Abstract
Alcohol is the most commonly used psychoactive drug, often taken in conjunction with opioid drugs. Since both alcohol and opioids can induce CNS depression, it is often assumed that alcohol potentiates the known hypoxic effects of opioid drugs, thus contributing to coma and death during opioid overdose. To address this supposition, we used oxygen sensors to examine the effects of alcohol on brain oxygenation and hypoxic responses induced by intravenous heroin in awake, freely moving rats. To eliminate robust sensory effects of alcohol following its oral or intraperitoneal delivery, alcohol was administered directly into the stomach via chronically implanted intragastric catheters at human relevant doses. Alcohol delivered at a 0.5 g/kg dose did not affect brain oxygen levels, except for a weak transient increase during drug delivery. This phasic oxygen increase was stronger at a 2.0 g/kg alcohol dose and followed by a weaker tonic increase. Since alcohol absorption from intragastric delivery is much slower and more prolonged than with intraperitoneal or intravenous injections, the rapid rise of brain oxygen levels suggests direct action of alcohol on sensory afferents in the stomach well before the drug physically reaches brain tissue via circulation. Despite slow tonic increases in brain oxygen, alcohol at the 2.0 g/kg dose strongly potentiates heroin-induced oxygen responses, increasing both the magnitude and duration of oxygen decrease. Therefore, under the influence of alcohol, the use of opioid drugs becomes much more dangerous, increasing brain hypoxia and enhancing the probability of serious health complications, including coma and death.
Keywords: Brain oxygen, Brain temperature, Opioid drugs, Neural activation, Peripheral vasoconstriction, Central vasodilation, Rats
1. Introduction
Alcohol is the most commonly used psychoactive drug, which is often co-administered with opioid drugs (Witkiewitz and Vowles, 2019). While alcohol at low doses can act as a CNS stimulant, at higher doses it can induce CNS depression, accompanied by respiratory depression that could result in systemic hypoxia (McIntosh and Chick, 2004; Imam 2010). Respiratory depression is also the most dangerous effect of opioid drugs at large doses (Baud, 2009; Pattinson, 2008); it is responsible for robust hypoxia that could result in coma and death (Jaffe et al., 1997). Considering the commonality of respiratory depressive effects in both alcohol and opioids, it is reasonable to assume that alcohol will potentiate the hypoxic effects of opioid drugs. This study was designed to address this issue by directly monitoring brain oxygen levels using oxygen sensors coupled with high-speed amperometry in freely moving rats.
While pulse oximetry is routinely used for monitoring changes in blood oxygen levels in human patients, hypoxic effects of drugs in small animals are usually assessed by whole-body plethysmography, which provides a measure of respiratory activity (rate and tidal volume). Although respiratory activity is the primary means for oxygen delivery into brain tissue, oxygen dissolved in the brain’s extracellular space is a more clinically relevant parameter and its decrease below physiological range is a direct measure of brain hypoxia during opioid overdose. The use of oxygen sensors coupled with fixed-potential amperometry makes it possible to monitor changes in brain oxygen levels in awake, freely moving rats (Kiyatkin, 2018). Another critical advantage of this technology is its second-scale temporal resolution that allows for the observation of rapid drug-induced oxygen changes under physiologically relevant conditions. These technical features are especially important for opioid drugs, which have distinct effects on brain oxygen levels depending on drug dose. While intravenous (iv) heroin at low reinforcing doses moderately increases brain oxygen levels due to cerebral vasodilation, at larger doses it induces rapid, dose-dependent oxygen decreases due to respiratory depression and subsequent drop in blood oxygen levels (Solis et al., 2017; Thomas et al., 2021).
While electrochemical assessments of brain oxygen responses induced by heroin following exposure to alcohol was the final goal of this study, we also used multi-site temperature recordings to assess the basic effects of alcohol on brain metabolic activity and the tone of skin vessels. As shown previously, temperature differences between a brain site and temporal muscle provide a measure of intra-brain heat production, a reliable index of metabolic activity (Kiyatkin, 2018). Temperature differences between skin and muscle temperature serves as a measure of peripheral vessel tone, indicating its constriction or dilation.
In contrast to other drugs, which can be delivered via subcutaneous, intraperitoneal or intravenous (iv) administration, alcohol is exclusively consumed by drinking and its physiological effects result from rapid diffusion into the blood stream from the stomach, which is densely vascularized. To mimic human conditions, in this study we employed a chronically implanted intra-gastric (ig) catheter for alcohol delivery. This type of drug delivery is free of administration-related stress and avoids local sensory excitatory effects that may be induced by other routes of drug administration.
This study consisted of two experiments conducted in freely moving rats. In the first thermorecording experiment, we examined the effects of ig alcohol in two doses on temperatures in the brain, temporal muscle, and subcutaneous space (skin). For comparison with previous studies, the nucleus accumbens (NAc), a deep, ventrally located structure that is critically involved in sensorimotor integration and drug reinforcement (Mogenson et al., 1980; Wise and Bozarth, 1987), was chosen as a representative brain site. Based on previous rat studies and the range of possible human consumption, we tested the effects of alcohol at two doses: low (0.5 g/kg) and high (2.0 g/kg). Temperature recordings in this experiment were coupled with monitoring locomotor activity. As a control stimulus, we tested the effects of tail-pinch—an arousing stimulus that induces locomotor activation and consistent changes in temperatures. The results of this experiment were essential in planning the second experiment and deciding which dose of alcohol was optimal to examine its effects on heroin responses.
In the second electrochemical experiment, we examined how alcohol affects NAc oxygen levels and NAc oxygen responses induced by iv heroin (0.2 mg/kg). Heroin at this dose maintains drug self-administration (Gerber and Wise, 1988) and induces well-defined oxygen responses, with the initial rapid decrease followed by a weaker and more prolonged increase (Solis et al., 2017). To test for sensor performance, rats were also exposed to tail-pinch, an arousing procedure which results in rapid and strong increases of NAc oxygen levels.
2. Materials and Methods
2.1. Subjects
11 adult male Long-Evans rats (Charles River Laboratories) weighing 440±40 g at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12–12 light-dark cycle with food and water available ad libitum. All procedures were approved by the NIDA-IRP Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23). Maximal care was taken to minimize the number of experimental animals and any possible discomfort or suffering at all stages of the study.
2.2. Overview of the study
In this study, we describe the results of two experiments. Rats for the first thermorecording experiment (n=4 rats) were chronically implanted with three thermocouple sensors and an ig catheter. In this experiment, we examined how alcohol at two doses affects locomotor activity and temperatures recorded from the NAc, temporal muscle, and skin in awake, freely moving rats. Rats for the second electrochemical experiment (n=7 rats) were chronically implanted with an oxygen sensor, ig catheter and iv jugular catheter. In this experiment, we examined how alcohol at one effective dose (determined by the first experiment) affects NAc oxygen responses induced by heroin (0.2 mg/kg). As a control stimulus with known patterns of temperature and oxygen responses, in both experiments we examined the effects of tail-pinch.
