Abstract
Objective:
To evaluate the effects of early treatment with CPAP on nutritional intake and in-hospital growth rates of extremely preterm (EPT) infants.
Study design:
EPT infants (24–0/7 to 27–6/7 weeks of gestation) enrolled in the Surfactant Positive Airway Pressure and Pulse Oximetry Trial (SUPPORT) were included. EPT infants who died before 36 weeks’ postmenstrual age (PMA) were excluded. The growth rates from birth to 36 weeks’ PMA and follow-up outcomes at 18–22 months’ corrected age of EPT infants randomized at birth to either early CPAP (intervention group) or early intubation for surfactant administration (control group) were analyzed.
Results:
810 of 1316 infants enrolled in SUPPORT (414 in intervention group, 396 in control group) had growth data analyzed. Median gestational age was 26 weeks and mean birthweight was 839 grams. Baseline characteristics, total nutritional intake, and in-hospital comorbidities were not significantly different between groups. In a regression model, growth rates between birth and 36 weeks’ PMA as well as growth rates during multiple intervals from birth to day 7, day 7 to14, day 14 to 21, day 21 to 28, day 28 to 32 weeks’ PMA, and 32 weeks’ PMA to 36 weeks’ PMA did not differ between treatment groups. Independent of treatment group, higher growth rates from day 21 to day 28 were associated with a lower risk of Bayley III cognitive score <85 at 18–22 months’ corrected age (P = .002).
Conclusions:
EPT infants randomized to early CPAP did not have higher in-hospital growth rates than infants randomized to early intubation.
Trial registration
Extremely preterm (EPT) infants have a higher probability of survival without a disability when clinicians prevent severe comorbidities and provide nutritional support to optimize growth outcomes1, 2–6.
Because early CPAP and a protocol-driven strategy created to limit mechanical ventilation attenuates respiratory morbidity in the first week after birth and reduces the use of postnatal steroids7, current clinical practice guidelines recommend early treatment with CPAP in EPT infants 27 weeks of gestation or less8. However, the effects of CPAP on weight gain expressed as growth rate and nutritional practices associated with improved neurodevelopmental outcomes in EPT infants treated with CPAP have not been investigated. Early CPAP could increase in-hospital growth rates by attenuating respiratory morbidity9, 10. Early CPAP might also reduce in-hospital growth rates by increasing caloric expenditure from work of breathing or by decreasing caloric intake from enteral sources due to frequent feeding interruptions and abdominal distension. The effect of early CPAP on growth rates of EPT infants has not been reported in a large, multicenter, randomized clinical trial.
Our specific aims were to determine whether in-hospital growth rates were higher in EPT infants randomized to early CPAP than in EPT infants randomized to early intubation for surfactant administration and to determine the association between in-hospital growth rates and adverse neurodevelopmental outcomes.
METHODS
This was a secondary analysis of the Surfactant Positive Airway Pressure and Pulse Oximetry Trial (SUPPORT) Postnatal Growth Study. A detailed description of the main trial and the growth secondary study have been published elsewhere7, 11, 12. Briefly, SUPPORT was a 2 × 2 factorial, multicenter randomized trial in which EPT infants with gestational ages between 240 and 276 weeks of gestation born at one of the NICHD Neonatal Research Network centers between February 2005 and February 2009 were randomized at birth to determine if early CPAP compared with early intubation for surfactant administration would increase survival without bronchopulmonary dysplasia (BPD) at 36 weeks’ postmenstrual age (PMA) and to determine if lower saturation targets compared with higher saturation targets would decrease mortality and neurodevelopmental impairment at 18–22 months’ corrected age. IRB approval was obtained at all the participating centers and antenatal, written informed consent was obtained before randomization. Infants enrolled in SUPPORT were eligible to participate in the SUPPORT postnatal growth study. When required by a local IRB, a separate written informed consent was obtained to collect supplementary anthropometric data and detailed 24-hour nutritional data at birth, postnatal day 7, postnatal day 14, postnatal day 21, postnatal day 28, and then at 32 and 36 weeks’ PMA12. Thus, the SUPPORT postnatal growth study included a subgroup of EPT infants with gestational ages between 24 and 27 weeks of gestation who were enrolled in SUPPORT and survived to 36 weeks’ PMA. Nutritional practices were not dictated by the study protocol. Enteral intake was calculated assuming an average composition of breast milk and reviewing product information of the different formulas prescribed during the study.
For this analysis, the exposure status was defined by randomization to either early CPAP and a protocol-driven strategy created to limit mechanical ventilation (intervention group) or early intubation for surfactant administration and a protocol-driven strategy created to favor mechanical ventilation (control group). The primary outcome was growth rate from birth to 36 weeks’ PMA or discharge, whichever occurred first, calculated using the exponential method5. Secondary outcomes included postnatal growth failure defined as weight <10th percentile at 36 weeks’ PMA or discharge using the Fenton growth curves13, 14 and cognitive delay defined as cognitive score <85 using the Bayley Scales of Infant and Toddler Development, Third Edition at 18 to 22 months’ CA. The Bayley III cognitive score was determined during a comprehensive neurodevelopmental assessment performed by annually certified examiners at an outpatient follow-up visit.
The following characteristics were studied in this analysis: in-hospital morbidities (intraventricular hemorrhage [IVH] grade 3 or 4, cystic periventricular leukomalacia [PVL], BPD defined as receipt of supplemental oxygen at 36 weeks’ PMA, necrotizing enterocolitis [NEC] stage 2 or greater, retinopathy of prematurity [ROP] stage 3 or greater, culture-proven sepsis, and culture-proven meningitis); maternal characteristics (education level, socioeconomic status, and history of hypertension or diabetes during pregnancy); and neonatal characteristics (early nutrition, sex, race, exposure to antenatal steroids, Apgar score at 5 minutes, and intrauterine patterns of growth defined by size at birth). The interaction between early CPAP and target saturations was not assessed in these analyses.
