Abstract
Background:
Ischemia with no obstructive coronary artery disease (INOCA) is common and has an adverse prognosis. We set out to describe the natural history of symptoms and ischemia in INOCA.
Methods:
CIAO-ISCHEMIA (Changes in Ischemia and Angina over One year in ISCHEMIA trial screen failures with INOCA) was an international cohort study conducted from 2014–2019 involving angina assessments (Seattle Angina Questionnaire [SAQ]) and stress echocardiograms 1-year apart. This was an ancillary study that included patients with history of angina who were not randomized in the ISCHEMIA trial. Stress-induced wall motion abnormalities were determined by an echocardiographic core laboratory blinded to symptoms, coronary artery disease (CAD) status and test timing. Medical therapy was at the discretion of treating physicians. The primary outcome was the correlation between changes in SAQ Angina Frequency score and change in echocardiographic ischemia. We also analyzed predictors of 1-year changes in both angina and ischemia, and compared CIAO participants with ISCHEMIA participants with obstructive CAD who had stress echocardiography before enrollment, as CIAO participants did.
Results:
INOCA participants in CIAO were more often female (66% of 208 vs. 26% of 865 ISCHEMIA participants with obstructive CAD, p<0.001), but the magnitude of ischemia was similar (median 4 ischemic segments [IQR 3–5] both groups). Ischemia and angina were not significantly correlated at enrollment in CIAO (p=0.46) or ISCHEMIA stress echocardiography participants (p=0.35). At 1 year, the stress echocardiogram was normal in half of CIAO participants and 23% had moderate or severe ischemia (≥3 ischemic segments). Angina improved in 43% and worsened in 14%. Change in ischemia over one year was not significantly correlated with change in angina (rho=0.029).
Conclusions:
Improvement in ischemia and improvement in angina were common in INOCA, but not correlated. Our INOCA cohort had a similar degree of inducible wall motion abnormalities to concurrently enrolled ISCHEMIA participants with obstructive CAD. Our results highlight the complex nature of INOCA pathophysiology and the multifactorial nature of angina.
Keywords: INOCA, stress testing, angina
Introduction
Among stable patients referred to cardiac catheterization for evaluation of suspected coronary artery disease (CAD), 20–65% have no obstructive stenoses.1, 2 When documented myocardial ischemia occurs with no coronary stenoses ≥50%, the diagnosis of Ischemia with No Obstructive Coronary Artery disease (INOCA) is established. This condition has been associated with (a) an increased risk of death, myocardial infarction and stroke, compared to the general population,1, 3–5 especially in those who remain symptomatic6 or have mild-moderate atherosclerosis,7,8 (b) persistent chest pain by 1 year in half of patients, with a 20% 5-year risk of hospitalization for chest pain, and (c) high healthcare costs, estimated at nearly $770,000 lifetime cost per patient.9 Despite these adverse outcomes, the underlying mechanisms responsible for symptoms and ischemia are unclear; impaired coronary flow reserve (CFR) and/or inducible coronary artery spasm have been documented in some patients.10–14 Event rates may be higher in patients with evidence of inducible ischemia, e.g., on stress echocardiography, though this was not the case in a large European series.7, 8, 15–17 Whether myocardial ischemia is solely responsible for angina symptoms in this population is uncertain. The treatment for patients with INOCA is highly variable in clinical practice, with guidelines focusing on symptom management.18,19, 20 Better understanding of the relationship between ischemia and health status (i.e., symptoms, physical functioning and quality of life) in patients with INOCA is needed.
To address this gap, we leveraged the enrollment process of the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial, which recruited patients with moderate or severe inducible ischemia. To assess trial eligibility, coronary CT angiography (CCTA) was performed in most participants, of whom 21% had no obstructive CAD meeting criteria for randomization, and were excluded.21 These participants were eligible for the Changes in Ischemia and Angina over One year among ISCHEMIA trial screen failures with no obstructive CAD on CCTA (CIAO-ISCHEMIA, “CIAO”) ancillary study, funded separately by the National Heart, Lung, and Blood Institute. The main objective of CIAO were to analyze the natural history of symptoms and ischemia in INOCA patients. We compared clinical characteristics of patients with INOCA to those with obstructive CAD, collected in a unified trial program. We also explored relationships among symptoms, ischemia, and medication use.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CIAO participants underwent stress echocardiography 1 year after the initial stress echocardiogram done to qualify participants for the ISCHEMIA trial. CIAO participants were also assessed for angina-related health status using the Seattle Angina Questionnaire at enrollment and at 6 months and 1 year from the initial stress echocardiogram.
We compared clinical characteristics, quality of life and stress echocardiographic findings between CIAO participants and the subset of ISCHEMIA participants who were randomized in ISCHEMIA after a CCTA demonstrated obstructive CAD (Figure 1). This comparison aimed to investigate how the associations of ischemia and symptoms might differ between patients with and without obstructive CAD. Within CIAO, we analyzed the relationships between symptoms, ischemia and atherosclerosis severity on CCTA, and examined changes in angina severity and ischemia severity over 1 year. We also compared the 1-year rate of clinical endpoint events between CIAO and ISCHEMIA participants.
Figure 1. CONSORT diagram.
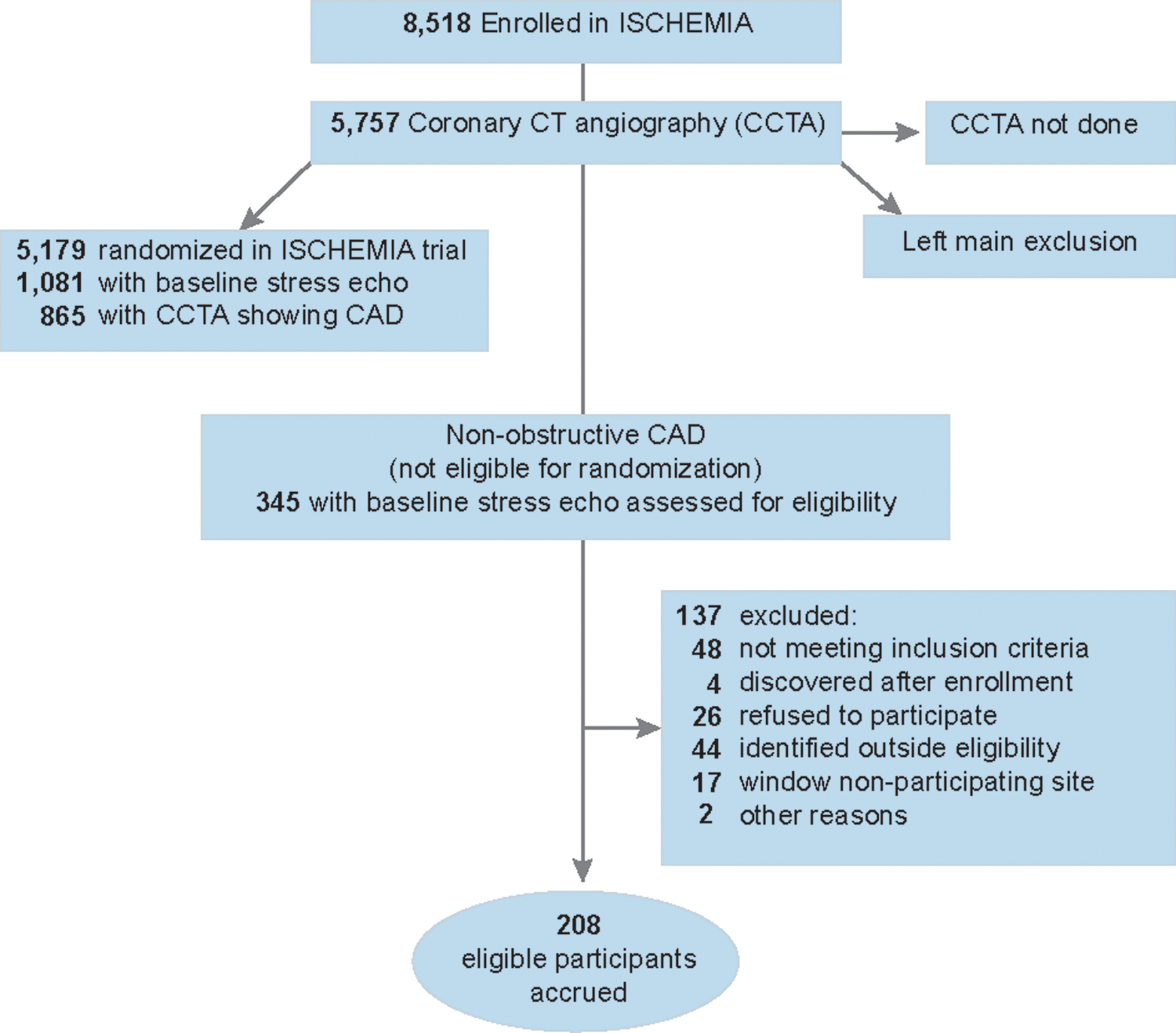
CIAO eligibility was restricted to stress echocardiography to avoid the additional radiation exposure required for stress nuclear imaging in follow up. Stress CMR was used infrequently in ISCHEMIA and was not included in CIAO to minimize heterogeneity. The most common reasons for clinical ineligibility were prior revascularization indicating history of obstructive CAD (n=12), normal stress echocardiogram (n=10) and no ischemic symptoms (n=7). ISCHEMIA stress echocardiography participants were enrolled between July 2012-January 2018 in 21 (of 37 total) countries. Most, but not all ISCHEMIA participants had CCTA done before randomization. Among 1,081 randomized participants who had stress echo, 865 had CCTA showing obstructive CAD and 216 did not undergo CCTA. Recruitment was completed when all candidates for CIAO enrollment among ISCHEMIA trial screen failures had been considered. SAQ was missing at baseline in 5 and at 1 year in 11. 1-year stress echocardiography was missing in 16 (not mutually exclusive from missing SAQ).
