Abstract
Osteocytes are dynamic, bone matrix-remodeling cells that form an intricate network of interconnected projections through the bone matrix, called the lacunar-canalicular system. Osteocytes are the dominant mechanosensory cells in bone and their mechanosensory and mechanotransductive functions follow their morphological form. During osteocytogenesis and development of the osteocyte lacunar-canalicular network, osteocytes must dramatically remodel both their cytoskeleton and their extracellular matrix. In this review, we summarize our current understanding of the mechanisms that govern osteocyte differentiation, cytoskeletal morphogenesis, mechanotransduction, and matrix remodeling. We postulate that the physiologic activation of matrix remodeling in adult osteocytes, known as perilacunar/canalicular remodeling (PLR) represents a re-activation of the developmental program by which the osteocyte network is first established. While much of osteocyte biology remains unclear, new tools and approaches make the present moment a particularly fruitful and exciting time to study the development of these remarkable cells.
Hezekiah’s Tunnel
The year was c.700 BC. The Assyrian empire was expanding, and King Sennacharib was about to complete his conquest of the fertile crescent. Hezekiah, king in Jerusalem, scrambled to prepare for a siege. But there was a problem. The Gihon spring that provided water to the hill-top city was outside the city walls, so Hezekiah devised a last-ditch plan: dig a tunnel to direct the water into the city. With time slipping away, workers dug from both ends, hoping to meet in the middle. Despite a few changes of direction, the engineers were successful; the pool filled with water, and the city successfully held off the siege.
This blind tunneling through rock, from both ends, meeting in the middle, also occurs more than a trillion times in each adult human skeleton in the osteocyte lacunarcanalicular network (Figure 1) [1]. But how do osteocytes accomplish this? And how does this complex network develop? In this review, we discuss our current understanding of how the osteocyte network develops, how osteocyte dendrites dig through the bone matrix, and highlight the putative role of mechanobiology in osteocyte morphogenesis.
Figure 1: Acid-etched human cortical bone, showing the complexity of the osteocyte canalicular network.
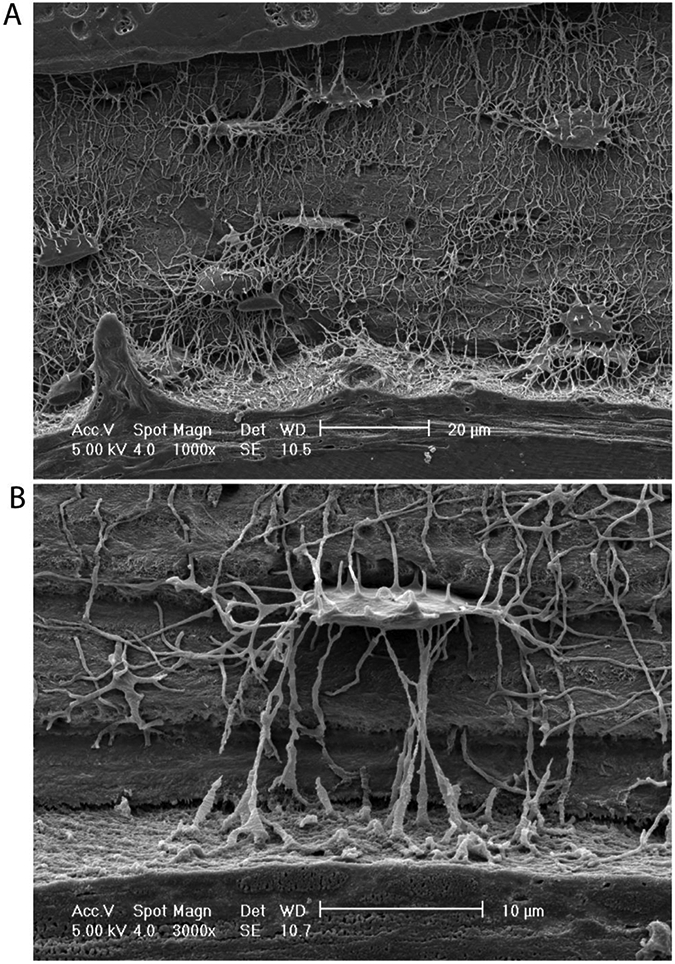
A) 1000x and B) 3000x magnified images courtesy of Lilian Plotkin and Lynda Bonewald (Indiana University School of Medicine), and Teresita Bellido (University of Arkansas for Medical Sciences).
Osteocyte characteristics
Osteocytes are the most abundant cell type in bone. There are approximately 40 billion osteocytes per adult skeleton, and they form an incredibly complex, interconnected network that permeates the mineralized bone matrix (Figure 1) [2-5]. Osteocytes reside in lacunae of 15-20 μm in diameter and extend dendritic arms to connect with neighboring osteocytes, blood vessels, surface cells, and bone marrow [2-4]. These dendritic processes pass through small tunnels called canaliculi whose diameter ranges from 250-300 nm. This complex system is called the lacunar-canalicular network [4,5]. The lacunar-canalicular network is filled with fluid to facilitate oxygen and nutrient transport to osteocytes [3]. The dendritic processes do not float freely in the liquid, but are anchored to the canalicular wall by tethering fibers [6]. Osteocytes are surrounded by a pericellular matrix (PCM), containing collagens and non-collagenous proteins, and are separated from the extracellular matrix by a 50-80 nm thick space that enables fluid flow and nutrient transport [7]. Although osteocytes share morphological characteristics (e.g., stellate shape), many factors such as location and bone compartment (i.e., cancellous vs. cortical), age, and the magnitude of typical local mechanical stimuli define their cellular shape and connectivity. For example, trabecular bone of the mandible features less-aligned canaliculi compared to diaphyseal cortical bone in mice [8,9]. Advanced aging reduces canalicular processes length and connectivity in human subjects [10]. Osteocytes in embryonic mouse long bones exhibit short, isotropic canaliculi with low connectivity, but lengthen and re-orient their canaliculi with the principal axis of the bone by six weeks of age [11]. Further, preventing ambulatory loading by sciatic nerve resection shortly after birth blunted this canalicular network adaptation, implicating mechanical forces in osteocyte morphogenesis [11]. In adult mice, comparing osteocytes across anatomical sites further suggests a role of the mechanical environment as long bone osteocytes feature more elongated cell bodies than the relatively spherical osteocytes in calvaria [12]. Together, these observations indicate that morphogenesis and adaptation of the osteocyte lacunar-canalicular are dynamic and may be regulated by mechanical cues.