Statistical Analyses
Baseline characteristics were compared using either t-test or Mann Whitney U test for continuous variables and χ2 test for categorical variables. T-test was used to compare enteral and parenteral intake between treatment groups. The association between the two treatment groups and growth rates during different intervals from birth to 36 weeks’ PMA (from birth to day 7, day 7 to14, day 14 to 21, day 21 to 28, day 28 to 32 weeks’ PMA, and 32 weeks’ PMA to 36 weeks’ PMA) was examined with a repeated measures regression model that included polynomial terms. This approach allowed the comparison of growth during specific intervals between treatment groups using one single model with greater power. The repeated measures regression model was unadjusted and adjusted for multiple birth clustering and trial stratification parameters (i.e., gestational age and center).
The association between in-hospital growth rates from birth to postnatal day 28 and cognitive delay at 18 to 22 months’ CA was examined in two steps. First, discriminant analyses were performed to determine which growth and nutritional measures known at or before each time point have a higher predictive value of Bayley III cognitive score <85. Then, the results of these discriminant analyses were used to inform which growth and nutritional measures at different points should be included in a robust Poisson regression model with cognitive delay as outcome variable and other clinical characteristics as covariates. This approach minimized problems related to multiple testing and avoided problems of collinearity between growth and nutritional measures at different time points from the same infant.
All statistical analyses were performed using SAS 9.4 (Cary, NC). Corrections for multiple comparisons were not included.
Previous data indicated that the mean growth rate among EPT infants is 13.5 ± 2.5 g/kg/day from birth to 36 weeks’ PMA12. To detect a small clinical difference of 1 g/kg/day between groups, we determined that at least 100 patients were needed in each comparison group (power 80% and two sided α = 0.05).
RESULTS
Of 1316 infants enrolled in SUPPORT from February 2005 through February 2009, 483 were enrolled before the Postnatal Growth Study was approved (April 2006). Of 810 infants enrolled in SUPPORT after April 2006 that simultaneously participated in the Postnatal Growth Study (97%), 681 infants (84%) survived to 36 weeks’ PMA and 597 infants (88% of survivors) had a Bayley-III assessment at 18–22 months’ corrected age [Figure; available at www.jpeds.com]
Figure.
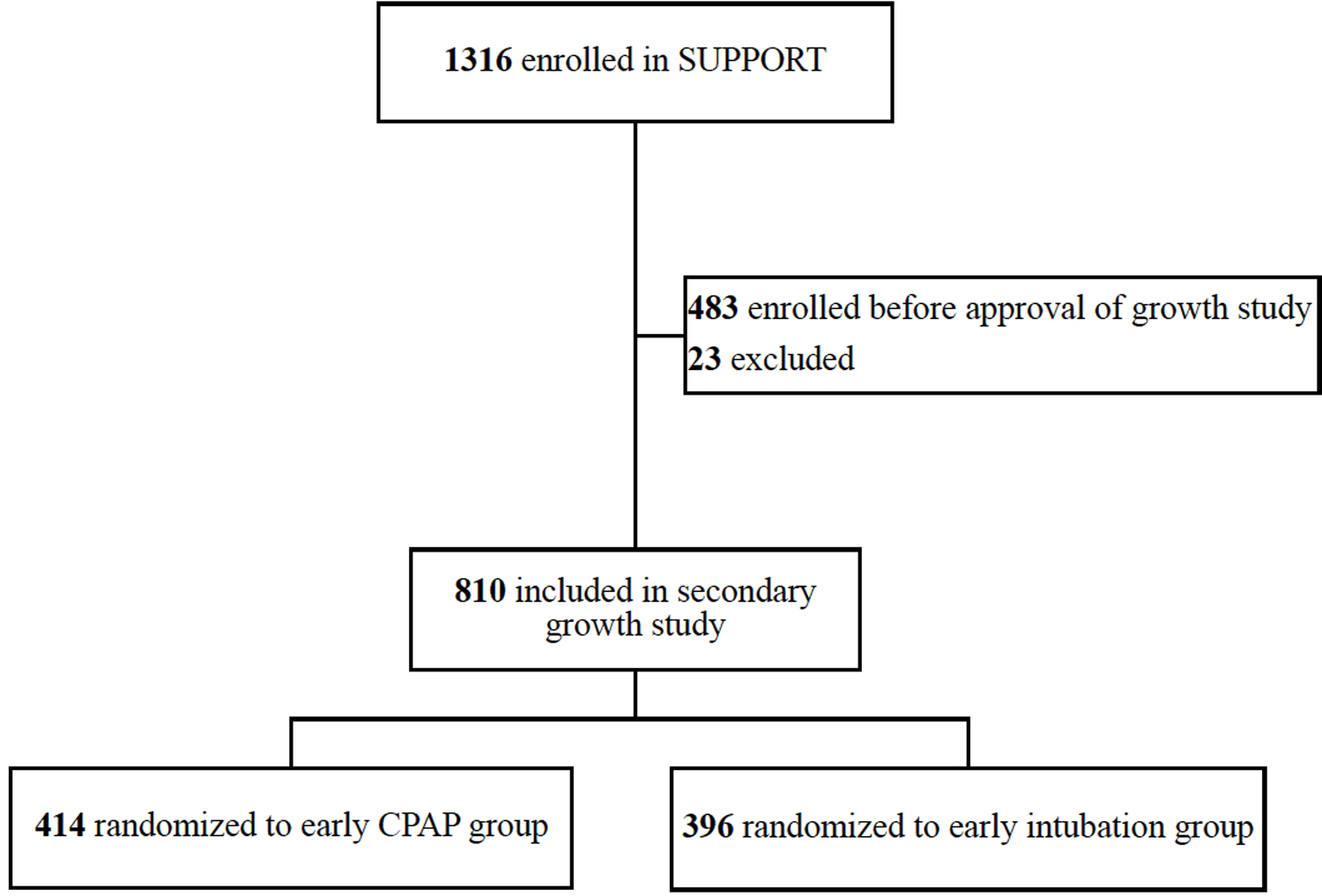
Participant flow diagram
Growth data for 810 EPT infants randomized to either early CPAP (n=414) or early intubation for surfactant administration (n=396) were analyzed (mean gestational age: 26.2 weeks; mean birth weight: 839 grams).
