Abstract
Objective:
To examine associations of dietary changes from childhood to adolescence with adolescent hepatic fat and whether the PNPLA3 rs738409 risk allele, a strong genetic risk factor for HF, modifies associations.
Study design:
Data were from 358 participants in the Exploring Perinatal Outcomes among CHildren (EPOCH) study, a longitudinal cohort in Colorado. Diet was assessed by food frequency questionnaire in childhood (~10 yrs) and adolescence (~16 yrs) and converted to nutrient densities. HF was assessed in adolescence by magnetic resonance imaging. Linear regression was used to test associations of dietary changes (Δ) from childhood to adolescence with adolescent HF.
Results:
Increases in fiber, vegetable protein and polyunsaturated fat intake from childhood to adolescence were associated with lower adolescent HF, and increases in animal protein were associated with higher HF [β(95%CI) per 5 unit increase on log-HF: −0.12(−0.21,−0.02) for Δfiber; −0.26(−0.45,−0.07) for Δvegetable protein; −0.18(−0.35,−0.02) for Δpolyunsaturated fat; 0.13(0.04,0.22) for Δanimal protein]. There was evidence of effect modification by PNPLA3 variant, whereby inverse associations of Δfiber and Δvegetable protein and positive associations of Δsaturated fat with adolescent HF were stronger in risk allele carriers. Conclusions were similar after adjusting for obesity in adolescence, but associations of Δsaturated fat with HF were attenuated to the null.
Conclusions:
Our results suggest that nutrient intake changes between childhood and adolescence, particularly decreases in fiber and vegetable protein and increases in saturated fat intake, interact with the PNPLA3 variant to predict higher HF in adolescence, and may be targets for reducing HF in high-risk youth.
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in children1, 2 and is strongly associated with obesity and the metabolic syndrome3, 4. NAFLD disproportionately affects certain groups, such as children of Hispanic ethnicity2, 5, in-part due to a higher frequency of certain genetic risk alleles associated with NAFLD, such as the patatin-like phospholipase domain-containing 3 (PNPLA3) gene6–10. NAFLD can progress to steatohepatitis and further to cirrhosis11, and is the foremost cause of liver transplants among young adults12.
The typical age of diagnosis for pediatric NAFLD is in adolescence2, likely due to a culmination of lifecourse exposures and biological and behavioral changes that occur during this time, interacting with underlying genetic predisposition; though, the exact mechanisms remain unknown. Notably, dietary behaviors often change during the transition from childhood to adolescence, as reported in a 2012 review13, due to greater independence, increased sedentary activities, and time spent away from home14, which may carry health risks. For example, studies have reported increased sugar consumption, especially from sugar sweetened beverages, in adolescents compared with younger children15–17, which in turn is associated with weight gain15.
Understanding whether these dietary changes are associated with NAFLD, especially in those with a genetic predisposition, would help in designing more effective dietary interventions for preventing NAFLD. The objective of this study was to examine whether dietary changes from childhood (~10 yrs) to adolescence (~16 yrs), specifically for macronutrients and fiber, are associated with HF levels in adolescence using data from a longitudinal cohort study in Colorado. We also explored whether associations were modified by the PNPLA3 rs738409 risk allele, a strong genetic risk factor that was previously associated with HF content in this cohort9.
METHODS:
The Exploring Perinatal Outcomes among CHildren (EPOCH) study is a prospective, multiethnic cohort of 604 mother/child pairs based in Colorado. Eligible participants were offspring of singleton pregnancies at a single hospital between 1992 and 2002, whose biological mothers were members of the Kaiser Permanente of Colorado Health Plan at the child’s delivery. Offspring were invited to complete two research visits: first in childhood between 6–12 years old (visit 1, mean: 10.4±1.5 years) and second in adolescence between 12–19 years old (visit 2, mean: 16.7±1.2 years). More details on the study population and methodology have been previously published18, 19. The eligible sample for this study was 399 offspring who underwent magnetic resonance imaging (MRI) at visit 2 to assess HF in adolescence. Among these participants, 16 were excluded for missing dietary data at visit 1 or visit 2 and 25 were excluded for having extreme energy intakes at visit 1 or visit 2 (defined as <800 or > 4000 kcal for boys and <500 kcal or >3500 kcal for girls)20, resulting in an analytical sample of 358 participants. The study was approved by Colorado Multiple Institutional Review Board. Mothers provided written informed consent and children >8 years provided written assent.
Dietary assessment (exposure):
Dietary intake at each visit was assessed using a modified version of the Block Kid’s Food Questionnaire, a semi-quantitative food frequency questionnaire that has been validated in youth as young as 8 years old21, 22. A detailed description of the development of the modified questionnaire has been published elsewhere23. Briefly, the questionnaire used the same format as the Block Questionnaire, but expanded the number of food groups queried (to 85 food lines), particularly focusing on adding foods with regional or cultural importance. The instrument was administered to participants by trained staff, in either a self-administered or structured interview format. Participants were able to ask questions if needed and research staff were able to request clarifications on participant answers and returned forms. Data collected from the questionnaire was analyzed by the Nutrition Data System for Research software (University of Minnesota, Minneapolis, MN, USA) at the University of North Carolina Nutrition Obesity Research Center to quantify intakes of nutrients and food groups.
Nutrient intakes in grams/day were converted to kcal/day and then to nutrient densities as a percentage of total energy intake (TEI). These conversions were done for total carbohydrates, protein, and fat, as well as specific types of macronutrients [starch and total sugar intake, animal and vegetable protein intake, and saturated fat (SFA), monounsaturated fat (MUFA), and polyunsaturated fat (PUFA)]. Dietary fiber intake was converted to grams per 1000 kcal/day. For all intakes, change values were calculated as visit 2 intake minus visit 1 intake. Plausibility of TEI was assessed using the Goldberg method24, 25, which compares individual ratios of reported TEI:basal metabolic rate (BMR) to a physical activity level constant. Specifically, we calculated BMR using Schofield equations and assumed a conservative physical activity constant of 1.55 based on World Health Organization (WHO) recommendations for light activity due to a lack of objective physical activity measures. The cut-off for under-reporting was the lower confidence limit calculated using the equation described by Black24; thus, participants with TEI:BMR<1.10 were categorized as under-reporters.
Hepatic fat assessment (outcome):
As previously described9, 26, offspring HF was measured by MRI only during the second research visit, when participants were adolescents. Briefly, MRIs were performed by trained technicians and research staff at the University of Colorado Anschutz Medical Campus using a breathold, 6-point MRI-proton density fat fraction (PDFF) technique27. HF was calculated from the mean pixel signal intensity data for each flip angle acquisition using the Osirix, Lipoquant plug-in28. For descriptive purposes in Table 1, participants were categorized as having NAFLD at visit 2 in adolescence based on a cut-off of HF>5.5%29. Given the low prevalence of clinical NAFLD in EPOCH at visit 2 (n=22 or 6%), we otherwise analyzed HF as a continuous variable.
