Abstract
In this study, we investigated the effect of a triple knockout of the genes alpha-1,3-galactosyltransferase (GGTA1), cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH), and alpha 1,3-galactosyltransferase 2 (A3GALT2) in Yucatan miniature pigs on human immune reactivity. We used the CRISPR/Cas9 system to create pigs lacking GGTA1 (GTKO) and GGTA1/CMAH/A3GALT2 triple gene knockout (TKO). The expression of all three xenoantigens was absent in TKO pigs, but there was no additional reduction in the level of Galα1,3Gal (αGal) epitopes expression in the A3GALT2 gene KO. Peripheral blood mononuclear cells (PBMCs), aorta endothelial cells (AECs), and cornea endothelial cells (CECs) were isolated from these pigs, and their ability to bind human IgM/IgG and their cytotoxicity in human sera were evaluated. Compared to wild type (WT) pigs, the level of human antibody binding of the PBMCs, AECs, and CECs of the transgenic pigs (GTKO and TKO) was significantly reduced. However, there were significant differences in human antibody binding between GTKO and TKO depending on the cell type. Human antibody binding of TKO pigs was less than that of GTKO on PBMCs but was similar between GTKO and TKO pigs for AECs and CECs. Cytotoxicity of transgenic pig (GTKO and TKO) PBMCs and AECs was significantly reduced compared to that of WT pigs. However, TKO pigs showed a reduction in cytotoxicity compared to GTKO pigs on PBMCs, whereas in AECs from both TKO and GTKO pigs, there was no difference. The cytotoxicity of transgenic pig CECs was significantly decreased from that of WT at 300 min, but there was no significant reduction in TKO pigs from GTKO. Our results indicate that genetic modification of donor pigs for xenotransplantation should be tailored to the target organ and silencing of additional genes such as CMAH or A3GALT2 based on GTKO might not be essential in Yucatan miniature pigs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11248-021-00271-w.
Keywords: CRISPR/Cas9, GGTA1, CMAH, A3GALT2, Antibody binding, Cytotoxicity
Introduction
Pigs are similar to humans with respect to their genetics, anatomy, and physiology, making them the best donor of biological materials for xenotransplantation (Butler et al. 2015; Cooper et al. 2017; Hryhorowicz et al. 2017). Despite this similarity, there is a considerable phylogenetic distance between pigs and humans, which can cause problems with the immune system following xenotransplantation (Hryhorowicz et al. 2017). To facilitate successful xenotransplantation using pig organs and tissues, the generation and characterization of transgenic pigs with reduced immunogenic properties are important (Niemann and Petersen 2016).
In pig-to-human xenotransplantation, Galα1,3Gal (αGal) is a major carbohydrate causing hyperacute rejections. Anti-Gal antibodies in human blood recognize the Gal antigen expressed in pig cells and activate a complement cascade, which eventually leads to cell lysis (Hryhorowicz et al. 2017; Niemann and Petersen 2016; Song and Kim 2013). Therefore, the removal of the αGal antigen from xenograft cell surfaces helps in producing transplantable organs. N-glycolylneuraminic acid (Neu5Gc), another carbohydrate xenoantigen, has been identified as another key antigen that is recognized by human antibodies and causes organ rejection (Wang et al. 2014). Neu5Gc is synthesized from N-acetylneuraminic acid (Neu5Ac) by cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH). It is expressed in the tissues of most mammals, including pigs and monkeys, but humans have an inactivated CMAH gene, so only Neu5Ac is present on human cells (Breimer 2011). Previous reports have suggested that GGTA1 gene knockout (GTKO) pigs had an increased expression of sialyltransferase-related genes and thus increased production of Neu5Gc. These findings suggest that CMAH gene knockout (KO) is necessary to prevent further rejection of xenografts (Park et al. 2012; Park et al. 2011). Kwon et al. generated biallelic CMAH KO pigs, and showed that the transcription level of non-Gal antigen-related genes decreased in CMAH KO pig cells (Kwon et al. 2013). Many studies have reported that GGTA1/CMAH double KO pig cells had significantly less antigenicity than GTKO pig cells (Li et al. 2015; Li et al. 2013; Lutz et al. 2013; Sato et al. 2014; Whitworth et al. 2014). Butler et al. reported that GGTA1/CMAH double KO pig livers consumed fewer human platelets in a liver perfusion model (Butler et al. 2016a). In recent years, genome engineering techniques, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR/Cas9), have been used to produce precisely targeted genetic modifications in pigs (Butler et al. 2015; Hai et al. 2014; Ryu et al. 2018; Zhang et al. 2017b). Estrada et al. created GGTA1/CMAH/β4GalNT2 triple KO pigs using CRISPR/Cas9, and cells from these transgenic pigs had reduced xenoreactive antibody binding in peripheral blood mononuclear cells (PBMCs). These authors suggest that the reduction of xenoantigens through the deletion of GGTA1/CMAH/β4GalNT2 might reduce the antibody barrier and increase the success of xenotransplantation (Estrada et al. 2015). With the development of gene editing technology, pig-to-nonhuman primate xenotransplantation studies have made considerable progress. Researchers have successfully transplanted kidneys from Gal and Sda double xenoantigen KO pigs into rhesus monkeys, with the transplants functioning for 435 days (Adams et al. 2018). GTKO/hCD46/hTBM pig hearts were heterotopically transplanted into baboons that then survived for 945 days (Mohiuddin et al. 2016). Längin et al. extended the survival of baboons receiving life-supporting heart transplants by up to 195 days using an optimized process to preserve pig hearts during transplantation (Langin et al. 2018).
Several researchers have reported that a residual amount of αGal epitope reactivity has been found in GTKO mice tissues and GTKO pig cells (Milland et al. 2006; Milland et al. 2005; Sharma et al. 2003), suggesting that this epitope might be synthesized by other galactotransferase enzyme family proteins (Butler et al. 2016b; Kuwaki et al. 2005; Sharma et al. 2003; Yamada et al. 2005). The Gal antigen is synthesized by glycoprotein GGTA1 or glycosphingolipids alpha 1,3-galactosyltransferase 2 (A3GALT2, also known as iGb3s). As with GGTA1 and CMAH genes, humans possess the A3GALT2 gene, but it is thought to be inactive owing to several mutations (Christiansen et al. 2008). Some studies have suggested that isoglobotriosylceramide (iGb3, synthesized by A3GALT2) could be another source of αGal and that the A3GALT2 gene must also be deleted for complete removal of αGal (Christiansen et al. 2008; Milland et al. 2005). On the other hand, recent studies have shown the evidence that A3GALT2 is unlikely to be a xenoantigen for xenotransplantation (Butler et al. 2016b; Sanderson et al. 2013; Tahiri et al. 2013). Shao et al. suggested that the A3GALT2 gene can affect the expression of the Gal epitope, but the effect on its immunological properties in A3GALT2 KO mice may be very weak (Shao et al. 2018). Butler et al. showed that the A3GALT2 gene could change the renal glycosphingolipid profile but had no effect on antibody binding, or the cytotoxicity of porcine PBMCs in baboon and human sera (Butler et al. 2016b). However, iGb3 has been identified as an endogenous ligand, which is recognized by the invariant natural killer T (iNKT) cells in mice and human (Mattner et al. 2005; Zhou et al. 2004). In addition, it has been suggested that the expression of iGb3 on pig cells may trigger the activation of natural killer T (NKT) cells on adaptive immune cells (Christiansen et al. 2008). As the role of iGb3 on the development and function of NKT cells is still not clear, a study of A3GALT2 KO in pigs is required.
