Abstract
Introduction:
Molecular pathological epidemiology (MPE) is an integrative disciplinary area examining the relationships between various exposures and pathogenic signatures of diseases. In line with the accelerating advancements in MPE, social science and its health-related interdisciplinary areas have also developed rapidly. Accumulating evidence indicates the pathological role of social-demographic factors. We therefore initially proposed social MPE in 2015, which aims to elucidate etiological roles of social-demographic factors and address health inequalities globally. With the ubiquity of molecular diagnosis, there are ample opportunities for researchers to utilize and develop the social MPE framework.
Areas covered:
Molecular subtypes of breast cancer have been investigated rigorously for understanding its etiologies rooted from social factors. Emerging evidence indicates pathogenic heterogeneity of neurological disorders such as Alzheimer’s disease. Presenting specific patterns of social-demographic factors across different molecular subtypes should be promising for advancing the screening, prevention, and treatment strategies of those heterogeneous disease. This article rigorously reviewed literatures investigating differences of race/ethnicity and socioeconomic status across molecular subtypes of breast cancer and Alzheimer’s disease to date.
Expert Opinion:
With advancements of the multi-omics technologies, we foresee a blooming of social MPE studies, which can address health disparities, advance personalized molecular medicine, and enhance public health.
Keywords: Alzheimer’s disease, breast cancer, health inequality, heterogeneity, laboratory medicine, molecular pathological epidemiology, precision medicine, prevention, social science, social epidemiology
1. Outline
The intention of this paper is to revisit social-MPE framework that was initially proposed in 2015. The first section, “Molecular pathological epidemiology in the modern era of precision medicine”, provides the definition and main components of the general MPE framework. The second section, “Integration of social science and MPE”, presents the latest concept of the social MPE framework. The third and fourth sections are to illustrate the application of social MPE in neoplasms (breast cancer as an example) and non-neoplastic diseases (Alzheimer’s disease as an example), which were the main focus and novelty of the current study, followed by the conclusion section.
2. Molecular pathological epidemiology in the modern era of precision medicine
Pathology, which is a basic field of biomedical science, analyzes tissue morphologies and cellular molecular features of diseases to predict treatment and intervention outcome1. Epidemiology, which is the study of the distribution and determinants of disease in specified populations, provides conceptual and analytical frameworks for examining the associations between various exposures and health-related outcomes2. Although diverse technologies are employed by pathologists and epidemiologists alike, both pathology and epidemiology embrace a common goal of elucidating the etiologies and pathologies of diseases to promote population health3.
Due to the increasing availability of molecular pathological diagnostic tools and the breakneck development of laboratory technologies, the field of molecular pathology has advanced rapidly in the past few decades4. Nowadays, incorporation of molecular pathology into epidemiological research frameworks is becoming a mainstream in cancer epidemiology5. For example, several attempts have been made to generate a molecular classification of gastric cancer in order to facilitate the early detection of gastric cancer as well as the development of personalized treatment6,7. By integrating molecular pathology and epidemiology, our previous study has identified a panel of 13 mRNA to predict the overall survival of gastric cancer patients, which might be useful in clinical practice for informing personalized treatment options8. The integration of molecular pathology and epidemiology has been beneficial to both subjects. As a method-based discipline, epidemiology develops and standardizes analytical strategies to elucidate the association between exposures and outcomes, which provides both reproducibility and the potential of applying epidemiological method like causal inference toolbox (i.e., inverse probability weighting, marginal structural model, g-computation, and so on) to pathological studies9–13. Reciprocally, epidemiologists who utilize molecular pathology approaches can not only link exposures to molecular pathological biomarkers but also advance and refine disease classification systems and molecular mechanisms underlying the pathogenesis of the disease14,15, which further prompt the individualized therapeutic and preventive strategies16.
In parallel with the increasing incorporation between molecular pathology and epidemiology, molecular pathological epidemiology (MPE) has been proposed as an integrative research discipline which employs molecular pathological biomarkers to subclassify diseases and to shed light on interindividual differences with regard to specified epidemiological determinants of human population health5,17,18. The field of MPE is versatile, having integrated other scientific disciplines related to pathology such as microbiology and immunology5,19–21. The MPE paradigm conceptually stands on the unique disease principle and the disease continuum theory22,23. The unique disease principle is based on the concept that human diseases result from substantially complex interplay of alterations in epigenomes, transcriptomes, proteomes, metabolomes, microbiomes and interactomes, the combination of which is unique to each individual. Thus, each disease process in each human being should be distinctive from “the same disease process (under the traditional epidemiology paradigm)” in other people. The unique disease principle highlights the distinctiveness of disease pathogenesis within each human being22. The disease continuum theory emphasizes that different disease entities might have pathologically overlapping features and related etiologies23. For example, benign lymphoproliferative diseases have overlapping clinicopathological features with malignant lymphomas24. Moreover, neoplastic diseases often cause para-neoplastic syndromes with symptoms which may often be observed in non-neoplastic disease conditions23. Therefore, pathophysiology of seemly diverse diseases in every single person within a specific study population should be considered as a combination of multiple phenotypes23. The integrated MPE model allows us to explore the novel molecular biomarkers, which were unavailable in the conventional epidemiology studies. Moreover, the MPE method can offer us a deeper understanding on the mechanisms underlying differential associations of risk factors with certain diseases subtypes through adding the molecular pathological signatures into the causal chains, which play a critical role in the precision medicine initiative25. In line with the short-term milestone of the precision medicine initiative, there is an abundance of molecular pathological diagnostic tests on tumor specimens to study. Thus, utilization of the MPE method has been prevalent in cancer epidemiological research. But in fact, MPE can be readily applied to both neoplastic and non-neoplastic disease showing considerable interpersonal heterogeneity23,26. The importance of the MPE framework and its relevance have been discussed and emphasized in the international meetings27–30 and the literature31–47.
3. Integration of social science and MPE
Social science is a discipline that studies societies and the relationships among individuals within those societies48. Social science (e.g., sociology) and its health-related interdisciplinary areas (social epidemiology) have also expanded significantly and concurrently with the booming development of MPE49,50. Social-demographic factors such as socioeconomic status/position, income inequality, social support and capital, neighborhood quality, gender and race/ethnicity can influence lifestyle and other exposure status of individuals and therefore determine human health49,51. As an interdisciplinary area of social science and epidemiology, social epidemiology is concerned with the way that social structures, institutions, and relationships influence health52. Social epidemiology is a method-based subject, which has sometimes adapted theories and analytical strategies from other fields of social science to cast light on population health-related questions50,53–59. One crucial goal of social epidemiology is to understand the biological mechanism of the interested social risk factors upon population health outcomes52.
Increasing evidence has indicated the pathological role of social-demographic factors, which have been linked to individuals’ genetic or epigenetic alterations60,61. Social-demographic factors can exert influence on human disease outcome through managing lifestyle and other risk factors of individuals62,63. Herein, to deepen our knowledge on how social-demographic factors can affect disease pathogenic procedure and to better address health disparities through regulating social-demographic factors, it is imperative to incorporate social epidemiology (or social science in a broad manner) into MPE paradigm (referred to as “Social MPE”49) (Figure 1). Social MPE aims to offer a unified framework not only to examine the interpersonal heterogeneity of the disease pathologies in regard to the social disparities but also to explore the underlying pathological mechanism linking the social-demographic factors to disease development, which would both fulfill the need of precision medicine and meet the promising path of social epidemiology. For instance, in order to estimate the effect magnitudes of social inequalities on human health outcomes, we can compare the associations between social determinates and various molecular subtypes of human diseases.
Figure 1.
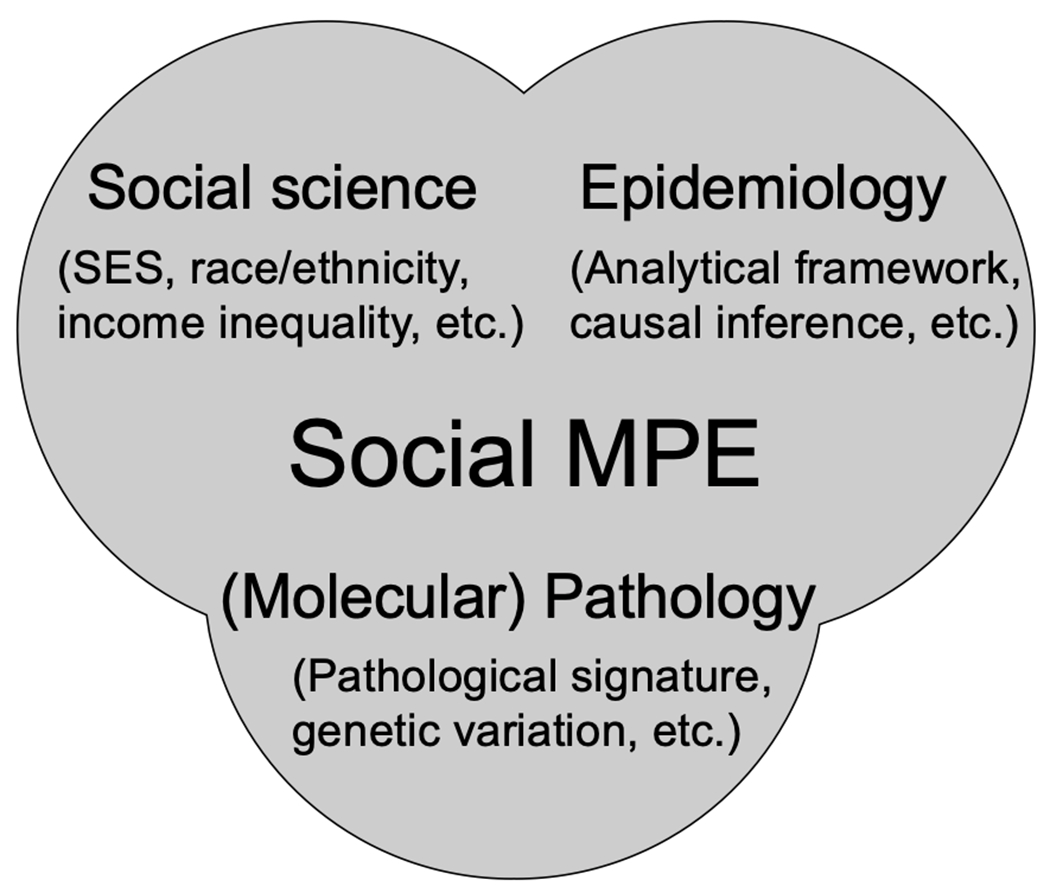
The framework of social molecular pathological epidemiology (MPE). Social MPE is the integration of (molecular) pathology, epidemiology, and social science. The social MPE framework enables us to detangle the underlying mechanisms of social-demographic factors on disease pathogenic progress.
By integrating social science and MPE studies, the social MPE framework enables us to detangle the underlying mechanisms of social-demographic factors on disease pathogenic progress, to better understand inter-personal disease heterogeneity, and to estimate the effect magnitude of social-demographic factors on certain disease molecular subtypes. Moreover, social MPE studies can expand our horizons from individual levels to aggregate levels and therefore address the social disparities in global contexts. Although the MPE framework has been commonly employed in the neoplastic diseases (e.g., as colorectal, lung, and prostate cancers) due to the widespread of the molecular diagnosis, it can be readily applied to both neoplastic and non-neoplastic diseases hosting substantial interpersonal heterogeneity.
