Abstract
Background:
Allogeneic hematopoietic cell transplant (allo-HCT) remains the only potentially curative therapeutic modality for patients with primary or secondary myelofibrosis (MF). However, many patients are considered ineligible for allo-HCT and transplant-related mortality can be substantial. Data on the efficacy and safety of allo-HCT are mixed and largely derived from retrospective studies.
Objective:
To synthesize the available evidence on the safety and efficacy of allo-HCT in MF and to identify patient, disease, and transplant characteristics with prognostic impact on outcomes of patients with MF undergoing allo-HCT.
Methods:
For this systematic review and meta-analysis Cochrane Library, Google Scholar, Ovid Medline, Ovid Embase, PubMed, Scopus, and Web of Science Core Collection were searched from inception to October 11, 2020 for studies on allo-HCT in MF. Random-effects models were used to pool response rates for the co-primary outcomes of 1-year, 2-year, and 5-year overall survival (OS). Rates of non-relapse mortality and acute and chronic graft-versus-host-disease (GVHD) were studied as secondary endpoints. Subgroup analyses on the effect of conditioning regimen intensity, baseline dynamic international prognostic scoring system (DIPSS) score, and patient age were performed. The study protocol has been registered on PROSPERO (CRD42020188706).
Results:
Forty-three studies with 8739 patients were identified and included in this meta-analysis. Rates of 1-year, 2-year, and 5-year OS were 66.7% (95% confidence interval [CI]: 63.5–69.8%), 64.4% (57.6–70.6%), and 55.0% (51.8–58.3%), respectively. Rates of 1-year, 2-year, and 5-year non-relapse mortality were 25.9% (23.3–28.7%), 29.7% (24.5–35.4%), and 30.5% (25.9–35.5%), respectively. The combined rate of graft failure was 10.6% (95% CI: 8.9 – 12.5%) with primary and secondary graft failure occurring in 7.3% (95% CI: 5.7 – 9.4%) and 5.9% (95% CI: 4.3 – 8.0%) of patients, respectively. Rates of acute and chronic graft-versus-host disease were 44.0% (95% CI: 39.6–48.4%; grade III/IV: 15.2%) and 46.5% (95% CI: 42.2–50.8%; extensive or moderate/severe: 26.1%), respectively. Subgroup analyses did not show any significant difference between conditioning regimen intensity (myeloablative vs reduced-intensity), median patient age, and proportion of DIPSS-intermediate-2/high patients. The quality of the evidence is limited by the absence of randomized clinical trials in the field and the heterogeneity of patient and transplant characteristics across included studies.
Conclusion:
Given the poor prognosis of patients not receiving transplant and in the absence of curative non-transplant therapies, our results support consideration of allo-HCT for eligible patients with MF.
Introduction:
Together with essential thrombocythemia (ET) and polycythemia vera (PV), primary myelofibrosis (MF) belongs to the heterogenous group of BCR-ABL1-negative myeloproliferative neoplasms (MPN). While the prognosis for individual patients is variable, MF has been associated with inferior survival compared to both healthy, age-matched controls and patients with PV and ET.1, 2 Registry data from the United States reported a median age of diagnosis for primary MF of 69 years and a median overall survival (OS) of 3.6 years.2 With the identification of driver mutations in janus kinase 2 (JAK2), calreticulin (CALR), and myeloproliferative leukemia virus (MPL), which are present in about 90% of MF patients, novel therapies targeting JAK signaling have been developed and risk stratification, counseling, and treatment selection of MF patients has become increasingly individualized.3, 4 Even though novel therapies improve symptoms and decrease spleen size their ability to improve survival remains limited.
As the prognosis of MF patients varies substantially based on patient (e.g. age and symptom burden) and disease characteristics (e.g. extent of peripheral blood cytopenias, degree of fibrosis, karyotype, and presence of certain high-risk mutations), various risk stratification tools such as the international prognostic scoring system (IPSS), the dynamic IPSS (DIPSS) and the mutation-enhanced IPSS (MIPSS-70) have been developed and are recommended for an individualized treatment selection.5–9 Using DIPSS for risk stratification, Kroeger et al. have shown that patients with intermediate-2 or high-risk MF experience a survival benefit with allogeneic hematopoietic cell transplant (allo-HCT) compared to non-transplant treatment strategies.10 Based on those data, the European Leukemia Net (ELN) and the National Comprehensive Cancer Network (NCCN) recommend consideration of allo-HCT for patients with higher-risk MF (i.e. patients with intermediate-2 or high risk by IPSS/DIPSS), as it constitutes the only potentially curative therapeutic option.7 However, allo-HCT is associated with a significant risk of short-term and long-term morbidity and mortality due to both transplant complications and relapse, which mandates careful patient selection based on patient characteristics (e.g. age, performance status) and donor availability.11, 12 Several questions regarding the optimal timing, conditioning regimen and donor source as well as the role of prior therapies including splenectomy and JAK inhibitors remain unresolved.12, 13 As a result, only a small proportion of patients with transplant indications based on disease risk proceed to this potentially curative treatment.
Although allo-HCT has been performed in MF patients for decades, high-quality evidence from randomized clinical trials is scarce and data on the safety and efficacy of allo-HCT in MF are heterogenous due to variations in study design, patient selection, and transplant characteristics. We therefore conducted a systematic review and meta-analysis to objectively evaluate the efficacy and safety of allo-HCT in both primary and secondary MF.
Methods:
Search strategy:
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines.14 We searched Cochrane Library, Google Scholar, Ovid Medline, Ovid Embase, PubMed, Scopus, and Web of Science Core Collection from inception to October 11, 2020. Databases were searched using a combination of controlled vocabulary and free text terms for relevant studies on the efficacy and safety of allo-HCT in patients with primary and secondary MF. Details of the full search strategy are listed in the supplemental materials. The study protocol has been registered on PROSPERO (CRD42020188706).
Study selection:
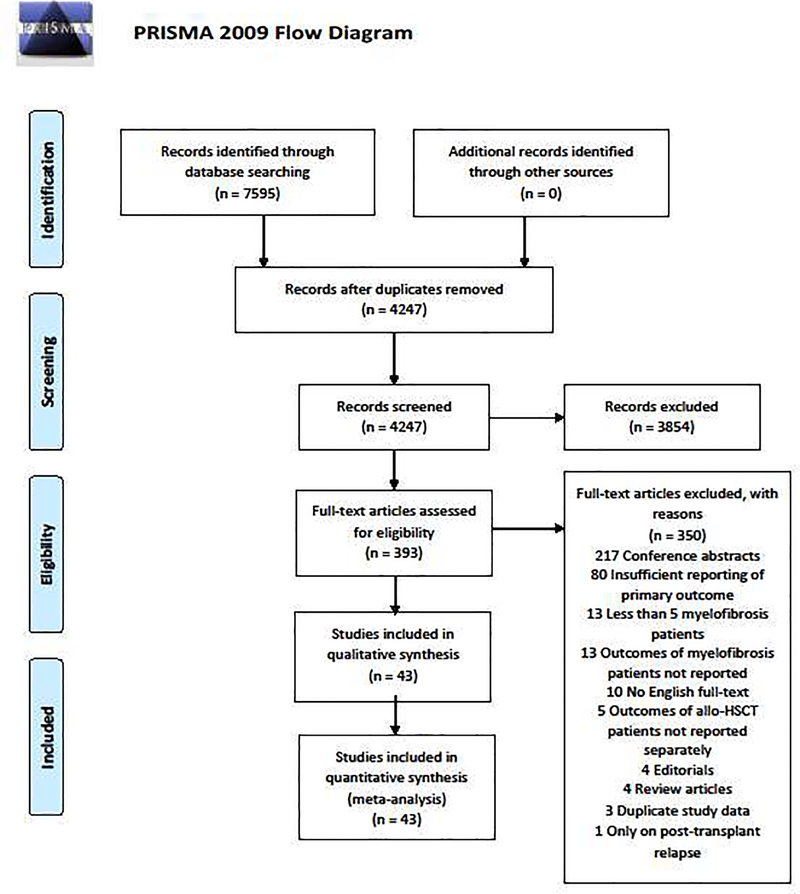
After removal of duplicates, studies were screened for eligibility based on title and abstract. Potentially eligible studies were subsequently reviewed for inclusion by full-text review. Studies were excluded as outlined in Figure 1 and in the supplemental materials. All studies were reviewed by two reviewers independently regarding inclusion in the meta-analysis (JPB and AS or SV) with conflicts being resolved by adjudication by a third reviewer (MS) or consensus discussion. The study selection process is illustrated in Figure 1.
Figure 1: PRISMA diagram.
Our search strategy identified 4247 unique citations of which 3854 were excluded based on title and abstract review. The remaining 393 records were reviewed as full-texts and 43 studies were included in this meta-analysis after the application of various exclusion criteria.
