Abstract
Botulinum neurotoxins (BoNTs) are proteins produced by bacteria of the Clostridium family. Upon oral ingestion, BoNT causes the neuroparalytic syndrome botulism. There are seven serotypes of BoNT (serotypes A-G); BoNT-A and BoNT-B are the botulinum toxin serotypes utilized for therapeutic applications. Treatment with BoNT injections is used to manage chronic medical conditions across multiple indications. As with other biologic drugs, immunogenicity after long-term treatment with BoNT formulations may occur, and repeated use can elicit antibody formation leading to clinical nonresponsiveness. Thus, approaching BoNT treatment of chronic conditions with therapeutic formulations that minimize stimulating the host immune response while balancing patient responsiveness to therapy is ideal. Immunogenicity is a clinical limitation in many settings that use biologic drugs for treatment, and clinically relevant immunogenicity reduction has been achieved through engineering smaller protein constructs and reducing unnecessary formulation components. A similar approach has influenced the evolution of BoNT formulations. Three BoNT-A products and one BoNT-B product have been approved by the Food and Drug Administration (FDA) for therapeutic use: onabotulinumtoxinA, abobotulinumtoxinA, incobotulinumtoxinA, and rimabotulinumtoxinB; a fourth BoNT-A product, daxibotulinumtoxinA, is currently under regulatory review. Additionally, prabotulinumtoxinA is a BoNT-A product that has been approved for aesthetic indications but not therapeutic use. Here, we discuss the preclinical and clinical immunogenicity data that exist within the scientific literature and provide a perspective for considering immunogenicity as a key factor in choice of BoNT formulation.
Keywords: AbobotulinumtoxinA, Antibodies, Biologics, Clinical response, IncobotulinumtoxinA, Neutralizing, OnabotulinumtoxinA, Second generation
Key Summary Points
| Immunogenicity is a frequent clinical barrier seen with the use of many biologic drugs, including botulinum toxins utilized for therapeutic applications; repeated use can lead to the development of neutralizing antibodies (nAbs) that may affect treatment outcomes |
| Common strategies for reducing the immunogenicity of biologic drugs and the prevalence of neutralizing antibodies include engineering smaller proteins and reducing contaminants or unnecessary formulation components |
| For botulinum neurotoxin (BoNT) formulations utilized for therapeutic applications, incobotulinumtoxinA is the most purified; preclinical and clinical data suggest it has reduced immunogenicity compared with other formulations |
| BoNT therapy is often lifelong in patients with chronic conditions; thus, the potential for immunogenicity and risk of reducing nAb production should be considered when making treatment decisions regarding BoNT formulation |
Introduction
Botulinum neurotoxin (BoNT) injections are used to manage chronic medical conditions across multiple indications and aesthetic applications, including symptomatic relief of blepharospasm, cervical dystonia, various types of focal muscle spasticity, and temporary improvement of dynamic facial lines [1–3]. The therapeutic use of BoNT in chronic conditions is potentially lifelong, and, given the bacterial origins of BoNT, repeated exposure can elicit antibody formation leading to clinical nonresponsiveness [2, 3]. Reports of immunogenicity after long-term treatment with some BoNT formulations are increasingly emergent [4–13], with varying outcomes dependent on factors such as diagnosis, BoNT formulation, prior BoNT treatment, neurotoxin complex protein load, injection session dose, treatment duration, and length of reinjection interval [3, 14–16].
Multiple BoNTs are approved for use in numerous countries worldwide; first-generation BoNT formulations contain a core neurotoxin plus complexing accessory clostridial proteins, whereas second-generation BoNTs lack complexing, accessory clostridial proteins as a result of their removal during purification [17–21]. These BoNT formulations are associated with varying incidence rates for development of neutralizing antibodies (nAbs) that bind to BoNT and may reduce efficacy or duration of clinical response [17–20]. Additionally, the US Food and Drug Administration (FDA) has issued guidance documents on assessing immunogenicity of therapeutic protein products and recommends a “risk-based approach to evaluating and mitigating immune responses to, or adverse immunologically related responses associated with, therapeutic protein products” [22, 23]. This emerging body of evidence [17–20] and current guidelines [22, 23] emphasize the importance of approaching BoNT treatment of chronic conditions with protocols to minimize the immune response and maximize patient responsiveness.
Immunogenicity of biologic drugs is a clinical limitation in many settings, and observing how other therapies have evolved could provide insights for reducing immunogenicity in BoNT treatment paradigms [24–26]. This article reviews the existing literature and does not contain any new studies with human participants or animals performed by any of the authors. In this review, we describe the basic science of immunogenicity as a potential clinical barrier to the efficacy of biologic therapies and its effect on the evolution of BoNT formulations. We summarize available nonclinical and clinical evidence of immunogenicity and clinical nonresponsiveness associated with different BoNT formulations and discuss whether there is a lower risk of immunogenicity with a second-generation BoNT formulation, incobotulinumtoxinA. Finally, we discuss potential areas of research to address current knowledge gaps and provide an immunologic perspective for considering immunogenicity as a factor in choosing a BoNT formulation.
Immunogenicity and Clinical Limitations of Biologic Drugs
Immunogenicity is the ability of any molecule, including foreign proteins or biologic drugs, to provoke a host immune response [27]. Any biologic drug, such as a recombinant therapeutic protein, gene therapy vector, or vaccine, has the potential to become a target of the immune system, particularly if administered repeatedly or at a high dose [27, 28]. For example, protein-based vaccines are designed to trigger adaptive immunity and the development of specific antibodies to potential pathogens to exert their effect [29]. However, immunogenicity is undesirable when the production of antidrug antibodies or other immune processes lead to a loss of therapeutic effectiveness of a biologic drug, which can occur through direct neutralization as well as alteration in pharmacokinetics [27].
Immunogenicity in Response to Biologic Drugs
The recognition of biologics and induction of an immune response are mediated by a network of immune cells, including antigen presenting cells (APCs), T cells, and B cells (Fig. 1) [30]. Dendritic cells are a type of APC that engulfs foreign proteins, such as biologics, by capturing them through various cell-surface receptors, including Toll-like receptors (TLRs), Fc receptors, and members of the C-type lectin family [31]. Dendritic cells can then process the captured biologic and display peptide fragments on the cell surface via the major histocompatibility complex (MHC) [30, 31]. Peptide-MHC complexes are presented to and recognized by T cells, which in turn can stimulate B cells to produce antigen-specific antibodies [30, 31]. The activation of T cells is also dependent on the presence and binding of costimulatory molecules, including cytokines, that are produced when the APCs are activated by stimulation of surface receptors (e.g., TLRs). Subsequent activation and expansion of B cells can result in long-lasting and abundant production of antibodies through memory B cells or plasma cells, a hallmark of adaptive immunity [30, 31]. Additionally, antibodies against biologics can be generated through T-cell independent pathways, where aggregates of the biologic can directly bind and stimulate B cells to produce antibodies [31, 32]. Some of the antibodies produced through either T cell-dependent or T cell-independent pathways are nAbs that can inhibit the activity and nullify the therapeutic effect of the biologic [27].
Fig. 1.
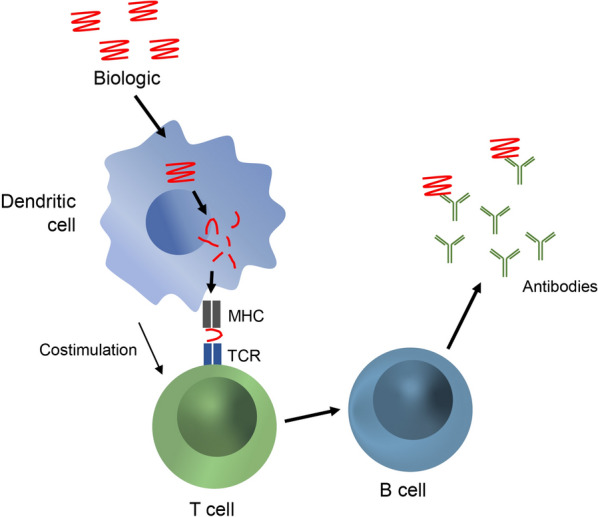
Immunogenicity in response to biologic drugs. Dendritic cells engulf biologics that bind to various cell surface receptors [27, 31]. Biologics are processed and presented as peptide fragments on the cell surface via the major histocompatibility complex (MHC) [30, 31]. Peptide-MHC complexes are presented to and recognized by T cells, which stimulate B cells to clonally expand and produce antigen-specific antibodies [30, 31]. Costimulation by molecules such as cytokines is required for complete activation of T cells. Subsequent expansion of B cells results in long-lasting and abundant production of antibodies [30, 31]. TCR, T-cell receptor
The immunogenicity can be influenced by several key factors, including molecular weight, structural complexity, posttranslational modifications, and features of the amino acid sequence [27, 33]. However, smaller molecules that may not be immunogenic alone can bind to larger endogenous proteins and be recognized by the immune system, leading to activation of dendritic cells and an adaptive immune response [34]. Biologics that form large aggregates may also be able to interact with and activate B cells [31, 35]. Additionally, other components of the biologic formulation, such as excipients (e.g., surfactants) and contaminants (e.g., host cell proteins, including bacterial flagellin) present in injected treatments, may stimulate the immune system and result in an increase in neutralizing antibodies against a biologic due to an unintended adjuvant effect [26, 36]. This adjuvant effect has been suggested to initiate an innate immune response after exposure to flagellin via TLR5 [9, 37].
