Abstract
BACKGROUND:
Poor mercaptopurine (6MP) adherence (mean adherence rate < 90%) increases the relapse risk among children with acute lymphoblastic leukemia (ALL). 6MP adherence remains difficult to measure in real time. Easily measured patient-level factors could identify patients at risk for poor adherence.
METHODS:
The authors measured 6MP adherence via electronic monitoring for 6 months per patient. Using data from month 3, they created a risk prediction model for 6MP nonadherence in 407 children with ALL (mean age, 7.7 ± 4.4 years); they used receiver operating characteristic analyses in the training set (n = 250) and replicated this in the test set (n = 157).
RESULTS:
Age, race/ethnicity, 6MP dose intensity, absolute neutrophil count, 6MP ingestion patterns, and household structure were retained in the prediction model. The model yielded areas under the receiver operating characteristic curve (AUCs) of 0.79 (95% confidence interval [CI], 0.71-0.85) and 0.74 (95% CI, 0.63-0.85) in the training and test sets, respectively. The model performed better for those who were ≥12 years old (AUC, 0.79; 95% CI, 0.59-0.99) than those <12 years old (AUC, 0.70; 95% CI, 0.58-0.81). Using the predicted probability of nonadherence based on receiver operating characteristic analysis, the authors developed a binary risk classifier to classify patients with a high or low probability of nonadherence. The sensitivity and specificity of the binary risk classifier were 71% and 76%, respectively. Adjusted for clinical prognosticators, the risk of relapse was 2.2-fold higher (95% CI, 0.94-5.1; P = .07) among patients with a high probability of nonadherence in comparison with those with a low probability, as identified by the risk prediction model.
CONCLUSIONS:
The risk prediction model identified patients with a high probability of nonadherence and could be used in real time to personalize recommendations and interventions in the clinic.
Keywords: acute lymphoblastic leukemia, adolescent, child, medication adherence, mercaptopurine
LAY SUMMARY:
• The vast majority of children with acute lymphoblastic leukemia, the most common childhood cancer, are cured.
• The treatment of acute lymphoblastic leukemia includes taking an oral chemotherapy medicine (mercaptopurine) for approximately 2 years. Children who miss doses of this medicine (specifically children who take the medicine less than 90% of the time that it is prescribed) are more likely to suffer leukemia relapse.
• The authors of this article have measured mercaptopurine adherence with electronic bottle caps to determine characteristics of patients that predict nonadherence, and they have created a prediction tool that could allow physicians to identify and intervene with patients at high risk of nonadherence.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common cancer diagnosed in childhood.1 More than 95% of children with ALL successfully enter remission after 4 weeks of induction therapy; however, approximately 20% relapse within 5 years.1 Patients who relapse face a poorer prognosis, and second-line therapies are toxic.2 Durable first clinical remissions require a prolonged maintenance phase with daily self-administered oral mercaptopurine (6MP); low or varying 6MP exposure increases relapse risk.3,4 Factors influencing the adequacy of systemic 6MP exposure include the host’s pharmacogenetics, the physician-prescribed mercaptopurine dose intensity (6MPDI), and the patient’s adherence to 6MP.5–12 Using the Medication Event Monitoring System (MEMS) to measure 6MP adherence, we found that adherence rates below 90% were associated with up to a 3.9-fold higher risk of relapse after adjustments for clinical and sociodemographic factors.6–8 These findings suggest a critical need for pediatric oncology providers to identify patients with 6MP nonadherence in the clinic. However, self-reporting is unreliable, especially among nonadherers,9 and electronic monitoring (via MEMS) is logistically challenging in a clinical setting. This presents a need to develop a risk prediction tool that would use patient-level factors to identify individuals at high risk of 6MP nonadherence in real time in the clinic.
Several studies have identified patient-level factors associated with an increased risk of nonadherence (adolescence, a non-White racial/ethnic background, single-parent/multiple-children households, low parental income/education, and 6MP ingestion at varying times of the day).5–8,10 We have also reported a negative correlation between 6MPDI and adherence and between the absolute neutrophil count (ANC) and adherence.6 In this study, we test the hypothesis that these patient-level factors will allow the identification of nonadherent patients through the development of a risk prediction model.
