Abstract
Aims:
The role of ceramides in the pathogenesis of type 2 diabetes mellitus (T2DM) is incompletely characterized. Given that ceramides represent therapeutic targets to disrupt the euglycemia-T2DM transition, we aimed to characterize their association with prevalent and incident T2DM in a novel cohort.
Methods:
We examined the cross-sectional and longitudinal association of baseline ceramides with prevalent and incident T2DM among 1423 adults(47% women; median(range) baseline age 72(51–95) years) in the Mayo Clinic Study of Aging cohort. We examined the associations of ceramides with prevalent T2DM (adjusted odds ratio[95% confidence interval]) at baseline and incident T2DM (adjusted hazard ratio[95% confidence interval]) during median follow-up of 6.2 years, after adjusting for demographic and metabolic factors.
Results:
Among 1423 adults, there were 222 prevalent and 37 incident cases of T2DM. In cross-sectional analyses, higher levels of ceramide C16:0 were associated with lower odds of prevalent T2DM(aOR 0.84[0.71–0.99];P=0.03) whereas C18:0(aOR 1.27[1.06–1.42];P=0.01), C18:0/16:0(aOR 1.41[1.22–1.62]; P<0.001) and C18:0/24:0(aOR 1.22[1.05–1.41]; P=0.01) were associated with higher odds. In Cox hazard regression models, C18:0/16:0(aHR 1.63[1.26–2.10];P<0.001) and C18:0(aHR 1.53[1.12–2.08];P=0.01) were associated with increased risk of incident T2DM.
Conclusions:
In this prospective population-based cohort, ceramides were associated with prevalent T2DM(C16:0,C18:0, C18:0/C16:0 ratio, C18:0/C24:0 ratio) and incident T2DM (C18:0, C18:0/C16:0 ratio) and could suggest targets for the primary and secondary prevention of T2DM.
Keywords: type 2 diabetes, ceramides, incident diabetes, prevalent diabetes, inflammation
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with a high burden of morbidity and mortality among all races and ethnic groups [1,2]. The Diabetes Prevention Program showed that aggressive lifestyle intervention reduces incidence of T2DM by 58% compared with 31% by metformin [3]. Despite these and other studies, the metabolic abnormalities that precede and/or co-occur with T2DM are incompletely characterized, thereby limiting the development of interventions to prevent T2DM and its sequelae. In particular, the incidence and prevalence of diabetes is higher among middle and older aged adults (age ≥45 years) compared to younger adults; however, there are sparse data on novel therapeutic targets to disrupt the transition from prediabetes to T2DM among middle and older aged adults [4,5].
Ceramides are sphingolipids with roles in cell structural integrity and cell signaling [6,7]. Ceramides have a sphingosine backbone attached to a fatty acid and are precursors for complex sphingolipids [8]. Ceramides are pro-inflammatory and pro-apoptotic and serum concentrations increase with advancing age. However, the significance of this change for T2DM is unknown [9]. Collectively, animal and human studies implicate ceramides in the pathogenesis of T2DM through reduced insulin secretion, impaired insulin signaling, and impaired glucose transporter activity, among other pathways [10]. In addition to their role in T2DM pathogenesis, ceramides have also been implicated in the development of T2DM sequelae including cardiovascular disease, nephropathy, and neuropathy. Ceramides may promote these sequelae through lipid and inflammatory pathways, but also through non-overlapping, novel, incompletely characterized pathways. To this end, there is emerging interest to characterize the associations between ceramides and prevalent or incident T2DM, especially among middle and older aged adults. Although a few human studies have suggested associations between plasma ceramides and T2DM, these have primarily been cross-sectional studies or small longitudinal studies of middle-aged adults [10,11]. An improved understanding of ceramides and T2DM risk may guide the development of novel pharmacologic interventions for the primary and secondary prevention of T2DM [12,13].
Therefore, the objective of this study was to examine the association of plasma ceramides with prevalent and incident T2DM among participants in the Mayo Clinic Study of Aging (MCSA), a prospective cohort of middle and older aged adults. This study addresses knowledge gaps on ceramide profiles in middle and older aged adults, a group with a high burden of T2DM and with limited information on ceramide profiles.
2. METHODS
2.1. Study design and setting
The Mayo Clinic Study of Aging (MCSA) is a prospective population-based cohort study of residents in Olmsted County, Minnesota, USA, to assess the burden of and risk factors for mild cognitive impairment, as previously described [14,15]. The MCSA initially enrolled residents aged 70–89 years and was expanded in 2012 to include residents aged 50 years and older. Residents were recruited using a stratified random sampling design of sex and 10-year age strata.
2.2. Population
The present study included 1423 participants with information on prevalent T2DM at the time of enrollment and assayed plasma ceramides. We categorized participants as having prevalent T2DM (n = 222; cases) or being T2DM-free (n = 1201; controls). The study was approved by the Mayo Clinic and Olmsted Medical Centers Institutional Review Boards. Participants provided written informed consent.
2.3. Baseline characteristics
At the time of enrollment, participants provided demographic information (e.g., age and sex). During the in-clinic visit, height (cm), weight (kg), and blood pressure (mm Hg) were measured. Body mass index (BMI, kg/m2) was calculated from height and weight measurements. Medical conditions and medications were obtained from the Rochester Epidemiology Project (REP) medical records-linkage system [16,17]. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic ≥90 mm Hg on ≥2 occasions, or treatment for hypertension. Dyslipidemia was defined by presence of at least one criterion: total cholesterol >200 mg/dL, high-density lipoprotein <40 mg/dL (men) or <50 mg/dL (women), serum triglycerides ≥150 mg/dL, or use of lipid-lowering medication. Vascular burden score was a composite of congestive heart failure, myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, ischemic heart disease, coronary angioplasty, and coronary artery disease. The score ranged from 0 – 7 (one point for each condition), which we dichotomized as absence (score 0) or presence (score ≥1) of vascular burden. Medications examined included metformin, statins (atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin) and non-statin cholesterol lowering (bile acid sequestrants, ezetimibe, fibric acid, nicotinic acid, omega 3 fatty acids).