There were no statistically significant differences in baseline characteristics or in-hospital morbidities between groups [Table I]. The outcome of NEC was slightly higher in the early CPAP group, but this finding was not statistically significant (14 % vs. 10%; p=0.07). The total nutritional intake expressed as kcal/kg/day did not differ between the groups [Table 2]. The mean protein intake during the first 28 days after birth was approximately 2.9 g/kg/day in both groups. The mean caloric intake at day 28 was 5 kcal/kg/day lower in the early CPAP group, but this finding was not statistically significant (p=0.07).
Table 1.
Demographics and clinical characteristics
| Characteristics | Early CPAP group N=414 | Early intubation group N=396 | P-value |
|---|---|---|---|
| Maternal demographics | |||
| Age in years, mean (SD) | 26.7 (6.3) | 27.0 (6.5) | 0.53 |
| Married marital status, n (%) | 186 (45) | 158 (40) | 0.14 |
| Mother’s education: HS or greater, n (%) | 83 (25) | 76 (26) | 0.99 |
| Medical insurance | 0.09 | ||
| Public insurance, n (%) | 211 (52) | 230 (59) | |
| Private insurance, n (%) | 162 (40) | 136 (35) | |
| Other insurance, n (%) | 34 (8) | 23 (6) | |
| Multiple gestation, n (%) | 106 (26) | 96 (24) | 0.65 |
| Hypertension, n (%) | 94 (23) | 107 (27) | 0.16 |
| Diabetes, n (%) | 17 (4) | 28 (7) | 0.07 |
| ANS exposure, n (%) | 401 (97) | 378 (96) | 0.38 |
| Neonatal demographics | |||
| Birth weight in grams, mean (SD) | 841.6 (182.2) | 836.7 (194.8) | 0.95 |
| Gestational age in weeks, mean (SD) | 26.2 (1.1) | 26.2 (1.1) | 0.97 |
| SGA, n (%) | 156 (53) | 150 (54) | 0.70 |
| Female, n (%) | 194 (47) | 169 (43) | 0.23 |
| Black race, n (%) | 179 (43) | 159 (40) | 0.37 |
| Weight z-score at birth, mean (SD) | 0.0 (1.0) | −0.0 (1.1) | 0.50 |
| In-hospital morbidity | |||
| Apgar score at 5 minutes, median (IQR) | 7 (6, 8) | 7 (6, 8) | 0.55 |
| Intraventricular hemorrhage [IVH] grade 3 or 4, n (%) | 65 (16) | 54 (14) | 0.38 |
| Periventricular leukomalacia [PVL], n (%) | 17 (4) | 19 (5) | 0.66 |
| Bronchopulmonary dysplasia [BPD], n (%) | 148 (42) | 142 (44) | 0.62 |
| Necrotizing enterocolitis [NEC], n (%) | 59 (14) | 40 (10) | 0.07 |
| Retinopathy of prematurity [ROP] stage 3 or greater, n (%) | 40 (12) | 37 (13) | 0.93 |
| Meningitis, n (%) | 15 (4) | 17 (4) | 0.62 |
Table 2.