MATERIALS AND METHODS
Study Design and Participants
This report is a secondary analysis of data from the Children’s Oncology Group (COG) AALL03N1 study. Patients at 1 of 83 participating COG sites (Supporting Table 1) were enrolled in the original prospective study.6–8 All sites obtained institutional review board approval and written informed consent before enrolling patients. For this study, eligible patients were ≤21 years old at their ALL diagnosis, were in their first remission, and were receiving oral 6MP as part of maintenance chemotherapy. All patients had completed at least 6 months of maintenance therapy at study enrollment and were required to have a minimum of 6 months remaining on maintenance.
Electronic Monitoring of 6MP Adherence
The study called for 6 months of adherence monitoring (Supporting Fig. 1) using MEMS (AARDEX Group, Belgium/Switzerland). MEMS uses microelectronic technology to record the date and time of each bottle opening (Supporting Fig. 2). Patients were instructed to take all doses of 6MP from the MEMS bottle. MEMS data were downloaded at the end of the study (Supporting Fig. 2).
Medication-Taking Habits
Patients or parents completed a questionnaire on 6MP consumption habits. One of the questions asked, “Do you take your 6MP at the same time each day?” The responses were “yes,” “no,” and “don’t know.” Patients/parents completed the questionnaire on days 29, 57, 113, and 141; respondents answering “no” on any of these questionnaires were considered to have taken 6MP at varying times of the day.
Demographics
The following were obtained at study entry via a questionnaire: self-reported race/ethnicity, household structure (nuclear family vs single parent/single child vs single parent/multiple children), mother as a full-time caregiver (yes/no), annual household income, and parental education.
Health Care Provider Reports
Participating institutions submitted monthly reports for each patient; these reports detailed the prescribed 6MP dose for each day of the preceding month and dates when the prescriber held the 6MP dose for toxicity or illness. The participating institutions also submitted monthly ANC values obtained as part of the routine monthly clinic visit.
6MPDI
Providers titrated the prescribed dose on the basis of drug toxicity (eg, neutropenia, thrombocytopenia, and liver toxicity) every 4 weeks. 6MPDI was calculated as a ratio of the prescribed dose to the planned dose of 75 mg/m2/d.
6MP Pharmacogenetics and Red Cell Metabolites
The thiopurine methyltransferase (TPMT) genotype was assessed at study entry.13 Red cell thioguanine nucleotide (TGN) levels (pmol/8 × 108 red cells) were assayed at 6 consecutive monthly time points per patient and averaged.14,15 The proportion of patients with metabolite levels obtained at each of the 6 time points ranged from 86.5% to 90.7%.
Statistical Analysis
Program and coding
We used SAS (version 9.4; SAS Institute, Inc, Cary, North Carolina) for our statistical analyses. P values ≤ .05 were considered to be statistically significant.
Development of the individual risk prediction model for 6MP nonadherence
The MEMS-based adherence rate was defined as the ratio of the number of days with MEMS cap openings (X) to the number of days that 6MP was prescribed (N), and it was reported as a percentage (X/N × 100); days when 6MP was held by the prescriber were removed from the denominator (N). Adherence rates were calculated for each month of study. We selected month 3 data for developing the risk prediction model because that month had the least amount of missing data; this yielded 407 evaluable patients.
Using receiver operating characteristic analyses, we developed a prediction model for 6MP nonadherence (monthly MEMS adherence rate < 90%). The cohort was divided into a training set (n = 250) for model development and a test set (n = 157) for validation via stratified random sampling (stratified by race/ethnicity, sex, age at study participation, and 6MP nonadherence). Variables evaluated for inclusion in the model included sociodemographics (age, sex, race/ethnicity, annual household income, mother as full-time caregiver, maternal/paternal education, and household structure), clinical variables (TPMT genotype, time from start of maintenance, ANC, 6MPDI, and red cell TGN level), and behavioral variables (taking 6MP at the same time of day vs varying time of day). Guided by the change in the area under the receiver operating characteristic curve (AUC), we used logistic regression with backward variable selection to derive a prediction model in the training set; variables that affected the AUC by >2% were retained. The model was replicated in the test set. In addition, we examined the performance of the model by using data from study months 1, 2, 4, and 5 as well as data from 2 age groups (<12 and ≥12 years at study). Multiple imputation (N = 20) was used to impute data for missing values. We generated a binary risk classifier (using a cut point for predicted probabilities of 6MP nonadherence between 0.1 and 0.9) in the test set to classify patients with a high or low probability of nonadherence. We followed patients for 5 years to examine the cumulative incidence of relapse among those with high and low probabilities of nonadherence.