The primary outcome was the correlation between change in angina and the change in the number of ischemic segments on stress echocardiography over 1 year. The study was approved by the NYU Grossman School of Medicine Institutional Review Board, and by each site’s local institutional review board or ethics committee. All patients provided informed consent.
Participant Eligibility
CIAO.
Patients with chronic stable ischemia who were ineligible for ISCHEMIA due to non-obstructive atherosclerosis or entirely normal arteries on CCTA were considered for CIAO if they had ischemic symptoms before or during stress echocardiography21 (Figure 1). Ischemic symptoms could include chest pain or anginal equivalent symptoms such as shortness of breath. We restricted CIAO to patients who had stress echocardiography to eliminate the potential for heterogeneity from different stress testing modalities at various sites.
ISCHEMIA trial comparator group.
Inclusion criteria for the ISCHEMIA trial appear in Table I in the Supplement.22 For comparison to patients enrolled in CIAO, we considered ISCHEMIA trial participants randomized to either arm who had been enrolled after a qualifying stress echocardiogram and who had at least 50% stenosis of a coronary artery (“obstructive CAD”) on CCTA. This approach maximized comparability of ischemia testing between patients with and without obstructive CAD. This comparison was included here to put into context the CIAO results with findings in patients with obstructive CAD who had the same type of qualifying stress test, stress echocardiography.
Stress Echocardiographic Ischemia Assessment
Stress echocardiograms were interpreted at the Massachusetts General Hospital core laboratory, blinded to clinical parameters, stress ECG and CCTA results. The core laboratory was blinded as to whether participants were enrolled in ISCHEMIA or CIAO, which was possible because the same study number scheme was used for both. Further, CIAO 1-year stress echocardiograms were analyzed while the ISCHEMIA trial was still enrolling, hence the core laboratory was unaware if a test was a CIAO or ISCHEMIA qualifying stress test or a CIAO follow-up examination. Ischemia severity was defined by the number of segments with stress-induced moderate or severe wall motion abnormalities.23 The ISCHEMIA trial protocol did not include repeat stress testing. Stress wall motion score index was calculated as follows: each of 16 left ventricular segments was associated a wall motion score on post-stress imaging (1 = normal or hyperkinetic, 2 = hypokinetic, 3 = akinetic, 4 = dyskinetic) and scores were averaged over the segments.24
To assess inter- and intra-observer variability at the core laboratory, the core laboratory director read 62 stress echocardiograms (36 from enrollment, 26 follow-up) a second time, and a second reader interpreted 12 stress echocardiograms, all while blinded to the initial reading. We tested the null hypothesis of no systematic differences in the inter- and intra-observability agreement measurements using McNemar’s exact test.25 Intra-observer agreement at the core laboratory was 96.8% for positive vs. negative stress echocardiography, and 95.1% for moderate or severe vs. mild ischemia. There was no evidence for systematic differences between readings (p=0.50 for positive vs. negative and p=0.25 for moderate or severe ischemia). Inter-observer agreement for positive vs. negative stress echocardiography and for moderate or severe vs. mild ischemia was 100%.
Health Status Assessments
CIAO participants had assessment of their angina at enrollment, 6 months and at 1 year using the 7-item Seattle Angina Questionnaire (SAQ), which quantifies patients’ symptoms of angina, physical limitations due to angina and angina-related quality of life over the past month.26 The 3 domains are averaged to define a SAQ Summary Score capturing all domains in the 4 weeks preceding enrollment. The 6-month visit was not required. Scores range from 0–100 with higher scores indicating fewer symptoms and better health status. The Rose Dyspnea Questionnaire and EQ-5D were also obtained.27, 28
Clinical Outcomes
We collected the following site-reported events over 1 year of follow up: myocardial infarction, unstable angina, heart failure, stroke, resuscitated cardiac arrest, death, chest pain hospitalization. These events were defined as in the ISCHEMIA trial29 with the addition of chest pain hospitalization, which was defined as an overnight stay in a hospital with the discharge diagnosis of chest pain.
Statistical Analyses
We computed descriptive statistics of baseline clinical characteristics, medications, stress test results and symptoms, presented as median and inter-quartile range (IQR), or frequency and percentage, as appropriate. Categories for each variable are shown in the tables. We evaluated differences in study variables between patients with INOCA and obstructive CAD using the Wilcoxon rank-sum test or Student’s t-test (as appropriate) for continuous variables, and the Pearson’s chi-square test or McNemar’s test (as appropriate) for categorical variables. Findings on stress echocardiography and symptoms were similarly compared between enrollment and 1 year, and enrollment, 6 months, and 1 year, respectively. Correlations were measured using Spearman correlation coefficients.
To assess the relationship of symptoms, degree of ischemia, and medication usage in CIAO participants, we examined change in SAQ Summary Score and the SAQ Angina Frequency and Physical Limitation subscale scores as binary outcomes based on clinically relevant definitions of improvement (≥5 points for summary score and ≥10 for subscale scores)30 and continuously, measured as the difference between scores at follow-up and enrollment. Similarly, we examined ischemia on stress echocardiography in a continuous and a binary outcome setting. We calculated the change in the number of ischemic segments as the difference between 1 year and enrollment. We defined binary variables of improvement: reduction by at least 2 ischemic segments; and normalization, defined as zero ischemic segments at 1 year.
For continuously measured SAQ scores (at enrollment, 6 months and 1 year), we used linear mixed models with patient as the cluster variable to estimate associations with the change in number of ischemic segments over 1 year. We fit three sets of models in which we sequentially adjusted for covariates. In the first, we adjusted for visit timing, the number of ischemic segments at enrollment and the change in the number of ischemic segments over time. In the second, we controlled for hypothesized confounders, namely, age, sex, hypertension, diabetes, and segment stenosis score as a measure of non-obstructive atherosclerosis severity on CCTA.31 Finally, we included medications which, to mitigate the limitations of small sample sizes, we grouped into the following classes: beta blockers, anti-anginal medications other than beta blockers (e.g., calcium channel blockers, long-acting nitrates and ranolazine), angiotensin converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB), and statins. Then, we constructed categories to represent patterns of use. Each medication class was defined as a binary variable equal to 1 if a patient was always on medications in the class over follow-up, or started taking them by the 1-year visit; and 0 if a patient never used the medication class during the study period, or stopped it by the 1-year visit. Binary outcomes of improvement in symptoms were analogously analyzed using logistic regression.
For continuously measured number of ischemic segments (measured at 1 year), we used linear regression modeling of the change from enrollment and logistic regression modeling of the binary outcomes of improvement. In initial modeling, we assessed the association of the number of ischemic segments with medication usage by including the medication classes (as previously defined) simultaneously. Then, we extended the models to adjust for patient-level risk factors as noted above.
For regression analyses, we created multiply imputed datasets using classification and regression tree methods in the MICE package in R32 and pooled results from each dataset together using Rubin’s formula. Descriptive analyses include available data, whereas results from regression analyses are based on the multiply imputed datasets.
Power was projected at 80% to detect a difference in median SAQ Summary Score of ≥8.5 points between groups of participants with vs. without ischemia improvement over 1 year, for 198 paired assessments, with an assumed standard deviation of 20 based on the prior literature.33 Ischemia improvement was defined as reduction by at least 2 in the number of ischemic segments from enrollment to 1 year. The null hypothesis was that change in SAQ would not be correlated with ischemia improvement in CIAO participants, and the alternative hypothesis was that these two change parameters would be significantly correlated. Alpha was set at 0.05, with two-sided testing for all analyses.
The time to event was calculated from ISCHEMIA enrollment for both CIAO and ISCHEMIA participants, since this was a time point common to both cohorts.
Results
Patient Characteristics: CIAO (INOCA) and ISCHEMIA (Obstructive CAD)
There were 208 eligible INOCA participants enrolled in CIAO between July 2014-July 2018 at 39 sites in 11 countries (Figure 1). These were compared to 865 ISCHEMIA randomized trial participants with obstructive CAD on study CCTA who underwent echocardiographic stress testing.
INOCA participants were much more likely to be female than obstructive CAD participants who qualified by stress echocardiography (66% vs. 26%, p<0.001, Table 1). INOCA participants were younger (median 63 years, interquartile range [IQR 56–70] years vs. 66 years [IQR 59–72], p=0.001) and were more likely to be non-Hispanic Whites and less likely to be non-Hispanic Asians. INOCA participants were less likely to have diabetes, prior MI and current or former smoking history but more likely to have history of depression. On study CCTA, 23% had no evident atherosclerosis and 77% had non-obstructive atherosclerosis.
Table 1.