Social MPE may help elucidate etiologies of recent trends and changes in incidence of certain diseases in the context of economical and lifestyle development of modern human societies. A notable example of such trends is a recent increase in (so-called “early-onset”) cancers that occur in many different organs of adults before age 50 years. The reasons of this trend remain uncertain. Because of increasing impacts of cancer burden in young individuals, the USA National Cancer Institute designated “What are the underlying causes of the unexplained rising incidence in certain early-onset cancers?” as the top 2020 “Provocative Question”. Certain malignancies such as colorectal, endometrial, esophageal, pancreatic, and thyroid cancers had shown increases in their incidence since the 1950s, when many countries started showing extensive industrialization and economic growth accompanied by modern diet and lifestyle changes. Notably, incidence of these cancers in adults before age 50 has shown a delayed rise since the 1980s and 1990s. This phenomenon has led to the hypothesis that early life exposures may play etiological roles in those early-onset cancers, as they may put individuals at higher cancer risk for decades before clinically detectable cancers develop64. It is considered that the rise of early-onset cancers is likely tied to modern diet and lifestyle factors (especially in early life), which are in turn tightly linked to social and economic factors. Hence, to elucidate the etiologies of early-onset cancers, the approaches of lifecourse MPE and social MPE need to be integrated49,51.
Hereafter, we illustrate the social MPE approach using the breast cancer as an example for neoplasms and the Alzheimer’s disease as an example for non-neoplastic diseases to elaborate the application of social MPE framework.
4. The roles of race/ethnicity and socioeconomic status in breast cancer heterogeneity
Breast cancer is the most common cancer and the leading cause of cancer death among females worldwide65. It is also the most common and the second leading cause of cancer death among US women66. Based on the combined status of ESR1 (estrogen receptor 1) overexpression, PGR (progesterone receptor) overexpression, and ERBB2 (HER-2) overexpression (or high-copy gain), breast cancer can be approximately divided into four major molecular subtypes, including luminal A (ERBB2−, either ESR1+ or PGR+), luminal B (ERBB2+, either ESR1+ or PGR+), ERBB2-enriched (ERBB2+, ESR1−, PGR−), and triple-negative (ERBB2−, ESR1−, PGR−)67–69. Due to its molecular heterogeneity, examination of ESR1 (ER), PGR (PR), and ERBB2 (HER-2) statuses has been incorporated into the routine clinical care of breast cancer in the US70,71 (note that we comply with standardized protein nomenclature recommended by an international panel of experts72).
In addition, US population-based cancer registries were required to report the expression status of ESR1, PGR, and ERBB2 since 201069. In 2019, a U.S. nationwide study utilized population-based incidence data of invasive breast cancers female aged ≥ 20 with diagnosis between 2012 to 2016, which were collaboratively collected by the SEER program and the Centers for Disease Control and Prevention’s National Program of Cancer Registries66. It reported that luminal A subtype, the least aggressive breast cancer subtype, was the most common subtypes (66%) in all racial/ethnic groups66. Moreover, treatment strategies for breast cancer varied across different molecular subtypes73. For instance, recommended systemic therapy for nonmetastatic breast cancer differs across molecular subtypes: patients with luminal A subtype receive endocrine therapy and sometimes chemotherapy, patients with ERBB2+ subtypes receive ERBB2-targeted therapy (ERBB2-enriched) and additional endocrine therapy (luminal B), and patients with triple-negative subtypes receive chemotherapy only73. As significant differences in demographic and clinical features across breast cancer subtypes have been reported74,75, breast cancer was exemplified here as a representative of neoplasm to demonstrate utility of the social MPE paradigm. To search for articles investigating the role of race/ethnicity in breast cancer heterogeneity, “race”, “ethnicity”, “subtype”, “estrogen receptor”, “progesterone receptor”, “human epidermal growth factor receptor 2”, and “breast cancer” were included as key words for the literature research. For socioeconomic status in breast cancer heterogeneity, “socioeconomic status”, “subtype”, “estrogen receptor”, “progesterone receptor”, “human epidermal growth factor receptor 2”, and “breast cancer” were included as key words for research. The literature searches were conducted through PubMed. We identified 668 articles and found 12 articles being most relevant to the aim of exploring the association between race/ethnicity and breast cancer molecular subtypes74,76–86. In relation to socioeconomic status (SES), a total of 81 articles were identified, with four of them being most relevant87–90.
One recent study published in 2020 comprehensively explored the heterogeneity of the association between racial/ethnic groups and incidence of breast cancer molecular subtypes83. They included women diagnosed with invasive breast cancer between 2010 and 2015 from the 18 SEER registries in the US83. Compared with non-Hispanic Whites, African Americans, Hispanics, and Asian/Pacific Islanders were associated with increased incidence of luminal B, ERBB2-enriched, and triple-negative subtypes of breast cancer but not the luminal A subtype83. Asian/Pacific Islanders were associated with increased incidence of luminal B and ERBB2-enriched subtypes and decreased incidence of the triple-negative subtype compared with the luminal A subtype83. Based on data from the National Cancer Data Base, which is a national hospital-based cancer registry capturing nearly 73% of newly diagnosed breast cancer patients in the US, a study included women diagnosed with invasive breast cancer between 2010 and 2011 in the US76. Compared with the non-Hispanic Whites, they consistently reported the stronger association for African Americans and Hispanics with the increased incidence of triple-negative subtypes but not the luminal A subtype76. In addition, they found that non-Hispanic Asian/Pacific Islanders were associated with increased incidence of the ERBB2-enriched subtype76. Based on data of women diagnosed with invasive breast cancer and hosted in The Cancer Genome Atlas (TCGA), researchers reported that African Americans were associated with increased incidence of triple-negative and ERBB2-enriched subtypes but not luminal A subtypes78. In summary, compared with non-Hispanic Whites, positive associations have been consistently reported by three or more previous studies between African Americans and the increased incidence of triple-negative subtypes74,76,78,81–83, Hispanics and the increased incidence of triple-native subtypes74,76,79,83, and Asian/Pacific Islanders and the increased incidence of luminal B subtypes74,77,83 (Table 1).
Table 1.
Studies evaluating the roles of race/ethnicity and socioeconomic status in breast cancer heterogeneity
| Author, journal, year | Study population | Study design, number of participants, and follow-up | Results |
|---|---|---|---|
| Auguste et al., PLoS One, 2017 | Women diagnosed with invasive breast cancer between 2003 and 2013 recorded by the Breast Cancer Registry in Côte-d’Or, France | Case-control study, n = 4,553 | No significant association was observed between SES and breast cancer molecular subtypes in French women. |
| Howlader et al., JNCI-J. Natl. Cancer Inst, 2014 | Women diagnosed with invasive breast cancer in 2010 from the 17 SEER registries in the US | Case-control study, n = 57,483 | Compared with non-Hispanic Whites, African Americans and Hispanics tended to develop triple-negative (African Americans: OR = 2.0, 95% CI = 1.8-2.2; Hispanics: OR = 1.3, 95% CI = 1.2-1.5) and ERBB2-enriched subtypes (African Americans: OR = 1.4, 95% CI = 1.2-1.6; Hispanics: OR = 1.4, 95% CI = 1.2-1.6) but not the luminal A subtype. Non-Hispanic Asian/Pacific Islanders tended to develop luminal B (OR = 1.2, 95% CI = 1.1 to 1.4) and ERBB2-enriched (OR = 1.8, 95% CI = 1.5-2.1) but less likely to develop triple-negative subtypes (OR = 0.8, 95% CI = 0.7-0.9) than the luminal A subtype. |
| Huo et al., JAMA Oncol, 2017 | Women diagnosed with invasive breast cancer and hosted in The Cancer Genome Atlas | Case-control study, n = 930 | Compared with non-Hispanic Whites, African Americans tended to develop triple-negative (OR = 3.80, 95% CI = 2.46-5.87) and ERBB2-enriched subtypes but not the luminal A subtype (OR = 2.22, 95% CI = 1.10-4.47). |
| Kong et al., JAMA Netw. Open, 2020 | Women diagnosed with invasive breast cancer between 2010 and 2015 from the 18 SEER registries in the US | Case-control study, n = 239,211 | Compared with non-Hispanic Whites, African Americans, Hispanics, and American Indian/Alaska Natives tended to develop luminal B (African Americans: OR = 1.28, 95% CI = 1.23-1.34; Hispanics: OR = 1.20, 95% CI = 1.15-1.25; American Indian/Alaska Natives: OR = 1.36, 95% CI = 1.16-1.59), ERBB2-enriched (African Americans: OR = 1.64, 95% CI = 1.55-1.74; Hispanics: OR = 1.41, 95% CI = 1.33-1.50; American Indian/Alaska Natives: OR = 1.47, 95% CI = 1.17-1.85), and triple-negative subtypes (African Americans: OR = 2.4, 95% CI = 2.31-2.48; Hispanics: OR = 1.28, 95% CI = 1.23-1.34; American Indian/Alaska Natives: OR = 1.26, 95% CI = 1.07-1.49) but not the luminal A subtype. Asian/Pacific Islander tended to develop luminal B (OR = 1.19, 95% CI = 1.14-1.25) and ERBB2-enriched (OR = 1.66, 1.56-1.76) but less likely to develop triple-negative subtypes (OR =0.91, 95% CI = 0.87-0.96) than the luminal A subtype. |
| Kulkarni et al., Cancer Health Disparities, 2019 | Women diagnosed with invasive breast cancer between 2008 and 2013 recorded by the New Jersey State Cancer Registry | Cohort study, n = 32,770 | Compared with non-Hispanic Whites, African Americans tended to have the worse overall survival with luminal A (HR = 1.64, 95% CI = 1.41-1.91), luminal B (HR = 1.54, 95% CI = 1.10-2.15), and triple-negative subtypes (HR = 1.28, 95% CI = 1.05-1.56). |
| Lawrenson et al., Cancer Causes Control, 2017 | Women diagnosed with stage I-III breast cancer between 2000 and 2013 recorded by the combined Waikato and Auckland Breast Cancer Registries in New Zealand | Cohort study, n = 9,015 | Compared with non-Māori/Pacific women, Māori and Pacific women with luminal A subtype tended to have the worse breast cancer-specific survival (HR = 1.52, 95% CI = 1.06-2.18; HR = 1.55, 95% CI = 1.04-2.31, respectively). |
| Linnenbringer et al., Breast Cancer Res Treat, 2020 | Non-Hispanic Whites and African Americans diagnosed with Triple-negative and luminal A subtypes between 2006 and 2014 recorded by the California Cancer Registry | Case-control study, n = 81,499 | Non-Hispanic Whites having higher neighborhood median household income were less likely to develop triple-negative subtypes than the luminal A subtype (OR = 0.99, 95% CI = 0.98-0.99). No significant association has been observed for African Americans. |
| Liu et al., Cancers, 2019 | Women aged ≥ 20 diagnosed with ductal carcinoma in situ from 1990 to 2015 with a median follow-up of 90 months from the 17 SEER registries in the US | Cohort study, n = 163,892, median follow-up = 90 months | Compared with non-Hispanic Whites, African Americans were more likely to develop triple-negative subtypes but not the luminal A subtype (HR = 1.99, 95% CI = 1.44-2.75). |
| Martínez et al., Breast Cancer Res Treat, 2017 | Non-Hispanic White and Hispanic female California residents aged ≥ 20 diagnosed with invasive breast cancer between 2004 and 2014 | Case-control study, n = 129,488 | Compared with non-Hispanic Whites, Hispanics tended to develop triple-negative (OR = 1.29, 95% CI = 1.23-1.35), ERBB2-enriched (OR = 1.19, 95% CI = 1.14-1.25), and luminal B subtypes (OR = 1.39, 95% CI = 1.31-1.48) but not the luminal A subtype. |
| Parise et al., Breast Cancer Res Treat, 2017 | Women diagnosed with Triple-negative and luminal A subtypes between 2000 and 2014 recorded by the California Cancer Registry | Case-control study, n = 108,372 | Non-Hispanic Whites and Hispanics having the lowest quintile of SES were more likely to develop triple-negative subtypes but not the luminal A subtype compared with those having the highest quintile of SES (OR = 1.15, 95% CI = 1.04-1.27; OR = 1.33, 95% CI = 1.05-1.68, respectively). No significant association has been observed for African Americans and Asian/Pacific Islander. |
| Parise et al., Cancer Epidemiol., 2014 | Women diagnosed with invasive breast cancer between 2000 and 2011 recorded by the California Cancer Registry | Case-control study, n = 225,441 | Compared with non-Hispanics Whites, Asian/Pacific Islanders tended to develop luminal B subtypes but not the luminal A subtype (OR = 1.17, 95% CI = 1.04-1.31). |
| Qin et al., Cancer Epidemiol. Biomarkers Prev, 2020 | African Americans diagnosed with invasive breast cancer between 2005 and 2017 in the Women’s Circle of Health and Women’s Circle of Health Follow-up Study | Case-control study, n = 1,220 | Compared with census tracts characterized by high-SES neighborhoods (T3), African Americans living in census tracts with intermediate- (T2) and low-SES neighborhoods (T1) tended to develop triple-negative subtypes but not the luminal A subtype (OR = 1.81, 95% CI = 1.20-2.71; OR = 1.95, 95% CI = 1.27-2.99, respectively; p-trend = 0.001). |
| Sineshaw et al., Breast Cancer Res Treat, 2014 | Women diagnosed with invasive breast cancer between 2010 and 2011 hosted in the National Cancer Data Base | Case-control study, n = 260,577 | Compared with non-Hispanics Whites, African Americans and Hispanics tended to develop triple-negative but not the luminal A subtype (African Americans: OR = 1.84, 95% CI = 1.77-1.92; Hispanics: OR = 1.17, 95% CI = 1.11-1.24). Non-Hispanic Asian/Pacific Islanders tended to develop ERBB2-enriched but not the luminal A subtype (OR = 1.45, 95% CI = 1.31-1.61). |
| Troester et al., JNCI-J. Natl. Cancer Inst, 2018 | African American and non-Hispanic White females diagnosed with invasive breast cancer from the Carolina Breast Cancer Study Phase 3 (2008-2013) | Case-control study, n = 980 | Compared with non-Hispanic Whites, African Americans tended to develop triple-negative but not the luminal A subtype (OR = 1.93, 95% CI = 1.27-2.93). |
| Zhao et al., Breast Cancer Res Treat, 2020 | African American and non-Hispanic White females diagnosed with invasive breast cancer between 1993 and 2019 at the University of Chicago Comprehensive Cancer Center | Cohort study, n = 2,795, median follow-up = 6.9 years | Compared with non-Hispanic Whites, African Americans with luminal A and ERBB2-enriched subtypes tended to have the worse overall survival (HR = 1.56, 95% CI = 1.22-2.00; HR = 1.26, 95% CI = 0.84-1.88, respectively), the worse recurrence-free survival (HR = 1.53, 95% CI = 1.22-1.91; HR = 3.00, 95% CI = 1.36-6.60, respectively), and the worse breast cancer-specific survival (HR = 2.37, 95% CI = 1.60-3.50; HR = 4.17, 95% CI = 1.35-12.88, respectively). African Americans with the luminal A subtype tended to have the worse time-to-recurrence survival (HR = 1.67, 95% CI = 1.20-2.34). |
Note: subtype definitions are as follows: luminal A = ERBB2 (HER2)-negative and [either ESR1 (ER)+ or PGR (PR)+]; luminal B = ERBB2+ and (either ESR1+ or PGR+); ERBB2-enriched = ERBB2+, ESR1-negative, PGR-negative; triple negative = ERBB2-negative, ESR1-negative, PGR-negative. We comply with standardized protein nomenclature recommended by an international expert panel40.