RESULTS
Findings are reported in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines (Supporting Table 2).16 Table 1 summarizes the sociodemographic and clinical characteristics and 6MP intake practices for the 407 study participants. The mean age at study participation was 7.7 years (standard deviation, ±4.4 years). Males constituted 68% of the cohort; 35% were White, 34% were Hispanic, 16% were African American, and 15% were Asian. The mean time from the start of maintenance to the end of study month 3 was 415 days (range, 139-897 days). Eighty-five percent of the patients lived in a nuclear family household, 5% lived in a single-parent/single-child household, and 10% lived in a single-parent/multiple-children household. Ninety-four percent of the patients carried a TPMT wild-type genotype. Thirty-three percent of the cohort did not take 6MP at the same time daily. Twenty-eight percent of the patients were non-adherers (mean adherence rate < 90% for month 3). The training set (used for model development) and the test set (used for validation) were comparable with respect to patient sociodemographics, clinical characteristics, and 6MP ingestion practices (Table 2).
TABLE 1.
Characteristics of the Study Population at 3 Months (N = 407)
| Characteristic | Value |
|---|---|
| Age at study participation | |
|
| |
| Mean (SD), y | 7.7 (4.4) |
|
| |
| Time from start of maintenance therapy | |
|
| |
| Mean (range), d | 415 (139-897) |
|
| |
| Race/ethnicity, No. (%) | |
|
| |
| Non-Hispanic White | 143 (35) |
| Hispanic | 138 (34) |
| Asian | 62 (15) |
| African American | 64 (16) |
|
| |
| Sex, No. (%) | |
|
| |
| Male | 276 (68) |
|
| |
| Age group, No. (%) | |
|
| |
| ≥12 y | 68 (17) |
|
| |
| Household income (annual), No. (%)a | |
|
| |
| <$50,000 (US dollars) | 226 (59) |
|
| |
| Mother as full-time caregiver, No. (%)a | |
|
| |
| Yes | 192 (49) |
|
| |
| Maternal education, No. (%)a | |
|
| |
| College graduate or formal training | 223 (57) |
| High school plus some college | 50 (13) |
| ≤High school | 122 (31) |
|
| |
| Paternal education, No. (%)a | |
|
| |
| College graduate or formal training | 233 (60) |
| High school plus some college | 48 (12) |
| ≤High school | 108 (28) |
|
| |
| Household structure, No. (%)a | |
|
| |
| Nuclear family | 339 (85) |
| Single parent/single child | 20 (5) |
| Single parent/multiple children | 39 (10) |
|
| |
| NCI risk groups, No. (%)a | |
|
| |
| High risk | 160 (40) |
|
| |
| Leukemia cytogenetics, No. (%)a | |
|
| |
| Normal | 195 (51) |
| Favorable | 171 (44) |
| Unfavorable | 19 (5) |
|
| |
| TPMT genotype, No. (%) | |
|
| |
| Wild type | 382 (94) |
|
| |
| 6MP ingestion pattern, No. (%)a | |
|
| |
| Taking 6MP at the same time of day | 270 (67) |
|
| |
| Absolute neutrophil counta | |
|
| |
| Mean (SD) | 2.04 (1.38) |
|
| |
| 6MP dose intensity | |
|
| |
| Mean (SD) | 0.82 (0.28) |
|
| |
| Red cell TGN level (pmol/8 x 108 red cells) | |
|
| |
| Mean (SD) | 155 (88) |
|
| |
| Mean adherence rates, No. (%) | |
|
| |
| <95% | 148 (36) |
| <90% | 115 (28) |
Abbreviations: 6MP, mercaptopurine; NCI, National Cancer Institute; SD, standard deviation; TGN, thioguanine nucleotide; TPMT, thiopurine methyltransferase.
Data were missing for the following: annual household income (n = 23), mother as full-time caregiver (n = 14), maternal education (n = 12), paternal education (n = 18), household structure (n = 9), NCI risk groups (n = 2), cytogenetics (n = 22), taking 6MP at the same time of day (yes/no; n = 1), and absolute neutrophil count (n = 19).
TABLE 2.
