Abstract
Chronic graft-versus-host disease (GVHD) can be associated with significant morbidity, in part because of nonreversible fibrosis, which impacts physical functioning (eye, skin, lung manifestations) and mortality (lung, gastrointestinal manifestations). Progress in preventing severe morbidity and mortality associated with chronic GVHD is limited by a complex and incompletely understood disease biology and a lack of prognostic biomarkers. Likewise, treatment advances for highly morbid manifestations remain hindered by the absence of effective organ-specific approaches targeting “irreversible” fibrotic sequelae and difficulties in conducting clinical trials in a heterogeneous disease with small patient numbers. The purpose of this document is to identify current gaps, to outline a roadmap of research goals for highly morbid forms of chronic GVHD including advanced skin sclerosis, fasciitis, lung, ocular and gastrointestinal involvement, and to propose strategies for effective trial design. The working group made the following recommendations: (1) Phenotype chronic GVHD clinically and biologically in future cohorts, to describe the incidence, prognostic factors, mechanisms of organ damage, and clinical evolution of highly morbid conditions including long-term effects in children; (2) Conduct longitudinal multicenter studies with common definitions and research sample collections; (3) Develop new approaches for early identification and treatment of highly morbid forms of chronic GVHD, especially biologically targeted treatments, with a special focus on fibrotic changes; and (4) Establish primary endpoints for clinical trials addressing each highly morbid manifestation in relationship to the time point of intervention (early versus late). Alternative endpoints, such as lack of progression and improvement in physical functioning or quality of life, may be suitable for clinical trials in patients with highly morbid manifestations. Finally, new approaches for objective response assessment and exploration of novel trial designs for small populations are required.
Keywords: Chronic graft-versus-host disease, Allogeneic hematopoietic cell transplantation, Consensus, Lung, Sclerosis, Gastrointestinal tract, Ocular, Skin
Chronic graft-versus-host disease (GVHD) may be associated with significant morbidity, in part due to development of non-reversible fibrosis (eye, sclerosis, lung) with detrimental impact on physical functioning (sclerotic skin manifestations, lung) and survival (lung, gastrointestinal) [1,2]. Progress in prevention of long-term severe morbidity and mortality associated with chronic GVHD is limited by a complex and incompletely understood disease biology and lack of prognostic biomarkers associated with a highly morbid future course. Treatment advances for these highly morbid manifestations are limited because of both the difficulty in conducting clinical trials in a heterogeneous disease with small patient numbers and the absence of effective organ-specific approaches targeting “irreversible” fibrotic sequelae.
PURPOSE OF THIS DOCUMENT
The goal of this working group is to address gaps and outline a roadmap of research goals including suggestions on trial design for frequent, highly morbid forms of chronic GVHD, namely advanced skin sclerosis and fasciitis, and lung, ocular, and gastrointestinal (GI) involvement.
SUMMARY OF RECOMMENDATIONS
Future studies should phenotype chronic GVHD clinically and biologically, to describe the incidence, prognostic factors, mechanisms of organ damage, and clinical evolution of highly morbid manifestations including long term effects of morbid forms in children. Longitudinal multicenter studies with common definitions (diagnostic and inclusion criteria, documentation of organ involvement and endpoints with sufficient follow up) and research sample collections are needed (Figures 1 and 2).
Develop new approaches for early identification and treatment of highly morbid forms of chronic GVHD, especially biologically targeted treatments, with a special focus on prevention and treatment of fibrotic changes.
Establish primary endpoints for clinical trials addressing each highly morbid manifestation in relationship to the time point of intervention (early versus late). Alternative endpoints, such as lack of progression and improvement in physical functioning or quality of life, can provide compelling evidence of clinical benefit in clinical trials to evaluate treatment of highly morbid manifestations.
Explore novel trial designs for small populations, emphasizing the need for objective endpoints.
Figure 1.
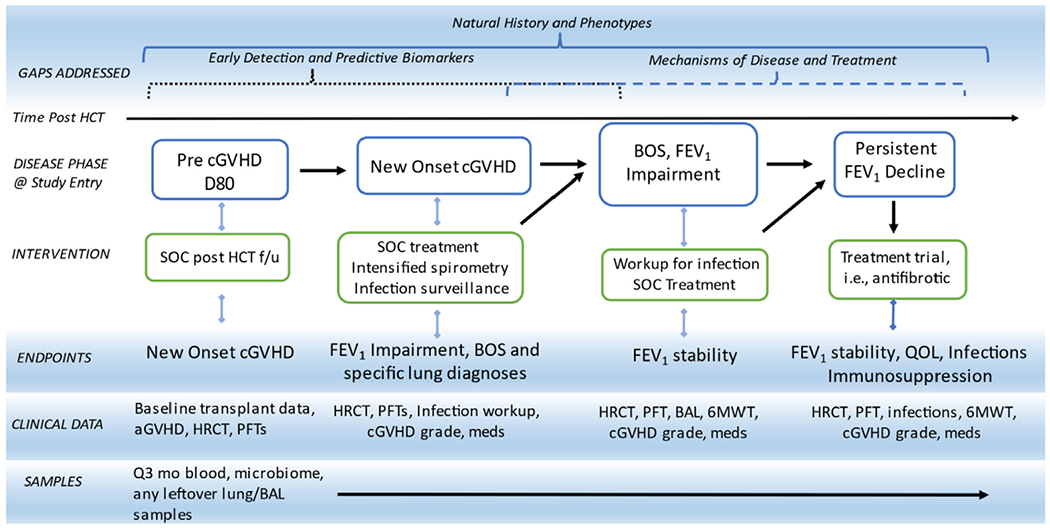
Potential Longitudinal Trial Design Proposal for Highly Morbid Manifestations of chronic GVHD using BOS as example. The proposed study approach aims to simultaneously address identified fundamental knowledge gaps in several domains, including (1) description of clinical evolution and clinical phenotypes, (2) early detection and predictive biomarker discovery, (3) mechanisms of disease through translational work, and (4) evaluation of novel treatments. High-risk patients are enrolled at a pre-diagnosis phase based on biomarker and clinical risk factors and followed over time through phases of chronic GVHD. Patients may also enter the longitudinal cohort at the time of chronic GVHD diagnosis, and if they develop a highly morbid manifestation, they are followed in that specific cohort category and may be enrolled on clinical trials. Longitudinal clinical data and serial tissue samples and specimens will be collected. In this Figure, lung disease is used as an example for the enrollment entry, interventions, endpoints, and data and samples to be collected. This schema can be easily expanded to reflect skin, GI, and other manifestations with relevant data collection and treatment agents. SOC indicates standard of care; f/u, follow-up; HRCT, high-resolution chest tomography; BAL, bronchoalveolar lavage; 6MWT, 6-minute walk test.
Figure 2.
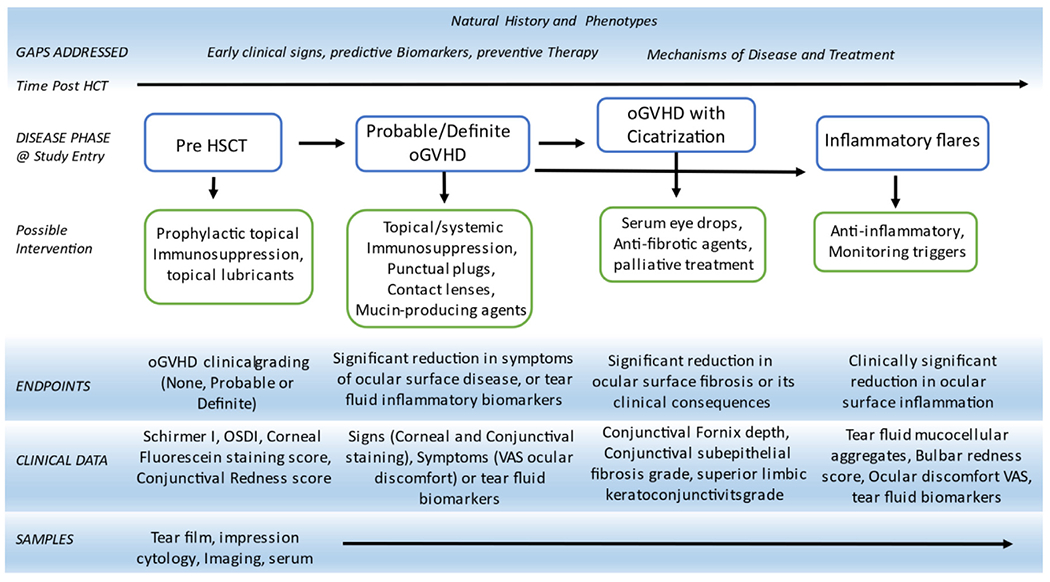
Potential longitudinal trial design proposal for oGVHD. OSDI indicates ocular surface disease index; VAS, visual analog scale.
METHODS
Each working group was created to encourage global engagement in the topic [3]. Groups worked individually to review the relevant literature and create the initial draft of the paper, which was circulated for review and comment by the Steering Committee. Two iterative rounds of comments from the Steering Committee were collected before the November 2020 Consensus Conference with appropriate manuscript revisions. Based on additional comments from Conference participants and a 30-day public comment period, the paper was further revised for submission.
SCLEROSIS OF SKIN AND FASCIA
Current clinical knowledge
Skin is the organ most frequently affected by chronic GVHD. Although inflammatory disease manifestations characterized by erythematous or lichen planus-like clinical presentations are often responsive to therapy, management options for fibrotic disease remain limited although responses have been reported in superficial and deep sclerosis.
Sclerotic chronic GVHD (ScGVHD) at onset of disease occurs infrequently [4] but long-standing chronic GVHD is likely to advance to sclerosis, with 20% of patients having sclerosis after 3 years of chronic GVHD therapy. The prevalence of sclerosis exceeds 50% among those with severe disease [4–7]. ScGVHD can manifest as localized disease (morphea-like), diffuse involvement, deep sclerosis, panniculitis, or fasciitis without epidermal manifestations. ScGVHD may cause joint contractures, skin breakdown, neuropathy (including small fiber neuropathy [8], nerve compression syndrome and painful muscle cramping [9]), myopathy as a consequence of fascial compression, and vascular insufficiency that contributes to poor wound healing.
Pathophysiology
Fibrosis represents the terminal step of an unchecked inflammatory alloreactivity cascade. The role of T cells in chronic skin GVHD development is well defined and supported by defined genetic risk factors [10], but their role in an established sclerotic response is unknown. ScGVHD biopsy specimens demonstrate variable degrees of CD4+ and CD8+ T cell infiltration with unknown clonal architecture [11–14]; and infiltrating T cells may represent bystanders or effectors depending on biopsy timing [11,12]. In systemic sclerosis (SSc) [15], as well as chronic GVHD [16,17] impaired function of regulatory T cells has been reported, and IL-2 treatment, which expands regulatory T cells, has shown efficacy in advanced chronic GVHD [18]. Agonist platelet—derived growth factor-receptor (PDGF-R) antibodies [19] or other antibodies targeting surface antigens [20] could have a role in severe fibrotic forms of chronic GVHD. Poor correlation of chronic GVHD severity, lack of damage in grafted donor skin indicating host specificity, and limited response to PDGF-R inhibitors in patients with these antibodies argue against the broader relevance of these findings [21,22]. A possible mechanistic role of B cells in ScGVHD has been suggested with improvement of sclerosis after B cell depletion [23]. Additional work is needed in chronic GVHD, to determine how B cells might promote fibrosis since definitive evidence linking antibody-dependent mechanisms to human ScGVHD is lacking [24].
Recently, distinct dermal myeloid cell populations were identified in human skin [25]. In animal models, macrophages contribute to development of fibrosis in both transforming growth factor beta (TGFβ)-dependent and -independent fashion and their pathogenic role in chronic GVHD is increasingly recognized [26–28]. Relevant for ScGVHD, myeloid-sourced TGFβ [29,30] promotes fibrosis through positive regulation of fibroblast proliferation and differentiation into myofibroblasts [31] and stimulation of extracellular matrix overproduction [30]. In addition, macrophage-derived TGFβ promotes epithelial mesenchymal transition in models of lung fibrosis [32]. Partial epithelial mesenchymal transition is involved in normal wound healing, although its disruption in the inflammatory environment can promote pathologic fibrosis in lung and skin [33]. Although fibroblasts represent critical mediators of fibrotic tissue injury, little is known about their homeostasis during chronic GVHD.
TGFβ is a keystone pathway in many fibrotic disorders and has a documented role in preclinical ScGVHD [29,30]. In patients, higher TGFβ levels are associated with adverse outcomes taking into account the challenges of correlating expression and activity [34,35]. However, TGFβ exerts distinct effects on post-transplantation complications early and late after transplantation [30], has pleiotropic roles in different compartments [36], and activates distinct downstream signaling pathways [36], making it challenging as a therapeutic target. Type I interferon responses feature prominently in SSc skin fibrosis and ScGVHD as well [37,38], tightly linking adaptive and innate immune crosstalk in initiation and persistence of ScGVHD, with possible therapeutic implications. The recent approval of tocilizumab in pulmonary manifestations of SSc highlights the importance of IL-6 in the pathophysiology of fibrosis and this cytokine may also play a role in ScGVHD [39–41 ].
Other interconnected pathways, commonly influenced by TGFβ, often create a feed-forward loop promoting aberrant tissue remodeling such as the developmental (morphogen) pathways, particularly Hedgehog, Wnt, and Notch, which are involved in fibrotic disorders [33,42–44]. Active Hedgehog signaling has been observed in the skin of patients with ScGVHD, and its targeting in preclinical models modulated collagen production by myofibroblasts and reduced fibrosis [45]. Hedgehog inhibitors have been tested in chronic GVHD with some efficacy, although their use is hindered by significant toxicities [46,47]. Recent data in ScGVHD suggested an immunomodulatory role of morphogen pathways with broad effects on adaptive immunity promoting chronic GVHD [46,48], thus providing an added impetus for clinical translation. The endocannabinoid system is involved in multiple inflammatory and fibrotic disorders, with opposing roles for signaling through cannabinoid receptor 1 (CB1R; profibrogenic) and cannabinoid receptor 2 (CB2R; antifibrotic/anti-inflammatory), and agents targeting these receptors (CB1R antagonists, CB2R agonists) are already in clinical trials [49–52]. Of the many immune mechanisms that contribute to development of chronic GvHD, it will be important to elucidate whether different immune pathways lead to specific clinical presentations.