Nutritional intake
| Outcome | Early CPAP group | Early intubation group | P-value |
|---|---|---|---|
| Age birth weight was first regained in days, median (IQR) | 12.0 (9.0,15.0) | 11.0 (9.0,15.0) | 0.30 |
| Duration of parenteral nutrition in days, median (IQR) | 24.0 (14.0,37.0) | 23.0 (15.0,36.0) | 0.84 |
| Age at first enteral feeding in days, median (IQR) | 4.0 (3.0,7.0) | 4.0 (3.0,7.0) | 0.60 |
| Age at full enteral feeding in days, median (IQR) | 24.0 (16.0,36.0) | 23.0 (17.0,32.0) | 0.48 |
| Any breast milk use in the first month, n (%) | 340 (88) | 320 (87) | 0.57 |
| Total energy intake (kcal/kg/day) | |||
| Day 7 | 83.4 (24.1) | 82.5 (23.5) | 0.49 |
| Day 14 | 91.9 (24.8) | 90.1 (26.1) | 0.30 |
| Day 21 | 92.2 (35.6) | 94.3 (30.0) | 0.43 |
| Day 28 | 94.2 (30.1) | 98.9 (28.0) | 0.07 |
| 32 weeks’ PMA | 103.9 (27.7) | 105.5 (29.4) | 0.41 |
| 36 weeks’ PMA | 108.4 (33.1) | 110.1 (36.9) | 0.59 |
| Energy from protein intake (kcal/kg/day) | |||
| Day 7 | 13.0 (3.3) | 13.2 (3.3) | 0.66 |
| Day 14 | 12.1 (4.9) | 12.4 (4.8) | 0.71 |
| Day 21 | 10.7 (5.1) | 10.9 (5.2) | 0.85 |
| Day 28 | 10.1 (4.8) | 10.3 (5.1) | 0.98 |
| 32 weeks’ PMA | 9.9 (5.1) | 9.9 (5.1) | 0.98 |
| 36 weeks’ PMA | 10.4 (4.9) | 10.5 (5.0) | 0.73 |
| Energy from carbohydrate intake (kcal/kg/day) | |||
| Day 7 | 40.0 (13.3) | 39.5 (12.8) | 0.77 |
| Day 14 | 38.9 (11.5) | 39.1 (15.4) | 0.97 |
| Day 21 | 38.8 (14.5) | 38.1 (14.7) | 0.33 |
| Day 28 | 38.2 (12.9) | 38.2 (12.0) | 0.76 |
| 32 weeks’ PMA | 38.7 (12.4) | 37.5 (10.9) | 0.30 |
| 36 weeks’ PMA | 38.4 (12.3) | 37.8 (11.4) | 0.87 |
| Energy from fat intake (kcal/kg/day) | |||
| Day 7 | 32.3 (16.1) | 31.9 (15.0) | 0.69 |
| Day 14 | 44.9 (21.0) | 42.3 (19.8) | 0.21 |
| Day 21 | 49.2 (26.8) | 50.3 (22.1) | 0.36 |
| Day 28 | 51.7 (23.2) | 54.1 (21.4) | 0.19 |
| 32 weeks’ PMA | 58.5 (20.3) | 58.5 (20.3) | 0.98 |
| 36 weeks’ PMA | 60.3 (19.9) | 60.6 (20.4) | 0.95 |
Primary outcome
The mean (SD) growth rate from birth to 36 weeks’ PMA was not different in the early CPAP vs. early intubation group (13.2 ± 2.4 g/kg/day (2.4) vs. 13.2 ± 2.6 g/kg/day, p= 0.76). There were no significant differences in growth rates during any interval from birth to 36 weeks’ PMA between treatment groups (from birth to postnatal day 7, from postnatal day 7 to 14, from postnatal day 14 to 21, from postnatal day 21 to 28, from postnatal day 28 to 32 weeks’ PMA, and from 32 weeks’ PMA to 36 weeks’ PMA) [Table 3].
Table 3.
Growth rates of extremely preterm infants randomized to either early CPAP or surfactant at birth
| Growth rate (g/kg/day) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| Early CPAP group Mean (SE) | Early intubation group Mean (SE) | P-value | Early CPAP group Mean (SE) | Early intubation group Mean (SE) | P-value | |
| From birth to day 7 | −9.45 (0.87) |
−9.69 (0.83) |
0.88 | −9.27 (0.87) |
−9.55 (0.80) |
0.81 |
| From day 7 to day 14 | 13.77 (0.75) |
14.65 (0.79) |
0.61 | 13.91 (0.75) |
14.72 (0.78) |
0.45 |
| From day 14 to day 21 | 12.27 (0.60) |
12.39 (0.63) |
0.86 | 12.31 (0.59) |
12.37 (0.63) |
0.94 |
| From day 21 to day 28 | 14.06 (0.71) |
13.29 (0.73) |
0.16 | 14.08 (0.73) |
13.25 (0.73) |
0.41 |
| From day 28 to 32 weeks’ PMA | 13.36 (0.80) |
14.60 (0.46) |
0.45 | 13.34 (0.76) |
14.40 (0.46) |
0.24 |
| From 32 weeks’ PMA to 36 weeks’ PMA | 15.72 (0.27) |
15.67 (0.24) |
0.82 | 15.64 (0.31) |
15.34 (0.28) |
0.46 |
| From 36 weeks’ PMA to 18–22 months | 2.67 (0.02) |
2.60 (0.02) |
0.07 | 2.50 (0.14) |
2.38 (0.17) |
0.54 |
Secondary outcomes
Postnatal growth failure occurred in 306 of 574 infants (53%). Early CPAP was not associated with a higher or lower risk of postnatal growth failure (p=0.70). There were no significant differences in length and head circumference measurements at multiple time points from birth to 36 weeks’ PMA between treatment groups [Table 4;available at www.jpeds.com].
Table 4.
