Abstract
Background and Purpose
CD200, a highly glycosylated protein primarily expressed on neurons in the central nervous system (CNS), binds with its receptor CD200R to form an endogenous inhibitory signal against immune responses. However, little is known about the effect of neuronal CD200 signaling in cerebral ischemia. The aim of this study was to investigate how neuronal CD200 signaling impacts post-stroke inflammation and the ischemic injury.
Methods
CD200 tma1lf/fl:Thy1CreER mice were treated with tamoxifen (TMX) to induce conditional gene knockout (ICKO) of neuronal CD200. The mice were subjected to a 60-min transient middle cerebral artery occlusion (MCAO). Stroke outcomes, apoptotic cell death, immune cell infiltration, microglia activation and other inflammatory profiles were evaluated at 3 and 7 days after stroke.
Results
Infarct volumes were significantly larger, and behavioral deficits more severe in ICKO vs. control mice at 3 d after MCAO. TUNEL assay also revealed a significant increase in apoptotic neuronal death in CD200 ICKO mice. An enhancement in lymphocytic infiltration and microglial pro-inflammatory responses were revealed by flow cytometry at 3 and 7 days after stroke in ICKO mice, accompanied by an increased microglial phagocytosis activity. Plasma pro-inflammatory cytokine (TNFα and IL-1β) levels significantly increased at 3d, and IL-1β/IL-6 levels increased at 7d in ICKO vs. control animals. ICKO led to significantly lower baseline level of CD200 both in brain and plasma.
Conclusion
Neuronal CD200 inhibits pro-inflammatory responses and is protective against stroke injury.
Keywords: CD200, Neuroprotection, ischemic stroke, Neuroinflammation
Introduction
The inflammatory response to ischemic stroke is a fundamental pathophysiological process that significantly contributes to ischemic brain injury.1, 2 Under normal conditions, neurons cross talk with both resident and circulating immune cells through either membrane-bound signaling molecules or soluble factors to maintain cerebral homeostasis.3, 4 After ischemia, vast amounts of neurons die and trigger post-stroke inflammation. The post-stroke immune response causes secondary ischemic damage, and peaks within days of the stroke onset and lasts for months, potentially providing a longer time window for intervention compared to energy failure, oxidative stress5, 6 or calcium excitotoxicity.7, 8 Holding pro-inflammatory responses in check can mitigate stroke injury and has significant translational potential.
CD200 (OX-2) is a highly glycosylated membrane protein mainly expressed on the cell surface of neurons and endothelia in CNS.9 CD200 was shown to exert its immunosuppressive and anti-inflammatory activities through interaction with CD200 receptor (CD200R), which belongs to the immunoglobulin superfamily and is expressed by myeloid cells and lymphocytes.10–12 The CD200-CD200R signaling maintains immune cells in a quiescent state, and disruption of this interaction leads to pro-inflammatory activation of myeloid cells/lymphocytes and consequently pathological outcomes.10 The contribution of neuronal CD200 signaling to stroke recovery has not been well studied. In the present study, we hypothesized that the neuronal CD200 signaling is protective in acute stroke and investigated the role of neuronal CD200 by using an inducible conditional knock out mouse model. We demonstrate that the neuronal CD200 signaling functions as a critical regulator of the immune response to ischemic brain injury.
Methods
The data that support the findings of this study are available from the authors upon reasonable request.
Animals
To generate neuronal CD200 inducible conditional knockout (CD200 ICKO) mice, we crossed CD200 tma1 floxed mice with Thy1-Cre (thy1CreER) mice (Jackson Laboratory) in the C57BL/6 background. All the breeding pairs were housed in pathogen-free rooms and had access to food and water ad libitum. All mice were kept and generated under normal light-dark-cycles. As no sex difference in neuronal CD200 was noted, male (6–8 weeks old) CD200tma1fl/fl/Thy1creER mice received injection (ip) of tamoxifen (TMX) (75mg/Kg BW) to induce Cre/LoxP mediated CD200 conditional gene deletion, or received corn oil (Sigma) injection as vehicle (VEH) controls. The injection was given a single dose every day for five consecutive days. All the mice were subjected to stroke/sham surgery 2 weeks after the last injection. All procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals and were approved by the Institutional Animal care and use committee of the University of Texas Health Science Center Houston.
Ischemic stroke model
Cerebral ischemia was induced by 60-minute reversible middle cerebral artery occlusion (MCAO) under isoflurane anesthesia as previously described.2, 10, 13 Details of the model are in the online-only supplementary material.
Behavior testing
Neurological deficit scores (NDS), corner test, and spontaneous alternation in Y-maze were assessed as previously reported1, 2; for details, see the online-only supplementary material.
Immunohistochemistry, Cresyl Violet (CV), TUNEL staining
Brain sections were stained with mouse anti-NeuN (1:500, R&D Systems, MN, USA), rat anti-CD200 (1:300, abcam, USA), mouse anti-von Willebrand Factor (VWF, sc-365712, Santa Cruz biotechnology Inc, TX, USA), and rabbit anti-glial fibrillary acidic protein (GFAP) (1:500, Cell Signaling Technology # 80788). Brain images were obtained on Leica DMi8 confocal microscope. At day 3, infarct was evaluated by performing CV staining as described previously.14, 15 TUNEL assay was performed according to manufacture instruction (In Situ Cell Death Detection Kit, Fluorescein, Millipore Sigma). For details, see the online-only supplementary material.
Flow cytometry (FC) and Phagocytosis assay
Leukocytes from the ipsilateral brain tissue were prepared as previously described.16–18 Isolated leukocytes were stained with antibodies for cell membrane and intracellular markers. Microglial phagocytosis was evaluated in isolated microglia with bio-particles. Details of these assays were provided in the online-only supplementary material.
Determination of CD200 and cytokine Levels
The levels of CD200 in mouse brain, plasma and in the supernatants of primary neuronal culture were measured by a sandwich ELISA kit according to manufacture instruction ((# EK1185, Mouse CD200 PicoKine ELISA Kit, Boster Biological, Pleasanton, CA, USA.). Other plasma cytokine levels (IL-1β, TNF-α, IL-6, IL-10, IL-4) were measured by commercially available specific multiplex quantitative Bio-Plex Pro™ Mouse Cytokine 23-plex assay according to manufacturer instructions (# M60009RDPD, Bio-Rad Laboratories, Hercules, CA).
Primary neuronal culture and Oxygen-Glucose Deprivation (OGD)
Culture of primary neurons was performed as described previously.19, 20 Oxygen-glucose deprivation (OGD) was slightly modified from a previously described method.21 After OGD, CD200 levels in cell culture supernatant was evaluated as described in.19 For details please see the online-only supplementary material.
Splenic CD3+ isolation, co-culture with primary neurons, and CD200-CD200R binding assay
CD3+ T cells were isolated from the spleen of a 7-week old naïve male C57BL/6 mouse using the MojoSort™ Mouse CD3 T Cell Isolation Kit according to manufacture instruction (# 480024 BioLegend). The purity of isolated CD3+ cells was confirmed by flow cytometry. The isolated cells were plated at 1 × 106 cell per T-75 flask, and rested for 5–7 days before co-culture with cortical neurons, and followed by ICC. CD200-CD200R binding assay was performed by using recombinant mouse CD200R1 Fc chimera peptide_2554-CD (R&D Systems) and biotinylated recombinant mouse CD200-Fc Chimera (carrier-free)_786004 (BioLegend). For details please see the online-only supplementary material.
Statistical Analysis
Data from individual experiments were presented as mean ± SD and assessed by Student t test or one-/two-way ANOVA with Tukey post-hoc test for multiple comparisons and Hold-Sidak test for paired comparisons (GraphPad Prism Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant. The ordinal data of the NDS was analyzed with Mann-Whitney U test. Investigators were blinded to mouse strains for stroke surgery, behavioral testing, infarct, and inflammation analysis.
Results
Stroke outcomes in CD200 ICKO mice.