Gaps in knowledge and unmet needs; highest priorities
The pivotal role of immune injury in the initial steps of fibrosis is well accepted. However, the mechanisms responsible for the shift from active inflammation to feed-forward loops of dysregulated tissue remodeling remains unknown. Understanding this transition is essential to devise approaches with optimal therapeutic indices that minimize immunosuppression. Both skin and peripheral blood samples should be queried to identify abnormalities along the disease continuum to inform preclinical modeling with a goal of defining the mechanistic relevance of the observations. Optimized preclinical ex vivo approaches could be well suited for the latter (e.g., to evaluate the effect of TGFβ and TGFβ pathway inhibitors on sclerotic skin fibroblasts). Deeper interrogation should use -omics methods and novel tissue diagnostic approaches such as multiplex immunohistochemistry/immunofluorescence, which can be enhanced by artificial intelligence (machine and deep learning) to offer a spatial perspective into the disease process and facilitate the development of novel biomarker signatures.
Clinical trials need more robust and sensitive endpoints. It is particularly challenging to precisely quantify the evolution and the extent of deep-seated (subcutaneous and fascial) disease to assess response and the current organ-based grading system is poorly suited to detect responses in established sclerosis. Given this limitation, functional improvement (e.g., improved joint mobility documented by P-ROM and physician global and skin and joint tightening scale per the 2014 National Institutes of Health [NIH] Consensus) could be considered an ScGVHD response, even if skin-specific scoring remains unchanged. Data supporting such an approach have been published [53], and bedside validation in ScGVHD should be actively pursued. Imaging biomarkers that have been suggested include high-frequency ultrasonography and magnetic resonance imaging, but rapid, safe, less costly, and accessible clinical assessment tools are needed (Table 1) [54,55]. Gene expression biomarkers in SSc skin correlated highly with changes of the modified Rodnan skin score and have been utilized to support response assessment in several clinical trials in that disease [56–59], but its use in ScGVHD requires additional exploration.
Table 1.
Potential Objective Assessment Tools to Assess Skin Sclerosis in Chronic GVHD
| Modality | Advantages | Disadvantages | Use in ScGVHD |
|---|---|---|---|
| High-frequency ultrasound, acoustic radiation force impulse, shear wave elasticity imaging, ultrasound surface wave elastography | Bedside use, easy to assess multiple sites, allows rapid comparability to previous images | Cost, requires training, requires marking of target area for repeat assessment; edema from active inflammation may confound imaging | CS [237] CS [54] CS [238] CS [239] CS [240] |
| Durometer | Bedside use, affordable, small, hand-held device, easy to use, provides numerical readout | “Anvil effect” from underlying bony structures, less sensitive for deep-seated disease; reproducibility requires consistent technique | CS [241] |
| MRI, MRI/positron emission tomography | Detection of deep-seated, sub-clinical involvement; useful for detecting active fascial inflammation; does not require marking of target area | Cost, inconvenient for patients, unclear if responsive to small improvements in fibrosis | CS [55] CS [242] CS [243] |
| Optical coherence tomography/elastography | High-resolution imaging, including capability to assess local blood flow | Limited depth of penetration | CR [244] |
| Laser doppler flowmetry | 2D flow map of skin perfusion; can assess dynamic changes; monitoring potential for compromised acral sites of sclerotic chronic GVHD | Affected by ambient temperature; movement, pressure or other contact with skin will influence perfusion | CR [245] |
| Suction probe (Cutometer, Dermaflex, Nimble) | Devices measure stiffness and elasticity; have been used in clinical assessment of morphea and systemic sclerosis | Affected by many variables, including sun damage, water balance, age, body location; does not capture changes in subcutaneous fat/fascia; remission may not result in return of elasticity | CS [237] |
| Myoton | Hand-held device, detects changes in tissue oscillation (skin stiffness and other properties) after a mechanical impulse | Requires adherence to measurement protocols and knowledge of muscular anatomy. Results depend on underlying muscle tone, patient positioning | CS [246] CS [247] CS [241] |
CR indicates case report; CS, case series; MRI, magnetic resonance imaging;.
Translation of knowledge accrued from organ fibrosis (e.g., SSc and idiopathic pulmonary fibrosis) to ScGVHD should be accelerated and is critical to improvement in patient care. Some agents have already demonstrated promise in chronic GVHD (e.g. belumosudil, a rho-associated coiled coil protein kinase-2 (ROCK2) inhibitor [60]), whereas many others have yet to be explored (e.g., connective tissue growth factor or cannabinoid receptor—directed therapies) (Table 2). Theoretically, avoiding unnecessary immunosuppression and side effects is possible with topical delivery methods [61], but most are formulated to be effective only against superficial skin conditions affecting the epidermis and papillary dermis. Effective topical delivery in ScGVHD may be hampered by increased dermal thickness and possibly by lower permeability. Strategies to improve drug delivery include physical approaches (microneedles, laser, iontophoresis), particle-based drug carriers (lipid-based, nanoparticles) and chemical approaches (permeation modifiers, prodrugs) [62]. Precision medicine with engineered cell therapies targeting fibrosis have been explored in other diseases [63] and could be considered in ScGVHD. Multitargeting approaches may be helpful to prevent evolution to sclerosis and to enhance safety without compromising efficacy [64].
Table 2.
Candidate Therapeutic Agents in Sclerotic Chronic GVHD
| Target | Drug(s) | Target Cellular Subsets | Clinical Development Status | References |
|---|---|---|---|---|
| Connective tissue growth factor/cellular communication network factor 2 | Pamrevlumab (FG-3019) | Fibroblasts | Phase 3–Idiopathic Pulmonary Fibrosis (NCT01890265) | [248, 249] |
| Autotaxin | Ziritaxestat (GLPG-1690) | Fibroblasts | Phase 3–Idiopathic Pulmonary Fibrosis (NCT03733444, NCT03711162) | [250] |
| Heatshock protein (HSP47) | ND-L02-s0201 | Fibroblasts | Phase 2–Idiopathic Pulmonary Fibrosis (NCT03538301) | [61, 251, 252] |
| Pentraxin 2 (agonist) | PRM-151 | Fibroblasts Macrophages |
Phase 2–Idiopathic Pulmonary Fibrosis (NCT02550873) Phase 2–Myelofibrosis (NCT01981850) |
[253, 254] |
| CB2R (agonist) | Lenabasum (Ajulemic acid) | Fibroblasts T cells Macrophages |
Phase 3–Systemic Sclerosis (NCT03398837) | [52] |
| CB2R/Peroxisome proliferator-activated receptor (Dual Agonist) | EHP-101 | Fibroblasts Endothelial cells Macrophages |
Phase 2–Systemic Sclerosis (NCT04166552) | [255] |
| CB1R/inducible nitric oxide synthase (dual antagonist) | MRI-1867 | Fibroblasts T cells Macrophages |
Phase 1 | [49, 256, 257] |
| Oncostatin M (antagonist) | GSK2330811 | Fibroblasts Endothelial cells T cells Macrophages |
Phase 2–Systemic Sclerosis (NCT03041025) | [258, 259] |
| TGFβ | AVID200 | Fibroblasts T cells Macrophages |
Phase 1–Myelofibrosis (NCT03895112) Phase 1–Systemic Sclerosis (NCT03831438) | [260] |
| IL-6R | Tocilizumab | Fibroblasts T cells Macrophages | Phase 3–Systemic Sclerosis (NCT02453256) Phase 2–Steroid dependent immune related adverse events (NCT04375228) |
[261–263] |
| CSF-1R | Axatilimab (SNDX-6352) | Macrophages | Phase 2–Chronic GVHD (NCT03604692) | [26] |
| ROCK2 | Belumosudil (KD025) | T cells Macrophages | Phase 2–Chronic GVHD (NCT03640481, NCT02841995) Phase 2–Systemic Sclerosis (NCT03919799) |
[264] |
| Interferon receptor type 1 | Anifrolumab | T cells Macrophages | Phase 3–Systemic Lupus Erythematosus (NCT02446899) Phase 2–Rheumatoid Arthritis (NCT03435601) |
[265] |
CB2R indicates cannabinoid receptor.
Highest Priorities and Roadmap for progress for ScGVHD
Longitudinal multicenter studies to evaluate pathologic cell populations in lesional and unaffected skin and peripheral blood, and cytokine and chemokine responses, should be done to identify early signatures for subsequent fibrosis and to identify additional target pathways.
Capitalize on the enhanced resolution of next generation sequencing strategies, including single-cell RNA-, assay for transposase-accessible chromatin (ATAC)-, TCR-, and BCR-sequencing to query skin biopsies to provide biological insight into the mediators of ScGVHD in individual patients, address the degree of temporal and clinical disease heterogeneity, and the origins (recipient versus donor) and phenotype of clonally expanded T- and B-cell populations. These investigations could be complemented by new proteomic technologies such as multiplexed ion beam imaging by time of flight (MIBI-TOF) [65] combined with nonlinear dimensionality reduction analysis approaches (visualization of t-distributed stochastic neighbor embedding [tSNE]/[viSNE]).
Analyze differences in expression and the spatial distribution of mediators and targets within the epidermis, dermis, subcutaneous fat, and fascia in an effort to understand differences in clinical presentations and identify interventions that could be personalized.
Test emerging therapies being developed for organ fibrosis and supported by biological insights in ScGVHD, focusing on early intervention. Promising candidates are listed in Table 2. Combination therapies targeting multiple pathways active in fibrosis should be considered to augment efficacy while minimizing toxicities aiming to stop or potentially reverse fibrotic changes.
Develop novel cost-effective tools for better measurement and documentation of change in skin sclerosis for clinical trials. Refinements of the current 2014 clinical response criteria are needed for skin sclerosis/fascia manifestations.
PULMONARY INVOLVEMENT
Current clinical knowledge
Bronchiolitis obliterans syndrome (BOS) is the only formally recognized manifestation of lung chronic GVHD, with an incidence of 3% to 10% of allogeneic hematopoietic cell transplant (HCT) recipients [66–68], and 14% [69] in those with chronic GVHD. Although the histologic entity of obliterative bronchiolitis is the diagnostic lesion of lung GVHD, clinical diagnosis is largely based on pulmonary function studies that cannot be performed in children under age 7 [70]. Risk factors for onset include antecedent respiratory viral infections [71,72] and impaired lung function early after transplantation [68,73]. Worse prognosis is associated with early onset after transplantation and severe impairment of forced expiratory volume first second (FEV1) at diagnosis. Contemporary series show a 2-year survival rate of 70% after BOS diagnosis [74], and 5-year survival remains low at approximately 50%, highlighting the need for novel prevention and treatment strategies [68].
Pathophysiology
The pathology of BOS is characterized by fibrotic narrowing and obstruction of small airways, likely the shared outcome of immune and non-immune mediated injury to the airway epithelium. A fundamental knowledge gap, however, lies in understanding the triggers of lung epithelial cell injury and subsequent mechanisms of altered immune and fibrotic responses that result in obliterative bronchiolitis. Mechanisms being explored in other disease contexts include airway stem cell depletion [75] and acquisition of a persistent inflammatory airway epithelial cell phenotype [76,77]. The immune dysregulation associated with BOS after lung allograft or HCT appears to involve oligoclonal expansion of CD4+ T cells, reduced T regulatory cells, and higher levels of IL-17 and IL-8 [78]. In one murine model, alternatively activated macrophages drove BOS, supported clinically by evidence of leukotriene production, and polarized CD4+ immune activation [26]. In another preclinical model, donor B cells contribute to airway pathology through local antibody production. In this model, genetic disruption of germinal center formation, which is supported by T follicular helper cells [79], reduced pulmonary dysfunction [80]. These mechanistic insights have not yet been confirmed in humans, although biomarker studies support a prominent role of B cells with significantly elevated CD19+CD21low B cells and high soluble B-cell activating factor levels [81]. The role of the microbiome, which is influential in other airway diseases, should be investigated [82].
Physiological subtypes
Defining clinical phenotypes of BOS remains a significant knowledge gap that hampers our ability to identify patients at risk for morbidity and death from lung GVHD. Current NIH spirometric criteria used for BOS diagnosis defines this disorder based upon airflow obstruction. Although this definition encompasses most cases of BOS, it is unlikely to reflect the full spectrum of physiologic and histologic manifestations of BOS, nor does it facilitate identification of early disease [83–85]. A concerning pattern is reduced FEV1 and forced vital capacity (FVC) with normal FEV1/FVC ratio [83], potentially reflecting impaired exhalation caused by air trapping by small airways obstruction, resulting in a pattern that suggests restriction. This pattern underlines the need for a complete evaluation including lung volumes and a high-resolution chest computed tomography scanning in expiration. An open question remains whether lymphocytic bronchiolitis, which is responsive to anti-inflammatory agents [84], represents early phase of disease, a distinct subtype of BOS, or is a separate entity from BOS. Although some patients demonstrate stability of FEV1 after clinical recognition, this plateau could be due to treatment, a distinct biology, or the stage of the disease at diagnosis [69,74]. More significantly, the clinical and biological risk factors for progressive refractory lung function decline are not known.
The association of chronic GVHD with restrictive lung impairment remains ill-defined for HCT survivors and is not currently recognized as a chronic GVHD manifestation. Restrictive allograft syndrome is a phenotype of chronic lung allograft dysfunction (CLAD) in lung transplantation recipients, and is defined by a reduction in FVC or total lung capacity (TLC) with persistent lung infiltrates and carries a worse prognosis than classic BOS [86–89]. Although a similar entity is suspected to occur after HCT, confounding diagnoses for restrictive physiology and the lack of validated diagnostic criteria in the context of chronic GVHD have been barriers to recognition [89]. Although restriction may be due to known interstitial lung disease entities including organizing pneumonia, restrictive lung impairment in chronic GVHD can also be caused by extraparenchymal processes including truncal sclerosis [90], respiratory muscle weakness [85,91], or pleural effusions. In severe BOS, histological evidence of interstitial pathology is often associated with the bronchiolar lesions [85], suggesting that interstitial abnormalities, in addition to airway pathology, are part of the spectrum of lung chronic GVHD. Table 3 summarizes the spectrum of lung abnormalities, diagnostic criteria and association with chronic GVHD after HCT.
Table 3.
