Abstract
Background and purpose:
Overnight shifts of fluid from lower to upper compartments exacerbate obstructive sleep apnea (OSA) in some OSA populations. Given the high prevalence of OSA after stroke, decreased mobility and use of IV fluids among hospitalized stroke patients, and improvement in OSA in the months after stroke, we hypothesized that overnight fluid shifts occur and are associated with OSA among patients with subacute ischemic stroke.
Methods:
Within a population-based project, we performed overnight sleep apnea tests (ApneaLink Plus) during ischemic stroke hospitalizations. Prior to sleep that evening, and the following morning prior to rising from bed, we assessed neck and calf circumference, and leg fluid volume (bioimpedance spectroscopy). The average per subject overnight change in the three fluid shift measurements was calculated and compared with zero. Linear regression was used to test the crude association between each of the three fluid shift measurements and the respiratory event index (REI).
Results:
Among the 292 participants, mean REI was 24 (SD=18). Within individuals, calf circumference decreased on average by 0.66 cm (SD 0.75 cm, p<0.001), leg fluid volume decreased by a mean of 135.6 ml (SD 132.8 ml, p<0.001), and neck circumference increased by 0.20 cm (SD 1.71 cm, p=0.07). In men, when the overnight change of calf circumference was negative, an IQR (0.8 cm) decrease in calf circumference overnight was significantly associated with a 25.1% increase in REI (p=0.02); the association was not significant in women. The relationship between overnight change in leg fluid volume and REI was U shaped.
Conclusions:
This population-based, multicenter, cross-sectional study showed that in hospitalized ischemic stroke patients, nocturnal rostral fluid shifts occurred, and two of the three measures were associated with greater OSA severity. Interventions that limit overnight fluid shifts should be tested as potential treatments for OSA among patients with subacute ischemic stroke.
Keywords: Stroke, Sleep Apnea, Obstructive, Shifts, Fluid
Subject terms: Ischemic Stroke, Cerebrovascular Disease/Stroke, Risk Factors
Obstructive sleep apnea (OSA) represents an important disease in stroke patients given its high prevalence1 and association with worse outcomes including poor functional and cognitive outcomes,2 higher mortality,3 and greater risk of recurrent stroke.4 The pathophysiology of post-stroke OSA is poorly understood and its treatment is challenging due to limited tolerance of standard treatments in stroke patients.5, 6 New potential treatment targets that lend themselves to simple, tolerable interventions are needed. Nocturnal rostral fluid shifts may represent one such novel treatment target. Overnight redistribution of fluid from the legs to more rostral compartments such as the neck and thorax is believed to contribute to OSA in non-obese men,7 those with heart failure,8 venous insufficiency,9 end stage renal disease,10 and drug-resistant hypertension.11 Rostral fluid shifts are associated with decreased pharyngeal airway size,12 increased upper airway collapsibility,13 and worse sleep apnea, measured by the apnea-hypopnea index (AHI, number of events per hour of sleep).7, 8, 11 Furthermore, daytime administration of positive pressure to the lower extremities attenuates these fluid shifts and reduces AHI.9, 12, 14 Although to our knowledge, rostral fluid shifts have not been previously investigated after stroke, stroke patients may be particularly affected by fluid redistribution because standard treatment of acute ischemic stroke involves administration of intravenous fluids.15 Furthermore, due to weakness and discoordination, decreased physical activity is common after stroke, especially in hospitalized patients, a factor that may contribute to daytime pooling of fluid.
The purpose of this study was to assess for nocturnal rostral fluid shifts in stroke patients and to test their association with OSA severity. We hypothesized that within individuals overnight, neck circumference would increase, calf circumference would decrease, and leg fluid volume would decrease, and that these changes would be associated with greater OSA severity. Identification of nocturnal rostral fluid shifts and their association with OSA severity, in patients with recent stroke, could identify a novel potential treatment target.
Materials and Methods
The data that support the findings of this study are not available to other investigators as this process is not covered by the informed consent document. A STROBE checklist was completed and can be found in the supplemental materials (Table I). This study was conducted within the Brain Attack Surveillance in Corpus Christi (BASIC) sleep apnea projects. Methods for BASIC16 and its associated sleep apnea projects17 have been detailed previously. In BASIC, active and passive surveillance identifies acute ischemic stroke cases from all 7 hospitals in Nueces County, Texas. Patients who are younger than 45 years and those who are not residents of Nueces County are excluded. Those who qualify are offered enrollment into BASIC which includes an in-person baseline interview (incorporating the Berlin Questionnaire in reference to risk for OSA in the pre-stroke state) and follow-up assessments for clinical outcomes, based on in-home visits, including at 3 months. Between November 2015 and August 2018, patients who qualified for BASIC were offered enrollment into the current study if they were not pregnant, did not use oxygen, were not mechanically ventilated or using positive pressure ventilation, and were still hospitalized or institutionalized at the time of enrollment (to allow for access to subjects before rising from bed). The study was approved by the Institutional Review Boards of the University of Michigan and the two Corpus Christi hospital systems. Written informed consent was obtained for each subject from the patient or a legally authorized representative.
Procedures
On the evening of testing, the subject completed hourly logs about body position (time in bed, time in chair, and time on feet) and use of pneumatic compression devices on the legs retrospectively for the prior daytime and prospectively overnight. If the subject was not able to complete these, family or nursing assistance was sought. Most recent height and weight were abstracted from the medical record.
Body fluid measurements, including neck and calf circumference and leg fluid volume, were obtained at night, after retiring to bed, and the following morning, before rising from bed using standard techniques.7 Pillows were removed and bed angle was made flat for all recordings. If this was not possible, the nighttime incline and pillow use were replicated in the morning. Neck and calf circumferences were assessed with a tape measure. The exact position of the tape was marked so it could be replicated the following morning. Leg fluid measurements were made via bioimpedance spectroscopy (BIS) using the SFB7 device (ImpediMed, Inc). This procedure is highly accurate, reliable, and previously validated,18–21 and allows for calculation of fluid volume based on electrical current resistance. Resistance and reactance values were obtained for the right and left leg using dual tab electrodes placed on the ipsilateral thigh and ankle. Segmental fluid volumes were calculated based on the geometry of a tapered cylinder, using thigh and ankle circumferences and the distance between the electrodes (leg length). Whole-body BIS measurements including whole-body water, extracellular fluid, intracellular fluid, fat free mass, and fat mass were taken using electrodes placed on the right hand and ankle. The leg most affected and least affected by stroke was recorded based on self-report. BIS measurements were not performed in those with a pacemaker, defibrillator, or bilateral leg prosthesis. In cases where a metallic orthopedic implant was present, that limb was excluded from BIS measurement, and whole-body BIS measurements were made using the other side. BIS measurements were excluded in subjects with a BMI>35 as the values may be technically inaccurate.22–24 If BIS and calf circumference measurements were available for both legs, the side least affected by the stroke, based on self-report, was selected.
OSA was assessed with the well-validated ApneaLink Plus device21 during the intervening night between the nighttime and morning fluid assessments. The ApneaLink Plus device is a portable monitor that measures nasal pressure, oxygen saturation, respiratory effort, and pulse. The ApneaLink software scored the apneic events using its default settings that were applied during validation studies. Following this, a registered polysomnographic technologist edited the recording for mis-scored events and to correct the start/stop times for presumed wakefulness. The software then tabulated the sum of apneas (obstructive and central) plus hypopneas per hour of recording to calculate the respiratory event index (REI). After stroke, the vast majority of respiratory events are obstructive.1 The REI approximates the AHI, which uses time asleep as the denominator.