We first confirmed CD200 deletion from neurons by performing NeuN and CD200 immunofluorescence staining in brain slices from CD200 ICKO mice. We found that almost none of the NeuN positive cells express CD200 in Tamoxifen treated mice (TMX); however, NeuN and CD200 were colocalized in vehicle treated mice (Sup. Fig. IA). We confirmed that TMX injection had minimal effect on the expression of CD200 level in endothelial or astrocytic cells as we found there was no difference in CD200 immunofluorescence intensity in VWF or GFAP positive cells between TMX and VEH treated mice (Sup. Fig. IB–D), demonstrating that the ICKO only affects neuronal but not endothelial or astrocytic CD200.
Next, we examined brain infarct at 3 days after stroke in both VEH and TMX treated mice. Quantitative data showed the TMX treated (CD200 ICKO) mice had significantly larger infarct in the cortex (p = 0.0172), striatum (p = 0.0161), and ipsilateral hemisphere (p = 0.0191) than the VEH treated (CD200 intact) mice (Fig. 1A). We also performed functional behavior tests after 3d of stroke. The NDS significantly increased in TMX group compared to the VEH control (p = 0.025, Fig. 1A lower left). In the corner test, the proportion of right turns was significantly higher in TMX vs. VEH stroke group (p = 0.0011, Fig. 1A lower right). There was no difference in Y-maze test between TMX and VEH groups (data not shown). We also evaluated the effect of CD200 deletion on neuronal apoptosis near the peri-infarct area. DNA damage is one of the main features of apoptosis and can be visualized with TUNEL staining.22 The nuclear morphology of apoptotic neuronal cells was evaluated with TUNEL counterstained with DAPI. There was no difference in TUNEL positive cells between sham VEH and TMX treated mice; however, in the MCAO groups, TMX mice showed significantly more TUNEL positive cells compared to the VEH control (p = 0.001, Fig. 1B). These data indicated that neuronal CD200 signaling is protective against ischemic injury.
Figure 1.
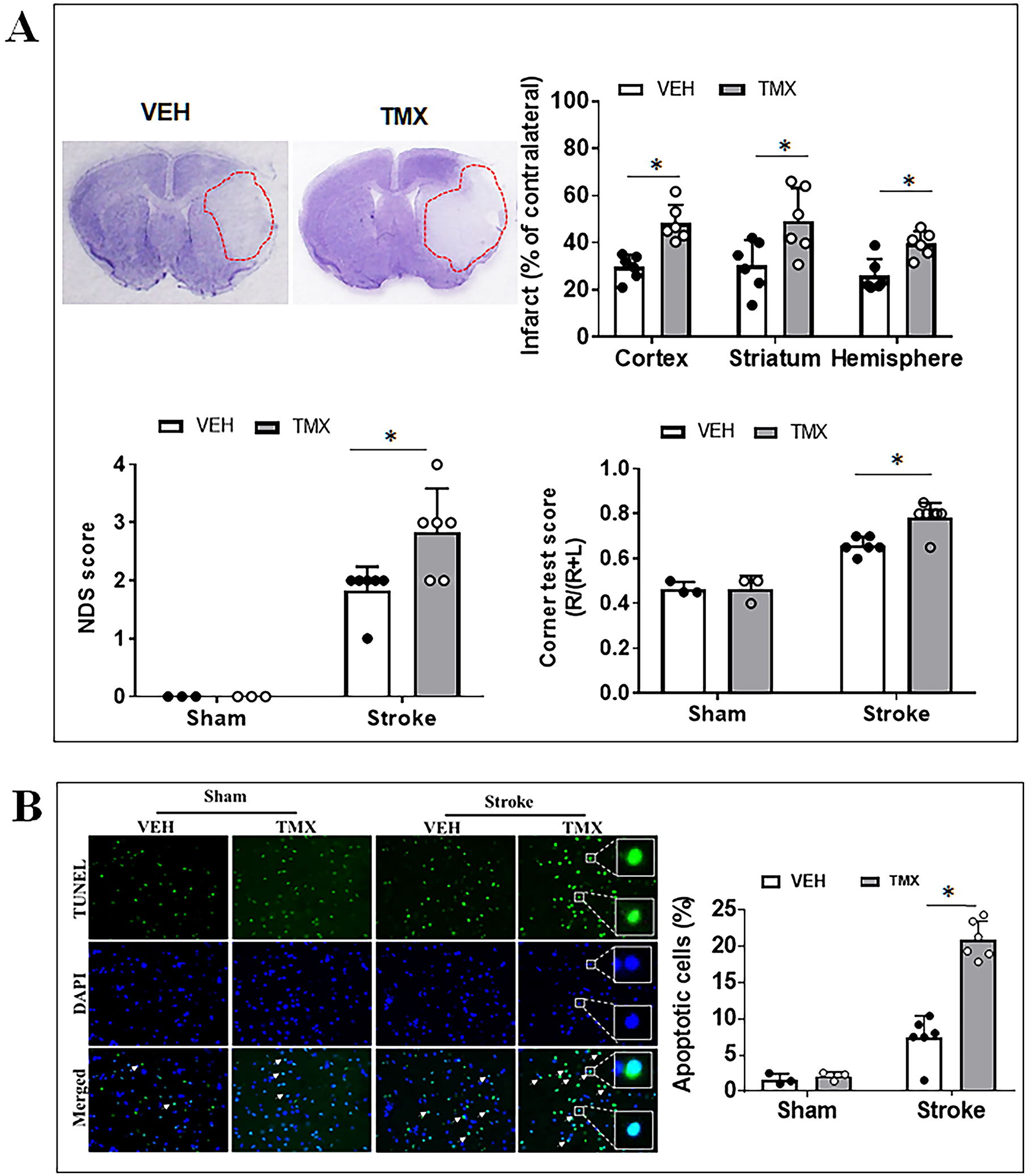
Effects of neuronal CD200 signaling on stroke outcomes and neuronal apoptosis at 3 days after stroke. (A) Representative images of brain slices stained with cresyl violet (CV) and quantification of brain infarct volumes, NDS, and corner test scores in TMX vs. VEH mice. (B) TUNEL labelled cell apoptosis in peri-infarct area of TMX and VEH treated mice and the quantification of percent of TUNEL positive cells in ipsilateral hemispheres Each dot represents the average of % apoptotic cells from eight 20× fields/animal in the peri-infarct area. N=3 for sham and 6–7 for stroke per group; *P < 0.05.
CD200 ICKO mice had more infiltration of leukocytes into the ischemic brain.
To evaluate the effect of neuronal CD200 on peripheral immune cell infiltration in the ischemic brain, we quantified infiltrating leukocytes in sham and stroke mice brains with flow cytometry (FC). Total peripheral myeloid cells were gated as CD45highCD11b+, and lymphocytes as CD45highCD11b− (Fig. 2A). Among the peripheral myeloid cells, monocytes were further gated as CD45highCD11b+Ly6C+Ly6G−, and neutrophils as CD45highCD11b+Ly6G+. Quantitative data showed that there was significantly more lymphocyte infiltration in the TMX vs. VEH treated mice brains at 3 days (p = 0.003, Fig. 2B) and 7 days (p = 0.0182, Fig. 2C) after MCAO. More neutrophil infiltration was also seen in TMX vs. VEH brains at 7 days (Fig. 2C).
Figure 2.