Osteocyte mechanosensation and mechanotransduction
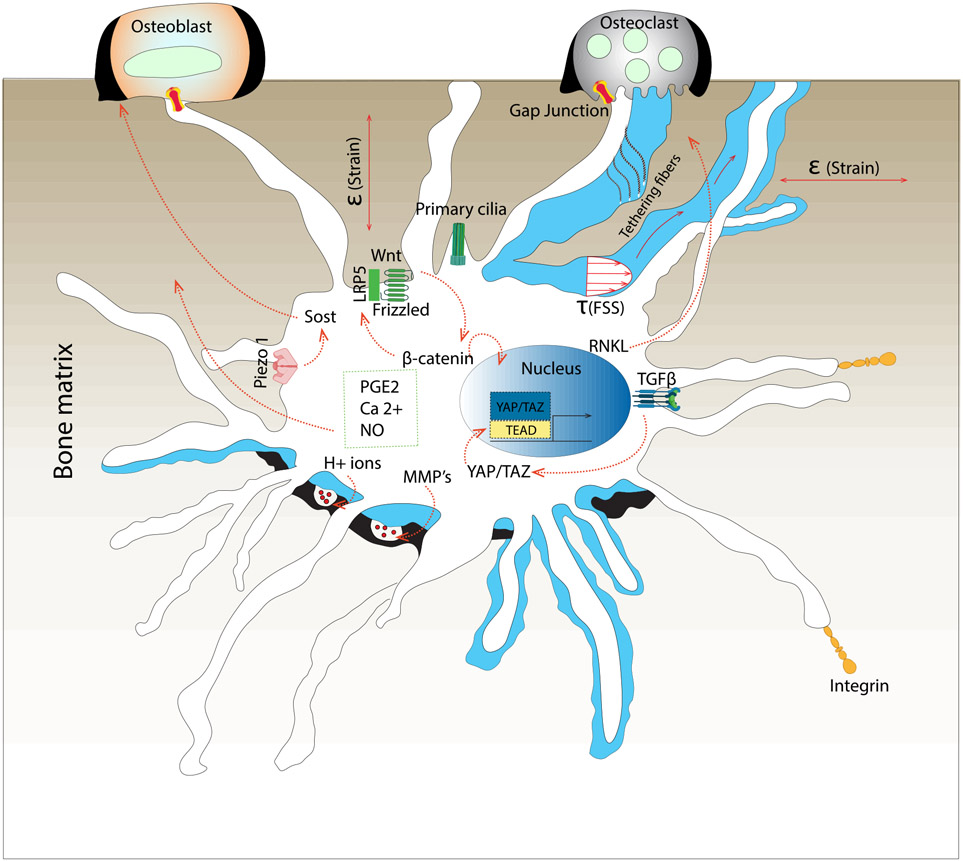
Osteocytes are the primary mechanosensing cells in bone (Figure 2). Their abundance, architecture, and connectivity provide osteocytes unique and dynamic sensitivity to mechanical stimuli in the skeleton [13-16]. Osteocytes are exposed to a variety of mechanical stimuli, including fluid shear stress and direct matrix deformation. Under direct perilacunar bone matrix deformation, the osteocyte lacuna can act as a strain concentrator to promote osteocyte mechanotransduction [17]. Likewise, fluid flow through the canalicular system may further amplify mechanical signals as even small inhomogeneous matrix deformations can produce pressure differentials, resulting in both shear stress on the osteocyte cell membrane and drag forces on the tethering elements that attach the dendrites to the canalicular wall [18]. Primary cilia may also function as "cellular antennae" that enable the osteocyte to sense mechanical signals [19]. Osteocyte mechanotransduction translates these mechanical cues into biochemical signals that communicate to osteoblasts and osteoclasts and direct bone remodeling [20-22]. Demonstrating the functional roles of osteocytes in bone physiology, Tatsumi and colleagues conditionally ablated osteocytes using diphtheria toxin (D.T.), and observed bone fragility, microfractures, osteoblastic dysfunction, and resistance to unloading-induced bone loss [23].
Figure 2:
Mechanisms of osteocyte mechanosensation and mechanotransduction and communication with osteoblast and osteoclasts.
Osteocyte mechanotransduction translates mechanical cues to biochemical signals. The mechanisms that mediate mechanotransduction in osteocytes are reviewed thoroughly in other articles [14,24-30]. Briefly, osteocytes transduce mechanical cues by activation of a concert of signaling pathways. Osteocytes transduce mechanical signals at the membrane through multiple signals, including matrix adhesions and calcium channels such as Piezo and TRPV4 [31-33]. Intracellularly, these signals are transmitted by pathways that activate transcriptional regulators, including β-catenin and YAP/TAZ [4,31,34,35]. For example, the osteocyte-secreted signaling protein, sclerostin, antagonizes Wnt/β-catenin signaling by binding to the Wnt co-receptors, LRP5/6, to prevent bone anabolism. In response to mechanical stimulation, osteocytes both decrease Sost mRNA expression and rapidly degrade the sclerostin protein to enable bone formation [33,36-38]. Interestingly, bones from lactating mice exhibit greater ex vivo load-induced β-catenin expression and sclerostin suppression than bones from virgin controls, suggesting a greater response to loading during lactation [39,40]. Other intracellular mediators of mechanotransduction include intracellular calcium (Ca2+), adenosine triphosphate (ATP), prostaglandin E2 (PGE2), and nitric oxide (NO) [13,41-43], the latter of which serve also as second messengers to promote differentiation and induce bone formation [44-46].
In addition to experimental methods, multiscale computational approaches have contributed to our understanding of the osteocyte mechanical environment and mechanotransduction. For instance, osteocytic strain-amplification was identified using theoretical and computational approaches to provide a physically-consistent solution to the paradox that physiologic tissue-level strains are too small to initiate the cellular responses [6,47-49]. Such multiscale modeling approaches can also be applied to the osteocyte cytoskeleton and mechanotransduction [50]. Multiscale finite element analyses recapitulate the location-dependent osteocytic mechano-response depending on anatomic location [51].
Osteocyte mechanosensory function follows morphological form
Osteocyte mechanosensation and mechanotransduction arise from the osteocyte’s unique stellate shape and interconnected architecture. The osteocyte is made up of an ellipsoid cell body and many long dendritic processes, both of which may contribute to mechanosensation. Direct in vitro stimulation data suggest that the dendritic processes are more mechanosensitive than the cell body, though the cell body also has important roles in mechanotransduction. Adachi et al. measured intracellular calcium transients in response to local mechanical deformation of either the cell body or of the dendrites [52]. Intracellular calcium wave propagation was faster and was induced at smaller deformations when stimulation was applied to the dendrites than to the cell body [52]. Similarly, Thi et al. showed that osteocyte-like cells respond to fluid flow through αvβ3 integrin attachment sites in the dendritic processes but not in the cell body [53]. However, Burra et al. observed a significant cell body response to mechanical stimulation through connexin 43 (Cx43) hemichannel opening while dendritic hemichannel opening was minimal under the same stimulus [54]. Together, these observations show that osteocyte morphology is critical to their mechanosensory function.