Analysis:
The average per subject overnight change in the three fluid shift measurements was calculated and compared with zero. Multivariable linear regression was used to identify potential predictors of each overnight change variable. Variables considered are found in Table II. Continuous variables were centered around their respective mean, including age, BMI, daytime hours spent in chairs, waist circumference, and daytime hours wearing sequential compression devices. Linear regression was used to test the crude association between each of the three fluid shift measurements (overnight change within person) separately and REI. Due to its skewed distribution, REI was log transformed (log(REI+1)). Interactions with sex were assessed given an a priori suspicion based on the literature.25 A sex by fluid shift interaction was found for overnight change in calf circumference (p=0.06) but not overnight change in leg fluid volume or neck circumference, and therefore only the calf circumference models were stratified by sex. Residuals plots were assessed to determine the appropriate functional form of variables. Splines, log transformations, and quadratic terms were used as appropriate. A quadratic term was added to the overnight change in leg fluid model, and the overnight change in calf circumference was treated with a linear spline with one knot. Examination of overnight neck circumference showed little deviation from a linear association and therefore was modeled linearly. In secondary models, additional adjustments were made for potential confounders including age, ethnicity, nighttime measurements, and overnight change in total body water (TBW). Interaction terms for overnight change in the fluid shift measurement by nighttime fluid shift measurement interaction were assessed in each model but excluded from final models due to a lack of statistical significance (p>0.20). In exploratory analysis, in three separate linear regression models, the adjusted R2 was calculated for REI (outcome) and nighttime extracellular fluid (ECF), baseline waist circumference, and most recent weight recorded in the medical record prior to ApneaLink testing. Outliers for overnight change in the fluid shift values were removed prior to analyses after visual inspection of plots as follows: 99th percentile and values >10cm for neck circumference, <−4cm and >2.5cm for calf circumference, <−600ml and a single value of 679ml for leg fluid volume. Missing data were addressed with case-wise deletion. Statistical software R was used for all analyses.
Results
Of the 292 study participants, 161 (55%) were men, 85 (29%) were non-Hispanic white, 175 (60%) were Mexican American, 30 (10%) were other race/ethnicity, and two had unknown race/ethnicities (Figure I). Baseline characteristics are found in table 1 (n=292). Median ApneaLink Plus recording evaluation time was 8.2 hours (IQR: 6.6, 9.0). Mean REI was 24 (SD=18). Analysis of calf and neck circumferences included n=260 after exclusion of other and unknown race/ethnicities due to their small numbers. For analysis of leg fluid volume, n=137 were available after limitation to only Mexican Americans and non-Hispanic whites and those with a BMI ≤35 (Figure I).
Table 1.
Baseline characteristics of ischemic stroke participants (n=292).
| All | Men | Women | |||||
| Categorical variables | Missing | Count | Proportion | Count | Proportion | Count | Proportion |
| Hypertension | 7 | 247 | 0.85 | 137 | 0.55 | 110 | 0.45 |
| Diabetes | 7 | 163 | 0.56 | 83 | 0.51 | 80 | 0.49 |
| Atrial fibrillation | 7 | 31 | 0.11 | 13 | 0.42 | 18 | 0.58 |
| High cholesterol | 8 | 136 | 0.47 | 78 | 0.57 | 58 | 0.43 |
| Coronary disease | 9 | 87 | 0.3 | 55 | 0.63 | 32 | 0.37 |
| Congestive heart failure | 7 | 29 | 0.1 | 12 | 0.41 | 17 | 0.59 |
| Current smoking | 8 | 118 | 0.4 | 79 | 0.67 | 39 | 0.33 |
| History of stroke/TIA | 7 | 113 | 0.39 | 59 | 0.52 | 54 | 0.48 |
| Alcohol abuse | 8 | 9 | 0.03 | 7 | 0.78 | 2 | 0.22 |
| Berlin questionnaire in reference to pre-stroke state (high risk) | 13 | 132 | 0.45 | 66 | 0.50 | 66 | 0.50 |
| REI>=5 | 54 | 216 | 0.74 | 118 | 0.55 | 98 | 0.45 |
| All | Men | Women | |||||
| Continuous variables | Missing | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 0 | 65.62 | 11.74 | 63.70 | 10.32 | 67.98 | 12.93 |
| Body mass index (kg/m2) | 1 | 29.69 | 6.49 | 29.40 | 5.11 | 30.05 | 7.88 |
| In chair, daytime (7 am – 9 pm), hrs | 1 | 5.63 | 3.71 | 5.70 | 3.81 | 5.53 | 3.60 |
| Sequential compression devices, daytime (7 am – 9 pm), hrs | 0 | 1.52 (78.4% no use) | 3.74 | 1.56 (79.5% no use) | 3.80 | 1.48 (77.1% no use) | 3.68 |
| Sequential compression devices, overnight (9pm – 6 am), hrs | 3 | 1.16 (85.5% no use) | 2.99 | 1.23 (84.4% no use) | 3.04 | 1.07 (86.8% no use) | 2.93 |
| NIH stroke scale score | 9 | 4.64 | 4.90 | 4.16 | 4.37 | 5.22 | 5.42 |
| Waist circumference, cm | 2 | 181.70 | 242.46 | 174.85 | 233.37 | 190.13 | 253.85 |
| Calf circumference, cm (nighttime) | 7 | 34.86 | 4.38 | 35.47 | 4.10 | 34.13 | 4.60 |
| Leg fluid volume, ml (nighttime) | 108 | 2062.45 | 524.43 | 2260.89 | 519.49 | 1767.48 | 372.76 |
| Neck circumference, cm (nighttime) | 7 | 41.35 | 5.19 | 43.78 | 4.54 | 38.38 | 4.34 |
| Respiratory Event Index (REI, number per hour of recording) | 54 | 23.82 | 17.67 | 26.34 | 18.45 | 20.84 | 16.27 |
| Central apnea index (CAI) | 54 | 2.34 | 4.60 | 2.74 | 4.61 | 1.86 | 4.56 |
Overnight fluid shifts
All three fluid measurements changed or showed a trend in the expected direction overnight. Within individuals, calf circumference decreased on average by 0.66 cm (SD 0.75 cm, p<0.001), leg fluid volume decreased on average by a mean of 135.6 ml (SD 132.8 ml, p<0.001), and neck circumference increased by 0.20 cm (SD 1.71 cm, p=0.07). Figure 1 shows the histograms of the overnight fluid shifts. No differences in overnight change in the fluid shift measurements existed by sex (data not shown). In multivariable models to predict overnight change in each of the three fluid shift measurements, only time spent in a chair during the day was associated with an overnight decrease in calf circumference and decrease in leg fluid volume (Table II).
Figure 1.