2.3. Surgical preparations for temperature and electrochemical experiments
A total of four surgical procedures (implantation of thermocouple sensors, implantation of electrochemical sensor, implantation of iv catheter; implantation of ig sensor) were conducted in rats described in this study. For the first experiment, rats underwent two surgical procedures during the same session, while three procedures were conducted during the same session for the second experiment.
Surgical procedures for the thermorecording experiment have been described in detail elsewhere (Brown and Kiyatkin, 2005; Bola and Kiyatkin, 2017). Briefly put, under general anesthesia (Equithesin, a mixture of sodium pentobarbital and chloral hydrate), rats were chronically implanted with three copper-constantan thermocouple electrodes in the NAc shell, temporal muscle, and subcutaneously (Skin) along the nasal ridge with the tip ~15 mm anterior to bregma. Target coordinates for the right NAc were: AP +1.2 mm, ML ±0.8 mm, and DV −7.2–7.6 mm from the skull surface, according to coordinates from the rat brain atlas (Paxinos and Watson, 1998). The probes were secured with dental acrylic to three stainless steel screws threaded into the skull. Next, rats were the implanted with an ig catheter. The night prior to the surgery, rats were deprived of food to ensure the stomach was empty. During the surgery, a small incision is made on the abdomen after tissue infiltration by a local anesthetic (2% lidocaine). The muscle is bluntly cut to access the abdominal cavity and held open using eye speculums. The stomach is isolated, and a small incision was made on the forestomach, which is the area that has the lowest vascularization. A catheter is placed into the incision and secured with two sutures around the catheter to prevent its removal from mechanical stress. Once the catheter is secure, the wound is sutured. Rats were allowed a minimum of 5 days of post-operative recovery and at least 3 daily habituation sessions (~6 h each) to the recording environment. The ig catheter was flushed daily with water.
Surgical procedures for the electrochemical experiment have also been described in detail elsewhere (Solis et al., 2017). Under the same anesthesia, rats were implanted with a Pt-Ir oxygen sensor (Model 7002–02; Pinnacle Technology, Inc., Lawrence, KS, USA) into the NAc shell, using the same target coordinates as the first experiment. The sensor was secured with dental acrylic to three stainless steel screws threaded into the skull. Following this procedure, rats were implanted with an ig catheter similar to that of the first experiment and an iv catheter. Both catheters ran subcutaneously to the head mount and connected to this mount using dental acrylic. Rats were allowed a minimum of 5 days for post-operative recovery and at least 3 daily habituation sessions (~6 h each) to the recording environment. During recovery, both catheters were flushed daily with 0.2 ml heparinized saline (for iv catheter) or water (for ig catheter) to maintain their patency.
2.4. Electrochemical detection of oxygen
Pinnacle oxygen sensors consist of an epoxy-sheathed disc electrode that is ground to a fine surface using a diamond-lapping disc. These sensors are prepared from Pt-Ir wire 180 μm in diameter, with a sensing area of 0.025 mm2 at the tip. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. Dissolved oxygen is reduced on the active surface of these sensors, which is held at a stable potential of −0.6 V vs. the reference electrode. The current from the sensor is relayed to a computer via a potentiostat (Model 3104, Pinnacle Technology) and recorded at 1-s intervals, using PAL software utility (Version 1.5.0, Pinnacle Technology).
Oxygen sensors were calibrated at 37°C by the manufacturer (Pinnacle Technology) according to a standard protocol described elsewhere (Bolger et al., 2011) and the results of these calibrations were analyzed in-house. The sensors produced linear current changes with increases in oxygen concentrations within the wide range of previously reported brain oxygen concentrations (0–40 μM). Substrate sensitivity of oxygen sensors varied from 0.92 nA/1 μM to 1.38 nA/1μM. Oxygen sensors were also tested by the manufacturer for their selectivity toward other electroactive substances, including dopamine (0.4 μM) and ascorbate (250 μM), none of which had significant effects on reduction currents.
2.5. Experimental procedures for electrochemical and temperature experiments
At the beginning of each experiment, rats were lightly anesthetized (<2 min) with isoflurane. This allowed the electrochemical or temperature sensors to be connected to the recording instruments via an electrically shielded flexible cable and a multi-channel electrical swivel. The injection ports of the jugular and/or ig catheter were connected to catheter extensions mounted on the recording cord. This allowed for stress- and cue-free drug delivery of alcohol and heroin from outside the cage. Testing began approximately 60–90 min after the temperature or electrochemical sensors were connected to the recording instruments and respective baseline temperature or electrochemical currents were stabilized.
In the first experiment, 4 rats were recorded during repeated daily sessions (n=14). We examined changes in temperature and locomotor activity induced by 1-min tail-pinch and two ig injections of alcohol at 0.5 and 2.0 g/kg in 20% solution. After stabilization of temperature baselines, rats were first exposed to 1-min tail-pinch and then, after 60 min, received a low-dose alcohol injection delivered in ~1 ml volume over ~1.3 min). After 90 min, rats received the second, larger dose of alcohol delivered in ~4 ml volume over ~3.5 min. Post-injection recording continued for 120 min. For the tail-pinch trial, a wooden clothespin was manually attached to the base of the tail and removed 1 min later.
In electrochemical experiments (n=7 rats), we examined changes in NAc oxygen levels induced by (a) 1-min tail-pinch; (b) two injections of alcohol (0.5 and 2 g/kg); and (c) three iv injections of heroin (0.2 mg/kg) following ig administration of either alcohol at one dose (2 g/kg) or water. The latter tests were conducted in 5 rats recorded during 14 daily sessions (n=6 after alcohol and n=8 after water) on subsequent days. The effects of alcohol on NAc oxygen levels were tested in 4 rats (6 sessions), two of which were intact and two other were tested in a previous group during the first recording session. The effects of tail-pinch were tested in all 7 rats at least once in each rat; this was the first stimulus of a session. The timing intervals between tail-pinch and two alcohol administrations were similar to those of the first experiment (60, 90, and 120 min). In sessions where we tested the effects of alcohol or water on heroin responses, time intervals following their administration were 20 min with heroin injections delivered at 60-min inter-injection intervals. To decrease the duration of ig administrations, alcohol was delivered as 40% solution.
In our electrochemical experiments, we aimed to maintain similar experimental protocols for each rat, but in some sessions deviated from this protocol due to technical problems with recording quality of oxygen sensors or/and patency of both catheters. Therefore, the number of tests in each group varied. In tests with heroin injections following alcohol or water, each rat was tested with both alcohol and water and these administrations were counter-balanced (alcohol-water and water-alcohol).