Pulmonary Syndromes After Allogeneic HCT
| Entity | Established in definition of lung GVHD | PFT pattern | High Resolution Chest CT Findings | Lung Histology | Comment |
|---|---|---|---|---|---|
| Bronchiolitis obliterans syndrome | Yes | Fixed obstructive pattern: FEV1 decline >10%, FEV1/FVC < LLN. Elevated residual volume or residual volume/Total Lung Capacity. FEV1/FVC > LLN with preserved TLC may be seen. DLCO may be normal or reduced. | Signs of air trapping (mosaic attenuation on expiratory phase) or bronchiolitis (centrilobular micronodules, bronchial wall thickening) or late sequalae (bronchiectasis) | Obliterative bronchiolitis: partial or complete fibroproliferative occlusion of terminal small bronchioles, lymphocytic bronchiolitis may also be seen | Subtypes of BOS have been defined based on timing of onset after HCT, initial tempo of onset, FEV1 decline, histology, response to therapy, and prognosis. |
| Restrictive impairment caused by ILD entities* | |||||
| Organizing pneumonia [267] | No, but evidence supports association with acute and chronic GVHD. | Restrictive impairment with reduced TLC with FEV1/FVC > LLN most common. Obstructive or mixed pattern may be seen. Reduced DLCO. | Patchy and peribronchial infiltrates or consolidation, and reticular ground glass opacities, often predominant in upper lobes and periphery [268] | Bronchiolar and alveolar granulation tissue | Bronchoscopy should be performed to rule out infection. Clinical diagnosis often made without lung histology and is empirically based on steroid-responsiveness |
| Nonspecific interstitial pneumonia [266] | No | Reduced TLC and DLCO | Confluent bilateral lower lobe ground glass opacities, bronchiectasis and lower lobe volumes loss, classically sparing the subpleural area | Diffuse alveolar wall thickening by uniform fibrosis; interstitial inflammation | Bronchoscopy should be performed to rule out infection |
| Pleuroparenchymal pulmonary fibroelastosis [266,269] | No | Reduced TLC and DLCO, occasionally obstructive and restrictive pattern. Progressive and severe impairment over time | Upper lobe fibrosis with subpleural and pleural thickening, loss of lung volume, and lower lobe traction bronchiectasis | Subpleural and pleural fibroelastic proliferation with minimal inflammation | Diagnosis is usually made by typical chest CT findings |
| Restrictive impairment not attributed to ILD† | |||||
| Truncal sclerosis | No. Sclerosis due to chronic GVHD is an indirect cause of ventilatory impairment | Reduced TLC; RV/TLC may be elevated but usually does not necessarily indicate small airways disease | No parenchymal infiltrates. Parametric response mapping shows low inspiratory volumes. | N/A. | |
| Respiratory muscle weakness | No. Weakness may result from chronic GVHD-related myositis or prolonged steroid use to treat chronic GVHD. | Concomitant reduction in FVC and FEV1, reduced TLC with relative sparing of RV. Reduced supine FVC. Maximal inspiratory and expiratory pressures may be reduced. | Low lung volumes, normal parenchyma. If diaphragmatic weakness or paralysis is suspected, a fluorographic sniff test may show reduced diaphragmatic excursion | N/A. Evidence of myositis in a peripheral muscle. | Diagnosis of exclusion |
RAS has been defined for lung transplantation [89] as a manifestation of chronic allograft dysfunction. BOS is the obstructive form of chronic lung allograft destruction after lung transplantation. RAS after lung transplantation is defined by restrictive physiology and persistent pulmonary infiltrates that represent heterogeneous histology. A similar syndrome of restrictive impairment as a manifestation of alloimmunity in the context of chronic GVHD may also exist; however, the epidemiologic associations and definitions remain to be determined. It is possible that ILD entities that occur in the context of chronic GVHD could be considered as an “RAS-like” condition, or “restrictive alloimmune syndrome” after HCT.
ILD indicates interstitial lung disease; RAS, restrictive allograft syndrome.
Multiple entities, as per the ATS/ERS classification of ILD may occur after HCT, beyond what is listed here [85,266]. If restrictive impairment is seen on PFT (i.e., reduced FVC with preserved FEV1/FVC and reduced TLC), high resolution chest CT should be performed to evaluate for ILD and other entities.
These entities are secondary to extrathoracic consequences of chronic GVHD.
Treatment
Treatment for manifest BOS is aimed at stabilizing lung function, which reflects the sobering observations that diagnosis is usually made at a later stage of disease, and no therapies that reverse the end-stage lesion of obliterative bronchiolitis have been established. Nonetheless, efforts at early recognition and intervention may be able to reverse BOS [92]. The combination of inhaled corticosteroids (fluticasone), azithromycin, and montelukast, with or without a long-acting bronchodilator, has been established as organ-specific therapy for BOS [93,94]. Observations of potential impaired graft-versus-leukemia effects associated with prophylactic azithromycin given in the early posttransplant period raised concerns about its use to treat BOS [95]. A subsequent analysis of patients treated for manifest BOS did not show an increased risk for relapse [96]. Despite these treatments, lung function continues to decline in a significant proportion of BOS patients [97], and intensified immunosuppression contributes to lung infections, which in turn worsen lung function supporting the need for antimicrobial prophylaxis and pulmonary rehabilitation [98,99]. Agents that are currently under investigation have shown utility in other chronic lung conditions such as pulmonary fibrosis and include inhaled immunosuppressants [100] and anti-inflammatory or antifibrotic agents [101].
Highest priorities and roadmap for progress in pulmonary chronic GVHD
- Understand the onset and evolution of lung GVHD. A prospective longitudinal multicenter patient study cohort including adults and children followed up from the time of onset of chronic GVHD, is essential to comprehensively identify biologic triggers (e.g., viral infections), enable discovery of biomarkers for early diagnosis, provide biospecimens for translational mechanistic studies and microbiome analysis, and define lung GVHD subtypes (Figure 1).
- Serial pulmonary function tests (PFTs) and chest computed tomography [90,102] with quantitative lung imaging techniques (e.g., parametric response mapping) are clinical tools that could be implemented immediately as part of clinical care to delineate phenotypes and physiologic biomarkers that associate with BOS. Machine learning approaches that combine serial data from PFTs, imaging, and clinical risk factors might identify scenarios that predict high risk [103]. Additional modalities including hyperpolarized xenon-129 magnetic resonance imaging for the early detection of small airways disease in children should be further explored [104].
- The creation of a shared lung-specific biorepository will support biomarker discovery and mechanistic studies. Given the inherent challenges of procuring surgical lung tissue, universal protocols should be implemented to systematically collect excess bronchoalveolar lavage and lung biopsy specimens obtained during clinical care. Less invasive means of sampling airway epithelium, e.g., bronchial brushings, and development of validated serum or plasma-based assays should be explored [102]. Coupling these samples with carefully annotated clinical databases will be critical.
Test strategies for early diagnosis and novel treatments in clinical trials. Early diagnostic strategies coupled with preemptive treatment with targeted agents should be evaluated to avert severe BOS forms and potentially reverse obstruction before progression to advanced fibrosis. Novel therapies for established BOS need to be tested in clinical trials that are informed by knowledge of BOS evolution and an understanding of pathogenesis and biomarkers of response, which is possible only with a longitudinal prospective cohort. Clinically relevant endpoints include FEV1 stability (or lack of progression of FEV1 decline), infectious exacerbations, exercise tolerance, quality of life, reduction of systemic steroid use, and overall survival.
GASTROINTESTINAL INVOLVEMENT
Current clinical knowledge
Historically, the intestine has been less commonly affected by chronic GVHD, which may be partly explained by lack of documentation of GI involvement in the past. The 2005 NIH consensus (updated in 2014) provided for the first time a definition and severity grading for GI manifestations in the context of chronic GVHD [105]. The 2014 consensus requires the presence of other diagnostic or distinctive manifestations to distinguish chronic GVHD from acute GVHD diagnosis in patients with upper and lower GI symptoms (loss of appetite, diarrhea) that are typical of acute GVHD. However, the 2014 NIH organ scoring of chronic GVHD does not distinguish between the site of GI involvement (esophagus, upper GI, and lower GI), although the response criteria provide a more detailed framework for reporting and grading these manifestations [105,106]. Applying the NIH criteria, the respective incidence rates of esophageal, upper GI, and lower GI involvement are 16%, 20%, and 13%, according to a cross-sectional analysis from the Chronic GVHD Consortium [107]. Most importantly, intestinal involvement is associated with greater risk of non-relapse mortality and patients with histologically confirmed severe lower GI involvement as part of chronic GVHD are usually treated with regimens recommended for management of acute GVHD [108–110]. Of note, esophageal web or strictures or stenosis of the upper to mid third part are the only manifestations regarded as diagnostic signs of chronic GVHD [105]. Major limitations in diagnosis and management of GI symptoms such as nausea, loose stool and anorexia include multiple potential causes unrelated to GVHD [111] (i.e., maldigestion, toxic effects of medication, autonomic nervous system dysfunction, bacterial overgrowth, endocrinological sequelae, etc.). Histopathology may not be able to completely resolve diagnostic uncertainty due to limited sampling, patchy involvement and nonspecific histological abnormalities in mild cases [112,113].
Risks factors for intestinal involvement in chronic GVHD remain to be elucidated. Ethnicity, genetic diversity, environmental differences, diet, antibiotic use, supportive care, and microbiota or microbe-derived metabolites may all influence GI GVHD [114–118]. Age is a potential risk factor because children appear to be particularly susceptible to late GI-acute GVHD which affects up to 25% of pediatric transplant recipients [119] and can persist to and beyond the diagnosis of chronic GVHD. A small study showed that increased relative abundance of butyrogenic bacteria after the onset of acute GVHD was associated with subsequent steroid-refractory acute GVHD or chronic GVHD [118] indicating the need for further investigations of dysbiosis and antibiotic strategies and their association with GI-chronic GVHD and other manifestations of the disease [117]. GI manifestations of chronic GVHD may have complex causes but are rarely directly fatal, and the mechanisms that increase the subsequent risk for nonrelapse mortality remain to be elucidated.
Pathophysiology
In many tissues chronic GVHD is characterized by atrophy and destruction with subsequent fibrosis, but intestinal fibrosis is a rare GI-manifestation of chronic GVHD [120,121]. Intestinal epithelium is the most rapidly self-renewing tissue in adults; intestinal epithelial cells are continuously regenerated from intestinal stem cells, which are key to the regeneration of damaged intestinal epithelium [122]. Tissues having squamous epithelium (e.g., skin, mouth, esophagus, and vagina) and tissues having cuboidal epithelium (e.g., sweat, lacrimal, and salivary glands) appear to be more prone to dysregulated fibrosis in chronic GVHD than those having columnar epithelium (e.g., stomach, intestine, and trachea). Animal studies have demonstrated that both intestinal stem cells and their Paneth cell niche are impacted in acute GVHD, with impaired regeneration of the injured epithelium [123–129]. The rapid and potent repair ability of the intestine may protect against early fibrotic processes that often accompany repair processes in other tissues. Profiling of immune cell populations and plasma markers at day 100 after HCT demonstrated biological differences between chronic GVHD and late-onset acute GVHD [130].
Highest priorities and roadmap for progress in gastrointestinal chronic GVHD
Enforcement of the NIH 2014 classification terminology distinguishing acute from chronic GVHD within and across studies [112,131–136] since current longitudinal observational and clinical trials revealed a significant number of incorrectly classified patients [119]. According to the NIH 2014 terminology any patient developing diagnostic or distinctive signs of chronic GVHD during treatment of acute GVHD should be classified as having chronic GVHD with documentation of all manifestations. The presence and severity of individual GI manifestations (esophagus, upper GI, lower GI) should be also recorded and the use of the 2014 response criteria form to document individual GI manifestations is necessary at the time of diagnosis and in response to therapeutic strategies. Electronic tools such as the GVHD App may assist [137]. Adhering to these terminology will allow more reliable future studies of GI GVHD.
Develop diagnostic tools (i.e., blood and/or histopathological biomarkers among others) to differentiate GVHD from other causes of GI-symptoms.
Generate experimental and clinical models able to address the role of dysbiosis and intestinal inflammation in chronic GVHD involving organ manifestations outside the GI tract.
Collect blood, stool and or other body sites samples (e.g., saliva) and GI biopsies in either longitudinal observational cohorts or interventional clinical trials to allow development of biomarkers [138–140], through metabolomic alterations and microbiome compositions with sufficient sampling. These studies should include follow-up of acute GVHD trials.
OCULAR INVOLVEMENT
Current clinical knowledge
Chronic ocular GVHD (oGVHD) is one of the most frequent, rapidly-progressive organ manifestations with characteristic inflammatory, immune dysregulatory and fibrotic pathophysiological mechanisms [31,141–143]. Ocular GVHD is usually diagnosed between 5-24 months after HCT [144–146], and it can severely impact quality of life and vision [147,148] because of severe symptoms such as burning, dryness [105,149–151], and loss of visual function [152]. Pre-existing dry-eye disease (DED) and Meibomian gland disease as a consequence of chemotherapies or possibly irradiation increases the risk for later oGVHD [153,154]. Early after transplantation, some patients already have impaired tear quantity and quality [155], yet eye involvement is recognized only after damage exceeds the eye’s ability to compensate. Most importantly, oGVHD is not another form of DED, and approaches and therapies for DED may fail in oGVHD. Table 4 summarizes the differences between DED and oGVHD.
Table 4.