Demographics and clinical history in INOCA participants in CIAO and in ISCHEMIA stress echocardiography participants with obstructive CAD on coronary CT angiography
| INOCA N=208 |
Obstructive CAD N=865 | p | |
|---|---|---|---|
| Age, years (median (Q1,Q3)) | 62.5 (56.0, 70.0) | 65.7 (59.4, 71.7) | 0.001 |
| Female Sex | 137 (66%) | 221 (26%) | <0.001 |
| Race/Ethnicity | N=208 | N=824 | |
| Non-Hispanic White (n, %) | 151 (73%) | 434 (53%) | <0.001 |
| Non-Hispanic Asian (n, %) | 14 (7%) | 151 (18%) | |
| Non-Hispanic Black (n, %) | 10 (5%) | 17 (2%) | |
| Hispanic (n, %) | 25 (12%) | 217 (26%) | |
| Other (n, %) | 8 (4%) | 5 (0.6%) | |
| Body Mass Index, median (Q1,Q3) | n=195 28.7 (25.4, 32.9) |
n=852 28.1 (25.6, 31.4) |
0.09 |
| Estimated glomerular filtration rate (ml/min), median (Q1,Q3) | 86 (76,97) | 86 (72,100) | 0.52 |
| Hypertension n (%) | 132/207 (64%) | 585/857 (68%) | 0.25 |
| Diabetes (%) | 40/208 (19%) | 281/865 (33%) | <0.001 |
| Cigarette Smoking (n, %) | n=181 | n=864 | |
| Never Smoked (%) | 107 (59%) | 380 (44%) | 0.001 |
| Former Smoker (%) | 53 (29%) | 348 (40%) | |
| Current Smoker (%) | 21 (12%) | 136 (16%) | |
| Prior MI (%) | 4/208 (2%) | 129/860 (15%) | <0.001 |
| Prior HF (%) | 5/207 (2%) | 21/865 (2%) | >0.99 |
| Valvular heart disease (%) | 9/204 (4%) | 21/809 (3%) | 0.26 |
| Atrial fibrillation or flutter (%) | 9/206 (4%) | 19/864 (2%) | 0.13 |
| Chronic lung disease (%) | 9/206 (4%) | 69/865 (8%) | 0.10 |
| Malignancy (%) | 8/206 (4%) | 48/864 (6%) | 0.43 |
| Anxiety (%) | 27/206 (13%) | NA | NA |
| Depression (%) | 40/206 (19%) | 80/861 (9%) | <0.001 |
| Gastroesophageal reflux disease (%) | 33/206 (16%) | NA | NA |
| Cardiac catheterization before ISCHEMIA enrollment (%) | 9/208 (4%) | 214/865 (25%) | <0.001 |
| Time between stress echo and enrollment in ISCHEMIA, days, median (Q1,Q3) | n=206 10 (3–23) |
n=865 11 (4–26) |
0.61 |
| Time between enrollment in ISCHEMIA and CIAO, days, median (Q1,Q3) | n=208 41 (24–91) |
NA | NA |
| Number of segments with stress-induced moderate or severe wall motion abnormalities, median (Q1,Q3) | 4 (3–5) | 4 (3–5) | 0.012 |
| Stress wall motion score index | N=206 1.2 (1.1, 1.2) |
N=865 1.3 (1.1,1.5) |
<0.001 |
| Ischemia Location | n=208 | n=865 | |
| Anterior | 91 (44%) | 304 (35%) | 0.027 |
| Inferior | 87 (42%) | 662 (77%) | <0.001 |
| Lateral | 68 (33%) | 571 (66%) | <0.001 |
| Stress type | |||
| Exercise | 167/205 (82%) | 621/760 (82%) | >0.99 |
| Symptoms during stress | n=200 | n=721 | <0.001 |
| No | 91 (45.5%) | 285 (40%) | 0.15 |
| Yes | 109 (54.5%) | 436 (61%) | |
| Limiting Exertional Chest pain | 21 (19%) | 122 (29%) | 0.06 |
| Non-Limiting Exertional Chest pain | 22 (20%) | 138 (32%) | 0.02 |
| Dyspnea | 49 (45%) | 123 (29%) | 0.002 |
| Claudication | 1 (1%) | 4 (1%) | >0.99 |
| Other | 15 (14%) | 33 (8%) | 0.08 |
| Unknown | 1 (1%) | 6 (1%) | >0.99 |
NA=not applicable
Indications for initial stress testing in CIAO participants (not mutually exclusive; total is greater than 100%) were: typical angina (n=104, 50%), atypical chest pain (n=67, 32%), shortness of breath (n=102, 49%), discomfort in the arm, neck, jaw or throat (n=17, 8%), abdominal discomfort (n=6, 3%), fatigue (n=29, 14%), nausea or other gastrointestinal upset (n=5, 2%), sweating (n=9, 4%), screening with no symptoms (n=4, 2%), other (n=3, 1%).
Indication for initial stress testing was not collected in ISCHEMIA participants.
Indications for Stress Echocardiography
The most common indication for initial stress testing in CIAO was chest pain, in 77%. Indications for initial stress testing were (not mutually exclusive): typical chest pain (n=104, 50%), atypical chest pain (n=67, 32%), shortness of breath (n=102, 49%), discomfort in the arm, neck, jaw or throat (n=17, 8%), abdominal discomfort (n=6, 3%), fatigue (n=29, 14%), nausea or other gastrointestinal upset (n=5, 2%), sweating (n=9, 4%), screening with no symptoms (n=4, 2%), other (n=3, 1%, Table 1). The indication for initial stress testing was not collected in ISCHEMIA participants. Only 18 stress echocardiograms were performed for screening or other reasons.
Medication Use at Enrollment
At the time of enrollment, 122 INOCA participants (59%) were taking a beta blocker (Table II in the Supplement) and 57 (28%) any antianginal medication other than beta blocker. At enrollment, 41% were taking ACEI or ARB, 82% a statin, and 78% an antiplatelet agent or anticoagulant. Laboratory values are shown in Table II in the Supplement.
Stress Echocardiography Findings at Enrollment
The median number of ischemic segments on the qualifying stress echocardiogram was 4 (IQR 3–5, Table 2, Figure 2) in both INOCA and obstructive CAD participants from ISCHEMIA, with more obstructive CAD participants having very severe ischemia (p=0.012, Figure 2). Anterior ischemia was frequent, and was less common with obstructive CAD (35% vs. 44% of INOCA, p=0.027). The enrollment test utilized exercise in 167 (82%) INOCA patients and 621 (82%) CAD patients (Table 1). A sample INOCA case showing severe ischemia at enrollment is shown in Videos A and B.
Table 2.
Findings on stress echocardiography at enrollment and at 1 year in INOCA patients in CIAO
| INOCA enrollment N=208 |
INOCA 1-year N=194 |
p enrollment vs. 1 year | |
|---|---|---|---|
| Number of ischemic segments, median (Q1,Q3) | 4 (3,5) | 0 (0,2) | <0.001 |
| Stress wall motion score index, median (Q1, Q3) | N=206 1.2 (1.1, 1.2) |
N=192 1.0 (1.0, 1.1) |
<0.001 |
| Type of stress* | n=205 | n=193 | 0.013 |
| Dobutamine (%) | 40 (100%) | 4 (22%) | |
| Exercise (%) | 167 (81%) | 149 (77%) | |
| Peak heart rate | n=201 148 (137,158) |
n=192 144 (131,157) |
0.18 |
| % of maximal predicted heart rate achieved, median (Q1,Q3) | 91.6 (86.3, 98.6) | 89.5 (85.0, 98.7) | 0.014 |
| Peak heart rate ≥ 85% of maximal predicted heart rate | 163 (81%) | 144 (75%) | 0.049 |
| Baseline systolic blood pressure | n=194 140 (123,150) |
n=193 133 (120,149) |
0.16 |
| Baseline diastolic blood pressure | n=165 80 (76,89) |
n=193 80 (72,89) |
0.003 |
| Peak systolic blood pressure | n=195 170 (150,190) |
n=193 170 (150,191) |
0.57 |
| Peak diastolic blood pressure | n=167 83 (79,93) |
n=193 84 (73,104) |
0.17 |
| Exercise testing parameters | n=167 | n=149 | |
| Standard Bruce protocol (%) | 116 (69%) | 112 (75%) | 0.50 |
| Average time on standard Bruce protocol (seconds), median (Q1, Q3) | n=116 364 (282, 503) |
n=110 410 (302, 511.5) |
0.003 |
| Peak METs achieved | n=157 7.0 (6.0, 9.3) |
n=123 7.8 (6.3,10.1) |
0.065 |
| ST segment depression present (%) | 60/206 (29%) | 41/194 (21%) | <0.001 |
| Peak severity of ST segment depression, mm, median (Q1, Q3) | n=60 1.00 (1.00,1.70) |
n=41 1.00 (1.00,1.50) |
0.92 |
| Echocardiographic location of ischemia | n=208 | n=194 | |
| Anterior ischemia (%) | 91 (44%) | 28 (15%) | <0.001 |
| Moderate anterior ischemia (≥3 ischemic segments in anterior territory, n) | 48 (23%) | 12 (6%) | <0.001 |
| Lateral ischemia (n) | 68 (33%) | 12 (6%) | <0.001 |
| Inferior ischemia (n) | 87 (42%) | 25 (13%) | <0.001 |
| Normal study, % | 15 (7.2%) | 107 (55%) | <0.001 |
| Symptoms during stress | n=200 | n=193 | |
| No | 91 (45%) | 123 (64%) | <0.001 |
| Yes | 109 (55%) | 70 (36%) | |
| Limiting Exertional Chest pain | 21 (19%) | 4 (5.7%) | <0.001 |
| Non-Limiting Exertional Chest pain | 22 (20%) | 16 (23%) | 0.049 |
| Dyspnea | 49 (45%) | 36 (51%) | 0.073 |
| Claudication | 1 (1%) | 0 (0%) | NA |
| Other | 15 (14%) | 29 (15%) | 0.17 |
| Unknown | 1 (0.9%) | 0 (0%) | NA |
2 participants who had qualifying exercise stress echocardiography underwent dobutamine stress echocardiography in follow up, and 2 participants who had qualifying dobutamine stress
Median time from initial to follow up stress echocardiography 320 days (Q1,Q3 261–357). There were no complications of study stress testing.