2.6. Histological verification of electrode placements
When electrochemical and temperature recordings were completed, rats were deeply anesthetized with isoflurane, decapitated, the brains were extracted, and stored in 10% formalin solution. Later, the brains were sectioned and analyzed for verification of the locations of cerebral implants and tissue damage. During post-mortem examination, all rats were also checked for proper location of ig catheter and no leaking of both the stomach surface and catheter connection to the injection port.
2.7. Data analysis
Temperature data were recorded at 10-s quantification bins and averaged as the change in 1-min values. These data were analyzed as (a) absolute changes in each recording location, (b) relative changes with one-min values set as a pre-event baseline; and (c) changes in NAc-Muscle and Skin-Muscle differential, to provide information on the effects on brain metabolic activity and skin vessel tone (Kiyatkin, 2018). Locomotor data were analyzed with 1-min time resolution. Electrochemical data was analyzed with both slow (1-min) and rapid (10-s) resolution. Because each individual sensor differed in substrate sensitivity, currents were converted into concentrations of μM oxygen according to sensitivity calibrations provided by the manufacturer. Data were then converted into % changes in concentration of oxygen. Values from one min prior to the event of interest were averaged and used to set the 100% baseline.
One-way repeated measure ANOVAs (followed by Fisher LSD post-hoc tests) were used to evaluate statistical significance of stimulus- and drug-induced changes in both temperature parameters and oxygen reduction currents. Between-group (heroin after alcohol vs. heroin after water) differences were represented as area under the curve calculated for the time interval of mean oxygen decrease. To determine this parameter, we summated all the points within the decreasing interval, where these values were then averaged to determine the means and standard errors for heroin-induced oxygen decrease. These mean values were statistically evaluated using Student’s t-test. We also used two-way repeated measure ANOVAs to verify significance of differences in heroin-induced oxygen responses following ig delivery of alcohol or water. Quantitative results of statistical evaluations are shown in the Results section and in Supplementary Materials.
3. Results
Data presented in this study were obtained in 11 rats with histologically verified locations of brain thermorecording and electrochemical sensors in medial segment of the NAc. The results of histological work are shown in Fig. 1S in Supplementary Materials.
3.1. Alcohol-induced changes in temperature parameters and locomotion
As shown in Figure 1, alcohol delivered during quiet resting conditions induced weak changes in temperature parameters with no effects on locomotor activity. At the small dose (0.5 g/kg), there were no significant changes in NAc and muscle temperature (F13,767=0.71 and 1.17, p=0.95 and 0.18, respectively) and a weak biphasic effect on skin temperature (F13,767=1.88, p<0.05), with the initial decrease followed by an increase. Brain-Muscle differential tonically decreased after a transient increase during the injection (F13,767=2.27, p<0.001), while the Skin-Muscle differential showed variable changes around baseline (F12,767=1.88, p<0.01).
Fig. 1. Changes in temperature and locomotion induced by tail-pinch and alcohol (0.5 and 2.0 g/kg) delivered intra-gastrically in awake, freely moving rats.
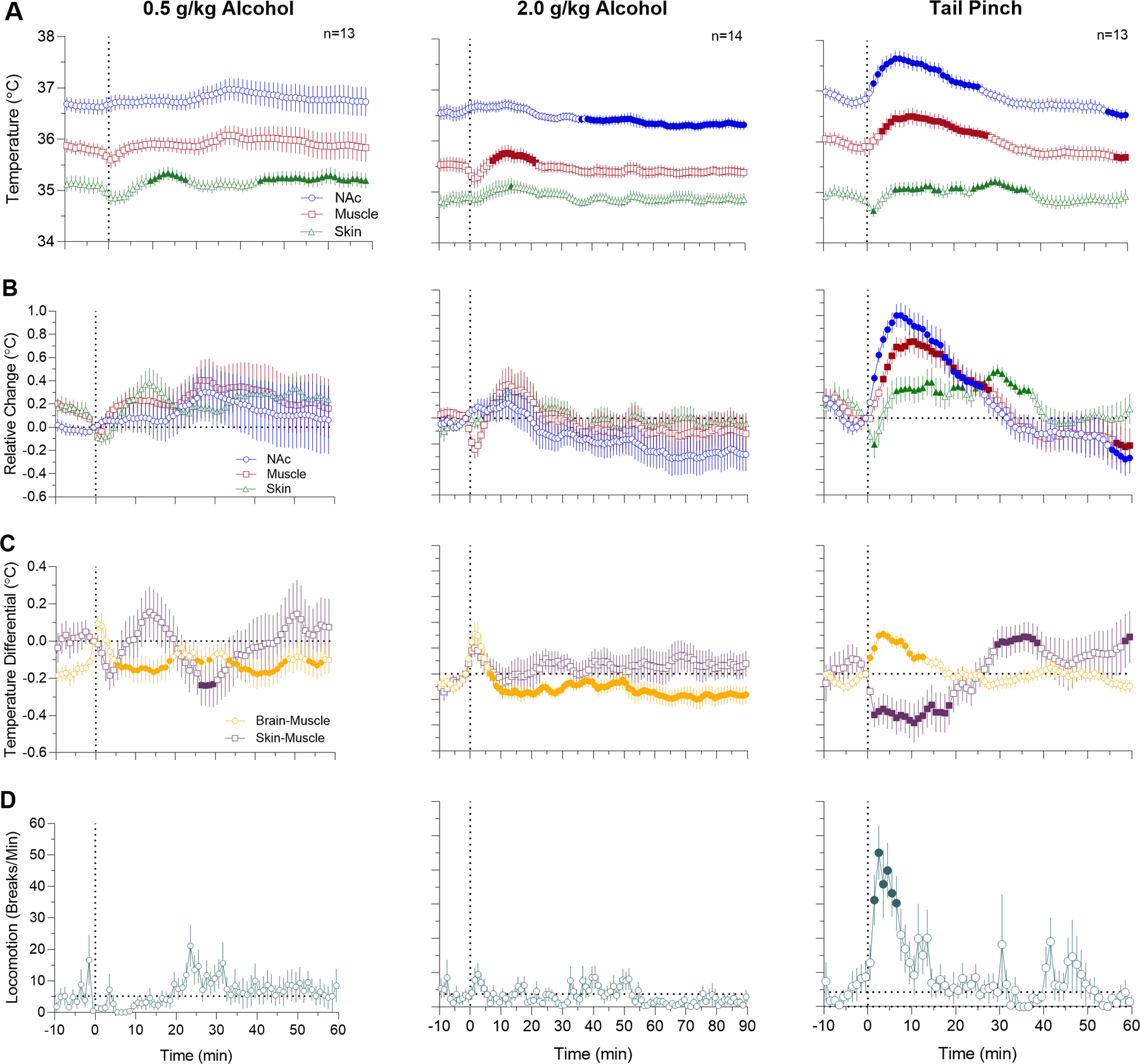
A - absolute temperature changes; B - relative temperature changes; C - changes in brain-muscle and skin-muscle differentials; and D - changes in locomotion. Filled symbols mark values significantly different from the pre-event baseline (p<0.05). Vertical dotted lines (0, min) show the onset of either alcohol administration or tail-pinch. See the text and Supplemental Materials for quantitative results of statistical evaluations.