Differences Between Chronic Ocular GVHD and Dry Eye Disease
| Dry-eye disease (DED) | Ocular GVHD (oGVHD) | Clinical trial endpoint consideration in oGVHD | ||
|---|---|---|---|---|
| Cause | ||||
| Known immunological mechanisms | Autoimmune Th17, CD4+/CD8+ T-cell activation through extrinsic or intrinsic triggers, unknown antigen | Migration and activation of donor hematopoietic /mesenchymal stem cells | Inclusion of participants before onset of disease possible | |
| Meibomian gland dysfunction (MGD) | Caused by numerous factors (aging, rosacea, drugs) leading to evaporation caused by MGD | Caused by chemotherapy and oGVHD, leading to evaporation | MGD as secondary endpoint | |
| Fibrosis | Not typical for dry-eye disease (see below) | Early activation of fibroblasts and macrophages | Fibrosis as clinical endpoint feasible assessed by slit lamp microscopy of fornix and tarsal conjunctiva | |
| Other causes | Numerous: systemic drugs, contact lens wear, aging, etc. | Presumed: chemotherapy and/or conditioning procedures | Pre-treatments and underlying oncological disease, origin of donor cells, should be considered during stratification | |
| Time course | Onset mostly unknown, slow progress in a majority of cases, over years to decades | Rapid manifestation after HCT, progresses within weeks to months | Preventive clinical trials vs. therapeutic clinical trials feasible | |
| Impact on visual function | Mild to severe impact, blinding disease very rare | Mostly severe; if untreated, often blinding disease | Primary endpoint | |
| Clinical findings (selection of typical findings) | ||||
| Tear production | Reduced in aqueous deficient DED and in overlap (mostly slow onset) | Reduced (fast onset, rapid progression) | Secondary endpoint | |
| Blepharitis | Mostly mild/moderate | Mostly severe | Secondary endpoint | |
| Meibomian gland dysfunction | Up to 80% in DED | Up to 100% in oGVHD | Unsuitable endpoint, as currently unclear mechanism | |
| Corneal and conjunctival intravital staining | Mild to severe | Mostly severe | Due to higher severity different grading systems needed to assess treatment success using staining as endpoint | |
| Conjunctival redness | Mild to severe | Mostly severe | Secondary endpoint, detection and grading systems should be validated | |
| Fibrosis | Rare finding, associated with severe rosacea, atopic keratoconjunctivitis or ocular cicatricial pemphigoid | Frequent finding | Primary or secondary endpoint, detection and grading systems need to be validated | |
| Filamentary keratitis | Rare finding, only in severe cases, mostly Sjögren Syndrome | Common finding, presumably related to activation of innate immune system | Primary or secondary endpoint | |
| Superior bulbar and limbal keratoconjunctivitis | Rare finding, distinct entity not typically related to DED | Frequent finding | Secondary clinical endpoint | |
| Intraocular involvement | Not related to DED | Intraocular involvement reported | Secondary endpoint in subgroup analysis is possible | |
| Correlation between signs and symptoms | Low correlation: strong symptoms, weak clinical signs | Low correlation: weak symptoms, strong clinical signs | Development of suitable symptom questionnaires for oGVHD is necessary |
Ocular GVHD mainly presents as ocular surface disease demonstrating features such as blepharitis, Meibomian gland disease, qualitative and quantitative alteration of tear film, loss of goblet cells, corneal and conjunctival epitheliopathy, corneal vascularization and fibrosis of ocular tissues including conjunctiva and lacrimal glands [144,156–159]. In addition, a few reports have described intraocular involvement including choroid and retina [160,161]. However, no specific signs that are currently diagnostic for oGVHD, although certain combinations of findings, such as conjunctival subepithelial scarring and superior bulbar and limbal keratoconjunctivitis are frequently present [143,162–164]. Without early diagnosis and appropriate treatment, oGVHD progresses towards loss of visual function by complete loss of aqueous tear production and tear film stability, and scarring of the cornea. The impaired epithelial barrier can lead to complications such as infection, corneal ulceration and melting, and endophthalmitis. High-risk corneal transplants fail frequently presumably due to immunological rejection and impaired tear production, eventually resulting in loss of the eye [165–168].
The 2013 International Chronic Ocular GVHD Consensus Group (ICOGVHD 2013) Diagnostic Criteria filled an existing gap by adding recommendations for specific examinations to be performed by eye care specialists [150,169] to previous NIH consensus criteria [170]. The 2013 classification facilitates diagnosis of oGVHD by providing a structured clinical approach for distinguishing definite oGVHD from probable or “none” categories. However, the 2013 classification is not designed to detect preclinical oGVHD or to assess severity, and it does not translate into the NIH 0-3 eye score. Other grading systems have been suggested and validated [171], but they have not yet been established internationally.
Pathophysiology
Conditioning chemotherapy, radiation and infection often precede the onset of oGVHD and may induce homing signals for mobilization and migration of circulating bone marrow derived hematopoietic cells and mesenchymal stromal/stem cells into the microenvironment of the ocular surface and lacrimal gland. However, it is not understood how innate and adaptive immune mechanisms are triggered and how these common mechanisms initiate oGVHD only in selected patients. Studies show increased concentrations of ICAM-1, IL-1β, IL-6, IL-8 [172,173], neutrophil extracellular traps (NETs) [142], extracellular DNA [174,175] and decreased concentrations of lactoferrin [176], DNAse [175], IL-7, and epidermal growth factor (EGF) [173] in the tear film. In lacrimal glands affected by oGVHD, early fibrosis and myxedematous tissue may herald a rapidly progressive fibrosis [143] with activated fibroblasts already infiltrating into the lacrimal gland. Stromal fibroblasts in the lacrimal gland and conjunctiva interact with pathogenic T cells and antigen-presenting cells including macrophages [143,177], resulting in the proliferation and activation of fibroblasts through cytokines, such as IL-4, IL-6, and IL-17 [178,179]. Macrophages and fibroblasts activated through both the classical immunological and sterile inflammatory pathways involving NETs [142] and extracellular DNA from the damaged tissue [175], activation of the endoplasmic reticulum stress pathway [180] and the tissue renin angiotensin system [181] synthesize an excessive amount of extracellular matrix, resulting in rapid interstitial inflammation and fibrosis [179,182,183]. The limited knowledge about key pathological mechanisms translates into the current lack of biomarkers for early diagnosis of oGVHD and the absence of effective topical and systemic anti-inflammatory, antifibrotic, and possibly preventive therapies.
Information from animal models and clinical analyses
Several animal models have been used to study biology, onset, time course, and therapies for oGVHD [177,184–189]. These models showed that donor derived T cells infiltrate the cornea and lacrimal glands and lead to an oGVHD phenotype [187,189] with subsequent fibrosis. Perez et al introduced a scoring system for murine models of oGVHD [184]. Several preclinical studies tested potential therapeutics such as siRNA [190], bromodomain inhibitors [191], rebamipide [192], vascular adhesion protein-1 [193], and a spleen tyrosine kinase inhibitor [194]. Because clinical signs in oGVHD are also present in isolated forms in other ocular disease (e.g., conjunctival fibrosis in ocular cicatricial pemphigoid or chronic allergic keratoconjunctivitis), it may be necessary to use such models [195,196] as comparators in experimental studies to distinguish organ-specific chronic GVHD pathologies from secondary, damage-related disease.
Gaps, highest priorities, and roadmap for progress in oGVHD
Currently, no treatments have been specifically approved for treatment of oGVHD, in part because the clinical evolution of oGVHD is largely undefined and the innate and adaptive immune mechanisms that trigger and sustain oGVHD are incompletely understood. Furthermore, oGVHD clinical trials are challenging because of the lack of well-defined and specific primary efficacy outcome measures, and small sample sizes. Clinical metrics, such as the Schirmers test or intravital staining of the ocular surface, that are established for diagnosing generic DED, should be amended with specific oGVHD metrics and defined for better application as clinical endpoints. Gaps in clinical management include uncertainty regarding whether to perform baseline examinations before HCT and then refer patients for scheduled reevaluations or for evaluations only as needed after HCT. Another uncertainty is whether to start oGVHD treatment with aggressive anti-inflammatory and immunosuppressive topical therapy followed by tapering based on improvement (step-down treatment) or to start with lubrication therapy followed by escalation based on progression or lack of improvement (step-up treatment).
HIGHEST PRIORITIES AND ROADMAP FOR PROGRESS IN CHRONIC OCULAR GVHD
Establish early diagnostic criteria (clinical signs and biomarkers) distinguishing oGVHD from other forms of DED so that appropriate interventions can be promptly instituted. This revision requires a better understanding of the immunopathology derived from appropriate animal models for oGVHD that mimic the human situation as closely as possible. These animal models should also be used to identify therapeutic targets and to enable preclinical testing of promising drug candidates and identification of functional connections between organ systems that are sequentially or simultaneously affected by chronic GVHD.
Identify biomarkers associated with active oGVHD at the earliest possible time points. As the eye is easily accessible, tear film or impression cytology can be tested. Besides cytokines and genetic markers, optical biomarkers may be useful, including optical coherence tomography or confocal microscopy that can be used noninvasively.
Develop and validate efficacy outcome measures that are specific for oGVHD clinical trials. Preferential primary outcome measures are corneal fluorescein staining and ocular discomfort measured by visual analog scale or ocular surface disease index until more appropriate measures that assess specific interventions (e.g., punctal plugs, contact lenses, serum eye drops, amniotic membrane transplantation) are identified. Such measures should distinguish ophthalmologist-driven tools from assessments that can be done in the hematologist’s office. Clinical trial designs that circumvent the challenges imposed by limitations of small sample size are also needed (Figure 2).
Conduct eye-targeted studies, for example, (a) punctal occlusion or not; (b) referral as-needed for eye care versus prescheduled frequent follow ups; (c) step down (start treating aggressively then taper) versus step up (escalate treatment based on response).
Evaluate systemic treatment options for efficacy in oGVHD. Currently oGVHD is treated with topical interventions independently of other organ manifestations despite obvious similarities in the pathophysiology. A systematic analysis of ocular effects of systemic immunosuppression is needed.
OTHER MORBID CONDITIONS
Other conditions that are either part of NIH-defined chronic GVHD or occur in association with chronic GVHD require further research efforts. These include genital involvement, which occurs more frequently than reported in large registries due to the lack of routine screening [197], oral manifestations that impair quality of life and may increase the risk for secondary malignancies [198], isolated fasciitis [199], and wasting syndrome not explained by GI manifestations. Although these are NIH consensus-defined conditions, limited understanding of organ-specific pathophysiology prevents the development of targeted treatment approaches. Moreover, associated syndromes seen with chronic GVHD [200,201], such as polyserositis, which occurs infrequently but is difficult to treat [202], immune mediated cytopenias and renal complications (e.g., glomerulonephritis, nephrotic syndrome) require more study. All have in common the lack of knowledge of their incidence, their specific pathophysiology and relationship in the context of chronic GVHD.
In addition, other potential organs may also be targeted by chronic GVHD, but the exact relationship has not been established. For example, central nervous system dysfunction is reported by a significant percentage of long-term survivors mainly as cognitive dysfunction [203]. It remains to be established whether cognitive dysfunction is caused by cumulative neurotoxicity and acute GVHD, as demonstrated in experimental models and clinical investigations [204–206], or whether chronic GVHD further contributes. Rare cases of chronic GVHD with acute disseminated encephalomyelitis have been reported [161,207]. Similarly, peripheral nervous system dysfunction is seen in a high proportion of chronic GVHD patients [8,9,208], but a relationship to alloimmunity has not been established. Autonomic nervous system dysfunction with dry mouth or eyes, dry skin, obstipation, diarrhea, and sweating disturbances are of interest due to overlap with symptoms of chronic GVHD. For example, impaired sensitivity of the ocular surface has been reported after HCT [209]. Endothelial dysfunction could be part of the pathophysiology of chronic GVHD in a variety of organs based on experimental [210–212] and clinical evidence [213,214] and may contribute to long term cardiovascular morbidity and mortality [215,216] Therefore additional study is warranted.
OTHER KNOWLEDGE GAPS
An additional gap is the limited knowledge of the age-dependent disease features and associated morbidity and mortality of chronic GVHD in children and individuals older than 70 years, 2 populations that are especially vulnerable to comorbidity induced by chronic GVHD. Although chronic GVHD in children may be difficult to diagnose, chronic GVHD manifestations involving the lungs and eyes [119] and other sequelae may have significant life-long consequences [217–219]. In older patients, chronic GVHD has been associated with decreased physical functioning [220], but the detailed contributions of chronic GVHD to mortality and potential insights for prevention and treatment of chronic GVHD in older patients are unknown. Moreover, while preliminary data indicate that racial and ethnic background are associated with long term outcomes including GVHD, large studies are lacking [114,116,221]. Finally, clinical care of morbid forms of chronic GVHD requires long-term care engagement of a multidisciplinary team [222,223]. Development and evaluation of survivorship care structures to provide access to multidisciplinary subspecialty care taking into account the socioeconomic and travel situations of individual patients remain an urgent research need [224].
STUDY DESIGN CONSIDERATIONS
Because of the rare incidence and limited prevalence of the highly morbid conditions, feasibility is a concern in clinical trials, and novel approaches to clinical investigation are needed [225–228]. Careful selection of endpoints that can reliably demonstrate objective clinically significant benefit with a realistic number of patients is critical. Studies should be designed with attention to sample size, statistical power, and control of bias. A detailed discussion of innovative trial designs is beyond the scope of this paper, but we offer the following recommendations.
Careful consideration of eligibility criteria utilizing enrichment strategies based on diagnostic criteria, phenotype, or biomarkers [229] may identify a smaller but more informative study population where a drug effect can be observed [230]. Patients not meeting the eligibility criteria may be treated in observational cohorts.
Some established chronic GVHD manifestations may be permanent, and a worthy goal could be “stable disease or improved trajectory” (reflecting prevention of new damage) or functional or symptom improvement instead of partial or complete response. These endpoints require acceptance that lack of worsening or improved patient functioning or patient-reported outcomes are meaningful clinical benefits in a given patient even if chronic GVHD organ function does not measurably improve. Lack of worsening can be documented in comparison to concurrent or historical controls [231] or the patient’s prior trajectory if well documented in real time.
Although a nonrandomized single arm study, without concurrent controls, may seem attractive, this design is necessarily less precise, and outcomes are less definitive. Alternatives to consider include use of historical controls or using each patient as their own control. Single case experimental design or N-of-1 trials may be the most feasible option for the very rare highly morbid forms of chronic GVHD. In such trials, each individual participant serves as their own control, and may receive multiple interventions in a crossover fashion. Multiple N-of-1 studies may then be combined in a meta-analysis. Of note, efforts should be made to document the course and response using objective response measures focused on clinically meaningful changes.
Efficiency of study design should be optimized. The more complex designs are adaptive [232–234], with the design being modified during the conduct of the study according to pre-specified rules to increase efficiency. For example, a Bayesian approach [235] is a statistical inference framework for leveraging existing data from different sources, synthesizing evidence of different types, including retrospective data and information gained during the conduct of the study. In particular, the data deficits of “small” clinical trials can be mitigated by incorporating past information. The combination of observed data and prior opinion is governed by Bayes’ theorem and can result in smaller sample sizes needed to reach conclusions. The major criticisms of the Bayesian approach are the uncertainty regarding the prior probability and the subjective interpretation of results since formal significance testing is not required, although this problem could be addressed by using independent or blinded assessors.
Optimize data analysis strategies, for example, by using more efficient continuous outcomes when the sample size is small. Consider longer duration of studies and use covariate adjustment, such as statistical stratification. Consider whether the distribution is likely to be parametric (modeled by a probability distribution that has a fixed set of parameters) or non-parametric when designing the analysis plan.
When multiple agents are available, consider efficient study designs to rank the agents and eliminate less-effective ones through futility or selection designs.