Table 1:
Characteristics of the sample of multi-ethnic youth, overall and by clinical nonalcoholic fatty liver disease (NAFLD) diagnosis* at visit 2 (adolescence) (n=358)
| Overall (n=358) | Non-NAFLD (n=336) | NAFLD* (n=22) | |||||
|---|---|---|---|---|---|---|---|
| Variable: | SD or % | SD or % | SD or % | pa | |||
| Male sex, n (%) | 47.2% | 46.7% | 54.6% | 0.48 | |||
| Hispanic ethnicity, n (%) | 36.9% | 34.2% | 77.3% | <0.001 | |||
| Offspring Traits at visit 1 (~10 yrs): | |||||||
| Age (years), mean (SD) | 1.5 | 1.5 | 1.6 | 0.08 | |||
| BMI z-score, mean (SD) | 1.20 | 1.18 | 1.08 | <0.001 | |||
| BMI z-score ≤1.0, n (%) | 70.4% | 72.6% | 36.4% | <0.001 | |||
| BMI z-score >1.0 to ≤2, n (%) | 23.2% | 22.6% | 31.8% | ||||
| BMI z-score ≥2.0, n (%) | 6.4% | 4.8% | 31.8% | ||||
| Physical activityb,c, mean (SD) | 0.31 | 0.31 | 0.27 | 0.27 | |||
| Pubertal Stagec, n (%) | |||||||
| Pre-Pubertal (Tanner=1) | 44.0% | 43.3% | 54.6% | 0.30 | |||
| Pubertal (Tanner=2–4) | 56.0% | 56.7% | 45.5% | ||||
| TEI Under-reporter, n (%) | 22.4% | 20.8% | 45.5% | 0.007 | |||
| Offspring Traits at visit 2 (~16 yrs): | |||||||
| Age (years), mean (SD) | 1.2 | 1.2 | 1.3 | 0.06 | |||
| BMI z-score, mean (SD) | 1.12 | 1.07 | 0.82 | <0.001 | |||
| BMI z-score ≤1.0, n (%) | 68.7% | 72.6% | 9.0% | <0.001 | |||
| BMI z-score >1.0 to ≤2, n (%) | 23.7% | 22.3% | 45.5% | ||||
| BMI z-score ≥2.0, n (%) | 7.5% | 5.1% | 45.5% | ||||
| Physical activityb,c, mean (SD) | 0.40 | 0.40 | 0.43 | 0.54 | |||
| Pubertal Stagec, n (%) | |||||||
| Pubertal (Tanner=2–4) | 45.0% | 45.2% | 40.9% | 0.69 | |||
| Post-Pubertal (Tanner=5) | 55.0% | 54.8% | 59.1% | ||||
| Hepatic Fat (%)d, median (IQR) | 1.2–2.5 | 1.2–2.4 | 6.9–11.1 | <0.001 | |||
| Under-reporter, n (%) | 66.2% | 64.6% | 90.9% | 0.01 | |||
| Maternal Traits, n (%): | |||||||
| Education, Some college or more | 81.6% | 81.9% | 77.3% | 0.59 | |||
| Maternal pre-pregnancy obesityc | 20.8% | 18.6% | 61.5% | <0.001 | |||
| Maternal diabetes in pregnancy | 18.2% | 18.2% | 18.2% | 0.99 | |||
| Maternal smoking in pregnancy | 7.3% | 7.4% | 4.6% | 0.61 | |||
| Risk Allele Frequency e | |||||||
| PNPLA3 rs738409 (G) | 0.28 | 0.27 | 0.47 | 0.02 | |||
P-values calculated by Student’s test for continuous variables or Chi-square test for continuous variables
Measured as average mean expenditure in metabolic equivalents over 3 days based on physical activity questionnaire.
Data was missing for the following traits: n=6 participants missing physical activity at visit 1; n=9 participants missing physical activity at visit 2; n=1 participant missing pubertal stage at visit 1; n=86 participants missing maternal pre-pregnancy BMI/obesity.
Reporting medians and IQRs due to non-normal distribution for hepatic fat. P-value calculated by Mann-Whitney test.
Based on the sub-sample (n=308) who underwent genotyping. To calculate p-values by Chi-square test, we rounded imputed values to the nearest integer and categorized participants based on number of risk alleles (0, 1, or 2).
NAFLD diagnosis at visit 2 was defined as hepatic fat>5.5% measured by magnetic resonance imaging.
Abbreviations: TEI, total energy intake; BMI, body mass index; HFF, hepatic fat fraction
Genetic assessments (effect modifier):
Standard approaches were used for genotyping as described in detail by Stanislawski et al, which investigated the relationship between genetic factors and HF as a continuous measure among participants in the same cohort.9 Briefly, DNA was extracted from stored peripheral venous blood samples using the QIAamp kit (Qiagen, Germantown, MD). DNA quantification and purity assessment were performed using a NanoDrop spectrophotometer and a Qubit fluorometer (Thermo Scientific, Wilmington, DE). For the EPOCH cohort, genotyping occurred in two batches: the first batch was performed using the Illumina Infinium Omni2.5–8 v1.1 BeadChip (for n=226 samples), and the second batch was performed using the Illumina Multi-Ethnic Global Array v1.0 (for n=130 samples). All quality control and filtering steps were performed using PLINK 1.9 (https://www.cog-genomics.org/plink/1.9), which were previously described in detail30. Participants with >5% missing genotypes and SNPs with >2% missing genotypes were excluded, and genetic data were imputed to the 1000 Genomes Phase 3 (v5) multi-ethnic reference panel. We also calculated principal components (PCs) to assess global ancestry, batch effects, and any residual relatedness for SNPs that were directly genotyped and passed quality control thresholds on both platforms. In this prior study of EPOCH, we evaluated associations between a set of five SNPs (PNPLA3 rs738409, GCKR rs1260326, PPP1R3B rs4240624, LYPLAL1 rs12137855, and NCAN rs2228603) and adolescent HF, and found that only the PNPLA3 risk allele was associated with HF content. Thus, for the purpose of the present analysis, we focused only on the PNPLA3 rs738409 risk allele as a potential effect modifier of associations of nutrient intake changes with adolescent HF.
Other covariate assessments:
Participant sex and race/ethnicity were self-reported at the first research visit. For analyses, we grouped participants as Hispanic or non-Hispanic ethnicity due to small sample sizes for non-Hispanic black and non-Hispanic other. Height and weight were measured at both visits in light clothing without shoes. Age- and sex-adjusted body mass index (BMI) z-scores were calculated using the WHO growth reference31. Pubertal stage was assessed by self-reported Tanner staging of pubic hair for boys and breast development for girls32. Physical activity was assessed by 3 day physical activity recall questionnaire33, which has been validated against accelerometry34, and used to calculate average energy expenditure over three days in metabolic equivalents (METs). Offspring were categorized as having exposure to maternal diabetes mellitus (DM) during pregnancy if the mother had a physician diagnosis of gestational DM during pregnancy or DM prior to pregnancy, which was ascertained from a medical records database as previously described18. Maternal pre-pregnancy BMI was calculated using the last measured weight in the medical record before pregnancy and height collected at the first research visit, and categorized as obesity based on pre-pregnancy BMI≥30 kg/m2. Maternal education and smoking during pregnancy were assessed at the first research visit via questionnaire.
