Abstract
Background:
Residual confounding is a major concern for causal inference in observational studies on air pollution–autism spectrum disorder (ASD) associations. This study is aimed at assessing confounding in these associations using negative control exposures.
Methods:
This nested case–control study included all children diagnosed with ASD (detected through 31 December 2016) born during 2007-2012 in Israel and residing in the study area (N= 3,843), and matched controls of the same age (N= 38,430). We assigned individual house-level exposure estimates for each child. We estimated associations using logistic regression models, mutually adjusted for all relevant exposure periods (pre-pregnancy, pregnancy, and postnatal). We assessed residual confounding using post-outcome negative control exposure at age 28-36 months.
Results:
In mutually adjusted models we observed positive associations with ASD for postnatal exposures to NOx (odds ratio [OR] per interquartile range, 95% confidence interval [CI]: 1.19, 1.02-1.38) and NO2 (1.20, 1.00-1.43), and gestational exposure to PM2.5-10 (1.08, 1.01-1.15). The result for the negative control period was 1.04, 0.99-1.10 for PM2.5, suggesting some residual confounding, but no associations for PM2.5-10 (0.98, 0.81-1.18), NOx (1.02, 0.84-1.25) or NO2 (0.98, 0.81-1.18), suggesting no residual confounding.
Conclusions:
Our results further support a hypothesized causal link with ASD that is specific to postnatal exposures to traffic-related pollution.
Keywords: autism spectrum disorder, ASD, air pollution, negative control
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by persistent deficits in social communication and social interaction, and restricted, repetitive patterns of behavior, interests, or activities.1 ASD has become a major public health concern posing considerable social, economic, and medical burden, given the profound life-long dysfunction it produces. Environmental exposures, independently of, or interacting with genetic factors, may play an important role in the development of ASD.2-5 Accumulating evidence suggests that the brain and the central nervous system may be adversely affected by air pollutants.6,7 Meta-analyses and reviews of the literature8-14 support the notion that air pollution is associated with elevated risk of ASD, while making some reservations that the evidence is not consistent.
Accumulating research on the hypothesized role of air pollution in autism spectrum disorder (ASD) suggests various possible windows of susceptibility before, during, and after pregnancy that act through distinct exposure routes and biologic mechanisms.15 During the pre-pregnancy period, accumulation of de novo mutations may lead to aberrant brain development.16 During gestation, mechanisms such as maternal immune activation and systemic inflammation or penetration of ultrafine particles to the foetal brain may induce neuro-inflammation,17 which may result in ASD.18 In the early postnatal exposure period, the infant directly inhales polluted air so that neuro-inflammation may be induced through systemic inflammation or particle penetration into the brain, possibly through the olfactory route.19 A comprehensive investigation of the role of air pollutants in ASD should take into account all these exposure periods, given that each of them represents a distinct mechanistic pathway.
Various epidemiologic studies demonstrated associations with ASD of pre- to postnatal exposures to common air pollutants, such as particulate matter and traffic-related air pollution (nitrogen dioxide: NO2 and nitrogen oxides: NOx, which are often used as markers of traffic-related pollution in epidemiologic studies). Evidence for such associations with PM2.5 (particulate matter with aerodynamic diameter ≤2.5 μm), PM10 (≤10 μm), and traffic-related air pollution in particular, has been inconclusive. Models with only one exposure period (pre-pregnancy, pregnancy, or post-pregnancy) to PM2.5, PM10, or traffic markers resulted in positive associations with ASD in most studies but null associations in others.8-10 In addition, any association found with a certain exposure period, when other exposure periods are not in the model, may have resulted from a causal association of a different exposure period due to the high correlation among these exposures. To increase the likelihood that the association with a specific exposure period is not confounded by an effect in another period, all relevant periods should be included in the final models.20
Two population-based studies from Denmark and Israel included pre-pregnancy, pregnancy, and post-pregnancy exposures, mutually adjusted in the same model.21,22 The Danish case–control study examined associations of ASD with NO2, SO2, PM2.5, and PM10, among all Danish children with ASD born during 1989–2013 and respective controls. The Israeli study implemented a nested case–control design using a national database and examining associations of NO2 with ASD for the entire population born 2005-2009 in central Israel. In the mutually adjusted models for all three period exposures to NO2, similar positive associations were found in both studies in the postnatal exposure period and null associations during the entire pregnancy period.21,22
Beyond the correlation among exposure periods, apparent inconsistencies in the literature8-10 may result from residual confounding - a prominent concern for causal inference in observational studies.23 Several policy and review papers suggested to address this concern in order to elucidate the causal effect of air pollution on risk of ASD,8-13,24 but none of the air pollution - ASD studies so far was designed to specifically estimate residual confounding. One method to assess whether residual confounding exists or not is using negative control exposures. An ideal negative control exposure should be known a priori as not causing the outcome under study and should be impacted similarly by the potential unobserved confounders (“U-comparability”).20,25-28
The main aim of this study is to strengthen the ability to derive causal conclusions by examining, for the first time, the relationship between air pollution and ASD with negative control exposures that test for residual confounding. This assessment is based on negative control exposures, with future exposures that cannot possibly have caused the outcome in question (which already occurred) but are expected to be impacted by the same unobserved confounders in a similar way to the possibly causal exposures.20,25-28
Methods
Study design and study population
This is a nested case–control study. The case group contained all children diagnosed with ASD (detected through 31 December 2016) born during the years 2007-2012, alive at age 4, and residing in the area covered by the exposure models (N= 3,843). Ten controls per case were individually matched by year of birth (N= 38,430) from all children without ASD, with the same inclusion criteria. We conducted this matching to enable us to identify the negative control exposure period for the controls. Since the exposure period for the secondary negative control is after the diagnosis of ASD, it is undefined for controls, who by definition do not have a diagnosis. Therefore, the matching allowed us to use the same period in the controls as the matched case for the secondary negative control exposure (the post-diagnosis period of the case). The study was approved by the ethics committee of the Hebrew University of Jerusalem (approval # 09112015).