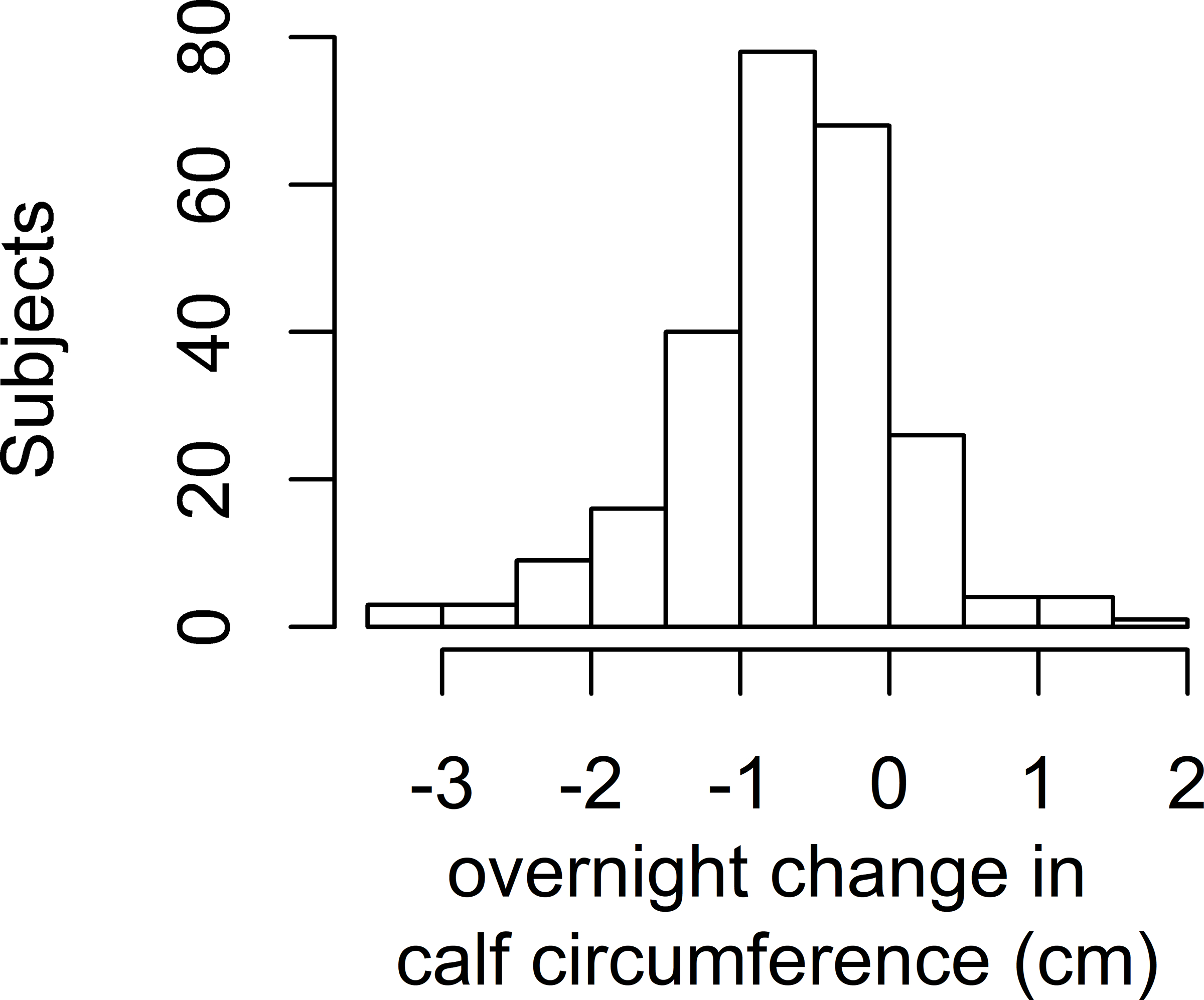
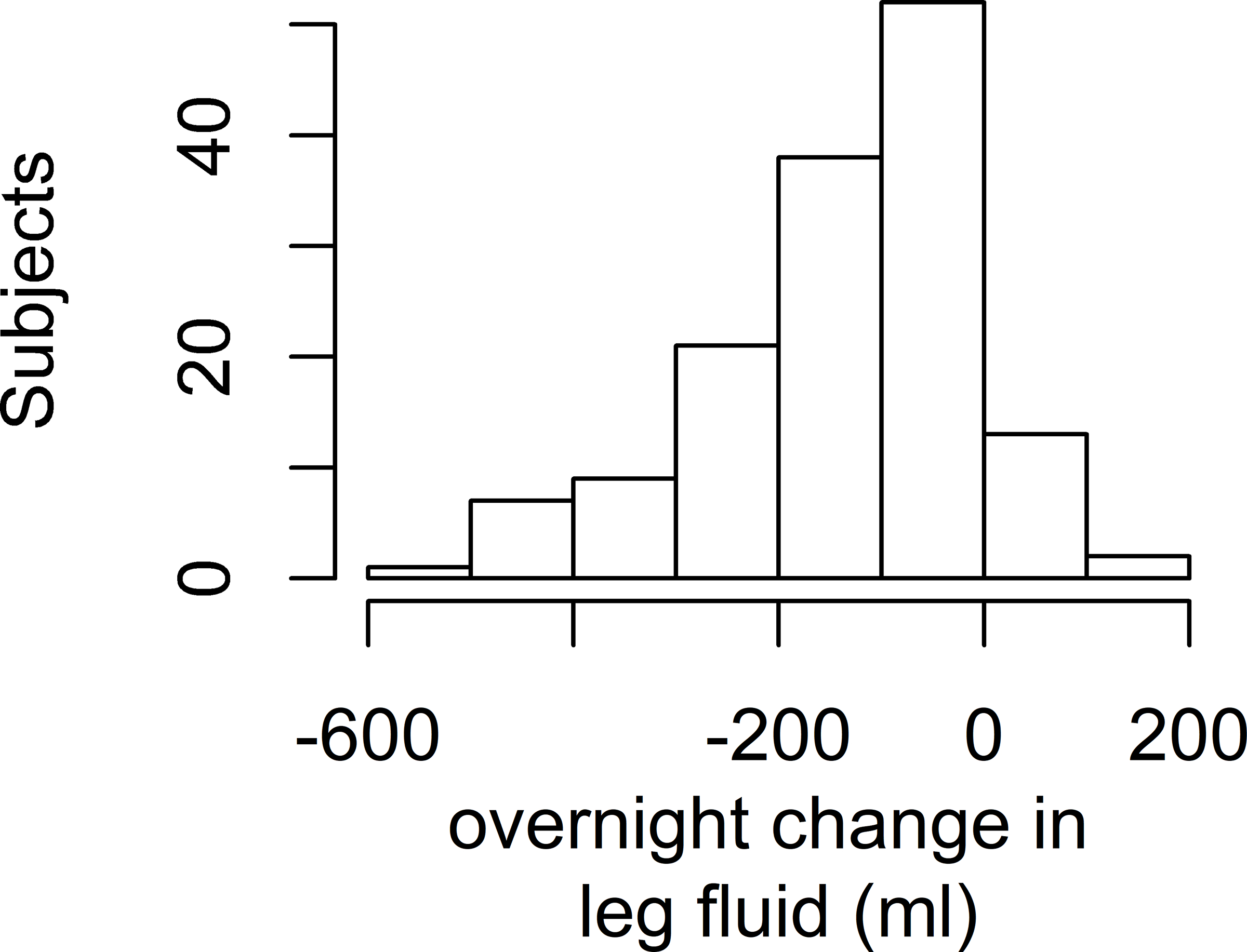
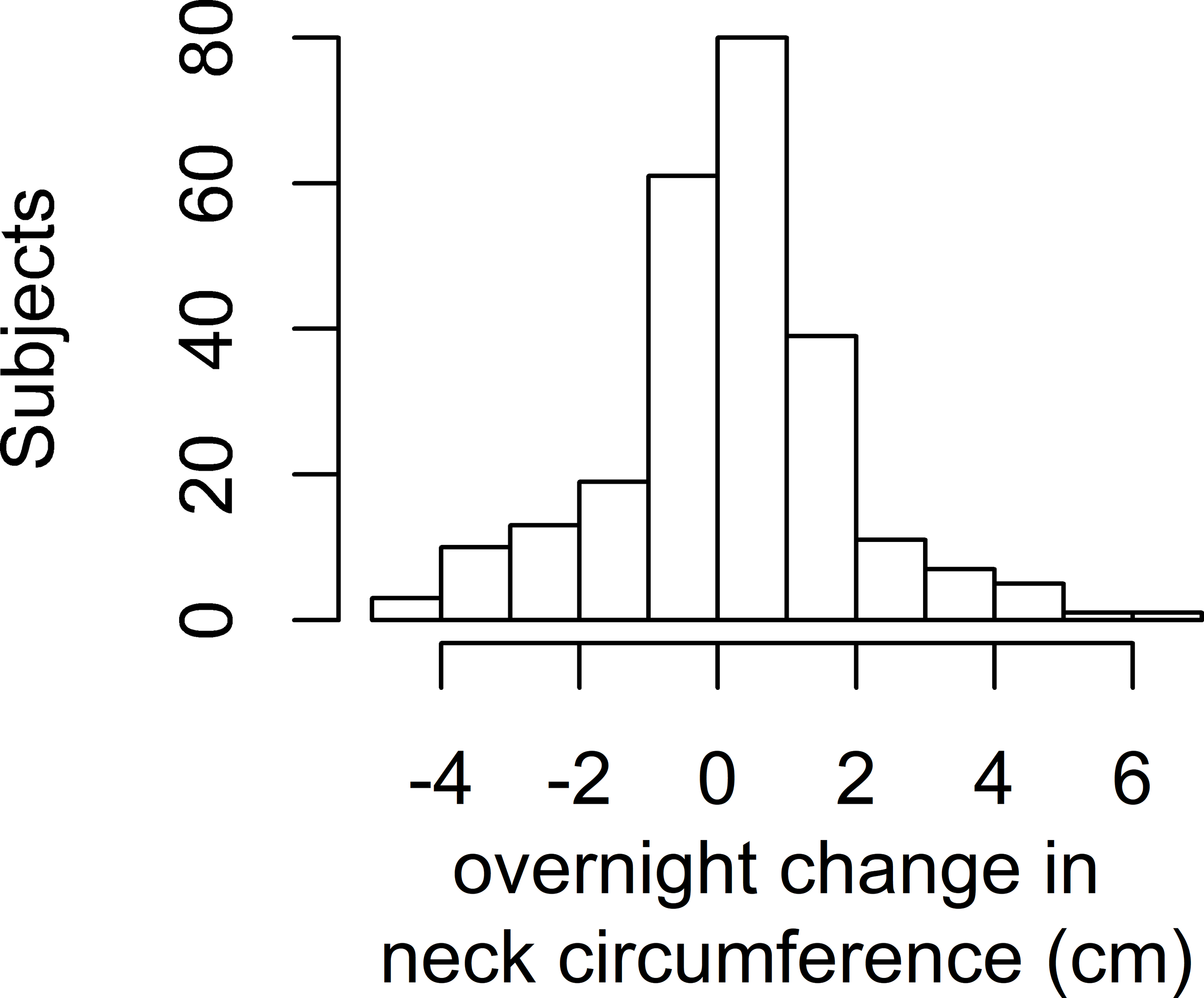
Histograms of Overnight Fluid Shifts
Figure 1a: Histogram of overnight change in calf circumference
Figure 1b: Histogram of overnight change in leg fluid volume
Figure 1c: Histogram of overnight change in neck circumference
Relationship between overnight fluid shift measurements and OSA severity
Calf circumference:
A nonlinear association with overnight change in calf circumference and log(REI+1) was modeled by including a piecewise linear spline with one knot located at zero, which segregated the association into negative and positive components. Within each component, a linear association between the overnight change in calf circumference and log(REI+1) was found. In addition, because an interaction term between sex and overnight change in calf circumference was borderline significant (p=0.06), sex stratified models are presented for calf circumference. As shown in Figure 2a, for men, higher magnitudes of change (either increase or decrease overnight) in calf circumference were associated with increased REI. For the purpose of interpretation, the results are based on a back transformation of the outcome to the original scale. This gives the percent increase (or decrease) in the REI for every one-unit increase in the overnight change in calf circumference. Specifically, when the overnight change of calf circumference was negative, an IQR (0.8 cm) decrease in calf circumference overnight was significantly associated with a 25.1% higher REI (p=0.02). In contrast, when