Abstract
Cilostazol is a phosphodiesterase III inhibitor with a long track record of safety that is FDA and EMA approved for the treatment of claudication in patients with peripheral arterial disease. In addition, cilostazol has been approved for secondary stroke prevention in several Asian countries based on trials that have demonstrated a reduction in stroke recurrence among patients with non-cardioembolic stroke. The onset of benefit appears after 60–90 days of treatment, which is consistent with cilostazol’s pleiotropic effects on platelet aggregation, vascular remodeling, blood flow, and plasma lipids. Cilostazol appears safe and does not increase the risk of major bleeding when given alone or in combination with aspirin or clopidogrel. Adverse effects such as headache, gastrointestinal symptoms and palpitations, however, contributed to a 6% increase in drug discontinuation among patients randomized to cilostazol in a large secondary stroke prevention trial (CSPS.com). Due to limitations of prior trials, such as open label design, premature trial termination, large loss to follow-up, lack of functional or cognitive outcome data, and exclusive enrollment in Asia, the existing trials have not led to a change in clinical practice or guidelines in Western countries. These limitations could be addressed by a double-blind placebo-controlled randomized trial conducted in a broader population. If positive, it would increase the evidence in support of long-term treatment with cilostazol for secondary prevention in the millions of patients worldwide who have suffered a non-cardioembolic ischemic stroke.
History and Mechanism of Action of Cilostazol
In the 1980s, Takao Nishi’s lab at the Japanese pharmaceutical company Otsuka synthesized the quinolone derivative cilostamide, which inhibited platelet aggregation and had vasodilatory properties, but caused persistent tachycardia.1 With modification of an amide side chain, the Otsuka researchers created cilostazol, which had the desired effects of cilostamide and a reduced risk of tachycardia (Figure 1). Cilostazol was first marketed in Japan in 1988 as Pletaal and subsequently as Pletal in the United States in 1999 and the United Kingdom in 2001.2 In the United States and Europe, cilostazol is only approved for intermittent claudication in patients with peripheral arterial disease (PAD). Japanese regulators approved cilostazol for PAD as well as prevention of recurrent ischemic stroke in 2003,3 as have several other countries in Asia. At present, cilostazol is available worldwide in both branded and generic formulations, and is available in the United States as a generic medication for less than $10 a month and in Germany for as little as €5 a month.4–6
Figure 1.

The molecular structure of cilostazol.
Cilostazol is a phosphodiesterase III (PDE3) inhibitor that is metabolized by the hepatic cytochrome P450 system and is independent of the cyclooxygenase (aspirin) or platelet P2Y12 receptor (clopidogrel) pathways.7,8 The active metabolites of cilostazol reversibly inhibit PDE3, which mitigates degradation of cyclic adenosine monophosphate (cAMP) and leads to inhibition of platelet reactivity and aggregation. Cilostazol also prevents adenosine uptake into cells, further augmenting cAMP.9,10 Cilostazol’s cAMP-mediated inhibition of platelet reactivity and aggregation is comparable to that of aspirin and ticlopidine, yet cilostazol has a lower incidence of hemorrhagic complications.11–14 Unlike aspirin and ticlopidine, which block extracellular membrane receptors for their antiplatelet effect,15 cilostazol functions intracellularly and does not prolong overall bleeding time.16–20 A comparison of the mechanism of action, common and major adverse events, and out-of-pocket cost of common antiplatelet medications used in stroke prevention is shown in Table 1.
Table 1.
Comparison of the mechanism of action, common and major adverse events, and out-of-pocket cost of common anti-platelet medications used in stroke prevention.
| Medication | Mechanism of action | Most common adverse events* | Major adverse events | Monthly cost** |
|---|---|---|---|---|
| Cilostazol | Reversible intracellular PDE3 inhibition | Headache, diarrhea, palpitation, dizziness, tachycardia | Contraindicated in patients with heart failure | $9.00 |
| Dipyridamole | Reversible intracellular PDE5 inhibition | Headache, dizziness, abdominal distress, angina pectoris, nausea | Major bleeding (when combined with aspirin) | $73.54 |
| Aspirin | Irreversible extracellular inhibition of COX | Dyspepsia, rash, minor bleeding, epistaxis | Major bleeding, upper gastrointestinal ulcers | $2.47 |
| Clopidogrel | Irreversible extracellular inhibition of P2Y12 | Bruising, minor bleeding, epistaxis | Major bleeding | $15.00 |
| Ticagrelor | Irreversible extracellular inhibition of P2Y12 | Dyspnea, ventricular pause, nausea, dizziness, transient creatinine increase, minor bleeding | Major bleeding, bradyarrhythmia, thrombotic thrombocytopenic purpura | $373.90 |
| Prasugrel | Irreversible extracellular inhibition of P2Y12 | Hypertension, headache, nausea, minor bleeding, epistaxis | Fatal bleeding, thrombotic thrombocytopenic purpura | $32.36 |
According to Lexicomp (online.lexi.com).
According to goodrx.com, in the United States, without insurance, at Walmart.
In addition to platelet inhibition, cilostazol enhances vasodilatation, prevents intimal hyperplasia and proliferation of vascular smooth muscle cells, lowers blood pressure, and may increase cerebral blood flow, improve myelin repair, and enhance astrocyte-to-neuron energy supply.21–27 The vasodilatory effects of cilostazol appear to be mediated by endothelial production of nitric oxide (which may be deficient in many patients with stroke, particularly lacunar stroke) in response to elevated concentrations of cAMP23–25,28, as well as the chronic inhibition of vascular smooth muscle cell proliferation that leads to more compliant arteries.29–32 This vascular remodeling occurs because cAMP reduces the activity of platelet derived growth factor, which regulates vascular smooth muscle proliferation.29–31 Cilostazol’s vasodilatory and arterial remodeling effects lead to a mild (2–4 mm Hg) reduction in systolic blood pressure,33 but hypotension is seen in <1% of patients.34 Patients taking cilostazol have an increase in heart rate, typically on the order of 6 beats per minute, but the effect has not been associated with tachyarrhythmia.35,36
Because PDE3 is found throughout the human body, the effects of cilostazol are not limited to platelets and endothelial cells.37 Higher levels of intracellular cAMP reduce the expression of interleukin-6, which can increase the activity of lipoprotein lipase.38,39 Consequently, cilostazol therapy is associated with a 16–29% decrease in triglycerides and a 12–13% increase in high-density lipoprotein cholesterol.40–42 Additional pleiotropic effects that have been proposed include a decrease in systemic inflammation, improved stroke recovery, reduction of amyloid-related neurotoxicity, prevention of restenosis of cardiac stents, and prevention of diabetic nephropathy.37,43–53 Another potential role for cilostazol is in the prevention of dementia or cognitive decline21,27,54–56, particularly in patients with chronic microvascular disease who may benefit from the effects of cilostazol on myelin repair and astrocyte-to-neuron energy supply.13,26,27
Other Phosphodiesterase Inhibitors
Other PDE3 inhibitors include milrinone, vesnarinone and amrinone. These drugs have been used to treat severe congestive heart failure that is refractory to first-line medications.57,58 Milrinone, the most commonly used, inhibits both PDE3 and PDE4, increases cardiac index, has more cardiac side effects than cilostazol, and has been associated with sudden cardiac death and an increase in all-cause mortality with chronic use.59,60 Milrinone is therefore only used for short durations and not a viable alternative to cilostazol for stroke or cardiovascular disease prevention.61
Cilostazol is sometimes compared to dipyridamole, which inhibits PDE5 and has been combined with aspirin for secondary stroke prevention.62 While cilostazol and dipyridamole are both PDE inhibitors with similar systemic side effects (Table 1), their effects within the platelet are unique because they inhibit different PDEs.63 For example, inhibition of PDE5 (dipyridamole) increases cyclic guanosine monophosphate while inhibition of PDE3 increases cAMP. Outside platelets, the drugs also have different pleotropic effects since PDE5 is only expressed by platelets, smooth muscle cells, and the lung, while PDE3 is more widely expressed throughout the human body.1 Because of these differences, results of clinical trials with dipyridamole do not provide insight into cilostazol’s effect on secondary stroke prevention.
