Abstract
Background:
The ISCHEMIA trial postulated that patients with stable coronary artery disease (CAD) and moderate or severe ischemia would benefit from revascularization. We investigated the relationship between severity of CAD and that of ischemia and trial outcomes, overall and by management strategy.
Methods:
5,179 patients with moderate or severe ischemia were randomized to an initial invasive or conservative management strategy. Blinded, core-laboratory-interpreted coronary CT angiography (CCTA) was used to assess anatomic eligibility for randomization. Extent and severity of CAD were classified using the modified Duke Prognostic Index (n=2,475, 48%). Ischemia severity was interpreted by independent core laboratories (nuclear, echocardiography, magnetic resonance imaging, exercise tolerance testing, n=5,105, 99%). We compared 4-year event rates across subgroups defined by severity of ischemia and CAD. The primary endpoint for this analysis was all-cause mortality. Secondary endpoints were myocardial infarction (MI), cardiovascular (CV) death or MI, and the trial primary endpoint (CV death, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest).
Results:
Relative to mild/no ischemia, neither moderate nor severe ischemia was associated with increased mortality (moderate ischemia hazard ratio (HR) 0.89, 95% confidence interval [CI] 0.61– 1.30, severe ischemia HR 0.83, 95% CI 0.57–1.21, p=0.33). Nonfatal MI rates increased with worsening ischemia severity (HR for moderate ischemia 1.20 (95% CI 0.86–1.69) vs. mild/no ischemia; HR for severe ischemia 1.37, 95% CI 0.98–1.91, p=0.04 for trend, p=NS after adjustment for CAD). Increasing CAD severity was associated with death (HR 2.27, 95% CI 1.37–3.75) and MI (HR 1.69, 95% CI 1.17–2.45) for the most vs. least severe CAD subgroup. Ischemia severity did not identify a subgroup with treatment benefit on mortality, MI, the trial primary endpoint, or CV death or MI. In the most severe CAD subgroup (n=659), the 4-year rate of CV death or MI was lower in the invasive strategy group (difference 6.3%, 95% CI 0.2%−12.4%), but 4-year all-cause mortality was similar.
Conclusions:
Ischemia severity was not associated with increased risk after adjustment for CAD severity. More severe CAD was associated with increased risk. Invasive management did not lower all-cause mortality at 4 years in any ischemia or CAD subgroup.
Clinical Trial Registration:
ClinicalTrials.gov identifier: NCT01471522; https://clinicaltrials.gov/ct2/show/NCT01471522
Keywords: ischemia, coronary artery disease, revascularization, PCI, CABG
Introduction
The foundational premise of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial was that patients with moderate or severe ischemia would be at higher risk for events and would therefore have greater potential to benefit from an invasive management strategy incorporating complete revascularization than patients with less severe ischemia.1 This hypothesis was based largely on observational data demonstrating that patients’ risk for cardiovascular death was directly related to degree of ischemia and that those with >10% left ventricular (LV) ischemia who were selected for revascularization had lower risk of cardiovascular death than patients with the same degree of ischemia who were not selected for revascularization.2 This relationship was reversed for patients with <10% LV ischemia. Observational data using stress echocardiography also suggested that revascularization might be most beneficial in patients with more extensive ischemia.3 However, analysis of outcomes among participants in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial found that severity of CAD was associated with adverse outcomes but extent of ischemia was not.4
The ISCHEMIA trial randomized participants with moderate or severe ischemia on stress testing to an initial invasive strategy of routine cardiac catheterization and revascularization, if feasible, along with guideline-directed medical therapy consisting of pharmacologic and lifestyle measures (INV), or to a conservative strategy of optimal medical therapy alone, with cardiac catheterization deferred unless there was failure of medical therapy (CON).1 Stress testing with several different commonly available modalities was permitted for eligibility. Most participants underwent blinded coronary CT angiography (CCTA) before randomization to exclude any enrolled participants who either did not have obstructive CAD or had at least 50% stenosis in the left main coronary artery. Primary results of the ISCHEMIA trial found no statistical evidence of a benefit of the invasive strategy on the primary or major secondary endpoints in this stable ischemic heart disease population.5
Leveraging the randomization in ISCHEMIA, we sought to validate prior observational studies suggesting risk stratification based on the magnitude of ischemia or extent of CAD could identify patients who might benefit more from revascularization. We set out to determine whether estimates of severity of CAD and/or ischemia were independently associated with increased risk of all-cause mortality, myocardial infarction or other cardiovascular outcomes in the ISCHEMIA trial cohort, and whether there was any heterogeneity of treatment effect in trial participants based on their severity of CAD on CCTA or inducible ischemia on stress testing.
Methods
Deidentified data and a data dictionary will be available starting March 30, 2022. Methods of data sharing will be determined based on the National Institutes of Health data sharing policy and in discussion with the National Institutes of Health and the National Heart, Lung, and Blood Institute program officer.
Detailed methods of the ISCHEMIA trial, baseline characteristics and primary results have been reported previously.1, 5–7 IRB approval was obtained at each site, and all participants provided informed consent.
Test performance and interpretation
The trial protocol included definitions of moderate and severe ischemia for each of the following testing modalities performed before study enrollment: stress nuclear myocardial perfusion imaging (“nuclear”), stress echocardiography (“echo”), stress cardiac magnetic resonance imaging perfusion testing (“CMR”) and non-imaging exercise tolerance testing (“ETT”). For stress imaging, both exercise and pharmacologic stress were permitted. Tests were interpreted at dedicated stress core laboratories according to published guidelines,8–11 without knowledge of detailed coronary anatomy or detailed clinical characteristics. Severity of ischemia was categorized by independent core laboratories using previously defined criteria (Table 1).1 Most randomized participants underwent CCTA (76%), which was interpreted by a separate independent core laboratory without access to stress test findings. Enrolled participants with kidney impairment (estimated glomerular filtration rate <60 ml/min) or with known coronary anatomy were not required to undergo CCTA. For participants randomized without a study CCTA who underwent a prior CCTA within 1 year of enrollment, those images were requested and, when available, were interpreted by the core laboratory and included in this analysis (n = 130).
Table 1.
Definitions of Ischemia and Anatomic Subgroups
| Ischemia Severity | INV group | CON group | |
|---|---|---|---|
| Severe |
|
All modalities: 1382 Nuclear: 474 (34.3%) Echo: 305 (22.1%) CMR: 69 (5.0%) ETT: 534 (38.6%) |
All modalities: 1415 Nuclear: 494 (34.9%) Echo: 321 (22.7%) CMR: 83 (5.9%) ETT: 517 (36.5%) |
| Moderate |
|
All modalities: 873 Nuclear: 596 (68.3%) Echo 178 (20.4%) CMR: 44 (5.0%) ETT 55 (6.3%) |
All modalities: 829 Nuclear: 607 (73.2%) Echo: 138 (16.6%) CMR: 38 (4.6%) ETT: 46 (5.5%) |
| Mild |
|
All modalities: 300 Nuclear: 199 (66.3%) Echo: 64 (21.3%) CMR: 12 (4.0%) ETT 25 (8.3%) |
All modalities: 306 Nuclear: 185 (60.5%) Echo: 73 (23.9%) CMR: 11 (3.6%) ETT 37 (12.1%) |
| None |
|
||
| Anatomic Severity of CAD (modified Duke Prognostic Index) | N=1234 | N=1241 | |
| 6 | 3-vessel severe stenosis (≥70%) or 2-vessel severe stenosis with proximal LAD | N=343 | N=316 |
| 5 | 2-vessel severe stenosis not including the proximal LAD, 1-vessel severe proximal LAD, or 3-vessel moderate stenosis (≥50%) | N=439 | N=455 |
| 4 | 2-vessel moderate stenosis or 1-vessel severe stenosis other than proximal LAD | N=361 | N=382 |
| 3 | 1-vessel moderate stenosis (≥50%) | N=91 | N=88 |
Duke categories 1 and 2 (non-obstructive CAD or normal arteries) and 7 (left main stenosis ≥50%) were excluded from analysis, because these findings were not consistent with eligibility for randomization after coronary CT angiography.
Segmental interpretation of the CCTA was carried out according to Society of Cardiovascular Computed Tomography (SCCT) guidelines, with each segment coded as demonstrating no stenosis, 1–24% stenosis, 25–49% stenosis, 50–69% stenosis or 70–100% stenosis, if the segment was interpretable.12 Percent diameter stenosis was represented by ratio of the maximum lumen diameter at the site of maximal obstruction divided by the lumen diameter at the most proximal normal reference vessel x 100. Any coronary artery bypass grafts were noted and stenosis within them quantified. The modified Duke prognostic index, the segment stenosis score and the segment involvement score were calculated as previously described.13 The modified Duke prognostic index categorizes CAD according to extent, location and stenosis severity (Table 1).
CCTA was considered not evaluable for severity of CAD as defined by the modified Duke prognostic index if certain segments designated a priori by the core laboratory could not be interpreted for stenosis severity according to the categories listed above, for example, the mid RCA, due to artifact such as cardiac motion. The primary role of CCTA in the ISCHEMIA trial was to assess participant eligibility for randomization to the invasive or the conservative management strategy based on the presence of obstructive CAD and the absence of left main stenosis ≥50%. As published, 76% of participants underwent CCTA;14 65% of CCTA studies were interpretable for number of vessels diseased using a 70% stenosis threshold. Thus 48% of participants had CCTA interpretable for modified Duke prognostic index.