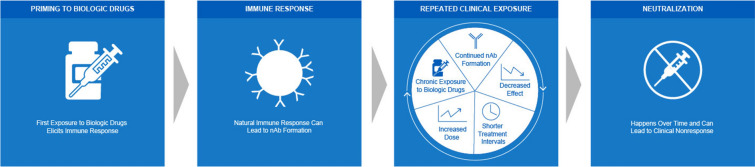
Following the initial priming of the adaptive immune response, repeated exposure to the antigen may result in a faster and stronger response [30, 38]. In the case of biologic drug therapies, including BoNT therapy, repeated clinical exposure can potentially provoke a continual immune response and formation of nAbs, resulting in a spectrum of clinical nonresponsiveness outcomes [31, 39–42]. Common signs of clinical resistance include increasing the frequency or dose of the drug administered to elicit results, complete nonresponse, and partial nonresponse (Fig. 2) [25, 42, 43].
Fig. 2.
Immunogenicity drives clinical nonresponsiveness. Repeated clinical exposure to biologic drugs can provoke a continual immune response and formation of nAbs leading to clinical resistance [30, 31, 39]. Common signs of clinical resistance include increasing the frequency or dose of the drug administered to elicit results. Over time, this can lead to clinical nonresponse [25, 42, 43]. nAb, neutralizing antibody
Clinical Challenges of Immunogenicity to Biologic Drugs
Biologic drugs have revolutionized treatment of many conditions across all areas of medicine [25]. Nevertheless, immunogenicity is a key clinical challenge associated with the use of many biologic therapies [30]. The formation of nAbs and antibodies against formulation contaminants has been observed in several therapeutic areas, including Fabry disease [24], rheumatoid arthritis [25], insulin-dependent diabetes [44], and asthma [26], among others (also seen in inflammatory bowel disease, psoriasis, and psoriatic arthritis). In Fabry disease, a rare X-linked disorder, enzyme replacement therapy (ERT) can lead to dose-related development of nAbs, which in turn limits treatment efficacy and results in disease progression, loss of renal function, and adverse cardiovascular outcomes [24, 45, 46]. However, data from patients with Fabry disease and renal transplants have shown that administration of immunosuppressant drugs before ERT prevents the formation of nAbs, suggesting that immunomodulation prior to ERT in patients at risk for clinically significant antibody development may be a promising approach to nAb management [24, 47].
The formation of nAbs is a common concern in patients with rheumatoid arthritis receiving biologic tumor necrosis factor α (TNF-α) inhibitors, and secondary loss of response may require switching to a treatment with an alternative mechanism of action [25, 48]. Approximately 30% to 40% of patients discontinue use of biologic TNF-α inhibitors because of nonresponse or intolerance [48], and the risk of nAb development can vary across TNF-α inhibitors, in part because of structural differences between antibody constructs. A reduction in immunogenicity has been achieved by developing smaller, more targeted fusion proteins rather than using large chimeric or fully humanized antibodies [25, 49, 50].
Similarly, treatment for diabetes has evolved over time to address immunogenicity issues in insulin formulations related to host-derived (e.g., bovine, porcine, human) structural differences, insulin purity, formulation additives (e.g., zinc, protamine, surfen), and drug aggregation [44]. Treatment with initial insulin formulations showed both insulin-specific nAbs and antibodies to other drug components [44]. However, replacing impure animal insulins with highly purified porcine insulins and, more recently, recombinant and semisynthetic human insulin preparations has vastly reduced—although not completely eliminated—the occurrence of immunogenicity [44, 51, 52].
As seen with early insulin formulations, immunogenicity to formulation additives and contaminants presents additional clinical challenges that have emerged in other therapeutic applications of biologic drugs [26]. Initial trials of lebrikizumab, an investigational treatment for asthma, were conducted with a formulation that contained a Chinese hamster ovary (CHO) cell protein contaminant, which provoked a measurable immune response in ~ 90% of patients. As a result, the ongoing phase 3 studies were converted to phase 2b and were no longer considered pivotal. Further purification of material was required, and drug manufacturing protocols were adjusted to reduce the CHO contamination, which led to a reduction in immunogenicity in subsequent trials [26].
Immunogenicity is a common clinical barrier to therapy with many biologic drugs, and it has influenced the evolution of biologic treatments across multiple disease states. Some common strategies to reduce general immunogenicity and the prevalence of nAbs include engineering smaller proteins and reducing contaminants or unnecessary formulation components [31]. These general themes can inform the understanding of immunogenicity of BoNT therapy.
Immunogenicity and Evolution of Botulinum Neurotoxin Biologic Therapy
Botulinum Neurotoxin Structure and Function
BoNTs are proteins produced by bacteria of the Clostridium family, which upon oral ingestion cause the neuroparalytic syndrome botulism. There are seven serotypes of BoNT (serotypes A–G) with different toxicities but similar structures. In all serotypes, the bacterial complex comprises a core ~ 150-kDa neurotoxin surrounded by a group of associated accessory proteins (Fig. 3A, B) [53, 54]. These accessory proteins assemble into a supramolecular structure that supports the dual function of protecting the core neurotoxin from low pH conditions upon oral ingestion and facilitating gastrointestinal absorption [54].
Fig. 3.
Molecular structures of botulinum neurotoxin type A in A supramolecular complex and B purified core neurotoxin. A BoNT-A is a supramolecular complex consisting of hemagglutinin proteins, a nontoxic nonhemagglutinin protein, and the core neurotoxin [54, 64], with a molecular weight of up to ~ 900 kDa as produced by the Clostridum bacteria [57]. The structural model shown here (representing ~ 760 kDa) was determined using recombinant protein and cryogenic electron microscopy [57] and is currently the largest assembly for which three-dimensional structural information is available. B Purified BoNT-A formulations contain only the core neurotoxin (~ 150 kDa) [54, 64]. Images rendered with PyMol (Schrödinger, Inc.) using atomic coordinates from the Protein Data Bank with the following accession codes: 3WIN, 3V0A, 3V0B, 3V0C, and 4LO7 [56, 57, 120]. BoNT-A, botulinum neurotoxin type A
At low pH, the core neurotoxin is surrounded by an assembly of one nontoxic nonhemagglutinin (NTNH) protein plus a complex of hemagglutinin (HA) proteins [54, 55]. The ~ 140 kDa NTNH protein plays a key role in protecting BoNT from protease digestion and low pH degradation in the stomach. However, at a neutral pH in the small intestine, the “pH-sensor” residues of the NTNH protein induce a conformational change releasing the core neurotoxin [56]. The HA proteins (HA1, HA2, and HA3) mediate cell-surface binding and translocation across the intestinal epithelium. The NTNH protein, core neurotoxin, and HA complex assemble to form the final supramolecular structure [55, 57].
The core neurotoxin itself is formed of a heavy (100 kDa) chain and a light (50 kDa) chain linked by a disulfide bond [54, 55]. The role of the heavy chain is to bind to presynaptic cholinergic terminals in the neuromuscular junction to gain cell entry and mediate translocation of the dissociated light chain to the cell cytoplasm. The light chain is a zinc metalloprotease that cleaves specific target soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins to prevent vesicle fusion and subsequent release of acetylcholine (ACh) causing flaccid paralysis [55]. The seven BoNT serotypes cleave different components of the SNARE complex to achieve this effect. BoNT-A and BoNT-E cleave synaptosomal-associated protein 25 (SNAP-25); BoNT-B, BoNT-D, BoNT-F, and BoNT-G cleave synaptobrevin; and BoNT-C cleaves both SNAP-25 and syntaxin [58]. Blocking ACh release is the mechanism of action of therapeutic BoNT formulations, and BoNT-A and BoNT-B are the botulinum toxin serotypes currently in clinical use [17–20].