Length and head circumference gain velocities in extremely preterm infants randomized to early CPAP or surfactant
| Length gain velocity (cm/week) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| Early CPAP group Mean (SE) | Early intubation group Mean (SE) | P-value | Early CPAP group Mean (SE) | Early intubation group Mean (SE) | P-value | |
| From birth to day 7 | 0.013 (0.050) | 0.014 (0.048) | 0.41 | 0.013 (0.003) | 0.014 (0.003) | 0.92 |
| From day 7 to day 14 | 0.021 (0.039) | 0.027 (0.048) | 0.30 | 0.022 (0.002) | 0.027 (0.003) | 0.19 |
| From day 14 to day 21 | 0.020 (0.031) | 0.020 (0.036) | 0.37 | 0.020 (0.002) | 0.021 (0.002) | 0.80 |
| From day 21 to day 28 | 0.021 (0.030) | 0.026 (0.039) | 0.38 | 0.022 (0.002) | 0.026 (0.003) | 0.15 |
| From day 28 to 32 weeks PMA | 0.021 (0.030) | 0.020 (0.043) | 0.96 | 0.022 (0.002) | 0.020 (0.003) | 0.60 |
| From 32 weeks PMA to 36 weeks PMA | 0.025 (0.016) | 0.025 (0.016) | 0.88 | 0.026 (0.001) | 0.025 (0.001) | 0.75 |
| Head circumference gain velocity (cm/week) | ||||||
| Unadjusted | Adjusted | |||||
| Early CPAP group Mean (SE) | Early intubation group Mean (SE) |
P-value | Early CPAP group Mean (SE) |
Early intubation group Mean (SE) |
P-value | |
| From birth to day 7 | −0.014 (0.052) | −0.007 (0.051) | 0.77 | −0.012 (0.003) | −0.006 (0.003) | 0.18 |
| From day 7 to day 14 | 0.026 (0.049) | 0.018 (0.055) | 0.15 | 0.027 (0.003) | 0.018 (0.003) | 0.05 |
| From day 14 to day 21 | 0.026 (0.047) | 0.030 (0.043) | 0.71 | 0.026 (0.003) | 0.030 (0.003) | 0.34 |
| From day 21 to day 28 | 0.027 (0.045) | 0.031 (0.050) | 0.46 | 0.027 (0.003) | 0.031 (0.003) | 0.45 |
| From day 28 to 32 weeks PMA | 0.030 (0.029) | 0.027 (0.040) | 0.86 | 0.030 (0.002) | 0.027 (0.003) | 0.31 |
| From 32 weeks PMA to 36 weeks PMA | 0.031 (0.014) | 0.032 (0.013) | 0.42 | 0.032 (0.001) | 0.031 (0.001) | 0.38 |
Cognitive delay (i.e., Bayley-III cognitive score <85) occurred in 161 of 597 infants (27%). Higher growth rates from postnatal day 21 to 28 (p=0.002) were associated with a lower risk of cognitive delay [Table 5]. Postnatal growth failure at 36 weeks’ PMA was not associated with a higher risk of cognitive delay (p=0.19). None of the other pre or post-discharge growth rates estimated over multiple intervals between birth and 18–22 month’s CA were strong predictors of cognitive delay.
Table 5.
Relationship between treatment arm, growth, and nutritional characteristics within the first month and cognitive outcomes at 2 years of age*
| Characteristics | Cognitive delay (BSID III < 85) | |||
|---|---|---|---|---|
| Unadjusted RR | P-value | Adjusted RR | P-value | |
| Treatment (CPAP vs Intubation) | 0.84 (0.64, 1.09) | 0.19 | 0.71 (0.52, 0.97) | 0.03 |
| Growth rate from 21 to 28 days | 0.87 (0.82, 0.92) | <0.01 | 0.89 (0.82, 0.96) | <0.01 |
| Enteral intake at 7 days | 0.90 (0.84, 0.97) | <0.01 | 0.94 (0.83, 1.06) | 0.30 |
| Enteral intake at 14 days | 0.95 (0.91, 1.00) | 0.05 | 1.01 (0.93, 1.10) | 0.76 |
| Carbohydrate intake at 7 days | 0.84 (0.74, 0.95) | 0.01 | 0.93 (0.74, 1.18) | 0.56 |
| Fat intake at 14 days | 0.91 (0.84, 0.98) | 0.01 | 0.94 (0.84, 1.05) | 0.28 |
| Fat intake at 21 days | 0.88 (0.83, 0.93) | <0.01 | 0.91 (0.84, 0.97) | <0.01 |
Variables listed in the table were found to best fit the final model using discriminant analyses with all growth rates and nutritional measures within the first month after birth. Results for continuous variables reflect the risk ratio for every 10 units. The adjusted Poisson regression model included treatment arm, center, gestational age, maternal education, and severe IVH.
DISCUSSION
In this secondary analysis, we characterized growth rates of extremely preterm infants 24 to 27 weeks of gestation randomized to early CPAP or early intubation. During multiple intervals between birth and 36 weeks’ PMA, EPT infants randomized to early CPAP and a protocol-driven strategy created to limit mechanical ventilation did not have higher in-hospital growth rates than infants randomized to early intubation and a protocol-driven strategy created to favor mechanical ventilation. The trend toward decreased caloric intake from postnatal day 21 to 28 observed in the early CPAP group did not result in lower growth rates. Similarly, EPT infants randomized to early CPAP did not have a higher risk of postnatal growth failure at 36 weeks’ PMA. Independent of treatment group, higher growth rates between postnatal day 21 and day 28 were associated with a lower risk of cognitive delay at 18 to 22 months’ CA. Postnatal growth failure defined as weight <10th percentile at 36 weeks’ PMA was not associated with a delay in cognitive function at 18 to 22 months’ CA after adjustment for potential confounders.
This study provides longitudinal data on growth rates of infants randomized to early CPAP in a large, multicenter, randomized clinical trial, a reliable source of clinical evidence. We chose growth rate as the primary outcome of the study because comparing growth rates between EPT infants and healthy fetuses of the same PMA is more informative than comparing growth percentiles (ie, postnatal growth failure)15, 16. Although the number of neonatal trials reporting postnatal growth failure at 36 weeks’ PMA as a primary outcome of growth has increased, the number of studies suggesting an association between postnatal growth failure and neurodevelopmental impairment (NDI) has decreased17. Similar to recent reports, including our comparative analysis of growth outcomes according to the saturation target arm of SUPPORT12, we could not identify a significant association between the outcome of postnatal growth failure at 36 weeks’ PMA and the outcome of cognitive delay at 18 to 22 months’ CA using the Fenton growth curves13, 14 to define postnatal growth failure at 36 weeks’ PMA17.