Quality assessment:
Two investigators (JPB and AS or SV) extracted data independently using a standardized data-extraction form. Downs and Black checklists, as published previously, were used by one author to assess study quality with cross-check of a random subset of studies performed by another author.15–18
Definition of endpoints:
We chose 1-year, 2-year and 5-year OS as the co-primary outcome to reflect both the short-term mortality related to allo-HCT itself (1-year and 2-year OS) and the long-term mortality related to the underlying disease (5-year OS). Key secondary outcomes included the rates of non-relapse mortality (NRM) and relapse-free survival (RFS)/progression-free survival (PFS) at various timepoints (1-year, 2-year, 5-year). Safety endpoints were the rate of overall acute and chronic graft-versus-host disease (GVHD) and the incidence of ≥grade 3 acute GVHD and extensive or moderate/severe chronic GVHD as well as graft failure (primary, secondary, and total). Prespecified subgroup analyses were performed to assess the impact of conditioning regimen intensity (myeloablative vs reduced-intensity), median patient age (<50 years vs 50–59 years vs ≥60 years), and underlying disease risk based on DIPSS risk categories (<50% patients with DIPSS intermediate-2/high vs 50–74% vs ≥75%).
Statistical analysis:
Random-effects models were used to pool 1-year, 2-year, and 5-year OS rates as well as for the safety endpoints. Heterogeneity of studies was determined using Cochran Q and I2 indices and was graded as low, moderate, and substantial for I2 indices of 30%, 30–60%, and >60%, respectively. Subgroup analyses and univariate meta-regression analyses were performed using the same analytic approach. Comprehensive Meta-Analysis (CMA version 2.2, Biostat) was used for all analyses.
Results:
Results of literature search:
Our search strategy identified 4247 studies after duplicate removal. Following title and abstract review, 393 studies were assessed as full-texts for eligibility and 43 studies were included in the meta-analysis after applying prespecified exclusion criteria as outlined in the supplemental materials. Figure 1 provides the PRISMA diagram of the study selection process. Studies were primarily excluded based on insufficient reporting of the prespecified primary endpoints and publication as conference abstracts (Supplemental material).
Description of included studies:
We included 43 studies in the meta-analysis with a total of 8739 patients with MF who had undergone allo-HCT. There were 38 retrospective studies,19–56 one prospective study,57 and four phase II clinical trials.58–61 There were 19 single center 22–25, 27, 32, 37–39, 42, 47–49, 52–54, 57–59 and 13 multicenter studies.19–21, 26, 33, 35, 36, 40, 50, 51, 55, 60, 61 Additionally, we included 11 studies that were derived from well-annotated registries such as the European Society of Blood and Marrow Transplantation Registry (EBMTR) or the Center for International Blood and Marrow Transplant Research (CIBMTR).28–31, 34, 41, 43–46, 56 Table 1 summarizes key study characteristics of the individual included studies. Only a single study exclusively enrolled pediatric patients,36 while four additional studies included both pediatric and adult patients.30, 32, 43, 48 The mean median age of patients among included studies was 53 years (range: 8 months – 62 years). Four studies included only patients with primary MF,24, 30, 31, 36 one study enrolled only patients with secondary MF,28 and the remainder reported outcomes of patients with both primary and secondary MF. Rates of patients with progression to AML at the time of transplant were inconsistently reported but ranged from 0% to 27%. DIPSS scores were not reported by 18 studies.20, 21, 23–26, 30, 36, 37, 39, 41–44, 53, 57, 60 Among studies reporting DIPSS scores, the percentage of patients with intermediate-2 or high risk DIPSS scores ranged from 25% to 100%.27, 54 Conditioning regimens used were variable among included studies with three 23, 30, 36 and 14 studies using exclusively myeloablative (MAC) or reduced-intensity conditioning (RIC) regimens 19, 21, 22, 24, 25, 31, 42, 47, 53, 54, 57, 59–61, respectively. Two studies enrolled patients receiving either MAC or RIC and reported outcomes separately.20, 41 The remainder of studies used various combinations of MAC, RIC, and nonmyeloablative conditioning regimens but did not provide outcomes stratified by conditioning intensity. Donor sources as well as the pre-transplant treatment histories including prior splenectomy were highly variable precluding a meta-analysis.
Table 1:
Study characteristics of included studies
| Author (ref) | Year of publication | Study population | Transplant characteristics | Outcomes |
|---|---|---|---|---|
| Anderson23 | 1997 | 13 patients median age: 40 (R: 18–49) Primary/Secondary MF: 62%/38% DIPSS int-2/high: not reported |
Conditioning: 100% MAC Donor source: 62% MRD; 8% MMRD; 23% MUD; 8% haplo GVHD prophylaxis: 92% CsA/MTX; 8% MTX |
Survival: 2-year OS: 77% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 548% (15% grade III/IV); cGVHD: 46% (46% extensive) Graft failure: total 0% |
| Guardiola30 | 1999 | 55 patients median age: 42 (R: 4–53) Primary MF: 100% DIPSS int-2/high: not reported |
Conditioning: 100% MAC Donor source: 89% MRD; 6% MMRD; 6% MUD GVHD prophylaxis: 78% CsA/MTX; 7% CsA/MTX/steroid; 9% T-cell depletion; 15% other |
Survival: 5-year OS: 47%; 1-year NRM: 27% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 60% (33% grade III/IV); cGVHD: 60% (36% extensive) Graft failure: total 9.1% (9.1% primary; secondary not reported) |
| Daly26 | 2003 | 25 patients median age: 49 Primary/Secondary MF: 76%/24% DIPSS int-2/high: not reported |
Conditioning: not reported Donor source: 52% MRD; 8% MMRD; 40% MUD GVHD prophylaxis: 100% CsA/MTX |
Survival: 2-year OS: 41%; 1-year NRM: 48% RFS/EFS/PFS/DFS: 2-year PFS: 37% GVHD: aGVHD: 52% (16% grade III/IV); cGVHD: 59% (35% extensive) Graft failure: total 9.1% (9.1% primary; secondary not reported) |
| Kerbauy37 | 2007 | 104 patients median age: 49 (R: 18–70) Primary/Secondary MF: 66%/29% DIPSS int-2/high: not reported |
Conditioning: 91% MAC, 9% nonmyeloablative Donor source: 53% MRD; 4% MMRD; 35% MUD; 9% MMUD GVHD prophylaxis: 86% CsA/MTX; 10% CsA/MMF; 5% other |
Survival: 5-year OS: 61%; 5-year NRM: 34% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 64% (21% grade III/IV); cGVHD: 84% (59% extensive) Graft failure: total 6.9% (primary not reported; 6.9% secondary) |
| Patriaca44 | 2008 | 100 patients median age: 49 (R: 21–68) Primary/Secondary MF: 82%/18% DIPSS int-2/high: not reported |
Conditioning: 48% MAC, 52% RIC Donor source: not reported GVHD prophylaxis: 70% CsA/MTX; 10% CsA; 2% CsA/MMF; 18% CsA/MTX/ATG |