Immunogenicity and Evolution of BoNT Formulations: Nonclinical Data
As is possible for other biologic drugs, both the BoNT core neurotoxin and the associated accessory proteins have the potential to be immunogenic [35]. Detoxified BoNT extracts and catalytically inactive BoNT proteins have been used for many years to develop vaccines against botulism [59, 60]. Administration of these vaccines results in production of antibodies that can neutralize the toxic effects of BoNT [59–62]. For example, antibodies targeting the core neurotoxin that successfully inhibit its neuronal binding or uptake or its catalytic activity have the potential to be neutralizing [35, 59].
Immunogenicity of Accessory Proteins
The accessory proteins in BoNT formulations have a nontherapeutic role and rapidly dissociate from the core neurotoxin at neutral pH [54, 63, 64]. Thus, the total clostridial protein load (inclusive of accessory proteins and the core neurotoxin) and composition may determine the relative immunogenicity of each BoNT formulation [1].
There is compelling evidence that accessory clostridial proteins, particularly HA-1, act as adjuvants to the immune response [65–71] and that this activation of the immune system can facilitate the development of therapeutically relevant nAbs against the BoNT core neurotoxin [1, 2]. In mice, injection with the core neurotoxin of the BoNT complex alone has low immunogenicity. In contrast, when HA proteins (especially HA-1 and HA-3b) are injected also, antibody production is significantly increased [69]. Additionally, immunization of rabbits with the full inactivated BoNT complex results in production of antibodies with a greater neutralizing effect compared with antibodies induced by immunization with the core neurotoxin alone [66]. These data support an adjuvant effect of accessory proteins as injections were administered in a neutral pH buffer in which the BoNT complex would be dissociated [66, 69].
In addition, accessory proteins may induce other immune-mediated effects. In vitro assays show that in the presence of BoNT accessory proteins, neuronal cells increase production of inflammatory cytokines such as IL-6 and TNF-α [71]. Accessory proteins also bind to multiple nonneuronal cell types, including fibroblasts, lymphoblasts, and skeletal muscle cells [71]. In contrast, the core neurotoxin does not bind to nonneuronal cell types and does not induce cytokine release [71].
On the basis of available preclinical data, a BoNT formulation containing as little clostridial protein as possible is desirable as it may avoid stimulating the host immune response leading to nAb formation and clinical nonresponse [1]. Three BoNT-A products are approved by the FDA for therapeutic use: onabotulinumtoxinA (onaBoNT-A; Botox®; Allergan Pharmaceuticals), abobotulinumtoxinA (aboBoNT-A; Dysport®; Ipsen Biopharm Ltd; Galderma Ltd), and incobotulinumtoxinA (incoBoNT-A; Xeomin®; Merz Pharmaceuticals GmbH) [17–19]; a fourth BoNT-A product, daxibotulinumtoxinA (daxiBoNT-A; Revance Therapeutics), is currently under regulatory review for a nontherapeutic indication [72]. These products vary in the amount of accessory proteins and the excipients (e.g., albumin; Table 1) [17–19]. PrabotulinumtoxinA is a BoNT-A product that has only been investigated and approved for use in the treatment of glabellar facial lines and is not included in our discussion of therapeutic applications [21, 73]. Additionally, one BoNT-B formulation, rimabotulinumtoxinB (rimaBoNT-B; Myobloc®; Solstice Neurosciences, LLC), is FDA approved and contains the core neurotoxin and the accessory proteins [20]. The different protein loads, excipients, and other characteristics of these BoNT formulations may affect their immunogenicity potential [9, 43].
Table 1.
Characteristics of current first- and second-generation BoNT-A preparations [64, 74, 75, 79, 94, 116–118]
| First-generation BoNT-Aa | Second-generation BoNT-Ab | |||
|---|---|---|---|---|
| Parameter | OnabotulinumtoxinA | AbobotulinumtoxinA | IncobotulinumtoxinA | DaxibotulinumtoxinAc |
| Molecular weight of bacterial protein, kDa | ~ 900 | ~ 300–500d | ~ 150 | ~ 150 |
| Accessory proteins present | Yes | Yes | No | No |
| Total protein/vial | 5 ng/100 U | 4.87 ng/500 U | 0.44 ng/100 U | NA |
| Total core neurotoxin protein/100 MU, ng | 0.73 | 0.65 | 0.44 | NA |
| Active neurotoxin protein/100 MU, ng | 0.44 | 0.44 | 0.44 | NA |
| Inactive neurotoxin protein/100 MU, nge | 0.29 | 0.21 | 0 | NA |
| pH after reconstitution | 7.4 | 7.4 | 7.4 | NA |
| Excipients |
HSA NaCl |
HSA Lactose |
HSA Sucrose |
RTP004 peptide Polysorbate-20 Buffers Sugar |
BoNT, botulinum neurotoxin; FDA, Food and Drug Administration; HSA, human serum albumin; MU, mouse unit; NA, not available; NaCl, sodium chloride
aFirst-generation BoNT-A formulations contain core neurotoxins and accessory botulinum proteins; only formulations approved or under investigation for therapeutic applications are represented
bSecond-generation BoNT-A formulations contain only the therapeutic neurotoxin without accessory proteins or other bacterial substances such as flagellin
cCurrently undergoing FDA review; full details on the formulation are not yet available
dFormulation is a mixture of species, with 300 and 500 kDa being the most common
eValues for inactive neurotoxin are approximate and were estimated in Frevert et al. 2010 and then reported in Kerscher et al. 2019 [64, 74]
BoNT-A Formulations with Accessory Proteins
OnabotulinumtoxinA (onaBoNT-A) was the first BoNT-A formulation to be approved by the FDA in 1989 [17]. The initial formulation contained a large proportion of inactivated core neurotoxin plus clostridial proteins, and up to 17% of patients developed anti-BoNT antibodies [2]. In 1997, a less immunogenic formulation containing a reduced quantity of inactivated neurotoxin was manufactured (Fig. 4A) [2]. Currently, onaBoNT-A contains 0.73 ng of core neurotoxin protein (a mixture of active protein and inactive/denatured toxoid) and ~ 4.3 ng of additional accessory protein (Fig. 4, Table 1) [1, 10, 74, 75].
Fig. 4.
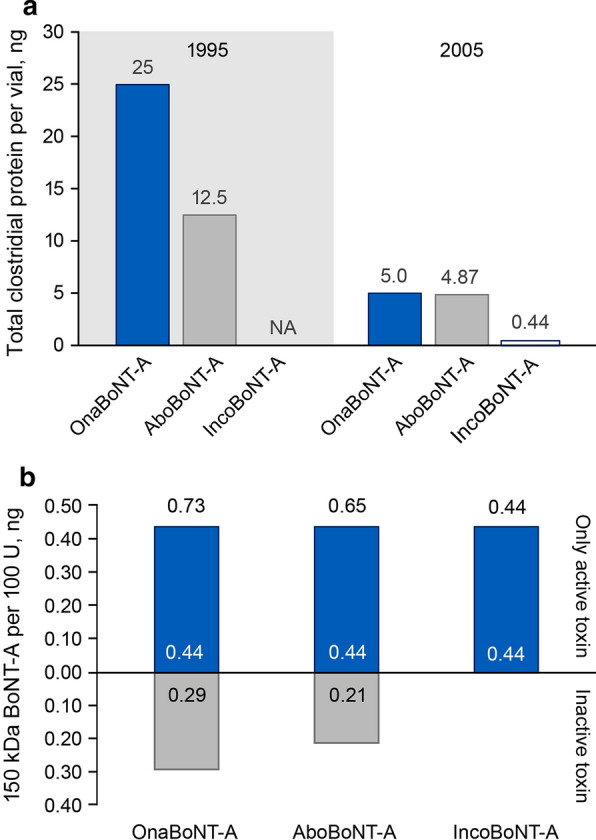
Total clostridial protein and active core neurotoxin in BoNT-A formulations. A Changes in total clostridial protein content in onaBoNT-A [1, 121], aboBoNT-A [1, 74], and incoBoNT-A [1] formulations over time. Current formulations are presented in the 2005 panel. B Total core BoNT-A neurotoxin in onaBoNT-A, aboBoNT-A, and incoBoNT-A formulations separated into denatured/inactive toxin and active neurotoxin components [64, 74, 75]. aboBoNT-A, abobotulinumtoxinA; BoNT-A, botulinum neurotoxin type A; incoBoNT-A, incobotulinumtoxinA; NA, not available; onaBoNT-A, onabotulinumtoxinA
In vivo evidence indicates that anti-BoNT antibodies are produced in response to onaBoNT-A injections, and more frequent dosing is associated with higher antibody levels [76]. Furthermore, in rabbits given nine doses of onaBoNT-A or incoBoNT-A (at 2- to 8-week intervals), nAbs were detected in 20% of onaBoNT-A-treated animals. In contrast, no nAbs were detected in animals treated with the incoBoNT-A formulation containing only the core neurotoxin, suggesting an adjuvant effect of the accessory proteins and high core neurotoxin concentration in onaBoNT-A [77].
