 8 (5) |
| ADHD medication use | 15 (96) | 25 (3) | 7 (2) | 19 (12) |
| ADHD† | 15 (97) | 25 (31) | 10 (3) | 24 (13) |
| Anxiety | ||||
| (parent report) | 15 (98) | 8 (10) | 10 (3) | 10 (6) |
| (teacher report) | 14 (88) | 11 (14) | 10 (3) | 19 (12) |
| Depression | ||||
| 16 (100) | 11 (14) | 23 (7) | 11 (7) | |
| (teacher report) | 15 (96) | 15 (18) | 23 (7) | 32 (20) |
| Composite neurodevelopmental burden | ||||
| No cognitive impairment, CP, ASD, or epilepsy | 76 (487) | 69 (86) | 40 (12) | 38 (24) |
| No cognitive impairment; one or more of CP, ASD, or epilepsy | 4 (26) | 6 (7) | 13 (4) | 6 (4) |
| Cognitive impairment | 20 (128) | 25 (31) | 47 (14) | 56 (35) |
| Maximum Column Total | 641 | 124 | 30 | 63 |
Abbreviations: ASD- autism spectrum disorder; CP- cerebral palsy; GMFCS- gross motor function classification system; IVH- intraventricular hemorrhage; SRS- social responsiveness scale; WMD- white matter damage, defined as cerebral echolucency or late moderate-to-severe ventriculomegaly
Cognitive function group derived from latent profile analysis, using scores on 9 variables measuring verbal and nonverbal IQ (DAS-II Verbal and Nonverbal Reasoning scales), working memory (DAS-II Recall of Digits Backwards, Recall Sequential Order), concept generation and mental flexibility (NEPSY-II Animal Sorting), auditory attention and set switching (NEPSY-II Auditory Attention, Response Set), and simple inhibition and inhibition shifting (NEPSY-II Inhibition Inhibition and Inhibition Switching). Sample sizes for cognitive function for each ultrasound finding were: Neither IVH nor WMD- 632; Isolated IVH- 122; Isolated WMD- 29; IVH and WMD- 61