Statistical analyses
Descriptive statistics were performed to summarize characteristics of the sample overall and according to NAFLD status at visit 2. Means and standard deviations or medians and interquartile ranges (IQRs) were reported for continuous variables and compared between groups using the Student T-tests or Mann-Whitney tests, respectively. Counts and frequencies were reported for categorical variables and compared between groups using Chi-square tests. Linear regression models were constructed to examine associations of nutrient intake changes from visit 1 to visit 2 with HF at visit 2. Prior to analysis, HF was natural log-transformed to ensure that residuals were normally distributed. Histograms of the distribution of HF before and after log-transformation are shown in Figure 1 (available at www.jpeds.com). Models were adjusted for covariates in a stepwise manner, whereby Model 1 was adjusted for potential confounders selected a priori, including offspring age, sex, race/ethnicity, maternal education (high school or less or some college or more), exposure to maternal DM during pregnancy, maternal smoking during pregnancy, baseline nutrient intake at visit 1, change in TEI (visit 2-visit 1), and TEI plausibility at visit 1 (acceptable reporter or under reporter). Model 2 was adjusted for Model 1 covariates plus BMI z-score at visit 2 to assess if associations were independent of concurrent obesity. We reported the β-coefficients and 95% confidence intervals (CIs) for associations of a 5 unit increase in each nutrient intake on adolescent log-HF. For interpretation, we also reported results after back-transformation in supplementary tables, where β-coefficients and 95% CIs reflect the ratio of geometric means for adolescent HF per 5 unit increase in intakes.
Figure 1: Online Only.
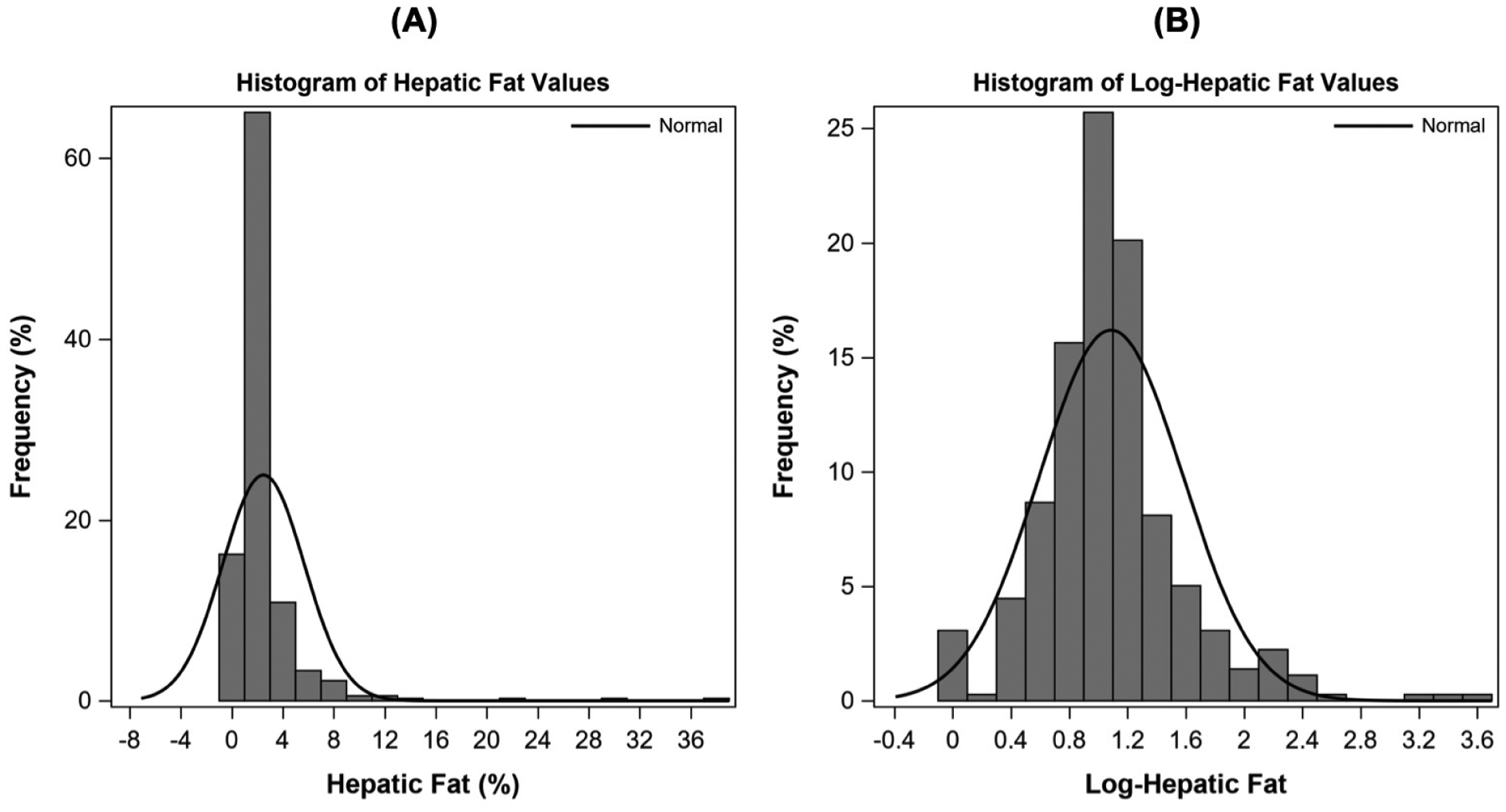
Histograms showing the distribution of hepatic fat values at visit 2 (adolescence, ~16 yrs) in the sample of multi-ethnic youth, before and after natural log-transformation (n=358). The decision to natural log-transform hepatic fat for all analyses was also based on ensuring a normal distribution of residuals in linear regression models.
We tested for effect modification by the PNPLA3 rs738409 risk allele, as well as offspring sex and race/ethnicity, using product terms. Stratified estimates were reported based on P-interaction<0.05. We also performed several sensitivity analyses. First, we re-ran analyses after excluding participants with obesity to determine whether associations were only driven by these individuals. We also examined whether results differed when we adjusted Model 1 for additional covariates at visit 1 or visit 2, which were only available in a sub-sample, including maternal pre-pregnancy obesity, pubertal stage, and self-reported physical activity level, or when we additionall adjusted for TEI plausibility at visit 2. All analyses were carried out using SAS statistical software (v9.4, Cary, NC, USA).
RESULTS:
Characteristics of the sample of 358 participants included in analyses, according to NAFLD status at visit 2, are shown in Table I. A total of 22 participants (6.1%) were categorized as having NAFLD (defined as HF>5.5%). Among participants with NAFLD vs. without NAFLD, there were higher percentages of Hispanic children (77.3% vs. 34.2% respectively) and exposure to maternal pre-pregnancy obesity in utero (61.5% vs. 18.6%) (Table 1). Participants with NAFLD also had higher mean BMI z-score at visit 1 (1.27 vs. 0.13, respectively) and visit 2 (1.82 vs. 0.29, respectively) (Table 1). All other characteristics were similar between groups. The frequency of the PNPLA3 rs738409 risk allele is also shown in Table 1, which was higher in participants with NAFLD at visit 2 (0.47) compared with those without NAFLD (0.28).