Characteristics of the Study Population (Model Development and Validation Subgroups)
| Characteristic | Training Set (n = 250) | Test Set (n = 157) | P |
|---|---|---|---|
| Age at study participation | |||
|
| |||
| Mean (SD), y | 6.1 (4.4) | 6.1 (4.3) | .93 |
|
| |||
| Time from start of maintenance therapy | |||
|
| |||
| Mean (SD), d | 404 (156) | 436 (167) | .053 |
|
| |||
| Race/ethnicity, No. (%) | |||
|
| |||
| Non-Hispanic White | 90 (35) | 53 (35) | .95 |
| Hispanic | 85 (33) | 53 (35) | |
| Asian | 40 (16) | 22 (15) | |
| African American | 42 (16) | 22 (15) | |
|
| |||
| Sex, No. (%) | |||
|
| |||
| Male | 171 (67) | 105 (70) | .47 |
|
| |||
| Age group, No. (%) | |||
|
| |||
| ≥12 y | 47 (18) | 21 (14) | .26 |
|
| |||
| Household income (annual), No. (%) | |||
|
| |||
| <$50,000 (US dollars) | 141 (58) | 85 (60) | .66 |
|
| |||
| Mother as full-time caregiver | |||
|
| |||
| Yes | 118 (47) | 74 (51) | .44 |
|
| |||
| Maternal education, No. (%) | |||
|
| |||
| College graduate or formal training | 138 (55) | 85 (58) | .78 |
| High school plus some college | 31 (12) | 19 (13) | |
| ≤High school | 80 (32) | 42 (29) | |
|
| |||
| Paternal education, No. (%) | |||
|
| |||
| College graduate or formal training | 145 (58) | 88 (62) | .53 |
| High school plus some college | 34 (14) | 14 (10) | |
| ≤High school | 69 (28) | 39 (38) | |
|
| |||
| Household structure, No. (%) | |||
|
| |||
| Nuclear family | 216 (85) | 123 (85) | .88 |
| Single parent/single child | 12 (5) | 8 (6) | |
| Single parent/multiple children | 25 (10) | 14 (10) | |
|
| |||
| NCI risk groups, No. (%) | |||
|
| |||
| High risk | 101 (39) | 59 (40) | .98 |
|
| |||
| Cytogenetics, No. (%) | |||
|
| |||
| Normal | 131 (54) | 64 (45) | .27 |
| Favorable | 101 (41) | 70 (50) | |
| Unfavorable | 12 (5) | 7 (5) | |
|
| |||
| TPMT genotype, No. (%) | |||
|
| |||
| Wild type | 243 (95) | 139 (93) | .44 |
|
| |||
| 6MP ingestion pattern, No. (%) | |||
|
| |||
| Taking 6MP at the same time of day | 172 (67) | 98 (66) | .81 |
|
| |||
| Absolute neutrophil count | |||
|
| |||
| Mean (SD) | 1.99 (1.23) | 2.12 (1.60) | .42 |
|
| |||
| 6MP dose intensity | |||
|
| |||
| Mean (SD) | 0.83 (0.29) | 0.81 (0.26) | .54 |
|
| |||
| Red cell TGN level (pmol/8 x 108 red cells) | |||
|
| |||
| Mean (SD) | 155 (86) | 154 (91) | .92 |
|
| |||
| Mean adherence rates, No. (%) | |||
|
| |||
| <95% | 92 (36) | 56 (37) | .76 |
| <90% | 74 (29) | 41 (27) | .75 |
Abbreviations: 6MP, mercaptopurine; NCI, National Cancer Institute; SD, standard deviation; TGN, thioguanine nucleotide; TPMT, thiopurine methyltransferase.
Supporting Table 3 reports sample characteristics by 6MP adherence status. Compared with adherers, nonadherers were more likely to be Hispanic (44% vs 30%) or African American (25% vs 12%; P < .0001), more likely to have a household composed of a single parent with multiple children (20% vs 6%, P < .001), and less likely to take 6MP at the same time each day (54% vs 71%; P = .0007). Nonadherers on average had a higher ANC (2.42 vs 1.89; P = .002) and a higher 6MPDI (0.90 vs 0.79; P = .001).