In this study, we generated GGTA1/CMAH/A3GALT2 triple knockout (TKO; TKO pigs mean silencing a gene not β4galNT2 but A3GALT2 along with GGTA1 and CMAH) pigs using CRISPR/Cas9 and examined the effect of the elimination of these genes on pig-to-human immune reactivity.
Materials and methods
Animals and chemicals
All animal care and use procedure protocols were approved by the Institutional Animal Care and Use Committee of Optipharm, Inc., Life Science Institute (IACUC approval No. OPT-140103-1). All pigs were housed in the animal facility at Optipharm, Inc., Korea, in a specific pathogen-free environment. The room was maintained at 24 °C ± 2 °C and 12 h light/12 h dark cycles. Filtered air and water, and sterilized feed were supplied. All pigs in this study were blood type O Yucatan miniature pigs, except for the surrogate gilts (Landrace/Yorkshire/Duroc cross-breed). For further experiments, six- to seven-week-old healthy pigs, GTKO, TKO, and wild type (WT) were humanely euthanized, and their organs harvested. The euthanasia was performed by intravenous injection of 2 mmol/kg potassium chloride solution under general anesthesia, and a veterinarian certified the death of the animals. Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Establishment of transgenic cell lines
For the establishment of the GTKO cell lines, a pair of oligos specific for the targeted site of GGTA1 (5′-GCAAATACATACTTCATGGT-3′) were hybridized using a thermal cycler. Ear fibroblast cells derived from Yucatan miniature pigs were transfected with Cas9-GFP-GGTA1 plasmids using electroporation (Amaxa 4D, Lonza, Walkersville, MD, USA). After 48 h, transfected cells were selected using FACS AriaII (BD Bioscience, San Diego, CA, USA) and seeded on a 10 cm culture dish. Single cell clusters were transferred to 96-well plates, and serial amplification was performed on colonies from the 10 cm culture dish. To establish the TKO cell lines, the guide RNA sequences for CMAH (5′-AACTCCTGAACTACAAGGCT-3′) and A3GALT2 (5′-GACGTGGATCAGCACTTCAG-3′) were propagated. GTKO cells were transfected with both Cas9-GFP-CMAH and Cas9-GFP-A3GALT2 vectors to establish the TKO cell lines using Lipofectamine 3000 (Invitrogen, San Diego, CA, USA) according to the manufacturer’s protocol. All cell lines were then produced using the procedure described below.
Analysis of CRISPR/Cas9-induced mutations in nuclear donor cells and cloned piglets
Genomic DNA from transgenic cells and cloned piglets were extracted using DNeasy extraction kits (Qiagen, Hilden, Germany). PCR was performed using GGTA1-, CMAH-, and A3GALT2-specific primer sets (S1 Table). The primer sets were designed to include the target sites and produced amplicons of 496 base pairs for GGTA1, 328 base pairs for CMAH, and 344 base pairs for A3GALT2. The PCR conditions for GGTA1 were as follows: initial denaturation step of 95 °C for 5 min; 95 °C for 30 s, 61 °C for 30 s, and 72 °C for 30 s; for 35 cycles; and final extension at 72 °C for 5 min. The PCR conditions for CMAH were as follows: initial denaturation step of 95 °C for 5 min; 95 °C for 30 s, 52 °C for 30 s, and 72 °C 30 s; for 35 cycles; and final extension at 72 °C for 5 min. For A3GALT2, the PCR conditions were as follows: initial denaturation step of 95 °C for 5 min; 95 °C for 30 s, 60 °C for 30 s, and 72 °C 30 s; for 35 cycles; and final extension at 72 °C for 5 min. The PCR products were confirmed by sequencing (Solgent, Daejeon, Korea).
Somatic cell nuclear transfer (SCNT) and embryo transfer (ET)
SCNT was performed as described in our previous study (Choi et al. 2017). Ovaries were obtained from a local slaughterhouse (Farmstory Hannaeng, Cheongju, Korea). Immature oocytes were cultured in M199 (Gibco, Waltham, MA, USA) based maturation medium for 42–44 h at 38.5 °C under 5% CO2 in air. Matured oocytes at the MII stage were enucleated by aspirating the first polar body, chromosomes, and the adjacent cytoplasm with a fine glass pipette in M199 medium (supplemented with 0.3% bovine serum albumin (BSA) and 7.5 μg/mL cytochalasin B). A single donor cell at the G0/G1 stage of the cell cycle was injected into the perivitelline space. The reconstructed oocytes were fused in 0.3 M mannitol medium (BTX, Holliston, MA, USA). Fused embryos were cultured in porcine zygote medium (PZM-3) supplemented with 0.4% BSA. Embryos were surgically transferred to oviducts of a surrogate gilt on day 0 or 1 of estrus. All surrogate gilts were anesthetized with an intravenous injection of Alfaxan (0.5 mg/kg; Jurox Pty. Ltd., NSW, Australia) and Domitor (10 μg/kg; Orion Pharma, Berkshire, UK), and anesthesia was maintained using inhaled isoflurane (Hana Pharm, Hwasung, Korea) at concentrations of < 5%. Pregnancy was monitored at day 28 after ET using an ultrasound scanner (PA60A; Samsung Medison, Seoul, Korea). The cloned piglets were delivered by natural birth.
Culture of porcine AECs and CECs
Porcine AECs and CECs were isolated, as previously described, with slight modifications (Kim et al. 2016; Lee et al. 2016a). Porcine aortas were digested with collagenase. The cells were washed with Dulbecco’s phosphate buffered saline (DPBS, Invitrogen), and then cultured in porcine endothelial cell culture medium (Lonza, Basel, Switzerland). Corneal tissues were dissected and incubated with 0.25% trypsin-EDTA (Invitrogen) for 20 min at 37 °C. CECs were peeled off and cultured in M199 medium (Gibco) containing 10% fetal bovine serum (Hyclone, UT, USA).
Isolation of PBMCs
Animals over 12 months old were used for blood collection. Blood from GTKO, TKO, and WT pigs was collected in EDTA tubes (BD Bioscience). PBMCs were separated from the whole blood using Ficoll-Paque PLUS (GE, UK) according to the manufacturer’s protocol. Separated PBMCs were washed with PBS (Gibco). Isolated PBMCs were then used immediately for human complement-mediated cytotoxicity or human antibody binding assays.
Immunofluorescence assay (IFA) from organ tissues
Tissues were fixed using 4% paraformaldehyde and made into paraffin blocks. Tissue sections were used for the immunofluorescence analysis of two antigens. αGal was stained with isolectin GS-IB4, Alexa Fluor® 594 conjugate (Life Technologies, Carlsbad, CA, USA). Neu5Gc was stained with chicken anti-Neu5Gc antibody (BioLegend, San Diego, CA, USA) and goat anti-chicken fluorescein isothiocyanate (FITC) (Abcam, Cambridge, UK) following the manufacturer’s protocols. The tissues were imaged using a confocal laser scanning microscope (Zeiss, Oberkochen, Germany).