Associations of nutrient intake changes in childhood with HF in adolescence:
Mean change values for each nutrient intake are summarized in Table 2 (available at www.jpeds.com). Estimates for associations of nutrient intake changes from visit 1 (childhood) to visit 2 (adolescence) with log-transformed HF at visit 2 are shown in Table 3. Increases in dietary fiber, vegetable protein, and PUFA intake were associated with significantly lower log-HF at visit 2 (Table 3). based on Model 1 adjusted for potential confounding variables. In contrast, increases in animal protein intake were associated with higher HF at visit 2 (Table 3). For dietary fiber, we examined whether associations differed for soluble versus insoluble fiber and found that increases in both types of fiber were inversely associated with log-HF at visit 2, but estimates for soluble fiber crossed the null (Table 3). We also examined whether results differed by type of PUFA and found that the association was attenuated and less precise for omega-3 PUFAs, but similar for all other PUFAs (calculated as total - omega-3 PUFAs) (Table 3). All results were similar after additionally adjusted for BMI z-score at visit 2 in Model 2 (Table 3), suggesting associations were independent of concurrent obesity at the time HF was assessed. Estimates for the above associations after back-transformation are summarized in Table 4 (available at www.jpeds.com).
Table 2 (Online Only):
Means and standard deviations for nutrient intakes at visit 1 (childhood, ~10 yrs) and visit 2 (adolescence, ~16 yrs) in the sample of multi-ethnic youth, overall and by nonalcoholic fatty liver disease (NAFLD) diagnosis at visit (n=358)
| Full sample (n=358) | Non-NAFLD (n=336) | NAFLD (n=22) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutrient (%TEI or g/1000 kcal) | pb | Pb | Pb | ||||||
| Energy | <0.001 | 0.003 | 0.039 | ||||||
| Total Carbohydrates | <0.001 | <0.001 | 0.29 | ||||||
| Starch | 0.41 | 0.49 | 0.67 | ||||||
| Sugar | <0.001 | <0.001 | 0.57 | ||||||
| Total Fiber | 0.003 | <0.001 | 0.10 | ||||||
| Soluble fiber | 0.003 | <0.001 | 0.13 | ||||||
| Insoluble fiber | 0.004 | <0.001 | 0.10 | ||||||
| Total Protein | <0.001 | <0.001 | 0.11 | ||||||
| Animal Protein | 0.14 | 0.35 | 0.19 | ||||||
| Vegetable Protein | <0.001 | <0.001 | 0.93 | ||||||
| Total Fat | <0.001 | <0.001 | 0.68 | ||||||
| Saturated Fat | 0.23 | 0.38 | 0.93 | ||||||
| Monounsaturated Fat | <0.001 | <0.001 | 0.54 | ||||||
| Polyunsaturated Fat | 0.04 | 0.01 | 0.47 | ||||||
| Other PUFA | 0.007 | 0.02 | 0.49 | ||||||
| Omega-3 PUFAc | 0.06 | 0.004 | 0.60 | ||||||
Nutrient intakes reported as a percentage of TEI, except for energy (kcal/d) and dietary fiber (g/1000 kcal).
Calculated using paired t-tests to compare intakes at visit 1 vs. intakes at visit 2. Bold black indicates p<0.05.
Estimated as total PUFA intake – omega-3 PUFA intake.
There were no significant difference (p<0.05) between non-NAFLD and NAFLD participants for the nutrient intake at each time point (visit 1 or 2) based on Student’s t-test.
Abbreviations: NAFLD, nonalcoholic fatty liver disease; TEI, total energy intake; PUFA, polyunsaturated fat
Table 3:
Associations of nutrient intake changes from visit 1 (childhood) to visit 2 (adolescence) with log-transformed hepatic fat at visit 2 in the sample of multi-ethnic youth (n=358)
| Model 1a | Model 2b | ||||
|---|---|---|---|---|---|
| △ Nutrient Intake (% TEI or g/1000 kcal) | Mean △ Intakec: | p | p | ||
| Total Carbohydrates | −2.20 (7.98) | 0.35 | 0.70 | ||
| Starch | −0.21 (5.07) | 0.91 | 0.52 | ||
| Sugar | −2.49 (8.02) | 0.79 | 0.87 | ||
| Total Fiber | 0.64 (3.90) | 0.02 | 0.02 | ||
| Soluble fiber | 0.21 (1.31) | 0.06 | 0.07 | ||
| Insoluble fiber | 0.42 (2.65) | 0.02 | 0.02 | ||
| Total Protein | 0.62 (2.78) | 0.09 | 0.13 | ||
| Animal Protein | 0.22 (3.31) | 0.007 | 0.01 | ||
| Vegetable Protein | 0.39 (1.50) | 0.008 | 0.008 | ||
| Total Fat | 1.75 (6.39) | 0.92 | 0.48 | ||
| Saturated Fat | 0.13 (2.76) | 0.14 | 0.34 | ||
| Monounsaturated Fat | 1.09 (3.00) | 0.67 | 0.36 | ||
| Polyunsaturated Fat | 0.22 (1.78) | 0.03 | 0.008 | ||
| Other PUFAe | 0.19 (1.73) | 0.03 | 0.007 | ||
| Omega-3 PUFA | 0.03 (0.20) | 0.89 | 0.68 | ||
Model 1: Adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (visit 1), change in TEI (visit 2-visit 1), and TEI plausibility at visit 1.
Model 2: Adjusted for Model 1 covariates and BMI z-score at visit 2.
Mean change values calculated as visit 2-visit 1.
β coefficients are estimate the effect on log-hepatic fat for a +5 unit increase in each nutrient as a percentage of TEI, except for dietary fiber, which is assessed as g/1000 kcal. Bold black indicates p<0.05.
Calculated as total PUFA intake – omega-3 PUFA intake.
Abbreviations: HF, hepatic fat; TEI, total energy intake; BMI, body mass index; PUFA, polyunsaturated fat.
Table 4 (Online Only):
Back-transformed estimates for associations of nutrient intake changes from visit 1 (childhood) to visit 2 (adolescence) with log-transformed hepatic fat at visit 2 in the sample of multi-ethnic youth (n=358)
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| △ Nutrient Intake (%TEI or g/1000 kcal) | p-value | p-value | ||
| Total Carbohydrates | 0.35 | 0.70 | ||
| Starch | 0.91 | 0.52 | ||
| Sugar | 0.79 | 0.87 | ||
| Total Fiber | 0.02 | 0.02 | ||
| Soluble fiber | 0.06 | 0.07 | ||
| Insoluble fiber | 0.02 | 0.02 | ||
| Total Protein | 0.09 | 0.13 | ||
| Animal Protein | 0.007 | 0.01 | ||
| Vegetable Protein | 0.008 | 0.008 | ||
| Total Fat | 0.92 | 0.48 | ||
| Saturated Fat | 0.14 | 0.34 | ||
| Monounsaturated Fat | 0.67 | 0.36 | ||
| Polyunsaturated Fat (PUFA) | 0.03 | 0.008 | ||
| Other PUFAd | 0.03 | 0.007 | ||
| Omega-3 PUFA | 0.89 | 0.68 | ||
Model 1: Adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (visit 1), change in TEI (visit 2-visit 1), and TEI plausibility at visit 1.
Model 2: Adjusted for Model 1 covariates and BMI z-score at visit 1.
β coefficients have been back-transformed and represent the geometric mean ratio for hepatic fat per +5 unit increase per nutrient as a percentage of TEI, except for dietary fiber, which is assessed as g/1000 kcal. Bold black indicates p<0.05.