Case ascertainment
The Israeli National Insurance Institute (NII) is a governmental institution, which provides (among other social security benefits) a child disability benefit for any Israeli family whose child has a confirmed ASD diagnosis.29 This benefit is provided regardless of income or other socioeconomic characteristics, independent of eligibility for, or actual use of, services. To receive the benefit, strict criteria must be met to verify the diagnosis, which are based on DSM-IV-TR or DSM 5, for diagnoses conducted until 2013 or afterward, respectively. We validated ASD case status in the database in a previous study, with 97% of the children with ASD detected correctly.29
Exposure assessment
Daily estimates of PM2.5 and PM10 concentrations were available from spatiotemporally resolved hybrid models at 1km resolution, incorporating satellite remote sensing products and meteorologic and land use data,30 with a cross-validated R2 of 0.72 for PM2.5 and 0.79 for PM10.30 PM2.5-10 concentrations were calculated as the difference between PM10 and PM2.5 concentrations (eAppendix 1). Half-hourly estimates of NOx and NO2 at a 500 m resolution were available from the Optimized Dispersion Model, which uses air quality monitoring data to calibrate a non-linear empiric model based on vehicle-mounted Global Positioning System receivers and meteorologic data.31-33 The models’ cross-validated performance measures in estimating weekly NOx and NO2 concentrations were R2=0.67 and R2=0.75, respectively32,33 (eAppendix 2).
We assigned individual-level average exposure estimates for each child according to the official maternal address at birth, birth date, and gestational age at birth. We attempted to geocode all the addresses at the house level and calculated average concentrations per each exposure period at the relevant grid point in each exposure model. For children whose addresses were geocoded at the locality level only (13% of the cases and 17% of the controls), exposure estimates were averaged for the entire locality area. Missing data of gestational age at birth (2.3% of the cases and 1.9% of the controls) were imputed with the mode (40 weeks).
Negative Control
We used the exposure at the age of 28-36 months as our primary negative control exposure, assuming that by that age, critical phases in possible atypical brain development initiating ASD (such as axonal pruning and synaptogenesis in specific cortical areas) had already been completed.34-36 Therefore, we assume that this negative control exposure, calculated for all cases and controls as the exposure at their birth address when they were at age 28-36 months, cannot influence the outcome in question. We also argue that this exposure is impacted by very similar spatial components that influence the perinatal exposures, since it is based on the same address.
Possible confounding by temporal factors was controlled by adjustment for year and calendar month of birth. Theoretically, an ideal exposure period for a negative control would have been after the diagnosis of ASD had been determined. Therefore, we have also conducted a secondary negative control analysis in which the 9-months post-diagnosis exposure period was used as a negative control. However, despite the theoretical advantage of this negative control period, it has two main disadvantages. First, NII data of the age at diagnosis might be prone to some inaccuracy. This is because the age of diagnosis as documented in NII may not reflect the exact date of diagnosis when NII confirmed the claim more than one year after the diagnosis. Second, the period that immediately follows the diagnosis overlaps with the first 9-month of life for 6.7% of the cases. These are the reasons that we defined the post-diagnosis negative control as secondary.
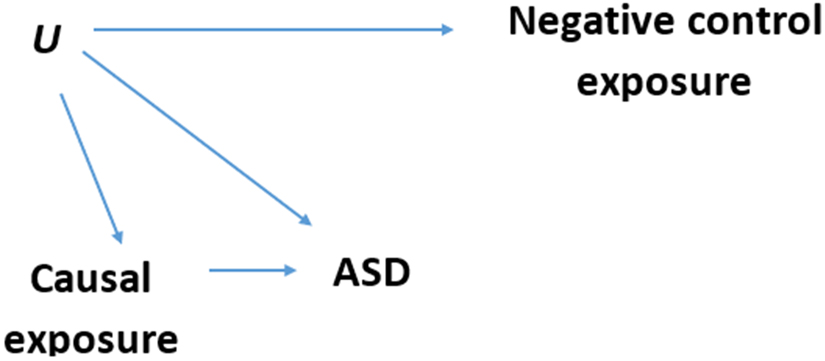
The directed acyclic graph (DAG) in Figure 1 demonstrates our assumptions about the causal structure created by the negative control approach.37 Residual confounding exists if there is an unobserved variable (U) that is influencing both the possibly causal exposures and the risk of ASD. Since variables that vary temporally are controlled by adjustment for year and month of birth, emergence of residual confounding is expected, if at all, only from variables that vary spatially.
Figure 1. Negative control exposure Directed Acyclic Graph (DAG).
