Abstract
Background:
Many neuroactive steroids induce sedation/hypnosis by potentiating γ-aminobutyric acid (GABAA) currents. However, we previously demonstrated that an endogenous neuroactive steroid epipregnanolone [(3β,5β)-3-hydroxypregnan-20-one] (EpiP) exerts potent peripheral analgesia and blocks T-type calcium currents while sparing GABAA currents in rat sensory neurons. This study seeks to investigate the behavioral effects elicited by systemic administration of EpiP and to characterize its use as an adjuvant agent to commonly used general anesthetics (GAs).
Methods:
Here, we utilized electroencephalographic (EEG) recordings to characterize thalamocortical oscillations, as well as mouse genetics with wild-type (WT) and different knockout (KO) models of T-channel isoforms to investigate potential sedative/hypnotic and immobilizing properties of EpiP.
Results:
Consistent with increased oscillations in slower EEG frequencies, EpiP induced an hypnotic state in WT mice when injected alone intra-peritoneally (i.p.) and effectively facilitated anesthetic effects of isoflurane (ISO) and sevoflurane (SEVO). The CaV3.1 (Cacna1g) KO mice demonstrated decreased sensitivity to EpiP-induced hypnosis when compared to WT mice, whereas no significant difference was noted between CaV3.2 (Cacna1h), CaV3.3 (Cacna1i) and WT mice. Finally, when compared to WT mice, onset of EpiP-induced hypnosis was delayed in CaV3.2 KO mice but not in CaV3.1 and CaV3.3 KO mice.
Conclusion:
We posit that EpiP may have an important role as novel hypnotic and/or adjuvant to volatile anesthetic agents. We speculate that distinct hypnotic effects of EpiP across all three T-channel isoforms is due to their differential expression in thalamocortical circuitry.
Keywords: low-voltage-activated, calcium, thalamus, righting reflex, withdrawal reflex, isoflurane
INTRODUCTION
It is generally accepted that many neuroactive steroids (neurosteroids) induce sedation/hypnosis by potentiating neuronal GABAA currents (Belelli and Lambert, 2005; Ziemba et al., 2018). However, we previously demonstrated that an endogenous neurosteroid (Liu et al., 2003) [(3β,5β)-3-hydroxypregnan-20-one] blocks the low voltage-activated (LVA) or T-type family of voltage-gated calcium channels (VGCCs) in sensory neurons, but unlike many other neurosteroids lacks any GABA-mimetic properties (Ayoola et al., 2014). Furthermore, our previous studies have established that the antinociceptive effects of EpiP in rats and mice are mediated, at least in part, by the inhibition of multiple classes of VGCCs when the drug was injected locally into hind paws (Ayoola et al., 2014) and intrathecally (Joksimovic et al., 2019). However, virtually nothing is known about the role of VGCC in possible sedative/hypnotic effects of this neurosteroid. This is important since VGCCs are essential for the regulation of synaptic transmission and excitability in the neuronal sleep pathway including the thalamus. Indeed, both human and animal studies in vivo have indicated that the thalamus is deactivated during anesthesia (Alkire et al., 2008; Mashour and Alkire, 2013). T-type voltage-gated calcium channels (T-channels) are crucial for the rhythmic oscillations between mutually interconnected cortical neurons (Ctx), inhibitory GABAergic nucleus reticularis thalami neurons (nRT), and glutamatergic relay neurons in the ventrobasal (VB) thalamic nucleus. Furthermore, a synthetic neurosteroid analogue of EpiP that also lacks GABA-mimetic properties ([3β-OH; (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile]) inhibits T-currents in the nRT (Joksovic et al., 2007), VB thalamus (Atluri et al., 2018), as well as central medial thalamic (CeM) nucleus (Timic Stamenic et al., 2021). Importantly, we have shown that 3β-OH also displays strong sedative/hypnotic properties in rat pups (Atluri et al., 2018), adult rats (Joksimovic et al., 2020) and adult mice (Timic Stamenic et al., 2021). Hence, this current work seeks to investigate the potential sedative/hypnotic properties of EpiP and to characterize its use as an adjuvant agent to commonly used volatile GAs such as isoflurane (ISO) and sevoflurane (SEVO). Our first objective was to use behavioral studies correlated with electroencephalographic (EEG) recordings and systemically injected doses of EpiP to characterize its potential sedative/hypnotic effects in mice. Second, we set out to compare and contrast the effects of EpiP in the wild-type (WT) mice versus mice lacking specific T-channel isoforms, henceforth referred to as CaV3.1 (Cacna1g), CaV3.2 (Cacna1h) and CaV3.3 (Cacna1i) KO mice.
MATERIALS AND METHODS
Animals
Experimental procedures with animals were performed according to the guidelines approved by University of Colorado Anschutz Medical Campus. Treatments of mice adhered to guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data. For our behavioral studies we used adult male mice that were 2–6 months old. All mice were kept in a 14 hour light/10 dark cycle with free access to food and water. Heating pad and pulse oximetry were used in all experiments to assure normothermia and normal oxygenation of animals. Temperatures were taken rectally during the experiments involving ISO and SEVO. Male C57BL/6 J (wild type – WT) mice were obtained from the Jackson laboratory (USA) and were used as controls in all experiments. CaV3.1 KO mice (Ricken BioResources Centre, Japan) were generated on C57BL/6 background as previously documented (Petrenko et al., 2007). The CaV3.2 KO mice were generated as described previously using a mixed C57BL6J/129S3 background (Chen et al., 2003) whose off-springs were backcrossed into C57BL6J mice for at least 6 generations (Orestes et al., 2009). Finally, the CaV3.3 KO mice were developed on a C57BL/6 background as recently described (Ghoshal et al., 2020). Although we used only male mice for this study, we are aware of reports that some neurosteroids may exhibit sex-dependent hypnotic effects in rodents (Erickson et al., 2019). However, we feel that studies of possible differences in male and female mice to EpiP-induced hypnosis are beyond the scope of this study.
Our methods for the assessment of anesthetic endpoints such as loss of righting reflex (LORR), loss of withdrawal response (LOWR) and electroencephalographic (EEG) recordings are described in detail elsewhere (Feseha et al., 2020; Timic Stamenic et al., 2021).
Neurosteroid preparation
EpiP (Figure 1A) was dissolved in stock solution of 15% cyclodextrin in H2O to yield the desired concentration for intra-peritoneal (i.p.) injections. All doses of EpiP solution were prepared on the same day as they were injected into the mice. Mice were weighed and injected with the appropriate volume of EpiP in 15% cyclodextrin to achieve desired dose. EpiP doses ranged from 25 mg/kg to 120 mg/kg.
Figure 1 -. EpiP is a dose-dependent hypnotic agent.
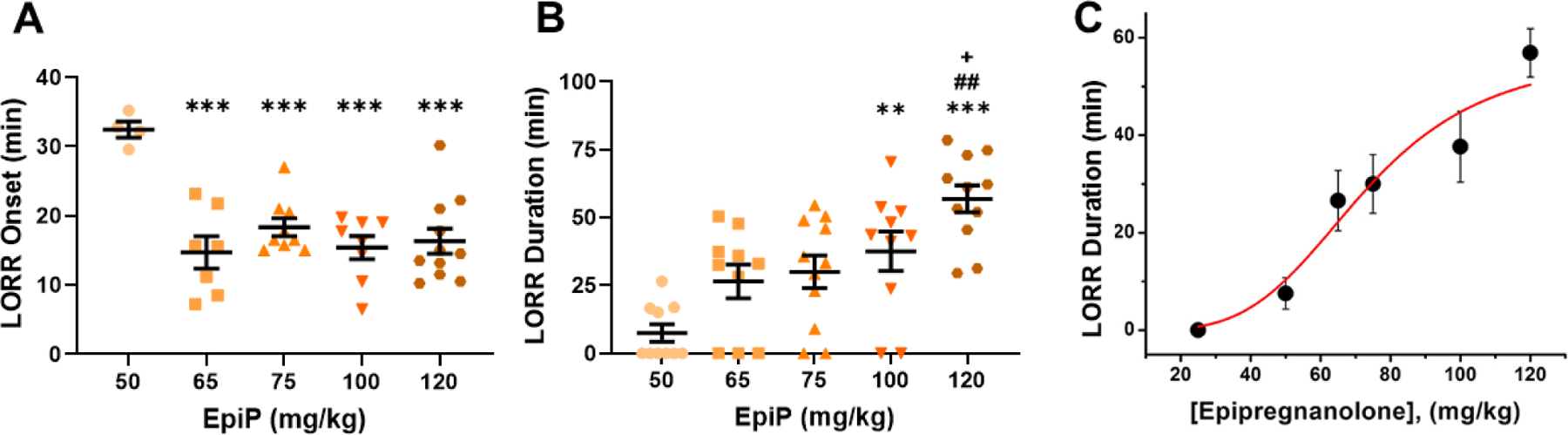
A. WT mice demonstrated a dose-dependent decrease in time to LORR onset with increasing EpiP dose (one way ANOVA F(4,34) = 9.494, p < 0.001). This effect was prominently seen in doses higher than 50 mg/kg. Specifically, we found a significant decrease in time to LORR onset from 50 mg/kg to 65 mg/kg, 75 mg/kg, 100 mg/kg, and 120 mg/kg, (p <0.001, <0.001, <0.001, and <0.001, respectively). B. LORR duration exhibited a dose-dependent response to EpiP (one way ANOVA F(4,34) = 10.000, p < 0.001). Statistical analysis yielded between 50 mg/kg and 100 mg/kg as well as 120 mg/kg (p = 0.003, <0.0001); between 65 mg/kg and 120 mg/kg (p = 0.002); and finally 75 mg/kg and 120 mg/kg (p = 0.006). C. Average duration of LORR is plotted against the injected dose of EpiP (same data as panel B of this figure). Solid red line is best fit of the data points using Hill-Langmuir equation yielding estimated ED50 of 72.53 ± 4.00 mg/kg and slope n of 4.05 ± 0.98. Fit was constrained to the maximal duration of 56.91 minutes achieved with dose of 120 mg/kg. *vs 50 mg/kg EpiP, #vs 65 mg/kg EpiP, +vs 75 mg/kg EpiP
EEG Data Acquisition and Spectral Analysis
Synchronized, time locked video and EEG signals were recorded using the Pinnacle system (Pinnacle Technology Inc., Lawrence, KS, USA). The EEG signals were amplified (100×) and digitized at a sampling frequency rate of 2000 Hz (high pass filter 0.5 Hz and low pass filter 500 Hz) and stored on a hard disk for offline analysis. The electrodes (one depth coated tungsten in CeM [anteroposterior – AP: −1.35 mm, mediolateral – MD: 0 and dorsoventral – DV: −3.6 mm], and two screw-type cortical [AP: −1 mm, MD: ± 3mm, DV: 0]) were implemented under continuous 2.5 vol% ISO anesthesia. Banamine® – Merck (intraperitoneal (i.p.) 2.5 mg/kg) was applied right after surgery and every 24 h for 48 h. Seven to ten days after surgery WT male animals (n = 11) were put in recording chamber ((H × W × L) 15.2 × 16.5 × 31.1 cm) and EEG were recorded 30 min before (baseline recordings) and 60 min after i.p. injection of neurosteroid. To compare spectra, 5 min of signal in baseline and during 100 mg/kg i.p. of neurosteroid were analyzed. All spectral analysis was carried out using the LabChart 8 and Origin 2018 software. The relative (%) power in following frequency ranges was calculated: δ (0.5–4 Hz), θ (4–8 Hz), α (8–13 Hz), β (13–30 Hz) and low γ (30–50 Hz). After completion of experiments, mice were anesthetized with ketamine (100 mg/kg i.p.) and electrolytic lesions were made by passing 5 μA current for 1 s (5 times). Mice were anesthetized additionally with ISO and perfused with ice-cold 0.1M phosphate buffer containing 1% of potassium-ferrocyanide. The brains were extracted, kept in 4% formalin (PFA) for 2 days and sliced (100–150 μm) using a vibrating micro slicer (Laica VT 1200 S). Photos of coronal slices with electrode location conformation were obtained using bright-field Zeiss stereoscope and Zen Blue software.