Estimated as total PUFA intake – omega-3 PUFA intake.
Abbreviations: TEI, total energy intake; PUFA, polyunsaturated fat; BMI, body mass index.
Examining potential effect modification by genetic risk and demographics:
We found evidence of effect modification by the PNPLA3 rs738409 risk allele for associations of fiber (p-interaction=0.007), vegetable protein (p-interaction=0.005), and SFA intake (p-interaction=0.039) with HF at visit 2. In model 1 adjusted for potential confounders, inverse associations of changes in fiber and vegetable protein intake and positive associations for change in SFA intake with log-HF at visit 2 were stronger and only significant in carriers of the risk allele compared with non-carriers (Table 5 and Figure 2). Associations followed a similar pattern for soluble versus insoluble fiber (Table 5). In Model 2, conclusions were similar when models were additionally adjusted for BMI z-score at visit 2, but associations of change in SGA intake with log-HF were attenuated (Table 5), suggesting partial mediation by concurrent obesity at visit 2. Back-transformed estimates are shown in Table 6 (available at www.jpeds.com). We also found evidence of effect modification by Hispanic ethnicity for associations of change in fiber intake with HF at visit 2 (p-interaction=0.029), whereby inverse associations with log-HF were stronger and only significant in Hispanic participants [β (95% CI): −0.22 (−0.36, −0.08) per 5 g/1000 kcal fiber, p=0.002] versus non-Hispanic [−0.08 (−0.18, 0.02) per 5 g/1000 kcal fiber, p=0.13]. Estimates were not appreciably changed after additionally adjusting for BMI z-score at visit 2 (not shown).
Table 5:
Associations of select nutrient intake changes from visit 1 (childhood) to visit 2 (adolescence) with log-transformed hepatic fat at visit 2 in the sample of multi-ethnic youth stratified by PNPLA3 rs738409 risk allele (n=308)
| Model 1a | Model 2b | ||||
|---|---|---|---|---|---|
| △ Nutrient Intake (%TEI or g/1000 kcal) | # Risk Alleles | p-value | p-value | ||
| Total Fiber | |||||
| 2 | <0.001 | 0.001 | |||
| Soluble Fiber | |||||
| 2 | 0.003 | 0.005 | |||
| Insoluble Fiber | |||||
| 2 | <0.001 | <0.001 | |||
| Vegetable Protein | |||||
| 2 | <0.001 | <0.001 | |||
| Saturated Fat | |||||
| 2 | 0.02 | 0.07 | |||
Model 1: Adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (T1), change in TEI (T2-T1), and TEI plausibility at T1.
Model 2: Adjusted for Model 1 covariates and BMI z-score at T2.
β coefficients are estimate the effect on log-hepatic fat for a +5 unit increase in each nutrient as a percentage of TEI, except for dietary fiber, which is assessed as g/1000 kcal. Bold black indicates p<0.05.
Abbreviations: TEI, total energy intake; BMI, body mass index.
Figure 2:
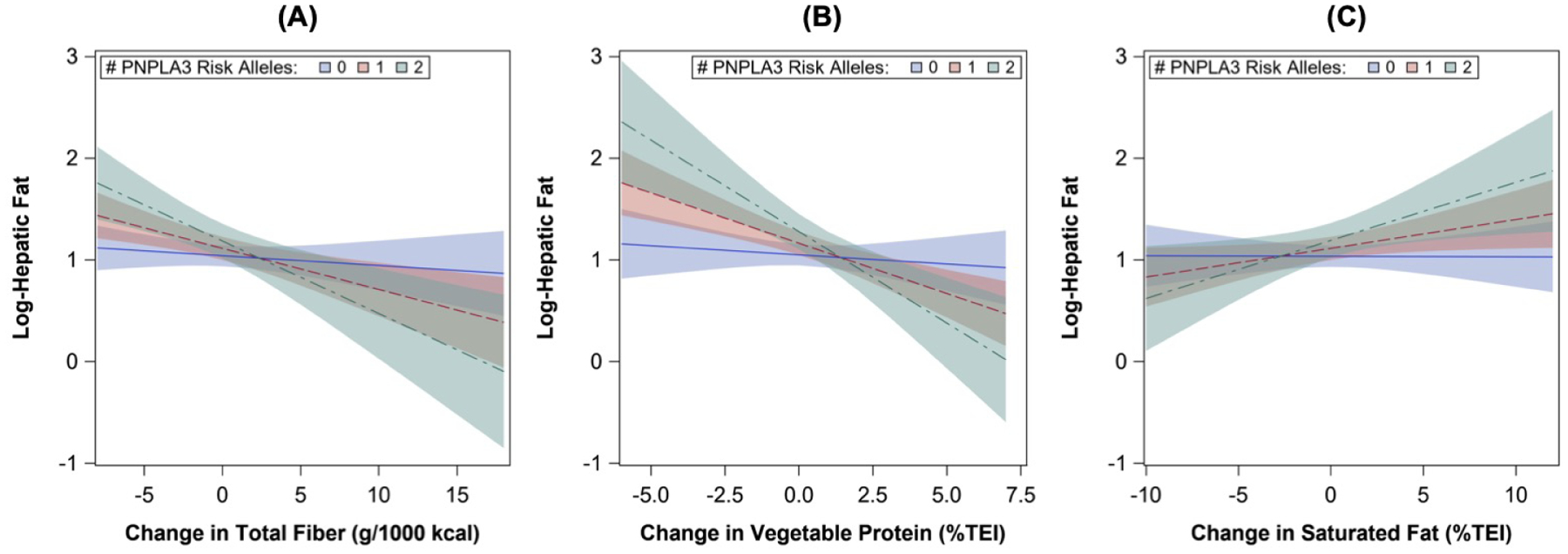
Trend lines for associations of nutrient intake changes from childhood to adolescence with HF in adolescence according to PNPLA3 (rs738409) risk allele. Trend lines were estimated from linear regression models adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (visit 1), change in TEI (visit 2-visit 1), and TEI plausibility at visit 1. Abbreviations: HF, hepatic fat; TEI, total energy intake.
Table 6 (Online Only):
Back-transformed estimates for associations of select nutrient intake changes from visit 1 (childhood) to visit 2 (adolescence) with log-transformed hepatic fat at visit 2 in the sample of multi-ethnic youth, stratified by PNPLA3 rs738409 risk allele (n=308)
| Model 1a | Model 2b | ||||
|---|---|---|---|---|---|
| △ Nutrient Intake (%TEI or g/1000 kcal) | # Risk Alleles | p-value | p-value | ||
| Total Fiber | |||||
| 2 | <0.001 | 0.001 | |||
| Soluble Fiber | |||||
| 2 | 0.003 | 0.005 | |||
| Insoluble Fiber | |||||
| 2 | <0.001 | <0.001 | |||
| Vegetable Protein | |||||
| 2 | <0.001 | <0.001 | |||
| Saturated Fat | |||||
| 2 | 0.02 | 0.07 | |||
Model 1: Adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (visit 1), change in TEI (visit 2-visit 1), and TEI plausibility at visit 1.
Model 2: Adjusted for Model 1 covariates and BMI z-score at visit 1.