Outcomes
The primary endpoint for this analysis was all-cause mortality. Secondary endpoints were myocardial infarction (MI), cardiovascular (CV) death or MI (trial major secondary endpoint), and the trial primary endpoint (CV death, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest). Endpoint definitions have been previously published.1
Statistical Methods
Ischemia and CAD severity as predictors of outcomes.
For analyses of ischemia severity in relation to outcomes, we included all randomized participants with available stress test interpretations. We categorized participants as having severe, moderate, mild or no ischemia based on core laboratory interpretation (Table 1). We compared ischemia to outcomes using these categories for all participants combined and, as a sensitivity analysis, for each modality separately. For analyses of extent and severity of CAD in relation to outcomes (referring to both the severity of stenosis and the number of vessels or segments affected, and termed CAD severity throughout this paper for simplicity), only randomized participants with interpretable CCTA for the modified Duke prognostic index were included. We analyzed several measures of CAD severity including modified Duke prognostic index, the number of vessels diseased based on 50% and 70% diameter stenosis thresholds, segment stenosis score and segment involvement score. The outcomes assessed were all-cause mortality, MI, the trial primary composite endpoint (cardiovascular [CV] death, myocardial infarction [MI], or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest), cardiovascular (CV) death and MI, CV death, spontaneous MI (type 1, 4b or 4c), peri-procedural MI (type 4a or 5), hospitalization for unstable angina, and hospitalization for heart failure. To permit adjustment for covariates, the associations between categories of ischemia and outcomes, and categories of CAD severity and outcomes were assessed in a series of Cox proportional hazards models with assigned management strategy incorporated as a stratum variable to account for violation of the model’s underlying proportional hazards assumption due to up front risk related to procedures in the invasive group that diminished over time.5 Ischemia and CAD severity were included as a series of categorical indicator variables. In order to control for other potentially related prognostic factors, we also included the following baseline covariates in the models: age, sex, geographical region, diabetes, hypertension, smoking, estimated glomerular filtration rate (eGFR), body mass index (BMI), ejection fraction, prior MI, heart failure, NYHA class II, prior revascularization, Seattle Angina Questionnaire Angina Frequency subscale score at baseline (SAQ-AF), and new or increasing angina. To test for a linear trend between the ischemia measurements and outcomes, and the CAD measurements and outcomes, the same series of Cox models described above were repeated with the ischemia or CAD categorical indicators replaced by continuous variables; the p-values from the Wald test are reported.
To understand whether the observed association between CAD and outcomes was independent of ischemia severity, we added ischemia severity to the CAD model. We also performed a sensitivity analysis in which CAD was added to the ischemia model for outcomes. This latter analysis was considered supplemental because not all participants had available CAD severity data, given that not all had CCTA.
Ischemia and CAD severity as randomized strategy effect modifiers.
After assessing associations between ischemia, CAD severity, and outcomes, we performed a series of analyses to assess whether differences in cumulative event rates for initial invasive versus conservative strategy varied by levels of ischemia or CAD severity (i.e., heterogeneity of treatment effect). All analyses were based on intention to treat. Based on observed associations between all measures of CAD severity and all-cause mortality and MI, randomized group comparisons as a function of CAD severity were limited to the modified Duke prognostic index. The modified Duke prognostic index was selected a priori by the steering committee and performed at least as well as other measures in prediction of all-cause mortality and of MI. To explore heterogeneity of treatment effect, event rate curves for each randomized group were estimated non-parametrically and plotted within subgroups defined by ischemia categories and levels of the modified Duke prognostic score. In light of crossing event rate curves, the treatment effect cannot be fully captured by any single number. For assessing the presence of a management strategy interaction with ischemia and CAD severity we focused on 4-year event rates and we assessed whether differences in 4-year event rates for invasive minus conservative were consistent across levels of ischemia and the Duke prognostic score. Differences between treatment groups are presented with 95% confidence intervals, without adjustment for multiple comparisons.
In a sensitivity analysis to further explore the potential for heterogeneity of treatment effect, we re-estimated event rate curves for each treatment group in a semi-parametric Cox modeling framework, both with and without the following covariates: age, sex, eGFR, EF, heart failure, diabetes and the number of vessels with 70% stenosis. To account for non-proportional hazards in these models, treatment group was modeled as a stratum variable. To account for the competing risk of non-cardiovascular death in these analyses, we separately modeled the cause-specific hazards for endpoint events and competing events. These cause-specific results were then combined into an appropriate estimate of the event rate of interest. Event rate curves adjusting for covariates were obtained by first predicting a curve for each individual patient under invasive treatment and again under conservative treatment and then averaging across patients. Two approaches were used to account for uncertainty in these analyses. First, we used bootstrap resampling to calculate approximate 95% confidence intervals around the difference in 4-year event rates across treatments. Second, we used Bayesian Markov Chain Monte Carlo to evaluate the posterior probability that the true 4-year event rate for the invasive group is lower than the conservative group by amounts ranging from 0 to 5 percentage points. The Bayesian analysis was implemented with a flexible piecewise-constant baseline hazard approximation and non-informative priors, as previously described.5
Missing data.
Covariate data were nearly complete with no variable other than ejection fraction missing in more than 1.5% of patients and the majority of variables missing in fewer than 1.0% of patients. To make full use of the available covariate data, we imputed missing ejection fraction values (10.1%) to the average value across 100 imputed data sets, imputed other continuous variables to the median value, and imputed categorical variables to the most common category.
Results
Patient Population
Among 5,179 randomized participants in the ISCHEMIA trial, core laboratory-determined ischemia severity was available for analysis in 5,105 (99%), among whom nuclear imaging was the most commonly used stress test modality. Of those, CCTA-defined extent and stenosis severity of CAD based on the modified Duke prognostic index (hereafter referred to as “severity of CAD”) was available for analysis in 2,475 (48%, Figure I in the Supplement). Participants for whom severity of CAD was not available either did not undergo CCTA (n=1,244) or had CCTA that was not interpretable for number of vessels diseased due to at least one uninterpretable key segment (n=1,343). Baseline characteristics of the randomized ISCHEMIA cohort subdivided by ischemia severity appear in Tables I–II in the Supplement, and by CAD severity in III–IV in the Supplement. In general, participants with more extensive ischemia and those with more severe CAD were more likely to be male. Patients with more severe CAD were more likely to be of Asian race and less likely to be of white race. As previously reported, more severe ischemia and more severe CAD were correlated.15 Characteristics of patients who did or did not have interpretable CCTA for severity of CAD appear in Table V of the Supplement. In general, participants who had CCTA interpretable for severity of CAD based on the modified Duke prognostic index were more likely to be male, to have hypertension and to have stress echocardiography as the qualifying stress test, and were more likely to have ETT as the qualifying stress test than participants randomized with a CCTA not interpretable for severity of CAD.
Ischemia severity and risk of outcomes
Increasing ischemia severity was not associated with an increase in the 4-year event rates for all-cause mortality or the five-component primary endpoint (Figure 1). For all-cause mortality, relative to mild or no ischemia as determined by the stress core laboratories, the hazard ratio (HR) for moderate ischemia was 0.89 (95% confidence interval [CI] 0.61–1.30), and for severe ischemia was 0.83 (95% CI 0.57–1.21), p=0.33. This finding was also evident for each individual stress testing modality (nuclear, echo, CMR, ETT; Tables VI–VIII in the Supplement). More severe ischemia was associated with higher risk of MI (Figure 1): relative to mild/no ischemia, the HR for moderate ischemia was 1.20 (95% CI 0.86–1.69) and HR for severe ischemia was 1.37 (95% CI 0.98–1.91), p=0.04. After adjustment for CAD, this association was no longer significant (Figure II in the Supplement). There was no significant association between severity of ischemia and risk of cardiovascular (CV) death, the major secondary trial outcomes of CV death or MI, or hospitalization for heart failure, hospitalization for unstable angina, spontaneous MI or peri-procedural MI (Figure III in the Supplement).
Figure 1. Association between Ischemia Severity and Outcomes.