Expression of A3GALT2 and αGal epitopes
The expression of A3GALT2 and αGal epitopes in pig cells was analyzed using FACS analysis with A3GALT2 antibody, a synthetic polyclonal antibody against pig A3GALT2 in rabbits, and anti-αGal antibody (Enzo Life Sciences, NY, USA). AECs and PBMCs were stained with mouse anti-αGal epitope antibody for 2 h at room temperature and goat anti-mouse Dylight 488 (Abcam) for 1 h on ice. AECs were stained with A3GALT2 antibody for 2 h at room temperature and goat anti-rabbit Dylight 488 (Abcam) for 1 h on ice. Flow cytometry was performed using BD FACS CantoII (BD biosciences, CA, USA), and the results were analyzed using FlowJo software (version 10; https://www.flowjo.com/).
Antibody binding of IgM and IgG to pig cells
The binding of human antibody to pig cells was evaluated as previously described (Hara et al. 2008). Isolated PBMCs and harvested AECs and CECs were incubated in 10% normal goat serum for 30 min at 4 °C. Cells were then washed twice with DPBS. The washed cells were incubated in 10% normal human serum (NHS; EMD Millipore, Burlington, MA, USA) or DPBS (negative control) for 30 min (PBMCs) or 2 h (AECs and CECs) at 4 °C. Cells were washed with DPBS and incubated with FITC-conjugated goat anti-human IgM (μ chain-specific) and IgG (γ chain-specific, Invitrogen) antibodies for 1 h at 4 °C. Binding of human IgM and IgG to PBMCs, AECs, and CECs was detected using a flow cytometer (FACS CantoII, BD) and quantified using relative mean fluorescence intensity (MFI), calculated as follows: Relative MFI = (MFI value of target)/(MFI value of negative control).
Human complement-mediated cytotoxicity assay
We conducted a complement-dependent cytotoxicity assay of the PBMCs, AECs, and CECs collected from TKO, GTKO, and WT pigs. Cells were counted using an EVE cell counter (Nanoentek, Seoul, Korea) and incubated in 50% pooled complement human serum (Innovative Research, Peary Court Novi, MI, USA) for 300 min. After incubation, human serum was removed by centrifugation. The cells were then resuspended in 100 μL of cell culture media. To estimate the viability of the cells, 10 μL of CCK-8 solution (Dojindo, Kumamoto, Japan) was added, and the cells were then incubated for another 2 h. Cytotoxicity was analyzed using absorbance at a wavelength of 450 nm recorded using a microplate reader (Infinite M200 pro NanoQuant, Tecan, Mannedorf, Switzerland).
Statistical analysis
Statistical analysis was conducted using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA). One-way or two-way (for cytotoxicity) analysis of variance (ANOVA) followed by Bonferroni post-hoc testing was used to evaluate significance.
Results
Generation of GTKO and TKO fibroblasts
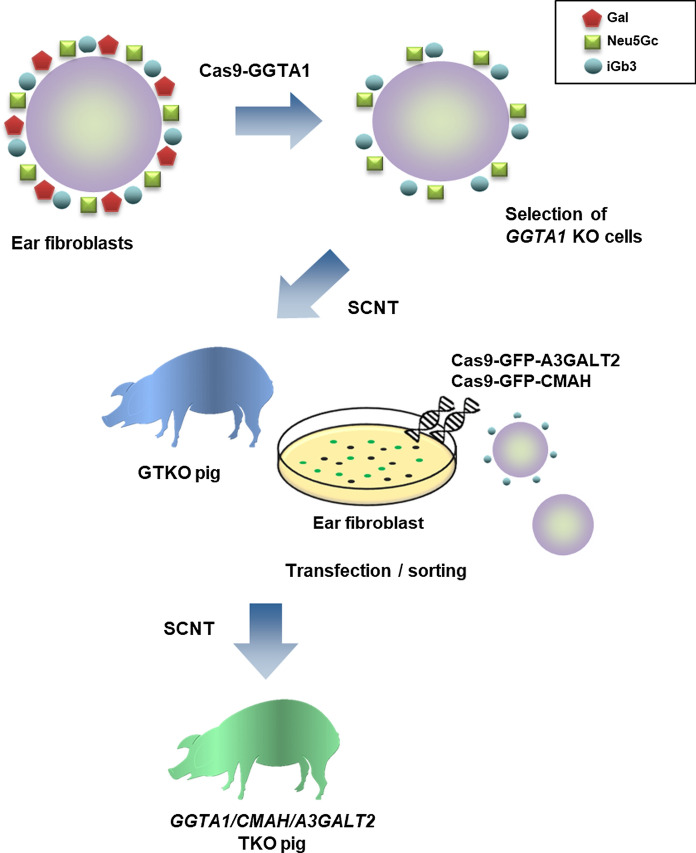
Figure 1 outlines the process used to produce genetically modified pigs. For the GGTA1 gene, sgRNAs were designed to target exon 9 as shown in Fig. 2a. Porcine ear fibroblast cells were transfected with the CRISPR/Cas9 vector for GGTA1 KO, and 13 GGTA1 mutant colonies were selected (13/21, 61.9%; Table 1 and Fig. S1a). Of these mutants, #226 was selected as the source of donor cells for SCNT using DNA sequencing (Fig. 3a). This procedure was repeated for the other two genes with sgRNAs designed to target exon 9 of CMAH and exon 4 of the A3GALT2 genes (Fig. 2b, c). The ear fibroblast cells isolated from GTKO piglets were simultaneously transfected with the CRISPR/Cas9 vectors for CMAH and A3GALT2 KO. Subsequently, 16 colonies carrying mutations in both CMAH and A3GALT2 (16/19, 84.2%) were identified and confirmed by DNA sequencing (Table 1 and Fig. S1b, c).
Fig. 1.
Workflow of the generation of GGTA1, CMAH, and A3GALT2 gene KO pigs using CRISPR/Cas9 technology. Ear fibroblast cells of Yucatan miniature pigs were transfected with a Cas9-GGTA1 vector. We produced cloned GGTA1 KO (GTKO) piglets using GS-IB4 lectin negative cells as nuclear donors for SCNT. We then simultaneously targeted the CMAH and A3GALT2 genes using CRISPR/Cas9 in ear fibroblast cells from a GTKO piglet. SCNT was performed with these transfected cells, and GGTA1/CMAH/A3GALT2 triple gene KO (TKO) piglets were produced.
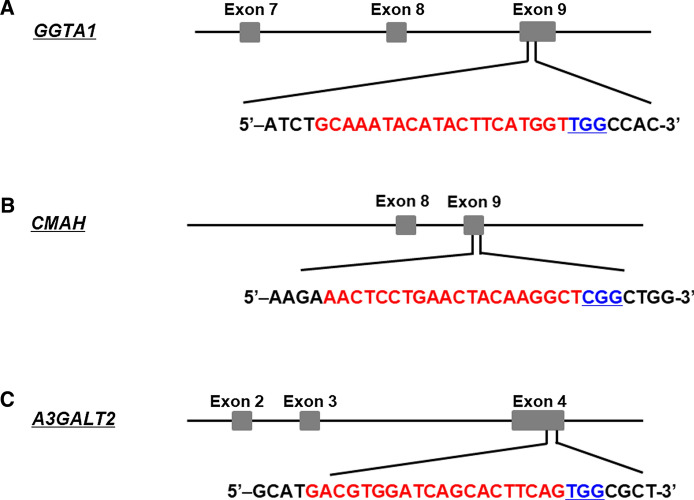
Fig. 2.