β coefficients have been back-transformed and represent the geometric mean ratio for hepatic fat per +5 unit increase per nutrient as a percentage of TEI, except for dietary fiber, which is assessed as g/1000 kcal. Bold black indicates p<0.05.
Abbreviations: TEI, total energy intake; BMI, body mass index.
Sensitivity analyses:
We performed a sensitivity analysis where we excluded participants with obesity at either visit to assess whether these participants were driving our results. As shown in Table 7 (available at www.jpeds.com) and Table 8 (available at www.jpeds.com) for the full sample and stratified by the PNPLA3 rs738409 risk allele, respectively, most results were unchanged in terms of directionality and significance, but the magnitude of several estimates was attenuated slightly. We also examined whether results differed after adjusting for other potential confounders, including maternal pre-pregnancy obesity exposure, pubertal stage at visit 1 or visit 2, and physical activity at visit 1 or visit 2, and found that no associations were appreciably changed (available upon request). Results were also similar if we additionally adjusted for TEI plausibility at visit 2 (available upon request).
Table 7 (Online Only):
Associations of nutrient intake changes from visit 1 (childhood) to visit 2 (adolescence) with log-transformed hepatic fat at visit 2 in the sample of multi-ethnic youth, excluding participants with obesity at visit 1 or visit 2 (n=322)
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| △ Nutrient Intake (% TEI or g/1000 kcal) | p | p | ||
| Total Carbohydrate | 0.25 | 0.48 | ||
| Starch | 0.83 | 0.47 | ||
| Sugar | 0.49 | 0.57 | ||
| Total Fiber | 0.07 | 0.09 | ||
| Soluble Fiber | 0.26 | 0.29 | ||
| Insoluble Fiber | 0.04 | 0.05 | ||
| Total Protein | 0.19 | 0.26 | ||
| Animal Protein | 0.03 | 0.05 | ||
| Vegetable Protein | 0.02 | 0.03 | ||
| Total Fat | 0.73 | 0.91 | ||
| Saturated Fat | 0.03 | 0.10 | ||
| Monounsaturated Fat | 0.87 | 0.62 | ||
| Polyunsaturated Fat | 0.06 | 0.03 | ||
| Other PUFAd | 0.03 | 0.03 | ||
| Omega-3 PUFA | 0.77 | 0.97 | ||
Model 1: Adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (visit 1), change in TEI (visit 2-visit 1), and TEI plausibility at visit 1.
Model 2: Adjusted for Model 1 covariates and BMI z-score at visit 2.
β coefficients are estimate the effect on log-hepatic fat for a +5 unit increase in each nutrient as a percentage of TEI, except for dietary fiber, which is assessed as g/1000 kcal. Bold black indicates p<0.05. Bold black italic indicate p<0.10.
Calculated as total PUFA intake – omega-3 PUFA intake.
Abbreviations: HF, hepatic fat; TEI, total energy intake; BMI, body mass index; PUFA, polyunsaturated fat.
Table 8 (Online Only):
Associations of select nutrient intake changes from visit 1 to visit 2 with log-transformed hepatic fat at visit 2 in the sample of multi-ethnic youth stratified by PNPLA3 rs738409 risk allele, excluding participants with obesity at visit 1 or visit 2 (n=3279)
| Model 1a | Model 2b | ||||
|---|---|---|---|---|---|
| △ Nutrient Intake (%TEI or g/1000 kcal) | # Risk Alleles | p-value | p-value | ||
| Total Fiber | 0 | 0.15 | 0.21 | ||
| 1 | 0.01 | 0.01 | |||
| 2 | 0.02 | 0.02 | |||
| Soluble Fiber | 0 | 0.40 | 0.51 | ||
| 1 | 0.04 | 0.07 | |||
| 2 | 0.05 | 0.07 | |||
| Insoluble Fiber | 0 | 0.11 | 0.15 | ||
| 1 | 0.004 | 0.01 | |||
| 2 | 0.01 | 0.02 | |||
| Vegetable Protein | 0 | 0.40 | 0.50 | ||
| 1 | 0.001 | 0.001 | |||
| 2 | 0.005 | 0.002 | |||
| Saturated Fat | 0 | 0.28 | 0.40 | ||
| 1 | 0.02 | 0.06 | |||
| 2 | 0.06 | 0.12 | |||
Model 1: Adjusted for offspring sex, age, Hispanic ethnicity, maternal education, maternal DM, maternal smoking, baseline intake (T1), change in TEI (T2-T1), and TEI plausibility at T1.
Model 2: Adjusted for Model 1 covariates and BMI z-score at T2.
β coefficients are estimate the effect on log-hepatic fat for a +5 unit increase in each nutrient as a percentage of TEI, except for dietary fiber, which is assessed as g/1000 kcal. Bold black indicates p<0.05. Bold black italic indicate p<0.10.
Abbreviations: TEI, total energy intake; BMI, body mass index.
DISCUSSION:
In this study, we examined associations of nutrient intake changes during the transition from childhood to adolescence with hepatic fat levels in adolescence and whether genetic background modifies these associations. This was based on a well-characterized, multi-ethnic, longitudinal cohort in the U.S. (Colorado), where intake was assessed as nutrient densities relative to total energy intake, and HF was assessed by MRI. Collectively, our results suggest that increases in dietary fiber, vegetable protein, and PUFA intake, and decreases in animal protein intake from childhood to adolescence may be protective against higher HF in adolescence. Examination of effect modification by genetic risk of HF revealed that inverse associations of fiber and vegetable protein and positive associations of saturated fat with HF were markedly stronger in carriers of the PNPLA3 rs738409 variant. In contrast, the positive association between change in animal protein and adolescent HF in the sample overall was similar regardless of genetic profile. Importantly, most associations were independent of concurrent BMI z-score at the time of HF assessment, suggesting a pathway between these nutrients and HF that is independent of obesity.
Most epidemiological evidence of associations between specific nutrient intakes and HF is limited to cross-sectional studies, which cannot establish temporality. This may explain inconsistent findings in the literature, with some studies showing that certain nutrient intakes are associated with higher HF35–38, such as carbohydrates (especially refined carbohydrates and sugars) and saturated fat, and others reported no associations between these nutrient intakes and HF39–41. Among the few longitudinal investigations conducted thus far, one study from the UK found no associations between energy-adjusted macronutrient intakes at any age in childhood (3, 7, or 13 years) and ultrasound-measured HF at 17 yrs42. Similarly, a longitudinal study by Perng et al, which was based on the same EPOCH cohort as this analysis, found no associations between dietary pattern scores (derived from principal components analysis) in childhood (visit 1) and HF in adolescence (visit 2); whereas, greater adherence to a prudent diet pattern at visit 2 was associated with lower HF at visit 226. Together with the results of our study, this suggests that dietary shifts from childhood to adolescence, especially changes characterized by a divergence from a ‘healthy’ diet pattern, such as decreases in fiber, vegetable protein, and PUFAs and increases in animal protein, are more strongly associated with higher HF in adolescence compared with nutrient intakes or dietary patterns in early childhood.