Trials of Cilostazol for Secondary Stroke Prevention
With the exception of a small trial (n=57) in the United Kingdom,27 all cilostazol trials for stroke and cardiovascular disease prevention have been conducted in Asia.13 These trials have typically used a cilostazol dose of 100 mg BID. The most important and largest randomized clinical trials are CSPS (2000), CSPS 2 (2010), PICASSO (2018), and CSPS.com (2019).33,64,65 In CSPS, 1,095 patients (65.6% male) who had a non-cardioembolic stroke in the prior 1–6 months were randomized to cilostazol 100 mg BID or placebo in a double-blind design. The primary outcome was ischemic stroke, which occurred less frequently in the cilostazol versus placebo arm (3.37% vs. 5.78% annually, relative risk reduction 0.42, 95% CI 0.09–0.63). Bleeding complications were similar in both arms.65 The PICASSO trial of 1,534 ischemic stroke patients with a history of intracerebral hemorrhage or imaging evidence of two or more cerebral microbleeds also found that cilostazol did not increase the risk of intracerebral hemorrhage.66
In CSPS 2, 2,672 patients who had a non-cardioembolic stroke within the prior 26 weeks were randomized to cilostazol 100 mg BID or aspirin 81 mg daily in a double-blind design and followed for a mean of 29 months. The majority of patients (65%) had a lacunar stroke as the index event and 72% were male.64 The primary outcome in CSPS 2 was stroke, including intraparenchymal and subarachnoid hemorrhage, which occurred less frequently in the cilostazol arm (2.8%) compared to the aspirin arm (3.7%), with a hazard ratio for cilostazol of 0.74 (95% CI 0.56–0.98).
The CSPS.com trial enrolled 1,879 high-risk patients (cervical/intracranial artery stenosis or multiple vascular risk factors) with a non-cardioembolic stroke in the prior 8–180 days. The trial was unblinded and randomized patients who were receiving standard-of-care treatment with aspirin or clopidogrel to cilostazol 100 mg BID versus no additional medication.33 The primary outcome was first recurrence of a symptomatic ischemic stroke and the median follow-up duration was 1.4 years. In CSPS.com, cilostazol was highly effective at preventing recurrent stroke when added to aspirin or clopidogrel monotherapy (hazard ratio 0.49, 95% CI 0.31–0.76). Subgroup analyses showed relatively equal efficacy among all major patient subgroups, with the exception of sex. Female patients experienced less benefit (hazard ratio 0.82, 95% CI 0.37–1.84) than men (hazard ratio 0.40, 95% CI 0.23–0.68), but this difference was not significant (interaction term sex*treatment p=0.13). Since women only represented 30% of the cohort, this could have been due to low power. Cilostazol had a nearly identical risk reduction in the 547 patients with intracranial artery stenosis (hazard ratio 0.48, 95% CI 0.27–0.86) and the 1,177 patients without (hazard ratio=0.47, 95% CI 0.23–0.95, p value for interaction=0.95). In the subgroup of patients with lacunar stroke (n=925), cilostazol had a hazard ratio of 0.41 (95% CI 0.21–0.81).
The most recent and comprehensive meta-analysis of cilostazol for the prevention of ischemic stroke and major adverse cardiovascular events (MACE) in stroke survivors showed that “cilostazol reduced recurrent ischemic stroke, recurrent hemorrhagic stroke, death, and major adverse cardiovascular events compared with control, in the presence or absence of aspirin, or when compared directly with aspirin.”13 (Figure 2) An important finding from this meta-analysis is that cilostazol was most beneficial in trials that treated patients for 6 months or longer. This is consistent with the Kaplan-Meier analysis for the prevention of any stroke in CSPS 2 and ischemic stroke in CSPS.com (Figure 3), which shows that the curves for the cilostazol and control arms separate 60–90 days after randomization. It is also consistent with the neutral results of the ADS trial, which randomized 1,201 patients with non-cardioembolic stroke within 48 hours of symptom onset to cilostazol plus aspirin versus aspirin alone and followed patients for 3 months.67 During this relatively short follow-up period, cilostazol did not reduce stroke recurrence. The delayed onset of benefit is likely a result of an accumulation of the pleiotropic effects of cilostazol over time.
Figure 2.

Meta-analysis combinations13 of cilostazol versus controls for the outcomes of ischemic stroke (top), major adverse cardiovascular events (middle), and hemorrhagic stroke (bottom), showing odds ratios below 1, consistent with the benefit of cilostazol.
*ASA: aspirin, CLP: clopidogrel, CLZ: cilostazol.
Figure 3.
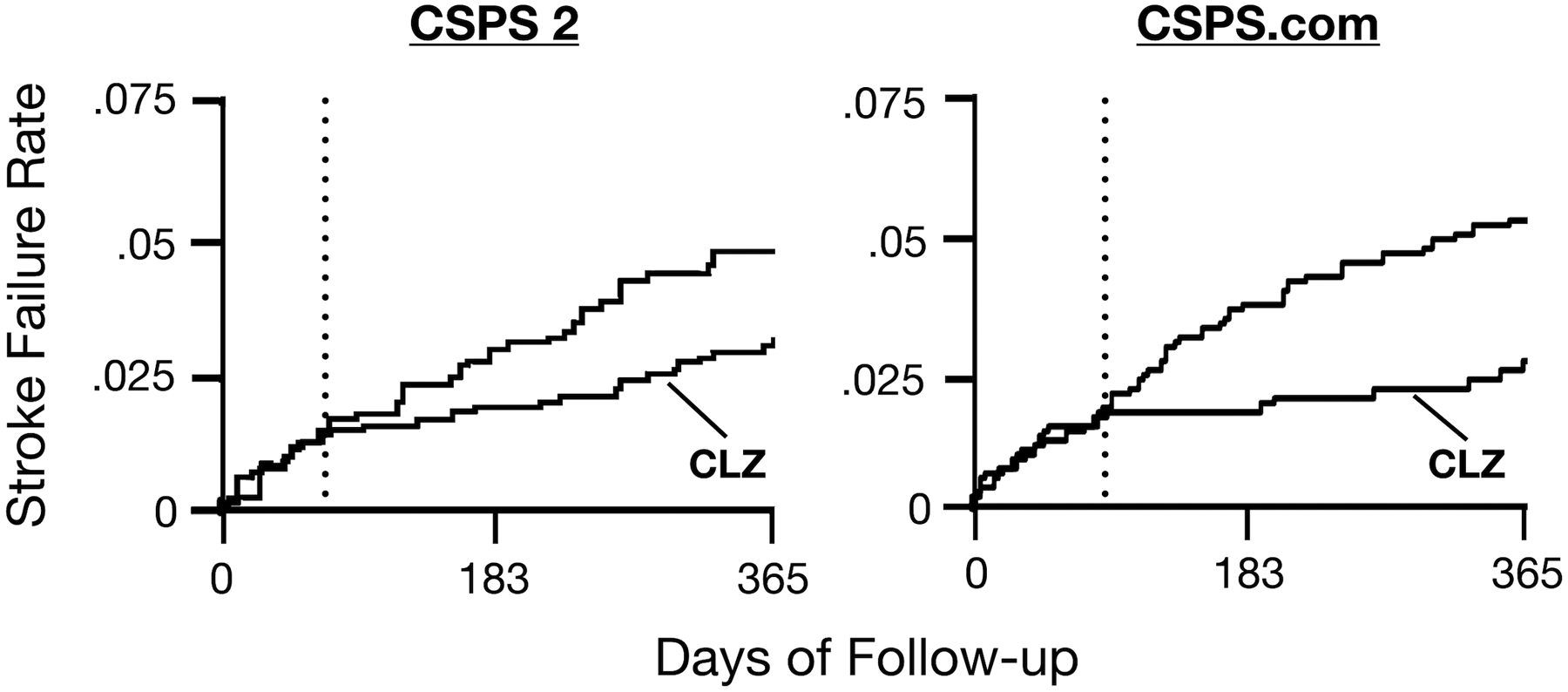
Kaplan-Meier curves for CSPS 2 (left) and CSPS.com (right), showing the failure rate for stroke events in the first year of follow-up and lower rate for patients randomized to cilostazol (CLZ).