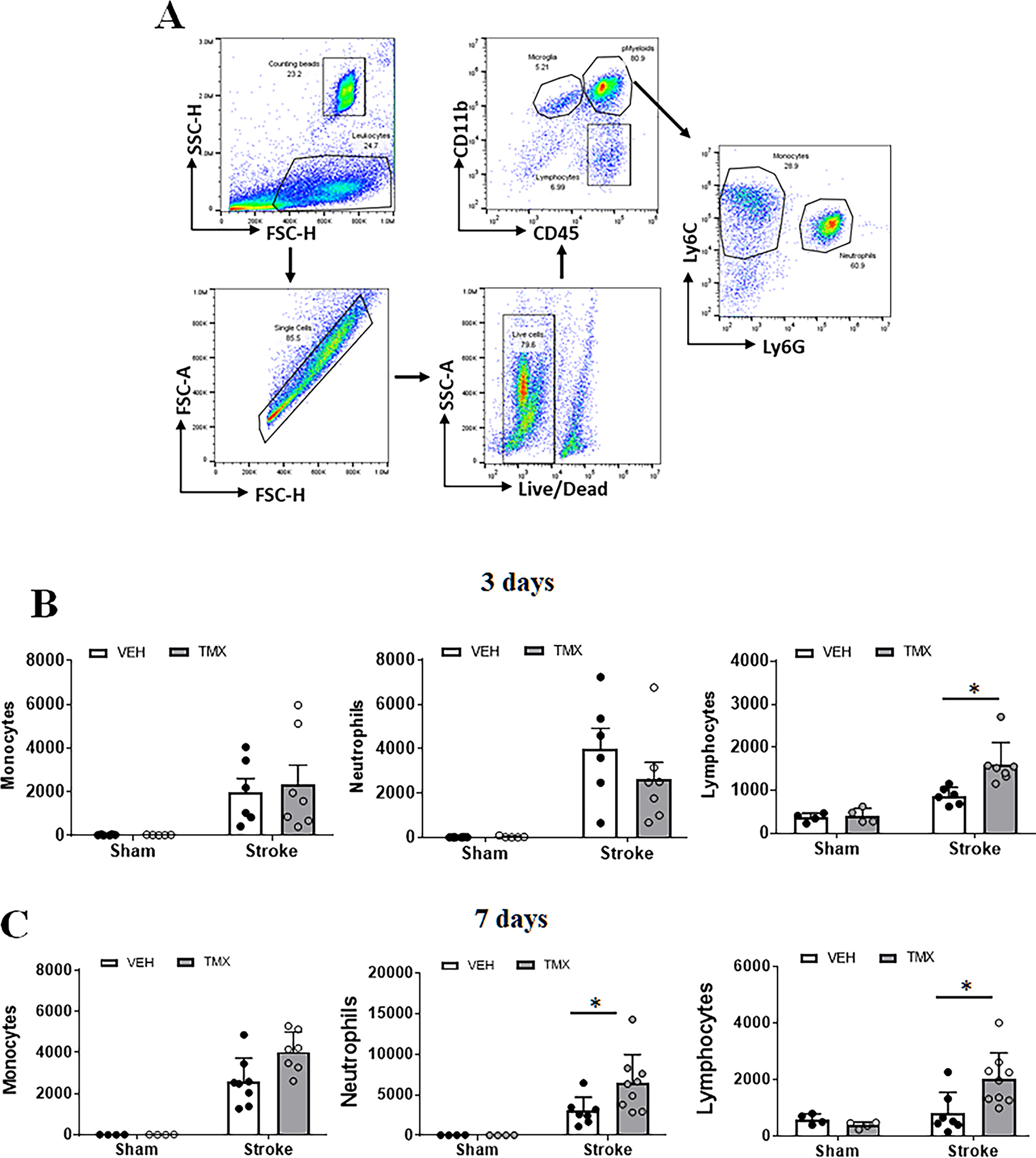
Peripheral immune cell infiltration in ischemic brains at 3 and 7 days after stroke. (A) Representative dot plots depict the gating strategy used to differentiate the microglia (CD45intermediateCD11b+), peripheral myeloid cells (CD45highCD11b+) (pMyeloid), and lymphocytes (CD45highCD11b−). In pMyeloid cells, monocytes are further gated as CD45highCD11b+Ly6C+Ly6G−, and neutrophils as CD45highCD11b+Ly6G+ Ly6C−. Absolute counts of infiltrating monocytes, neutrophils, and lymphocytes at 3d (B) and 7d (C) after stroke were shown. N=4–5 sham and 6–8 stroke animals per group; *P < 0.05.
Microglia are more activated in ischemic brains of TMX vs. VEH treated mice.
We next examined microglial activation after stroke with FC, as microglial activation is a key element in initiating and perpetuating inflammatory responses to ischemia. CD68 is a well-established marker of phagocytic pro-inflammatory activation.22, 23 We gated microglia as CD45intermediateCD11b+, and quantified the median fluorescence intensity (MFI) of CD68 and CD206 in microglia. Gating strategy of microglia is in Fig. 2A. Our data revealed that the MFI of CD68 significantly increased in TMX vs. VEH treated microglia 3 (p = 0.023, Fig.3A) and 7 days (p = 0.003, Fig.3B) after stroke. In contrast, there was no difference in CD206 (an anti-inflammatory marker)1, 2 MFI between the two groups (Fig.3 A, B). Ex vivo assays was performed on freshly isolated microglia by FC from the ischemic brains at 7 days after MCAO to evaluate microglial phagocytosis activity. The gating strategy of the isolated microglia can be found in Sup. Fig. II. Our data demonstrated a significant increase in the phagocytic activity of microglia (MFI of phagocytic beads) in TMX treated mice compared to VEH controls (p < 0.0001, Fig. 3C). These data suggest that CD200 signaling dampens microglial pro-inflammatory response to ischemic stroke.
Figure 3.
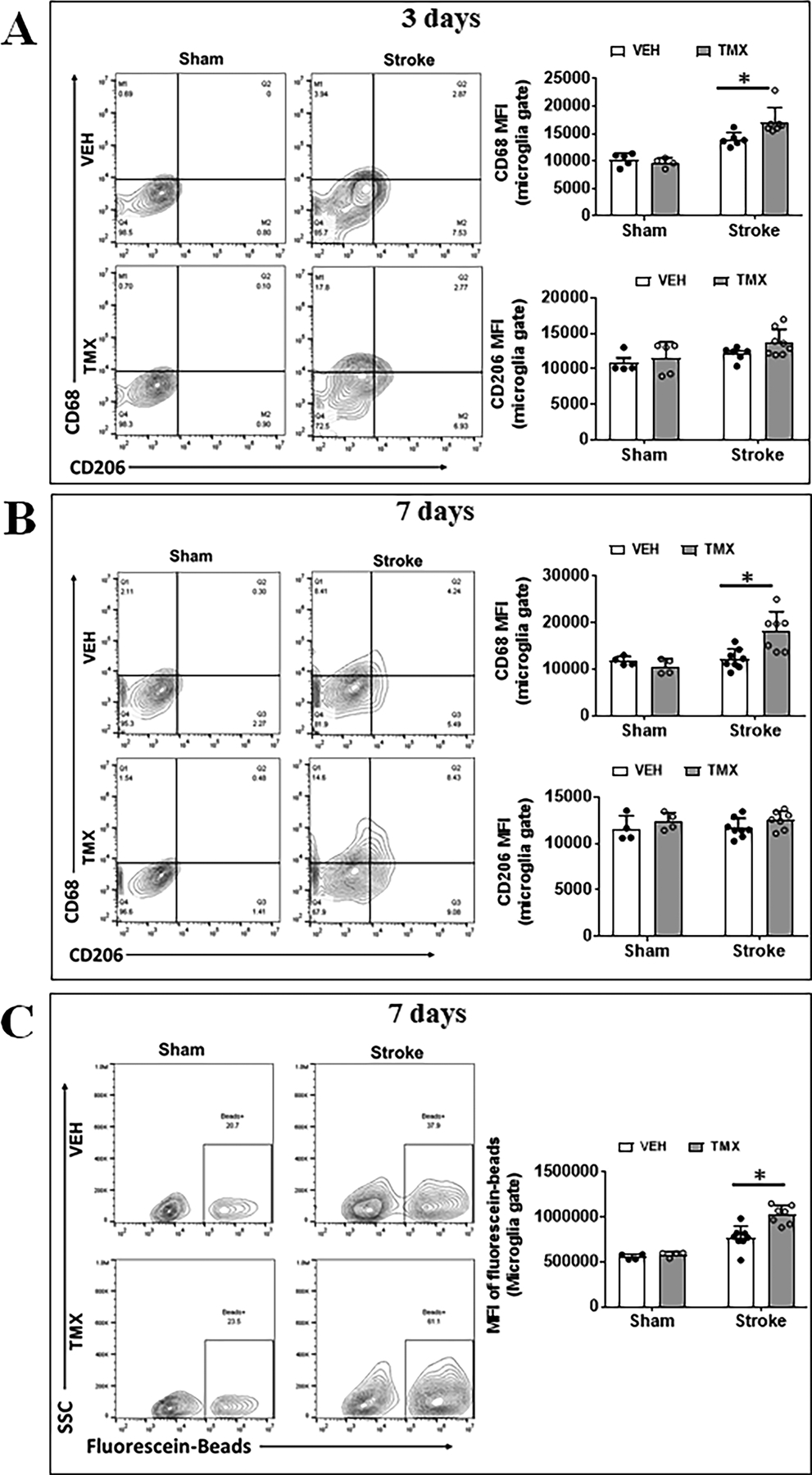
Microglial activation after stroke in TMX and VEH treated mice. Representative FC plots of CD68/CD206 expressed on microglia at 3d (A) and 7d (B) after MCAO. Quantitative expression of CD68 and CD206 was measured as mean fluorescence intensity (MFI). (C) Representative plots illustrate the phagocytic activity of microglia at 7d after MCAO, which was quantified by MFI of bio-particles in microglia. N=6–8 stroke/4–5 sham animals per group; *P < 0.05.
