Abstract
Background:
Obesity is a chronic, relapsing, and progressive disease. Obesity is associated with poor health-related quality of life (HRQoL) in survivors of breast cancer.
Methods:
In this 2×2 factorial trial, 351 survivors of breast cancer with overweight or obesity were randomized to one of four treatment groups for 52 weeks: control, exercise alone, diet alone, or exercise plus diet. HRQoL endpoints were measured at baseline and week 52 using the Medical Outcomes Survey-Short Form (SF)-36. Repeated measures analysis of covariance quantified the estimated treatment difference (ETD).
Results:
At baseline, participants had a mean (SD) age of 59.4 (8.7) years, body mass index of 34.0 (5.9) kg/m2, and 71 (20.2%) participants self-reported fair or poor general health. After 52 weeks, compared to control, exercise plus diet improved the physical health summary score [ETD: 5.39 (95% CI: 0.55, 10.22)]; exercise alone [ETD: −1.91 (95% CI: −6.60, 2.79)] and diet alone [ETD: 3.16 (95% CI: −1.52, 7.83)] did not change the physical health summary score. Compared to control, exercise alone [ETD: −0.27 (95% CI: −6.60, 2.79)], diet alone [ETD: 3.25 (95% CI: −1.41, 7.91)], and exercise plus diet [ETD: 1.75 (95% CI: −2.90, 6.39)] did not change the mental health summary score. Exercise alone did not impact any HRQoL subscale; diet alone improved the vitality subscale; exercise plus diet improved the physical functioning, role—physical, and vitality subscales.
Conclusion:
In survivors of breast cancer with overweight or obesity, exercise plus diet improved select HRQoL endpoints at week 52.
Keywords: randomized trial, obesity, weight loss, exercise, survivorship
Precis:
Obesity is associated with poor health-related quality of life in survivors of cancer. In survivors of breast cancer with overweight or obesity, exercise plus diet improved select health-related quality of life endpoints at week 52.
INTRODUCTION
Obesity is a chronic, relapsing, and progressive disease.1 At the time of breast cancer diagnosis, one-in-three patients has obesity.2 After breast cancer diagnosis, 50–80% of patients experience additional weight gain.3 The prevalence of obesity in survivors of breast cancer exceeds that of the general population; the annual increase in obesity prevalence is 2.3% in women with no cancer history versus 3.0% in survivors of breast cancer (P < 0.001).4
Health-related quality of life (HRQoL) is a key component of cancer survivorship.5 Among women without cancer, obesity and weight gain are associated with poor HRQoL,6, 7 and these patterns have been observed in survivors of breast cancer.8, 9 Poor HRQoL in survivors of breast cancer is associated with an increased risk of cancer recurrence and death.10-12
Randomized trials of multimodal lifestyle weight loss interventions that include diet modification, increased physical activity, and lifestyle modification instruction improve HRQoL in survivors of breast cancer.13-15 Randomized trials of exercise alone also improve HRQoL, independent of weight loss.16, 17 The comparative effectiveness of distinct lifestyle modalities for improving HRQoL in survivors of breast cancer therefore remains unclear.
These observations provided the rationale to test the hypothesis that exercise alone, diet alone, and the combination of exercise plus diet would improve HRQoL in survivors of breast cancer with overweight or obesity. This trial used a 2×2 factorial design, which permitted examination of the independent and combined effect of exercise and diet on HRQoL. This trial was part of the Transdisciplinary Research on Energetics and Cancer (TREC) consortium that was sponsored by the National Cancer Institute (NCI).18
METHODS
Study Design
This study was a 52-week, randomized, 2×2 factorial trial. The study was conducted in accordance with Good Clinical Practice and the ethical principles originating in the Declaration of Helsinki. The Institutional Review Board of the University of Pennsylvania approved the protocol and the informed consent document. All participants provided written informed consent and approval from their physician to participate in the study. The study is registered on ClinicalTrials.gov as NCT01515124. The trial design is described in detail.19 The primary endpoint of breast cancer-related lymphedema interlimb volume difference has been reported elsewhere.20
Participants
Participants were recruited from a single metropolitan area using direct to patient mailings from cancer registry databases, flyers placed in oncology clinics, and informational sessions conducted in collaborating hospitals.21 Eligible participants had stage I-III breast cancer; completed surgery, chemotherapy, radiotherapy, and targeted therapy ≥6 months before study enrollment (concurrent endocrine therapy was allowed); had a body mass index (BMI) of 25–50 kg/m2; had breast cancer related lymphedema, defined using the Common Terminology Criteria for Adverse Events (CTCAE; version 4)22 or a prior clinical diagnosis of lymphedema; and were aged 18–80 years. In addition, eligible participants had no evidence of residual or recurrent cancer; no medical conditions that would prohibit engaging in exercise or diet; were not engaging in any weightlifting exercise or ≥3 bouts of aerobic activity weekly over the prior 52 weeks; were not using any medications for weight loss; had no weight loss ≥4.5 kg in the previous 12 weeks; and had no history of bariatric or metabolic surgery.