Assessment of hypnotic behavior in mice
We measured hypnosis by assessing the loss of righting reflex (LORR). After movement of mice to experimental table we waited 30 minutes to establish baseline habituation. Experimental mice received i.p. injections of EpiP and were then kept in a separate cage for 15 minutes prior to being transferred to a clear plastic cage. This cage was intermittently gently tilted to roll a given mouse onto its back. Loss of righting reflex was measured as inability to right self within 30 seconds. We also measured Gain of Righting Reflex (GORR) to assess duration of the loss of righting reflex. For some experiments WT mice injected with saline as control were placed in anesthetic chambers equilibrated at 0.5% atm of ISO. Consequently, ISO was increased by 0.1% atm every 10 minutes and the concentration at which LORR occurred was noted. Next, mice were injected with 25 mg/kg EpiP and then placed in the anesthetic chamber to be subjected to the same ISO conditions as control.
Assessment of immobility
We measured the sensitivity to mechanical stimulation by assessing the loss of withdrawal response (LOWR). For LOWR, an alligator clip covered with airway tubing was used on the proximal 1/3 tail and LOWR was considered when there was no withdrawal for a minimum of 30-seconds. After movement of mice to experimental table we waited 30 minutes to establish baseline habituation. Experimental mice received i.p. injections of EpiP and were then kept in a separate cage for 15 minutes. During this time a clear plastic anesthetic chamber was equilibrated to desired concentration of ISO at 0.5% or 0.9 % atm, or SEVO at 1.0 % atm in ~97% oxygen. After 15 minutes, injected mice were transferred to the anesthetic chamber. Chamber gas level was constantly measured via a Datex Ohmeda Capnomac Ultima. Every 10 min, ISO or SEVO was manually increased by 0.1% atm. Loss of withdrawal reflex was measured as lack of tail withdrawal response to the tail clamp within 30 seconds. After each trial, rectal temperature was recorded.
Data Analysis
Statistical analysis was performed using one-way and two-way repeated measure (RM) ANOVA as well as Student unpaired and paired two-tailed t-test where appropriate. Where interaction between factors after one way or two-way RM ANOVA was significant, Sidak’s or Bonferroni’s multiple comparisons test were used. Significance was accepted with p values < 0.05. Statistical and graphical analysis was performed using GraphPad Prism 8.00 software (GraphPad Software, La Jolla, CA, USA) and Origin 2018 (OriginLab, Northampton, MA, USA). LFPs were analyzed using LabChart 8 (AD Instruments, Dunedin, New Zealand).
In order to generate dose-response curve for LORR, male mice were injected intra-peritoneally (i.p.) with 25, 50, 65, 75, 100 and 120 mg/kg 3β-OH of EpiP and duration of LORR and percentage of mice with LORR was determined. The ED50 was calculated using the Hill-Langmuir equation as follows: PE ([EpiP]) = PEmax / (1+(ED50 / [EpiP])h), where PEmax is the maximal percent of animals with LORR or maximal duration of LORR, ED50 is the dose that produces 50% effect, and h is the apparent Hill-Langmuir coefficient that defines the slope of the curve. The fitted values are reported with > 95% linear confidence limits.
RESULTS
EpiP is an effective hypnotic on its own and also significantly lowers the concentration of common volatile GAs needed to induce hypnosis and immobility in the WT mice
We began by seeking to characterize the sedative/hypnotic properties of EpiP (EpiP). To accomplish this, we injected escalating doses of EpiP intra-peritoneally (i.p.) to WT mice and assessed their LORR. At a dose of 50 mg/kg, we found that the onset of LORR was about 30 minutes, while at doses higher than 50 mg/kg, time to onset of hypnosis became progressively faster and remained stable at about 15 minutes (Figure 1A). In contrast, LORR duration increased gradually from 10 to 60 minutes with escalating doses without any apparent saturation of effect (Figure 1B). The effective dose at which 50% of mice lost righting reflex (ED50) was estimated to be about 72 mg/kg for the duration of LORR (Figure 1C). During hypnosis induced by all of the doses of EpiP tested, mice displayed withdrawal response to tail clamp (data not shown). Over all behavioral experiments conducted in this study only two WT mice died - one after receiving 65 mg/kg EpiP, the other after administration of 100 mg/kg EpiP.
We next investigated how i.p. injections of sub-hypnotic dose of EpiP at 25 mg/kg may affect the requirement of a prototypical volatile anesthetic ISO necessary to cause hypnosis in WT mice (Figure 2A). We found that WT mice exposed to a sub-hypnotic dose of EpiP had LORR at a significantly lower concentration of ISO than those that received vehicle injection by about 30 percent (Figure 2A, treatment effect p < 0.0001). Experimental chambers were kept warm with heating pads. During control experiments with vehicle the mean recorded temperature of the mice was 35.3°C while the mean SpO2 was 95%. During the experimental trial with EpiP, the average temperature and SpO2 was 35.4°C and 95.7%, respectively.
Figure 2 -. EpiP significantly lowers ISO concentration necessary to induce hypnosis and lowers ISO and SEVO anesthetic concentration in WT mice.
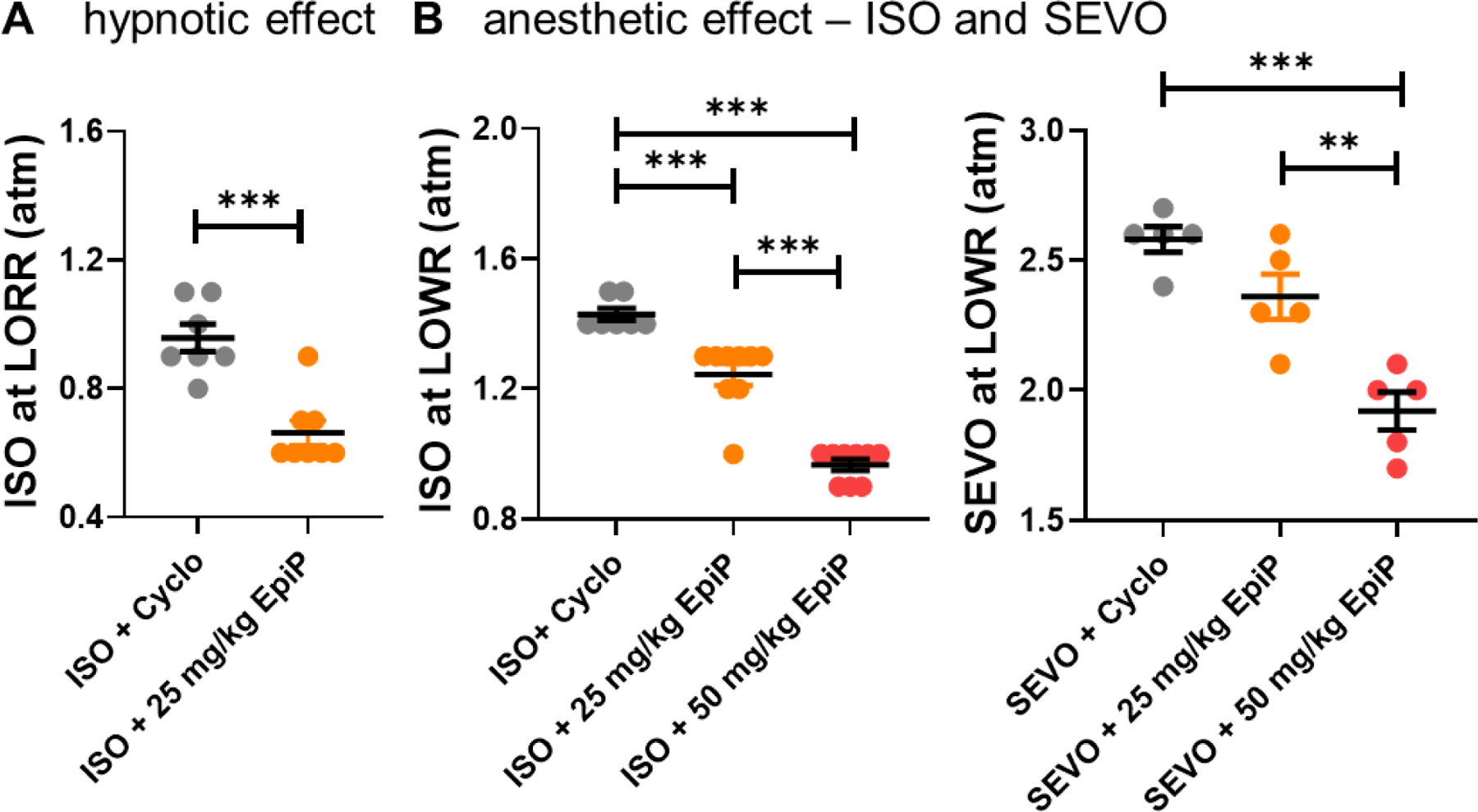
A. A low dose of EpiP yielded a significant decrease in concentration of ISO necessary to induce LORR (unpaired two-tailed t-test t(13) = 5.20, p < 0.001). Animals given a sub-hypnotic dose of 25 mg/kg i.p injection of EpiP required 0.295 atm (95% CI +/− 0.058) less ISO than animals given vehicle alone to cause LORR. B. EpiP lowered the anesthetic concentration of ISO necessary to immobilize WT mice and inhibit the LOWR in a dose-dependent manner - left (one way ANOVA F(2,22) = 82.43, p < 0.001, post hoc presented on Figure). At 25 mg/kg of EpiP lowered ISO requirements for LOWR by 0.185 atm. At the dose of 50 mg/kg, EpiP further lowered the ISO requirement by 0.462 atm. Similarly EpiP lowered the anesthetic concentration of SEVO necessary to immobilize WT mice and inhibit the LOWR in a dose-dependent manner - right (one way ANOVA F(2,12) = 22.00, p < 0.001, post hoc presented on Figure).