Risk Prediction Model for 6MP Nonadherence
Table 3 details the variables retained in the risk prediction model as well as the magnitude of effect. These variables included the following: age at study, race/ethnicity, ANC, 6MPDI, household structure (nuclear family, single parent/single child, or single parent/multiple children), and 6MP ingestion pattern (6MP taken at the same or varying times of the day). The model yielded AUCs of 0.79 (95% confidence interval [CI], 0.71-0.85) and 0.74 (95% CI, 0.63-0.85) in the training and test sets, respectively. The AUC curves for the training and test sets are shown in Figure 1. The following variables were also evaluated and were not associated with the 6MP adherence status: TPMT genotype, red cell TGN level, sex, time from start of maintenance, annual household income, having a mother as a full-time caregiver, and maternal/paternal education.
TABLE 3.
Magnitude of the Association of Variables With Nonadherence to Oral 6MP
| Adherence Model |
|||
|---|---|---|---|
| Variablea | OR | 95% CI | P |
| Age at study (per year increase) | 1.09 | 1.02-1.17 | .01 |
| Hispanicb | 3.31 | 1.48-7.44 | .004 |
| Asianb | 2.57 | 0.93-7.15 | .07 |
| African Americanb | 4.90 | 1.85-12.99 | .001 |
| Absolute neutrophil count | 1.39 | 1.11-1.74 | .004 |
| 6MP dose intensityc | 11.21 | 2.75-45.74 | .0008 |
| Single parent/single childd | 0.67 | 0.15-2.99 | .6 |
| Single parent/multiple childrend | 3.66 | 1.35-9.92 | .01 |
| 6MP ingestion patterne | 0.63 | 0.33-1.20 | .2 |
Abbreviations: 6MP, mercaptopurine; CI, confidence interval; OR, odds ratio.
Includes variables retained in the risk prediction model.
Compared with non-Hispanic Whites.
OR per 1-unit increase in the absolute neutrophil count or 6MP dose intensity.
Compared with a nuclear family.
Ingested at the same time of day versus varying time of day.
Figure 1.
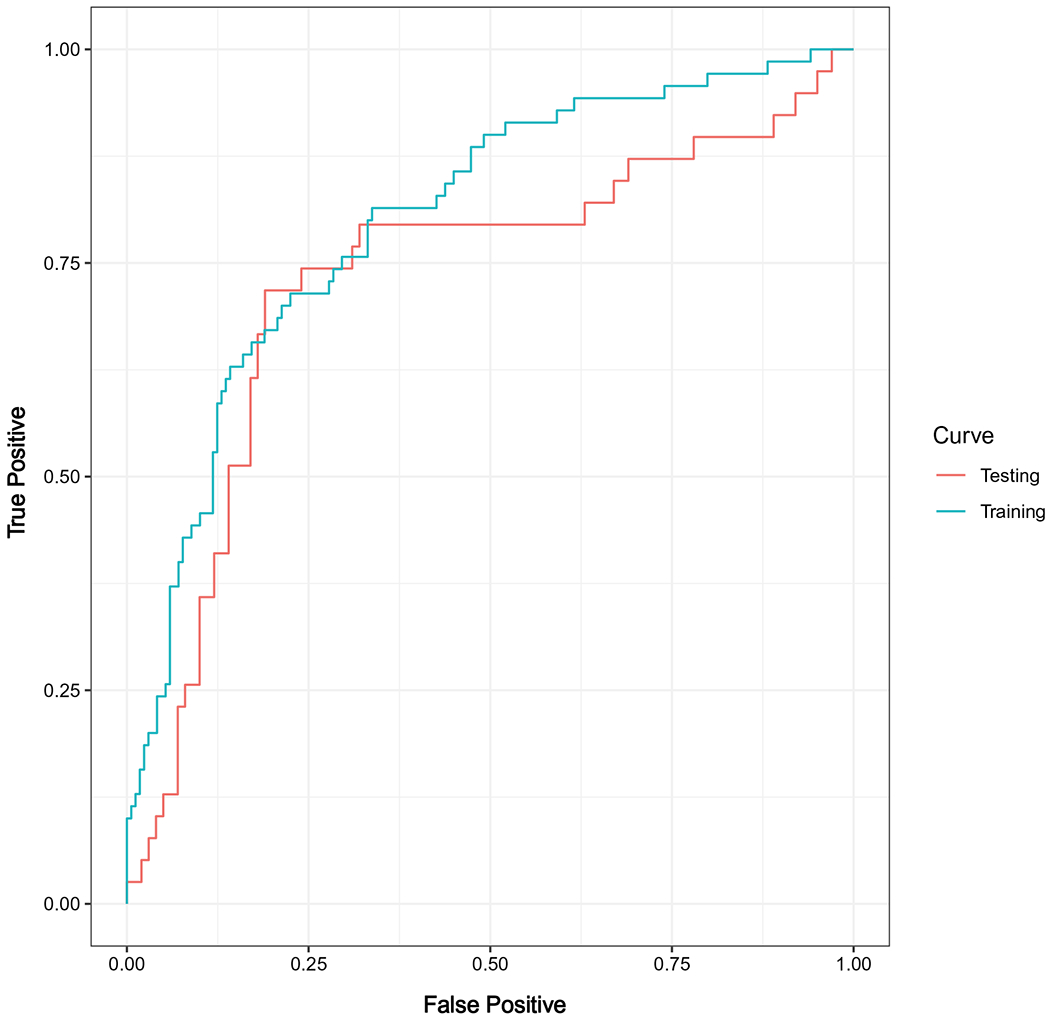
Receiver operating characteristic curves for the training and test sets.