Conversely, osteocyte mechano-activation may also alter osteocyte morphology. This has been observed both in cell culture and animal models. In vitro, for example, Ponik et al. observed a dramatic increase in the number of dendrites in MLO-Y4 osteocyte-like cells after exposing the cells to 24 hours of oscillatory fluid flow. [32]. In vivo, osteocytes align with the direction of principal mechanical stresses across anatomical sites, with increased alignment in bones that experience more loading [12]. During development and growth, reducing ambulatory loading can impair osteocyte morphogenesis. Sugawara et al. performed sciatic nerve resection in 5-day-old mice and observed development of rounded-shape osteocytes at 6 weeks of age, in contrast to spindleshaped osteocytes in mice allowed to develop normally [11]. Similarly, Britz et al. performed sciatic nerve resection on 3-week-old rats and observed decreased lacunar density and volume at week 30 [55]. Sasaki et al. found that osteocytes in bone formed around mechanically-loaded orthopaedic implants exhibited greater ellipticity and dendrite density than in non-loaded implants [56]. Together, these observations suggest that osteocyte morphogenesis is influenced by the mechanical environment. However, whether osteocytes are capable of remodeling their matrix to re-orient the lacunae or canaliculi in response to adult bone loading remains an open question.
Osteocyte morphogenesis and the cytoskeleton
Osteocyte morphology is supported by the osteocyte cytoskeleton. The cytoskeleton forms a mechanical link between the extracellular environment and the intracellular compartment and is a central effector of mechanotransduction. Cytoskeletal filaments consist of three main types: actin filaments, microtubules, and intermediate filaments. Filament length, crosslinking geometry, binding proteins, and the cytoskeletal components' mechanical properties determine the cytoskeletal network's mechanical characteristics and the osteocytes [57]. Kamioka et al. showed that actin filaments abundant in isolated osteocyte processes and extend toward the end of the processes compared to intermediate filaments that were most abundant in the proximal end of the dendrites [45]. Actin filaments facilitate mechanotransduction by allowing the direct transmission of the mechanical signals from the dendrites to the cell body [58,59]. The microtubule cytoskeleton is also important in osteocyte mechanotransduction. For example, Lyons et al. identified a microtubule-dependent mechanotransduction pathway by which fluid shear stress (FSS) de-tyrosinates and stabilizes microtubules to induce reactive oxygen and calcium signaling to reduce sclerostin abundance in cultured osteocyte-like cells [60]. This rapid reduction in sclerostin abundance is effected by lysosomal degradation of sclerostin protein [38]. Together, these observations point to the cytoskeleton as important contributors to osteocyte shape.
Osteocyte morphogenesis requires biologic regulation of the cytoskeleton. Osteocyte morphology differs dramatically from that of the cuboidal osteoblasts from which they differentiate, including a dramatic rearrangement of the cytoskeleton (Figure 3). In osteoblasts, the microtubules radiate from the perinuclear microtubule organizing center and extend throughout the cell body. As osteoblasts differentiate into osteocytes, the microtubule filaments extend toward the immature dendrites; however, in mature osteocytes in vivo, the microtubule filaments are primarily localized in the cell body rather than the processes [61]. For example, actin bundling proteins such as fimbrin, α-actinin and villin are abundant in osteocyte dendrites but absent from osteoblast stress fibers [58][59]. Similarly, motor proteins that regulate cytoskeletal tension are expressed highly in osteocytes in vivo [62], but classic methods to directly measure cytoskeletal tension are difficult to perform on osteocytes in vivo due to their architecture and location in the mineralized bone matrix. However, cytoskeletal properties and dynamics can be studied using osteocyte-like cells in vitro. Cell culture models suggest that the significant cytoskeletal dissimilarities between osteoblasts and osteocytes contribute to their differential mechanosensitivity. For example, Ponik et al. compared the morphological and biochemical response to unidirectional and oscillatory fluid flow in MC3T3-E1 osteoblasts and MLO-Y4 osteocyte-like cells. They noticed that the MLO-Y4cells required a prolonged time to form and organize their stress fibers under unidirectional fluid flow. Whereas, in osteoblasts, the time needed for the formation and organization of the stress fibers under similar fluid flow was shorter [63]. In vivo, however, the density and organization of cytoskeletal elements in the dendrites combined with the dynamic mechanical environment of the canalicular system support unique mechanoresponsiveness [6]. Despite this understanding, the regulatory mechanisms that mediate cytoskeletal remodeling during osteocyte morphogenesis and the extent to which the cytoskeleton directs or is directed by osteocyte differentiation are still unclear.
Figure 3: Osteocyte morphogenesis requires dramatic reorganization of cytoskeletal architecture during osteoblast-osteocyte differentiation.
The extent to which these cytoskeletal changes are required for or consequent to osteocytogenesis remains an open question.
Osteocyte development and differentiation
Osteocytes differentiate from osteoblasts (Figure 3). During the final stages of bone formation, osteoblasts undergo three potential fates: 1) to embed in the bone matrix to become osteocytes, 2) to become bone lining cells or inactive osteoblasts, 3) to die (apoptosis). The type of bone, age, and animal species determine the proportion of the osteoblasts undergoing any of these fates [64]. However, the causes that determine which osteoblasts embed and become osteocytes are still unclear. This transition coincides with a dramatic change in morphology from cuboidal osteoblasts to highly dendritic cells and a reduction in the cell body size of approximately 70% [65]. Three cell types can be distinguished during this transition: osteoblastic osteocytes (Type 1), osteoid-osteocytes (Type 2), and osteocytes surrounded by mineralized matrix (Type 3) [66]. The transition process was once thought to be a passive process by which a new generation of osteoblasts covered existing osteoblasts on the bone surface, which would then decrease their matrix deposition rate and become trapped by the secretion of their neighboring cells [67-69]. However, it is now clear that osteocytes attain their unique morphology through an active invasive process. Zhao et al. observed collagen cleavage at the peri-osteocytic extracellular matrix, demonstrating that this remodeling during embedding was mediated by Matrix Metalloproteinases (MMP) [70]. Holmbeck et al., showed that collagen fibril cleavage along the processes is mediated by an MMP type collagenase (MT1-MMP/MMP14) [71]. The establishment of live imaging techniques in recent years has further confirmed that the transitional process is active. For example, using time-lapse intravital imaging of DMP1-GFP-labeled cells, the Dallas group observed osteocytically-differentiating osteoblasts migrating along the formed bone surface accompanied by extension of dendritic processes [3].