Survival: 1-year OS: 59%; 1-year NRM: 35%; 5-year OS: 31% RFS/EFS/PFS/DFS: 5-year RFS: 28% GVHD: aGVHD: 41%; cGVHD: 43% (11% extensive) Graft failure: total 12.0% (12.0% primary; secondary not reported) |
| Kroger60 | 2009 | 103 patients median age: 55 (R: 32–68) Primary/Secondary MF: 61%/39% DIPSS int-2/high: not reported |
Conditioning: 100% RIC Donor source: 32% MRD; 68% MUD GVHD prophylaxis: 100% CsA/MTX |
Survival: 5-year OS: 67%; 1-year NRM: 16% RFS/EFS/PFS/DFS: 5-year DFS: 51% GVHD: aGVHD: 27% (11% grade III/IV); cGVHD: 49% (24% extensive) Graft failure: total 1.9% (1.9% primary; secondary not reported) |
| Alchalby21 | 2010 | 162 patients median age: 56 (R: 32–73) Primary/Secondary MF: 65%/35% DIPSS int-2/high: not reported |
Conditioning: 100% RIC Donor source: not reported GVHD prophylaxis: not reported |
Survival: 5-year OS: 62%; 1-year NRM: 22% RFS/EFS/PFS/DFS: 5-year DFS: 46% GVHD: aGVHD: 22% (6% grade III/IV); cGVHD: 38% (20% extensive) Graft failure: total 12.3% (4.3% primary; 8.0% secondary) |
| Bacigalupo24 | 2010 | 46 patients median age: 51 (R: 24–67) Primary MF: 100% DIPSS int-2/high: not reported |
Conditioning: 100% RIC Donor source: 65% MRD; 4% MMRD; 30% MUD GVHD prophylaxis: 100% CsA/MTX |
Survival: 5-year OS: 45%; 5-year NRM: 24% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 37%; cGVHD: 30% (30% extensive) Graft failure: not reported |
| Nagi42 | 2011 | 11 patients median age: 51 (R: 46–62) Primary/Secondary MF: 55%/45% DIPSS int-2/high: not reported |
Conditioning: 100% RIC Donor source: 18% MRD; 55% MUD; 27% MMUD GVHD prophylaxis: 100% CsA/alemtuzumab |
Survival: 2-year OS: 46%; 2-year NRM: 54% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 27%; cGVHD: 9% (9% extensive) Graft failure: total 9.1% (9.1% primary; secondary not reported) |
| Abelsson20 | 2012 | 92 patients median age: not reported Primary/Secondary MF: 76%/24% DIPSS int-2/high: not reported |
Conditioning: 44% MAC, 54% RIC Donor source: 51% MRD; 49% MUD GVHD prophylaxis: not reported |
RIC cohort: 5-year OS: 59%; 28% aGVHD (11% grade III/IV) MAC cohort: 5-year OS: 49%; aGVHD: 76% (17% grade III/IV) Graft failure: total 14.1% (14.1% primary; secondary not reported) |
| Ditschkowski27 | 2012 | 76 patients median age: 51 (R: 22–67) Primary/Secondary MF: 62%/38% DIPSS int-2/high: 25% |
Conditioning: 59% MAC; 41% RIC Donor source: 36% MRD; 4% MMRD; 43% MUD; 17% MMUD GVHD prophylaxis: 61% CsA/MTX; 22% CsA/ATG; 17% CsA/alemtuzumab |
Survival: 5-year OS: 53%; 1-year NRM: 26% RFS/EFS/PFS/DFS: 5-year RFS: 50% GVHD: aGVHD: 31% (12% grade III/IV); cGVHD: 54% (24% extensive) Graft failure: total 5.3% (4.0% primary; 1.3% secondary) |
| Nivison-Smith43 | 2012 | 57 patients median age: 47 (R: 16–71) Primary/Secondary MF: 86%/14% DIPSS int-2/high: not reported |
Conditioning: 32% MAC; 68% RIC Donor source: 32% MRD; 68% MUD GVHD prophylaxis: 38% CsA/MTX; 18% CsA/MTX/steroid; 6% CsA/MMF; 35% other; 7% none |
Survival: 1-year OS: 72%; 5-year OS: 58%; 1-year NRM: 25% RFS/EFS/PFS/DFS: 5-year DFS: 57% GVHD: aGVHD: 37% Graft failure: total 12.3% (12.3% primary; secondary not reported) |
| Scott48 | 2012 | 170 patients median age: 51.5 (R: 12–79) Primary/Secondary MF: 59%/40% DIPSS int-2/high: 60% |
Conditioning: 89% MAC; 11% RIC Donor source: 51% MRD; 2% MMRD; 39% MUD; 11% MMUD GVHD prophylaxis: 78% CsA/MTX; 7% CsA/MTX/steroid; 9% T-cell depletion; 14.5% other |
Survival: 1-year OS: 74%; 5-year OS: 57%; 1-year NRM: 26% RFS/EFS/PFS/DFS: 5-year RFS: 57% GVHD: aGVHD: 68% (18% grade III/IV); cGVHD: 63% (55% extensive) Graft failure: total 7.2% (2.4% primary; 4.8% secondary) |
| Hussein36 | 2013 | 8 patients median age: 0.7 (R: 0.1–1.5) Primary MF: 100% DIPSS int-2/high: not reported |
Conditioning: 100% MAC Donor source: 50% MRD; 12.5% MUD; 25% MMUD; 12.5% haplo GVHD prophylaxis: 75% CsA/MTX; 25% CsA/MMF |
Survival: 5-year OS: 100% RFS/EFS/PFS/DFS: 5-year RFS: 100% GVHD: aGVHD: 50% (13% grade III/IV); cGVHD: 63% (25% extensive) Graft failure: total 0% |
| Gupta31 | 2014 | 233 patients median age: 55 (R: 19–79) Primary MF: 100% DIPSS int-2/high: 38% |
Conditioning: 100% RIC Donor source: 48% MRD; 5% MMRD; 19% MUD; 13% MMUD; 13% UC GVHD prophylaxis: 53% CsAbased; 45% Tac-based; 2% other |
Survival: 1-year OS: 62%; 5-year OS: 47%; 1-year NRM: 18% RFS/EFS/PFS/DFS: 5-year PFS: 27% GVHD: aGVHD: 37% (19% grade III/IV); cGVHD: 51% Graft failure: total 16.0% (16.0% primary; secondary not reported) |
| Robin46 | 2014 | 35 patients median age: 54 (R: 28–63) Primary/Secondary MF: 57%/43% DIPSS int-2/high: 49% |
Conditioning: 100% RIC Donor source: 100% UC GVHD prophylaxis: 97% calcineurin inhibitor-based |
Survival: 2-year OS: 44%; 2-year NRM: 35% RFS/EFS/PFS/DFS: 2-year EFS: 30% GVHD: aGVHD: 29% (9% grade III/IV); cGVHD: 39% (6% extensive) Graft failure: total 40.0% (not differentiated) |
| Shanavas49 | 2014 | 27 patients median age: 61 (R: 30–70) Primary/Secondary MF: 63%/34% DIPSS int-2/high: 82% |
Conditioning: 26% MAC; 74% RIC Donor source: 59% MRD; 30% MUD; 11% MMUD GVHD prophylaxis: 74% CsA/MMF; 19% CsA/alemtuzumab; 4% CsA/MMF/alemtuzumab; 4% CsA/MMF/ATG |
Survival: 1-year OS: 77%; 2-year OS: 56%; 1-year NRM: 23% RFS/EFS/PFS/DFS: 2-year PFS: 46% GVHD: aGVHD: 48% (12% grade III/IV); cGVHD: 66% Graft failure: total 0% |
| Slot50 | 2015 | 53 patients median age: 60 (R: 36–69) Primary/Secondary MF: 64%/36% DIPSS int-2/high: 44% |
Conditioning: 36% nonmyeloablative; 64% RIC Donor source: 34% MRD; 42% MUD; 15% MMUD; 7% UC GVHD prophylaxis: 4% CsA; 56% CsA/MMF; 11% CsA/mycophenolic acid; 6% CsA/steroid; 16% CsA/MMF/steroid; 2% mycophenolic acid; 2% none |
Survival: 2-year OS: 49%; 2-year NRM: 43% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 63%; cGVHD: 49% Graft failure: total 28.3% (22.6% primary; 5.7% secondary) |
| Shanavas55 | 2016 | 100 patients median age: 59 (R: 32–72) Primary/Secondary MF: 57%/43% DIPSS int-2/high: 54% |
Conditioning: 44% MAC; 56% RIC Donor source: 36% MRD; 50% MUD GVHD prophylaxis: not reported |
Survival: 2-year OS: 61%; 2-year NRM: 28% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 37% (16% grade III/IV); cGVHD: 48% (23% extensive) Graft failure: total 8.0% (4.0% primary; 4.0% secondary) |
| Kroger35 | 2017 | 169 patients median age: 58 (R: 18–75) Primary/Secondary MF: 65%/27% DIPSS int-2/high: 73% |
Conditioning: 2% MAC; 98% RIC Donor source: 21% MRD; 49% MUD; 30% MMUD GVHD prophylaxis: not reported |
Survival: 5-year OS: 56%; 1-year NRM: 28% RFS/EFS/PFS/DFS: 5-year PFS: 48% Graft failure: not reported |
| Lestang39 | 2017 | 34 patients median age: 57 (R: 41–66) Primary/Secondary MF: 65%/35% |
Conditioning: 82% MAC; 15% RIC Donor source: 42% MRD; 39% MUD; 12% MMUD; 7% UC GVHD prophylaxis: not reported |
Survival: 5-year OS: 52%; 2-year NRM: 30% RFS/EFS/PFS/DFS: 5-year PFS: 40% GVHD: aGVHD: 41% (9% grade III/IV); cGVHD: 42% (27% extensive) Graft failure: total 10.0% (10.0% primary; secondary not reported) |