Abbreviation: CI, confidence interval; HR, hazard ratio; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic status.
By including African American and non-Hispanic White females diagnosed with invasive breast cancer between 1993 and 2019 at the University of Chicago Comprehensive Cancer Center, one cohort study found that African Americans with luminal A and ERBB2-enriched subtypes were associated with the worse overall survival, the worse recurrence-free survival, and the worse breast cancer-specific survival compared with the non-Hispanic Whites85. The positive association between African Americans and worse overall survival with luminal A has been consistently reported by another cohort study, which included women diagnosed with invasive breast cancer between 2008 and 2013 recorded by the New Jersey State Cancer Registry84. They further suggested that, compared with non-Hispanic Whites, African Americans tended to have the worse overall survival of luminal B and triple-negative subtypes. In addition to studies conducted in the US, researchers in New Zealand included women diagnosed with stage I-III breast cancer between 2000 and 2013 according to Waikato and Auckland Breast Cancer Registries80. They revealed that Māori and Pacific women with the luminal A subtype tended to have the worse breast cancer-specific survival compared with non-Māori/Pacific women80. Taken together, current evidence on differential associations of race/ethnicity with breast cancer prognosis according to molecular subtypes has been scarce. Given the significant subtype heterogeneity within the association between race/ethnicity and breast cancer risk, more studies are warranted to explore the heterogenous patterns for the prognosis of breast cancer in relation to different racial/ethnic groups (Table 1).
Racial/ethnic differences in breast cancer subtypes hold the promise of the incorporation of social-demographic factors into the MPE framework. However, to what extent of differences attributable to social race/ethnicity or biological race/ethnicity have not been thoroughly studied91. For example, BRCA1 is a tumor suppressor gene and its loss-of-function alterations account for nearly 70% of triple-negative subtypes92. Although strong evidence has revealed that African Americans have the substantially higher incidence of triple-negative subtypes compared with non-Hispanic Whites, several studies have continuously showed that the incidence of germline BRCA1 mutations is lower than that among women of European descent93,94. This indicated that additional genetic mechanisms apart from germline mutation of BRCA1 may promote the carcinogenesis of triple-negative subtypes among African Americans. By comparing the gene expression profile of triple-negative subtypes between African Americans and non-Hispanic Whites, various results have been generated. For example, one study reported the similar gene expression pattern between these two groups and further concluded that the triple-negative subtypes in African Americans were not a unique disease compared to those in non-Hispanic Whites95. Whereas another study demonstrated a panel of gene signature featured by increased loss of BRCA1 expression, increased activation of insulin-like growth factor 1 receptor and increased expression of vascular endothelial growth factor-activated genes in African Americans compared with non-Hispanic Whites96. In addition to the biological race, these differences may be attributable to the disparities in health and co-morbidity of diseases as well as the access to screening test91. Studies from Finland has indicated the difference of molecular subtype distribution between screening-detected and non-screening detected breast cancer, which partially accounted for the better outcome of screening-detected cancer97. In the US, African American women experience deficiency of breast cancer screening tests and longer period between screening mammograms and follow-up, which might affect the stage of presentation as well as the survival of African American women with triple-negative subtypes98–100. Moreover, emerging evidence also suggested that health disparities might drive aggressive biology in African Americans with triple-negative subtypes91. In light of the widespread of the molecular diagnosis of breast cancer, future studies are warranted to explore the underlying biological mechanisms of breast cancer molecular subtypes, which may help us differentiate the influence of disparity and biology.
Lower SES has been a risk factor for both the incidence and prognosis of breast cancer101,102. Neighborhood-level SES, which was defined by education, unemployment characteristics, median household income, proportion of the population living 200% below the Federal Poverty Level, median rent, and median housing value of census tract of residence, has been largely utilized for studies based on cancer registry data88,103. By including women diagnosed with triple-negative and luminal A subtypes between 2000 and 2014 recorded by the California Cancer Registry, researchers revealed that compared with non-Hispanic Whites and Hispanics having the highest quintile of SES, those having the lowest quintile of SES were associated with increased incidence of triple-negative subtypes but not luminal A subtypes88. Solely employing the neighborhood median household outcome as the representative of the neighborhood SES, another study replicated the inverse association between high-SES neighborhoods and the increased incidence of triple-negative subtypes in non-Hispanic White females89. By including African Americans diagnosed with invasive breast cancer between 2005 and 2017 in the Women’s Circle of Health and Women’s Circle of Health Follow-up Study, a recent study published in 2020 indicated that, compared with census tracts characterized by high-SES neighborhoods, African Americans living in census tracts with intermediate- and low-SES neighborhoods tended to develop triple-negative subtypes90. In addition to studies carried out in the US, researchers in France included women diagnosed with invasive breast cancer between 2003 and 2013 recorded by the Breast Cancer Registry in Côte-d’Or87. No significant association between SES and any molecular subtypes of breast cancer was observed in French women87 (Table 1).
In summary, studies aiming to interrogate the association between SES and molecular subtypes of breast cancer are still scarce. Conflicted association between SES and incidence rate of the triple-negative subtype among African Americans has also been observed88–90. Researchers have argued that the spurious null or even inverse association between SES and triple-negative subtype might be explained by obesity, which was not specific for any population group and would be induced by low consumption of healthy foods and sedentary behavior among low SES individuals104,105.
5. The roles of race/ethnicity and socioeconomic status in Alzheimer’s disease
Alzheimer’s disease, the most prevalent cause of dementia worldwide, is a neurodegenerative disease distinguished by two pathologies: β-amyloid plaque deposition and neurofibrillary tangles of hyperphosphorylated microtubule associated protein tau (MAPT), causing reduced memory, language, executive and visuospatial, personality, and behavior functions106. Currently, there are four verifiable subtypes in Alzheimer’s disease, characterized by neurofibrillary tangle spread, the distribution of accumulated MAPT pathology that impedes neural transport and communication systems within neurons107. These subtypes include typical Alzheimer’s disease, which has balanced neurofibrillary tangle counts in the hippocampus and association cortex; limbic-predominant Alzheimer’s disease, which mainly has neurofibrillary tangle counts in the hippocampus; hippocampal-sparing Alzheimer’s disease, which mainly has neurofibrillary tangle counts in the association cortex; and minimal atrophy, which has nominal counts of grey matter atrophy108. While relatively extensive bodies of research have corroborated the utility of applying social MPE in neoplastic diseases such as gallbladder, colorectal, and breast cancer in identifying social risk factors related to disease etiology109, there is a paucity in literature applying the paradigm to non-neoplastic diseases. Socially dependent heterogeneity in Alzheimer’s disease incidence, biomarker pathology, and intervention trajectory110,111 make Alzheimer’s disease useful in representing non-neoplastic diseases. Moreover, Alzheimer’s disease is an especially interesting non-neoplastic disease to evaluate through the social MPE lens because it is a subtype of dementia and itself has multiple subtypes108,112.
To retrieve articles investigating the role of race/ethnicity in Alzheimer’s disease heterogeneity, we included the following key words: “race”, “ethnicity,” “subtype,” “incidence”, “heterogeneity”, “Alzheimer’s disease,” “dementia,” “biomarkers,” “molecular pathology,” “medicine,” and “treatment”. For socioeconomic status in Alzheimer’s disease, we included the following key words: “socioeconomic status”, “education,” “subtype,” “heterogeneity”, “Alzheimer’s disease,” “dementia,” “biomarkers,” “molecular pathology,” “medicine,” “and “treatment.” The literature searches were conducted through PubMed. We identified 469 articles and found seven articles being most relevant to the aim of exploring the association between race/ethnicity and Alzheimer’s disease molecular subtypes113–119. In relation to SES, a total of 345 articles were identified, with six of them being most relevant120–125. As a result of the review, we showed that social-demographic factors such as race and SES were both highly implicated in differences in Alzheimer’s disease incidence, biomarker presentations, and neuropathology. We then suggested how race and SES might affect subtype incidence, warranting further study, and we maintained the need to employ the social MPE paradigm to advance precision medicine and equitable research practices.