The other first-generation BoNT-A formulation abobotulinumtoxinA (aboBoNT-A) contains less clostridial protein than the current formulation of onaBoNT-A; however, accessory proteins make up ~ 30% of the total clostridial protein content (Table 1; Fig. 4) [1, 74]. The aboBoNT-A formulation also contains the non-BoNT clostridial flagella remnant (flagellin), which has been shown to activate TLR5, initiating an innate immune response [9, 37]. Given its immune stimulating properties, flagellin has been utilized as a vaccine adjuvant and may contribute to the immunogenicity of aboBoNT-A [9, 37, 78].
BoNT-A Formulations Without Accessory Proteins
Second-generation BoNT-A formulations (incoBoNT-A and the investigational product daxiBoNT-A) contain only the therapeutic neurotoxin without accessory proteins or other bacterial substances such as flagellin (Table 1, Fig. 4) [1, 18, 64, 74, 75, 79]. The absence of accessory proteins in incoBoNT-A has no effect on efficacy [1, 80]. The formulation of daxiBoNT-A is unusual in that it does not contain human serum albumin (HSA) and instead contains a proprietary, HIV-derived 5-kDa stabilizing peptide and polysorbate 20 [79, 81]. The adjuvant effects of these excipients—particularly the novel HIV-derived peptide that has not been used in any other drug formulation approved by the FDA—are not yet known.
Immunogenicity and Clinical Nonresponsiveness During BoNT Treatment: Clinical Data
BoNT treatment can cause an adaptive immune response with repeated injections leading to nAb formation over time [7, 10]. This is clinically relevant because nAb formation is strongly associated with secondary nonresponse (treatment resistance) [82–86]. There are multiple clinical risk factors for immunogenicity during BoNT treatment related to treatment parameters, patient characteristics, and choice of BoNT formulation [2, 3, 14].
BoNT Formulations: Immunogenicity Data
Development of nAbs is possible with all formulations of BoNT; however, the rate of nAb development and occurrence of clinical resistance appear to be at least partially dependent on the BoNT formulation and correlated to the protein content in the formulation [43]. Incidence rates vary by indication and, in pivotal trials supporting FDA approvals of BoNT formulations in clinical use, nAbs developed in patients treated with rimaBoNT-B, onaBoNT-A, aboBoNT-A, or incoBoNT-A (in patients all of whom had been pretreated with onaBoNT-A or aboBoNT-A; Table 2) [17–20]. It is also important to note that there are differences in the relative sensitivity of assays used by BoNT manufacturers to detect the presence of nAbs, which can complicate making direct comparisons [2]. Pivotal studies supporting FDA approval of onaBoNT-A, aboBoNT-A, and rimaBoNT-B utilize the mouse protection assay (MPA); in contrast, most studies of incoBoNT-A use the mouse hemidiaphragm assay (MHDA), which is at least five times more sensitive than the MPA and nevertheless revealed the lowest rates of nAb formation (Table 2) [2, 43]. Additionally, the reported incidence rates of nAbs in product labeling are based on data from short-term clinical trials (~ 2 years) and may not reflect real-world data given there may be a cumulative effect of repeated BoNT use over time [43].
Table 2.
Incidence rates of development of nAbs and clinical nonresponsiveness with current BoNT formulations
| First generation | Second generation | |||
|---|---|---|---|---|
| BoNT-Aa | BoNT-B | BoNT-A | ||
| OnabotulinumtoxinA | AbobotulinumtoxinA | RimabotulinumtoxinB | IncobotulinumtoxinA | |
| Patients with nAbs in pivotal clinical trials | 0.0%-1.9% [17] | 0.0–3.6% [19] | 10–18% [20] | 0–1.8% [18] |
| Patients with nAbs in real-world studies [3, 5] | 1.5–7.0% | 1.7–6.0% | 42.4% | 0.0–0.5% |
| Reports of clinical resistance/nonresponse | Yes | Yes [90–92] | Yes | No |
| Formulation notes | Reduced protein load from original formulation [119] (i.e., reduced clostridial protein impurities and inactive BoNT-A) [74] | Contains flagellin with potential adjuvant properties [9, 37]; contains complexing proteins [74] | Contains complexing proteins | No complexing proteins; no inactive toxoids [74]; no patients with secondary nonresponse |
However, similar trends are seen in real-world studies with long-term follow-up analysis showing reduced nAb presence in patients treated with incoBoNT-A [5, 82, 87]. A retrospective meta-analysis suggests more prevalent nAbs across indications in patients treated with onaBoNT-A (~ 1.5%) or aboBoNT-A (~ 1.7%) compared with incoBoNT-A (0.5%) [5]. While overall prevalence is low, there was a considerably higher rate of nAb development among patients who were identified as demonstrating secondary nonresponse in this meta-analysis [5]. Among such patients, nAbs were reported in 32.5% of those treated with onaBoNT-A and 56.7% of those treated with aboBoNT-A [5]. Importantly, no patients treated with incoBoNT-A demonstrated secondary nonresponse [5].
BoNT-A Formulations with Accessory Proteins
Occurrence of nAbs after treatment with onaBoNT-A or aboBoNT-A has been reported in patients with cervical dystonia, spasticity, and other indications [3, 9, 12, 82, 88, 89]. In a cross-sectional study of patients with facial hemispasm, blepharospasm, cervical dystonia, other dystonia, and spasticity, nAbs were reported with use of both onaBoNT-A (7%) and aboBoNT-A (6%) but not incoBoNT-A [3]. These findings are consistent with a recent retrospective cohort study of long-term BoNT treatment across indications, which showed nAb-induced treatment failure in patients who received onaBoNT-A (4%) or aboBoNT-A (16%) but not in those who received incoBoNT-A [82]. Furthermore, there is evidence from case studies of BoNT-A use for aesthetic indications of nAb development and secondary nonresponsiveness over time with both onaBoNT-A and aboBoNT-A [90–92]. In general, incidences of nAb development and secondary nonresponsiveness are lower in aesthetic indications, which may reflect the lower doses and minimal long-term data [9, 92].
BoNT-A Formulations Without Accessory Proteins
The development of antibodies and likelihood of clinical nonresponse are reduced with formulations of BoNT without accessory proteins [43]. No toxin-naive patients treated with incoBoNT-A developed neutralizing antibodies, based on the sensitive MHDA assay [16]. Furthermore, no reports of clinical nonresponse exist in the medical literature for patients who were toxin-naive when they received incoBoNT-A [2, 16]. In a recent study examining two patient cohorts, those treated exclusively with incoBoNT-A did not show any signs of secondary treatment failure, whereas those previously treated with other BoNT formulations were more likely to develop such signs [16]. This study also showed that switching to incoBoNT-A after secondary treatment failure with another BoNT formulation helped patients begin to recover responsiveness to treatment; development of nAbs only occurred in two patients previously treated with aboBoNT-A [16]. Additionally, pooled analysis data from pivotal clinical studies across aesthetic indications reported no lack of treatment response due to development of nAbs [93].
Out of > 2600 patients treated with incoBoNT-A in pivotal clinical trials across all approved indications, nAbs developed in only nine adult (5 with unknown and 4 with negative nAb status at baseline) and four pediatric (nAb status unknown at baseline) patients pretreated with either onaBoNT-A or aboBoNT-A, and none exhibited secondary treatment failure due to nAbs [18]. It is promising that no incoBoNT-A–treated children developed resistance during clinical trials [18], as these patients potentially require lifelong treatment with BoNT starting from an early age and are potentially at greater risk to develop an immune response.
An investigational BoNT-A formulation daxibotulinumtoxinA has been evaluated in phase 3 clinical trials for aesthetic use, but long-term data are not yet available, and immunogenicity outcomes are yet to be established [94].
Treatment Parameters
Multiple treatment parameters affect BoNT immunogenicity. Most importantly, during a potential life-long treatment, prevalence of nAbs increases with chronic BoNT use—cumulative dose, repeated injections, and total treatment duration [2, 6, 7, 13]. Other parameters, such as protein load, injection session dose, and length of reinjection interval, have also been demonstrated to be clinically relevant to BoNT immunogenicity [3, 6, 13, 15].
Patient Characteristics
The rate of nAb development in clinical practice may be higher under certain conditions; several studies have suggested prevalence rates of ~ 15–20% in patients with cervical dystonia [3, 5, 6] after long-term treatment vs. ~ 1–6% in patients with limb spasticity [5, 7, 89]. The reasons for these differences are not well understood but may be related to variations in underlying pathophysiology and dosing/administration requirements [2]. Conditions that require more frequent administration or higher doses appear to be associated with a greater risk of immunogenicity [3, 6, 13, 89].