| Wolschke53 | 2017 | 136 patients median age: 58 (R: 32–75) Primary/Secondary MF: 66%/29% |
Conditioning: 100% RIC Donor source: 19% MRD; 49% MUD; 32% MMUD GVHD prophylaxis: not reported |
Survival: 5-year OS: 60% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 38% (18% grade III/IV) Graft failure: not reported |
| Abd Kadir19 | 2018 | 159 patients median age: 59 (R: 28–74) Primary/Secondary MF: 68%/32% DIPSS int-2/high: 68% |
Conditioning: 31% MAC; 69% RIC Donor source: 15% MRD; 54% MUD; 31% MMUD GVHD prophylaxis: 18% CsA/MTX; 75% CsA/MMF; 1% Tac/MMF; 5% other |
Survival: 2-year OS: 86%; 2-year NRM: 23% RFS/EFS/PFS/DFS: 2-year PFS: 62% GVHD: not reported Graft failure: total 4.4% (3.1% primary; 1.3% secondary) |
| Kuykendall38 | 2018 | 17 patients median age: 62 (R: 42–72) Primary/Secondary MF: not reported DIPSS int-2/high: 83% |
Conditioning: not reported Donor source: not reported GVHD prophylaxis: not reported |
Survival: 2-year OS: 67% RFS/EFS/PFS/DFS: not reported GVHD: not reported Graft failure: not reported |
| Tefferi52 | 2018 | 67 patients median age: 55 (R: 19–68) Primary/Secondary MF: 64%/36% DIPSS int-2/high: 79% |
Conditioning: 18% MAC; 82% RIC Donor source: 45% MRD; 46% MUD GVHD prophylaxis: 45% Tac/MTX+/−ATG; 22% Tac/MMF/ATG; 30% CsA/MTX |
Survival: 5-year OS: 62% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 68%; cGVHD: 44% (33% moderate/severe) Graft failure: total 4.5% (4.5% primary; secondary not reported) |
| Ali22 | 2019 | 110 patients median age: 55 (R: 29–72) Primary/Secondary MF: 53%/43% DIPSS int-2/high: 52% |
Conditioning: 100% RIC Donor source: 46% MRD; 40% MUD; 14% MMUD GVHD prophylaxis: 91% Tac/sirolimus-based; 4% Tac-based; 5% CsA/MMF-based |
Survival: 5-year OS: 65%; 5-year NRM: 17% RFS/EFS/PFS/DFS: 5-year PFS: 60% GVHD: aGVHD: 45% (17% grade III/IV); cGVHD: 59% extensive Graft failure: total 1.8% (1.8% primary; secondary not reported) |
| Barabanshikova57 | 2019 | 20 patients median age: 51 (R: 32–64) Primary/Secondary MF: 65%/35% DIPSS int-2/high: not reported |
Conditioning: 100% RIC Donor source: 15% MRD; 55% MUD; 10% MMUD; 20% haplo GVHD prophylaxis: 100% PTCy/ruxolitinib |
Survival: 2-year OS: 85%; 2-year NRM: 15% RFS/EFS/PFS/DFS: 2-year EFS: 72% GVHD: aGVHD: 25% (25% grade III/IV); cGVHD: 40% (20% extensive) Graft failure: total 15.0% (15.0% primary; secondary not reported) |
| Barabanishikova25 | 2019 | 8 patients median age: not reported Primary/Secondary MF: not reported DIPSS int-2/high: not reported |
Conditioning: 100% RIC Donor source: 25% MRD; 38% MUD; 38% haplo GVHD prophylaxis: 100% PTCy |
Survival: 2-year OS: 90% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 38% (25% grade III/IV); cGVHD: 13% (0% extensive) Graft failure: total 12.5% (12.5% primary; secondary not reported) |
| Gagelmann28 | 2019 | 159 patients median age: 59 (R: 33–75) Primary/Secondary MF: 0%/100% DIPSS int-2/high: 53% |
Conditioning: 16% MAC; 84% RIC Donor source: 37% MRD; 63% MUD GVHD prophylaxis: not reported |
Survival: 2-year OS: 61% RFS/EFS/PFS/DFS: not reported GVHD: not reported Graft failure: not reported |
| Gupta61 | 2019 | 21 patients median age: 59 (R: 39–70) Primary/Secondary MF: 48%/52% DIPSS int-2/high: 76% |
Conditioning: 100% RIC Donor source: 33% MRD; 67% MUD GVHD prophylaxis: 100% calcineurin inhibitor +/− ATG |
Survival: 2-year OS: 63%; 2-year NRM: 28% RFS/EFS/PFS/DFS: 2-year PFS: 59% GVHD: aGVHD: 57% (14% grade III/IV); cGVHD: 76% (19% extensive) Graft failure: total 14.3% (4.8% primary; 9.5% secondary) |
| Helbig32 | 2019 | 44 patients median age: 49 (R: 14–67) |
Conditioning: 2% MAC, 98% RIC Donor source: 43% MRD; 57% MUD |
Survival: 2-year OS: 54% RFS/EFS/PFS/DFS: not reported |
|
Primary/Secondary MF: 68%/32% DIPSS int-2/high: 84% |
GVHD prophylaxis: 100% CsA/MTX +/− ATG |
GVHD: aGVHD: 47% (7% grade III/IV); cGVHD: 36% Graft failure: total 2.3% (2.3% primary; secondary not reported) |
||
| Mannina40 | 2019 | 18 patients median age: 59 (R: 43–67) Primary/Secondary MF: 78%/22% DIPSS int-2/high: 72% |
Conditioning: 33% MAC, 67% RIC Donor source: 39% MRD; 50% MUD; 11% MMUD GVHD prophylaxis: not reported |
Survival: 5-year OS: 84% RFS/EFS/PFS/DFS: 5-year RFS: 84% GVHD: aGVHD: 72% (11% grade III/IV); cGVHD: 50% (6% moderate) Graft failure: 0% |
| McLornan41 | 2019 | 2224 patients (14–43 RIC and 781 MAC) median age: 57.5 (RIC) and 52.8 (MAC) Primary/Secondary MF: 65%/35% (RIC) and 83%/17% (MAC) DIPSS int-2/high: not reported |
Conditioning: 35% MAC, 65% RIC Donor source: not reported GVHD prophylaxis: not reported |
Survival: 5-year OS: 51% for RIC and 53% for MAC; 1-year NRM: 26% for RIC and 25.5% for MAC RFS/EFS/PFS/DFS: not reported GVHD: not reported Graft failure: total 12.0% (12.0% primary; secondary not reported) |
| Raj45 | 2019 | 56 patients median age: 57 (R: 38–72) Primary/Secondary MF: 75%/25% DIPSS int-2/high: not reported |
Conditioning: 70% MAC, 30% RIC Donor source: not reported GVHD prophylaxis: 79% PTCy, 21% other |
Survival: 2-year OS: 56%; 2-year NRM: 38% RFS/EFS/PFS/DFS: 2-year RFS: 43% GVHD: aGVHD: 28% (9% grade III/IV); cGVHD: 42% (6% extensive) Graft failure: total 22.0% (9.0% primary; 13.0% secondary) |
| Salas47 | 2019 | 37 patients median age: 60 (R: 18–69) Primary/Secondary MF: not reported DIPSS int-2/high: 76% |
Conditioning: 100% RIC Donor source: 24% MRD; 46% MUD; 11% MMUD, 19% haplo GVHD prophylaxis: 100% CsA/PTCy/ATG |
Survival: 2-year OS: 66%; 1-year NRM: 23% RFS/EFS/PFS/DFS: 2-year RFS: 50% GVHD: aGVHD: 35% (11% grade III/IV); cGVHD: 27% (22% extensive) Graft failure: total 16.2% (not differentiated) |
| Tamari51 | 2019 | 101 patients median age: 59 (R: 30–73) Primary/Secondary MF: 61%/28% DIPSS int-2/high: 56% |
Conditioning: 18% MAC, 82% RIC Donor source: 46% MRD; 52% MUD GVHD prophylaxis: not reported |
Survival: 2-year OS: 61%; 5-year OS: 52%; 2-year NRM: 26% RFS/EFS/PFS/DFS: 2-year RFS: 56% GVHD: not reported Graft failure: not reported |
| Gowin29 | 2020 | 551 patients median age: 51 (R: 20–69) Primary/Secondary MF: 84%/16% DIPSS int-2/high: 34% |
Conditioning: 51% MAC, 7% nonmyeloablative, 41% RIC Donor source: 38% MRD; 47% MUD; 15% MMUD GVHD prophylaxis: not reported |
Survival: 5-year OS: 55% RFS/EFS/PFS/DFS: not reported GVHD: not reported Graft failure: not reported |
| Hernandez-Boluda33 | 2020 | 197 patients median age: 58 (R: 52–62) Primary/Secondary MF: 56%/22% DIPSS int-2/high: 74% |
Conditioning: 33% MAC, 67% RIC Donor source: 43% MRD; 1% MMRD; 32% MUD; 13% MMUD; 9% haplo, 3% UC GVHD prophylaxis: 54% CsAbased, 40% Tac-based; 6% sirolimus-based; 28% PTCy; 24% ATG |
Survival: 5-year OS: 51%; 1-year NRM: 27% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 55% (23% grade III/IV); cGVHD: 40% (20% extensive) Graft failure: total 5.1% (not differentiated) |
| Hernandez-Boluda34 | 2020 | 2916 patients median age: 56 Primary/Secondary MF: 74%/26% DIPSS int-2/high: 61% |