Each arrow in the DAG represents an assumption of a causal relationship, and the lack of an arrow represents an assumption of no causal relationship. Causal exposure = possibly causal periods of exposure: 9 months before pregnancy, entire pregnancy period, and 9 months after birth; Negative control exposure = non-causal period of exposure (post-outcome): at age 28-36 months (or for the secondary negative control: 9 months after diagnosis); ASD = autism spectrum disorder (the outcome). U = unobserved confounder(s); An association between the negative control exposure and the outcome persists with adjustment for possibly causal exposures if and only if the arrow U → ASD exists. Such an association is not causal. The path that creates it is (in DAGs terminology) a backdoor path (from the outcome through the possibly causal exposure and U to the negative control exposure) that serves to assess residual confounding in the primary analyses.
According to DAG theory, once we fit a regression model predicting ASD and adjust for measured confounders as well as all possibly causal exposures, an association between the negative control and the outcome is present if and only if the arrow U → ASD exists. This is because otherwise, these variables are statistically independent (“D-separated” in DAG terminology).25,26,38 Any association found between the negative control exposure and the outcome in this model, would imply residual confounding in the association between the possibly causal exposure and ASD.
Statistical analyses
We estimated associations between exposures and ASD with logistic regression models. First, we calculated odds ratios (OR) and 95% confidence intervals (CI) in separate single-pollutant, single-exposure period models, with and without adjustment for potential confounders. Next, we fitted models that include pre-pregnancy (9 months before pregnancy), entire pregnancy, postnatal (9 months after birth) and the negative control exposure period (9 months, at age 28-36 months). In sensitivity analyses, we subsequently restricted the sample to subjects with complete gestational age data, house-level geocoding, or localities larger than 10,000 inhabitants. An additional sensitivity analysis further adjusted for parental ages.
The DAG presented in eAppendix 3 demonstrates possible backdoor paths biasing the crude exposure–outcome association. This DAG represents possible confounding of the air pollution – ASD association, by several factors of concern for confounding. We built the DAG by adding common ancestors of the exposure and the outcome and then adding variables that partially or entirely mediate the causal relations we draw. We also used variables used or mentioned in previous studies of air pollution and ASD but did not automatically add them to the DAG. Accordingly, we adjusted our models for the following potential confounders (for which we have available data): year and calendar month of birth, population group, census-tract socioeconomic (SES) index, and household wage (as reported to the National Insurance from Israel tax authorities). The Population group variable included three categories: Israeli Arabs, ultra-orthodox Jews, and the general population. The census tract-standardized SES index is a composite measure calculated and published by the Israel Central Bureau of Statistics (as of the 2008 census)39 which we categorized into sextiles. Once we adjusted for these confounders, we blocked these confounding paths (eAppendix 4). We used R statistical software version 3.6.140 for all our analyses.
Results
The Table 1 presents the characteristics of the study population. As expected, the percentage of boys is much higher among the cases (83%) in comparison to the controls (51%). Cases and controls also differed by paternal age, population group, wage, and socioeconomic index. The average age at diagnosis of the cases in our study is 2.06 years and the interquartile range is 2 years (Quartile 1=1.0 year, Quartile 3=3.0 years).
Table 1.
Birth and sociodemographic characteristics of the study population
| Characteristic | Cases (N=3,843) | Controls (N=38,430) | |||
|---|---|---|---|---|---|
| Mean / n | SD / % | Mean / n | SD / % | ||
| Maternal age at birth, years (Mean, SD) | 31.3 | 5.4 | 30.3 | 5.4 | |
| Paternal age at birth, years (Mean, SD) | 34.4 | 6.2 | 33.1 | 6.1 | |
| Gestational age at birth, weeks (Mean, SD) | 38.5 | 2.3 | 39.0 | 1.9 | |
| Sex, n (%) | Girls | 658 | 17 | 18,942 | 49 |
| Boys | 3185 | 83 | 19,488 | 51 | |
| Population group, n (%) | General population | 3,443 | 90 | 30,279 | 79 |
| Ultra-orthodox | 194 | 5 | 5,306 | 14 | |
| Israeli Arabs | 206 | 5 | 2,845 | 7 | |
| Socioeconomic index (sextiles), n (%) | Sextile 1(lowest) | 176 | 5 | 3,809 | 10 |
| Sextile 2 | 450 | 12 | 5,473 | 14 | |
| Sextile 3 | 622 | 16 | 5,474 | 14 | |
| Sextile 4 | 667 | 17 | 6,202 | 16 | |
| Sextile 5 | 890 | 23 | 7,393 | 19 | |
| Sextile 6 (highest) | 976 | 25 | 9,306 | 24 | |
| Missing | 62 | 2 | 773 | 2 | |
| Wage (USD/month), n (%) | Not Working | 370 | 10 | 5,047 | 13 |
| 0-1,500 | 620 | 16 | 7,412 | 19 | |
| 1,500-3,000 | 957 | 25 | 8,531 | 22 | |
| 3,000-4,000 | 756 | 20 | 6,471 | 17 | |
| 4,000+ | 1,140 | 30 | 10,969 | 29 | |
| Year of birth, n (%) | 2007 | 589 | 15 | 5,890 | 15 |
| 2008 | 633 | 16 | 6,330 | 16 | |
| 2009 | 723 | 19 | 7,230 | 19 | |
| 2010 | 742 | 19 | 7,420 | 19 | |
| 2011 | 657 | 17 | 6,570 | 17 | |
| 2012 | 499 | 13 | 4,990 | 13 | |
| Geocoding level, n(%) | House-level | 3,328 | 87 | 31,912 | 83 |
| Locality-level | 515 | 13 | 6,518 | 17 | |
Controls matched to cases by year of birth (10 controls per case). Percentages may not add up to 100 because of rounding. ASD = Autism spectrum disorder; IA = Israeli Arabs; UOJ = Ultra-orthodox Jews; GP = General population; Wage = household wage. converted by rate of US dollars (USD) 1 = NIS 4; socioeconomic index was measured at the census small statistical area level. SD indicates standard deviation.