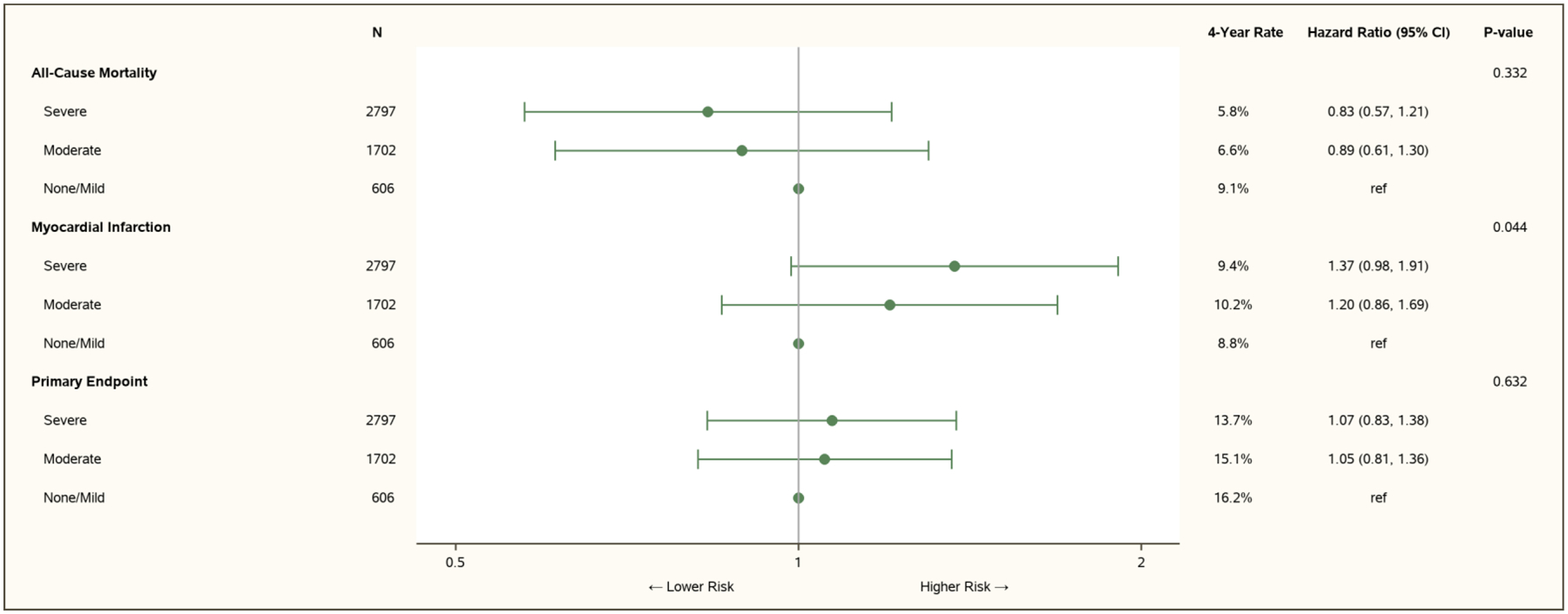
See Table 1 for details of ischemia severity categories. Covariates for adjustment included: age, sex, region (North America and Europe, Asia, Other), diabetes, hypertension, current smoking, prior MI, heart failure or New York Heart Association Class II, prior revascularization, angina frequency (Seattle Angina Questionnaire angina frequency subscale score ≤60, 61–90, 91–100), new or increasing angina, eGFR, ejection fraction and body-mass index.
CAD severity and risk of outcomes
Among the 2,475 participants with CCTA data, there was a graded association between the severity of CAD and all-cause mortality (Figure 2; with ischemia severity excluded from the model, in Figure IV in the Supplement). More severe CAD was associated with increased risk of MI, including both spontaneous and peri-procedural MI subtypes (Figure IV in the Supplement). More severe CAD was also associated with higher risk of CV death, the trial primary composite endpoint and CV death or MI, but not with hospitalization for heart failure or unstable angina. Results were similar when considering other measures of CAD severity incorporating stenosis severity and extent of disease, including the number of vessels diseased, the segment stenosis score or the segment involvement score (Tables VIII–X in the Supplement), and when restricting analysis to participants with moderate or severe ischemia as determined by the core laboratories (Figure V in the Supplement). Proximal LAD stenosis ≥70% was not associated with increased risk of death (Table IX in the Supplement), MI (Table X in the Supplement) or CV death (Table XI in the Supplement).
Figure 2. Association between CAD Severity and Outcomes.
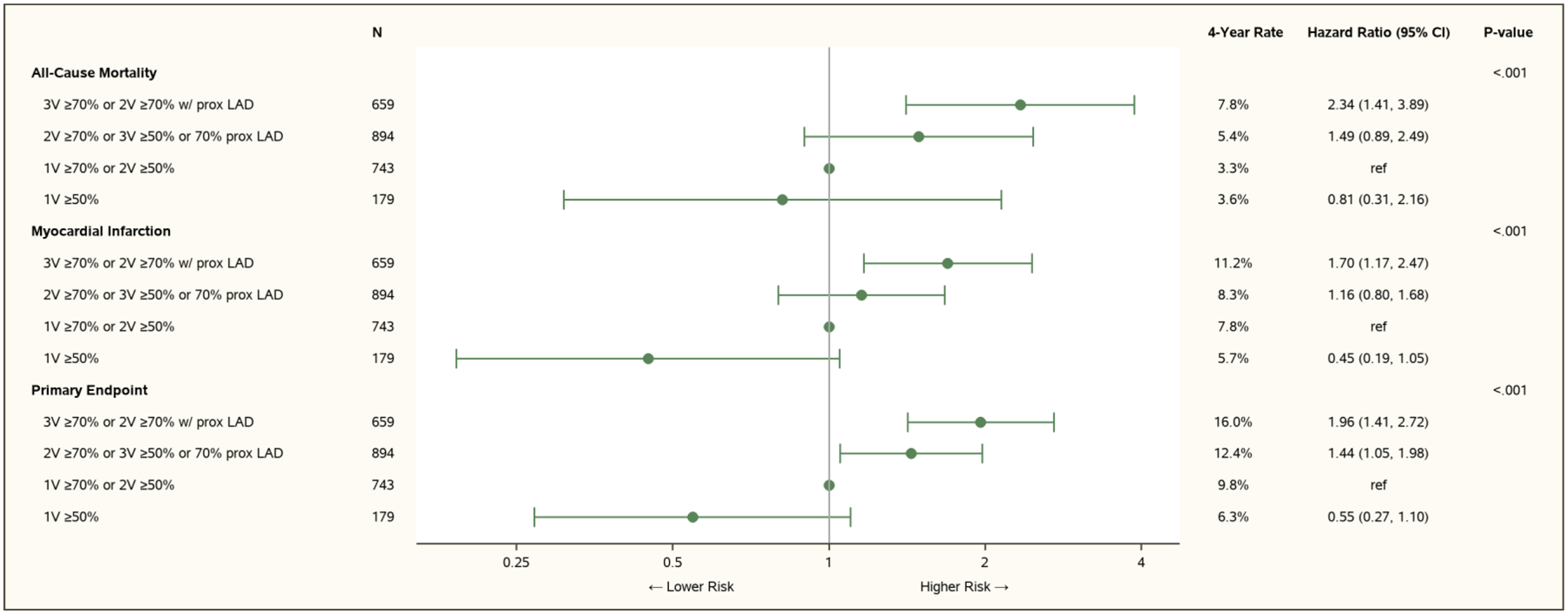
V denotes vessel. Covariates for adjustment included: ischemia severity, age, sex, region (North America and Europe, Asia, Other), diabetes, hypertension, current smoking, prior MI, heart failure or New York Heart Association Class II, prior revascularization, angina frequency (Seattle Angina Questionnaire angina frequency subscale score ≤60, 61–90, 91–100), new or increasing angina, eGFR, ejection fraction and body-mass index.
Cardiac catheterization, revascularization and medication use in subgroups defined by severity of ischemia and severity of CAD, by randomized management strategy assignment
The use of cardiac catheterization and revascularization during follow-up was low in participants assigned to the conservative strategy, in all subgroups defined by ischemia and CAD severity (at most 33%, Tables XII and XIII in the Supplement).
Among conservative strategy-assigned participants, the 4-year rate of revascularization during follow-up was lowest in those with single vessel moderate CAD (12.4%), and highest in those with 3-vessel CAD with ≥70% stenosis or 2-vessel CAD with ≥70% stenosis including the proximal LAD (Duke 6 subgroup; 23.5%, Table XIII in the Supplement).
In general, the first revascularization procedure was more likely to be coronary artery bypass grafting (CABG) when there was more severe ischemia or CAD, though most invasive strategy participants in all CAD subgroups underwent PCI (Tables XIV and XV in the Supplement). For example, in the invasive strategy group, the proportion of participants undergoing CABG was 2.2% in those with single vessel moderate CAD, 29.8% in the Duke 5 subgroup and 32.7% in those in the Duke 6 subgroup.
Medication use at enrollment and at the last study visit in subgroups defined by ischemia severity appear in Table XVI in the Supplement and, by CAD, in Table XVII in the Supplement. Participants with more severe CAD and those with more severe ischemia were more likely to receive high-intensity statin therapy, beta blockade and long-acting nitrates both at baseline and at 12 months after randomization. Patients with more severe ischemia were less likely to receive angiotensin converting enzyme inhibitors or angiotensin receptor blockers than those with less severe ischemia.
Management strategy group comparisons in subgroups defined by severity of ischemia
Four-year rates of all-cause mortality, MI (including the subtypes of spontaneous or peri-procedural MI), CV death or MI, the trial primary composite endpoint, CV death, hospitalization for heart failure or hospitalization for unstable angina in any ischemia subgroup are presented in Table 2, Figure 3, and Table XVII and Figure VI in the Supplement. No statistical evidence of a difference between treatment groups was identified for any ischemia subgroup, but power for subgroup analyses is inherently limited.
Table 2.