Cilostazol Adverse Events, Interactions, and Contraindications
A meta-analysis of eight randomized, placebo-controlled trials of cilostazol among patients with intermittent claudication (n=2,702), identified headache, diarrhea, and abnormal stools as the most common side effects of cilostazol 100 mg BID, which were increased over the placebo arm rates by 19%, 10%, and 10%, respectively.42 Palpitations, dizziness, and tachycardia were also common.68 The adverse events of cilostazol reported in a meta-analysis of secondary stroke prevention trials are listed in Table 2.13 These side effects likely contributed to the increased rate of drug discontinuation in the cilostazol arm of the CSPS.com trial (9.8%) compared to the control arm (3.5%).69 A suggested approach to minimize cilostazol discontinuation is to begin therapy with a lower dose (50 mg twice daily) and titrate to the full 100 mg twice daily dose after 2–4 weeks.70
Table 2.
Adverse events in patients taking cilostazol compared to controls taking antiplatelet therapy.
| Adverse event | Cilostazol (n, %) |
Control (n, %) |
Odds ratio (95% CI) |
|---|---|---|---|
| Major Bleeding | 79/4211, 1.9% |
102/4176, 2.4% |
0.73 (0.54–0.99) |
| Headache | 743/4804, 15.5% |
413/4778, 8.6% |
2.00 (1.76–2.28) |
| Dizziness | 349/3419, 10.2% |
292/3418, 8.5% |
1.22 (1.04–1.44) |
| Palpitations | 281/4566, 6.2% |
124/4581, 2.7% |
2.29 (1.87–2.80) |
| Diarrhea | 303/2434, 12.5% |
126/2403, 5.2% |
2.42 (1.99–2.96) |
| Constipation | 189/2334, 8.1% |
268/2330, 11.5% |
0.68 (0.56–0.82) |
| Nausea | 76/1548, 4.9% |
53/1547, 3.4% |
1.47 (1.02–2.11) |
Cilostazol has potential drug interactions with medications that affect the hepatic P450 enzymes CYP3A4 and CYP2C19. In the United States Food and Drug Administration (FDA) labelling for cilostazol, CYP3A4 and CYP2C19 inhibitors, which decrease cilostazol clearance, such as macrolide antibiotics, diltiazem, azoles, omeprazole, fluoxetine, and sertraline, are identified as having a drug-drug interaction.34 A reasonable approach for patients on these medications would be to decrease the cilostazol dose to 50 mg BID, which is consistent with the labelling for PAD patients.34 Although atorvastatin, fluvastatin, lovastatin, and simvastatin could theoretically decrease cilostazol levels by inhibiting CYP2C19, the effect is not clinically significant.34 Cilostazol has a half-life of 14–15 hours and needs to be dosed twice daily. Cilostazol’s absorption is increased with high-fat meals, which almost doubles the peak effect and reduces the half-life to 5 hours, so it should not be taken within two hours of a meal.71
Because other oral phosphodiesterase inhibitors (e.g. milrinone and vesnarinone – see paragraph above on phosphodiesterase inhibitors for more detail) have caused increased mortality in patients with severe heart failure,60,72 the FDA placed a boxed warning on cilostazol that contraindicates its use in patients with heart failure, which is consistent with a similar contraindication from the European Medicines Agency (EMA).34,73 However, the increase in mortality with milrinone or similar drugs was relatively small (relative risk in a Cochrane Review: 1.17, 95% CI 1.06–1.30)60,74,75, an analysis of the Cilostazol Safety Database did not find an increase in mortality with cilostazol in heart failure patients, and other studies of administrative claims have likewise failed to show an association between cilostazol and mortality among patients with heart failure.74,76,77
Cilostazol’s pharmacokinetics do not appear to be meaningfully altered by patient age or sex,78,79 and data from PAD patients does not suggest that race or ethnicity impacts the effectiveness of cilostazol for reducing claudication symptoms.68,80,81 Genetic polymorphisms in hepatic P450 enzymes can affect cilostazol pharmacokinetics, but not to the degree seen with platelet P2Y12 receptor inhibitors such as clopidogrel.82–84 Although mild hepatic impairment has little effect on cilostazol levels,34 more severe hepatic impairment warrants caution as cilostazol has not been studied in that patient population and its metabolism could be reduced, leading to elevated serum levels.85 Dose adjustment is not recommended in patients with renal impairment, and dialysis is unlikely to affect levels given the very high (≥95%) protein binding of cilostazol.85
Clinical Guideline Recommendations Regarding the Use of Cilostazol
A 2014 Cochrane review of fifteen double-blind randomized trials with a total of 3,718 PAD patients showed that cilostazol 100 mg BID increased walking distance prior to claudication compared to both placebo and to cilostazol 50 mg BID.68 The American Heart Association/American College of Cardiology guideline on peripheral arterial disease states that “cilostazol is an effective therapy to improve symptoms and increase walking distance in patients with claudication” (Class IA recommendation).86 Based off this robust and consistent data, cilostazol has an indication for symptomatic treatment of intermittent claudication in the United States, Japan, Korea, China, the United Kingdom, and many other countries.5
In Japan, cilostazol was recommended as a first line antiplatelet agent for the prevention of recurrent ischemic stroke in 2015.87 In the upcoming 2021 Japan Stroke Society prevention guideline, the addition of cilostazol to treatment with either aspirin or clopidogrel will be recommended as a reasonable option for patients with non-cardioembolic stroke or TIA who have cervical/intracranial artery stenosis or multiple vascular risk factors. Secondary stroke prevention guidelines in Asia give a high level of evidence recommendation for cilostazol in all subtypes of non-cardioembolic stroke.88 In the United States, the 2014 American Heart Association secondary prevention guideline gives cilostazol a Class IIb (Level of Evidence C) recommendation specific to only patients with “stroke or TIA attributable to 50% to 99% stenosis of a major intracranial artery”, which states that “the data are insufficient to make a recommendation regarding the usefulness of … cilostazol.”89 European Stroke Organization guidelines do not mention cilostazol for secondary prevention.90
Cilostazol Prescription Patterns for Stroke Prevention in Western Countries
To understand if, despite the lack of guideline endorsement, neurologists in the United States were prescribing cilostazol, we performed a retrospective analysis of the publicly available Medicare Part D Prescriber Files from 2017–2018.91 We included providers designated as “Neurology” and calculated how many prescribed cilostazol and, as a comparator, clopidogrel. In 2017, only 76/12,995 (0.6%) neurologists prescribed cilostazol at least once to a Medicare Part D beneficiary, while 4,601/12,995 (35.4%) prescribed clopidogrel at least once. In 2018, the number of neurologists who prescribed cilostazol was even lower at 65/13,206 (0.5%), while 4,491/13,206 (34.0%) prescribed clopidogrel at least once.