The median, mean and interquartile range width (IQRw) of PM2.5 exposures were 22.3, 22.6, and 2.6 μg/m3, respectively, and of PM2.5-10 exposures: 31.0, 30.8, and 6.8 μg/m3, respectively. NOx and NO2 mean exposures were 22.7 (median, 23.4; IQRw, 11.7) ppb, and 14.2 (median, 14.4; IQRw, 5.6) ppb, respectively (see eAppendix 5 for density plots). Single-pollutant correlations between exposures over different time periods were high only for NOx (r = 0.77-0.95) and NO2 (r = 0.82-0.95) (eAppendix 6). Multi-pollutant correlations were high for NOx and NO2 only (r = 0.96, eAppendix 7).
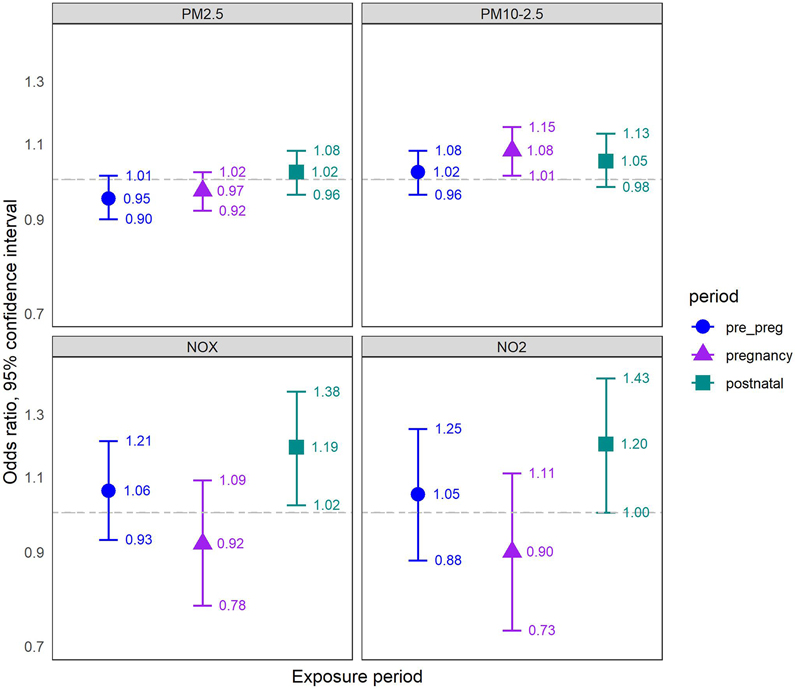
In unadjusted models with one exposure period only, we found positive associations for PM2.5-10, NOx and NO2 with ASD, and mostly null associations for PM2.5 (eAppendix 8). Upon adjustment for potential confounders we found similar associations, with somewhat stronger associations for PM2.5-10 (eAppendix 9). When we mutually adjusted for all pre- to postnatal exposure periods, we found positive associations with ASD only with postnatal exposures to either NOx (OR per IQRw = 1.19, 95% CI: 1.02-1.38), or NO2 (OR = 1.20, 95% CI: 1.00-1.43), and gestational exposure to PM2.5-10 (OR = 1.08, 95% CI: 1.01-1.15) (Figure 2).
Figure 2. Air pollution - ASD Associations: Results of mutually adjusted logistic regression models.
Associations are presented as ORs (95% CI) per IQRw increase in air-pollutant concentrations for ASD, referring to IQRw of each pollutant exposure during pregnancy (2.55 μg/m3 for PM2.5, 6.79 μg/m3 for PM2.5-10, 11.7 ppb for NOx, 5.56 ppb for NO2). All models refer to a matched sample comprised of 3,843 cases and 38,430 controls (10 controls matched per one case, by year of birth), and are adjusted for year and calendar month of birth, population group, census-tract socioeconomic index, and wage; and mutually adjusted for pre-pregnancy, pregnancy and postnatal exposures. Pre_preg = pre-pregnancy exposure, 9 months before pregnancy; pregnancy = entire pregnancy exposure; postnatal = postnatal exposure, 9 months after birth; ORs = odds ratios; CI = Confidence interval; IQRw = interquartile range width; ASD = autism spectrum disorder.