Serum cytokine levels after stroke in CD200 ICKO vs. CD200 intact mice.
Serum cytokine levels are reflective of the degree of immune responses to ischemic injury.1, 24 To investigate how CD200 deletion affects serum cytokine levels, we measured pro-inflammatory (IL-1β, TNF-α, and IL-6) and anti-inflammatory (IL-10, and IL-4) cytokine levels in the serum of sham and stroke mice with MultiPlex at 3 and 7 days after MCAO. As expected, in both the TMX and VEH cohorts, levels of all cytokines significantly increased after stroke. When the comparison was made between TMX vs. VEH groups, we observed no difference in the levels of these cytokines (Fig. 4A&B) between the two sham groups, both of which were equivalently low. However, in the 3d MCAO group (Fig. 4A), TNFα (p=0.0001) and IL-1β (p= 0.0002) levels were significantly higher in TMX vs. VEH mice; IL-6 level showed an increase trend in TMX vs. VEH stroke mice, and there was no difference in IL-10 and IL-4 cytokine levels between the two groups. Similar results were seen in the 7d MCAO group (Fig. 4B): IL-1β and IL-6 levels were significantly increased in TMX vs. VEH stroke mice. However, we observed an increased IL-4 level in VEH vs. TMX mice at 7d, suggesting the ICKO suppressed the anti-inflammatory cytokine which begins to increase at the sub-acute phase of stroke. The data further suggested that the CD200 signaling mainly impacts the pro-inflammatory responses in the acute stroke.
Figure 4.
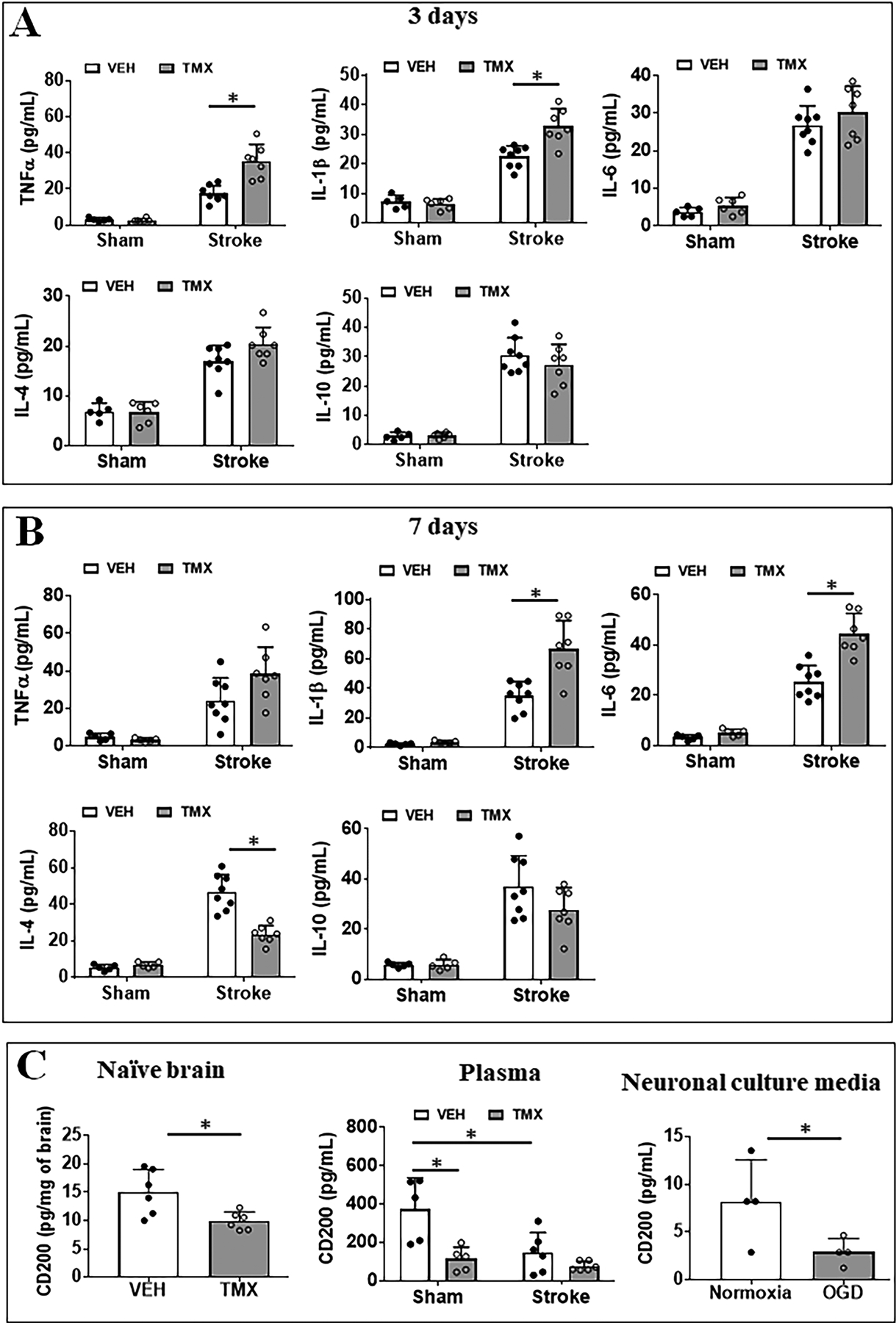
Plasma cytokine levels after ischemic injury. Pro-inflammatory (TNFα, IL-1β and IL-6) and anti-inflammatory (IL-10 and IL-4) cytokine levels (pg/mL) were measured in the plasma of VEH and TMX treated sham/stroke mice at 3d (A) and 7d (B). N=5–6 sham and 6–7 stroke animals per group; *P < 0.05. (C) CD200 levels in naïve brains (left), in the plasma (middle) of TMX and VEH treated mice, and in neuronal culture medium after OGD (right). N=5–6 sham and 6–8 stroke animals per group; *P < 0.05. For neuronal culture medium, N=4 independent OGD experiments; *P < 0.05.