the overnight change in calf circumference was positive, an IQR (0.8 cm) increase in calf circumference overnight was associated with a 56.5% higher REI but this association was not significant (p=0.19). In men, after adjustment for age, ethnicity, nighttime calf circumference, and overnight change in TBW, a similar trend was found; higher magnitudes of change (either increase or decrease over night) in calf circumference were associated with higher REI (Table 2). In women, there was no association between change in calf circumference and log(REI+1) and no other variables tested were associated with REI (Figure 2b, Table 2).
Figure 2.
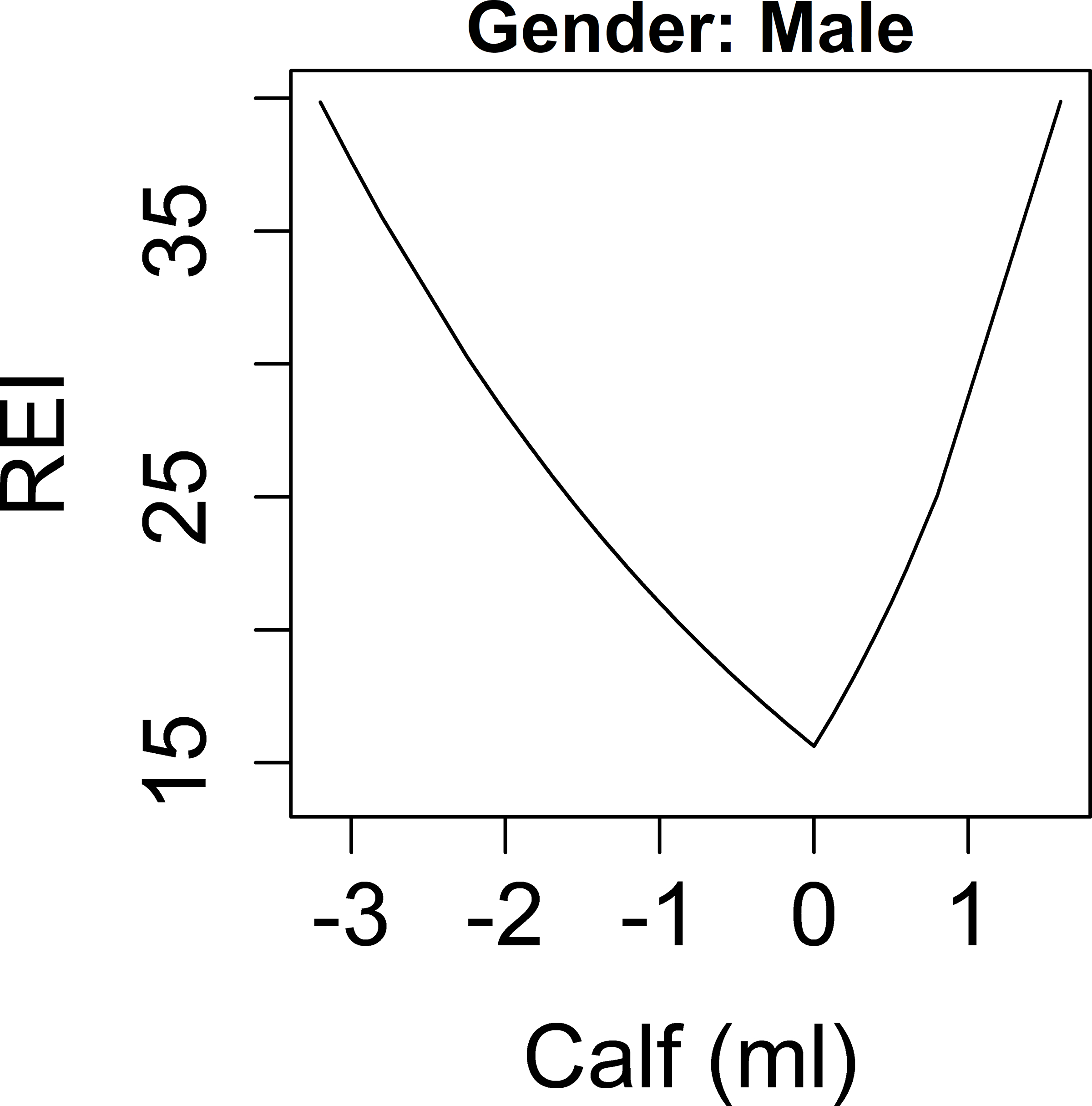
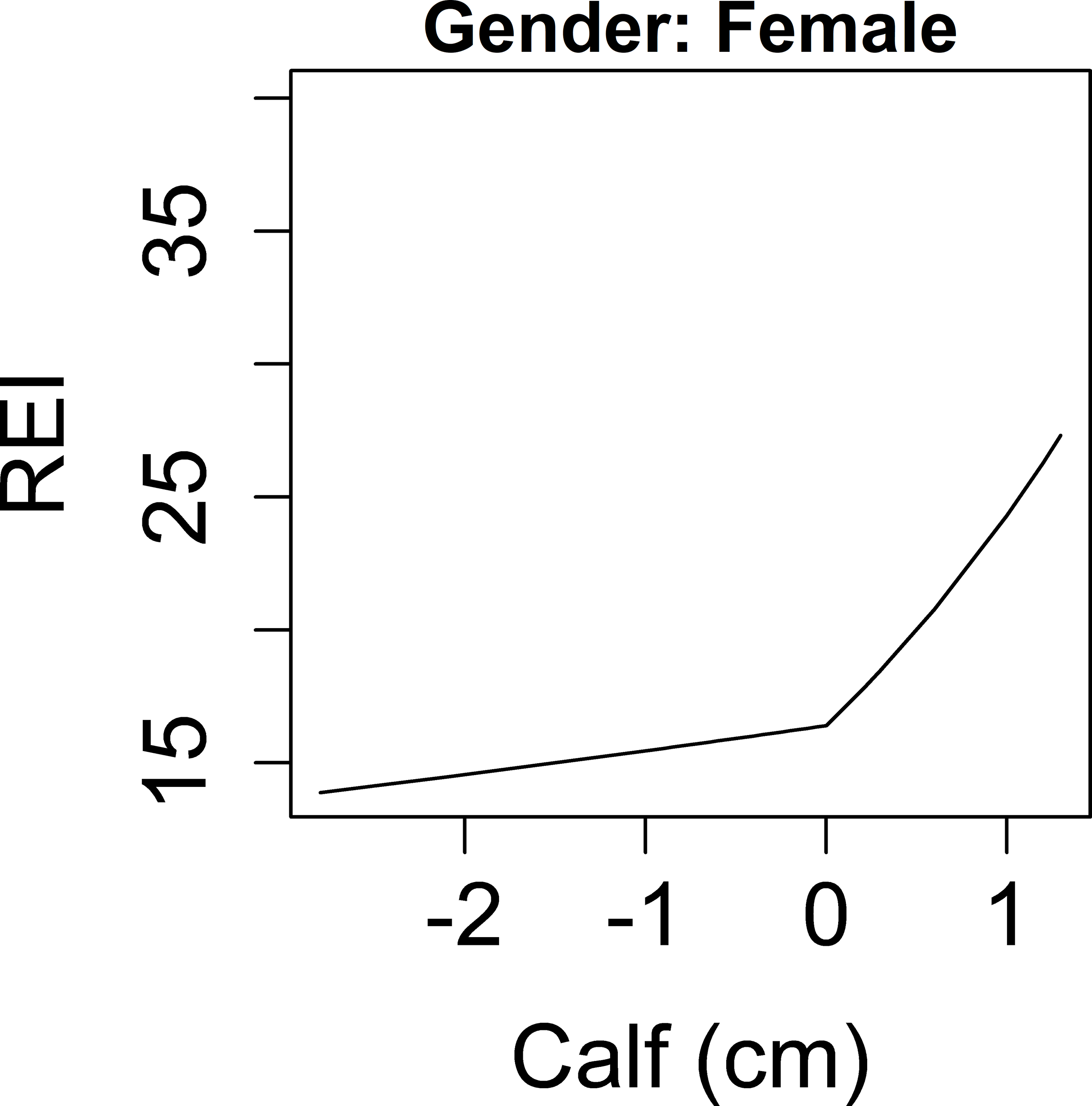
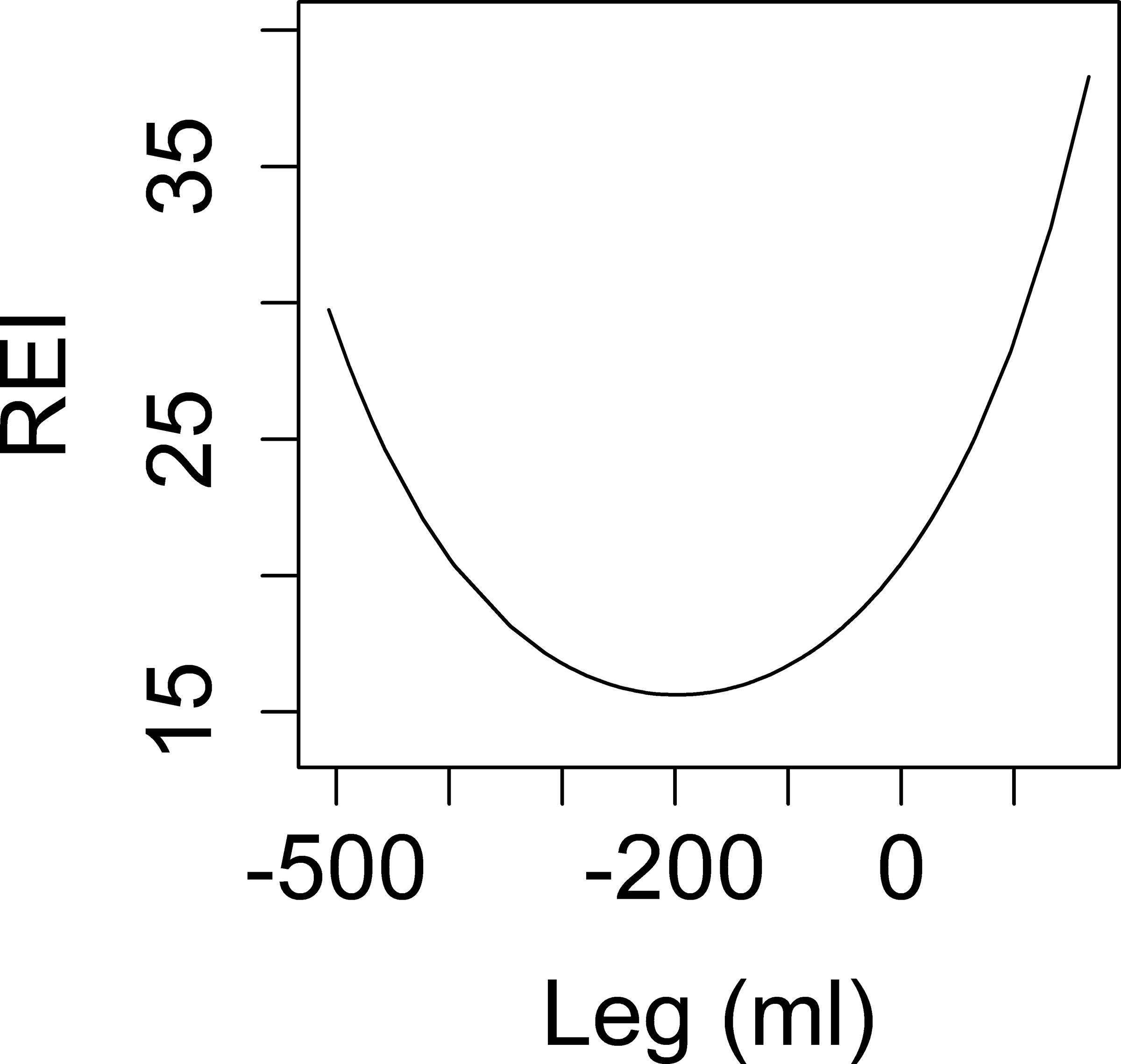
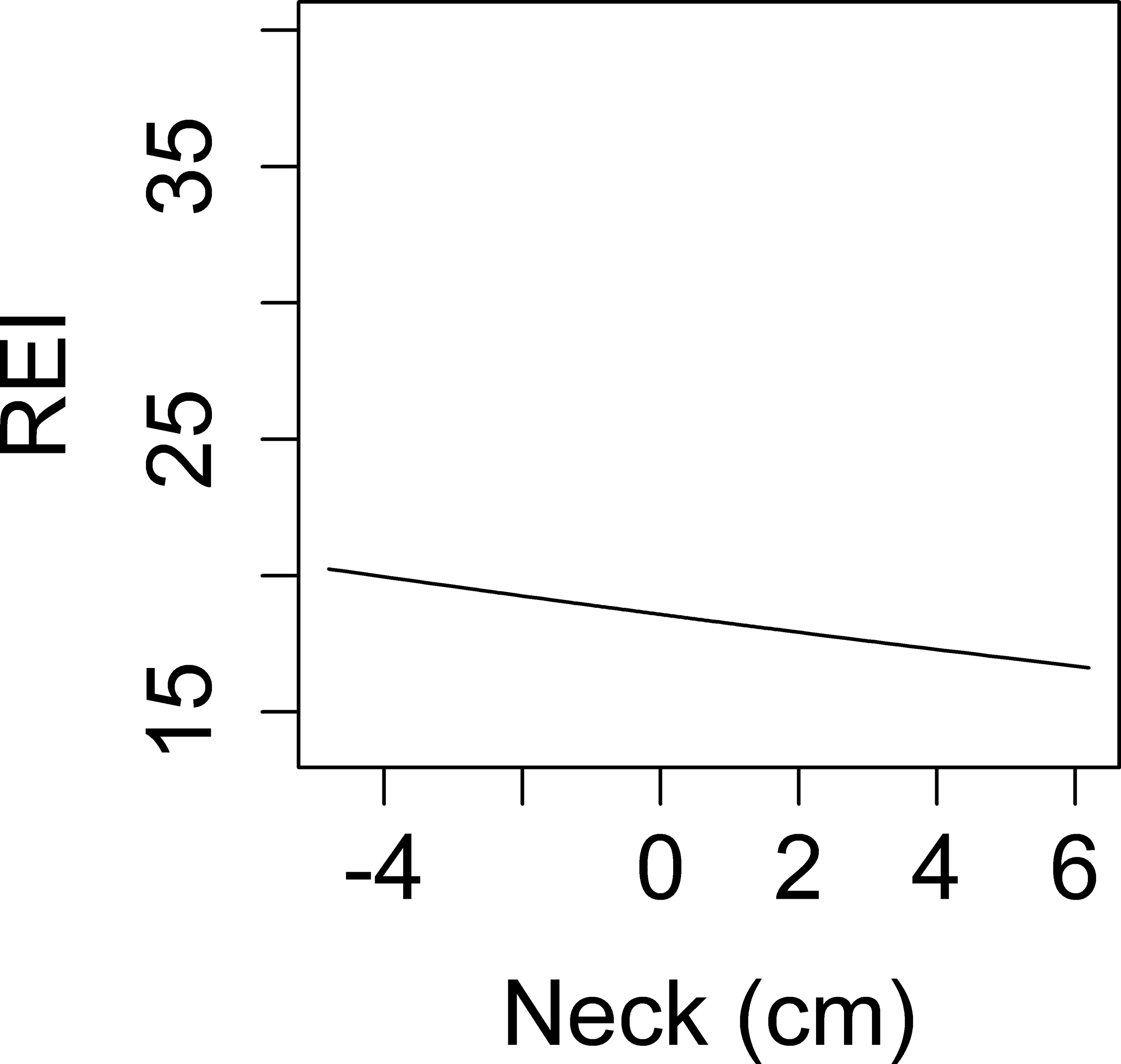
Associations between overnight change in fluid shift measurements and REI. Y-axis: predicted values of REI. X-axis: overnight change in: calf circumference in men (a), calf circumference in women (b), leg fluid volume (c), neck circumference (d).
Table 2.
Multivariable model : association between overnight change in calf circumference and log(REI+1). To account for the non-linear effects, a linear spline regression with one knot located at zero was fitted for overnight change in calf circumference, which segregates the change in calf circumference into the negative and positive components. Within each partition, a linear association between the overnight change in calf circumference and log(REI+1) is reported.
| Male (n=106) | ||||
| term | estimate | SE | statistic | p value |