Dynamics of osteocyte development
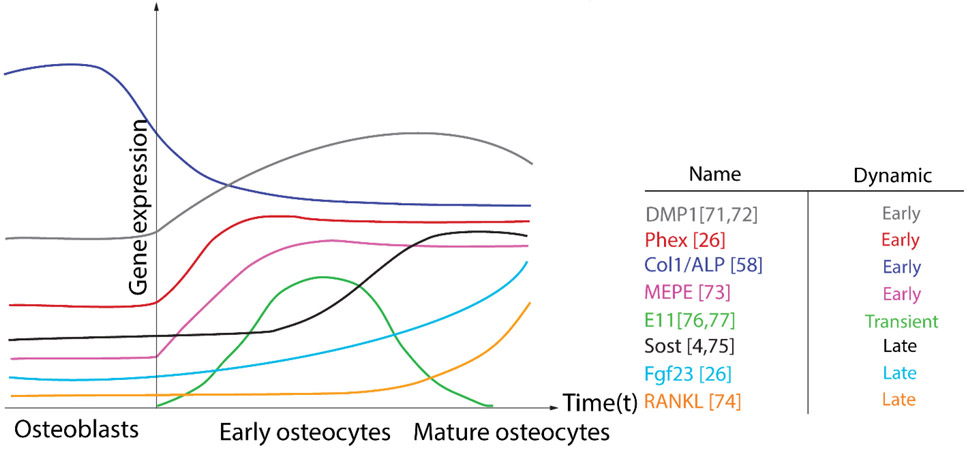
Osteoblasts differentiate into osteocytes by dynamic regulation of transcriptional programs. Some osteoblastic genes are downregulated with osteocyte differentiation; others are upregulated. Downregulated genes include Type I Collagen and alkaline phosphatase (ALP), while upregulated genes include dentin matrix protein (DMP1), fibroblast growth factor 23 (FGF23), extracellular matrix phosphoglycoprotein (MEPE), and phosphate regulating endopeptidase homolog X-linked (PHEX) (Figure 4). Many of these genes are particularly regulated by mechanotransduction in osteocytes, including DMP1, MEPE, and PHEX [26]. For example, mechanical loading of mouse alveolar bone causes localization of DMP1 protein to the osteocytes canalicular wall [72,73]. Interestingly, a similar mechanical loading approach, reveals distinct regulation of MEPE compared to DMP1 [74]. These observations suggest unique interacts between cellular state, context, mechanical environment, and inducible gene expression in osteocytes. Besides genes associated with matrix deposition and mineralization, osteocytes are also a significant source of the pro-osteoclastic cytokine, RANKL, which is negatively regulated by fluid shear stress (FSS) and is upregulated with osteocytic differentiation [75]. Similarly, Sost, which encodes sclerostin, a negative regulator of osteoblast activity, is induced in mature osteocytes and is robustly suppressed by mechanical loading [4][76]. In addition to genes that more stably mark cell of the osteoblast vs. osteocyte differentiation state, some genes are also transiently expressed, only during the transition. The most well-described of these genes is E11/gp33, also known as podoplanin. E11 is localizes to nascent osteocytic dendritic processes, which have begun to embed in the bone matrix. Upon osteocyte maturation, E11 expression effectively disappears [77,78]. Like many other osteocyte genes, E11 is regulated by loading; for example, fluid shear stress (FSS) induction of E11 expression has been suggested as one of the fundamental mechanisms leading to dendritic elongation [79]. Identification of the transient signals expressed uniquely during the osteoblast-osteocyte transition may be a particularly fruitful approach to understanding osteocytogenesis. Still, much remains to be discovered about the mechanisms by that control osteocyte morphogenesis and the development of the lacunar-canalicular system. Yet, similarities between canalicular network development and adult osteocytic remodeling may provide some clues.
Figure 4: Osteocyte differentiation requires dynamic gene expression.
Genes associated with osteocytogenesis include both upregulated (DMP1, Phex, MEPE) and downregulated (Col1/ALP) early osteocyte genes, transient genes (E11/gp38), and late genes (Sost, Fgf23, RANKL).
Perilacunar/canalicular remodeling: re-activation of a developmental program?
We postulate that perilacunar/canalicular remodeling (PLR) in adult osteocytes is a reactivation of the developmental program by which the canalicular network is first established. Mature osteocytes dynamically remodel their extracellular matrix to mobilize calcium for lactation and to regulate bone matrix quality [2,5,80]. We pose the hypothesis, yet to be tested, that the mechanisms by which new osteocytes form their interconnected dendritic network in the bone matrix and the mechanisms by which mature osteocytes remodel that same network are functionally equivalent. Indeed, the first descriptions of “osteocyte osteolysis” in the 1960s were made by observing the morphology and peri-osteocytic matrix acidification of chick embryo osteocytes [81,82]. Functional and mechanistic convergence is also supported by recent studies of osteocytic (re)modeling. During both canalicular network development and adult PLR, osteocytes actively alter their own connectivity and their extracellular matrix collagen organization and mineralization [71,83]. Recent data from Marc Wein and colleagues support a molecular and functional link between osteocyte morphogenesis and canalicular remodeling [84]. They show that the transcription factor osterix-1, which is encoded by the Sp7 gene, regulates osteocyte expression of osteocrine (Ostn) to promote dendrite formation during development [84]. Sp7 deletion impaired canalicular network morphogenesis, but, remarkably, systemic injection of adenoviral particles to exogenously express osteocrin in 3-week-old Sp7 knockout mice rescued dendrite formation by 6 weeks of age [84]. These data identify a developmental program can be re-activated post-weaning to induce dramatic canalicular remodeling.
Adult perilacunar/canalicular remodeling occurs in both male and female bones, but is sexually dimorphic [85]. In female rats, loss of estrogen by experimental ovariectomy (OVX), increases lacunar size compared to non-OVX controls [86]. Dole et al. showed that TGFβ regulation of PLR is sexually dimorphic, such that osteocyte-conditional TGFβ receptor II deletion in male mice impaired PLR and caused bone fragility, but female mice were protected from TGFβ-dependent defects in PLR and bone quality [85]. In adults, PLR is most prominently observed during lactation [39,40,87,88], but is also activated by exercise [89] and fracture repair [90]. During lactation, osteocytes actively degrade the mineralized matrix to mobilize calcium and meet the demands of milk production [2]. Osteocyte lacunar size increases in lactating mice but is reversed by osteocytic mineral deposition after weaning when the resorption pressure is lifted [88]. Although the mechanisms remain unclear, parathyroid hormone (PTH) is a key mediator of lactation-induced PLR [39]. Lactation elevates levels of PTH-related Peptide (PTHrP) in the maternal circulation to induce bone resorption [91]. Mechanistically, continuous PTH signaling upregulates ATPase H+ Transporting V0 Subunit D2 (ATP6V0D2), a proton pump on the cell membrane acidifying the extracellular environment [88]. During lactation, PTHrP promotes ATP6V0D2 expression to acidify and demineralize the perilacunar matrix [2]. In contrast, intermittent PTH(1-34) administration promotes bone formation and alters perilacunar matrix remodeling to promote fatigue resistance [92]. Physiologically, exercise produces intermittent endogenous PTH release to promote bone formation and perilacunar matrix remodeling [89]. Notably, PTH signaling is critical for embryonic and post-natal bone development [93,94], but mapping the multifactorial roles of PTH signaling between osteocyte morphogenesis and adult PLR requires further study. Enzymes and membrane pumps such as Acp5/TRAP, the Na+/H+ exchanger and the domain containing 2 (NHEDC2) contribute to matrix degradation and demineralization [88] and may explain the early observations of periosteocyte acidification in embryonic bone explants [81,82,95]. The collagen-degrading enzymes, matrix-type 1 MMP. (MT1-MMP/MMP14), MMP13, and Cathepsin K (Ctsk) are critical to form the canalicular network in development [70,71,83] and mediate PLR by degradation and remodeling of the organic matrix [35,83]. Lactation enhances Ctsk expression [96], and Ctsk deletion from osteocytes increases PTHrP levels and prevents the decrease in PTH that is induced by lactation [97]. Taken together, these studies of bone development and adaptation suggest convergent mechanisms for osteocyte-mediated matrix remodeling.