Selection of the primary endpoint depends on the mechanism of action and targeted manifestations (e.g., if an antifibrotic agent is tested to target sclerotic lesions, response of inflammatory manifestations may be captured only as a secondary endpoint). However, all systemic and topical agents given and all changes in organs should be recorded. Evaluation of agents given systemically, even if targeted to a single specific organ manifestation, requires documentation of all other organ manifestations since broader effects cannot be excluded. The same documentation of systemic immunosuppression is needed in studies that evaluate topical agents. Protocols should specify how non-study systemic and topical agents are handled and how responses in nontargeted organs are interpreted. In addition, efficacy measures developed for studies of comparable diseases other than chronic GVHD may also be evaluated in chronic GVHD [236]. Last but not least, predictive biomarkers indicating response to specific treatments should be developed and validated.
Efforts should be made to enhance access of children, older patients and racial and ethnic minorities to clinical trials since these characteristics are relevant covariables. Inclusion of pediatric cohorts into adult trials should be considered if feasible.
Rare manifestations (e.g., glomerulonephritis, restrictive lung disease) mimicking well-characterized immune-mediated diseases outside the transplantation setting may be potentially included in basket trials that include nontransplant patients, acknowledging potential variations in pathophysiology.
CONCLUSIONS
The need to identify approaches for effective treatment and prevention of highly morbid manifestations has emerged as one of the most important future goals in the field. During the next 3 years, identification of new diagnostic tools including biomarkers of all types and clinical risk factors will be crucial to prevent highly morbid complications. In the next 3 to 7 years, we expect that a better understanding of local tissue pathophysiology will lead to identification of therapeutic targets. Eventually, organ-specific therapeutic clinical studies will be necessary. Careful study design recognizing the small size of the eligible population and designating appropriate endpoints will increase the likelihood of informative results.
ACKNOWLEDGMENTS
Special acknowledgement goes to the Meredith Cowden GVHD foundation, France Lymphome Espoir, NBMTLink; Anthony Nolan, National Marrow Donor Program, BMT InfoNet and other patient advocacy groups for partnering and collaboration. Thanks go to all working groups and consensus conference participants, professional societies, US government agencies and stakeholders in the field of hematopoietic stem cell transplantation for the generous donation of their work, time, talents and expertise. We especially acknowledge the ASTCT and EBMT for their roles in the dissemination, education and implementation of the concepts and best practices evolving from this project. The authors thank Eneida Nemecek for contribution of the cutaneous section and Sarah Anand for support of the pulmonary section. Special thanks go to the independent external peer reviewers who provided they comments and critiques to the 2020 NIH Chronic GVHD Consensus Project: Nicolaus Kröger, M.D. Professor & Clinical Director of the Department of Stem Cell Transplantation, University of Hamburg, Hamburg, Germany, President EBMT; Ryotaro Nakamura, M.D. Professor & Director of the Center for Stem Cell Transplantation City of Hope Cancer Center, Duarte, California; John DiPersio, MD, PhD, Chief, Division of Oncology; Director, Center for Gene and Cellular Immunotherapy; Deputy Director, Siteman Cancer Center, Washington University School of Medicine, St. Louis, Missouri; Mark Juckett, MD, Professor & Director of the Blood and Marrow Transplant Program, University of Wisconsin, Madison, Wisconsin; George Chen, MD, Associate Professor of Medicine, University at Buffalo, Buffalo, New York; Rafael Duarte, MD, PhD, FRCP, Head of Department of Hematology and Director of the Hematopoietic Transplant Program, Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain; Franco Locatelli, M.D. Professor of Pediatrics Università Sapienza, Roma, Head of the Department of Pediatric Hematology and Cell and Gene Therapy, IRCCS Ospedale Pediatrico Bambino Gesù, Roma, Italy; Areej El-Jawahri, MD, Associate Professor, Director of the Bone Marrow Transplant Survivorship Program, and Associate Director of the Cancer Outcomes Research and Education Program at Massachusetts General Hospital, Boston, Massachusetts; Robert Soiffer, MD, Professor, Chief of the Division of Hematologic Malignancies, Chair of the Executive Committee for Clinical Programs, Vice Chair for the Department of Medical Oncology, Chief of the Division of Hematologic Malignancies, Dana Farber Cancer Institute, Boston, Massachusetts; Daniel Weisdorf, MD, Professor of Medicine & Deputy Director of Clinical Science and Translational Science Institute; Director, Clinical and Translational Research Services, University of Minnesota, Minneapolis, Minnesota; Keith Sullivan. MD, Professor of Medicine, Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, North Carolina; Catherine Lee, MD, Assistant Professor, Hematology and Hematologic Malignancies, Huntsman Cancer Institute - University of Utah, Salt Lake City, Utah; Jose Antonio Perez-Simon, MD, Professor of Hematology, University of Seville, Head of Department of Hematology, University Hospital Virgen del Rocio and Vice director of the Biomedical Research Institute of Seville (IBIS), Seville, Spain; Doris Ponce, MD, Associate Professor of Medicine, Hematologic Oncologist, Memorial Sloan-Kettering Cancer Center, New York City, New York; Andrew Harris, MD, Pediatric Hematologist-Oncologist & Assistant Professor of Pediatrics, Pediatric BMT and Cellular Therapy Program, University of Utah/Primary Children’s Hospital, Salt Lake City, Utah.
The opinions expressed are those of the authors and do not represent the position of the National Cancer Institute, the National Institutes of Health, or the United States Government.
Financial disclosure:
Supported by the Intramural Program of the National Cancer Institute — Center for Cancer Research, the NIH Intramural and Extramural Research Programs Institutes and Centers. D.W. is a consultant for Novartis, Incyte, Syndax, Pfizer, and Behring; receives honoraria from Mallinckrodt, MACO, Takeda, and Neovii. V.R. is a consultant for Regeneron. R.L. is a consultant for Pfizer, Bristol Myers Squibb, Boehringer-Ingelheim, Formation, Sanofi, Boehringer-Mannheim, Merck and Genentech/Roche; receives research support from Corbus, Formation, Moderna, Regeneron, Pfizer, and Kinksa. R.C. is co-inventor on U.S. patents covering hybrid CB1R/inducible nitric oxide synthase antagonists. S.L. receives research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda; is a member of a steering committee for Incyte. O.P. receives honoraria or travel support from Astellas, Gilead, Jazz, MSD, Neovii Biotech, Novartis, Pfizer, and Therakos; research support from Gilead, Incyte, Jazz, Neovii Biotech, and Takeda; is an advisory board member of Jazz, Gilead, MSD, Omeros, Shionogi, and SOBI. T.T. receives grants from Kyowa Kirin, Chugai, Sanofi, Astellas, Teijin Pharma, Fuji Pharma, and Nippon Shinyaku; honoraria from Novartis, Merck, Kyowa Kirin, Takeda, Pfizer, Bristol-Myers Squibb, and Janssen. B.R.B. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics; research funding from BlueRock Therapeutics and Rheos Medicines; is a steering committee member for Kadmon Corporation; is a cofounder of Tmunity Therapeutics. A.B. receives research grants from SOS Oxygen; honoraria from Zambon, Shire, Pfizer, and Gilead. A.S. receives consulting fees from Psioxus Therapeutics. S.J. is a consultant for Ocugen Inc., and Neutrolis Inc.; has personal financial interest in Advaite, Inc., and Selagine Inc.; holds patents US9867871B2 and PCT/US19/60566. P.S. is a consultant for GSK, Bausch+Lomb, Ursapharm Arzneimittel GmbH; receives research funding from Roche. Y.O. has a patent in Japan (patent no. 4966019; Name; Topical application and oral intake of tranilast for the treatment of chronic GVHD-related dye eye disease) and patent application number JP 2017-018643 published as JPA2017-178922, application number JP2018-510646 published as WO2017/175808 and application number JP 2019-004730 published as JPA2020-111548. Z.K.L. has U.S. patent filed by Glia LLC on oGVHD treatment; receives research funding from Glia LLC. T.D-N. receives honoraria from Alcon/Novartis, Santen, Alimera Sciences, and Bayer. D.R.S. is a consultant for Dompé and Roche; receives financial support from Dompé and Novartis. S.R.McC. receives grant/research/clinical trial support from Gilead Sciences, Aprea Therapeutics, and McCabe Fund. P.J.M. is on the advisory boards for Mesoblast and Rigel Pharmaceuticals Inc.; receives honoraria from Janssen. J.L.T. receives research support from Boehringer Ingelheim, CareDx, and AstraZeneca. J.P. has consulting and advisory board membership for Syndax, CTI Biopharma, Amgen, Regeneron, Incyte; receives clinical trial support from Novartis, Amgen, Takeda, Janssen, Johnson & Johnson, Pharmacyclics, Abbvie, CTI Biopharma, BMS. D.R.C. is a consultant for Fresenius Kabi and Incyte; a non-promotional speaker for Mallinckrodt. E.H. is on the advisory board for Novartis, Medac, and Maat-Pharma; receives honoraria from Novartis, Neovii. C.C. receives consulting honoraria from Incyte, Jazz, CareDx, Mesoblast, Syndax, Omeros, Pfizer. H.T.G. receives honoraria for presentations in scientific meetings and consultations from Novartis, Celgene/BMS, Sanofi, Janssen, and Therakos. S.S. serves on the advisory board for Rigel Pharmaceuticals, Inc. R.R.J. receives consultant role fees from Merck, Karius, and Microbiome DX; advisory member role fees from Seres, Kaleido, MaaT Pharma, Prolacta, and LISCure; patent licensing fees from Seres. A.M.H. holds intellectual property related to Interleukin-22. B.D.S. receives consulting honoraria fromJanssen/Legend, Celgene, Kite/Gilead, and BMS; is an advisory board member for In8bio. G-S.C. is a consultant for Janssen Pharmaceutica. S.P. holds intellectual property on Biomarkers and assay to detect chronic graft versus host disease
APPENDIX.
2020 NATIONAL INSTITUTES OF HEALTH CONSENSUS DEVELOPMENT PROJECT ON CRITERIA FOR CLINICAL TRIALS IN CHRONIC GVHD STEERING COMMITTEE
Chairs:
Steven Pavletic, MD, MS, National Cancer Institute; Stephanie J. Lee, MD, MPH, Fred Hutchinson Cancer Research Center; Kirk R. Schultz, MD, University of British Columbia; Daniel Wolff, MD, University of Regensburg
Members:
Hildegard Greinix, MD, University of Graz; Sophie Paczesny, MD, University of South Carolina; Bruce Blazar, MD, University of Minnesota; Stefanie Sarantopoulos, MD, PhD, Duke University; Joseph Pidala, MD, PhD, Moffitt Cancer Center; Corey Cutler, MD, MPH, FRCPC, Dana Farber Cancer Institute; Gerard Socie, MD, PhD, St. Louis Hospital, Paris; Paul Martin, MD, Fred Hutchinson Cancer Research Center; Meredith Cowden, MA, LPCC-S, Cowden Foundation
MORBID FORMS OF CHRONIC GVHD WORKING GROUP 4
Co-Chairs:
Daniel Wolff, MD, University of Regensburg; Sophie Paczesny, MD, University of South Carolina
Members:
Guang-Shing Cheng, MD, Fred Hutchinson Cancer Research Center; Vedran Radojcic, MD, University of Utah; Bianca Santomasso, MD, PhD, MSKCC; Ervina Bilic, MD, PhD, University of Zagreb; Sandeep Jain, MD, University Illinois; Takanori Teshima, MD, PhD, Hokkaido University; Olaf Penack, MD, PhD, Charite Berlin; David Jacobsohn, MD, ScM, Children's National; Linda M. Griffith, MD, MHS, PhD, Division of Allergy Immunology and Transplantation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA; Donna Przepiorka, MD, PhD, US Food and Drug Administration
REFERENCES
- 1.Grube M, Holler E, Weber D, Holler B, Herr W, Wolff D. Risk Factors and Outcome of Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation-Results from a Single-Center Observational Study. Biol Blood Marrow Transplant. 2016;22:1781–1791. [DOI] [PubMed] [Google Scholar]
- 2.Wood WA, Chai X, Weisdorf D, et al. Comorbidity burden in patients with chronic GVHD. Bone Marrow Transplant. 2013;48:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavletic SZ, Martin PJ, Schultz KR, Lee SJ. The future of chronic graft-versus-host disease: introduction to the 2020 National Institutes of Health Consensus Development Project Reports. Transplant Cell Ther. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Inamoto Y, Storer BE, Petersdorf EW, et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013;121:5098–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martires KJ, Baird K, Steinberg SM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118:4250–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detrait MY, Morisset S, Peffault de Latour R, et al. Pre-transplantation risk factors to develop sclerotic chronic GvHD after allogeneic HSCT: a multicenter retrospective study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Bone Marrow Transplant. 2015;50:253–258. [DOI] [PubMed] [Google Scholar]
- 7.Uhm J, Hamad N, Shin EM, et al. Incidence, risk factors, and long-term outcomes of sclerotic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1751–1757. [DOI] [PubMed] [Google Scholar]
- 8.Bilic E, Delimar V, Desnica L, et al. High prevalence of small- and large-fiber neuropathy in a prospective cohort of patients with moderate to severe chronic GvHD. Bone Marrow Transplant. 2016;51:1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus PD, Wolff D, Grauer O, et al. Muscle cramps and neuropathies in patients with allogeneic hematopoietic stem cell transplantation and graft-versus-host disease. PLoS One. 2012;7:e44922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inamoto Y, Martin PJ, Flowers MED, et al. Genetic risk factors for sclerotic graft-versus-host disease. Blood. 2016;128:1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrie JL, Kmieciak M, Sabo RT, et al. Distinct oligoclonal T cells are associated with graft versus host disease after stem-cell transplantation. Transplantation. 2012;93:949–957. [DOI] [PubMed] [Google Scholar]
- 12.Yew PY, Alachkar H, Yamaguchi R, et al. Quantitative characterization of T-cell repertoire in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015;50:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruggen MC, Klein I, Greinix H, et al. Diverse T-cell responses characterize the different manifestations of cutaneous graft-versus-host disease. Blood. 2014;123:290–299. [DOI] [PubMed] [Google Scholar]
- 14.Hill GR, Olver SD, Kuns RD, et al. Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood. 2010;116:819–828. [DOI] [PubMed] [Google Scholar]
- 15.Ugor E, Simon D, Almanzar G, et al. Increased proportions of functionally impaired regulatory T cell subsets in systemic sclerosis. Clin Immunol. 2017;184:54–62. [DOI] [PubMed] [Google Scholar]
- 16.Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127:646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–241. [DOI] [PubMed] [Google Scholar]