Ischemia location does not add to 100% because participants could have ischemia in multiple coronary territories. Ischemia in each of the anterior, inferior or lateral locations required two segments with stress-induced wall motion abnormalities in the territory as defined by the American Society of Echocardiography segmentation model. Moderate anterior ischemia was defined as at least 3 segments with stress-induced wall motion abnormalities in the left anterior descending coronary artery territory.
Figure 2. Ischemia severity, angina severity and their correlation in INOCA and CAD at enrollment.
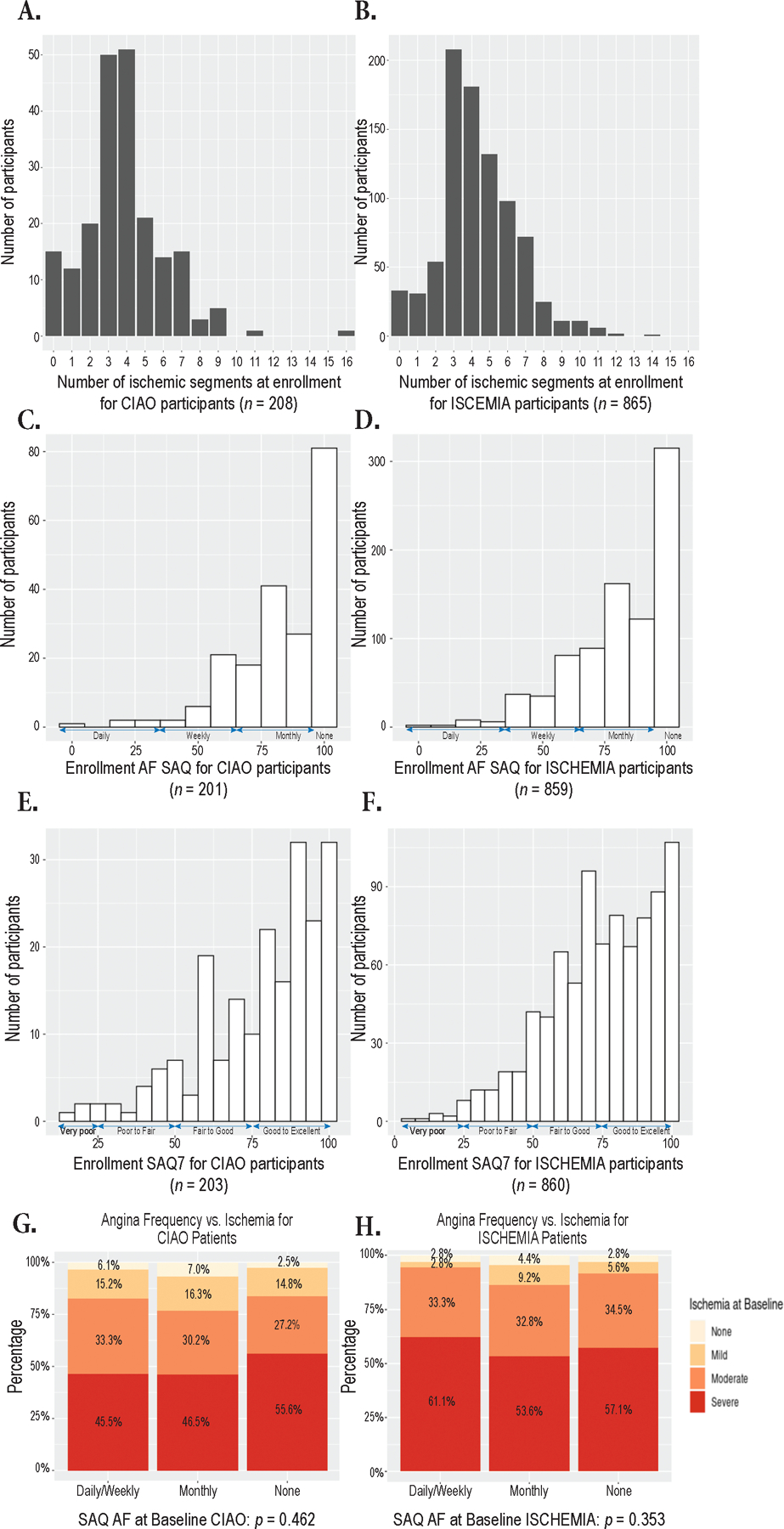
Panels A–B. Distribution of the number of ischemic segments on enrollment stress echocardiography for CIAO participants with INOCA (A) and ISCHEMIA participants with CAD on study coronary CT angiogram (B).
Panels C–D. Distribution of Angina Frequency (AF) scores on the Seattle Angina Questionnaire (SAQ) at enrollment in participants with INOCA (C) and CAD (D)
Panels E–F. Distribution of Seattle Angina Questionnaire (SAQ) summary scores at enrollment in participants with INOCA (E) and CAD (F).
Panels G–H. Distribution of ischemia severity categories as compared with SAQ AF score categories in participants with INOCA (G) and CAD (H). When SAQ AF was analyzed as a continuous variable, there was also no significant correlation: rho=0.105, p=0.15 for INOCA and rho=0.027, p=0.42, respectively.
Only ISCHEMIA participants who qualified for the trial based on stress echocardiography and underwent coronary CT angiography showing at least 1 vessel with ≥50% stenosis are included here. Number of ischemic segments reflects core laboratory determination. Ischemia severity was mild if there were 1–2 ischemic segments, moderate if there were 3 ischemic segments, and severe if there were 4 or more ischemic segments. Participants in ISCHEMIA were permitted to enroll in CIAO before core laboratory confirmation of ischemia severity, to be consistent with the main trial, so some INOCA participants had less than 3 ischemic segments per core laboratory assessment. The interpretation of SAQ scores is depicted on the figure. The median time between stress echocardiography and angina assessment was similar for INOCA and CAD participants (Table 1). ISCHEMIA stress echocardiography participants with CAD had similar angina frequency (median 90, IQR 70–100, p=0.22, Figure 2 and Table 3) but poorer SAQ summary scores (ISCHEMIA median 77, IQR 63–92, CIAO median 83, IQR 66–93, p=0.019) at baseline. Median overall health status was rated by INOCA participants as 75 (IQR 60,81) on a scale of 100 on the EQ-5D, indicating, on average, good health status. There was also no correlation between SAQ summary score or angina frequency score and number of ischemic segments when considering SAQ scores as continuous rather than categorical measures.
Health Status at Enrollment
At enrollment, the median SAQ summary score was higher in INOCA than obstructive CAD participants, though both groups had good baseline health status (Figure 2, Table 3). Angina frequency was similar over the 4 weeks preceding enrollment, with 16% of INOCA participants having angina weekly or more often vs. 20% of those with CAD, and 40% reporting no angina in the month prior to enrollment in INOCA vs. 37% in obstructive CAD, p=0.62 (Table 3). Of note, even those INOCA patients reporting no angina in the 4 weeks preceding enrollment had ischemic symptoms either before or during the stress echocardiogram. Similarly, we have previously reported that in ISCHEMIA, 90% of participants had a history of angina, but 35% reported no angina in the 4 weeks preceding enrollment.34
Table 3.