There were several studies showing racially significant differences in dementia subtype incidence. In one longitudinal cohort study, Alzheimer’s disease and vascular dementia, a subtype of dementia typically caused by reduced blood flow to the brain and leading to memory impairment as well as loss of executive functioning, were both found to exist at higher rates among African American individuals as compared to their non-Hispanic White counterparts126. Within the Cardiovascular Health Study cohort, 34.7 per 1,000 African Americans were found to have Alzheimer’s disease as compared to 19.2 per 1,000 non-Hispanic Whites113. In addition, 27.2 per 1,000 African Americans were found to have vascular dementia as compared to 14.6 per 1,000 non-Hispanic Whites113. In another study, researchers from the Medical University of South Carolina evaluated the differential effect of stroke index on dementia subtypes between African Americans and non-Hispanic Whites114. The study concluded that African Americans were more likely to be diagnosed with dementia 5 years after an ischemic stroke regardless of the dementia subtype114. However, unlike other dementia subtypes evaluated, the risk for Alzheimer’s disease among African Americans with intervening strokes, defined as an additional stroke after the index stroke, was reduced when compared to that for non-Hispanic Whites114. This indicates that non-stroke cerebrovascular diseases, cardiovascular diseases, and metabolic diseases that lead to cognitive impairment may exist at a greater rate in African Americans before stroke than in non-Hispanic Whites before stroke114. In addition, intervening strokes may precipitate all dementia subtypes at a greater rate in African Americans than in non-Hispanic Whites, with the exception of Alzheimer’s disease subtype114.
A recent research study marks the first attempt at implicating race as a demographic factor integral in etiological heterogeneity, modifying cerebrospinal fluid Alzheimer’s disease biomarker levels and the relationship between white matter hyperintensity and cognition115. After discovering that African Americans had significantly lower levels of total MAPT (t-tau) and MAPT with phosphorylation at Threonine 181 (p-tau181), biomarkers for Alzheimer’s disease in cerebrospinal fluid, researchers concluded that diagnostic techniques relying on normative datasets of MAPT pathology could prelude underdiagnosis of Alzheimer’s disease in minority populations115,127. Another study also related the lower levels of MAPT (t-tau) and MAPT with phosphorylated Threonine at 181 (so-called p-tau181) in cerebrospinal fluid to the presence of the APOE ε4 allele, an allele defined by the rs7412 and rs429358 single nucleotide polymorphisms (SNPs) and highly implicated in Alzheimer’s disease incidence116. This study revealed a race by APOE ε4 relationship on MAPT (t-tau) and MAPT with phosphorylated Threonine at 181 (p-tau181), wherein African Americans who carried the APOE ε4 had significantly lower MAPT (t-tau) and MAPT with phosphorylated Threonine at 181 (p-tau181) concentrations compared to non-Hispanic White carriers116. Since African American non-carriers of APOE ε4 did not have significantly different MAPT (t-tau) and MAPT with phosphorylated Threonine at 181 (p-tau181) concentrations as non-Hispanic Whites non-carriers of APOE ε4, researchers concluded that racial differences in genetic components must be considered in the amyloid/tau/neurodegeneration model for diagnosing Alzheimer’s disease116,128. One review article on the various subtypes of Alzheimer’s disease noted that more MAPT-related pathology was associated with hippocampal-sparing Alzheimer’s disease, while less MAPT-related pathology was associated with limbic-predominant Alzheimer’s disease129. It also noted that APOE ε4 noncarriers were more likely to develop hippocampal-sparing Alzheimer’s disease, while APOE ε4 carriers were more likely to develop limbic-predominant and typical Alzheimer’s disease119. As such, the study published in 2019 accurately predicted the alignment of APOE ε4 allele presence with MAPT pathology concentration on the clinical subtype manifestation116,129.
These findings have complicated the recent trend towards MAPT-targeting Alzheimer’s disease treatments, many of which have reached clinical trial stages130. For example, lithium chloride, a drug that has been shown to diminish MAPT phosphorylation in clinical trials for individuals with Alzheimer’s disease, may be less effective for African American individuals as compared to non-Hispanic White individuals due to differential levels in MAPT with phosphorylated Threonine at 181 (p-tau181) biomarkers130. Other possible Alzheimer’s diseases treatments such as small interfering RNA and antisense oligonucleotides, which were suspected to diminish MAPT expression, might also be less effective for African American individuals as compared to non-Hispanic Whites, due to differential levels in MAPT (t-tau) biomarkers131. More research is needed to evaluate racial heterogeneity in the associated biological mechanisms underlying these drugs’ efficacies.
In addition to heterogeneous cerebrospinal fluid biomarker concentrations, researchers identified heterogeneous functional connectivity within the default mode network, an imaging biomarker far more available than other diagnostic biomarkers of interest117,132. In non-Hispanic White populations, decreased default mode network connectivity between the precuneus and lateral temporal cortex and between the precuneus and the temporal pole was associated with greater cognitive impairment117. However, in African American populations, increased default mode network connectivity between both of these respective regions was associated with greater cognitive impairment117. These findings elucidated the advantage of using racially cognizant biomarkers in complement with traditional biomarker diagnostic tools such as positron emission tomography, cerebrospinal fluid, and resting state functional magnetic resonance imaging 115,117,132,133. Doing so will mitigate under-diagnosis of Alzheimer’s disease and refine clinical trial interpretations, thus advancing racialized precision medicine115. Monolithic clinical diagnostic consequences can also be diminished by increasing representation of racial minorities in diagnostic data, acknowledging racial biomarking impacts in diagnostic practices, and investigating the efficacy of novel race conscious diagnostic methods such as plasma MAPT with phosphorylated Threonine at 181 (pTau181) to APP (amyloid beta precursor protein; so-called Aβ1-42) ratio127.
In addition to differences in pathological mechanisms between African American and non-Hispanic White individuals, post-mortem autopsies comparing neuropathologies of African American and non-Hispanic White individuals showed that African American individuals were significantly more likely to have mixed Alzheimer’s disease pathology. In one study, 71% of African American individuals with Alzheimer’s disease dementia compared to 51% of non-Hispanic White individuals with Alzheimer’s disease dementia showed Alzheimer’s disease pathology in addition to at least one other Alzheimer’s disease related pathology: Lewy bodies and/or macroscopic and microinfarcts118. Compounding risk in developing multiple Alzheimer’s disease-related pathologies, African American individuals were also more likely to have comorbid cardiovascular disease than their non-Hispanic White counterparts119. In the same study, researchers seeking to ameliorate these comorbidities found African American subjects were 60% more likely to drop out of Alzheimer’s disease clinical trials119. These points informed several insights regarding Alzheimer’s disease precision research and Alzheimer’s disease precision treatment. Firstly, any pharmacological treatment that exclusively treats Alzheimer’s disease might be less effective for African American individuals—considering both multiple Alzheimer’s disease related pathologies and comorbidities. Secondly, there is an urgent need to advance retention-strategies, prioritize trusting relationships between physician-scientists and racial minorities, and work to bolster race MPE research by dismantling systems of racial harm resembling the infamous Tuskegee Study in Alzheimer’s disease clinical trials119,131,134. Finally, there is a continuing need to study the relationship between race and Alzheimer’s disease subtype incidence. This can eventually evolve into identifying most practical yet effective racial biomarker correlates, exploring how biological race or social-demographic factors associated with race influence Alzheimer’s disease pathology, and advancing precision medicine from race MPE frameworks.
Social MPE also has considerable utility in relating SES to heterogeneous Alzheimer’s disease outcome and molecular pathology. In the analysis of the viability of SES MPE (MPE research related to socioeconomic status) in non-neoplastic Alzheimer’s disease, we operationalized SES as materials goods, occupation, and educational opportunity and attainment135. Verified through Mendelian randomization analyses, educational attainment and completing university have been shown to modify pathways in Alzheimer’s disease, reduce genetic variants, and diminish the likelihood of developing Alzheimer’s disease136. Specifically, less educated individuals, defined in one study as having attended primary school only, were over 3 times more likely to have developed Alzheimer’s disease as compared to more educated individuals, defined as having attended intermediate or university level education120. In the same study, individuals with low occupational-based SES, operationalized through a socioeconomic occupation classification system involving education requirements, blue-collar versus white-collar categorization, and skills involved for the occupation, were 1.6 times more likely to develop Alzheimer’s disease120. The protective effect of education attainment and occupation has been seen across multiple dementia subtypes including vascular dementia as well as aforementioned Alzheimer’s disease120. In an evaluation of dementia subtype prevalence in a cross-sectional study in China, researchers found an increasing protective effect against Alzheimer’s disease and vascular dementia with increased years of education, noting a slightly more potent protective effect of education against Alzheimer’s disease as compared to vascular dementia 121. Moreover, with respect to the farm laborer occupation, occupations such as non-farm laborer, official, and professional protected against both Alzheimer’s disease and vascular dementia121. Complementing the results of this study, another research study following Roman Catholic nuns revealed that uneducated nuns were nearly twice as likely to lose cognitive function as compared to educated nuns122. This research study is particularly useful because it explicates how individuals living in similar adult conditions can be significantly more predisposed to Alzheimer’s disease based on SES related factors before joining the congregation122.
What is critical to SES MPE in Alzheimer’s disease is the special consideration of life course epidemiology, which posits that early harmful exposures contribute to downstream disease onset well into late adulthood (especially in the case of Alzheimer’s disease)110,136. This paradigm has mostly been applied to critical theory models, the prototypical example being nutrition perturbations or other biosocial-demographic factors disrupting cell division and leading to greater coronary disease incidence later in life137. In the case of Alzheimer’s disease, early life SES, as defined in one study as parental education, parental occupation, family size, country literacy rate, and country education rate, was associated with baseline cognition level during adulthood, but unrelated to cognitive decline and Alzheimer’s disease incidence123. These findings suggested that metrics of SES unrelated to individual educational attainment, may not significantly impact Alzheimer’s disease incidence. However, early lifetime SES’ interaction with baseline cognition during adulthood as well as individual education attainment’s interaction with Alzheimer’s disease incidence maintained critical theory models.
An individual’s education attainment was also highly implicated in the molecular pathway leading to Alzheimer’s disease onset. According to a recent study that analyzed data from the Alzheimer’s Disease Neuroimaging Initiative, the extent of education was correlated with total brain volume in individuals with mild cognitive impairment diagnoses124. Seeing as mild cognitive impairment is the symptomatic stage that precedes Alzheimer’s disease diagnosis, education reduces the likelihood of Alzheimer’s disease onset through brain reserve mechanisms124,125. Interestingly, paraffin-embedded brain samples coupled with clinical data showed that education was not associated with neuropathologies such as cortical atrophy, hippocampal neurotic plaques, atherosclerosis, and Barak stages125,138. This conferred the cognitive reserve theory, which posited that education had no statistically significant effect on the aggregation of neuropathological biomarkers, but rather diminished the effect of these neuropathological biomarkers, possibly linked to the increased brain weight124,125. The diminishing effect of neuropathological biomarkers was most evident in the increased pathology required to cause cognitive impairment in more educated individuals as compared to less educated individuals139. Further, more educated individuals were more likely to develop hippocampal-sparing Alzheimer’s disease, and less educated individuals were more likely to develop minimal-atrophy Alzheimer’s disease129. This could be attributed to education protecting the hippocampus, which increased clinical burden of pathologies affecting the posterior cortex instead of the hippocampus119. Treatment based on the cognitive reserve theory currently include non-pharmaceutical interventions such as aerobic exercise, cognitive stimulation, and social stimulation, which increase brain-derived neurotrophic factor (BDNF)140. In addition to ascertaining heterogeneous pharmacological treatments by education differences, SES MPE in Alzheimer’s disease should work to study and address social, political, and economic institutions that maintain educational disparity.
Admittedly, the explanation underlying how the cognitive reserve theory disrupts brain degradation was murky. Even more, the connection between SES and the biological mechanisms responsible for increased Alzheimer’s disease incidences were even less clear112. However, current research, in addition to linking SES to Alzheimer’s disease incidence and cognitive reserve theories, linked SES to heterogeneous comorbidities associated with Alzheimer’s disease. For example, low SES was associated with depression comorbidity, while high SES is associated with hypertension comorbidity141. As such, physicians must consider how adverse effects of pharmacological treatment interact with SES-associated comorbidities. Cholinesterase inhibitors, one of the most common Alzheimer’s disease treatments, have been cited to cause hypertension, which might adversely interact with high SES individuals who are at greater risk to hypertension comorbidity142. On the other hand, treating Alzheimer’s disease comorbidities such as depression through pharmacological options such as tricyclic antidepressants might be less effective in individuals of low SES due to interaction with Alzheimer’s disease143. Thus, SES-related precision medicine requires robust communication pipelines between various specialist health practitioners and general health practitioners. Further, more research is required to ascertain the biological mechanisms underlying SES-related incidence, the cognitive reserve theory, and associated comorbidities144.