Molecular regulation of osteocytic remodeling
Recent studies are beginning to shed light on the osteocyte-intrinsic mechanisms that enable osteocyte morphogenesis and perilacunar/canalicular remodeling. For example, Alliston and colleagues found that osteocyte TGF-β signaling mediates bone adaptation to mechanical loads [99] and mediates PLR [98]. Specifically, consistent with their prior observation that global MMP13 deletion disorganized collagen matrix [83], osteocyte-conditional TGF-β receptor deletion impaired bone matrix organization and canalicular network density and connectivity (Figure 5A and C) [98. In our pursuit of the molecular mediators of mechanotransduction in osteocytes, we found that osteocyte-conditional deletion of the mechanosensitive transcriptional regulators, Yes-associated protein (YAP) and Transcriptional co-activator with PDZ-binding motif (TAZ) produced a nearly-identical phenotype, with disorganized collagen matrix organization and disrupted canalicular network development [35] (Figure 5B and D). Together, these observations suggest a role for osteocyte TGF-β signaling in mechanoregulation of osteocyte development, though causative relationships remain to be established. Notably all of these findings were made using constitutive knockout mouse models, resulting in a disruption of lacunar-canalicular network morphogenesis. Decoupling the mechanisms of osteocyte development from adult PLR and osteocyte-intrinsic mechanotransduction will require further research using inducible genetic models and rescue experiments.
Figure 5: Convergent consequences of osteocyte-conditional deletion of TGF-β receptor II and YAP/TAZ.
A) Second harmonic generated (SHG) images of tibial cortical bone to show the collagen matrix in WT and MMP-13-deficient bone adapted with permission from Ref. [83]. B) Second harmonic generated (SHG) images of femoral cortical bone to show the collagen matrix in WT and Ocy-conditional YAP/TAZ KO mice adapted with permission from Ref. [35] C) Silver staining of mouse femoral cortical bone canalicular networks in W.T. and Ocy-conditional TβRII KO mice adapted with permission from Ref. [98].. D) Silver staining of mouse femoral cortical bone canalicular networks in WT and Ocy-conditional YAP/TAZ KO mice, adapted with permission from Ref. [35].
Conclusion
Like King Hezekiah's engineers, who dug through the bedrock to let the springwater flow, osteocytes are dynamic cells that tunnel through mineralized bone matrix to form a network of fluid-filled tunnels. These canalicular tunnels communicate a wide variety of signals, from mechanical to endocrine. Osteocyte mechanosensory function follows morphological form, which are both effected and supported by the cytoskeleton. Osteocytic differentiation from osteoblasts and the development of the lacunar-canalicular network requires dynamic signaling and both cytoskeletal and matrix remodeling. This osteocyte-mediated remodeling can be re-activated by physiologic events in adult osteocytes in perilacunar/canalicular remodeling. We postulate that PLR represents the re-activation of this developmental program, though further research on these remarkable cells is required. Given the emerging roles of osteocytes as direct regulators of both bone quality and systemic organ crosstalk, osteocytes represent an exciting frontier for therapeutic intervention.
Highlights.
Osteocytes are the dominant mechanosensory cells in bone and their mechanosensory and mechanotransductive functions follow their morphological form.
In this review, we summarize our current understanding of the mechanisms that govern osteocyte differentiation, cytoskeletal morphogenesis, mechanotransduction, and matrix remodeling.
We postulate that the physiologic activation of matrix remodeling in adult osteocytes, known as perilacunar/canalicular remodeling (PLR) represents a re-activation of the developmental program by which the osteocyte network is first established.
Acknowledgements:
This work was supported by NIH/NIAMS grants R21 AR071559 (to J.D.B.) and P30 AR069619 (to J.D.B.) and ), and the Center for Engineering Mechanobiology (CEMB), an NSF Science and Technology Center, under grant agreement CMMI: 15-48571.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Buenzli PR, Sims NA, Quantifying the osteocyte network in the human skeleton, Bone. 75 (2015) 144–150. 10.1016/j.bone.2015.02.016. [DOI] [PubMed] [Google Scholar]
- [2].Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, Wysolmerski J, Bonewald LF, Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation., J. Bone Miner. Res 27 (2012) 1018–29. 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang K, Le L, Chun BM, Tiede-Lewis LM, Shiflett LA, Prideaux M, Campos RS, Veno PA, Xie Y, Dusevich V, Bonewald LF, Dallas SL, A Novel Osteogenic Cell Line That Differentiates Into GFP-Tagged Osteocytes and Forms Mineral With a Bone-Like Lacunocanalicular Structure, J. Bone Miner. Res 34 (2019) 979–995. 10.1002/jbmr.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dallas SL, Prideaux M, Bonewald LF, The osteocyte: An endocrine cell … and more, Endocr. Rev 34 (2013) 658–690. 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Qing H, Bonewald LF, Osteocyte remodeling of the perilacunar and pericanalicular matrix., Int. J. Oral Sci 1 (2009) 59–65. 10.4248/ijos.09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Y, McNamara LM, Schaffler MB, Weinbaum S, A model for the role of integrins in flow induced mechanotransduction in osteocytes, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 15941–15946. 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB, Attachment of osteocyte cell processes to the bone matrix, Anat. Rec 292 (2009) 355–363. 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lai X, Price C, Modla S, Thompson WR, Caplan J, Kirn-Safran CB, Wang L, The dependences of osteocyte network on bone compartment, age, and disease, Bone Res. 3 (2015) 1–11. 10.1038/boneres.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alemi AS, Mazur CM, Fowler TW, Woo JJ, Knott PD, Alliston T, Glucocorticoids cause mandibular bone fragility and suppress osteocyte perilacunar-canalicular remodeling, Bone Reports. 9 (2018) 145–153. 10.1016/j.bonr.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Püschel K, Djuric M, Amling M, Busse B, Osteocytic canalicular networks: Morphological implications for altered mechanosensitivity, ACS Nano. 7 (2013) 7542–7551. 10.1021/nn401360u. [DOI] [PubMed] [Google Scholar]
- [11].Sugawara Y, Kamioka H, Ishihara Y, Fujisawa N, Kawanabe N, Yamashiro T, The early mouse 3D osteocyte network in the presence and absence of mechanical loading, Bone. 52 (2013) 189–196. 10.1016/j.bone.2012.09.033. [DOI] [PubMed] [Google Scholar]
