Abstract
To assess the expected clinical and financial benefits of rapid reporting of microbiology results, we compared patients whose cultured samples were processed in the normal manner to patients whose samples were processed more rapidly due to a minor change in work flow. For the samples tested in the rapid-reporting time period, the vast majority of bacterial identification and antimicrobial susceptibility testing (AST) results were verified with the Vitek system on the same day that they were available. This time period was called rapid AST (RAST). For RAST, a technologist on the evening shift verified the data that became available during that shift. For the control time period, cultures were processed in the normal manner (normal AST [NAST]), which did not include evening-shift verification. For NAST, the results for approximately half of the cultures were verified on the first day that the result was available. The average turnaround time for the reporting of AST results was 39.2 h for RAST and 44.4 h for NAST (5.2 h faster for RAST [P = 0.001]). Subsequently, physicians were able to initiate appropriate antimicrobial therapy sooner for patients whose samples were tested as part of RAST (P = 0.006). The mortality rates were 7.9 and 9.6% for patients whose samples were tested as part of RAST and NAST, respectively (P = 0.45). The average length of stay was 10.7 days per patient for RAST and 12.6 days for NAST, a difference of 2.0 days less for RAST (P = 0.006). The average variable cost was $4,927 per patient for RAST and $6,677 for NAST, a difference of $1,750 less per patient for RAST (P = 0.001). This results in over $4 million in savings in variable costs per year in our hospital.
Earlier studies have shown that antimicrobial susceptibility testing (AST) did not significantly influence a physician’s choice of appropriate antimicrobial therapy (1a, 2, 6, 8, 9). However, because of advances in technology, results of AST can now be available substantially sooner than they were in the past. Because of this, it seems axiomatic to most microbiologists that faster reporting of results will have both clinical and financial benefits. However, only a rare study has objectively addressed these issues (11). In a large, prospective study Doern and colleagues (5) documented the impressive benefits of rapid reporting of bacterial identification and AST.
Because of managed care issues, it is imperative that clinical microbiologists and pathologists effectively demonstrate the impacts of their contributions on patient care. In this study, we assessed the expected benefits (such as decreased morbidity, mortality, and costs) by comparing patients whose cultured samples were processed in the normal manner to patients whose samples were processed more rapidly due to a minor change in work flow.
(Part of this study was presented at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998 [1].)
MATERIALS AND METHODS
Study design.
Memorial Medical Center is a 500-bed community teaching hospital for the Southern Illinois University School of Medicine. In a historical cohort analysis during a 7-month period, we examined data for two groups of inpatients for whom AST was performed. One group consisted of patients whose cultured samples were processed more rapidly than normal; the control group consisted of patients whose samples were processed in the normal manner. In the normal manner, generally between 8 and 9 a.m., bacterial isolates were inoculated in the Vitek system (bioMerieux, St. Louis, Mo.), an instrument which performs identification of bacteria and AST. During the day shift (7:00 a.m. to 3:30 p.m.) approximately half of the Vitek reports were ready to be verified and were verified. Verification is a process in which a technologist reviews the accuracy of the bacterial identification and AST data. If the data are acceptable, it is verified electronically in the Vitek system. All verified data from the Vitek system automatically download to the laboratory information system (LIS). Verified data are available to physicians via computer access to the LIS at any time. At 10 p.m. each night (once every 24 h), a printed copy (cumulative report) of all laboratory reports (including microbiology results) is generated and is available to the physicians making rounds the following morning. In the normal manner, reports which were not yet ready to be verified on the day shift were verified between 7 and 9 a.m. the next day. Samples processed in this manner comprised the control group. The samples in this group are referred to as those which underwent normal AST (NAST). Samples received for culture from 21 May 1997 to 30 September 1997 (excluding samples received in 12 days in July which were included in the rapid AST [RAST] group) were included in the NAST group. All patients whose samples were cultured in this time period were included in the NAST group, whether the results for their samples were verified on the first day that the results were ready for verification or on the second day. For the RAST group, the procedure was exactly the same as that described above, except that a technologist on the second (evening) shift was scheduled to verify the reports which became ready for verification after the day shift was over. Evening-shift verification was done at about 8:30 p.m. and took less than 1 h. Data which were verified on the second shift were included in the cumulative report generated at 10 p.m. All patients whose samples were cultured in this time period were included in this group, whether the results for their samples were verified on the first day that the results were ready (during the day or evening shift) or on the second day. Samples received from 20 July 1997 to 31 July 1997 and from 27 October 1997 to 16 December 1997 were included in the RAST group. Both NAST and RAST groups included samples from patients whose results were verified before and after 10:00 p.m., the difference being that for the patients whose samples were in the NAST group, approximately 50% of the results were verified before 10:00 p.m. on the first day that results were available, whereas for the patients whose samples were in the RAST group more than 90% of the results were verified before 10 p.m.