Temperature effects induced by alcohol at 2 g/kg dose were also relatively weak, but significant. In this case, NAc temperature tonically decreased (F12,1092=3.13, p<0.001), muscle temperature showed a down-up change (F12,1092=3.21, p<0.01), while skin temperature remained stable with a tendency to increase (F12,1092=1.34, p<0.05). Due to different temperature dynamics in each recording location, NAc-Muscle differential showed a biphasic response (F12,1092=3.88, p<0.001) with a rapid but transient increase during the injection and tonic decrease thereafter. The Skin-Muscle differential did not show a significant time effect (F12,1092=1.06, p=0.33), but transiently increased during alcohol delivery.
These weak effects of alcohol sharply contrasted with the effects of tail-pinch. Consistent with our previous studies (Kiyatkin, 2018), tail-pinch induced significant increases in NAc and muscle temperatures (F12,732=21.4 and 15.0, both p<0.001), weaker biphasic (down-up) change in skin temperature (F12,732=3.87, p<0.01) and strong locomotor activation (F10,610=3.80, p<0.001). The temperature increase in the NAc was more rapid and stronger than in temporal muscle, resulting in a significant rise of NAc-Muscle differential (F12,732=6.75, p<0.001). The Skin-Muscle differential rapidly decreased for ~25 min following tail-pinch and then showed a rebound-like increase (F12,732=8.39).
3.2. Alcohol-induced changes in NAc oxygen levels
In contrast to our expectations, alcohol delivered to quietly resting rats at both doses had relatively weak effects on NAc oxygen levels (Fig. 2). At a lower dose (0.5 g/kg), NAc oxygen levels remained unchanged (F5,300=1.48, p=0.25), but a transient increase was seen during alcohol delivery. The effect of alcohol at a 2 g/kg dose was stronger but remained non-significant as analyzed for 60 min after alcohol delivery (F5,300=1.74, p=0.22). In this case, NAc oxygen levels initially rapidly increased and then tonically increased again for the entire analysis intervals. Consistent with our previous studies (Solis et al., 2017), tail-pinch increased NAc oxygen levels (F9,549=4.01, p<0.01); the response peaked at 1–2 min and returned to baseline at ~10 min after the stimulus onset.
Fig. 2. Changes in NAc oxygen levels induced by tail-pinch (A) and alcohol (B, 0.5 and C, 2.0 g/kg) delivered intra-gastrically in awake, freely moving rats.
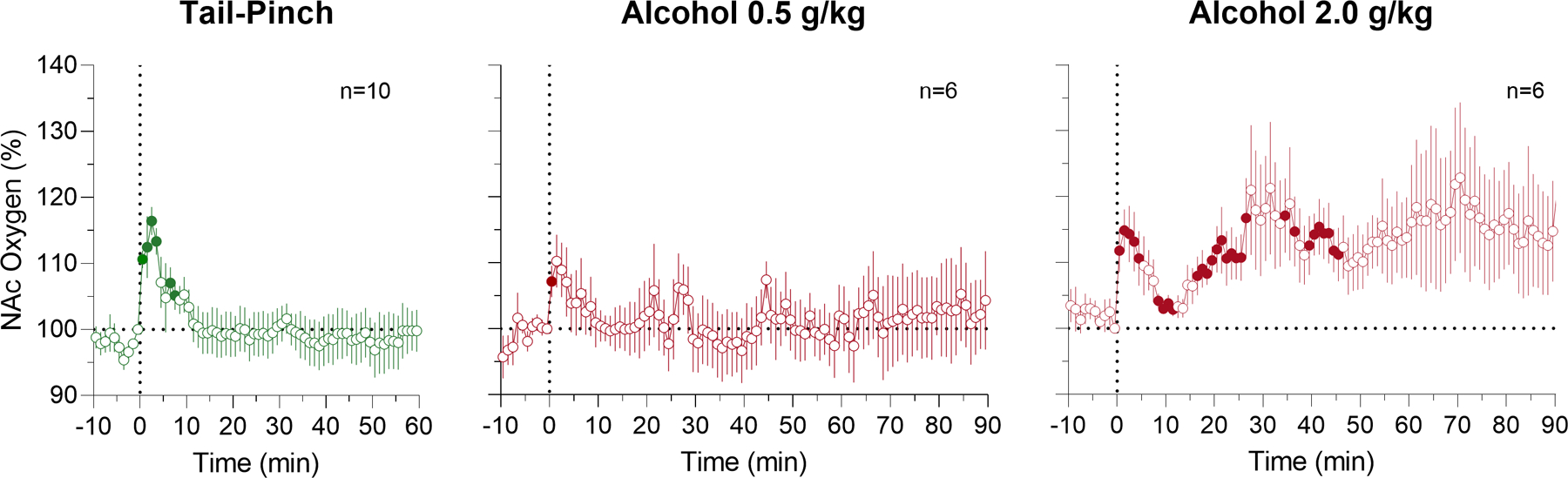
Filled symbols mark values significantly different from the pre-event baseline (p<0.05). Vertical dotted lines (0, min) show the onset of either alcohol administration or tail-pinch. See the text and Supplemental Materials for quantitative results of statistical evaluations.
3.3. Effects of alcohol on NAc oxygen responses induced by heroin
When analyzed with 1-min temporal resolution (Fig. 3), ig administration of both alcohol (2 g/kg) and water did not result in significant changes of NAc oxygen (F5,105=1,17, p=0.35 and F7,147=1.56, p=0.24, respectively). However, NAc levels slightly increased during administration of both alcohol and water. While they returned to baseline in water group, levels remained increased until the 20th min, when the rat received the first heroin injection.
Fig. 3. Changes in NAc oxygen levels induced by iv heroin (0.2 mg/kg) after intragastric administration of alcohol (2.0 g/kg) or water in awake, freely moving rats analyzed at slow time-resolution.