Conditioning: 35% MAC, 65% RIC Donor source: 37% MRD; 63% MUD GVHD prophylaxis: not reported |
Survival: 5-year OS: 50%; 1- NRM: 26% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 46% (14% grade III/IV); cGVHD: 42% (24% moderate) Graft failure: total 11.1% (not differentiated) |
| Lwin56 | 2020 | 142 patients median age: 56 (R: 26–69) Primary/Secondary MF: 66%/34% DIPSS int-2/high: 58% |
Conditioning: 17% MAC, 83% RIC Donor source: 52% MRD GVHD prophylaxis: 57% CsA/MTX; 15% CsA/MTX/steroid; 9% CsA or tac + MMF; 16% other |
Survival: 5-year OS: 57%; 1-year NRM: 25% RFS/EFS/PFS/DFS: 5-year PFS: 44% GVHD: aGVHD: 21% (9% grade III/IV); cGVHD: 29% (18% moderate) Graft failure: total 12.7% (6.3% primary; 6.3% secondary) |
| Morozova59 | 2020 | 20 patients median age: 51 (R: 32–64) Primary/Secondary MF: 70%/30% DIPSS int-2/high: 90% |
Conditioning: 100% RIC Donor source: 15% MRD; 55% MUD; 10% MMUD; 20% haplo GVHD prophylaxis: 100% PTCy + ruxolitinib |
Survival: 2-year OS: 85%; 2-year NRM: 15% RFS/EFS/PFS/DFS: 2-year EFS: 72% GVHD: aGVHD: 25% (15% grade III/IV); cGVHD: 40% (20% moderate) Graft failure: total 5.0% (5.0% primary; secondary not reported) |
| Salit58 | 2020 | 28 patients median age: 56 (R: 34–68) Primary/Secondary MF: 54%/46% DIPSS int-2/high: 58% |
Conditioning: 82% MAC, 18% RIC Donor source: 50% MRD GVHD prophylaxis: Tac + MTX or MMF |
Survival: 2-year OS: 86% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 70% (15% grade III/IV); cGVHD: 35% (29% moderate/severe) Graft failure: total 0% |
| Yoon54 | 2020 | 35 patients median age: 55 (R: 34–72) Primary/Secondary MF: 77%/23% DIPSS int-2/high: 100% |
Conditioning: 100% RIC Donor source: 46% MRD; 29% MUD; 14% MMUD; 11% haplo GVHD prophylaxis: CsA or tac + MTX +/− ATG |
Survival: 2-year OS: 60%; 2-year NRM: 30% RFS/EFS/PFS/DFS: not reported GVHD: aGVHD: 54% (34% grade III/IV); cGVHD: 46% (33% moderate) Graft failure: total 2.9% (0% primary; 2.9% secondary) |
aGVHD – acute graft-versus-host disease; ATG – anti-thymocyte globulin; cGVHD – chronic graft-versus-host disease; CsA – cyclosporin A; DFS – disease-free survival; EFS – event-free survival; haplo – haploidentical; MAC – myeloablative conditioning; MF – myelofibrosis; MMF – mycophenolate mofetil; MMRD – mismatched related donor; MMUD – mismatched unrelated donor; MRD – matched related donor; MTX – methotrexate; MUD – matched unrelated donor; NRM – non-relapse mortality; OS – overall survival; PTCy – post-transplant cyclophosphamide; R – range; RFS – relapse-free survival; RIC – reduced intensity conditioning; Tac – tacrolimus; UC – umbilical cord
Assessment of study quality:
Generally, quality of the evidence was limited by the absence of randomized clinical trials and the retrospective design of most studies. Only the studies by Gowin et al. and Lestang et al. used a control population of MF patients who did not undergo allo-HCT without a formal matching process between transplant and non-transplant patients.29, 39 Using a Downs and Black checklist, scores ranged from 16 to 23, with the study by Gupta et al. scoring highest.15, 61 A detailed quality assessment using Downs and Black checklist is provided in Supplemental Table 1.
Survival outcomes following allo-HCT for myelofibrosis:
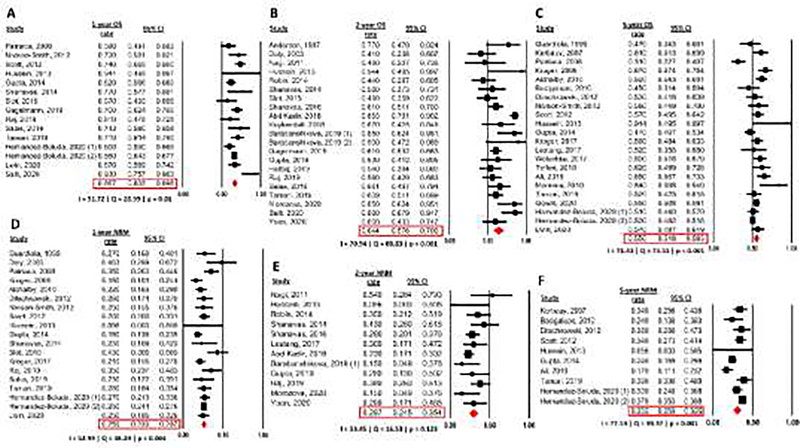
Rates of 1-year, 2-year, and 5-year OS were reported by 15, 21, and 22 studies, respectively (Figure 2 A–C). 1-year, 2-year, and 5-year OS rates were 66.7% (95% confidence interval [CI]: 63.5–69.8%), 64.4% (95% CI: 57.6–70.6%), and 55.0% (95% CI: 51.8–58.3%). Study heterogeneity was significant for all OS endpoints and was rated as moderate for 1-year OS (Cochran’s Q=28.99; p=0.01; I2=51.72%) and as substantial for 2-year (Cochran’s Q=68.83; p<0.001; I2=70.94%), and 5-year OS (Cochran’s Q=73.51; p<0.001; I2=71.43%).
Figure 2: Overall Survival (OS) and Non-relapse mortality (NRM) rates.
(A) 1-year OS rate
(B) 2-year OS rate
(C) 5-year OS rate
(D) 1-year NRM rate
(E) 2-year NRM rate
(F) 5-year NRM rate
Non-relapse mortality (NRM) is a major driver of mortality especially in the first year following allo-HCT for MF. Rates of 1-year, 2-year, and 5-year NRM were 25.9% (95% CI: 23.3–28.7%), 29.7% (95% CI: 24.5–35.4%), and 30.5% (95% CI: 25.9–35.5%), and were reported by 19, 12, and 10 studies, respectively (Figure 2 D–F). Study heterogeneity was significant for 1-year and 5-year NRM and was rated as moderate for 1-year NRM (Cochran’s Q=38.29; p=0.004; I2=52.99%) and 2-year NRM (Cochran’s Q=16.53; p=0.123; I2=33.45%), and substantial for 5-year NRM (Cochran’s Q=39.37; p<0.001; I2=77.14%).
Relapse rates:
Relapse-free survival (RFS) and progression-free survival (PFS) were reported by seven 27, 36, 40, 44, 47, 48, 51 and 10 studies,19, 22, 26, 31, 35, 39, 45, 49, 56, 61 respectively. However, not all studies reported RFS and PFS for all timepoints, which limited our ability to conduct a meta-analysis. Additionally, we had to rely on the definitions of RFS and PFS used by the original studies.
Among evaluable studies, rates of 1-year, 2-year, and 5-year RFS were 65.3% (95% CI: 56.5–73.1%), 56.2% (95% CI: 41.6–69.8%), and 53.6% (95% CI: 39.9–66.9%), respectively (Supplemental Figure 1 A–C). Study heterogeneity was significant for 1-year and 5-year RFS but not for 2-year RFS and was rated as moderate for 1-year RFS (Cochran’s Q=9.98; p=0.041; I2=59.9%) and 2-year RFS (Cochran’s Q=3.69; p=0.157; I2=49.91%), and substantial for 5-year RFS (Cochran’s Q=31.77; p<0.001; I2=84.26%).
Rates of 1-year PFS, 2-year PFS, and 5-year PFS were 56.9% (95% CI: 41.4–71.2%), 50.6% (95% CI: 39.7–61.4%), and 43.5% (95% CI: 31.9–55.8%), respectively (Supplemental Figure 1 D–F). Study heterogeneity was significant and substantial for 1-year PFS (Cochran’s Q=25.78; p<0.001; I2=88.36%), 2-year PFS (Cochran’s Q=10.49; p=0.033; I2=61.85%), and 5-year PFS (Cochran’s Q=37.5; p<0.001; I2=89.33%).
Incidence of acute and chronic graft-versus-host disease:
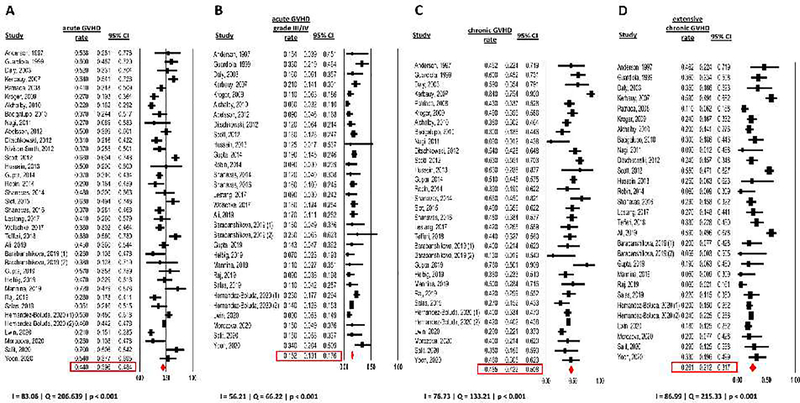
Regimens used for GVHD prophylaxis were variable among included studies (Table 1) and were not reported by 13 studies. Incidence rates of acute and chronic GVHD were reported by 36 and 32 studies with 5334 and 4962 patients, respectively. Acute GVHD occurred in 44.0% of patients (95% CI: 39.6–48.4%) with grade III/IV acute GVHD being reported in 15.2% of patients (95% CI: 13.1–17.6%). Of note, 26 studies only reported incidence rates of grade II-IV acute GVHD.21, 22, 24–27, 30, 31, 37, 39, 42–46, 48, 49, 52, 53, 55–60 Heterogeneity was significant for both all grade acute GVHD and grade III/IV acute GVHD rate and was rated as substantial for acute GVHD rate (Cochran’s Q=206.64; p<0.001; I2=83.06%) and moderate for grade III/IV acute GVHD (Cochran’s Q=66.22; p<0.001; I2=56.21%) (Figure 3 A and B).
