Abstract
Ionotropic glutamate receptors of the NMDA and AMPA subtypes transduce excitatory signaling on neurons in the prefrontal cortex (PFC) in support of cognitive flexibility. Cognitive flexibility is reliably observed to decline at advanced ages, coinciding with changes in PFC glutamate receptor expression and neuronal physiology. However, the relationship between age-related impairment of cognitive flexibility and changes to excitatory signaling on distinct classes of PFC neurons is not known. In this study, one cohort of young adult (4 months) and aged (20 months) male F344 rats were characterized for cognitive flexibility on an operant set-shifting task. Expression of the essential NMDAR subunit, NR1, was correlated with individual differences in set-shifting abilities such that lower NR1 in the aged PFC was associated with worse set-shifting. In contrast, lower expression of two AMPAR subunits, GluR1 and GluR2, was not associated with set-shift abilities in aging. As NMDARs are expressed by both pyramidal cells and fast-spiking interneurons (FSI) in PFC, whole-cell patch clamp recordings were performed in a second cohort of age-matched rats to compare age-associated changes on these neuronal subtypes. Evoked excitatory postsynaptic currents were generated using a bipolar stimulator while AMPAR vs. NMDAR-mediated components were isolated using pharmacological tools. The results revealed a clear increase in AMPA/NMDA ratio in FSIs that was not present in pyramidal neurons. Together, these data indicate that loss of NMDARs on interneurons in PFC contributes to age-related impairment of cognitive flexibility.
1. Introduction
Executive functions encompass higher-order cognitive processes that guide goal-directed behavior. Cognitive flexibility, the ability to modify behavioral strategies in accord with shifting contingencies, is an integral aspect of executive function that normally attains its maximal capacity in early adulthood, contemporaneous with the maturation of the prefrontal cortex (PFC; reviewed in (Diamond, 2013)). Advanced aging is characterized by a decline in cognitive flexibility, which can be assessed across species via “set-shifting” tasks (Barense et al., 2002; Beas et al., 2013; Boone et al., 1993; Floresco et al., 2008; Hernandez et al., 2017; Lacreuse et al., 2018; Moore et al., 2003; Nieves-Martinez et al., 2012; Rhodes, 2004; Ridderinkhof et al., 2002; Tomm et al., 2018) and reviewed in (Bizon et al., 2012; McQuail et al., 2018). Attenuated N-methyl-D-aspartate receptor (NMDAR) signaling in aging may be one contributor to age-related cognitive deficits as acutely blocking NMDARs with MK-801, phencyclidine or ketamine reliably impairs set-shifting in young adult rats (Blot et al., 2015; Darrah et al., 2008; Egerton et al., 2005; Jett et al., 2017; Nikiforuk et al., 2010; Stefani et al., 2003; Stefani and Moghaddam, 2010, 2005). Consistent with this view, NMDARs are known to decline in PFC aging across humans and rats (Dickstein et al., 2013; Dyall et al., 2007; Hellström-Lindahl and Court, 2000; Magnusson, 1998; Magnusson et al., 2007, 2005; Magnusson and Cotman, 1993; McQuail et al., 2016; Migani et al., 2000; Mitchell and Anderson, 1998; Piggott et al., 1992; Wenk et al., 1991). Critically, activation of PFC NMDARs during cognitively demanding tasks depends on permissive contributions from α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), which may also diminish with age and are implicated in cognitive flexibility (Jett et al., 2017; Magnusson and Cotman, 1993; McQuail et al., 2016; Stefani et al., 2003; Wang et al., 2013). While these data make a compelling case that age-related changes in glutamatergic NMDARs and AMPARs contribute to cognitive dysfunction, relatively little is known about whether such changes are localized to specific types of neurons.
Non-competitive NMDAR antagonists that produce set-shifting deficits in rodent models are known to suppress activity of GABAergic interneurons in medial PFC (mPFC), resulting in increased, but desynchronized, activity of pyramidal neurons (Homayoun and Moghaddam, 2007; Kargieman et al., 2007; Hervig et al., 2016). In particular, fast-spiking interneurons (FSIs) target and potently inhibit somatic compartments of pyramidal neurons, contributing to feedforward inhibition, gain control, and the generation of gamma oscillations (Bartos et al., 2007; Cardin et al., 2009; Cruikshank et al., 2012; Delevich et al., 2015; Doischer et al., 2008; Ferguson and Gao, 2018; Isaacson and Scanziani, 2011; Mann and Paulsen, 2007; Sohal et al., 2009). Consistent with a vital role for inhibitory regulation of PFC-dependent cognitive flexibility, blocking GABAARs with their selective antagonist bicuculline impairs set-shifting whereas the GABABR agonist baclofen improves set-shifting in young adult rats (Enomoto et al., 2011; Beas et al., 2016). This relationship is conserved in the aging brain as intra-mPFC infusion of baclofen effectively reverses impaired set-shifting in aged rats (Beas et al., 2017). These cellular and behavioral pharmacology data are suggestive that diminished NMDAR signaling on aging FSIs may be particularly consequential to impaired cognitive flexibility. The goals of the present study were two-fold. Our first goal was to determine the relationship between individual differences in cognitive flexibility of aging rats and PFC protein levels of ionotropic glutamate receptor (iGluR) subunits. The second goal was to determine whether the relevant age-associated changes were specific to either PFC pyramidal neurons or FSIs.
2. Materials and Methods
2.1. Subjects
Young adult (4 months, n=23) and aged (22 months, n=27) male Fischer 344 (F344) rats were obtained from the National Institute on Aging’s Aging Rodent Colony maintained by Charles River Laboratories. All animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited vivarium facility in the McKnight Brain Institute at the University of Florida. The facility was maintained at a consistent temperature of 25°C with a 12-hour light/dark cycle (lights on at 0700 h) with free access to food and water except as otherwise noted. All animal procedures were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines. Rats used for set-shifting and western blot experiments comprised young adult (n=8) and aged (n=15) rats that were drawn from a larger cohort tested for set-shift abilities and used in previous studies (Beas et al., 2017; McQuail et al., 2016). Rats used for in vitro electrophysiological experiments were from a separate cohort of behaviorally naïve young adult (n=15) and aged (n=12) rats.
2.2. Relationship Between Set-Shifting in Aged Rats and Expression of NMDARs in PFC.
2.2.1. Operant Testing Apparatus:
All behavioral testing was performed using 8 identical operant test chambers (30.5 × 25.4 × 30.5 cm, Coulbourn Instruments, Whitehall, PA) constructed with metal front and back walls, transparent Plexiglas side walls, and a floor made of steel rods (0.4 cm diameter) spaced 1.1 cm apart. The front wall contained a central, recessed trough connected to a food pellet dispenser. The trough was outfitted with a 1.12 W lamp and a photobeam sensor. Retractable levers were mounted on either side of the food-delivery trough and a 1.12 W cue light was located 3.8 cm above each lever. Each operant chamber was enclosed within a sound-attenuating cubicle that contained a 1.12 W house light mounted on the rear wall. All chambers were connected to a PC running Graphic State 3.01 software (Coulbourn Instruments) to automate behavioral testing and data collection.