Since neurons are the primary cells that express CD200 in the CNS, we wondered if neurons can secret CD200 into the blood circulation to bind to CD200R expressed on circulating leukocytes. We first examined the base line level of CD200 in both TMX and VEH mice brains. Our data demonstrate a significant decrease in CD200 baseline level in the TMX treated naïve brains compared to their VEH treated counterparts (p = 0.027, Fig. 4C). Next, we examined the plasma level of CD200 in these mice 3d after MCAO with ELISA. Interestingly, the quantitative analysis showed the plasma level of CD200 protein was significantly decreased in TMX injected compared to VEH sham mice, indicating that deletion of neuronal CD200 alters circulating CD200 levels. We also observed a significant decrease in serum CD200 after stroke in Vehicle treated mice. This suggests that neurons are a major source of circulating CD200 as the stroke injury causes death of vast amount of neurons, leading to a significant loss of CD200. The level of CD200 did not significantly change in TMX mice after stroke, probably due to a floor effect of CD200 ICKO from neurons (Fig. 4C). To confirm this finding, we performed primary neuronal culture, used OGD as an in vitro ischemia model, and examined CD200 levels in the culture medium. Consistent with our in vivo results, we also observed a significant decrease of soluble CD200 levels in the culture supernatant after OGD (Fig. 4C).
Administration of CD200Fc during the acute phase of stroke improves outcomes.
To confirm the protective effect of CD200 during the acute phase of stroke, CD200Fc or mouse IgG2a (control) was administered in C57BL/6 mice before and after stroke (the method was provided in the online-only supplementary material). Stroke outcomes were evaluated at 3d of MCAO, and we found that CD200Fc-treated mice had a smaller infarct in the ipsilateral cortex (p = 0.026) and hemisphere (p = 0.001) than the IgG treated mice (Fig. 5A). Consistent with the infarct data, CD200Fc-treated mice showed improved NDS compared to control mice (p = 0.0214, Fig. 5A), although there was no significant difference in corner test scores between the two groups. We also performed flow cytometry in CD200 Fc treated mice to examine microglial expression of cell membrane and intracellular markers. As shown in Fig. 5B, the anti-inflammatory marker CD206 was significantly higher expressed in ischemic microglia in CD200 Fc treated vs. control mice. However, CD200 Fc ischemic microglia expressed significantly less intracellular pro-inflammatory markers (IL-1β, TNFα) compared to controls after stroke (Fig. 5C). The intracellular anti-inflammatory markers (IL-10, IL-4) did not show differences between the two groups (Sup. Fig. III). These data confirmed that the CD200 signaling is protective against ischemic injury by quenching the inflammatory response.
Figure 5.
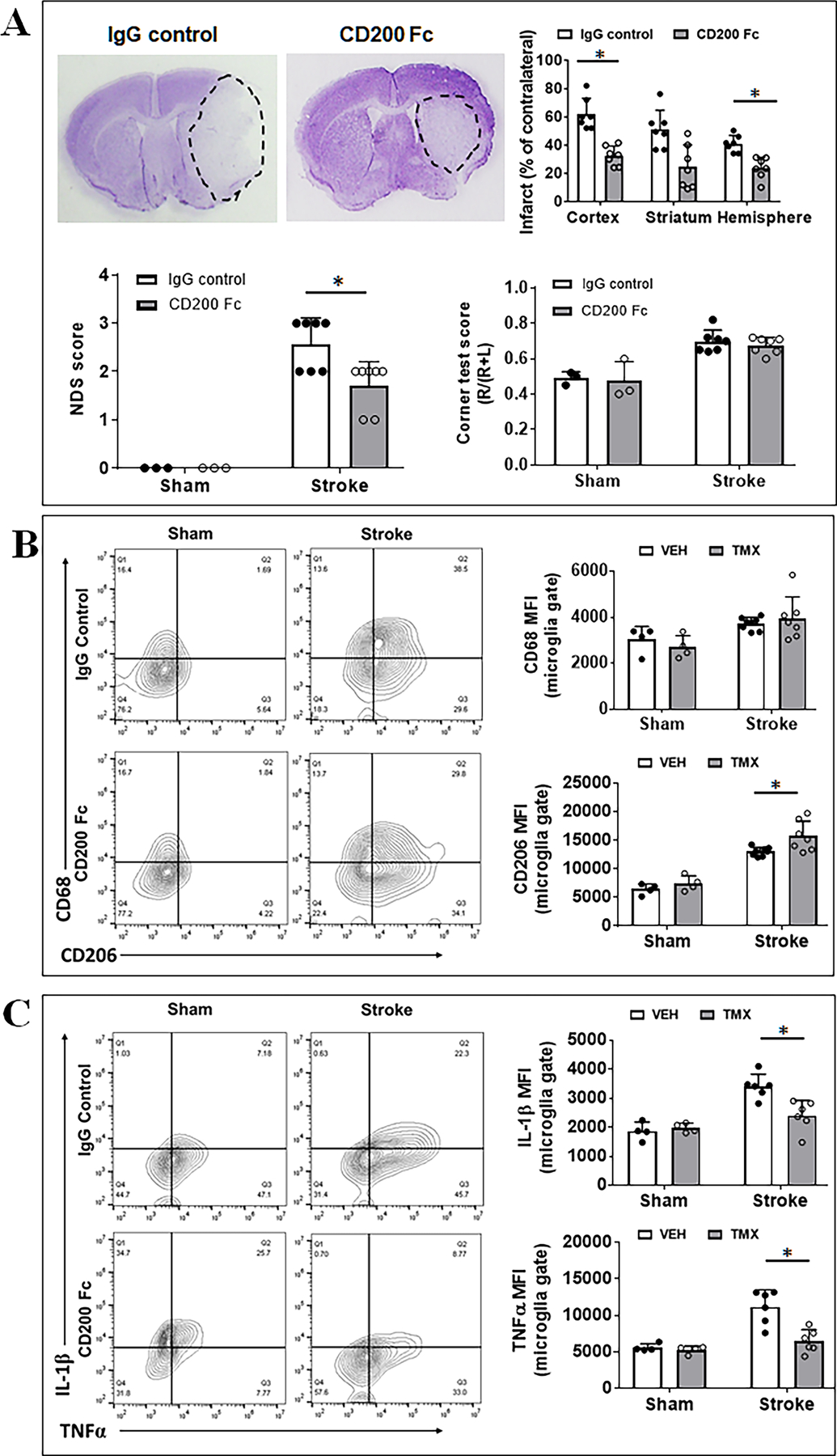
Stroke outcomes and flow cytometry in CD200 Fc treated mice. (A) Representative images of brain slices stained with cresyl violet (CV) and quantification of brain infarct volumes, NDS, and corner test scores in CD200Fc vs. IgG treated mice. N=3 for sham and 6–7 for stroke per group; *P < 0.05. (B) Microglial expression of cell membrane markers CD68/CD206 measured as mean fluorescence intensity (MFI). (C) Microglial expression of intracellular markers IL-1β/TNFα. For (B) and (C), n=4/sham group and n=6/stroke group; *P < 0.05.
Neuronal CD200 interaction with lymphocytic CD200R
Neuronal CD200 functions through interactions with CD200R expressed on leukocytes. To confirm the interaction, we performed neuron and T cell co-culture to examine the actual ligand-receptor binding with confocal microscoping. Fig. 6A shows the purity of T cells (CD45+CD3+) separated from a C57BL/6 mouse spleen by flow cytometry. These T cells express CD200R1(the primary receptor that binds to CD200 in CD200R family)25, 26, and the cultured primary neurons express CD200 (Fig. 6B). After a co-culture of T cell and neurons, ICC was performed. Fig. 6C showed CD3 and MAP2 (a neuronal marker) were colocalized (upper panel), and CD200R1 from T cells bound to CD200 on neurons (lower panel). To further confirm the interaction, we additionally performed ligand-receptor binding assay, and found CD200 binds to CD200R in a concentration dependent manner (Fig. 6D).
Figure 6.