| (Intercept) | −1.90 | 0.94 | −2.00 | 0.05 |
| Age | 0.03 | 0.01 | 3.90 | 0.0002 |
| Mexican American: non-Hispanic white | 0.29 | 0.16 | 1.70 | 0.09 |
| Nighttime calf circumference | 0.08 | 0.02 | 4.30 | <0.0001 |
| Overnight increase in calf circumference (negative part) | −0.20 | 0.11 | −1.80 | 0.08 |
| Overnight increase in calf circumference (positive part) | 0.60 | 0.38 | 1.60 | 0.12 |
| Overnight increase in total body water | 0.03 | 0.01 | 2.80 | 0.01 |
| Female (n=84) | ||||
| Term | estimate | SE | statistic | p value |
| (Intercept) | 1.00 | 1.00 | 1.00 | 0.32 |
| Age | 0.01 | 0.01 | 1.49 | 0.14 |
| Mexican American: non-Hispanic white | 0.28 | 0.18 | 1.58 | 0.12 |
| Nighttime calf circumference | 0.03 | 0.02 | 1.30 | 0.20 |
| Overnight increase in calf circumference (negative part) | 0.10 | 0.14 | 0.75 | 0.46 |
| Overnight increase in calf circumference (positive part) | 0.30 | 0.34 | 0.87 | 0.39 |
| Overnight increase in total body water | −0.02 | 0.01 | −1.61 | 0.11 |
Leg fluid:
As shown in Figure 2c, the relationship between overnight change in leg fluid volume and REI was U shaped, such that both very positive and very negative values of overnight change in leg fluid volume were associated with higher REI. Specifically, when the value of overnight change was less than −200 ml, lower values of overnight change in leg fluid volume were associated with higher REI. In contrast, when the value of overnight change was greater than −200 ml, higher values of overnight change in leg fluid volume were associated with higher REI. A similar association remained after adjustment for age, ethnicity, nighttime