2.2.2. Shaping of Operant Procedures:
The design of the set-shifting task was modified from (Floresco et al., 2008) and has been adapted by our lab to characterize age-related impairment of set-shifting in F344 rats (Beas et al., 2013, 2017). Rats were food-restricted to 85% of free-feeding body weight over a 5-day period. On the final day before the start of shaping, each rat was given five 45-mg food pellets (PJAI, Test Diet, Richmond, IN) in the home cage to reduce neophobia to this food reward. Shaping procedures comprised four stages. In Stage 1, rats received a 64-minute session of magazine training, involving 38 deliveries of a single food pellet with an inter-trial interval (ITI) of 100 ± 40 s. In Stage 2, one lever (left or right, counterbalanced across groups) was inserted into the test chamber and lever presses were reinforced on a fixed-ratio 1 (FR1) schedule. After pressing the lever 50 times in 30 min, rats were reinforced to press the opposite lever on an FR1 schedule. In Stage 3, rats received 90 trials designed to train them to press the levers immediately after their insertion into the test chamber. Each 20 s trial began with the house light being illuminated and insertion of a single lever (either left or right, randomly selected within each pair of trials) into the test chamber where it remained for a maximum of 10 s. A lever press in this time window caused the lever to retract, a single food pellet to be delivered, and the house light to remain on for an additional 4 s. If a rat failed to press the lever within 10 s, the lever was retracted and the house light turned off, and the trial was scored as an omission. Rats received at least 4 daily sessions in this stage, and training proceeded until rats reached a criterion of fewer than 10 omissions out of the 90 trials in a single session. In Stage 4 each rat’s side bias (i.e., preference for one lever over the other) was determined. Each trial consisted of two phases. In phase one, the house light was illuminated and both levers were inserted into the test chamber. A press on either lever caused both levers to retract and a single food pellet to be delivered. In phase two, both levers were again inserted, but only a press on the lever that was not pressed in phase one resulted in food delivery. A press on the same lever chosen in the first phase caused the levers to be retracted and the house light to be extinguished. After a “correct” response in this second phase of a trial, a new trial was initiated, whereas after an “incorrect” response, the second phase was repeated until the rat made a “correct” response. The session ended after a total of 45 completed trials. The side associated with the greatest number of total responses across this phase of testing was considered a rat’s biased side.
2.2.3. Initial Discrimination (Illuminated Cue Light):
On the day after the side bias determination session, rats were trained to discriminate the location of the illuminated cue light (Fig. 1A). Each 20 s trial began with illumination of either the left or right cue light (randomized within each pair of trials). After 3 s, the house light was illuminated while both levers were inserted into the chamber. A press on the lever below the illuminated cue light (a correct response) resulted in delivery of a single food pellet while the cue light was extinguished, the levers retracted and the house light remained illuminated for 4 s. Pressing the lever beneath the non-illuminated cue light (an incorrect response) or failure to respond within 10 s (omission) led to retraction of both levers and all lighting was extinguished. The criterion for acquisition for the cue light discrimination was 8 consecutive correct trials after completing a minimum of 30 total trials (excluding omissions). Rats performed a maximum of 120 trials per session and rats not reaching criterion within a session received additional days of testing as needed.
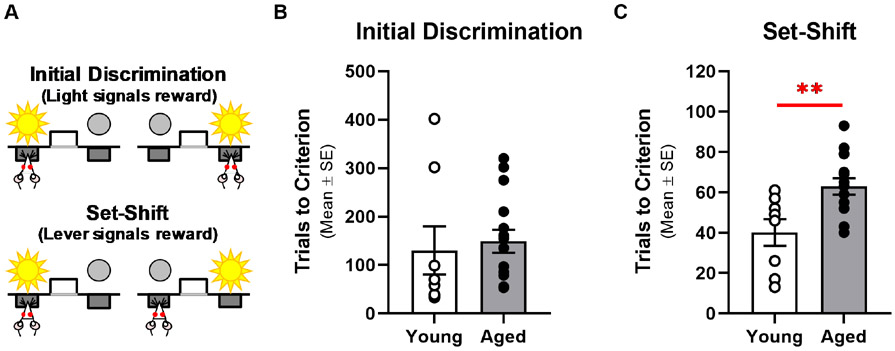
Fig. 1. Aging Impairs Cognitive Flexibility.
A: The automated set-shifting task used in this study involves learning an initial discrimination followed by a set-shift rule. In the initial discrimination (top panel), the rule is to press the lever beneath the illuminated cue light regardless of the left versus right location. In the set-shift phase (bottom panel), the rule is to press only the left or right lever (counterbalanced for each rat’s side-bias) regardless of the position of the illuminated cue light. B: Young adult (n=8; open circles) and aged (n=15: filled circles; x-axis) rats require a similar number of trials (y-axis) to acquire the initial rule. C: Aged rats take more trials to learn the new rule imposed in the set-shift phase of the task. **p<0.01.
2.2.4. Set-Shift Discrimination (Left or Right Lever):
The next day after achieving criterion performance on the cue light discrimination, the task contingencies were shifted to reinforce lever presses only on the left or right lever (each rat was reinforced to press its own non-preferred lever as determined in Stage 4 of shaping procedures; Fig. 1A). This rule shift challenged each rat to disregard the location of the illuminated cue light and attend only to the lever to consistently obtain food rewards. As in the initial discrimination, criterion performance was defined as 8 consecutive correct trials (excluding omissions). If rats did not attain criterion within a single session (120 trials/session), they received additional sessions as needed on subsequent days.
2.2.5. Analysis of Behavioral Data:
Data collected using Graphic State were exported and converted into Excel file format. The chief index of performance was the total number of trials to reach criterion on the initial and set-shift discrimination phases of testing. Performance was compared between young and aged rats by means of independent samples t-tests using IBM SPSS Statistics, version 28. For these and all subsequent statistical comparisons, p < 0.05 was considered significant.
2.2.6. Preparation of Tissue Homogenates:
Rats were restored to ad libitum feeding for at least one week before being sacrificed by decapitation. The brain was removed from the skull and the mPFC was dissected on an ice-cold plate and frozen on dry ice. Tissues were stored at −80° C until used for the preparation of membrane fractions as in (McQuail et al., 2012). Frozen mPFC tissue was weighed, thawed, and homogenized in ice-cold hypotonic buffer (50 mM HEPES, pH 7.4, 1 mM EDTA and 1 mM EGTA) with protease inhibitors (Thermo Fisher Scientific, Waltham, Massachusetts, USA) using a glass-Teflon Dounce homogenizer. The membrane fraction was collected by centrifuging homogenates at 14,000 rpm for 20 minutes at 4° C. The membrane fraction was washed by resuspending the pellet in 20 mL of the same buffer without protease inhibitors and incubated on ice for 30 minutes followed by centrifugation at 16,500 rpm for 15 minutes at 4° C. The final pellet was resuspended in 50 mM HEPES, pH 7.4, and total protein concentration was determined using the Pierce BCA Kit (Rockford, IL, USA). Aliquots were stored at −80° C until used for Western blotting.
2.2.7. SDS-PAGE and Immunoblotting:
Membrane proteins were solubilized, denatured and reduced in Laemmli sample buffer with 5% (vol/vol) β-mercaptoethanol (BioWorld, Dublin, OH, USA) with heating at 95° C for 5 min. A total of 5 μg of protein per lane was electrophoretically separated on a 4%–15% Tris-HCl gel at 200 V for 35 min then transferred to nitrocellulose membranes using a wet transfer apparatus for 40 min at 200 V (Bio-Rad, Hercules, CA, USA). Blots were washed 3 times with Tris-buffered saline (TBS; pH 7.4) then blocked for 1 h in blocking buffer (Rockland, Gilbertsville, PA, USA). Blots were then incubated overnight at 4° with primary antibodies raised against NR1 (1:2000; AB9864 from Millipore), GluR1 (1:1000; ABN241 from Millipore) and GluR2 (1:5000; MABN71 from Millipore) diluted in blocking buffer supplemented with 0.1% Tween-20. The dilution used for each antibody was previously optimized to produce a linear range of detection for 1.25-10 μg of total mPFC protein and non-specific staining was not evident in control experiments where the primary antibodies were omitted (McQuail et al., 2016; and see Supplemental Methods for details). Blots were then washed 3 times with TBS, incubated with either donkey-anti-rabbit IgG or donkey-anti-mouse IgG conjugated to IRDye 680RD or IRDye 800CW, (LI-COR Biosciences, Lincoln, NE, USA) and diluted 1:15,000 or 1:20,000. Following 3 additional TBS washes, blots were scanned on an Odyssey imaging system (LI-COR Biosciences). Samples were assayed in triplicate; the loading position of the sample was varied between gels/experiments in a pseudorandom fashion to control for technical variation in the electroblotting procedure. Level of β-tubulin (1:1000; MCA-1B12 from EnCor Biotechnology, Gainesville, FL, USA) was probed in every experiment to verify equality of loading across conditions.