ADHD defined as at least two of the following: screen positive for ADHD on the parent-reported Child Symptom Inventory-4 (CSI-4), screen positive on the teacher-reported CSI-4, and physician diagnosis of ADHD, as reported by the parent
Figure 2:
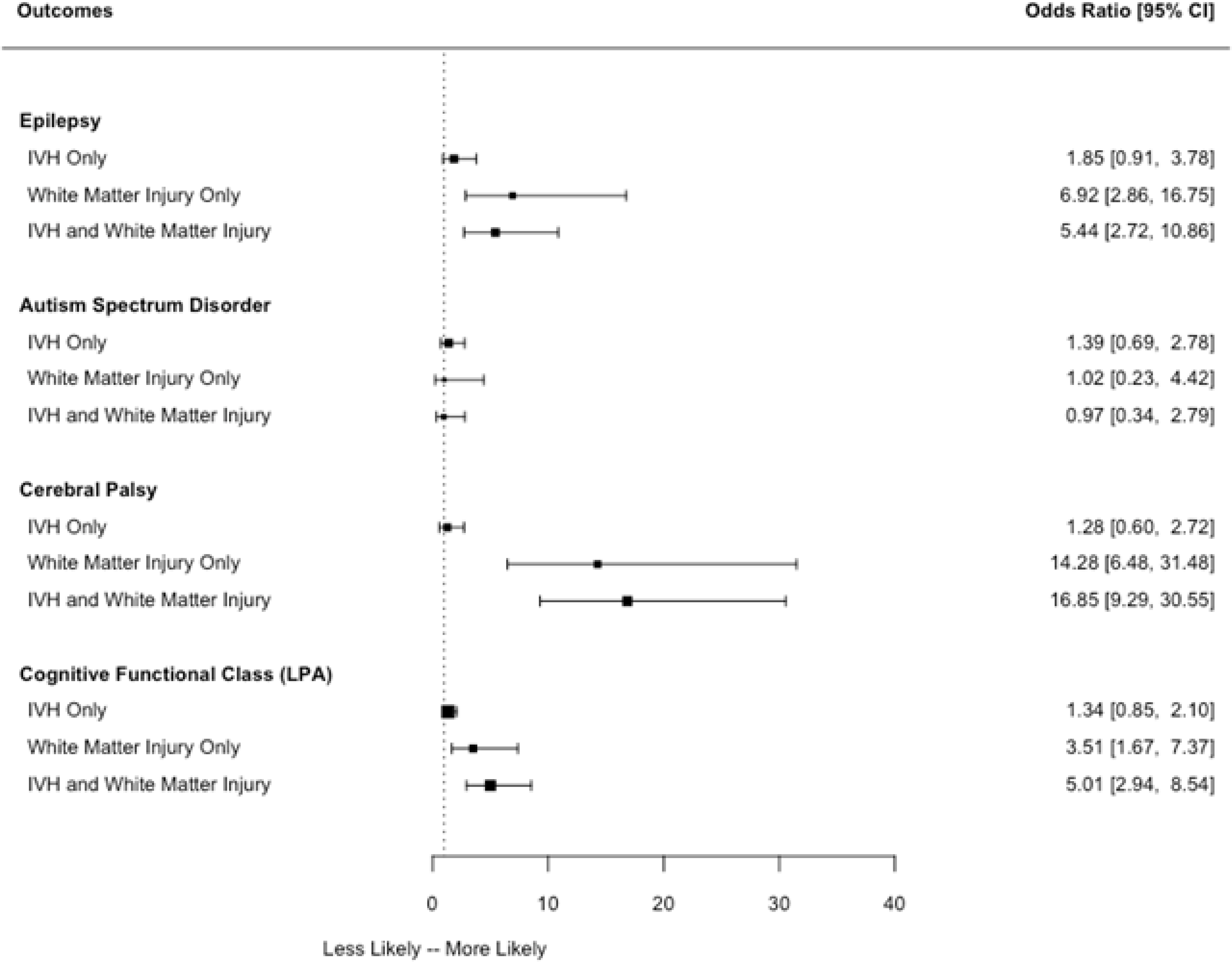
Association between ultrasound findings and neurodevelopmental outcomes. Adjusted odds ratios are adjusted for gestational age, birthweight z-score, sex, maternal education, bronchopulmonary dysplasia, sepsis, necrotizing enterocolitis (Bell Stage 2 or 3), and severe retinopathy of prematurity. Abbreviations: CI- confidence interval; IVH- intraventricular hemorrhage; WMD- white matter damage defined as cerebral echolucency or late moderate-to-severe ventriculomegaly
Table 6; online:
Association between ultrasound findings and neurodevelopmental outcomes. For each outcome listed in the left-most column, the top row shows unadjusted odds ratios; the second row shows odds ratios adjusted for gestational age, birthweight z-score, sex, maternal education, bronchopulmonary dysplasia, sepsis, necrotizing enterocolitis (Bell Stage 2 or 3), and severe retinopathy of prematurity; the third row shows adjusted odds ratios estimated with generalized estimation equations; with each multiple birth considered as a cluster.