To explore if patients in the United States with ischemic stroke are being prescribed cilostazol more frequently since CSPS.com, we used data from TriNetX, a global federated health research network providing access to electronic medical records from approximately 69.5 million patients.92 The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No Protected Health Information (PHI) or Personal Data is made available and IRB approval is not required. This analysis included patients diagnosed with ischemic stroke (ICD-10-CM I63)93 from 2017 to the end of 2020. In 2017–18 there were 249,621 patients diagnosed with ischemic stroke, of which 2,490 received cilostazol (1.0%) and 81,455 received clopidogrel (32.6%). In 2019–20, 255,514 patients were diagnosed with ischemic stroke, of which 2,516 received cilostazol (1.0%) and 88,759 received clopidogrel (34.7%). Though this sample is not fully representative, these data suggest that cilostazol prescriptions in United States stroke survivors have not increased since the positive results of the CSPS.com trial were presented in February 2019.
In Europe, the EMA has only recommended cilostazol for claudication symptoms in PAD patients and in 2013 further restricted the indication to patients who had failed lifestyle changes and do not have comorbid cardiovascular disease.73 A study of five European health agency databases from 2002–2012 examined new off-label prescriptions of cilostazol. In the United Kingdom only 1% of off-label cilostazol prescriptions were for cerebrovascular disease, in Spain it was 0.3–2.1%, in Sweden it was 0.3%, and in Germany it was 4.8%.94 These data suggest that in Europe, like in the United States, cilostazol is not widely used in patients with cerebrovascular disease.
Barriers to Cilostazol for Stroke Prevention in the United States and Europe
The fact that the major cilostazol trials conducted thus far have not led to a change in practice patterns or guideline recommendations in Western countries might be because of limitations in their design, surprisingly large effect sizes, and the patients that were studied (exclusively Asians and predominantly male participants). Another factor that might give physicians pause before adopting long-term dual antiplatelet therapy (DAPT) with cilostazol is that prior trials of long-term DAPT after ischemic stroke have not shown an acceptable risk/benefit profile. The MATCH trial, which randomized 7,599 patients with recent ischemic stroke or TIA to either DAPT (aspirin plus clopidogrel) or clopidogrel monotherapy, showed no reduction in the primary MACE outcome (ischemic stroke, myocardial infarction, vascular death, or rehospitalization for acute ischemia including TIA, angina pectoris, or worsening of peripheral arterial disease) during a mean follow-up of 18 months.95 While there was a nonsignificant 7.1% (95% CI −8.5–20.4) relative risk reduction in ischemic stroke, the rate of life-threatening bleeding was 1.3% higher in the DAPT arm compared to the clopidogrel monotherapy arm (p<0.001).
The SPS3 trial compared DAPT (aspirin plus clopidogrel) to aspirin monotherapy in 3,020 patients with recent symptomatic lacunar ischemic stroke.96 The primary outcome was recurrent stroke, including hemorrhagic stroke, and patients were followed for a mean of 3.4 years. Like MATCH, SPS3 showed a nonsignificant lower rate of recurrent ischemic stroke with DAPT (hazard ratio 0.82, 95% CI 0.63–1.09) that was offset by almost twice the rate of major hemorrhage and death with DAPT. The ESPRIT trial compared aspirin plus dipyridamole to aspirin alone (30–325mg daily) in 2,739 patients with recent ischemic stroke who were followed for a mean of 3.5 years.97 The primary outcome in ESPRIT was a MACE composite, which was reduced by 20% (HR 0.80, 95% CI 0.66–0.98). The PRoFESS trial compared aspirin plus dipyridamole to clopidogrel in 20,332 patients with recent ischemic stroke who were followed for a mean of 2.5 years.62 In PRoFESS, aspirin plus dipyridamole was not associated with a significant reduction in recurrent stroke (HR 1.01, 95% CI 0.92–1.11) and resulted in an increase in major hemorrhagic events (hazard ratio 1.15, 95% CI 1.00 to 1.32).
Given the failure of long-term DAPT in large randomized controlled secondary stroke prevention trials, there has been reluctance to test novel long-term DAPT combinations. In recent years, clinical trials have instead focused on short-term DAPT therapy started within days after stroke onset, to balance the secondary stroke prevention benefit with hemorrhagic risk (CHANCE, POINT, and THALES).98–100
While these trials have demonstrated benefit from short-term DAPT after ischemic stroke, they do not address the risk of recurrent ischemic events that are experienced by patients beyond the first month. The PRoFESS and MATCH trials show that stroke patients continue to accumulate MACE events at a nearly linear rate of 4–8% per year during long-term follow-up (Figure 4). Cilostazol could reduce this rate by 34% based on the results of a recent meta-analysis, or 48% based on the results of CSPS.com.13,33 Long-term cilostazol combined with aspirin or clopidogrel does not increase bleeding risk beyond that of either aspirin or clopidogrel alone, and has considerably less risk than the long-term combination of aspirin and clopidogrel.13,95 Thus, the safety and efficacy profile of cilostazol could complement current post-stroke DAPT strategies. After completion of a short (3 weeks to 3 months) course of DAPT with aspirin and clopidogrel or ticagrelor, cilostazol could subsequently be added indefinitely to single antiplatelet treatment.
Figure 4.

The rate of major adverse cardiovascular events (MACE) during follow-up in the PRoFESS and MATCH trials.
Ongoing and Future Trials of Cilostazol
There is ongoing research with cilostazol. LACI-2 (ISRCTN 14911850, EudraCT 2016-002277-35) is studying long-term cilostazol and isosorbide mononitrate in a partial factorial PROBE design after lacunar ischemic stroke in the UK (target n=400)70 and the COMCID trial in Japan is evaluating cilostazol’s effects on cognitive function in patients with mild cognitive impairment at baseline (target n=200).54,91 However, there are no ongoing trials that will provide Level 1 evidence of cilostazol for preventing recurrent stroke and MACE. Positive results of a large placebo-controlled trial conducted outside of Asia and with adequate enrollment of both women and men and careful stroke subtyping is needed to change guidelines in Western countries and determine if cilostazol should become standard of care for secondary prevention after non-cardioembolic stroke.