We next examined ability of EpiP in combination with ISO to inhibit withdrawal repose to tail clamp in WT mice as assessed with LOWR (a measure of full anesthetic effect). We found that EpiP significantly decreased the ISO concentration required to achieve LOWR in a dose-dependent manner up to 40% (Figure 2B left; treatment effect p < 0.0001). For example, when exposed to ISO alone, mice had LOWR at an average of about 1.4% atm. With the addition of 25 mg/kg EpiP, the same mice stopped withdrawing at an average of about 1.2% atm. When 50 mg/kg EpiP was given, the concentration of ISO necessary to induce LOWR further descended to an average of about 1.0% atm. Recorded temperature and oxygen saturation vitals for control, 25 mg/kg EpiP trial, and 50 mg/kg EpiP trial were as follows: 35.3°C, 96.7%; 35.6 °C, 98.1%; 35.1°C, 97%.
Similarly, we found that i.p. injections of EpiP at 25 mg/kg and 50 mg/kg lowered SEVO concentration required to achieve LOWR in dose-dependent manner up to 30% (Figure 2B right).
Characterisation of thalamocortical oscillations in vivo during systemic administration of EpiP in WT mice
Next, we sought to characterize thalamocortical oscillations in vivo using a sub-maximal dose of 100 mg/kg i.p. of EpiP, which effectively induced LORR in most of the WT mice. After stable baseline recording for 30 minutes, we saw a sustained increase in total power in the 60 minute period following EpiP injections in all frequencies in comparison to vehicle injection (Figure 3A–E). Note that although we saw small but significant differences between vehicle and EpiP experiment in baseline’s total power in α and β frequency range (Figure 3C and D), analysis of baseline’s relative power did not show differences between two cohorts (data not shown). Representative heat maps from baseline and 30 minutes after EpiP are shown on Figure 4A. Note that 15 min after injections of EpiP (mean value for time to LORR) animals showed an increase in total power of all frequency bands except the γ frequency range (Figure 4B left) without differences in relative power (Figure 4B right). Additionally, 30 min after neurosteroid injection the increase in total power in the EpiP group was still present (Figure 4C, left), with an increase in the δ and β relative power and a decrease in the θ and γ relative frequency range (Figure 4C, right). At time point of 60 min after EpiP injection the increase in total power was still present (Figure 4D left) with the relative differences in δ (increased from baseline) and γ (decreased from baseline) oscillations (Figure 4D right). Statistical analysis of relative power at the time points of 15, 30 and 60 minutes after EpiP injection revealed statistically significant decrease in γ frequency range 60 minutes after EpiP injection in comparison to 15 min time point (data not shown).
Figure 3. Time course of total EEG power change after vehicle or EpiP injections.
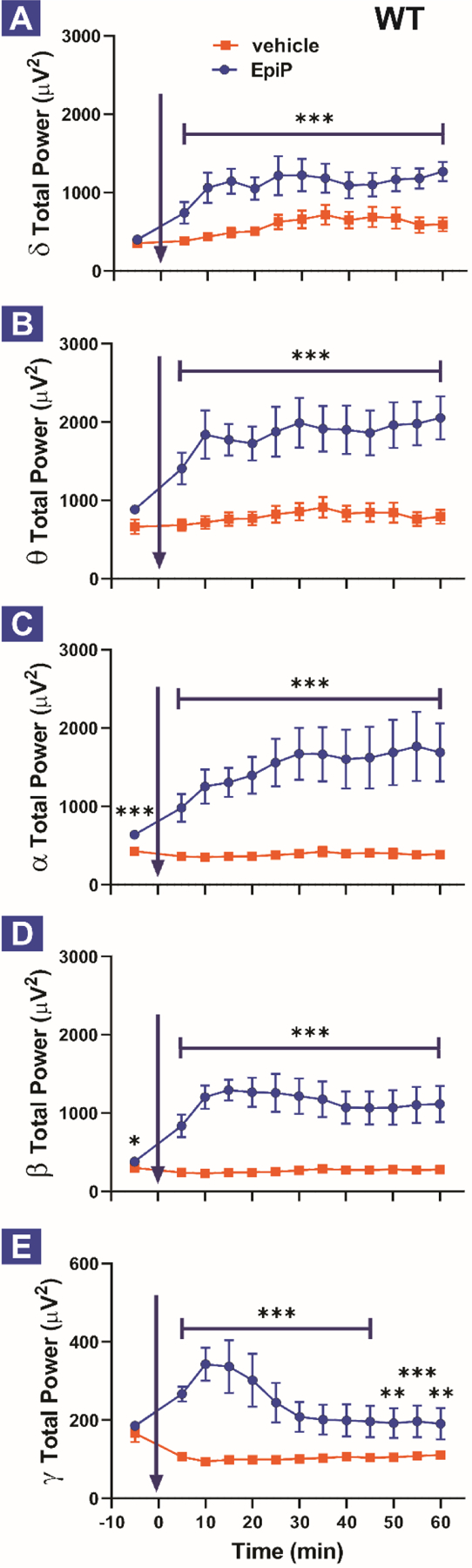
Total δ (A), θ (B), α (C), β (D) and γ (E) power during baseline recordings and 60 min after vehicle or neurosteroid i.p. injection (in 5-min bins). A. EpiP-injected animals had more absolute power in δ frequency range (0.5–4 Hz) in comparison to control (vehicle) (two way RM ANOVA, Interaction F(11,110) = 0.592, p = 0.832; EpiP F(1,10) = 26.47, p < 0.001, Time F(11,110) = 2.459, p = 0.009). B. EpiP-injected animals had more absolute power in θ frequency range (4–8 Hz) (two way RM ANOVA, Interaction F(11,110) = 0.621, p = 0.808; EpiP F(1,10) = 38.57, p < 0.001, Time F(11,110) = 1.413, p = 0.177). C. EpiP-injected animals had more absolute power in α frequency range (8–13 Hz) (two way RM ANOVA, Interaction F(11,110) = 1.130, p = 0.345; EpiP F(1,10) = 21.54, p < 0.001, Time F(11,110) = 1.492, p = 0.145). Note that baseline power in α frequency range was higher during baseline in EpiP experiment (paired t-test t(10) = 5.989, p < 0.001). D. EpiP-injected animals had more absolute power in β frequency range (13–30 Hz) (two way RM ANOVA, Interaction F(11,110) = 1.113, p = 0.358; EpiP F(1,10) = 31.48, p < 0.001, Time F(11,110) = 0.984, p = 0.465). Note that baseline power in β frequency range was higher during baseline in EpiP experiment (paired t-test t(10) = 2.326, p = 0.042). E. EpiP-injected animals had more absolute power in γ frequency range (30–50 Hz) in comparison to control (two way RM ANOVA, Interaction F(11,110) = 8.414, p < 0.001; EpiP F(1,10) = 14.05, p = 0.004, Time F(11,110) = 5.716, p < 0.001, post hoc presented on Figure). Analysis of recordings from 11 animals, the same animals were injected with vehicle on Day 1 and 24 hours later (Day 2) they were injected with EpiP 100 mg/kg.
Figure 4. Total and relative EEG power during baseline recordings, 15, 30 and 60 min after neurosteroid injections.