AST results were reported as MICs with interpretations of susceptible, intermediate, or resistant. Cascading of cephalosporins and aminoglycosides was used. Selective reporting of results of susceptibility to various antimicrobial agents was done according to the guidelines recommended by a multidisciplinary team composed of pharmacists, microbiologists, pathologists, and physicians, including infectious disease specialists. During the course of the study, physicians were not aware of any change of practice in microbiology because they were already accustomed to having access to AST results within 48 h after receipt of the sample in the laboratory for approximately half of their patients (the normal manner). During the RAST period, access to these data for more than the usual number of their patients was not noted or brought to their attention.
Parameters analyzed.
Turnaround time was estimated by subtracting the time that the LIS received verification of the first AST result for a sample from the time that the sample was initially received in the laboratory. All patients (inpatients and outpatients) for whose samples AST was performed during the given time periods were used in the analysis of turnaround time.
Costs (not charges) were obtained from the clinical data management team. Total costs were the sum of fixed direct, variable direct, and fixed indirect costs. Costs attributable to specific departments (e.g., the laboratory) were the sum of fixed direct and variable direct costs. Fixed costs are those costs which do not change with an individual patient, such as overhead and costs of administration. Variable costs are those costs which are associated directly with patient care such as supplies used for a patient or laboratory or radiological tests performed with samples from a patient.
Matching.
Since the patients were different in the two time periods, matching by diagnosis-related group (DRG) was done. All categories of DRGs for patients in the RAST group were examined, and those categories of DRGs which matched the DRGs with patients in the NAST group were included in the study. The NAST group had 523 patients; the RAST group had 242 patients. DRG-matched patients were used in the analysis of mortality, length of stay, and cost and the evaluation of antimicrobial therapy.
Evaluation of therapy with antimicrobial agents.
Medical records for 75 patients in the DRG-matched group for RAST and 75 patients in the DRG-matched group for NAST were examined to determine which patients were receiving appropriate antimicrobial therapy and when it was ordered. The patients in this evaluation were randomly selected by including every second entry from a list of patients in the RAST group made on 17 November 1997 and every seventh entry for the patients in the NAST group.
Statistical analysis.
All analyses were performed by a doctorate-level biostatistician with the computer program SPSS (Statistical Package for Social Sciences, Inc., Chicago, Ill.). DRG severity was determined by relative weights from the Health Care Financing Administration published in the Federal Register (7). The higher the relative weight for a DRG, the greater the severity of disease. There was no statistical difference in the severity of disease or the age of the patients in the NAST and RAST groups, so direct comparison between the two groups by a two-tailed t test for mortality, length of stay in the hospital, and costs (not charges) and for comparison of the patterns of activity when physicians ordered antimicrobial therapy was appropriate. When appropriate, the t tests were corrected for unequal variances by the Levene test (4).
Controlling for unequal distributions of various DRG categories for RAST and NAST groups.
The means for each individual DRG group were used to control for the potential of unequal distribution in the NAST and RAST groups of patients with different DRG categories. That is, if just the mean of the total of all DRGs were compared for the two groups, there may have been for the NAST group (which generally had larger numbers of patients in a DRG than the RAST group) bias from excessive representation by patients with a DRG category associated with a high cost of care or a prolonged length of stay. In order to eliminate this bias, the average of each separate DRG from the two groups was used to calculate the average costs. For instance, if 22 patients in the NAST group with the DRG for “coronary bypass with catheterization” had an average cost of $22,225, then $22,225 was used as a component in the final calculation for the average cost for the entire NAST group. Similarly, if nine patients in the RAST group with the same DRG had an average cost of $19,825, then $19,825 was used as a component in the final calculation for the cost for the entire RAST group. This method of calculating the average cost and length of stay for the NAST and RAST groups eliminated any bias due to the unequal distribution of the two groups in DRGs of different severities.