Although month 3 study data were used to generate the model, we tested the utility of the risk prediction model in the remaining study months. The AUCs were as follows: month 1, 0.63; month 2, 0.72; month 4, 0.71; and month 5, 0.69. The risk prediction model performed better in the older cohort (AUC for ≥12 years, 0.79; 95% CI, 0.59-0.99) than the younger cohort (AUC for <12 years, 0.70, 95% CI, 0.58-0.81; Table 4).
TABLE 4.
AUC Analysis for Replication of the Prediction Model for Study Months 1, 2, 4, and 5 and by Age Group
| Adherence Model |
||
|---|---|---|
| Subgroup | AUC | 95% CI |
| Study month | ||
|
| ||
| Month 1 | 0.6323 | 0.5471-0.7176 |
| Month 2 | 0.7182 | 0.6509-0.7855 |
| Month 4 | 0.7135 | 0.6533-0.7737 |
| Month 5 | 0.6879 | 0.6228-0.7531 |
|
| ||
| Age at study | ||
|
| ||
| <12 y | 0.6954 | 0.5812-0.8097 |
| ≥12 y | 0.7902 | 0.5931-0.9873 |
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Binary Risk Classifier
Using the predicted probability of nonadherence (range, 0.1-0.9) from the receiver operating characteristic analysis, we developed a binary risk classifier to classify patients as having a high or low risk of nonadherence. Supporting Table 4 summarizes the performance of the binary classifier at various cut points of the predicted probability of nonadherence. We chose the predicted probability of 0.3 as the cut point to maximize the sensitivity and specificity of the classifier. The sensitivity and specificity of the binary risk classifier were 71% and 76%, respectively. The 5-year cumulative incidence of relapse was 11.9% for patients classified to be at high risk of 6MP nonadherence with the binary risk classifier and 4.5% for those classified to be at low risk of nonadherence (P = .006; Fig. 2). After adjustments for the National Cancer Institute risk status, those at high risk for 6MP nonadherence had a 2.2-fold increased risk of leukemia relapse (95% CI, 0.94-5.07; P = .07). Blast cytogenetics were also evaluated in the multivariable model, but they did not alter the magnitude of association between the binary risk classifier and the relapse risk, so they were not included.
Figure 2.

Cumulative incidence of acute lymphoblastic leukemia relapse by the risk of 6MP nonadherence. 6MP indicates mercaptopurine; CI, confidence interval.
DISCUSSION
It is well established that nonadherence to oral 6MP increases the risk of relapse in children with ALL.6–8 Recent research has shown that targeted interventions including education coupled with personalized text message reminders and directly supervised therapy improve 6MP adherence among adolescents with low baseline adherence.17 Furthermore, we know that certain practices, particularly taking 6MP at varying times of the day, are associated with poor adherence.10 However, it is challenging to identify nonadherent patients in the clinic, and this precludes the clinician’s ability to institute interventions or counsel patients and families in real time. Although MEMS remains the gold standard for measuring medication adherence, it is logistically impractical to use in a clinic setting because it requires a data download from the microprocessor chip in the medication bottle cap, an analysis of bottle openings with respect to the doses held by the prescriber, and an interpretation of the results. The day-to-day utility of MEMS is further limited by the additional cost of the MEMS bottles and the associated software. To address this gap, we developed a validated risk prediction model for 6MP nonadherence. The risk prediction model is data-derived and contains routinely available patient-level factors.