- [12].Vatsa A, Breuls RG, Semeins CM, Salmon PL, Smit TH, Klein-Nulend J, Osteocyte morphology in fibula and calvaria - Is there a role for mechanosensing?, Bone. 43 (2008) 452–458. 10.1016/j.bone.2008.01.030. [DOI] [PubMed] [Google Scholar]
- [13].Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH, Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts - correlation with prostaglandin upregulation, Biochem. Biophys. Res. Commun 217 (1995) 640–648. 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- [14].Fritton SP, Weinbaum S, Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction, Annu. Rev. Fluid Mech 41 (2009) 347–374. 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Klein-Nulend J, Van Der Plas A, Semeins CM, Ajubi NE, Erangos JA, Nijweide PJ, Burger EH, Sensitivity of osteocytes to biomechanical stress in vitro, FASEB J. 9 (1995) 441–445. 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- [16].Wang L, Solute Transport in the Bone Lacunar-Canalicular System (LCS), Curr. Osteoporos. Rep 16 (2018) 32–41. 10.1007/s11914-018-0414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J, Osteocyte lacunae tissue strain in cortical bone, J. Biomech 39 (2006) 1735–1743. 10.1016/j.jbiomech.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han Y, Cowin SC, Schaffler MB, Weinbaum S, Mechanotransduction and strain amplification in osteocyte cell processes, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 16689–16694. 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR, Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 13325–13330. 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Robling AG, Turner CH, Mechanical signaling for bone modeling and remodeling., Crit. Rev. Eukaryot. Gene Expr 19 (2009) 319–38. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3743123&tool=pmcentrez&rendertype=abstract (accessed October 19, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Robling AG, Castillo AB, Turner CH, BIOMECHANICAL AND MOLECULAR REGULATION OF BONE REMODELING, Annu. Rev. Biomed. Eng 8 (2006) 455–498. 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- [22].Marotti G, Ferretti M, Remaggi F, Palumbo C, Quantitative evaluation on osteocyte canalicular density in human secondary osteons, Bone. 16 (1995) 125–128. 10.1016/8756-3282(95)80022-I. [DOI] [PubMed] [Google Scholar]
- [23].Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K, Targeted Ablation of Osteocytes Induces Osteoporosis with Defective Mechanotransduction, Cell Metab. 5 (2007) 464–475. 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [24].Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, Danzig J, Robling AG, Mechanobiology of the skeleton., Sci. Signal 2 (2009) pt3. 10.1126/scisignal.268pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Geoghegan IP, Hoey DA, McNamara LM, Integrins in Osteocyte Biology and Mechanotransduction., Curr. Osteoporos. Rep 17 (2019) 195–206. 10.1007/s11914-019-00520-2. [DOI] [PubMed] [Google Scholar]
- [26].Uda Y, Azab E, Sun N, Shi C, Pajevic PD, Osteocyte Mechanobiology, Curr. Osteoporos. Rep 15 (2017) 318–325. 10.1007/s11914-017-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li X, Kordsmeier J, Xiong J, New Advances in Osteocyte Mechanotransduction., Curr. Osteoporos. Rep 19 (2021) 101–106. 10.1007/s11914-020-00650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S, Mechanosensation and transduction in osteocytes., Bone. 54 (2013) 182–90. 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- [29].Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH, Aging, Osteocytes, and Mechanotransduction., Curr. Osteoporos. Rep 15 (2017) 401–411. 10.1007/s11914-017-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Plotkin LI, Speacht TL, Donahue HJ, Cx43 and mechanotransduction in bone., Curr. Osteoporos. Rep 13 (2015) 67–72. 10.1007/s11914-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, Xiong J, Stimulation of piezo1 by mechanical signals promotes bone anabolism, Elife. 8 (2019) 1–22. 10.7554/eLife.49631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, Zhang X, Zhou Y, Yadav PS, Lin J, Wu N, Zhao L, Huang D, Zhou S, Su P, Yang Y, Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin, Elife. 9 (2020). 10.7554/eLife.52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Williams KM, Leser JM, Gould NR, Joca HC, Lyons JS, Khairallah RJ, Ward CW, Stains JP, TRPV4 calcium influx controls sclerostin protein loss independent of purinergic calcium oscillations., Bone. 136 (2020) 115356. 10.1016/j.bone.2020.115356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, Bonewald LF, Johnson ML, Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading, J. Bone Miner. Res 29 (2014) 705–715. 10.1002/jbmr.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kegelman CD, Coulombe JC, Jordan KM, Horan DJ, Qin L, Robling AG, Ferguson VL, Bellido TM, Boerckel JD, YAP and TAZ Mediate Osteocyte Perilacunar/Canalicular Remodeling, J. Bone Miner. Res 35 (2020) 196–210. 10.1002/jbmr.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Burgers TA, Williams BO, Regulation of Wnt/β-catenin signaling within and from osteocytes, Bone. 54 (2013) 244–249. 10.1016/j.bone.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T, Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading, Bone. 50 (2012) 209–217. 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gould NR, Williams KM, Joca HC, Torre OM, Lyons JS, Leser JM, Srikanth MP, Hughes M, Khairallah RJ, Feldman RA, Ward CW, Stains JP, Disparate bone anabolic cues activate bone formation by regulating the rapid lysosomal degradation of sclerostin protein, Elife. 10 (2021). 10.7554/eLife.64393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu XS, Wang L, de Bakker CMJ, Lai X, Mechanical Regulation of the Maternal Skeleton during Reproduction and Lactation, Curr. Osteoporos. Rep 17 (2019) 375–386. 10.1007/s11914-019-00555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hemmatian H, Jalali R, Semeins CM, Hogervorst JMA, van Lenthe GH, Klein-Nulend J, Bakker AD, Mechanical Loading Differentially Affects Osteocytes in Fibulae from Lactating Mice Compared to Osteocytes in Virgin Mice: Possible Role for Lacuna Size, Calcif. Tissue Int 103 (2018) 675–685. 10.1007/s00223-018-0463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kringelbach TM, Aslan D, Novak I, Ellegaard M, Syberg S, Andersen CKB, Kristiansen KA, Vang O, Schwarz P, Jørgensen NR, Fine-tuned ATP signals are acute mediators in osteocyte mechanotransduction, Cell. Signal 27 (2015) 2401–2409. 10.1016/j.cellsig.2015.08.016. [DOI] [PubMed] [Google Scholar]