- 20.Wang KS, Kim HT, Nikiforow S, et al. Antibodies targeting surface membrane antigens in patients with chronic graft-versus-host disease. Blood. 2017;130:2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen GL, Arai S, Flowers MED, et al. A phase 1 study of imatinib for corticosteroid-dependent/refractory chronic graft-versus-host disease: response does not correlate with anti-PDGFRA antibodies. Blood. 2011;118:4070–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spies-Weisshart B, Schilling K, Bohmer F, Hochhaus A, Sayer HG, Scholl S. Lack of association of platelet-derived growth factor (PDGF) receptor autoantibodies and severity of chronic graft-versus-host disease (GvHD). J Cancer Res Clin Oncol. 2013;139:1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arai S, Pidala J, Pusic I, et al. A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clinical Cancer Research. 2016;22:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita T, Kobayashi T, Mizumaki K, et al. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv. 2018;4:eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue D, Tabib T, Morse C, Lafyatis R. Transcriptome landscape of myeloid cells in human skin reveals diversity, rare populations and putative DC progenitors. J Dermatol Sci. 2020;97:41–49. [DOI] [PubMed] [Google Scholar]
- 26.Alexander KA, Flynn R, Lineburg KE, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Paz K, Flynn R, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-beta production. Blood. 2017;129:2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamakawa T, Ohigashi H, Hashimoto D, et al. Vitamin A-coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood. 2018;131:1476–1485. [DOI] [PubMed] [Google Scholar]
- 29.McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol. 1999;163:5693–5699. [PubMed] [Google Scholar]
- 30.Banovic T, MacDonald KP, Morris ES, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106:2206–2214. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald KP, Blazar BR, Hill GR. Cytokine mediators of chronic graft-versus-host disease. J Clin Invest. 2017;127:2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Fu X, Chen X, Han X, Dong P. M2 macrophages induce EMT through the TGF-β/Smad2 signaling pathway. Cell Biol Int. 2017;41:960–968. [DOI] [PubMed] [Google Scholar]
- 33.Distler JHW, Györfi A-H, Ramanujam M, Whitfield ML, Königshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15:705–730. [DOI] [PubMed] [Google Scholar]
- 34.Wu JM, Thoburn CJ, Wisell J, Farmer ER, Hess AD. CD20, AIF-1, and TGF-beta in graft-versus-host disease: a study of mRNA expression in histologically matched skin biopsies. Modern Pathol. 2010;23:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyrcz-Krzemien S, Helbig G, Zielinska P, Markiewicz M. The kinetics of mRNA transforming growth factor beta1 expression and its serum concentration in graft-versus-host disease after allogeneic hemopoietic stem cell transplantation for myeloid leukemias. Med Sci Monitor. 2011;17:CR322–CR328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banovic T, Macdonald KPA, Morris ES, et al. TGF-β in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106:2206–2214. [DOI] [PubMed] [Google Scholar]
- 37.Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakim FT, Memon S, Jin P, et al. Upregulation of IFN-Inducible and Damage-Response Pathways in Chronic Graft-versus-Host Disease. J Immunol. 2016;197:3490–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanna D, Denton CP,Jahreis A, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387:2630–2640. [DOI] [PubMed] [Google Scholar]
- 40.Roofeh D, Lin CJF, Goldin J, et al. Tocilizumab Prevents Progression of Early Systemic Sclerosis Associated Interstitial Lung Disease. Arthritis Rheumatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kattner AS, Holler E, Holler B, et al. IL6-receptor antibody tocilizumab as salvage therapy in severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Ann Hematol. 2020;99:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dees C, Tomcik M, Zerr P, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011;70:1304–1310. [DOI] [PubMed] [Google Scholar]
- 43.Dees C, Zerr P, Tomcik M, et al. Inhibition of notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheumatism. 2011;63:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Shen L, Dreissigacker K, et al. Targeting of canonical WNT signaling ameliorates experimental sclerodermatous chronic graft-versus-host disease. Blood. 2021. [DOI] [PubMed] [Google Scholar]
- 45.Zerr P, Palumbo-Zerr K, Distler A, et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood. 2012;120:2909–2917. [DOI] [PubMed] [Google Scholar]
- 46.Radojcic V, Lee C, Pletneva M, Hicks K, Sarantopoulos S, Couriel D. Hedgehog blockade in the treatment of steroid-refractory sclerodermatous chronic graft-versus-host disease. Bone Marrow Transplantation. 2019;54:305–306. [Google Scholar]
- 47.DeFilipp Z, Nazarian RM, El-Jawahri A, et al. Phase 1 study of the Hedgehog pathway inhibitor sonidegib for steroid-refractory chronic graft-versus-host disease. Blood Adv. 2017;1:1919–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radojcic V, Flynn RP, Chung J, et al. Notch signaling mediated by Dll1/4 notch ligands controls the pathogenesis of both multi-organ system non-sclerodermatous and sclerodermatous chronic graft-versus-host disease. Blood. 2016;128:805.27268084 [Google Scholar]
- 49.Cinar R, Iyer MR, Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol Ther. 2020;208:107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan CY, Zhou V, Sauber G, et al. Signaling through the type 2 cannabinoid receptor regulates the severity of acute and chronic graft versus host disease. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan CY, Zhou V, Sauber G, et al. Signaling through the type 2 cannabinoid receptor regulates the severity of acute and chronic graft-versus-host disease. Blood. 2021;137:1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiera R, Hummers L, Chung L, et al. Safety and efficacy of lenabasum in a phase 2 randomized, placebo-controlled trial in adults with systemic sclerosis. Arthritis & Rheumatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curtis LM, Ostojic A, Venzon D, et al. A randomized phase-2 trial of pomalidomide in subjects failing prior therapy of chronic graft-versus-host disease. Blood. 2021;137:896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlöber P, Leiter U, Friedrich W, et al. Chronic cutaneous sclerodermoid graft-versus-host disease: evaluation by 20-MHz sonography. J Eur Acad Dermatol Venereol. 2003;17:402–407. [DOI] [PubMed] [Google Scholar]
- 55.Clark J, Yao L, Pavletic SZ, et al. Magnetic resonance imaging in sclerotic-type chronic graft-vs-host disease. Arch Dermatol. 2009;145:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mantero JC, Kishore N, Ziemek J, et al. Randomised, double-blind, placebo-controlled trial of IL1-trap, rilonacept, in systemic sclerosis.A phase I/II biomarker trial. Clin Exp Rheumatol. 2018;36(Suppl 113):146–149. [PubMed] [Google Scholar]
- 57.Lafyatis R, Mantero JC, Gordon J, et al. Inhibition of beta-catenin signaling in the skin rescues cutaneous adipogenesis in systemic sclerosis: a randomized, double-blind, placebo-controlled trial of C-82. J Invest Dermatol. 2017;137:2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice LM, Ziemek J, Stratton EA, et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2015;67:3004–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rice LM, Padilla CM, McLaughlin SR, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jagasia M, Salhotra A, Bachier CR, et al. KD025 for patients with chronic graft-versus-host disease (cGVHD)—long-term follow-up of a phase 2a study (KD025-208). Blood. 2019;134:872. [Google Scholar]
- 61.Yamakawa T, Ohigashi H, Hashimoto D, et al. Vitamin A—coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood. 2018;131:1476–1485. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Feng X, Meng S. Site-specific drug delivery in the skin for the localized treatment of skin diseases. Expert Opin Drug Deliv. 2019;16:847–867. [DOI] [PubMed] [Google Scholar]
- 63.Aghajanian H, Kimura T, Rurik JG, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Distler A, Lang V, Del Vecchio T, et al. Combined inhibition of morphogen pathways demonstrates additive antifibrotic effects and improved tolerability. Ann Rheum Dis. 2014;73:1264–1268. [DOI] [PubMed] [Google Scholar]
- 65.Keren L, Bosse M, Thompson S, et al. MIBI-TOF: a multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci Adv. 2019;5:eaax5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. [DOI] [PubMed] [Google Scholar]
- 67.Arora M, Cutler CS, Jagasia MH, et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bergeron A, Chevret S, Peffault de Latour R, et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2018:51. [DOI] [PubMed] [Google Scholar]
- 69.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood MarrowTransplant. 2011;17:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loeb JS, Blower WC, Feldstein JF, Koch BA, Munlin AL, Hardie WD. Acceptability and repeatability of spirometry in children using updated ATS/ERS criteria. Pediatr Pulmonol. 2008;43:1020–1024. [DOI] [PubMed] [Google Scholar]
- 71.Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193:1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheshadri A, Chemaly RF, Alousi AM, et al. Pulmonary impairment after respiratory viral infections is associated with high mortality in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2019;25:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jamani K, He Q, Liu Y, et al. Early post-transplantation spirometry is associated with the development of bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng GS, Storer B, Chien JW, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc. 2016;13:1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swatek AM, Lynch TJ, Crooke AK, et al. Depletion of airway submucosal glands and TP63(+)KRT5(+) basal cells in obliterative bronchiolitis. Am J Respir Crit Care Med. 2018;197:1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao W, Wang S, Duleba M, et al. Regenerative metaplastic clones in COPD lung drive inflammation and fibrosis. Cell. 2020;181:848–864.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanaudenaerde BM, Wuyts WA, Geudens N, et al. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am J Transplant. 2007;7:76–82. [DOI] [PubMed] [Google Scholar]
- 79.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuzmina Z, Krenn K, Petkov V, et al. CD19(+)CD21(low) B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121:1886–1895. [DOI] [PubMed] [Google Scholar]
- 82.Combs MP, Wheeler DS, Luth JE, et al. Lung microbiota predict chronic rejection in healthy lung transplant recipients: a prospective cohort study. Lancet Respir Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergeron A, Godet C, Chevret S, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant. 2013;48:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meignin V, Thivolet-Bejui F, Kambouchner M, et al. Lung histopathology of non-infectious pulmonary complications after allogeneic haematopoietic stem cell transplantation. Histopathology. 2018;73:832–842. [DOI] [PubMed] [Google Scholar]
- 86.Belloli EA, Wang X, Murray S, et al. Longitudinal forced vital capacity monitoring as a prognostic adjunct after lung transplantation. Am J Respir Crit Care Med. 2015;192:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Todd JL, Jain R, Pavlisko EN, et al. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. [DOI] [PubMed] [Google Scholar]
- 89.Glanville AR, Verleden GM, Todd JL, et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38:483–492. [DOI] [PubMed] [Google Scholar]
- 90.Sharifi H, Lai YK, Guo H, et al. Machine learning algorithms to differentiate among pulmonary complications after hematopoietic cell transplant. Chest. 2020;158:1090–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greer M, Riise GC, Hansson L, et al. Dichotomy in pulmonary graft-versus-host disease evident among allogeneic stem-cell transplant recipients undergoing lung transplantation. Eur Respir J. 2016;48:1807–1810. [DOI] [PubMed] [Google Scholar]
- 92.Kitko CL, Pidala J, Schoemans HM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report [e-pub ahead of print April 9, 2021]. Transplant Cell Ther. 2021. 10.1016/j.jtct.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bergeron A, Chevret S, Chagnon K, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–1249. [DOI] [PubMed] [Google Scholar]
- 94.Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bergeron A, Chevret S, Granata A, et al. Effect of azithromycin on airflow decline-free survival after allogeneic hematopoietic stem cell transplant: the ALLOZITHRO Randomized ClinicalTrial. JAMA. 2017;318:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng GS, Bondeelle L, Gooley T, et al. Azithromycin use and increased cancer risk among patients with bronchiolitis obliterans after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hefazi M, Langer KJ, Khera N, et al. Extracorporeal photopheresis improves survival in hematopoietic cell transplant patients with bronchiolitis obliterans syndrome without significantly impacting measured pulmonary functions. Biol Blood Marrow Transplant. 2018;24:1906–1913. [DOI] [PubMed] [Google Scholar]
- 98.Tran J, Norder EE, Diaz PT, et al. Pulmonary rehabilitation for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1250–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi HE, Lim SN, Lee JH, Park SH. Comprehensive pulmonary rehabilitation in patients with bronchiolitis obliterans syndrome: a case series. Respir Med Case Rep. 2020;31:101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iacono A, Wijesinha M, Rajagopal K, et al. A randomised single-centre trial of inhaled liposomal cyclosporine for bronchiolitis obliterans syndrome post-lung transplantation. ERJ Open Res. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du J, Paz K, Thangavelu G, et al. Invariant natural killer T cells ameliorate murine chronic GVHD by expanding donor regulatory T cells. Blood. 2017;129:3121–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng GS, Selwa KE, Hatt C, et al. Multicenter evaluation of parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Am J Transplant. 2020;20:2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barbosa EJM Jr, Lanclus M, Vos W, et al. Machine learning algorithms utilizing quantitative CT features may predict eventual onset of bronchiolitis obliterans syndrome after lung transplantation. Acad Radiol. 2018;25:1201–1212. [DOI] [PubMed] [Google Scholar]
- 104.Walkup LL, Myers K, El-Bietar J, et al. Xenon-129 MRI detects ventilation deficits in paediatric stem cell transplant patients unable to perform spirometry. Eur Respir J. 2019:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21. 389–401 e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21:984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pidala J, Chai X, Kurland BF, et al. Analysis of gastrointestinal and hepatic chronic graft-versus-host [corrected] disease manifestations on major outcomes: a chronic graft-versus-host [corrected] disease consortium study. Biol Blood Marrow Transplant. 2013;19:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inamoto Y, Martin PJ, Storer BE, et al. Association of severity of organ involvement with mortality and recurrent malignancy in patients with chronic graft-versus-host disease. Haematologica. 2014;99:1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pidala J, Vogelsang G, Martin P, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wolff D, Ayuk F, Elmaagacli A, et al. Current practice in diagnosis and treatment of acute graft-versus-host disease: results from a survey among German-Austrian-Swiss hematopoietic stem cell transplant centers. Biol Blood Marrow Transplant. 2013;19:767–776. [DOI] [PubMed] [Google Scholar]