| Outcome | Ultrasound findings | |||
|---|---|---|---|---|
| Neither IVH nor WMD | Isolated IVH | Isolated WMD | IVH and WMD | |
| Cerebral palsy | ||||
| Crude OR | 1.0 (ref) | 1.28 (0.60, 2.72) | 14.28 (6.48, 31.48) | 16.85 (9.29, 30.55) |
| Adjusted OR | 1.0 (ref) | 1.19 (0.54, 2.61) | 18.63 (7.37, 47.06) | 13.43 (7, 25.78) |
| GEE | 1.0 (ref) | 1.04 (0.46, 2.35) | 17.65 (6.88, 45.32) | 14.08 (6.96, 28.49) |
| Autism | ||||
| Crude OR | 1.0 (ref) | 1.39 (0.69, 2.78) | 1.02 (0.23, 4.42) | 0.97 (0.34, 2.79) |
| Adjusted OR | 1.0 (ref) | 1.24 (0.59, 2.6) | 0.74 (0.09, 5.88) | 0.58 (0.19, 1.77) |
| GEE OR | 1.0 (ref) | 1.28 (0.61, 2.68) | 0.83 (0.15, 4.57) | 0.63 (0.21, 1.88) |
| Epilepsy | ||||
| Crude OR | 1.0 (ref) | 6.92 (2.86, 16.75) | 5.44 (2.72, 10.86) | |
| Adjusted OR | 1.0 (ref) | 1.5 (0.68, 3.3) | 7.56 (2.85, 20.06) | 4.89 (2.31, 10.35) |
| GEE OR | 1.0 (ref) | 1.57 (0.71, 3.51) | 7.46 (2.85, 19.5) | 4.28 (1.98, 9.28) |
| Cognitive Impairment | ||||
| Crude OR | 1.0 (ref) | 1.34 (0.85, 2.1) | 3.51 (1.67, 7.37) | 5.01 (2.94, 8.54) |
| Adjusted OR | 1.0 (ref) | 1.21 (0.73, 1.98) | 5.07 (2.13, 12.02) | 4.49 (2.49, 8.11) |
| GEE OR | 1.0 (ref) | 1.3 (0.78, 2.17) | 5.56 (2.2, 14.07) | 4.79 (2.53, 9.06) |
Abbreviations: GEE- generalized estimation equation IVH- intraventricular hemorrhage; OR- odds ratio; WMD- white matter damage defined as cerebral echolucency or late moderate-to-severe ventriculomegaly
Neurological disorders and quality of life:
Neonatal WMD was associated with an increased frequency of cerebral palsy, epilepsy, and low quality of life score at age 10 years (Table 5). Approximately 50% of children with WMD had cerebral palsy compared with only 6% of children without IVH or WMD. WMD was also associated with an increased risk of epilepsy (unadjusted OR 6.9 for WMD, OR for WMD with IVH 5.4) and an increased risk of low quality of life score. The frequency of epilepsy was slightly more than double among those with isolated IVH, as compared with those with neither IVH nor WMD, but this association was not statistically significant after adjustment for potential confounders. No association was found between isolated IVH and the frequency of cerebral palsy or low quality of life.
Psychiatric outcomes:
The psychiatric outcomes associated with ultrasound abnormalities were ADHD, which was identified more frequently among infants with IVH (OR 1.6, 95% CI 1.1, 2.5) and teacher-reported depression, which was about twice as likely among children with WMD compared with those without WMD (Table 5). Although the frequency of non-ASD social impairment was higher among children with WMD, this difference was not statistically significant. In a sensitivity analysis we separately evaluated associations between ASD and ventriculomegaly and between ASD and echolucency. The unadjusted OR for ventriculomegaly was 1.8 (0.9, 3.7) and the unadjusted OR for echolucency was 1.1 (0.4, 3.2).
Test characteristics:
CUS findings had low sensitivity (10–21%) for identification of children who developed either neurologic or cognitive impairments (Table 7). Specificity was high (95–98%) for the finding of WMD for identification of children who would not develop neurological or cognitive impairments. Positive and negative likelihood ratios were around 1 for the finding of IVH, indicating that this finding does not add information relative to neurodevelopmental prognosis. In contrast, the positive likelihood ratios for WMD ranged between 3.3 (1.6, 6.5) and 8.2 (4.0, 16.7), suggesting that this finding does provide useful information when present.
Table 7:
Test Characteristics for ultrasound findings to predict neurodevelopmental outcome. Test negative group consists of infants with neither IVH nor WMD. Test positive group consists of infants with ultrasound abnormality listed in the left-most column.
| Prediction of neurological disorder† | ||||
| Sensitivity | Specificity | +LR | −LR | |
| Isolated IVH | 21 (14, 29) | 85 (82, 87) | 1.4 (0.9, 2.0) | 0.93 (0.85, 1.03) |
| Isolated WMD | 16 (10, 24) | 98 (96, 99) | 8.2 (4.0, 16.7) | 0.85 (0.8, 0.9) |
| IVH + WMD | 27 (20, 36) | 95 (93, 97) | 6.0 (3.8, 9.6) | 0.8 (0.7, 0.9) |
| Prediction of cognitive impairment | ||||
| Sensitivity | Specificity | +LR | −LR | |
| Isolated IVH | 20 (14, 27) | 85 (82, 87) | 1.3 (0.9, 1.8) | 0.95 (0.87, 1.03) |
| Isolated WMD | 10 (6, 16) | 97 (95, 98) | 3.3 (1.6, 6.5) | 0.9 (0.88, 0.98) |
| IVH + WMD | 21 (15, 29) | 95 (93, 97) | 4.2 (2.6, 6.6) | 0.8 (0.76, 0.90) |
Abbreviations: IVH- intraventricular hemorrhage; LR- likelihood ratio; WMD- white matter damage defined as cerebral echolucency or late moderate-to-severe ventriculomegaly
Neurological disorder refers to presence of cerebral palsy, autism spectrum disorder, and/or epilepsy
Although information about parenchymal echodensities was not considered in the primary analysis because of the lower reliability of this ultrasound finding, when parenchymal echodensity was included as a criterion for WMD, we observed similar associations between WMD and adverse neurodevelopmental outcomes (Table 8 and Table 9; available at www.jpeds.com).