2.4. Ascertainment of T2DM
T2DM was ascertained by medical record abstraction using the REP medical records-linkage system [17]. T2DM was defined by the presence of at least one criterion (1) fasting blood glucose ≥126 mg/dL (7.0mmoL/L) on 2 separate occasions, (2) documented insulin and/or oral hypoglycemic medication with a history of diabetes, (3) hemoglobin A1c ≥6.5%, or (4) physician diagnosis of diabetes, as previously described [16]. We excluded blood glucose readings obtained during a non-fasting state, concomitant with intravenous infusion, or during hospitalization. We excluded participants with type 1 diabetes mellitus, and instances when insulin was initiated in the hospital and continued only up to 2 weeks after hospital discharge.
2.5. Ceramide measurement
During the in-clinic visit, participants provided a fasting blood sample that was centrifuged, aliquoted, and stored at −80°C until biomarker measurement. For participants in this study, plasma ceramides were measured from 2008–2015, as previously described [18]. Liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) analysis of ceramides was performed using an AB Sciex quadrupole mass spectrometer 6500 (Sciex, Framingham, MA) equipped with an ESI probe and interfaced with the Agilent 1290 infinity liquid chromatography system (Agilent, Palo Alto, CA). The ultra-performance liquid chromatography (UPLC) system consisted of an Agilent 1290 binary pump, thermostat, thermostatted column compartment (TCC), and sampler. The injection volume was 10 μL for extracted samples. Ceramides were separated with a Poroshell 120 EC- C8 column, 2.1×50 mm, 2.7 μm (Agilent, Palo Alto, CA). Mobile phase A was water:methanol:formic acid:ammonium formate (45/55/0.5%/5 mM by v/v). Mobile phase B was acetonitrile:methanol:formic acid:ammonium formate (50/50/0.5%/5 mM by v/v). The valve, sample loop, and needle were washed with acetonitrile: methanol (50/50 by v/v) for 20 sec. Mass spectrometric analyses were performed online using electrospray ionization tandem mass spectrometry in the positive mode.
Samples were prepared using a Biomek FX (Beckman Coulter, Brea, CA). A small amount of plasma sample was added to a 2-mL 96-well plate, followed by an internal standard mixture. Ceramides were extracted using 1 phase extraction with methanol-dichloromethane. Lipid levels were quantified by the ratio of the ceramides to internal standard. Calibration curves were obtained by serial dilution of a mixture of lipid standards and expressed in ng/ml. Pure synthetic ceramide standards were purchased from Avanti Lipids. Isotope labeling synthetic standards were synthesized internally at Eli Lilly and Company). As previously described, we examined ceramides C14:0, C16:0, C18:0, C18:1, C20:0, C22:0, C24:0, C24:1, and C26:0 as well as the following ceramide ratios: C16:0/24:0, C18:0/24:0, C24:1/24:0, and C18:0/C16:0 [19].
2.6. Prevalent T2DM
We evaluated the association of characteristics with prevalent T2DM, defined by T2DM diagnosed prior to MCSA enrollment. At enrollment, 1201 participants were T2DM-free and 222 had prevalent T2DM. The association of characteristics with prevalent T2DM was evaluated using logistic regression models (described in section 2.8.).
2.7. Incident T2DM
T2DM-free participants (n = 1201) were followed prospectively for incident T2DM, defined as T2DM diagnosed after MCSA enrollment. Of these, 73 participants were administratively censored, to yield a cohort of 1128 participants. Incident T2DM was determined by review of the medical record on a rolling schedule every 15 – 24 months. For this study, medical records were last reviewed in March 2020. The association of characteristics with incident T2DM was evaluated using Cox regression models (described in section 2.8.).
2.8. Statistical analysis
We compared baseline demographic characteristics by baseline T2DM using descriptive statistics. Kruskal-Wallis tests were used to compare medians for continuous measurements, Chi-square test for categorical measurements, and Spearman’s rho to assess correlations. The ceramides and ceramide ratios were z-scored with a mean of 0 and SD of 1 to allow comparison across the ceramide species and ratios. We used logistic regression to evaluate the cross-sectional association between ceramides and odds of prevalent T2DM. Among those without T2DM at baseline, we fit Cox proportional hazard models to examine the association between the plasma ceramides or ratios and risk of incident T2DM, using age as the time scale. We developed two models: model 1 adjusted for age and sex; model 2 adjusted additionally for hypertension, dyslipidemia, and vascular burden. The associations between ceramides (per SD increment in z-scores) and T2DM are reported as adjusted odds ratio and 95% confidence interval (aOR [95% CI]) for prevalent T2DM and as adjusted hazard ratios (aHR [95% CI]) for incident T2DM. Statistical analysis was completed using SAS 9.4 (SAS Institute Inc, Cary, NC) and R, with statistical significance established at a two-tailed alpha of 0.05.
2.9. Data sharing
Study data are available upon reasonable request.
3. RESULTS
3.1. Participant characteristics and prevalent T2DM
The study population included 222 participants with prevalent T2DM at baseline and 1201 without (Table 1). Participants with prevalent T2DM had a higher median age, a higher proportion of men, a higher median BMI, and a higher proportion of participants with hypertension but similar median systolic blood pressure. The proportion of participants with dyslipidemia and use of metformin and cholesterol-lowering medications (statins and non-statins) was higher among those with T2DM compared to those without. The results were generally similar when stratified by metformin use (Supplement Table 1) and age <70 years (Supplement Table 2) and age ≥70 years (Supplement Table 3).
Table 1 -.