- 111.Kida A, McDonald GB. Gastrointestinal, hepatobiliary, pancreatic, and iron-related diseases in long-term survivors of allogeneic hematopoietic cell transplantation. Semin Hematol. 2012;49:43–58. [DOI] [PubMed] [Google Scholar]
- 112.Shulman HM, Cardona DM, Greenson JK, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kreft A, Mottok A, Mesteri I, et al. Consensus diagnostic histopathological criteria for acute gastrointestinal graft versus host disease improve interobserver reproducibility. Virchows Arch. 2015;467:255–263. [DOI] [PubMed] [Google Scholar]
- 114.Kanda J, Brazauskas R, Hu ZH, et al. Graft-versus-host disease after hlamatched sibling bone marrow or peripheral blood stem cell transplantation: comparison of North American Caucasian and Japanese Populations. Biol Blood Marrow Transplant. 2016;22:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Inamoto Y, Kimura F, Kanda J, et al. Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica. 2016;101:1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inamoto Y, White J, Ito R, et al. Comparison of characteristics and outcomes of late acute and NIH chronic GVHD between Japanese and white patients. Blood advances. 2019;3:2764–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Markey KA, Schluter J, Gomes AL, et al. Microbe-derived short chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Golob JL, DeMeules MM, Loeffelholz T, et al. Butyrogenic bacteria after acute graft-versus-host disease (GVHD) are associated with the development of steroid-refractory GVHD. Blood advances. 2019;3:2866–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cuvelier GDE, Nemecek ER, Wahlstrom JT, et al. Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood. 2019;134:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spencer GD, Shulman HM, Myerson D, Thomas ED, McDonald GB. Diffuse intestinal ulceration after marrow transplantation: a clinicopathologic study of 13 patients. Hum Pathol. 1986;17:621–633. [DOI] [PubMed] [Google Scholar]
- 121.Tordjman M, Ouachee M, Bonnard A, et al. Small bowel stenosis: a manifestation of chronic graft-versus-host disease in children? Hum Pathol. 2018;72:174–179. [DOI] [PubMed] [Google Scholar]
- 122.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- 123.Takashima S, Kadowaki M, Aoyama K, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hayase E, Hashimoto D, Nakamura K, et al. R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J Exp Med. 2017;214:3507–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanash AM, Dudakov JA, Hua G, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120:223–231. [DOI] [PubMed] [Google Scholar]
- 128.Fu YY, Egorova A, Sobieski C, et al. T cell recruitment to the intestinal stem cell compartment drives immune-mediated intestinal damage after allogeneic transplantation. Immunity. 2019;51. 90–103 e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takashima S, Martin ML, Jansen SA, et al. T cell-derived interferon-gamma programs stem cell death in immune-mediated intestinal damage. Sci Immunol. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schultz KR, Kariminia A, Ng B, et al. Immune profile differences between chronic GVHD and late acute GVHD: results of the ABLE/PBMTC 1202 studies. Blood. 2020;135:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sung AD, Hassan S, Cardona DM, et al. Late gastrointestinal complications of allogeneic hematopoietic stem cell transplantation in adults. Biol Blood Marrow Transplant. 2018;24:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Akpek G, Chinratanalab W, Lee LA, et al. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46–51. [DOI] [PubMed] [Google Scholar]
- 133.Rieger K, Gunther U, Erben U, et al. Confocal endomicroscopy in diagnosis of intestinal chronic graft-versus-host disease. Hematol Oncol. 2018;36:291–298. [DOI] [PubMed] [Google Scholar]
- 134.Salomao M, Dorritie K, Mapara MY, Sepulveda A. Histopathology of graft-vs-host disease of gastrointestinal tract and liver: an update. Am J Clin Pathol. 2016;145:591–603. [DOI] [PubMed] [Google Scholar]
- 135.Milano F, Shulman HM, Guthrie KA, Riffkin I, McDonald GB, Delaney C. Late-onset colitis after cord blood transplantation is consistent with graft-versus-host disease: results of a blinded histopathological review. Biol Blood Marrow Transplant. 2014;20:1008–1013. [DOI] [PubMed] [Google Scholar]
- 136.Shimoji S, Kato K, Eriguchi Y, et al. Evaluating the association between histological manifestations of cord colitis syndrome with GVHD. Bone Marrow Transplant. 2013;48:1249–1252. [DOI] [PubMed] [Google Scholar]
- 137.Schoemans HM, Goris K, Van Durm R, et al. Accuracy and usability of the eGVHD app in assessing the severity of graft-versus-host disease at the 2017 EBMT annual congress. Bone Marrow Transplant. 2018;53:490–494. [DOI] [PubMed] [Google Scholar]
- 138.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kohler N, Zeiser R. Intestinal microbiota influence immune tolerance post allogeneic hematopoietic cell transplantation and intestinal GVHD. Front Immunol. 2018;9:3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft-versus-host disease: a task force report from the national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:211–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.An S, Raju I, Surenkhuu B, et al. Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: Implications for novel biomarkers and therapeutic strategies. Ocul Surf. 2019;17:589–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ogawa Y, Yamazaki K, Kuwana M, et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001;42:111–119. [PubMed] [Google Scholar]
- 144.Ogawa Y, Okamoto S, Wakui M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999;83:1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Na KS, Yoo YS, Mok JW, Lee JW, Joo CK. Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015;50:1459–1464. [DOI] [PubMed] [Google Scholar]
- 146.Shikari H, Amparo F, Saboo U, Dana R. Onset of ocular graft-versus-host disease symptoms after allogeneic hematopoietic stem cell transplantation. Cornea. 2015;34:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun YC, Chai X, Inamoto Y, et al. Impact of ocular chronic graft-versus-host disease on quality of life. Biol Blood Marrow Transplant. 2015;21:1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saboo US, Amparo F, Abud TB, Schaumberg DA, Dana R. Vision-related quality of life in patients with ocular graft-versus-host disease. Ophthalmology. 2015;122:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tung CI. Current approaches to treatment of ocular graft-versus-host disease. International ophthalmology clinics. 2017;57:65–88. [DOI] [PubMed] [Google Scholar]
- 150.Rapoport Y, Freeman T, Koyama T, et al. Validation of International Chronic Ocular Graft-Versus-Host Disease (GVHD) Group diagnostic criteria as a chronic ocular GVHD-specific metric. Cornea. 2017;36:258–263. [DOI] [PubMed] [Google Scholar]
- 151.Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Survey of ophthalmology. 2013;58:233–251. [DOI] [PubMed] [Google Scholar]
- 152.Shimizu E, Aketa N, Yazu H, et al. Corneal higher-order aberrations in eyes with chronic ocular graft-versus-host disease. Ocul Surf. 2020;18:98–107. [DOI] [PubMed] [Google Scholar]
- 153.Ban Y, Ogawa Y, Ibrahim OM, et al. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis. 2011;17:2533–2543. [PMC free article] [PubMed] [Google Scholar]
- 154.Engel LA, Wittig S, Bock F, et al. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplant. 2015;50:961–967. [DOI] [PubMed] [Google Scholar]
- 155.Steven P, Faust C, Holtick U, et al. Adverse environmental conditions are a risk factor for ocular GvHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:1851–1853. [DOI] [PubMed] [Google Scholar]
- 156.Hessen M, Akpek EK. Ocular graft-versus-host disease. Current opinion in allergy and clinical immunology. 2012;12:540–547. [DOI] [PubMed] [Google Scholar]
- 157.Kerty E, Vigander K, Flage T, Brinch L. Ocular findings in allogeneic stem cell transplantation without total body irradiation. Ophthalmology. 1999;106:1334–1338. [DOI] [PubMed] [Google Scholar]
- 158.Nassiri N, Eslani M, Panahi N, Mehravaran S, Ziaei A, Djalilian AR. Ocular graft versus host disease following allogeneic stem cell transplantation: a review of current knowledge and recommendations. J Ophthalmic Vis Res. 2013;8:351–358. [PMC free article] [PubMed] [Google Scholar]
- 159.Pathak M, Diep PP, Lai X, Brinch L, Ruud E, Drolsum L. Ocular findings and ocular graft-versus-host disease after allogeneic stem cell transplantation without total body irradiation. Bone Marrow Transplant. 2018;53:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mirza N, Zierhut M, Korn A, et al. Graft versus self (GvS) against T-cell autoantigens is a mechanism of graft-host interaction. Proc Natl Acad Sci U S A. 2016;113:13827–13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blecha C, Angstwurm K, Wolff D, et al. Retinal involvement in a patient with cerebral manifestation of chronic graft-versus-host-disease. Oncol Res Treat. 2015;38:532–534. [DOI] [PubMed] [Google Scholar]
- 162.Sivaraman KR, Jivrajka RV, Soin K, et al. Superior limbic keratoconjunctivitis-like inflammation in patients with chronic graft-versus-host disease. Ocul Surf. 2016;14:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kheirkhah A, Coco G, Satitpitakul V, Dana R. Subtarsal fibrosis is associated with ocular surface epitheliopathy in graft-versus-host disease. Am J Ophthalmol. 2018;189:102–110. [DOI] [PubMed] [Google Scholar]
- 164.Kusne Y, Temkit M, Khera N, Patel DR, Shen JF. Conjunctival subepithelial fibrosis and meibomian gland atrophy in ocular graft-versus-host disease. Ocul Surf. 2017;15:784–788. [DOI] [PubMed] [Google Scholar]
- 165.Stevenson W, Shikari H, Saboo US, Amparo F, Dana R. Bilateral corneal ulceration in ocular graft-versus-host disease. Clin Ophthalmol. 2013;7:2153–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tarnawska D, Wylegala E. Corneal grafting and aggressive medication for corneal defects in graft-versus-host disease following bone marrow transplantation. Eye (Lond). 2007;21:1493–1500. [DOI] [PubMed] [Google Scholar]
- 167.Heath JD, Acheson JF, Schulenburg WE. Penetrating keratoplasty in severe ocular graft versus host disease. Br J Ophthalmol. 1993;77:525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Plattner K, Goldblum D, Halter J, Kunz C, Koeppl R, Gerber-Hollbach N. Osteo-odonto-keratoprosthesis in severe ocular graft versus host disease. Klin Monbl Augenheilkd. 2017;234:455–456. [DOI] [PubMed] [Google Scholar]
- 169.Ogawa Y, Kim SK, Dana R, et al. International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: proposed diagnostic criteria for chronic GVHD (Part I). Sci Rep. 2013;3:3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 171.Blecha C, Wolff D, Holler B, et al. Verification of the new grading scale for ocular chronic graft-versus-host disease developed by the German-Austrian-Swiss consensus conference on chronic GVHD. Ann Hematol. 2016;95:493–499. [DOI] [PubMed] [Google Scholar]
- 172.Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- 173.Hu B, Qiu Y, Hong J. Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease(GVHD). Ocul Surf. 2020;18:298–304. [DOI] [PubMed] [Google Scholar]
- 174.Tibrewal S, Sarkar J, Jassim SH, et al. Tear fluid extracellular DNA: diagnostic and therapeutic implications in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:8051–8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sonawane S, Khanolkar V, Namavari A, et al. Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:8253–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sonobe H, Ogawa Y, Yamada K, et al. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocul Surf. 2019;17:160–166. [DOI] [PubMed] [Google Scholar]
- 177.Yamane M, Ogawa Y, Mukai S, et al. Functional role of lacrimal gland fibroblasts in a mouse model of chronic graft-versus-host disease. Cornea. 2017. in press. [DOI] [PubMed] [Google Scholar]
- 178.Yamane M, Sato S, Shimizu E, et al. Senescence-associated secretory phenotype promotes chronic ocular graft-versus-host disease in mice and humans. FASEB J. 2020. in press. [DOI] [PubMed] [Google Scholar]
- 179.Ogawa Y, Morikawa S, Okano H, et al. MHC-compatible bone marrow stromal/stem cells trigger fibrosis by activating host T cells in a scleroderma mouse model. eLife. 2016;5:e09394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mukai S, Ogawa Y, Urano F, Kudo-Saito C, Kawakami Y, Tsubota K. Novel treatment of chronic graft-versus-host disease in mice using the ER stress reducer 4-phenylbutyric acid. Sci Rep. 2017;7:41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yaguchi S, Ogawa Y, Shimmura S, et al. Angiotensin II type 1 receptor antagonist attenuates lacrimal gland, lung, and liver fibrosis in a murine model of chronic graft-versus-host disease. PLoS One. 2013;8:e64724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ogawa Y, Shimmura S, Kawakita T, Yoshida S, Kawakami Y, Tsubota K. Epithelial mesenchymal transition in human ocular chronic graft-versus-host disease. The American journal of pathology. 2009;175:2372–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ogawa Y, Kodama H, Kameyama K, et al. Donor fibroblast chimerism in the pathogenic fibrotic lesion of human chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2005;46:4519–4527. [DOI] [PubMed] [Google Scholar]
- 184.Perez VL, Barsam A, Duffort S, et al. Novel scoring criteria for the evaluation of ocular graft-versus-host disease in a preclinical allogeneic hematopoietic stem cell transplantation animal model. Biol Blood Marrow Transplant. 2016;22:1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ogawa Y. Ocular diseases and epithelial mesenchymal transition. Nippon Ganka Gakkai Zasshi. 2016;120:743–745. [PubMed] [Google Scholar]
- 186.Martinez-Carrasco R, Sanchez-Abarca LI, Nieto-Gomez C, et al. Assessment of dry eye in a GVHD murine model: approximation through tear osmolarity measurement. Exp Eye Res. 2017;154:64–69. [DOI] [PubMed] [Google Scholar]
- 187.Herretes S, Ross DB, Duffort S, et al. Recruitment of donor T cells to the eyes during ocular GVHD in recipients of MHC-matched allogeneic hematopoietic stem cell transplants. Invest Ophthalmol Vis Sci. 2015;56:2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He J, Yamane M, Shibata S, et al. Ocular surface and tear film characteristics in a sclerodermatous chronic graft-versus-host disease mouse model. Cornea. 2018;37:486–494. [DOI] [PubMed] [Google Scholar]
- 189.Hassan AS, Clouthier SG, Ferrara JL, et al. Lacrimal gland involvement in graft-versus-host disease: a murine model. Invest Ophthalmol Vis Sci. 2005;46:2692–2697. [DOI] [PubMed] [Google Scholar]