Table 8; online.
Frequencies of neurodevelopmental outcomes by neonatal cranial ultrasound finding defining white matter damage as cerebral echolucency, cerebral echodensity, or moderate to severe ventriculomegaly; IVH – intraventricular hemorrhage.
| Ultrasound findings Percent (Ns) | ||||
|---|---|---|---|---|
| Outcome | Neither IVH nor WMD | IVH without WMD | WMD without IVH | IVH and WMD |
| Cerebral palsy | 5 (34) | 9 (7) | 23 (18) | 31 (34) |
| Gross motor function classification system level > 0 | 2 (14) | 3 (2) | 13 (10) | 16 (18) |
| Epilepsy | 4 (27) | 10 (8) | 18 (14) | 15 (17) |
| Pediatric quality of life < 70 | 20 (123) | 19 (15) | 38 (28) | 47 (48) |
| Neurodevelopmental burden | ||||
| No cognitive impairment, cerebral palsy, ASD, or epilepsy | 75 (466) | 65 (52) | 39 (30) | 48 (53) |
| No cognitive impairment; one or more of: cerebral palsy, ASD, or epilepsy | 6 (40) | 8 (6) | 19 (15) | 12 (13) |
| Cognitive impairment* | 19 (115) | 28 (22) | 42 (32) | 41 (45) |
| TOTAL | 621 | 80 | 77 | 111 |
Cognitive impairment is LPA score indicating moderate or severe impairment.
Abbreviations: WMD - White matter injury defined as cerebral echolucency, cerebral echodensity, or moderate to severe ventriculomegaly; IVH – intraventricular hemorrhage
Table 9; online.
Frequencies of psychiatric outcomes by neonatal cranial ultrasound finding defining white matter damage as cerebral echolucency, cerebral echodensity, or moderate to severe ventriculomegaly; IVH – intraventricular hemorrhage.
| Ultrasound findings Percent (Ns) | ||||
|---|---|---|---|---|
| Outcome | Neither IVH nor WMD | IVH without WMD | WMD without IVH | IVH and WMD |
| Autism spectrum disorder | 5 (34) | 6 (5) | 14 (11) | 10 (11) |
| SRS ≥65 among children with IQ ≥ 85, excluding those with ASD¶ | 13 (57) | 18 (9) | 32 (11) | 21 (11) |
| ADHD | 15 (93) | 24 (19) | 19 (14) | 25 (26) |
| Anxiety (parent report) | 15 (95) | 10 (8) | 17 (23) | 9 (9) |
| Depression (parent report) | 16 (97) | 9 (7) | 17 (13) | 15 (15) |
| TOTAL | 621 | 80 | 77 | 111 |
Abbreviations: WMD - White matter injury defined as cerebral echolucency, cerebral echodensity, or moderate to severe ventriculomegaly; IVH – intraventricular hemorrhage
n=425, 51, 34, 52 for participants with, respectively, neither IVH nor WMI, IVH, WMI, IVH and WMI
DISCUSSION
WMD, as indicated by the presence of moderate to severe ventricular enlargement or cerebral white matter echolucency on neonatal cranial ultrasound, is predictive of adverse cognitive and neurological outcomes ten years later. In addition, WMD was associated with depression as reported by the child’s school teacher and ADHD, and one form of WMD, ventriculomegaly, was associated with ASD. In the absence of ultrasound indicators of WMD, isolated neonatal IVH is not predictive of adverse neurodevelopmental outcomes in middle childhood. Although increased risk of adverse neurodevelopmental outcomes was observed in children with WMD in this study, it is reassuring that almost one half of children with neonatal WMD did not have cognitive impairment, and nearly one-third of these children were considered free of neurodevelopmental burden, with no evidence of cognitive impairment, cerebral palsy, ASD, or epilepsy, at age 10 years. These findings, as well as our previous reports on the relationship between CUS findings and neurodevelopmental outcomes at two years of age,(6, 7, 40) suggest that of the various forms of neonatal brain injury identifiable with ultrasound, cerebral WMD is the most important predictor of long-term neurodevelopmental outcome.