6. Challenges in social MPE research
Since the novel framework of social MPE was introduced in 2015, an increasing but still limited number of studies have explored molecular heterogeneity within diseases in relation to diverse social-demographic factors, with only part of them having primary goals of applying the social MPE paradigm90,145. There exist several challenges in this inter-disciplinary field. First of all, the number of specimens available for molecular pathological tests is still limited, which prevents us from answering research questions of interest due to the deficient sample size. However, thanks to the research initiative on precision medicine, continuing efforts have been invested to examine molecular heterogeneity of multiple diseases, especially of neoplasms, which met the short-term goal of precision medicine8,25. Moreover, increasingly technological advances facilitate the analyses of various omics, including genomics, epigenomics, transcriptomics, proteomics, metabolomics, metagenomics, microbiome, immunomics, interactomics, etc23. In the past decade, there has been rapid and thorough development in high-throughput methods, ranging from traditional real-time polymerase chain reaction to more composite systems (e.g., next-generation sequencing) in the field of genomics57. Similarly, due to the advancement of next-generation sequencing method which can explore multiple facets of chromatin biology (e.g., DNA methylation, histone modification), the field of epigenomics evolved rapidly146. Likewise, the rapid development of high-throughput DNA sequencing technologies has given rise to the establishment of RNA-Seq in the field of transcriptomics147. In addition, the growth of next-generation sequencing endows increasing power of integrating genomics-transcriptomics and epigenomics-transcriptomics148. In the field of proteomics, to assess the host-pathogen interactions by quantifying protein expression, modification, and secretion, mass-spectrometry have been developed with higher sensitivity compared to protein microarrays149. The protein-protein interactions, as an example of interactome, are beneficiary of the advancement in proteomics150. Additionally, technical advancement in the fields of genomics, transcriptomics, and proteomics have been integrated with the discipline of immunology to gain a deep understanding of the mechanism of immune system functions151. Metabolomics, thought to be the closest representative of phenotype, provides a good understanding of the state of cellular and biological processes at different stages of growth or under disease conditions. To overcome the limitation of a large amount of uncharacterized metabolites and imprecise statistical validation for large-scale spectral assignments, novel approaches have been developed (e.g., integration of CSI:FingerID in SIRIUS4152, novel integrative metabolomics platforms153, and metabolic reaction network-based annotation154). Metagenomics is the study of a collection of genetic material (genomes) from a mixed community of organisms (usually refers to microbial communities). Due to the advancement of high-throughput approach, metagenomics sequencing has been employed to identify uncultured bacteria as well as novel viruses, which cannot be done by shotgun sequencing155. Likewise, the next-generation sequencing approach offers a more effective way to study complex microbial systems than ever before156. In parallel with the aforementioned increasing trend in molecular pathological tests and technical advancement, a growing body of publicly available datasets containing large-scale -omics profiles have been emerging, representing invaluable sources for the research community of the social MPE discipline157,158. For instance, TCGA hosted enormous genomics/epigenetics profile (i.e., DNA sequence alterations, mRNA expression, DNA methylation, copy number variation, and so on) and the clinicopathological annotations of the cancer patients. By using TCGA, researchers could explore the association between different racial groups and the onset or multiple prognostic outcomes of molecular subtypes of breast cancer or any other cancers hosting considerable interpersonal heterogeneity, which should favor the management of patients with different races. Second, as molecular pathological tests are normally expensive, the prevalence of those tests might be considerably different between developed and developing countries. Therefore, social MPE studies might enlarge health disparities in the global context159,160. Also, the generalizability of the conclusions drawn from the resource-rich populations might be questionable due to the potential effect measure modifiers such as SES and health disparity status. Third, there are few experts with interdisciplinary knowledge of both social science and MPE. The training programs which could provide adequate professional level training in pathology, statistics, sociology, social science, and bioinformatics are also rare161. Moreover, in conformity with the limited number of experts and training programs, the current conceptual framework of MPE have not adequately integrated the social-demographic factors. Therefore, MPE studies might not be easily and smoothly conducted as should be expected. Collaboration between experts from different disciplines could be one solution to overcome this barrier. Fourth, current support for interdisciplinary science is scarce, which might be attributed to the less experts with transdisciplinary training, who could fairly evaluate the true value of interdisciplinary research162. Challenges in general MPE studies have been discussed detailly elsewhere3,18.
7. Conclusions
The integrative approach of social MPE, which has been originally proposed by our previous study and updated in the current study, enables us to bridge the knowledge gap of the underlying biological mechanisms of how social-demographic factors shape population health outcomes49. After publishing our initial paper on social MPE in 2015, we have seen a growing body of research applying the social MPE frameworks in non-neoplastic diseases as we mentioned above, which strengthens our former statement that social MPE could be applied to any disease49. With the advancement and ubiquity of high-throughput sequencing and microarray technologies, further social MPE research should and will be conducted, thereby fulfilling our ultimate goal of promoting population health in the global context through implementation of health policies based on evidence from social MPE studies. We also call for appropriate training programs in the integrative field of social epidemiology and molecular pathology. It is ideal to conduct social MPE research by one expert with transdisciplinary knowledge, which is beneficial for developing novel concepts and frameworks5.
Table 2.
Studies evaluating the roles of race/ethnicity and socioeconomic status in Alzheimer’s disease heterogeneity
| Author, journal, year | Study population | Study design, number of participants, and follow-up | Results |
|---|---|---|---|
| Barnes et al., Neurology, 2015 | Deceased African American and non-Hispanic White individuals with Alzheimer’s disease dementia from the Rush Alzheimer’s Disease Clinical Core | Case-control study, n = 122 | Compared with non-Hispanic Whites with Alzheimer’s disease, African Americans with Alzheimer’s disease tended to develop mixed pathology which could include Alzheimer’s disease + lewy bodies, Alzheimer’s disease + infarct, or Alzheimer’s disease + lewy bodies + infarct (OR = 2.4, 95% CI = 1.10-5.20). |
| Brayne et al., Brain, 2010 | Participants interviewed to establish dementia diagnoses and their brain donations from Medical Research Council Cognitive Function and Ageing Study, Cambridge City Over-75s Cohort study (CC75C; Vantaa 85+ | Case-control study, n = 872 | Compared with individuals with less formal education, individuals with more formal education per year tended to not have dementia diagnoses (OR = 0.89; 95% CI = 0.83-0.94). No significant association was observed between education and neurodegenerative or neurovascular pathology. Compared with individuals with less formal education, individuals with more formal education tended to have greater brain weight (OR = 1.14, 95% CI =1.06-1.24). |
| Clark et al., J Stroke Cerebrovasc Dis, 2018 | Non-Hispanic Whites and African Americans with a diagnosis of ischemic stroke prior to 2010 | Cohort study, n = 68,758, follow-up = 5 years | Compared with non-Hispanic Whites with intervening stroke, African Americans with intervening stroke were less likely to receive Alzheimer’s disease diagnosis and more likely to receive vascular dementia diagnosis (HR = .84, 95% CI = 0.71-0.98; HR = 1.67, 95% CI = 1.46-1.90, respectively). Compared with non-Hispanic Whites without intervening stroke, African Americans without intervening stroke were more likely to receive Alzheimer’s disease diagnosis and vascular dementia diagnosis (HR = 1.38, 95% CI = 1.29-1.48; HR = 2.02, 95% CI = 1.86-1.20, respectively). |
| Fitzpatrick et al., J Am Geriatr Soc, 2004 | Non-Hispanic Whites and African Americans dementia free Cardiovascular Health Studies participants between 1992 and 1994 | Cohort study, n = 3,602, mean follow-up = 5.4 years | Compared with non-Hispanic Whites, African Americans tended to develop Alzheimer’s disease subtype (African Americans: age-adjusted IR per 1,000 = 34.7; non-Hispanic Whites: age-adjudged IR per 1,000 = 19.2). Compared with non-Hispanic Whites, African Americans tended to develop vascular dementia subtype (African Americans: age-adjusted IR per 1,000 = 27.2; non-Hispanic Whites: age-adjusted IR per 1,000 = 14.6). |
| Howell et al., Alzheimers Res Ther, 2017 | Older Americans to undergo clinical, neuropsychological, genetic, mild cognitive impairment, and cerebrospinal fluid analysis from 2013 to 2015 at Emory University | Case-control study, n = 1,355 | Compared with non-Hispanic Whites with Alzheimer’s disease, African Americans with Alzheimer’s disease tended to have lower levels of MAPT (t-tau) and p-tau181 biomarkers in cerebrospinal fluid, which is related to increased limbic-predominant Alzheimer’s disease subtype (African Americans: mean MAPT (t-tau), pg/mL = 72.8; non-Hispanic Whites: mean MAPT (t-tau), pg/mL = 103.8; African Americans: mean p-tau181, pg/mL = 25.3; non-Hispanic Whites: mean p-tau181, pg/mL = 34.2). |
| Karp et al., Am J Epidemiol, 2004 | Initially nondemented subjects aged ≥ 75 years from the Kungsholmen Project, Stockholm, Sweden followed-up between 1987 and 1993 | Cohort study, n = 931, follow-up = 3 years | Compared with individuals of high education attainment (>7 years), individuals of low education attainment (2-7 years) tended to have clinically diagnosed Alzheimer’s disease (RR = 3.4, 95% CI = 2.0-6.0) Compared with individuals of high occupation SES, individuals of low occupation SES tended to have clinically diagnosed Alzheimer’s disease (1.6, 95% CI = 1.0-2.5). |
| Kennedy et al., Am J Geriatr Psychiatry, 2017 | Subjects with baseline data for comorbid disorders taken from a meta-database of 18 studies from the Alzheimer’s Disease Cooperative Study and the Alzheimer’s Disease Neuroimaging Initiative | Case-control study, n = 5,164 | Compared with non-Hispanic Whites with Alzheimer’s disease, mild cognitive impairment, or normal cognition, African Americans with Alzheimer’s disease, mild cognitive impairment, or normal cognition tended to have cardiovascular comorbidities (OR = 2.10, 95% CI = 1.71-2.57). Compared with non-Hispanic Whites with Alzheimer’s disease, mild cognitive impairment, or normal cognition, African Americans with Alzheimer’s disease, mild cognitive impairment, or normal cognition tended to drop out of clinical trials (OR = 1.60, 95% CI = 1.15-2.21). |
| Misiura et al., Transl Neurodegener, 2020 | African American and Non-Hispanic White individuals over the age of 64 with varying diagnoses on the spectrum of Alzheimer’s disease according to consensus criteria | Case-control study, n = 137 | Decrease in cognitive performance is observed with decreased default mode network connectivity between midline core subsystem and dorsomedial subsystem for African Americans, while decrease in cognitive performance is observed with increased default mode network connectivity between midline core subsystem and dorsomedial subsystem for non-Hispanic Whites. |
| Morris et al., JAMA Neurol, 2019 | African American and Non-Hispanic White individuals enrolled from January 1, 2004, to December 31, 2015 at the Knight Alzheimer Disease Research Center at Washington University | Case-control study, n = 1,255 | Compared with non-Hispanic White carriers of the aAPOE ε4 allele, African American carriers of the APOE ε4 allele had lower mean (SE) concentrations of MAPT (t-tau) biomarkers in cerebrospinal fluid (African Americans: mean MAPT (t-tau) (SE), pg/mL = 269.67 (43.73); non-Hispanic Whites: mean MAPT (t-tau) (SE) = 463.54 (20.32); P < 0.001) Compared with non-Hispanic White carriers of the APOE ε4 allele, African American carriers of the APOE ε4 allele had lower mean (SE) concentrations of p-tau181 biomarkers in cerebrospinal fluid (African Americans: mean p-tau181 (SE), pg/mL = 48.77 (6.23) Non-Hispanic Whites: mean p-tau181 (SE), pg/mL = 74.98 (2.78); P < 0.001. There were no significant racial differences in MAPT (t-tau) and p-tau181 concentration for individuals without an APOE ε4 allele. |
| Snowdon et al., Journal of Clinical Epidemiology, 1989 | Roman Catholic nuns sharing similar lifestyle conditions but different educational attainment from the Mankato, Minnesota Province | Case-control study, n = 247 | Compared with high-educated individuals (individuals with at least a bachelor’s degree), low-educated individuals (individuals with less than a bachelor’s degree tended to be more cognitively impaired (OR = 2.11, 95% CI = 0.88-5.03). |
| Wada et al., J Alzheimers Dis, 2018 | Participants aged between 55 and 90 years and diagnosed as healthy controls, having mild cognitive impairment, or having Alzheimer’s disease. | Case-control study, n = 825 for education and amyloid-β deposition analysis, n = 1,304 for education and brain metabolism analysis | No significant association between education and both amyloid-β deposition (n = 825) and brain metabolism (n = 1,304) for individuals with Alzheimer’s disease and mild cognitive impairment. |
| Wilson et al., NED, 2005 | Catholic nuns, priests and brothers from the Religious Orders Study followed-up periodically from 1994 with ongoing follow-ups since date of study | Cohort study, n = 999, mean follow-up number = 6.6 | No significant association between early life SES to cognitive decline and Alzheimer’s disease incidence. |
| Zhang et al., Neuroepidemiology, 2006 | Community residents ≥ 55 years in Beijing, Shanghai, Chengdu and Xian with diagnoses of Alzheimer’s disease or vascular dementia collected from 1997-1998 | Cohort study, n = 34,807, follow-up = 6 months | Compared with individuals with <1 year of education, individuals with 1-6 years of education, individuals with 7-12, and individuals with 12+ years of education had increasing protection to Alzheimer’s disease (OR = 0.3, 95% CI = 0.2-0.4; OR = 0.2, 95% CI = 0.2-0.4; OR = 0.2 95% CI = 0.1-0.3 to Alzheimer’s disease respectively) and increasing protection to vascular dementia (OR = 0.7, 95% CI = 0.5-0.9; OR = 0.6, 95% CI = 0.3-0.9; OR = 0.3, 95% CI = 0.1-0.8 respectively). Compared with farm laborers, non-farmer laborers, officials, and professionals had greater protection to Alzheimer’s disease (OR = 0.6, 95%CI = 0.4-0.8, OR = 0.3, 95% CI = 0.2-0.5, and OR = 0.2, 95% CI = 0.1-0.3 respectively) and greater protection to vascular dementia (OR = 0.8, CI = 0.5-1.2, OR = 0.7, 95% CI = 0.4-1.4, and OR = 0.1 95% CI = 0.1-0.4 respectively). |
Abbreviation: CI, confidence interval; IR, incidence rate; OR, odds ratio; SE, standard error; SES, socioeconomic status.