- [42].Jing D, Baik AD, Lu XL, Zhou B, Lai X, Wang L, Luo E, Guo XE, In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading, FASEB J. 28 (2014) 1582–1592. 10.1096/fj.13-237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ, Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes, Am. J. Physiol. - Endocrinol. Metab 276 (1999). 10.1152/ajpendo.1999.276.1.e171. [DOI] [PubMed] [Google Scholar]
- [44].Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J, Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation, Biochem. Biophys. Res. Commun 348 (2006) 1082–1088. 10.1016/j.bbrc.2006.07.146. [DOI] [PubMed] [Google Scholar]
- [45].Nagata T, Kaho K, Nishikawa S, Shinohara H, Wakano Y, Ishida H, Effect of prostaglandin E2 on mineralization of bone nodules formed by fetal rat calvarial cells, Calcif. Tissue Int 55 (1994) 451–457. 10.1007/BF00298559. [DOI] [PubMed] [Google Scholar]
- [46].Raisz LG, Effects of prostaglandin E2 on bone formation in cultured fetal rat calvariae: role of insulin-like growth factor-I, Endocrinology. 133 (1993) 1504–1510. 10.1210/en.133.4.1504. [DOI] [PubMed] [Google Scholar]
- [47].Han Y, Cowin SC, Schaffler MB, Weinbaum S, Mechanotransduction and strain amplification in osteocyte cell processes, Proc. Natl. Acad. Sci 101 (2004) 16689–16694. 10.1073/PNAS.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].You L, Cowin SC, Schaffler MB, Weinbaum S, A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix, J. Biomech 34 (2001) 1375–1386. 10.1016/S0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- [49].Rath Bonivtch A, Bonewald LF, Nicolella DP, Tissue strain amplification at the osteocyte lacuna: A microstructural finite element analysis, J. Biomech 40 (2007) 2199–2206. 10.1016/j.jbiomech.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kardas D, Nackenhorst U, Balzani D, Computational model for the cell-mechanical response of the osteocyte cytoskeleton based on self-stabilizing tensegrity structures, Biomech. Model. Mechanobiol 12 (2013) 167–183. 10.1007/s10237-012-0390-y. [DOI] [PubMed] [Google Scholar]
- [51].Vaughan TJ, Verbruggen SW, McNamara LM, Are all osteocytes equal? Multiscale modelling of cortical bone to characterise the mechanical stimulation of osteocytes, Int. j. Numer. Method. Biomed. Eng 29 (2013) 1361–1372. 10.1002/CNM.2578. [DOI] [PubMed] [Google Scholar]
- [52].Adachi T, Aonuma Y, Tanaka M, Hojo M, Takano-Yamamoto T, Kamioka H, Calcium response in single osteocytes to locally applied mechanical stimulus: Differences in cell process and cell body, J. Biomech 42 (2009) 1989–1995. 10.1016/j.jbiomech.2009.04.034. [DOI] [PubMed] [Google Scholar]
- [53].Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC, Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αvβ3 integrin, Proc. Natl. Acad. Sci. U. S. A 110 (2013)21012–21017. 10.1073/pnas.1321210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX, Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 13648–13653. 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Britz HM, Carter Y, Jokihaara J, Leppänen OV, Järvinen TLN, Belev G, Cooper DML, Prolonged unloading in growing rats reduces cortical osteocyte lacunar density and volume in the distal tibia, Bone. 51 (2012) 913–919. 10.1016/j.bone.2012.08.112. [DOI] [PubMed] [Google Scholar]
- [56].Sasaki M, Kuroshima S, Aoki Y, Inaba N, Sawase T, Ultrastructural alterations of osteocyte morphology via loaded implants in rabbit tibiae., J. Biomech 48 (2015) 4130–4141. 10.1016/j.jbiomech.2015.10.025. [DOI] [PubMed] [Google Scholar]
- [57].Klein-Nulend J, Bacabac RG, Bakker AD, Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton., Eur. Cell. Mater 24 (2012) 278–91. http://www.ncbi.nlm.nih.gov/pubmed/23007912 (accessed March 6, 2016). [DOI] [PubMed] [Google Scholar]
- [58].Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS, Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections, J. Bone Miner. Res 13 (1998) 1555–1568. 10.1359/jbmr.1998.13.10.1555. [DOI] [PubMed] [Google Scholar]
- [59].Kamioka H, Sugawara Y, Honjo T, Yamashiro T, Takano-Yamamoto T, Terminal Differentiation of Osteoblasts to Osteocytes Is Accompanied by Dramatic Changes in the Distribution of Actin-Binding Proteins, J. Bone Miner. Res 19 (2004) 471–478. 10.1359/JBMR.040128. [DOI] [PubMed] [Google Scholar]
- [60].Lyons JS, Joca HC, Law RA, Williams KM, Kerr JP, Shi G, Khairallah RJ, Martin SS, Konstantopoulos K, Ward CW, Stains JP, Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Murshid SA, Takano-Yamamoto T, Kamioka H, Differential distribution of microtubules in immature osteocytes in vivo, J. Oral Biosci 60 (2018) 98–101. 10.1016/j.job.2018.08.001. [DOI] [Google Scholar]
- [62].Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I, Identification of differentially expressed genes between osteoblasts and osteocytes, Bone. 45 (2009) 682–692. 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ponik SM, Triplett JW, Pavalko FM, Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles, J. Cell. Biochem 100 (2007) 794–807. 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- [64].Dallas SL, Bonewald LF, Dynamics of the transition from osteoblast to osteocyte, in: Ann. N. Y. Acad. Sci, Blackwell Publishing Inc., 2010: pp. 437–443. 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bonewald LF, Osteocytes, INC, 2021. 10.1016/b978-0-12-813073-5.00007-1. [DOI] [Google Scholar]
- [66].Palumbo C, Palazzini S, Zaffe D, Marotti G, Osteocyte Differentiation in the Tibia of Newborn Rabbit: An Ultrastructural Study of the Formation of Cytoplasmic Processes, Cells Tissues Organs. 137 (1990) 350–358. 10.1159/000146907. [DOI] [PubMed] [Google Scholar]
- [67].Franz-Odendaal TA, Hall BK, Witten PE, Buried alive: How osteoblasts become osteocytes, Dev. Dyn 235 (2006) 176–190. 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- [68].Marotti G, Canè V, Palazzini S, Palumbo C, Structure-function relationships in the osteocyte., Undefined. (1990). [Google Scholar]
- [69].F.N. Nefussi JR, Sautier JM, Nicolas V, How osteoblasts become osteocytes: a decreasing matrix forming process, J Biol Buccale. 19 (1991) 75–82. [PubMed] [Google Scholar]