The mortality rates represent all the deaths in DRG-matched patients in the NAST and RAST groups; no DRG group averages were used. The mortality rate is a crude rate for the NAST and RAST groups.
RESULTS
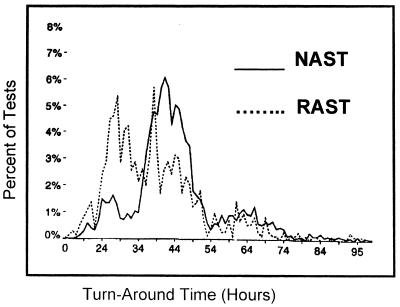
The average turnaround time for the reporting of AST results for all patients, outpatients as well as inpatients (including non-DRG matches), for the RAST group was 39.2 h; it was 44.4 h for all patients in the NAST group. The turnaround time for the RAST group was 5.2 h faster than that for the NAST group (P = 0.001). A chi-square analysis indicated that for a larger portion of patients in the RAST group than patients in the NAST group the results were verified within the first 1 to 2 days (P < 0.001) (Fig. 1).
FIG. 1.
Distribution of turnaround times for patients in the RAST and NAST groups.
The most frequent diagnoses included kidney and urinary tract infections, rehabilitation, wound debridement and skin grafting, coronary bypass with cardiac catheterization, cerebral vascular disorder, respiratory infection and inflammation, septicemia, heart failure, and shock. Although the number of patients in the NAST group was greater than the number of patients in the RAST group, the distributions of the patients in the various DRGs were generally similar in both groups and paralleled the distribution of all patients that year at Memorial Medical Center. There was no statistical difference between the age or the severity code of the DRGs between patients in the RAST and NAST groups.
Having determined that the two patient groups were not statistically different except for the shortened AST turnaround time, it was of interest to compare DRG-matched patients in the RAST and NAST groups with respect to impact on mortality, length of stay in the hospital, costs, and orders and timing for antimicrobial agents.
The mortality rate for the RAST group was 7.9%; that for the NAST group was 9.6% (the difference was not statistically significant). The average lengths of stay for all inpatients at Memorial Medical Center for various months in 1997 were as follows: June, 5.93 days; July, 6.23 days; August, 6.15 days; September, 6.06 days; October, 6.04 days; November, 6.28 days; and December, 5.96 days. The average length of stay of a patient in the RAST group was 10.7 days; that of a patient in the NAST group was 12.6 days. This was a statistically significant difference of 2.0 days less for the RAST group (P = 0.006). The average total cost for a patient was $13,227 for the RAST group and $15,622 for the NAST group. This was a statistically significant difference of $2,395 less per patient for the RAST group (P = 0.04). The average variable cost for a patient was $4,927 for the RAST group and $6,677 for the NAST group. This was a statistically significant difference in variable costs of $1,750 less per patient for the RAST group (P = 0.001) (Table 1).
TABLE 1.
Summary of parameters examined for patients in RAST and NAST groups
| Parametera | Mean for RAST group | SD for RAST group | Mean for NAST group | SD for NAST group | Mean difference | 95% Confidence interval
|
|
|---|---|---|---|---|---|---|---|
| RAST group | NAST group | ||||||
| Turnaround time (h) (1282, 2231) | 39.2 | 14.0 | 44.4 | 12.7 | 5.2b | 38.5, 40 | 43.9, 44.9 |
| Mortality rate (%) (242, 523) | 7.9 | 27.0 | 9.6 | 29.4 | 1.7c | 4.4, 11.3 | 7, 12.1 |
| Length of stay (days) (242, 523) | 10.7 | 7.5 | 12.6 | 12.1 | 2.0d | 9.7, 11.6 | 11.6, 13.7 |
| Average total cost ($) (242, 523) | 13,227 | 13,321 | 15,622 | 18,163 | 2,395e | 11,541, 14,914 | 14,062, 17,182 |
| Average variable cost ($) (242, 523) | 4,927 | 5,660 | 6,677 | 8,385 | 1,750b | 4,210, 5,644 | 5,956, 7,397 |
Numbers in parentheses are number of patients in RAST group, number of patients in NAST group.
P = 0.001.
P = 0.45.
P = 0.006.