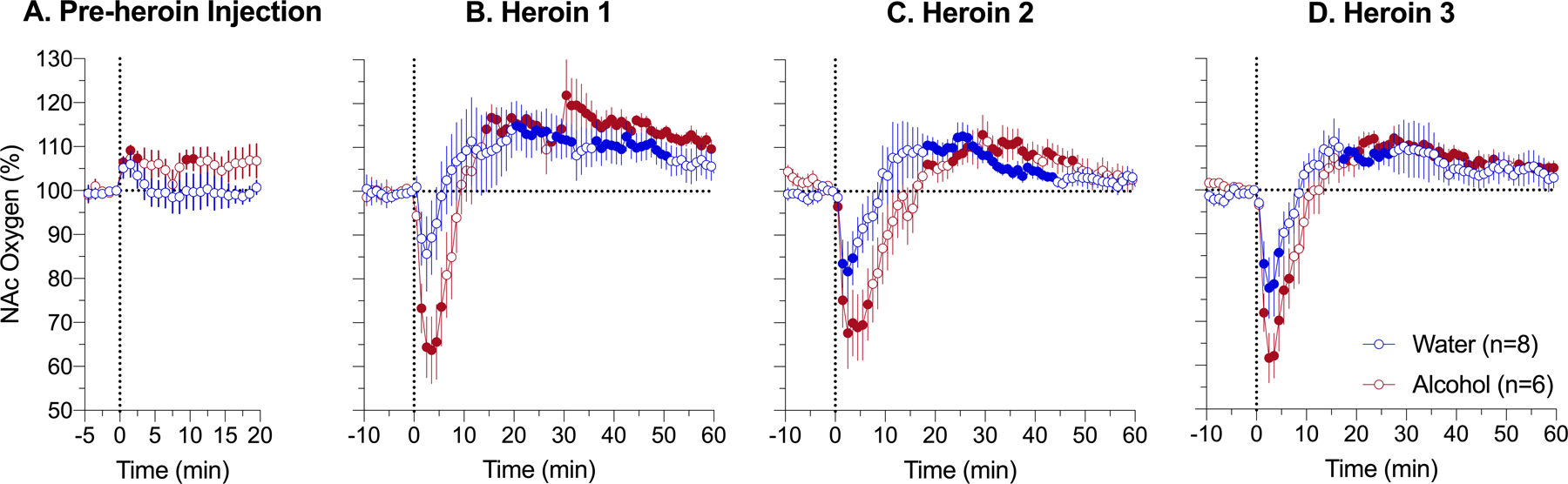
A - changes induced by administration of alcohol (2.0 g/kg) and water; B, C and D – changes induced by heroin delivered at 20, 80, and 140 min after intragastric administration of alcohol or water. Filled symbols mark values significantly different from pre-event baseline (p<0.05). Vertical dotted lines (0, min) show the onset of ig alcohol delivery (A) and iv heroin injection (B-D). See the text and Supplemental Materials for quantitative results of statistical evaluations.
Heroin injections, after both alcohol and water administration, caused a biphasic oxygen response where oxygen levels rapidly decreased, reached nadir at 3–4 min, and then increased more tonically above baseline. In the water group, the time-course of NAc oxygen responses was similar in each of three heroin injections, where oxygen levels initially decreased to 75–85% of baseline and increased thereafter to 14–20% above baseline. Due to high variability, the effect of the first heroin injection in this group was weakest and did not reach statistical significance (F7,427=2.91; p=0.08). While the time-course of heroin-induced NAc oxygen responses in the alcohol group was similar to that in the water group, the initial decrease in alcohol group was clearly stronger and more prolonged than in the water group for all three heroin injections (alcohol 1: F5,305=10.68, p<0.01, water 1:F7,427=2.91, p=0.08; alcohol 2: F5,305=7.38, p<0.01; water 2: F7,427=3.98, p<0.05; alcohol 3: F5,305=13.43, p<0.001; water 3: F7,427=5.38, p<0.01). In this case, NAc oxygen levels dropped to 60–65% of baseline and decreases were evident for 9–14 min post-injection. Like the control group, the effects of heroin were similar for all three injections.
Between-group differences in the heroin-induced NAc oxygen decrease were evident analyzing their areas under the curve. In this case, heroin-induced oxygen hypoxia was larger for all injections in the alcohol group (alcohol 1: 207.83±62.34 and water 1: 43.38±38.79, t=2.23, p<0.05; alcohol 2: 261.55±71.05 and water 2: 86.06±42.78, t=2.12, p<0.05 and alcohol 3: 217.79±65.47; water 3: 101.35±39.30, t=1.52). The difference was largest for the first heroin injection, slightly weaker for the second injection, and smallest for the third injection, where it was non-significant. Similar results were obtained by using 2-way repeated measure ANOVA, which revealed significant interactions for all three heroin injections (F61,732=2.31, p<0.0001; F61,732=1.73, p<001; F61,732=1.62, p<0.01, respectively). The effect was strongest for the first heroin injection and gradually decreased for the second and third injections.
More precise pictures of the heroin-induced oxygen response with clearer differences between alcohol and water groups were obtained in high time-resolution analyses (Fig. 4). For this analysis, the same data analyzed before with 1-min time resolution, were re-analyzed with 10-s quantification bin. The effect of time was similarly significant for each injection in both groups (alcohol 1: F5,605=20.69, p<0.001; water 1: F7,847=0.01, p<0.01; alcohol 2: F5,605=5.49, p<0.05; water 2: F7,847=5.52, p<0.01; alcohol 3: F5,605=16.4, p<0.001; water 3: F7,847=9.20, p<0.01) and the decrease was clearly stronger and more prolonged in the alcohol group. To represent these changes, we calculated area under the curve for heroin-induced oxygen decrease. This change was clearly larger in the alcohol group for all three heroin injections (alcohol 1, 2, 3: 1231±386, 1570±4.31, 1308±345 vs. water 1, 2, 3: 268±231, 606±296, 612±230). In terms of the extent of change, heroin-induced hypoxia following alcohol delivery was as a mean 2.77-fold stronger than in control conditions, when the rats received water. Following this analysis, we also found that ig injection of both alcohol and water induced weak but significant immediate increase in NAc oxygen, which returned to baseline in the water group but remained increased in the alcohol group (alcohol: F5,155=1.40, p=0.29 and water: F7,217=4.34, p<0.05).
Fig. 4. Changes in NAc oxygen levels induced by iv heroin (0.2 mg/kg) after intragastric administration of alcohol (2.0 g/kg) or water in awake, freely moving rats analyzed at rapid time-resolution.