Randomization and Blinding
Participants were assigned to one of four treatment groups in a 1:1:1:1 ratio for 52 weeks: control, exercise alone, diet alone, or exercise plus diet. All study participants were provided with two custom-fitted compression garments. Participants were stratified by age at study enrollment, the number of lymph nodes resected during breast cancer surgery, receipt of radiotherapy, lymphedema severity, and body mass index. Participants were assigned to treatment group with minimization, which uses an adaptive stratified sampling procedure to balance baseline prognostic factors.23 Participants were not blinded to treatment assignment.
Control Treatment Condition
Participants assigned to the control group were advised to ask their physician regarding what types of exercise or diet would be safe and effective. No specific guidance regarding exercise or diet was provided.
Exercise Treatment Condition
Participants assigned to the exercise group performed a combination of in-person and home-based exercise. In-person exercise was supervised by an exercise oncology professional and occurred weekly in the first six weeks of the study, and once per month thereafter in groups of 2–6 participants. Consistent with recommendations from the American College of Sports Medicine,24 exercise modalities included resistance and aerobic activity. Participants performed twice-weekly resistance exercise using adjustable dumbbell weights (PowerBlock, Inc). The resistance program was adapted from the ‘Physical Activity and Lymphedema’ (PAL) trial,25, 26 that included nine exercises performed twice per week. Moderate-intensity aerobic exercise was prescribed four to six days per week to a goal of 180 minutes weekly. Adherence to home-based exercise was self-reported using standardized reporting logs. Additional details of the exercise treatment plan are published elsewhere.19
Diet Treatment Condition
Participants assigned to the diet group attended 24 weekly sessions led by a registered dietitian in groups of 2–12 participants. The goal of the diet was to induce a 10% loss of body weight. Weekly nutritional counseling sessions included a weigh-in, review of the week, and behavioral modification lesson (e.g., self-monitoring, goal setting, stimulus control). During the first 20 weeks, participants followed a meal replacement program (Nutrisystem, Inc) that included seven daily servings of fruits and vegetables, consistent with the American Cancer Society recommendations.27 During weeks 21–24, the focus shifted to applying the behavioral modification techniques to food shopping and preparation. During weeks 24–52, the groups met in-person monthly for additional behavioral modification lessons (e.g., problem-solving, relapse prevention). Behavior modification lessons were adapted from the ‘Improving the Management of Obesity in Primary Care’ (POWER-UP) trial.28 Additional details of the diet treatment plan are published elsewhere.19
Combined Exercise Plus Diet Treatment Condition
Participants assigned to the exercise plus diet group started with six weeks of exercise instruction. At week seven, participants received the diet intervention in addition to the exercise intervention. Thereafter, participants received the exercise and diet interventions concurrently.
Quality-of-Life Outcomes
HRQoL was assessed at baseline and week 52 using the Medical Outcome Survey Short Form (SF)-36.29 The SF-36 includes eight subscales that form two composite summary domains. The eight subscales include 1) physical functioning (limitations in physical activities because of physical health problems); 2) role—physical (limitations in usual role activities because of physical health problems); 3) bodily pain (intensity of bodily pain or discomfort); 4) general health (overall health perceptions); 5) vitality (energy and fatigue); 6) social functioning (limitations in social activities due to physical or emotional problems); 7) role—emotional (limitations in usual role activities because of emotional problems); and 8) mental health (phycological distress and well-being). These subscale scores are aggregated into the composite summary domains of physical health (subscales 1–4) and mental health (subscales 5–8). Scores for each scale range from 0–100, with higher values indicating better HRQoL.
Other Measures
Demographic characteristics, including age, race, and education, were self-reported. Clinical characteristics, including time since breast cancer diagnosis, cancer stage, number of resected lymph nodes, and treatment types were abstracted from a combination of pathology reports and other physician records. Arm volume was measured by perometry.30 Adverse events were assessed using the Common Terminology Criteria for Adverse Events, version 4.
Statistical Analysis
The sample size was selected to provide statistical power to detect change in the primary endpoint of breast cancer-related lymphedema interlimb volume difference.20 Based on estimates from the Nutrition and Exercise in Women (NEW) trial,31 this study had 80% statistical power to detect standardized mean difference effect sizes of ≥0.2 for the self-reported physical and mental health summary scores using two-sided tests with a 5% Type I error rate.