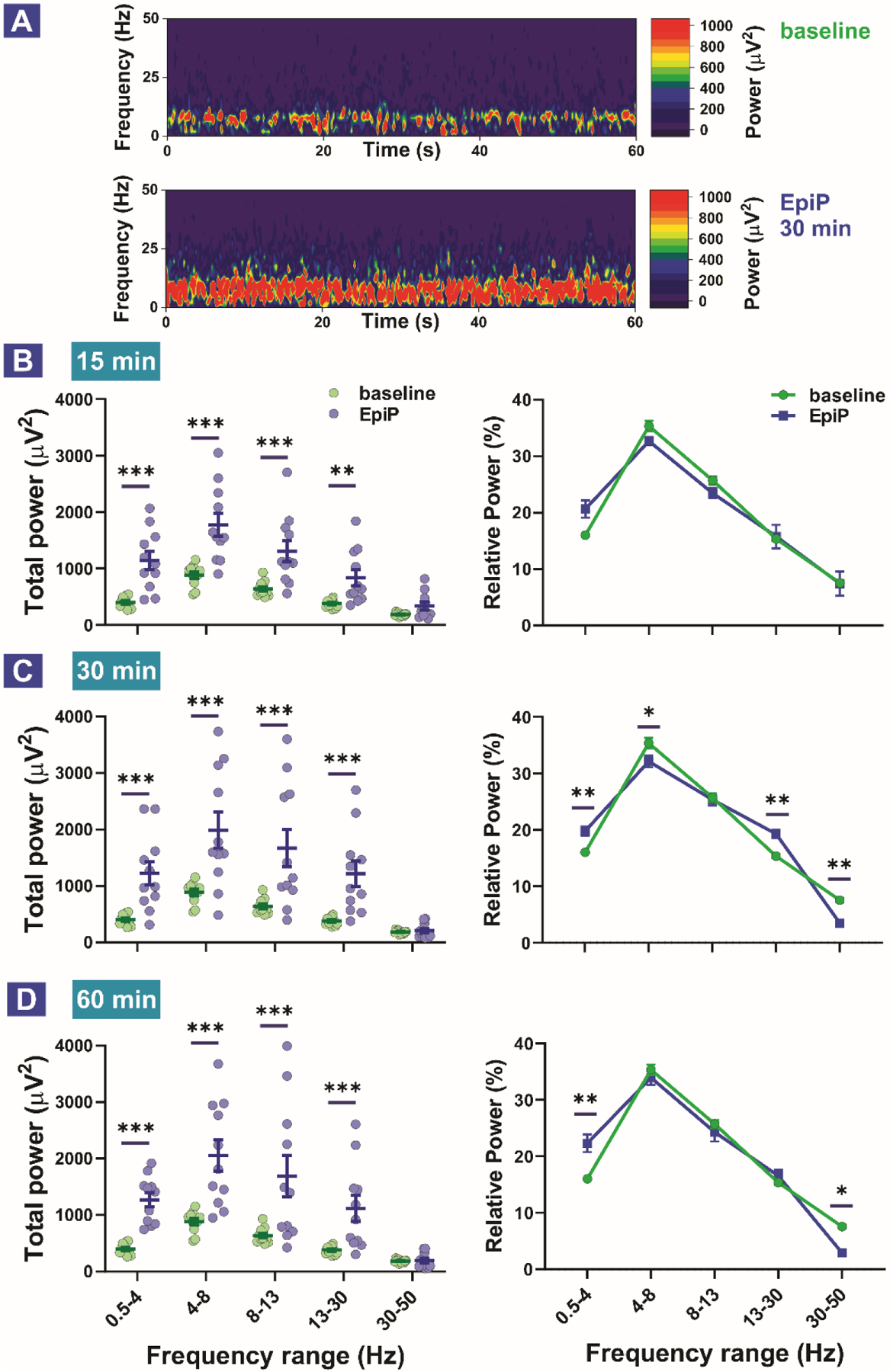
A. Representative heat maps during baseline recordings and 30 minutes after EpiP injection. B. Total (left) and relative (right) power 15 min after i.p. injection of the neurosteroid. Analysis of total power revealed an increase in the δ, θ, α and β frequency ranges (two way RM ANOVA, Interaction F(4,40) = 6.517, p < 0.001; EpiP F(1,10) = 29.180, p < 0.001, Frequency F(4,40) = 42.24, p < 0.001; post-hoc presented on Figure). Analysis of relative power revealed no statistically significant change between baseline and EpiP (two way RM ANOVA, Interaction F(4,40) = 2.569, p = 0.05; EpiP F(1,10) = 0.639, p = 0.442, Frequency F(4,40) = 102.7, p < 0.001; post-hoc revealed trend in δ frequency range (p = 0.07)). C. Total (left) and relative (right) power 30 min after i.p. injection of the neurosteroid. Analysis of total power revealed an increase in the δ, θ, α and β frequency ranges (two way RM ANOVA, Interaction F(4,40) = 11.85, p < 0.001; EpiP F(1,10) = 13.95, p = 0.004, Frequency F(4,40) = 44.24, p < 0.001; post-hoc presented on Figure). Analysis of relative power revealed rise in δ and β and drop in θ and γ relative power (two way RM ANOVA, Interaction F(4,40) = 12.94, p < 0.001; EpiP F(1,40) = 2.386, p = 0.153, Frequency F(4,40) = 295.1, p < 0.001; post-hoc presented on Figure). D. Total (left) and relative (right) power 60 min after i.p. injection of the neurosteroid. Analysis of total power revealed an increase in the δ, θ, α and β frequency ranges (two way RM ANOVA, Interaction F(4,40) = 10.85, p < 0.001; EpiP F(1,10) = 16.11, p = 0.002, Frequency F(4,40) = 43.53, p < 0.001; post-hoc presented on Figure). Analysis of relative power revealed rise in δ and drop γ relative power (two way RM ANOVA, Interaction F(4,40) = 6.44, p < 0.001; EpiP F(1,40) = 0.563, p = 0.47, Frequency F(4,40) = 204.3, p < 0.001; post-hoc presented on Figure). Analysis of recordings was averaged from 11 animals. *p < 0.05, **p < 0.01, ***p < 0.001
Comparison of sensitivity of different mouse mutants to EpiP-induced hypnosis
To study the potential roles of 3 different T-channel isoforms in EpiP-induced hypnosis, we compared the sensitivities of different KO mice and WT mice to a range of escalating doses of EpiP, measured as fraction of animals that underwent LORR. Figure 5 summarizes these results and indicates that when compared to the WT mice (ED50 about 54 mg/kg), the dose-response curve for CaV3.1 KO mice is shifted to the right (ED50 about 67 mg/kg) indicating resistance to neurosteroid hypnosis. In contrast, the dose-response curves for CaV3.2 KO mice and CaV3.3 KO mice only slightly deviated from the curve for WT mice.
Figure 5 -. Different sensitivity of T-channel isoform-specific knockout mice to EpiP-induced hypnosis.
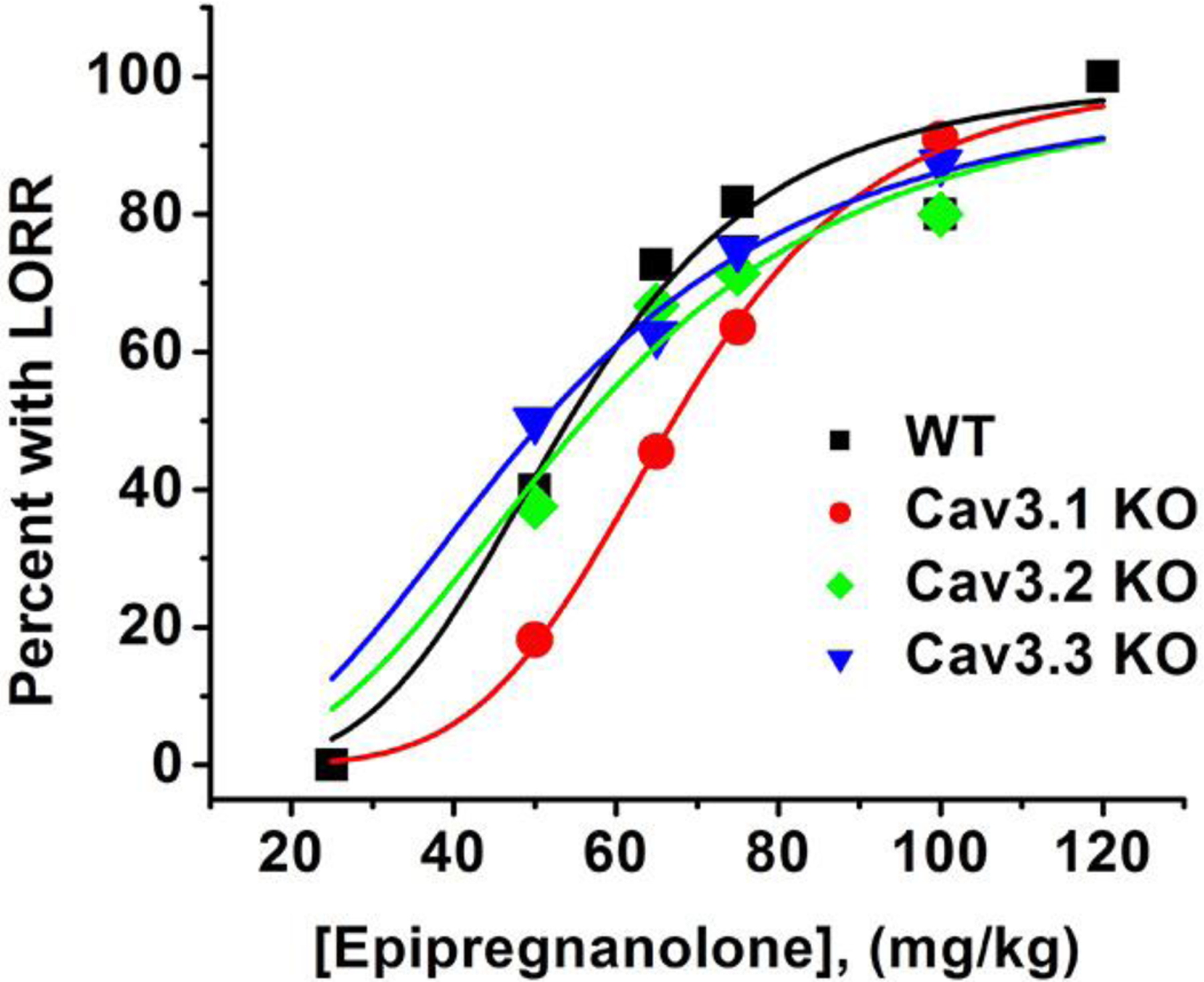
This graph shows best fit (solid lines) of dose-response curves to escalating doses of EpiP to percent of mice with LORR using Hill-Langmuir equation. All fits are constrained to 100% maximal effect. Estimated ED50 values in mg/kg and slope of the curve n and (SEM) were as follows: WT mice ED50 54.1 ± 2.8, n 4.2 ± 0.9; CaV3.1 KO mice ED50 67.1 ± 0.5, n 5.3 ± 0.2; CaV3.2 KO mice ED50 56.1 ± 3.2, n 3.0 ± 0.7; CaV3.3 KO mice ED50 51.1 ± 1.8, n 2.7 ± 0.3. Total of mice included to generate the data was 11 for WT mice and CaV3.1 KO mice, and 8 for CaV3.2 KO mice and CaV3.3 KO mice.
Characterization of hypnotic effect of EpiP in CaV3.1 KO mice
We next performed further analysis of LORR experiments in WT and mutant mice, starting with the CaV3.1 KO mice. Our escalating injected doses (in mg/kg) included in all mutants were 50, 65, 75 and 100. Interestingly, we did not find a dose-dependent effect of EpiP on LORR onset which remained at about 20 minutes (Figure 6A). However, we established that EpiP did indeed induce a dose-dependent duration of hypnosis in CaV3.1 KO mice with LORR lasting to about 40 minutes with the highest dose (Figure 6B). We next compared CaV3.1 KO and WT mice across all doses. Hypnotic induction as determined by LORR onset was not significantly different between WT and CaV3.1 KO mice (Figure 6C), but at the middle doses 65 mg/kg and 75 mg/kg i.p. CaV3.1 KO mice exhibited shorter LORR duration by about 3-fold when compared to WT mice (Figure 6D). Overall, we found that the CaV3.1 KO mice manifested significantly decreased duration, but minimal change in induction of hypnotic behavior when compared to the WT mice.
Figure 6 -. Knockout of the CaV3.1 channel confers resistance to the hypnotic effects of EpiP.