Figure 3: GVHD rate during study duration.
(A) acute GVHD
(B) acute GVHD grade III/IV
(C) chronic GVHD
(D) extensive or moderate/severe chronic GVHD
Chronic GVHD occurred in 46.5% of patients (95% CI: 42.2–50.8%) with extensive or moderate/severe chronic GVHD being reported in 26.1% of patients (95% CI: 21.2–31.7%). Heterogeneity was significant and substantial for both overall chronic GVHD (Cochran’s Q=133.21; p<0.001; I2=76.73%) and extensive or moderate/severe chronic GVHD rates (Cochran’s Q=215.33; p<0.001; I2=86.99%) (Figure 3 C and D).
Incidence of graft failure:
Rates of graft failure were reported by 36 studies of which 31 and 13 studies reported the incidence of primary and secondary graft failure separately (Table 1). The combined rate of graft failure was 10.6% (95% CI: 8.9 – 12.5%) with significant and substantial heterogeneity across studies (Cochran’s Q = 102.69; p<0.001; I2 = 65.92%). The rate of primary graft failure was 7.3% (95% CI: 5.7 – 9.4%) with significant and substantial heterogeneity (Cochran’s Q = 82.43; p<0.001; I2 = 63.61%). Secondary graft failure occurred in 5.9% (95% CI: 4.3 – 8.0%) of patients with no significant heterogeneity across the included studies (Cochran’s Q = 15.95; p=0.194; I2 = 24.76%) (Supplemental Figure 2 A–C).
Subgroup analyses:
As several factors such as patient age and prognosis of the underlying MF based on DIPSS score are essential when evaluating a patient for allo-HCT, we conducted subgroup analyses to evaluate the impact of median patient age and percentage of patients with intermediate-2/high risk by DIPSS.
In the absence of individual patient-level data, we compared 1-year, 2-year, and 5-year OS rates among studies with a median patient age <50 years, 50–59 years, and ≥60 years. There was no statistically significant difference in any of these comparisons (Supplemental Figure 3).
When comparing 1-year, 2-year and 5-year OS among studies with <50% patients with DIPSS intermediate-2/high, 50–74%, and ≥75%, respectively, there were no statistically significant differences in any comparison (Supplemental Figure 4). However, the number of studies in each group was small.
Finally, we evaluated the influence of conditioning regimen intensity on survival outcomes by comparing studies in which all patients received either myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC). There was no statistically significant difference in terms of 2-year and 5-year OS between studies using only MAC or RIC conditioning (Supplemental Figure 5).
Adverse events:
Due to the long timespan of publication dates of included studies, reporting and grading of adverse events other than acute and chronic GVHD was heterogenous across studies. We were unable to quantitatively assess rates of adverse events in a meta-analysis. However, rates of 100-day mortality were reported by seven studies and can serve as a surrogate for transplant-related complications.26, 36, 43, 47, 49, 58, 61 One hundred day post-allo-HCT mortality rates ranged from 0% in the study by Hussein et al.36 to 20% in the study by Daly et al.26
Discussion:
In this systematic review and meta-analysis of 43 studies with 8739 patients with primary or secondary MF who underwent allo-HCT we found rates of 1-year, 2-year, and 5-year OS of 66.7% (95% CI: 63.5–69.8%), 64.4% (95% CI: 57.6–70.6%), and 55.0% (95% CI: 51.8–58.3%), respectively. As allo-HCT is the only potentially curative therapeutic modality for MF at this point and should be considered for eligible patients with higher-risk MF (e.g. intermediate-2 or high risk by DIPSS),62 the survival outcomes found in our meta-analysis compare favorably to the natural history of MF with a median OS of 4 years for intermediate-2 and 1.5 years for high risk based on DIPSS.6 However, MF patients undergoing allo-HCT are highly selected based on age, performance status and disease characteristics. Our results are in line with several studies not included in our meta-analysis showing 5-year OS rates of 50–60% and 32–47% among the DIPSS intermediate-2 and high-risk patient subset, respectively.10, 63 The survival benefit of allo-HCT in primary MF patients with intermediate-2 and high-risk disease who are <65 years of age compared to non-transplant therapies has been shown in a matched retrospective cohort study with a relative risk of death of 0.55 (95% CI, 0.36–0.83; p=0.005) for intermediate-2 risk, and 0.37 (95% CI, 0.21–0.66; p=0.0007) for high-risk DIPSS patients.10 We were unable to include those studies in our meta-analysis as they only reported outcomes stratified by DIPSS category and not for the entire patient cohort. However, the consistency of our results with those studies supports the external validity of our analyses.
As outcomes were only infrequently reported separately by DIPSS category among the studies included in our meta-analysis, we used a subgroup analysis comparing studies based on the proportion of patients with intermediate-2 and high-risk MF, which did not reveal any differences in survival outcomes between subgroups. This could suggest that the adverse outcomes in patients with higher DIPSS scores might be abrogated by allo-HCT. However, it is important to note that the number of included studies in each subgroup is small and baseline patient and transplant characteristics are heterogenous which may have introduced unmeasured confounders. Consistent with this observation a large recent transplant registry study demonstrated an OS benefit with allo-HCT even in DIPSS intermediate-1 patients, albeit at the cost of early NRM.29
Risks and benefits of allo-HCT need to be balanced carefully. In our meta-analysis rates of NRM at 1-year, 2-years, and 5-years were 25.9% (95% CI: 23.3–28.7%), 29.7% (95% CI: 24.5–35.4%), and 30.5% (95% CI: 25.9–35.5%), respectively. Additionally, any grade acute and chronic GVHD occurred in 44.0% (95% CI: 39.6–48.4%) and 46.5% of patients (95% CI: 42.2–50.8%), respectively, which highlights that allo-HCT in this setting can be associated with significant complications. Improvement in GVHD prophylaxis strategies and decrease in NRM post allo-HCT in recent years has been reported.64 In parallel to mechanistic drug discoveries the number of allo-HCTs performed for MF continues to increase thanks to more frequent use of RIC regimens and improvements in supportive care with the median age at transplant approaching 60 years with RIC regimens.34, 41 This highlights the need for an individualized approach to patient selection, timing of allo-HCT and discussion of non-transplant strategies in the treatment of MF.
Discovery of high-risk genetic mutations such as ASXL1, SRSF2, U2AF1, EZH2, and IDH1/2 together with further improvement of cytogenetic risk stratification led to the development of risk stratification tools such as the MIPSS70+ and GIPSS.5, 65–68 Additionally, patients with CALR type 1 mutations appear to have a more favorable disease course.69–72 Among the studies included in this meta-analysis, mutational testing was only available for a subset of studies and only the study by Mannina et al. reported specific survival outcomes for a genetically defined subgroup (patients with MPL mutation).40
While allo-HCT remains the only curative therapeutic modality for MF at this point, the JAK inhibitor ruxolitinib has been shown to improve symptom burden, spleen size, and OS and has become the standard of care for higher-risk MF patients who are not transplant candidates. However, it is also commonly used by patients prior to allo-HCT.73, 74 The role of ruxolitinib in patients who are transplant-eligible is ill-defined. Due to the immunomodulatory effects, higher rates of engraftment and improvements in performance status and spleen size, pre-transplant ruxolitinib has been hypothesized to improve outcomes.55 In six studies included in this meta-analysis all patients had received ruxolitinib prior to transplant with outcomes being generally in line with non-ruxolitinib studies.25, 38, 57–59, 61 Ruxolitinib has also been shown to be an effective treatment for steroid-refractory acute GVHD but drug-induced cytopenias and a potential to reduce graft-versus-leukemia effect might limit its role during the post-transplant period.61, 75 Issues regarding patient selection as well as safety and efficacy of peri-transplant ruxolitinib remain unresolved and are being addressed in ongoing clinical trials (e.g. NCT04384692, NCT03333187, NCT03427866).