Statistical analyses and reporting of HRQoL endpoints were guided by the recommendations provided from the Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium (SISAQOL).32 The statistical analysis included all participants who were randomly assigned, and all available in-trial data at week 52 were included in accordance with the intention-to-treat principle. In-trial at week 52 included both intervention-compliant and retrieved participants who prematurely discontinued their assigned intervention. Missing data at week 52 were imputed using predictive mean matching multiple imputation with 20 imputation copies.33
The primary contrast quantified the effect of each of the three intervention groups (exercise alone, diet alone, and exercise plus diet) compared to the control group. HRQoL endpoints were analyzed using a repeated measures analysis of covariance model that included group-by-time interaction terms, the baseline value of the dependent variable, and stratification factors used in the minimization allocation procedure.34 The adjusted between-group mean difference was quantified as the estimated treatment difference (ETD) with corresponding 95% confidence intervals (CI). The 95% confidence intervals were not adjusted for multiplicity; thus, results should be interpreted accordingly. When statistically significant effects were identified, the standardized mean difference effect size (Cohen’s d) was calculated to quantify the magnitude of treatment effect. Values of d of 0.2, 0.5, and 0.8 represent small, medium, and large treatment effects, respectively.35 Radar plots were created to visually depict longitudinal changes in HRQoL endpoints between the control and each intervention group.36 Correlational analyses between change in body mass index and HRQoL endpoints will be published separately. All statistical testing was two-sided. Analyses were done using Stata/MP v.15.1 (StataCorp, LLC).
RESULTS
Trial Population
Between December 2011 and April 2015, 351 participants were randomized, with endpoint data collection ending in May 2016. Study participants had a mean (SD) age of 59.4 (8.7) years, 133 (38%) were non-white race, and 165 (47%) completed a four-year college degree (Table 1). At baseline, the mean BMI was 34.0 (5.9) kg/m2, and 71 (20.2%) participants self-reported fair or poor general health.
Table 1.
Baseline characteristics by randomized group
| Characteristic | Control (n=90) |
Exercise (n=87) |
Diet (n=87) |
Exercise & Diet (n=87) |
|---|---|---|---|---|
| Age, y | 59.0 (8.5) | 59.1 (8.1) | 59.4 (9.2) | 60.0 (9.0) |
| Race, n (%) | ||||
| White | 66 (73.3%) | 50 (57.5%) | 52 (59.8%) | 50 (57.5%) |
| Black | 22 (24.4%) | 36 (41.4%) | 32 (36.8%) | 32 (36.8%) |
| Other | 2 (2.2%) | 1 (1.1%) | 3 (3.3%) | 5 (5.8%) |
| Education, n (%) | ||||
| High school or less | 19 (21.1%) | 15 (17.2%) | 12 (13.8%) | 18 (20.7%) |
| Some college | 28 (31.1%) | 29 (33.3%) | 36 (41.4%) | 29 (33.3%) |
| College degree or more | 43 (47.8%) | 43 (49.4%) | 39 (44.9%) | 40 (46.0%) |
| Time since cancer diagnosis, mo. | 97.5 (61.3) | 92.6 (64.3) | 89.9 (66.9) | 87.8 (61.7) |
| Cancer stage, n (%) | ||||
| Ductal carcinoma in situ | 10 (11.1%) | 6 (6.9%) | 5 (5.8%) | 3 (3.4%) |
| I | 19 (21.1%) | 24 (27.6%) | 17 (19.5%) | 14 (16.0%) |
| II | 23 (25.6%) | 24 (27.6%) | 29 (33.3%) | 28 (32.2%) |
| III | 16 (17.8%) | 13 (14.9%) | 20 (23.0%) | 21 (24.1%) |
| Unknown | 22 (24.4%) | 20 (23.0%) | 16 (18.4%) | 21 (24.1%) |
| No. of nodes removed, n | 12.7 (9.5) | 12.6 (9.4) | 12.5 (9.8) | 12.0 (8.3) |
| Cancer treatments, n (%) | ||||
| Chemotherapy | 74 (82.2%) | 65 (74.7%) | 71 (81.6%) | 79 (90.8%) |
| Radiotherapy | 73 (81.1%) | 73 (83.9%) | 69 (79.3%) | 73 (83.9%) |
| Tamoxifen | 10 (11.1%) | 10 (11.5%) | 10 (11.5%) | 6 (6.9%) |
| Aromatase inhibitor | 31 (34.4%) | 25 (28.7%) | 22 (25.3%) | 24 (27.6%) |
| Arm volume difference, % | 9.6 (14.4) | 8.8 (16.6) | 8.7 (13.5) | 7.6 (13.7) |
| Body mass index, kg/m2 | 34.0 (5.7) | 34.0 (5.7) | 33.8 (5.6) | 34.2 (6.3) |
| Self-rated general health, n (%) | ||||
| Excellent | 4 (4.4%) | 1 (1.1%) | 4 (4.6%) | 1 (1.1%) |
| Very good | 22 (24.4%) | 25 (28.7%) | 21 (24.1%) | 20 (23.0%) |
| Good | 48 (53.3%) | 43 (49.4%) | 44 (50.6%) | 47 (54.0%) |
| Fair | 15 (16.7%) | 18 (20.7%) | 17 (19.5%) | 18 (20.7%) |
| Poor | 1 (1.1%) | 0 (0.0%) | 1 (1.1%) | 1 (1.1%) |
Values are mean ± standard deviation or n (%). Percentages may not sum to 100.0% due to rounding error.