2.2.8. Analysis of Protein Expression Data:
Integrated intensity was measured for each immuno-reactive band using ImageStudio Software (LI-COR) and normalized for level of β-tubulin, which was not different between age groups (ts(21)<0.7, ps>0.5; data not shown). Protein level is presented as the percentage of average expression in young rats (i.e. mean of young group is defined as 100%) and compared between age groups by means of independent-samples t-tests performed in IBM SPSS Statistics, version 28; p<0.05 was considered significant for these and all other analyses. Relationships between protein expression and behavioral performance were assessed using bivariate correlations between protein level in young or aged rats and the number of trials to achieve criterion on the initial discrimination and set-shift phases of testing.
2.3. Effect of Age on Glutamatergic Inputs to Pyramidal Neurons and Interneurons in PFC
2.3.1. Whole Cell Recording and Analysis:
Coronal brain slices (300 μm) containing the mPFC were prepared from young and aged rats according to our previously published methods (Carpenter et al., 2016). Slices were transferred to a recording chamber where they were perfused at 2 ml/min with Mg2+-free artificial cerebrospinal fluid (ACSF; 129 mM NaCl, 3 mM KCl, 25 mM NaHCO3, 1.2 mM NaH2PO4, 2.4 mM CaCl2, and 11 mM D-glucose) that was saturated with 95% O2/5% CO2 and maintained at 30 ± 2 °C. The patch electrode was filled with an internal solution (130 mM K-Gluconate, 10 mM KCl, 5 mM NaCl, 2 mM MgCl2, 2 mM Na2-ATP, 0.3 mM Na3-GTP, 0.1 mM EGTA, 10 mM HEPES, and 10 mM phosphocreatine) that was supplemented with 1 mM 4,4'-dinitrostilbene-2,2'-disulfonic acid, a chloride channel blocker to eliminate GABAA receptor-mediated currents in patched cells. Internal solutions were pH adjusted to 7.3 with CsOH or KOH, and were volume adjusted to 285-300 mOsm. All recordings were made using a MultiClamp 700B amplifier (Molecular Devices), a Digidata 1440A digitizer (Molecular Devices), and Clampex 10.2 software (Molecular Devices). All data was sampled at 20 kHz. Voltage-clamp recordings were lowpass filtered at 2 kHz and current-clamp recordings were lowpass filtered at 10 kHz. Offline analysis of all electrophysiological data was performed using custom software written in OriginC (Originlab, Northampton, MA) by CJF.
2.3.2. Identification and characterization of mPFC neurons:
Pyramidal neurons or interneurons were initially selected for whole-cell recording based on morphological characteristics apparent when observed using infrared differential interference contrast (IR-DIC) imaging under 40× magnification with an Olympus BW51WI microscope. Pyramidal neurons had a large pyramidal soma, with a clear primary apical dendrite projecting toward the pial surface. Interneurons had clearly rounder somata and lacked a primary apical dendrite. Further identification and classification of neuron type was corroborated by analysis of both passive and active electrophysiological properties.
Passive electrophysiological properties examined were whole-cell capacitance and input resistance. Both properties were calculated from data obtained by delivering a series of 50 msec steps to −80 mV in cells voltage clamped at −70 mV. Specifically, input resistance was calculated as Rm = (dV-(Ra*Iss))/Iss, where dV is the change in voltage delivered during the step, Iss is the steady state current observed at the end of the step, and Ra is the access resistance, calculated by dividing dV by the instantaneous current observed immediately after initiation of the voltage step. Whole-cell capacitance (Cm) was calculated as τ (1/Ra+1/Rm), where τ is the time constant obtained by fitting the whole-cell capacitive transients produced by the voltage step, using data observed between 10% and 90% of their peak amplitude, to a monoexponential function.
To examine active electrophysiological properties, cells were delivered a series of 0.5 sec long current pulses while current clamped at I=0. Amplitude of these pulses ranged from −100 pA to > 500 pA. The maximum firing rate was defined as the maximum frequency of action potentials observed during any current step. Fast-spiking interneurons (FSIs) were separated from other interneurons observed based on their ability to fire at frequencies in excess of 40 Hz. Other active properties reported were obtained from an analysis of the first derivative of the first action potential observed during the first current step that was suprathreshold. These include the maximum depolarization velocity (dVmax), the maximum repolarization velocity (dVmin), and the time between these points (dVdVdeltaT).
2.3.3. Measurement of AMPA/NMDA ratio from evoked responses:
To generate evoked postsynaptic currents, a theta glass electrode was filled with magnesium free ACSF, connected to a TTL controlled constant current stimulus isolator (World Precision Instruments, Sarasota, FL, USA), and placed in the mPFC. Stimuli lasting 0.1 msec were delivered at a frequency of 0.1 Hz. Stimulus intensity was adjusted separately in each cell to elicit an evoked response of ~75% of the maximum possible amplitude. Evoked responses were measured for 5 minutes to establish baseline. Once a stable 5-minute baseline was obtained, an AMPAR antagonist (DNQX, 20 μM) was bath applied for 10 minutes, followed by an NMDAR antagonist (APV, 40 μM), for an additional 10 minutes. Average evoked responses were then calculated from data obtained during the last 3 minutes in each drug condition. Specifically, the isolated AMPAR current was obtained by subtracting the average evoked response observed in DNQX from the average evoked response observed during the baseline period, while the isolated NMDAR current was obtained by subtracting the average evoked response observed in APV from the average evoked response observed in DNQX. The reported AMPA/NMDA ratio is calculated from the baseline subtracted peak amplitude of the isolated currents.
2.3.4. Statistics:
Passive properties, active properties, and AMPA/NMDA ratio obtained from young vs. aged neurons (or from combined pyramidal neurons vs. FSIs) were all compared using a standard two-tailed, two-sample, unpaired Student’s t-test. In cases where source data had unequal variance, Welch’s correction was applied.
3. Results
3.1. Age-related deficits in cognitive flexibility associated with lower level of NR1 in medial prefrontal cortex.
Young adult and aged rats did not differ in number of trials to acquire the initial discrimination (see Methods) compared to young adults (t(21)= −0.392, p=0.699; Fig. 1B). In contrast, aged rats were impaired relative to young adults in their ability to modify learned behavior as revealed by the greater number of trials needed to reliably shift their response strategy to conform to a new rule (t(21)=−3.075, p=0.006; Fig. 1C). In this same cohort of rats, mPFC expression of the essential NMDAR subunit, NR1 (t(21)=2.973, p=0.007; Fig. 2B), and the GluR2 AMPAR subunit (t(21)=2.828, p=0.010) were significantly lower in aged compared to young adults while expression of GluR1 AMPAR subunit was marginally lower in aged (t(21)=1.950, p=0.065; Fig 2B).
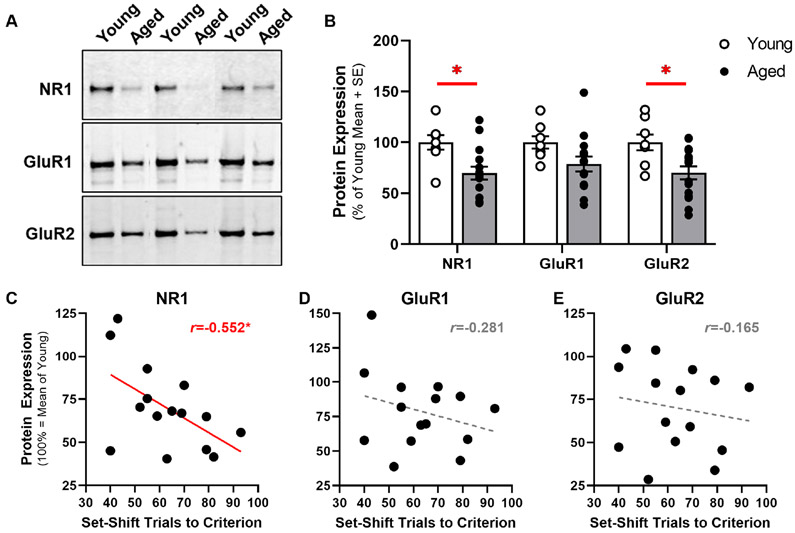
Fig. 2. Lower Expression of NR1 in Aged mPFC Correlates with Set-Shifting Deficits.