The risk prediction model performed well in discriminating patients who were nonadherent to 6MP from those who were adherent. When developing the binary classifier, we wanted to maximize the sensitivity of the test while ensuring that the specificity exceeded 70%. We selected a cut point of ≥0.3 for the predicted probability of nonadherence to achieve these goals; this yielded a sensitivity of 71% and a specificity of 76%. The binary risk classifier showed excellent discrimination of patients at high risk of relapse. With adjustments for the National Cancer Institute risk status, those at high risk of 6MP nonadherence were 2.2-fold more likely to relapse than those at low risk of nonadherence.
The variables retained in this predictive model (age, race/ethnicity, ANC, 6MPDI, household structure, and 6MP ingestion pattern) are largely consistent with the previously described factors associated with nonadherence.6–8,18–20 Patient age was retained as a predictor for nonadherence, and this is consistent with prior studies describing the unique challenges faced by adolescents and young adults.8,18,20 Patients from a racial/ethnic minority background are at risk for nonadherence.7,8 Race likely mediates nonadherence through specific sociodemographic characteristics and serves as a proxy for other barriers to adherence.7 In the building of our predictive model, race was retained over maternal/paternal education, parental income, and having a mother as a full-time caregiver. This suggests that the mechanisms through which race is associated with nonadherence are diverse and possibly vary across races and ethnicities.
As part of the standard management approach for patients with persistently high ANC levels, physicians increase the dose intensity of 6MP during maintenance cycles. Because nonadherent patients are likely to have a high ANC level (because of missed 6MP doses), their 6MPDI is often high and is likely reflective of physician responses to the high ANC level. Indeed, in a previous study, we showed that declining 6MP adherence rates correlated with rising 6MPDI as well as rising ANC.6
In a previous study, we used grounded theory to identify facilitators and barriers to adherence based on interviews with patients with ALL and their caregivers. One of the facilitators associated with 6MP adherence included “taking control” (mastering the mechanics of administration, establishing routines, and making medication nonnegotiable).19 Consistent with this theoretical framework, our risk prediction model found that patients from single-caregiver/multiple-children households (less able to “take control” because of competing responsibilities) and those who reported taking medications at varying times of the day were more likely to be nonadherent to 6MP. One third of the sample reported taking 6MP at varying times of the day; because of the association of this practice with nonadherence, all patients should be counseled to develop a routine to take 6MP at the same time of day to promote adherence.
When we replicated the risk prediction model in various study months, month 1 had the lowest AUC (0.63) in comparison with study months 2, 4, and 5 (AUC, 0.69-0.72). This finding is due to higher patient adherence to 6MP at the study start in comparison with subsequent months; this makes it more challenging to predict nonadherence in month 1.8 Likewise, the risk prediction model performed better among older children (AUC, 0.79 for those ≥12 years old vs 0.70 for those <12 years old) as expected because of the higher proportion of nonadherers among adolescents.
We must place our findings in the context of the study’s limitations. Although MEMS is the gold standard for measuring medication adherence, it captures bottle opening and not necessarily medication ingestion. Because of the time period of this cohort study, information regarding the impact of the minimal residual disease status on the risk of relapse is not available. Although the patients were enrolled at various time points from the start of maintenance (beyond the initial 6 months), our previous studies indicated that the time from the start of maintenance therapy was not associated with adherence.6 These limitations notwithstanding, the strengths of this analysis include the availability of a large, geographically and racially diverse patient population with electronically monitored 6MP adherence. Given the size and diversity of our sample, we were able to apply a stratified random sampling technique when we were creating the training and test sets.
This analysis addresses an important challenge faced by clinicians in identifying children with ALL at risk for 6MP nonadherence. The risk prediction model retained risk factors known to be associated with 6MP nonadherence (ie, age, race/ethnicity, ANC, 6MPDI, household structure, and 6MP ingestion pattern) and effectively identified those at risk for nonadherence. Although information regarding a patient’s household structure and 6MP ingestion routine may not be available in the medical record, oncologists can easily obtain this information from the family during the office visit.