At week 52, HRQoL endpoint data was provided by 244 (70%) participants (Figure 1). Participants who did not provide endpoint data had lower social functioning subscale scores [multivariable-adjusted odds ratio (OR): 1.02 per one-point decrease (95% CI: 1.00, 1.05)] and lower mental health subscale scores [OR: 1.02 per one-point decrease (95% CI: 1.00, 1.04)]; no other measured baseline factors, including randomized group assignment (P=0.30), were associated with missing week 52 endpoint data.
Figure 1.
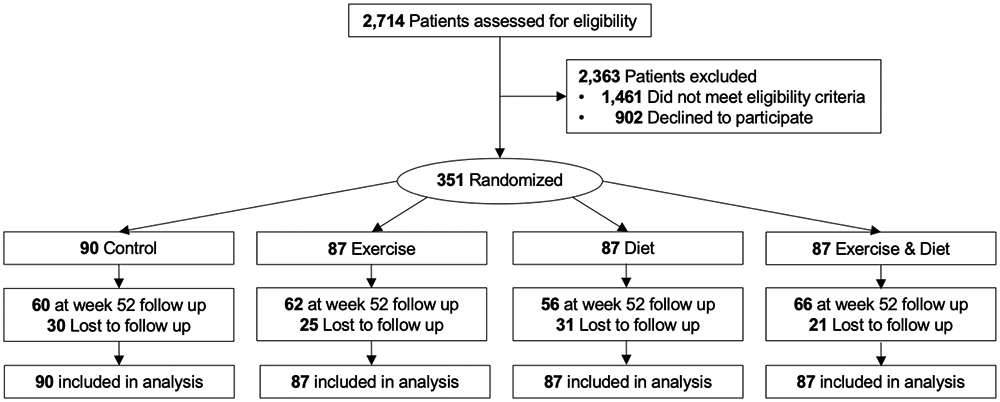
Flow of participants and ascertainment of health-related quality of life endpoint measures at week 52 by randomized group
Composite Summary Domains
Compared to control, exercise plus diet improved the self-reported physical health summary score [ETD: 5.39 (95% CI: 0.55, 10.22); d: 0.33 (95% CI: 0.04, 0.63); Table 2]; exercise alone [ETD: −1.91 (95% CI: −6.60, 2.79)] and diet alone [ETD: 3.16 (95% CI: −1.52, 7.83)] did not change the physical health summary score. Compared to control, exercise alone [ETD: −0.27 (95% CI: −5.02, 4.47)], diet alone [ETD: 3.25 (95% CI: −1.41, 7.91)], and exercise plus diet [ETD: 1.75 (95% CI: −2.90, 6.39)] did not change the self-reported mental health summary score.
Table 2.
Change in Medical Outcomes Survey-Short Form (SF)-36 by randomized group
| Outcome | Randomized Group |
Baseline Mean (SD) |
Mean Change (SE) |
Intervention Main Effect (95% CI) |
|---|---|---|---|---|
| Physical Health Summary Score | Control | 63.2 (21.3) | 2.40 (1.73) | 0.00—Reference |
| Exercise | 61.0 (20.3) | 0.49 (1.75) | −1.91 (−6.60, 2.79) | |
| Diet | 65.5 (20.3) | 5.56 (1.64)a | 3.16 (−1.52, 7.83) | |
| Exercise & Diet | 61.7 (19.1) | 7.79 (1.70)a | 5.39 (0.55, 10.22)b | |
| Mental Health Summary Score | Control | 69.8 (20.2) | 2.14 (1.71) | 0.00—Reference |
| Exercise | 68.3 (18.9) | 1.87 (1.73) | −0.27 (−5.02, 4.47) | |
| Diet | 70.4 (22.3) | 5.39 (1.68)a | 3.25 (−1.41, 7.91) | |
| Exercise & Diet | 70.4 (17.4) | 3.89 (1.68)a | 1.75 (−2.90, 6.39) | |
| Physical Functioning | Control | 67.5 (23.7) | 1.86 (1.73) | 0.00—Reference |
| Exercise | 66.6 (18.0) | 1.44 (1.74) | −0.42 (−5.23, 4.40) | |
| Diet | 67.6 (21.5) | 5.65 (1.82)a | 3.79 (–1.16, 8.74) | |
| Exercise & Diet | 67.1 (19.1) | 6.93 (1.72)a | 5.08 (0.28, 9.87)b | |
| Role–Physical | Control | 59.2 (41.6) | 2.60 (3.77) | 0.00—Reference |
| Exercise | 54.6 (41.1) | −2.05 (3.88) | −4.65 (−15.42, 6.12) | |
| Diet | 64.6 (40.3) | 7.26 (3.94) | 4.66 (−6.34, 15.66) | |
| Exercise & Diet | 52.9 (40.2) | 14.55 (3.89)a | 11.96 (1.51, 22.40)b | |
| Bodily Pain | Control | 70.9 (21.3) | 1.98 (1.95) | 0.00—Reference |
| Exercise | 65.3 (25.9) | 1.68 (1.89) | −0.31 (−5.54, 4.93) | |
| Diet | 70.8 (20.8) | 2.80 (1.94) | 0.82 (−4.50, 6.14) | |
| Exercise & Diet | 68.3 (21.6) | 4.13 (1.88)a | 2.14 (−3.28, 7.57) | |