Generation of GGTA1, CMAH, and A3GALT2 gene KO transgenic cell lines. Schematic diagram of the CRISPR/Cas9 system targeting pigs. a GGTA1, b CMAH, and c A3GALT2 locus. The target site was in exon 4 of A3GALT2 and exon 9 of GGTA1 and CMAH genes. The red highlight indicates sgRNA targeting sites, and the blue underlined highlight indicates protospacer adjacent motif (PAM).
Table 1.
Targeting efficiency of CRISPR/Cas9
| Target gene | Target cells | Sex | Cell cloned with mutations (%) |
|---|---|---|---|
| GGTA1 | WT ear cells | Male | 13/21 (61.9) |
| CMAH/A3GALT2 | GTKO ear cells | Male | 16/19 (84.2) |
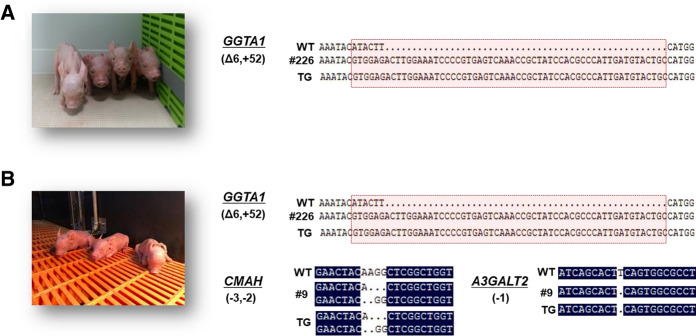
Fig. 3.
Generation of CRISPR/Cas9-induced GTKO and TKO cloned piglets. DNA sequence analysis and photographs of a GTKO and b TKO piglets. The DNA sequences of the cloned piglets showed a mutation identical to the sequences of the nuclear donor cells. Deletion, insertion, and shift mutations of the base pairs are indicated by dots (∙), plus (+), and delta (Δ), respectively.
Production of GTKO and TKO pigs by SCNT
A total of 401 reconstructed embryos derived from the GGTA1 KO cells were transferred into two surrogate recipients. One surrogate became pregnant and gave birth to five piglets, of which one piglet was stillborn and four piglets were live born (Table 2). All piglets were biallelic for the GGTA1 gene KO as determined by DNA sequencing (Fig. 3a). Flow cytometry analysis and western blot assay of the ear cells showed that the GTKO piglets did not express αGal (Fig. S2). We then attempted to generate cloned pigs using the selected TKO donor cells. A total of 718 reconstructed embryos were transferred into five recipient gilts and four became pregnant. All surrogate recipients carried to full term. Two piglets were stillborn, and eight piglets were live born (Table 2). The genotypes of the cloned piglets were determined using PCR and sequencing. All of the cloned piglets contained deletions of the GGTA1, CMAH, and A3GALT2 genes like the donor cells (Fig. 3a, b).
Table 2.
Efficiency of SCNT using transgenic donor cells
| Donor cells | No. of recipients | No. of transferred embryos (embryos per transfer) | Pregnancy (%)a | Delivery (%) | Live born (still born) | Cloning efficiencyb |
|---|---|---|---|---|---|---|
| GTKO | 2 | 401 (200) | 1 (50) | 1 (50) | 4 (1) | 1.2 |
| TKO | 5 | 718 (144) | 4 (80) | 4 (100) | 8 (2) | 1.3 |
aPregnant animals/total recipients
bFetuses and piglets/total embryos transferred
Expression of αGal, Neu5Gc and A3GALT2
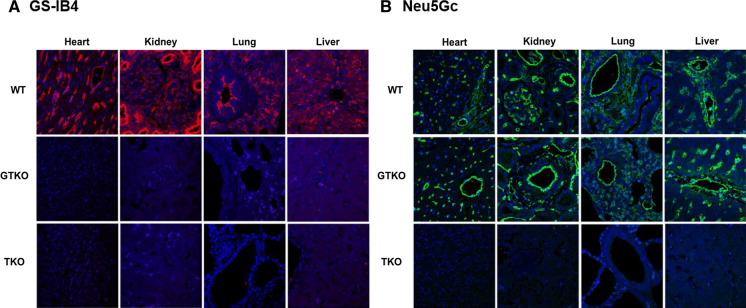
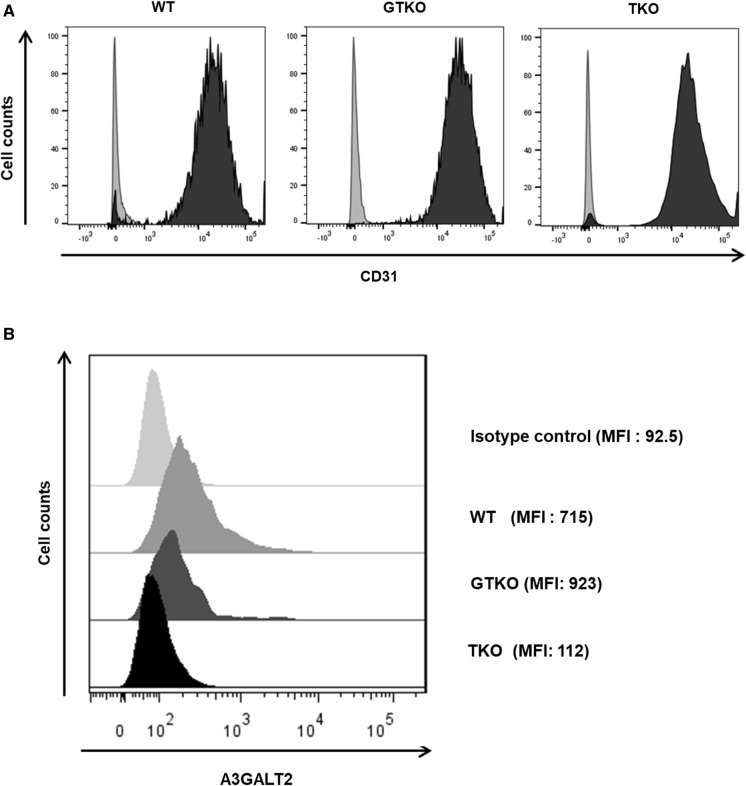
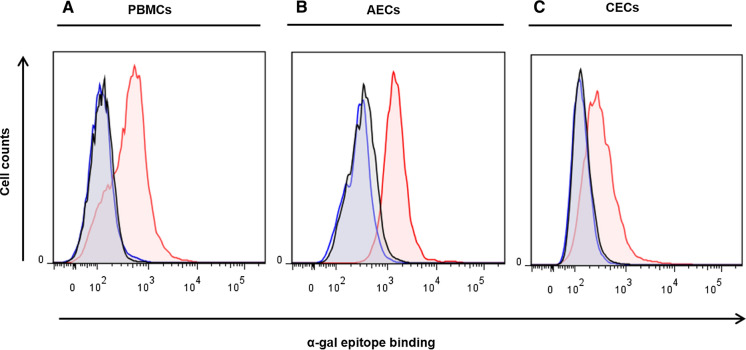
The expression of αGal and Neu5Gc antigens was examined in the heart, kidney, lung, and liver from GTKO, TKO, and WT pigs using immunofluorescence (IFA). αGal was expressed at a high level in the cardiac muscle, capillary endothelia, kidney tubules, pulmonary alveoli, and hepatocytes in a WT pig but was not detected in any of the transgenic pig tissues. However, we could not identify an additional reduction of αGal in TKO pig organ tissues when compared with GTKO pigs using the IFA assays (Fig. 4a). Neu5Gc was primarily expressed in the capillaries and vessels of all tissues in the WT and GTKO pigs (Fig. 4b). Neither αGal nor Neu5Gc was observed in any of the tissues from a TKO pig (Fig. 4a, b). We also examined the expression of A3GALT2 in various organ tissues of GTKO, TKO, and WT pigs and found no visible difference among them (data not shown). We then compared the expression of A3GALT2 from GTKO, TKO, and WT pigs at the cellular level (AECs) using FACS analysis. As shown in Fig. 5, the expression level of A3GALT2 in the TKO pig AECs was approximately eight-fold lower than that in AECs from WT or GTKO pigs (Fig. 5). To identify correlations between the level of αGal epitope expression and the silencing of the A3GALT2 gene, we evaluated αGal expression in pig PBMCs, AECs, and CECs using anti-Gal antibody. The expression of the αGal epitope in PBMCs, AECs, and CECs in all of the transgenic pigs was decreased from that in WT pigs. There were no differences in αGal expression in any of the cell types of GTKO and TKO pigs (Fig. 6).