Symptoms at enrollment, 6 months and 1 year in CIAO with INOCA and at randomization in ISCHEMIA stress echocardiography participants with CAD on coronary CT angiography
| Obstructive CAD at enrollment (N=865) | INOCA at enrollment (N=203) | INOCA 6 months (N=160) | INOCA 1 year (N=197) | P (INOCA across all 3 time points) | |
|---|---|---|---|---|---|
| SAQ-7, median (Q1,Q3) | 78 (64,92) | 83 (66,93)* | 92 (76,100) | 90 (77,100) | <0.001 |
| Seattle angina questionnaire angina frequency score – median (Q1,Q3) | N=859 100 (90, 100) |
N=201 90 (70,100)* |
100 (80,100) | 100 (80,100) | |
| No angina in last month (SAQ AF = 100 at the time of CIAO enrollment, %) | 534 (62%) | 81 (40%) | 94 (59%) | 117 (59%) | |
| Monthly angina (SAQ AF = 61–99 at the time of CIAO enrollment, %) | 293 (34%) | 86 (43%) | 55 (34%) | 64 (32%) | |
| Weekly angina (SAQ AF = 31–60 at the time of CIAO enrollment, %) | 31 (3.6%) | 29 (14%) | 11 (6.9%) | 15 (8%) | |
| Daily angina (SAQ AF = 0–30 at the time of CIAO enrollment, %) | 5 (0.6%) | 5 (2%) | 0 (0%) | 1 (1%) | |
| Seattle angina questionnaire physical limitation score – median (Q1,Q3) | N=806 100 (67, 100) |
N=190 92 (75,100) |
N=154 92 (75,100) |
N=184 100 (75,100) |
<0.001 |
| SAQ quality of life score, median (Q1,Q3) | N=859 63 (50, 88) |
N=201 75 (50, 100) |
N=160 88 (63, 100) |
N=196 88 (63, 100) |
<0.001 |
| Time since first diagnosis of angina (months) | N=716 8 (3,36) |
N=145 8 (3,18) |
|||
| Dyspnea present by Rose Dyspnea Questionnaire | 504/847 (60%) | 130/202 (64%)* | 96 (60%) | 114 (58%) | <0.001 |
| Overall health score, median out of 100 (Q1,Q3) | N=842 80 (70, 90) |
N=196 75 (60, 81)* |
N=158 80 (65, 85) |
N=196 80 (70, 90) |
<0.001 |
P<0.05 for comparison between obstructive CAD and INOCA participants at the time of enrollment. For SAQ-7, p=0.04; for SAQ AF and Rose Dyspnea Scale, p<0.001; for overall health score, p=0.01.
Characteristics of INOCA patients based on the presence or absence of any atherosclerosis on CCTA
INOCA patients with any atherosclerosis on CCTA were older and had lower eGFR than those without any atherosclerosis, and were more likely to have diabetes, with no difference in SAQ scores at enrollment (Table III in the Supplement). There were no differences in stress test parameters between these groups (Table IV in the Supplement).
Association of Inducible Ischemia and Health Status in Participants with and without Obstructive CAD at Enrollment
At enrollment, there was no significant correlation between the number of ischemic segments on stress echocardiography and SAQ Angina Frequency subscale score for either INOCA or obstructive CAD participants (Figure 2). There was also no significant correlation between SAQ Summary Score and the number of ischemic segments in INOCA (rho=0.066, p=0.37) or obstructive CAD participants (rho=0.055, p=0.11).
Longitudinal Changes in Health Status with INOCA
At 1 year, the SAQ Angina Frequency subscale score improved by ≥10 points in 82 (43%) of INOCA participants, with 117 (59%) reporting no angina in the prior month (Table 3). Angina frequency worsened from enrollment to 1 year by ≥10 points in 27 (14%). At 1 year, SAQ7 summary score improved by ≥5 points in 96 (50%) INOCA participants and worsened by ≥5 points in 39 (20%). Angina-related health status improved from enrollment to 6 months, without significant incremental improvement from 6 months to 1 year.
Medication use at 1 year is shown in Table II in the Supplement. The median number of anti-anginal agents was 1 at enrollment and at 1 year. Beta blockers were used in the majority of participants at all time points. The use of antianginal medications other than beta blockers tended to increase over time, while use of statins decreased. There was no significant change from enrollment to 1 year in the use of ACEI/ARBs or in use of antiplatelet agents.
Longitudinal Changes in Ischemia in INOCA
Among 192 CIAO participants who completed the 1-year stress echocardiogram, half had normal stress echocardiography. At 1 year, the median number of ischemic segments was 0 (IQR 0–2). Moderate or severe ischemia was present in 44 (23%). The distribution of change in ischemia from enrollment to 1 year is shown in Figure 3. 132 (68%) had reduction of at least 2 ischemic segments. 40 (21%) of 1-year stress echocardiograms were abnormal and had not improved by at least 2 ischemic segments.
Figure 3. Change in number of ischemic segments from enrollment to 1 year in INOCA.
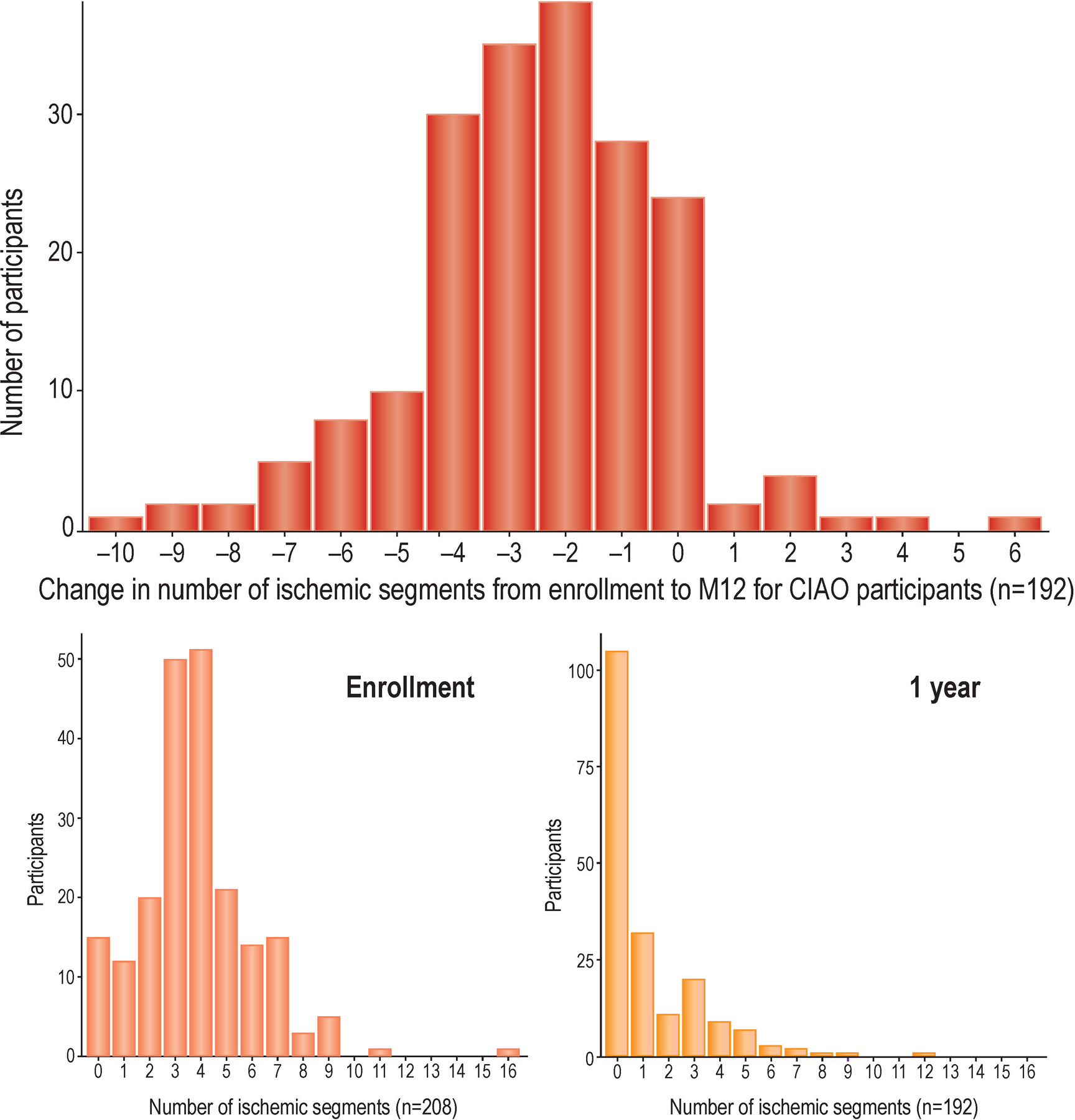
Panel A. Change in the number of ischemic segments on stress echocardiography from enrollment to 1 year.
Panel B. Number of ischemic segments at enrollment.
Panel C. Number of ischemic segments at 1 year.
Correlation between Change in Ischemia and Change in Health Status
Participants with 1-year improvement of at least 2 ischemic segments on stress echocardiography had similar 1-year SAQ summary scores to those without this degree of improvement (median 92 [IQR 81–97] vs. 89 [IQR 70–100], p=0.56). There was also no difference between these groups in angina frequency at 1 year (100 [IQR 90–100] vs. 100 [IQR 80–100], p=0.75) and 1-year SAQ physical limitation scores (100 [IQR 83–100] vs. 100 [IQR 67–100], p=0.23).
There was no relationship between 1-year change in angina and 1-year change in ischemia in CIAO as measured by the number of segments on stress echocardiography with stress-induced wall motion abnormalities (Figure 4).
Figure 4. Change in angina vs. change in ischemia in INOCA, in relation to 1-year stress echo findings.