A: Representative immunoreactive bands in mPFC homogenates from young adult and aged rats. To prepare this image, three independent pairs of immunoreactive bands (one young and one aged sample, electrophoretically separated in adjacent lanes of the same gel) were selected from each experiment. The same pairs of samples are shown after detection with antibody raised against NR1 (top panel), GluR1 (middle panel) or GluR2 (bottom panel). ImageStudio acquisition parameters, including laser intensity and pixel resolution were identical across experiments. Acquired images were then imported into ImageJ using the BioFormats Plug-in; brightness and contrast were adjusted equally for each pair of bands. The final panel was organized/annotated using Microsoft PowerPoint. B: Expression of NR1 and GluR2 in mPFC (y-axis) was significantly lower in aged rats (n=15; filled bars) compared to young (n=8; open bars); expression of GluR1 tended to be lower in aged relative to young. C: Expression of NR1 in aged mPFC was negatively associated (r=−0.552, p=0.03) with the number of trials to attain criterion performance on the set-shift phase of the task (n=15). D&E: There was no reliable relationship between GluR1 or GluR2 and performance on the set-shift phase of the task. For panels C-E, each filled circle represents a single aged rat plotted as a function of protein expression (100% = mean expression of young adult; y-axis) and set-shift trials to criterion (x-axis). A solid red line illustrates a significantly non-zero line of best fit and dashed gray lines illustrate non-significant lines of best fit. Inset, Pearson’s r. *p<0.05.
To avoid conflating main effects of age on behavioral and biochemical outcomes, bivariate correlations were performed separately for aged and young adult rats. In aged rats, the number of trials needed to achieve criterion performance on the set-shift phase of the task was inversely related to expression of NR1 in mPFC (r=−0.552, p=0.033; Fig. 2C). In contrast, correlations between set-shift performance and GluR1 (r=−0.281, p=0.311; Fig. 2D) or GluR2 (r=−0.165, p=0.558; Fig. 2E) were not statistically significant. In young adults, there was a significant association between set-shift performance and expression of GluR1 (r=−0.737, p=0.037) whereas associations between set-shift and NR1 (r=−0.204, p=0.627) or GluR2 (r=−0.220, p=0.600) were not significant. The relevance of select iGluR subunits to set-shift abilities in young adult or aged rats appears to be specific as performance on the initial discrimination was not correlated with iGluR subunits in either age group (Table 1).
Table 1.
Correlations Between Performance on Set-Shifting Task and Expression of Ionotropic Glutamate Receptor Subunits in mPFC of Young Adult and Aged Rats
| Young (n=8) | Aged (n=15) | |||||||
|---|---|---|---|---|---|---|---|---|
|
Initial
Discrimination |
Set-Shift |
Initial
Discrimination |
Set-Shift | |||||
| r | p | r | p | r | p | r | p | |
| NR1 | −0.070 | 0.870 | −0.204 | 0.627 | 0.043 | 0.880 | −0.552* | 0.033 |
| GluR1 | 0.432 | 0.285 | −0.737* | 0.037 | 0.135 | 0.631 | −0.281 | 0.311 |
| GluR2 | 0.463 | 0.248 | −0.220 | 0.600 | 0.088 | 0.755 | −0.165 | 0.558 |
p<0.05
3.2. Aging has minimal impact on passive or active electrophysiological properties of either pyramidal neurons or fast-spiking interneurons in Layer 2/3 of the medial prefrontal cortex.
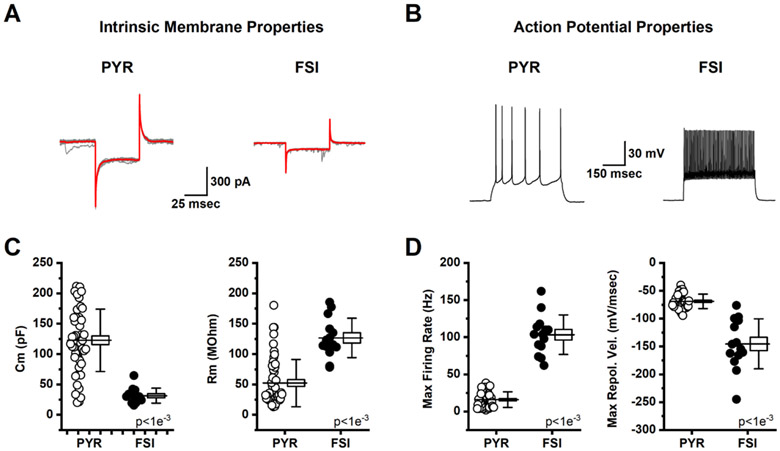
We evaluated the effect of age on passive and active electrophysiological properties of both pyramidal neurons and FSIs found in Layer 2/3 of the mPFC (see Methods). For pyramidal neurons, aging had no effect on whole-cell capacitance, input resistance, maximum firing frequency, or maximum depolarization velocity observed during an action potential (Table 2). That said, the maximum repolarization velocity was significantly increased, from −64.3 ± 3.0 mV/msec to −72.44 ± 2.33 mV/msec (t(42)=2.14, p=0.04), and this produced a trend towards decreased action potential width, defined as the time between maximum observed rate of depolarization and repolarization (t(42)=1.91, p=0.06). For FSIs, aging had no effect on any passive or active electrophysiological property examined (Table 3). Due to the minimal effect of aging on passive and active electrophysiological properties, data were combined across age, but separated by cell type (Fig. 3). This analysis is presented to indicate that pyramidal cells and FSIs in the mPFC can be robustly separated not just by their morphology as apparent under IR-DIC, but also by their whole-cell capacitance, input resistance, maximum firing frequency, and maximum repolarization velocity.
Table 2.
Active and Passive Electrophysiological Properties of Pyramidal Neurons in mPFC of Young and Aged Rat
| Passive Properties | Young (n=15,26) | Aged (n=12,25) | t(49) | p |
|---|---|---|---|---|
| Capacitance | 125.98 ± 11.14 | 119.13 ± 9.23 | 0.47 | 0.64 |
| Input Resistance | 56.23 ± 9.06 | 48.03 ± 6.03 | 0.75 | 0.46a |
| Active Properties | Young (n=12,20) | Aged (n=12,24) | t(42) | p |
| dVmax | 250.52 ± 15.51 | 267.52 ± 8.63 | −0.96 | 0.35a |
| dVmin | −64.33 ± 3.06 | −72.44 ± 2.33 | 2.14 | 0.04* |
| dVdV deltaT | 0.82 ± 0.06 | 0.69 ± 0.04 | 1.91 | 0.06 |
| max Freq | 14.91 ± 2.67 | 16.36 ± 1.90 | −0.45 | 0.65 |
Numbers of observations are (rats, cells). Data are mean ± standard error.
p<0.05.
Welch’s correction applied.
Table 3.
Active and Passive Electrophysiological Properties of Fast-Spiking Interneurons in mPFC of Young and Aged Rat
| Passive Properties | Young (4,6) | Aged (5,8) | t(12) | p |
|---|---|---|---|---|
| Capacitance | 25.83 ± 3.06 | 35.92 ± 4.93 | −1.60 | 0.14 |
| Input Resistance | 130.60 ± 17.37 | 123.47 ± 8.93 | 0.39 | 0.70 |
| Active Properties | Young (4,6) | Aged (5,8) | t(12) | p |
| dVmax | 218.00 ± 18.01 | 207.25 ± 19.58 | 0.39 | 0.70 |
| dVmin | −126.19 ± 9.99 | −159.61 ± 18.57 | 1.44 | 0.18 |
| dVdV deltaT | 0.52 ± 0.02 | 0.44 ± 0.04 | 1.51 | 0.16 |
| max Freq | 94.33 ± 7.86 | 110.00 ± 10.78 | −1.10 | 0.29 |
Numbers of observations are (rats, cells). Data are mean ± standard error.