Fig. 4.
Immunofluorescence analysis of GTKO, TKO, and WT pigs. Tissue sections of the heart, kidney, lung, and liver from GTKO, TKO, and WT pigs were stained with a GS-IB4 lectin b anti-Neu5Gc antibodies. Expression of αGal and Neu5Gc antigens was widespread in WT pig tissues. All tissues from GTKO pigs were negative for αGal, but TKO pigs were negative for both αGal and Neu5Gc antigens (nuclei, blue; Gal, red; Neu5Gc, green, magnification, × 400).
Fig. 5.
Comparison of A3GALT2 expression in AECs from GTKO, TKO, and WT pigs. a Endothelial cell marker CD31 was expressed on AECs from all types of pigs. Gray histograms represent isotype control, and black histograms represent CD31. b The expression level of A3GALT2 on TKO pig AECs was significantly decreased from that of GTKO or WT pigs (MFI; mean fluorescence intensity).
Fig. 6.
Comparison of αGal epitope expression on various pig cells. αGal epitope expression on a PBMCs, b AECs, and c CECs from all transgenic pigs was decreased from that of WT. However, there were no differences in the expression of αGal between GTKO and TKO pig cells (WT, red; GTKO, blue; TKO, black).
Human IgM/IgG binding to pig cells
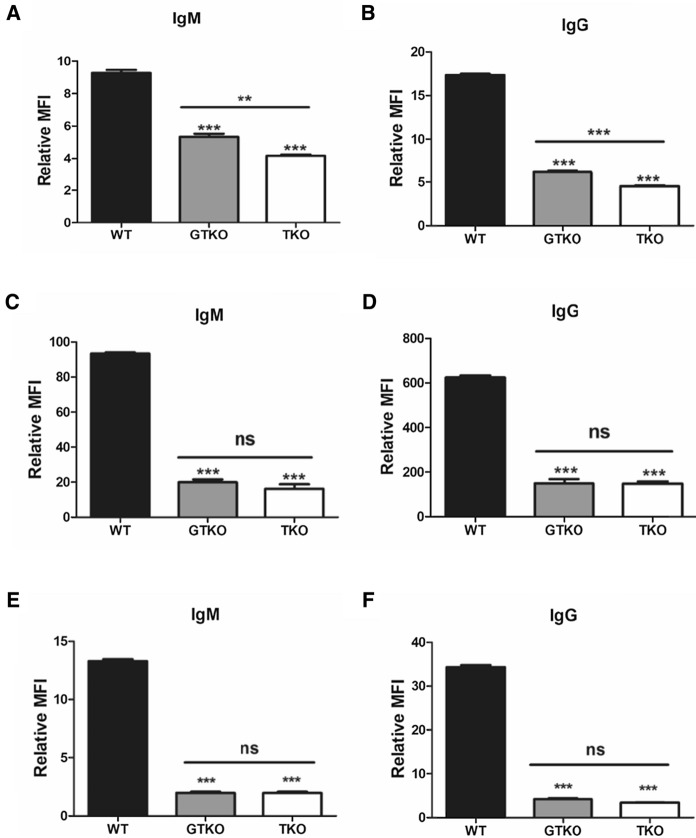
To investigate the immunoreactivity of pig-to-human xenotransplantation, we compared the IgM and IgG binding of human antibodies in the PBMCs, AECs, and CECs isolated from GTKO, TKO, and WT pigs (Fig. 7). IgM and IgG binding to the PBMCs of GTKO and TKO pigs were significantly reduced when compared to that of the WT (Fig. 7a, b, ***P < 0.001). IgM and IgG binding to PBMCs from TKO pigs were significantly lower than that in GTKO pigs (**P < 0.01, ***P < 0.001). IgM and IgG binding to AECs and CECs in all transgenic pigs was significantly decreased when compared to WT pigs (Fig. 7c, d, e, f, ***P < 0.001). However, there were no differences in IgM or IgG binding to AECs and CECs between transgenic pigs.
Fig. 7.
Human IgM and IgG binding to GTKO, TKO, and WT pig PBMCs (a, b), AECs (c, d), and CECs (e, f). a, b Human IgM/IgG binding of PBMCs was significantly decreased in transgenic pigs when compared to WT (***P < 0.001 vs. WT). There were also significant differences in IgM/IgG binding between GTKO and TKO pigs (**P < 0.01; ***P < 0.001). c, d Human IgM/IgG binding of AECs was significantly reduced in all transgenic pigs compared to that in WT (***P < 0.001 vs. WT). However, there was no further reduction in IgM and IgG binding in TKO pigs when compared to that in GTKO pigs. e, f Human IgM/IgG binding of CECs in GTKO and TKO pigs was markedly reduced when compared to that in WT pigs (***P < 0.001 vs. WT), but there were no significant differences in IgM/IgG binding between GTKO and TKO pigs. Experiments were performed in quadruplicate.