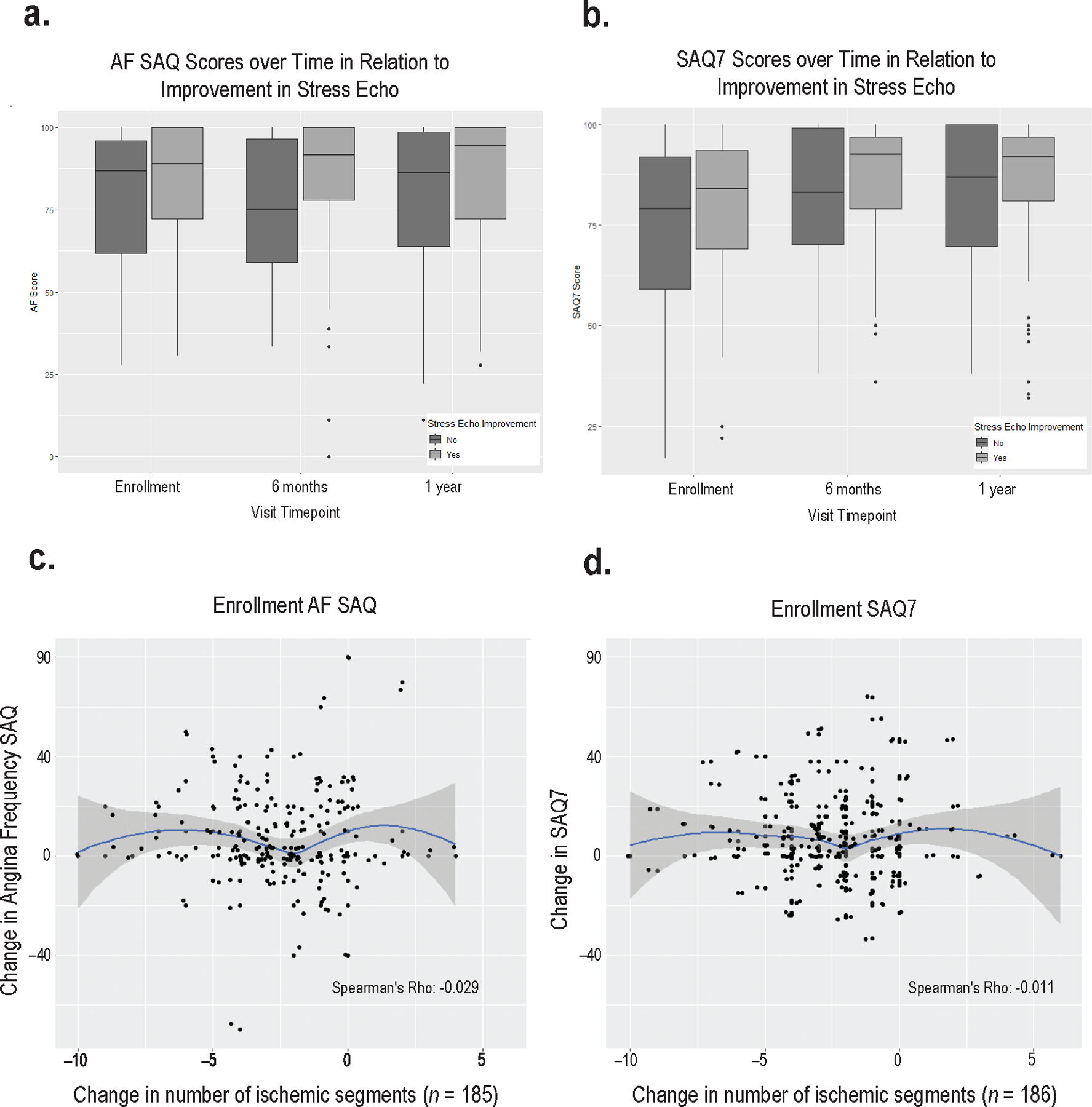
Panels A and B show SAQ scores at enrollment (SAQ AF score, panel A, and SAQ summary score, panel B), 6 months, and 1 year in relation to improvement in the number of ischemic segments on stress echocardiography by at least 2 segments from enrollment to 1 year.
Panels C and D. Correlation between change in the number of ischemic segments and change in SAQ angina frequency (C); and change in SAQ summary score (D). The median SAQ summary score was 90 (IQR 77–100) and median angina frequency score was 100 at 1 year (IQR 80–100, p<0.001 across all 3 time points, Table 3). Overall health status was rated similarly to that at enrollment, median 80 of 100 (IQR 70–90, p=<0.001 across all 3 time points). There was no correlation between the SAQ physical limitation subscale score and the number of ischemic segments at enrollment (rho =−0.03, p=0.69). There was no significant correlation between the SAQ summary score or any subscale score at 1 year and the number of ischemic segments at 1 year.
Additional analyses explored the correlation between change in ischemia and angina among subgroups defined by exercise stress echocardiography at baseline, peak heart rate >85% of the maximum predicted, stress testing indication of typical chest pain, symptoms elicited during initial stress echocardiogram and SAQ <100 at enrollment, but revealed no statistically significant correlation between changes in ischemia and angina in any of these subgroups.
Multivariable Analysis of Changes in Health Status and Ischemia, Including Medication Use
On multivariable analysis, change in number of ischemic segments from enrollment to 1 year was not statistically significantly associated with any health status outcomes (improvement in SAQ summary score of ≥5 points: OR 0.93 per segment more ischemia at 1 year as compared to enrollment, 95% CI 0.79–1.09, p=0.37; improvement in SAQ Angina Frequency subscale score of ≥10 points: OR 0.85, 95% CI 0.71–1.02, p=0.08; improvement in SAQ Physical Limitation subscale score of ≥10 points: OR 0.99, 95% CI 0.82–1.20, p=0.96) (Table V in the Supplement). When analyzing change in SAQ from enrollment as a continuous variable, we did not observe any association with change in the number of ischemic segments (Table VI in the Supplement).
Compared to patients without diabetes, patients with diabetes experienced 2.7 times the odds of improvement by ≥2 ischemic segments, adjusting for other risk factors (Table VII in the Supplement). Diabetes was also associated with reduction in the number of ischemic segments over time when analyzed as a continuous variable (Table VIII in the Supplement).
We did not observe evidence of association of angina-related health status with change in number of ischemic segments, age, history of hypertension or diabetes, severity of atherosclerosis on CCTA or medications (Table V in the Supplement) or in the models of continuous change in symptoms over time (Table VI in the Supplement). Male sex was associated with lower likelihood of change in SAQ scores over time.
Clinical Outcomes
Over 1 year in the CIAO cohort, the following clinical events occurred: 1 MI, 2 unstable angina hospitalizations, 1 heart failure hospitalization, 5 chest pain hospitalizations and no resuscitated cardiac arrests or deaths. Only one event occurred per participant. The 1-year cumulative event rate for the composite of death, MI, or hospitalization for heart failure or unstable angina was 2.06 per 100 participants (95% CI 0.68–4.88). This was lower than the cumulative event rate in the ISCHEMIA stress echo obstructive CAD comparator group, 6.15 (95% CI 4.68–7.89), p<0.0001 for comparison (Figure I in the Supplement). The 1-year cumulative event rate for the composite of the above events plus chest pain hospitalization was also lower in CIAO as compared to ISCHEMIA stress echo participants: 3.99 (95% CI 1.87–7.36) vs. 8.59 (95% CI 6.81–10.6), p=0.007 (Figure II in the Supplement).
Discussion
Given the prevalence of INOCA and limited evidence regarding how to manage patients with INOCA, we sought to describe the natural history of ischemia and health status, and the correlation between the two, in a well-characterized clinical cohort. In this international, multi-center observational study, patients with INOCA and those with obstructive CAD were selected using the same stress test and clinical eligibility criteria, and all stress echocardiograms were interpreted by the same, blinded core laboratory. In half of INOCA cases, the 1-year stress echocardiogram was normal, and in 23%, there was moderate or severe ischemia at 1 year. Angina frequency decreased by a clinically meaningful amount in 43%, despite little change in anti-anginal medications. There was no significant correlation between change in ischemia and change in angina.
The most striking difference between INOCA and obstructive CAD cohorts was the female predominance of INOCA. INOCA and obstructive CAD patients had a largely similar number of ischemic segments on stress echocardiography at enrollment. INOCA patients were slightly younger, with lower rates of diabetes, prior MI and smoking history and higher frequency of depression. INOCA patients had similar angina frequency, but overall angina-related quality of life was worse in patients with obstructive CAD. Angina was predominantly mild, owing to exclusion of patients with an unacceptable degree of angina from the ISCHEMIA trial and therefore from the CIAO study.
INOCA may be due to microvascular dysfunction causing supply-demand mismatch, inadequate vasodilation in response to increased demand and/or epicardial or microvascular coronary spasm.35 These entities may occur in the presence or absence of coronary atherosclerosis. Their dynamic nature over time may explain the substantial improvement in ischemia and angina in our INOCA patients. For example, changes in coronary flow reserve (CFR) over time in INOCA patients have been demonstrated in previous studies, with and without anti-anginal treatment.36, 37,38 Coronary artery spasm, present in 39% of INOCA patients in the Coronary Microvascular Angina (CorMicA) study, is inherently variable in relation to circadian rhythms, temperature and other factors.10, 39 Exercise stress testing is frequently abnormal in cases of epicardial spasm and microvascular spasm.40, 41 The lack of correlation between change in ischemia on stress echocardiography and change in angina severity is consistent with a smaller study showing no relationship between 1-year changes in coronary flow reserve and angina severity.42 Our observations may reflect peak disease activity at the time of initial stress testing. Clinicians often order stress tests when symptoms are new or worsening. Our observed improvement in angina over time is consistent with prior studies, including within placebo arms in clinical trials.43 It is also possible that INOCA patients experienced less angina over time due in part to reassurance by their physicians owing to the absence of obstructive CAD, or due to lifestyle modifications. It remains possible that at least some patients included in CIAO had false positive stress testing and therefore did not have INOCA.