Characteristics of participants categorized by baseline prevalence of T2DM.
| Characteristics | Controls (T2DM-free) n = 1201 | Cases (Prevalent T2DM) n = 222 | P value |
|---|---|---|---|
| Age, years | 71.3 (50.7 – 93.0) | 74.8 (51.6 – 95.3) | <0.001 |
| Men | 614 (51.1) | 140 (63.1) | 0.001 |
| Body mass index (kg/m2) | 27.4 (16.2 – 55.7) | 30.5 (19.4 – 53.4) | <0.001 |
| Systolic blood pressure (mm Hg) | 139 (87 – 200) | 140 (93 – 191) | 0.47 |
| Hypertension | 668 (55.6) | 199 (89.6) | <0.001 |
| Dyslipidemia | 914 (76.1) | 216 (97.3) | <0.001 |
| Vascular burden | 280 (23.3) | 108 (48.6) | <0.001 |
| Medications | |||
| Metformin | 4 (0.3) | 116 (52.3) | <0.001 |
| Statin | 513 (42.7) | 181 (81.5) | <0.001 |
| Non-statin cholesterol lowering | 69 (5.7) | 37 (16.7) | <0.001 |
| Ceramides (ng/mL) | |||
| C14:0 | 9.5 (1.8 – 127.2) | 7.7 (1.5 – 54.1) | <0.001 |
| C16:0 | 87.3 (24.7 – 198.7) | 80.1 (34.9 – 175.7) | <0.001 |
| C18:0 | 73.4 (4.9 – 235.9) | 75.4 (13.6 – 245.5) | 0.16 |
| C18:1 | 2.8 (0.1 – 27.1) | 2.4 (0.4 – 30.3) | 0.03 |
| C20:0 | 223.4 (38.1 – 572.7) | 221.8 (73.8 – 728.6) | 0.51 |
| C23:0 | 415.4 (37.6 – 987.5) | 431.7 (155.5 – 828.0) | 0.37 |
| C24:0 | 1230.2 (102.0 – 3238.3) | 1118.3 (286.2 – 2329.7) | <0.001 |
| C24:1 | 309.7 (124.2 – 711.6) | 292.1 (108.4 – 732.0) | 0.01 |
| C16:0/24:0 | 0.07 (0.03 – 0.89) | 0.07 (0.03 – 0.15) | 0.97 |
| C18:0/16:0 | 0.83 (0.09 – 2.76) | 0.94 (0.22 – 2.23) | <0.001 |
| C18:0/24:0 | 0.06 (0.01 – 0.56) | 0.07 (0.02 – 0.31) | <0.001 |
| C24:1/24:0 | 0.26 (0.08 – 2.55) | 0.27 (0.11 – 0.80) | 0.07 |
Data presented as median (range) for age, body mass index, systolic blood pressure, and ceramides, and as frequency (%) for others.
Percentages may not add to 100 due to rounding.
Data were missing for body mass index (n = 10) and systolic blood pressure (n = 11).
Statistical significance established at two-tailed P< 0.05 from Kruskal-Wallis (continuous variables) and Chi-square (categorical variables) tests.
3.2. Plasma ceramides and prevalent T2DM
The median plasma concentration of ceramides C14:0, C16:0, C18:1, C24:0, C24:1 was lower in cases compared with controls, whereas C18:0/16:0 and C18:0/24:0 ratios were higher (Table 1). None of the other ceramide levels or ratios differed by T2DM status.
Spearman correlations between ceramide levels and ceramide ratios by T2DM are shown in Figure 1. Ceramide C18:0 showed positive correlation with C20:0 (rho 0.8 for cases and 0.71 for controls) and C24:1 (rho 0.57 for cases and 0.61 for controls). The correlation coefficients were generally similar among participants with and without prevalent T2DM.
Fig. 1.
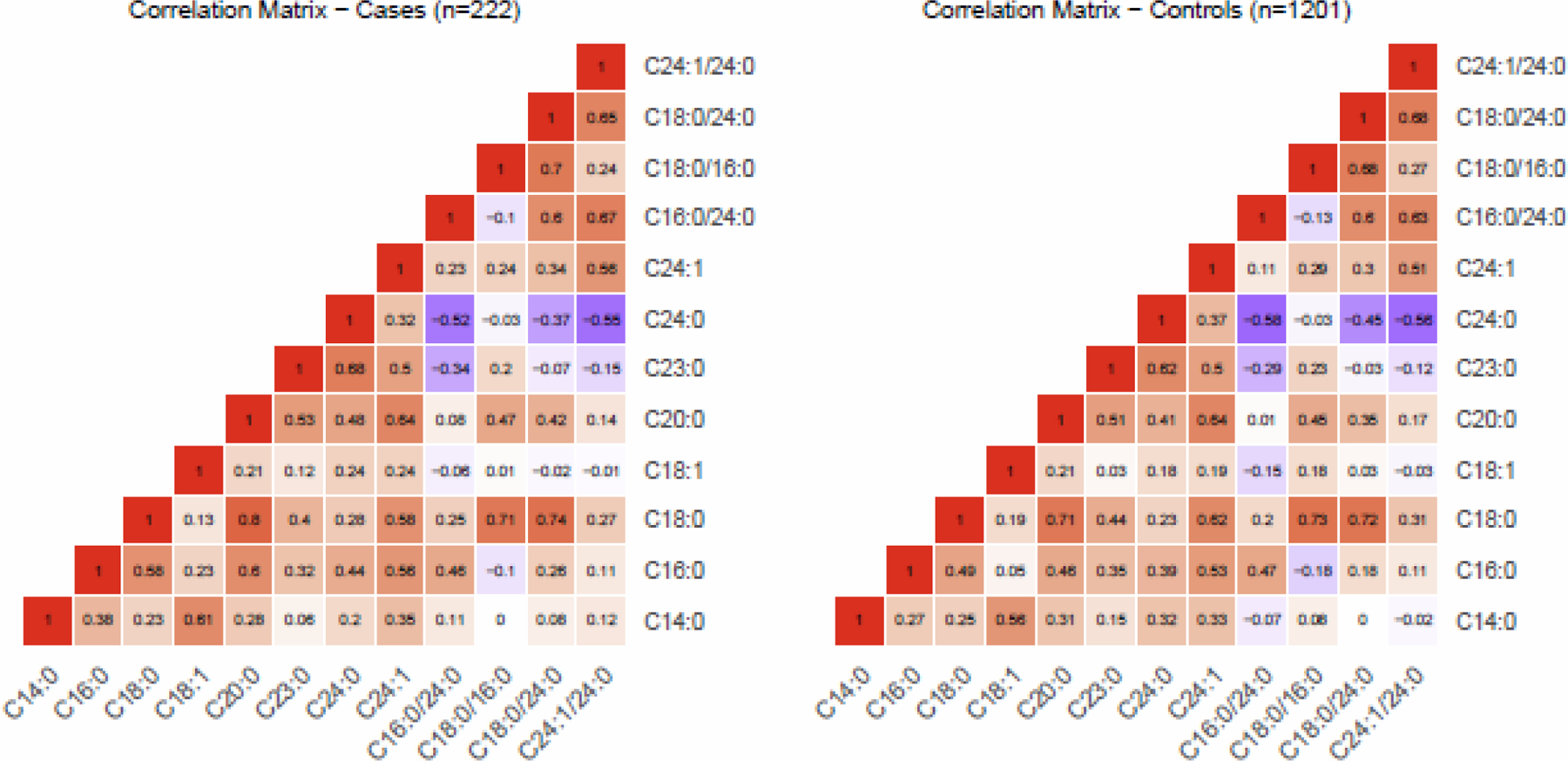
Spearman correlation matrix of plasma ceramides and ceramide ratios among participants with baseline prevalent type 2 diabetes mellitus (left panel) and without (right panel).