| General Health | Control | 55.4 (21.8) | 3.14 (1.57)a | 0.00—Reference |
| Exercise | 57.4 (17.8) | 1.41 (1.57) | −1.72 (−6.02, 2.57) | |
| Diet | 58.9 (20.4) | 4.47 (1.63)a | 1.33 (−3.25, 5.92) | |
| Exercise & Diet | 58.4 (19.6) | 6.16 (1.57)a | 3.02 (−1.29, 7.34) | |
| Vitality | Control | 48.9 (21.0) | 3.24 (1.81) | 0.00—Reference |
| Exercise | 47.1 (20.3) | 4.38 (1.79)a | 1.14 (−3.97, 6.25) | |
| Diet | 50.2 (22.2) | 8.97 (1.83)a | 5.73 (0.70, 10.76)b | |
| Exercise & Diet | 49.0 (19.8) | 8.69 (1.86)a | 5.45 (0.39, 10.51)b | |
| Social Functioning | Control | 80.7 (24.6) | 0.34 (2.00) | 0.00—Reference |
| Exercise | 78.4 (21.1) | 0.32 (1.98) | −0.02 (−5.51, 5.48) | |
| Diet | 79.6 (25.0) | 4.37 (2.08)a | 4.03 (−1.67, 9.72) | |
| Exercise & Diet | 79.4 (20.8) | 2.62 (2.02) | 2.28 (−3.43, 8.00) | |
| Role–Emotional | Control | 74.4 (37.4) | 4.06 (3.69) | 0.00—Reference |
| Exercise | 72.0 (37.3) | 0.21 (3.65) | −3.84 (−13.82, 6.13) | |
| Diet | 75.9 (37.2) | 6.38 (3.76) | 2.32 (−8.06, 12.70) | |
| Exercise & Diet | 76.2 (33.3) | 2.53 (3.51) | −1.53 (−11.50, 8.44) | |
| Mental Health | Control | 75.1 (17.5) | 1.32 (1.38) | 0.00—Reference |
| Exercise | 75.7 (14.4) | 2.67 (1.32)a | 1.35 (−2.53, 5.22) | |
| Diet | 75.8 (18.1) | 2.75 (1.40) | 1.43 (−2.41, 5.27) | |
| Exercise & Diet | 77.1 (14.1) | 2.20 (1.33) | 0.88 (−2.87, 4.62) |
Note: Unobserved data were multiply imputed using predictive mean matching and analyzed using a repeated measures analysis of covariance model. Models are adjusted for the baseline value of the dependent variable, and randomization stratification factors including age, receipt of radiotherapy, number of lymph nodes resected, lymphedema severity, and body mass index.
P<0.05 compared with baseline (within group).
P<0.05 compared with control.
Physical Health Subscales
Compared to control, exercise plus diet improved the self-reported physical functioning subscale score [ETD: 5.08 (95% CI: 0.28, 9.87); d: 0.31 (95% CI: 0.02, 0.61); Figure 2]; exercise alone [ETD: −0.42 (95% CI: −5.23, 4.40)] and diet alone [ETD: 3.79 (95% CI: −1.16, 8.74)] did not improve the physical functioning subscale score. Compared to control, exercise plus diet improved the self-reported role—physical subscale score [ETD: 11.96 (95% CI: 1.51, 22.40); d: 0.33 (95% CI: 0.04, 0.63)]; exercise alone [ETD: −4.65 (95% CI: −15.42, 6.12)] and diet alone [ETD: 4.66 (95% CI: −6.34, 15.66)] did not improve the role—physical subscale score. Compared to control, exercise alone [ETD: −0.31 (95% CI: −5.54, 4.93)], diet alone [ETD: 0.82 (95% CI: −4.50, 6.14)], and exercise plus diet [ETD: 2.14 (95% CI: −3.28, 7.57)] did not improve the bodily pain subscale score. Compared to control, exercise alone [ETD: −1.72 (95% CI: −6.02, 2.57)], diet alone [ETD: 1.33 (95% CI: −3.25, 5.92)], and exercise plus diet [ETD: 3.02 (95% CI: −1.29, 7.34)] did not improve the general health subscale score.