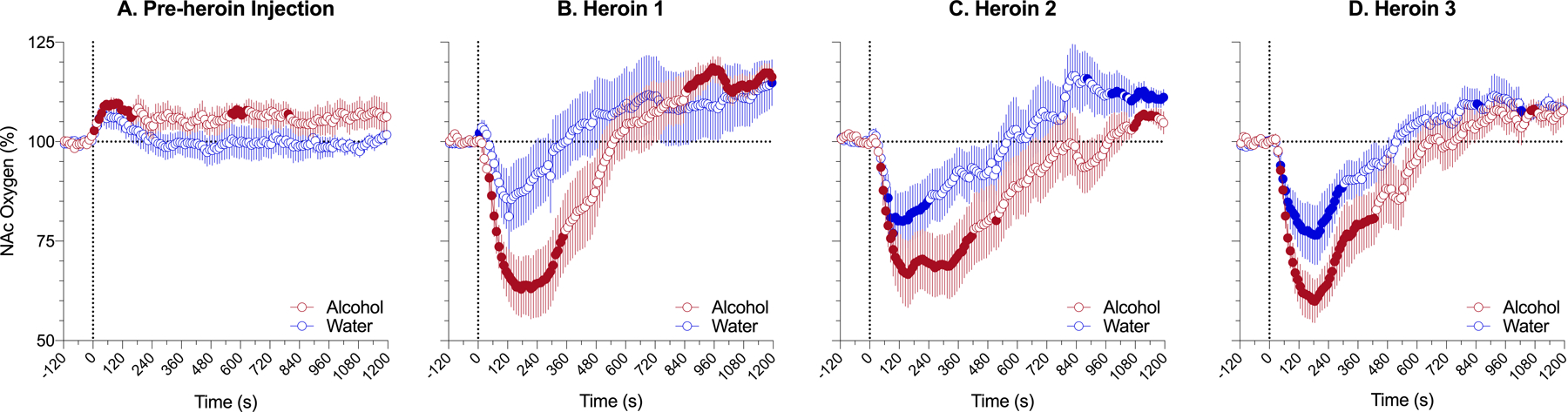
A - changes induced by administration of alcohol (2.0 g/kg) and water; B, C, D - changes induced by heroin delivered 20, 80, and 140 min after intragastric administration of alcohol or water. Filled symbols mark values significantly different from pre-event baseline (p<0.05). Vertical dotted lines (0, min) show the onset of ig alcohol delivery (A) and iv heroin injections (B-D). See the text and Supplemental Materials for quantitative results of statistical evaluations.
4. Discussion
Multiple clinical reports revealed that many patients admitted in emergency departments with a diagnosis of opioid overdose and symptoms of oxygen deficiency have different amounts of alcohol in their blood. Alcohol and opioids are also often found in post-mortem blood samples. Since both opioid drugs and alcohol are usually viewed as CNS depressants, these findings may indicate that alcohol can potentiate respiratory depressant effects of opioids, thus resulting in systemic hypoxia, coma and death. The goal of this study was to examine how alcohol in human-relevant doses and routes of administration affects brain oxygen responses induced by iv heroin, a representative opioid drug of abuse. This study is a logical extension of our previous work examining brain hypoxic effects of different opioid drugs by using oxygen sensors coupled with high-speed amperometry in awake, freely moving rats (Kiyatkin, 2018). In contrast to plethysmography that can detect drug-induced changes in breathing rate and tidal volumes, electrochemical oxygen sensors permit direct monitoring of fluctuations in dissolved oxygen of the brain’s extracellular space, a functionally and clinically relevant parameter. Moreover, these measurements could be conducted with second-scale temporal resolution under physiologically relevant conditions of multi-session animal experiment. This distinction between these monitored parameters is important because, under some specific conditions, inhibition of respiratory activity could co-exist with brain hyperoxia. As recently reported (Thomas et al., 2021), iv heroin at low reinforcing doses (>50 μg/kg) induces a modest increase of brain oxygen levels, while subcutaneous oxygen levels show stronger and more prolonged decreases reflecting respiratory depression.
Our electrochemical experiments were proceeded by multi-site temperature monitoring. As an important homeostatic parameter that reflects metabolic neural activity and affects multiple neural functions, brain temperature should be affected by alcohol. While this knowledge is important by itself, multi-site thermorecordings could provide critical information about the effects of drugs on metabolic neural activity and tone of skin vessels.
The third novel feature of this study was the use of chronic ig catheter that allowed delivery of alcohol into the stomach under stress- and cue-free conditions, when the recorded animal is in a quiet resting “sleep-like” conditions. However, the primary reason for using this approach was the need to eliminate strong sensory effects, which exist with any other way of alcohol delivery. Oral alcohol delivery in rats is exceptionally stressful and alcohol injected intra-peritonially stimulates afferents of sensory fibers that abundantly innervate the abdominal cavity. These influences are especially important for brain oxygen, which shows rapid and relatively large fluctuations elicited even by weak sensory stimuli (i.e., sound, tail-pinch, social interaction, etc.).
To examine the effects of alcohol on temperature and locomotor effects we chose two doses (0.5 and 2.0 g/kg), which are equivalent to 87 and 350 ml of vodka (40% ethyl alcohol). While these doses exceed the range of typical human consumption, they are much lower than LD50 (7–12 g/kg; Wiberg et al., 1970) for oral administration in rats. The issue of equipotentiality of animal and human doses is very controversial but rats are typically less sensitive to toxic effects of drugs than humans. Calculations based on body surface area and LD50 for per oral alcohol delivery suggest that equipotent doses of alcohol in rats are about 4–6 times higher than those in humans (Gable, 2004). Therefore, doses of alcohol used in this study appear to be within human-relevant range.
4.1. Effects of alcohol
Although it is generally believed that alcohol, as a CNS depressant, should induce respiratory depression, which leads to brain hypoxia, direct experimental support is limited and the existing data are controversial. Human observations revealed that the effects of alcohol with oral delivery even at relatively large doses are relatively minor, with both increases (Bellwille et al., 1976; Setnik et al., 2014), decreases (Michaels et al., 1983; McCauley et al., 1988), or no changes in respiratory activity (Johnstone and Witt, 1972; van der Schrier et al., 2017). Relatively minor inhibitory effects of alcohol on breathing were also found in rats using whole-body plethysmography (Ren et al., 2012). In this latter study, alcohol at the 2 g/kg dose with intraperitoneal delivery modestly decreased tidal volumes and minute ventilation without any effect on rate of breathing. Based on the results of human observations, it was suggested that alcohol has dose-dependent effects, stimulating breathing at low doses and inhibiting breathing at very large doses.
Contrary to our initial expectations, alcohol delivered to quietly resting rats at a 0.5 g/kg dose induced weak temperature effects, with a tendency for increasing NAc, muscle, and skin temperature, and it had no effects on both NAc oxygen levels and animal locomotion. The only change was the immediate effect during alcohol delivery, when NAc oxygen transiently increased for several minutes. At this time interval, NAc-Muscle differential phasically increased and Skin-Muscle differential phasically decreased. While these changes were small and only significant only for NAc oxygen, these temperature changes suggest transient metabolic neural activation and skin vasoconstriction—the effect seen at a larger scale with tail-pinch, a typical arousing stimulus. Therefore, it appears that alcohol at low doses has weak stimulatory effects during its delivery.