calf circumference, and overnight change in TBW (Table 3). In addition, the relationship between nighttime, rather than overnight, leg fluid and REI was reverse U shaped, such that both very positive and very negative values of nighttime leg fluid volume were associated with lower REI. Higher age and Mexican American ethnicity were also associated with higher REI.
Table 3.
Multivariable model (n=117): association between overnight change in leg fluid volume and log(REI+1). Because the relationship between overnight change in leg fluid volume and log(REI+1) was U shaped (Figure 2c), overnight change in leg fluid volume was modeled to include a quadratic term.
| Term | estimate | SE | statistic | p value |
|---|---|---|---|---|
|
| ||||
| (Intercept) | −1.87 | 1.43 | −1.31 | 0.19 |
|
| ||||
| Age | 0.02 | 0.01 | 3.93 | 0.00015 |
|
| ||||
| Mexican American: non-Hispanic white | 0.33 | 0.16 | 2.10 | 0.04 |
|
| ||||
| Women:men | −0.09 | 0.18 | −0.51 | 0.61 |
|
| ||||
| Nighttime leg fluid * | 0.02 | |||
| 25th percentile of nighttime leg fluid (1633.31) | 1.33 | |||
| Median of nighttime leg fluid (1987.02) | 1.60 | |||
| 75th percentile of nighttime leg fluid (2354.50) | 1.72 | |||
|
| ||||
| Overnight change in leg fluid † | 0.02 | |||
| 25th percentile of overnight change in leg fluid (−203.04) | −2.29 | |||
| Median of overnight change in leg fluid (−116.93) | −2.18 | |||
| 75th percentile of overnight change in leg fluid (−43.05) | −2.01 | |||
|
| ||||
| Overnight increase in total body water | −0.01 | 0.02 | −0.34 | 0.74 |
Evaluated at various values of nighttime leg fluid.