Human complement-dependent cytotoxicity
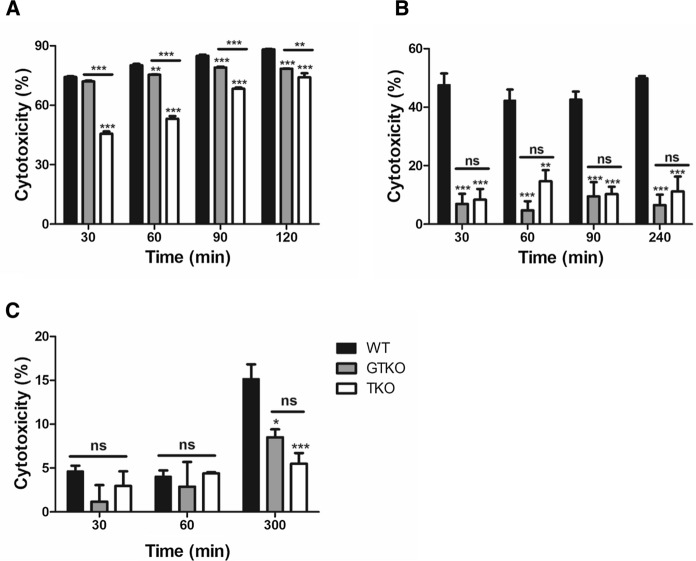
We assessed the ability of human serum to cause complement-mediated lysis of PBMCs, AECs, and CECs from GTKO, TKO and WT pigs. The pig cells were incubated with 50% pooled normal human complement serum for 300 min. The cytotoxicity of PBMCs from TKO pigs was significantly decreased when compared to that of WT or GTKO pigs at all time points (Fig. 8a, (**P < 0.01, ***P < 0.001 vs. WT or GTKO). The cytotoxicity of PBMCs from GTKO pigs was significantly decreased from that of WT pigs at 60, 90, and 120 min (**P < 0.01, ***P < 0.001 vs. WT). The cytotoxicity of AECs from all transgenic pigs was significantly decreased from that of WT pigs at all time points (Fig. 8b. **P < 0.01, ***P < 0.001 vs. WT). However, there was no difference in the cytotoxicity of AECs from GTKO and TKO pigs at any time point. The cytotoxicity of CECs from transgenic pigs was significantly decreased from that of WT pigs at 300 min (Fig. 8c, *P < 0.05, ***P < 0.001 vs. WT). However, there was no difference in the cytotoxicity of CECs from GTKO and TKO at any time point.
Fig. 8.
Cytotoxicity of PBMCs, AECs, and CECs from GTKO, TKO, and WT pigs. a The cytotoxicity of GTKO pigs on PBMCs was significantly lower than that on WT pigs at 60, 90, and 120 min (**P < 0.01; ***P < 0.001 vs. WT), and there was further significant reduction in the cytotoxicity of TKO pig compared to that of GTKO or WT pigs (**P < 0.01; ***P < 0.001) at all time points. b The cytotoxicity of transgenic pigs on AECs was significantly decreased compared to that of WT pigs at all time points (**P < 0.01; ***P < 0.001 vs. WT), but there was no difference in cytotoxicity between GTKO and TKO pig AECs. c The cytotoxicity of transgenic pigs on CECs was significantly reduced from WT at 300 min (*P < 0.05; ***P < 0.001 vs. WT), but there was similar cytotoxicity between GTKO and TKO pigs. Experiments were performed in quadruplicate.
Discussion
Pig organs and tissues offer a promising solution to the problem of shortages of organs for transplantation into humans. However, because pigs and humans are different species, human antibodies recognize pig cells as xenogenic antigens, thereby triggering the rejection of transplanted tissues (Hryhorowicz et al. 2017). To facilitate the clinical application of xenotransplantation, understanding the antibody-mediated rejection of transplanted organs is essential (Butler et al. 2016b). Researchers have identified several xenoantigens from pigs and have demonstrated that modifications to the genome of donor pigs using genetic engineering techniques such as ZFN, TALEN, and CRISPR/Cas9 are valuable for generating pig organs that have lower immunogenicity (Hryhorowicz et al. 2017; Niemann and Petersen 2016).
In this study, we generated GGTA1/CMAH/A3GALT2 TKO pigs using CRISPR/Cas9 genome editing and investigated the effect of this triple gene KO. The CRISPR/Cas9 system is known to be highly efficient for editing genes, is easy, relatively straightforward, and rapid, and can be used to target multiple genes simultaneously (Mehravar et al. 2019). Recently, one-step genome editing using intracytoplasmic microinjection of fertilized zygotes with a CRISPR/Cas9 vector has been successful (Petersen et al. 2016). However, direct injection of CRISPR/Cas9 molecules can cause the system to continue to operate at various stages of embryo development, resulting in genetic mosaicism of transgenic animals (Mehravar et al. 2019; Sato et al. 2018). For these reasons, we used the SCNT system to generate GTKO and TKO pigs in this study. Although the cloning efficiencies were less than 2% (Table 2), the piglets that were produced were genetically identical to the donor cells (Fig. 3).
The expression of αGal was barely detectable in all transgenic pigs. Neu5Gc was not expressed in TKO pigs (Fig. 4). Previous studies in other animals have shown that iGb3 levels are species- and tissue-specific. iGb3 is only detected in the thymus and murine dorsal root ganglia of rats and is not detectable in humans, pigs, or other tissues of mice (Christiansen et al. 2008; Speak et al. 2007). However, A3GALT2 mRNA is ubiquitous within pig tissues and endothelial cells (Puga Yung et al. 2012). Therefore, we confirmed the expression of the A3GALT2 protein in the AECs of TKO pigs using a synthetic polyclonal antibody instead of iGb3 (Fig. 5). We found that the AECs of TKO pigs rarely express A3GALT2 more than GTKO or WT pigs. This result indicated that three targeted genes were disrupted completely by the CRISPR/Cas9 constructs (Figs. 4, 5).
However, there were no significant differences in IB4 binding in tissues from GTKO and TKO pigs (Fig. 4a). We could not detect any differences in IB4 binding at a cellular level between GTKO and TKO pigs (Fig. S3). Similarly, Butler et al. showed that IB4 binding in PBMCs, spleen and lymph node cells did not differ between GTKO and GGTA1/A3GALT2 DKO pigs and suggested that silencing of the A3GALT2 gene did not affect IB4 binding (Butler et al. 2016b). Galili et al. demonstrated that because the binding affinity of the lectin and αGal epitope is low, the detection of the relatively small amount of αGal epitope on cell surfaces may be insufficient to allow an observer to draw any conclusions (Galili et al. 1998). Therefore, we further attempted to detect the αGal epitope on the pig cells (PBMCs, AECs and CECs) using αGal epitope monoclonal antibody. However, the level of αGal epitope expression was already close to zero in GTKO pigs, and we could not measure any additional reduction in its expression associated with A3GALT2 KO (Fig. 6). Shao et al. showed that αGal epitope expression in various organs of A3GALT2 KO mice decreased from 21.74 to 5.19% compared to WT mice (Shao et al. 2018). It is possible that the A3GALT2 gene contributes to αGal epitope expression in mice rather than in pigs.
We evaluated human antibody binding and human complement-mediated cytotoxicity of different cell types from the pigs. Human preformed natural antibodies bind to pig organs or tissues during pig-to-human xenotransplantation. Antibody deposition causes complement-mediated injury of the grafts, leading to thrombosis, interstitial hemorrhage, and edema, all of which disrupts graft function (Cooper et al. 2015). We examined the binding potential of the most important human antibodies, namely, IgM and IgG, and cytotoxicity to PBMCs, AECs, and CECs isolated from GTKO, TKO, and WT pigs. The human antibody binding ability of PBMCs, AECs, and CECs from the transgenic pigs was significantly reduced when compared to that of WT pigs, but these results were different in TKO and GTKO pigs depending on the cell type (Fig. 7). In the PBMCs of TKO pigs, the binding of human antibodies was significantly lower than that in GTKO pigs (Fig. 7a, b). The cytotoxicity of PBMCs from TKO pigs was markedly reduced from those of GTKO or WT pigs at all time points (Fig. 8a). As we did not examine GGTA1/CMAH DKO pigs, it is not clear whether the reduction in antibody binding and cytotoxicity in the TKO pigs is caused by CMAH KO alone or whether there is an additional effect from the A3GALT2 KO. However, because we could not observe additional reductions in αGal expression in TKO cells when compared with GTKO, we can conclude that these results were due to the CMAH gene KO.