Article Highlights.
Social MPE is an integrative research discipline that relates social-demographic factors to heterogeneities in molecular pathological biomarkers and associated disease subtypes.
Parallel advances in social sciences and MPE generate increasing opportunities to center social MPE frameworks in research and practice, which will deepen precision medicine initiatives.
By evaluating breast cancer through the social MPE framework, the current study adds to evidence suggesting heterogeneous associations between race/ethnicity and socioeconomic status on neoplastic disease subtypes.
By evaluating the role or race/ethnicity and socioeconomic status on Alzheimer’s Disease etiology, this is the first study summarizing the application of social MPE framework on a non-neoplastic disease.
We recommend transdisciplinary pathology training programs and increasing representation of minority populations in research studies to diminish health disparities and ensure the benefits of social MPE are equitably distributed.
Expert opinion.
Integration of molecular pathological epidemiology and social science can be a robust and promising approach to shed light on the etiology and pathology of diseases. Since we initially proposed the social MPE framework in 2015, we have witnessed a considerable proportion of studies explicitly or implicitly utilizing the social MPE paradigm to deal with the inter-person variation in neoplastic diseases with regard to the social factors (e.g., race/ethnicity and SES). Although most of the relevant publications have been targeting the neoplasms, a growing body of evidence has indicated the contribution of social MPE paradigm in non-neoplastic diseases that host considerable interindividual heterogeneity in the disease phenotype. This extension of research focus from neoplasms to both neoplastic and non-neoplastic disease is existing and may hopefully drive the precision medicine. In the next several years, more and more molecular pathological data of disease are expected to be collected not only from cancer registries, but also from hospitals. In company with the explosive expansion of digitized pathological and other clinical data, more pathologists with computation expertise will be in great demand. Herein, pathology training programs that provide pathologists the opportunity to learning principle of epidemiology and data science may be helpful for bridging the gap between pathology and data science as well as solving the increasing issue of non-reproducibility in research. As artificial intelligence has been studied to support decision-making in clinical medicine, hopefully, it may help reduce the measurement errors and therefore enhance the precision of disease outcomes as well as advance the utilization of social MPE framework in population health science. In addition to the outcome measurement, we were also concerned with the inconsistent measurements of the social factors, for example, various measurements exist for SES, which we believe is part of the reason leading to the diverse associations between social factors and molecular subtypes of diseases across studies. Therefore, a uniform workflow to measure social factors is needed for ensuring the comparability across studies. What is more, given that MPE is not a disease-based but method-based discipline, which can give rise to a number of frontiers like social-MPE, such as pharmacology-MPE, nutritional-MPE, and microbiology-MPE. As social factors, for example, the SES, could affect the intake of medicine and nutrition that perform a key role in the etiologies of most diseases, increasing effort, for example, developing novel paradigms, frameworks, and methodologies in these aforementioned and related disciplines, can be useful for explaining the identified associations and therefore advancing our understanding of the findings from the social-MPE. We foresee an increasing trend of social MPE studies which aim to present the differences of social-demographic factors across various molecular subtypes. Disease entities originally defined by organ site hosting substantial interpersonal heterogeneity may be accordingly divided into molecular subtypes, which will broaden our understanding of the disease etiology and pathology. Consequently, disease control, prevention, and treatment strategy will be tailored and advanced for certain sub-population (e.g., minorities, population with lower SES). Along with the emerging of social MPE studies, health disparities should be alleviated in the global context.
Funding
This work was supported in part by a USA National Institutes of Health grant (R35 CA197735 to S.O.; R01 CA248857 to S.O.).
Abbreviations:
- MPE
molecular pathological epidemiology
- SEER
Surveillance, Epidemiology, and End Results
- SES
socioeconomic status
- SNP
single nucleotide polymorphism
- TCGA
the Cancer Genome Atlas
Footnotes
Use of standardized official symbols: Authors use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products, including APOE, APP, BDNF, BRCA1, ERBB2, ESR1, MAPT, and PGR; all of which are described at www.genenames.org. The gene symbols are italicized while protein symbols and non-official colloquial protein names are non-italicized.
Declaration of Interest
A Nishi is a consultant to Urbanic & Associates. Y Yamamoto is the Founder and CEO, Kyoto Angel Fund, Inc. in Japan. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Rothstein WG Pathology: The Evolution of a Specialty in American Medicine. Medical Care 17, 975–988 (1979). [PubMed] [Google Scholar]
- 2.Rothman KJ, Greenland S & Lash TL Modern epidemiology. (Lippincott Williams & Wilkins, 2008). [Google Scholar]
- 3.Ogino S et al. Interdisciplinary education to integrate pathology and epidemiology: towards molecular and population-level health science. Am J Epidemiol 176, 659–667, doi: 10.1093/aje/kws226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurnit KC et al. Precision Oncology Decision Support: Current Approaches and Strategies for the Future. Clin Cancer Res 24, 2719–2731, doi: 10.1158/1078-0432.Ccr-17-2494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogino S, Nowak JA, Hamada T, Milner DA Jr. & Nishihara R Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol 14, 83–103, doi: 10.1146/annurev-pathmechdis-012418-012818 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Provides the up-to-date information of the MPE field and discusses current knowledge gaps and need for transdisciplinary education and training.
- 6.Deng N et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61, 673–684, doi: 10.1136/gutjnl-2011-301839 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209, doi: 10.1038/nature13480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai J et al. Whole Genome Messenger RNA Profiling Identifies a Novel Signature to Predict Gastric Cancer Survival. Clin Transl Gastroenterol 10, e00004, doi: 10.14309/ctg.0000000000000004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seaman SR & White IR Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 22, 278–295, doi: 10.1177/0962280210395740 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Lukowsky LR et al. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol 8, 619–628, doi: 10.2215/cjn.04810512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawlor DA, Harbord RM, Sterne JA, Timpson N & Davey Smith G Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27, 1133–1163, doi: 10.1002/sim.3034 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Kuller LH Epidemiologists of the Future: Data Collectors or Scientists? Am J Epidemiol 188, 890–895, doi: 10.1093/aje/kwy221 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Kuller LH Epidemiology: Then and Now. American Journal of Epidemiology 183, 372–380, doi: 10.1093/aje/kwv158 (2015). [DOI] [PubMed] [Google Scholar]
- 14.O’Malley KJ et al. Measuring diagnoses: ICD code accuracy. Health Serv Res 40, 1620–1639, doi: 10.1111/j.1475-6773.2005.00444.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishihara R et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol 178, 84–100, doi: 10.1093/aje/kws431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyskens FL Jr. et al. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J Natl Cancer Inst 108, doi: 10.1093/jnci/djv309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogino S & Stampfer M Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst 102, 365–367, doi: 10.1093/jnci/djq031 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogino S, Chan AT, Fuchs CS & Giovannucci E Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 60, 397–411, doi: 10.1136/gut.2010.217182 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Explains MPE as a single unified field that provides the solid rational and enormous opportunities for the integration with other disciplines
- 19.Ogino S et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut 67, 1168–1180, doi: 10.1136/gutjnl-2017-315537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Introduces inmmunology-MPE model, one of the many transdisplines integrating MPE and other disciplines, as an exempliery model to study environment-tumour-immune interactions, and effective immunoprevention and immunotherapy strategies for precision medicine.
- 20.Hamada T, Nowak JA, Milner DA Jr., Song M & Ogino S Integration of microbiology, molecular pathology, and epidemiology: a new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J Pathol 247, 615–628, doi: 10.1002/path.5236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mima K et al. The microbiome, genetics, and gastrointestinal neoplasms: the evolving field of molecular pathological epidemiology to analyze the tumor-immune-microbiome interaction. Hum Genet 140, 725–746, doi: 10.1007/s00439-020-02235-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol 26, 465–484, doi: 10.1038/modpathol.2012.214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Provides the conceptual foundation of MPE, that is, the unique disease principle.
- 23.Ogino S et al. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology 27, 602–611, doi: 10.1097/ede.0000000000000471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Explains that MPE can be readily applied to non-neoplastic diseases, which leads the innovation part of the current paper, that is to summarize the application of social MPE framework in Alzheimer’s disease.