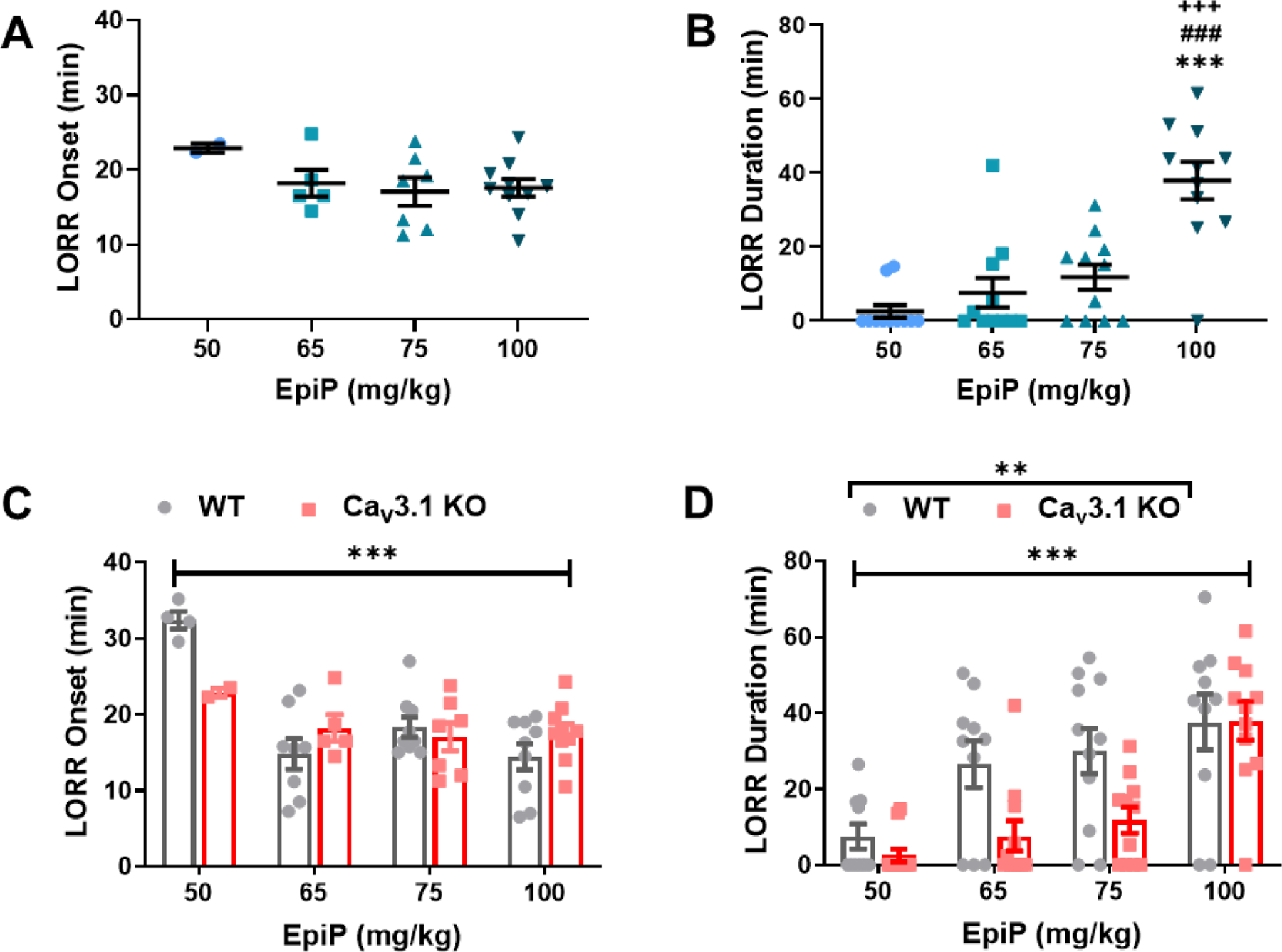
A. We found weak dose-dependent changes in LORR onset in CaV3.1 KO mice (one-way ANOVA F(3,20) = 1.116, p = 0.3661). B. EpiP caused a dose-dependent hypnosis in CaV3.1 KO mice (one-way ANOVA F(3,40) = 17.840, p < 0.001). Bonferroni’s multiple comparison test shows that there was a statistically significant difference between 50 mg/kg and 100 mg/kg (p < 0.001); 65 mg/kg and 100 mg/kg (p < 0.001); and 75 mg/kg and 100 mg/kg (p < 0.001). C. No difference in LORR onset between CaV3.1 KO and WT mice was measured (two-way ANOVA Interaction F(3,46) = 3.388, p = 0.026; Dose F(3,46) = 9.971, p < 0.001, Genotype F(1,46) = 0.612, p = 0.438). D. The CaV3.1 KO mice demonstrate an overall shorter LORR duration than WT mice (two-way ANOVA Interaction F(3,77) = 1.967, p = 0.126; Dose F(3,77) = 15.370, p < 0.001, Genotype F(1,77) = 9.263, p = 0.003). The WT data shown here is the same as those from Figure 1. *vs 50 mg/kg EpiP, #vs 65 mg/kg EpiP, +vs 75 mg/kg EpiP
Characterization of hypnotic effect of EpiP in CaV3.2 KO mice
In contrast to CaV3.1 KO mice, we found that CaV3.2 KO mice exhibited progressively faster induction from about 30 minutes with the dose of 50 mg/kg i.p. to about 15 minutes with the dose of 100 mg/kg of EpiP (Figure 7A). The CaV3.2 KO mice also exhibited dose-dependent increase in duration of LORR in response to i.p. injections of escalating doses of EpiP with a maximal effect of about 60 minutes (Figure 7B). Interestingly, at 65 mg/kg, we observed that WT mice experienced a significantly earlier time to LORR compared to CaV3.2 KO mice by about 40% (Figure 7C). In contrast, no significant difference in overall response was observed between CaV3.2 KO and WT mice in LORR duration across all doses tested (Figure 7D). Overall, we found that the CaV3.2 KO mice manifested significantly delayed induction at one moderate dose but no different duration of hypnosis than the WT mice across all doses.
Figure 7 -. EpiP exerts dose-dependent hypnosis in CaV3.2 KO mice that shows delayed induction but same duration when compared to WT mice.
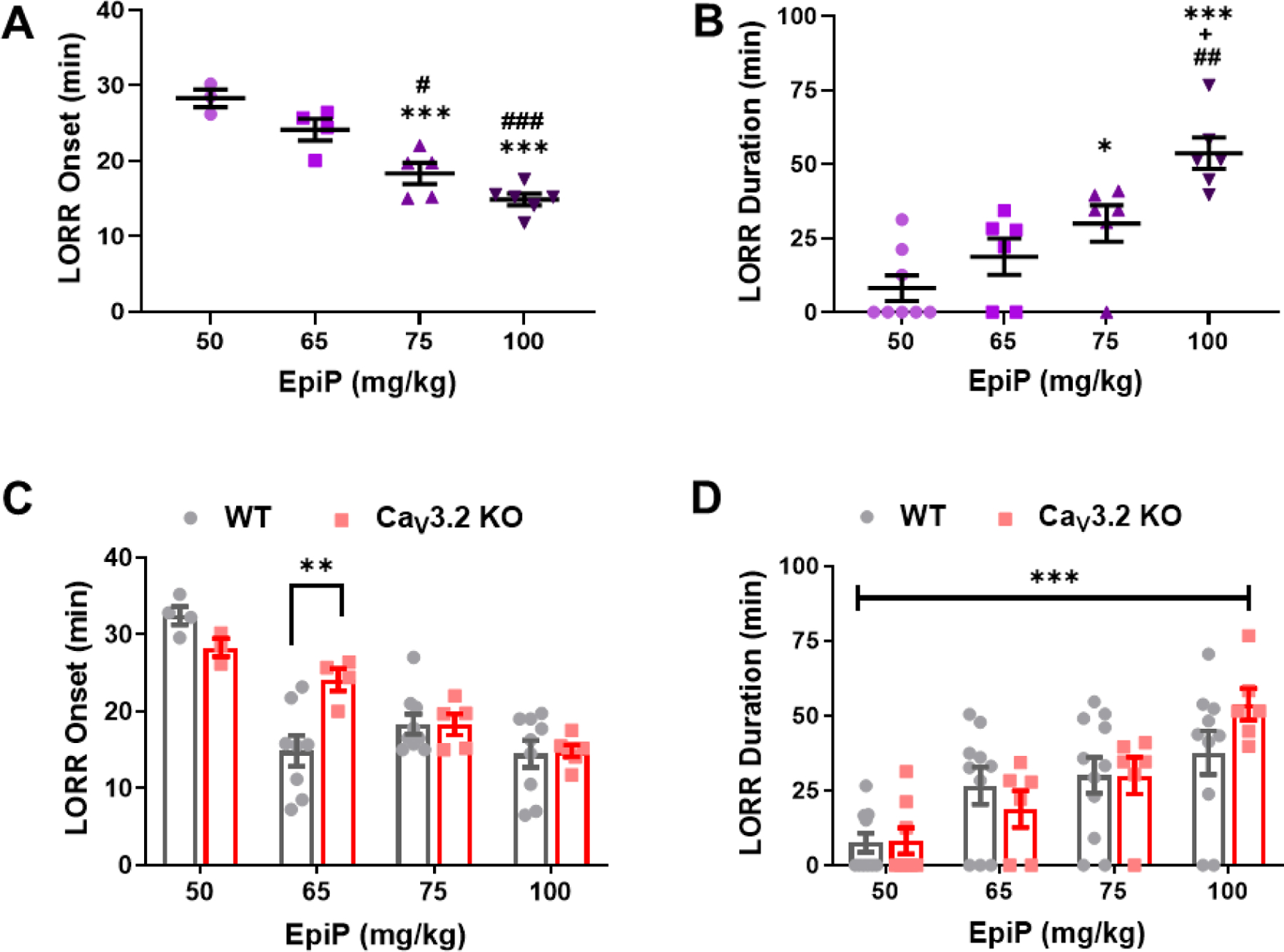
A. CaV3.2 KO mice exhibited dose-dependent onset of LORR changes in response to EpiP (one-way ANOVA F(3,14) = 23.380, p < 0.001). Multiple comparisons analysis indicates that there was a significant difference between 50 mg/kg and both 75 mg/kg and 100 mg/kg (p < 0.001, p < 0.001); as well as between 65 mg/kg and both 75 mg/kg and 100 mg/kg (p = 0.024, p < 0.001). B. EpiP generated a dose-dependent hypnosis in CaV3.2 KO mice (one-way ANOVA F(3,22) = 13.330, p < 0.001). Post-hoc analysis showed a statistically significant difference between 50 mg/kg and both 75 mg/kg (p = 0.046) and 100 mg/kg (p < 0.001); 65 mg/kg and 100 mg/kg (p = 0.0014); and finally between 75 mg/kg and 100 mg/kg (p = 0.040). C. No overall difference in LORR onset between CaV3.2 KO and WT mice (two-way ANOVA Interaction F(3,40) = 4.483, p = 0.008; Dose F(3,40) = 22.870, p < 0.001, Genotype F(1,40) = 1.131, p = 0.294). Post-hoc analysis with Bonferroni’s multiple comparisons demonstrated that WT mice had earlier onset of LORR at 65 mg/kg (p = 0.0028). D. LORR Duration in CaV3.2 KO male mice was not significantly different from WT mice (two-way ANOVA Interaction F(3,59) = 1.288, p = 0.287; Dose F(3,59) = 13.780, p < 0.001, Genotype F(1,59) = 0.258, p = 0.613). The WT data shown here is the same as those from Figure 1. *vs 50 mg/kg EpiP, #vs 65 mg/kg EpiP, +vs 75 mg/kg EpiP
Characterization of hypnotic effect of EpiP in CaV3.3 KO mice
Next, we asked if the CaV3.3 KO mice displayed a dose-dependent hypnotic response to EpiP, as with the previously tested genotypes (Figure 8A, B). In contrast to CaV3.1 KO mice, but similar to the CaV3.2 KO mice, we found that CaV3.3 KO mice exhibited progressively faster induction from about 30 minutes with the dose of 50 mg/kg i.p. to about 10 minutes with the dose of 100 mg/kg of EpiP (Figure 8A). The CaV3.3 KO mice also exhibited a dose-dependent increase in duration of LORR in response to i.p. injections of escalating doses of EpiP with a maximal effect of about 70 minutes (Figure 8B). However, in contrast to previously tested CaV3.1 and CaV3.2 KO mice, no significant genotype-dependent effects were seen in LORR onset or duration (Figure 8C, D). We conclude that CaV3.3 KO male mice do not manifest a differential hypnotic response to EpiP at a moderate dose compared to WT male mice. However, we noted that KO mice had a trend towards prolonged LORR of about 40% when compared to the WT at a higher dose of 100 mg/kg (Figure 8D).