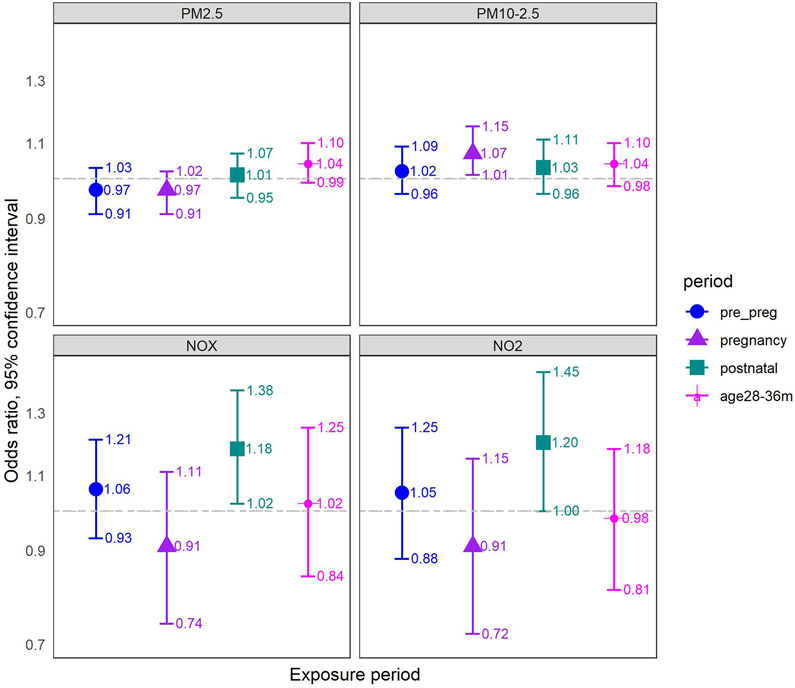
When we added the negative control exposure period to the model, the associations for gestational and postnatal exposures remained largely unaltered (Figure 3). For example, for gestational exposure to PM2.5-10 we found an OR of 1.07 (95% CI: 1.01-1.15), and for postnatal exposure to NOx we found an OR of 1.18 (95% CI: 1.02-1.38). When examining the negative control exposures in these models, we found the following associations (OR per IQRw) for PM2.5: 1.04, 0.99-1.10, and PM2.5-10: 1.04, 0.98-1.10, and null associations for NOx (1.02, 0.84-1.25) and NO2 (0.98, 0.81-1.18). In the secondary analysis of the post-outcome negative control exposure period (9-month post-diagnosis), we found fairly similar associations for the negative control exposures, except for weak, imprecise associations for NOx and NO2 rather than complete null as in the main negative control analyses (eAppendix 10).
Figure 3. Air pollution - ASD Associations: Results of negative control at age 28-36 months, mutually adjusted logistic regression models.
Associations are presented as ORs (95% CI) per IQR increase in air-pollutant concentrations for ASD, referring to IQR of each pollutant exposure during pregnancy (2.55 μg/m3 for PM2.5, 6.79 μg/m3 for PM2.5-10, 11.7 ppb for NOx, 5.56 ppb for NO2). All models refer to a matched sample comprised of 3,843 cases and 38,430 controls (10 controls matched per one case, by year of birth), and are adjusted for year and calendar month of birth, population group, census-tract socioeconomic index, and wage; and mutually adjusted for pre-pregnancy, pregnancy, postnatal exposures and a negative control exposure at age 28-36 months. Pre_preg = pre-pregnancy exposure, 9 months before pregnancy; pregnancy = entire pregnancy exposure; postnatal = postnatal exposure, 9 months after birth; age28-36m = exposure at age 28-36 months; ORs = odds ratios; CI = Confidence interval; IQRw = interquartile range width; ASD = autism spectrum disorder.
In sensitivity analyses we restricted the sample to subjects with complete gestational age data (eAppendix 11), house-level geocoding (eAppendix 12) or localities with >10,000 inhabitants (eAppendix 13), and found similar associations. An additional sensitivity analysis with further adjustment for parental ages did not alter the results (eAppendix 14).
Discussion
To our knowledge, this is the first study to examine associations between ASD and air pollution with a negative control approach. We have shown that in models with one exposure period only, all exposures (except for PM2.5) over all periods are positively associated with ASD in Israel. Our mutually adjusted models, co-adjusting for all three exposure periods, emphasize the need for this adjustment to limit confounding by an effect of the exposure in another developmental period.
While we mutually adjust for various highly correlated exposures, such co-adjustment does not, in and of itself, bias effect estimates and is necessary if leaving them out would cause a bias.41 A negative control exposure is almost by definition correlated with other exposures because it is the shared drivers of the exposures and the negative control that makes the negative control useful. Thus, the highly correlated exposures must be included in the model together because if one of them has a causal effect on the outcome, the negative control exposure will also look associated with the outcome without it in the model. This is despite not having a causal effect, because it correlates with an exposure that does have a causal effect. Previous studies from Denmark21 and Israel22 also implemented such mutual adjustment for all three exposure periods (some with high correlations among themselves). In both studies, entire pregnancy exposure to NO2 was not associated with ASD, whereas the pre-pregnancy exposure in the Israeli study was slightly associated with ASD (results for pre-pregnancy were not presented in the Danish study).
Our mutually adjusted models show no associations of PM2.5 with ASD in either the gestational or postnatal exposure period, whereas the Danish paper suggested positive associations in the postnatal period, but slightly negative in the gestational period.21 Ritz et al. obtained similar results in that study for PM10, whereas we found positive associations of PM2.5-10 in both pregnancy and postnatal periods. The slightly negative associations with ASD for gestational exposures to PM2.5 and PM10 in the Danish study may be explained by a live-birth bias,42 for which we see no indication in relation to PM gestational exposures in Israel.