Fig. 3. Active and passive electrophysiological properties reliably distinguish pyramidal neurons from fast-spiking interneurons in the mPFC.
A: Representative traces from a pyramidal cell (PYR, left) and a fast-spiking interneuron (FSI, right) voltage clamped at −70 mV illustrate the response to a series of 50 msec voltage steps to −80 mV. Individual traces are illustrated in grey and the average trace for each cell is illustrated in red. Whole-cell capacitance and input resistance were calculated in each cell from these data as described in the methods. B: Sample data obtained in current clamp illustrating the response of a representative pyramidal cell (left) and fast-spiking interneuron (right) to a 300 pA x 500 msec current step. C: Summary data indicating that capacitance was significantly larger, and input resistance was significantly lower, in pyramidal cells (open circles) vs. fast-spiking interneurons (filled circles). D: Summary data indicating that the maximum firing rate achieved was lower, and that the maximum rate of repolarization from the action potential peak was slower, in pyramidal cells (open circles) vs. fast-spiking interneurons (closed circles). For box plots in C-D, the horizontal line through the middle of the box indicates the population mean, the box size indicates the standard error, and the whiskers indicate the standard deviation. Inset text provides p-values.
3.3. Aging increases AMPA/NMDA ratio as observed in fast-spiking interneurons, but not pyramidal cells.
In order to evaluate the effect of age on AMPA/NMDA ratio in L2/3 pyramidal neurons we used a bipolar stimulator constructed from theta glass to generate evoked EPSCs in control conditions, after bath application of DNQX, and after bath application of both DNQX and APV. The AMPA and NMDA receptor mediated components of the evoked response were then isolated from those data as described in the methods and as illustrated in Fig. 4A-B.
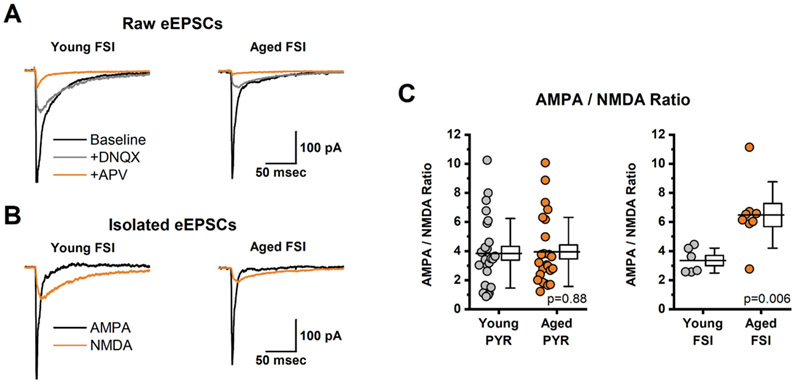
Fig. 4. Aging Increases AMPA/NMDA Ratio in Fast-Spiking Interneurons but not pyramidal cells of mPFC.
A: Illustrates average evoked excitatory postsynaptic currents (eEPSCs) in a representative FSI from a young animal (left) and from and aged animal (right). Data were collected sequentially in baseline conditions (black trace), in the presence of DNQX (grey trace), and in the presence of DNQX + APV (orange trace). B: Illustrates the isolated AMPA- and NMDA-receptor mediated components of the eEPSC (black and orange trace, respectively), calculated from the data illustrated in panel A, for both a young and aged FSI (left, right, respectively, see Methods for additional details). C: Aging had no effect on AMPA/NMDA ratio in pyramidal neurons (left panel) but was associated with a significant increase in AMPA/NMDA ratio in FSIs (right panel). For box plots in each panel, the horizontal line through the middle of the box indicates the population mean, the box size indicates the standard error, and the whiskers indicate the standard deviation. Inset text provides p-values.
When recording from pyramidal neurons, the stimulator used to generate evoked currents was placed in Layer 1, in Layer 2/3, or in Layer 5 (n=22, 13, and 16, respectively). Stimulator placement (combined across age) had no impact on observed AMPA/NMDA ratio in pyramidal neurons (L1: 3.7 ± 0.5, L2/3: 4.2 ± 0.77, L5: 4.0 ± 0.53, F(2,48)=0.19, p=0.82, 1-way ANOVA), and age had no effect on observed AMPA/NMDA ratio (regardless of stimulator placement, Table 4). Based on these observations, the AMPA/NMDA ratio data obtained from mPFC pyramidal neurons were combined across stimulator placement, compared across age, and presented in Fig. 3D (left panel, t(49)=−0.15, p=0.88). This analysis further reinforces the conclusion that age has no effect on AMPA/NMDA ratio as observed in Layer 2/3 pyramidal neurons (Table 4).
Table 4.
AMPA/NMDA Ratio of Pyramidal Neurons by Stimulator Position
| Stimulator Position (young, aged; cells) |
Young | Aged | df | t | p |
|---|---|---|---|---|---|
| Layer 1 (14, 8) | 3.61 ± 0.54 | 3.78 ± 1.04 | 20 | −0.15 | 0.87 |
| Layer 2/3 (5, 8) | 4.47 ± 1.80 | 3.96 ± 0.71 | 11 | 0.27 | 0.80a |
| Layer 5 (7, 9) | 3.88 ± 0.72 | 4.09 ± 0.80 | 14 | −0.19 | 0.86 |
| Combined (26, 25) | 3.85 ± 0.47 | 3.95 ± 0.47 | 49 | −0.15 | 0.88 |
Data are mean ± standard error.
Welch’s correction applied.
In sharp contrast to results obtained in pyramidal neurons, we found that age produced a clear increase in AMPA/NMDA ratio as observed in Layer 2/3 FSIs (t(9.4) =−3.58, p=0.006), Fig. 3D, right panel). For these experiments, the stimulator used to generate the evoked responses was placed in Layer 2/3. Interestingly, a comparison of AMPA/NMDA ratio between young pyramidal cells and young FSIs also yielded no significant difference (Young PYR: 3.85 ± 0.47, Young FSI: 3.34 ± 0.35, n=26, 6, t(23.6)=0.86, p=0.40).
4. Discussion
Cognitive flexibility, or the ability to update behavioral strategies in relation to shifting contingencies in the environment, complements other aspects of executive function, such as attentional control, behavioral inhibition and working memory, to coordinate goal-directed behaviors, which critically depend on the PFC. The present study selectively links age-related deficits in cognitive flexibility to loss of NMDARs from the PFC and, further, reveals an age-related increase in AMPA/NMDA ratio of synaptically evoked responses that was observed in PFC FSIs, but not pyramidal cells.
In humans, PFC-dependent cognitive flexibility declines with advancing age (Ashendorf and McCaffrey, 2008; Robbins et al., 1998; Terry and Sliwinski, 2012; Volkow et al., 1998). In rodents, cognitive flexibility depends on the mPFC and may be evaluated through set-shifting tasks implemented across a variety of modalities and experimental settings (Birrell and Brown, 2000; Floresco et al., 2008; Ragozzino et al., 1999). While decline of hippocampus-dependent spatial memory is also a reliable feature of rodent brain aging (Barnes et al., 1997; Bizon et al., 2009; Gallagher et al., 1993; McQuail and Nicolle, 2015), individual differences in set-shift performance do not correlate hippocampal-dependent learning and memory as evaluated in the Morris water maze (Barense et al., 2002; Beas et al., 2013; Nieves-Martinez et al., 2012). Further, analysis of errors committed by aged rats during the set-shift phase of the task reveals that slower acquisition of the new rule is due to perseverative errors made in response to the previously reinforced stimulus (i.e. location of the illuminated light; (Beas et al., 2013, 2017) and see also (Beas et al., 2016)), mirroring the pattern of behavioral deficits produced by mPFC inactivation (Floresco et al., 2008). Collectively, these data indicate that performance on the operant set-shifting task is a sensitive and specific behavioral probe for naturally occurring, age-related decline of cognitive flexibility that depends on the mPFC.