Our study also revealed several gene-nutrient interactions associated with HF in adolescence, especially for the PNPLA3 rs738409 allele, which is the strongest genetic risk factor for HF and confers susceptibility likely by impairing triglyceride hydrolysis43, 44. Specifically, we found that an increase in SFA intake from childhood to adolescence was associated with higher adolescent HF in risk allele carriers. As shown by Luukkonen et al, a link between excess SFA intake and HF is likely explained by upregulation adipose tissue lipolysis and subsequently the hepatic fatty acid pool45, which contributes to HF deposition. It is plausible this SFA-induced lipolysis exacerbates disruptions in hepatic lipid metabolism in carriers of the PNPLA3 variant. We also found that increases in ‘healthy’ nutrients, especially fiber and vegetable protein, were more protective against adolescent HF in carriers of the risk allele, suggesting that higher intakes of these nutrients may attenuate the adverse effect of the PNPLA3 variant on HF. Relative to the literature on this topic, it should be noted that other pediatric studies have reported an interaction between PNPLA3 and carbohydrate/sugar intake on HF deposition39, 46, which was not observed in our study. It is possible that this discrepancy was due to differences in sample characteristics; in particular, the EPOCH cohort is multi-ethnic and generally healthy, compared with the other samples that found this interaction, which had more homogenous ancestries (Hispanic or Italian) and higher rates of obesity, NAFLD, and/or other metabolic alterations.
This study has limitations and strengths. We relied on self-reported dietary data, which may be prone to recall and social desirability biases. This may lead to under-reporting, especially in children with obesity47, and for certain food groups, such as sugary beverages48. At the same time, we used an FFQ that has been validated in children to assess long-term dietary habits, which is directly relevant to chronic disease risk, and examined within-person changes in intake, which limited potential bias. We also adjusted for plausibility of energy intake at both times, which did not alter findings; thus, we believe measurement error had a limited impact. We only tested the PNPLA3 risk allele as an effect modifier due to previous associations with HF in this cohort30, but not all genetic variants that have been reported in the literature. Larger studies will be needed to assess diet-by-gene interactions in a more global manner. Because HF was only assessed at visit 2 in adolescence, we were unable to assess parallel changes in HF from childhood to adolescence. Thus, studies with repeated measures of HF across childhood and adolescence are also needed to test this. The sample size of participants with clinically-defined NAFLD was low in EPOCH (n=22 or 6.1%), but similar to prevalence estimates in other general population studies (7.6%, as reported by Anderson et al1). Thus, by examining associations with HF as a continuous outcome in adolescence in a relatively healthy, general risk population, our findings could be used to inform strategies for NAFLD prevention in the future. The sample also included multiple racial/ethnic groups and individuals across the spectrum of BMI categories, including lean participants in addition to participants with overweight or obesity, which increases the generalizability of our findings, especially given most associations were similar even when we excluded participants with obesity in sensitivity analyses. Other strengths include the use of state-of-the-art MRI to assess HF as a continuous measure, the integration of offspring genotypic information to elucidate nutrient-by-gene interactions, and the extensive phenotypic assessments performed at two visits, which enabled us to control for and stratify by key variables.
Our findings may have important clinical and public health implications. Although currently the mainstay treatment for NAFLD is lifestyle management to promote weight loss49, there has been insufficient data to identify an ‘optimal’ dietary and/or exercise prescription for improving NAFLD outcomes50, 51. Further, evidence from pediatric interventions has shown that it is very challenging to achieve and maintain meaningful weight loss52, 53. Thus, there is need to develop more targeted and sustainable strategies for preventing HF in high-risk children, as well as treating or reducing HF in those already diagnosed. The findings from this study suggest that nutrient intake changes from childhood to adolescence, especially decreases in fiber, vegetable protein, and PUFAs and increases in animal protein and saturated fat, are associated with higher HF deposition in adolescence, independent of potential intrauterine and postnatal confounding variables. Over time, higher HF in adolescence may be associated with higher risk of NAFLD in adulthood and its associated hepatic and extrahepatic co-morbidities. Longitudinal studies with repeated measures of HF starting earlier in childhood will be needed to fully elucidate the relationship between the dietary intakes throughout childhood and adolescence and HF deposition. In addition, intervention studies will be needed to test whether targeting these dietary factors is an effective strategy for reducing or preventing HF deposition in youth.
Supplementary Material
Acknowledgments
Funded by the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) (R01-DK068001). C.C. was supported by NIDDK (T32-DK07658). W.P. is supported by the Colorado Clinical and Translational Sciences Institute (KL2-TR002534). The authors declare no conflicts of interest.
Abbreviations:
- NAFLD
nonalcoholic fatty liver disease
- PNPLA3
patatin-like phospholipase domain-containing 3
- SNP
single nucleotide polymorphism
- EPOCH
Exploring Perinatal Outcomes in Childhood Health
- TEI
total energy intake
- SFA
saturated fat
- MUFA
monounsaturated fat
- PUFA
polyunsaturated fat
- BMR
basal metabolic rate
- WHO
world health organization
- MRI
magnetic resonance imaging
- BMI
body mass index
- MET
metabolic equivalent
- DM
diabetes mellitus
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. [DOI] [PubMed] [Google Scholar]
- [3].Cali AM, Caprio S. Ectopic fat deposition and the metabolic syndrome in obese children and adolescents. Horm Res. 2009;71Suppl 1:2–7. [DOI] [PubMed] [Google Scholar]
- [4].Wicklow BA, Wittmeier KD, MacIntosh AC, Sellers EA, Ryner L, Serrai H, et al. Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Diabetes Care. 2012;35:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. J Pediatr. 2019;207:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, the NC. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santoro N, Kursawe R, D’Adamo E, Dykas DJ, Zhang CK, Bale AE, et al. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stanislawski MA, Shaw J, Litkowski E, Lange EM, Perng W, Dabelea D, et al. Genetic Risk for Hepatic Fat among an Ethnically Diverse Cohort of Youth: The Exploring Perinatal Outcomes among Children Study. J Pediatr. 2020;220:146–53.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goran MI, Walker R, Le KA, Mahurkar S, Vikman S, Davis JN, et al. Effects of PNPLA3 on liver fat and metabolic profile in hispanic children and adolescents. Diabetes. 2010;59:3127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alkhouri N, Hanouneh IA, Zein NN, Lopez R, Kelly D, Eghtesad B, et al. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl Int. 2016;29:418–24. [DOI] [PubMed] [Google Scholar]
- [12].Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J Clin Gastroenterol. 2018;52:339–46. [DOI] [PubMed] [Google Scholar]