3.3. Association of ceramides with prevalent T2DM
In models adjusting for age and sex (model 1), each z-score increase of ceramides C18:0 and the C18:0/16:0 and C18:0/24:0 ratios was associated with higher odds of T2DM. In contrast, each z-score increase of ceramides C14:0, C16:0, and C24:0 was associated with lower odds (Table 2). After adjustment for additional covariates in model 2, the associations remained with the exception that higher levels of ceramides C14:0 and C24:0 were no longer associated with lower odds of T2DM. In model 2, each z-score increase in the ceramide C18:0/C16:0 ratio was associated with a 41% higher odds of prevalent T2DM (aOR 1.41 [1.22 – 1.62]; P< 0.001). Ceramide C18:0 was associated with a 23% higher odds of prevalent T2DM (aOR 1.23 [1.06 – 1.42]; P= 0.01), which was similar to the 22% higher odds of the C18:0/24:0 ratio (aOR 1.22 [1.05 – 1.41]; P= 0.01), but of somewhat higher magnitude than the 16% lower odds of C16:0 with prevalent T2DM (aOR 0.84 [0.71 – 0.99]; P= 0.03).
Table 2 -.
Association of plasma ceramides with baseline prevalence of T2DM.
| Ceramides | Cases vs. Controls Odds ratio (95% CI) Model 1 | P value | Cases vs. Controls Odds ratio (95% CI) Model 2 | P value |
|---|---|---|---|---|
| C14:0 | 0.78 (0.65 – 0.93) | 0.007 | 0.84 (0.71 – 1.01) | 0.06 |
| C16:0 | 0.73 (0.62 – 0.86) | <0.001 | 0.84 (0.71 – 0.99) | 0.03 |
| C18:0 | 1.27 (1.10 – 1.45) | 0.001 | 1.23 (1.06 – 1.42) | 0.01 |
| C18:1 | 0.88 (0.75 – 1.03) | 0.12 | 0.95 (0.81 – 1.12) | 0.54 |
| C20:0 | 1.08 (0.94 – 1.25) | 0.28 | 1.10 (0.95 – 1.28) | 0.21 |
| C23:0 | 1.07 (0.93 – 1.24) | 0.35 | 1.12 (0.97 – 1.30) | 0.13 |
| C24:0 | 0.80 (0.69 – 0.93) | 0.005 | 0.88 (0.75 – 1.03) | 0.11 |
| C24:1 | 0.86 (0.74 – 1.01) | 0.06 | 0.92 (0.79 – 1.08) | 0.32 |
| C16:0/24:0 | 0.93 (0.77 – 1.12) | 0.44 | 0.94 (0.80 – 1.11) | 0.45 |
| C18:0/16:0 | 1.58 (1.38 – 1.80) | <0.001 | 1.41 (1.22 – 1.62) | <0.001 |
| C18:0/24:0 | 1.33 (1.15 – 1.53) | <0.001 | 1.22 (1.05 – 1.41) | 0.01 |
| C24:1/24:0 | 1.07 (0.94 – 1.22) | 0.30 | 1.02 (0.90 – 1.16) | 0.75 |
Odds ratio (95% confidence interval) of ceramides per standard deviation (SD) increment in z-score for cases (prevalent T2DM) compared with controls (T2DM-free).
Model 1: adjusted for age and sex.
Model 2: additionally adjusted for hypertension, dyslipidemia, and vascular burden.
3.4. Participant characteristics and incident T2DM
During median (range) follow-up of 6.2 (1.0 –11.3) years, the incidence of T2DM was 3.3% (n = 37/1128). Participants with incident T2DM had higher baseline BMI (29.5 [25.3 – 43.2] vs 27.3 [17.3 – 55.7] kg/m2), and higher vascular burden (37.8% vs 22.4%) compared to the referent group that remained T2DM-free (Table 3). Plasma median ceramide C18:0/16:0 and C18:0/24:0 ratios were higher for participants with incident T2DM compared to the referent group (Table 3).
Table 3 -.