An alternative to a large RCT is an observational study that compares outcomes between patients who are prescribed cilostazol and those who are not. The limitation of such a design is that there are only small numbers of post-stroke patients who are prescribed cilostazol and that prescription patterns are likely confounded by indication.101 Another alternative is an open-label cluster randomized trial, but such a design would still be costly while not providing the highest level evidence that is lacking. In contrast, a well-designed randomized placebo-controlled trial of cilostazol could provide this evidence. To be most informative such a trial should have the following features: 1) broad inclusion criteria in terms of stroke etiology, 2) stratified randomization (e.g. according to stroke etiology, gender, race/ethnicity, and duration since index stroke), 3) a relatively long follow-up duration (median of at least 2 years) to assess both early and long-term effects, 4) assessment of multiple patient-centric outcomes (e.g. recurrent stroke, dependency, MACE, cognition, and death), and 5) a large enough sample to have sufficient power to detect a relatively modest treatment effect (e.g. a 20% risk reduction for recurrent stroke or MACE would be a clinically relevant finding even though a meta-analysis of prior studies suggests that cilostazol reduces the rate of MACE by 34%). Because cilostazol is currently generic and inexpensive, industry is unlikely to fund such a trial.
The cost of cilostazol may increase dramatically if a pharmaceutical company pursues a branded formulation and is granted market exclusivity by the FDA.102 A cautionary tale is the anti-inflammatory medication colchicine, which is derived from the autumn crocus plant and was used by ancient Egyptians for rheumatism and joint swelling as far back as 1500 BC and as a tablet extract in 19th century Europe.103,104 Generic colchicine continued to be prescribed and was endorsed in guidelines for gout and other indications until in 2009 the FDA granted a Philadelphia pharmaceutical company market exclusivity for a branded form (Colcrys) based off improved labelling and a small trial with 185 gout patients.105–107 The result was an overnight 5,289% price increase, a subsequent decrease in the use of colchicine, and 50 fold increase in the cost of colchicine for Medicare.105,108,109 A 2010 editorial in the New England Journal of Medicine aptly stated “an alternative solution, probably much less expensive, would be for the FDA or the National Institutes of Health to fund trials that address outstanding questions related to widely available drugs such as colchicine”107 and, we would argue, cilostazol.
Conclusion
Cilostazol is a phosphodiesterase III inhibitor with a long track record of safety. In Asian populations, its long-term use has been shown to reduce recurrent stroke and MACE when used alone or in combination with aspirin or clopidogrel for secondary stroke prevention. Cilostazol has a delayed onset of benefit for stroke prevention, which is consistent with its pleotropic effects on platelet aggregation, vascular remodeling, vasoreactivity, and the plasma lipid profile. Given the low cost of cilostazol and the encouraging data from Asian trials, that suggest a very robust treatment effect, it is imperative that a confirmatory trial be conducted in a Western population to demonstrate generalized efficacy. If positive, it would provide the required evidence to support the long-term use of cilostazol for secondary stroke prevention in the millions of patients worldwide who have suffered a non-cardioembolic stroke.
Sources of Funding:
Dr. de Havenon is funded by NIH-NINDS K23NS105924; Dr. Johnston by NIH-NCATS UL1TR003015, NIH-NCATS KL2TR00316, NIH-NINDS U01 NS086872; Dr. Sheth by NIH-NINDS U01NS106513, R01NS11072, R01NR018335, R03NS112859, U24NS107215, U24NS107136, and American Heart Association 17CSA33550004; Dr. Toyoda by the Japan Agency for Medical Research and Development (AMED) 20lk0201094h0002, 20lk0201109h0001. JM Wardlaw is funded by the UK Dementia Research Institute (funded by the UK Medical Research Council, Alzheimer’s Research UK and Alzheimer’s Society) and the Fondation Leducq (16 CVD 05). The research reported in this publication was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures:
Drs. de Havenon, Sheth, and Lansberg report having submitted a concept synopsis to the NINDS of a randomized controlled trial of cilostazol for secondary stroke prevention. Dr. de Havenon has investigator-initiated funding from AMAG and Regeneron pharmaceuticals. Dr. Karen Johnston has investigator-initiated funding from Rivanna Medical, LLC, is on DSMBs for Biogen and is a consultant to Diffusion Pharmaceuticals, Neurotrauma Science LLC, and the FDA. Dr. S. Claiborne Johnston reports research support from AstraZeneca and receives Drub Placebo for NIH-sponsored randomized trial from Sanofi. Dr. Sheth reports funding from Biogen, Novartis, Bard, Hyperfine, Astrocyte, Alva Health, NControl, and is DSMB Chair for Zoll. Dr. Toyoda reports personal fees from Daiichi-Sankyo, Bayer Yakuhin, Bristol-Myers-Squibb, Takeda, and Nippon Boehringer-Ingelheim. Dr. Shoamanesh reports funding from Bayer AG, Daiichi Sankyo, Servier Canada, Portola Pharmaceuticals and Bristol-Myers Squibb. Dr. Wardlaw reports being primary investigator of the LACI-2 trial, which is funded by the British Heart Foundation.
Non-standard Abbreviations and Acronyms
- PDE3
Phosphodiesterase III
- cAMP
Cyclic adenosine monophosphate
References
- 1.Nishi T, Kimura Y, Nakagawa K. [Research and development of cilostazol: an antiplatelet agent]. Yakugaku Zasshi. 2000;120:1247–1260. [DOI] [PubMed] [Google Scholar]
- 2.Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003;9:2289–2302. [DOI] [PubMed] [Google Scholar]
- 3.Pharmaceutical Business Products [Internet].Otsuka Pharmaceutical Co., Ltd. [cited2021 Feb 26];Available from: https://www.otsuka.co.jp/en/pharmaceutical-business/products/ [Google Scholar]
- 4.Cilostazol Prices, Coupons & Savings Tips [Internet].GoodRx. [cited2020 Oct 1];Available from: https://www.goodrx.com/cilostazol
- 5.Cilostazol: Drug information - UpToDate [Internet]. [cited 2021 Feb 26];Available from: https://www.uptodate.com/contents/cilostazol-drug-information?search=pletal&source=panel_search_result&selectedTitle=1~33&usage_type=panel&kp_tab=drug_general&display_rank=1
- 6.www.shop-apotheke.com. Cilostazol-Elpen 100 mg 56 St - shop-apotheke.com [Internet]. www.shop-apotheke.com. [cited2021 Apr 13];Available from: https://www.shop-apotheke.com/arzneimittel/10941689/cilostazol-elpen-100-mg.htm [Google Scholar]
- 7.Hiratsuka M, Hinai Y, Sasaki T, Konno Y, Imagawa K, Ishikawa M, Mizugaki M. Characterization of human cytochrome p450 enzymes involved in the metabolism of cilostazol. Drug Metab Dispos. 2007;35:1730–1732. [DOI] [PubMed] [Google Scholar]
- 8.PubChem. Cilostazol [Internet]. [cited 2020 Oct 1];Available from: https://pubchem.ncbi.nlm.nih.gov/compound/2754
- 9.Liu Y, Fong M, Cone J, Wang S, Yoshitake M, Kambayashi J. Inhibition of adenosine uptake and augmentation of ischemia-induced increase of interstitial adenosine by cilostazol, an agent to treat intermittent claudication. J Cardiovasc Pharmacol. 2000;36:351–360. [DOI] [PubMed] [Google Scholar]