Evaluated at various values of overnight change in leg fluid volume.
Neck circumference:
A linear association between overnight change in neck circumference and REI was found. With back transformation to the original REI, an IQR (1.55 cm) increase in neck circumference overnight was associated with a 2.7% lower REI, but the association was non-significant (p=0.60). In a multivariable model, greater age and greater nighttime neck circumference were associated with REI, but overnight change in neck circumference was not (Table 4).
Table 4.
Multivariable model (n=188): association between overnight change in neck circumference and log(REI+1). To account for the non-linear effects, a linear spline regression with one knot located at zero was fitted for overnight change in neck circumference, which segregates the calf circumference into the negative and positive parts. Within each partition, a linear association between the overnight change in neck circumference and log(REI+1) is reported.
| Term | estimate | SE | statistic | p value |
|---|---|---|---|---|
| (Intercept) | −1.70 | 0.68 | −2.49 | 0.01 |
| Age | 0.02 | 0.00 | 4.17 | <0.0001 |
| Mexican American: non-Hispanic white | 0.10 | 0.12 | 0.90 | 0.37 |
| Women:men | 0.15 | 0.13 | 1.17 | 0.24 |
| Nighttime neck circumference | 0.08 | 0.01 | 6.30 | <0.0001 |
| Overnight increase in neck circumference | 0.03 | 0.03 | 1.01 | 0.31 |
| Overnight increase in total body water | 0.01 | 0.01 | 1.48 | 0.14 |
Relationship between total body fluid and OSA
Table III presents the association between intracellular fluid volume, extracellular fluid volume, and total fluid volume, measured at night just prior to sleep apnea testing. Each measure was positively associated with log(REI+1). Overnight changes in these three measurements were not associated with log(REI+1) (Table III).
Prediction of REI by ECF, weight, and waist circumference
The highest adjusted R2 was for waist circumference (0.1226), followed by nighttime ECF (0.06692), and then weight (0.005627).
Discussion
This population-based, multicenter study of ischemic stroke patients with prospective data collection produced several key findings: (1) mean calf circumference and leg fluid volume decreased and neck circumference increased overnight as anticipated, (2) two of the three fluid shift measurements, overnight decrease in calf circumference and leg fluid volume, were associated with greater OSA severity in stroke patients, (3) an overnight decrease in calf circumference was associated with greater OSA severity in men but not in women, and (4) nighttime measures of total body fluid were associated with OSA severity.
As has been identified in other types of patients, such as the general OSA population7 and persons with fluid overload states,10, 25 this study confirmed that in ischemic stroke patients, overnight fluid displaces from lower to upper compartments, as evidenced by changes in calf circumference, leg fluid volume, and neck circumference. The absolute changes in calf and neck circumference identified in the current study were smaller than in the prior studies, and the leg fluid volume shifts were in keeping with the lower end of those identified in the prior studies.10, 25 Nighttime leg fluid volumes were smaller than others have reported,11 such that less fluid may have been available for overnight redistribution. Although we originally hypothesized that stroke may be associated with greater leg pooling due to intravenous fluid treatment for stroke care and decreased mobility, stroke hospitalizations may be associated with greater time in bed during the day resulting in decreased pooling and less substantial rostral fluid shifts.
Unlike some prior work in other populations, we did not find a linear association between overnight rostral fluid shifts and OSA severity, although one previous study did report a non-linear association.25 We did find that when leg fluid volume decreased or increased overnight, these changes were associated with an increase in OSA severity (U-shaped relationship). Similarly, among men we found a V-shaped relationship between overnight change in calf circumference and OSA severity; however, this was only borderline significant with adjustment. We did not anticipate these findings, but unlike prior work, our subjects were inpatients who received intravenous fluids overnight, which could have resulted in both lower extremity fluid accumulation overnight and increased OSA severity. We do not have information on the amount of fluids received. We do have data on overnight change in total body fluid, but accounting for overnight changes in TBW did not alter the finding of non-linear associations. Differences between populations and smaller fluid shifts, perhaps as a result of greater daytime recumbent posture resulting in less fluid accumulation, may account for the differences between our study and prior findings. Our study was approximately 10 times larger than the prior studies, so lack of power relative to the prior work is unlikely to account for the difference.
In our data, adjustment for confounders did attenuate the association between calf circumference and OSA severity. Unlike other studies, because of our much larger sample size, we were able to adjust for other potential confounders such as demographics and baseline calf circumference. Still larger studies in other populations may be needed to tease out the importance of potential confounding. Not surprisingly, an increase in overnight TBW, in the adjusted calf circumference model, was associated with OSA severity. These findings are in keeping with increases in AHI after surgery,26 and the association between overnight saline administration and increases in AHI in older men.27 Our findings have potential implications for IV fluid treatment of stroke patients.
Some of the previous investigations found an association between overnight fluid shifts in men and not women.25 We also found a sex difference in the association between overnight calf circumference and OSA severity, with significant findings in men (borderline significant after adjustment) and non-significant findings in women. We did not find a lower volume of overnight fluid shift in women as found in one other study.25 Sex differences in response to fluid displacement could relate to anatomical differences in fat distribution or differences in airway collapsibility.
The associations found between OSA severity and nighttime total body fluid measurements, neck circumference, and calf circumference warrant further consideration. Total body fluid, extracellular fluid, intracellular fluid, calf circumference, and neck circumference may be markers of body size or total fluid overload states, both known correlates of REI. Our exploratory analyses, in which waist circumference accounted for greater variance in REI than ECF, suggest that body size may be a more significant driver than fluid overload. Alternatively, abdominal girth may lower lung volumes and thereby increase airway collapsibility. Further investigations based on spectroscopic data may help differentiate which of these two conditions is represented by body fluid measures in their association with REI.
Strengths of this study include its large sample size for this type of physiological work, well-characterized cohort, population-based sampling, and performance in a real-world clinical setting. Limitations include the use of a home sleep apnea test instead of the gold standard, in-laboratory polysomnography. Measurements during a stroke hospitalization may have been confounded by clinical care such as administration of intravenous fluids or diuretics. Although we did not collect information about volume of intravenous fluids, urinary output, other body fluid output, or diuretic administration, and could not collect information about insensible losses, we did measure TBW status via bioimpedance. We attempted to examine time spent in the seated position during the day, which would be expected to increase overnight fluid shifts, and use of lower extremity compression devices. However, these measures were based on self-report and may not have been highly reliable. Nonetheless, the association between time spent in a chair and overnight decrease in leg circumference and fluid volume raises the possibility that daytime activities, such as physical therapy, can be manipulated to reduce overnight fluid shifts. Furthermore, our results may not generalize to stroke patients with greater use of lower extremity compression devices during the day (expected to decrease overnight fluid shifts) or overnight (expected to increase overnight fluid shifts). The lack of a control group does not permit us to determine whether our findings are attributable to the stroke, the hospitalization, or other factors. We do not have validated data on infarction location, and brainstem location has been associated with a higher prevalence of OSA in this community.17 This should not have confounded our results, however, as infarction location is not likely associated with fluid shifts. As the vast majority of post-stroke OSA is undetected prior to stroke, we are unable to determine whether our results pertain more to patients with pre-existing or new post-stroke OSA. Regardless, our findings remain relevant to post-stroke care. We also did not retest participants to determine whether their OSA severity changed over the months post stroke, and therefore are unable to assess whether fluid shifts predict post-stroke OSA improvement.