P = 0.04.
The most logical explanation for the differences between the two groups was that physicians had access to crucial bacterial identification and AST data earlier for the RAST group and were able to order appropriate antimicrobial therapy earlier for patients in this group. Therefore, a review of the medical records for 75 patients in the RAST group and 75 patients in the NAST group was done to determine whether physicians prescribed appropriate antimicrobial therapy sooner for patients in the RAST group. Our focus was the earliest possible time that physicians could respond to the result of AST on the written cumulative report, which would be within 48 h of receipt of the sample in the laboratory. If the earlier reporting of AST results for the RAST group were to have an effect, one would expect to see appropriate antimicrobial therapy started earlier for patients in the RAST group. In fact, the percentage of the patients for whom appropriate antimicrobial therapy was initiated within 48 h of receipt of their samples in the laboratory was 94% for the RAST group but only 77% for the NAST group. This difference between patients in the RAST and NAST groups was statistically significant (P = 0.006).
DISCUSSION
Because managed care often results in the downsizing of clinical microbiology laboratories, it is essential that the contribution of microbiology data to patient outcomes be recognized by administrators (3). This study confirms the clinical and financial benefits of rapid reporting of bacterial identification and AST results in the setting of a community teaching hospital. By having a technologist on the evening shift verify the results of microbiology reports not available by the end of the day shift, bacterial identifications and AST were available in the LIS and in the cumultative reports sooner than they had been previously. This, in turn, resulted in physicians having access to crucial information sooner, enabling them to initiate antimicrobial therapy or switch to a more appropriate antimicrobial agent sooner. Statistically significant decreases in turnaround time, length of stay, and hospital costs resulted. Of particular note is the statistically significant difference in variable costs of $1,750 less per patient for patients in the RAST group (P = 0.001). Administrators consider these variable costs responsible for the actual cost savings realized by the hospital.
Each year our hospital has an estimated 2,394 different inpatients for whom AST is done. By having a technologist verify data on a later shift, the hospital could expect to save $4,189,500 per year ($1,750 in variable cost savings per patient × 2,394 patients).
Analysis of a subset of the departmental costs included in the overall and variable costs also showed the same trend, that of decreased costs associated with patients in the RAST group. Namely, the average cost of pharmacy services was $811 for the RAST group and $1,196 for the NAST group, a difference of $385 less for the RAST group. The average cost of imaging was $285 for the RAST group and $354 for the NAST group, a difference of $69 less for the RAST group. The average costs of laboratory and microbiology for the RAST group were $592 and $92, respectively; for the NAST group they were $633 and $103, respectively.
A large prospective study by Doern and colleagues (5), who used techniques similar to ours, showed a cost savings of $4,194 per patient for their RAST group; our study showed a cost savings of $2,395 per patient. Explanations for this difference include the facts that their study and control groups differed from ours and that different components possibly went into the category of “total costs” at the two medical centers.
Interestingly, Doern et al. (5) found no significant differences in the length of stay between his NAST and RAST groups, but our study did show a statistically significant decrease in the length of stay for patients in the RAST group.
Doern et al. (5) showed a statistically significant decreased mortality rate for patients in their RAST group; we did not. Mortality rates for our patients in both the RAST and NAST groups were in the same range as that for patients in their RAST group (8.8%). Other than examining mortality rates, our study did not examine clinical outcomes directly, but an indirect assessment of this is length of stay. If a patient is discharged from the hospital sooner, it is reasonable to assume that the earlier discharge reflects a more favorable clinical outcome or lack of disease. If patients in the RAST group and a given DRG have decreased lengths of stay, one can assume that they got better faster than patients in the NAST group who had longer lengths of stay.
The most likely reason for the clinical and financial benefits for the RAST group was that physicians were able to switch to appropriate antimicrobial therapy sooner for the RAST group. Appropriate antimicrobial therapy was ordered earlier for more patients in the RAST group than patients in the NAST group, and this difference was statistically significant. These findings are consistent with the results of a study by Schifman et al. (10) that evaluated the impact of early intervention on patients with discordant AST results and antibiotic therapy.