Genetic differences in the control of immune responses indicate that patients exhibit variable speed and magnitude of immune reactions and patterns of nAb generation [36, 95–97]. Furthermore, not all nAbs are the same—variations in target binding site and binding affinity result in antibodies generated against BoNT that vary in their neutralizing effects [2, 96]. Thus, there is not an absolute correlation between appearance of nAbs and treatment resistance, and there does not seem to be a particular threshold for nAb titer above which clinical resistance occurs [2]. However, investigations of such a threshold have been limited. Often, secondary treatment failure may be observed after an initial positive response over several treatment cycles [2, 12].
Immunologists’ Perspective: Immunogenicity Should Be a Key Factor in Choice of BoNT Therapy
BoNT use may have a cumulative immunogenic effect over time in patients with lifelong conditions, such as cervical dystonia [13, 98], as well as in patients who receive multiple BoNT-A treatments for a range of different indications (both aesthetic and therapeutic) during their lifetime. Both the FDA [22] and the European Medicines Agency (EMEA) [99] recommend evaluating and mitigating adverse immunologically related responses associated with therapeutic protein products and encourage risk reduction. Therefore, where efficacy and safety are comparable, a BoNT formulation that is potentially less likely to cause immunogenicity should be considered as a first-line therapy [16, 43].
While they are not interchangeable, clinical study data comparing onaBoNT-A and incoBoNT-A demonstrate similar efficacy and safety when used at similar doses across multiple indications [80, 100–103]. Thus, initiating treatment with a second-generation BoNT-A formulation that has lower potential immunogenicity (incoBoNT-A) may reduce the risk of nAb production and future treatment failure [16, 80]. For patients who had begun treatment with a more immunogenic BoNT formulation (onaBoNT-A or aboBoNT-A), switching to a less immunogenic formulation may be an appropriate choice as the process of developing nAbs can begin very early—before clinical signs of resistance are apparent [12, 43, 104–108]. It is particularly important to proactively and systematically recognize signs of clinical resistance, such as increased dosage and shortened injection intervals [12, 42, 43]. In such cases, changing to a less immunogenic formulation is especially warranted and would ideally occur early enough to prove effective in restoring an optimal clinical response [104, 109].
Switching to a BoNT-B formulation is undesirable from an immunologic perspective, given that BoNT-B has a higher immunogenicity than BoNT-A (Table 2) [110, 111]. Additionally, patients who change from BoNT-A to BoNT-B show a reduced response to BoNT-B over time [111, 112], and resistance can develop within a few cycles of treatment [85, 113, 114]. Overall, in the absence of an ability to test nAbs commercially, available evidence regarding differences between BoNT formulations in immunogenic potential and changes in clinical responsiveness over time should be used to inform treatment decisions.
Data Gaps and Unknowns
Despite the evidence that accessory clostridial proteins can act as adjuvants to the immune response [65–71] and in vivo data linking the first-generation BoNT-A formulations to the formation of nAbs [76, 77], unanswered questions remain relating to the immunogenicity of BoNT formulations. Further study is needed to elucidate the different effects of core neurotoxin alone and accessory proteins on the immune system, including the role of specific cytokines, TLRs, and other innate immune or pattern recognition markers. It is largely unknown whether inactive denatured toxin (such as that used in BoNT vaccines [59, 60] and present in onaBoNT-A and aboBoNT-A) [74] has any effect on nAb production, although it is of clear concern from a therapeutic effect standpoint. However, initial evidence strongly suggests that nAbs are reduced in formulations that lack accessory proteins (incoBoNT-A) [11, 16, 18], and additional larger studies are needed to confirm this correlation.
Perhaps the largest data gap is the lack of long-term data in pediatric patients who often receive lifelong treatment and may be at higher risk of chronic inflammation and other potential complications due to repeated immune system stimulation. Some data suggest that in pediatric patients treated for spasticity with onaBoNT-A or aboBoNT-A, the likelihood of an immune response increased with number of treatments [86]. However, identifying signs of clinical resistance in pediatric patients is complicated by the fact that they are still growing and may have different trajectories of their underlying disease state compared with adult patients; accordingly, use of increased doses of BoNT over time or earlier waning of clinical effect—often signs of clinical resistance in adults—may not be similarly informative in children. Even so, longitudinal real-world studies to determine development of nAbs and to identify practical assessments of clinical nonresponsiveness in pediatric patients would be highly informative for developing effective treatment paradigms in chronic conditions treated with BoNT. This is also true for other patient populations, and real-world studies could help determine if there is a cutoff for nAb titer related to lack of efficacy in patients who receive multiple injections.
It is important to note that nAb testing offers a single snapshot in time of a patient’s antibody titer, but titers may exhibit temporal variations between injections. Thus, regular nAb testing would be helpful, although the current practical limitations, such as cost and high volume of patient serum required for testing, remain a barrier to implementation [2]. Availability of an affordable commercial nAb test would help to address these current challenges. In the meantime, utilizing other clinically useful tools, such as the ninhydrin sweat test, unilateral brow injection test, and extensor digitorum brevis test, to screen for potential nAbs in patients receiving BoNT are sometimes beneficial [2, 107, 115].
Limitations
All biologic drugs and therapeutic proteins, including BoNT formulations, can be recognized as foreign by the immune system and, therefore, have the potential for immunogenicity. Detecting immunogenicity via nAb formation is assay dependent given the variability in sensitivity and specificity (sensitivity discussed in Clinical Data section). Assessing the incidence of nAb positivity may be influenced by factors such as assay methodology, handling of samples and timing of collection, concurrent use of medications, and underlying disease pathology. Therefore, directly comparing the incidence of nAbs across BoNT formulations may be misleading. Other limitations of the data presented in this review include the lack of a commercially available quantitative assay to measure nAbs and a lack of studies comparing BoNT formulations with a standardized nAb assay.
Conclusions
Immunogenicity is a common clinical barrier seen with the use of many biologic drugs. Repeated exposure to biologic therapies, including BoNT, can provoke a continual immune response, leading to formation of nAbs that can result in a spectrum of clinical outcomes (e.g., reduced efficacy and/or treatment failure) [31, 39–42]. In some cases, mitigation of general immunogenicity and nAb formation in response to biologic therapies have been achieved through engineering smaller proteins and reducing contaminants or unnecessary formulation components [31]. Specifically, accessory clostridial proteins in BoNT formulations, particularly HA-1, may act as adjuvants to the immune response [65–71]. Additionally, protein content of BoNT-A formulations influences nAb formation—higher protein content is correlated with increased nAb induction [43].
Compared with onaBoNT-A and aboBoNT-A, the second-generation BoNT formulation incoBoNT-A contains only the therapeutic neurotoxin (150 kDa) and lacks accessory proteins or other potential adjuvants (e.g., flagellin) [1, 9, 37]. Additionally, incoBoNT-A demonstrates strong clinical efficacy and safety, as well as low immunogenicity across a range of indications [16, 80, 100–102, 108]. Given that BoNT therapy is often lifelong, the potential for immunogenicity and risk of reducing nAb production should be considered when making treatment decisions regarding BoNT formulation.
Acknowledgements
Funding
Writing and editorial assistance was funded by Merz Pharmaceuticals GmbH and its affiliate, Merz Pharmaceuticals, LLC. Journal publication, including the Rapid Service fees, was funded by Merz Pharmaceuticals, LLC.
Medical Writing and Editorial Assistance
Writing and editorial assistance was provided under the direction of the authors by MedThink SciCom with support from Katie Veleta, PhD.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Warner Carr, Neal Jain, and J. Wesley Sublett contributed to the conception, drafting, and critical revision of this article. Warner Carr, Neal Jain, and J. Wesley Sublett read and approved the final version.
Disclosures
Warner Carr, Neal Jain, and J. Wesley Sublett have served as paid consultants for Merz Pharmaceuticals GmbH. Additionally, Dr. Carr has served as a paid consultant for Merz Pharmaceuticals, LLC.