The effects of alcohol at a larger dose were stronger but they partially mimic the effects seen with low doses. In this case, NAc levels showed the initial rapid and transient increase during alcohol delivery that followed by a weak and prolonged tonic increase thereafter. Similarly, the NAc-Muscle differential increased during alcohol delivery suggesting the immediate stimulatory effect on brain metabolic activity. The second tonic oxygen increase was associated with weak decreases in brain temperature and NAc-Muscle differential, suggesting tonic inhibitory effect on brain metabolic activity.
Therefore, we can conclude that ig alcohol delivery induces two effects: an immediate, transient neural activation followed by a more delayed and prolonged inhibition that is associated with a tonic decrease in NAc temperature and tonic increase in NAc oxygen. The appearance of such rapid neural effect, where its duration was close to the duration of the injection itself, cannot be explained by the direct action of alcohol in the brain because of its slower absorption after ig delivery and relatively late peaks in blood levels (Holt, 1981). It is unclear how slowly, but human data assessing differences in pharmacodynamics following different administration routes suggest that blood drug levels peak at 30–60 min after ig administration, much slower and at much lower levels than with iv or ip administration (Finch et al., 1974; Baraona et al., 2001). As shown in our study with electrochemical measurements of brain glucose, there is a definite onset latency from the start of ig glucose injection and its appearance in brain tissue (Kiyatkin and Wakabayashi, 2015). In this study, glucose was detectable in brain tissue at ~6 min from the start of ig injection and peaked at ~18–24 min, while it was detectable in brain at 1–2 min and peaked at 4–5 min after iv injection. Therefore, it may be assumed that this rapid effect is triggered by the direct action of alcohol on neural elements of the stomach.
The stomach is densely innervated by sensory fibers, ascending to the CNS within the parasympathetic and sympathetic nervous systems. About 90% of all vagal fibers to the stomach are afferent. Sensory fibers within sympathetic nerves innervating stomach reach the spinal cord via celiac ganglion and superior cervical ganglion. These sensory elements are nonmyelinated C fibers and they are sensitive to chemical, mechanical and temperature stimuli (Khurana and Petras, 1991; Furness et al., 2014; Gebhart, 1996). Therefore, we can assume that alcohol delivered intra-gastrically interact with these receptors in stomach walls and these stimulation results in afferent signal to the CNS inducing neural activation. It is not known which types of receptors can be stimulated by alcohol but chemoreceptors and mechanoreceptors that are sensitive to pressure and stomach extension could be the major candidates. It is impossible to exclude activation of thermoreceptors because alcohol was delivered into stomach at 22–23°C, where normal temperature is within 36–37°C. In support of possible involvement of mechano- and thermoreceptors, ig injection of water also induced an immediate rise in NAc oxygen, which had a similar pattern but was smaller and more transient than that induced by alcohol. Further analytical studies are necessary to substantiate these still hypothetical mechanisms.
The second component of NAc oxygen response, a tonic increase, was also an unexpected finding and its mechanisms remain unclear. Interestingly, this change was associated with decreases in brain temperature and brain-muscle differentials, possible correlates of central inhibitory action of alcohol. It is possible that this effect results from cerebral vasodilation and increased cerebral blood flow, the known effects of alcohol shown in multiple human studies (Mathew et al., 1986; Gundersen, 2013; Marxen, 2014). While oxygen diffusion is gradient-dependent, cerebral vasodilation and increased cerebral flow will enhance oxygen entry from arterial blood.
4.2. Effects of alcohol on NAc oxygen responses induced by heroin
Consistent with our previous studies (Solis et al., 2017), iv heroin injected at 0.2 mg/kg dose induced a biphasic brain oxygen response, with the initial rapid decrease followed by more delayed and weaker increase. These responses remained relatively stable for all three heroin injections delivered after water administration. These responses also showed the same biphasic pattern following ig alcohol administration, but the initial oxygen decrease was stronger and more prolonged for each subsequent heroin injection. Calculations of the area under the curve for oxygen decreases revealed that the initial brain hypoxia becomes 2 to 3-fold stronger under the influence of alcohol than in water control. Therefore, consistent with clinical observations (Johnstone and Witt, 1973; Levine et al., 1995), alcohol clearly potentiates heroin-induced brain hypoxia. It is pretty evident that such an enhancement of hypoxic effects of opioid drugs results from respiratory depression and subsequent decrease in blood oxygen levels. Alcohol-induced potentiation of respiratory activity was shown by monitoring ventilation in human volunteers with oxycodone, another widely used opioid drug (van der Schrier et al., 2017). In this study, alcohol (0.5–1.0 g/l) induced minimal changes in respiration but potentiated respiratory depressive effects of oxycodone (20 mg), strongly increased incidents of apnea, and enhanced a drop in oxycodone-induced oxygen saturation.
4.3. Conclusions and Functional Implications
The use of high-speed monitoring of brain oxygen levels in freely moving rats and intragastric alcohol delivery allowed us to reveal several novel findings that were unexpected from our original hypothesis. First, instead of supposed decreases, alcohol induced relatively weak bimodal effects on brain oxygen levels with a dose-dependent phasic increase during alcohol delivery and tonic increase thereafter. The phasic oxygen increase coexisted of transient increases in brain temperature and brain-muscle differentials, suggests neural activation. Thus, it appears that alcohol intra-gastrically delivered in rats at modest doses does not induce brain hypoxia and even slightly increased basal brain oxygen levels. Phasic oxygen increase during alcohol delivery was also an unexpected finding, which suggests that alcohol begins to act in the stomach and neural signals from the stomach rapidly reach the CNS via sensory pathways preceding much slower delivery of alcohol in brain tissue after its absorption and travel via circulation.
Based on numerous clinical and preclinical studies, we expected that alcohol will potentiate hypoxic responses induced by iv heroin. This was the case, and pre-treatment with alcohol strongly increased heroin-induced brain hypoxia, by making it stronger and more prolonged. However, this was not a simple summation of two effects as suggested by other studies, but a true potentiation, indicating different underling mechanisms. While future studies are necessary to uncover these mechanisms, this study indicates the danger of using alcohol in conjunction with opioid drugs. Finally, despite all advantages of animal experimentations in clarifying health hazards of opioid drug use, they cannot fully mimic real-life human conditions. Drug users may have silent pre-existing medical issues, heroin and oxycodone available in the market is often contaminated by more potent opioids such as fentanyl or carfentanil, and the use of opioids is often preceded by or coupled with the use of other neuroactive drugs.
Supplementary Material
Acknowledgements and Funding:
The study was supported by the Intramural Research Program of the NIH, NIDA (NIH Grant 1ZIADA000566-10 for Dr. Eugene A. Kiyatkin).