Four-Year Cumulative Event Rates by Ischemia Severity and Treatment Assignment
| Number of Events | 4- Year Event Rate | |||||
|---|---|---|---|---|---|---|
| INV | CON | INV | CON | Difference (95% CI) | Interaction P-value | |
| All-Cause Mortality | 0.56 | |||||
| None/Mild | 18 | 19 | 8.3% (4.9%, 12.8%) | 9.9% (5.8%, 15.2%) | −1.6% (−7.7%, 4.5%) | |
| Moderate | 45 | 42 | 6.7% (4.9%, 8.9%) | 6.5% (4.7%, 8.7%) | 0.1% (−2.7%, 3.0%) | |
| Severe | 60 | 59 | 6.1% (4.7%, 7.8%) | 5.6% (4.2%, 7.1%) | 0.6% (−1.6%, 2.7%) | |
| MI | 0.86 | |||||
| None/Mild | 20 | 23 | 8.4% (5.1%, 12.6%) | 9.2% (5.9%, 13.4%) | −0.8% (−6.1%, 4.5%) | |
| Moderate | 75 | 69 | 9.6% (7.6%, 11.9%) | 10.9% (8.5%, 13.6%) | −1.3% (−4.6%, 2.1%) | |
| Severe | 102 | 123 | 8.7% (7.1%, 10.5%) | 10.1% (8.4%, 11.9%) | −1.4% (−3.8%, 1.1%) | |
| CV Death or MI | 0.86 | |||||
| None/Mild | 32 | 35 | 13.7% (9.3%, 18.9%) | 15.5% (10.7%, 21.1%) | −1.8% (−8.9%, 5.3%) | |
| Moderate | 90 | 92 | 11.5% (9.3%, 14.0%) | 14.4% (11.6%, 17.4%) | −2.8% (−6.6%, 0.9%) | |
| Severe | 131 | 159 | 11.5% (9.6%, 13.5%) | 13.4% (11.5%, 15.5%) | −1.9% (−4.8%, 0.9%) | |
| Trial Primary Endpoint | 0.99 | |||||
| None/Mild | 38 | 39 | 15.6% (11.1%, 20.8%) | 16.9% (11.9%, 22.6%) | −1.3% (−8.5%, 6.0%) | |
| Moderate | 108 | 110 | 13.8% (11.4%, 16.5%) | 16.5% (13.7%, 19.7%) | −2.7% (−6.6%, 1.2%) | |
| Severe | 144 | 174 | 12.6% (10.7%, 14.8%) | 14.7% (12.6%, 16.8%) | −2.0% (−5.0%, 0.9%) | |
| CV Death | 0.85 | |||||
| None/Mild | 13 | 15 | 5.9% (3.1%, 10.0%) | 8.0% (4.4%, 13.0%) | −2.1% (−7.6%, 3.5%) | |
| Moderate | 28 | 32 | 4.1% (2.8%, 5.9%) | 4.9% (3.4%, 6.8%) | −0.7% (−3.1%, 1.6%) | |
| Severe | 39 | 49 | 3.7% (2.6%, 5.0%) | 4.5% (3.3%, 6.0%) | −0.9% (−2.7%, 0.9%) | |
| Spontaneous MI | 0.93 | |||||
| None/Mild | 14 | 20 | 6.3% (3.5%, 10.3%) | 7.8% (4.8%, 11.7%) | −1.5% (−6.4%, 3.4%) | |
| Moderate | 45 | 61 | 6.1% (4.4%, 8.1%) | 9.6% (7.4%, 12.3%) | −3.6% (−6.6%, −0.5%) | |
| Severe | 63 | 101 | 5.7% (4.4%, 7.3%) | 8.3% (6.8%, 10.0%) | −2.6% (−4.8%, −0.4%) | |
The trial primary endpoint was the composite of CV death, MI and hospitalization for heart failure, unstable angina or resuscitated cardiac arrest.
Figure 3. Ischemia Severity and Outcomes by Treatment Group.
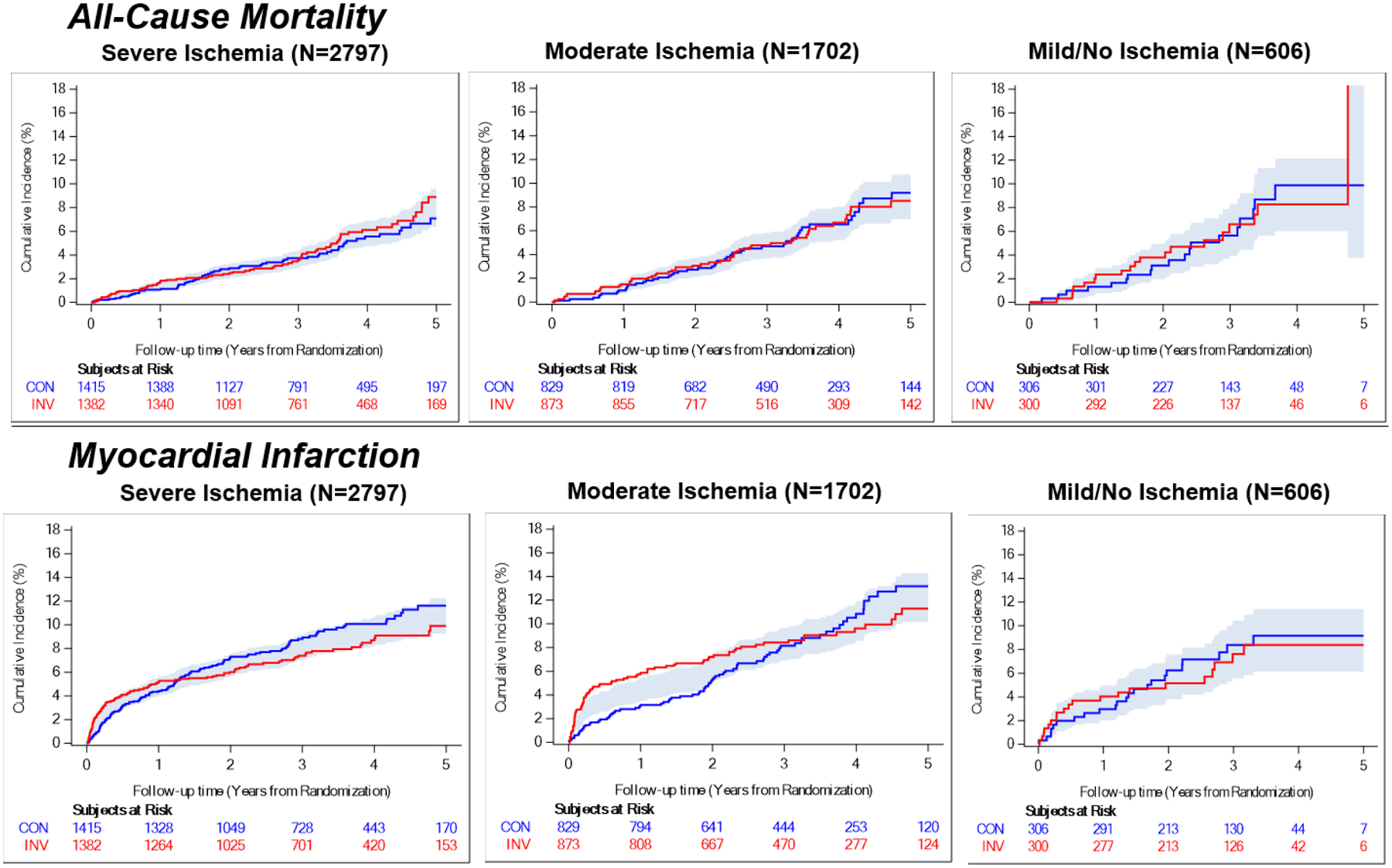
Shading indicates the half-width of confidence bands for the difference between treatment groups. CON = conservative strategy, INV = invasive strategy.
Management strategy group comparisons in subgroups defined by severity of CAD
Four-year rates of all-cause mortality are shown in Table 3 and Figure 4, MI in Figure 5, the major secondary outcome of cardiovascular death or MI in Figure VII of the supplement and the trial primary composite endpoint in Figure 8 of the Supplement. Given that 76% of the randomized trial cohort underwent CCTA and that 65% of those participants had adequate image quality in all key segments of the major coronary arteries as required for assignment of the modified Duke prognostic index, only 48% of randomized participants were included in the analysis of CAD severity and outcomes. In the subgroup of participants with Duke score 6, the four-year estimated rate of all-cause mortality was 7.7% in the invasive group and 7.7% in the conservative group (difference −0.0%, 95% CI −4.9% to 4.8%). In the same subgroup, the four-year estimated rate of MI was 9.2% in the invasive group and 13.4% in the conservative group (difference −4.2%, 95% CI −9.5% to 1.0%). The four-year estimated rate of the major secondary endpoint CV death or MI in this subgroup was 11.6% in the invasive group and 17.9% in the conservative group (difference −6.3%, 95% CI −12.4% to −0.2%). The CAD-by-treatment-strategy interaction p value for 4-year event rates was >0.05 for all endpoints evaluated. There was no statistical evidence of a difference between randomized management strategy groups identified for any other CAD subgroup. Peri-procedural MI was infrequent (Table XVIII in the Supplement).