In Israel, transportation has a limited contribution to ambient PM2.5 concentrations. Long-range transport of particles, including dust storms - which are common in Israel due to its location at the margins of the global dust belt43 - affects PM in Israel more than local sources.44 The relatively low correlations between the NOx/NO2 and PM2.5/PM2.5-10 exposures are also evidence for these conditions (eAppendix 6). In addition, traffic emissions contribute to the ultrafine fraction - for which NOx is a good proxy45 - but monitoring particle mass rather than number concentrations obscures this contribution. The lack of association of postnatal exposure to PM2.5 with ASD in our study, as opposed to the positive association found in the Danish study in the postnatal exposure, may be explained by the different composition and sources of PM2.5 in Israel and Denmark.46
Our negative control analyses suggest that residual confounding is present in all exposures to PM2.5 and PM2.5-10, except for gestational exposure to PM2.5-10. In contrast, we argue that the NOx and NO2 negative control analyses do not indicate residual confounding with these exposures, increasing the plausibility of a causal link between postnatal exposure to traffic-related air pollution and ASD.
Most studies so far have focused mainly on the gestational exposure period,17,18,47-49 whereas little has been studied about neuro-developmental mechanisms of early postnatal exposure to air pollution that may lead to ASD. This is true for animal studies as well, since most of them were focused on exposure in developmental periods which are equivalent to the third trimester in humans.50 During the early postnatal period, infants directly inhale polluted air, while the natural barriers - blood-brain, nasal, and lung, as well as the immune system - are still developing.19,50,51 Penetration of air pollutants into the brain induces an innate immune response, as evidenced by blood and cerebrospinal fluid inflammatory cytokines levels, which may lead to neuro-inflammation and neural tissue damage. Ultrafine particles can penetrate more easily to the brain either through the bloodstream or through the olfactory route.19
Our findings should be considered in light of several limitations. Our exposure estimates were based on maternal address at birth (with no available data about other addresses). These addresses may not necessarily represent the actual residence during pre-pregnancy, pregnancy, and the early postnatal period. In this context, we expect birth address errors to be non-differential with respect to ASD, and thus more likely to bias the associations to the null. Birth addresses are even less likely to represent real exposures during the negative control period, since it is further away from birth. However, actual residence in the birth address should not be a concern for a negative control exposure, since this exposure is only used to test for the persistence of unobserved confounding. Moreover, had we used the real addresses during the negative control period, we might have biased the associations with this exposure period, since the diagnosis of ASD may encourage migration to areas with better services.
Exposure measurement error might also occur since we based our exposure assessment on residential addresses, whereas the actual exposure of the study population was also away from the home address. This source of error is assumed to be non-differential with respect to ASD. Therefore, we expect it to bias the estimates towards the null. On the other hand, individual behavioral factors (related to the time one spends at home or away from home) and indoor pollution do not affect the exposure models. Thus, such exposure model estimates are likely to be protected from residual confounding by behavioral factors.52 On top of that, any measurement error should be the same in all exposure periods since the exposure models assess exposures by environmental and spatial data only. While any measurement error could influence results, it would do it equally in the different exposure periods (as long as those periods have the same length), thus not interfering with detecting period-specific effects.
Another limitation of our study is that our follow-up ended in 2016, possibly increasing outcome misclassification among our most recent birth cohorts. However, even the youngest birth cohort of 2012 were followed up 4.5 years on average, whereas the average age at diagnosis in our study population is ~ 2 years. In addition, our secondary negative control – exposure during the 9-months after ASD diagnosis – relied heavily on the validity of the diagnosis dates reported to the National Insurance. However, unlike the diagnosis itself, we were unable to validate diagnosis dates, and lack of validity of these dates may have influenced the results of this secondary analysis. This was of concern especially because, for some of the cases, the documented diagnosis age was just a few months, overlapping with the possibly causal exposure at 9 months after birth.
A possible choice to combine the advantages of our two negative control choices is to use the 9 months after the diagnosis of the last children in the cohort to ensure the negative control exposure falls into a period after diagnosis for all children. However, this approach is not a feasible option in our data since the negative control periods extend beyond the period covered by the air pollution models for those diagnosed in recent years, which would have caused us to lose many cases. In addition, setting the negative control exposure in a much later period than the possibly causal exposure periods may impair the validity of that negative control assessment. This may happen since the association of such late negative control exposure with ASD may not be “U-comparable” with the association in question due to the time lapse between the periods. In this sense, a negative control period closer to the possibly causal periods is superior to a negative control in a more distant period, as the former better reflects possible confounding structures during those periods.
This study has several strengths. First, we have used an innovative methodologic approach to study air pollution and ASD, using an ideal negative control exposure period. Second, we used a national, comprehensive dataset of ASD cases and a population-based random sample of matched controls. Third, we used high-resolution, validated spatiotemporal air pollution models to examine pre-pregnancy, pregnancy, and early postnatal exposures.
Our study has important public health implications, as it decreases the concern of residual confounding in the relationship between traffic-related pollution and ASD. These findings call for special attention to protect infants from exposure to traffic-related pollution. This is possible, for example, by increasing awareness of caregivers and through better urban planning and control of emission at the source level. We encourage future research aiming at the mechanism underlying the effect of postnatal exposure to air pollution on the risk of ASD.