This study focused on ultrasound findings after the first two postnatal weeks, when cerebral WMD is more readily identified than at earlier ages.(41) In the first two postnatal weeks, clinicians may use information about the long-term impact of severe cranial ultrasound abnormalities to inform parental decisions about goals of care, including redirection towards comfort care.(42, 43) In the current study, our goal was to describe relationships that are more pertinent to counseling families close to the time of discharge from neonatal intensive care, when prognostic information can be used to identify children most in need of early intervention therapies.
Our finding that cerebral WMD predicts adverse neurodevelopmental outcomes at school age agrees with the NICHD Neonatal Research Network Neuro study observation that findings of cystic periventricular leukomalacia or ventriculomegaly in extremely preterm newborns were associated with lower IQ and higher rates of moderate-to-severe disability at 6–7 years of age.(12) Similarly, in a cohort of very preterm children in France, neonatal WMD, but not isolated germinal matrix/intraventricular hemorrhage, was associated with increased risk of neurodisabilities, as reported on questionnaires completed by parents and health departments.(44) Our finding of an association between ASD and one form of WMD, ventricular enlargement, agrees with findings from the Neonatal Brain Hemorrhage Study(9) except that we found a smaller relative risk that was not quite statistically significant.
Low-grade IVH was not associated with an intellectual outcome in the cohort of low birth weight children enrolled in the Infant Health and Development Program in the 1980s.(45) In contrast to our findings, Vohr et al reported that even when not accompanied by ultrasound evidence of WMD, IVH was associated with an increased risk of intelligence quotient less than 70 at 16 years of age.(10) All these studies are birthweight-defined cohorts and therefore may have an overrepresentation of growth restricted infants whereas the ELGAN cohort is defined by gestational age.
In a study of CUS-outcome relationships in a cohort of extremely preterm infants, grade 2 IVH, as defined in the Papile grading system(46), was associated with an increased risk of cerebral palsy, in contrast to the current study. This observation could be explained by the inclusion, in the group with grade 2 IVH, of infants with the ultrasound finding of periventricular leukomalacia, which likely represents WMD; in contrast, we evaluated IVH as a separate ultrasound finding. Failure to account for the co-occurrence of IVH and WMD has been noted as a limitation of the Papile grading system.(47)
Strengths of our study include the large sample of individuals selected on the basis of gestational age, the relatively low rate of cohort attrition, extensive efforts to standardize and maximize the reliability of ultrasound interpretation, and the comprehensive assessment of neurodevelopment at school age by examiners who were not aware of study participants’ ultrasound findings. Limitations include the loss to follow up of 11% of the targeted sample and the failure to obtain optimal images of the cerebellum, limiting our ability to detect cerebellar lesions that the available evidence suggests are strongly associated with later neurodevelopmental impairment.(48, 49). In addition, CUS cannot identify all infants with WMD. Others have described CUS indicators as only detecting the “tip of the iceberg” of perinatal brain injury related to prematurity.(50, 51) Magnetic resonance imaging (MRI) is more sensitive for detection of white matter injury,(52–54) although it is not clear whether the greater sensitivity of MRI actually translates to improved prediction of clinically important outcomes, and ultrasound remains widely used during neonatal intensive care.(12, 55–58)
In summary, WMD was strongly associated with neurodevelopmental impairments identified at school age, including cognitive impairment, cerebral palsy, and epilepsy, but not with psychiatric disorders such as autism spectrum disorder, ADHD, or anxiety. Nonetheless, even among children with ultrasound-identified WMD, nearly one half did not have cognitive impairment and over one third were free from each of the four major neurodevelopmental disorders we studied. This finding implies that when counselling the family of an extremely preterm infant about prognosis, cautious optimism could be appropriate even for infants whose cranial ultrasound is indicative of cerebral white matter damage. Lastly, our findings serve as a reminder that developmental surveillance during childhood is needed even for those with normal ultrasound findings, in whom rates of cognitive impairment, cerebral palsy, epilepsy, and ASD are higher than in the general population of school-age children.