The human antibody binding and cytotoxicity of AECs were similar in GTKO and TKO pigs (Fig. 7c, d, and Fig. 8b). Both the antibody binding and cytotoxicity of the AECs were dramatically reduced by just a GGTA1 single gene KO compared with WT pigs. As AECs were cultured in a medium containing 2% of fetal bovine serum (FBS), we additionally confirmed whether FBS induced false-positive Neu5Gc expression using FACS analysis (Wang et al. 2016). As a result, AECs of TKO pigs were not expressed Neu5Gc differs from GTKO and WT pigs (Fig. S4). The effects of additional gene silencing such as CMAH and A3GALT2 based on GTKO in AECs, unlike PBMCs, do not appear to be significant. PBMCs are used primarily in assays related to the immune reactivity of xenogeneic antigens because they contain a range of immune cells, such as lymphocytes, monocytes, and macrophages (Pourahmad and Salimi 2015). However, it seems that the source of the cells used to demonstrate the effectiveness of xenoantigens in vitro should be target organ specific. For studies of solid organ xenotransplantation, porcine AECs have mainly been used to detect humoral immune responses in vitro. Zhang et al. suggested that assays based on pig renal microvascular endothelial cells (RMECs) are more useful than AECs to detect immune responses for kidney xenotransplantation because RMECs are more immunogenic than AECs (Zhang et al. 2017a).
In previous reports, GTKO pig organs increased the survival of pig-to-primate transplants, but graft failure did eventually occur (Kuwaki et al. 2005; Yamada et al. 2005). This observation suggests that non-Gal antigens contribute to acute vascular rejection responses (Ezzelarab et al. 2005; Kwon et al. 2013). Many researchers have shown that both GGTA1 and CMAH DKO pigs could reduce the humoral rejection response to xenotransplantation more than GTKO pigs using pig PBMCs, AECs, and RBCs (Hara et al. 2008; Lee et al. 2016a; Lee et al. 2016b; Lutz et al. 2013). It is possible that our results using AECs were different because we used different pig breeds. Previous reports were based on the use of cross-breed pigs, such as Landrace, Yorkshire, Chester white, Large White, or Duroc. However, we used Yucatan miniature pigs. Actually, we compared human antibody binding on PBMCs between commercial cross-breed (Landrace/Yokshire/Duroc) and WT Yucatan miniature pigs. IgM binding was similar between the two groups, but IgG binding in Yucatan miniature pigs was lower than that in cross-breed pigs (commercial cross-breeds vs. Yucatan, IgM 566: 431, IgG 2,564: 1,567, n = 3). This result indicates that Yucatan miniature pigs have lower non-gal antigen than cross-breed pigs, and xenoantigens might differ depending on the breed of pig.
Previous studies reported that the humoral and cellular responses to GTKO, GTKO/hCD46, or DKO (GGTA1/CMAH)/hCD46 pig CECs were dramatically reduced compared with WT pig CECs, but there were no significant differences among the CECs of transgenic pigs (Hara et al. 2011; Lee et al. 2016a; Lee et al. 2016b). As in previous reports, the level of human IgM/IgG binding in CECs was similar between GTKO and TKO transgenic pigs (Fig. 7c). Furthermore, the cytotoxicity of CECs was significantly reduced in transgenic pigs over WT at 300 min, and there was no difference between GTKO and TKO pigs (Fig. 8c). Cornea is an immune-privileged tissue (Hori et al. 2019; Yoon et al. 2021), and CECs appear to have less immunogenicity than PBMCs or AECs. These results appear to indicate that a GGTA1 single gene knockout is sufficient for corneal xenotransplantation.
Collectively, our data suggest that the genetic modification of donor pigs for xenotransplantation should differ depending on the target organ and silencing of additional genes such as CMAH or A3GALT2 based on GTKO might not be essential in Yucatan miniature pigs. However, our study has limitations due to the use of in vitro testing. Further study should focus on the characterization of Yucatan miniature pigs and the effects of genetically modified pig-to-nonhuman primate organ transplantation. Although A3GALT2 KO pigs rarely had an effect on pig-to-human immune reactivity in our study, it could provide more information on the role of iGb3 on NKT cell activity in preclinical test. Recently, preclinical studies have reported that pigs with a CMAH gene knocked out express new xenoantigens called “forth xenoantigen” in pig-to-Old World NHP organ transplantation (Cooper et al. 2020; Cui et al. 2020; Yamamoto et al. 2020). This finding indicates that it is, as yet, almost impossible to confirm the effect of CMAH KO in preclinical tests. If the deletion of the CMAH gene is not needed in Yucatan miniature pigs, they could be an ideal model animal for the study of xenotransplantation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Jeong-Woong Lee from KRIBB for his assistance in obtaining the CRISPR/Cas9 plasmid and synthesized A3GALT2 antibodies. The authors would also like to thank Youn-Sang Kweon and SeongHoon Kim for their technical support around animal management.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number : HI20C0056).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent for publication
The participant has consented to the submission of the case report to the journal.