Historically, myeloablative busulfan-based conditioning regimens have been the standard of care for allo-HCT in MF, which have been associated with higher rates of NRM and GVHD.30, 37 The wider use of RIC has led to an increase in transplant-eligible patients and a reduction of NRM.60 While no prospective clinical trial data comparing MAC and RIC conditioning exist, OS outcomes with RIC and MAC appear to be comparable both in our meta-analysis and in large retrospective registry studies although confounding by indication and other baseline patient and transplant characteristics cannot be excluded.41 However, based on the study by McLornan et al. which showed better relapse-free and GVHD-free, relapse-free survival with MAC compared to RIC, MAC should still be considered in younger and fitter patients.41
While our systematic review and meta-analysis included 43 studies with 8739 patients and yielded robust results, limitations exist. First, patient and transplant characteristics were variable among the included studies leading to moderate to substantial heterogeneity in our analyses. Second, due to the absence of randomized controlled trials, confounding and selection bias cannot be excluded, which may also explain the absence of statistically significant differences in our subgroup analyses by age, DIPSS intermediate-2/high risk, and conditioning regimen intensity. Third, genetic data were only available for a subset of studies, which precluded an assessment of the impact of certain molecular and cytogenetic alterations on outcomes after allo-HCT in MF. We were not able to analyze the impact of donor source, prior therapies (including splenectomy and JAK inhibitors), and GVHD prophylaxis on transplant outcomes due to significant variability between studies and the quality of the primary literature.
Conclusion:
This is the first published systematic review and meta-analysis on the efficacy and safety of allo-HCT for the treatment of primary and secondary MF. We identified 43 studies with 8739 patients and showed rates of 1-year, 2-year, and 5-year OS of 66.7%, 64.4%, and 55.0%, respectively. Given the poor prognosis of patients not undergoing allo-HCT and in the absence of curative non-transplant therapies, our results support consideration of allo-HCT for eligible patients with higher-risk MF. Subgroup analyses did not show any significant difference between conditioning regimen intensity (MAC vs RIC), median patient age, and proportion of DIPSS-intermediate-2/high patients. Additional studies are necessary to enhance patient selection (e.g. by incorporation of molecular markers) and to optimize pre- and post-transplant strategies (e.g. pre-transplant ruxolitinib, conditioning regimens, and donor selection).
Supplementary Material
Article Highlights:
Allo-HCT can be curative for myelofibrosis but data on safety and efficacy is mixed
Meta-analysis of 43 studies: 1- and 5-year OS of 66.7% and 55.0% with allo-HCT
Non-relapse mortality (1-year 25.9%) and GVHD (acute 44.0%; chronic 50.8%) pose challenges
No influence of conditioning regimen intensity, DIPSS score, and median patient age
Allo-HCT should be considered for eligible myelofibrosis patients
Acknowledgments:
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award (CCITLA). Maximilian Stahl received funding from the MSKCC Clinical Scholars T32 Program under award number 2T32 CA009512–31 and support from an ASCO/Conquer Cancer Foundation Young Investigator Award. Research reported in this publication was supported by the NCI of the National Institutes of Health under Award Number P30 CA016359 and Cancer Center Support Grant/Core Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest: N.A.P. consulted for and received honoraria from Alexion, Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene, Bristol-Myers Squib, CTI biopharma and PharmaEssentia. N.A.P. received research funding (all to the institution) from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Jazz Pharmaceuticals, Pfizer, Astex Pharmaceuticals, CTI biopharma, Celgene, Genentech, AI Therapeutics, Samus Therapeutics, Arog Pharmaceuticals and Kartos Therapeutics. L.G. has received research support from Bristol-Myers Squib. M.S.T. has received research funding from Abbvie, Cellerant, Orsenix, ADC Therapeutics, and Biosight. M.S.T. has received honoraria for M.S.T. has received research funding from AbbVie, Cellerant, Orsenix, ADC Therapeutics, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen. M.S.T. has received honoraria for advisory board membership from AbbVie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, Jazz Pharmaceuticals, Roche, Biosight, Novartis, Innate Pharmaceuticals, Kura, and Syros Pharmaceuticals. M.S.T. received royalties from UpToDate. R.K.R has received consulting fees from Bristol-Myers Squibb, Constellation, Incyte, Celgene, Promedior, CTI, Jazz Pharmaceuticals, Blueprint, Stemline, and research funding from Incyte, Constellation, Stemline. A.M.Z. received research funding (institutional) from Celgene/BMS, Abbvie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene/Cardiff oncology, Incyte, Takeda, Novartis, Amgen, Aprea, and ADC Therapeutics. A.M.Z participated in advisory boards, and/or had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene/BMS, Jazz, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Trovagene/Cardiff Oncology, Takeda, Ionis, Amgen, Janssen, Epizyme, Syndax, Gilead, Kura, Aprea, Janssen, and Tyme. A.M.Z served on clinical trial committees for Novartis, Abbvie, Geron, Gilead, Kura, and Celgene/BMS. A.M.Z received travel support for meetings from Pfizer, Novartis, and Cardiff Oncology None of these relationships were related to the development of this manuscript. All other authors report no relevant disclosures/competing interests.
Footnotes
Disclaimer: Part of this work has been submitted for presentation at the 2021 annual meeting of the American Society of Clinical Oncology
Data sharing statement: Original data from the studies included in this meta-analysis have been previously published. Primary data and research methodology can be requested from the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–2513; quiz 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shallis RM, Wang R, Davidoff A, Ma X, Podoltsev NA, Zeidan AM. Epidemiology of the classical myeloproliferative neoplasms: The four corners of an expansive and complex map. Blood Rev. 2020:100706. [DOI] [PubMed] [Google Scholar]
- 3.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667–679. [DOI] [PubMed] [Google Scholar]
- 4.Gangat N, Tefferi A. Myelofibrosis biology and contemporary management. Br. J. Haematol;n/a. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J. Clin. Oncol 2018;36:310–318. [DOI] [PubMed] [Google Scholar]
- 6.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115:1703–1708. [DOI] [PubMed] [Google Scholar]
- 7.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Guglielmelli P, Lasho TL, et al. MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for Primary Myelofibrosis. J. Clin. Oncol 2018;36:1769–1770. [DOI] [PubMed] [Google Scholar]
- 10.Kröger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125:3347–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin R, Gupta V. Myelofibrosis: to transplant or not to transplant? Hematology. American Society of Hematology. Education Program. 2016;2016:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol 2021;96:145–162. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120:1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 15.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl M, Bewersdorf JP, Giri S, Wang R, Zeidan AM. Use of Immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis. Haematologica. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bewersdorf JP, Giri S, Tallman MS, Zeidan AM, Stahl M. Leukapheresis for the management of hyperleukocytosis in acute myeloid leukemia-A systematic review and meta-analysis. Transfusion. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J. Clin. Epidemiol 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 19.Shahnaz Syed Abd Kadir S, Christopeit M, Wulf G, et al. Impact of ruxolitinib pretreatment on outcomes after allogeneic stem cell transplantation in patients with myelofibrosis. Eur. J. Haematol 2018;101:305–317. [DOI] [PubMed] [Google Scholar]
- 20.Abelsson J, Merup M, Birgegård G, et al. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant. 2012;47:380–386. [DOI] [PubMed] [Google Scholar]
- 21.Alchalby H, Badbaran A, Zabelina T, et al. Impact of JAK2V617F mutation status, allele burden, and clearance after allogeneic stem cell transplantation for myelofibrosis. Blood. 2010;116:3572–3581. [DOI] [PubMed] [Google Scholar]
- 22.Ali H, Aldoss I, Yang D, et al. MIPSS70+ v2.0 predicts long-term survival in myelofibrosis after allogeneic HCT with the Flu/Mel conditioning regimen. Blood Adv. 2019;3:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson JE, Sale G, Appelbaum FR, Chauncey TR, Storb R. Allogeneic marrow transplantation for primary myelofibrosis and myelofibrosis secondary to polycythaemia vera or essential thrombocytosis. Br. J. Haematol 1997;98:1010–1016. [DOI] [PubMed] [Google Scholar]
- 24.Bacigalupo A, Soraru M, Dominietto A, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45:458–463. [DOI] [PubMed] [Google Scholar]
- 25.Barabanshikova MV, Zubarovsky IN, Savrasov VM, et al. Splenectomy following JAK1/JAK2 inhibitor therapy in patients with myelofibrosis undergoing allogeneic stem cell transplantation. Hematol. Oncol. Stem Cell Ther. 2019;12:140–145. [DOI] [PubMed] [Google Scholar]
- 26.Daly A, Song K, Nevill T, et al. Stem cell transplantation for myelofibrosis: a report from two Canadian centers. Bone Marrow Transplant. 2003;32:35–40. [DOI] [PubMed] [Google Scholar]