- 190.Ohigashi H, Hashimoto D, Hayase E, et al. Ocular instillation of vitamin A-coupled liposomes containing HSP47 siRNA ameliorates dry eye syndrome in chronic GVHD. Blood advances. 2019;3:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Copsel SN, Lightbourn CO, Barreras H, et al. BET bromodomain inhibitors which permit treg function enable a combinatorial strategy to suppress GVHD in pre-clinical allogeneic HSCT. Front Immunol. 2018;9:3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shamloo K, Barbarino A, Alfuraih S, Sharma A. Graft versus host disease-associated dry eye: role of ocular surface mucins and the effect of rebamipide, a mucin secretagogue. Invest Ophthalmol Vis Sci. 2019;60:4511–4519. [DOI] [PubMed] [Google Scholar]
- 193.Mukai S, Ogawa Y, Kawakami Y, Mashima Y, Tsubota K. Inhibition of vascular adhesion protein-1 for treatment of graft-versus-host disease in mice. FASEB J. 2018;32:4085–4095. [DOI] [PubMed] [Google Scholar]
- 194.Poe JC, Jia W, Di Paolo JA, et al. SYK inhibitor entospletinib prevents ocular and skin GVHD in mice. JCI Insight. 2018;3:(19) e122430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ahadome SD, Abraham DJ, Rayapureddi S, et al. Aldehyde dehydrogenase inhibition blocks mucosal fibrosis in human and mouse ocular scarring. JCIInsight. 2016;1:e87001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reyes NJ, Mathew R, Saban DR. Induction and characterization of the allergic eye disease mouse model. Methods Mol Biol. 2018;1799:49–57. [DOI] [PubMed] [Google Scholar]
- 197.Frey Tirri B, Hausermann P, Bertz H, et al. Clinical guidelines for gynecologic care after hematopoietic SCT. Report from the international consensus project on clinical practice in chronic GVHD. Bone Marrow Transplant. 2015;50:3–9. [DOI] [PubMed] [Google Scholar]
- 198.Treister N, Duncan C, Cutler C, Lehmann L. How we treat oral chronic graft-versus-host disease. Blood. 2012;120:3407–3418. [DOI] [PubMed] [Google Scholar]
- 199.Oda K, Nakaseko C, Ozawa S, et al. Fasciitis and myositis: an analysis of muscle-related complications caused by chronic GVHD after allo-SCT. Bone Marrow Transplant. 2009;43:159–167. [DOI] [PubMed] [Google Scholar]
- 200.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53:1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Buxbaum NP, Pavletic SZ. Autoimmunity following allogeneic hematopoietic stem cell transplantation. Front Immunol. 2020;11:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leonard JT, Newell LF, Meyers G, et al. Chronic GvHD-associated serositis and pericarditis. Bone Marrow Transplant. 2015;50:1098–1104. [DOI] [PubMed] [Google Scholar]
- 203.Scherwath A, Schirmer L, Kruse M, et al. Cognitive functioning in allogeneic hematopoietic stem cell transplantation recipients and its medical correlates: a prospective multicenter study. Psychooncology. 2013;22:1509–1516. [DOI] [PubMed] [Google Scholar]
- 204.Hartrampf S, Dudakov JA, Johnson LK, et al. The central nervous system is a target of acute graft versus host disease in mice. Blood. 2013;121:1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mathew NR, Vinnakota JM, Apostolova P, et al. Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia. J Clin Invest. 2020;130:1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kaliyaperumal S, Watkins B, Sharma P, et al. CD8-predominant T-cell CNS infiltration accompanies GVHD in primates and is improved with immunoprophylaxis. Blood. 2014;123:1967–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Harvey CM, Gottipati R, Schwarz S, et al. Acute disseminated encephalomyelitis following allo-SCT: central nervous system manifestation of GVHD. Bone Marrow Transplant. 2014;49:854–856. [DOI] [PubMed] [Google Scholar]
- 208.Grauer O, Wolff D, Bertz H, et al. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain. 2010;133:2852–2865. [DOI] [PubMed] [Google Scholar]
- 209.Wang Y, Ogawa Y, Dogru M, et al. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant. 2010;45:1077–1083. [DOI] [PubMed] [Google Scholar]
- 210.Deschaumes C, Verneuil L, Ertault-Daneshpouy M, et al. CD95 ligand-dependent endothelial cell death initiates oral mucosa damage in a murine model of acute graft versus host disease. Lab Invest. 2007;87:417–429. [DOI] [PubMed] [Google Scholar]
- 211.Janin A, Deschaumes C, Daneshpouy M, et al. CD95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood. 2002;99:2940–2947. [DOI] [PubMed] [Google Scholar]
- 212.Sostak P, Reich P, Padovan CS, Gerbitz A, Holler E, Straube A. Cerebral endothelial expression of adhesion molecules in mice with chronic graft-versus-host disease. Stroke. 2004;35:1158–1163. [DOI] [PubMed] [Google Scholar]
- 213.Biedermann BC, Sahner S, Gregor M, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. [DOI] [PubMed] [Google Scholar]
- 214.Tardieu M, Rybojad M, Peffault de Latour R, et al. Localized edema with sclerodermatous evolution: a possible form of skin chronic graft-versus-host disease associated with endothelial activation. Blood. 2013;122:463–465. [DOI] [PubMed] [Google Scholar]
- 215.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. [DOI] [PubMed] [Google Scholar]
- 216.Clavert A, Peric Z, Brissot E, et al. Late complications and quality of life after reduced-intensity conditioning allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:140–146. [DOI] [PubMed] [Google Scholar]
- 217.Reinfjell T, Tremolada M, Zeltzer LK. A review of demographic, medical, and treatment variables associated with health-related quality of life (HRQOL) in survivors of hematopoietic stem cell (HSCT) and bone marrow transplantation (BMT) during childhood. Front Psychol. 2017;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee CJ, Kim S, Tecca HR, et al. Late effects after ablative allogeneic stem cell transplantation for adolescent and young adult acute myeloid leukemia. Blood advances. 2020;4:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bhatt NS, Brazauskas R, Tecca HR, et al. Post-transplantation employment status of adult survivors of childhood allogeneic hematopoietic cell transplant: a report from the Center for International Blood and Marrow Transplant Research (CIBMTR). Cancer. 2019;125:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.El-Jawahri A, Pidala J, Inamoto Y, et al. Impact of age on quality of life, functional status, and survival in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Solomon SR, Zhang X, Holland HK, Morris LE, Solh M, Bashey A. Superior survival of black versus white patients following post-transplant cyclophosphamide-based haploidentical transplantation for adults with hematologic malignancy. Biol Blood Marrow Transplant. 2018;24:1237–1242. [DOI] [PubMed] [Google Scholar]
- 222.Muhsen IN, Bar M, Savani BN, Estey EH, Hashmi SK. Follow-up issues in survivors of hematologic malignancies—current stance and future perspectives. Blood Rev. 2020;44:100674. [DOI] [PubMed] [Google Scholar]
- 223.Majhail NS, Mau LW, Chitphakdithai P, et al. Transplant center characteristics and survival after allogeneic hematopoietic cell transplantation in adults. Bone Marrow Transplant. 2020;55:906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Majhail NS, Mau LW, Chitphakdithai P, et al. National Survey of Hematopoietic Cell Transplantation Center Personnel, Infrastructure, and Models of Care Delivery. Biol Blood MarrowTransplant. 2015;21:1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: a way out of a conundrum. BMJ. 1995;311:1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Janosky JE. The ethics of underpowered clinical trials. JAMA. 2002;288:2118. author reply 2119. [PubMed] [Google Scholar]
- 227.Philippidis A Orphan drugs, big pharma. Hum Gene Ther. 2011;22:1035–1038. [DOI] [PubMed] [Google Scholar]
- 228.Administration FaD. Rare Diseases: Common Issues in Drug Development. In: FDA, ed 2019.
- 229.Administration FaD. Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products. In: FDA, ed 2019.
- 230.Administration FaD. BEST (Biomarker, Endpoints, and other Tools) Resource. In: FDA, ed 2020.
- 231.Administration FaD. Rare Diseases: Natural History Studies for Drug Development. In: FDA, ed 2019.
- 232.Administration FaD. Adaptive Designs for Clinical Trials of Drugs and Biologics. In: FDA, ed 2019.
- 233.Berry DA. Adaptive clinical trials in oncology. Nat Rev Clin Oncol. 2011;9:199–207. [DOI] [PubMed] [Google Scholar]
- 234.Berry DA. The brave new world of clinical cancer research: Adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Administration FaD. Guidance for the use of Bayesian statistics in medical device clinical trials. In: FDA, ed 2010.
- 236.Khanna D, Berrocal VJ, Giannini EH, et al. The American College of Rheumatology Provisional Composite Response Index for clinical trials in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2016;68:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Osmola-Mańkowska A, Silny W, Dańczak-Pazdrowska A, et al. Assessment of chronic sclerodermoid Graft-versus-Host Disease patients, using 20 MHz high-frequency ultrasonography and cutometer methods. Skin Res Technol. 2013;19:e417–e422. [DOI] [PubMed] [Google Scholar]
- 238.Lee SY, Cardones AR, Doherty J, Nightingale K, Palmeri M. Preliminary results on the feasibility of using ARFI/SWEI to assess cutaneous sclerotic diseases. Ultrasound Med Biol. 2015;41:2806–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang X, Zhou B, Osborn T. Ultrasound surface wave elastography for assessing scleroderma. . Ultrasound in medicine & biology. 2020;46:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cardones AR, Hall RP 3rd, Sullivan KM, et al. Quantifying skin stiffness in graft-versus-host disease, morphea, and systemic sclerosis using acoustic radiation force impulse imaging and shear wave elastography. J Invest Dermatol. 2021;141(4):924–927.e2. [DOI] [PubMed] [Google Scholar]
- 241.Chen F, Wang L, Vain A, et al. Interobserver reproducibility of the myoton and durometer devices to measure skin stiffness and hardness in chronic cutaneous graft-versus-host disease patients. Blood. 2019;134:4515. [Google Scholar]
- 242.Horger M, Bethge W, Boss A, et al. Musculocutaneous chronic graft-versus-host disease: MRI follow-up of patients undergoing immunosuppressive therapy. Am J Roentgenol. 2009;192:1401–1406. [DOI] [PubMed] [Google Scholar]
- 243.Sauter AW, Schmidt H, Mantlik F, et al. Imaging findings and therapy response monitoring in chronic sclerodermatous graft-versus-host disease: preliminary data of a simultaneous PET/MRI approach. Clin Nucl Med. 2013;38:e309–e317. [DOI] [PubMed] [Google Scholar]
- 244.Deegan AJ, Talebi-Liasi F, Song S, et al. Optical coherence tomography angiography of normal skin and inflammatory dermatologic conditions. Lasers Surg Med. 2018;50:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Neid T, Danz B, Eismann R, et al. [Sclerodermiform chronic graft-versus-host disease after allogenic peripheral blood stem-cell transplantation]. Dtsch Med Wochenschr. 2009;134:1106–1109. [DOI] [PubMed] [Google Scholar]
- 246.Baker LX, Chen F, Ssempijja Y, et al. 867 Direct mechanical measurements of skin to quantify evolution of sclerotic disease. J Invest Dermatol. 2020;140:S113. [Google Scholar]
- 247.Chen F, Dellalana LE, Gandelman JS, Vain A, Jagasia MH, Tkaczyk ER. Non-invasive measurement of sclerosis in cutaneous cGVHD patients with the handheld device Myoton: a cross-sectional study. Bone Marrow Transplant. 2019;54:616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Richeldi L, Ferńandez Pérez ER, Costabel U, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8:25–33. [DOI] [PubMed] [Google Scholar]
- 249.Makino K, Makino T, Stawski L, Lipson KE, Leask A, Trojanowska M. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res Ther. 2017;19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Castelino FV, Bain G, Pace VA, et al. An autotaxin/lysophosphatidic acid/interleukin-6 amplification loop drives scleroderma fibrosis. Arthritis Rheumatol. 2016;68:2964–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zabludoff S, Liu Y, Liu J, et al. Late breaking abstract - ND-L02-s0201 treatment leads to efficacy in preclinical IPF models. Eur Respir J. 2017;50:PA881. [Google Scholar]
- 252.Ogawa Y, Razzaque MS, Kameyama K, et al. Role of heat shock protein 47, a collagen-binding chaperone in Lacrimal Gland Pathology in Patients with cGVHD. Invest Ophthalmol Vis Sci. 2007;48:1079. [DOI] [PubMed] [Google Scholar]
- 253.Pilling D, Gomer RH. The development of serum amyloid P as a possible therapeutic. Front Immunol. 2018;9:2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Verstovsek S, Hasserjian RP, Pozdnyakova O, et al. PRM-151 in myelofibrosis: efficacy and safety in an open label extension study. Blood. 2018;132:686. [Google Scholar]
- 255.Garcia-Martin A, Garrido-Rodriguez M, Navarrete C, et al. EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis. Biochem Pharmacol. 2018;157:304–313. [DOI] [PubMed] [Google Scholar]
- 256.Cinar R, Gochuico BR, Iyer MR, et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight. 2017;2(8):e92281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Marquart S, Zerr P, Akhmetshina A, et al. Inactivation of the cannabinoid receptor CB1 prevents leukocyte infiltration and experimental fibrosis. Arthritis Rheum. 2010;62:3467–3476. [DOI] [PubMed] [Google Scholar]
- 258.Reid J, Zamuner S, Edwards K, et al. In vivo affinity and target engagement in skin and blood in a first-time-in-human study of an anti-oncostatin M monoclonal antibody. Br J Clin Pharmacol. 2018;84:2280–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Stawski L, Trojanowska M. Oncostatin M and its role in fibrosis. Connect Tissue Res. 2019;60:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lafyatis R, Spiera R, Domsic R, et al. THU0329 safety, target engagement, and initial efficacy of AVID200, a first-in-class potent and isoform-selective inhibitor of TGF-beta 1 and 3, in patients with diffuse cutaneous systemic sclerosis (DCSSC): a phase 1 dose escalation study. Ann Rheum Dis. 2020;79:394–395. [Google Scholar]
- 261.Denton CP, Ong VH, Xu S, et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann RheumDis. 2018;77:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Khan K, Xu S, Nihtyanova S, et al. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann Rheum Dis. 2012;71:1235–1242. [DOI] [PubMed] [Google Scholar]
- 263.Drobyski WR, Pasquini M, Kovatovic K, et al. Tocilizumab for the Treatment of Steroid Refractory Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2011;17:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Flynn R, Paz K, Du J, et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood. 2016;127:2144–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211–221. [DOI] [PubMed] [Google Scholar]
- 266.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–3828. [DOI] [PubMed] [Google Scholar]
- 268.Pipavath SN, Chung JH, Chien JW, Godwin JD. Organizing pneumonia in recipients of hematopoietic stem cell transplantation: CT features in 16 patients. J Comput Assist Tomogr. 2012;36:431–436. [DOI] [PubMed] [Google Scholar]
- 269.Bondeelle L, Gras J, Michonneau D, et al. Pleuroparenchymal fibroelastosis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:982–986. [DOI] [PubMed] [Google Scholar]