Table 3.
Four-Year Cumulative Event Rates by Coronary Artery Disease Severity and Treatment Assignment
| Number of Events | 4- Year Event Rate | |||||
|---|---|---|---|---|---|---|
| INV | CON | INV | CON | Difference (95% CI) | Interaction P-value | |
| All-Cause Mortality | 0.89 | |||||
| One-vessel CAD ≥50% | 2 | 2 | 2.7% (0.5%, 8.8%) | 4.4% (0.7%, 13.6%) | −1.6% (−8.9%, 5.6%) | |
| One-vessel CAD ≥70% or two-vessel ≥50% | 9 | 9 | 3.7% (1.7%, 6.8%) | 3.0% (1.4%, 5.4%) | 0.7% (−2.5%, 3.9%) | |
| Two-vessel CAD ≥70% or three-vessel ≥50% or 70% proximal LAD | 18 | 17 | 5.8% (3.4%, 9.1%) | 5.0% (2.9%, 7.9%) | 0.8% (−3.0%, 4.6%) | |
| Three-vessel CAD ≥70% or two-vessel ≥70% including proximal LAD | 20 | 20 | 7.7% (4.8%, 11.5%) | 7.7% (4.7%, 11.7%) | −0.0% (−4.9%, 4.8%) | |
| MI | 0.49 | |||||
| One-vessel CAD ≥50% | 2 | 4 | 2.2% (0.4%, 7.1%) | 8.7% (2.5%, 19.9%) | −6.5% (−15.8%, 2.8%) | |
| One-vessel CAD ≥70% or two-vessel ≥50% | 25 | 21 | 8.3% (5.4%, 12.1%) | 7.3% (4.6%, 11.0%) | 1.0% (−3.7%, 5.6%) | |
| Two-vessel CAD ≥70% or three-vessel ≥50% or 70% proximal LAD | 27 | 36 | 6.8% (4.5%, 9.8%) | 9.6% (6.7%, 13.0%) | −2.8% (−6.9%, 1.3%) | |
| Three-vessel CAD ≥70% or two-vessel ≥70% including proximal LAD | 27 | 39 | 9.2% (6.1%, 12.9%) | 13.4% (9.7%, 17.7%) | −4.2% (−9.5%, 1.0%) | |
| CV Death or MI | 0.33 | |||||
| One-vessel CAD ≥50% | 3 | 4 | 3.3% (0.9%, 8.6%) | 8.7% (2.5%, 19.9%) | −5.4% (−14.9%, 4.2%) | |
| One-vessel CAD ≥70% or two-vessel ≥50% | 26 | 25 | 8.8% (5.7%, 12.8%) | 8.7% (5.6%, 12.5%) | 0.2% (−4.7%, 5.1%) | |
| Two-vessel CAD ≥70% or three-vessel ≥50% or 70% proximal LAD | 38 | 48 | 10.2% (7.2%, 13.9%) | 12.8% (9.5%, 16.7%) | −2.6% (−7.5%, 2.3%) | |
| Three-vessel CAD ≥70% or two-vessel ≥70% including proximal LAD | 34 | 50 | 11.6% (8.1%, 15.7%) | 17.9% (13.4%, 22.8%) | −6.3% (−12.4%, −0.2%) | |
| Trial Primary Endpoint | 0.49 | |||||
| One-vessel CAD ≥50% | 4 | 5 | 4.5% (1.5%, 10.4%) | 8.2% (2.7%, 17.6%) | −3.6% (−12.3%, 5.1%) | |
| One-vessel CAD ≥70% or two-vessel ≥50% | 28 | 30 | 9.4% (6.2%, 13.4%) | 10.1% (6.9%, 14.1%) | −0.7% (−5.8%, 4.4%) | |
| Two-vessel CAD ≥70% or three-vessel ≥50% or 70% proximal LAD | 43 | 51 | 11.5% (8.3%, 15.3%) | 13.2% (9.9%, 17.0%) | −1.7% (−6.6%, 3.3%) | |
| Three-vessel CAD ≥70% or two-vessel ≥70% including proximal LAD | 39 | 53 | 13.3% (9.5%, 17.6%) | 18.8% (14.3%, 23.9%) | −5.6% (−11.9%, 0.7%) | |
| CV Death | 0.42 | |||||
| One-vessel CAD ≥50% | 1 | 1 | 1.1% (0.1%, 5.4%) | 2.7% (0.2%, 12.1%) | −1.6% (−7.2%, 4.1%) | |
| One-vessel CAD ≥70% or two-vessel ≥50% | 4 | 4 | 1.6% (0.5%, 4.1%) | 1.3% (0.4%, 3.3%) | 0.3% (−1.9%, 2.5%) | |
| Two-vessel CAD ≥70% or three-vessel ≥50% or 70% proximal LAD | 14 | 14 | 4.2% (2.3%, 6.9%) | 3.7% (2.1%, 6.1%) | 0.5% (−2.6%, 3.5%) | |
| Three-vessel CAD ≥70% or two-vessel ≥70% including proximal LAD | 11 | 17 | 3.7% (1.9%, 6.4%) | 6.7% (3.9%, 10.5%) | −3.0% (−7.0%, 1.0%) | |
| Spontaneous MI | 0.51 | |||||
| One-vessel CAD ≥50% | 1 | 2 | 1.1% (0.1%, 5.4%) | 5.7% (1.0%, 16.8%) | −4.5% (−12.6%, 3.5%) | |
| One-vessel CAD ≥70% or two-vessel ≥50% | 17 | 21 | 6.0% (3.5%, 9.6%) | 7.3% (4.6%, 11.0%) | −1.3% (−5.7%, 3.1%) | |
| Two-vessel CAD ≥70% or three-vessel ≥50% or 70% proximal LAD | 15 | 27 | 4.1% (2.2%, 6.7%) | 7.0% (4.6%, 10.0%) | −2.9% (−6.4%, 0.6%) | |
| Three-vessel CAD ≥70% or two-vessel ≥70% including proximal LAD | 15 | 30 | 5.4% (3.1%, 8.6%) | 10.2% (7.0%, 14.0%) | −4.8% (−9.3%, −0.3%) | |
The trial primary endpoint was the composite of CV death, MI and hospitalization for heart failure, unstable angina or resuscitated cardiac arrest.
Figure 4. Anatomic Severity of Coronary Artery Disease and All-Cause Mortality by Treatment Group.
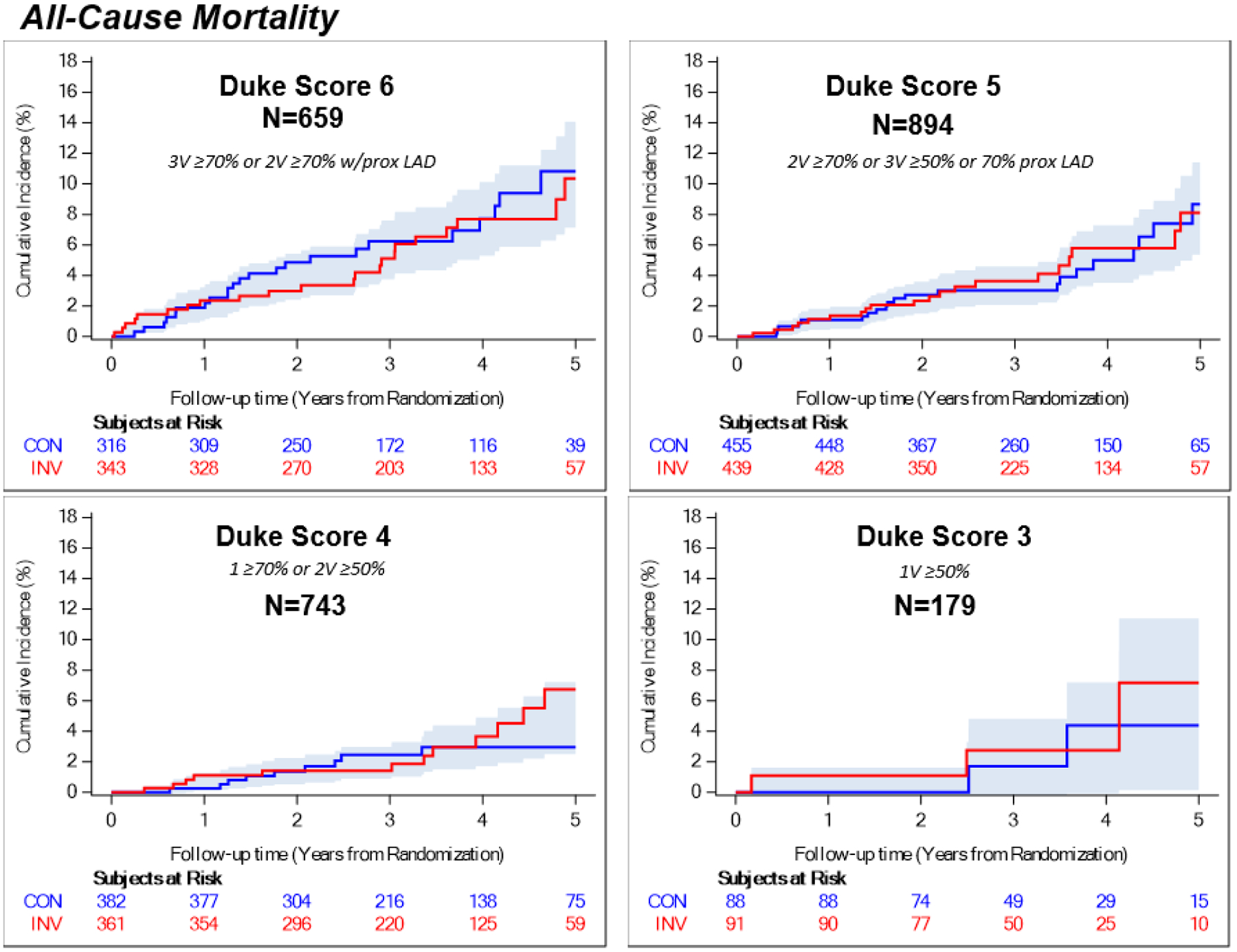
Shading indicates the half-width of confidence bands for the difference between treatment groups. CON = conservative strategy, INV = invasive strategy, V=vessel.