Baseline characteristics of participants categorized by incident T2DM.
| Participants followed prospectively for incident T2DM (n = 1128) | |||
|---|---|---|---|
| Characteristics | T2DM-free n = 1091 | Incident T2DM n = 37 | P value |
| Follow-up period, years | 6.2 (1.0 – 11.3) | 2.6 (1.0 – 8.7) | <0.001 |
| Age, years | 71.3 (50.7 – 93.0) | 72.3 (52.0 – 85.4) | 0.69 |
| Men | 557 (51.1) | 22 (59.5) | 0.31 |
| Body mass index (kg/m2) | 27.3 (17.3 – 55.7) | 29.5 (25.3 – 43.2) | <0.001 |
| Systolic blood pressure (mm Hg) | 139 (87 – 200) | 137 (117 – 172) | 0.94 |
| Hypertension | 600 (55.0) | 23 (62.2) | 0.39 |
| Dyslipidemia | 823 (75.4) | 30 (81.1) | 0.43 |
| Vascular burden | 244 (22.4) | 14 (37.8) | 0.03 |
| Medications | |||
| Metformin | 3 (0.3) | 1 (2.7) | 0.01 |
| Statin | 461 (42.3) | 20 (54.1) | 0.15 |
| Non-statin cholesterol lowering | 62 (5.7) | 3 (8.1) | 0.53 |
| Ceramides (ng/mL) | |||
| C14:0 | 9.5 (1.8 – 127.2) | 7.4 (3.3 – 48.6) | 0.07 |
| C16:0 | 88.0 (24.7 – 198.7) | 82.3 (41.9 – 119.6) | 0.08 |
| C18:0 | 73.1 (4.9 – 212.2) | 85.9 (28.9 – 184.2) | 0.06 |
| C18:1 | 2.8 (0.4 – 27.1) | 2.0 (0.6 – 9.9) | 0.74 |
| C20:0 | 223.7 (38.1 – 572.7) | 218.0 (76.4 – 468.6) | 0.92 |
| C23:0 | 415.8 (37.6 – 987.5) | 381.9 (253.0 – 736.1) | 0.74 |
| C24:0 | 1234.6 (102.0 – 3238.3) | 1282.1 (564.1 – 2431.1) | 0.50 |
| C24:1 | 308.1 (130.1 – 711.6) | 324.5 (172.1 – 480.9) | 0.86 |
| C16:0/24:0 | 0.07 (0.03 – 0.89) | 0.07 (0.04 – 0.13) | 0.42 |
| C18:0/16:0 | 0.83 (0.09 – 2.76) | 0.97 (0.52 – 2.05) | 0.002 |
| C18:0/24:0 | 0.06 (0.01 – 0.56) | 0.07 (0.03 – 0.14) | 0.03 |
| C24:1/24:0 | 0.25 (0.09 – 2.55) | 0.28 (0.12 – 0.46) | 0.41 |
Data presented as median (range) for follow-up period, age, body mass index, systolic blood pressure, and ceramides, and as frequency (%) for others. Of T2DM-free participants (n = 1201; Table 1), we excluded participants with administrative censoring (n = 73) to yield 1128 participants.
Percentages may not add to 100 due to rounding.
Data were missing for body mass index (n = 6) and systolic blood pressure (n = 9).
Statistical significance established at two-tailed P< 0.05 from Kruskal-Wallis (continuous variables) and Chi-square (categorical variables) tests.
Among ceramides, each z-score increase in the C18:0/16:0 ratio was associated with a 1.6-fold higher risk of incident T2DM in model 1 (aHR 1.63 [1.27 – 2.10]; P< 0.001) and model 2 (aHR 1.63 [1.26 – 2.10]; P< 0.001) (Table 4). Similarly, each z-score increase in ceramide C18:0 was associated with a 1.5-fold higher risk of incident T2DM in model 1 (aHR 1.49 [1.10 – 2.01]; P= 0.01) and model 2 (aHR 1.53 [1.12 – 2.08)]; P= 0.01). Increasing ceramide C18:0/24:0 ratio was also associated with risk of incident T2DM in model 1 (aHR 1.19 [1.01 – 1.40]; P= 0.04), but the association was attenuated and no longer significant after further adjustment in model 2 (aHR 1.15 [0.97 – 1.36]; P= 0.11).
Table 4 -.
Association of baseline plasma ceramides with risk of incident T2DM.
| Ceramides | Incident T2DM Hazard ratio (95% CI) n = 37/1128 Model 1 | P value | Incident T2DM Hazard ratio (95% CI) n = 37/1128 Model 2 | P value |
|---|---|---|---|---|
| C14:0 | 0.90 (0.62 – 1.30) | 0.56 | 0.92 (0.63 – 1.33) | 0.65 |
| C16:0 | 0.74 (0.51 – 1.09) | 0.13 | 0.78 (0.53 – 1.16) | 0.22 |
| C18:0 | 1.49 (1.10 – 2.01) | 0.01 | 1.53 (1.12 – 2.08) | 0.01 |
| C18:1 | 0.97 (0.68 – 1.39) | 0.88 | 1.01 (0.71 – 1.44) | 0.97 |
| C20:0 | 1.20 (0.87 – 1.66) | 0.27 | 1.26 (0.90 – 1.75) | 0.18 |
| C23:0 | 1.02 (0.74 – 1.42) | 0.90 | 1.07 (0.78 – 1.47) | 0.69 |
| C24:0 | 0.91 (0.65 – 1.28) | 0.59 | 0.98 (0.70 – 1.39) | 0.92 |
| C24:1 | 1.00 (0.71 – 1.39) | 0.97 | 1.05 (0.75 – 1.47) | 0.79 |
| C16:0/24:0 | 0.87 (0.54 – 1.40) | 0.57 | 0.84 (0.52 – 1.36) | 0.48 |
| C18:0/16:0 | 1.63 (1.27 – 2.10) | <0.001 | 1.63 (1.26 – 2.10) | <0.001 |
| C18:0/24:0 | 1.19 (1.01 – 1.40) | 0.04 | 1.15 (0.97 – 1.36) | 0.11 |
| C24:1/24:0 | 1.05 (0.83 – 1.33) | 0.69 | 1.02 (0.80 – 1.29) | 0.88 |
Cox proportional hazard ratio (95% confidence interval) for risk of incident T2DM per standard deviation (SD) increment in z-score of ceramides.