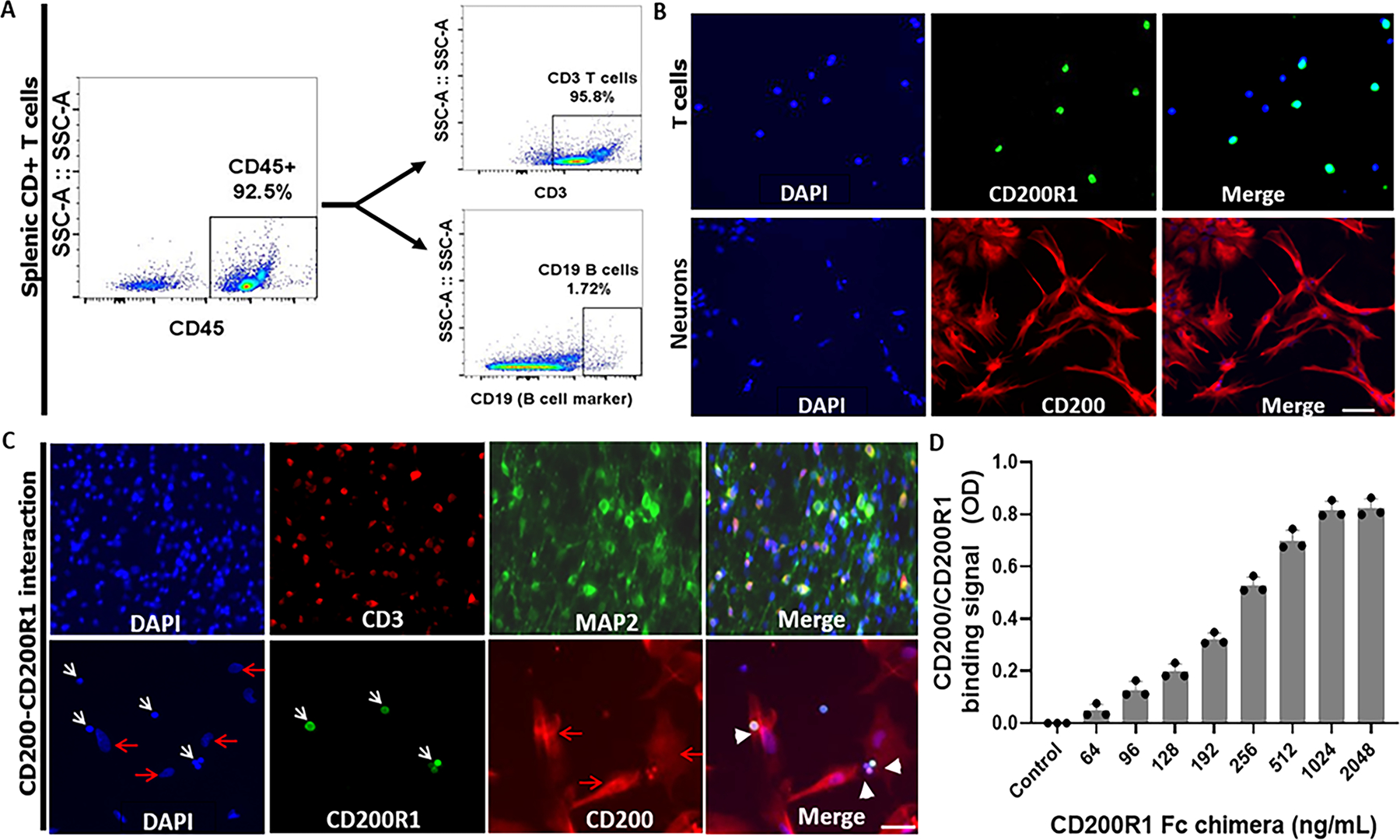
Interaction of neuronal CD200 with lymphocytic CD200R1. (A) The purity of CD45+CD3+ T cells from spleens of C57BL/6 mice were confirmed by flow cytometry. (B) Representative ICC images showed splenic CD3+ T cells express CD200R1 (upper panels; green) and cultured primary neurons express CD200 (lower panels; red). (C) Neurons interact with CD3+ T cells. Upper panels, colocalization of CD3 (marker for T cells) and MAP2 (marker for neurons). Lower panels, colocalization of CD200 ligand on neurons and CD200R1 on T cells. White arrows indicate T cells; red arrows indicate neurons; white arrow heads indicate the interaction of CD200 with CD200R. Scale bar=100 μm. (D) ELISA binding assay of mouse biotinylated CD200Fc chimera protein with mouse CD200R1 chimera protein (captured by anti-CD200R1 antibody) immobilized on a Nunc™ MaxiSorp™ ELISA plate and in a concentration dependent manner (n = 4 assays per group). Control ELISA wells were without CD200R1 Fc chimera protein.
Discussion
The present study demonstrated several key findings which support our hypothesis that neuronal CD200 signaling is essential to limit post-stroke inflammation and ischemic injury. Firstly, neuronal CD200 signaling mainly targets the pro-inflammatory response to acute stroke, evidenced by the data of microglial activation and circulating cytokines (Fig. 3&4). Secondly, neuronal CD200 is protective against ischemic injury and promotes functional recovery after stroke. This is consistent with previous studies showing CD200 signaling is beneficial in neurodegenerative diseases.27, 28 Thirdly, neuronal CD200 preferably targets the circulating lymphocytes and inhibits their infiltration in the ischemic brain. This is consistent with our previous report that lymphocytes robustly express the ligand receptor CD200R.10 Lastly, neurons secrete soluble CD200 into the blood circulation and are the primary source of circulating CD200. Of note, these findings were attributed to the neuronal CD200 signaling, as our ICKO model based on Thy1-CreER recombination only affects the CD200 expression in neurons but not endothelia or astrocytes (Sup.Fig.I), although it was reported these cells are also Thy1 positive. To our knowledge, the present study is the first report that has mechanistically investigated the involvement of neuronal CD200 signaling in post-stroke inflammation.
The CD200-CD200R signaling axis has been described as an “inflammation brake” in experimental allergic encephalomyelitis (EAE) and other neurodegenerative diseases.28–32 In stroke, the ischemic injury not only causes neuronal death but also induces inflammatory responses characterized by the activation of immune cells and release of cytokines/chemokines.33 Post-stroke inflammation plays a fundamental role in the progress of ischemic infarction and causes secondary neuronal damage.2, 34 In the CNS CD200 was primarily expressed by neurons,35 and the loss of neurons in the ischemic brain may lift the “brake” of CD200-CD200R inhibitory signaling and enable a more robust inflammatory response. It is controversial as to which immune cell population the CD200 binds to as a brake. Studies with other CNS disease models have suggested neuronal CD200 interacts with CD200R expressed on microglia to dampen inflammatory responses.36, 37 However, our study10 and others have found adult microglia express near null levels of CD200R.35, 38 The present study did show an enhanced inflammatory response in microglia demonstrated by an increased level of CD68 and phagocytic activity in CD200 ICKO vs. VEH stroke mice (Fig. 3). We hypothesize that the enhanced pro-inflammatory phenotype of microglia is secondary to the loss of CD200-CD200R brake between neurons and other immune cells. Our previous study used a different transgenic animal model, i.e. CD200R global knockout mice, and found the similar neurobehavior deficits and inflammatory responses after stroke as in the present study, indicating the protective role of CD200-CD200R signaling. This is consistent with a previous study39 which showed injection of exogenous recombinant CD200 protein into the cerebral ventricle reduced the expressions of Iba-1, IL-1β, and TNF-α at 48 h after permanent MCAO. Interestingly, the authors also reported a negative correlation between brain CD200 levels and neuronal death.
Our previous study demonstrated that lymphocytes were the predominant cell type expressing CD200R in the ischemic brain, with moderate expression in infiltrating monocytes.10 The present study found the deletion of CD200 signal from neurons exacerbates lymphocytic cell infiltration, but has no effect on the infiltration of monocytes (Fig. 2), suggesting the neuronal CD200 signaling has cell specific affinity and mainly binds to CD200R on lymphocytes. Although the mobilization of lymphocytes has long been considered a chronic inflammatory response,40, 41 the infiltration of lymphocytes in the ischemic brain has been extensively reported at the acute stage of stroke.42, 43 Lymphocytes can be divided into many subtypes, including CD4+/CD8+ T cells, B cells, natural killer cells, etc.44, 45 Our latest study has found the intracellular levels of TNFα and IFNγ in CD8+ T cells (but not in CD4+ cells or microglia) were increased after the disruption of CD200-CD200R signaling, and these changes mirrored the levels of these cytokines in whole brain. This is consistent with the findings in the present study that showed robust infiltration of lymphocytes and increased levels of circulating pro-inflammatory cytokines (TNFα, IL-1β, IL-6) (Fig. 5) in CD200 ICKO vs. control mice. Our in vitro data further confirmed the interaction of neuronal CD200 ligand with lymphocytic CD200R (Fig.6). Of note, the CD200-CD200R signaling functions on both central (brain) and peripheral inflammatory responses. Our previous study has found that CD200R global knockout mice developed more severe spontaneous bacterial infections of the lung after stroke compared to WT mice.10 Further studies are underway to investigate how CD200-CD200R signaling affects T cell activation in both the central (brain) and peripheral immune response to stroke.