Ethics approval
Approval was obtained from the ethics committee of Optipharm, Inc., Life Science Institute (IACUC approval No. OPT-140103-1).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams AB, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018;268:564–573. doi: 10.1097/SLA.0000000000002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer ME. Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalT-KO era. Xenotransplantation. 2011;18:215–228. doi: 10.1111/j.1399-3089.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- Butler JR, Ladowski JM, Martens GR, Tector M, Tector AJ. Recent advances in genome editing and creation of genetically modified pigs. Int J Surg. 2015;23:217–222. doi: 10.1016/j.ijsu.2015.07.684. [DOI] [PubMed] [Google Scholar]
- Butler JR, et al. Silencing porcine CMAH and GGTA1 genes significantly reduces xenogeneic consumption of human platelets by porcine livers. Transplantation. 2016;100:571–576. doi: 10.1097/TP.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JR, et al. Silencing the porcine iGb3s gene does not affect Galalpha3Gal levels or measures of anticipated pig-to-human and pig-to-primate acute rejection. Xenotransplantation. 2016;23:106–116. doi: 10.1111/xen.12217. [DOI] [PubMed] [Google Scholar]
- Choi K, et al. Production of heterozygous alpha 1,3-galactosyltransferase (GGTA1) knock-out transgenic miniature pigs expressing human CD39. Transgenic Res. 2017;26:209–224. doi: 10.1007/s11248-016-9996-7. [DOI] [PubMed] [Google Scholar]
- Christiansen D, et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008;6:e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DKC, Ekser B, Tector AJ. Immunobiological barriers to xenotransplantation. Int J Surg. 2015;23:211–216. doi: 10.1016/j.ijsu.2015.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DKC, et al. Pig kidney xenotransplantation: progress toward clinical trials. Clin Transplant. 2020 doi: 10.1111/ctr.14139. [DOI] [PubMed] [Google Scholar]
- Cooper DKC, et al. Regulation of clinical xenotransplantation-time for a reappraisal. Transplantation. 2017;101:1766–1769. doi: 10.1097/TP.0000000000001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, et al. Evidence for GTKO/beta4GalNT2KO pigs as the preferred organ-source for old world nonhuman primates as a preclinical model of xenotransplantation. Transplant Direct. 2020;6:e590. doi: 10.1097/TXD.0000000000001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada JL, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzelarab M, Ayares D, Cooper DK. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005;83:396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- Galili U, LaTemple DC, Radic MZ. A sensitive assay for measuring alpha-Gal epitope expression on cells by a monoclonal anti-Gal antibody. Transplantation. 1998;65:1129–1132. doi: 10.1097/00007890-199804270-00020. [DOI] [PubMed] [Google Scholar]
- Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24:372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Investig Ophthalmol Vis Sci. 2011;52:5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, Cooper DK. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- Hori J, Yamaguchi T, Keino H, Hamrah P, Maruyama K. Immune privilege in corneal transplantation. Prog Retinal Eye Res. 2019;72:100758. doi: 10.1016/j.preteyeres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Hryhorowicz M, Zeyland J, Slomski R, Lipinski D. Genetically modified pigs as organ donors for xenotransplantation. Mol Biotechnol. 2017 doi: 10.1007/s12033-017-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim J, Jeong HJ, Lee HJ, Kim MK, Wee WR. Biophysico-functional compatibility of Seoul National University (SNU) miniature pig cornea as xenocorneal graft for the use of human clinical trial. Xenotransplantation. 2016;23:202–210. doi: 10.1111/xen.12234. [DOI] [PubMed] [Google Scholar]
- Kuwaki K, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- Kwon DN, et al. Production of biallelic CMP-Neu5Ac hydroxylase knock-out pigs. Sci Rep. 2013;3:1981. doi: 10.1038/srep01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langin M, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. doi: 10.1038/s41586-018-0765-z. [DOI] [PubMed] [Google Scholar]
- Lee W, et al. Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs. Xenotransplantation. 2016;23:137–150. doi: 10.1111/xen.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, et al. Expression of NeuGc on pig corneas and its potential significance in pig corneal. Xenotransplant Cornea. 2016;35:105–113. doi: 10.1097/ICO.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation. 2015;22:20–31. doi: 10.1111/xen.12131. [DOI] [PubMed] [Google Scholar]
- Li P, Estrada JL, Burlak C, Tector AJ. Biallelic knockout of the alpha-1,3 galactosyltransferase gene in porcine liver-derived cells using zinc finger nucleases. J Surg Res. 2013;181:e39–45. doi: 10.1016/j.jss.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Lutz AJ, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Mehravar M, Shirazi A, Nazari M, Banan M. Mosaicism in CRISPR/Cas9-mediated genome editing. Dev Biol. 2019;445:156–162. doi: 10.1016/j.ydbio.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J Immunol. 2006;176:2448–2454. doi: 10.4049/jimmunol.176.4.2448. [DOI] [PubMed] [Google Scholar]
- Milland J, Christiansen D, Sandrin MS. Alpha 1,3-galactosyltransferase knockout pigs are available for xenotransplantation: are glycosyltransferases still relevant? Immunol Cell Biol. 2005;83:687–693. doi: 10.1111/j.1440-1711.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- Mohiuddin MM, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H, Petersen B. The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 2016;25:361–374. doi: 10.1007/s11248-016-9934-8. [DOI] [PubMed] [Google Scholar]
- Park JY, et al. alpha1,3-galactosyltransferase deficiency in germ-free miniature pigs increases N-glycolylneuraminic acids as the xenoantigenic determinant in pig-human xenotransplantation. Cell Reprogram. 2012;14:353–363. doi: 10.1089/cell.2011.0083. [DOI] [PubMed] [Google Scholar]
- Park JY, et al. Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity. J Biomed Biotechnol. 2011;2011:560850. doi: 10.1155/2011/560850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B, et al. Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes. Xenotransplantation. 2016;23:338–346. doi: 10.1111/xen.12258. [DOI] [PubMed] [Google Scholar]
- Pourahmad J, Salimi A. Isolated human peripheral blood mononuclear cell (PBMC), a cost effective tool for predicting immunosuppressive effects of drugs and xenobiotics. Iran J Pharm Res IJPR. 2015;14:979. [PMC free article] [PubMed] [Google Scholar]
- Puga Yung GL, Li Y, Borsig L, Millard AL, Karpova MB, Zhou D, Seebach JD. Complete absence of the alphaGal xenoantigen and isoglobotrihexosylceramide in alpha1,3galactosyltransferase knock-out pigs. Xenotransplantation. 2012;19:196–206. doi: 10.1111/j.1399-3089.2012.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J, Prather RS, Lee K. Use of gene-editing technology to introduce targeted modifications in pigs. J Anim Sci Biotechnol. 2018;9:5. doi: 10.1186/s40104-017-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JP, et al. CD1d protein structure determines species-selective antigenicity of isoglobotrihexosylceramide (iGb3) to invariant NKT cells. Eur J Immunol. 2013;43:815–825. doi: 10.1002/eji.201242952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, et al. Timing of CRISPR/Cas9-related mRNA microinjection after activation as an important factor affecting genome editing efficiency in porcine oocytes. Theriogenology. 2018;108:29–38. doi: 10.1016/j.theriogenology.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Sato M, et al. The combinational use of CRISPR/Cas9-based gene editing and targeted toxin technology enables efficient biallelic knockout of the alpha-1,3-galactosyltransferase gene in porcine embryonic fibroblasts. Xenotransplantation. 2014;21:291–300. doi: 10.1111/xen.12089. [DOI] [PubMed] [Google Scholar]
- Shao A, Xu L, Wu X, Liu S, Lu Y, Fan C. Gal epitope expression and immunological properties in iGb3S deficient mice. Sci Rep. 2018;8:15433. doi: 10.1038/s41598-018-33032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, et al. Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75:430–436. doi: 10.1097/01.TP.0000053615.98201.77. [DOI] [PubMed] [Google Scholar]
- Song KH, Kim CH. Sialo-xenoantigenic glycobiology. New York: Springer; 2013. pp. 1–10. [Google Scholar]
- Speak AO, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiri F, Li Y, Hawke D, Ganiko L, Almeida I, Levery S, Zhou D. Lack of iGb3 and isoglobo-series glycosphingolipids in pig organs used for xenotransplantation: implications for natural killer T-cell biology. J Carbohydr Chem. 2013;32:44–67. doi: 10.1080/07328303.2012.741637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014;21:376–384. doi: 10.1111/xen.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, et al. Immunogenicity of renal microvascular endothelial cells from genetically modified pigs. Transplantation. 2016;100:533–537. doi: 10.1097/TP.0000000000001070. [DOI] [PubMed] [Google Scholar]
- Whitworth KM, et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol Reprod. 2014;91:78. doi: 10.1095/biolreprod.114.121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout) Sci Rep. 2020;10:9771. doi: 10.1038/s41598-020-66311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CH, Choi HJ, Kim MK. Corneal xenotransplantation: Where are we standing? Prog Retinal Eye Res. 2021;80:100876. doi: 10.1016/j.preteyeres.2020.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. Potential antigens involved in delayed xenograft rejection in a Ggta1/Cmah Dko pig-to-monkey. Model Sci Rep. 2017;7:10024. doi: 10.1038/s41598-017-10805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. Generation of complement protein C3 deficient pigs by CRISPR/Cas9-mediated gene targeting. Sci Rep. 2017;7:5009. doi: 10.1038/s41598-017-05400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.