Figure 8 -. EpiP induces dose-dependent hypnosis in CaV3.3 KO mice that is not significantly different from WT mice.
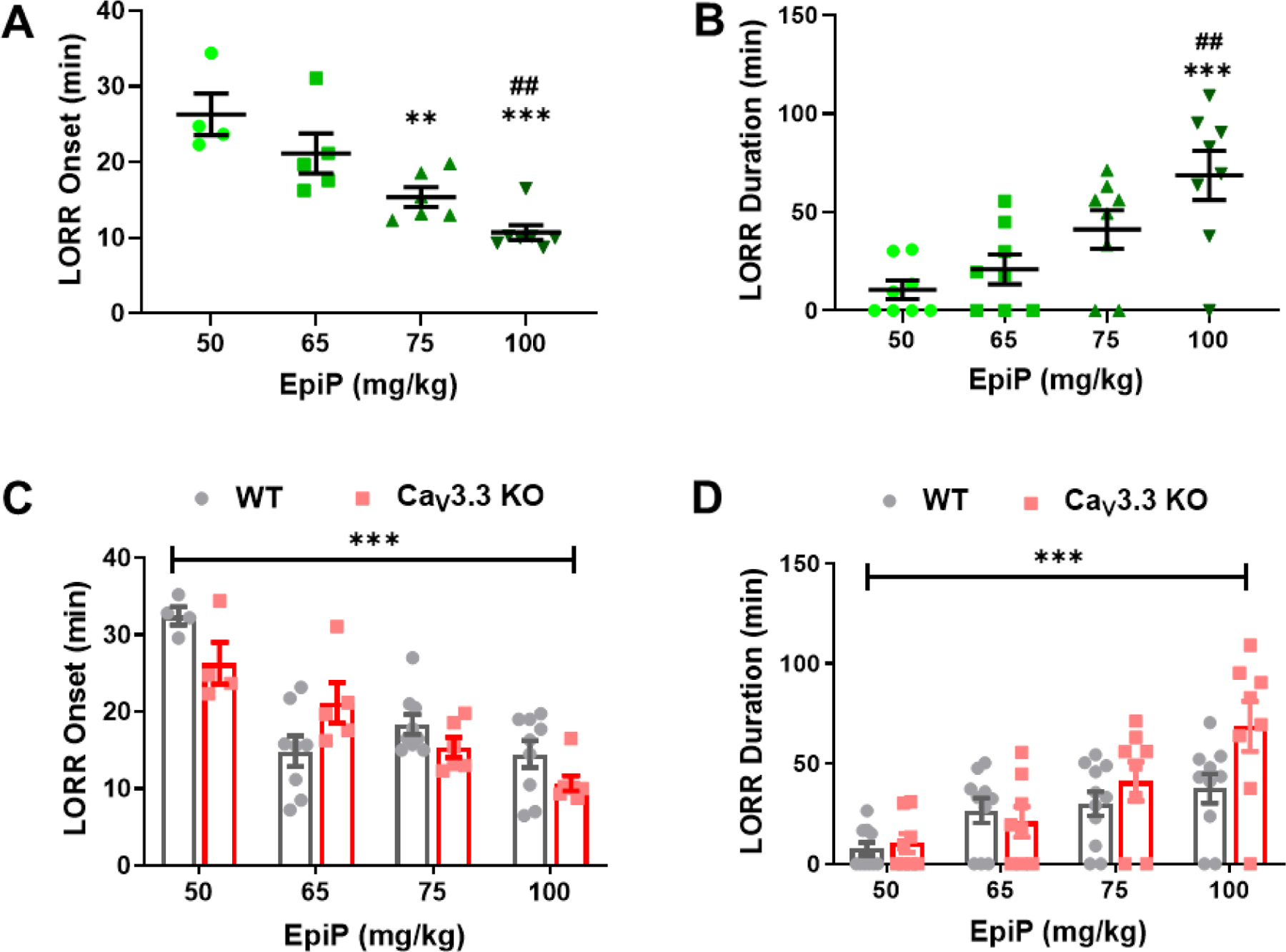
A. Increasing doses of EpiP caused a decreasing time to LORR in a dose-dependent fashion (one-way ANOVA F(3,18) = 13.580, p < 0.001). Post-hoc analysis illustrated significance between 50 mg/kg and both 75 mg/kg (p = 0.005) as well as 100 mg/kg (p < 0.001); and 65 mg/kg and 100 mg/kg (p = 0.003). B. CaV3.3 KO mice demonstrated a dose-dependent hypnosis duration in response to EpiP (one-way ANOVA F(3,28) = 7.906, p < 0.001). Bonferroni’s multiple comparisons specifically showed significant differences between 50 mg/kg and 100 mg/kg (p < 0.001); and between 65 mg/kg and 100 mg/kg (p = 0.006). C. The CaV3.3 KO mice exhibited no difference in LORR onset from WT mice (Interaction F(3,44) = 4.168, p = 0.011; Dose F(3,44) = 24.440, p < 0.001, Genotype F(1,44) = 1.556, p = 0.219). D. We did not find a significant difference in overall LORR duration between CaV3.3 KO and WT mice. (Interaction F(3,65) = 2.230, p = 0.093; Dose F(3,65) = 12.730, p < 0.001, Genotype F(1,65) = 3.616, p = 0.062). Despite the overall insignificant finding, there appears to be a trend indicating that CaV3.3 KO mice showed longer LORR duration than WT mice at the highest dose. The WT data shown here is the same as those from Figure 1. *vs 50 mg/kg EpiP, #vs 65 mg/kg EpiP, +vs 75 mg/kg EpiP
Differences in thalamocortical oscillations in vivo during systemic administration of EpiP in WT and CaV3.1 KO mice
Our data with LORR indicate that among all 3 mutant mice of different isoforms of T-channels, only CaV3.1 KO mice showed resistance to EpiP-induced hypnosis. Hence, we interrogated effects of i.p. injections of EpiP at 100 mg/kg on thalamocortical oscillations in CaV3.1 KO mice and compared results to the WT cohort at time points of 15 minutes (Figure 9A), 30 minutes (Figure 9B), and 60 minutes (Figure 9C) following injections. Left panels on Figure 9 represent total power of different frequency bands for WT and KO groups, while right panels depict normalized differences of two cohorts from pre-injection baselines at the same time points. We noticed that at all three time points, mutant mice had significantly decreased total power in δ, θ, and α bands up to 4-fold when compared to the WT mice. Furthermore, we found decreased in baseline- normalized δ oscillations in mutant mice at the time points of 15 minutes (Figure 9A, right panel) and 60 minutes (Figure 9C, right panel), as well as increased in β oscillations in mutant mice when compared to WT group after 15 minutes post injections (Figure 9A, right panel). We conclude that mutant mice have different EEG signature when compared to WT group during administration of hypnotic dose of EpiP.
Figure 9. Total and relative EEG power differences between WT and CaV3.1 KO animals 15, 30 and 60 min after neurosteroid injections.