To date, the relationship of neuronal CD200 signaling with peripheral CD200R expressing leukocytes has not been studied, as most studies have focused on neuron-microglial interactions. The neuroimmune cross-talk between neurons and peripheral leukocytes happens via two avenues: soluble factors4 and direct contact with infiltrating leukocytes. The involvement of neuronal soluble factors in immune cell activation has been increasingly reported; these include anti-inflammatory cytokines,46 chemokines and neurotrophins,47 neuropeptides,48 and neurotransmitters.49 It was found that soluble CD200 (sCD200) plays a critical role in the pathophysiology of many diseases.50–53 Consistently in the present study, we administered soluble CD200 Fc to WT mice and also induced protective effects (Fig. 5). Furthermore, our data showed that neurons are the primary source of circulating sCD200 as the neuronal CD200 ICKO mice had significantly lower levels of sCD200 compared to the CD200 intact mice; the ischemic insult significantly decreased sCD200 as seen in both in vitro and in vivo assays (Fig. 4C). The sCD200 secreted from neurons may bind to CD200R expressed on lymphocytes to quench lymphocytic activation and prevent them from infiltrating into the ischemic brain. Neuronal CD200 may also directly bind to adjacent infiltrating lymphocytes from the disrupted BBB after stroke and lower the lymphocytic activation level. The interaction between neurons and lymphocytes through CD200-CD200R signaling warrants further investigation.
Thy-1 is a widely accepted neuronal biomarker,54–56 and Thy1-CreER mice has been extensively used to generate inducible conditional knockout of neuronal genes.57, 58 However, it has also been reported that Thy1 was expressed not only by neurons, but also by endothelia, thymocytes, peripheral T cells, myoblasts, epidermal cells, and keratinocytes.56, 59, 60 By using the Thy1-CreER mice in the present study, we found the TMX induction affects the neuronal CD200 expression but not the endothelial or astrocytic levels; nevertheless, we cannot rule out the effect of CD200 deletion in other Thy1 positive cells. Ongoing studies in this lab are using an additional neuronal conditional knockout animal model, enolase-2 cre mouse, to study the impact of neuronal CD200 signaling on post-stroke inflammation. Another caveat of this study is that we only examined the immune response and stroke outcomes at the acute stages of stroke (3d and 7d) in young mice. It is important to also evaluate the effect of the neuronal CD200 signaling at the chronic phase of stroke and in older animals, which is underway in the lab.
In conclusion, our data demonstrated that neuronal CD200 signaling is critical for the control of post-stroke inflammation and limits stroke injury. The effect is unlikely achieved through a direct interaction between neurons and microglia as microglia express near null CD200R,10 but rather through the dampening of lymphocytic activation after stroke. The role of CD200 signaling is unique in post-stroke inflammation, as it primarily inhibits pro-inflammatory responses but not the anti-inflammatory response. Our observations provide new insights into the effect of the CD200 signaling on neuroinflammation not only in stroke, but also in other CNS disorders.
Supplementary Material
Acknowledgements
Drs Mamun, McCullough, Liu contributed to the study conception and design. Material preparation, experiments, data collection and analysis were performed by Dr Mamun, Dr Ngwa, Dr Qi, Pedram Honarpisheh, Saumil Datar, Romana Sharmeen, Yan Xu. Dr. Mamun drafted the first version of the manuscript, and all authors commented on previous versions and Dr. Liu edited/finalized the manuscript. All authors read and approved the final version of the manuscript.
Sources of Funding
This work was supported by funding from the National Institutes of Health: R01NS093042/R01NS108779 (Fudong Liu) and R37 NS096493 (Louise McCullough).
Nonstandard Abbreviations and Acronyms
- CNS
central nervous system
- CV
cresyl violet
- FC
flow cytometry
- ICKO
inducible conditional knock out
- MCAO
middle cerebral artery occlusion
- MFI
mean fluorescence intensity
- NDS
neurological deficit score
- OGD
oxygen-glucose deprivation
- TMX
tamoxifen
- VEH
vehicle
Footnotes
Disclosures
None of the authors have a conflict of interest relevant to this work.
References
- 1.Al Mamun A, Chauhan A, Yu H, Xu Y, Sharmeen R, Liu F. Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Eur J Neurosci. 2018;47:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R, Hazen AL, Li J, Aronowski JA, McCullough LD, et al. Microglial irf5-irf4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A. 2020;117:1742–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional microglia-neuron communication in health and disease. Frontiers in cellular neuroscience. 2018;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. Journal of neuroinflammation. 2012;9:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirley R, Ord EN, Work LM. Oxidative stress and the use of antioxidants in stroke. Antioxidants (Basel). 2014;3:472–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, Yong Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188 [DOI] [PubMed] [Google Scholar]
- 8.Wu QJ, Tymianski M. Targeting nmda receptors in stroke: New hope in neuroprotection. Molecular brain. 2018;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Shen Y, Tang Y, Gu Y. The role of n-glycosylation of cd200-cd200r1 interaction in classical microglial activation. J Inflamm (Lond). 2018;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritzel RM, Al Mamun A, Crapser J, Verma R, Patel AR, Knight BE, Harris N, Mancini N, Roy-O’Reilly M, Ganesh BP, et al. Cd200-cd200r1 inhibitory signaling prevents spontaneous bacterial infection and promotes resolution of neuroinflammation and recovery after stroke. Journal of neuroinflammation. 2019;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rijkers ES, de Ruiter T, Baridi A, Veninga H, Hoek RM, Meyaard L. The inhibitory cd200r is differentially expressed on human and mouse t and b lymphocytes. Molecular immunology. 2008;45:1126–1135 [DOI] [PubMed] [Google Scholar]
- 12.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the cd200 receptor. Journal of immunology (Baltimore, Md. : 1950). 2006;176:191–199 [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Schafer DP, McCullough LD. Ttc, fluoro-jade b and neun staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. Journal of neuroendocrinology. 2012;24:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: Sex chromosome complement vs. Gonadal hormones. Aging. 2016;8:1432–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Mamun A, Yu H, Romana S, Liu F. Inflammatory responses are sex specific in chronic hypoxic-ischemic encephalopathy. Cell Transplant. 2018;27:1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauhan A, Al Mamun A, Spiegel G, Harris N, Zhu L, McCullough LD. Splenectomy protects aged mice from injury after experimental stroke. Neurobiology of aging. 2018;61:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Mamun A, Yu H, Mirza MA, Romana S, McCullough LD, Liu F. Myeloid cell irf4 signaling protects neonatal brains from hypoxic ischemic encephalopathy. Neurochem Int. 2019;127:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano G, Costa LG. Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. Methods in molecular biology (Clifton, N.J.). 2011;758:13–27 [DOI] [PubMed] [Google Scholar]
- 20.Das G, Gupta V, Khan J, Mukherjee D, Ghosh S. Generation of neurospheres from mixed primary hippocampal and cortical neurons isolated from e14-e16 sprague dawley rat embryo. Journal of visualized experiments : JoVE. 2019 [DOI] [PubMed] [Google Scholar]
- 21.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of amp-activated protein kinase provides neuroprotection in stroke. The Journal of biological chemistry. 2005;280:20493–20502 [DOI] [PubMed] [Google Scholar]