Compliance with Ethics Guidelines
This article reviews the existing literature and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1.Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15:1–9. doi: 10.1007/s40268-014-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frevert J, Dressler D. Clinical relevance of immunoresistance to botulinum therapy. In: Rosales RL, Dressler D, editors. Botulinum toxin therapy manual for dystonia and spasticity. IntechOpen; 2016. pp. 33–49. [Google Scholar]
- 3.Albrecht P, Jansen A, Lee JI, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. 2019;92:e48–e54. doi: 10.1212/WNL.0000000000006688. [DOI] [PubMed] [Google Scholar]
- 4.Srinoulprasert Y, Wanitphakdeedecha R. Antibody-induced botulinum toxin treatment failure: a review and novel management approach. J Cosmet Dermatol. 2020;19:2491–2496. doi: 10.1111/jocd.13637. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri M, Leodori G, Fernandes RM, et al. Neutralizing antibody and botulinum toxin therapy: a systematic review and meta-analysis. Neurotox Res. 2016;29:105–117. doi: 10.1007/s12640-015-9565-5. [DOI] [PubMed] [Google Scholar]
- 6.Hefter H, Rosenthal D, Moll M. High botulinum toxin-neutralizing antibody prevalence under long-term cervical dystonia treatment. Mov Disord Clin Pract. 2016;3:500–506. doi: 10.1002/mdc3.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathevon L, Declemy A, Laffont I, Perennou D. Immunogenicity induced by botulinum toxin injections for limb spasticity: a systematic review. Ann Phys Rehabil Med. 2019;62:241–251. doi: 10.1016/j.rehab.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Yablon SA, Brashear A, Gordon MF, et al. Formation of neutralizing antibodies in patients receiving botulinum toxin type A for treatment of poststroke spasticity: a pooled-data analysis of three clinical trials. Clin Ther. 2007;29:683–690. doi: 10.1016/j.clinthera.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm (Vienna) 2013;120:275–290. doi: 10.1007/s00702-012-0893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012;26:e1–9. doi: 10.2165/11599840-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hefter H, Hartmann C, Kahlen U, Moll M, Bigalke H. Prospective analysis of neutralising antibody titres in secondary non-responders under continuous treatment with a botulinumtoxin type A preparation free of complexing proteins-a single cohort 4-year follow-up study. BMJ Open. 2012;2:e000646. doi: 10.1136/bmjopen-2011-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hefter H, Spiess C, Rosenthal D. Very early reduction in efficacy of botulinum toxin therapy for cervical dystonia in patients with subsequent secondary treatment failure: a retrospective analysis. J Neural Transm (Vienna) 2014;121:513–519. doi: 10.1007/s00702-013-1127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hefter H, Rosenthal D, Bigalke H, Moll M. Clinical relevance of neutralizing antibodies in botulinum toxin long-term treated still-responding patients with cervical dystonia. Ther Adv Neurol Disord. 2019;12:1756286419892078. doi: 10.1177/1756286419892078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213–217. doi: 10.1002/mds.870090216. [DOI] [PubMed] [Google Scholar]
- 15.Chohan N, Hilton P, Brown K, Dixon L. Efficacy and duration of response to botulinum neurotoxin A (onabotulinumA) as a treatment for detrusor overactivity in women. Int Urogynecol J. 2015;26:1605–12. doi: 10.1007/s00192-015-2751-4. [DOI] [PubMed] [Google Scholar]
- 16.Hefter H, Brauns R, Ürer B, Rosenthal D, Albrecht P. Effective long-term treatment with incobotulinumtoxin (Xeomin®) without neutralizing antibody induction: a monocentric, cross-sectional study. J Neurol. 2020;267:1340–1347. doi: 10.1007/s00415-019-09681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botox [package insert]. Dublin, Ireland: Allergan, Inc; 2021.
- 18.Xeomin [package insert]. Frankfurt am Main, Germany: Merz Pharmaceuticals GmbH; 2020.
- 19.Dysport [package insert]. Wrexham, UK: Ipsen Biopharm Ltd; 2020.
- 20.Myobloc [package insert]. Rockville, MD: Solstice Neurosciences, LLC; 2019.
- 21.Jeuveau [package insert]. Newport Beach, CA: Evolus; 2019.
- 22.Center for Drug Evaluation and Research Administration, US Food and Drug Administration. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products; 2014. https://www.fda.gov/media/85017/download. Accessed 27 July 2021.
- 23.Center for Drug Evaluation and Research Administration, US Food and Drug Administration. Immunogenicity testing of therapeutic protein products—developing and validating assays for anti-drug antibody detection; 2019. https://www.fda.gov/media/119788/download. Accessed 27 July 2021.
- 24.van der Veen SJ, van Kuilenburg ABP, Hollak CEM, Kaijen PHP, Voorberg J, Langeveld M. Antibodies against recombinant alpha-galactosidase A in Fabry disease: subclass analysis and impact on response to treatment. Mol Genet Metab. 2019;126:162–168. doi: 10.1016/j.ymgme.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707–718. doi: 10.1038/nrrheum.2017.187. [DOI] [PubMed] [Google Scholar]
- 26.Fischer SK, Cheu M, Peng K, et al. Specific immune response to phospholipase B-like 2 protein, a host cell impurity in lebrikizumab clinical material. AAPS J. 2017;19:254–263. doi: 10.1208/s12248-016-9998-7. [DOI] [PubMed] [Google Scholar]
- 27.Boehncke WH, Brembilla NC. Immunogenicity of biologic therapies: causes and consequences. Expert Rev Clin Immunol. 2018;14:513–523. doi: 10.1080/1744666X.2018.1468753. [DOI] [PubMed] [Google Scholar]
- 28.Pichler WJ, Adam J, Daubner B, Gentinetta T, Keller M, Yerly D. Drug hypersensitivity reactions: pathomechanism and clinical symptoms. Med Clin North Am. 2010;94:645–664. doi: 10.1016/j.mcna.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leach MW, Rottman JB, Hock MB, Finco D, Rojko JL, Beyer JC. Immunogenicity/hypersensitivity of biologics. Toxicol Pathol. 2014;42:293–300. doi: 10.1177/0192623313510987. [DOI] [PubMed] [Google Scholar]
- 31.Sethu S, Govindappa K, Alhaidari M, Pirmohamed M, Park K, Sathish J. Immunogenicity to biologics: mechanisms, prediction and reduction. Arch Immunol Ther Exp. 2012;60:331–344. doi: 10.1007/s00005-012-0189-7. [DOI] [PubMed] [Google Scholar]
- 32.Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front Immunol. 2020;11:1951. doi: 10.3389/fimmu.2020.01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doevendans E, Schellekens H. Immunogenicity of innovative and biosimilar monoclonal antibodies. Antibodies. 2019;8:21. doi: 10.3390/antib8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faulkner L, Meng X, Park BK, Naisbitt DJ. The importance of hapten-protein complex formation in the development of drug allergy. Curr Opin Allergy Clin Immunol. 2014;14:293–300. doi: 10.1097/ACI.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 35.Critchfield J. Considering the immune response to botulinum toxin. Clin J Pain. 2002;18(suppl):S133–S141. doi: 10.1097/00002508-200211001-00004. [DOI] [PubMed] [Google Scholar]
- 36.Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci. 2011;100:354–387. doi: 10.1002/jps.22276. [DOI] [PubMed] [Google Scholar]
- 37.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy K. Janeway’s immunobiology. 8. New York: Garland Science, Taylor & Francis Group, LLC; 2012. [Google Scholar]
- 39.Herrmann J, Mall V, Bigalke H, Geth K, Korinthenberg R, Heinen F. Secondary non-response due to development of neutralising antibodies to botulinum toxin A during treatment of children with cerebral palsy. Neuropediatrics. 2000;31:333–334. doi: 10.1055/s-2000-12955. [DOI] [PubMed] [Google Scholar]
- 40.Przydacz M, Golabek T, Chlosta P. How to assess and predict success or failure of intra-detrusor injections with onabotulinumtoxinA. Adv Clin Exp Med. 2019;28:555–567. doi: 10.17219/acem/90764. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira JJ, Bhidayasiri R, Colosimo C, Marti MJ, Zakine B, Maisonobe P. Survey of practices employed by neurologists for the definition and management of secondary non-response to botulinum toxin in cervical dystonia. Funct Neurol. 2012;27:225–230. [PMC free article] [PubMed] [Google Scholar]
- 42.Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 2009;32:213–218. doi: 10.1097/WNF.0b013e3181914d0a. [DOI] [PubMed] [Google Scholar]
- 43.Samadzadeh S, Ürer B, Brauns R, et al. Clinical implications of difference in antigenicity of different botulinum neurotoxin type A preparations: clinical take-home messages from our research pool and literature. Toxins. 2020;12:499. doi: 10.3390/toxins12080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schernthaner G. Immunogenicity and allergenic potential of animal and human insulins. Diabetes Care. 1993;16(suppl 3):155–165. doi: 10.2337/diacare.16.3.155. [DOI] [PubMed] [Google Scholar]