Figure 2.
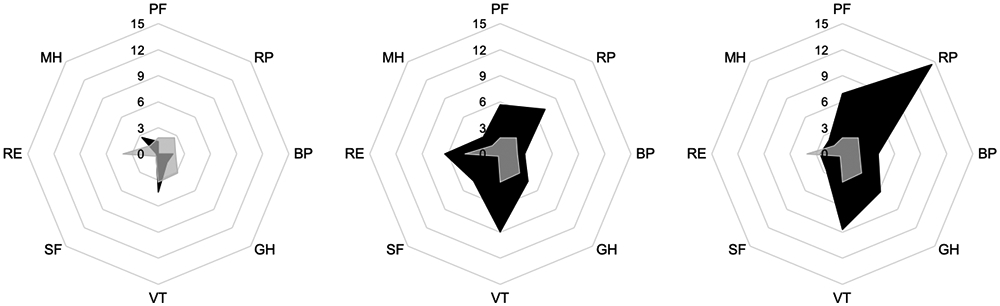
Radar chart of change in Medical Outcomes Survey-Short Form (SF)-36 subscales comparing the control group (grey shade) with exercise alone (red shade; left panel), diet alone (yellow shade; middle panel), and exercise plus diet (blue shade; right panel)
Each point around the octagon represents an SF-36 subscale, each octagonal ring represents a three-point change in the outcome measure, the lines of each colored shape intersect the octagonal rings that correspond to the change from baseline to week 52. PF, physical functioning; RP; role—physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role—emotional; MH, mental health. The negative value of role physical for exercise (−2.05) was truncated to zero to facilitate comparison between groups.
Mental Health Subscales
Compared to control, diet [ETD: 5.73 (95% CI: 0.70, 10.76); d: 0.34 (95% CI: 0.04, 0.63)] and exercise plus diet [ETD: 5.45 (95% CI: 0.39, 10.51); d: 0.32 (95% CI: 0.02, 0.61)] improved the self-reported vitality subscale score; exercise alone [ETD: 1.14 (95% CI −3.97, 6.25)] did not improve the vitality subscale score. Compared to control, exercise alone [ETD: −0.02 (95% CI: −5.51, 5.48)], diet alone [ETD: 4.03 (95% CI: −1.67, 9.72)], and exercise plus diet [ETD: 2.28 (95% CI: −3.43, 8.00)] did not improve the social functioning subscale score. Compared to control, exercise alone [ETD: −3.84 (95% CI: −13.82, 6.13)], diet alone [ETD: 2.32 (95% CI: −8.06, 12.70)], and exercise plus diet [ETD: −1.53 (95% CI: −11.50, 8.44)] did not improve the role—emotional subscale score. Compared to control, exercise alone [ETD: 1.35 (95% CI: −2.53, 5.22)], diet alone [ETD: 1.43 (95% CI: −2.41, 5.27)], and exercise plus diet [ETD: 0.88 (95% CI: −2.87, 4.62)] did not improve the mental health subscale score.
Adverse Events
No serious or unexpected adverse events were reported; nonserious adverse events have been described.20
DISCUSSION
In this trial of 351 survivors of breast cancer with overweight or obesity, 52 weeks of exercise plus diet improved HRQoL endpoints that related to overall physical health, and subscales related to physical functioning, limitations because of physical health problems, and energy and fatigue. Diet alone improved the subscale related to energy and fatigue. Exercise alone had no impact on HRQoL endpoints.
Obesity and weight gain are associated with poor HRQoL.8, 9 Among 661 survivors of breast cancer in the Health, Eating, Activity, and Lifestyle (HEAL) cohort, obesity was associated with statistically significantly poorer SF-36 physical health summary scores, and a poorer physical functioning subscale score.8 Furthermore, over 30 months, compared to participants who were weight stable, those who gained ≥5% of their baseline body weight reported a deterioration in the physical health summary score as well as a decline in the physical functioning and role—physical subscale scores.8 Our data indicate that the negative effects of obesity and weight gain on HRQoL in survivors of breast cancer can be reversed with a combination of exercise plus diet.
Poor HRQoL in survivors of breast cancer is associated with an increased risk of cancer recurrence and death.10-12 Among 2,967 survivors of breast cancer in the Women’s Healthy Eating and Living (WHEL) study, participants with a poorer SF-36 physical health summary score at baseline had a 42% higher risk of experiencing breast cancer recurrence or a second breast cancer, and a 37% higher risk of death from any cause, as compared with participants with a better physical health summary score.11 In the WHEL cohort, participants who reported poorer physical health summary scores were more likely to have obesity and engage in less physical activity.11 These findings highlight the potential clinical importance of the HRQoL improvements that were observed with exercise plus diet in the current trial.