Acknowledgments
Supported by grants from the National Institute of Neurological Disorders and Stroke (U01NS040069 [to A.L.] and R01NS040069 [to K.K.]); the Office of the NIH Director (5UH3OD023348–05 [to T.O.]); and the National Institute of Child Health and Human Development (5R01HD092374–04 [to T.O.]).
List of Abbreviations:
- ADHD
attention deficit hyperactivity disorder
- ADI-R
Autism Diagnostic Interview-Revised
- ADOS-2
Autism Diagnostic Observation Schedule-2
- ASD
autism spectrum disorder
- CI
confidence interval
- CSI-4
Child Symptom Inventory-4
- CUS
cranial ultrasound
- DAS-II
Differential Ability Scales-II
- ELGAN
Extremely Low Gestational Age Newborn
- IQ
intelligence quotient
- IVH
intraventricular hemorrhage
- LPA
Latent profile analysis
- NEPSY-II
Developmental NEuroPSYchological Assessment-II
- NICU
neonatal intensive care unit
- NICHD
National Institute of Child Health and Human Development
- OR
odds ratio
- SCQ
Social Communication Questionnaire
- SRS
Social Responsiveness Scale
- WMD
white matter damage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Johnson S, Fawke J, Hennessy E, Rowell V, Thomas S, Wolke D, et al. Neurodevelopmental Disability Through 11 Years of Age in Children Born Before 26 Weeks of Gestation. Pediatrics. 2009;124(2):E249–E57. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2009;94(4):F283–F9. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric Disorders in Extremely Preterm Children: Longitudinal Finding at Age 11 Years in the EPICure Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(5):453–63. [PubMed] [Google Scholar]
- 4.Stewart AL, Reynolds EO, Hope PL, Hamilton PA, Baudin J, Costello AM, et al. Probability of neurodevelopmental disorders estimated from ultrasound appearance of brains of very preterm infants. Dev Med Child Neurol. 1987;29(1):3–11. [DOI] [PubMed] [Google Scholar]
- 5.Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Dev Med Child Neurol. 1999;41(12):826–33. [DOI] [PubMed] [Google Scholar]
- 6.O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122(3):e662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuban KC, Allred EN, O’Shea TM, Paneth N, Pagano M, Dammann O, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto-Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics. 1995;95(2):249–54. [PubMed] [Google Scholar]
- 9.Movsas TZ, Pinto-Martin JA, Whitaker AH, Feldman JF, Lorenz JM, Korzeniewski SJ, et al. Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. J Pediatr. 2013;163(1):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vohr BR, Allan W, Katz KH, Schneider K, Tucker R, Ment LR. Adolescents born prematurely with isolated grade 2 haemorrhage in the early 1990s face increased risks of learning challenges. Acta Paediatr. 2014;103(10):1066–71. [DOI] [PubMed] [Google Scholar]
- 11.Hollebrandse NL, Spittle AJ, Burnett AC, Anderson PJ, Roberts G, Doyle LW, et al. School-age outcomes following intraventricular haemorrhage in infants born extremely preterm. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):4–8. [DOI] [PubMed] [Google Scholar]
- 12.Hintz SR, Vohr BR, Bann CM, Taylor HG, Das A, Gustafson KE, et al. Preterm Neuroimaging and School-Age Cognitive Outcomes. Pediatrics. 2018;142(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KCK, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Human Development. 2009;85(11):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuban KC, Joseph RM, O’Shea TM, Allred EN, Heeren T, Douglass L, et al. Girls and Boys Born before 28 Weeks Gestation: Risks of Cognitive, Behavioral, and Neurologic Outcomes at Age 10 Years. J Pediatr. 2016;173:69–75 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuban K, Adler I, Allred EN, Batton D, Bezinque S, Betz BW, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol. 2007;37(12):1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vries LS, Regev R, Pennock JM, Wigglesworth JS, Dubowitz LM. Ultrasound evolution and later outcome of infants with periventricular densities. Early Hum Dev. 1988;16(2–3):225–33. [DOI] [PubMed] [Google Scholar]