- 10.Sun B, Le SN, Lin S, Fong M, Guertin M, Liu Y, Tandon NN, Yoshitake M, Kambayashi J-I. New mechanism of action for cilostazol: interplay between adenosine and cilostazol in inhibiting platelet activation. J Cardiovasc Pharmacol. 2002;40:577–585. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda Y, Kikuchi M, Murakami H, Satoh K, Murata M, Watanabe K, Ando Y. Comparison of the inhibitory effects of cilostazol, acetylsalicylic acid and ticlopidine on platelet functions ex vivo. Randomized, double-blind cross-over study. Arzneimittelforschung. 1987;37:563–566. [PubMed] [Google Scholar]
- 12.Goto S Cilostazol: Potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atherosclerosis Supplements. 2005;6:3–11. [DOI] [PubMed] [Google Scholar]
- 13.Caroline McHutchison, Blair Gordon W, Appleton Jason P, Chappell Francesca M, Doubal Fergus, Bath Philip M, Wardlaw Joanna M Cilostazol for Secondary Prevention of Stroke and Cognitive Decline. Stroke. 2020;51:2374–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panchal HB, Shah T, Patel P, Albalbissi K, Molnar J, Coffey B, Khosla S, Ramu V. Comparison of on-treatment platelet reactivity between triple antiplatelet therapy with cilostazol and standard dual antiplatelet therapy in patients undergoing coronary interventions: a meta-analysis. J Cardiovasc Pharmacol Ther. 2013;18:533–543. [DOI] [PubMed] [Google Scholar]
- 15.Rondina MT, Weyrich AS. Targeting Phosphodiesterases in Anti-platelet Therapy. Handb Exp Pharmacol. 2012;225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JB, Araki H, Lefer AM. Thromboxane A2, prostacyclin and aspirin: effects on vascular tone and platelet aggregation. Circulation. 1980;62:V19–25. [PubMed] [Google Scholar]
- 17.Dobesh PP, Stacy ZA, Persson EL. Pharmacologic therapy for intermittent claudication. Pharmacotherapy. 2009;29:526–553. [DOI] [PubMed] [Google Scholar]
- 18.Yasunaga K, Mase K. Clinical effects of oral cilostazol on suppression of platelet function in patients with cerebrovascular disease. Arzneimittelforschung. 1985;35:1186–1188. [PubMed] [Google Scholar]
- 19.Igawa T, Tani T, Chijiwa T, Shiragiku T, Shimidzu S, Kawamura K, Kato S, Unemi F, Kimura Y. Potentiation of anti-platelet aggregating activity of cilostazol with vascular endothelial cells. Thromb Res. 1990;57:617–623. [DOI] [PubMed] [Google Scholar]
- 20.Tani T, Sakurai K, Kimura Y, Ishikawa T, Hidaka H. Pharmacological manipulation of tissue cyclic AMP by inhibitors. Effects of phosphodiesterase inhibitors on the functions of platelets and vascular endothelial cells. Adv Second Messenger Phosphoprotein Res. 1992;25:215–227. [PubMed] [Google Scholar]
- 21.Sakurai H, Hanyu H, Sato T, Kume K, Hirao K, Kanetaka H, Iwamoto T. Effects of cilostazol on cognition and regional cerebral blood flow in patients with Alzheimer’s disease and cerebrovascular disease: A pilot study. Geriatrics & Gerontology International. 2013;13:90–97. [DOI] [PubMed] [Google Scholar]
- 22.Lee S-J, Lee JS, Choi MH, Lee SE, Shin DH, Hong JM. Cilostazol improves endothelial function in acute cerebral ischemia patients: a double-blind placebo controlled trial with flow-mediated dilation technique. BMC Neurology. 2017;17:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda U, Ikeda M, Kano S, Kanbe T, Shimada K. Effect of cilostazol, a cAMP phosphodiesterase inhibitor, on nitric oxide production by vascular smooth muscle cells. Eur J Pharmacol. 1996;314:197–202. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Houchi H, Minami A, Sakamoto S, Tsuchiya K, Niwa Y, Minakuchi K, Nakaya Y. Endothelium-dependent relaxation by cilostazol, a phosphodiesteras III inhibitor, on rat thoracic aorta. Life Sci. 2001;69:1709–1715. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto A, Miyakoda G, Hirose Y, Mori T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis. 2006;189:350–357. [DOI] [PubMed] [Google Scholar]
- 26.Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke. 2015;10:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair GW, Appleton JP, Flaherty K, Doubal F, Sprigg N, Dooley R, Richardson C, Hamilton I, Law ZK, Shi Y, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: The LACunar Intervention-1 (LACI-1) trial, a randomised clinical trial. EClinicalMedicine. 2019;11:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Ishikawa T, Hagiwara M, Onoda K, Itoh H, Hidaka H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology. 1988;36:313–320. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi S, Morishita R, Matsushita H, Nakagami H, Taniyama Y, Nakamura T, Aoki M, Yamamoto K, Higaki J, Ogihara T. Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension. 2000;35:237–243. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi S, Oida K, Fujiwara R, Maeda H, Hayashi S, Takai H, Tamai T, Nakai T, Miyabo S. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992;20:900–906. [DOI] [PubMed] [Google Scholar]
- 31.Hadrava V, Kruppa U, Russo RC, Lacourcière Y, Tremblay J, Hamet P. Vascular smooth muscle cell proliferation and its therapeutic modulation in hypertension. Am Heart J. 1991;122:1198–1203. [DOI] [PubMed] [Google Scholar]
- 32.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel J-B. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovascular Research. 2012;95:194–204. [DOI] [PubMed] [Google Scholar]
- 33.Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, Sakai N, Okada Y, Tanaka K, Origasa H, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2019;18:539–548. [DOI] [PubMed] [Google Scholar]
- 34.Cilostazol - FDA prescribing information, side effects and uses [Internet].Drugs.com. [cited 2021 Feb 26];Available from: https://www.drugs.com/pro/cilostazol.html
- 35.Kwon B-J, Lee S-H, Kim D-B, Park H-J, Jang S-W, Ihm S-H, Kim H-Y, Seung K-B. A randomized comparison study assessing the impact of cilostazol on the heart rate and arrhythmias by 24-hour ambulatory holter electrocardiographic monitoring after drug-eluting stent implantation for coronary artery disease. J Atheroscler Thromb. 2015;22:152–164. [DOI] [PubMed] [Google Scholar]
- 36.Appleton JP, Blair GW, Flaherty K, Law ZK, May J, Woodhouse LJ, Doubal F, Sprigg N, Bath PM, Wardlaw JM. Effects of Isosorbide Mononitrate and/or Cilostazol on Hematological Markers, Platelet Function, and Hemodynamics in Patients With Lacunar Ischaemic Stroke: Safety Data From the Lacunar Intervention-1 (LACI-1) Trial. Front. Neurol [Internet].2019. [cited 2021 Mar 3];10. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2019.00723/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asal NJ, Wojciak KA. Effect of cilostazol in treating diabetes-associated microvascular complications. Endocrine. 2017;56:240–244. [DOI] [PubMed] [Google Scholar]
- 38.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab. 2004;89:5577–5582. [DOI] [PubMed] [Google Scholar]
- 39.Zitnik RJ, Zheng T, Elias JA. cAMP inhibition of interleukin-1-induced interleukin-6 production by human lung fibroblasts. Am J Physiol. 1993;264:L253–260. [DOI] [PubMed] [Google Scholar]
- 40.Lee TM, Su SF, Hwang JJ, Tseng CD, Chen MF, Lee YT, Wang SS. Differential lipogenic effects of cilostazol and pentoxifylline in patients with intermittent claudication: potential role for interleukin-6. Atherosclerosis. 2001;158:471–476. [DOI] [PubMed] [Google Scholar]