Summary
This population-based study of prospectively collected data pertaining to overnight fluid shifts and OSA provides new insights into the pathophysiology of post-stroke OSA, a very common and highly consequential condition. The novel confirmation of nocturnal rostral fluid shifts in hospitalized stroke patients together with discovery of an association between some measures of overnight fluid shifts and OSA severity raise the possibility that interventions to limit overnight fluid shifts could attenuate post-stroke OSA severity. Furthermore, efforts to reduce general fluid overload might also be tested.
Supplementary Material
Figure I: Flow diagram
Table I: STROBE Statement checklist
Table II: Predictors of overnight increase in fluid shift variables
Table III: Bivariate associations (n=192) between nighttime and overnight change in total body fluid measurements
Acknowledgments:
This study was performed in the Corpus Christi Medical Center and CHRISTUS Spohn hospitals, CHRISTUS Health system, in Corpus Christi, Texas.
Sources of Funding:
The project was funded by the NIH (R01 HL123379, R01 HL126700, R01 NS038916). The funding source played no role in the decision to submit this analysis for consideration or in the interpretation of data.
Piper: received funding from a Natural Sciences and Engineering Research Council of Canada Discovery grant
Chervin: Dr. Chervin has consulted for Zansors; serves as an editor and author for UpToDate; edited a book published by Cambridge University Press; and has produced copyrighted material, patents, and patents pending, owned by the University of Michigan, focused on assessment or treatment of sleep disorders. He has served on the Boards of Directors for the American Academy of Sleep Medicine, Associated Professional Sleep Societies, and International Pediatric Sleep Association; and receives support from NIH.
Non-standard Abbreviations and Acronyms:
- AHI
apnea-hypopnea index
- BIS
bioimpedance spectroscopy
- BASIC
Brain Attack Surveillance in Corpus Christi
- ECF
extracellular fluid
- OSA
obstructive sleep apnea
- REI
respiratory event index
- TBW
total body water
Footnotes
Disclosures:
Brown: none
Yadollahi: none
He: none
Xu: none
Case: none
Lisabeth: none
References
- 1.Johnson K, Johnson D. Frequency of sleep apnea in stroke and tia patients: A meta-analysis. J Clin Sleep Med 2010;6:131–137 [PMC free article] [PubMed] [Google Scholar]
- 2.Lisabeth LD, Sanchez BN, Lim D, Chervin RD, Case E, Morgenstern LB, Tower S, Brown DL. Sleep-disordered breathing and poststroke outcomes. Ann Neurol 2019;86:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, Franklin KA. Obstructive sleep apnea is a risk factor for death in patients with stroke: A 10-year follow-up. Arch Intern Med 2008;168:297–301 [DOI] [PubMed] [Google Scholar]
- 4.Brown DL, Shafie-Khorassani F, Kim S, Chervin RD, Case E, Morgenstern LB, Yadollahi A, Tower S, Lisabeth LD. Sleep-disordered breathing is associated with recurrent ischemic stroke. Stroke. 2019;50:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palombini L, Guilleminault C. Stroke and treatment with nasal cpap. Eur J Neurol 2006;13:198–200 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep disordered breathing after stroke. A randomized controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, Bradley TD. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Resp Crit Care Med 2009;179:241–246 [DOI] [PubMed] [Google Scholar]
- 8.Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, Mak S, Bradley TD. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–1605 [DOI] [PubMed] [Google Scholar]
- 9.Redolfi S, Arnulf I, Pottier M, Lajou J, Koskas I, Bradley T, Similowski T. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med 2011;184:1062–1066 [DOI] [PubMed] [Google Scholar]
- 10.Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: Relationship with obstructive sleep apnea. Nephrol Dial Transplant 2012;27:1569–1573 [DOI] [PubMed] [Google Scholar]
- 11.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–1082 [DOI] [PubMed] [Google Scholar]
- 12.Friedman O, Bradley TD, Logan AG. Influence of lower body positive pressure on upper airway cross-sectional area in drug-resistant hypertension. Hypertension. 2013;61:240–245 [DOI] [PubMed] [Google Scholar]
- 13.Su MC, Chiu KL, Ruttanaumpawan P, Shiota S, Yumino D, Redolfi S, Haight JS, Bradley TD. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir Physiol Neurobiol 2008;161:306–312 [DOI] [PubMed] [Google Scholar]
- 14.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol 2011;175:390–393 [DOI] [PubMed] [Google Scholar]
- 15.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, QURESHI AI, Rosenfield K, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:870–947 [DOI] [PubMed] [Google Scholar]
- 16.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al Wabil A, et al. Excess stroke in mexican americans compared with non-hispanic whites: The brain attack surveillance in corpus christi project. Am J Epidemiol 2004;160:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown D, McDermott M, Mowla A, De Lott L, Morgenstern L, Kerber K, Hegeman G, Smith M, Garcia N, Chervin R, et al. Brainstem infarction and sleep-disordered breathing in the basic sleep apnea study. Sleep Med 2014;15:887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon JR, Smith AE, Tobkin SE, Lockwood CM, Kendall KL, Graef JL, Roberts MD, Dalbo VJ, Kerksick CM, Cramer JT, et al. Total body water changes after an exercise intervention tracked using bioimpedance spectroscopy: A deuterium oxide comparison. Clin Nutr 2009;28:516–525 [DOI] [PubMed] [Google Scholar]
- 19.Moon JR, Stout JR, Smith AE, Tobkin SE, Lockwood CM, Kendall KL, Graef JL, Fukuda DH, Costa PB, Stock MS, et al. Reproducibility and validity of bioimpedance spectroscopy for tracking changes in total body water: Implications for repeated measurements. Br J Nutr 2010;104:1384–1394 [DOI] [PubMed] [Google Scholar]
- 20.Moon JR, Tobkin SE, Roberts MD, Dalbo VJ, Kerksick CM, Bemben MG, Cramer JT, Stout JR. Total body water estimations in healthy men and women using bioimpedance spectroscopy: A deuterium oxide comparison. Nutr Metab. 2008;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng S, Chan T, To K, Ngai J, Tung A, Ko F, Hui D. Validation of a portable recording device (apnealink) for identifying patients with suspected obstructive sleep apnea syndrome (osas). Intern Med J 2009;39:757–762 [DOI] [PubMed] [Google Scholar]
- 22.Kushner RF, Gudivaka R, Schoeller DA. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am J Clin Nutr 1996;64:423s–427s [DOI] [PubMed] [Google Scholar]
- 23.Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20−−94 years. Nutrition. 2001;17:248–253 [DOI] [PubMed] [Google Scholar]
- 24.Kyle UG, Genton L, Mentha G, Nicod L, Slosman DO, Pichard C. Reliable bioelectrical impedance analysis estimate of fat-free mass in liver, lung, and heart transplant patients. JPEN. Journal of parenteral and enteral nutrition. 2001;25:45–51 [DOI] [PubMed] [Google Scholar]
- 25.Kasai T, Motwani SS, Yumino D, Mak S, Newton GE, Bradley TD. Differing relationship of nocturnal fluid shifts to sleep apnea in men and women with heart failure. Circulation: Heart Failure. 2012;5:467–474 [DOI] [PubMed] [Google Scholar]
- 26.Lam T, Singh M, Yadollahi A, Chung F. Is perioperative fluid and salt balance a contributing factor in postoperative worsening of obstructive sleep apnea? Anesthesia and analgesia. 2016;122:1335–1339 [DOI] [PubMed] [Google Scholar]
- 27.Yadollahi A, Gabriel JM, White LH, Taranto Montemurro L, Kasai T, Bradley TD. A randomized, double crossover study to investigate the influence of saline infusion on sleep apnea severity in men. Sleep. 2014;37:1699–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure I: Flow diagram
Table I: STROBE Statement checklist
Table II: Predictors of overnight increase in fluid shift variables
Table III: Bivariate associations (n=192) between nighttime and overnight change in total body fluid measurements