Figure 5. Anatomic Severity of Coronary Artery Disease and Myocardial Infarction by Treatment Group.
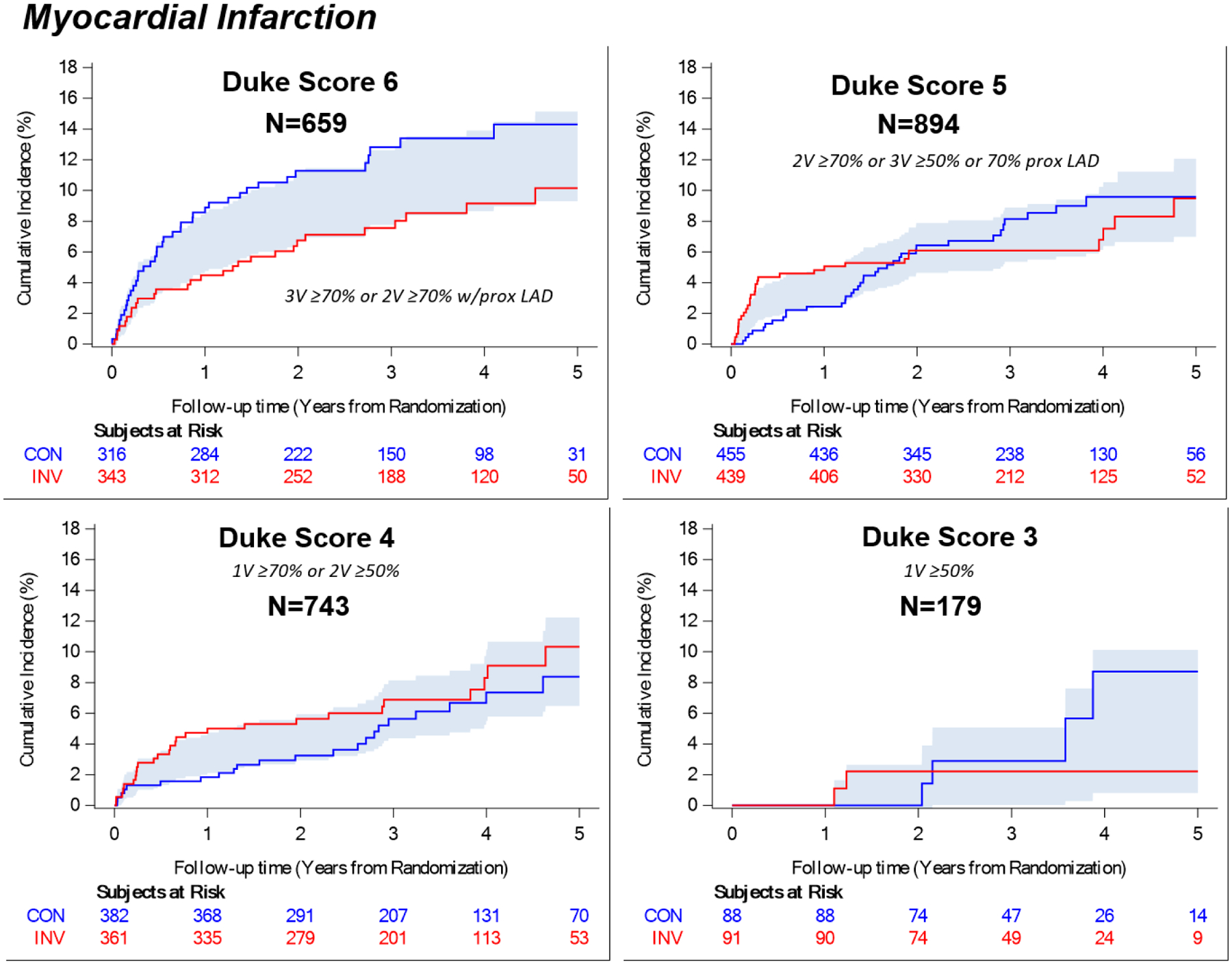
Shading indicates the half-width of confidence bands for the difference between treatment groups. CON = conservative strategy, INV = invasive strategy, V=vessel. The trial primary endpoint was a composite of CV death, MI, hospitalization for heart failure, hospitalization for unstable angina, and resuscitated cardiac arrest.
We conducted several sensitivity analyses of the relationship between CAD severity and outcomes by treatment group. Results were similar when analysis was restricted to patients with moderate or severe ischemia as determined by the core laboratories (Table XIX in the Supplement, Figures IX–X in the Supplement). Results were also similar when CAD was categorized according to the number of arteries with at least 70% stenosis on CCTA (Figures XI–XII and Table XX in the Supplement). When we modeled outcomes using semi-parametric Cox regression, the confidence interval around the difference in 4-year rates for invasive versus conservative management among patients in the Duke score 6 subgroup favored the invasive group for MI (difference −6.8%, 95% CI −12.4% to −1.3%), CV death or MI (difference −8.7%, 95% CI −14.8% to −2.4%), and the trial composite primary endpoint (difference −7.6%, 95% CI −14.0% to −1.1%). However, these were not adjusted for multiple comparisons. Modeling results were similar when adjusting or not adjusting for baseline covariates. In Bayesian analyses, posterior probability of a 5 percentage point difference in 4-year all-cause mortality favoring the invasive strategy group was 6% for the Duke score 6 subgroup and was ≤2% for all other CAD subgroups (Table XXI in the Supplement). The posterior probability that the 4-year event rate of MI was lower by at least 5 percentage points in the invasive group compared with the conservative group was 68% for the Duke score 6 subgroup and was ≤25% for all other CAD subgroups (Table XXII in the Supplement). For CV death or MI, the posterior probability of a 5 percentage point difference favoring the invasive strategy group was 88% for the Duke score 6 subgroup and was ≤20% for all other CAD subgroups (Table XXIII in the Supplement). Additional Bayesian analyses are presented in Tables XXIV–XXV in the Supplement.
Discussion
In our large stable ischemic heart disease trial cohort, CAD severity was a highly significant predictor of all-cause mortality, MI, the trial primary 5-component composite endpoint, CV death or MI, and other individual trial endpoints, independent of ischemia severity and other clinical predictors. In contrast, core laboratory-determined ischemia severity was associated with only one trial outcome, MI, and this was no longer significant after including CAD severity in the model. We did not detect significant heterogeneity of treatment effect in the 4-year rates of all-cause mortality, MI or the primary endpoint in relation to ischemia severity. In the subgroup of 659 participants with the most severe CAD, there was no difference in 4-year all-cause mortality rates, but the rate of CV death or MI was lower among invasive strategy participants. In light of the relatively small size of the subgroup, the absence of a graded relationship between CAD extent and the likelihood of benefit, the absence of a mortality difference, and the fact that only about half of randomized participants had CCTA interpretable for extent and severity of CAD for technical reasons, we cannot definitively conclude that those with the most extensive CAD benefit from an invasive strategy.
Ischemia severity and outcomes
ISCHEMIA selected participants based primarily on the presence of moderate or severe stress-induced ischemia, though approximately 15% had less than moderate ischemia based on core laboratory review. We included a narrow range of ischemia by design, and ischemia severity within this high range did not discriminate risk further. This was true overall and when considering each stress test modality used to qualify patients for the trial individually. More severe ischemia was associated with more severe CAD in cross sectional analysis of ISCHEMIA at baseline.15 In line with our findings, ischemia severity was not associated with risk of adverse outcomes in the COURAGE trial, while extent of CAD was.4 It should be noted that patients who were perceived by physicians to be at highest clinical risk were less likely to be considered by clinicians for trial enrollment. Patients with left main coronary artery disease were excluded from the trial. In the PROMISE trial, moderate or severe abnormalities on functional testing among patients with suspected cardiac chest pain were associated with increased risk of cardiovascular death or MI.16 By trial design, in PROMISE, unlike ISCHEMIA, the clinical assessment of ischemic risk did not influence enrollment. Patients with milder degrees of myocardial ischemia were not considered by sites for enrollment in ISCHEMIA, resulting in a narrower range of ischemic responses than in PROMISE. Future analyses will delve into the relationships between modality-specific parameters and outcomes, exercise capacity and outcomes, and the relative impact of ischemia and CAD on exercise capacity.