In CIAO, we did not perform invasive testing of coronary flow in response to vasoactive stimuli, but such testing is typically recommended only for patients with persistent symptoms not responsive to medical therapy.44 Invasive testing in unselected patients referred for cardiac catheterization and found to have nonobstructive CAD demonstrated objective abnormalities in the majority of patients tested in the CorMicA trial (89%), similar to results of testing in the Women’s Ischemia Syndrome Evaluation (WISE, 78%) and a Mayo Clinic cohort (66%).10,11,12 A positive stress test increased the likelihood of microvascular dysfunction or spasm, but typical rather than atypical chest pain did not.10, 40, 41 Interestingly, the majority of our patients in CIAO and in ISCHEMIA did not report limiting chest pain during their initial stress test. Based on this literature, the ischemia severity required for study entry, and the high reproducibility of blinded stress echocardiographic assessments at the ISCHEMIA core laboratory, we believe our INOCA participants had true myocardial ischemia. However, we cannot prove this in the absence of invasive testing.
The pathophysiology of angina is complex, with contributions not only from conditions that limit blood flow at the epicardial vessel and/or microvascular level but also the intensity of daily physical and mental activities that may provoke angina, diastolic function, myocardial wall stress, autonomic nervous system function, patient-specific pain sensitivity, oxygen carrying capacity and other factors, including patient sex.45 We found no significant correlation between ischemia severity on stress echocardiography and angina frequency or overall angina-related health status at enrollment in patients selected for moderate or severe ischemia, either with INOCA or with obstructive CAD. Data from the WISE Study have also shown similar angina frequency and quality of life in INOCA patients with and without ischemia on noninvasive testing, and in those with CAD.46 It is possible that some patients with persistent ischemia on stress echocardiography undertook lifestyle changes (such as reducing physical activity or emotional stress to avoid angina), leading to a disconnect between changes in day-to-day symptoms and objective, inducible wall motion abnormalities of this degree on stress echocardiography in follow up. For example, the majority of patients with and without obstructive CAD did not report limiting chest pain during their initial stress test. In support of this hypothesis is the correlation we observed between improvements in ischemia and the SAQ physical limitation subscale over time. Conversely, changes such as adaptation to exercise or changes in microvascular function may lead to improvement in inducible ischemia on stress echocardiography, even as emotional stress may continue to provoke symptoms. In the case of episodic coronary spasm, a lack of correlation between ischemia at the time of stress testing and angina severity over the preceding four weeks is logical given the episodic nature of the disorder. Additional research into these relationships in patients with and without obstructive CAD is needed.
We explored the relationship between medication use, improvement in angina and improvement in ischemia, recognizing the limitations of our small sample size and the observational nature of our study. We did not identify any medication class associated with improvement in angina-related health status or ischemia severity over 1 year, but power is limited and our comparisons were not randomized. The ACEI quinapril was previously shown in a small randomized trial to reduce angina in women with INOCA, mediated in that study at least in part by improvement in coronary flow reserve over 16 weeks.47 ACEI/ARB and statin therapy are the subject of an ongoing large clinical trial in INOCA (NCT03417388). Diabetes was associated with improvement in ischemia on stress echocardiography over 1 year in this study, for unclear reasons.
The rate of major adverse cardiovascular events (MACE) over 1 year in our cohort, approximately 2%, is consistent with meta-analyses of outcomes in INOCA48 and with the event rates in prior reports showing up to fourfold increased risk of cardiovascular events in patients with symptoms prompting angiography showing no obstructive CAD as compared to healthy people in the community, and lower risk in INOCA than in patients with obstructive CAD.3, 5, 17, 49–51 The rate of MACE or chest pain hospitalization was approximately 4% in one year, consistent with the 20% rate of chest pain hospitalization over 5 years in the WISE, in which associated high healthcare costs were high.9 Analysis of event rates in CIAO was considered exploratory based on limited sample size and 1-year duration of follow up.
Our study must be interpreted in the context of several limitations. The link with the parent ISCHEMIA trial is both a strength and limitation: inclusion of many centers increases generalizability, and interpretation of stress tests at a core laboratory blinded to obstructive CAD status and timing is a strength. The CIAO sample is relatively large and incorporated systematic, longitudinal assessments. However, patients in CIAO were excluded from ISCHEMIA randomization in ISCHEMIA and therefore did not undergo invasive testing to confirm ischemia or the lack of ≥50% stenosis. Angina was less frequent than in CorMicA because we excluded patients with an unacceptable degree of angina from ISCHEMIA (and thus CIAO), to focus on patients who would be suitable for randomization. ISCHEMIA participants were not required to have anginal symptoms, but 90% did.21 Enrollment per site was low, reflecting the requirement for moderate or severe ischemia as in the ISCHEMIA trial. The SAQ is phrased in terms of chest pain, chest tightness or angina, and it is possible that some patients with atypical ischemic symptoms did not convey the full severity of their symptoms in their responses. However, the SAQ has been validated in women with ischemic heart disease and in patients with myocardial infarction with no obstructive CAD (MINOCA).52 The SAQ assesses symptoms over the preceding month, but there was typically more than 1 month between stress testing and angina assessment; interim changes in symptoms could have biased our results to the null. There could have been regression to the mean in angina over time. Analysis of events is considered exploratory based on our small sample size. Finally, this was an observational, non-randomized study. We did not specify medical therapy in the protocol or collect doses, so analyses incorporating medical therapy are limited by the potential for confounding. Future analyses of ISCHEMIA study data will evaluate predictors of no obstructive CAD on study CCTA in a larger sample incorporating multiple stress test modalities.
In conclusion, in this well-characterized, predominantly female cohort of patients with significant ischemia on stress echocardiography as determined by a blinded core laboratory, predominantly mild angina, and no obstructive CAD, half had resolution of stress-induced wall motion abnormalities on repeat stress echocardiography at 1 year and symptoms improved in 43%. There was no significant correlation between change in angina and change in ischemia on stress echocardiography. Further research is needed to improve understanding of mechanisms and define how best to manage symptoms in patients with INOCA to improve patient-centered outcomes.
Supplementary Material
Clinical Perspective.
What is new?
Patients with moderate or severe ischemia on stress echocardiography and no obstructive CAD in this study (INOCA) were more often female, but had largely similar ischemia compared to obstructive CAD patients (median 4 segments).
Half of 1-year INOCA stress echocardiograms were normal and 23% had moderate or severe ischemia at 1 year (≥3 ischemic segments). Angina improved in 43% and worsened in 14%.
Ischemia and angina were not correlated in those with INOCA or with obstructive CAD at baseline, and change in ischemia was not correlated with change in angina in INOCA patients.
What are the clinical implications?
It is common for ischemia and angina to improve in INOCA, but the two may not be linked, because pathophysiology of angina is complex.
Ischemia severity on stress echocardiography is not an adequate surrogate for symptom severity either in patients with INOCA or in patients with obstructive CAD. Clinicians should focus on symptom management in order to maximize patients’ quality of life.
Acknowledgements
Disclaimer: Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Funding:
NIH grants R01HL119153, U01HL105907, U01HL105462, U01HL105561, U01HL105565
Non-standard Abbreviations and Acronyms
- CIAO
Changes in Ischemia and Angina over One year among ISCHEMIA trial screen failures with no obstructive CAD on CCTA
- CCTA
coronary computed tomography angiography
- CorMicA
Coronary Microvascular Angina
- INOCA
ischemia with no obstructive coronary artery disease
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- MINOCA
myocardial infarction with non-obstructive coronary arteries
- SAQ
Seattle Angina Questionnaire
Footnotes
Conflict of Interest Disclosures
Dr. Harmony Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from BioTelemetry, outside the submitted work
Dr. Michael Picard reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. John Spertus reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; personal fees from Bayer, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Amgen, personal fees from Janssen, personal fees from United Healthcare, grants from American College of Cardiology, outside the submitted work; In addition, Dr. Spertus has a patent Copyright to Seattle Angina Questionnaire with royalties paid and Board of Directors for Blue Cross Blue Shield of Kansas City and Equity in Health Outcomes Sciences
Dr. Jesus Peteiro grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Jose Luis Lopez Sendon reports grants from National Heart, Lung and Blood Institute during the conduct of the study; he also reports grants from Merck, Pfizer, and Boehringer Ingelheim outside the submitted work
Dr. Roxy Senior reports National Heart, Lung and Blood Institute during the conduct of the study; he also reports speaker fees from Lantheus Medical Imaging, Boston, Mass, Bracco, Milan, Italy, Philips Healthcare, Eindhoven, Holland
Dr. Mohammad El-Hajjar reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Jelena Celutkiene reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Michael Shapiro reports grants from National Heart, Lung and Blood Institute during the conduct of the study; He serves as Scientific Advisory Board for Regeneron and Amgen.