- 45.Lenders M, Neußer LP, Rudnicki M, et al. Dose-dependent effect of enzyme replacement therapy on neutralizing antidrug antibody titers and clinical outcome in patients with Fabry disease. J Am Soc Nephrol. 2018;29:2879–2889. doi: 10.1681/ASN.2018070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauhin W, Lidove O, Amelin D, et al. Deep characterization of the anti-drug antibodies developed in Fabry disease patients, a prospective analysis from the French multicenter cohort FFABRY. Orphanet J Rare Dis. 2018;13:127. doi: 10.1186/s13023-018-0877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenders M, Oder D, Nowak A, et al. Impact of immunosuppressive therapy on therapy-neutralizing antibodies in transplanted patients with Fabry disease. J Intern Med. 2017;282:241–253. doi: 10.1111/joim.12647. [DOI] [PubMed] [Google Scholar]
- 48.Rubbert-Roth A, Szabó MZ, Kedves M, Nagy G, Atzeni F, Sarzi-Puttini P. Failure of anti-TNF treatment in patients with rheumatoid arthritis: the pros and cons of the early use of alternative biological agents. Autoimmun Rev. 2019;18:102398. doi: 10.1016/j.autrev.2019.102398. [DOI] [PubMed] [Google Scholar]
- 49.van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2013;9:164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 50.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–79. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Cernea S, Raz I. Insulin therapy: future perspectives. Am J Ther. 2020;27:e121–e132. doi: 10.1097/MJT.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 52.Radermecker RP, Scheen AJ. Allergy reactions to insulin: effects of continuous subcutaneous insulin infusion and insulin analogues. Diabetes Metab Res Rev. 2007;23:348–355. doi: 10.1002/dmrr.714. [DOI] [PubMed] [Google Scholar]
- 53.Dembek ZF, Smith LA, Rusnak JM. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep. 2007;1:122–134. doi: 10.1097/DMP.0b013e318158c5fd. [DOI] [PubMed] [Google Scholar]
- 54.Kutschenko A, Bigalke H, Wegner F, Wohlfarth K. The role of human serum albumin and neurotoxin associated proteins in the formulation of BoNT/A products. Toxicon. 2019;168:158–163. doi: 10.1016/j.toxicon.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Benefield DA, Dessain SK, Shine N, Ohi MD, Lacy DB. Molecular assembly of botulinum neurotoxin progenitor complexes. Proc Natl Acad Sci U S A. 2013;110:5630–5635. doi: 10.1073/pnas.1222139110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu S, Rumpel S, Zhou J, et al. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science. 2012;335:977–981. doi: 10.1126/science.1214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K, Gu S, Jin L, et al. Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog. 2013;9:e1003690. doi: 10.1371/journal.ppat.1003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol. 2001;8(suppl 5):21–29. doi: 10.1046/j.1468-1331.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 59.Webb RP, Smith LA. What next for botulism vaccine development? Expert Rev Vaccines. 2013;12:481–492. doi: 10.1586/erv.13.37. [DOI] [PubMed] [Google Scholar]
- 60.Yu CH, Song DH, Choi JY, et al. A mutated recombinant subunit vaccine protects mice and guinea pigs against botulinum type A intoxication. Hum Vaccin Immunother. 2018;14:329–336. doi: 10.1080/21645515.2017.1405201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shearer JD, Vassar ML, Swiderski W, Metcalfe K, Niemuth N, Henderson I. Botulinum neurotoxin neutralizing activity of immune globulin (IG) purified from clinical volunteers vaccinated with recombinant botulinum vaccine (rBV A/B) Vaccine. 2010;28:7313–7318. doi: 10.1016/j.vaccine.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 62.Khouri JM, Motter RN, Arnon SS. Safety and immunogenicity of investigational recombinant botulinum vaccine, rBV A/B, in volunteers with pre-existing botulinum toxoid immunity. Vaccine. 2018;36:2041–2048. doi: 10.1016/j.vaccine.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 63.Eisele K-H, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57:555–565. doi: 10.1016/j.toxicon.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 64.Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, Maas C, Frevert J. IncobotulinumtoxinA: a highly purified and precisely manufactured botulinum neurotoxin type A. J Drugs Dermatol. 2019;18:52–7. [PubMed] [Google Scholar]
- 65.Sharma SK, Singh BR. Immunological properties of Hn-33 purified from type A Clostridium botulinum. J Nat Toxins. 2000;9:357–362. [PubMed] [Google Scholar]
- 66.Kukreja R, Chang T-W, Cai S, et al. Immunological characterization of the subunits of type A botulinum neurotoxin and different components of its associated proteins. Toxicon. 2009;53:616–624. doi: 10.1016/j.toxicon.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Sayadmanesh A, Ebrahimi F, Hajizade A, Rostamian M, Keshavarz H. Expression and purification of neurotoxin-associated protein HA-33/A from Clostridium botulinum and evaluation of its antigenicity. Iran Biomed J. 2013;17:165–170. doi: 10.6091/ibj.1216.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahmut N, Inoue K, Fujinaga Y, et al. Characterisation of monoclonal antibodies against haemagglutinin associated with Clostridium botulinum type C neurotoxin. J Med Microbiol. 2002;51:286–294. doi: 10.1099/0022-1317-51-4-286. [DOI] [PubMed] [Google Scholar]
- 69.Lee J-C, Yokota K, Arimitsu H, et al. Production of anti-neurotoxin antibody is enhanced by two subcomponents, HA1 and HA3b, of Clostridium botulinum type B 16S toxin-haemagglutinin. Microbiology. 2005;151(pt 11):3739–3747. doi: 10.1099/mic.0.28421-0. [DOI] [PubMed] [Google Scholar]
- 70.Bryant AM, Cai S, Singh BR. Comparative immunochemical characteristics of botulinum neurotoxin type A and its associated proteins. Toxicon. 2013;72:126–132. doi: 10.1016/j.toxicon.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Sun Y, Yang W, Lindo P, Singh BR. Type A botulinum neurotoxin complex proteins differentially modulate host response of neuronal cells. Toxicon. 2014;82:52–60. doi: 10.1016/j.toxicon.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Carruthers J, Solish N, Humphrey S, et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: a phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol Surg. 2017;43:1321–1331. doi: 10.1097/DSS.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 73.Beer KR, Shamban AT, Avelar RL, Gross JE, Jonker A. Efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results from 2 identical phase III studies. Dermatol Surg. 2019;45:1381–1393. doi: 10.1097/DSS.0000000000001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10:67–73. doi: 10.2165/11584780-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dressler D, Mander G, Fink K. Measuring the potency labelling of onabotulinumtoxinA (Botox®) and incobotulinumtoxinA (Xeomin®) in an LD50 assay. J Neural Transm. 2012;119:13–15. doi: 10.1007/s00702-011-0719-1. [DOI] [PubMed] [Google Scholar]
- 76.Sutphin DD, Chun J, Hill W, et al. Type A botulinum toxin-induced antibody production: a murine model of antibody response. Aesthet Surg J. 2009;29:414–418. doi: 10.1016/j.asj.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Jost WH, Blümel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (XEOMIN®) in focal dystonia. Drugs. 2007;67:669–683. doi: 10.2165/00003495-200767050-00003. [DOI] [PubMed] [Google Scholar]
- 78.Cui B, Liu X, Fang Y, Zhou P, Zhang Y, Wang Y. Flagellin as a vaccine adjuvant. Expert Rev Vaccines. 2018;17:335–349. doi: 10.1080/14760584.2018.1457443. [DOI] [PubMed] [Google Scholar]
- 79.Carruthers JD, Fagien S, Joseph JH, et al. DaxibotulinumtoxinA for injection for the treatment of glabellar lines: results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2) Plast Reconstr Surg. 2020;145:45–58. doi: 10.1097/PRS.0000000000006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park J-Y, Sunga O, Wanitphakdeedecha R, Frevert J. Neurotoxin impurities: a review of threats to efficacy. Plast Reconstr Surg Glob Open. 2020;8:e2627. doi: 10.1097/GOX.0000000000002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glogau RG, Waugh JM. Preclinical transcutaneous flux experiments using a macromolecule transport system (MTS) peptide for delivery of botulinum toxin type A. In: Poster presented at: 66th Annual Meeting of the American Academy of Dermatology; February 1–5, 2008; San Antonio.