CAD severity and outcomes
The extent of CAD as assessed by coronary angiography has long been known to predict adverse outcomes, dating back to early revascularization trials employing bypass surgery and persisting in the era of guideline-based medical therapy.17–20 In addition, studies of larger cohorts who underwent CCTA have demonstrated that more extensive and severe CAD is associated with worse prognosis.13, 16, 21 ISCHEMIA builds on these studies by demonstrating a graded association between CAD severity and adverse outcomes, derived from a larger sample size of participants who had more extensive CAD on CCTA. Left main CAD was also associated with poorer prognosis in prior studies, but could not be evaluated in this study because patients with significant left main CAD were excluded from randomization and follow-up. CAD remained associated with all-cause mortality, MI and the trial primary composite outcome even after adjustment for other clinical variables, including ischemia on stress testing. This likely reflects an inherent relationship between the burden of atherosclerosis and the likelihood of plaque instability causing fatal and nonfatal events. Of note, in ISCHEMIA, there was a broad representation of CAD severity, including 79% with multi-vessel CAD overall and 63% in this analysis with multiple vessels affected by ≥70% stenosis.
Ischemia and management strategy effects
There was no significant variation in the effect or the randomized management strategy on 4-year outcomes based on ischemia severity. The rates of cardiac catheterization and revascularization in the conservative strategy groups were low, at most 33%.
CAD and management strategy effects
The overall trial results extend the findings of COURAGE and the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D). Randomization in the ISCHEMIA trial before cardiac catheterization reduced the potential for enrollment bias related to anatomic suitability for revascularization. There was no signal of a mortality benefit overall or in any subgroup based on a severity of ischemia or CAD. There was a lower 4-year rate of CV death or MI among participants assigned to the invasive strategy within the most severe anatomic CAD subgroup (those with 3-vessel CAD with ≥70% stenosis or 2-vessel CAD with ≥70% stenosis, including in the proximal LAD), driven by lower rates of spontaneous MI; albeit with no adjustment for multiple testing. In the overall trial cohort, there was also a lower 4-year rate of CV death or MI among participants assigned to the invasive strategy, but the confidence interval of the difference in 5-year rates between the invasive and conservative strategy groups included zero (difference, −2.3 percentage points; 95% CI, −5.0 to 0.4). Results of the current analysis were consistent across multiple sensitivity analyses. The posterior probability of a difference of at least 5 percentage points favoring the invasive strategy group in the Duke 6 subgroup was 6% for all cause mortality, 68% for MI and 88% for CV death or MI, while the same probability was low for all these endpoints in all other CAD subgroups. Our ability to draw conclusions in subgroups is limited by low power and by smaller sample sizes, and the possibility of a spurious association cannot be excluded.
Older data derived from randomized trials of bypass surgery vs. no bypass surgery did show variation in the mortality benefit with bypass surgery based on CAD severity, leading to anatomy-based recommendations which are still in effect today, for revascularization in stable ischemic heart disease patients to improve survival.22, 23 The relationship between coronary anatomy and potential benefit from revascularization on survival was not replicated in BARI 2D, but power was limited in that study as well, and there was an interaction between angiographic complexity of CAD and treatment effect within the CABG stratum.24, 25 As expected, the use of CABG as the first revascularization procedure was more common among participants with more extensive CAD as compared with participants with less extensive CAD. However, the use of CABG as the first revascularization procedure was similar in the Duke 5 and Duke 6 subgroups, suggesting that differences in results between the Duke 5 and Duke 6 subgroups are not solely attributable to the use of CABG for revascularization. Analysis of outcomes among participants in the COURAGE trial showed no interaction between core-laboratory-interpreted ischemia severity or CAD severity with outcomes by assignment to a strategy of routine percutaneous coronary revascularization or a strategy of initial optimal medical therapy alone.4 Importantly, in the ISCHEMIA trial there were no differences between treatment strategies in all-cause mortality or CV death in any subgroup defined by CAD severity.
Pathophysiologic Considerations
Our results underscore the fundamental vascular biology of coronary atherosclerosis, specifically, that the vast majority of severe flow-limiting plaques remain quiescent and do not cause cardiovascular events. It is possible that the risk conferred by more severe and extensive CAD as measured by the modified Duke prognostic index simply reflects a greater overall atherosclerotic burden. Within each coronary plaque, whether flow-limiting or not, there may be heterogeneous areas at risk to destabilize, and most of these are not severely obstructive.26 Medical therapy reduces the pathobiologic risk of the plaque destabilizion, regardless of whether a plaque includes a severe flow-limiting obstruction. This conceptual model is particularly relevant when considering the effects of revascularization, which is directed at ischemia-producing severe arterial narrowing but does not treat areas of adjacent plaque that may remain at risk of destabilization.
The method of revascularization in the invasive group, percutaneous or surgical, was not randomized in ISCHEMIA. This was instead determined locally by a heart team including invasive and non-invasive cardiologists, surgeons and the patient. Approximately 25% of patients in the invasive group who had revascularization underwent coronary artery bypass grafting, with a higher proportion receiving bypass surgery in subsets with more extensive CAD. Our results align with the findings of the BARI 2D trial bypass surgery randomized stratum, in which 52% of patients with diabetes had three-vessel CAD. There was no difference in mortality between those randomized to CABG versus initial medical therapy alone, though the CABG stratum was not powered for mortality.27 In BARI 2D, there was a lower rate of cardiac death or MI within the subgroup with the most extensive CAD in the CABG stratum, but not the PCI stratum.20
Potential implications for use of testing in patients suspected to have stable ischemic heart disease
Our study was not designed as a randomized trial of different testing strategies on outcomes. Instead, we blinded detailed study CCTA results to treating physicians, unlike the SCOTHEART and PROMISE studies. Among our trial population, selected for at least moderate ischemia, CCTA was superior to stress testing for risk prediction. The presence of obstructive CAD does not provide certainty that CAD is the cause of symptoms such as chest pain, since many patients have extensive CAD without symptoms, including some patients in this trial. Conversely, some patients with core laboratory confirmed ischemia had no obstructive CAD on CCTA (INOCA). Therefore, stress testing will continue to help clinicians and patients define whether ischemic heart disease is the cause of symptoms in a particular patient, and may be necessary even after CCTA for this purpose.
Limitations
This analysis is limited by the relatively short duration of follow-up, median 3.2 years. Extended follow-up of the ISCHEMIA trial cohort for mortality is ongoing. Outcomes are estimated with a statistical margin of error, and power for comparisons of the randomized management strategy within subgroups was limited. Subgroup-specific estimates have the disadvantage of being less precise, but they have the potential to shed light on treatment effects that differ depending on patient characteristics. Anatomy was defined by noninvasive CCTA rather than conventional invasive angiography, limiting the potential for selection bias in enrollment and ascertainment bias related to knowledge of coronary anatomy. In some participants, clinicians had knowledge of the severity of CAD prior to randomization. Patients with left main coronary artery stenosis were excluded from the trial. In general, CCTA and invasive angiography provide similar but not identical results, and recommendations in SIHD guidelines are based on conventional invasive angiography.14 Detailed plaque characterization was not available for this analysis, but is an active area of investigation and results are forthcoming. CCTA was not performed in all participants, mostly due to renal dysfunction. Some CCTA studies (35%) had uninterpretable segments that prevented scoring of the modified Duke prognostic index and number of vessels diseased at the 70% stenosis threshold. Ischemia testing was performed with mutliple modalities, increasing applicability to real world practice, but introducing heterogeneity. Physicians may have been unlikely to enroll patients with very severe ischemia, such as those with a fall in blood pressure with exercise, or those perceived by clinicians to be at highest risk. ISCHEMIA was an unblinded trial. There was no adjustment for multiple comparisons. Patients with an unacceptable degree of angina were excluded, as were patients with left main disease, recent ACS or heart failure, or EF less than 35%. One-fifth of patients assigned to the invasive strategy did not undergo revascularization, though the most common reason for this was no obstructive CAD at angiography. Heterogeneity of health status outcomes by severity of ischemia and CAD will be reported separately.
Conclusions
Ischemia severity was not associated with increased risk of adverse outcomes after adjustment for CAD severity. More severe CAD was associated with increased risk for all-cause mortality, but the invasive strategy did not lower that risk at 4 years.
Supplementary Material
Clinical Perspective.
What is new?
In the ISCHEMIA trial of stable ischemic heart disease (SIHD), severity of coronary artery disease (CAD), but not ischemia severity, predicted 4-year mortality.
CAD severity, but not ischemia severity, was independently predictive of 4-year myocardial infarction (MI) risk.
There was no evidence of a difference between treatment groups in 4-year rates of mortality, MI or the trial primary or major secondary endpoint in any ischemia subgroup.
In the subgroup with the most severe CAD (n=659), there was no difference between treatment groups in 4-year mortality rates, but the CV death or MI rate was lower among invasive-strategy-assigned participants.
What are the clinical implications?
Among patients with stable CAD, coronary CT angiography (CCTA) may be superior to stress testing for risk prediction.