Efforts are currently underway to build an online risk prediction tool for clinical use that would allow a provider to enter a patient’s individual characteristics in real time and output the probability of a patient being nonadherent, which would be accompanied by tailored recommendations for that patient (eg, the output could include a recommendation to counsel the patient on taking 6MP at a standard time of day or on developing a routine for 6MP intake). This online risk prediction tool will allow clinicians to tailor recommendations accordingly for patients at risk for nonadherence and emphasize practices associated with adherence, including the importance of developing a routine and having an adult assume primary responsibility for ensuring 6MP ingestion.
Supplementary Material
FUNDING SUPPORT
This work was supported in part by the National Cancer Institute of the National Institutes of Health (R01CA096670 [principal investigator Smita Bhatia], U10CA098543, U10CA095861, R37CA36401, CA21765, GM92666, CA156449, M01-RR00043, U10CA180886, U10CA180899, U10CA098413, UG1CA189955, and T32CA047888), the St. Baldrick’s Foundation, and the American Lebanese Syrian Associated Charities.
CONFLICT OF INTEREST DISCLOSURES
Yanjun Chen discloses employment with Edwards Lifesciences. Wendy Landier discloses research funding from Merck Sharp & Dohme. William E. Evans reports being an Scientific Adisory Board member for the Princess Máxima Centre for Child Oncology and a board member for BioSkryb. Mary V. Relling reports receiving research funding and payments or honoraria from Servier and participating on a data safety monitoring board or advisory board for the National Human Genome Research Institute and on a board for Pharmacogenomics Global Research Network; she also reports that her spouse is on a board for BioSkryb. The other authors made no disclosures.
Footnotes
This study was presented in part at the annual meeting of the American Society for Clinical Oncology on May 29, 2020.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. [DOI] [PubMed] [Google Scholar]
- 2.Raetz EA,Bhatla T Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–136. [DOI] [PubMed] [Google Scholar]
- 3.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323:17–21. [DOI] [PubMed] [Google Scholar]
- 4.Toyoda Y, Manabe A, Tsuchida M, et al. Six months of maintenance chemotherapy after intensified treatment for acute lymphoblastic leukemia of childhood. J Clin Oncol. 2000;18:1508–1516. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Hageman L, Chen Y, et al. A randomized trial of a mercaptopurine (6MP) adherence-enhancing intervention in children with acute lymphoblastic leukemia (ALL): a COG ACCL1033 study. J Clin Oncol. 2019;37:10007. [Google Scholar]
- 6.Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: a Children’s Oncology Group study. JAMA Oncol. 2015;1:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124:2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic White children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landier W, Chen Y, Hageman L, et al. Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: a Children’s Oncology Group study. Blood. 2017;129:1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landier W, Hageman L, Chen Y, et al. Mercaptopurine ingestion habits, red cell thioguanine nucleotide levels, and relapse risk in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group study AALL03N1. J Clin Oncol. 2017;35:1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod HL, Krynetski EY, Relling MV, Evans WE. Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia. 2000;14:567–572. [DOI] [PubMed] [Google Scholar]
- 12.Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yates CR, Krynetski EY, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–614. [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Hon YY, Chu Y, Van de Poll ME, Relling MV. Assay of 6-mercaptopurine and its metabolites in patient plasma by high-performance liquid chromatography with diode-array detection. J Chromatogr B Biomed Sci Appl. 1999;732:459–468. [DOI] [PubMed] [Google Scholar]
- 15.Dervieux T, Chu Y, Su Y, Pui CH, Evans WE, Relling MV. HPLC determination of thiopurine nucleosides and nucleotides in vivo in lymphoblasts following mercaptopurine therapy. Clin Chem. 2002;48:61–68. [PubMed] [Google Scholar]
- 16.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia S, Hageman L, Chen Y, et al. Effect of a daily text messaging and directly supervised therapy intervention on oral mercaptopurine adherence in children with acute lymphoblastic leukemia: a randomized clinical trial. JAMA Netw Open. 2020;3:e2014205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butow P, Palmer S, Pai A, Goodenough B, Luckett T, King M. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4800–4809. [DOI] [PubMed] [Google Scholar]
- 19.Landier W, Hughes CB, Calvillo ER, et al. A grounded theory of the process of adherence to oral chemotherapy in Hispanic and Caucasian children and adolescents with acute lymphoblastic leukemia. J Pediatr Oncol Nurs. 2011;28:203–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrady ME, Williams SN, Davies SM, Pai AL. Adherence to outpatient oral medication regimens in adolescent hematopoietic stem cell transplant recipients. Eur J Oncol Nurs. 2014;18:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