The HRQoL benefits of exercise plus diet in the current trial are similar to that of the Nutrition and Exercise for Women (NEW) trial, a 2×2 factorial design of 52 weeks of exercise and diet in postmenopausal women aged 50–75 years with overweight or obesity.31 These findings are also consistent with randomized trials of multimodal lifestyle weight loss interventions that combine exercise, diet, and behavioral therapy in survivors of cancer.13-15 The HRQoL benefits of exercise plus diet were generally limited to dimensions of physical health, but not mental health, which is similar to the general population.37, 38
The mechanisms through which exercise and diet improve HRQoL may be mediated, in part, by changes in both cardiopulmonary fitness and weight loss.31, 39 The Sex Hormone and Physical Exercise (SHAPE-2) trial demonstrated that a 6–7% weight loss achieved by diet only or mainly by exercise both improve HRQoL endpoints in postmenopausal women aged 50–69 years, however exercise-induced weight loss achieved modestly larger HRQoL benefits, as compared to diet-induced weight loss.40 Exercise improves cardiovascular fitness and induces weight loss in a dose dependent manner; thus, it is possible that the dose of exercise prescribed in the current study was of insufficient volume to induce meaningful changes in HRQoL endpoints,41 as we did not observe statistically significant improvements in cardiorespiratory fitness.20
There are limitations to this trial. This trial was designed to examine the effects of two different lifestyle modalities, exercise and diet, for which it is challenging to blind study participants to treatment assignment. Missing endpoint data at week 52 was more common in participants who reported poorer social functioning and mental health subscale scores at baseline, which has been reported in other longitudinal studies of survivors of cancer.42 The rates of missing endpoint data, however, did not differ among randomized groups. Nonetheless, additional research is needed to maximize adherence and retention to lifestyle interventions in this population. Due to our inability to blind participants to treatment assignment, concern may exist that participants are unable to provide unbiased estimates of their HRQoL, however data to support this viewpoint are limited.43 The study sample was not recruited on the basis of reporting poor HRQoL at baseline, which may limit our ability to detect treatment effects. Moreover, our understanding of the treatment effect in populations with poor HRQoL at baseline is limited. All study participants had breast cancer-related lymphedema, which is associated with poorer HRQoL.44
There are strengths to this trial. The randomized design and use of two distinct interventions that are both hypothesized to improve HRQoL endpoints allowed for a time- and cost-efficient estimation of treatment effects. The large sample size allowed us to detect relatively small treatment effect differences. The diversity of the study sample with respect to race, education, and time since breast cancer diagnosis, improves the generalizability of study findings. The exercise and diet interventions utilized a variety of resources that are commercially available, including home-based exercise weights and meal replacement programs.
In survivors of breast cancer with overweight or obesity, the combination of exercise plus diet produced improvements in select HRQoL endpoints at week 52. The combination of exercise plus diet may be the optimal lifestyle prescription to improve HRQoL in survivors of breast cancer with overweight or obesity. These findings support the need for practice and policy changes to assure the availability of effective, affordable, and feasible multimodal lifestyle interventions in survivors of breast cancer with overweight or obesity.27
Funding:
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U54-CA155850, UL1-TR001878, P30-CA016520, P30-CA006927. Compression garments were donated by BSN Medical, and discounted meal replacements were provided by Nutrisystem, Inc. Dr. Brown is supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R00-CA218603 and R25-CA203650, the National Institute of General Medicine Sciences of the National Institutes of Health under Award Number U54-GM104940, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30-DK072476, the Susan G. Komen Foundation, and the American Institute for Cancer Research. Dr. Sarwer is supported by the National Institute for Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01-DK108628. Dr. Sturgeon is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1-TR002014, UL1-TR000003, and KL2-TR002015. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: Dr. Brown reports receiving grants from the National Institutes of Health, American Institute for Cancer Research, and the Susan G. Komen Foundation. Dr. Sarwer reports receiving grants from the National Institutes of Health and receiving personal fees from Ethicon and Novo Nordisk. Dr. Troxel reports receiving grants from the National Institutes of Health. Dr. DeMichele reports receiving grants from Novartis, Pfizer, Genentech, Calithera, and Menarini. Dr. Sturgeon reports receiving grants from the National Institutes of Health. Dr. Denlinger reports receiving grants from Agios Pharmaceuticals, Amgen Pharmaceuticals, Array BioPharma, Astra Zeneca, Bristol Myer Squibb, BeiGene, Genmab A/S, Loxo Oncology, and Zymeworks Inc. and honoraria from Bristol Myer Squibb, Merck, Exelixis, Taiho Oncology, and BeiGene. Dr. Schmitz reports receiving grants from the National Institutes of Health and nonfinancial support from BSN Medical, personal fees from Klose Training, and a licensed patent for a Strength After Breast Cancer course. No other disclosures were reported.