- 41.Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, Gordon IL, Bortey EB, Forbes WP Effect of the Novel Antiplatelet Agent Cilostazol on Plasma Lipoproteins in Patients With Intermittent Claudication. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1942–1947. [DOI] [PubMed] [Google Scholar]
- 42.Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. American Journal of Cardiology. 2002;90:1314–1319. [DOI] [PubMed] [Google Scholar]
- 43.Motta NAV da, Brito FCF de. Cilostazol exerts antiplatelet and anti-inflammatory effects through AMPK activation and NF-kB inhibition on hypercholesterolemic rats. Fundamental & Clinical Pharmacology. 2016;30:327–337. [DOI] [PubMed] [Google Scholar]
- 44.Motta NAV, Autran LJ, Brazão SC, Lopes R de O, Scaramello CBV, Lima GF, Brito FCF de. Could cilostazol be beneficial in COVID-19 treatment? Thinking about phosphodiesterase-3 as a therapeutic target. International Immunopharmacology. 2021;92:107336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chattipakorn SC, Thummasorn S, Sanit J, Chattipakorn N. Phosphodiesterase-3 inhibitor (cilostazol) attenuates oxidative stress-induced mitochondrial dysfunction in the heart. J Geriatr Cardiol. 2014;11:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang W-H, Lin F-H, Lee C-H, Kuo F-C, Hsieh C-H, Hsiao F-C, Hung Y-J. Cilostazol effectively attenuates deterioration of albuminuria in patients with type 2 diabetes: a randomized, placebo-controlled trial. Endocrine. 2014;45:293–301. [DOI] [PubMed] [Google Scholar]
- 47.Douglas John S, Holmes David R, Kereiakes Dean J, Grines Cindy L, Block Elizabeth, Ghazzal Ziyad MB, Morris Douglas C, Liberman Henry, Parker Karen, Jurkovitz Claudine, et al. Coronary Stent Restenosis in Patients Treated With Cilostazol. Circulation. 2005;112:2826–2832. [DOI] [PubMed] [Google Scholar]
- 48.Godinho J, de Oliveira JN, Ferreira EDF, Zaghi GGD, Bacarin CC, de Oliveira RMW, Milani H. Cilostazol but not sildenafil prevents memory impairment after chronic cerebral hypoperfusion in middle-aged rats. Behavioural Brain Research. 2015;283:61–68. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto S, Shimodozono M, Miyata R, Kawahira K. Effect of Cilostazol Administration on Cerebral Hemodynamics and Rehabilitation Outcomes in Poststroke Patients. International Journal of Neuroscience. 2011;121:271–278. [DOI] [PubMed] [Google Scholar]
- 50.Thomas Zeller, Dietmar Trenk. Cilostazol. Circulation 2013;127:2261–2263.23652862 [Google Scholar]
- 51.Ono K, Tsuji M. Pharmacological Potential of Cilostazol for Alzheimer’s Disease. Front. Pharmacol [Internet].2019[cited2021 Mar 1];10. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2019.00559/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka M, Saito S, Inoue T, Satoh-Asahara N, Ihara M. Potential Therapeutic Approaches for Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Int J Mol Sci [Internet].2020. [cited 2021 Mar 1];21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7139812/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maki T, Okamoto Y, Carare RO, Hase Y, Hattori Y, Hawkes CA, Saito S, Yamamoto Y, Terasaki Y, Ishibashi-Ueda H, et al. Phosphodiesterase III inhibitor promotes drainage of cerebrovascular β-amyloid. Ann Clin Transl Neurol. 2014;1:519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saito S, Kojima S, Oishi N, Kakuta R, Maki T, Yasuno F, Nagatsuka K, Yamamoto H, Fukuyama H, Fukushima M, et al. A multicenter, randomized, placebo-controlled trial for cilostazol in patients with mild cognitive impairment: The COMCID study protocol. Alzheimers Dement (N Y). 2016;2:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J-Y, Lee H, Yoo HB, Choi J-S, Jung H-Y, Yoon EJ, Kim H, Jung Y-H, Lee H-Y, Kim YK. Efficacy of Cilostazol Administration in Alzheimer’s Disease Patients with White Matter Lesions: A Positron-Emission Tomography Study. Neurotherapeutics. 2019;16:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tai S-Y, Chien C-Y, Chang Y-H, Yang Y-H. Cilostazol Use Is Associated with Reduced Risk of Dementia: A Nationwide Cohort Study. Neurotherapeutics. 2017;14:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmerman J, Lee JP, Cahalan M. 25 - Vasopressors and Inotropes[Internet]. In: Hemmings HC, Egan TD, editors. Pharmacology and Physiology for Anesthesia (Second Edition). Philadelphia: Elsevier; 2019. [cited 2021 Feb 26]. p. 520–534.Available from: https://www.sciencedirect.com/science/article/pii/B9780323481106000259 [Google Scholar]
- 58.Amsallem E, Kasparian C, Haddour G, Boissel JP, Nony P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst Rev. 2005;CD002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shakur Y, Fong M, Hensley J, Cone J, Movsesian MA, Kambayashi J-I, Yoshitake M, Liu Y. Comparison of the effects of cilostazol and milrinone on cAMP-PDE activity, intracellular cAMP and calcium in the heart. Cardiovasc Drugs Ther. 2002;16:417–427. [DOI] [PubMed] [Google Scholar]
- 60.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. [DOI] [PubMed] [Google Scholar]
- 61.Ayres JK, Maani CV. Milrinone [Internet]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [cited 2021 Feb 27]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK532943/ [Google Scholar]
- 62.Sacco RL, Diener H-C, Yusuf S, Cotton D, Ôunpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, et al. Aspirin and Extended-Release Dipyridamole versus Clopidogrel for Recurrent Stroke. New England Journal of Medicine. 2008;359:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. British Journal of Clinical Pharmacology. 2011;72:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. The Lancet Neurology. 2010;9:959–968. [DOI] [PubMed] [Google Scholar]
- 65.Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, Shinohara Y, Itoh E, Matsuda T, Sawada T, et al. Cilostazol stroke prevention study: A placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis. 2000;9:147–157. [DOI] [PubMed] [Google Scholar]
- 66.Kim BJ, Lee E-J, Kwon SU, Park J-H, Kim Y-J, Hong K-S, Wong LKS, Yu S, Hwang Y-H, Lee JS, et al. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. The Lancet Neurology. 2018;17:509–518. [DOI] [PubMed] [Google Scholar]
- 67.Aoki J, Iguchi Y, Urabe T, Yamagami H, Todo K, Fujimoto S, Idomari K, Kaneko N, Iwanaga T, Terasaki T, et al. Acute Aspirin Plus Cilostazol Dual Therapy for Noncardioembolic Stroke Patients Within 48 Hours of Symptom Onset. J Am Heart Assoc [Internet].2019. [cited 2021 Mar 4];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6761671/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Cilostazol for intermittent claudication. Cochrane Database of Systematic Reviews [Internet].2014. [cited 2020 Oct 1];Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003748.pub4/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hankey Graeme J CSPS.com Trial of Adding Cilostazol to Antiplatelet Therapy to Reduce Recurrent Stroke. Stroke. 2020;51:696–698. [DOI] [PubMed] [Google Scholar]
- 70.Wardlaw J, Bath PMW, Doubal F, Heye A, Sprigg N, Woodhouse LJ, Blair G, Appleton J, Cvoro V, England T, et al. Protocol: The Lacunar Intervention Trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease. Eur Stroke J. 2020;5:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bramer SL, Forbes WP. Relative Bioavailability and Effects of a High Fat Meal on Single Dose Cilostazol Pharmacokinetics. Clin Pharmacokinet. 1999;37:13–23. [DOI] [PubMed] [Google Scholar]