The first finding of our study is that individual differences in age-related decline of cognitive flexibility reliably covary with level of the essential NMDAR subunit, NR1, in PFC. Loss of NMDARs from the aged PFC has been observed in humans (Hellström-Lindahl and Court, 2000; Piggott et al., 1992), monkeys (Dickstein et al., 2013; Hof et al., 2002, p. 1; Wenk et al., 1991), rats (Dyall et al., 2007; McQuail et al., 2016; Mitchell and Anderson, 1998; Wenk et al., 1991) and mice (Magnusson, 1998; Magnusson et al., 2007, 2005; Magnusson and Cotman, 1993; Migani et al., 2000). The current study greatly extends the relevance of these prior observations to PFC-dependent cognition by determining that age-related impairment of cognitive flexibility is coupled to individual differences in loss of NR1 from the aging mPFC. Critically, we observed that level of NR1 was specifically associated with performance during the set-shift phase of the task, when aged rats were challenged to modify or inhibit responses that are no longer appropriate, and not related to acquisition of the initial rule. This association in aged rats is consistent with behavioral pharmacology data from young adult rats that demonstrates intra-mPFC infusion of the NMDAR antagonist AP5 impairs set-shifting, but not initial learning (Jett et al., 2017). Given these data that clearly implicate a vital role for NMDARs in neural processes that underlie cognitive flexibility, the relationship between age-related loss of NR1 from PFC and diminished ability to flexibly modify behavior supports the conclusion that loss of NMDARs is a key mediator of specific PFC cognitive deficits that emerge with age.
The second finding of our study is that any potential loss of AMPARs from the aging PFC is not related to individual differences in cognitive flexibility. Activation of NMDARs relies, in part, on depolarizing influences from AMPARs during performance of PFC-dependent cognitive tasks (Wang et al., 2013). Consistent with the concept that AMPAR activation is upstream from NMDARs, blocking AMPARs in the rat mPFC impairs set-shifting (Jett et al., 2017; Stefani et al., 2003). In the current study, we detected a significant age-dependent loss of GluR2 subunits coupled with a similar trend towards lower expression of GluR1 subunits. However, individual differences in expression of GluR1 and GluR2 were not related to impaired set-shifting, nor initial discrimination, of aged rats. These new results are highly consistent with our prior study of iGluR subunits within the aged mPFC wherein marginally lower levels of AMPAR subunits were not associated with degree of age-related decline in PFC-dependent working memory (McQuail et al., 2016). As such, the current biochemical data support the notion that PFC AMPARs may be susceptible to decline with age but that any possible reductions do not meaningfully contribute to age-related changes in PFC-dependent cognition. Furthermore, the lack of association between cognitive flexibility and AMPAR subunits in aging individuals is in sharp contrast to the reliable correlation with loss of NMDARs to PFC-dependent cognition noted above and in other studies (i.e. (McQuail et al., 2016).
The third finding of our study is that intrinsic electrophysiological properties of pyramidal neurons and FSIs in Layer 2/3 of PFC are minimally affected by aging. We did observe an increase in repolarization velocity in aged pyramidal neurons, that likely contributed to a marginal decrease in action potential width, and may speak to potential age-related changes in a fast after hyperpolarization (Shao et al., 1999). That said, there were no significant changes in any other passive or active properties of pyramidal neurons examined. Similarly, we noted no effect of aging on passive or active properties of mPFC FSIs. Across species, the cellular and functional integrity of PFC neurons is essential for normal cognitive flexibility (Birrell and Brown, 2000; Bissonette et al., 2008; Dias et al., 1996; Floresco et al., 2008; Moore et al., 2009; Pantelis et al., 1999). Stereological investigation of the aging primate dorsolateral PFC (dlPFC) indicates that there is a partial loss of neurons specific to Area 8A (anterior to arcuate sulcus) that is not evident in adjacent Area 46 (surrounding the principal sulcus; (Smith et al., 2004)). Similarly, stereologically estimated total numbers of neurons in the rodent mPFC reveal moderate age-related decrements (~15-25% loss), though there is disagreement in whether these cellular changes localize to the dorsal (i.e. cingulate) or the ventral mPFC (i.e. prelimbic and infralimbic cortices; (Stranahan et al., 2012; Yates et al., 2008)). Regardless, changes in neuron number in the aging mPFC appear to affect pyramidal neurons and interneurons similarly (Stranahan et al., 2012), and there also appears to be proportional effects of aging on excitatory and inhibitory synapses (Bañuelos et al., 2014; Beas et al., 2017; McQuail et al., 2016; Peters et al., 2008). Consistent with that observation, most physiological studies in rodents suggest minimal/modest effect on the balance of inhibitory and excitatory fast synaptic transmission (Bories et al., 2013; Carpenter et al., 2016). By contrast, analyses of primate dlPFC pyramidal neurons provides mixed evidence for age-related changes in cell activity that could be construed as reflecting diminished excitability on the one hand, namely decreased frequency of sEPSCs and increased frequency of sIPSCs (Luebke et al., 2004), or enhanced excitability on the other, specifically greater evoked firing (Chang et al., 2005; Luebke and Amatrudo, 2012). Collectively these data suggest that age related changes in E/I balance in the mPFC may depend heavily on changes in function of receptor systems beyond those that report the majority of fast spontaneous EPSCs and IPSCs. Indeed, prior work from our group implicates age-related changes in tonic inhibition carried by extrasynaptic GABA receptors (Bañuelos et al., 2014; Beas et al., 2017; Carpenter et al., 2016). As highlighted here and in our prior study (McQuail et al., 2016), changes in NMDAR function may comprise another such mechanism.
In that regard, the fourth finding from our study is that aging alters the AMPA/NMDA ratio of eEPSCs observed in PFC FSIs, but not pyramidal neurons. To the best of our knowledge, this represents the first direct physiological examination of how aging effects excitatory transmission to non-pyramidal neurons in the mPFC. Parvalbumin-expressing (PV+) FSIs regulate pyramidal neuron spiking, promote synchronicity in neural networks, and orchestrate the complex network dynamics proposed to underlie cognition (reviewed in (Ferguson and Gao, 2018; McQuail et al., 2015)). While aging is affiliated with a slowing of gamma frequency in the mPFC (Insel et al., 2012), experimental treatment with ketamine, PCP or MK-801 increases frontal gamma in rats, monkeys and humans (Goonawardena et al., 2016; Hakami et al., 2009; Lazarewicz et al., 2010; Rivolta et al., 2015). These differences could relate to FSI-specific NMDAR-signaling deficits that emerge naturally with age versus generalized consequences of inhibiting NMDARs on multiple cell types in PFC. Supportive of this view, PV+ cell-specific NR1-knockout (NR1-KO) mice present with a selective deficit in the ability to update, but not to maintain, memoranda during performance of a PFC-dependent task (Carlén et al., 2012). Critically, the agreement between the effects of aging on NMDAR activity in mPFC FSIs and behavioral deficits following targeted NR1-KO from PV+ cells converge to support a mechanism wherein NMDAR signaling on FSIs is vitally important to normal cognitive flexibility and reduced NMDAR signaling on FSIs is implicated in cognitive declines that emerge at advanced ages. Finally, it is worth highlighting that substantial data from both the current and prior studies suggest that the larger AMPA/NMDA ratio reported here in aged FSIs is likely produced primarily by loss of functional NMDARs, and not by an increase in expression of AMPARs (Dyall et al., 2007; McQuail et al., 2016; Mitchell and Anderson, 1998; Wenk et al., 1991).
5. Conclusions
These new, descriptive data strongly suggest that diminished NMDAR signaling specific to FSIs contributes to later-life decline of PFC-dependent cognition. The apparent specificity of this deficit may explain why non-selective NMDAR potentiators produce modest-to-mixed effects on cognition in aging (Baxter et al., 1994; Billard and Rouaud, 2007; Burgdorf et al., 2011; McQuail et al., 2016; Panizzutti et al., 2014). Consequently, new studies of NMDAR signaling on FSIs may inform the development of truly effective therapeutics. The practicality of targeting NMDARs on FSIs is bolstered by recent molecular findings that PV+ cells in PFC are highly enriched for expression of grin2d, which encodes the NR2D isoform (Garst-Orozco et al., 2020). The emerging relevance of these data to cognition are supported by studies that show NR2D-KO in the mouse brain reduces sensitivity to the behavioral deficits induced by MK-801 (Ide et al., 2019; Sapkota et al., 2016) and that a positive allosteric modulator of NR2D is able to reverse MK-801-induced behavioral deficits (Suryavanshi et al., 2014). Collectively, we speculate that targeting NR2D-NMDARs could provide a means to the determine the mechanism by which distributed NMDAR signaling on FSIs contributes to the age-related decline of cognitive flexibility and, possibly, to rectify NMDAR-mediated cognitive impairments.