Model 1: adjusted for age and sex.
Model 2: additionally adjusted for hypertension, dyslipidemia, and vascular burden.
4. DISCUSSION
In this prospective study of middle and older aged adults, we described the associations of plasma ceramides with both prevalent and incident T2DM. Ceramide C18:0/C16:0 ratio and C18:0 were associated with a ~1.3-fold higher odds of baseline prevalent T2DM and a ~1.5-fold higher risk of incident T2DM. A higher ceramide C16:0 level was associated with a lower odds of prevalent T2DM whereas an increasing C18:0/24:0 ratio was associated with higher odds. This study builds on the growing literature of ceramides and T2DM with implications for the primary and secondary prevention of T2DM in middle and older aged adults.
Studies on ceramides and incident T2DM have yielded mixed results [10–12,20–25]. In a study on 163 metabolites and incident T2DM, ceramides C16:1 and C18:2 were associated with a lower incidence of T2DM [12]. The addition of ceramides C16:1, C18:2, and other metabolites to a diabetes risk score improved model discrimination for incident T2DM [12]. The PREDIMED trial, which examined Mediterranean diets for primary cardiovascular prevention, reported that sphingomyelin d18:1/18:0 was associated with a reduced risk of incident T2DM [25]. In contrast, a recent population-based study of 76 sphingolipids showed an increased risk between ceramides (e.g., d18:1/18:1, d18:1/20:0, d18:1/20:1, d18:1/22:1) and incident T2DM [11]. In the present study, higher levels of C18:0 and ceramide C18:0/16:0 ratio were associated with a higher risk of incident T2DM, but we did not find associations between the other ceramides and T2DM. The association of ceramides with T2DM may vary based on genetic factors, lifestyle, and race/ethnic groups, and may, in part, explain differences across studies [24,26]. In the present study, the population of predominantly older White adults with a high burden of hypertension, dyslipidemia, and vascular disease, differed from other study populations of younger, healthier adults of diverse races and ethnicities. The role of ceramides and pathways that increase risk of T2DM should be investigated in other diverse populations.
Ceramides have also been implicated in the development of T2DM sequelae [6,7,10,27–33]. In the Framingham Offspring Study, a higher C16:0/24:0 ratio was associated with a higher risk of heart failure [27]. These results agreed with findings from the Cardiovascular Health Study, in which, higher plasma concentrations of ceramide C16:0 and sphingomyelin 16 (d18:1/16:0) were associated with increased risk of heart failure, whereas ceramide C22:0 and sphingomyelin 20 (d18:1/20:0), sphingomyelin 22 (d18:1/22:0), and sphingomyelin 24 (d18:1/24:0) were associated with lower risk [32]. In the present study, >80% of participants with baseline prevalent T2DM were on cholesterol-lowering medication, and ceramides (C16:0, C18:0; C18:0/C24:0) associated with prevalent T2DM may reflect a ceramide profile associated with T2DM sequalae. In this context, ceramide-based risk scores have been developed to predict risk of incident and recurrent cardiovascular disease [28,33]. In our cohort, many participants had a high burden of hypertension; in participants with T2DM, these comorbidities increase risk for kidney disease and cardiovascular outcomes [34]. The significance of ceramide profiles and ceramide-based risk scores to T2DM sequelae (e.g., renal and cardiovascular outcomes) may guide secondary prevention among adults with T2DM.
In this study, there was a higher proportion of men among participants with vs. without baseline prevalent T2DM. Although we adjusted for gender and comorbidities in model 2, this study highlights the importance of gender in the development and management of T2DM and comorbidities. In this context, the REgistro POliterapie SIMI (REPOSI) prospective registry of hospitalized patients 65 years or older has highlighted the complex interplay of gender, comorbidities, and frailty [35,36]. Strategies to reduce the risk of T2DM should consider gender-based prevalence of comorbidities and preferences for management.
Ceramides may exert biological effects through inflammatory and/or lipid pathways [37]. Animal models suggested that inhibition of tumor necrosis factor (TNF) α improves insulin resistance, although a meta-analysis showed positive correlation of T2DM with interleukin (IL)-6 and C-reactive protein, but not with TNFα and IL-1β [38,39]. The CANTOS trial, which randomized participants to canakinumab (IL-1β inhibitor) showed neutral effect on the prespecified endpoint of incident T2DM, leading to speculation on the role of inflammation or, at least, IL-1β mediated pathways in T2DM [40]. In the present study, most participants with prevalent T2DM were on metformin and cholesterol-lowering medication, which could alter the profile and level of ceramides. The possibility that ceramides modulate metabolic pathways independent of inflammatory biomarkers and ‘traditional lipids’ such as low-density lipoprotein and high-density lipoprotein has led to speculation that ceramides may represent a novel pharmacologic target to modulate the trajectory for T2DM and its sequelae [6,8,37]. In support of the role of ceramides in T2DM pathogenesis, a murine knock-out model of CerS1, the gene that synthesizes ceramide C18:0, showed improved glucose sensitivity [41]. Such models could reveal the effect of altering inflammatory and/or lipid pathways on glucose metabolism and risk of developing T2DM.
Several studies have examined strategies to regulate ceramide levels and function. The Framingham Offspring Study showed that higher quality of diet (e.g., Mediterranean diet) attenuated the association between serum ceramides and cancer mortality, whereas sugar-sweetened beverages increased concentrations of ceramides associated with adverse metabolic health [42,43]. In prospective studies, nut consumption, which is associated with lower risk of T2DM, was positively associated with sphingomyelin d18:1/C22:0 and d18:1/C24:0, and ceramide C24:0, and lower levels of C-reactive protein and IL-6 [44,45]. Studies have explored pharmacologic approaches to target ceramide pathways linked to diabetes, cardiovascular disease, depression, and hematologic disease [46,47]. Whether or not findings from different disciplines could translate into improved outcomes for T2DM is unknown.