Dr. Patricia Pellikka reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Khaled Alfakih reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Khaled Abdul-Nour reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Michel Khouri reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Leonid Bershtein reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Mark de Belder reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Keong Kian Poh reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. John Beltrame reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dennis Kunichoff reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Yi Li reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. James Min reports grants from National Heart, Lung, and Blood Institute , during the conduct of the study; other from CLEERLY INC., grants and other from GE HEALTHCARE, other from ARINETA, outside the submitted work
Dr. Jerome Fleg reports no conflict of interest
Dr. David Maron grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Judith Hochman reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; other from AstraZeneca Pharamceuticals LP, other from Arbor Pharmaceuticals LLC, other from Abbott Vascular, other from Medtronic Inc, other from St. Jude Medical Inc, other from Volcano Corp, other from Merck Sharp & Dohme Corp, other from Omron Healthcare Inc, other from Amgen Inc, during the conduct of the study
References
- 1.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H and Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. European Heart Journal. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. [DOI] [PubMed] [Google Scholar]
- 3.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine. 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairey Merz CN, Pepine CJ, Walsh MN and Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S and Prescott E. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PloS one. 2014;9:e93170–e93170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, Rogers WJ, Mankad S, Sharaf BL, Bittner V, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. European Heart Journal. 2006;27:1408–1415. [DOI] [PubMed] [Google Scholar]
- 7.Sicari R, Palinkas A, Pasanisi EG, Venneri L and Picano E. Long-term survival of patients with chest pain syndrome and angiographically normal or near-normal coronary arteries: the additional prognostic value of dipyridamole echocardiography test (DET). European Heart Journal. 2005;26:2136–2141. [DOI] [PubMed] [Google Scholar]
- 8.Lin FY, Shaw LJ, Dunning AM, LaBounty TM, Choi J-H, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, et al. Mortality Risk in Symptomatic Patients With Nonobstructive Coronary Artery Disease: A Prospective 2-Center Study of 2,583 Patients Undergoing 64-Detector Row Coronary Computed Tomographic Angiography. Journal of the American College of Cardiology. 2011;58:510–519. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Merz CNB, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, et al. The Economic Burden of Angina in Women With Suspected Ischemic Heart Disease. Circulation. 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 10.Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. Ischemia and No Obstructive Coronary Artery Disease: Prevalence and Correlates of Coronary Vasomotion Disorders. Circulation Cardiovascular interventions. 2019;12:e008126–e008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RD, Petersen JW, Mehta PK, Wei J, Johnson BD, Handberg EM, Kar S, Samuels B, Azarbal B, Kothawade K, et al. Prevalence of Coronary Endothelial and Microvascular Dysfunction in Women with Symptoms of Ischemia and No Obstructive Coronary Artery Disease Is Confirmed by a New Cohort: The NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD). J Interv Cardiol. 2019;2019:7169275–7169275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO and Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovascular interventions. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 13.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC and Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). Journal of the American College of Cardiology. 2012;59:655–62. [DOI] [PubMed] [Google Scholar]
- 14.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC and Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson BD, Shaw LJ, Buchthal SD, Merz CNB, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease - Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–2999. [DOI] [PubMed] [Google Scholar]
- 16.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G and Bairey Merz CN. Coronary Microvascular Reactivity to Adenosine Predicts Adverse Outcome in Women Evaluated for Suspected Ischemia. Results From the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. Journal of the American College of Cardiology. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly CA, De Stavola B, Sendon JL, Tavazzi L, Boersma E, Clemens F, Danchin N, Delahaye F, Gitt A, Julian D, et al. Predicting prognosis in stable angina--results from the Euro heart survey of stable angina: prospective observational study. Bmj. 2006;332:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston N, Schenck-Gustafsson K and Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J. 2011;32:1331–6. [DOI] [PubMed] [Google Scholar]
- 19.Members C, Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, et al. ACC/AHA Guidelines for the Management of Patients With Unstable Angina and Non–ST-Segment Elevation Myocardial Infarction: Executive Summary and Recommendations. Circulation. 2000;102:1193–1209. [DOI] [PubMed] [Google Scholar]
- 20.Fox K, Garcia MAA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, et al. Guidelines on the management of stable angina pectoris: executive summary. European Heart Journal. 2006;27:1341–1381. [DOI] [PubMed] [Google Scholar]
- 21.Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Alexander KP, Senior R, Boden WE, Stone GW, Goodman SG, Lopes RD, et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical TrialBaseline Characteristics of Participants in the ISCHEMIA StudyBaseline Characteristics of Participants in the ISCHEMIA Study. JAMA Cardiology. 2019;4:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group ITR, Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR and Sawada SG. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2020;33:1–41.e8. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 25.Watson PF and Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73:1167–79. [DOI] [PubMed] [Google Scholar]
- 26.Chan PS, Jones PG, Arnold SA and Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circulation Cardiovascular quality and outcomes. 2014;7:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose G and Blackburn H. Cardiovascular survey methods. East African medical journal. 1969;46:220–227. [PubMed] [Google Scholar]
- 28.Group TE. EuroQol-a new facility for the measurement of health-related quality of life. Health policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 29.Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. American Heart Journal. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonnell M and Fihn SD. Development and evaluation of the Seattle Angina questionnaire: A new functional status measure for coronary artery disease. Journal of the American College of Cardiology. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 31.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS and Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. Journal of the American College of Cardiology. 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 32.van Buuren S and Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. 2011. 2011;45:67. [Google Scholar]
- 33.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, et al. Effect of PCI on quality of life in patients with stable coronary disease. The New England journal of medicine. 2008;359:677–87. [DOI] [PubMed] [Google Scholar]
- 34.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. The New England journal of medicine. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taqueti VR and Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options. J Am Coll Cardiol. 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelsen MM, Rask AB, Suhrs E, Raft KF, Høst N and Prescott E. Effect of ACE-inhibition on coronary microvascular function and symptoms in normotensive women with microvascular angina: A randomized placebo-controlled trial. PloS one. 2018;13:e0196962–e0196962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CNB, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). American Heart Journal. 2011;162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah NR, Cheezum MK, Veeranna V, Horgan SJ, Taqueti VR, Murthy VL, Foster C, Hainer J, Daniels KM, Rivero J, et al. Ranolazine in Symptomatic Diabetic Patients Without Obstructive Coronary Artery Disease: Impact on Microvascular and Diastolic Function. Journal of the American Heart Association. 2017;6:e005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasue H, Nakagawa H, Itoh T, Harada E and Mizuno Y. Coronary artery spasm—Clinical features, diagnosis, pathogenesis, and treatment. Journal of cardiology. 2008;51:2–17. [DOI] [PubMed] [Google Scholar]
- 40.Cheng C-W, Yang N-I, Lin K-J, Hung M-J and Cherng W-J. Role of coronary spasm for a positive noninvasive stress test result in angina pectoris patients without hemodynamically significant coronary artery disease. Am J Med Sci. 2008;335:354–362. [DOI] [PubMed] [Google Scholar]
- 41.Ong P, Athanasiadis A, Hill S, Schäufele T, Mahrholdt H and Sechtem U. Coronary microvascular dysfunction assessed by intracoronary acetylcholine provocation testing is a frequent cause of ischemia and angina in patients with exercise-induced electrocardiographic changes and unobstructed coronary arteries. Clinical cardiology. 2014;37:462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Franco A, Villano A, Di Monaco A, Lamendola P, Russo G, Stazi A, Scavone G, Nerla R, Sestito A, Lanza GA, et al. Correlation between coronary microvascular function and angina status in patients with stable microvascular angina. Eur Rev Med Pharmacol Sci. 2014;18:374–379. [PubMed] [Google Scholar]
- 43.Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben-Yehuda O, Katz A, Jones PG, Olmsted A, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). Journal of the American College of Cardiology. 2013;61:2038–45. [DOI] [PubMed] [Google Scholar]
- 44.Wei J, Cheng S and Bairey Merz CN. Coronary Microvascular Dysfunction Causing Cardiac Ischemia in Women. JAMA. 2019;322:2334–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, Picard MH, Kwong RY, Bairey-Merz CN, Cyr DD, et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020;5:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson MB, Kelsey SF, Matthews K, Shaw LJ, Sharaf BL, Pohost GM, Cornell CE, McGorray SP, Vido D and Bairey Merz CN. Symptoms, myocardial ischaemia and quality of life in women. European Heart Journal. 2003;24:1506–1514. [DOI] [PubMed] [Google Scholar]
- 47.Pauly D, Johnson B, Anderson R, Handberg E, Smith K, Cooper-DeHoff R, Sopko G, Sharaf B, Kelsey S, Merz C, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 2011;162:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radico F, Zimarino M, Fulgenzi F, Ricci F, Di Nicola M, Jespersen L, Chang SM, Humphries KH, Marzilli M and De Caterina R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39:2135–2146. [DOI] [PubMed] [Google Scholar]
- 49.Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S and Prescott E. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PloS one. 2014;9:e93170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine. 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herscovici R, Sedlak T, Wei J, Pepine Carl J, Handberg E and Bairey Merz CN. Ischemia and No Obstructive Coronary Artery Disease (INOCA): What Is the Risk? Journal of the American Heart Association. 2018;7:e008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel KK, Arnold SV, Chan PS, Tang Y, Jones PG, Guo J, Buchanan DM, Qintar M, Decker C, Morrow DA, et al. Validation of the Seattle angina questionnaire in women with ischemic heart disease. American heart journal. 2018;201:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