- 22.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: An immunohistochemical approach for identifying m1 and m2 macrophages. PloS one. 2013;8:e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber M, Moebius P, Büttner-Herold M, Amann K, Preidl R, Neukam FW, Wehrhan F. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas--an immunohistochemical study. British journal of cancer. 2015;113:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ormstad H, Aass HC, Lund-Sørensen N, Amthor KF, Sandvik L. Serum levels of cytokines and c-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. Journal of neurology. 2011;258:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatherley D, Cherwinski HM, Moshref M, Barclay AN. Recombinant cd200 protein does not bind activating proteins closely related to cd200 receptor. Journal of immunology (Baltimore, Md. : 1950). 2005;175:2469–2474 [DOI] [PubMed] [Google Scholar]
- 26.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, et al. Characterization of the cd200 receptor family in mice and humans and their interactions with cd200. Journal of immunology (Baltimore, Md. : 1950). 2003;171:3034–3046 [DOI] [PubMed] [Google Scholar]
- 27.Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of cd200 protects wlds mice from inflammation-mediated neurodegeneration. The American journal of pathology. 2007;170:1695–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XJ, Ye M, Zhang YH, Chen SD. Cd200-cd200r regulation of microglia activation in the pathogenesis of parkinson’s disease. J Neuroimmune Pharmacol. 2007;2:259–264 [DOI] [PubMed] [Google Scholar]
- 29.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, et al. Down-regulation of the macrophage lineage through interaction with ox2 (cd200). Science. 2000;290:1768–1771 [DOI] [PubMed] [Google Scholar]
- 30.Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of cd200 and cd200 receptor in alzheimer’s disease: A potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. Cd200-cd200r dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of parkinson’s disease. Journal of neuroinflammation. 2011;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernangomez M, Mestre L, Correa FG, Loria F, Mecha M, Inigo PM, Docagne F, Williams RO, Borrell J, Guaza C. Cd200-cd200r1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia. 2012;60:1437–1450 [DOI] [PubMed] [Google Scholar]
- 33.Vila N, Castillo J, Dávalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–675 [DOI] [PubMed] [Google Scholar]
- 34.Dihne M, Grommes C, Lutzenburg M, Witte OW, Block F. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke. 2002;33:3006–3011 [DOI] [PubMed] [Google Scholar]
- 35.Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules cd200 and cd200r in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol. 2009;68:159–167 [DOI] [PubMed] [Google Scholar]
- 36.Minas K, Liversidge J. Is the cd200/cd200 receptor interaction more than just a myeloid cell inhibitory signal? Crit Rev Immunol. 2006;26:213–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dentesano G, Serratosa J, Tusell JM, Ramon P, Valente T, Saura J, Carme S. Cd200r1 and cd200 expression are regulated by ppar-gamma in activated glial cells. Glia. 2014;62:982–998 [DOI] [PubMed] [Google Scholar]
- 38.Walker DG, Lue LF. Understanding the neurobiology of cd200 and the cd200 receptor: A therapeutic target for controlling inflammation in human brains? Future Neurol. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Zhang XJ, Zhang C, Chen R, Li L, He J, Xie Y, Chen Y. Loss of neuronal cd200 contributed to microglial activation after acute cerebral ischemia in mice. Neuroscience letters. 2018;678:48–54 [DOI] [PubMed] [Google Scholar]
- 40.Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory t cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. Journal of cerebral blood flow and metabolism : Journal of Cerebral Blood Flow and Metabolism. 2013;33:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvaraj UM, Stowe AM. Long-term t cell responses in the brain after an ischemic stroke. Discovery medicine. 2017;24:323–333 [PMC free article] [PubMed] [Google Scholar]
- 42.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857 [DOI] [PubMed] [Google Scholar]
- 43.Gill D, Veltkamp R. Dynamics of t cell responses after stroke. Current opinion in pharmacology. 2016;26:26–32 [DOI] [PubMed] [Google Scholar]
- 44.Chaplin DD. Overview of the immune response. The Journal of allergy and clinical immunology. 2010;125:S3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of t lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahl SM, Wen J, Moutsopoulos N. TGF-beta: A mobile purveyor of immune privilege. Immunological reviews. 2006;213:213–227. [DOI] [PubMed] [Google Scholar]
- 47.Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in cns diseases. Neuroscience. 2009;158:1122–1132 [DOI] [PubMed] [Google Scholar]
- 48.Reinke E, Fabry Z. Breaking or making immunological privilege in the central nervous system: The regulation of immunity by neuropeptides. Immunology letters. 2006;104:102–109 [DOI] [PubMed] [Google Scholar]
- 49.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘on’ and ‘off’ signals control microglia. Trends in neurosciences. 2007;30:596–602 [DOI] [PubMed] [Google Scholar]
- 50.Clark DA, Dmetrichuk JM, Dhesy-Thind S, Crowther MA, Arredondo JL. Soluble cd200 in secretory phase endometriosis endometrial venules may explain endometriosis pathophysiology and provide a novel treatment target. Journal of reproductive immunology. 2018;129:59–67 [DOI] [PubMed] [Google Scholar]
- 51.Lauzon-Joset JF, Marsolais D, Tardif-Pellerin É, Patoine D, Bissonnette EY. Cd200 in asthma. The international journal of biochemistry & cell biology. 2019;112:141–144 [DOI] [PubMed] [Google Scholar]
- 52.Sakthivel P, Breithaupt A, Gereke M, Copland DA, Schulz C, Gruber AD, Dick AD, Schreiber J, Bruder D. Soluble cd200 correlates with interleukin-6 levels in sera of copd patients: Potential implication of the cd200/cd200r axis in the disease course. Lung. 2017;195:59–68 [DOI] [PubMed] [Google Scholar]
- 53.Sari F, Gumuslu S, Cetinkaya R, Sarikaya M, Yalcin AD. High serum soluble cd200 levels in patients with autosomal dominant polycystic kidney disease. Journal of investigative medicine. 2017;65:784–786 [DOI] [PubMed] [Google Scholar]
- 54.Alić I, Kosi N, Kapuralin K, Gorup D, Gajović S, Pochet R, Dinko M. Neural stem cells from mouse strain thy1 yfp-16 are a valuable tool to monitor and evaluate neuronal differentiation and morphology. Neuroscience letters. 2016;634:32–41 [DOI] [PubMed] [Google Scholar]
- 55.Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, Rainnie DG, Ressler KJ. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. The Journal of neuroscience. 2013;33:10396–10404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinkovic P, Godinho L, Misgeld T. Generation and screening of transgenic mice with neuronal labeling controlled by thy1 regulatory elements. Cold Spring Harbor protocols. 2015;2015:875–882 [DOI] [PubMed] [Google Scholar]
- 57.Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G. Single-neuron labeling with inducible cre-mediated knockout in transgenic mice. Nature neuroscience. 2008;11:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herzer S, Hagan C, von Gerichten J, Dieterle V, Munteanu B, Sandhoff R, Hopf C, Nordström V. Deletion of specific sphingolipids in distinct neurons improves spatial memory in a mouse model of alzheimer’s disease. Frontiers in molecular neuroscience. 2018;11:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurisic G, Iolyeva M, Proulx ST, Halin C, Detmar M. Thymus cell antigen 1 (thy1, cd90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Exp Cell Res. 2010;316:2982–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haeryfar SM, Conrad DM, Musgrave B, Hoskin DW. Antibody blockade of thy-1 (cd90) impairs mouse cytotoxic t lymphocyte induction by anti-cd3 monoclonal antibody. Immunol Cell Biol. 2005;83:352–363 [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Bando Y, Vargas-Lowy D, Elyaman W, Khoury SJ, Huang T, Reif K, Chitnis T. Cd200r1 agonist attenuates mechanisms of chronic disease in a murine model of multiple sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2025–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swonger AK, Rech RH. Serotonergic and cholinergic involvement in habituation of activity and spontaneous alternation of rats in a y maze. J Comp Physiol Psychol. 1972;81:509–522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