- 17.Appleton RE, Lee RE, Hey EN. Neurodevelopmental outcome of transient neonatal intracerebral echodensities. Archives of disease in childhood. 1990;65(1 Spec No):27–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlman JM, Rollins N, Burns D, Risser R. Relationship between periventricular intraparenchymal echodensities and germinal matrix-intraventricular hemorrhage in the very low birth weight neonate. Pediatrics. 1993;91(2):474–80. [PubMed] [Google Scholar]
- 19.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Cognitive functioning at the age of 10 years among children born extremely preterm: a latent profile approach. Pediatr Res. 2017;82(4):614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuban KC, Allred EN, O’Shea M, Paneth N, Pagano M, Leviton A, et al. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153(4):466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuban KCK, O’Shea M, Allred E, Leviton A, Gilmore H, DuPlessis A, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. Journal of Child Neurology. 2005;20(10):829–31. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288(11):1357–63. [DOI] [PubMed] [Google Scholar]
- 23.Douglass LM, Kuban K, Tarquinio D, Schraga L, Jonas R, Heeren T, et al. A Novel Parent Questionnaire for the Detection of Seizures in Children. Pediatr Neurol. 2016;54:64–9 e1. [DOI] [PubMed] [Google Scholar]
- 24.Douglass LM, Heeren TC, Stafstrom CE, DeBassio W, Allred EN, Leviton A, et al. Cumulative Incidence of Seizures and Epilepsy in Ten-Year-Old Children Born Before 28 Weeks’ Gestation. Pediatr Neurol. 2017;73:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Western Psychological Services; 2003. [Google Scholar]
- 26.LeCouteur A, Lord C, Rutter M. The autism diagnostic interview- revised (ADI-R). Western Psychological Services; 2003. [Google Scholar]
- 27.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(9):1094–103. [DOI] [PubMed] [Google Scholar]
- 28.C L, M R, P D, S R, K G, S B. Autism Diagnostic observation schedule-2 (ADOS-2). Western Psychological Corporation; 2012. [Google Scholar]
- 29.Constantino JN. The quantitative nature of autistic social impairment. Pediatr Res. 2011;69(5 Pt 2):55R–62R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–33. [DOI] [PubMed] [Google Scholar]
- 31.Dvir Y, Frazier JA, Joseph RM, Mokrova I, Moore PS, O’Shea TM, et al. Psychiatric Symptoms: Prevalence, Co-occurrence, and Functioning Among Extremely Low Gestational Age Newborns at Age 10 Years. J Dev Behav Pediatr. 2019;40(9):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc Psychol. 2002;31(4):513–24. [DOI] [PubMed] [Google Scholar]
- 33.Scott MN, Hunter SJ, Joseph RM, O’Shea TM, Hooper SR, Allred EN, et al. Neurocognitive Correlates of Attention-Deficit Hyperactivity Disorder Symptoms in Children Born at Extremely Low Gestational Age. J Dev Behav Pediatr. 2017;38(4):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leviton A, Hunter SJ, Scott MN, Hooper SR, Joseph RM, O’Shea TM, et al. Observer variability identifying attention deficit/hyperactivity disorder in 10-year-old children born extremely preterm. Acta Paediatr. 2017;106(8):1317–22. [DOI] [PubMed] [Google Scholar]
- 35.Hirschberger RG, Kuban KCK, O’Shea TM, Joseph RM, Heeren T, Douglass LM, et al. Co-occurrence and Severity of Neurodevelopmental Burden (Cognitive Impairment, Cerebral Palsy, Autism Spectrum Disorder, and Epilepsy) at Age Ten Years in Children Born Extremely Preterm. Pediatr Neurol. 2018;79:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–12. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23(6):859–74; discussion 75–7,79–80. [DOI] [PubMed] [Google Scholar]