Most clinical guidelines recommend growth rates between 15 and 20 g/kg/day to prevent postnatal growth failure in preterm infants 23 to 36 weeks of gestation5. The proportion of infants with postnatal growth failure at 36 weeks’ PMA in this study was within previously described ranges. However, many EPT infants included in this study had in-hospital growth rates below 15 g/kg/day and lower cognitive scores at 18 to 22 months’ CA than EPT infants included in other studies18, 19. It remains unclear if higher growth rates that prevent postnatal growth failure can reduce the risk of cognitive delay without increasing the risk of adverse metabolic outcomes6. A recent study suggests that, among EPT infants, higher post-discharge growth rates are more strongly associated with adverse metabolic outcomes than higher in-hospital growth rates20.
Our sufficiently powered analysis indicates that infants treated with early CPAP and those treated with early intubation for surfactant administration have similar growth rates from birth to 36 weeks’ PMA. We observed a trend toward more NEC, subsequent feeding interruptions, and lower enteral intakes in the first month after birth in infants treated with CPAP. We suggest that these infants could benefit from nutritional practices that reduce the risk of NEC or feeding intolerance while optimizing energy intake. A large observational study suggested that each additional 1 kcal/kg/d of total energy intake is associated with a nearly 2% risk reduction of BPD and NDI in critically ill EPT infants10. It remains unclear if the trend toward increased risk of NEC in EPT infants treated with CPAP represents a cause-effect relationship between CPAP use and NEC or evidence that CPAP use increases the risk of abdominal distention, the number of feeding interruptions, and therefore, the clinicians’ concerns regarding the diagnosis of NEC. The association between early CPAP and NEC reported in this secondary analysis was similar to the association reported in SUPPORT (13% in the CPAP group and 10% in the intubation group).
One of the main limitations of this study is that we only included infants who survived through 2 years of age and underwent neurodevelopmental assessments. This approach systematically excluded critically ill infants with inadequate nutritional support or growth failure who died from severe comorbidities. It also excluded infants who survived but did not return for follow-up. Growth rates are often confounded by illness severity and comorbidities associated with extreme prematurity 9, 10. We attempted to overcome this limitation with a regression model that accounted for in-hospital comorbidities. However, establishing the mechanisms by which severity of illness affects nutritional practices, growth, and survival without a disability is complex10. Other limitations of this study were the specific gestational ages listed as inclusion criteria and the antenatal consent requirement for enrollment in SUPPORT. The possibility that nutritional practices may have varied greatly from center to center and attending to attending is also a limitation. We included the covariate center in all the adjusted models to address this limitation, but we did not quantify the amount of bias introduced by center variability. Another important limitation is that several nutritional strategies have changed since the completion of this trial. The use of donor human milk and liquid human milk fortifiers has been expanded, the age at first feeding and the time to full enteral nutrition have been reduced, and the guidelines to provide parenteral nutrition have been slightly modified. NEC and postnatal growth failure have also decreased overall in frequency, but the increasing survival rates observed among infants born at the limits of viability have increased the number of infants at risk.
The main strengths of this study were the multicenter study design, the sufficient power to detect true differences in longitudinal growth data between groups, the report of growth rates during multiple intervals, and the assessment of cognitive outcomes with standardized methods. Although most clinicians and researchers refer to growth rate as the change in weight from birth to hospital discharge, recent studies have confirmed that growth rates are variable throughout hospitalization21 and more likely to be higher in growth-restricted infants3, 22. We selected cognitive delay as one the outcomes of the study because Bayley III cognitive scores define most of the variability in the outcome of NDI19, 23–26. Cognitive outcomes at 2 years of age are also reliable predictors of poor cognitive skills at school age and then at early adulthood27.
In conclusion, randomization to early CPAP did not affect in-hospital growth rates relative to early intubation. Higher growth rates between postnatal day 21 and postnatal day 28 were associated with higher cognitive scores at 18 to 22 months’ CA. We speculate that optimizing nutrition by either reducing feeding interruptions or adjusting parenteral nutrition in EPT infants treated with CPAP could prevent morbidities and increase growth rates in this population.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network’s SUPPORT Trial through cooperative agreements (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880, UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68270, UG1 HD68278, UG1 HD68263, UG1 HD68284; UG1 HD87226, UG1 HD87229). A.S. received grant support from (K23 HD102554). The comments and views of the authors do not necessarily represent the views of NICHD, NHLBI, or the National Institutes of Health. A.S. received consulting fees from the Lockwood Group LLC for participation in advisory board meetings and filed a patent application for an instrumented bottle. W.C. serves on the board of directors of MEDNAX Services, Inc.
Abbreviations:
- PMA
Postmenstrual age
- CA
Corrected age
- CCS
Cognitive composite score
- NDI
Neurodevelopmental impairment.
Appendix
List of additional members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network
Alan H. Jobe, MD PhD, University of Cincinnati, Cincinnati, OH
Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine, Chicago, IL
Abbot R. Laptook, MD; William Oh, MD; Betty R. Vohr, MD; Angelita M. Hensman, RN BSN; Bonnie E. Stephens, MD; Barbara Alksninis, PNP; Dawn Andrews, RN; Kristen Angela, RN; Susan Barnett, RRT; Bill Cashore, MD; Melinda Caskey, MD; Kim Francis, RN; Dan Gingras, RRT; Regina A. Gargus, MD FAAP; Katharine Johnson, MD; Shabnam Lainwala, MD; Theresa M. Leach, MEd CAES; Martha R. Leonard, BA BS; Sarah Lillie, RRT; Kalida Mehta; James R. Moore, MD; Lucy Noel; Suzy Ventura; Rachel V. Walden; Victoria E. Watson, MS CAS, Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island, Providence, RI.
Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Nancy S. Newman, RN; Deanne E. Wilson-Costello, MD; Bonnie S. Siner, RN; Arlene Zadell RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD, Case Western Reserve University, Rainbow Babies & Children’s Hospital, Cleveland, OH.
Kurt Schibler, MD; Edward F. Donovan, MD; Kimberly Yolton, PhD; Vivek Narendran, MD MRCP; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA, Cincinnati Children’s Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital, Cincinnati, OH.
Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Patricia Ashley, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sharon F. Freedman, MD; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; William F. Malcolm, MD; David K. Wallace, MD MPH, Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital, Durham, NC.
Barbara J. Stoll, MD; Susie Buchter, MD; Anthony J. Piazza, MD; David P. Carlton, MD; Ira Adams-Chapman, MD (deceased); Linda Black, MD; Ann M. Blackwelder, RNC BS MS; Sheena Carter, PhD; Elisabeth Dinkins, PNP; Sobha Fritz, PhD; Ellen C. Hale, RN BS CCRC; Amy K. Hutchinson, MD; Maureen Mulligan LaRossa, RN; Gloria V. Smikle, PNP MSN, Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital, Atlanta, GA.
Stephanie Wilson Archer, MA, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
James A. Lemons, MD; Anna M. Dusick, MD FAAP; Leslie D. Wilson, BSN CCRC; Faithe Hamer, BS; Ann B. Cook, MS; Dianne E. Herron, RN; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP, Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services, Indianapolis, IN.
Mary Anne Berberich, PhD; Carol J. Blaisdell, MD; Dorothy B. Gail, PhD; James P. Kiley, PhD, National Heart, Lung, and Blood Institute, Bethesda, MD.
W. Kenneth Poole, PhD; Marie G. Gantz, PhD; Jamie E. Newman, PhD MPH; Betty K. Hastings; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; James W. Pickett II, BS; Dennis Wallace, PhD; Kristin M. Zaterka-Baxter, RN BSN, RTI International, Research Triangle Park, NC.
David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; M. Bethany Ball, BS CCRC; Barbara Bentley, PsychD MSEd; Elizabeth F. Bruno, PhD; Alexis S. Davis, MD MS; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP; Jean G. Kohn, MD MPH; Melinda S. Proud, RCP; Renee P. Pyle, PhD; Nicholas H. St. John, PhD; Hali E. Weiss, MD. Stanford University and Lucile Packard Children’s Hospital, Palo Alto, CA.
Ivan D. Frantz III, MD; John M. Fiascone, MD; Elisabeth C. McGowan, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA. Tufts Medical Center, Floating Hospital for Children, Boston, MA.
Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Myriam Peralta-Carcelen, MD MPH; Vivien A. Phillips, RN BSN; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Maria Hopkins, PhD; Kristen C. Johnston, MSN CRNP; Sara Krzywanski, MS; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Richard V. Rector, PhD; Leslie Rodriguez, PhD; Amanda Soong, MD; Sally Whitley, MA OTR-L FAOTA; Sheree York, PT DPT MS PCS. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama, Birmingham, AL
Neil N. Finer, MD; Maynard R. Rasmussen, MD; Paul R. Wozniak, MD; Yvonne E. Vaucher, MD MPH; Wade Rich, RRT; Kathy Arnell, RNC; Rene Barbieri-Welge; Ayala Ben-Tall; Renee Bridge, RN; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Elaine Ito; Meghan Lukasik; Deborah Pontillo; Donna Posin, OTR/L MPA; Cheryl Runyan; James Wilkes; Paul Zlotnik, University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women, San Diego, CA.
John A. Widness, MD; Jonathan M. Klein, MD; Tarah T. Colaizy, MD MPH; Karen J. Johnson, RN BSN; Michael J. Acarregui, MD; Diane L. Eastman, RN CPNP MA. University of Iowa, Iowa City, IA.
Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Maria Calejo, MEd; Alexis N. Diaz, BA; Silvia M. Frade Eguaras, BA; Andrea Garcia, MA; Kasey Hamlin-Smith, PhD; Michelle Harwood Berkowits, PhD; Sylvia Hiriart-Fajardo, MD; Elaine O. Mathews, RN; Helina Pierre, BA; Arielle Riguard, MD; Alexandra Stroerger, BA. University of Miami, Holtz Children’s Hospital, Miami, FL.
Robin K. Ohls, MD; Janell Fuller, MD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN; Jean Lowe, PhD; Rebecca Montman, BSN. University of New Mexico Health Sciences Center, Albuquerque, NM.
Nirupama Laroia, MD; Dale L. Phelps, MD; Gary J. Myers, MD; Gary David Markowitz, MD; Linda J. Reubens, RN CCRC; Diane Hust, MS RN CS; Lisa Augostino; Julie Babish Johnson, MSW; Erica Burnell, RN; Harris Gelbard, MD PhD; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Jonathan Mink, MD PhD; Carlos Torres, MD; David Wang, MD; Kelley Yost, PhD, University of Rochester Medical Center, Golisano Children’s Hospital, Rochester, NY.
Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Roy J. Heyne, MD; Sally S. Adams, MS RN CPNP; James Allen, RRT; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, PsyD PA-C; Melissa H. Lepps, RN; Linda A. Madden, RN CPNP; Melissa Martin, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Araceli Solis, RRT; Lizette E. Torres, RN; Catherine Twell Boatman, MS CIMI; Diana M Vasil, RNC-NIC; Kerry Wilder, RN. University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas, Dallas, TX.
Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Nora I. Alaniz, BS; Patricia W. Evans, MD; Beverly Foley Harris, RN BSN; Charles Green, PhD; Margarita Jiminez, MD MPH; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; Margaret L. Poundstone, RN BSN; Stacy Reddoch, BA; Saba Siddiki, MD; Patti L. Pierce Tate, RCP; Laura L. Whitely, MD; Sharon L. Wright, MT (ASCP). McGovern Medical School at The University of Texas Health Science Center at Houston and Children’s Memorial Hermann Hospital, Houston, TX.
Bradley A. Yoder, MD; Roger G. Faix, MD; Shawna Baker, RN; Karie Bird, RN; Jill Burnett, RN; Laura Cole, RN; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Mike Steffens, PhD; Kimberlee Weaver-Lewis, RN BSN; Karen Zanetti, RN. University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center, Salt Lake City, UT.
T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Nancy J. Peters, RN CCRP; Barbara G. Jackson, RN BSN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD. Wake Forest University, Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center, Winston-Salem, NC.
Athina Pappas, MD; Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Elizabeth Billian, RN MBA; Laura A. Goldston, MA; Mary Johnson, RN BSN. Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan, Ann Arbor, MI.
Richard A. Ehrenkranz, MD (deceased); Vineet Bhandari, MD DM; Harris C. Jacobs, MD; Pat Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Christine G. Butler, MD; Nancy Close, PhD; Walter Gilliam, PhD; Sheila Greisman, RN; Elaine Romano, MSN; Joanne Williams, RN BSN. Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital, New Haven, CT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
REFERENCES
- [1].Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Embleton ND, Morgan C, King C. Balancing the risks and benefits of parenteral nutrition for preterm infants: can we define the optimal composition? Arch Dis Child Fetal Neonatal Ed. 2015;100:F72–5. [DOI] [PubMed] [Google Scholar]
- [3].Martin CR, Brown YF, Ehrenkranz RA, O’Shea TM, Allred EN, Belfort MB, et al. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics. 2009;124:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107:270–3. [DOI] [PubMed] [Google Scholar]
- [5].Fenton TR, Anderson D, Groh-Wargo S, Hoyos A, Ehrenkranz RA, Senterre T. An Attempt to Standardize the Calculation of Growth Velocity of Preterm Infants-Evaluation of Practical Bedside Methods. J Pediatr. 2018;196:77–83. [DOI] [PubMed] [Google Scholar]
- [6].Harding JE, Cormack BE, Alexander T, Alsweiler JM, Bloomfield FH. Advances in nutrition of the newborn infant. Lancet. 2017;389:1660–8. [DOI] [PubMed] [Google Scholar]
- [7].Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Committee on F, Newborn, American Academy of P. Respiratory support in preterm infants at birth. Pediatrics. 2014;133:171–4. [DOI] [PubMed] [Google Scholar]
- [9].Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Seminars in Perinatology. 2003;27:302–10. [DOI] [PubMed] [Google Scholar]
- [10].Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Navarrete CT, Wrage LA, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Growth Outcomes of Preterm Infants Exposed to Different Oxygen Saturation Target Ranges from Birth. J Pediatr. 2016;176:62–8e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC pediatrics. 2003;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].American Academy of Pediatrics Committee on Nutrition: Nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976–86. [PubMed] [Google Scholar]
- [16].Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91. [DOI] [PubMed] [Google Scholar]
- [17].Fenton TR, Cormack B, Goldberg D, Nasser R, Alshaikh B, Eliasziw M, et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J Perinatol. 2020;40:704–14. [DOI] [PubMed] [Google Scholar]
- [18].Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014;75:670–4. [DOI] [PubMed] [Google Scholar]
- [19].Spencer-Smith MM, Spittle AJ, Lee KJ, Doyle LW, Anderson PJ. Bayley-III Cognitive and Language Scales in Preterm Children. Pediatrics. 2015;135:e1258–65. [DOI] [PubMed] [Google Scholar]
- [20].Vohr BR, Heyne R, Bann CM, Das A, Higgins RD, Hintz SR, et al. Extreme Preterm Infant Rates of Overweight and Obesity at School Age in the SUPPORT Neuroimaging and Neurodevelopmental Outcomes Cohort. J Pediatr. 2018;200:132–9e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rochow N, Raja P, Liu K, Fenton T, Landau-Crangle E, Gottler S, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res. 2016;79:870–9. [DOI] [PubMed] [Google Scholar]
- [22].Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. [DOI] [PubMed] [Google Scholar]
- [23].Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW, Victorian Infant Collaborative G. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164:352–6. [DOI] [PubMed] [Google Scholar]
- [24].Vohr BR. Neurodevelopmental outcomes of extremely preterm infants. Clin Perinatol. 2014;41:241–55. [DOI] [PubMed] [Google Scholar]
- [25].Doyle LW. Are Neurodevelopmental Outcomes of Infants Born Extremely Preterm Improving Over Time? Pediatrics. 2018;141. [DOI] [PubMed] [Google Scholar]
- [26].Adams-Chapman I, Heyne RJ, DeMauro SB, Duncan AF, Hintz SR, Pappas A, et al. Neurodevelopmental Impairment Among Extremely Preterm Infants in the Neonatal Research Network. Pediatrics. 2018;141. [DOI] [PMC free article] [PubMed]
- [27].Linsell L, Johnson S, Wolke D, O’Reilly H, Morris JK, Kurinczuk JJ, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population-based cohort study. Arch Dis Child. 2018;103:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