- 82.Walter U, Mühlenhoff C, Benecke R, et al. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology. 2020;94:e2109–e2120. doi: 10.1212/WNL.0000000000009444. [DOI] [PubMed] [Google Scholar]
- 83.Wanitphakdeedecha R, Kantaviro W, Suphatsathienkul P, et al. Association between secondary botulinum toxin A treatment failure in cosmetic indication and anti-complexing protein antibody production. Dermatol Ther. 2020;10:707–720. doi: 10.1007/s13555-020-00397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulte-Baukloh H, Bigalke H, Miller K, et al. Botulinum neurotoxin type A in urology: antibodies as a cause of therapy failure. Int J Urol. 2008;15:407–15. doi: 10.1111/j.1442-2042.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 85.Dressler D, Bigalke H. Antibody-induced failure of botulinum toxin type B therapy in de novo patients. Eur Neurol. 2004;52:132–135. doi: 10.1159/000081463. [DOI] [PubMed] [Google Scholar]
- 86.Herrmann J, Geth K, Mall V, et al. Clinical impact of antibody formation to botulinum toxin A in children. Ann Neurol. 2004;55:732–735. doi: 10.1002/ana.20098. [DOI] [PubMed] [Google Scholar]
- 87.Rahman E, Alhitmi HK, Mosahebi A. Immunogenicity to botulinum toxin type A: a systematic review with meta-analysis across therapeutic indications. Aesthet Surg J. 2021 doi: 10.1093/asj/sjab058. [DOI] [PubMed] [Google Scholar]
- 88.Brin MF, Comella CL, Jankovic J, Lai F, Naumann M. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–1360. doi: 10.1002/mds.22157. [DOI] [PubMed] [Google Scholar]
- 89.Müller K, Mix E, Adib Saberi F, Dressler D, Benecke R. Prevalence of neutralising antibodies in patients treated with botulinum toxin type A for spasticity. J Neural Transm. 2009;116:579–585. doi: 10.1007/s00702-009-0223-z. [DOI] [PubMed] [Google Scholar]
- 90.Cohen JL, Scuderi N. Safety and patient satisfaction of abobotulinumtoxinA for aesthetic use: a systematic review. Aesthet Surg J. 2017;37(suppl 1):S32–S44. doi: 10.1093/asj/sjx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36(suppl 4):2182–2187. doi: 10.1111/j.1524-4725.2010.01710.x. [DOI] [PubMed] [Google Scholar]
- 92.Torres S, Hamilton M, Sanches E, Starovatova P, Gubanova E, Reshetnikova T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2014;7:11–17. doi: 10.2147/CCID.S51938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coleman WP, III, Sattler G, Weissenberger P, Hast MA, Hanschmann A. Safety of incobotulinumtoxinA in the treatment of facial lines: results from a pooled analysis of randomized, prospective, controlled clinical studies. Dermatol Surg. 2017;43(suppl 3):S293–S303. doi: 10.1097/DSS.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 94.Bertucci V, Solish N, Kaufman-Janette J, et al. DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2) J Am Acad Dermatol. 2020;82:838–845. doi: 10.1016/j.jaad.2019.06.1313. [DOI] [PubMed] [Google Scholar]
- 95.Atassi MZ. Molecular basis of immunogenicity to botulinum neurotoxins and uses of the defined antigenic regions. Toxicon. 2015;107(pt A):50–58. doi: 10.1016/j.toxicon.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 96.Dolimbek BZ, Aoki KR, Steward LE, Jankovic J, Atassi MZ. Mapping of the regions on the heavy chain of botulinum neurotoxin A (BoNT/A) recognized by antibodies of cervical dystonia patients with immunoresistance to BoNT/A. Mol Immunol. 2007;44:1029–1041. doi: 10.1016/j.molimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 97.Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins. 2019;11:491. doi: 10.3390/toxins11090491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ni J, Wang X, Cao N, Si J, Gu B. Is repeat botulinum toxin A injection valuable for neurogenic detrusor overactivity—a systematic review and meta-analysis. Neurourol Urodyn. 2018;37:542–553. doi: 10.1002/nau.23354. [DOI] [PubMed] [Google Scholar]
- 99.European Medicines Agency. Guideline on Immunogenicity Assessment of Therapeutic Proteins; 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf. Accessed 27 July 2021.
- 100.Wabbels B, Reichel G, Fulford-Smith A, Wright N, Roggenkämper P. Double-blind, randomised, parallel group pilot study comparing two botulinum toxin type A products for the treatment of blepharospasm. J Neural Transm. 2011;118:233–239. doi: 10.1007/s00702-010-0529-x. [DOI] [PubMed] [Google Scholar]
- 101.Saad J, Gourdeau A. A direct comparison of onabotulinumtoxina (Botox) and incobotulinumtoxinA (Xeomin) in the treatment of benign essential blepharospasm: a split-face technique. J Neuroophthalmol. 2014;34:233–236. doi: 10.1097/WNO.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 102.Roggenkämper P, Jost WH, Bihari K, Comes G, Grafe S. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm. 2006;113:303–312. doi: 10.1007/s00702-005-0323-3. [DOI] [PubMed] [Google Scholar]
- 103.Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santamato A, Ranieri M, Panza F, et al. Effectiveness of switching therapy from complexing protein-containing botulinum toxin type A to a formulation with low immunogenicity in spasticity after stroke: a case report. J Rehabil Med. 2012;44:795–797. doi: 10.2340/16501977-1009. [DOI] [PubMed] [Google Scholar]
- 105.Grosset DG, Tyrrell EG, Grosset KA. Switch from abobotulinumtoxinA (Dysport®) to incobotulinumtoxinA (Xeomin®) botulinum toxin formulation: a review of 257 cases. J Rehabil Med. 2015;47:183–186. doi: 10.2340/16501977-1895. [DOI] [PubMed] [Google Scholar]
- 106.Dressler D, Pan L, Adib SF. Antibody-induced failure of botulinum toxin therapy: re-start with low-antigenicity drugs offers a new treatment opportunity. J Neural Transm. 2018;125:1481–1486. doi: 10.1007/s00702-018-1911-3. [DOI] [PubMed] [Google Scholar]
- 107.Kranz G, Sycha T, Voller B, Kranz GS, Schnider P, Auff E. Neutralizing antibodies in dystonic patients who still respond well to botulinum toxin type A. Neurology. 2008;70:133–136. doi: 10.1212/01.wnl.0000287087.99612.e5. [DOI] [PubMed] [Google Scholar]
- 108.Hefter H, Hartmann CJ, Kahlen U, Samadzadeh S, Rosenthal D, Moll M. Clinical improvement after treatment with incobotulinumtoxinA (XEOMIN®) in patients with cervical dystonia resistant to botulinum toxin preparations containing complexing proteins. Front Neurol. 2021;12:636590. doi: 10.3389/fneur.2021.636590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shinn JR, Nwabueze NN, Patel P, Norton C, Ries WR, Stephan SJ. Contemporary review and case report of botulinum resistance in facial synkinesis. Laryngoscope. 2019;129:2269–2273. doi: 10.1002/lary.27709. [DOI] [PubMed] [Google Scholar]
- 110.Jankovic J, Hunter C, Dolimbek BZ, et al. Clinico-immunologic aspects of botulinum toxin type B treatment of cervical dystonia. Neurology. 2006;67:2233–2235. doi: 10.1212/01.wnl.0000249308.66959.43. [DOI] [PubMed] [Google Scholar]
- 111.Bentivoglio AR, Del Grande A, Petracca M, Ialongo T, Ricciardi L. Clinical differences between botulinum neurotoxin type A and B. Toxicon. 2015;107(pt A):77–84. doi: 10.1016/j.toxicon.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Factor SA, Molho ES, Evans S, Feustel PJ. Efficacy and safety of repeated doses of botulinum toxin type B in type A resistant and responsive cervical dystonia. Mov Disord. 2005;20:1152–1160. doi: 10.1002/mds.20531. [DOI] [PubMed] [Google Scholar]
- 113.Hefter H, Samadzadeh S, Moll M. Transient improvement after switch to low doses of rimabotulinumtoxinB in patients resistant to abobotulinumtoxinA. Toxins. 2020;12:677. doi: 10.3390/toxins12110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dressler D, Bigalke H, Benecke R. Botulinum toxin type B in antibody-induced botulinum toxin type A therapy failure. J Neurol. 2003;250:967–969. doi: 10.1007/s00415-003-1129-6. [DOI] [PubMed] [Google Scholar]
- 115.Voller B, Moraru E, Auff E, et al. Ninhydrin sweat test: a simple method for detecting antibodies neutralizing botulinum toxin type A. Mov Disord. 2004;19:943–947. doi: 10.1002/mds.20073. [DOI] [PubMed] [Google Scholar]
- 116.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69:200–235. doi: 10.1124/pr.116.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dressler D, Benecke R. Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil. 2007;29:1761–1768. doi: 10.1080/09638280701568296. [DOI] [PubMed] [Google Scholar]
- 118.Ferrari A, Manca M, Tugnoli V, Alberto L. Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal. Funct Neurol. 2018;33:7–18. doi: 10.11138/FNeur/2018.33.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dover JS, Monheit G, Greener M, Pickett A. Botulinum toxin in aesthetic medicine: myths and realities. Dermatol Surg. 2018;44:249–260. doi: 10.1097/DSS.0000000000001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amatsu S, Sugawara Y, Matsumura T, Kitadokoro K, Fujinaga Y. Crystal structure of Clostridium botulinum whole hemagglutinin reveals a huge triskelion-shaped molecular complex. J Biol Chem. 2013;288:35617–35625. doi: 10.1074/jbc.M113.521179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology. 2003;60:1186–1188. doi: 10.1212/01.WNL.0000055087.96356.BB. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.