- 24.Martinez-Cabriales SA, Walsh S, Sade S & Shear NH Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol 34, 59–73, doi: 10.1111/jdv.15931 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Collins FS & Varmus H A new initiative on precision medicine. N Engl J Med 372, 793–795, doi: 10.1056/NEJMp1500523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Field AE, Camargo CA Jr. & Ogino S The merits of subtyping obesity: one size does not fit all. Jama 310, 2147–2148, doi: 10.1001/jama.2013.281501 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Kuller LH, Bracken MB, Ogino S, Prentice RL & Tracy RP The role of epidemiology in the era of molecular epidemiology and genomics: Summary of the 2013 AJE-sponsored Society of Epidemiologic Research Symposium. Am J Epidemiol 178, 1350–1354, doi: 10.1093/aje/kwt239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epplein M et al. Challenges and opportunities in international molecular cancer prevention research: An ASPO Molecular Epidemiology and the Environment and International Cancer Prevention Interest Groups Report. Cancer Epidemiol Biomarkers Prev 23, 2613–2617, doi: 10.1158/1055-9965.Epi-14-0848 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell PT et al. Proceedings of the third international molecular pathological epidemiology (MPE) meeting. Cancer Causes Control 28, 167–176, doi: 10.1007/s10552-016-0845-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell PT et al. Proceedings of the fourth international molecular pathological epidemiology (MPE) meeting. Cancer Causes Control 30, 799–811, doi: 10.1007/s10552-019-01177-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes LAE, Simons C, van den Brandt PA, van Engeland M & Weijenberg MP Lifestyle, Diet, and Colorectal Cancer Risk According to (Epi)genetic Instability: Current Evidence and Future Directions of Molecular Pathological Epidemiology. Curr Colorectal Cancer Rep 13, 455–469, doi: 10.1007/s11888-017-0395-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rescigno T, Micolucci L, Tecce MF & Capasso A Bioactive Nutrients and Nutrigenomics in Age-Related Diseases. Molecules 22, doi: 10.3390/molecules22010105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waluga M, Zorniak M, Fichna J, Kukla M & Hartleb M Pharmacological and dietary factors in prevention of colorectal cancer. J Physiol Pharmacol 69, doi: 10.26402/jpp.2018.3.02 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Carr PR et al. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analyses. Ann Oncol 29, 825–834, doi: 10.1093/annonc/mdy059 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Benelli R, Venè R & Ferrari N Prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2), a complex target for colorectal cancer prevention and therapy. Transl Res 196, 42–61, doi: 10.1016/j.trsl.2018.01.003 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Luo K et al. Fusobacterium nucleatum, the communication with colorectal cancer. Biomed Pharmacother 116, 108988, doi: 10.1016/j.biopha.2019.108988 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Gunter MJ et al. Meeting report from the joint IARC-NCI international cancer seminar series: a focus on colorectal cancer. Ann Oncol 30, 510–519, doi: 10.1093/annonc/mdz044 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang ST et al. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J Gastroenterol 26, 562–597, doi: 10.3748/wjg.v26.i6.562 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jatho A, Tran BT, Cambia JM, Nanyingi M & Mugisha NM Cancer Risk Studies and Priority Areas for Cancer Risk Appraisal in Uganda. Ann Glob Health 86, 78, doi: 10.5334/aogh.2873 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Chen M, Ye X & Poon K Role and potential clinical utility of ARID1A in gastrointestinal malignancy. Mutation Research/Reviews in Mutation Research 787, 108360, doi: 10.1016/j.mrrev.2020.108360 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Advani SM et al. Epidemiology and Molecular-Pathologic Characteristics of CpG Island Methylator Phenotype (CIMP) in Colorectal Cancer. Clin Colorectal Cancer, doi: 10.1016/j.clcc.2020.09.007 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Bai J, Chen H & Bai X Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J Clin Lab Anal 35, e23810, doi: 10.1002/jcla.23810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Cai S & Ma Y Association between Fusobacterium nucleatum and colorectal cancer: Progress and future directions. J Cancer 9, 1652–1659, doi: 10.7150/jca.24048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan L et al. Advances in prognostic markers for colorectal cancer(). Expert Rev Mol Diagn 19, 313–324, doi: 10.1080/14737159.2019.1592679 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Song C, Kong Y, Huang L, Luo H & Zhu X Big data-driven precision medicine: Starting the custom-made era of iatrology. Biomed Pharmacother 129, 110445, doi: 10.1016/j.biopha.2020.110445 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Amitay EL et al. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer 122, 1604–1610, doi: 10.1038/s41416-020-0803-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamal Y, Schmit SL, Frost HR & Amos CI The tumor microenvironment of colorectal cancer metastases: opportunities in cancer immunotherapy. Immunotherapy 12, 1083–1100, doi: 10.2217/imt-2020-0026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon K & Blackman D A guide to understanding social science research for natural scientists. Conserv Biol 28, 1167–1177, doi: 10.1111/cobi.12326 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Nishi A et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn 16, 11–23, doi: 10.1586/14737159.2016.1115346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Initially proposes the definition and framework of social MPE and serves as the intellectual basis for the current study.
- 50.Nishi A Evolution and social epidemiology. Soc Sci Med 145, 132–137, doi: 10.1016/j.socscimed.2015.08.015 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Nishi A et al. Lifecourse epidemiology and molecular pathological epidemiology. Am J Prev Med 48, 116–119, doi: 10.1016/j.amepre.2014.09.031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawachi I & Subramanian SV Social epidemiology for the 21st century. Soc Sci Med 196, 240–245, doi: 10.1016/j.socscimed.2017.10.034 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Diez-Roux AV Multilevel analysis in public health research. Annu Rev Public Health 21, 171–192, doi: 10.1146/annurev.publhealth.21.1.171 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Ogden J Psychosocial theory and the creation of the risky self. Soc Sci Med 40, 409–415, doi: 10.1016/0277-9536(94)00150-r (1995). [DOI] [PubMed] [Google Scholar]
- 55.McKinlay JB & Marceau LD To boldly go. Am J Public Health 90, 25–33, doi: 10.2105/ajph.90.1.25 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Susser M Should the epidemiologist be a social scientist or a molecular biologist? Int J Epidemiol 28, S1019–1022, doi: 10.1093/oxfordjournals.ije.a019905 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Gasperskaja E & Kučinskas V The most common technologies and tools for functional genome analysis. Acta Med Litu 24, 1–11, doi: 10.6001/actamedica.v24i1.3457 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krieger N Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med 39, 887–903, doi: 10.1016/0277-9536(94)90202-x (1994). [DOI] [PubMed] [Google Scholar]
- 59.The triple helix: gene, organism and environment. Nat Med 6, 1206, doi: 10.1038/81288 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Stringhini S et al. Life-course socioeconomic status and DNA methylation of genes regulating inflammation. Int J Epidemiol 44, 1320–1330, doi: 10.1093/ije/dyv060 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Knight JM et al. Low Socioeconomic Status, Adverse Gene Expression Profiles, and Clinical Outcomes in Hematopoietic Stem Cell Transplant Recipients. Clin Cancer Res 22, 69–78, doi: 10.1158/1078-0432.Ccr-15-1344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansson L, Thelle DS, Solvoll K, Bjørneboe GE & Drevon CA Healthy dietary habits in relation to social determinants and lifestyle factors. Br J Nutr 81, 211–220, doi: 10.1017/s0007114599000409 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Northstone K, Emmett P & Rogers I Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. Eur J Clin Nutr 62, 471–479, doi: 10.1038/sj.ejcn.1602741 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akimoto N et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol, doi: 10.1038/s41571-020-00445-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424, doi: 10.3322/caac.21492 (2018). [DOI] [PubMed] [Google Scholar]
- 66.DeSantis CE et al. Breast cancer statistics, 2019. CA Cancer J Clin 69, 438–451, doi: 10.3322/caac.21583 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Anderson WF, Rosenberg PS, Prat A, Perou CM & Sherman ME How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst 106, doi: 10.1093/jnci/dju165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perou CM et al. Molecular portraits of human breast tumours. Nature 406, 747–752, doi: 10.1038/35021093 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Kohler BA et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 107, djv048, doi: 10.1093/jnci/djv048 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson WF, Rosenberg PS & Katki HA Tracking and evaluating molecular tumor markers with cancer registry data: HER2 and breast cancer. J Natl Cancer Inst 106, doi: 10.1093/jnci/dju093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discuss needs to treat breast cancer as a molecularly pathological heterogeneous disease.
- 71.Clarke CA et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst 104, 1094–1101, doi: 10.1093/jnci/djs264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujiyoshi K et al. Opinion: Standardizing gene product nomenclature-a call to action. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2025207118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waks AG & Winer EP Breast Cancer Treatment: A Review. Jama 321, 288–300, doi: 10.1001/jama.2018.19323 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Howlader N et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106, doi: 10.1093/jnci/dju055 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardner K The Science of Cancer Health Disparities: A Young Discipline with an Old Heritage. Am J Pathol 188, 268–270, doi: 10.1016/j.ajpath.2017.08.037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sineshaw HM et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010–2011). Breast Cancer Res Treat 145, 753–763, doi: 10.1007/s10549-014-2976-9 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Parise C & Caggiano V Disparities in the risk of the ER/PR/HER2 breast cancer subtypes among Asian Americans in California. Cancer Epidemiol 38, 556–562, doi: 10.1016/j.canep.2014.08.001 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Huo D et al. Comparison of Breast Cancer Molecular Features and Survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol 3, 1654–1662, doi: 10.1001/jamaoncol.2017.0595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martínez ME et al. Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non-Hispanic white women. Breast Cancer Res Treat 166, 185–193, doi: 10.1007/s10549-017-4389-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lawrenson R et al. Treatment and survival disparities by ethnicity in New Zealand women with stage I-III breast cancer tumour subtypes. Cancer Causes Control 28, 1417–1427, doi: 10.1007/s10552-017-0969-9 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Troester MA et al. Racial Differences in PAM50 Subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst 110, 176–182, doi: 10.1093/jnci/djx135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y, West R, Weber JD & Colditz GA Race and risk of subsequent aggressive breast cancer following ductal carcinoma in situ. Cancer 125, 3225–3233, doi: 10.1002/cncr.32200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kong X et al. Variation in Breast Cancer Subtype Incidence and Distribution by Race/Ethnicity in the United States From 2010 to 2015. JAMA Netw Open 3, e2020303, doi: 10.1001/jamanetworkopen.2020.20303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kulkarni A et al. Breast Cancer Incidence and Mortality by Molecular Subtype: Statewide Age and Racial/Ethnic Disparities in New Jersey. Cancer Health Disparities 3, e1–e17, doi: 10.9777/chd.2019.1012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao F et al. Racial disparities in survival outcomes among breast cancer patients by molecular subtypes. Breast Cancer Res Treat, doi: 10.1007/s10549-020-05984-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Telli ML et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat 127, 471–478, doi: 10.1007/s10549-010-1173-8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Auguste A et al. Breast cancer subtype of French women is not influenced by socioeconomic status: A population-based-study. PLoS One 12, e0170069, doi: 10.1371/journal.pone.0170069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parise CA & Caggiano V Risk factors associated with the triple-negative breast cancer subtype within four race/ethnicities. Breast Cancer Res Treat 163, 151–158, doi: 10.1007/s10549-017-4159-y (2017). [DOI] [PubMed] [Google Scholar]
- 89.Linnenbringer E et al. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among Black and White women. Breast Cancer Res Treat 180, 437–447, doi: 10.1007/s10549-020-05545-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin B et al. Neighborhood social environmental factors and breast cancer subtypes among Black women. Cancer Epidemiol Biomarkers Prev, doi: 10.1158/1055-9965.Epi-20-1055 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R & Seewaldt VL Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 15, 248–254, doi: 10.1038/nrc3896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cleator S, Heller W & Coombes RC Triple-negative breast cancer: therapeutic options. Lancet Oncol 8, 235–244, doi: 10.1016/s1470-2045(07)70074-8 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Nanda R et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. Jama 294, 1925–1933, doi: 10.1001/jama.294.15.1925 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Olopade OI et al. Breast cancer genetics in African Americans. Cancer 97, 236–245, doi: 10.1002/cncr.11019 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Sturtz LA, Melley J, Mamula K, Shriver CD & Ellsworth RE Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer 14, 62, doi: 10.1186/1471-2407-14-62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindner R et al. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One 8, e71915, doi: 10.1371/journal.pone.0071915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sihto H et al. Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clin Cancer Res 14, 4103–4110, doi: 10.1158/1078-0432.Ccr-07-5003 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Warnecke RB, Campbell RT, Vijayasiri G, Barrett RE & Rauscher GH Multilevel Examination of Health Disparity: The Role of Policy Implementation in Neighborhood Context, in Patient Resources, and in Healthcare Facilities on Later Stage of Breast Cancer Diagnosis. Cancer Epidemiol Biomarkers Prev 28, 59–66, doi: 10.1158/1055-9965.Epi-17-0945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molina Y, Silva A & Rauscher GH Racial/Ethnic Disparities in Time to a Breast Cancer Diagnosis: The Mediating Effects of Health Care Facility Factors. Med Care 53, 872–878, doi: 10.1097/mlr.0000000000000417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vona-Davis L & Rose DP The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 18, 883–893, doi: 10.1089/jwh.2008.1127 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Bradley CJ, Given CW & Roberts C Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst 94, 490–496, doi: 10.1093/jnci/94.7.490 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Keegan TH et al. Racial/ethnic and socioeconomic differences in short-term breast cancer survival among women in an integrated health system. Am J Public Health 105, 938–946, doi: 10.2105/ajph.2014.302406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yost K, Perkins C, Cohen R, Morris C & Wright W Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12, 703–711, doi: 10.1023/a:1011240019516 (2001). [DOI] [PubMed] [Google Scholar]
- 104.Banegas MP et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat 144, 625–634, doi: 10.1007/s10549-014-2882-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pavela G, Lewis DW, Locher J & Allison DB Socioeconomic Status, Risk of Obesity, and the Importance of Albert J. Stunkard. Curr Obes Rep 5, 132–139, doi: 10.1007/s13679-015-0185-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weller J & Budson A Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res 7, doi: 10.12688/f1000research.14506.1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Metaxas A & Kempf SJ Neurofibrillary tangles in Alzheimer’s disease: elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen Res 11, 1579–1581, doi: 10.4103/1673-5374.193234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferreira D, Pereira JB, Volpe G & Westman E Subtypes of Alzheimer’s Disease Display Distinct Network Abnormalities Extending Beyond Their Pattern of Brain Atrophy. Front Neurol 10, 524, doi: 10.3389/fneur.2019.00524 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discuss needs to treat Alzheimer’s disease as a molecularly pathological heterogeneous disease.