- 38.Højsgaard SH U; Yan J The R Package geepack for Generalized Estimating Equations Journal of Statistical Software. 2006;15(2):1–11. [Google Scholar]
- 39.Yan J Yet another package for generalized estimating equations. R News. 2002;2:12–4. [Google Scholar]
- 40.O’Shea TM, Allred EN, Kuban KC, Hirtz D, Specter B, Durfee S, et al. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol. 2012;27(1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.deVries LS, Eken P, Dubowitz LMS. The Spectrum of Leukomalacia Using Cranial Ultrasound. Behavioural Brain Research. 1992;49(1):1–6. [DOI] [PubMed] [Google Scholar]
- 42.Mann PC, Woodrum DE, Wilfond BS. Fuzzy images: Ethical implications of using routine neuroimaging in premature neonates to predict neurologic outcomes. J Pediatr. 2013;163(2):587–92. [DOI] [PubMed] [Google Scholar]
- 43.Sheehan JW, Pritchard M, Heyne RJ, Brown LS, Jaleel MA, Engle WD, et al. Severe intraventricular hemorrhage and withdrawal of support in preterm infants. J Perinatol. 2017;37(4):441–7. [DOI] [PubMed] [Google Scholar]
- 44.Marret S, Marchand-Martin L, Picaud JC, Hascoet JM, Arnaud C, Roze JC, et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 2013;8(5):e62683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ann Wy P, Rettiganti M, Li J, Yap V, Barrett K, Whiteside-Mansell L, et al. Impact of intraventricular hemorrhage on cognitive and behavioral outcomes at 18 years of age in low birth weight preterm infants. J Perinatol. 2015;35(7):511–5. [DOI] [PubMed] [Google Scholar]
- 46.Papile LA, Munsickbruno G, Schaefer A. Relationship of Cerebral Intraventricular Hemorrhage and Early-Childhood Neurologic Handicaps. Journal of Pediatrics. 1983;103(2):273–7. [DOI] [PubMed] [Google Scholar]
- 47.Leviton A, Kuban K, Paneth N. Intraventricular haemorrhage grading scheme: time to abandon? Acta Paediatrica. 2007;96(9):1254–6. [DOI] [PubMed] [Google Scholar]
- 48.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr., Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–93. [DOI] [PubMed] [Google Scholar]
- 49.Villamor-Martinez E, Fumagalli M, Alomar YI, Passera S, Cavallaro G, Mosca F, et al. Cerebellar Hemorrhage in Preterm Infants: A Meta-Analysis on Risk Factors and Neurodevelopmental Outcome. Front Physiol. 2019;10:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holling EE, Leviton A. Characteristics of cranial ultrasound white-matter echolucencies that predict disability: a review. Dev Med Child Neurol. 1999;41(2):136–9. [DOI] [PubMed] [Google Scholar]
- 51.Leviton A, Gilles F. Ventriculomegaly, delayed myelination, white matter hypoplasia, and “periventricular” leukomalacia: How are they related? Pediatr Neurol. 1996;15:127–36. [DOI] [PubMed] [Google Scholar]
- 52.Whyte HE, Blaser S. Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology. 2013;55Suppl 2:3–11. [DOI] [PubMed] [Google Scholar]
- 53.de Vries LS, Benders MJ, Groenendaal F. Imaging the premature brain: ultrasound or MRI? Neuroradiology. 2013;55Suppl 2:13–22. [DOI] [PubMed] [Google Scholar]
- 54.Anderson PJ, Cheong JL, Thompson DK. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin Perinatol. 2015;39(2):147–58. [DOI] [PubMed] [Google Scholar]
- 55.El-Dib M, Massaro AN, Bulas D, Aly H. Neuroimaging and neurodevelopmental outcome of premature infants. Am J Perinatol. 2010;27(10):803–18. [DOI] [PubMed] [Google Scholar]
- 56.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–94. [DOI] [PubMed] [Google Scholar]
- 57.Rademaker KJ, Uiterwaal CS, Beek FJ, van Haastert IC, Lieftink AF, Groenendaal F, et al. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed. 2005;90(6):F489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horsch S, Skiold B, Hallberg B, Nordell B, Nordell A, Mosskin M, et al. Cranial ultrasound and MRI at term age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010;95(5):F310–4. [DOI] [PubMed] [Google Scholar]