- 27.Ditschkowski M, Elmaagacli AH, Trenschel R, et al. Dynamic International Prognostic Scoring System scores, pre-transplant therapy and chronic graft-versus-host disease determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica. 2012;97:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagelmann N, Eikema DJ, de Wreede LC, et al. Comparison of Dynamic International Prognostic Scoring System and MYelofibrosis SECondary to PV and ET Prognostic Model for Prediction of Outcome in Polycythemia Vera and Essential Thrombocythemia Myelofibrosis after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant 2019;25:e204–e208. [DOI] [PubMed] [Google Scholar]
- 29.Gowin K, Ballen K, Ahn KW, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4:1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guardiola P, Anderson JE, Bandini G, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Société Française de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood. 1999;93:2831–2838. [PubMed] [Google Scholar]
- 31.Gupta V, Malone AK, Hari PN, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol. Blood Marrow Transplant 2014;20:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helbig G, Wieczorkiewicz-Kabut A, Markiewicz M, et al. Splenic irradiation before allogeneic stem cell transplantation for myelofibrosis. Med. Oncol 2019;36:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernández-Boluda JC, Pereira A, Alvarez-Larran A, et al. Predicting Survival after Allogeneic Hematopoietic Cell Transplantation in Myelofibrosis: Performance of the Myelofibrosis Transplant Scoring System (MTSS) and Development of a New Prognostic Model. Biol. Blood Marrow Transplant 2020;26:2237–2244. [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Boluda JC, Pereira A, Kröger N, et al. Determinants of survival in myelofibrosis patients undergoing allogeneic hematopoietic cell transplantation. Leukemia. 2020. [DOI] [PubMed] [Google Scholar]
- 35.Kröger N, Panagiota V, Badbaran A, et al. Impact of Molecular Genetics on Outcome in Myelofibrosis Patients after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017;23:1095–1101. [DOI] [PubMed] [Google Scholar]
- 36.Hussein AA, Hamadah T, Domm J, Al-Zaben A, Frangoul H. Allogeneic hematopoietic stem cell transplantation for infants with idiopathic myelofibrosis. Pediatr. Transplant 2013;17:815–819. [DOI] [PubMed] [Google Scholar]
- 37.Kerbauy DM, Gooley TA, Sale GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol. Blood Marrow Transplant 2007;13:355–365. [DOI] [PubMed] [Google Scholar]
- 38.Kuykendall AT, Shah S, Talati C, et al. Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann. Hematol 2018;97:435–441. [DOI] [PubMed] [Google Scholar]
- 39.Lestang E, Peterlin P, Le Bris Y, et al. Is allogeneic stem cell transplantation for myelofibrosis still indicated at the time of molecular markers and JAK inhibitors era? Eur. J. Haematol 2017;99:60–69. [DOI] [PubMed] [Google Scholar]
- 40.Mannina D, Gagelmann N, Badbaran A, et al. Allogeneic stem cell transplantation in patients with myelofibrosis harboring the MPL mutation. Eur. J. Haematol 2019;103:552–557. [DOI] [PubMed] [Google Scholar]
- 41.McLornan D, Szydlo R, Koster L, et al. Myeloablative and Reduced-Intensity Conditioned Allogeneic Hematopoietic Stem Cell Transplantation in Myelofibrosis: A Retrospective Study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant 2019;25:2167–2171. [DOI] [PubMed] [Google Scholar]
- 42.Nagi W, Lim ZY, Krishnamurthy P, et al. Alemtuzumab based reduced intensity conditioning allogeneic haematopoietic stem cell transplantation for myelofibrosis. Leuk. Res 2011;35:998–1000. [DOI] [PubMed] [Google Scholar]
- 43.Nivison-Smith I, Dodds AJ, Butler J, et al. Allogeneic hematopoietic cell transplantation for chronic myelofibrosis in Australia and New Zealand: older recipients receiving myeloablative conditioning at increased mortality risk. Biol. Blood Marrow Transplant 2012;18:302–308. [DOI] [PubMed] [Google Scholar]
- 44.Patriarca F, Bacigalupo A, Sperotto A, et al. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica. 2008;93:1514–1522. [DOI] [PubMed] [Google Scholar]
- 45.Raj K, Eikema DJ, McLornan DP, et al. Family Mismatched Allogeneic Stem Cell Transplantation for Myelofibrosis: Report from the Chronic Malignancies Working Party of European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant 2019;25:522–528. [DOI] [PubMed] [Google Scholar]
- 46.Robin M, Giannotti F, Deconinck E, et al. Unrelated cord blood transplantation for patients with primary or secondary myelofibrosis. Biol. Blood Marrow Transplant 2014;20:1841–1846. [DOI] [PubMed] [Google Scholar]
- 47.Salas MQ, Lam W, Law AD, et al. Reduced-intensity conditioning allogeneic transplant with dual T-cell depletion in myelofibrosis. Eur. J. Haematol 2019;103:597–606. [DOI] [PubMed] [Google Scholar]
- 48.Scott BL, Gooley TA, Sorror ML, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 2012;119:2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shanavas M, Messner HA, Atenafu EG, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis using fludarabine-, intravenous busulfan- and low-dose TBI-based conditioning. Bone Marrow Transplant. 2014;49:1162–1169. [DOI] [PubMed] [Google Scholar]
- 50.Slot S, Smits K, van de Donk NW, et al. Effect of conditioning regimens on graft failure in myelofibrosis: a retrospective analysis. Bone Marrow Transplant. 2015;50:1424–1431. [DOI] [PubMed] [Google Scholar]
- 51.Tamari R, Rapaport F, Zhang N, et al. Impact of High-Molecular-Risk Mutations on Transplantation Outcomes in Patients with Myelofibrosis. Biol. Blood Marrow Transplant 2019;25:1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tefferi A, Partain DK, Palmer JM, et al. Allogeneic hematopoietic stem cell transplant overcomes the adverse survival effect of very high risk and unfavorable karyotype in myelofibrosis. Am. J. Hematol 2018;93:649–654. [DOI] [PubMed] [Google Scholar]
- 53.Wolschke C, Badbaran A, Zabelina T, et al. Impact of molecular residual disease post allografting in myelofibrosis patients. Bone Marrow Transplant. 2017;52:1526–1529. [DOI] [PubMed] [Google Scholar]
- 54.Yoon JH, Min GJ, Park SS, et al. HLA-mismatched donor and high ferritin level showed poor clinical outcomes after allogeneic hematopoietic cell transplantation in patients with advanced myelofibrosis. Ther Adv Hematol. 2020;11:2040620720936935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shanavas M, Popat U, Michaelis LC, et al. Outcomes of Allogeneic Hematopoietic Cell Transplantation in Patients with Myelofibrosis with Prior Exposure to Janus Kinase ½ Inhibitors. Biol. Blood Marrow Transplant 2016;22:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lwin Y, Kennedy G, Gottlieb D, et al. Australasian Trends in Allogeneic Stem Cell Transplantation for Myelofibrosis in the Molecular Era: A Retrospective Analysis from the Australasian Bone Marrow Transplant Recipient Registry. Biol. Blood Marrow Transplant 2020;26:2252–2261. [DOI] [PubMed] [Google Scholar]
- 57.Barabanshikova MV, Morozova EV, Moiseev IS, et al. Pharmacokinetics of ruxolitinib administrated before and after allo-HSCT in patients with myelofibrosis. Cell. Ther. Transplant 2019;8:32–35. [Google Scholar]
- 58.Salit RB, Scott BL, Stevens EA, Baker KK, Gooley TA, Deeg HJ. Pre-hematopoietic cell transplant Ruxolitinib in patients with primary and secondary myelofibrosis. Bone Marrow Transplant 2020;55:70–76. [DOI] [PubMed] [Google Scholar]
- 59.Morozova EV, Barabanshikova MV, Moiseev IS, et al. A Prospective Pilot Study of Graft-versus-Host Disease Prophylaxis with Post-Transplantation Cyclophosphamide and Ruxolitinib in Patients with Myelofibrosis. Acta Haematol. 2020:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Kröger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–5270. [DOI] [PubMed] [Google Scholar]
- 61.Gupta V, Kosiorek HE, Mead A, et al. Ruxolitinib Therapy Followed by Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation for Myelofibrosis: Myeloproliferative Disorders Research Consortium 114 Study. Biol. Blood Marrow Transplant 2019;25:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kröger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–2133. [DOI] [PubMed] [Google Scholar]
- 63.Alchalby H, Yunus DR, Zabelina T, et al. Risk models predicting survival after reduced-intensity transplantation for myelofibrosis. Br. J. Haematol 2012;157:75–85. [DOI] [PubMed] [Google Scholar]
- 64.Penack O, Peczynski C, Mohty M, et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4:6283–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tefferi A, Guglielmelli P, Nicolosi M, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018;32:1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tefferi A, Guglielmelli P, Pardanani A, Vannucchi AM. Myelofibrosis Treatment Algorithm 2018. Blood Cancer J. 2018;8:72–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. [DOI] [PubMed] [Google Scholar]
- 68.Tefferi A, Nicolosi M, Mudireddy M, et al. Revised cytogenetic risk stratification in primary myelofibrosis: analysis based on 1002 informative patients. Leukemia. 2018;32:1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–1477. [DOI] [PubMed] [Google Scholar]
- 70.Tefferi A, Nicolosi M, Mudireddy M, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am. J. Hematol 2018;93:348–355. [DOI] [PubMed] [Google Scholar]
- 71.Panagiota V, Thol F, Markus B, et al. Prognostic effect of calreticulin mutations in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation. Leukemia. 2014;28:1552–1555. [DOI] [PubMed] [Google Scholar]
- 72.Tefferi A, Lasho TL, Tischer A, et al. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood. 2014;124:2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verstovsek S, Mesa RA, Gotlib J, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J. Hematol. Oncol 2017;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med 2020;382:1800–1810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.