Supplementary Material
Source of Funding:
This study was funded by the US National Institutes of Health grants R21 ES026900 and R01 AG065276.
Footnotes
Conflicts of interests:
None declared.
References
- 1.American Psychological Association (APA). Diagnostic and Statistical Manual of Mental Disorders: Neurodevelopmental Disorders. Fifth Edit. American Psychiatric Publishing; 2013. doi: 10.1176/appi.books.9780890425596.dsm01 [DOI] [Google Scholar]
- 2.Lyall K, Schmidt RJ, Hertz-Picciotto I. Environmental Factors in the Preconception and Prenatal Periods in Relation to Risk for ASD. Handb Autism Pervasive Dev Disord Fourth Ed Assessment, Interv Policy, Futur. 2014:424–456. [Google Scholar]
- 3.Kim YS, Leventhal BL. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry. 2015;77(1):66–74. doi: 10.1016/j.biopsych.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balestrieri E, Arpino C, Matteucci C, et al. HERVs Expression in Autism Spectrum Disorders. PLoS One. 2012;7(11):e48831. doi: 10.1371/journal.pone.0048831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sealey LA, Hughes BW, Sriskanda AN, et al. Environmental factors in the development of autism spectrum disorders. Environ Int. 2016;88:288–298. doi: 10.1016/j.envint.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 6.Narayan KMV, Ali MK, Koplan JP. Global Noncommunicable Diseases — Where Worlds Meet. N Engl J Med. 2010;363(13):1196–1198. doi: 10.1056/NEJMp1002024 [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Goldberg MS, Viileneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23(4):243–297. doi: 10.1515/reveh.2008.23.4.243 [DOI] [PubMed] [Google Scholar]
- 8.Chun H, Leung C, Wen SW, McDonald J, Shin HH. Maternal exposure to air pollution and risk of autism in children: A systematic review and meta-analysis. Environ Pollut. 2020;256:113307. doi: 10.1016/J.ENVPOL.2019.113307 [DOI] [PubMed] [Google Scholar]
- 9.Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ Res. 2016;151:763–776. doi: 10.1016/j.envres.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 10.Lam J, Sutton P, Kalkbrenner A, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):1–27. doi: 10.1371/journal.pone.0161851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiwara T, Morisaki N, Honda Y, Sampei M, Tani Y. Chemicals , Nutrition , and Autism Spectrum Disorder : A Mini-Review. 2016;10(April):1–7. doi: 10.3389/fnins.2016.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C, Zhao W, Deng K, Zhou V, Zhou X, Hou Y. The association between air pollutants and autism spectrum disorders. Environ Sci Pollut Res. 2017;24(19):15949–15958. doi: 10.1007/s11356-017-8928-2 [DOI] [PubMed] [Google Scholar]
- 13.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43(2):443–464. doi: 10.1093/ije/dyt282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suades-González E, Gascon M, Guxens M, Sunyer J. Air pollution and neuropsychological development: A review of the latest evidence. Endocrinology. 2015;156(10):3473–3482. doi: 10.1210/en.2015-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275–1297. doi: 10.1007/s00018-018-2988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso-Gonzalez A, Rodriguez-Fontenla C, Carracedo A. De novo mutations (DNMs) in autism spectrum disorder (ASD): Pathway and network analysis. Front Genet. 2018;9(September). doi: 10.3389/fgene.2018.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulanger-Bertolus J, Pancaro C, Mashour GA. Increasing role of maternal immune activation in neurodevelopmental disorders. Front Behav Neurosci. 2018;12(October):1–6. doi: 10.3389/fnbeh.2018.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa LG, Cole TB, Dao K, Chang Y-C, Coburn J, Garrick J. Neurotoxicity of air pollution: Role of neuroinflammation. In: Advances in Neurotoxicology Volume 3, 2019. Vol 3. Academic Press; 2019:195–221. doi: 10.1016/bs.ant.2018.10.007 [DOI] [Google Scholar]
- 19.Brockmeyer S, D’Angiulli A. How air pollution alters brain development: The role of neuroinflammation. Transl Neurosci. 2016;7(1):24–30. doi: 10.1515/tnsci-2016-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Curr Environ Heal Reports. 2015;2(4):430–439. doi: 10.1007/s40572-015-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritz B, Liew Z, Yan Q, et al. Air pollution and autism in Denmark. Environ Epidemiol. 2018;2(4):e028. doi: 10.1097/EE9.0000000000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz R, Levine H, Pinto O, Broday DM, Yuval, Weisskopf MG. Traffic-Related Air Pollution and Autism Spectrum Disorder: A Population-Based Nested Case-Control Study in Israel. Am J Epidemiol. 2018;187(4):717–725. doi: 10.1093/aje/kwx294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nørgaard M, Ehrenstein V, Vandenbroucke JP. Confounding in observational studies based on large health care databases: Problems and potential solutions – a primer for the clinician. Clin Epidemiol. 2017;9:185–193. doi: 10.2147/CLEP.S129879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.USEPA USEPA. Integrated Science Assessment for Particulate Matter.; 2019. [PubMed] [Google Scholar]