- [70].Zhao W, Byrne MH, Wang Y, Krane SM, Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen, J. Clin. Invest 106 (2000) 941–949. 10.1172/JCI10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR, The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone, J. Cell Sci 118 (2005) 147–156. 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- [72].Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D, Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo., J. Bone Miner. Res 18 (2003) 807–17. 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- [73].Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE, Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism, Nat. Genet 38 (2006) 1310–1315. 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gluhak-Heinrich J, Pavlin D, Yang W, MacDougall M, Harris SE, MEPE expression in osteocytes during orthodontic tooth movement, Arch. Oral Biol 52 (2007) 684–690. 10.1016/j.archoralbio.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, Balcells-Camps M, Kronenberg HM, Babij P, Pajevic PD, The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro, J. Biol. Chem 290 (2015) 16744–16758. 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH, Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin., J. Biol. Chem 283 (2008) 5866–75. 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- [77].Bellido T, Plotkin LI, Bruzzaniti A, Bone Cells, in: Basic Appl. Bone Biol, Elsevier, 2019: pp. 37–55. 10.1016/b978-0-12-813259-3.00003-8. [DOI] [Google Scholar]
- [78].Prideaux M, Loveridge N, Pitsillides AA, Farquharson C, Extracellular Matrix Mineralization Promotes E11/gp38 Glycoprotein Expression and Drives Osteocytic Differentiation, PLoS One. 7 (2012) e36786. 10.1371/journal.pone.0036786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF, E11/gp38 Selective Expression in Osteocytes: Regulation by Mechanical Strain and Role in Dendrite Elongation, Mol. Cell. Biol 26 (2006) 4539–4552. 10.1128/mcb.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Alliston T, Biological Regulation of Bone Quality, Curr. Osteoporos. Rep 12 (2014) 366–375. 10.1007/s11914-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bélanger LF, Bélanger C, Semba T, Technical approaches leading to the concept of osteocytic osteolysis., Clin. Orthop. Relat. Res 54 (1967) 187–196. 10.1097/00003086-196709000-00020. [DOI] [PubMed] [Google Scholar]
- [82].Zambonin Zallone AZ, Teti A, Nico B, Primavera MV, Osteoplastic activity of mature osteocytes evaluated by H-proline incorporation., Basic Appl. Histochem 26 (1982) 65–7. [PubMed] [Google Scholar]
- [83].Tang SY, Herber R-P, Ho SP, Alliston T, Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance, J. Bone Miner. Res 27 (2012) 1936–1950. 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang JS, Kamath T, Mirzamohammadi F, Rotter D, Hojo H, Castro CD, Patel R, Govea N, Enishi T, da Silva Martins J, Bruce M, Brooks DJ, Bouxsein ML, Tokarz D, Lin CP, Abdul A, Macosko EZ, Fiscaletti M, Munns CF, Fujiwara M, Kronenberg HM, Wein MN, Title: Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin 1 2, BioRxiv. (2021) 2021.03.22.436056. 10.1101/2021.03.22.436056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dole NS, Yee CS, Mazur CM, Acevedo C, Alliston T, TGFβ Regulation of Perilacunar/Canalicular Remodeling Is Sexually Dimorphic, J. Bone Miner. Res 35 (2020) 1549–1561. 10.1002/jbmr.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sharma D, Ciani C, Marin PAR, Levy JD, Doty SB, Fritton SP, Alterations in the osteocyte lacunar-canalicular microenvironment due to estrogen deficiency, Bone. 51 (2012)488–497. 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tsourdi E, Jähn K, Rauner M, Busse B, Bonewald LF, Physiological and pathological osteocytic osteolysis, J. Musculoskelet. Neuronal Interact 18 (2018) 292–303. [PMC free article] [PubMed] [Google Scholar]
- [88].Jähn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, Dallas SL, Bonewald LF, Osteocytes Acidify Their Microenvironment in Response to PTHrP In Vitro and in Lactating Mice In Vivo., J. Bone Miner. Res 32 (2017) 1761–1772. 10.1002/jbmr.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gardinier JD, Al-Omaishi S, Morris MD, Kohn DH, PTH signaling mediates perilacunar remodeling during exercise, Matrix Biol. 52–54 (2016) 162–175. 10.1016/J.MATBIO.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Osipov B, Paralkar MP, Emami AJ, Cunningham HC, Tjandra PM, Pathak S, Langer HT, Baar K, Christiansen BA, Sex Differences in Systemic Bone and Muscle Loss Following Femur Fracture in Mice, J. Orthop. Res (2021) jor.25116. 10.1002/jor.25116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kovacs CS, Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery., Physiol. Rev 96 (2016) 449–547. 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- [92].Gardinier JD, Al-Omaishi S, Rostami N, Morris MD, Kohn DH, Examining the influence of PTH(1-34) on tissue strength and composition., Bone. 117 (2018) 130–137. 10.1016/j.bone.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fan Y, Hanai J, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, Baron R, Bronson RT, Horowitz MC, Wu JY, Bilezikian JP, Dempster DW, Rosen CJ, Lanske B, Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate, Cell Metab. 25 (2017) 661–672. 10.1016/J.CMET.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sinha P, Aarnisalo P, Chubb R, Poulton IJ, Guo J, Nachtrab G, Kimura T, Swami S, Saeed H, Chen M, Weinstein LS, Schipani E, Sims NA, Kronenberg HM, Wu JY, Loss of G s α in the Postnatal Skeleton Leads to Low Bone Mass and a Blunted Response to Anabolic Parathyroid Hormone Therapy, J. Biol. Chem 291 (2016) 1631–1642. 10.1074/jbc.M115.679753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wergedal JE, Baylink DJ, Distribution of acid and alkaline phosphatase activity in undemineralized sections of the rat tibial diaphysis., J. Histochem. Cytochem 17 (1969) 799–806. 10.1177/17.12.799. [DOI] [PubMed] [Google Scholar]
- [96].Clarke MV, Russell PK, Findlay DM, Sastra S, Anderson PH, Skinner JP, Atkins GJ, Zajac JD, Davey RA, A role for the calcitonin receptor to limit bone loss during lactation in female mice by inhibiting osteocytic osteolysis, Endocrinology. 156 (2015) 3203–3214. 10.1210/en.2015-1345. [DOI] [PubMed] [Google Scholar]
- [97].Lotinun S, Ishihara Y, Nagano K, Kiviranta R, Carpentier VT, Neff L, Parkman V, Ide N, Hu D, Dann P, Brooks D, Bouxsein ML, Wysolmerski J, Gori F, Baron R, Cathepsin K–deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression, J. Clin. Invest 129 (2019) 3058–3071. 10.1172/JCI122936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S, Evans DS, Lang TF, Zhang B, Ritchie RO, Mohammad KS, Alliston T, Osteocyte-Intrinsic TGF-β Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling., Cell Rep. 21 (2017) 2585–2596. 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Nguyen J, Tang SY, Nguyen D, Alliston T, Load regulates bone formation and Sclerostin expression through a TGFβ-dependent mechanism., PLoS One. 8 (2013) e53813. 10.1371/journal.pone.0053813. [DOI] [PMC free article] [PubMed] [Google Scholar]