Within the narrow range of ischemia severity enrolled in the trial, ischemia severity may not identify SIHD patients who benefit from invasive management over 4 years.
Stress testing may be necessary to determine whether ischemia is likely to be the cause of symptoms in a particular patient, even after CCTA.
CCTA may be a more efficient method than ischemia testing for risk stratification and may inform decision making for potential benefits of an invasive approach for patients with severe CAD.
Acknowledgements:
Conflict of Interest Disclosures:
Dr.Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from Siemens, non-financial support from BioTelemetry, outside the submitted work
Dr. Shaw reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Min reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; other from CLEERLY INC., grants and other from GE HEALTHCARE, other from ARINETA, outside the submitted work
Courtney Page reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Berman reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; other from GE, CSMC, Bayer and grants from Heartflow outside the submitted work
Dr. Chaitman reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; personal fees from Merck, NovoNordisk, Lilly, Johnson and Johnson, Daiichi Sankyo, Imbria, Xylocor, Sanofi, Tricida, and Xylocor, outside the submitted work
Dr. Picard reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Kwong reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. O’Brien reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study
Zhen Huang reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study
Dr. Mark reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; grants from HeartFlow, Merck, Oxygen Therapeutics, Eli Lilly & Company, AstraZeneca, Bristol Myers Squibb and personal fees from Novo Nordisk, Cytokinetics, and CeleCor, outside the submitted work
Dr. Nath reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study
Dr. Dwivedi reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study
Dr. Smanio reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Stone reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Held reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study
Dr. Keltai reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study
Dr. Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbott Vascular; serves as an Advisory board for Abbott Vascular, Pfizer, Amgen, Biotronik, Meril and Reata, outside the submitted work; serves as a Board of Directors for American Heart Association (unpaid).
Dr. Newman reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Spertus reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; personal fees from Bayer, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Amgen, personal fees from Janssen, personal fees from United Healthcare, grants from American College of Cardiology, personal fees from Blue Cross Blue Shield of Kansas City, outside the submitted work; In addition, Dr. Spertus has a patent Copyright to Seattle Angina Questionnaire with royalties paid and Equity in Health Outcomes Sciences.
Dr. Stone reports grants and personal fees from the National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Terumo, Amaranth, and Shockwave; personal fees and other from Valfix; personal fees from TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, and Sirtex; personal fees and other from Ancora and Qool Therapeutics; other from Cagent, Applied Therapeutics, Biostar family of funds, and MedFocus family of funds; personal fees and other from SpectraWave; personal fees from MAIA Pharmaceuticals; personal fees and other from Orchestra Biomed; other from Aria; personal fees from Vectorious; and other from Cardiac Success, outside the submitted work.
Dr. Maron reports grants from National Heart, Lung and Blood Institute during the conduct of the study;
Dr. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; St. Jude Medical, Inc.; Volcano Corporation; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc.; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Funding Sources:
NIH grants U01HL105907; U01HL105462; U01HL105561; U01HL105565
This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences and by grants from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Amgen Inc; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck Sharp & Dohme Corp. and Sunivion Pharmaceuticals
Non-standard Abbreviations and Acronyms
- BARI-2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes trial
- BMI
body mass index
- CCTA
coronary CT angiography
- CMR
stress cardiac magnetic resonance imaging perfusion testing
- CON
Conservative Strategy
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation
- ECHO
stress echocardiography
- eGFR
estimated glomerular filtration rate
- ETT
non-imaging exercise tolerance testing
- INOCA
ischemia have no obstructive CAD on CCTA
- INV
Invasive Strategy
- ISCHEMIA
The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- LAD
left anterior descending artery
- Nuclear
stress nuclear myocardial perfusion imaging
- PROMISE
Prospective Multicenter Imaging Study for Evaluation of Chest Pain
- Proximal LAD [pLAD]
Proximal left anterior descending coronary artery
- RCA
Right coronary artery
- SAQ-AF
Seattle Angina Questionnaire Angina Frequency
- SCCT
Society of Cardiovascular Computed Tomography
- SCOT-HEART
Scottish COmputed Tomography of the HEART Trial
- SIHD
stable ischemic heart disease
Footnotes
Publisher's Disclaimer: Disclaimer: The manuscript contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
References
- 1.Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. American Heart Journal. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hachamovitch R, Hayes SW, Friedman JD, Cohen I and Berman DS. Comparison of the Short-Term Survival Benefit Associated With Revascularization Compared With Medical Therapy in Patients With No Prior Coronary Artery Disease Undergoing Stress Myocardial Perfusion Single Photon Emission Computed Tomography. 2003;107:2900–2907. [DOI] [PubMed] [Google Scholar]
- 3.Yao SS, Bangalore S and Chaudhry FA. Prognostic implications of stress echocardiography and impact on patient outcomes: an effective gatekeeper for coronary angiography and revascularization. J Am Soc Echocardiogr. 2010;23:832–9. [DOI] [PubMed] [Google Scholar]
- 4.Mancini GBJ, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, Maron DJ, Teo K, Sedlis SP, Chaitman BR, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovascular interventions. 2014;7:195–201. [DOI] [PubMed] [Google Scholar]
- 5.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. The New England journal of medicine. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Alexander KP, Senior R, Boden WE, Stone GW, Goodman SG, Lopes RD, et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical TrialBaseline Characteristics of Participants in the ISCHEMIA StudyBaseline Characteristics of Participants in the ISCHEMIA Study. JAMA Cardiology. 2019;4:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE Jr., Boden WE, Weintraub WS, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. The New England journal of medicine. 2020;382:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR and Sawada SG. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2020;33:1–41.e8. [DOI] [PubMed] [Google Scholar]
- 9.Dorbala S, Ananthasubramaniam K, Armstrong IS, Chareonthaitawee P, DePuey EG, Einstein AJ, Gropler RJ, Holly TA, Mahmarian JJ, Park M-A, et al. Single Photon Emission Computed Tomography (SPECT) Myocardial Perfusion Imaging Guidelines: Instrumentation, Acquisition, Processing, and Interpretation. J Nucl Cardiol. 2018;25:1784–1846. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002;106:1883–92. [DOI] [PubMed] [Google Scholar]
- 11.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic R and Board of Trustees Task Force on Standardized P. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. Journal of Cardiovascular Magnetic Resonance. 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GBJ, Nieman K, Pontone G and Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. Journal of Cardiovascular Computed Tomography. 2014;8:342–358. [DOI] [PubMed] [Google Scholar]
- 13.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS and Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 14.Mancini GBJ, Leipsic J, Budoff MJ, Hague CJ, Min JK, Stevens SR, Reynolds HR, O’Brien SM, Shaw LJ, Manjunath CN, et al. Coronary CT Angiography Followed by Invasive Angiography in Patients With Moderate or Severe Ischemia-Insights From the ISCHEMIA Trial. JACC Cardiovasc Imaging. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds HR, Shaw LJ, Min J, Mark D, Spertus JA, Berman DS, Chaitman B, Picard M, Kwong RY, Page CB, et al. CORONARY ANATOMY, ISCHEMIA AND ANGINA: ASSOCIATIONS AT BASELINE IN THE ISCHEMIA TRIAL. Journal of the American College of Cardiology. 2020;75:21. [Google Scholar]
- 16.Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain. Circulation. 2017;135:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR Jr., Chaitman BR, Kaiser GC, Alderman E and Killip T 3rd. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90:2645–57. [DOI] [PubMed] [Google Scholar]
- 18.Mark DB, Nelson CL, Califf RM, Harrell FE Jr., Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR and et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–25. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub WS, Hartigan PM, Mancini GBJ, Teo KK, Maron DJ, Spertus JA, Chaitman BR, Shaw LJ, Berman D and Boden WE. Effect of Coronary Anatomy and Myocardial Ischemia on Long-Term Survival in Patients with Stable Ischemic Heart Disease. Circ Cardiovasc Qual Outcomes. 2019;12:e005079. [DOI] [PubMed] [Google Scholar]
- 20.Brooks MM, Chaitman BR, Nesto RW, Hardison RM, Feit F, Gersh BJ, Krone RJ, Sako EY, Rogers WJ and Garber AJ. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2012;126:2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. Journal of the American College of Cardiology. 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 22.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. Circulation. 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R and et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–70. [DOI] [PubMed] [Google Scholar]
- 24.Brooks MM, Chaitman BR, Nesto RW, Hardison RM, Feit F, Gersh BJ, Krone RJ, Sako EY, Rogers WJ, Garber AJ, et al. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2012;126:2115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeno F, Brooks MM, Nakagawa K, Kim M-K, Kaneda H, Mitsutake Y, Vlachos HA, Schwartz L, Frye RL, Kelsey SF, et al. SYNTAX Score and Long-Term Outcomes. The BARI-2D Trial. 2017;69:395–403. [DOI] [PubMed] [Google Scholar]
- 26.Varshney AS, Coskun AU, Siasos G, Maynard CC, Pu Z, Croce KJ, Cefalo NV, Cormier MA, Fotiadis D, Stefanou K, et al. Spatial relationships among hemodynamic, anatomic, and biochemical plaque characteristics in patients with coronary artery disease. Atherosclerosis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. The New England journal of medicine. 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