The procedures and work flow used in this study are fairly typical of those used in other, similar institutions. A critical reassessment of the routine work flow and a definition of priorities and goals by the microbiology staff were necessary to accomplish rapid reporting of information from the microbiology laboratory. Most likely, the aspect that facilitated the impact of this project was the fact that physicians had more microbiology reports on their morning rounds. Despite the fact that results from the microbiology laboratory are available in the LIS several hours sooner than when they appear on the written cumulative report, in our hospital, most attending physicians do not access this information during the day unless their patient is critically ill. This is because there is no set time for new information to become available, so busy clinicians would have to interrupt their schedules several times a day to gain access to new LIS data for their patients. This would be extremely time-consuming and therefore unfeasible. In addition, most attending physicians do not even have access to the hospital LIS during the day because their offices do not have on-line access to the hospital LIS. Even in teaching hospitals, many residents who are based within the hospital often do not frequently access the LIS during the day for microbiology reports; they often rely solely on cumulative reports for the majority of their information from the microbiology laboratory.
The change in work flow necessary to verify evening Vitek system reports had the disadvantage that technologists on the evening shift needed to be trained and available for approximately 1 h each night to verify the reports. However, this disadvantage was offset by the impressive clinical and financial gains. Fortunately, it is not necessary to have an experienced microbiologist verify evening reports from the Vitek system. No problems were encountered as a result of having a technologist who was not a microbiologist verify the evening reports.
In summary, work-flow techniques were altered so that bacterial identification and AST results were verified from the Vitek system on the first day that they were available. This caused a turnaround time for reports on bacterial identification and AST to be an average of 5.2 h faster than usual (P = 0.001). This resulted in the majority of the bacterial identification and AST results being printed on the cumulative report within 48 h of receipt of the sample in the laboratory. Subsequently, physicians were able to initiate appropriate antimicrobial therapy statistically sooner. Statistically significant differences resulted, namely, a decreased length of stay of 2.0 days per patient and a decreased variable cost of $1,750 per patient for the RAST group, thus potentially saving the hospital over $4 million per year.
ACKNOWLEDGMENT
We gratefully acknowledge the work of Jennifer Dietrich.
REFERENCES
- 1.Barenfanger J, Drake C, Kacich G. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Benefits of rapid reporting in microbiology, C-461; p. 208. [Google Scholar]
- 1a.Barnes M P. Influence of laboratory reports on prescribing of antimicrobials for urinary tract infection. J Clin Pathol. 1980;33:481–483. doi: 10.1136/jcp.33.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campo L, Mylotte J M. Use of microbiology reports by physicians in prescribing antimicrobial agents. Am J Med Sci. 1988;296:392–398. doi: 10.1097/00000441-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Check W. Managed care deeply affecting clinical microbiology. ASM News. 1998;64:495–500. [Google Scholar]
- 4.Dawson-Saunders B, Trapp R. Biostatistics. 2nd ed. Norwalk, Conn: Appleton and Lange; 1994. [Google Scholar]
- 5.Doern G, Vautour R, Gaudet M, Levy B. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol. 1994;32:1757–1762. doi: 10.1128/jcm.32.7.1757-1762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granato P A. Impact of same day tests versus traditional overnight testing. Diagn Microbiol Infect Dis. 1993;16:237–243. doi: 10.1016/0732-8893(93)90116-o. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz L, editor. St. Anthony’s diagnosis related group guidebook for 1997. Reston, Va: St. Anthony’s Publishing Inc.; 1997. [Google Scholar]
- 8.Lundberg G D. Acting on significant laboratory results. JAMA. 1981;245:1762–1763. doi: 10.1001/jama.1981.03310420052033. [DOI] [PubMed] [Google Scholar]
- 9.Matsen J. Rapid reporting of results—impact on patients, physicians, and the laboratory. In: Tilton R C, editor. Rapid methods in automated microbiology. Washington, D.C: American Society for Microbiology; 1981. pp. 98–102. [Google Scholar]
- 10.Schifman R, Pindur A, Bryan J A. Laboratory practices for reporting bacterial susceptibility tests that affect antibiotic therapy. Arch Pathol Lab Med. 1997;121:1168–1170. [PubMed] [Google Scholar]
- 11.Trenholme G M, Kaplan R L, Karakusis P H, Stine T, Furher J, Landan W, Levin S. Clinical impact of rapid identification and susceptibility testing of bacterial blood culture isolates. J Clin Microbiol. 1989;27:1342–1345. doi: 10.1128/jcm.27.6.1342-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]