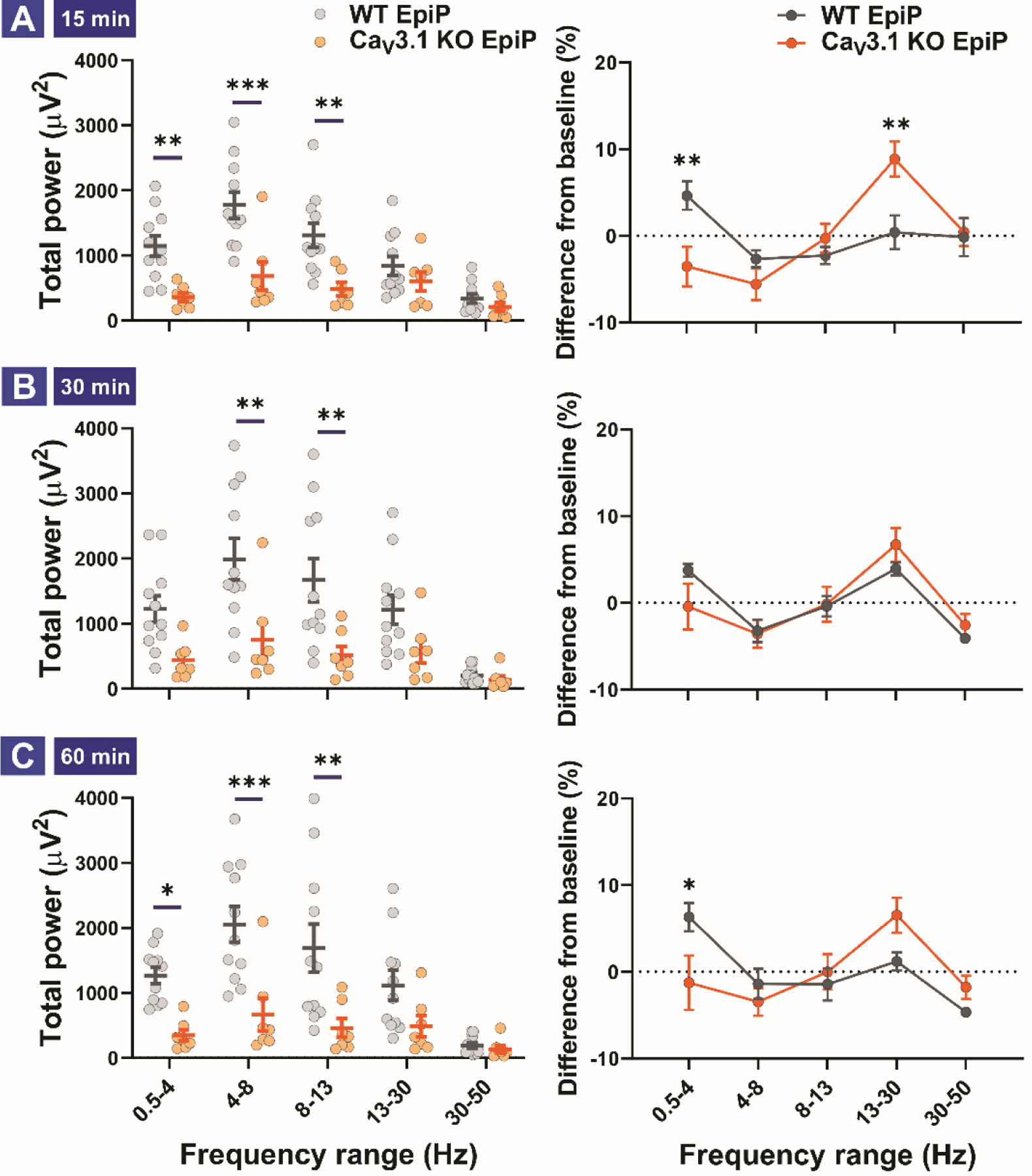
A. Total (left) and relative (right) power 15 min after i.p. injection of the neurosteroid. Analysis of total power revealed an increase in the δ, θ and α frequency ranges in WT animals in comparison to mutant mice (two way RM ANOVA, Interaction F(4,64) = 6.78, p < 0.001; Frequency F(4,64) = 19.09, p < 0.001, Genotype F(1,16) = 11.96, p = 0.003; post-hoc presented on Figure). Analysis of difference from baseline (relative power) revealed δ rise in WT and β rise in mutant animals (two way RM ANOVA, Interaction F(4,64) = 4.652, p = 0.002; Frequency F(4,64) = 4.976, p = 0.001, Genotype F(1,16) = 1.025, p = 0.326; post-hoc presented on Figure). B. Total (left) and relative (right) power 30 min after i.p. injection of the neurosteroid. Analysis of total power revealed an increase in the θ and α frequency ranges (two way RM ANOVA, Interaction F(4,64) = 6.86, p < 0.001; Frequency F(4,64) = 24.76, p < 0.001, Genotype F(1,16) = 6.807, p = 0.019; post-hoc presented on Figure). Analysis of differences from baseline (relative power) revealed no change between baseline and EpiP. C. Total (left) and relative (right) power 60 min after i.p. injection of the neurosteroid. Analysis of total power revealed an increase in the δ, θ and α frequency ranges in WT animals (two way RM ANOVA, Interaction F(4,64) = 7.781, p < 0.001; Frequency F(4,64) = 22.04, p < 0.001, Genotype F(1,16) = 9.197, p = 0.008; post-hoc presented on Figure). Analysis of difference from baseline (relative power) revealed rise in δ on WT animals (two way RM ANOVA, Interaction F(4,64) = 3.30, p = 0.016; Frequency F(4,64) = 5.020, p = 0.001, Genotype F(1,16) = 0.108, p = 0.747; post-hoc presented on Figure). Analysis of recordings was averaged from 11 (WT) and 7 (CaV3.1 KO) animals. *p < 0.05, **p < 0.01, ***p < 0.001
DISCUSSION
It is well known that the neuroactive steroids are potent modulators of neuronal ion channels in the central nervous system and consequently induce hypnosis, analgesia, cognitive and anti-depressant effects (reviewed in (Jevtovic-Todorovic et al., 2009; Zorumski et al., 2013)), with main focus being on direct modulation of GABAA receptors (Zorumski et al., 2013). However, we reported that EpiP potently inhibited CaV3.2 T-type currents and high-voltage-activated (HVA) subtypes of VGCCs in rat sensory neurons with a similar potency (Ayoola et al., 2014) (Joksimovic et al., 2019). Although we did not directly investigate the ability of EpiP to inhibit CaV3.1 and CaV3.3 currents, our data strongly suggest that EpiP is a non-selective blocker of different classes of VGCCs. Other reports also confirmed a lack of effect of EpiP on the native neuronal GABAA receptors in CNS (Poisbeau et al., 1997; Weir et al., 2004). However, another study using recombinant GABAA channels has reported that at higher concentrations EpiP and other 5β-reduced steroids may also act as noncompetitive blockers of GABAA receptors (Wang et al., 2002). Our current study strongly suggests that different isoforms of T-channels contribute to different endpoints of hypnosis induced by EpiP in mice. However, it is generally accepted that GAs and sedative agents affect multiple ion channels that mediate important clinical effects such as hypnosis (Chau, 2010; Hemmings et al., 2005; Rudolph and Antkowiak, 2004). Hence, while our study points to the importance of T-channels, we cannot exclude other potential molecular targets. According to this multi-site hypothesis, the importance of a particular molecular target depends on the specific anesthetic and the specific endpoint in consideration such as hypnosis, immobility and analgesia. However, sensitivity of other voltage-gated ion channels to EpiP is not currently known. Deciphering other potential targets that may work in concert with T-channels to underlay EpiP-induced hypnosis remains an important area of future investigations.
To our knowledge, this work is the first to characterize the hypnotic properties of EpiP in wild-type rodents, as well as in various T-channel isoform knockouts. We found that EpiP is an efficacious dose-dependent hypnotic when given alone and that it significantly lowers the required concentration of ISO required to induce hypnosis and immobility. Notably, at a very low dose of 25 mg/kg when administered alone, EpiP failed to induce hypnosis in any of the injected mice. However, when we injected EpiP before administration of ISO, it effectively lowered the requirement for ISO to induce LORR and LOWR. Consequently, we showed that a hypnotic dose of EpiP of 50 mg/kg even further decreased the required amount of ISO to induce LOWR in mice and similarly in SEVO experiments. This indicates that even low doses of EpiP can significantly alter necessary ISO and SEVO concentrations. Since LORR and LOWR are behavioral surrogate measurements for the clinical effects of GAs, these results indicate that EpiP may be useful in lowering required amounts of GAs used to induce hypnosis and surgical anesthesia, respectively. Furthermore, most known GAs can induce developmental neurotoxicity in different species including non-human primates (Jevtovic-Todorovic, 2016; Yu et al., 2020). Hence, future studies of mechanisms of hypnosis and neurodevelopmental effects of endogenous molecules like EpiP are warranted.
We observed a rise in total power in all EEG frequencies after i.p. injection of EpiP in WT mice. For slower frequency bands (δ, θ and α), as well as β oscillations, the rise in total power remained unchanged during all 60 minutes of recording. Detailed analysis of total power at the time point of 15 minutes after injections, which corresponds to the average LORR in WT animals (with 100 mg/kg), and 30 min after EpiP revealed increase in all bands except γ frequencies. More importantly, change in relative power 15 min after EpiP revealed a large rise in β and consequent drop in α frequency oscillations. The rise in relative power for β frequencies was still present 30 min after EpiP injection, as well as an additional rise in δ and drop in γ frequencies. Our results are in accordance with described EEG changes observed after administration of other known sedative/hypnotic drugs. For example, it has been described that benzodiazepine hypnotics alter EEG by reducing δ and increasing β activity (Feinberg et al., 2000). Additionally, GAs that potentiate GABAA receptors first induce sedation/hypnosis with characteristic rise in β oscillation followed by an increase in slow and δ oscillations which are dominant during deeper levels of hypnosis/anesthesia (Akeju and Brown, 2017). Consistent with this idea, we found that CaV3.1 KO mice showed resistance to hypnosis after injections of EpiP, as well as significant decrease in δ oscillations at 15 minutes and at 60 minutes time points when compared to the WT mice. Moreover, 30 min after EpiP we also observed a decrease in γ oscillations that could also contribute to its sedative/hypnotic effect, having in mind the role of high frequency oscillations in attention and memory processes (Jensen et al., 2007). These data are also in agreement with our recent study with 3β-OH which induced hypnosis in WT animals with a similar EEG signature (Timic Stamenic et al., 2021).
Differential responses to EpiP based on T-channel expression were also noted. For example, CaV3.1 KO mice showed overall resistance to hypnosis with a particularly prominent effect with a moderate dose of EpiP (65 mg/kg) as evidenced by about a 3-fold shorter duration of LORR when compared to the WT mice. In contrast to CaV3.1 KO mice, CaV3.2 KO and CaV3.3 KO mice did not show significantly different duration of LORR when compared to WT mice. Although CaV3.2 KO mice did not differ from the WT cohort in duration of hypnosis, it uniquely exhibited a delayed onset of LORR after a moderate dose of EpiP was injected. This is consistent with our recent data concerning male CaV3.2 KO mice that also showed delayed induction with that 3β-OH and no effect on LORR duration when compared to WT mice (Joksimovic et al., 2020).
Furthermore, we reported that male CaV3.1 KO mice showed resistance to hypnosis with 3β-OH when compared to WT mice (Timic Stamenic et al., 2021). The agreement between those data and the results of the current study suggests that 5β-reduced neurosteroids in general share a common pattern of interaction with the various T-channel isoforms when inducing hypnosis. Similarly, different T-channel KO mice also showed distinct hypnotic phenotypes to volatile GAs such as ISO. For example, it has been reported that induction with ISO is delayed in both CaV3.1 (Petrenko et al., 2007) and CaV3.2 (Orestes et al., 2009) mutants, while it is faster in CaV3.3 (Feseha et al., 2020) KO mice when compared to WT mice. We propose that the opposing effects of T-channel isoforms in anesthetic hypnosis may stems from the distinct functional roles and different localization of T-channel isoforms in the thalamocortical circuitry. Indeed, the nRT is enriched in CaV3.2 on cell somas and CaV3.3 on dendrites, while the VB and CeM thalamic nuclei almost exclusively express the CaV3.1 isoform (Talley et al., 1999) (Joksovic et al., 2005). In further support of this proposal, studies with KO mice suggest different roles of T-channel isoforms in the synchronization of the thalamocortical circuit during sleep and in absence seizures (Cheong and Shin, 2013; Pellegrini et al., 2016). Alternatively, it is possible that phenotypes may, at least in part be related to different homeostatic compensatory mechanisms that are described in the thalamocortical circuitry of global KO mice of different VGCCs (Lee et al., 2014; Llinás et al., 2007; Song et al., 2004; Timic Stamenic et al., 2019; Zhang et al., 2002).