- [13].Madruga SW, Araújo CLP, Bertoldi AD, Neutzling MB. Manutenção dos padrões alimentares da infância à adolescência. Revista de Saúde Pública. 2012;46:376–86. [DOI] [PubMed] [Google Scholar]
- [14].Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Ann N Y Acad Sci. 2008;1135:265–79. [DOI] [PubMed] [Google Scholar]
- [15].Berkey CS, Rockett HRH, Field AE, Gillman MW, Colditz GA. Sugar-Added Beverages and Adolescent Weight Change. Obesity Research. 2004;12:778–88. [DOI] [PubMed] [Google Scholar]
- [16].Lytle LA, Seifert S, Greenstein J, McGovern P. How do children’s eating patterns and food choices change over time? Results from a cohort study. Am J Health Promot. 2000;14:222–8. [DOI] [PubMed] [Google Scholar]
- [17].Demory-Luce D, Morales M, Nicklas T, Baranowski T, Zakeri I, Berenson G. Changes in food group consumption patterns from childhood to young adulthood: the Bogalusa Heart Study. J Am Diet Assoc. 2004;104:1684–91. [DOI] [PubMed] [Google Scholar]
- [18].Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bellatorre A, Scherzinger A, Stamm E, Martinez M, Ringham B, Dabelea D. Fetal Overnutrition and Adolescent Hepatic Fat Fraction: The Exploring Perinatal Outcomes in Children Study. J Pediatr. 2018;192:165–70.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Willett WC. Overview of Nutritional Epidemiology. In: Willett WC, editor. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- [21].Block G, Murphy M, Roullet J, Wakimoto P, Crawford P, Block T. Pilot validation of a FFQ for children 8–10 years. Fourth International Conference on Dietary Assessment Methods 2000. [Google Scholar]
- [22].Cullen KW, Watson K, Zakeri I. Relative reliability and validity of the Block Kids Questionnaire among youth aged 10 to 17 years. J Am Diet Assoc. 2008;108:862–6. [DOI] [PubMed] [Google Scholar]
- [23].Mayer-Davis EJ, Nichols M, Liese AD, Bell RA, Dabelea DM, Johansen JM, et al. Dietary Intake among Youth with Diabetes: The SEARCH for Diabetes in Youth Study. Journal of the American Dietetic Association. 2006;106:689–97. [DOI] [PubMed] [Google Scholar]
- [24].Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24:1119–30. [DOI] [PubMed] [Google Scholar]
- [25].Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45:569–81. [PubMed] [Google Scholar]
- [26].Perng W, Harte R, Ringham BM, Baylin A, Bellatorre A, Scherzinger A, et al. A Prudent dietary pattern is inversely associated with liver fat content among multi-ethnic youth. Pediatric Obesity. 2021;16:e12758. [DOI] [PubMed] [Google Scholar]
- [27].Carreau A-M, Pyle L, Garcia-Reyes Y, Rahat H, Vigers T, Jensen T, et al. Clinical prediction score of nonalcoholic fatty liver disease in adolescent girls with polycystic ovary syndrome (PCOS-HS index). Clin Endocrinol (Oxf). 2019;91:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Smits LP, Coolen BF, Panno MD, Runge JH, Nijhof WH, Verheij J, et al. Noninvasive Differentiation between Hepatic Steatosis and Steatohepatitis with MR Imaging Enhanced with USPIOs in Patients with Nonalcoholic Fatty Liver Disease: A Proof-of-Concept Study. Radiology. 2016;278:782–91. [DOI] [PubMed] [Google Scholar]
- [29].Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. [DOI] [PubMed] [Google Scholar]
- [30].Kahali B, Halligan B, Speliotes EK. Insights from Genome-Wide Association Analyses of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual review of medicine. 1968;19:283–300. [DOI] [PubMed] [Google Scholar]
- [33].Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Medicine and science in sports and exercise. 1997;29:138–43. [DOI] [PubMed] [Google Scholar]
- [34].Pate RR, Ross R, Dowda M, Trost SG, Sirard JR. Validation of a 3-Day Physical Activity Recall Instrument in Female Youth. Pediatric Exercise Science. 2003;15:257–65. [Google Scholar]
- [35].Papandreou D, Rousso I, Malindretos P, Makedou A, Moudiou T, Pidonia I, et al. Are saturated fatty acids and insulin resistance associated with fatty liver in obese children? Clin Nutr. 2008;27:233–40. [DOI] [PubMed] [Google Scholar]
- [36].Mager DR, Patterson C, So S, Rogenstein CD, Wykes LJ, Roberts EA. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr. 2010;64:628–35. [DOI] [PubMed] [Google Scholar]
- [37].Papandreou D, Karabouta Z, Pantoleon A, Rousso I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite. 2012;59:939–44. [DOI] [PubMed] [Google Scholar]
- [38].Nier A, Brandt A, Conzelmann IB, Özel Y, Bergheim I. Non-Alcoholic Fatty Liver Disease in Overweight Children: Role of Fructose Intake and Dietary Pattern. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Davis JN, Lê KA, Walker RW, Vikman S, Spruijt-Metz D, Weigensberg MJ, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. 2010;92:1522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gibson PS, Lang S, Gilbert M, Kamat D, Bansal S, Ford-Adams ME, et al. Assessment of Diet and Physical Activity in Paediatric Non-Alcoholic Fatty Liver Disease Patients: A United Kingdom Case Control Study. Nutrients. 2015;7:9721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Arenaza L, Medrano M, Oses M, Huybrechts I, Díez I, Henriksson H, et al. Dietary determinants of hepatic fat content and insulin resistance in overweight/obese children: a cross-sectional analysis of the Prevention of Diabetes in Kids (PREDIKID) study. Br J Nutr. 2019;121:1158–65. [DOI] [PubMed] [Google Scholar]
- [42].Anderson EL, Howe LD, Fraser A, Macdonald-Wallis C, Callaway MP, Sattar N, et al. Childhood energy intake is associated with nonalcoholic fatty liver disease in adolescents. J Nutr. 2015;145:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Perttilä J, Huaman-Samanez C, Caron S, Tanhuanpää K, Staels B, Yki-Järvinen H, et al. PNPLA3 is regulated by glucose in human hepatocytes, and its I148M mutant slows down triglyceride hydrolysis. Am J Physiol Endocrinol Metab. 2012;302:E1063–9. [DOI] [PubMed] [Google Scholar]
- [44].Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci U S A. 2010;107:7892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Luukkonen PK, Sädevirta S, Zhou Y, Kayser B, Ali A, Ahonen L, et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41:1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nobili V, Liccardo D, Bedogni G, Salvatori G, Gnani D, Bersani I, et al. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at-risk adolescents. Genes Nutr. 2014;9:392-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rennie KL, Jebb SA, Wright A, Coward WA. Secular trends in under-reporting in young people. Br J Nutr. 2005;93:241–7. [DOI] [PubMed] [Google Scholar]
- [48].Krebs-Smith SM, Graubard BI, Kahle LL, Subar AF, Cleveland LE, Ballard-Barbash R. Low energy reporters vs others: a comparison of reported food intakes. Eur J Clin Nutr. 2000;54:281–7. [DOI] [PubMed] [Google Scholar]
- [49].Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children. J Pediatr Gastroenterol Nutr. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mann JP, Tang GY, Nobili V, Armstrong MJ. Evaluations of Lifestyle, Dietary, and Pharmacologic Treatments for Pediatric Nonalcoholic Fatty Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol. 2019;17:1457–76.e7. [DOI] [PubMed] [Google Scholar]
- [51].Gibson PS, Lang S, Dhawan A, Fitzpatrick E, Blumfield ML, Truby H, et al. Systematic Review: Nutrition and Physical Activity in the Management of Paediatric Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2017;65:141–9. [DOI] [PubMed] [Google Scholar]
- [52].O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents. JAMA. 2017;317:2427. [DOI] [PubMed] [Google Scholar]
- [53].van der Heijden LB, Feskens EJM, Janse AJ. Maintenance interventions for overweight or obesity in children: a systematic review and meta-analysis. Obes Rev. 2018;19:798–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