In our study, the incidence rate of T2DM was low and may result from enrolled participants being healthier than non-participants and/or participants/healthcare providers preference for infrequent T2DM testing. The robust REP data linkage system and manual review of medical records reduced errors associated with purely electronic data extraction, thereby, mitigating errors in data abstraction. In addition, the incident rate of T2DM in Olmsted County is incompletely described. Data from the Centers for Disease Control and Prevention suggest that the annual, age-adjusted incidence rates of diabetes in Olmsted County, from 2008–2015, range from 5.8%−7% [48]. However, these rates include type 1 and type 2 diabetes and all race/ethnic groups, which differs from the MCSA cohort.
The study has several strengths. We used a population-based sample with robust longitudinal follow-up of disease outcomes. Many baseline characteristics (e.g., medical conditions) were determined from medical records rather than self-report, thereby, reducing recall bias. The study focused on a cohort (middle and older aged adults) for whom there was sparse data on ceramide profiles and T2DM. Finally, this study described associations of ceramides with prevalent and incident T2DM, in contrast to previous studies that focused on either prevalent or incident T2DM, thereby, allowing for description of ceramide profiles relevant to the primary and secondary prevention of T2DM. The study also has limitations. Most participants were White, and the findings may not be generalizable to other races and ethnic groups. Participants may be healthier than non-participants; however, the MCSA used a population-based sampling frame design, thereby reducing potential bias in participant selection. The confidence interval for some characteristics was close to 1.0 and possibly influenced by the study power to identify associations with T2DM.
In summary, we characterized ceramide profiles among middle and older aged adults and described the association of C18:0 and C18:0/16:0 ratio with prevalent and incident T2DM. The findings build on previous studies and underscore the emerging role of ceramides in T2DM pathogenesis. This study has clinical implications as it identifies C18:0 and C18:0/16:0 as biomarkers that may improve T2DM risk prediction and stratification and as potential pharmacologic targets for T2DM prevention.
5. CONCLUSION
In this prospective study of 1423 middle and older aged adults in MCSA, we described the associations of ceramides with both prevalent and incident T2DM. In models adjusted for traditional metabolic factors, C18:0/16:0 ratio and C18:0 were associated with higher odds of prevalent T2DM and higher risk of incident T2DM. In addition, C16:0 was associated with lower odds of prevalent T2DM whereas C18:0/24:0 ratio was associated with higher odds. To our knowledge, there are sparse studies on the longitudinal association of ceramides and T2DM, and none specifically in older adults. In this context, the study addresses a knowledge gap and has implications for primary and secondary T2DM prevention in older adults. Future studies are required to examine the effect of lifestyle and pharmacologic interventions on ceramide levels and risk of T2DM.
Supplementary Material
ACKNOWLEDGEMENTS
Funding
SBD is supported by a career development award from the National Institutes of Health/National Institute on Minority Health and Health Disparities (K23 MD016230) and by the Robert and Elizabeth Strickland Career Development Award, Mayo Clinic, Rochester, MN, USA. The study was supported by grants from the National Institutes of Health/National Institute on Aging (R01 AG49704; U01 AG006786) and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Disclosure of Competing Interest
SBD, LRC, JAA, HHB: none
AV is the recipient of an investigator-initiated grant from Novo Nordisk. He has consulted for vTv Therapeutics and Zealand Pharmaceuticals. He receives research funding from the National Institutes of Health (NIH), USA.
MMM has consulted for Brain Protection Company and Biogen. She receives research funding from the NIH and Department of Defense (DOD), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].CDC. National Diabetes Statistics Report 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2020.
- [2].American Diabetes Association. 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl 1):S7–S14. doi: 10.2337/dc21-S001 [DOI] [PubMed] [Google Scholar]
- [3].Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care 2019;7(1):e000657. doi: 10.1136/bmjdrc-2019-000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care 2017;40(4):440–3. doi: 10.2337/dci17-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Summers SA. Could ceramides become the new cholesterol? Cell Metab 2018;27(2):276–80. doi: 10.1016/j.cmet.2017.12.003 [DOI] [PubMed] [Google Scholar]
- [7].Iqbal J, Walsh MT, Hammad SM, Hussain MM. Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol Metab 2017;28(7):506–18. doi: 10.1016/j.tem.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol 2017;13(2):79–91. doi: 10.1038/nrendo.2016.169 [DOI] [PubMed] [Google Scholar]
- [9].Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, et al. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell 2015;14(6):1014–23. doi: 10.1111/acel.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mandal N, Grambergs R, Mondal K, Basu SK, Tahia F, Dagogo-Jack S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J Diabetes Complications 2021;35(2):107734. doi: 10.1016/j.jdiacomp.2020.107734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yun H, Sun L, Wu Q, Zong G, Qi Q, Li H, et al. Associations among circulating sphingolipids, beta-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med 2020;17(12):e1003451. doi: 10.1371/journal.pmed.1003451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62(2):639–48. doi: 10.2337/db12-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 2016;39(5):833–46. doi: 10.2337/dc15-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388 [DOI] [PubMed] [Google Scholar]