- 72.Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, Gottlieb SO, McGrew F, DeMets DL, White BG. A Dose-Dependent Increase in Mortality with Vesnarinone among Patients with Severe Heart Failure. New England Journal of Medicine. 1998;339:1810–1816. [DOI] [PubMed] [Google Scholar]
- 73.Anonymous. Cilostazol-containing medicines [Internet].European Medicines Agency. 2018. [cited 2021 Apr 10];Available from: https://www.ema.europa.eu/en/medicines/human/referrals/cilostazol-containing-medicines
- 74.Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: The CASTLE study (Cilostazol: A Study in Long-term Effects). Journal of Vascular Surgery. 2008;47:330–336.e2. [DOI] [PubMed] [Google Scholar]
- 75.Classes of Heart Failure [Internet].www.heart.org. [cited 2021 Feb 27];Available from: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure
- 76.Wu C-K, Lin J-W, Wu L-C, Chang C-H. Risk of Heart Failure Hospitalization Associated With Cilostazol in Diabetes: A Nationwide Case–Crossover Study. Front Pharmacol [Internet].2019. [cited 2021 Feb 27];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6330376/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pratt CM. Analysis of the cilostazol safety database. Am. J. Cardiol 2001;87:28D–33D. [DOI] [PubMed] [Google Scholar]
- 78.Jung YS, Chae D, Park K. Population pharmacodynamics of cilostazol in healthy Korean subjects. Transl Clin Pharmacol. 2018;26:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee D, Lim LA, Jang SB, Lee YJ, Chung JY, Choi JR, Kim K, Park JW, Yoon H, Lee J, et al. Pharmacokinetic comparison of sustained- and immediate-release oral formulations of cilostazol in healthy Korean subjects: a randomized, open-label, 3-part, sequential, 2-period, crossover, single-dose, food-effect, and multiple-dose study. Clin Ther. 2011;33:2038–2053. [DOI] [PubMed] [Google Scholar]
- 80.Kallirroi Kalantzi, Nikolaos Tentolouris, Melidonis Andreas J., Papadaki Styliani, Peroulis Michail, Amantos Konstantinos A., Andreopoulos George, Bellos George I., Boutel Dimitrios, Bristianou Magdalini, et al. Efficacy and Safety of Adjunctive Cilostazol to Clopidogrel‐Treated Diabetic Patients With Symptomatic Lower Extremity Artery Disease in the Prevention of Ischemic Vascular Events. Journal of the American Heart Association. 2021;10:e018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, Forbes WP. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. Journal of Vascular Surgery. 1998;27:267–275. [DOI] [PubMed] [Google Scholar]
- 82.Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoo H-D, Cho H-Y, Lee Y-B. Population pharmacokinetic analysis of cilostazol in healthy subjects with genetic polymorphisms of CYP3A5, CYP2C19 and ABCB1. Br J Clin Pharmacol. 2010;69:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee H-I, Byeon J-Y, Kim Y-H, Lee C-M, Choi C-I, Jang C-G, Bae J-W, Lee YJ, Lee S-Y. Effects of CYP2C19 and CYP3A5 genetic polymorphisms on the pharmacokinetics of cilostazol and its active metabolites. Eur J Clin Pharmacol. 2018;74:1417–1426. [DOI] [PubMed] [Google Scholar]
- 85.Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs. 2003;3:117–138. [DOI] [PubMed] [Google Scholar]
- 86.Gerhard-Herman Marie D, Gornik Heather L, Barrett Coletta, Barshes Neal R, Corriere Matthew A, Drachman Douglas E, Fleisher Lee A., Fowkes Francis Gerry R., Hamburg Naomi M, Kinlay Scott, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toyoda K, Inoue M, Koga M. Small but Steady Steps in Stroke Medicine in Japan. J Am Heart Assoc. 2019;8:e013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JS, Kwon SU, Uchiyama S. Cilostazol research in Asia: can it be applied to European and American patients? International Journal of Stroke. 2015;10:1–9. [DOI] [PubMed] [Google Scholar]
- 89.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack. Stroke. 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 90.ESO Guideline Directory [Internet].European Stroke Organisation. [cited2021 Apr 9];Available from: https://eso-stroke.org/guidelines/eso-guideline-directory/
- 91.Medicare Provider Utilization and Payment Data: Part D Prescriber | CMS [Internet]. [cited 2020 Jan 20];Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Part-D-Prescriber
- 92.TriNetX [Internet].TriNetX. [cited2021 Feb 16];Available from: https://trinetx.com/coronavirus/
- 93.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of Diagnostic Codes for Acute Stroke in Administrative Databases: A Systematic Review. PLoS One [Internet].2015. [cited 2020 Feb 2];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4546158/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castellsague J, Perez‐Gutthann S, Calingaert B, Bui C, Varas‐Lorenzo C, Arana A, Prados‐Torres A, Poblador‐Plou B, Gonzalez‐Rubio F, Giner‐Soriano M, et al. Characterization of new users of cilostazol in the UK, Spain, Sweden, and Germany. Pharmacoepidemiology and Drug Safety. 2017;26:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diener H-C, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht H-J, MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 96.SPS3 Investigators, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N. Engl. J. Med 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. The Lancet. 2006;367:1665–1673. [DOI] [PubMed] [Google Scholar]
- 98.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. New England Journal of Medicine. 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. New England Journal of Medicine. 2020;383:207–217. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. New England Journal of Medicine. 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 101.Kyriacou DN, Lewis RJ. Confounding by Indication in Clinical Research. JAMA. 2016;316:1818–1819. [DOI] [PubMed] [Google Scholar]
- 102.Sahragardjoonegani B, Beall RF, Kesselheim AS, Hollis A. Repurposing existing drugs for new uses: a cohort study of the frequency of FDA-granted new indication exclusivities since 1997. Journal of Pharmaceutical Policy and Practice. 2021;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graham W, Roberts JB. Intravenous Colchicine in the Management of Gouty Arthritis *. Ann Rheum Dis. 1953;12:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Research & Therapy. 2006;8:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer H The High Price of FDA Approval [Internet]. Kaiser Health News. 2009. [cited 2021 Mar 2];Available from: https://khn.org/news/fda-approval/ [Google Scholar]
- 106.Research C for DE and. Colchicine (marketed as Colcrys) Information. FDA [Internet].2018. [cited 2021 Mar 2];Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/colchicine-marketed-colcrys-information [Google Scholar]
- 107.Kesselheim AS, Solomon DH. Incentives for Drug Development — The Curious Case of Colchicine. New England Journal of Medicine. 2010;362:2045–2047. [DOI] [PubMed] [Google Scholar]
- 108.2,000% Drug Price Surge Is a Side Effect of FDA Safety Program [Internet].Bloomberg.com.2015. [cited 2021 Mar 2];Available from: https://www.bloomberg.com/news/articles/2015-10-06/2-000-drug-price-surge-is-a-side-effect-of-fda-safety-program
- 109.Kesselheim AS, Franklin JM, Kim SC, Seeger JD, Solomon DH. Reductions in Use of Colchicine after FDA Enforcement of Market Exclusivity in a Commercially Insured Population. J Gen Intern Med. 2015;30:1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]