Supplementary Material
Highlights:
Aged rats are impaired on a set-shifting test of cognitive flexibility
Age-related set-shifting impairment correlates with lower expression of NR1 in PFC
Age increases AMPA/NMDA ratio on fast-spiking interneurons but not pyramidal neurons
Expression of AMPAR subunits in PFC is not associated with cognition in aging
Active and passive properties of PFC neurons are generally conserved with age
Acknowledgements:
We thank Miranda Schwabe, Kailey Simpson, Shannon Wall, and Lauren Vetere for assistance with behavioral testing, and Brandon Hellbusch for assistance with Western blotting. This work was supported by NIH grants F32AG051371 (JAM), K01AG061263 (JAM), P20GM109091 (JAM), P20GM103641 (JAM), NSF Graduate Research Fellowship Program DGE-0802270 (BSB), NIH/NICHD 2T32HD071866-06 (CMH), a McKnight Predoctoral Fellowship and the Pat Tillman Foundation (CMH), R01AG029421 (JLB), and the McKnight Brain Research Foundation (JLB).
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashendorf L, McCaffrey RJ, 2008. Exploring age-related decline on the Wisconsin Card Sorting Test. Clin. Neuropsychol 22, 262–272. 10.1080/13854040701218436 [DOI] [PubMed] [Google Scholar]
- Bañuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL, 2014. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J. Neurosci 34, 3457–3466. 10.1523/JNEUROSCI.5192-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG, 2002. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn. Mem. Cold Spring Harb. N 9, 191–201. 10.1101/lm.48602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL, 1997. Multistability of cognitive maps in the hippocampus of old rats. Nature 388, 272–275. 10.1038/40859 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Lanthorn TH, Frick KM, Golski S, Wan RQ, Olton DS, 1994. D-cycloserine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol. Aging 15, 207–213. [DOI] [PubMed] [Google Scholar]
- Beas B, Setlow B, Bizon J, 2013. Distinct manifestations of executive dysfunction in aged rats. Neurobiol. Aging 34, 2164–2174. 10.1016/j.neurobiolaging.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Banuelos C, Setlow B, Bizon JL, 2017. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neuroscience 345, 274–286. 10.1016/j.neuroscience.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL, 2016. Effects of acute administration of the GABA(B) receptor agonist baclofen on behavioral flexibility in rats. Psychopharmacology (Berl.) 233, 2787–2797. 10.1007/s00213-016-4321-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard J-M, Rouaud E, 2007. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur. J. Neurosci 25, 2260–2268. 10.1111/j.1460-9568.2007.05488.x [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ, 2000. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci 20, 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM, 2008. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci 28, 11124–11130. 10.1523/JNEUROSCI.2820-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL, 2012. Characterizing cognitive aging of working memory and executive function in animal models. Front. Aging Neurosci 4, 19. 10.3389/fnagi.2012.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH 2009. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol. Aging 30, 646–655. 10.1016/j.neurobiolaging.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot K, Kimura S-I, Bai J, Kemp A, Manahan-Vaughan D, Giros B, Tzavara E, Otani S, 2015. Modulation of hippocampus-prefrontal cortex synaptic transmission and disruption of executive cognitive functions by MK-801. Cereb. Cortex N. Y. N 199125, 1348–1361. 10.1093/cercor/bht329 [DOI] [PubMed] [Google Scholar]
- Boone KB, Ghaffarian S, Lesser IM, Hill-Gutierrez E, Berman NG, 1993. Wisconsin Card Sorting Test performance in healthy, older adults: relationship to age, sex, education, and IQ. J. Clin. Psychol 49, 54–60. [DOI] [PubMed] [Google Scholar]
- Bories C, Husson Z, Guitton MJ, Koninck YD, 2013. Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J. Neurosci 33, 1344–1356. 10.1523/JNEUROSCI.3258-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang X, Weiss C, Matthews E, Disterhoft JF, Stanton PK, Moskal JR, 2011. The N-methyl-d-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol. Aging 32, 698–706. 10.1016/j.neurobiolaging.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Rühlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai L-H, 2012. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 17, 537–548. 10.1038/mp.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter HE, Kelly KB, Bizon JL, Frazier CJ, 2016. Age-related changes in tonic activation of presynaptic versus extrasynaptic γ-amniobutyric acid type B receptors in rat medial prefrontal cortex. Neurobiol. Aging 45, 88–97. 10.1016/j.neurobiolaging.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-M, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI, 2005. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb. Cortex N. Y. N 199115, 409–18. 10.1093/cercor/bhh144 [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B, 2008. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav. Pharmacol 19, 225–234. 10.1097/FBP.0b013e3282feb0ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive functions. Annu. Rev. Psychol 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC, 1996. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav. Neurosci 110, 872–886. 10.1037//0735-7044.110.5.872 [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, Hof PR, 2013. Dendritic spine changes associated with normal aging. Neuroscience 251, 21–32. 10.1016/j.neuroscience.2012.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT, 2007. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol. Aging 28, 424–439. 10.1016/j.neurobiolaging.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA, 2005. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology (Berl.) 179, 77–84. 10.1007/s00213-004-2109-y [DOI] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB, 2011. Reducing Prefrontal Gamma-Aminobutyric Acid Activity Induces Cognitive, Behavioral, and Dopaminergic Abnormalities That Resemble Schizophrenia. Biol. Psychiatry, Schizophrenia: From Circuit Dysfunction to Treatment? 69, 432–441. 10.1016/j.biopsych.2010.09.038 [DOI] [PubMed] [Google Scholar]
- Ferguson BR, Gao W-J, 2018. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front. Neural Circuits 12, 37. 10.3389/fncir.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL, 2008. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav. Brain Res 190, 85–96. 10.1016/j.bbr.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M, 1993. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci 107, 618–626. [DOI] [PubMed] [Google Scholar]
- Garst-Orozco J, Malik R, Lanz TA, Weber ML, Xi H, Arion D, Enwright JF, Lewis DA, O’Donnell P, Sohal VS, Buhl DL, 2020. GluN2D-mediated excitatory drive onto medial prefrontal cortical PV+ fast-spiking inhibitory interneurons. PloS One 15, e0233895. 10.1371/journal.pone.0233895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Heiss J, Glavis-Bloom C, Trube G, Borroni E, Alberati D, Wallace TL, 2016. Alterations in High-Frequency Neuronal Oscillations in a Cynomolgus Macaque Test of Sustained Attention Following NMDA Receptor Antagonism. Neuropsychopharmacol. 41, 1319–1328. 10.1038/npp.2015.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O’Brien TJ, Pinault D, 2009. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PloS One 4, e6755. 10.1371/journal.pone.0006755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström-Lindahl E, Court JA, 2000. Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behav. Brain Res 113, 159–168. 10.1016/S0166-4328(00)00210-2 [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Vetere LM, Orsini CA, McQuail JA, Maurer AP, Burke SN, Setlow B, Bizon JL, 2017. Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344 × brown Norway hybrid rats. Neurobiol. Aging 60, 141–152. 10.1016/j.neurobiolaging.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervig ME, Thomsen MS, Kalló I, Mikkelsen JD, 2016. Acute phencyclidine administration induces c-Fos-immunoreactivity in interneurons in cortical and subcortical regions. Neuroscience 334, 13–25. 10.1016/j.neuroscience.2016.07.028 [DOI] [PubMed] [Google Scholar]
- Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, Morrison JH, 2002. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 928, 175–186. 10.1016/S0006-8993(01)03345-5 [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B, 2007. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci 27, 11496–11500. 10.1523/JNEUROSCI.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Ikekubo Y, Mishina M, Hashimoto K, Ikeda K, 2019. Cognitive Impairment That Is Induced by (R)-Ketamine Is Abolished in NMDA GluN2D Receptor Subunit Knockout Mice. Int. J. Neuropsychopharmacol 22, 449–452. 10.1093/ijnp/pyz025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA, 2017. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience 346, 284–297. 10.1016/j.neuroscience.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F, 2007. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc. Natl. Acad. Sci. U. S. A 104, 14843–14848. 10.1073/pnas.0704848104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Parr L, Chennareddi L, Herndon JG, 2018. Age-related decline in cognitive flexibility in female chimpanzees. Neurobiol. Aging 72, 83–88. 10.1016/j.neurobiolaging.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ, 2010. Ketamine modulates theta and gamma oscillations. J. Cogn. Neurosci 22, 1452–1464. 10.1162/jocn.2009.21305 [DOI] [PubMed] [Google Scholar]
- Luebke JI, Amatrudo JM, 2012. Age-related increase of sIAHP in prefrontal pyramidal cells of monkeys: relationship to cognition. Neurobiol. Aging 33, 1085–1095. 10.1016/j.neurobiolaging.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Chang Y-M, Moore TL, Rosene DL, 2004. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 125, 277–288. 10.1016/j.neuroscience.2004.01.035 [DOI] [PubMed] [Google Scholar]
- Magnusson KR, 1998. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech. Ageing Dev 104, 227–248. 10.1016/s0047-6374(98)00076-1 [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Bai L, Zhao X, 2005. The effects of aging on different C-terminal splice forms of the zeta1(NR1) subunit of the N-methyl-d-aspartate receptor in mice. Brain Res. Mol. Brain Res 135, 141–149. 10.1016/j.molbrainres.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Cotman CW, 1993. Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol. Aging 14, 197–206. 10.1016/0197-4580(93)90001-r [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R, 2007. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 8, 43. 10.1186/1471-2202-8-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Bañuelos C, LaSarge CL, Nicolle MM, Bizon JL, 2012. GABAB receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol. Aging 33, 1124.e1–1124.e12. 10.1016/j.neurobiolaging.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Simpson KL, Frazier CJ, Setlow B, Bizon JL, 2016. NR2A-Containing NMDARs in the Prefrontal Cortex Are Required for Working Memory and Associated with Age-Related Cognitive Decline. J. Neurosci 36, 12537–12548. 10.1523/JNEUROSCI.2332-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL, 2015. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol. Med 21, 450–460. 10.1016/j.molmed.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Johnson SA, Burke SN, Bizon JL, 2018. Chapter 17 - Rat Models of Cognitive Aging, in: Ram JL, Conn† PM (Eds.), Conn’s Handbook of Models for Human Aging (Second Edition). Academic Press, pp. 211–230. 10.1016/B978-0-12-811353-0.00017-8 [DOI] [Google Scholar]
- McQuail JA, Nicolle MM, 2015. Spatial reference memory in normal aging Fischer 344 × Brown Norway F1 hybrid rats. Neurobiol. Aging 36, 323–333. 10.1016/j.neurobiolaging.2014.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migani P, Magnone MC, Rossolini G, Piantanelli L, 2000. Excitatory amino acid receptors in the prefrontal cortex of aging mice. Neurobiol. Aging 21, 607–612. 10.1016/s0197-4580(00)00146-9 [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Anderson KJ, 1998. Age-related changes in [3H]MK-801 binding in the Fischer 344 rat brain. Neurobiol. Aging 19, 259–265. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB, 2003. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol. Aging 24, 125–134. 10.1016/s0197-4580(02)00054-4 [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB, 2009. Effects on executive function following damage to the prefrontal cortex in the rhesus monkey (Macaca mulatta). Behav. Neurosci 123, 231–241. 10.1037/a0014723 [DOI] [PubMed] [Google Scholar]
- Nieves-Martinez E, Haynes K, Childers SR, Sonntag WE, Nicolle MM, 2012. Muscarinic receptor/G-protein coupling is reduced in the dorsomedial striatum of cognitively impaired aged rats. Behav. Brain Res 227, 258–264. 10.1016/j.bbr.2011.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Gołembiowska K, Popik P, 2010. Mazindol attenuates ketamine-induced cognitive deficit in the attentional set shifting task in rats. Eur. Neuropsychopharmacol 20, 37–48. 10.1016/j.euroneuro.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Panizzutti R, Scoriels L, Avellar M, 2014. The co-agonist site of NMDA-glutamate receptors: a novel therapeutic target for age-related cognitive decline. Curr. Pharm. Des 20, 5160–5168. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW, 1999. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr. Res 37, 251–270. 10.1016/s0920-9964(98)00156-x [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI, 2008. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152, 970–981. 10.1016/j.neuroscience.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott MA, Perry EK, Perry RH, Court JA, 1992. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 588, 277–286. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP, 1999. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J. Neurosci 19, 4585–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MG, 2004. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol. Aging 19, 482–494. 10.1037/0882-7974.19.3.482 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Span MM, van der Molen MW, 2002. Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn. 49, 382–401. 10.1006/brcg.2001.1506 [DOI] [PubMed] [Google Scholar]
- Rivolta D, Heidegger T, Scheller B, Sauer A, Schaum M, Birkner K, Singer W, Wibral M, Uhlhaas PJ, 2015. Ketamine Dysregulates the Amplitude and Connectivity of High-Frequency Oscillations in Cortical-Subcortical Networks in Humans: Evidence From Resting-State Magnetoencephalography-Recordings. Schizophr. Bull 41, 1105–1114. 10.1093/schbul/sbv051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM, 1998. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J. Int. Neuropsychol. Soc. JINS 4, 474–490. 10.1017/s1355617798455073 [DOI] [PubMed] [Google Scholar]
- Sapkota K, Mao Z, Synowicki P, Lieber D, Liu M, Ikezu T, Gautam V, Monaghan DT, 2016. GluN2D N-Methyl-d-Aspartate Receptor Subunit Contribution to the Stimulation of Brain Activity and Gamma Oscillations by Ketamine: Implications for Schizophrenia. J. Pharmacol. Exp. Ther 356, 702–711. 10.1124/jpet.115.230391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF, 1999. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol 521Pt 1, 135–146. 10.1111/j.1469-7793.1999.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH, 2004. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J. Neurosci 24, 4373–4381. 10.1523/JNEUROSCI.4289-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B, 2003. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav. Neurosci 117, 728–737. 10.1037/0735-7044.117.4.728 [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B, 2010. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur. J. Pharmacol 639, 26–32. 10.1016/j.ejphar.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B, 2005. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav. Neurosci 119, 420–428. 10.1037/0735-7044.119.2.420 [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M, 2012. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J. Comp. Neurol 520, 1318–1326. 10.1002/cne.22790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryavanshi PS, Ugale RR, Yilmazer-Hanke D, Stairs DJ, Dravid SM, 2014. GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br. J. Pharmacol 171, 799–809. 10.1111/bph.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry CP, Sliwinski MJ, 2012. Aging and random task switching: the role of endogenous versus exogenous task selection. Exp. Aging Res 38, 87–109. 10.1080/0361073X.2012.637008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomm RJ, Tse MT, Tobiansky DJ, Schweitzer HR, Soma KK, Floresco SB, 2018. Effects of aging on executive functioning and mesocorticolimbic dopamine markers in male Fischer 344 × brown Norway rats. Neurobiol. Aging 72, 134–146. 10.1016/j.neurobiolaging.2018.08.020 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J, 1998. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry 155, 344–349. 10.1176/ajp.155.3.344 [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang C-J, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang X-J, Arnsten AFT, 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77, 736–749. 10.1016/j.neuron.2012.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC, 1991. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol. Aging 12, 93–98. 10.1016/0197-4580(91)90047-N [DOI] [PubMed] [Google Scholar]
- Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM, 2008. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 1218, 1–12. 10.1016/j.brainres.2008.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.