Abbreviations:
- ANOVA
analysis of variance
- CNS
central nervous system
- ig
intragastric
- iv
intravenous
- LD50
the dose of a toxic substance that is sufficient to kill 50% of a population of animals
- NAc
nucleus accumbens
Footnotes
Conflict of Interest: The Authors reports no conflict of interest
References
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW et al. 2001. Gender differences in pharmacokinetics of alcohol. Acoholism: Clinical and Experimental Research 25, 502–507. [PubMed] [Google Scholar]
- Baud J 2009. Mechanisms of opioid-induced overdose: experimental approach to clinical concerns. Ann. Pharm. Fr 67, 353–359. [DOI] [PubMed] [Google Scholar]
- Bellville JW, Swanson GD, Miyake T, Aqleh KA 1976. Respiratory stimulation observed following ethanol ingestion. West J. Med 124, 423–425. [PMC free article] [PubMed] [Google Scholar]
- Bola RA, Kiyatkin EA, 2017. Brain temperature effects of intravenous heroin: state dependency, environmental modulation, and the effects of dose. Neuropharmacology 126, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger FB, Bennett R Lowry JP. 2011. An in vitro characterization comparing carbon paste and Pt microelectrodes for real-time detection of brain tissue oxygen. 4028–4035. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA, 2005. Brain temperature changes and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psyhopharmacology 181, 299–308. [DOI] [PubMed] [Google Scholar]
- Finch JE, Kendall MJ, Mitchard M 1974. An assessment of gastric amptying by breathalyser. Br. J. Clin. Pharmac 1, 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB., Callaghan BP, Rivera LR., Cho HJ 2015. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol 817, 39–71. [DOI] [PubMed] [Google Scholar]
- Gable RS 2004. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 99, 686–696. [DOI] [PubMed] [Google Scholar]
- Gebhart GF 1996. Visceral polymodal receptors. Prog. Brain Res 113, 101–112. [PubMed] [Google Scholar]
- Gerber GJ, Wise RA 1988. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol. Biochem. Behav 32, 527–531. [DOI] [PubMed] [Google Scholar]
- Gundersen H, van Wageningen H, Gruner R 2013. Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol 48, 160–165. [DOI] [PubMed] [Google Scholar]
- Holt S 1981. Observation on the relation between alcohol absorption and the rate of gastric emptying. CMA Journal, 125, 267–298. [PMC free article] [PubMed] [Google Scholar]
- Imam I 2010. Alcohol and the central nervous system. Br. J. Hosp. Med. (Lon.) 71, 635–639. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Knapp CM, and Ciraulo DA (1997). Opiates: Clinical Aspects. In: Substance Abuse. A comprehensive Textbook (3rd Ed) edited by Lowinson JH, Ruis P, Millman RB Langrod JG. (pp. 158–165). Baltimore: et al. : Williams and Wilkins. [Google Scholar]
- Johnstone RE, Witt RL 1972. Respiratory effects of ethyl alcohol intoxication. JAMA 222, 486. [DOI] [PubMed] [Google Scholar]
- Johnstone RE, Reier C 1973. Acute respiratory effects of ethanol in man. Clin. Pharmacol. Ther 14, 501–508. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA 2018. Brain temperature: from physiology and pharmacology to neuropathology. Handb. Clin. Neurol 157, 483–504. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA 2018. Central and peripheral mechanisms underlying physiological and drug-induced fluctuations in brain oxygen in freely-moving rats. Front. Integr. Neurosci 12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA 2019. Respiratory depression and brain hypoxia induced by opioid drugs: morphine, oxycodone, heroin, and fentanyl. Neuropharmacology, 151, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wakabayashi KT 2015. Behavior-associated and post-consumption glucose entry into the nucleus accumbens extracellular space during glucose free-drinking intrained rats. Front Behav Neurosci. 9:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana RK, Petras JM 1991. Sensory innervation of the canine esophagus, stomach, and duodenum. Am.. J Anat. 192, 293–306. [DOI] [PubMed] [Google Scholar]
- Levine B, Green D, Smialek JE 1995. The role of ethanol in heroin death. J. Forensic Sci 40, 808–810. [PubMed] [Google Scholar]
- Marxen M, Gan G, Schwarz D, Mennigen E, Pihatch M, Zimmerman US 2014. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J. Cereb. Blood Flow Metab 34, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH 1986. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke 17, 1156–1159. [DOI] [PubMed] [Google Scholar]
- McCauley VB, Grunstein RR, Sullivan CE 1988. Ethanol-induced depression of hypoxic drive and reversal by naloxone—A sex difference. Am. Rev. Resp. Dis 137, 1406–1410. [DOI] [PubMed] [Google Scholar]
- McIntosh C, Chick J 2004. Alcohol and the nervous system. J. Neurol. Neurosurg. Psychiatry Suppl 3: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels TM, Light RW, Mahutte CK 1983. Naloxone reverses ethanol-induced depression of hypercapnic drive. Am. Rev. Resp. Dis 128, 823–826. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY 1980. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Pattinson KT 2008. Opioids and the control of respiration. Br. J. Anaesth 100, 747–758. [DOI] [PubMed] [Google Scholar]
- Paxinos G Watson C 1998. The Rat Brain in Stereotaxic Coordinates, 4th Edition. (Academic Press; ). [Google Scholar]
- Ren J, Ding X, Greer JJ 1985. Respiratory depression in rats induced by alcohol and barbiturates and rescue by ampakine CX717. J. Appl. Physiol 113, 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setnik B, Sokolowska M, Johnson F, Oldenhof J, Romach M 2014. Evaluation of the safety, pharmacodynamic, and pharmacokinetic effects following oral coadministration of immediate-release morphine with ethanol in healthy male participants. Hum. Psychopharmacol 29, 251–265. [DOI] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT & Kiyatkin EA 2017. Rapid physiological fluctuations in nucleus accumbens oxygen levels induced by arousing stimuli: Relationships with changes in brain glucose and metabolic brain activation. Front. Integr. Neurosci 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, Kiyatkin EA 2017. Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro, 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Perekopskiy D, Kiyatkin EA 2020. Cocaine added to heroin fails to affect heroin-induced hypoxia. Brain Res. 1746, 147008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Schrier R, Roozekranz M, Olofsen E, Aarts L, van Velzen M, de Joun M et al. 2017. Influence of ethanol on oxycodone-induced respiratory depression. Anesthesiology 126: 534–542. [DOI] [PubMed] [Google Scholar]
- Wiberg GS, Trenholm HL, Coldwell BB 1970. Increased ethanol toxicity in old rats: changes in LD50, in vivo and in vitro metabolism, and liver alcohol dehydrogenese activity. Toxicol. Appl. Pharmacol 16, 718–727. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA 1987. A psychomotor stimulant theory of addiction. Psychol. Rev 94, 469–492. [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE 2019. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemics: A critical review. Alcohol Clin. Exp. Res 42, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