In conclusion, this work is the first to report on the hypnotic properties of EpiP in rodents and the importance of neuronal T-channels in this effect. We speculate that the distinct hypnotic effects of EpiP across all T-channel isoforms are due to their differential expression in thalamocortical circuitry. This notion remains to be scrutinized in future experiments with isoform- and nucleus-specific conditional deletion of T-channels in the thalamus. We posit that endogenous neuroactive steroids that target neuronal T-channels may have an important role as novel hypnotics and/or adjuvants to anesthetic agents.
This study seeks to investigate hypnotic effects elicited by systemic administration of epipregnanolone [(3β,5β)-3-hydroxypregnan-20-one] (EpiP) and to characterize its use as an adjuvant agent to commonly used general anesthetics (GAs).
Consistent with increased oscillations in slower electroencephalogram (EEG) frequencies, EpiP induced an hypnotic state in wild type (WT0 mice when injected alone intra-peritoneally (i.p.) and effectively facilitated anesthetic effects of prototypical volatile agents such as isoflurane (ISO) and sevoflurane (SEVO).
Using mouse genetics we found that the CaV3.1 (Cacna1g) KO mice demonstrated decreased sensitivity to EpiP-induced hypnosis when compared to WT mice, whereas no significant difference was noted between CaV3.2 (Cacna1h), CaV3.3 (Cacna1i) and WT mice.
Furthermore, when compared to WT mice, onset of EpiP-induced hypnosis was delayed in CaV3.2 KO mice but not in CaV3.1 and CaV3.3 KO mice.
We conclude that EpiP may have an important role as novel hypnotic and/or adjuvant to volatile anesthetic agents and that distinct hypnotic effects of EpiP across all three T-channel isoforms may be due to their differential expression in thalamocortical circuitry.
Acknowledgements
This study was funded in part by grants from the National Institutes of Health (GRANT# R01GM102525 and R35GM141802 to S.M.T.) and funds from the Department of Anesthesiology and School of Medicine at Anschutz Medical Campus. We thank the University of Colorado Anschutz Medical Campus Rodent In Vivo Neurophysiology Core, which is partly supported by the NIH P30 NS048154 grant, for providing facilities to acquire and review video-EEG data. We thank Dr. Charles Adrian Handforth for donating breeding pairs of CaV3.1 knockout mice to our laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors received no compensation nor do they have any conflicting financial interests in regards to the work described in this manuscript.
References
- Akeju O, Brown EN, 2017. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr. Opin. Neurobiol 44, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G, 2008. Consciousness and anesthesia. Science 322, 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri N, Joksimovic SM, Oklopcic A, Milanovic D, Klawitter J, Eggan P, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V, 2018. A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br. J. Anaesth 120, 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola C, Hwang SM, Hong SJ, Rose KE, Boyd C, Bozic N, Park JY, Osuru HP, DiGruccio MR, Covey DF, Jevtovic-Todorovic V, Todorovic SM, 2014. Inhibition of CaV3.2 T-type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone. Psychopharmacology (Berl). 231, 3503–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, 2005. Neurosteroids : endogenous regulators of the GABAA receptor. Nat Rev Neurosci 6, 565–575. [DOI] [PubMed] [Google Scholar]
- Chau PL, 2010. New insights into the molecular mechanisms of general anaesthetics. Br. J. Pharmacol 161, 288–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, Ding K, Lo WHY, Qiang B, Chan P, Shen Y, Wu X, 2003. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann. Neurol 54, 239–243. [DOI] [PubMed] [Google Scholar]
- Cheong E, Shin HS, 2013. T-type Ca2+ channels in normal and abnormal brain functions. Physiol. Rev 93, 961–992. [DOI] [PubMed] [Google Scholar]
- Erickson RL, Blevins CE, De Souza Dyer C, Marx JO, 2019. Alfaxalone-xylazine anesthesia in laboratory mice (Mus musculus). J. Am. Assoc. Lab. Anim. Sci 58, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Maloney T, Campbell IG, 2000. Effects of hypnotics on the sleep EEG of healthy young adults: New data and psychopharmacologic implications. J. Psychiatr. Res 34, 423–438. [DOI] [PubMed] [Google Scholar]
- Feseha S, Timic Stamenic T, Wallace D, Tamag C, Yang L, Pan JQ, Todorovic SM, 2020. Global genetic deletion of CaV3.3 channels facilitates anaesthetic induction and enhances isoflurane-sparing effects of T-type calcium channel blockers. Sci. Rep 10, 21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal A, Uygun DS, Yang L, Mcnally JM, Lopez-huerta VG, Arias-garcia MA, Baeznieto D, Allen A, Fitzgerald M, Choi S, Zhang Q, Hope JM, Yan K, Mao X, Nicholson TB, Imaizumi K, Fu Z, Feng G, Brown RE, Strecker RE, Purcell SM, Pan JQ, 2020. Effects of a patient-derived de novo coding alteration of CACNA1I in mice connect a schizophrenia risk gene with sleep spindle de fi cits. Transl. Psychiatry 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL, 2005. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci 26, 503–510. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP, 2007. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, 2016. General Anesthetics and Neurotoxicity: How Much Do We Know? Anesthesiol. Clin 34, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Covey DF, Todorovic SM, 2009. Are neuroactive steroids promising therapeutic agents in the management of acute and chronic pain? Psychoneuroendocrinology 34, S178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic SL, Donald RR, Park J-Y, Todorovic SM, 2019. Inhibition of multiple voltage-gated calcium channels may contribute to spinally mediated analgesia by epipregnanolone in a rat model of surgical paw incision. Channels (Austin). 13, 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic SL, Joksimovic SM, Manzella FM, Asnake B, Orestes P, Raol YH, Krishnan K, Covey DF, Jevtovic-Todorovic V, Todorovic SM, 2020. Novel neuroactive steroid with hypnotic and T-type calcium channel blocking properties exerts effective analgesia in a rodent model of post-surgical pain. Br. J. Pharmacol 177, 1735–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksovic PM, Bayliss DA, Todorovic SM, 2005. Different kinetic properties of two T-type Ca2+ currents of rat reticular thalamic neurones and their modulation by enflurane. J. Physiol 566, 125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksovic PM, Covey DF, Todorovic SM, 2007. Inhibition of T-type calcium current in the reticular thalamic nucleus by a novel neuroactive steroid. Ann. N. Y. Acad. Sci 1122, 83–94. [DOI] [PubMed] [Google Scholar]
- Lee SE, Lee J, Latchoumane C, Lee B, Oh SJ, Saud ZA, Park C, Sun N, Cheong E, Chen CC, Choi EJ, Lee CJ, Shin HS, 2014. Rebound burst firing in the reticular thalamus is not essential for pharmacological absence seizures in mice. Proc. Natl. Acad. Sci. U. S. A 111, 11828–11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sjövall J, Griffiths WJ, 2003. Neurosteroids in Rat Brain: Extraction, Isolation, and Analysis by Nanoscale Liquid Chromatography-Electrospray Mass Spectrometry. Anal. Chem 75, 5835–5846. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Choi S, Urbano FJ, Shin HS, 2007. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc. Natl. Acad. Sci. U. S. A 104, 17819–17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour G. a, Alkire MT, 2013. Consciousness, anesthesia, and the thalamocortical system. Anesthesiology 118, 13–15. [DOI] [PubMed] [Google Scholar]
- Orestes P, Bojadzic D, Chow RM, Todorovic SM, 2009. Mechanisms and functional significance of inhibition of neuronal T-type calcium channels by isoflurane. Mol. Pharmacol 75, 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini C, Lecci S, Lüthi A, Astori S, 2016. Suppression of Sleep Spindle Rhythmogenesis in Mice with Deletion of CaV3.2 and CaV3.3 T-type Ca2+ Channels. Sleep 39, 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko AB, Tsujita M, Kohno T, Sakimura K, Baba H, 2007. Mutation of alpha1G T-type Calcium Channels in Mice Does Not Change Anesthetic Requirements for Loss of the Righting Reflex and Minimum Alveolar Concentration but Delays the Onset of Anesthetic Induction. Anesthesiology 106, 1177–1185. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R, 1997. Modulation of GABA(A) receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypophyseal coculture model. J. Physiol 500, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B, 2004. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci 5, 709–720. [DOI] [PubMed] [Google Scholar]
- Song I, Kim D, Choi S, Sun M, Kim Y, Shin HS, 2004. Role of the 1G T-Type Calcium Channel in Spontaneous Absence Seizures in Mutant Mice. J. Neurosci 24, 5249–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DDA, 1999. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci 19, 1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timic Stamenic T, Feseha S, Manzella FM, Wallace D, Wilkey D, Corrigan T, Fiedler H, Doerr P, Krishnan K, Raol YH, Covey DF, Jevtovic-Todorovic V, Todorovic SM, 2021. The T-type calcium channel isoform Cav3.1 is a target for the hypnotic effect of the anaesthetic neurosteroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile. Br. J. Anaesth 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timic Stamenic T, Feseha S, Valdez R, Zhao W, Klawitter J, Todorovic SM, 2019. Alterations in Oscillatory Behavior of Central Medial Thalamic Neurons Demonstrate a Key Role of CaV3.1 Isoform of T-Channels During Isoflurane-Induced Anesthesia. Cereb. Cortex 29, 4679–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng C-M, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S, 2002. 3β-Hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J. Neurosci 22, 3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir CJ, Ling ATY, Belelli D, Wildsmith JAW, Peters JA, Lambert JJ, 2004. The interaction of anaesthetic steroids with recombinant glycine and GABAAreceptors. Br. J. Anaesth 92, 704–711. [DOI] [PubMed] [Google Scholar]
- Yu W, Wu Z, Zhao P, 2020. Neurotoxicity effects of anesthetic exposure on the developing brain of non-human primates. Med. Hypotheses 140, 109647. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mori M, Burgess DL, Noebels JL, 2002. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J. Neurosci 22, 6362–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba AM, Szabo A, Pierce DW, Haburcak M, Stern AT, Nourmahnad A, Halpin ES, Forman SA, 2018. Alphaxalone Binds in Inner Transmembrane β+-α- Interfaces of α1β3γ2 γ-Aminobutyric Acid Type A Receptors. Anesthesiology 128, 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S, 2013. Neurosteroids, stress and depression: Potential therapeutic opportunities. Neurosci. Biobehav. Rev 37, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