- 109.Sharma A, Sharma KL, Gupta A, Yadav A & Kumar A Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 23, 3978–3998, doi: 10.3748/wjg.v23.i22.3978 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Logue MW et al. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol 68, 1569–1579, doi: 10.1001/archneurol.2011.646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garrett SL et al. Racial Disparity in Cerebrospinal Fluid Amyloid and Tau Biomarkers and Associated Cutoffs for Mild Cognitive Impairment. JAMA Netw Open 2, e1917363, doi: 10.1001/jamanetworkopen.2019.17363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stevens T et al. Islington study of dementia subtypes in the community. Br J Psychiatry 180, 270–276, doi: 10.1192/bjp.180.3.270 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Fitzpatrick AL et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 52, 195–204, doi: 10.1111/j.1532-5415.2004.52058.x (2004). [DOI] [PubMed] [Google Scholar]
- 114.Clark DG et al. Differential Impact of Index Stroke on Dementia Risk in African-Americans Compared to Whites. J Stroke Cerebrovasc Dis 27, 2725–2730, doi: 10.1016/j.jstrokecerebrovasdis.2018.05.048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Howell JC et al. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther 9, 88, doi: 10.1186/s13195-017-0315-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morris JC et al. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA Neurol 76, 264–273, doi: 10.1001/jamaneurol.2018.4249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Misiura MB et al. Race modifies default mode connectivity in Alzheimer’s disease. Transl Neurodegener 9, 8, doi: 10.1186/s40035-020-0186-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnes LL et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 85, 528–534, doi: 10.1212/wnl.0000000000001834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kennedy RE, Cutter GR, Wang G & Schneider LS Challenging Assumptions About African American Participation in Alzheimer Disease Trials. Am J Geriatr Psychiatry 25, 1150–1159, doi: 10.1016/j.jagp.2017.04.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karp A et al. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol 159, 175–183, doi: 10.1093/aje/kwh018 (2004). [DOI] [PubMed] [Google Scholar]
- 121.Zhang ZX et al. Socio-demographic variation of dementia subtypes in china: Methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology 27, 177–187, doi: 10.1159/000096131 (2006). [DOI] [PubMed] [Google Scholar]
- 122.Snowdon DA, Ostwald SK, Kane RL & Keenan NL Years of life with good and poor mental and physical function in the elderly. J Clin Epidemiol 42, 1055–1066, doi: 10.1016/0895-4356(89)90047-4 (1989). [DOI] [PubMed] [Google Scholar]
- 123.Wilson RS et al. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology 25, 8–14, doi: 10.1159/000085307 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Wada M et al. Effect of Education on Alzheimer’s Disease-Related Neuroimaging Biomarkers in Healthy Controls, and Participants with Mild Cognitive Impairment and Alzheimer’s Disease: A Cross-Sectional Study. J Alzheimers Dis 63, 861–869, doi: 10.3233/jad-171168 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Brayne C et al. Education, the brain and dementia: neuroprotection or compensation? Brain 133, 2210–2216, doi: 10.1093/brain/awq185 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Venkat P, Chopp M & Chen J Models and mechanisms of vascular dementia. Exp Neurol 272, 97–108, doi: 10.1016/j.expneurol.2015.05.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hampel H et al. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol 45, 30–40, doi: 10.1016/j.exger.2009.10.010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Riedel BC, Thompson PM & Brinton RD Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol 160, 134–147, doi: 10.1016/j.jsbmb.2016.03.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferreira D, Nordberg A & Westman E Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology 94, 436–448, doi: 10.1212/wnl.0000000000009058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee HE, Lim D, Lee JY, Lim SM & Pae AN Recent tau-targeted clinical strategies for the treatment of Alzheimer’s disease. Future Med Chem 11, 1845–1848, doi: 10.4155/fmc-2019-0151 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Babulal GM et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement 15, 292–312, doi: 10.1016/j.jalz.2018.09.009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hafkemeijer A, van der Grond J & Rombouts SA Imaging the default mode network in aging and dementia. Biochim Biophys Acta 1822, 431–441, doi: 10.1016/j.bbadis.2011.07.008 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Lee MH, Smyser CD & Shimony JS Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol 34, 1866–1872, doi: 10.3174/ajnr.A3263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shavers VL, Lynch CF & Burmeister LF Factors that influence African-Americans’ willingness to participate in medical research studies. Cancer 91, 233–236, doi: (2001). [DOI] [PubMed] [Google Scholar]
- 135.Winkleby MA, Jatulis DE, Frank E & Fortmann SP Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 82, 816–820, doi: 10.2105/ajph.82.6.816 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larsson SC et al. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. Bmj 359, j5375, doi: 10.1136/bmj.j5375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barker DJP The fetal origins of adult disease. Fetal and Maternal Medicine Review 6, 71–80, doi: 10.1017/S0965539500001005 (1994). [DOI] [Google Scholar]
- 138.Katzman R Education and the prevalence of dementia and Alzheimer’s disease. Neurology 43, 13–20, doi: 10.1212/wnl.43.1_part_1.13 (1993). [DOI] [PubMed] [Google Scholar]
- 139.Roe CM, Xiong C, Miller JP, Cairns NJ & Morris JC Interaction of neuritic plaques and education predicts dementia. Alzheimer Dis Assoc Disord 22, 188–193, doi: 10.1097/WAD.0b013e3181610fff (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012, doi: 10.1016/s1474-4422(12)70191-6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Camacho-Mercado CL, Figueroa R, Acosta H, Arnold SE & Vega IE Profiling of Alzheimer’s disease patients in Puerto Rico: A comparison of two distinct socioeconomic areas. SAGE Open Med 4, 2050312115627826, doi: 10.1177/2050312115627826 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clodomiro A et al. Somatic comorbidities and Alzheimer’s disease treatment. Neurol Sci 34, 1581–1589, doi: 10.1007/s10072-013-1290-3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cassano T et al. Pharmacological Treatment of Depression in Alzheimer’s Disease: A Challenging Task. Front Pharmacol 10, 1067, doi: 10.3389/fphar.2019.01067 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duthie A, Chew D & Soiza RL Non-psychiatric comorbidity associated with Alzheimer’s disease. Qjm 104, 913–920, doi: 10.1093/qjmed/hcr118 (2011). [DOI] [PubMed] [Google Scholar]
- 145.Scott LC, Mobley LR, Kuo TM & Il’yasova D Update on triple-negative breast cancer disparities for the United States: A population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer 125, 3412–3417, doi: 10.1002/cncr.32207 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carlberg C & Molnár F in Human Epigenomics 19–38 (Springer Singapore, 2018). [Google Scholar]
- 147.Shendure J & Ji H Next-generation DNA sequencing. Nature Biotechnology 26, 1135–1145, doi: 10.1038/nbt1486 (2008). [DOI] [PubMed] [Google Scholar]
- 148.Hawkins RD, Hon GC & Ren B Next-generation genomics: an integrative approach. Nat Rev Genet 11, 476–486, doi: 10.1038/nrg2795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aslam B, Basit M, Nisar MA, Khurshid M & Rasool MH Proteomics: Technologies and Their Applications. Journal of Chromatographic Science 55, 182–196, doi: 10.1093/chromsci/bmw167 (2017). [DOI] [PubMed] [Google Scholar]
- 150.Rain J-C et al. The protein–protein interaction map of Helicobacter pylori. Nature 409, 211–215, doi: 10.1038/35051615 (2001). [DOI] [PubMed] [Google Scholar]
- 151.De Sousa KP & Doolan DL Immunomics: a 21st century approach to vaccine development for complex pathogens. Parasitology 143, 236–244, doi: 10.1017/s0031182015001079 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Dührkop K et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods 16, 299–302, doi: 10.1038/s41592-019-0344-8 (2019). [DOI] [PubMed] [Google Scholar]
- 153.Chong J et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research 46, W486–W494, doi: 10.1093/nar/gky310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shen X et al. Metabolic reaction network-based recursive metabolite annotation for untargeted metabolomics. Nature Communications 10, 1516, doi: 10.1038/s41467-019-09550-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yadav BS, Yadav AK, Singh S, Singh NK & Mani A in Nanoscience and Biotechnology for Environmental Applications (eds Gothandam KM, Ranjan Shivendu, Dasgupta Nandita, & Lichtfouse Eric) 85–113 (Springer International Publishing, 2019). [Google Scholar]
- 156.Arnold JW, Roach J & Azcarate-Peril MA Emerging Technologies for Gut Microbiome Research. Trends in Microbiology 24, 887–901, doi: 10.1016/j.tim.2016.06.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu J et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 173, 400–416.e411, doi: 10.1016/j.cell.2018.02.052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barrett T et al. NCBI GEO: mining tens of millions of expression profiles--database and tools update. Nucleic Acids Res 35, D760–765, doi: 10.1093/nar/gkl887 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Quality pathology and laboratory diagnostic services are key to improving global health outcomes: improving global health outcomes is not possible without accurate disease diagnosis. Am J Clin Pathol 143, 325–328, doi: 10.1309/ajcp6k0dzcnvcsci (2015). [DOI] [PubMed] [Google Scholar]
- 160.Ayanian JZ & Carethers JM Bridging behavior and biology to reduce socioeconomic disparities in colorectal cancer risk. J Natl Cancer Inst 104, 1343–1344, doi: 10.1093/jnci/djs356 (2012). [DOI] [PubMed] [Google Scholar]
- 161.Ogino S & Nishihara R All Biomedical and Health Science Researchers, Including Laboratory Physicians and Scientists, Need Adequate Education and Training in Study Design and Statistics. Clin Chem 62, 1039–1040, doi: 10.1373/clinchem.2016.257873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bromham L, Dinnage R & Hua X Interdisciplinary research has consistently lower funding success. Nature 534, 684–687, doi: 10.1038/nature18315 (2016). [DOI] [PubMed] [Google Scholar]