REFERENCES
- 1.Bray GA, Kim KK, Wilding JPH, World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18: 715–723. [DOI] [PubMed] [Google Scholar]
- 2.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118: 5937–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12: 282–294. [DOI] [PubMed] [Google Scholar]
- 4.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34: 3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro CL. Cancer Survivorship. N Engl j Med. 2018;379: 2438–2450. [DOI] [PubMed] [Google Scholar]
- 6.Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf). 2005;27: 156–164. [DOI] [PubMed] [Google Scholar]
- 7.Fine JT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282: 2136–2142. [DOI] [PubMed] [Google Scholar]
- 8.Imayama I, Alfano CM, Neuhouser ML, et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res Treat. 2013;140: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118: 4024–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falagas ME, Zarkadoulia EA, Ioannidou EN, Peppas G, Christodoulou C, Rafailidis PI. The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast Cancer Res. 2007;9: R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saquib N, Pierce JP, Saquib J, et al. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. 2011;20: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv. 2013;7: 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301: 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32: 2231–2239. [DOI] [PubMed] [Google Scholar]
- 15.Demark-Wahnefried W, Colditz GA, Rock CL, et al. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Cancer Res Treat. 2015;154: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21: 1660–1668. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ML, Cartmel B, Harrigan M, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123: 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz KH, Gehlert S, Patterson RE, et al. TREC to WHERE? Transdisciplinary Research on Energetics and Cancer. Clin Cancer Res. 2016;22: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkels RM, Sturgeon KM, Kallan MJ, et al. The women in steady exercise research (WISER) survivor trial: The innovative transdisciplinary design of a randomized controlled trial of exercise and weight-loss interventions among breast cancer survivors with lymphedema. Contemp Clin Trials. 2017;61: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz KH, Troxel AB, Dean LT, et al. Effect of Home-Based Exercise and Weight Loss Programs on Breast Cancer-Related Lymphedema Outcomes Among Overweight Breast Cancer Survivors: The WISER Survivor Randomized Clinical Trial. JAMA Oncol. 2019;5: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturgeon KM, Hackley R, Fornash A, et al. Strategic recruitment of an ethnically diverse cohort of overweight survivors of breast cancer with lymphedema. Cancer. 2018;124: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health UDo, Services H. Common terminology criteria for adverse events (CTCAE) version 4.0. National Cancer Institute. 2009. [Google Scholar]
- 23.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clinical Pharmacology and Therapeutics. 1974;15: 443–453. [DOI] [PubMed] [Google Scholar]
- 24.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl j Med. 2009;361: 664–673. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304: 2699–2705. [DOI] [PubMed] [Google Scholar]
- 27.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62: 243–274. [DOI] [PubMed] [Google Scholar]
- 28.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl j Med. 2011;365: 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Medical care. 1995: AS264–AS279. [PubMed] [Google Scholar]
- 30.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer). Lymphology. 1997;30: 77–97. [PubMed] [Google Scholar]
- 31.Imayama I, Alfano CM, Kong A, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. The International Journal of Behavioral Nutrition and Physical Activity. 2011;8: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coens C, Pe M, Dueck AC, et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21: e83–e96. [DOI] [PubMed] [Google Scholar]
- 33.Little RJ. Missing-data adjustments in large surveys. Journal of Business & Economic Statistics. 1988;6: 287–296. [Google Scholar]
- 34.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, 2012. [Google Scholar]
- 35.Cohen J Statistical power analysis for the behavioural sciences (Rev. ed.). New York: Academic. 1977. [Google Scholar]
- 36.Saary MJ. Radar plots: a useful way for presenting multivariate health care data. J Clin Epidemiol. 2008;61: 311–317. [DOI] [PubMed] [Google Scholar]
- 37.Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15: 169–182. [DOI] [PubMed] [Google Scholar]
- 38.Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7: 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Gemert WA, van der Palen J, Monninkhof EM, et al. Quality of Life after Diet or Exercise-Induced Weight Loss in Overweight to Obese Postmenopausal Women: The SHAPE-2 Randomised Controlled Trial. PloS One. 2015;10: e0127520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsey I, de Rooij BH, Mols F, et al. Cancer survivors who fully participate in the PROFILES registry have better health-related quality of life than those who drop out. J Cancer Surviv. 2019;13: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson TM, Wagner JS, Basch E. Trustworthiness of Patient-Reported Outcomes in Unblinded Cancer Clinical Trials. JAMA Oncol. 2017;3: 738–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol. 2008;26: 5689–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]