- 25.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383–388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisskopf MG, Tchetgen EJT, Raz R. On the use of imperfect negative control exposures in epidemiologic studies. Epidemiology. 2016;27(3):365–367. doi: 10.1097/EDE.0000000000000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flanders WD, Klein M, Darrow LA, et al. A method for detection of residual confounding in time-series and other observational studies. Epidemiology. 2011;22(1):59–67. doi: 10.1097/EDE.0b013e3181fdcabe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flanders WD, Klein M, Darrow LA, et al. A method to detect residual confounding in spatial and other observational studies. Epidemiology. 2011;22(6):823–826. doi: 10.1097/EDE.0b013e3182305dac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raz R, Weisskopf MG, Davidovitch M, Pinto O, Levine H. Differences in Autism Spectrum Disorders Incidence by Sub-Populations in Israel 1992–2009: A Total Population Study. J Autism Dev Disord. 2015;45(4):1062–1069. doi: 10.1007/s10803-014-2262-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloog I, Sorek-Hamer M, Lyapustin A, et al. Estimating daily PM2.5 and PM10 across the complex geo-climate region of Israel using MAIAC satellite-based AOD data. Atmos Environ (1994). 2015;122:409–416. doi: 10.1016/j.atmosenv.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuval, Bekhor S, Broday DM. Data-driven nonlinear optimisation of a simple air pollution dispersion model generating high resolution spatiotemporal exposure. Atmos Environ. 2013;79:261–270. doi: 10.1016/J.ATMOSENV.2013.06.005 [DOI] [Google Scholar]
- 32.Yuval, Levy I, Broday DM. Improving modeled air pollution concentration maps by residual interpolation. Sci Total Environ. 2017;598:780–788. doi: 10.1016/J.SCITOTENV.2017.04.117 [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Yuval, Broday DM. A new modeling approach for assessing the contribution of industrial and traffic emissions to ambient NOx concentrations. Atmos Environ. 2018;173:173–184. doi: 10.1016/J.ATMOSENV.2017.11.006 [DOI] [Google Scholar]
- 34.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247–261. doi: [DOI] [PubMed] [Google Scholar]
- 35.Rice D, Barone S Jr. Critical Periods of Vulnerability for the Developing Nervous System : Evidence from Humans and Animal Models Critical Periods of Vulnerability for the Developing Nervous System : Evidence from Humans and Animal Models Development of the Brain in Utero. Environ Health Perspect. 2000;108(January):511–533. doi: 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti E, Calderoni S, Marchi V, Muratori F, Cioni G, Guzzetta A. The first 1000 days of the autistic brain: A systematic review of diffusion imaging studies. Front Hum Neurosci. 2015;9(March). doi: 10.3389/fnhum.2015.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. doi: 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 38.Pearl J Causality: Models, Reasoning, and Inference. Cambridge University Press; 2011. doi: 10.1017/CBO9780511803161 [DOI] [Google Scholar]
- 39.Israel Central Bureau of Statistics. CBS Socio-Economic Index.; 2013. http://www.cbs.gov.il/publications17/socio_eco13_1694/pdf/intro_e.pdf.
- 40.R Foundation. R: A Language and Environment for Statistical Computing. 2018. https://www.r-project.org/.
- 41.Schisterman EF, Perkins NJ, Mumford SL, Ahrens KA, Mitchell EM. Collinearity and Causal Diagrams: A Lesson on the Importance of Model Specification Enrique. Epidemiology. 2017;28(1):47–53. doi: 10.1097/EDE.0000000000000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raz R, Kioumourtzoglou M-A, Weisskopf MG. Live Birth Bias and Observed Associations between Air Pollution and Autism. Am J Epidemiol. August2018. doi: 10.1093/aje/kwy172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krasnov H, Katra I, Friger M. Increase in dust storm related PM 10 concentrations : A time series analysis of 2001 e 2015 *. Environ Pollut. 2016;213:36–42. doi: 10.1016/j.envpol.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 44.Abdeen Z, Qasrawi R, Heo J, et al. Spatial and temporal variation in fine particulate matter mass and chemical composition: The middle east consortium for aerosol research study. Sci World J. 2014;2014. doi: 10.1155/2014/878704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuval, Tritscher T, Raz R, Levi Y, Levy I, Broday DM. Emissions vs. turbulence and atmospheric stability: A study of their relative importance in determining air pollutant concentrations. Sci Total Environ. 2020;733:139300. doi: 10.1016/j.scitotenv.2020.139300 [DOI] [PubMed] [Google Scholar]
- 46.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371 [DOI] [PubMed] [Google Scholar]
- 47.Minakova E, Warner BB. Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res. 2018;110(20):1539–1550. doi: 10.1002/bdr2.1416 [DOI] [PubMed] [Google Scholar]
- 48.Woodward N, E. Finch C, E. Morgan T. Traffic-related air pollution and brain development. AIMS Environ Sci. 2015;2(2):353–373. doi: 10.3934/environsci.2015.2.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–516. doi: 10.1016/j.tins.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: Convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014. doi: 10.1155/2014/736385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunyer J The neurological effects of air pollution in children. doi: 10.1183/09031936.00073708 [DOI] [PubMed] [Google Scholar]
- 52.Weisskopf MG, Webster TF. Trade-offs of Personal Versus More Proxy Exposure Measures in Environmental Epidemiology. Epidemiology. 2017;28(5):635–643. doi: 10.1097/EDE.0000000000000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.