- [15].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30(1):58–69. doi: 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014;82(13):1132–41. doi: 10.1212/WNL.0000000000000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41(6):1614–24. doi: 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wennberg AMV, Hagen CE, Machulda MM, Knopman DS, Petersen RC, Mielke MM. The cross-sectional and longitudinal associations metween IL-6, IL-10, and TNFalpha and cognitive outcomes in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2019;74(8):1289–95. doi: 10.1093/gerona/gly217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fatemi F, Kantarci K, Graff-Radford J, Preboske GM, Weigand SD, Przybelski SA, et al. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology 2018;90(6):e466–e73. doi: 10.1212/WNL.0000000000004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chew WS, Torta F, Ji S, Choi H, Begum H, Sim X, et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight 2019;5. doi: 10.1172/jci.insight.126925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fretts AM, Jensen PN, Hoofnagle A, McKnight B, Howard BV, Umans J, et al. Plasma ceramide species are associated with diabetes risk in participants of the Strong Heart Study. J Nutr 2020;150(5):1214–22. doi: 10.1093/jn/nxz259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neeland IJ, Singh S, McGuire DK, Vega GL, Roddy T, Reilly DF, et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia 2018;61(12):2570–9. doi: 10.1007/s00125-018-4720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jensen PN, Fretts AM, Yu C, Hoofnagle AN, Umans JG, Howard BV, et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the Strong Heart Family Study. EBioMedicine 2019;41:44–9. doi: 10.1016/j.ebiom.2018.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen GC, Chai JC, Yu B, Michelotti GA, Grove ML, Fretts AM, et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr 2020;112(1):57–65. doi: 10.1093/ajcn/nqaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Razquin C, Toledo E, Clish CB, Ruiz-Canela M, Dennis C, Corella D, et al. Plasma lipidomic profiling and risk of type 2 siabetes in the PREDIMED Trial. Diabetes Care 2018;41(12):2617–24. doi: 10.2337/dc18-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buie JNJ, Hammad SM, Nietert PJ, Magwood G, Adams RJ, Bonilha L, et al. Differences in plasma levels of long chain and very long chain ceramides between African Americans and whites: an observational study. PLoS One 2019;14(5):e0216213. doi: 10.1371/journal.pone.0216213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nwabuo CC, Duncan M, Xanthakis V, Peterson LR, Mitchell GF, McManus D, et al. Association of circulating ceramides with cardiac structure and function in the community: the Framingham Heart Study. J Am Heart Assoc 2019;8(19):e013050. doi: 10.1161/JAHA.119.013050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020;41(3):371–80. doi: 10.1093/eurheartj/ehz387 [DOI] [PubMed] [Google Scholar]
- [29].Busik JV. Lipid metabolism dysregulation in diabetic retinopathy. J Lipid Res 2021;62:100017. doi: 10.1194/jlr.TR120000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides. Arterioscler Thromb Vasc Biol 2018;38(8):1933–9. doi: 10.1161/ATVBAHA.118.311199 [DOI] [PubMed] [Google Scholar]
- [31].Mundra PA, Barlow CK, Nestel PJ, Barnes EH, Kirby A, Thompson P, et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018;3(17). doi: 10.1172/jci.insight.121326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail 2019;12(7):e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hilvo M, Wallentin L, Ghukasyan Lakic T, Held C, Kauhanen D, Jylha A, et al. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J Am Heart Assoc 2020;9(10):e015258. doi: 10.1161/JAHA.119.015258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Minutolo R, Gabbai FB, Provenzano M, Chiodini P, Borrelli S, Garofalo C, et al. Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: pooled analysis of four cohort studies. Nephrol Dial Transplant 2018;33(11):1942–9. doi: 10.1093/ndt/gfy032 [DOI] [PubMed] [Google Scholar]
- [35].Marcucci M, Franchi C, Nobili A, Mannucci PM, Ardoino I, Investigators R. Defining Aging Phenotypes and Related Outcomes: Clues to Recognize Frailty in Hospitalized Older Patients. J Gerontol A Biol Sci Med Sci 2017;72(3):395–402. doi: 10.1093/gerona/glw188 [DOI] [PubMed] [Google Scholar]
- [36].Corrao S, Santalucia P, Argano C, Djade CD, Barone E, Tettamanti M, et al. Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med 2014;25(7):617–23. doi: 10.1016/j.ejim.2014.06.027 [DOI] [PubMed] [Google Scholar]
- [37].Chaurasia B, Summers SA. Ceramides in metabolism: key lipotoxic players. Annu Rev Physiol 2021;83:303–30. doi: 10.1146/annurev-physiol-031620-093815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013;36(1):166–75. doi: 10.2337/dc12-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016;86:100–9. doi: 10.1016/j.cyto.2016.06.028 [DOI] [PubMed] [Google Scholar]
- [40].Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol 2018;71(21):2392–401. doi: 10.1016/j.jacc.2018.03.002 [DOI] [PubMed] [Google Scholar]
- [41].Turpin-Nolan SM, Hammerschmidt P, Chen W, Jais A, Timper K, Awazawa M, et al. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep 2019;26(1):1–10 e7. doi: 10.1016/j.celrep.2018.12.031 [DOI] [PubMed] [Google Scholar]
- [42].Walker ME, Xanthakis V, Peterson LR, Duncan MS, Lee J, Ma J, et al. Dietary patterns, ceramide ratios, and risk of all-cause and cause-specific mortality: the Framingham Offspring Study. J Nutr 2020;150(11):2994–3004. doi: 10.1093/jn/nxaa269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Walker ME, Xanthakis V, Moore LL, Vasan RS, Jacques PF. Cumulative sugar-sweetened beverage consumption is associated with higher concentrations of circulating ceramides in the Framingham Offspring Cohort. Am J Clin Nutr 2020;111(2):420–8. doi: 10.1093/ajcn/nqz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288(20):2554–60. doi: 10.1001/jama.288.20.2554 [DOI] [PubMed] [Google Scholar]
- [45].Yu Z, Malik VS, Keum N, Hu FB, Giovannucci EL, Stampfer MJ, et al. Associations between nut consumption and inflammatory biomarkers. Am J Clin Nutr 2016;104(3):722–8. doi: 10.3945/ajcn.116.134205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab 2016;24(6):820–34. doi: 10.1016/j.cmet.2016.10.002 [DOI] [PubMed] [Google Scholar]
- [47].Kornhuber J, Muller CP, Becker KA, Reichel M, Gulbins E. The ceramide system as a novel antidepressant target. Trends Pharmacol Sci 2014;35(6):293–304. doi: 10.1016/j.tips.2014.04.003 [DOI] [PubMed] [Google Scholar]
- [48].Centers for Disease Control and Prevention. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html [accessed 9 July 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


